Abstract
Background
Impulsivity is a multifaceted construct that has recently been recognized as a factor contributing to enhanced vulnerability to drug abuse.
Objectives
In the present review, we focus on two facets of impulsivity (and tasks that measure them): (1) impulsive choice (delay discounting task) and (2) inhibitory failure (go/no-go, stop signal reaction time, and five-choice serial reaction time tasks). We also describe how performance on each of these tasks is associated with drug-related behavior during phases of drug abuse that capture the essential features of addiction (acquisition, escalation, and reinstatement of drug-seeking after drug access has terminated). Three hypotheses (H) regarding the relationship between impulsivity and drug abuse are discussed: (1) increased levels of impulsivity lead to drug abuse (H1), (2) drugs of abuse increase impulsivity (H2), and (3) impulsivity and drug abuse are associated through a common third factor (H3).
Conclusion
Impulsivity expressed as impulsive choice or inhibitory failure plays a role in several key transition phases of drug abuse. There is evidence to support all three nonexclusive hypotheses. Increased levels of impulsivity lead to acquisition of drug abuse (H1) and subsequent escalation or dysregulation of drug intake. Drugs of abuse may increase impulsivity (H2), which is an additional contributor to escalation/dysregulation. Abstinence, relapse, and treatment may be influenced by both H1 and H2. In addition, there is a relationship between impulsivity and other drug abuse vulnerability factors, such as sex, hormonal status, reactivity to nondrug rewards, and early environmental experiences that may impact drug intake during all phases of addiction (H3). Relating drug abuse and impulsivity in phases of addiction via these three hypotheses provides a heuristic model from which future experimental questions can be addressed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A growing number of studies using behavioral, neurobiological, and imaging techniques have confirmed a strong association between impulsivity and addictive behaviors such as drug abuse, binge eating, and gambling. The purpose of this review is to analyze the relation between impulsive behavior and drug abuse, an addictive behavior that has been studied most extensively. The main goals of this review are (1) to highlight the main measures of impulsive behavior that have been used in studies that have increased our knowledge of the connection between impulsivity and drug abuse, (2) to show how impulsivity may drive drug-seeking behavior during critical phases of drug abuse that are hallmarks of addiction, and (3) to examine fundamental questions about impulsivity and drug abuse in terms of three hypotheses (H): H1, impulsivity causes drug abuse; H2, drug abuse causes impulsivity; and H3, impulsivity is related to a third factor that controls drug abuse. To date, research findings concerning the relationship between impulsivity and drug abuse have been related to these three main hypotheses. It is essential to evaluate the results to date, select strong and consistent findings, and establish principles that will allow us to advance in designing future studies to further probe the key issues/hypotheses that are identified in this review.
A variety of definitions and measures of impulsivity have been used, and an analysis of the causes and consequences of impulsivity in drug abuse must take the operational definitions of impulsivity into account. Accordingly, impulsivity has been defined as the inability to stop a behavior that has negative consequences, preference for immediate over delayed gratification, tendency to engage in risky behaviors, heightened novelty-seeking, behaving without forethought or consideration of outcomes, being impatient when asked to wait, having a short attention span, and difficulty persisting at a particular activity (e.g., for reviews, see Evenden 1999; Mitchell 2004; Olmstead 2006). A variety of tools, such as questionnaires and operant conditioning tasks (i.e., tasks that require a subject to make a response, such as a lever press or key strike, to receive a contingent reinforcer), have been used to provide operational definitions of impulsivity, and there is not always reliability among these measures (e.g., Barratt and Patton 1983; Crean et al. 2000). This suggests that impulsivity is multidimensional and consists of several different and possibly independent features (e.g., Barratt and Patton 1983; Evenden 1999). There are no comprehensive animal or human laboratory models that take all features of impulsivity into account, but individual laboratory measures of many of the elements of impulsivity have been successfully used to examine the connection between impulsivity and drug abuse. Two facets of impulsivity seem to predominate in the drug abuse literature (Olmstead 2006), impulsive choice (choice of a small, immediate reinforcer over a large, delayed reinforcer) and impaired inhibition (inability to stop a prepotent behavior), and these will be the focus of our review.
Operational measures of impulsivity
This section describes the attempts to operationally define impulsive choice and impaired inhibition, and it demonstrates how various measures agree and translate from animals to humans. Operational tasks may measure a more circumscribed definition of impulsivity than questionnaires (Mitchell 1999), and this may be the reason that there is not always a consistent relationship between self-report and operational measures (e.g., de Wit et al. 2000; Reynolds et al. 2006a; Swann et al. 2002). However, it is also possible that limitations in individuals’ abilities to report the cognitive processes underlying their behavior (Nisbett and Wilson 1977) play a role in the inconsistency between self-report and operational measures of impulsivity. There are three main features of operational measures of impulsivity that allow for valid translation from human to animal subjects. First, the underlying processes needed for these tasks, such as attention and working memory, are present in both humans and animals. Second, the tasks should be relatively easy to administer to either species, and in many cases, they should require only a modest amount of training. Third, these tasks should measure the subject’s current state and not rely on introspective measures or recall of past events. The ability of these tasks to assess the subject’s current state is important because it allows for monitoring changes in impulsivity during various states of drug use.
Performance on laboratory measures of impulsive choice and inhibition are not necessarily related in humans (Dalen et al. 2004; Solanto et al. 2001; Sonuga-Barke 2003; Sonuga-Barke et al. 2003) or rats (Van den Bergh et al. 2006). There are most likely individual differences in the extent to which these two manifestations of impulsivity are present. An important focus of ongoing research is to identify how these operational measures relate to each other (if at all) and to determine what specific mechanisms underlie performance on each task. Thus, when studying impulsivity, it is important to use several models to obtain converging evidence regarding the underlying aspects of impulsivity that are associated with the behavior of interest. In this review, the delay discounting (DD) task will be discussed as an operational measure of impulsive choice, and the go/no-go, stop signal reaction time (SSRT), and five-choice serial reaction time (5CSRT) tasks will be discussed as operational measures of inhibitory control.
Impulsive choice
Drug abuse has been conceptualized as choosing a smaller, immediate reinforcer (e.g., immediate euphoric effects) over a larger reward that occurs in the future, such as good health, good relationships, or occupational success (de Wit and Richards 2004; Madden et al. 1997). In other words, drug abuse may occur because the value of the delayed consequences of drug abstinence is discounted or decreased in favor of the immediate drug effects. This aspect of impulsivity has been studied in animals and humans using DD procedures and with that task comparable results have been obtained across species (see review by de Wit and Richards 2004).
Delay discounting
In the DD paradigm, a subject is typically asked to choose between a small reinforcer delivered immediately and a larger reinforcer delivered after a delay. Human subjects are shown two options on a computer screen and asked to respond on the computer key that is associated with the reinforcer that they would prefer. The reinforcers are typically monetary, although hypothetical health outcomes (e.g., the onset of a serious drug-related illness) and drug use (e.g., Madden et al. 1997; Petry 2001) have also been used. For a discussion of the differences between discounting of hypothetical or actual rewards, see Bickel and Marsch (2001) and Kirby (1997). If actual reinforcers are used, one trial is chosen at random at the end of the session and the participant receives the reinforcer they chose on that particular trial. Immediate reinforcers are given at the end of the session, and delayed reinforcers are given after both the session and the specified delay has elapsed. In preclinical research, animals are trained to make an operant response, such as a lever press, to obtain a food, water, or drug reinforcer. A response on one device yields a small magnitude of the reinforcer immediately, whereas a response on the other device yields a larger magnitude of the reinforcer after a delay. In contrast to human research, reinforcers are given after each trial.
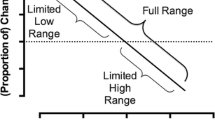
In both human and animal research, the delay at which the small, immediate reinforcer and the large, delayed reinforcer are chosen equally can be calculated, and it is referred to as the indifference point. To find an indifference point, the amount of the large reinforcer and the delay to its delivery are held constant and the amount of the small, immediate reinforcer is varied until subjects choose each alternative approximately equally (e.g., Madden et al. 1997; Richards et al. 1999b). The delay to the larger reinforcer is then changed and another indifference point is obtained. Graphing a typical plot of four to six indifference points on an x–y coordinate where the measure of the relative value of the reward (y-axis) is plotted as a function of the delay to the larger reinforcer (x-axis) yields a hyperbolic curve (see Fig. 1). This function is described by the equation V = A/(1 + kD) where V is the value of the reinforcer, A is the amount of the delayed reinforcer, D is the delay to the reinforcer, and k is used to describe the steepness of the curve (Mazur 1987). As the gradient of the curve becomes steeper, impulsivity increases (preference for the smaller immediate reinforcer increases). The discounting rate arises from a combination of the type of commodity offered (e.g., monetary, drug of choice, heath outcomes), whether the outcome is a loss or a gain, and the magnitude of the outcome (Baker et al. 2003; Bickel and Marsch 2001). The shape of the discount functions obtained in rats and humans are remarkably similar (i.e., both are hyperbolic), despite different reinforcer types, magnitudes, and delays (de Wit and Richards 2004); however, the discounting rate varies across species.
An example of the type of hyperbolic curve created in DD experiments. The curve is created by the equation V = A/(1 + kD) where V is value, A is the amount of the delayed reward, D is the delay to the reward, and k is used to describe the steepness of the curve. The y-axis represents the indifference point or the value of the smaller (or inferior) reinforcer at which both reinforcers were chosen equally
Performance on DD in pigeons and rodents is typically impulsive (Mazur and Logue 1978), whereas choice in human adults is typically more self-controlled (Logue 1988; Logue et al. 1986). Initial work with nonhuman primates (rhesus monkeys) self-administering i.v. cocaine suggests a low discounting rate with significant self-control that is comparable to humans (Woolverton et al. 2007). It is also apparent that animals and humans have differential sensitivities to changes in reinforcer magnitude. In humans, increasing reinforcer magnitude has consistently decreased impulsive choice (e.g., Baker et al. 2003; Johnson and Bickel 2002). However, results from animal studies are inconsistent. Increasing the amount of the delayed reinforcer decreased impulsive choice (Wade et al. 2000), had no effect (Green et al. 2004; Perry et al. 2007; Richards et al. 1997), or increased impulsive choice (Farrar et al. 2003; Woolverton et al. 2007).
Impaired inhibition
The go/no-go (Newman et al. 1985), SSRT (Logan et al. 1984), and 5CSRT (Carli et al. 1983; Robbins 2002) tasks have been used to measure inhibitory control. The go/no-go and SSRT tasks are similar in that they both measure inhibition of a prepotent response; however, the difference between them is that the go/no-go task requires subjects to either execute (go) or inhibit (no-go) a response and the SSRT task requires subjects to inhibit a response they have already initiated (stop). In the 5CSRT task, subjects are required to suppress responses until a stimulus signals that it is appropriate to respond. In studies not involving drugs, performance on the go/no-go and SSRT tasks is positively correlated in humans (r = 0.278, Reynolds et al. 2006a), suggesting that the tasks measure common underlying features. An advantage of the go/no-go and SSRT tasks is that they have been widely used in both human and nonhuman subjects, and the results have been concordant across species (e.g., de Wit and Richards 2004). A variant of the 5CSRT task has been used in humans (e.g., Sahakian et al. 1993); however, performance on this task has not yet been compared to other tasks or across species. It would be useful to conduct these experiments in an effort to understand whether these tasks measure similar aspects of inhibitory control. All three inhibitory tasks were sensitive to the effects of drugs of abuse (e.g., de Wit and Richards 2004; Fillmore 2003; Robbins 2002).
Go/no-go task
In the go/no-go task (Fig. 2), responding in the presence of a specific discriminative stimulus is reinforced. For example, human participants are shown repeated presentations of eight different numbers, four of which are designated “correct” numbers and the other four are designated “incorrect” numbers. They are required to respond to correct stimuli (go) and withhold responses to incorrect stimuli (no-go). Correct responses are reinforced with points, money, or social reinforcers and incorrect responses are either unreinforced or penalized. Similarly, in rats, a light or tone is typically the discriminative stimulus and a lever press is the appropriate behavior after the presentation of the discriminative stimulus to obtain a food reward. Food-restricted animals are reinforced with a food pellet after an appropriate response to a go stimulus and not reinforced during the no-go stimulus condition. Typically, human studies employ discrete-trial go/no-go tasks and animal studies use either continuous- or discrete-trial go/no-go tasks. In both continuous- and discrete-trial go/no-go tasks, errors of omission (withholding a response when a correct stimulus is presented) and errors of commission or “false alarms” (responding when an incorrect stimulus is presented) are the dependent measures. Impulsivity in this task is defined by the number of false alarms. Reaction time (RT) or the time it takes to make a response (response latency) can also be measured in this task, and that is another measure used to determine whether there are nonspecific effects of a particular manipulation on the time needed to activate a response.
Stop signal reaction time task
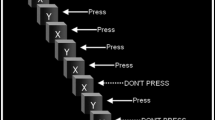
The SSRT task (see Fig. 3) measures the speed (or RT) at which subjects are able to inhibit a response that was previously maintained by a reinforcer (the stop RT). The stop RT is compared to the speed at which subjects perform a response (the go RT). This task is based on a “race” theory of impaired inhibition in which there is a ‘go’ process (the initial reaction time to execute a response) and a ‘stop’ process (the time needed to inhibit the response) and the ability to inhibit a behavior is a competition or race between these two processes (Logan et al. 1984). In human research, subjects are typically asked to respond on a keyboard as quickly as possible when a go signal (e.g., a specific letter of the alphabet) is presented on a computer screen. When a stop signal (e.g., a tone) is presented shortly after presentation of the go signal, subjects must inhibit the response they have begun or perform a different response (e.g., press a different key on the keyboard) to acknowledge the stop signal. In animal research, subjects perform one response (e.g., a lever press, nose poke) when a go signal (e.g., a light) is presented and a different response (e.g., press a different lever, nose poke) when a stop stimulus (e.g., a tone) is presented.
