Abstract
Rationale
Higher rates of delay discounting, or impulsive choice, may be related to relapse during abstinence reinforcement interventions for cigarette smoking, and a transdermal nicotine patch may attenuate delay discounting.
Objective
The objectives of this study are to assess the relation between delay discounting and smoking after nicotine deprivation in a laboratory model of abstinence reinforcement and the effects of a transdermal nicotine patch on discounting and smoking.
Materials and methods
Smokers with no self-reported intention to quit were randomly assigned to an active (14 mg) or placebo patch group (n = 15 per group). In each of three sessions, after a 3-h deprivation period, participants completed a delay discounting task, mood, and craving measures and finally engaged in a laboratory model of abstinence reinforcement. Three abstinence reinforcement conditions were presented in counterbalanced order across the three sessions. During the control session, monetary consequences were delivered every 30 s regardless of smoking. During the low ($5.00 available) and high ($20.00 available) sessions, participants could earn a progressively increasing amount of money for each 30 s period of abstinence.
Results
The low and high conditions significantly increased the latency to smoke relative to control and significantly decreased the amount of smoking. The nicotine patch decreased negative affect, but it did not significantly affect delay discounting or smoking. Individuals who smoked during the low and high conditions showed higher rates of discounting.
Conclusion
The patch did not attenuate delay discounting or smoking after a period of deprivation, but contingencies for abstinence significantly decreased smoking. Higher rates of delay discounting were related to smoking in a model of abstinence reinforcement treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Abstinence reinforcement therapy may be a feasible and effective alternative or complement to pharmacological treatments for cigarette smoking (Dallery and Glenn 2005; Dallery et al. in press; Higgins et al. 2004; Tidey et al. 2002; Wiseman et al. 2005). Under abstinence reinforcement procedures, rewards such as money or vouchers exchangeable for goods and services are provided contingent on objective evidence of drug abstinence (Higgins et al. 1994; Stitzer et al. 1977). A number of variables influence treatment effectiveness (Lussier et al. 2006). The schedule (Roll et al. 2006; Roll and Higgins 2000), magnitude (Dallery et al. 2001; Silverman et al. 1999), and duration (Lussier et al. 2005) of reinforcement have been shown to influence outcomes, and pharmacological agents may help (e.g., nicotine, bupropion) or hinder (e.g., alcohol, caffeine) treatment success. Furthermore, a growing body of research suggests a behavioral risk factor that may be associated with treatment outcome. This research has revealed that higher rates of delay discounting, or impulsive choice, is strongly associated with smoking (Bickel et al. 1999; Mitchell 1999; Ohmura et al. 2005; Reynolds 2006), and that deprivation from nicotine may increase discounting (Field et al. 2006; Mitchell 2004). It is unknown, however, whether higher rates of delay discounting increase the risk of relapse during treatment. The main goals of the present study were to develop and validate a human laboratory model of abstinence reinforcement and to evaluate whether rates of delay discounting would predict the decision to smoke during the laboratory model.
Although there are several views of impulsive choice, most behavioral accounts define it as choice for smaller, more immediate outcomes (e.g., drug effects) over larger delayed outcomes (e.g., health, discretionary income, work, leisure, etc.). One explanation for such preference holds that delayed rewards are discounted to a high degree (for a review, see Critchfield and Kollins 2001). That is, the rate at which the value of a reward decays with time may be greater in impulsive individuals than in self-controlled individuals. Further, the high rates of delay discounting exhibited by impulsive individuals may account for several important aspects of drug dependence. For example, impulsive individuals may discount the future aversive consequences associated with drug use and future positive consequences associated with abstinence, and this can form the basis for “loss of control” and relapse to smoking (Heather 1998; Herrnstein and Prelec 1992; Rachlin 1997).
