Abstract
Rationale
Sex differences have been reported in physiological and behavioral responses to cocaine, but it is unclear whether sex differences exist in conditioned-cued relapse to cocaine seeking after prolonged abstinence. Furthermore, the role of estrous cyclicity in conditioned-cued relapse has not been investigated.
Objective
We assessed the influence of sex and estrous cyclicity on conditioned-cued reinstatement of drug-seeking behavior in Sprague–Dawley rats.
Methods
Rats were trained to self-administer intravenous cocaine (unconditioned stimulus, US; 0.25, 0.4, 0.5, 0.6, or 1.0 mg/kg per infusion) paired with light+tone conditioned stimuli (CSs) and were subsequently tested for the ability of the CSs to reinstate extinguished cocaine seeking (i.e., nonreinforced lever responding).
Results
Females exhibited more responding on the cocaine-paired lever during self-administration and extinction than males. Subsequently, males exhibited equally robust conditioned-cued reinstatement of extinguished drug-seeking behavior independent of cocaine training dose. Males and females trained on 0.4–0.6 mg/kg cocaine reinstated to a similar extent. However, females trained on the lowest dose (0.25 mg/kg) exhibited less reinstatement than males, and the source of this effect was the absence of reinstatement in estrous females. In addition, independent of estrous state, females trained on the highest dose (1.0 mg/kg) exhibited less reinstatement than males.
Conclusions
While males and females are equally responsive to cocaine-paired CSs when the conditions for CS–US association are optimal, females appear to attribute less motivational significance to the CS when it presumably acquires weaker motivational salience because of (a) a low cocaine dose or (b) weaker CS–US contiguity due to the prolonged effects of a high cocaine dose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sex differences have been reported in drug-taking and drug-seeking behaviors for psychostimulants, such as cocaine, in human addicts and in laboratory animals. According to clinical studies, men are substantially more likely to have a drug-abuse or drug-dependence disorder than women (Brady and Randall 1999), but women progress more rapidly from casual use to drug dependence than men (McCance-Katz et al. 1999; Westermeyer and Boedicker 2000). Women also report higher rates of use and shorter drug-free periods (Griffin et al. 1989). However, women are more likely to seek treatment (Fiorentine et al. 1997) and enter treatment at an earlier age than men (Kosten et al. 1993). In terms of relapse to cocaine taking after a period of abstinence, some studies have suggested that gender differences exist in cocaine craving, with women being more susceptible to craving and relapse due to stressful events or depression (McKay et al., 1996; Elman et al. 2001). However, conflicting reports exist as to the nature of gender differences in relapse triggered by cocaine-paired environmental cues per se, with some studies indicating that in clinical populations female cocaine addicts are more (Robbins et al. 1999), less (Avants et al. 1995), or equally (Negrete and Emil 1992) susceptible to cocaine-paired cues relative to males. A recent report using positron emission tomography imaging suggests a physiological difference between men and women in response to cocaine-paired cues, in that females show significantly different patterns of regional cerebral blood flow in comparison with males (Kilts et al. 2004).
While it remains unclear as to what extent biological versus psychosocial factors account for gender differences in drug craving and relapse, the idea that sex differences exist in cocaine-induced behaviors receives strong support from preclinical studies using animal models. For example, female rats exhibit greater locomotor response to acute cocaine administration and greater sensitization of locomotor activity after repeated psychostimulant administration (Glick and Hinds 1984; van Haaren and Meyer 1991; Craft and Stratmann 1996; Hu and Becker 2003). Female rats acquire cocaine self-administration more rapidly than males (Lynch and Carroll 1999; Hu et al. 2004), and a larger proportion of drug-naive females learns to self-administer cocaine relative to males (Lynch and Carroll 1999). While female and male rats generally do not differ in cocaine intake on fixed ratio (FR) schedules of reinforcement, females exhibit greater breaking points on a progressive ratio (PR) schedule (Roberts et al. 1989). These results suggest that females may be more sensitive to the motivational effects of cocaine than males. Furthermore, motivation for cocaine in female rats varies as a function of estrous state (Roberts et al. 1989; Lynch et al. 2000), suggesting that cyclic hormonal changes likely contribute to the reported sex differences in cocaine-seeking behavior.
Studies to date have largely focused on sex differences in cocaine self-administration. A number of laboratories have shown that male rats exhibit robust reinstatement of cocaine-seeking behavior in the presence of cocaine-paired cues (de Wit and Stewart 1981; Fuchs et al. 1998; Meil and See 1996; Weiss et al. 2000). However, sex differences in relapse to cocaine seeking maintained by drug-paired conditioned cues have not been investigated. To examine whether sex differences exist in propensity for cue-induced relapse, the present study assessed conditioned-cued reinstatement of cocaine seeking in male and female Sprague–Dawley rats. Furthermore, since estrous state may influence responding in female rats, we also monitored estrous cyclicity across reinstatement test trials. For this purpose, we chose to use freely cycling females in order to ascertain differences related to estrous state without artificial manipulation of the estrous cycle by gonadectomy and subsequent hormone replacement. We hypothesized that estrous females would exhibit more robust conditioned-cued reinstatement of cocaine seeking than non-estrous females and males based on evidence that estrous females are more sensitive to the motivational effects of cocaine than females in other phases of the estrous cycle (Roberts et al. 1989; Lynch et al. 2000).
