Abstract
Rationale
Several studies with nonhumans and humans have shown that stimulants decrease impulsive choices on delay-to-reinforcement (self-control) procedures. Little is known, however, about the effects of the stimulant methylphenidate on choice for delayed reinforcers in humans.
Objectives
The present study was designed to investigate the effects of acute methylphenidate administrations on impulsive responding in adult humans on a delay-to-reinforcement task.
Methods
Eleven adult males with a history of criminal behavior but no history of attention–deficit hyperactivity disorder (ADHD) participated. Impulsive responding was measured using an adjusting-delay procedure in which subjects were presented with repeated choices between a small amount of money delivered after a short delay and a larger amount of money delivered after a delay that adjusted as a function of previous choices. Subjects were exposed to four experimental sessions each day of participation and 60 min prior to the first daily session received placebo or 0.15, 0.30, or 0.60 mg/kg methylphenidate. Stable choice patterns were re-established between each methylphenidate dose.
Results
Individuals differed in their sensitivity to methylphenidate, but in over half of the subjects methylphenidate decreased impulsive (i.e., increased the number of self-control choices) and increased the delay to the large reinforcer. The largest increases in self-control choices tended to occur at the 0.30-mg/kg and 0.60-mg/kg doses, and the effects often persisted across multiple daily sessions. In six subjects, under at least one methylphenidate dose, the number of impulsive choices decreased to zero.
Conclusions
Acute methylphenidate administrations tended to decrease the number of impulsive choices in adult humans on an adjusting-delay procedure, although there were substantial individual differences in the sensitivity of choice to methylphenidate. In no case, however, did methylphenidate increase impulsive choices. These results are consistent with several recent laboratory studies with nonhumans and humans showing that stimulants increase preference for large, delayed reinforcers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impulsiveness may underlie many socially unacceptable, maladaptive, and potentially harmful patterns of behavior (Evenden 1999; Logue 2000). A variety of drugs have therefore been investigated to determine their effects on impulsive responding, including central nervous system (CNS) stimulants. Clinical studies have shown that CNS stimulants significantly reduce ratings of problematic impulsive behavior in individuals with histories of attention–deficit hyperactivity disorder (ADHD) and conduct disorder (CD) (Barkley 1977; Campbell et al. 1999). Similar improvements in impulsive behavior following stimulant administration have been reported in adults with ADHD symptoms (Wender et al. 1985; Spencer et al. 1995). In addition, Klein et al. (1997) showed that stimulants improved ratings of impulsive and disruptive behaviors in children diagnosed with CD independent of whether they were diagnosed with ADHD.
The effects of stimulants on impulsive behavior have been investigated under laboratory conditions with a number of experimental procedures, including signal-detection procedures such as the matching familiar figures task (Rapport et al. 1988), the go–no-go task (Trommer et al. 1991), and the continuous performance task (Solanto et al. 1997), and response-inhibition procedures such as the stop-signal task (Tannock et al. 1989). On these tasks, impulsive responding is typically defined as inappropriate responses to non-target stimuli (commission errors) or failures to withhold or inhibit responses. Studies have shown that children with ADHD perform more poorly on these tasks than normal children, but that stimulants such as methylphenidate often improve performance and reduce the frequency of errors (Trommer et al. 1991; Riccio et al. 2001). Stimulants have also been shown to improve performance in normal children and adults on these tasks (Rapoport et al. 1980; Sostek et al. 1980), suggesting that the improvements in performance on these tasks following stimulant administration are not unique to individuals with ADHD diagnoses.
Another experimental procedure that has been used with a variety of species and populations to study impulsive responding is a delay-to-reinforcement procedure, called a self-control, delay-of-gratification, or delay-discounting task. Many variants of this procedure have been developed, but all involve presenting subjects with choices between small, immediate reinforcers and larger, but more delayed reinforcers. Preference for the small option is defined as impulsive, and preference for the large option is defined as self-controlled (Rachlin and Green 1972; Ainslie 1974; Logue 1988). Typically, choice in experimentally naive nonhumans is impulsive on these tasks (Mazur and Logue 1978), and choice in normal adults is self-controlled (Logue et al. 1986; Logue 1988). Children with ADHD (Sonuga-Barke et al. 1992; Solanto et al. 2001) and adults with histories of aggressive behavior (Cherek et al. 1997; Cherek and Lane 1999b) or substance abuse (Bickel and Marsch 2001) (behaviors typically defined as impulsive), however, often show greater impulsivity than matched controls.