Diagram of the SSRT task. Subjects are asked to respond as quickly as possible after a go signal. The go RT is the time between the go signal presentation and the subject’s response. On go/stop trials, subjects must inhibit their response or perform a different response after presentation of the stop signal. The go–stop signal interval is adjusted until the subject inhibits its responses on 50% of the trials, and the stop RT is calculated by subtracting the go–stop signal interval from the Go RT
In both human and animal research, the stop signal is randomly presented after the go signal on a proportion of trials. Reliably presenting the go signal on every trial produces a prepotency to respond, and subjects must overcome this tendency to suppress responses when the stop signal is presented. Impulsivity in this task is quantified as the stop RT.
Five-choice serial reaction time task
In the 5CSRT task (see Fig. 4), animals are required to withhold responding until a stimulus is presented that indicates that it is appropriate to respond (i.e responses will be reinforced). Each trial is initiated by a head-insertion response into a food magazine. After an intertrial interval (ITI), a brief stimulus light appears at the rear of one of five holes (Fig. 4 shows only activity at the active hole and its consequences). A nose-poke response in the hole in which the stimulus light appeared results in the presentation of food pellets in the food magazine. Errors of omission (failure to respond after the presentation of the light stimulus), errors of commission (responding in a hole that did not have a stimulus light on), and premature responses (responding during the ITI) are punished by a brief time out. After the time out, a subsequent trial can be initiated by a head insertion into the food magazine. Premature responses are used as the measure of impulsivity because they are considered to be inhibitory failures of prepotent responses.
Diagram of the 5CSRT task (adapted from Robbins 2002). Trials are initiated by a head insertion into a food magazine. After an intertrial interval, a stimulus light appears and a response is required within a specified time limit. Impulsivity is defined as the number of prepotent responses emitted
The effects of impulsivity during key phases of addiction
Tasks measuring both impulsive choice and impaired inhibition have been used to address the hypotheses (which are not mutually exclusive) regarding the relationship between impulsivity and drug abuse; for example, H1—that increased levels of impulsivity could lead to drug abuse and H2—that drugs of abuse may increase impulsivity during several key transition phases of the addiction process such as acquisition, escalation/dysregulation, abstinence, relapse, and treatment (see Fig. 5). The measures of impulsivity described above are especially sensitive to these critical transition phases of drug abuse. It is important to consider the relationship between impulsivity and drug-related behavior in each phase separately because addiction is a multistep, evolving process for which we have excellent laboratory models that are providing consistent findings across laboratories and experimental conditions. It should be noted that H1 and H2 are not mutually exclusive, and they can cooccur to various degrees in each of the phases.
Phases of drug abuse (adapted from Carroll et al. 2004). Impulsivity expressed as impulsive choice or inhibitory failure may play a role in each of these phases. In the acquisition phase, it is likely that increased levels of impulsivity lead to drug abuse (H1). Drugs of abuse may increase impulsivity (H2), resulting in escalation/dysregulation of drug intake, and the increases in impulsivity caused by drugs of abuse may continue despite drug cessation, resulting in shorter abstinence, reduced likelihood of treatment success, and greater likelihood of relapse. Individual differences in impulsivity may also influence abstinence, treatment, and relapse (H1). Alternatively, impulsivity and drug abuse may be associated through a third factor that influences drug intake during all phases of addiction (H3)
One phase we will not discuss in this review is the maintenance phase, because maintenance is typically studied under very limited access conditions, and impulsivity does not appear to be related to the patterns of steady, regular drug intake that occur under these conditions. For example, DD in rodents was not associated with differential drug intake under a fixed-ratio (FR) 1 schedule (Perry et al. 2008a) or a progressive-ratio (PR) schedule (Anker et al., in preparation). In this review, the critical transition phases that most reliably predict drug abuse are highlighted. In the first example, initiation of drug-taking behavior, we provide evidence that H1 is most applicable to explain how impulsive behavior predicts drug abuse. In the next phase, escalation, H1 and H2 are both relevant to escalation of drug intake, and finally, both H1 and H2 play a role in abstinence, relapse, and treatment. In addition, H3—impulsivity and drug abuse are associated through a third factor will be discussed in a subsequent section providing initial evidence on how other factors predisposing drug-seeking and drug-taking are related to impulsivity during key phases of addiction.
Acquisition
Acquisition of drug self-administration is defined as the transition from a single, initial drug use to continued, regular daily or weekly abuse (e.g., Campbell and Carroll 2000). This phase can be uniquely modeled in drug-naïve animals, and the hypothesis that impulsivity predates drug use (H1) may be the most applicable in this phase. For example, animals can be behaviorally screened as high or low on measures of impulsivity, and subsequent initiation of drug-taking can be studied. This would model human behavior in which individuals may make the impulsive choice to initiate drug use because they value the immediate euphoric effects of a drug over larger future benefits, such as personal, educational, social, and economic success or well-being (de Wit and Richards 2004; Madden et al. 1997). Alternatively, individuals with impaired inhibitory control may be unable to resist environmental cues (e.g., peer pressure) that lead them to abuse drugs (de Wit and Richards 2004).
Impulsive choice
Humans Consistent with theories that individuals may begin to abuse drugs because of greater devaluation of delayed rewards (H1), in a longitudinal study, impulsive choice at age 3 was correlated with adolescent social and personal problems that are associated with substance abuse, although it is important to note that other factors may have played a role (H3; Mischel et al. 1988; Wills et al. 1995). In humans, it is impossible to determine which came first, impulsive behavior or drug abuse (H1 vs H2); but there are correlational findings to suggest that impulsive behavior and drug abuse are related. Individuals who discounted delayed reinforcers began abusing alcohol, marijuana, and cigarettes at a younger age, and they abused a greater number of illicit drugs compared to individuals who discounted delayed reinforcers less (Kollins 2002). Adolescence is a period of enhanced impulsivity, risk-taking, and novelty-seeking behavior, and it is also a time when individuals are highly vulnerable to addiction (for reviews, see Chambers et al. 2003; Kelley et al. 2004). Higher levels of discounting were found in adolescent smokers compared to nonsmokers (Audrain-McGovern et al. 2004; Reynolds et al. 2007, but see Reynolds et al. 2003) and in heavy adolescent alcohol drinkers compared to light adolescent drinkers (Field et al. 2007). Adolescents who discounted more had lower grades and self-esteem and greater involvement with cigarettes, alcohol, and marijuana (Wulfert et al. 2002).
Several researchers have reported that, similar to adolescents, adult drug abusers discounted delayed reinforcers more than nonusers. For instance, opioid-dependent individuals discounted delayed monetary reinforcers more than nonusers (Kirby and Petry 2004; Kirby et al. 1999; Madden et al. 1997), and opioid-dependent individuals who shared needles discounted delayed reinforcers to an even greater extent than their opioid-dependent counterparts who did not share needles (Odum et al. 2000). Similarly, heavy social drinkers (Vuchinich and Simpson 1998) and alcoholics (Petry 2001, but see Kirby and Petry 2004) discounted monetary reinforcers more than light social drinkers and nonalcoholics, respectively. Cocaine abusers (Coffey et al. 2003; Heil et al. 2006; Kirby and Petry 2004), methamphetamine abusers (Hoffman et al. 2006; Monterosso et al. 2007), and cigarette smokers (Baker et al. 2003; Bickel et al. 1999; Heyman and Gibb 2006; Mitchell 1999; Reynolds et al. 2004b, but see Johnson et al. 2007; Ohmura et al. 2005) also discounted future monetary rewards to a greater extent than nonusers. To address H2 in humans, that drug ingestion elevated impulsive behavior, it would be necessary to show that elevated impulsivity during use returned to and remained at lower levels (equivalent to nonusers) in ex-users (e.g., Bickel et al. 1999). In contrast, a finding of continued elevation of impulsivity in ex-users may support H1 or H2, as an innate impulsivity trait and/or the previous drug use may be responsible. Even when longitudinal studies suggest H1, the possibility that a third factor covaries with impulsivity and drug abuse (H3) would also need to be carefully examined.
Animals
Preclinical models offer the longitudinal measures that are useful for distinguishing between H1 and H2 by studying the relationship between initial DD and subsequent initiation of drug intake. In carefully controlled laboratory studies using rodents, DD can be measured and compared to subsequent rates of drug self-administration. In one study, rats were allowed to choose between two food pellets delivered immediately and 12 pellets delivered after a 15-s delay (Poulos et al. 1995). Rats that selected the small, immediate reward on at least 75% of the trials (high impulsive) subsequently consumed more of a 12% ethanol solution than rats that chose the small, immediate reinforcer on 45–60% (medium impulsive) or 5–30% (low impulsive) of the trials (Poulos et al. 1995). In a second study, impulsive choice determined by a DD procedure predicted the subsequent rate of acquisition of cocaine self-administration. Rats selected for high impulsivity (HiI) acquired cocaine self-administration in greater numbers and at a faster rate than those selected for low levels (LoI) of impulsivity (Perry et al. 2005, 2008a). Together, these data support the hypothesis that impulsive choice predicts vulnerability to acquisition of drug intake (H1). To address H2, that drug self-administration increases impulsivity, a longitudinal study could be proposed in which groups with equal responding on a DD task for food would then be exposed to drug or vehicle self-administration, and they would later be retested on the DD food procedure when drug access had terminated. An increase in impulsivity in the drug-exposed group (vs vehicle-exposed) would indicate a change in impulsivity due to drug experience.
Impaired inhibition
Animals Impaired inhibition is also related to the propensity to initiate drug use. That is, current drug abusers have impaired inhibition compared to nonusers. For example, cocaine (Fillmore and Rush 2002; Li et al. 2006) and methamphetamine (Monterosso et al. 2005) abusers displayed inhibitory deficits on the SSRT task compared to nonusers. Cocaine users (Hester and Garavan 2004; Kaufman et al. 2003; Verdejo-Garcia et al. 2007) and alcoholics (Noel et al. 2007) also showed inhibitory deficits on a go/no-go task compared to controls; however, there were no differences between 3,4-methylendioxymethamphetamine (MDMA) or cannabis users and nonusers (Quednow et al. 2006). Cigarette smokers also showed decreased inhibitory control compared to nonsmokers, and the number of packs smoked per day was positively related to the number of inhibitory failures (Spinella 2002). In another study, no differences were found between smokers and nonsmokers (Dinn et al. 2004), but the lack of effect may have been due to low levels of participant smoking. Again, these correlational findings do not explain the causality of the results (H1 vs H2), and they emphasize the need for within-subject comparisons of users/ex-users vs nonusers that could be applied to H2 or a longitudinal examination of impulsivity before the onset of drug abuse that could be applied to H1. The results of the studies described above could also have been explained by a third factor (H3) such as cognitive deficits in working memory (Hester and Garavan 2004) and decision-making (Bechara et al. 2001) that may contribute to impaired inhibition in drug abusers.
Animals
Preclinical studies may be useful in determining the relationship between inhibitory control and other cognitive processes in the acquisition phase. In a recent preclinical study, rats selected for HiI based on 5CSRT task performance subsequently made significantly more responses in the nicotine-paired hole during acquisition of nicotine self-administration compared with LoI rats (Diergaarde et al. 2008). In another study, HiI rats (selected on the 5CSRT task) subsequently self-administered larger amounts of cocaine than LoI rats (Dalley et al. 2007a). Converging evidence also indicated that HiI rats also had fewer D2 dopamine receptors (vs LoI rats) in the ventral striatum, a part of the neurocircuitry thought to be involved in reward, movement, and response to novelty. However, caution may be warranted, as studies showing that impulsivity predicts cocaine self-administration (Dalley et al. 2007a; Perry et al. 2005) may be subject to alternative interpretations. For example, HiI rats may have greater reactivity to novelty or food-associated discriminative or conditioned/reinforcing stimuli (Uhl 2007)—other factors that are associated with cocaine self-administration (H3). Nevertheless, in both studies, neither impulsivity nor cocaine self-administration were correlated with locomotor activity (Dalley et al. 2007a; Perry et al. 2005). Together, the above sections provide preclinical evidence for the hypothesis that increased levels of impulsivity (expressed as both impulsive choice and inhibitory failure) predict vulnerability to drug abuse in the acquisition phase (H1), and methods for testing H1 and H2 in humans are suggested.
Escalation/dysregulation
Escalation or dysregulation of drug use is another critical phase and a hallmark of addictive behavior. It is thought to represent the switch from ‘control’ to ‘loss of control’ in addiction (e.g., Koob and Kreek 2007; Koob and Le Moal 2001). This phase is defined as a transition from low levels of controlled, regulated drug use to uncontrolled, dysregulated large levels of intake (Ahmed and Koob 1998, 1999) and binge drug use (Shaffer and Eber 2002), and it may also be driven by H1 or result in H2. In preclinical studies, this phase is typically modeled in animals by allowing extended access to drug self-administration (e.g., 5–12 h/day) because the pattern of drug self-administration that emerges approximates the features of bingeing or “out of control” drug-taking mentioned above (Ahmed and Koob 1998, 1999). An escalating or dysregulated pattern of drug abuse could reflect increased impulsivity attributable to the acute or chronic effects of the drug (H2), and chronic drug effects may be different in individuals with high and low baseline levels of impulsivity (H1).