Materials and methods
Subjects
Male (n=46, 275–300 g at the start of the experiment) and female (n=87, 250–275 g at the start of the experiment) Sprague–Dawley rats (Charles River, Wilmington, MA) were individually housed in a temperature- and humidity-controlled vivarium on a 12-h/12-h light/dark cycle. Rats were maintained on 25–30 g (males) or 20–25 g (females) rat chow (Harlan, Indianapolis, IN) per day, and ad libitum water. The housing and care of the rats followed the guidelines of the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996). Rats were given a minimum of 3 days for adaptation before the start of the experiment. To avoid the effects of sex pheromones, males and females were never tested in the same room or operant conditioning chambers on the same day.
Food training
Rats were trained to lever press on a fixed ratio 1 (FR 1) schedule of food reinforcement (45 mg pellets; Research Diets, New Brunswick, NJ) in sound-attenuated, operant conditioning chambers (30×20×24 cm high; Med Associates, St. Albans, VT) during a 16-h overnight food training session. The chambers were equipped with two retractable levers, a stimulus light above each lever, a food pellet dispenser, a house light, and a speaker connected to a tone generator (ANL-926, Med Associates). Experimental events and data collection were controlled by Schedule Manager software version 3.13 (Med Associates). During the session, each lever press on the right lever resulted in delivery of a food pellet only (i.e., active lever). Lever presses on the left lever had no programmed consequences (i.e., inactive lever). Rats were required to earn 100 pellets or more during the session and exhibit 50% more responding on the active lever than the inactive lever. Rats (∼1–2%) that did not reach this acquisition criterion were retrained on the following day. Following food training, food pellet dispensers were removed from the chambers.
Surgery
Rats were anesthetized, 48 h after food training, using a mixture of ketamine hydrochloride (Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (Phoenix Pharmaceutical, St. Joseph, MO; males 66 mg/kg and 1.33 mg/kg, respectively; females 33 mg/kg and 0.665 mg/kg, respectively, IP) followed by equithesin (Sigma, St. Louis, MO; males 0.5 ml/kg and females 0.25 ml/kg of a solution of 9.72 mg/ml pentobarbital sodium 42.5 mg/ml chloral hydrate and 21.3 mg/ml magnesium sulfate heptahydrate dissolved in a 44% propylene glycol, 10% ethanol solution; IP). Chronic indwelling catheters were constructed using a bent steel cannula with a screw-type connector (Plastics One, Roanoke, VA), SILASTIC tubing (10 cm, i.d. 0.64 mm, o.d. 1.19 mm; Dow Corning, Midland, MI), Prolite polypropylene monofilament mesh (Atrium Medical Corporation, Hudson, NH), and cranioplastic cement (Lang Dental Manufacturing, Wheeling, IL), as described previously (Fuchs and See 2002). The end of the catheter was inserted into the right jugular vein and was secured to surrounding tissue with suture. The catheter ran subcutaneously and exited on the rat’s back, posterior to the shoulder blades.
To extend catheter patency, the catheters were flushed once daily for 3 days following surgery with 0.1 ml of an antibiotic solution of cefazolin (10.0 mg/ml, Schein Pharmaceutical, Florham Park, NJ) dissolved in heparinized saline (70 U/ml; Elkins-Sinn, Cherry Hill, NJ). Thereafter, catheters were flushed with 0.1 ml of heparinized saline (10 U/ml) prior to each self-administration session, and with 0.1 ml of the cefazolin solution and 0.1 ml of heparinized saline after each session. Catheter patency was periodically verified by infusing 0.12 ml (males) or 0.04 ml (females) of methohexital sodium (10 mg/ml, IV; Eli Lilly, Indianapolis, IN), which produces a rapid loss of muscle tone only when administered intravenously.
Cocaine self-administration
Self-administration training was conducted during 2-h sessions during the rats’ light cycle. Rats were trained to press a lever according to a FR 1 schedule of cocaine reinforcement (cocaine hydrochloride; National Institute on Drug Abuse, Research Triangle Park, NC). Each infusion was followed by a 40-s time-out period, during which lever presses had no scheduled consequences. Cocaine was dissolved in sterile saline and delivered at a dose of 0.25, 0.4, 0.5, 0.6, or 1.0 mg/kg per 0.05-ml infusion in separate groups of rats (females n=16–18/dose; males n=7–12/dose). This dose range represents the descending limb of the cocaine dose–effect curve and encompasses the full range of cocaine training doses previously employed in reinstatement studies (Shalev et al. 2002). Daily sessions continued until the rats earned a minimum of seven (for the 1.0-mg/kg per infusion dose) or ten (0.25- to 0.6-mg/kg per infusion doses) infusions per session in a total of 10 days. Approximately 30% of rats required one to four additional training days to reach this criterion.
The house light was illuminated throughout each session. Lever presses on the right (active) lever resulted in a 2-s activation of the infusion pump (Model PHM-100, Med Associates) and a 5-s presentation of a stimulus complex, consisting of activation of the white stimulus light above the active lever and the tone generator (78 dB, 2 kHz). After each infusion, responses on the active lever had no consequences during the time-out period. During the sessions, responses on the left (inactive) lever had no programmed consequences, but were recorded.