Several studies with nonhumans have investigated the effects of stimulants on choice in reinforcer-delay tasks and have shown that stimulants sometimes reduce impulsive choices. For example, Richards et al. (1999) presented rats with choices between a delayed, constant amount of water and an immediate amount of water that varied in magnitude as a function of prior choices. Choice of the immediate option decreased the magnitude of the immediate option whereas choice of the delayed option increased it on the subsequent trial. Richards et al. showed that methamphetamine increased preference for the delayed option (i.e., increased the magnitude of the immediate option). Similar results were shown in a study with rats given d-amphetamine (Wade et al. 2000). Conversely, Charrier and Thiébot (1996) and Evenden and Ryan (1996) showed that d-amphetamine increased rather than decreased the number of impulsive choices in rats when responding was measured using non-adjusting, delay-to-reinforcement procedures. The variables responsible for these differences are uncertain, but several procedural differences may have contributed to the discrepant results (Richards et al. 1999).
To date, only one study has examined the effects of stimulants on choice in humans using a delay-to-reinforcement procedure. In a study with normal adults, de Wit et al. (2002) investigated the effects of d-amphetamine on performance using a variety of tasks designed to measure impulsivity, including a delay-discounting task. De Wit et al. showed that the highest dose of d-amphetamine significantly reduced delay discounting, i.e., increased self-control.
The aim of the present study was to investigate the effects of another stimulant, methylphenidate, on choice in adults on a reinforcer-delay (self-control) task. To study choice in individuals who would likely show frequent impulsive responses, individuals with histories of criminal behavior and substance abuse were recruited for participation. Although studies have shown that individuals with and without ADHD symptoms show comparable changes in performance following stimulant administration (Sostek et al. 1980; Rapoport et al. 1980), to minimize the possibility that the effects of methylphenidate on choice could be attributed to a specific behavioral deficiency characteristic of ADHD, individuals reporting previous ADHD diagnoses were excluded from participation.
Methods
Subjects
All procedures were reviewed and approved by the Institutional Review Board for the Health Science Center and informed consent was obtained from all subjects (and from parents or legal guardians of subjects below the age of 18 years) prior to participation. Potential volunteers responded to advertisements for research studies seeking individuals on parole or probation that were placed in free newspapers distributed in the Houston area. Only volunteers between the ages of 17 years and 26 years were recruited to facilitate assessment of ADHD symptoms in childhood (e.g., to increase the probability of contacting an older relative). Subjects reporting any medical or psychiatric illness were excluded. Subjects received a physical exam prior to participation. All subjects were screened for psychiatric illness using a mental status exam and the structured clinical interview for DSM-IV (SCID-P), a standardized psychiatric interview (First et al. 1996). Subjects were excluded for any axis-I disorder, except past substance abuse or dependence, although subjects who reported past substance abuse or dependence involving a stimulant were excluded. The SCID-II structured clinical interview was used to assess childhood CD by 15 years of age.
The final sample consisted of 11 male subjects between the ages of 17 years and 23 years (mean age was 20.1±1.6 years). None reported a history of ADHD or prior methylphenidate use. Five subjects met criteria for CD. All subjects had been arrested at least once for robbery, possession, delivery of a controlled substance, assault, fighting with a police officer, possession of an unlicensed weapon, endangering a child, or other crimes. Most subjects reported infrequent alcohol use; one reported consuming five alcoholic drinks per week. Four subjects reported smoking 6–15 cigarettes per day. None reported current illicit drug use. Five subjects met criteria for past substance dependence: marijuana (2), codeine (1), and multiple drug dependence (2, including alcohol, marijuana, codeine, phencyclidine, and benzodiazepines). The mean educational level was 12.1±0.94 years. Demographic information for all subjects is shown in Table 1 and Table 2.
Although no subject reported a history of ADHD, subjects were evaluated for possible ADHD symptoms in childhood using the Kiddie-Sads-Present and Lifetime Version (K-Sads-PL; Kaufman et al. 1997), worded in the past tense (which includes DSM IV-R criteria for ADHD diagnosis) and the Wender Utah Rating Scale (WURS; Ward et al. 1993). Subjects were screened for current symptoms of ADHD using the Conners Adult ADHD rating scales-self-report: long version (CAARS-S:L). When possible, informants (i.e., older relatives) were also contacted to evaluate past and current ADHD symptoms. For six subjects for whom informants could be contacted, informants completed the Conners Adult ADHD rating scales-observer-report: long version (CAARS-O:L) and responded to the K-Sads-PL, worded in past tense to retrospectively assess ADHD symptoms in childhood.
Scores on the WURS, CAARS-S:L ADHD index, and CAARS-O:L are shown in Table 1 and Table 2. On the WURS, one subject (2610) scored one point above the 46 point cutoff (out of a possible 75 points) used in previous research to help identify ADHD patients (Ward et al. 1993). On the CAARS-S:L, one subject (2737) scored above the 65th percentile on the ADHD index. Scores by the informant for this subject on the CAARS-O:L, however, were much lower. Subjects 2610 and 2705 reported ADHD symptoms on the K-Sads-PL that were consistent with DSM-IV criteria for childhood ADHD; but, for subject 2705, reports of ADHD symptoms in childhood and scores on the CAARS-O:L by the subject's mother did not indicate significant past or current AHDH symptoms. No other informant indicated that a subject showed significant ADHD symptoms in childhood or as an adult.