Escalation/dysregulation may occur because of a change in the ability to control responses, so that increased drug use is accompanied by decreased inhibitory control. Escalated/dysregulated patterns may also reflect a relative increase in the value of the immediate rewards of drug use compared to the delayed rewards associated with drug abstinence. In fact, when motivation for drugs was assessed by a PR schedule, dose–response functions (the willingness to work for a given dose) were elevated after escalation (Carroll et al. 2004; Roth and Carroll 2004; Wee et al. 2007), indicating increased reward value or decreased inhibitory control. It is important to consider whether impulsive individuals are more prone than those who are less impulsive to escalation of drug use (H1) and whether drug use increases impulsivity (H2) that leads to escalation because this phase is one of the most devastating in the development of drug abuse and it is where irreversible changes in patterns of drug-taking occur. We are unaware of studies of the effects of escalation of drug self-administration on impulsivity that would address H2 specifically. The possibility that acute or chronic administration of drugs may result in increased impulsivity has been examined in both humans and animal models and is discussed in subsequent sections. In addition, acute or chronic administration of therapeutic drugs that reduce impulsivity could be a means of preventing the escalation of drug self-administration and ultimately limiting the progression of this addictive behavior. Currently, there are only a few examples of attempts to limit the escalation process. For example, a corticotropin-releasing factor 1 (CRF1) receptor antagonist (Specio et al. 2008) and progesterone (Larson et al. 2007) reduced the escalation of i.v. cocaine self-administration in rats. Nondrug substitutes for drug also reduce escalation. Lenoir and Ahmed (2008) reported that access to sweetened water reduced escalated heroin consumption. The application for further studies of drug abuse is that prevention efforts could involve screening for impulsivity with standard, computerized tests for impulsive choice or inhibitory failure, and targeted prevention/intervention efforts could then be implemented.
Impulsive choice
We are unaware of any studies that have been conducted in humans to examine the relationship between impulsive choice and escalation/dysregulation of drug intake. Recent preclinical research with rats selected as HiI or LoI using a DD task with food reinforcers showed that HiI rats significantly escalated cocaine intake over repeated daily 6-h sessions compared with LoI rats that did not show significant escalation (see review by Carroll et al. 2008a). In addition, the HiI rats (but not LoI rats) also showed a significant pre–post increase in cocaine infusions during short-access (2 h) periods that occurred before and after the long access. These results were consistent with the acquisition data that showed greater acceleration of cocaine intake in HiI (vs LoI) rats (Perry et al. 2005), and together, the results support H1, suggesting that highly impulsive individuals are more prone to this accelerating phase of drug abuse.
Impaired inhibition
To date, the relationship between impaired inhibition and escalation of drug intake has not been examined in humans; however, rats screened for HiI using a 5CSRT task showed higher levels of cocaine intake compared to their LoI counterparts (Dalley et al. 2007a, b). The rats acquired self-administration of a maximum of 50 cocaine infusions in 5 h, and they were subsequently allowed to self-administer up to 150 infusions in 8 h. Rats screened as HiI showed greater escalation of cocaine intake than LoI, supporting H1. In addition, a recent report indicated that HiI rats were more likely to progress from initiation of drug taking to compulsive drug-seeking behavior, measured by animal models of three DSM-IV criteria (Belin et al. 2008). Addiction scores were higher in HiI vs LoI rats based on a go/no-go task, a PR schedule, and punished (shock) responding for cocains infusions. In summary, the responsiveness of escalation to impulsivity suggests that developing strategies to reduce impulsivity and/or escalation would successfully interrupt the addiction process.
Abstinence, relapse, and treatment
Individual levels of impulsivity are related to failures in the ability to maintain abstinence and overall treatment success (H1). In addition, if drugs of abuse cause long-term increases in impulsivity (H2) that persist beyond cessation of drug use, users will have a more difficult time maintaining abstinence, resulting in lower likelihood of treatment success. For example, individuals that scored high on questionnaire measures of impulsivity were more likely to drop out of treatment for cocaine abuse, and they remained in treatment for a shorter time than those with lower impulsivity scores (Moeller et al. 2001). Impulsive individuals also experience increased drug craving during withdrawal and greater likelihood of relapse. For instance, impulsive (as measured by a questionnaire) smokers exhibited increased craving in response to cigarette cues (Doran et al. 2007), and they relapsed more quickly (Doran et al. 2004) than less impulsive smokers.
In rodents, abstinence (extinction) and relapse (reinstatement) can be modeled using a procedure such as that developed by de Vries et al. (1998). This procedure is modeled after the human condition in which drug use ceases, resulting in an abstinence period. Subsequent exposure to cues or contexts previously associated with drug-taking typically elicits drug-seeking and relapse. Similarly, in animal models, subjects self-administer moderate doses of a drug under short-access (e.g., 2 h/day) conditions, and after about 2 weeks, the drug is replaced with saline, and responding on the previously drug-paired lever continues to be measured for approximately 2–3 weeks (extinction). Subsequently, an injection of saline or the previously self-administered drug is given (drug-primed reinstatement) and/or the animal is exposed to cues that were previously paired with the drug (cue-induced reinstatement), and responding on the lever previously paired with the drug is measured. Conditions that are reported to contribute to relapse in humans (e.g., stress, drug-associated cues, drug exposure) reinstate responding in this model (Shalev et al. 2002; Epstein et al. 2006). However, some aspects of this procedure deviate from the human condition, and its substitutability as a model for relapse in humans has been extensively discussed elsewhere (e.g., Epstein et al. 2006; Katz and Higgins 2003; Shaham and Miczek 2003; Shaham et al. 2003). For the purposes of this review, the reinstatement procedure is discussed as a useful and well-accepted model to study aspects of relapse that are difficult to study in humans. The topic of interest is whether impulsivity directly predicts reinstatement/relapse.
Impulsive choice
Humans In support of the hypothesis that impulsive individuals have higher rates of relapse or are more resistant to treatment than less impulsive smokers (H1), impulsive adolescents were less likely to achieve abstinence from smoking in a 4-week treatment program compared to adolescents that were less impulsive (Krishnan-Sarin et al. 2007). In a 1-year treatment study of pregnant and recently postpartum women using abstinence-contingent voucher-based incentives, greater DD at baseline was associated with greater likelihood of smoking relapse at 24 weeks postpartum (Yoon et al. 2007). In addition, smokers with higher rates of discounting were more likely to smoke in a laboratory model of abstinence reinforcement (Dallery and Raiff 2007).
It may be difficult to persuade drug abusers to abstain, even with voucher-based incentives, because they respond more impulsively to their drug of abuse than money. For example, opioid-dependent individuals (Giordano et al. 2002; Madden et al. 1997, 1999; Odum et al. 2000), crack-cocaine abusers (Coffey et al. 2003), and cigarette smokers (Bickel et al. 1999) discounted the value of hypothetical future receipt of their primary drug of abuse to a greater extent than money. Drug-dependent individuals also discounted the value of future changes in their health status (e.g., the onset of a serious drug-related illness) more than nonusers (e.g., Odum et al. 2000).
Drugs of abuse may cause long-term increases in impulsivity, but short-term drug deprivation in abusers can also increase impulsivity. For example, opiate users (Giordano et al. 2002) and cigarette smokers (Field et al. 2006) were more impulsive when drug-deprived than when drug-satiated. However, after a long-term period of abstinence from chronic smoking, ex-smokers discounted delayed money to the same extent as those who had never smoked, and both discounted less than current smokers (Bickel et al. 1999). Drug abstinence was also associated with lower levels of impulsivity in heroin abusers (Kirby and Petry 2004), but not in smokers (Field et al. 2006), cocaine abusers (Heil et al. 2006; Kirby and Petry 2004), or alcohol abusers (Kirby and Petry 2004). These results suggest that chronic abuse of some drugs increased impulsive choice (H2), and after drug abuse was terminated, impulsive behavior returned to a level similar to that of nonusers, while with other drugs, impulsivity was not reversed after an abstinence period. Contingency management studies indicate that vouchers can effectively compete with drug use, but drug-positive status at treatment entry predicts less success with voucher-based incentives (Stitzer et al. 2007a, b). In fact, drug-positive status at treatment entry predicts poorer response to treatment in general, not just with contingency management (e.g., Alterman et al. 1996, 1997; Ehrman et al. 2001; Kampman et al. 2001; Petry et al. 2004; Sofuoglu et al. 2003). Thus, if drug use increases impulsivity, impulsivity may underlie the relationship between drug-positive status at treatment entry and treatment success, and this has been shown in several studies (Krishnan-Sarin et al. 2007; Yoon et al. 2007).
Conditioned drug-augmented impulsivity may have also influenced relapse such that the drug abuser continues to exhibit impulsive behavior in the presence of drug- and impulsivity-related stimuli that previously signaled drug-induced impulsivity. Conditioned impulsivity may lead an individual to relapse because they are not able to inhibit drug-taking behavior after exposure to drug-related cues. The conditioned incentive value of drugs of abuse could play a role in this process, as the impulsive user may value the immediate rewarding effects of the drug over the positive long-term benefits of drug abstinence. With some forms of drug abuse, treating impulsivity in drug abusers could result in longer periods of abstinence and decreased likelihood of relapse.
Animals
To date, few animal studies have focused on the relationship between withdrawal from chronic drug administration and impulsive choice. In rats, chronic administration of cocaine increased impulsive choice that persisted 3 months after cocaine treatment ended (Simon et al. 2007). Repeated injections of nicotine also dose-dependently increased impulsivity (Dallery and Locey 2005), and rats continued to respond impulsively after discontinuation of nicotine injections, but impulsive choice gradually returned to baseline over an abstinence period. These results are similar to findings in humans (Bickel et al. 1999), suggesting that nicotine exposure can produce long-lasting, but reversible, increases in impulsive choice, but cocaine exposure produces long-term (>30 days) increases in impulsive choice (Heil et al. 2006; Kirby and Petry 2004).
Using the reinstatement paradigm, HiI female rats showed greater cocaine-primed reinstatement of cocaine-seeking behavior than LoI female rats (Perry et al. 2008a). In a separate study, HiI rats showed greater cue-induced reinstatement of nicotine-seeking behavior than LoI rats (Diergaarde et al. 2008). Withdrawal from chronic exposure to orally self-administered phenycyclidine resulted in increased impulsive choice for a palatable saccharin solution in male and female rhesus monkeys (see review by Carroll et al. 2008a). Together, these studies provide evidence that impulsivity can predict shorter duration of abstinence, reduced treatment efficacy, and increased likelihood of relapse (H1; Dallery and Raiff 2007; Diergaarde et al. 2008; Krishnan-Sarin et al. 2007; Perry et al. 2007b; Yoon et al. 2007). In addition, chronic exposure to drugs of abuse increases impulsivity (H2), and with some drugs, impulsivity remains elevated even after drug abuse ceases (e.g., Heil et al. 2006; Kirby and Petry 2004; Simon et al. 2007), which in turn could influence abstinence, treatment, and relapse.
Impaired inhibition
We are unaware of any human studies that have examined the role of inhibitory failure in abstinence, treatment, and relapse. However, conditioned drug-augmented impulsivity may influence relapse in that the drug abuser becomes conditioned not to inhibit their behavior in the presence of drug- and impulsivity-related stimuli. Accordingly, alcoholics showed greater impulsivity on the go/no-go task in response to alcohol-related stimuli compared to neutral stimuli (Noel et al. 2007).
Relapse in impulsive individuals may be due to individual differences in inhibition (difficulty preventing responses that previously led to drug abuse). For example, rats that showed high levels of responding during cocaine-primed reinstatement of cocaine-seeking behavior showed greater deficits in inhibitory control on the go/no-go task compared with rats that had lower levels of reinstatement responding (Deroche-Gamonet et al. 2004). Rats screened for HiI on the 5CSRT task showed greater resistance to extinction after nicotine self-administration compared to LoI rats; however, there were no differences between impulsivity groups in cue-induced reinstatement of nicotine-seeking behavior (Diergaarde et al. 2008). Le Dzung et al. (2008) found a neurobiological link between reinstatement and inhibitory failure. Inactivation of the median raphe nucleus via local muscimol injections resulted in increases in impulsive responding on the 5CSRT and reinstatement of alcohol-seeking behavior, suggesting that inhibitory failure and reinstatement are indeed related.
Withdrawal from chronic exposure to drugs of abuse may also contribute to impaired inhibition. On the 5CSRT task, withdrawal from methamphetamine and MDMA resulted in increased inhibitory failure (Dalley et al. 2007b); however, there were no increases in inhibitory failure after withdrawal from d-amphetamine, cocaine, heroin, or nicotine (Dalley et al. 2005a, b; Shoaib and Bizarro 2005). Based on these data, it is possible that individual differences in inhibitory failure predict abstinence, treatment, and relapse outcomes (H1), and that increased inhibitory failure during drug withdrawal may contribute to shorter abstinence periods, reduced likelihood of treatment success, and greater relapse. Future studies in both humans and animal models should focus on treating the underlying inhibitory failure as a strategy to reduce the probability of relapse.
The effects of drugs on impulsivity
Most of the discussion thus far has been concerned with how impulsivity affects drug abuse. There is a large body of literature showing the reciprocal phenomenon, that drugs affect impulsivity. Given the role of impulsivity on the key phases of drug abuse as described above, it is essential to understand how drugs influence the impulsive behavior that is in turn determining drug-seeking and drug-taking behavior that leads to addiction. Not only can drugs increase impulsivity, and impulsivity may, in turn, accelerate drug-seeking, leading to out of control behavior with associated morbidity and mortality, but drugs can reduce impulsivity, and they may be useful to medication development efforts to prevent escalation to out of control use or relapse after abstinence has occurred. The following section will summarize the effects of drugs on impulsive choice (see Table 1) and inhibitory failure (see Table 2), comparing human and rat studies and different classes of drugs that have abuse and therapeutic potential. If administration of drugs of abuse results in increased levels of impulsivity (H2), then there may be a greater likelihood of drug-seeking and drug-taking behavior at the various phases.