The rate at which a reward loses value with increases in delay can be described by a hyperbolic equation (Mazur 1987):
where V represents the current value of a delayed reward, A is the amount of the reward, D is the delay to the reward, and k is a free parameter that reflects the rate at which the reward loses value with increases in delay. Numerous research reports have revealed substantial individual differences in k, and these differences are consistently related to substance use. Higher ks are associated with use of nicotine (Bickel et al. 1999; Mitchell 1999), other licit (Vuchinich and Simpson 1998), and illicit substances (Kirby et al. 1999; Madden et al. 1997, 1999). Even within a group of substance users, higher ks may be related to increased use. In cigarette smokers, for example, Ohmura et al. (2005) demonstrated a positive association between rates of discounting and the frequency of nicotine self-administration, as well as the dose of nicotine administered.
Although it is logical that higher rates of delay discounting should increase risk of relapse (Field et al. 2006; Giordano et al. 2002), to our knowledge, there is no empirical support for such a relation. One study has indicated a predictive relation between a trait measure of impulsivity (the Barratt impulsivity scale, Doran et al. 2004) and time to relapse. In the present study, we assessed whether delay discounting was associated with smoking in a novel human laboratory model of abstinence reinforcement.
Although individuals differ in delay discounting, such behavior may be regarded as a behavioral tendency rather than a fixed trait—a tendency that may change with environmental and pharmacological conditions (Mazur 1987). One variable that has been shown to increase delay discounting is deprivation from an abused substance. For instance, Giordano et al. (2002) found that mild opioid deprivation increased delay discounting in dependent opiate users. These findings parallel another study showing that deprivation from nicotine may increase impulsive decision making in smokers (Field et al. 2006). In both studies, after deprivation, participants showed higher rates of discounting of both the delayed drug (cigarettes in the Field et al. study and heroin in the Giordano et al. study) and money. In light of these findings, an additional goal of the present study was to assess whether a transdermal nicotine patch would attenuate deprivation-induced increases in delay discounting of money relative to a placebo group. If deprivation from nicotine mediates increases in discounting, then nicotine replacement should prevent such increases.
Before each of three laboratory sessions, participants completed the delay discounting task and then engaged in the laboratory model of abstinence reinforcement. The model consisted of three conditions, and, in all conditions, opportunities to smoke were provided after a brief period of deprivation. In the control condition, no explicit contingencies were imposed on choices to smoke or abstain. In the other two conditions, participants could earn a progressively increasing amount of money for not smoking during successive 30 s intervals (technically, a differential reinforcement of other behavior, or DRO, contingency), and the two conditions differed in the total amount of money available (i.e., low and high magnitude conditions). The schedule of earnings, the DRO contingency, and the magnitude manipulation were chosen to increase the face validity and evaluate the predictive validity (e.g., Katz and Higgins 2003) of the model in relation to the results obtained from outpatient abstinence reinforcement interventions (Dallery et al. 2001; Roll et al. 2000; Silverman et al. 1999). Specifically, the schedule of progressive earnings and the DRO contingency were used because they are employed in outpatient abstinence reinforcement interventions. Similarly, the magnitude manipulation was used because increasing the magnitude of the alternative reinforcer has been shown to increase abstinence in laboratory (e.g., Roll et al. 2000) and clinical (e.g., Dallery et al. 2001; Silverman et al. 1999) settings.
Materials and methods
Participants
Thirty-two smokers completed the study; however, two participants were dropped from all analyses due to a computer malfunction (see Table 1 the for remaining participant characteristics). Participants were recruited via classified advertisements, word of mouth, and flyers posted throughout the community. Applicants were required to be between 18 and 60 years of age, report smoking at least one pack of cigarettes per day, and have no intention to quit smoking (selecting a 5 or less on a Likert scale ranging from 1 to 10, with 1 representing no intention to quit). If they satisfied these criteria, they were invited for a full screening (described below).
Setting and equipment
All sessions were completed in a small, well-ventilated room equipped with two PC computers, a couch and chair, television with VCR, magazines, and a one-way observation mirror. Plowshare topography hardware and software (Baltimore, MD) were installed on both computers. A cigarette mouthpiece was connected to the Plowshare equipment via a hose and was used to record number of puffs taken, puff volume, puff duration, and maximum flow. Microsoft Visual Basic 6.0 software was used to program experimental events during the delay discounting and abstinence reinforcement tasks.