Prior to participation, all subjects were tested on the immediate memory task (IMT; Dougherty et al. 1998) in which commission errors (false alarms) are used as a measure of impulsivity. Hit rates, false alarms, and values of d-prime (a measure of discriminability) for each subject are shown in Table 1 and Table 2. For false alarms, two subjects (2610 and 2705) scored more than one standard deviation above the group mean. At the end of the study, subjects completed the Barratt impulsivity scale-BIS 11 (Barratt 1985), the scores of which are also shown in Table 1 and Table 2. Subjects 2588 and 2737 scored one standard deviation above the group mean on measures of non-planning and attention, respectively.
Overall, across the K-SADS, WURS, CAARS-S:L, IMT, and BIS 11, no subject consistently scored higher or consistently showed more impulsive responding than others, although subjects 2610 and 2705 indicated some ADHD symptoms. Of these two subjects, only in subject 2705 was choice sensitive to methylphenidate and in both subjects choice patterns under placebo conditions and under methylphenidate conditions were consistent with choice patterns observed in the other subjects (see below).
To assess cognitive functioning, at the end of the study subjects were administered the Shipley Institute of Living Scale (Shipley Boyle 1967), a test of general intellectual aptitude that includes a 40-item vocabulary test and a 20-item abstraction test (subject 2491 did not return to complete the test). Shipley score estimates of Wechsler adult intelligence scale (WAIS) IQ correlate highly (0.76–0.87) with actual WAIS IQ scores (Zachary et al. 1985). Mean raw scores for the other ten subjects on the Shipley test were 45±12.2, with estimated mean WAIS scores of 99.8±9.5 (range 84–112).
Extraneous drug use
Each day in which subjects came into the laboratory an expired air sample and a urine sample were collected to monitor recent drug and alcohol use. The alcohol content of the expired air was measured using an Alcosensor III (Intoximeter, Model 3000, St. Louis, MO). The urine sample was subjected to a complete drug screen analysis using the enzyme multiple immunoassay technique—drug abuse urine assay (EMIT d.a.u. by SYLVA Corporation, Palo Alto, CA). Multiple detections of any drug in the subject's urine or alcohol in the air sample resulted in the removal of the subject from the study. Of the 11 subjects who completed the study, 2 tested positive for drugs (marijuana or benzodiazepines) on one occasion (data from those sessions were excluded from analyses). Urinalysis results were provided within 7 h.
Apparatus
During experimental sessions, subjects were seated in a 1.2-m by 1.8-m sound-attenuated chamber containing a VGA monitor and a 10×43×25-cm response panel. Three microswitch push buttons labeled "A", "B" and "C" were mounted horizontally on the top of the response panel 10 cm apart. The monitor and response panel were linked to a Pentium-based computer outside the chamber using a Med Associates interface card and a customized hardware/software system. This computer and interface controlled and recorded all experimental events.
Procedure
Subjects were presented with 50 choices between a small, immediate reinforcer (option A) and a larger, more delayed reinforcer (option B). Both the A and B letters appeared on the screen at the beginning of each trial. A single response on the A or B button disabled the alternative option and correlated letter and initiated the delay timer. At the end of the delay interval, the letter flashed off and on. A single response on the button corresponding to the letter on the screen added 5 cents (option A) or 15 cents (option B) to the counter and caused the letter to disappear. After 2 s, the two letters re-appeared on the screen signaling the beginning of the next trial.
The delay to money delivery on option A (small amount) was 5 s. At the beginning of each session, the delay to money delivery on option B (large amount) was 15 s. Each choice of the A option decreased the delay on the B option by 2 s on the next trial to a minimum of 7 s, and each choice of the B option increased its delay by 2 s on the next trial. If option B was chosen exclusively during a session, the delay on option B would increase to 113 s.
Because sessions terminated after a fixed number of trials rather than after a fixed amount of time, there was no monetary advantage for choosing the A option; only choices for the B (large) option would maximize earnings. Thus, choices for the small, immediate option (A) were operationally defined as impulsive, and choices for the larger, more delayed option (B) were operationally defined as self-controlled.
Subjects participated either two (Tues, Thur) or three (Mon, Wed, Fri) days a week. Urine and breath sample were obtained from subjects when they arrived in the laboratory at 0800 hours. Subjects participated in four impulsivity sessions per day at 0930 hours, 1100 hours, 1330 hours and 1500 hours. Subjects also participated in a laboratory task designed to measure aggressive responses (the point-subtraction aggression paradigm or PSAP; Cherek 1992) four sessions per day at 0900 hours, 1030 hours, 1300 hours, and 1430 hours. Results from these sessions are not reported here. PSAP sessions lasted 25 min and subjects were given a 5-min break outside the testing chamber between each PSAP and impulsivity session. Between sessions, subjects waited in a common area containing a television and magazines. Lunch was provided at 1200 hours. Impulsivity sessions ended after 50 trials, but the session length varied as a function of subjects' choices. If the self-control (B) option was preferred exclusively, the session was 53 min, and if the impulsive (A) option was preferred exclusively, the session was approximately 6 min. Subjects did not receive any information regarding session duration. At the end of each day of participation, subjects were paid in cash the total amount earned during all sessions.