Extinction and estrous cycle monitoring
Following self-administration training, rats underwent 2-h extinction sessions, during which responses were recorded on both levers, but had no programmed consequences (i.e., conditioned stimuli or cocaine were not presented). Extinction training continued for a minimum of 7 consecutive days plus for additional days, if necessary, until active lever responding fell to 25 responses per session or less on two consecutive days. All rats reached this extinction criterion within 7–13 days. Starting on extinction day 4, vaginal smears were taken daily, immediately prior to placement into the test chamber. Thus, the smears were taken at the same time each day (±30 min).
Samples were collected from the vaginal lumen using sterile, saline-dipped, cotton-tipped applicators (Fischer Scientific). The samples were smeared onto clean glass slides (Fischer Scientific). Smears were examined using a light microscope set at 10× magnification immediately after collection by an observer and re-examined after staining with Giemsa (Sigma) by a second observer blind to the rats’ group assignments. The inter-rater reliability between observers was 92%. The proestrous phase was defined as the presence of more than 75% of nucleated epithelial cells. The estrous phase (note: vaginal estrous as opposed to behavioral estrus) was defined as the presence of more than 75% of non-nucleated cornified epithelial cells. The metestrous (also known as diestrous I) phase was defined as the presence of approximately equal proportions of nucleated epithelial cells, non-nucleated cornified epithelial cells, and leukocytes. The diestrous (also known as diestrous II) phase was defined as a minimum amount of cells, including leukocytes and occasional epithelia (Feder 1981).
Conditioned-cued reinstatement of cocaine seeking
Once the rats reached the extinction criterion (see above), two reinstatement tests were conducted. Prior to each reinstatement test, vaginal smears were taken from females. Both males and females were then placed into the operant conditioning chamber for a 2-h test session. During the reinstatement test session, responses on the previously active lever resulted in 5-s presentations of the cocaine-paired light-tone stimulus complex in the absence of cocaine reinforcement. Each stimulus presentation was followed by a 40-s time-out period during which responses did not result in further stimulus presentations. Prior to the second reinstatement test, females underwent additional extinction sessions and daily vaginal smearing until they reached the extinction criterion (see above) and until they were in a phase of the estrous cycle that differed from the phase at the time of the first reinstatement test. Rats with ambiguous smears (e.g., in between two estrous phases) were not tested. This procedure yielded 5–15 observations per estrous phase subgroup (Table 1). The variability in the number of observations per subgroup was due to differences in estrous phase lengths (12–36 h per phase; Feder, 1981) and cocaine-induced abnormalities in cycling, which altered the probability to observe particular estrous states. Males (n=7–12 per training dose group) underwent additional extinction sessions until they reached the initial extinction criterion, plus the number of extinction days between the two test days was matched to that for the female rats.
Data analysis
Using SPSS software version 11.5 (SPSS, Chicago, IL), mixed-factors analyses of variance (ANOVA) were used to analyze active and inactive lever responses and drug infusions, with lever (active, inactive), day (self-administration day, extinction day, reinstatement test day), sex (male, female), and estrous phase (estrous, non-estrous, diestrous, metestrous, proestrous) as factors, where appropriate. Interaction effects were further investigated using simple main-effects tests (one-way ANOVA or t-test) and/or Tukey post-hoc tests, where appropriate. Heterogeneity of variance, as indicated by Mauchly’s test of sphericity, was corrected for using Greenhouse–Geisser adjusted degrees of freedom. Only statistically significant effects are noted below.
Results
Self-administration
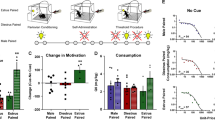
All groups of rats exhibited stable responding on the active lever during the previous three self-administration days. Both sex and cocaine training dose impacted the rate of responding selectively on the active lever (Fig. 1a). The ANOVA of lever presses indicated significant lever by sex (F1,122=7.895, P<0.01) and lever by cocaine training dose (F1,122=6.766, P<0.0001) interaction effects, as well as significant main effects of sex (F1,122=8.988, P<0.005), cocaine training dose (F1,122=7.764, P<0.0001), and lever (F1,122=202.641, P<0.0001). Independent of cocaine training dose, females exhibited more active lever presses than males (ANOVA sex simple main effect, P<0.05), whereas there was no difference between males and females in inactive lever responding (Fig. 1b). Furthermore, independent of sex, training dose-dependent differences occurred in active lever responding (Fig. 1c), which were characteristic of the descending limb of the inverted U-shaped cocaine dose–effect curve (Skjoldager et al. 1991). Collapsed across sex, the 0.5-mg/kg dose of cocaine elicited significantly less active lever responding than the 0.25-mg/kg dose (Tukey test, P<0.05). The 0.6-mg/kg dose of cocaine elicited significantly less active lever responding than the 0.25-mg/kg to 0.4-mg/kg doses (Tukey tests, P<0.01). Furthermore, the 1.0-mg/kg dose elicited significantly less active lever responding than the 0.25-mg/kg to 0.5-mg/kg doses (Tukey tests, P<0.01). There was no effect of cocaine training dose on responding on the inactive lever.