Impulsive choice
Humans
A number of studies with drugs from different classes support the hypothesis that drugs of abuse increase impulsive choice (a summary is presented in Table 1), and higher levels of drug intake have been related to greater DD. For example, higher levels of nicotine exposure in chonic smokers were correlated with greater discounting of delayed monetary reinforcers (Ohmura et al. 2005; Reynolds et al. 2004b). Heyman and Gibb (2006) showed that regular smokers discounted more than chippers (occasional smokers); however, Johnson et al. (2007) found that light smokers discounted monetary rewards similarly to heavy smokers. These data may support the hypothesis that drug abuse influences subsequent impulsivity measures (H2); however, it is also possible that individuals self-select nicotine intake based on their baseline levels of impulsivity (H1).
The acute effects of several drugs of abuse on DD have also been determined. Acute administration of the stimulants methylphenidate (Pietras et al. 2003) and d-amphetamine (de Wit et al. 2002) decreased impulsive choice in humans; however, administration of alcohol (Ortner et al. 2003; Richards et al. 1999b), diazepam (Reynolds et al. 2004a), and Δ9-tetrahydrocannabinol (THC; McDonald et al. 2003) did not change DD. A possible explanation for the negative findings is that the laboratory setting provides intoxicated participants with cues that inhibit impulsive choice (Olmstead 2006; Ortner et al. 2003). Because intoxicated individuals may focus on the most salient environmental cues (Steele and Josephs 1990), they could be responding to cues in the laboratory environment that cause them to inhibit the impulsive behavior that may normally be displayed outside the laboratory. It is also possible that the DD procedure lacks the sensitivity to measure the acute state changes expected after alcohol, diazepam, or THC administration (McDonald et al. 2003; Reynolds et al. 2006b; Reynolds and Schiffbauer 2004). Therefore, preclinical models may be useful to determine the acute effects of drugs of abuse on impulsivity.
Animals
Administration of psychostimulants had mixed effects on DD in rats (see Table 1). In agreement with data from human laboratory studies, methylphenidate (Pitts and McKinney 2005; van Gaalen et al. 2006b), atomoxetine (Robinson et al. 2007), and methamphetamine (Richards et al. 1999a) decreased impulsive choice, but amphetamine produced mixed effects on impulsive choice (Charrier and Thiebot 1996; Evenden and Ryan 1996; Isles et al. 2003; van Gaalen et al. 2006a, b; Wade et al. 2000; Winstanley et al. 2003) in rodents. There are several factors that could account for the discrepancies in stimulant-induced changes in impulsive choice: type of reinforcer offered; cues associated with the larger, delayed reinforcer; dosing regimens; species; and basal levels of impulsivity. For example, Wade et al. (2000) and Richards et al. (1999a) found stimulant-induced decreases in impulsivity using water reinforcers in water-deprived rats; whereas, Evenden and Ryan (1996) and Charrier and Thiebot (1996) found stimulant-induced increases in impulsivity using food reinforcers in food-restricted rats. The cues present during the delay between the response and the delivery of the larger, delayed reinforcer are also important factors because when the delays to the larger reinforcer were not signaled, stimulants increased impulsivity (Charrier and Thiebot 1996; Evenden and Ryan 1996); whereas, when the delays were cued, stimulants decreased impulsivity (Richards et al. 1999a; Wade et al. 2000). The larger, delayed reinforcer cue may become a conditioned reinforcer, thus enhancing responding after stimulant administration (e.g., Beninger et al. 1981; Hill 1970; Robbins 1978; Robbins et al. 1983). The differences noted in the above studies may be more attributable to stimulus control of behavior than impulsivity, and further studies designed to dissect these variables would be informative. Other variables such as dosing regimens (e.g., high vs low; Isles et al. 2003) and species (e.g., rat vs mouse) also influence the effects of stimulants on DD. The effects of stimulants on DD may also be dependent upon baseline performance. For example, acute administration of d-amphetamine increased impulsive choice in LoI rats, and it decreased impulsive choice in HiI rats (Perry et al. 2008b). In addition, methylphenidate acutely decreased impulsive choice in HiI rats, but it had no effect on LoI rats (Perry et al. 2008b).
Alcohol administration in rats produced a dose-dependent increase in impulsive choice (Evenden and Ryan 1999; Poulos et al. 1998), contrasting with the lack of alcohol-induced effects on DD in humans. Benzodiazepine administration produced mixed effects on DD in rats. Similar to humans, diazepam decreased impulsivity in one study (Evenden and Ryan 1996); however, it produced no change in another (Charrier and Thiebot 1996), and chlordiazepoxide increased impulsivity (Cardinal et al. 2000). Morphine also increased impulsive choice (Kieres et al. 2004; Pitts and McKinney 2005). Species differences may be accounted for by drug dosing or other procedural differences, such as reinforcer magnitude, the time until reinforcer is delivered (after experimental sessions in humans vs during experimental sessions in animals), or the range of reinforcers/delays experienced.
In addition to delineating the acute effects of drugs of abuse, preclinical models may also be better suited than human studies to explore the effects of chronic drug administration on impulsive choice. In rats, repeated administration of methamphetamine (Richards et al. 1999a) or cocaine (Logue et al. 1992; Paine et al. 2003) resulted in increased impulsive choice. Repeated injections of nicotine also dose-dependently increased impulsivity (Dallery and Locey 2005). Like acute effects, chronic drug effects may differ as a result of baseline levels of impulsivity. For example, HiI rats that received 10 daily injections of d-amphetamine became less impulsive; whereas, LoI rats receiving the same treatment became more impulsive (Perry and Bardo, unpublished data). In addition, HiI mice and rats exhibited greater locomotor sensitization after repeated exposure to ethanol (Mitchell et al. 2006) and d-amphetamine (Perry and Bardo, unpublished data) than LoI mice and rats, respectively, indicating that there are baseline-dependent changes in the drug-induced behavior of HiI and LoI rats.
Summary and future directions
In support of the hypothesis that drug use increases impulsivity (H2), alcohol and morphine acutely increased impulsive choice in rats. In addition, chronic administration of psychostimulants increased impulsivity in rats (supporting H2), and there is evidence that psychostimulant-induced changes in impulsivity may be baseline-dependent (H1). Thus, it is possible that escalation of drug intake results from a combination of H1 and H2. Longitudinal studies in humans would be helpful to assess the extent to which impulsive choice increases as drug use escalates and to determine whether interventions that reduce impulsive choice could also result in lower levels of drug use.
Impaired inhibition
Humans
Psychostimulants generally improve inhibitory control (decrease impulsivity) in humans with low baseline levels of inhibitory control. For example, in individuals with attention deficit hyperactivity disorder (ADHD), methylphenidate (Potter and Newhouse 2004; Tannock et al. 1989, 1995), atomoxetine (Chamberlain et al. 2007), and nicotine (Potter and Newhouse 2004) increased inhibitory control on the SSRT task. Amphetamine improved inhibitory control on the go/no-go task in individuals who initially showed poor levels of response inhibition (de Wit et al. 2000, 2002) and in chronic cocaine abusers (Fillmore et al. 2003), but it did not influence response inhibition on the SSRT task in healthy volunteers (Fillmore et al. 2005a). Cocaine (Fillmore et al. 2006, but see Fillmore et al. 2002) and MDMA (Ramaekers and Kuypers 2006) improved inhibitory control on the SSRT task in cocaine and MDMA users, respectively. Long-term stimulant abusers displayed neurological impairments, including deficits in inhibitory control (Fillmore and Rush 2002). Therefore, the stimulant-facilitated improvements in inhibitory control in cocaine and MDMA abusers is comparable to improvements in individuals with ADHD or those who had initially poor levels of inhibition. Thus, the results may represent rate-dependent effects or varying effects upon performance depending on whether the subject’s baseline response rate was low or high, such that there was an inverse relationship between the baseline response rate and the effects of the drug (e.g., Dews 1958; Dews and Wenger 1977; Kelleher and Morse 1968; Robbins and Sahakian 1979). This suggests that psychostimulants may decrease inhibitory control in individuals with high baseline levels of inhibitory control. There have been no systematic evaluations of this hypothesis, but it would be an important area for future study.
In contrast to psychostimulants, alcohol dose-dependently impaired inhibition (de Wit et al. 2000; Easdon et al. 2005; Fillmore and Blackburn 2002; Fillmore and Vogel-Sprott 1999; Marczinski et al. 2005; Marczinski and Fillmore 2003, 2005a, b; Mulvihill et al. 1997; Ramaekers and Kuypers 2006, but see Ortner et al. 2003). In addition, the expectation of alcohol-induced decreases in performance (Fillmore and Blackburn 2002), behavioral treatment (Fillmore and Vogel-Sprott 1999), and caffeine (Fillmore and Vogel-Sprott 1999) all reversed alcohol-induced impairments in inhibition, suggesting that behavioral or pharmacological interventions may also augment alcohol’s impulsivity-increasing effects. Similar to alcohol, benzodiazepines produced dose-dependent decreases in inhibitory control. While low doses of diazepam produced no changes in inhibition (Reynolds et al. 2004a), higher doses of diazepam (Acheson et al. 2006) and triazolam (Fillmore et al. 2001) impaired inhibition (see Table 2).
Unlike psychostimulants and sedative/hypnotics, the acute effects of THC on response inhibition appear to be task-dependent. A high dose of THC increased impulsive responding on the SSRT task; however, it did not affect responding on the go/no-go task (McDonald et al. 2003). It is possible that the inconsistencies between the SSRT task and the go/no-go task indicate that they are measuring different underlying processes or the tasks could differ in sensitivity or parametric features (Reynolds et al. 2006b). Given that performance on these two tasks is positively correlated in the absence of drugs (Reynolds et al. 2006a), the latter possibility may be more likely.
Animals
In animal studies, inhibitory responses to acute administration of drugs have been comparable to those obtained in humans (see Table 2). Consistent with the rate-dependency hypothesis (e.g., Dews 1958; Kelleher and Morse 1968; Robbins and Sahakian 1979), amphetamine (Eagle and Robbins 2003; Feola et al. 2000), methylphenidate (Eagle et al. 2007), and modafinil (Eagle et al. 2007) improved inhibition on the SSRT task in rats with poor baseline inhibitory control. Conversely, methylphenidate impaired inhibition on the SSRT task in rats with high baseline inhibitory control (Eagle et al. 2007). Regardless of baseline response rate, atomoxetine improved inhibition on the SSRT task (Robinson et al. 2007); whereas, alcohol impaired inhibition on the SSRT task (Feola et al. 2000), and cocaine impaired inhibition on the go/no-go (Paine and Olmstead 2004) and 5CSRT (van Gaalen et al. 2006a) tasks.
Unlike results from studies in which the go/no-go and SSRT tasks were employed, results from studies using the 5CSRT were largely mixed with some studies showing drug-induced increases in inhibitory failure and others showing drug-induced decreases. This was true of atomoxetine (Navarra et al. 2008; Robinson et al. 2007), methylphenidate (Bizarro et al. 2004; Navarra et al. 2008; Paine et al. 2007), amphetamine (Bizarro et al. 2004; Bizarro and Stolerman 2003; van Gaalen et al. 2006a), and nicotine (e.g., Bizarro et al. 2004; Blondel et al. 2000; Mirza and Stolerman 1998; van Gaalen et al. 2006a). Between-study differences in intertrial intervals, duration of stimulus presentation, and other procedural differences likely contributed to the discrepancies in these studies (Stolerman et al. 2000). It will be important for future research to determine experimental conditions that allow for comparison between the 5CSRT, go/no-go, and SSRT tasks. Together, the results from studies measuring inhibition in animal models typically provide comparable results to human research, implying construct validity for these tasks.
Summary and future directions
In summary, alcohol, diazepam, and triazolam in humans (and cocaine in rodents) acutely decreased inhibitory control, supporting the hypothesis that drug use increases impulsivity (H2). However, in both humans and rats, stimulants increased inhibitory control (decreased impulsivity) in impulsive individuals. In addition, acute administration of amphetamine, methylphenidate, atomoxetine, and nicotine in rodents produced mixed results on the 5CSRT task. Thus, it is unclear whether acute administration of drugs of abuse can increase impulsivity and enhance escalation/dysregulation of drug intake. Systematic studies of the effects of chronic administration of drugs of abuse on inhibitory control may provide evidence that drugs of abuse increase impulsivity (H2); however, we are unaware of studies that have addressed this question. There is need for these studies, as discovery of drugs that decrease impulsive behavior may be useful for harm reduction in users, and identification of drugs that increase impulsivity related to drug use could explain accelerated use and resistance to abstinence/treatment. In these cases, behavioral management of impulsive behavior may be of value.
Individual differences related to impulsivity and/or drug abuse
The third hypothesis under consideration (H3) suggests that impulsivity and drug abuse are associated through another factor (e.g., sex, hormonal status, general reactivity to rewards, early experiences) that interacts with and affects both impulsivity and drug abuse. Thus, impulsivity may not be independently influencing drug abuse, but it may covary with other factors that have a strong influence. In fact, impulsivity and other factors may have an additive influence on vulnerability to various aspects of drug abuse. This section focuses on factors that have been related to impulsivity and enhanced vulnerability to drug abuse with an emphasis on preclinical research. The vulnerability factors may be related to genetic or environmental conditions, such as sex and hormonal status, reactivity to nondrug rewards, and early life experiences (e.g., abuse/impoverished rearing conditions, prenatal drug exposure). In addition, these factors may be differentially related to different facets of impulsivity: impulsive choice or impaired inhibition. For example, extinction learning and aggression were both related to impulsive choice; however, neither was related to impaired inhibition (Van den Bergh et al. 2006).