Instructions
Prior to participation, subjects were provided with information about potential earnings, urine drug testing, breath alcohol testing, and psychiatric screening. Subjects were told that they could expect to earn an average of US $6.00 to $7.00 per hour and that additional bonuses would be provided for drug-free breath and urine samples, attendance, and for completing the study. The first day of participation, subjects were shown a diagram of the computer monitor and response panel and were read a set of scripted instructions (Cherek and Lane 1999a). While the instructions were read, the events were drawn on the diagram. The instructions stated that subjects must choose between the A or B option, and that when the letter of the selected option flashed, another response would add money to the counter. On the diagram, subjects were shown US $0.05 added to the counter when the A (small) option was chosen, and US $0.15 added to the counter when the B (large) option was chosen. No additional information regarding the procedure was provided, and portions of the instructions were repeated if a subject had any questions.
Methylphenidate
Placebo or methylphenidate was administered orally in #00 opaque capsules at 0830 hours, approximately 1 h before the first impulsivity session. The peak effects of methylphenidate occur approximately 1–2 h after administration (Patrick et al. 1987; Swanson and Volkow 2002). Thus, the peak effects were expected to occur during at least one of the four experimental sessions. Placebo doses were administered until the number of choices for the large option was judged stable by visual inspection (i.e., choices showed little session-to-session variability and no increasing or decreasing trends). Three doses of methylphenidate, 0.15, 0.30, and 0.60 mg/kg were then administered in an ascending sequence and stable choice patterns were re-established between each dose. Doses were separated by a minimum of 72 h (three calendar days), but in nearly every case doses were separated by at least six calendar days. The three doses used were within the range of doses used therapeutically for ADHD (Findling and Dogin 1998). At the end of each day, subjects were evaluated for signs of impairment and completed a side-effects questionnaire. Methylphenidate was well-tolerated and no adverse effects were reported.
Data analysis
The number of choices for the large option was used as the primary dependent measure. The mean delay to the large option per session was also measured. The peak effects of methylphenidate on choice and the effects of CD were analyzed using a repeated-measures ANOVA with one within-subject factor, dose (4), and one between-group factor, CD (CD+ vs CD−). Effects of session were evaluated using a repeated-measures ANOVA with two factors: session (4) and drug dose (4). Post-hoc analyses of significant main effects and interactions were conducted using the Tukey HSD procedure (Winer 1971).
Results
Because doses were administered in an ascending sequence, shifts in preference under placebo conditions would confound analyses of drug effects. Thus, a repeated-measures ANOVA was used to analyze the mean number of choices for the large option (i.e., the mean of the four daily sessions) across the three placebo days immediately preceding each methylphenidate dose. Two subjects (2563 and 2737) showed increases in self-control choices across the study (see below); but overall, there was no significant difference across placebo days (F 2,10=2.01, P=0.16).
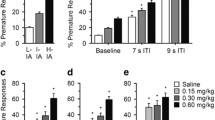
Figure 1 shows the mean number of choices for the large option (i.e., self-control choices) and the mean delay to the large option plotted as a percentage of the preceding placebo session for the daily sessions in which the peak effects of methylphenidate were observed. The sessions in which peak effects were observed (i.e., those showing the maximum change from placebo sessions) varied across subjects and occurred in the first, second, third, and fourth daily sessions in 9, 9, 3, and 12 cases, respectively. Although peak effects were not expected to occur in the fourth session of the day, because choice was often most impulsive in the fourth session (see below), the magnitude of the increase in self-control choices under methylphenidate was often largest in this session. Methylphenidate significantly increased self-control choices, i.e., decreased impulsive choices (F 3,27=3.33, P<0.04). The increase in self-control choices was clear in 7 of the 11 subjects under at least one dose. Post-hoc comparisons indicated that choices under the middle dose differed significantly from choices under placebo conditions (P<0.05). The difference between choices under the highest dose and placebo conditions approached but did not reach statistical significance (P<0.06). There was no significant effect of CD (F 1,9=1.16, P=0.31) and no CD by dose interaction (F 3,27=0.72, P=0.55). Methylphenidate also increased the mean delay to the large option, but the effect was not significant (F 3,27=2.75, P=0.06).
Figure 2 shows for each subject the number of choices for the large option across the four daily sessions under each methylphenidate dose and across the four daily sessions of the preceding placebo day. Mean values are also shown. In six subjects, under one or more methylphenidate doses, the number of choices for the large option increased to the maximum of 50 choices (i.e., impulsive choices decreased to zero). Furthermore, the number of choices for the large option often increased to 50 across multiple sessions. This increase occurred both in subjects who showed few (2452, 2457) and frequent (2482, 2491, 2563, and 2705) impulsive choices under placebo conditions.