Responses exhibited by male and female rats on the active and inactive levers during cocaine self-administration (mean of the last 3 days of training±SEM). During self-administration, active lever responses resulted in the delivery of a cocaine infusion (0.25–1.0 mg/kg per infusion) and simultaneous presentation of a light+tone stimulus complex. Each reinforced lever press was followed by a 40-s time-out period, during which active lever responses had no scheduled consequences. a Effects of cocaine training dose on lever responding in female (open symbols; n=16–18 per training dose) and male (filled symbols; n=7–12 per training dose) rats during self-administration. Large symbols indicate lever presses on the active lever and small symbols indicate lever presses on the inactive lever. The overall ANOVA of lever responding revealed statistically significant simple main effects of sex and cocaine dose, which are illustrated in panels b and c, respectively. b Sex differences in lever responding collapsed across cocaine dose. Open bars represent females and filled bars represent males. *Significant difference relative to males (ANOVA sex simple main effect, P<0.05). c Effects of training dose on active lever responding collapsed across sex. *Significant difference relative to the 0.25-mg/kg dose (Tukey test, *P<0.05). Daggers represent a significant difference relative to doses 0.25–0.4 mg/kg (Tukey tests, ††P<0.01). Double daggers represent a significant difference relative to doses 0.25–0.5 mg/kg (Tukey tests, ‡‡P<0.01)
A training dose-dependent effect was also observed on the mean number of infusions self-administered per session, and this effect was independent of sex (Fig. 2a–c). The ANOVA of cocaine infusions obtained averaged across the last 3 days of self-administration indicated a significant main effect of cocaine training dose (F4,122=17.999, P<0.0001) only. Thus, there was no sex difference in the amount of cocaine self-administered. However, independent of sex, rats self-administered fewer infusions of the 0.5-mg/kg dose than the 0.25-mg/kg dose of cocaine (Tukey test, P<0.01) and self-administered fewer infusions of the 0.6-mg/kg dose than the 0.25- to 0.4-mg/kg doses (Tukey tests, P<0.01). Rats also self-administered fewer infusions of the 1.0-mg/kg dose than the 0.25- to 0.5-mg/kg doses of cocaine (Tukey tests, P<0.01).
Cocaine intake in male and female rats during self-administration (mean of the previous 3 days±SEM). a Effects of cocaine training dose on the mean number of cocaine infusions self-administered by female (open symbols; n=16–18 per training dose) and male (filled symbols; n=7–12 per training dose) rats during self-administration. The ANOVA of cocaine infusions revealed a significant main effect of cocaine dose only, which is further illustrated in panel b. b Effects of training dose on the mean number of cocaine infusions self-administered, collapsed across sex. *Significant difference relative to the 0.25-mg/kg dose (Tukey test, **P<0.01). Daggers represent a significant difference relative to doses 0.25–0.4 mg/kg (Tukey tests, ††P<0.01). Double daggers represent a significant difference relative to 0.25–0.5 mg/kg (Tukey tests, ‡‡P<0.01). c Effects of cocaine training dose on absolute cocaine intake, defined as the mean daily milligrams per session cocaine intake collapsed across sex. There were no statistically significant sex differences in absolute cocaine intake. Note that rats did not perfectly titrate cocaine exposure as a function of training dose. *Significant difference relative to doses 0.4–1.0 mg/kg (Tukey test, *P<0.05). Dagger represents a significant difference relative to doses 0.25 mg/kg and 0.6 mg/kg (Tukey test, †P<0.05)
The mean daily milligram cocaine intake (“absolute cocaine intake” henceforth, Fig. 2c) also varied as a function of cocaine dose, but not sex, consistent with research indicating that rats do not perfectly titrate their blood levels of cocaine (Panlilio et al. 2003). The ANOVA of absolute cocaine intake revealed a significant training dose main effect (F4,122=10.235, P<0.0001) only. Collapsed across sex, rats trained on the 0.25-mg/kg training dose self-administered less cocaine than all other groups (Tukey test, P<0.05). Furthermore, rats trained on the 1.0-mg/kg training dose self-administered more cocaine than the groups trained on the 0.25-mg/kg or 0.6-mg/kg training doses (Tukey test, P<0.05).
Extinction
Removal of cocaine and conditioned cue reinforcement resulted in a gradual decrease in responding on the previously active lever and a transient increase in responding on the inactive lever. Females exhibited more responding during extinction than males, and this effect was independent of cocaine training dose (Fig. 3a). The ANOVA of active lever responses during the first 7 days of extinction indicated significant sex by extinction day (F6,732=4.091, P<0.0001) and cocaine training dose by extinction day (F24,732=1.593, P<0.05) interaction effects as well as significant main effects of sex (F1,122=7.793, P<0.01) and cocaine training dose (F4,122=1.561, P<0.05) only. Subsequent pair-wise comparisons indicated that females exhibited significantly more active lever presses than males on extinction days 1–4 (ANOVA sex simple main effect, P<0.05 to P<0.01). However, the sources of the significant cocaine training dose by extinction day interaction effect were not systematic (Tukey tests, P<0.05; Fig. 3b). The collection of estrous smears, starting on extinction day 4, did not appear to alter active lever pressing because there was no statistically significant change in responding on extinction day 4 relative to extinction day 3 (t(86)=1.869, P>0.05). The ANOVA of inactive lever responses during the first 7 days of extinction indicated a significant sex by extinction day (F6,732=4.487, P<0.0001) interaction effect and a significant sex main effect (F1,122=4.049, P<0.05) only. Subsequent pair-wise comparisons indicated that females exhibited more inactive lever presses on extinction day 1 (ANOVA sex simple main effects test, P<0.005) and day 2 (P<0.05) than males (Fig. 3b).