Sex and hormonal factors
Sex appears to be a major factor in human and animal drug consumption with females exhibiting greater drug-seeking behavior than males under a wide range of conditions (for reviews, see Carroll et al. 2004; Lynch et al. 2002; Lynch 2006; Roth et al. 2004). However, currently, more men report problems with drug abuse than women, but the gender gap appears to be closing (Substance Abuse and Mental Health Services Administration 2006). For example, men are more likely than women to have an initial opportunity to use drugs due to cultural factors, but once the opportunity to use occurs, women may be more likely than men to make a transition to continued abuse (Anglin et al. 1987; Brecht et al. 2004; Hernandez-Avila et al. 2004).
In preclinical models, females acquired drug self-administration faster than males (e.g., Carroll et al. 2000; Lynch and Carroll 1999), escalated their drug intake (Carroll et al. 2005; Roth and Carroll 2004), and regulated their drug intake less precisely (Lynch et al. 2000) showing more binge-like patterns (Morgan et al. 2002) than males. Females also maintained higher rates of responding during extinction and after drug-primed (but not cue-primed, Fuchs et al. 2005) reinstatement than males, suggesting that they are less likely to cease drug use and more likely to relapse than males (Lynch and Carroll 2000). Hormonal status (e.g., presence of estrogen/progesterone) plays a major role in sex differences in drug abuse. For example, in rats, estrogen facilitated acquisition of cocaine (Hu and Becker 2003; Jackson et al. 2006; Lynch et al. 2001) and heroin (Roth et al. 2002) self-administration, escalation of cocaine self-administration (Larson et al. 2007), and reinstatement of drug-seeking behavior (Larson et al. 2005), and progesterone attenuated estrogen’s effects on cocaine escalation (Larson et al. 2007) and reinstatement (Anker et al. 2007).
Several of these drug-related behaviors may be attributed to impulsivity (e.g., acquisition, escalation, extinction, reinstatement); however, clinical studies of sex differences in impulsivity produced mixed results. For example, women have lower (Kirby and Marakovic 1996), higher (Wallace 1979; Reynolds et al. 2006a), or the same (Fillmore and Weafer 2004; Reynolds et al. 2006b; Skinner et al. 2004) levels of impulsivity compared to males. Experimental conditions also play an important role in the determination of sex differences in impulsivity. In one study, women discounted delayed hypothetical reinforcers at a higher rate than men, but when real reinforcers were offered, men discounted at a higher rate than women (Heyman and Gibb 2006).
Age may be interrelated as well. In adolescents (but not adults), the relationship between impulsivity and drug abuse was stronger in males than females (Labouvie and McGee 1986). The interaction between impulsivity and sex may be drug-specific. Impulsivity was associated with higher levels of nicotine use in females, but in males, impulsivity was associated with heightened caffeine use (Waldeck and Miller 1997). Others have found no sex differences in the relationship between impulsivity and alcohol use (Nagoshi et al. 1991; Waldeck and Miller 1997). There is also evidence for sex differences in drug-induced changes in impulsivity. Men displayed more alcohol-induced inhibitory impairments than women in one study (Fillmore and Weafer 2004), but in other studies, there were no sex differences (Mulvihill et al. 1997; Reynolds et al. 2006a, b). Procedural differences may account for the discrepancies in these results; however, the lack of consistent sex differences in any of the above studies illustrates that further research with standard procedures is needed to characterize the relationship between sex, impulsivity, and drug abuse.
To date, few preclinical studies have examined sex differences in impulsivity. Using a go/no-go task, Jentsch and Taylor (2003) found that males had higher levels of responding during the no-go period than females, indicating higher levels of impulsivity. However, this interpretation may have been confounded by feeding conditions, as males and females were fed the same amount of food, despite the sex difference in body weights. Thus, the males had relatively more severe food restriction than the females and presumably greater motivation to respond for presentation of food. In a study conducted in our laboratory, there were no sex differences on a DD task for food or cocaine reinforcement; however, the results could be attributed to a floor effect because approximately 70% of the rats were impulsive on the task (Perry et al. 2008a). However, when we compared other studies of rats selectively bred for low saccharin intake (LoS) on the same task, there was less impulsivity and greater room for variation in impulsivity scores, and females were more impulsive than males (Perry et al. 2007). In another study using the high and low saccharin (HiS, LoS) rats and the go/no-go procedure for cocaine self-administration, a similar finding emerged. Female rats exceeded males on both go (self-administration) and no-go (inhibitory failure) responding in both the HiS and LoS lines (Anker and Carroll 2008). Van Haaren et al. (1988) also found that female rats discounted food reinforcers more than males. Thus, the data currently available suggest that females are more impulsive than males with respect to food and drug rewards.
Sex differences in impulsivity and drug abuse may be mediated by circulating gonadal hormones. For example, sham-operated males had the highest levels of impulsivity, while sham-operated females had the lowest, and gonadectomized males and females had intermediate levels of impulsive responding (Jentsch and Taylor 2003). These results suggest that gonadal hormones influence impulsivity; however, as mentioned above, feeding conditions were a confounding variable in this study. Svensson et al. (2000) also found that gonadectomy decreased impulsivity, and this effect was reversed in rats that received testosterone substitution after gonadectomy. Thus, two studies are in agreement that testosterone in males is related to higher levels of impulsivity; however, in a third study, there was no relationship between plasma testosterone and impulsivity in male rats (Van den Bergh et al. 2006). Results from another study suggested that testosterone’s effects may be baseline-dependent (Takahashi et al. 2006); that is, testosterone enhanced impulsive choice in nonimpulsive men, and it decreased impulsive choice in impulsive men (Takahashi et al. 2006). The role of female gonadal hormones in impulsivity remains to be determined. Future studies are needed to adequately understand the relationship between sex, gonadal hormones, impulsivity, and how the combination of these factors interacts with drug abuse.
Reactivity to rewards
Another determinant of the vulnerability to drug abuse (and possibly impulsivity) is reactivity to natural rewards, such as dietary (e.g., sweets, fats) substances (Carr 2002; Carroll 1999; Grigson 2002) or exercise, such as wheel running (Larson and Carroll 2005). For example, rats selectively bred for HiS consumed more ethanol than their LoS counterparts (Dess et al. 1998). HiS rats also acquired i.v. cocaine self-administration faster and in greater numbers than LoS rats (Carroll et al. 2002). HiS rats escalated their drug intake to higher levels and showed greater reinstatement of cocaine-seeking behavior than LoS rats (Perry et al. 2006). In addition, HiS females showed greater dysregulation of cocaine intake than LoS females (Carroll et al. 2007b) and higher rates of cocaine-induced locomotor activity (Carroll et al. 2007a). HiS males and females were also more impulsive on a DD task rewarded with food (Perry et al. 2007) and a go/no-go task rewarded by i.v. cocaine self-administration (Anker and Carroll 2008) compared to LoS males and females. These findings appear to be unidirectional, as HiI and LoI rats do not show differences in saccharin intake in a two-bottle choice test (Carroll et al. 2008b). In another study of reactivity to natural rewards and impulsivity, adolescent humans and rats were more impulsive than their adult counterparts, and they also showed a preference for sweeter sucrose solutions compared to adults, although there were no correlations between impulsivity measures and sucrose preferences (Vaidya et al. 2004). Excessive intake of palatable substances, such as sucrose or saccharin may be considered a form of impulsivity; however, further studies are necessary to explore the relationship between these addiction-prone behavioral phenotypes. The relationship between impulsivity and other phenotypes with high levels of reactivity to natural rewards may be revealed in future studies (e.g., high and low wheel runners), and this would indicate that impulsivity is related to a more fundamental construct.
Early environmental experiences
Traumatic experiences in early childhood, such as maltreatment or abuse have been associated with enhanced risk of subsequent alcohol and substance abuse (e.g., for review, see De Bellis 2002). (e.g., for reviews see Olmstead 2006; Spear and Molina 2005). Humans who have experienced detrimental early life experiences, such as abuse or prenatal exposure to drugs, also show high levels of impulsivity (for review, see Olmstead 2006). Similarly, in animal models, rats reared in an impoverished environment self-administered more amphetamine infusions under FR (Bardo et al. 2001) and PR (Green et al. 2002) schedules of reinforcement compared to rats reared in an enriched environment (but see Bardo and Dwoskin 2004; Olmstead 2006 for limitations on these findings). Rats reared in isolation also showed increased impulsive choice compared with rats reared in an enriched environment (Perry et al. 2008b). Others have reported no differences in impulsive choice (Adriani et al. 2006; Hellemans et al. 2005) or inhibition (Dalley et al. 2002; Hellemans et al. 2005) between rats that were socially or individually housed as adolescents. However, individually housed rats that experienced perinatal asphyxia were more impulsive as adults compared with socially housed rats that experienced perinatal asphyxia (Adriani et al. 2006). Earlier studies showed that animals (rats and monkeys) living in an environment with restricted food access (vs unlimited access) showed elevated drug self-administration and reinstatement of drug-seeking behavior (relapse, Carroll 1998; Campbell and Carroll 2000). Recent research with monkeys indicated that the restricted feeding condition resulted in increased impulsivity for orally delivered phencyclidine (PCP) compared to the unlimited food condition (Carroll et al. 2008a, b).
Another early life experience that predicts subsequent vulnerability to drug abuse is early (i.e., prenatal, fetal, or infantile) exposure to drugs of abuse (e.g., for reviews see Olmstead 2006; Spear and Molina 2005). In addition, chronic prenatal ethanol exposure in guinea pigs resulted in impaired inhibition (Olmstead 2006). Overall, these results suggest that impulsivity is influenced by adverse and early life experiences; however, stress may be a confounding factor. Stress is also associated with higher rates of acquisition of drug self-administration (Tidey and Miczek 1997) and impulsivity (Piazza and Le Moal 1998). Further research is needed to determine the relationship between stress, adversity, and impulsivity, and whether stress mediates the relationship between traumatic early experiences, impulsivity, and ultimately drug abuse.
In summary, impulsivity may be related to other factors (e.g., sex, reactivity to rewards, or early environmental experiences) that predict vulnerability to drug abuse (H3). A relationship between sex and impulsivity (F>M) is emerging. Now it will be important to examine the role of female gonadal hormones in impulsivity to determine its influence in other aspects of drug abuse. Reactivity to rewards (e.g., preference for a saccharin solution) and early environmental experiences (e.g., environmental enrichment) were related to impulsive choice and behavioral inhibition, and they were both related to vulnerability to drug abuse (H3). However, given that HiI and LoI rats do not differ in saccharin preference (Carroll et al. 2008b), it appears that impulsivity and reactivity to rewards are separate, but related constructs.
General discussion
A review of the literature suggests that impulsivity is a construct with multiple facets (e.g., Evenden 1999), and it is important to use several different measures of impulsivity to obtain converging evidence when assessing the effect of an experimental manipulation on impulsivity. The first goal of this review was to highlight the main measures of impulsive behavior that have increased our knowledge regarding the connection between impulsivity and drug abuse. To that end, we have described two aspects of impulsivity that have been associated with drug abuse: impulsive choice (measured by DD) and impaired inhibition (measured by the go/no-go, SSRT, and 5CSRT tasks). Although these definitions reflect different aspects of impulsivity, drug abusers show deficits in both impulsive choice and inhibition. Performance on tasks measuring impulsive choice and impaired inhibition is related to vulnerability during several phases of addiction (i.e., acquisition, escalation/dysregulation, abstinence, treatment, and relapse), and these tasks all appear to be sensitive to acute or chronic administration of drugs of abuse. It is important to note that the majority of the results from rodent models of impulsive choice and inhibition concur with findings in humans, indicating the face validity of the animal models. However, more research is needed to examine the variables controlling responding on these tasks (e.g., whether delays are cued in the DD task, ITI in the 5CSRT task). In addition, it remains unclear whether tasks proposed to study inhibitory control are related or whether they measure different aspects of inhibition. Clarifying the specific mechanisms that underlie performance on these tasks would greatly add to our understanding of current models of impulsivity and allow us to develop new models that more accurately capture the essence of several aspects of impulsivity.
A second goal of this review was to show how impulsivity may drive drug-seeking behavior during several phases of drug abuse, and to accomplish this, we have examined the relationship between impulsivity and drug abuse using three hypotheses (the third goal of this review). H1 states that increased levels of impulsivity expressed as impulsive choice or inhibitory failure leads to drug abuse, and H1 plays a role in acquisition, escalation/dysregulation, abstinence, treatment, and relapse. Drug abusers show deficits in impulsive choice and inhibition, although it is impossible to know whether differences in impulsivity caused or were caused by drug abuse. Preclinical models show that impulsive choice predicted elevated alcohol intake (Poulos et al. 1995), faster acquisition of cocaine self-administration (Perry et al. 2005), greater escalation of cocaine intake (Anker et al., in preparation), and greater drug- (Perry et al. 2008a) and cue-induced (Diergaarde et al. 2008) reinstatement of drug-seeking behavior. Impaired inhibition also predicted elevated cocaine self-administration (Dalley et al. 2007a), higher levels of responding during acquisition of nicotine self-administration (Diergaarde et al. 2008), and reinstatement of cocaine-seeking behavior (Deroche-Gamonet et al. 2004). Impulsive choice in humans predicted greater likelihood of relapse (Krishnan-Sarin et al. 2007; Yoon et al. 2007). With respect to H1, it will be important for future research to extend these initial findings in preclinical and human laboratory models by answering the following questions: (1) Does impulsive choice or impaired inhibition influence acquisition of drug-taking in humans? (2) How do baseline levels of impulsivity influence an individual’s response to drugs of abuse? (3) If impulsive choice and/or impaired inhibition predict greater likelihood of acquisition/escalation/relapse, will treating underlying impulsivity also reduce the likelihood of acquisition/escalation/relapse?