Number of choices for the large option across all four daily sessions under 0.15, 0.30 and 0.60 mg/kg methylphenidate (filled circles) and across all four daily sessions during the immediately preceding placebo day (open circles) for each subject. Mean choices are also shown with error bars indicating ±SEM
As shown in Fig. 2, in subjects whose choices were sensitive to methylphenidate, the largest drug effect occurred at different doses. Two subjects (2452, 2482) showed the largest increases in self-control choices at the 0.60-mg/kg doses, but showed little change at the 0.15-mg/kg dose. Conversely, subject 2491 showed a large increase in self-control choices at the 0.15-mg/kg and 0.30-mg/kg doses, but showed little change at the 0.60-mg/kg dose. Subjects 2583 and 2705 showed comparable increases under all doses. For subjects 2457 and 2563, the number of self-control choices increased across the experiment, and the largest proportional changes in self-control choices were observed at the 0.15-mg/kg or 0.30-mg/kg doses, respectively. Interestingly, for subject 2563, the increase in self-control choices was correlated with the administration of the 0.30-mg/kg methylphenidate dose. For this subject, during the four sessions (placebo day) preceding the 0.30-mg/kg dose, the large option was chosen on an average of 31.8 trials per session, but under 0.30 mg/kg methylphenidate the number of choices for the large option increased to an average of 49.3 trials per sessions and remained at an average of 46.9 trials for the remaining placebo sessions.
Figure 2 also illustrates the time course of methylphenidate's effect. Methylphenidate sometimes increased choices for the large option across multiple daily sessions and in some subjects (subjects 2491, 2563, and 2482) increased choices for the large option across all four daily sessions (i.e., up to 6.5 h after drug administration). Choice was often most impulsive in the fourth (final) session of the day under both placebo and methylphenidate conditions, however, presumably because impulsive choices shortened the session duration. There was a significant effect of session (F 3,30=7.41, P<0.001), but there was no significant effect of drug (F 3,30=1.89, P=0.15) or drug by session interaction (F 9,90=1.21, P=0.30). Analyses of the mean delays to the large option across all four daily sessions showed similar effects.
Discussion
Acute methylphenidate administrations tended to decrease impulsive responding (i.e., increase the number of self-controlled choices) in adult males on a reinforcer-delay task. Although there were individual differences in the sensitivity of choice to methylphenidate, with some subjects showing no change and some subjects showing large decreases in impulsive choices, in no case did methylphenidate consistently increase impulsive choices. The decrease in impulsivity is in accord with clinical and laboratory studies showing that methylphenidate decreased impulsive responding in children and adults with ADHD symptoms (Wender et al. 1985; Rapport et al. 1988). That methylphenidate increased self-control choices across multiple daily sessions in some subjects is also comparable with the results of a study by Solanto and Conners (1982) with ADHD children which showed that improvements in performance under methylphenidate on a signal-detection task persisted up to 5.8 h after methylphenidate administration. The present results are also consistent with studies with nonhumans showing that stimulants such as amphetamine and methamphetamine decreased impulsive choices on reinforcer-delay procedures (Richards et al. 1999; Cardinal et al. 2000; Wade et al. 2000), as well as a recent study with humans showing that d-amphetamine decreased delay discounting (de Wit et al. 2002), indicating that the effects of stimulants on impulsive choice generalize across several stimulant types, choice tasks, and subject populations.
The variables responsible for the individual differences in sensitivity to methylphenidate are unclear. Because the number of choices for the large option could not increase beyond 50 (the number of trials per session), it is difficult to determine whether the magnitude of the effects of methylphenidate was related to preference under placebo conditions. It is apparent in Fig. 2, however, that subjects who showed few choices (e.g., subject 2452) and subjects who showed frequent choices for the small option (e.g., subject 2705) under placebo conditions showed an increase in self-control choices under methylphenidate. A similar finding was reported by de Wit et al. (2002); the effects of d-amphetamine on delay discounting in humans were unrelated to delay discounting under placebo conditions. Although studies have shown that individuals with and without histories of childhood CD sometimes show different sensitivities to drugs (Cherek and Lane 2000; Cherek et al. 2002), and that ADHD children with and without histories of CD or aggressive-behavior problems sometimes show different changes in behavior when given methylphenidate (for a review see Hinshaw and Lee 2000), there was no relationship in the present study between the sensitivity of choice to methylphenidate and CD. Given the small number of subjects, however, no conclusions can be made regarding the relationship between CD and the behavioral effects of methylphenidate.
Results of clinical studies suggest that ADHD symptoms return to baseline levels when stimulant medications are discontinued (Brown et al. 1986). In the present study, the increases in self-control choices produced by methylphenidate were also temporary in most subjects; self-control choices decreased during subsequent placebo sessions. For subject 2563, however, methylphenidate may have produced an irreversible shift in preference toward the large, delayed option. It is possible that, for this subject, the exposure to greater session earnings produced by the increased number of self-control choices under methylphenidate maintained preference for the large option under subsequent placebo sessions.