Responses exhibited by male and female rats during extinction (mean of the first 7 days±SEM). The overall ANOVA indicated sex by extinction day and cocaine dose by extinction day effects, which are illustrated on panels a and b, respectively. a Sex differences in extinction responding on the previously active (large symbols) and inactive (small symbols) levers collapsed across cocaine training dose. Open symbols represent female rats (n=87) and filled symbols represent male rats (n=46). *Significant difference relative to responding exhibited by males on the respective lever (Tukey test, *P<0.05; **P<0.01). b Effects of cocaine training dose on extinction responding on the previously active lever collapsed across sex. *Significant difference relative to the 1.0-mg/kg dose (Tukey test, *P<0.05). Dagger represents a significant difference relative to the 0.25-mg/kg dose (Tukey test, †P<0.05)
Effects of sex and cocaine training dose on conditioned-cued reinstatement of cocaine seeking
The effects of the light+tone stimulus complex on reinstatement of cocaine seeking varied as a function of sex and cocaine training dose (Table 1 and Fig. 4a). First, separate ANOVAs were conducted for each cocaine training dose to examine whether responding was reinstated following exposure to the light+tone stimulus complex relative to the rat’s extinction baseline (i.e., responding on the last extinction day preceding the reinstatement test day). The extinction baseline values for the active and inactive levers were 10.65±0.48 (range=8.31–12.31) and 4.24±0.37 (range=2.56–6.06), respectively. Both males and females trained on the 0.25-mg/kg and 1.0-mg/kg doses of cocaine exhibited a significant increase in responding on the active lever on the reinstatement test day relative to the extinction baseline; however, the females trained at these doses of cocaine exhibited significantly fewer active lever responses than the males (ANOVA sex by test day by lever interaction, F1,52–56=7.764–10.451, P<0.005–0.01; followed by Tukey test, P<0.01). In contrast, both males and females trained on the 0.4-, 0.5-, and 0.6-mg/kg doses of cocaine exhibited a significant increase in responding on the active lever during the reinstatement test relative to the extinction baseline (ANOVA day×lever interaction effects, F1,48–52=36.066–44.905, P<0.0001, followed by ANOVA simple main effects tests, P<0.0001), and there was no difference in the number of active lever presses exhibited by males and females. Each ANOVA also revealed that there was no change in responding on the inactive lever on the reinstatement test day relative to the preceding extinction day.
Conditioned-cued reinstatement of extinguished cocaine-seeking behavior (mean±SEM) in male and female rats. During the reinstatement test, active lever responses resulted in 5-s presentations of the light+tone stimulus complex in the absence of cocaine reinforcement. a Effects of cocaine training dose and sex on responding during the reinstatement test session and the preceding extinction session (E, data collapsed across cocaine training dose). Open symbols represent female rats (n=16–18) and filled symbols represent male rats (n=7–12). Large symbols represent lever presses on the previously active lever and small symbols represent lever presses on the inactive lever. *Significant difference relative to responding on the preceding extinction day (ANOVA simple main effect test, **P<0.01–0.0001). Daggers represent a significant difference relative to males (Tukey test, ††P<0.01). b Influence of estrous state on responding during the reinstatement test session and the preceding extinction session. Triangles represent estrous females (n=9–15; Table 1) and diamonds represent non-estrous females (n=20–27). Note: estrous state refers to vaginal estrous as opposed to behavioral estrus. *Significant difference relative to responding on the preceding extinction day (ANOVA simple main effect test, **P<0.01–0.0001). Dagger represents a significant difference relative to non-estrous females (Tukey test, ††P<0.01)
An additional ANOVA of active lever presses exhibited on the reinstatement test day by rats trained on 0.25-mg/kg to 1-mg/kg doses of cocaine revealed a significant sex by cocaine dose interaction effect (F4,256=2.465, P<0.05) and a cocaine dose main effect (F4,256=3.810, P<0.005). There was no difference in the number of active lever responses exhibited by males as a function of cocaine training dose. In contrast, females trained on the 0.25-mg/kg and 1.0-mg/kg doses of cocaine exhibited less active lever responding than females trained on the 0.4-mg/kg dose (Tukey tests, P<0.05), which was also evident by a significant quadratic trend (F171=8.14, P<0.0001). A separate ANOVA of inactive lever presses revealed no sex or cocaine training dose effects.
Estrous cyclicity following cocaine self-administration
The mean cycle length, assessed prior to cocaine self-administration in a subset of the females (n=24), was determined to be 4.21±0.16 days. Cycle length was also assessed starting on extinction day 4 following cocaine self-administration (4.42±0.13 days) and was not significantly different in length relative to the pre-cocaine cyclicity as a function of cocaine training dose (F5,104=1.203, P>0.05). There was also no relationship between cycle length following cocaine self-administration and reinstatement magnitude (Pearson’s r(174)=0.16, P=0.835). Nevertheless, estrous cyclicity appeared abnormal in that particular phases of the cycle appeared to be occasionally longer or shorter than observed prior to cocaine self-administration in 45% of rats. In addition, a particular phase of the cycle was not observable in 28% of rats. These abnormalities in cycling were unrelated to cocaine training dose and did not involve the persistent absence of a particular phase of the cycle, rather they appeared to be unsystematic.