H2, that acute or chronic exposure to drugs of abuse increase impulsivity, is particularly relevant to escalation/dysregulation of drug intake and to abstinence, treatment, and relapse. To date, it is unclear whether a single drug exposure is sufficient to increase impulsivity, yet determining the influence of subtle procedural variations, such as dosing, behavioral history, and stimulus control on drug-induced impulsivity will undoubtedly clarify the existing results. Unlike acute dosing regiments, chronic dosing of nicotine (Dallery and Locey 2005), cocaine (Logue et al. 1992; Paine et al. 2003), and methamphetamine (Richards et al. 1999a) produced increases in impulsive choice that may contribute to escalation of intake (although there have not been any studies that have specifically examined this). We are unaware of any studies that have determined the effects of chronic administration of drugs on inhibitory control; however, these studies will be important for determining the role of chronic drug administration on inhibitory control and escalation/dysregulation. Additional work using self-administration in animal models or within-subject longitudinal comparisons in humans should examine impulsivity as drug intake progresses from low to high levels to further assess the role of H2 in escalation/dysregulation of drug intake.
The increases in impulsivity caused by chronic administration of drugs of abuse (H2) may continue despite drug cessation, resulting in shorter abstinence, faster relapse, and reduced likelihood of treatment success. For example, in both humans and rodents, nicotine (Bickel et al. 1999; Dallery and Locey 2005) and cocaine (Heil et al. 2006; Kirby and Petry 2004; Simon et al. 2007) exposure produced reversible increases in impulsive choice that persisted even after drug use was discontinued. Less is known about the relationship between inhibitory failure and withdrawal, but withdrawal from some drugs of abuse (i.e., methamphetamine, MDMA) increased impulsivity on the 5CSRT task (Dalley et al. 2007b), which may increase likelihood of subsequent relapse. A goal of future research in this area should be to determine how drug- or withdrawal-induced increases in impulsivity influence abstinence and treatment success, and whether reducing impulsivity would increase the length of abstinence and overall treatment success.
We have also presented evidence in support of H3, which suggests that impulsivity is associated with drug abuse through a third factor (e.g., sex, hormonal status, reactivity to rewards, early environmental experiences) that interacts with and affects both impulsivity and drug abuse during all phases of abuse. This hypothesis has been studied less frequently than H1 or H2; however, it appears that there is a relationship between impulsivity and sex (F>M). Reactivity to rewards and early environmental experiences also appears to be related to impulsivity, and predicts of vulnerability to drug abuse. Future studies with sensitive behavioral measures should examine the relationship between impulsivity, and phenotypes with high levels of reactivity to natural rewards. The relationship between stress, adversity, and impulsivity should also be studied to determine whether stress mediates the relationship between early experiences, impulsivity, and drug abuse.
It will be important for future research to continue to characterize the relationship between drug abuse and impulsivity using both human and animal models, several different drugs of abuse, and several different measures of impulsivity (i.e., DD, go/no-go, SSRT, and 5CSRT tasks). Factors that contribute to individual differences in impulsivity, such as behavioral history, motivation, neurobiology, and genetics should be studied to better understand the behavioral manifestations and neurobiology underlying impulsive behavior and drug abuse. For example, variants of the dopamine transporter, the dopamine D4 receptor, and the serotonin transporter have been linked to both impulsivity and alcoholism (Kreek et al. 2005), and understanding how each of these impacts impulsivity and alcoholism may yield new pharmacotherapies that decrease both impulsivity and drug abuse.
It may also be important to determine whether compulsive behavior is related to impulsivity and drug abuse. Compulsive behavior differs from impulsive behavior in that compulsive actions reduce anxiety (negative reinforcement); whereas, impulsive behaviors are thought to produce pleasure or gratification (positive reinforcement; American Psychiatric Association 2000). Certainly, both processes play a role in drug abuse (e.g., Le Moal and Koob 2007; Olmstead 2006). It has been suggested that impulsivity and compulsivity are on opposite ends of a continuum, and the initial phases of drug abuse are driven by impulsivity, while subsequent phases (e.g., escalation, relapse, reacquisition) are driven by compulsivity (e.g., Belin et al. 2008; Le Moal and Koob 2007). However, the relationship between impulsivity and compulsivity is complicated and both occur at different times or cooccur in the same individual (Grant and Potenza 2006). Therefore, it will be important to examine the relationships between compulsivity, impulsivity, and drug abuse.
Studying factors that contribute to individual differences in impulsivity may result in a greater understanding of how these forms of behavior contribute to critical phases of the addiction process. Future research should also focus on the relationship between impulsivity and other major vulnerability factors (e.g., sex, hormonal status, reactivity to rewards, early environmental experiences) and how the combination of these factors may influence drug abuse. Such research would result in the ability to recognize vulnerable phenotypes and focus prevention strategies on those with heightened vulnerability to drug abuse. It will also be important for researchers to determine whether present behavioral or pharmacological treatments decrease impulsivity and whether tasks that measure impulsivity would be useful in screening new treatments for drug abuse. The intent would be to find pharmacological and behavioral treatments that will decrease impulsivity and drug abuse simultaneously and to examine how effective behavioral and pharmacological treatments are in individuals differing in impulsivity.
References
Acheson A, Reynolds B, Richards JB, de Wit H (2006) Diazepam impairs behavioral inhibition but not delay discounting or risk taking in healthy adults. Exp Clin Psychopharmacol 14:190–198
Adriani W, Giannakopoulou D, Bokulic Z, Jernej B, Alleva E, Laviola G (2006) Response to novelty, social and self-control behaviors, in rats exposed to neonatal anoxia: modulatory effects of an enriched environment. Psychopharmacology 184:155–165
Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300
Ahmed SH, Koob GF (1999) Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacol 146:303–312
Alterman AI, McKay JR, Mulvaney FD, McLellan AT (1996) Prediction of attrition from day hospital treatment in lower socioeconomic cocaine-dependent men. Drug Alcohol Depend 40:227–233
Alterman AI, Kampman K, Boardman CR, Cacciola JS, Rutherford MJ, McKay JR, Maany I (1997) A cocaine-positive baseline urine predicts outpatient treatment attrition and failure to attain initial abstinence. Drug Alcohol Depend 46:79–85
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders. Text revision, 4th edn. American Psychiatric Association, Washington, DC
Anglin MD, Hser YI, McGlothlin WH (1987) Sex differences in addict careers. 2. Becoming addicted. Am J Drug Alcohol Abuse 13:59–71
Anker JJ, Larson EB, Gliddon LA, Carroll ME (2007) Effects of progesterone on the reinstatement of cocaine-seeking behavior in female rats. Exp Clin Psychopharmacol 15:472–480
Anker JJ, Carroll ME (2008) Impulsivity on a go/no-go for i.v. Cocaine and food in male and female rats selectivity bred for high and low saccharin intake. Behav Pharmacol (in press)
Audrain-McGovern J, Rodriguez D, Tercyak KP, Epstein LH, Goldman P, Wileyto EP (2004) Applying a behavioral economic framework to understanding adolescent smoking. Psychol Addict Behav 18:64–73
Baker F, Johnson MW, Bickel WK (2003) Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychology 112:382–392
Bardo MT, Dwoskin LP (2004) Biological connection between novelty- and drug-seeking motivational systems. Nebr Symp Motiv 50:127–158
Bardo MT, Klebaur JE, Valone JM, Deaton C (2001) Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacol 155:278–284
Barratt ES, Patton JH (1983) Impulsivity: cognitive, behavioral, and psychophysiological correlates. In: Zuckerman M (ed) Biological bases of sensation seeking, impulsivity, and anxiety. Erlbaum, Hillsdale, NJ, pp 77–122
Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE (2001) Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 39:376–389
Belin D, Mar AC, Dalley JW, Robbins RW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320:1652–1355
Beninger RJ, Hanson DR, Phillips AG (1981) The acquisition of responding with conditioned reinforcement: effects of cocaine, (+)amphetamine and pipradrol. Br J Pharmacol 74:149–154
Bickel WK, Marsch LA (2001) Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction 96:73–86
Bickel WK, Odum AL, Madden GJ (1999) Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacol 146:447–454
Bizarro L, Stolerman IP (2003) Attentional effects of nicotine and amphetamine in rats at different levels of motivation. Psychopharmacol 170:271–277
Bizarro L, Patel S, Stolerman IP (2003) Comprehensive deficits in performance of an attentional task produced by co-administering alcohol and nicotine to rats. Drug Alcohol Depend 72:287–295
Bizarro L, Patel S, Murtagh C, Stolerman IP (2004) Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine and methylphenidate. Behav Pharmacol 15:195–206
Blondel A, Simon H, Sanger DJ, Moser P (1999) The effect of repeated nicotine administration on the performance of drug-naïve rats in a five-choice serial reaction time task. Behav Pharmacol 10:665–673
Blondel A, Sanger DJ, Moser PC (2000) Characterisation of the effects of nicotine in the five-choice serial reaction time task in rats: antagonist studies. Psychopharmacol 149:293–305
Brecht M-L, O’Brien A, von Mayrhauser C, Anglin MD (2004) Methamphetamine use behaviors and gender differences. Addict Behav 29:89–106
Campbell UC, Carroll ME (2000) Acquisition of drug self-administration: environmental and pharmacological interventions. Exp Clin Psychopharmacol 8:312–325
Cardinal RN, Robbins TW, Everitt BJ (2000) The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacol 152:362–375
Carli M, Robbins TW, Evenden JL, Everitt BJ (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361–380
Carr KD (2002) Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav 76:353–364
Carroll ME (1998) Acquisition and reacquisition (relapse) of drug abuse: modulation by alternative reinforcers. NIDA Res Monogr 169:6–25
Carroll ME (1999) Interactions between food and addiction. In: Niesink R, Hoefakker R, Westera W, Jaspers R, Kornet L, Boobis S (eds) Neurobehavioral toxicology and addiction: food, drugs and environment. CRC, Boca Raton, FL, pp 286–311
Carroll ME, Roth ME, Voeller RK, Nguyen PD (2000) Acquisition of oral phencyclidine self-administration in rhesus monkeys: effect of sex. Psychopharmacol 149:401–408
Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK (2002) Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacol 161:304–313
Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP (2004) Sex and estrogen influence drug abuse. Trends Pharmacol Sci 25:273–279
Carroll ME, Batulis D, Landry K, Morgan AD (2005) Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacol 180:414–426
Carroll ME, Anderson MM, Morgan AD (2007a) Higher locomotor response to cocaine in female (vs. male) rats selectively bred for high (HiS) and low (LoS) saccharin intake. Pharmacol Biochem Behav 88:94–104
Carroll ME, Anderson MM, Morgan AD (2007b) Regulation of intravenous cocaine self-administration in rats selectively bred for high (HiS) and low (LoS) saccharin intake. Psychopharmacol 190:331–341
Carroll ME, Anker JJ, Mach JL, Newman JL, Perry JL (2008a) Delay discounting as a predictor of drug abuse. In: Madden GJ, Critehfield TS, Bickel WK (eds) Impulsivity: theory, science, and neiroscience of discounting. American Psychological Association, Washington, DC (in press)
Carroll ME, Morgan AD, Anker JJ, Perry JL (2008b) Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol (in press)
Chamberlain SR, del Campo N, Dowson J, Muller U, Clark L, Robbins TW, Sahakian BJ (2007) Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry 62:977–984
Chambers RA, Taylor JR, Potenza MN (2003) Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry 160:1041–1052
Charrier D, Thiebot MH (1996) Effects of psychotropic drugs on rat responding in an operant paradigm involving choice between delayed reinforcers. Pharmacol Biochem Behav 54:149–157
Coffey SF, Gudleski GD, Saladin ME, Brady KT (2003) Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol 11:18–25
Crean JP, de Wit H, Richards JB (2000) Reward discounting as a measure of impulsive behavior in a psychiatric outpatient population. Exp Clin Psychopharmacol 8:155–162
Dalen L, Sonuga-Barke EJ, Hall M, Remington B (2004) Inhibitory deficits, delay aversion and preschool AD/HD: implications for the dual pathway model. Neural Plast 11:1–11
Dallery J, Locey ML (2005) Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol 16:15–23
Dallery J, Raiff BR (2007) Delay discounting predicts cigarette smoking in a laboratory model of abstinence reinforcement. Psychopharmacol 190:485–496
Dalley JW, Theobald DEH, Pereira EA, Li PM, Robbins TW (2002) Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacol 164:329–340
Dalley JW, Laane K, Pena Y, Theobald DEH, Everitt BJ, Robbins TW (2005a) Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacol 182:579–587
Dalley JW, Theobald DEH, Berry D, Milstein JA, Laane K, Everitt BJ, Robbins TW (2005b) Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacol 30:525–537
Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007a) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315:1267–1270