The mean delay to the large option increased under methylphenidate, but the effect did not reach statistical significance. The lack of significant effect may be attributed in part to the greater variability in mean delays to the large option relative to number of choices, and to the use of number of choices rather than delays to assess stability. Although mean delays to the large option correlate with the number of choices for the large option, mean delays can vary to some extent depending on the pattern of choices even if the number of choices remains constant. It is possible that the effects of methylphenidate on the mean delay to the large option may have been more pronounced if the mean delay to the large option was used to evaluate stability.
The present procedure differed in one regard from more commonly used self-control procedures. Typically, in self-control procedures, post-reinforcer delays are programmed so that the delay to the onset of the next choice trial is identical following impulsive and self-controlled choices. In the present procedure, the post-reinforcer delays were constant (2 s). As a result, choice for the small option produced shorter session durations and a greater density of reinforcement than choice for the large option. Previous self-control research has shown that the small option is often preferred when such choices produce a greater density of reinforcement than choices for the large option (Logue et al. 1990; Sonuga-Barke et al. 1992; Ito and Nakamura 1998). One could argue, therefore, that in the present study, choices for the small option were not impulsive. In nearly every previous study that has shown that choice for the small option is influenced by reinforcement density, however, impulsive choices not only produced a greater density of reinforcement than self-controlled choices but also produced higher session earnings (but see Flora and Pavlik 1992). In the present study, because sessions ended after a fixed number of trials, choices for the small option produced lower session earnings: each choice for the small option reduced potential earnings by US $0.10. Furthermore, because session earnings were delivered at the end of each experimental day (i.e., after the fourth self-control session), only self-controlled choices could maximize daily reinforcement density. Thus, although choices for the small option produced a greater local density of reinforcement and shorter session durations than choices for the large option, because choices for the small option produced lower session earnings and lower total daily earnings (thereby indicating a weak control over choice by reinforcement amount), such choices may be defined as impulsive (Logue et al. 1990).
The behavioral mechanism by which stimulants affect choice in self-control procedures is uncertain. A number of studies have shown that stimulants increase the efficacy of conditioned reinforcers (Robbins 1975, 1978). Thus, increases in self-control may be attributed to a stimulant-produced increase in the conditioned-reinforcing value of stimuli paired with large, delayed reinforcers (Richards et al. 1999; Wade et al. 2000). To evaluate this possibility, Cardinal et al. (2000) presented rats with choices between small, immediate food deliveries and larger, more delayed food deliveries when delays to the large option were signaled or unsignaled. When delays were signaled, amphetamine increased preference for the large option, but when delays were unsignaled, amphetamine decreased preference for the large option. Cardinal et al. concluded that amphetamine influenced choice by increasing the conditioned-reinforcing effectiveness of the signal.
The increase in self-control choices in the present study may also be due to the effects of methylphenidate on conditioned reinforcement. Delays to money delivery were signaled (letters correlated with each choice option remained visible on the computer screen during the delays). Thus, one possibility is that methylphenidate differentially altered the conditioned-reinforcing effectiveness of the stimulus paired with the large option (i.e., the letter "B"). It is also possible that methylphenidate increased the conditioned-reinforcing effectiveness of money. Consistent with this interpretation, Wilkison et al. (1995) showed that, in children diagnosed with ADHD, methylphenidate administrations increased break-points on a progressive-ratio schedule of money delivery, indicating that methylphenidate increased the reinforcing value of money. Future research could evaluate these possibilities by investigating the effects of methylphenidate on choice when delays to money delivery are unsignaled, or when delays are signaled but reinforcers are unconditioned or consumable, i.e., do not need to be paired with other reinforcers to maintain their effectiveness. An increase in the efficacy of conditioned reinforcers is only one of several mechanisms by which stimulants may influence self-control choices, however, and stimulant effects on other psychological processes, such as delay sensitivity, timing, or the discriminative functions of stimuli, may also be important (but see discussions in Wade et al. 2000; Cardinal et al. 2000).
The primary pharmacological action of stimulants is to increase dopamine (DA) neurotransmission, by inhibiting DA re-uptake (e.g., methylphenidate), and/or by directly enhancing DA release (e.g., d-amphetamine; Patrick et al. 1987; Solanto 2002). For example, in normal adults, research has shown that, when taken orally, methylphenidate increases extracellular dopamine levels (Volkow et al. 2001). These and other related findings indicate that the effects of stimulants on impulsive choice are likely mediated by enhanced DA nuerotransmission (Solanto 1998). Results of a study by Wade et al. (2000), showing that a DA (specifically a D2 receptor) antagonist decreased preference for delayed reinforcers, whereas an indirect DA agonist (d-amphetamine) increased preference for delayed reinforcers, are consistent with this view. Stimulants also increase norepinephrine (NE) neurotransmission, however, and the finding that individuals with ADHD often show greater improvements when given stimulants that affect both DA and NE systems as opposed to DA systems alone suggests that enhanced NE activity may be an important component of stimulants' therapeutic effects (Solanto 1998; Kuczenski and Segal 2001). Thus, changes in NE activity as well as DA activity may be involved in the effects of stimulants on choice for delayed reinforcers. Additional research is therefore needed to clarify the behavioral effects of stimulant drugs in self-control procedures, determine the neurobiological actions that underlie their effects, and assess the relative contributions of dopamine and other neurotransmitter systems to choice for delayed reinforcers.