Effects of estrous phase and cocaine training dose on conditioned-cued reinstatement of cocaine seeking
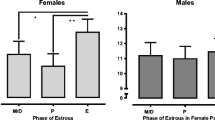
Estrous cyclicity did not appear to alter conditioned cue-induced reinstatement of cocaine seeking (Table 1). Separate ANOVAs were conducted for each cocaine training dose to examine the effects of estrous phase on reinstatement responding following exposure to the light+tone stimulus complex relative to the rat’s extinction baseline. These analyses failed to reveal any estrous main or interaction effects. Females in all phases of the estrous cycle exhibited a similar increase in active lever responding on the reinstatement test day relative to their extinction baseline, but did not exhibit a change in responding on the inactive lever (ANOVA lever by day interaction effects, F1,28–32=7.815–35.167, P<0.01–0.0001).
Since an initial aim of the study involved comparisons between estrous and non-estrous females, the data set was further analyzed (Fig. 4b). There was no significant difference between rats tested in the diestrous, metestrous, or proestrous state in responding on the active or inactive levers on the reinstatement test day (see above); therefore, responding exhibited by these groups of females was collapsed into a single group (“non-estrous,” henceforth). Separate ANOVAs were conducted for each cocaine training dose to examine whether responding was reinstated following exposure to the light+tone stimulus complex relative to the rat’s extinction baseline. Non-estrous females trained on the 0.25-mg/kg dose of cocaine exhibited a significant increase in responding on the active lever during the reinstatement test relative to the extinction baseline; however, estrous females trained at this dose of cocaine failed to reinstate and also exhibited fewer active lever responses than non-estrous females (ANOVA sex by test day by lever interaction, F1,32=5.899, P<0.05; followed by Tukey test, P<0.01). In contrast, both estrous and non-estrous females trained on the 0.4- to 1.0-mg/kg doses of cocaine exhibited an increase in responding on the active lever during the reinstatement test relative to the extinction baseline (ANOVA day by lever interaction effects, F1,30–34=18.839–37.009, P<0.0001) and there was no difference in the number of active lever presses exhibited by estrous and non-estrous females. There was also no difference between estrous and non-estrous females in inactive lever responding.
Discussion
Previous studies examined sex differences and the role of the estrous cycle in animal models of cocaine addiction, but they focused almost exclusively on differences during cocaine self-administration (Roberts et al. 1989; Grimm and See 1997; Lynch and Carroll 1999; Caine et al. 2004). The purpose of the current study was to determine whether sex differences occur in conditioned-cued reinstatement of extinguished drug-seeking behavior following prolonged withdrawal from cocaine self-administration. Our results show that, while male and female rats do not differ in their intake of cocaine during daily self-administration sessions, they do exhibit significant differences in conditioned-cued reinstatement, such that male rats are more susceptible to responding on a cocaine-paired lever in the presence of previously cocaine-paired stimuli.
Self-administration and extinction
In the present study, females exhibited more active lever responding for cocaine reinforcement than males, which was evident as an upward shift of the cocaine dose–effect curve. This effect, however, was due to females exhibiting an increase in responding on the active lever during the time-out period relative to males. Accordingly, there was no sex difference in the number of cocaine infusions self-administered, and both males and females showed a dose-dependent decrease in responding and in the number of infusions self-administered as the training dose was increased. These findings are consistent with previous reports suggesting the absence of sex differences in cocaine intake (Roberts et al. 1989; Lynch et al. 2000; Lynch and Carroll 2000; Lynch et al. 2001; Caine et al. 2004). Collectively, these findings suggest that males and females are equally sensitive to the rewarding effects of cocaine. In contrast, females may be somewhat more sensitive to the motivational effects of cocaine, as seen by the higher number of lever responses across doses. This effect is consistent with a report that females exhibit higher breaking points on a PR schedule of cocaine reinforcement (Roberts et al. 1989). Alternatively, increased active lever responding may be due to increased sensitivity to the psychomotor stimulant effects of cocaine, since females exhibit more locomotion and stereotypy in response to cocaine than males (Glick and Hinds 1984; van Haaren and Meyer 1991; Hu and Becker 2003). This possibility is mitigated, however, by the fact that no sex differences in inactive lever responses were seen, suggesting that females did not exhibit inadvertent lever presses on the active lever as a result of hyperactivity. It is also unlikely that the greater active lever responding in females than in males was due to sex differences in body weight, since food restriction and cocaine dosing were proportional to body weight.
Consistent with the possibility of increased motivation for cocaine, females also showed greater resistance to extinction when cocaine reinforcement was discontinued. Increased resistance to extinction was evident as greater active and inactive lever responding on extinction days 1–4 and 1–2, respectively, in females than in males. Robust inactive lever responding under extinction conditions may reflect the use of alternate response strategies for the procurement of reinforcement (Fuchs et al., 1998) or conditioned arousal (Stewart, 1992), which are considered evidence of a high degree of motivation. Importantly, the sex differences in lever responding during extinction were transient in the present study, and males and females reached similar extinction baselines prior to reinstatement testing.