Dalley JW, Laane K, Theobald DEH, Pena Y, Bruce CC, Huszar AC, Wojcieszek M, Everitt BJ, Robbins TW (2007b) Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacol 32:1195–1206
De Bellis MD (2002) Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinol 27:155–170
de Vries TJ, Schoffelmeer ANM, Binnekade R, Mulder AH, Vanderschuren LJMJ (1998) Drug-induced reinstatement of heroin- and cocaine-seeking behaviour following long-term extinction is associated with expression of behavioural sensitization. Eur J Neurosci 10:3565–3571
de Wit H, Richards JB (2004) Dual determinants of drug use in humans: reward and impulsivity. Nebr Symp Motiv 50:19–55
de Wit H, Crean J, Richards JB (2000) Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci 114:830–837
de Wit H, Enggasser JL, Richards JB (2002) Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacol 27:813–825
Deroche-Gamonet V, Belin D, Piazza PV (2004) Evidence for addiction-like behavior in the rat. Science 305:1014–1017
Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA (1998) Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol 16:275–278
Dews PB (1958) Studies on behavior. IV Stimulant actions of methamphetamine. J Pharmacol Exp Ther 122:137–147
Dews PB, Wenger GR (1977) Rate-dependency of the behavioral effects of amphetamine. In: Thompson T, Dews PB (eds) Advances in behavioral pharmacology, vol. 1. Academic, New York, pp 167–227
Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer ANM, de Vries TJ (2008) Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63:301–308
Dinn WM, Aycicegi A, Harris CL (2004) Cigarette smoking in a student sample: neurocognitive and clinical correlates. Addict Behav 29:107–126
Doran N, Spring B, McChargue D, Pergadia M, Richmond M (2004) Impulsivity and smoking relapse. Nicotine Tob Res 6:641–647
Doran N, Spring B, McChargue D (2007) Effect of impulsivity on craving and behavioral reactivity to smoking cues. Psychopharmacol 194:279–288
Eagle DM, Robbins TW (2003) Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci 117:1302–1317
Eagle DM, Tufft MR, Goodchild HL, Robbins TW (2007) Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacol 192:193–206
Easdon CM, Vogel-Sprott M (2000) Alcohol and behavioural control: impaired reponse inhibition and flexibility in social drinkers. Exp Clin Psychopharmacol 8:387–394
Easdon C, Izenberg A, Armilio ML, Yu H, Alain C (2005) Alcohol consumption impairs stimulus- and error-related processing during a go/no-go task. Brain Res Cogn Brain Res 25:873–883
Ehrman RN, Robbins SJ, Cornish JW (2001) Results of a baseline urine test predict levels of cocaine use during treatment. Drug Alcohol Depend 62:1–7
Epstein DH, Preston KL, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacol 189:1–16
Evenden JL (1999) Varieties of impulsivity. Psychopharmacol 146:348–361
Evenden JL, Ryan CN (1996) The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacol 128:161–170
Evenden JL, Ryan CN (1999) The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacol 146:413–421
Farrar AM, Kieres AK, Hausknecht KA, de Wit H, Richards JB (2003) Effects of reinforcer magnitude on an animal model of impulsive behavior. Behav Processes 64:261–271
Feola TW, de Wit H, Richards JB (2000) Effects of d-amphetamine and alcohol on a measure of behavioral inhibition in rats. Behav Neurosci 114:838–848
Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J (2006) Delay discounting and the behavioural economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacol 186:255–263
Field M, Christiansen P, Cole J, Goudie A (2007) Delay discounting and the alcohol Stroop in heavy drinking adolescents. Addiction 102:579–586
Fillmore MT (2003) Drug abuse as a problem of impaired control: current approaches and findings. Behav Cogn Neurosci Rev 2:179–197
Fillmore MT, Blackburn J (2002) Compensating for alcohol-induced impairment: alcohol expectancies and behavioral disinhibition. J Stud Alcohol 63:237–246
Fillmore MT, Rush CR (2002) Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend 66:265–273
Fillmore MT, Vogel-Sprott M (1999) An alcohol model of impaired inhibitory control and its treatment in humans. Exp Clin Psychopharmacol 7:49–55
Fillmore MT, Weafer J (2004) Alcohol impairment of behavior in men and women. Addiction 99:1237–1246
Fillmore MT, Rush CR, Kelly TH, Hays L (2001) Triazolam impairs inhibitory control of behavior in humans. Exp Clin Psychopharmacol 9:363–371
Fillmore MT, Rush CR, Hays L (2002) Acute effects of oral cocaine on inhibitory control of behavior in humans. Drug Alcohol Depend 67:157–167
Fillmore MT, Rush CR, Marczinski CA (2003) Effects of d-amphetamine on behavioral control in stimulant abusers: the role of prepotent response tendencies. Drug Alcohol Depend 71:143–152
Fillmore MT, Kelly TH, Martin CA (2005a) Effects of d-amphetamine in human models of information processing and inhibitory control. Drug Alcohol Depend 77:151–159
Fillmore MT, Rush CR, Hays L (2005b) Cocaine improved inhibitory control in a human model of response conflict. Exp Clin Psychopharmacol 13:327–335
Fillmore MT, Rush CR, Hays L (2006) Acute effects of cocaine in two models of inhibitory control: implications of non-linear dose effects. Addiction 101:1323–1332
Fuchs RA, Evans KA, Mehta RH, Case JM, See RE (2005) Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacol 179:662–672
Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ (2002) Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacol 163:174–182
Grant JE, Potenza MN (2006) Compulsive aspects of impulse-control disorders. Psychiatr Clin North Am 29:539–551
Green TA, Gehrke BJ, Bardo MT (2002) Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacol 162:373–378
Green L, Myerson J, Holt DD, Slevin JR, Estle SJ (2004) Discounting of delayed food rewards in pigeons and rats: is there a magnitude effect? J Exp Anal Behav 81:39–50
Grigson PS (2002) Like drugs for chocolate: separate rewards modulated by common mechanisms? Physiol Behav 76:389–395
Hahn B, Shoaib M, Stolerman IP (2002) Effects of dopamine receptor antagonists on nicotine-induced attentional enhancement. Behav Pharm 13:621–632
Heil SH, Johnson MW, Higgins ST, Bickel WK (2006) Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav 31:1290–1294
Hellemans KG, Nobrega JN, Olmstead MC (2005) Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: relationship to forebrain 5-HT1A receptor binding. Behav Brain Res 159:207–220
Hernandez-Avila CA, Rounsaville BJ, Kranzler HR (2004) Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend 74:265–272
Hester R, Garavan H (2004) Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci 24:11017–11022
Heyman GM, Gibb SP (2006) Delay discounting in college cigarette chippers. Behav Pharmacol 17:669–679
Hill RT (1970) Facilitation of conditioned reinforcement as a mechanism of psychomotor stimulation. In: Costa E, Garattini S (eds) Amphetamine and related compounds. Raven, New York, pp 781–795
Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH (2006) Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacol 188:162–170
Hu M, Becker JB (2003) Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci 23:693–699
Isles AR, Humby T, Wilkinson LS (2003) Measuring impulsivity in mice using a novel operant delayed reinforcement task: effects of behavioural manipulations and d-amphetamine. Psychopharmacol 170:376–382
Jackson LR, Robinson TE, Becker JB (2006) Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacol 31:129–138
Jentsch JD, Taylor JR (2003) Sex-related differences in spatial divided attention and motor impulsivity in rats. Behav Neurosci 117:76–83
Johnson MW, Bickel WK (2002) Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav 77:129–146
Johnson MW, Bickel WK, Baker F (2007) Moderate drug use and delay discounting: a comparison of heavy, light, and never smokers. Exp Clin Psychopharmacol 15:187–194
Kampman KM, Alterman AI, Volpicelli JR, Maany I, Muller ES, Luce DD, Mulholland EM, Jawad AF, Parikh GA, Mulvaney FD, Weinrieb RM, O’Brien CP (2001) Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychol Addict Behav 15:52–59
Katz JL, Higgins ST (2003) The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacol 168:21–30
Kaufman JN, Ross TJ, Stein EA, Garavan H (2003) Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci 23:7839–7843
Kelleher RT, Morse WH (1968) Determinants of the specificity of the behavioral effects of drugs. Ergeb Physiol 60:1–56
Kelley AE, Schochet T, Landry CF (2004) Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci 1021:27–32
Kieres AK, Hausknecht KA, Farrar AM, Acheson A, de Wit H, Richards JB (2004) Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacol 173:167–174
Kirby KN (1997) Bidding on the future: evidence against normative discounting of delayed rewards. J Exp Psychol Gen 126:54–70
Kirby KN, Marakovic NN (1996) Delay-discounting probabilistic rewards: rates decrease as amounts increase. Psychon Bull Rev 3:100–104
Kirby KN, Petry NM (2004) Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction 99:461–471
Kirby KN, Petry NM, Bickel WK (1999) Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen 128:78–87
Kollins SH (2002) Delay discounting is associated with substance use in college students. Addict Behav 28:1167–1173
Koob GF, Kreek MJ (2007) Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry 164:1149–1159
Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacol 24:97–129
Kreek MJ, Nielsen DA, Butelman ER, LaForge KS (2005) Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci 8:1450–1457
Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Cavallo DA, Carroll KM, Potenza MN (2007) Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend 88:79–82
Labouvie EW, McGee CR (1986) Relation of personality to alcohol and drug use in adolescence. J Consult Clin Psychol 54:289–293
Larson EB, Carroll ME (2005) Wheel running as a predictor of cocaine self-administration and reinstatement in rats. Pharmacol Biochem Behav 82:590–600
Larson EB, Roth ME, Anker JJ, Carroll ME (2005) Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav 82:98–108
Larson EB, Anker JJ, Gliddon LA, Carroll ME (2007) Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol 15:461–471
Le Dzung A, Funk D, Harding S, Juzytsch W, Li Z, Fletcher PJ (2008) Intra-median raphe nucleus (MRN) infusions of muscimol, a GABA-A receptor agonist reinstate alcohol seeking in rats: role of impulsivity and reward. Psychopharmacol 195:605–615
Le Moal M, Koob GF (2007) Drug addiction: pathways to the disease and pathophysiological perspectives. Eur Neuropsychopharmacol 17:37–93
Lenoir M, Ahmed SH (2008) Supply of a nondrug substitute reduces escalated heroin consumption. Neuropsychopharmacol (epub ahead of print)
Li CS, Milivojevic V, Kemp K, Hong K, Sinha R (2006) Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug Alcohol Depend 85:205–212
Logan GD, Cowan WB, Davis KA (1984) On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform 10:276–291
Logue AW (1988) Research on self-control: an integrating framework. Behav Brain Sci 11:665–709
Logue AW, Pena-Correal TE, Rodriguez ML, Kabela E (1986) Self-control in adult humans: variation in positive reinforcer amount and delay. J Exp Anal Behav 46:159–173
Logue AW, Tobin H, Chelonis JJ, Wang RY, Geary N, Schachter S (1992) Cocaine decreases self-control in rats: a preliminary report. Psychopharmacol 109:245–247
Lynch WJ (2006) Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol 14:34–41
Lynch WJ, Carroll ME (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacol 144:77–82
Lynch WJ, Carroll ME (2000) Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacol 148:196–200
Lynch WJ, Arizzi MN, Carroll ME (2000) Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacol 152:132–139
Lynch WJ, Roth ME, Mickelberg JL, Carroll ME (2001) Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 68:641–646
Lynch WJ, Roth ME, Carroll ME (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacol 164:121–137
Madden GJ, Petry NM, Badger GJ, Bickel WK (1997) Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol 5:256–262
Madden GJ, Bickel WK, Jacobs EA (1999) Discounting of delayed rewards in opioid-dependent outpatients: exponential or hyperbolic discounting functions? Exp Clin Psychopharmacol 7:284–293
Marczinski CA, Fillmore MT (2003) Dissociative antagonistic effects of caffeine on alcohol-induced impairment of behavioral control. Exp Clin Psychopharmacol 11:228–236
Marczinski CA, Fillmore MT (2005a) Alcohol increases reliance on cues that signal acts of control. Exp Clin Psychopharmacol 13:15–24
Marczinski CA, Fillmore MT (2005b) Compensating for alcohol-induced impairment of control: effects on inhibition and activation of behavior. Psychopharmacol 181:337–346
Marczinski CA, Abroms BD, Van Selst M, Fillmore MT (2005) Alcohol-induced impairment of behavioral control: differential effects on engaging vs. disengaging responses. Psychopharmacol 182:452–459
Mazur JE (1987) An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H (eds) Qualitative analyses of behavior: the effect of delay and of intervening events on reinforcement value. Erlbaum, Hillsdale, NJ, pp 55–73
Mazur JE, Logue A (1978) Choice in a self-control paradigm: effects of a fading procedure. J Exp Anal Behav 30:11–17
McDonald J, Schleifer L, Richards JB, de Wit H (2003) Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacol 28:1356–1365
Mirza NR, Stolerman IP (1998) Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology 138:266–274
Mischel W, Shoda Y, Peake PK (1988) The nature of adolescent competencies predicted by preschool delay of gratification. J Pers Soc Psychol 54:687–696
Mitchell S (1999) Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacol 146:455–464
Mitchell SH (2004) Measuring impulsivity and modeling its association with cigarette smoking. Behav Cogn Neurosci Rev 3:261–275
Mitchell SH, Reeves JM, Li N, Phillips TJ (2006) Delay discounting predicts behavioral sensitization to ethanol in outbred WSC mice. Alcohol Clin Exp Res 30:429–437
Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J (2001) The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat 21:193–198
Monterosso JR, Aron AR, Cordova X, Xu J, London ED (2005) Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend 79:273–277
Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED (2007) Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp 28:383–393
Morgan D, Brebner K, Lynch WJ, Roberts DC (2002) Increases in the reinforcing efficacy of cocaine after particular histories of reinforcement. Behav Pharmacol 13:389–396
Mulvihill LE, Skilling TA, Vogel-Sprott M (1997) Alcohol and the ability to inhibit behavior in men and women. J Stud Alcohol 58:600–605
Nagoshi CT, Wilson JR, Rodriguez LA (1991) Impulsivity, sensation seeking, and behavioral and emotional responses to alcohol. Alcohol Clin Exp Res 15:661–667
Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M (2008) Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry 32:34–41
Newman JP, Widom CS, Nathan S (1985) Passive avoidance in syndromes of disinhibition: Psychopathology and extraversion. J Pers Soc Psychol 48:1316–1327
Nisbett RE, Wilson TD (1977) Telling more than we can know: verbal reports on mental processes. Psychol Rev 84:231–259
Noel X, Van der Linden M, d’Acremont M, Bechara A, Dan B, Hanak C, Verbanck P (2007) Alcohol cues increase cognitive impulsivity in individuals with alcoholism. Psychopharmacol 192:291–298
Odum AL, Madden GJ, Badger GJ, Bickel WK (2000) Needle sharing in opioid-dependent outpatients: psychological processes underlying risk. Drug Alcohol Depend 60:259–266
Ohmura Y, Takahashi T, Kitamura N (2005) Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacol 182:508–515
Olmstead MC (2006) Animal models of drug addiction: where do we go from here? Q J Exp Psychol 59:625–653
Ortner CN, MacDonald TK, Olmstead MC (2003) Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol 38:151–156
Paine TA, Olmstead MC (2004) Cocaine disrupts both behavioural inhibition and conditional discrimination in rats. Psychopharmacol 175:443–450
Paine TA, Dringenberg HC, Olmstead MC (2003) Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res 147:135–147
Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA (2007) Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in sprague-dawley rats. Biol Psychiatry 62:687–693
Perry JL, Larson EB, German JP, Madden GJ, Carroll ME (2005) Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacol 178:193–201
Perry JL, Morgan AD, Anker JJ, Dess NK, Carroll ME (2006) Escalation of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacol 186:235–245
Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME (2007) Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav 86:822–837
Perry JL, Nelson SE, Carroll ME (2008a) Impulsive choice as a predictor of acquisition of i.v. cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmachol 16:165–177
Perry JL, Stairs DJ, Bardo MT (2008b) Delay discounting an environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res (epub ahead of print)
Petry NM (2001) Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacol 154:243–250
Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ (2004) Prize reinforcement contingency management for treating cocaine users: how low can we go, and with whom? Addiction 99:349–360
Piazza PV, Le Moal M (1998) The role of stress in drug self-administration. Trends Pharmacol Sci 19:67–74
Pietras CJ, Cherek DR, Lane SD, Tcheremissine OV, Steinberg JL (2003) Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacol 170:390–398
Pitts RC, McKinney AP (2005) Effects of methylphenidate and morphine on delay-discount functions obtained within sessions. J Exp Anal Behav 83:297–314
Potter AS, Newhouse PA (2004) Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacol 176:182–194
Poulos CX, Le AD, Parker JL (1995) Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol 6:810–814
Poulos CX, Parker JL, Le DA (1998) Increased impulsivity after injected alcohol predicts later alcohol consumption in rats: evidence for “loss-of-control drinking” and marked individual differences. Behav Neurosci 112:1247–1257
Quednow BB, Kuhn KU, Hoppe C, Westheide J, Maier W, Daum I, Wagner M (2006) Elevated impulsivity and impaired decision-making cognition in heavy users of MDMA (“Ecstasy”). Psychopharmacol 189(4):517–530
Ramaekers JG, Kuypers KP (2006) Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) on behavioral measures of impulsivity: alone and in combination with alcohol. Neuropsychopharmacol 31:1048–1055
Reynolds B, Schiffbauer R (2004) Measuring state changes in human delay discounting: an experiential discounting task. Behav Processes 67:343–356
Reynolds B, Karraker K, Horn K, Richards JB (2003) Delay and probability discounting as related to different stages of adolescent smoking and non-smoking. Behav Processes 64:333–344
Reynolds B, Richards JB, Dassinger M, de Wit H (2004a) Therapeutic doses of diazepam do not alter impulsive behavior in humans. Pharmacol Biochem Behav 79:17–24
Reynolds B, Richards JB, Horn K, Karraker K (2004b) Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav Processes 65:35–42
Reynolds B, Ortengren A, Richards JB, de Wit H (2006a) Dimensions of impulsive behavior: personality and behavioral measures. Pers Individ Diff 40:305–315
Reynolds B, Richards JB, de Wit H (2006b) Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question-based measure of delay discounting. Pharmacol Biochem Behav 83:194–202
Reynolds B, Patak M, Shroff P, Penfold RB, Melanko S, Duhig AM (2007) Laboratory and self-report assessments of impulsive behavior in adolescent daily smokers and nonsmokers. Exp Clin Psychopharmacol 15:264–271
Richards JB, Mitchell SH, de Wit H, Seiden LS (1997) Determination of discount functions in rats with an adjusting-amount procedure. J Exp Anal Behav 67:353–366
Richards JB, Sabol KE, de Wit H (1999a) Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacol 146:432–439
Richards JB, Zhang L, Mitchell SH, de Wit H (1999b) Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav 71:121–143
Robbins TW (1978) The acquisition of responding with conditioned reinforcement: effects of pipradrol, methylphenidate, d-amphetamine and nomifensine. Psychopharmacol 58:79–87
Robbins TW (2002) The 5-choice serial reaction time task: behavioral pharmacology and functional neurochemistry. Psychopharmacol 163:362–380
Robbins TW, Sahakian BJ (1979) “Paradoxical” effects of psychomotor stimulant drugs from the standpoint of behavioural pharmacology. Neuropharmacol 18:931–950
Robbins TW, Watson BA, Gaskin M, Ennis C (1983) Contrasting interactions of pipradrol, d-amphetamine, cocaine, cocaine analogs, apomorphine and other drugs with conditioned reinforcement. Psychopharmacol 80:113–119
Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW (2007) Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacol 33(5):1028–1037
Roth ME, Carroll ME (2004) Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav 78:199–207
Roth ME, Casimir AG, Carroll ME (2002) Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacol Biochem Behav 72:313–318
Roth ME, Cosgrove KP, Carroll ME (2004) Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev 28:533–546
Sahakian BJ, Owen AM, Morant NJ, Eagger SA, Boddington S, Crayton L, Crockford HA, Crooks M, Hill K, Levy R (1993) Further analysis of the cognitive effects of tetrahydroaminoacridine (THA) in Alzheimer’s disease: assessment of attentional and mnemonic function using CANTAB. Psychopharmacol 110:395–401
Shaffer HJ, Eber GB (2002) Temporal progression of cocaine dependence symptoms in the US National Comorbidity Survey. Addiction 97:543–554
Shaham Y, Miczek KA (2003) Reinstatement: toward a model of relapse. Psychopharmacol 168:1–2
Shaham Y, Shalev U, Lu L, de Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacol 168:3–20
Shalev U, Grimm JW, Shaham Y (2002) Neurobioloby of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42
Shoaib M, Bizarro L (2005) Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacol 178:211–222
Simon NW, Mendez IA, Setlow B (2007) Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci 121:543–549
Skinner MD, Aubin HJ, Berlin I (2004) Impulsivity in smoking, nonsmoking, and ex-smoking alcoholics. Addict Behav 29:973–978
Sofuoglu M, Gonzalez G, Poling J, Kosten TR (2003) Prediction of treatment outcome by baseline urine cocaine results and self-reported cocaine use for cocaine and opioid dependence. Am J Drug Alcohol Abuse 29:713–727
Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, Hechtman L, Hinshaw S, Turkel E (2001) The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. J Abnorm Child Psychol 29:215–228
Sonuga-Barke EJ (2003) The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev 27:593–604
Sonuga-Barke EJ, Dalen L, Remington B (2003) Do executive deficits and delay aversion make independent contributions to preschool attention-deficit/hyperactivity disorder symptoms? J Am Acad Child Adolesc Psych 42:1335–1342
Spear NE, Molina JC (2005) Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res 29:909–929
Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrila EP, Koob GF (2008) CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacol 196:473–482
Spinella M (2002) Correlations between orbitofrontal dysfunction and tobacco smoking. Addict Biol 7:381–384
Steele CM, Josephs RA (1990) Alcohol myopia. Its prized and dangerous effects. Am Psychol 45:921–933
Stitzer ML, Peirce J, Petry NM, Kirby K, Roll J, Krasnansky J, Cohen A, Blaine J, Vandrey R, Kolodner K, Li R (2007a) Abstinence-based incentives in methadone maintenance: interaction with intake stimulant test results. Exp Clin Psychopharmacol 15:344–350
Stitzer ML, Petry N, Peirce J, Kirby K, Killeen T, Roll J, Hamilton J, Stabile PQ, Sterling R, Brown C, Kolodner K, Li R (2007b) Effectiveness of abstinence-based incentives: interaction with intake stimulant test results. J Consult Clin Psychol 75:805–811
Stolerman IP, Mirza NR, Hahn B, Shoaib M (2000) Nicotine in an animal model of attention. Eur J Pharmacol 393:147–154
Substance Abuse and Health Services Administration (2006) Results from the 2005 National Survey on Drug Use and Health: National Findings. Office of Applied Studies, NSDUH Series H-30, DHHS Publication No. SMA 06-4194, Rockville, MD
Svensson AI, Soderpalm B, Engel JA (2000) Gonadectomy enhances shock-induced behavioral inhibition in adult male rats: implications for impulsive behavior. Pharmacol Biochem Behav 65:731–736
Swann AC, Bjork JM, Moeller FG, Dougherty DM (2002) Two models of impulsivity: relationship to personality traits and psychopathology. Biol Psychiatry 51:988–994
Takahashi T, Sakaguchi K, Oki M, Homma S, Hasegawa T (2006) Testosterone levels and discounting delayed monetary gains and losses in male humans. Neuro Endocrinol Lett 27:439–444
Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD (1989) Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol 17:473–491
Tannock R, Schachar R, Logan G (1995) Methylphenidate and cognitive flexibility: dissociated dose effects in hyperactive children. J Abnorm Child Psychol 23:235–266
Tidey JW, Miczek KA (1997) Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacol 130:203–212
Uhl G (2007) Premature poking: impulsivity, cocaine and dopamine. Nat Med 13:413–414
Vaidya JG, Grippo AJ, Johnson AK, Watson D (2004) A comparative developmental study of impulsivity in rats and humans: the role of reward sensitivity. Ann N Y Acad Sci 1021:395–398
Van den Bergh F, Spronk M, Ferreira L, Bloemarts E, Groenink L, Olivier B, Oosting R (2006) Relationship of delay aversion and response inhibition to extinction learning, aggression, and sexual behaviour. Behav Brain Res 175:75–81
van Gaalen MM, Brueggeman RJ, Bronius PFC, Schoffelmeer ANM, Vanderschuren LJMJ (2006a) Behavioral disinhibition requires dopamine receptor activation. Psychopharmacol 187:73–85
van Gaalen MM, van Koten R, Schoffelmeer A, Vanderschuren L (2006b) Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry 60:66–73
Van Haaren F, Van Hest A, Van De Poll NE (1988) Self-control in male and female rats. J Exp Anal Behav 49:201–211
Verdejo-Garcia AJ, Perales JC, Perez-Garcia M (2007) Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav 32:950–966
Vuchinich RE, Simpson CA (1998) Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol 6:292–305
Wade TR, de Wit H, Richards JB (2000) Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacol 150:90–101
Waldeck TL, Miller LS (1997) Gender and impulsivity differences in licit substance use. J Subst Abuse 9:269–275
Wallace CJ (1979) The effects of delayed rewards, social pressure, and frustration on the responses of opiate addicts. NIDA Res Monogr 25:6–25
Wee S, Wang Z, Woolverton WL, Pulvirenti L, Koob GF (2007) Effect of aripiprazole, a partial dopamine D(2) receptor agonist, on increased rate of methamphetamine self-administration in rats with prolonged session duration. Neuropsychopharmacol 32:2238–2247
Wills TA, Vaccaro D, Benson G (1995) Coping and competence in adolescent alcohol and drug use. In: Wallander JL, Lawrence J (eds) Adolescent health problems: behavioral perspectives. Advances in pediatric psychology. Guilford, New York, pp 160–178
Winstanley CA, Dalley JW, Theobald DE, Robbins TW (2003) Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacol 170:320–331
Woolverton WL, Myerson J, Green L (2007) Delay discounting of cocaine by rhesus monkeys. Exp Clin Psychopharmacol 15:238–244
Wulfert E, Block JA, Santa Ana E, Rodriguez ML, Colsman M (2002) Delay of gratification: impulsive choices and problem behaviors in early and late adolescence. J Pers 70:533–552
Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ (2007) Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp Clin Psychopharmacol 15:176–186
Acknowledgements
We would like to thank the following people for their comments on earlier versions of this manuscript: Justin Anker, Michael Bardo, Ph.D., Jonathan Gewirtz, Ph.D., Erin Larson, Ph.D., Joshua Lile, Ph.D., Sarah Nelson, Jennifer Newman, Ph.D., J. Bruce Overmier, Ph.D., Jason Ross, M.S., William Stoops, Ph.D., Mark Thomas, Ph.D., and Thomas Wooters, M.A. We also thank NIH/NIDA for grant support: R01 DA03240 and K05 DA15267 (MEC) and F31 DA020237 (JLP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perry, J.L., Carroll, M.E. The role of impulsive behavior in drug abuse. Psychopharmacology 200, 1–26 (2008). https://doi.org/10.1007/s00213-008-1173-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-008-1173-0