References
Ainslie GW (1974) Impulse control in pigeons. J Exp Anal Behav 21:485–489
Barkley RA (1977) A review of stimulant drug research with hyperactive children. J Child Psychol Psychiatry 18:137–165
Barratt ES (1985) Impulsiveness defined within a systems model of personality. In: Spielberger ED, Butcher JN (eds) Advances in personality assessment, vol 5. Erlbaum, Hillsdale, pp 113–132
Bickel WK, Marsch LA (2001) Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction 96:73–86
Brown RT, Borden KA, Wynne ME, Schleser R, Clingerman SR (1986) Methylphenidate and cognitive therapy with ADD children: a methodological reconsideration. J Abnorm Child Psychol 14:481–497
Campbell M, Cueva JE, Adams PB (1999) Pharmacotherapy of impulsive-aggressive behavior. In: Cloninger CR (ed) Personality and psychopathology. American Psychiatric Press, Washington, pp 431–455
Cardinal RN, Robbins TW, Everitt BJ (2000) The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology 152:362–375
Charrier D, Thiébot MH (1996) Effects of psychotropic drugs on rats responding in an operant paradigm involving choice between delayed reinforcers. Pharmacol Biochem Behav 54:149–157
Cherek DR (1992) The point subtraction aggression paradigm (PSAP). University of Texas Press, Houston
Cherek DR, Lane SD (1999a) Effects of d,l-fenfluramine on aggressive and impulsive responding in adult males with a history of conduct disorder. Psychopharmacology 146:473–481
Cherek DR, Lane SD (1999b) Laboratory and psychometric measurements of impulsivity among violent and nonviolent female parolees. Biol Psychiatry 46:273–280
Cherek DR, Lane SD (2000) Fenfluramine effects on impulsivity in a sample of adults with and without history of conduct disorder. Psychopharmacology 152:149–156
Cherek DR, Moeller FG, Dougherty DM, Rhoades H (1997) Studies of violent and nonviolent male parolees. II. Laboratory and psychometric measurements of impulsivity. Biol Psychiatry 41:523–529
Cherek DR, Lane SD, Pietras CJ, Sharon J, Steinberg JL (2002) Acute effects of baclofen, a γ-aminobutyric acid-B agonist, on laboratory measures of aggressive and escape responses of adult male parolees with and without a history of conduct disorder. Psychopharmacology 164:160–167
De Wit H, Enggasser JL, Richards JB (2002) Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology 27:813–825
Dougherty DM, Bjork JM, Marsh DM, Moeller FG (2000) A comparison between adults with conduct disorder and normal controls on a continuous performance test: differences in impulsive response characteristics. Psychol Rec 50:203–219
Evenden JL (1999) Varieties of impulsivity. Psychopharmacology 146:348–361
Evenden JL, Ryan CN (1996) The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology 128:161–170
Findling RL, Dogin JW (1998) Psychopharmacology of ADHD: children and adolescents. J Clin Psychiatry 59[Suppl 7]:42–49
First MB, Spitzer RL, Gibbon M, Williams JBW (1996) Structured clinical interview for DSM-IV axis I disorders. Biometrics Research Department. State Psychiatric Institute, New York
Flora SR, Pavlik WB (1992) Human self-control and the density of reinforcement. J Exp Anal Behav 57:201–208
Hinshaw SP, Lee SS (2000) Ritalin effects on aggression and antisocial behavior. In: Greenhill LL, Osman BB (eds) Ritalin: theory and practice, 2nd edn. Liebert, New York, pp 237–251
Ito M, Nakamura K (1998) Humans' choice in a self-control choice situation: sensitivity to reinforcer amount, reinforcer delay, and overall reinforcement density. J Exp Anal Behav 69:87–102
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997) Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988
Klein RG, Abikoff H, Klass E, Ganeles D, Seese LM, Pollack S (1997) Clinical efficacy of methylphenidate in conduct disorder with and without attention deficit hyperactivity disorder. Arch Gen Psychiatry 54:1073–1080
Kuczenski R, Segal DS (2001) Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther 296:876–883
Logue AW (1988) Research on self-control: an integrating framework. Behav Brain Sci 11:665–709
Logue AW (2000) Self-control and health behavior. In: Bickel WK, Vuchinich RE (eds) Reframing health behavior change with behavioral economics. Erlbaum, New Jersey, pp 167–192
Logue AW, Peña-Correal TE, Rodriguez ML, Kabela E (1986) Self-control in adult humans: variation in positive reinforcer amount and delay. J Exp Anal Behav 46:159–173
Logue AW, King GR, Chavarro A, Volpe JS (1990) Matching and maximizing in a self-control paradigm using human subjects. Learn Motivation 21:340–368
Mazur JE, Logue AW (1978) Choice in a "self-control" paradigm: effects of a fading procedure. J Exp Anal Behav 30:11–17