Sex differences in conditioned-cued reinstatement
As we have previously reported (See 2002), male rats show robust conditioned-cued reinstatement after extinction. To our knowledge, the present study represents the first comparison of conditioned-cued reinstatement after different training doses of cocaine in male and female rats, since previous studies have relied on a single training dose of cocaine. While males showed equivalent levels of conditioned cued reinstatement independent of cocaine training dose, females exhibited less conditioned-cued reinstatement than males when trained on either the lowest (0.25 mg/kg) or the highest (1.0 mg/kg) training doses of cocaine, and reinstatement was specific to the previously cocaine-paired lever in both males and females.
Conditioned-cued reinstatement is thought to be dependent on the acquisition of stimulus–reward associations (Kruzich et al. 2001), which is facilitated by strong CS–US overlap (Rescorla 1967). When the US is not intense enough (i.e., low cocaine training dose) or if it is prolonged (i.e., high cocaine training dose), which diminishes the CS–US contiguity, the conditions for associative learning are sub-optimal. This effect is equivalent to an increase in task difficulty on a cognitive task. Under these conditions, males exhibited more conditioned-cued reinstatement than females. The sources of these sex differences are unclear; however, they appear to be mediated by different mechanisms depending on cocaine training dose. Attenuated reinstatement in females trained on the lowest (0.25 mg/kg) cocaine dose depended on estrous state. Specifically, estrous females failed to exhibit significant reinstatement and exhibited less cocaine-seeking behavior than non-estrous females and males. This finding suggests that the source of the difference is related to the hormonal state in intact females at the time of reinstatement testing. Future studies will need to manipulate the level of various hormones in ovariectomized rats in order to determine the critical hormonal changes or interactions that may result in attenuated conditioned-cued reinstatement of cocaine seeking in female rats. In contrast to the effects observed at the 0.25-mg/kg cocaine training dose, female rats trained on the highest (1.0 mg/kg) training dose of cocaine exhibited less cocaine-seeking behavior than males independent of estrous state. This finding suggests that the source of these sex differences may be related to non-cycle-dependent differences in physiology, including sex differences in cocaine metabolism at the time of conditioning (i.e., during self-administration). Consistent with this hypothesis, the concentrations of norcocaine and ecgonine methyl ester, two active metabolites of cocaine, are significantly higher in females than in males, independent of hormonal status (Bowman et al. 1999). In addition, cocaine inhibits monoamine reuptake to a greater extent within the nucleus accumbens in females than males (Festa et al. 2004), which may be especially pronounced at higher doses of cocaine. Prolonged drug effects in turn likely reduce the CS–US contiguity and result in less motivational significance attributed to the CS in females than in males.
Estrous cyclicity and conditioned-cued reinstatement
It appears that sex hormones do not play a critical role in conditioned cue-induced motivation when rats self-administer training doses of cocaine higher than 0.25 mg/kg per infusion. In freely cycling females, hormone levels are at physiological concentrations and, notably, the cyclic change in hormone levels is unmanipulated. In contrast, in hormone-treated ovariectomized females, physiological hormone levels are approximated, but little or no attempt can be made to mimic the time-dependent changes in hormone levels, which are equally critical in determining the effects of these hormones on behavior. Thus, an important contribution of the present study was to characterize the conditions under which the hormonal state of the rats exerts critical influence on behavior. However, some effects may have been harder to detect using freely cycling female rats, since testing occurred in slightly different portions of a particular phase of the estrous cycle across subjects. Direct manipulation of hormones in ovariectomized rats reduces this source of variability; thus, this line of research will be an important future direction.
Estrous cycling was irregular, even though the average cycle length was approximately 4 days. This irregularity is congruent with reports showing that vaginal cytology becomes abnormal in rats that have self-administered cocaine (Roberts et al. 1989; Grimm and See 1997). Similar effects have been noted in human users, with reported cessation of the menstrual cycle during chronic cocaine use (Mello and Mendelson 1997). Importantly, the present study reveals that cycle abnormalities are prolonged following cessation of cocaine self-administration but do not predict the magnitude of reinstatement.
Application of the reinstatement model to test sex differences in relapse
The present study contributes to a growing recognition of sex and gender differences in cocaine addiction. Reinstatement of drug-seeking behavior can be elicited by stressors as well as drug priming, similar to exposure to drug-paired stimuli (Shalev et al., 2002), and emerging evidence suggests that sex differences may also exist in some of these other forms of drug reinstatement. For example, female rats exhibit more robust reinstatement of extinguished cocaine-seeking behavior in response to a cocaine priming injection than males (Lynch and Carroll 2000). Furthermore, we have recently observed greater cocaine-primed reinstatement in estrous females than in non-estrous females (Kippin et al., 2004). Thus, females may prove to be more susceptible to pharmacologically induced reinstatement, whereas males appear to be more susceptible to conditioned-cued reinstatement of cocaine-seeking behavior. Further application of the reinstatement model should provide useful insights into sex differences in the biological and behavioral features of relapse to drugs of abuse.