Patrick KS, Mueller RA, Gualtieri CT, Breese GR (1987) Pharmacokinetics and actions of methylphenidate. In: Meltzer HY (ed) Psychopharmacology: the third generation of progress. Raven Press, New York, pp 1387–1395
Rachlin H, Green L (1972) Commitment, choice and self-control. J Exp Anal Behav 17:15–22
Rapoport JI, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ (1980) Dextroamphetamine. Its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry 37:933–943
Rapport MD, Stoner G, DuPaul GJ, Kelly KL, Tucker SB, Schoeler T (1988) Attention deficit disorder and methylphenidate: a multilevel analysis of dose-response effects on children's impulsivity across settings. J Am Acad Child Adolesc Psychiatry 27:60–69
Riccio CA, Waldrop JJM, Reynolds CR, Lowe P (2001) Effects of stimulants on the continuous performance test (CPT): implications for CPT use and interpretation. J Neuropsychiatry Clin Neurosci 13:326–335
Richards JB, Sabol KE, de Wit H (1999) Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology 146:432–439
Robbins TW (1975) The potentiation of conditioned reinforcement by psychomotor stimulant drugs. A test of Hill's hypothesis. Psychopharmacologia 45:103–114
Robbins TW (1978) The acquisition of responding with conditioned reinforcement: effect of pipradrol, methylphenidate, d-amphetamine, and nomifensine. Psychopharmacology 58:79–87
Shipley Boyle B (1967) The Shipley institute of living scale. Western Psychological Services, Los Angeles, CA
Solanto MV (1998) Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res 94:127–152
Solanto MV (2002) Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Res 130:65–71
Solanto MV, Conners CK (1982) A dose-response and time-action analysis of autonomic and behavioral effects of methylphenidate in attention deficit disorder with hyperactivity. Psychophysiology 19:658–667
Solanto MV, Wender EH, Bartell SS (1997) Effects of methylphenidate and behavioral contingencies on sustained attention in attention-deficit hyperactivity disorder: a test of the reward dysfunction hypothesis. J Child Adolesc Psychopharmacol 7:123–136
Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, Hechtman L, Hinshaw S, Turkel E (2001) The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIHM multimodal treatment study of AD/HD. J Abnorm Child Psychol 29:215–228
Sonuga-Barke EJS, Taylor E, Sembi S, Smith J (1992) Hyperactivity and delay aversion. I. The effect of delay on choice. J Child Psychol Psychiatry 33:387–398
Sostek AJ, Buchsbaum MS, Rapoport JL (1980) Effects of amphetamine on vigilance performance in normal and hyperactive children. J Abnorm Child Psychol 8:491–500
Spencer T, Wilens T, Biederman J, Faraone SV, Ablon JS, Lapey K (1995) A double-blind, crossover comparison of methylphenidate and placebo in adults with childhood-onset attention-deficit hyperactivity disorder. Arch Gen Psychiatry 52:434–443
Swanson JM, Volkow ND (2002) Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res 130:73–78
Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD (1989) Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol 17:473–491
Trommer BL, Hoeppner JB, Zecker SG (1991) The Go-No Go test in attention deficit disorder is sensitive to methylphenidate. J Child Neurol 6[Suppl]:S128–S131
Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, Ding Y, Gatley SJ, Gifford A, Franceschi D (2001) Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 21:1–5
Ward MF, Wender PH, Reimherr FW (1993) The Wender Utah rating scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry 150:885–890
Wade TR, de Wit H, Richards JB (2000) Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology 150:90–101
Wender PH, Reimherr FW, Wood D, Ward M (1985) A controlled study of methylphenidate in the treatment of attention deficit disorder, residual type, in adults. Am J Psychiatry 142:547–552
Wilkison PC, Kircher JC, McMahon WM, Sloane HN (1995) Effects of methylphenidate on reward strength in boys with attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 34:897–901
Winer BJ (1971) Statistical principles in experimental design, 2nd edn. McGraw-Hill, New York
Zachary RA, Crumptom E, Spiegel DE (1985) Estimating WAIS-R IQ from the Shipley institute of living scale. J Clin Psychol 41:532–540
Acknowledgements
This research was supported by NIH grant DA 10552 from the National Institute on Drug Abuse. The authors wish to thank Sheila White, Jennifer Sharon and Ehren Bradbury for their assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pietras, C.J., Cherek, D.R., Lane, S.D. et al. Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacology 170, 390–398 (2003). https://doi.org/10.1007/s00213-003-1547-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1547-2