References
Avants SK, Margolin A, Kosten TR, Cooney NL (1995) Differences between responders and nonresponders to cocaine cues in the laboratory. Addict Behav 20:215–224
Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM (1999) Effects of sex and gonadectomy on cocaine metabolism in the rat. J Pharmacol Exp Ther 290:1316–1323
Brady KT, Randall CL (1999) Gender differences in substance use disorders. Psychiatr Clin North Am 22:241–252
Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK (2004) Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology 29:929–942
Craft RM, Stratmann JA (1996) Discriminative stimulus effects of cocaine in female versus male rats. Drug Alcohol Depend 42:27–37
de Wit H, Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 75:134–143
Elman I, Karlsgodt KH, Gastfriend DR (2001) Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse 27:193–202
Feder H (1981) Estrous cyclicity in mammals. In Adler N (ed) Neuroendocrinology of reproduction. Physiology and Behavior. Plenum Press, New York, pp 280–289
Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V (2004) Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 46:672–687
Fiorentine R, Anglin MD, Gil-Rivas V, Taylor E (1997) Drug treatment: explaining the gender paradox. Subst Use Misuse 32:653–678
Fuchs RA, See RE (2002) Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology 160:425–433
Fuchs RA, Tran-Nguyen LT, Specio SE, Groff RS, Neisewander JL (1998) Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology 135:151–160
Glick SD, Hinds PA (1984) Sex differences in sensitization to cocaine-induced rotation. Eur J Pharmacol 99:119–121
Griffin ML, Weiss RD, Mirin SM, Lange U (1989) A comparison of male and female cocaine abusers. Arch Gen Psychiatry 46:122–126
Grimm JW, See RE (1997) Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiol Behav 61:755–761
Hu M, Becker JB (2003) Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci 23:693–699
Hu M, Crombag HS, Robinson TE, Becker JB (2004) Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 29:81–85
Kilts CD, Gross RE, Ely TD, Drexler KP (2004) The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry 161:233–241
Kippin TE, Fuchs RA, Mehta RH, Case JM, See RE (2004) Sex and estrous cycle have opposite effects on cocaine-primed versus conditioned-cued reinstatement of cocaine-seeking in rats. Program No. 916.11 2004 Abstract Viewer/Itinerary Planner. Society for Neuroscience, Washington, DC
Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ (1993) Gender differences in cocaine use and treatment response. J Subst Abuse Treat 10:63–66
Kruzich PJ, Congleton KM, See RE (2001) Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav Neurosci 115:1086–1092
Lynch WJ, Carroll ME (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology 144:77–82
Lynch WJ, Carroll ME (2000) Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology 148:196–200
Lynch WJ, Arizzi MN, Carroll ME (2000) Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology 152:132–139
Lynch WJ, Roth ME, Mickelberg JL, Carroll ME (2001) Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 68:641–646
Meil WM, See RE (1996) Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol 7:754–763
McCance-Katz EF, Carroll KM, Rounsaville BJ (1999) Gender differences in treatment-seeking cocaine abusers—implications for treatment and prognosis. Am J Addict 8:300–311
McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI (1996) Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis 184:616–622
Mello NK, Mendelson JH (1997) Cocaine’s effects on neuroendocrine systems: clinical and preclinical studies. Pharmacol Biochem Behav 57:571–599
Negrete JC, Emil S (1992) Cue-evoked arousal in cocaine users: a study of variance and predictive value. Drug Alcohol Depend 30:187–192
Panlilio LV, Katz JL, Pickens RW, Schindler CW (2003) Variability of drug self-administration in rats. Psychopharmacology 167:9–19
Rescorla RA (1967) Pavlovian conditioning and its proper control procedures. Psychol Rev 74:71–80
Robbins SJ, Ehrman RN, Childress AR, O’Brien CP (1999) Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend 53:223–230
Roberts DC, Bennett SA, Vickers GJ (1989) The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology 98:408–411
See RE (2002) Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav 71:517–529
Shalev U, Grimm JW, Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54:1–42
Skjoldager P, Winger G, Woods JH (1991) Analysis of fixed-ratio behavior maintained by drug reinforcers. J Exp Anal Behav 56:331–343
Stewart J (1992) Conditioned stimulus control of the expression of sensitization of the behavioral activating effects of opiate and stimulant drugs. In: Gormezano I, Wasserman EA (eds) Learning and memory: the behavioral and biological substrates. Erlbaum, Hillsdale, NJ, pp 129–151
van Haaren F, Meyer ME (1991) Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav 39:923–927
Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O (2000) Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A 97:4321–4326
Westermeyer J, Boedicker AE (2000) Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse 26:523–535
Acknowledgements
We thank Drs. Rebecca Craft and Heather Bimonte-Nelson for generously sharing their protocols for staining and analyzing vaginal smears. We also thank Macon Parker and J. Matthew Edwards for providing excellent technical assistance. This research was supported by the Medical University of South Carolina Specialized Center for Research on Sex and Gender Factors Affecting Women’s Health P50 grant DA 16511.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuchs, R.A., Evans, K.A., Mehta, R.H. et al. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology 179, 662–672 (2005). https://doi.org/10.1007/s00213-004-2080-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-004-2080-7