Screening
Eligible participants attended a 1.5-h screening session. During the screening, participants completed the informed consent process (approved by the University of Florida Institutional Review Board), provided a urine sample for drug (Varian; Lake Forest, CA) and pregnancy testing (Calhoun Industries; Fort Smith, AZ), completed several questionnaires, and provided a breath carbon monoxide sample (CO; Bedfont piCO Smokerlyzer) after smoking one cigarette. To minimize practice effects during the experimental sessions and to assess the presence of group differences that may have existed before group assignment, participants completed the delay discounting task. During the screening session only, the delay discounting task consisted of choices between $10.00 delivered after a delay and an adjusting amount of money delivered immediately. A different amount was used during experimental sessions, but all other features of the task were similar to those described below. Participants were compensated $6.00 for completing the screening.
The questionnaires included a psychosocial history, the University of Rhode Island Change Assessment (URICA; Prochaska and DiClemente 1983), and the Fagerström test for nicotine dependence (FTND; Fagerström and Schneider 1989; Heatherton et al. 1991). The psychosocial history contained questions related to demographics, smoking history, prior drug use and abuse, general health, and medication use. The URICA is a self-report measure to assess motivation to change (i.e., motivation to change/quit smoking). Participants responded on a five-point Likert scale (1 = strongly disagree to 5 = strongly agree). The URICA contains four subscales that measure the stages of change: precontemplation, contemplation, action, and maintenance. The subscales are combined arithmetically to yield a continuous score that can be used to assess readiness to change, and a score of 9.3 or lower represents the precontemplation stage. The FTND is a six-item questionnaire assessing nicotine dependence. Scores can range from zero to ten with a score of zero representing very low dependence and a score of ten representing very high dependence.
Exclusion criteria were testing positive on the drug (methamphetamine, cocaine, morphine, benzodiazepines) or pregnancy tests, self-reported medical complications that would contraindicate the nicotine patch (e.g., heart disease), and self-reported clinically diagnosed mental illness (e.g., schizophrenia).
Experimental design
Eligible participants were randomly assigned to an active or placebo patch group (between-subject factor) and to one of the six possible orders of exposure to the three experimental conditions of the abstinence reinforcement model (within-subject factor). Participants were blind to their patch group assignment.
Procedure
Participants attended three experimental sessions on three separate days (see Fig. 1 for session schematic) and were compensated a minimum of $12.00 for each session (supplemental money could be earned during the abstinence reinforcement task as described below). Payments were made in the form of a lump sum check after the experiment had been completed (approximately 2 weeks later) and ranged from approximately $60 to $90, depending on how the participant responded during the session.
At the beginning of the session, participants smoked one cigarette of their preferred brand (supplied by the researcher). Immediately after smoking one cigarette, participants completed a craving questionnaire on the computer (Schuh and Stitzer 1995). The craving questionnaire consisted of the following four questions: (1) How pleasant would a cigarette be right now? (2) How much do you need a cigarette right now? (3) How much of an urge or desire do you have to smoke right now? and (4) How much do you want a cigarette right now? Answers were provided on a visual analog scale corresponding to a numerical value ranging between 0 (not at all) and 100 (extremely). Next, depending on participants’ patch assignments, a placebo patch (packaging tape) or a transdermal nicotine patch (14 mg Nicoderm CQ®; Palo Alto, CA) were adhered to their upper arm. Patches were concealed under a large bandage. Participants then provided a breath CO sample and were instructed to abstain from smoking for the following 3-h period. Participants were allowed to leave the smoking laboratory during this time.
Upon returning from the 3-h abstinence period, participants provided another breath CO sample. To continue the session, participants’ CO had to decrease by at least 20% from their previous post-cigarette CO (Middleton and Morice 2000). Next, participants completed the same craving questionnaire as described above, the profile of mood states (POMS; McNair et al. 1971) and an additional behavioral task (data not reported in the present article). The POMS is a 72-item survey consisting of one-word adjectives regarding current mood state. Participants responded on a five-point Likert scale (0 = not at all to 4 = extremely) and adjectives were grouped into seven categories (anxiety, depression, anger, vigor, fatigue, confusion, friendliness, elation).
After completing the surveys, participants began the delay discounting procedure. Participants clicked one of two response buttons presented on the computer screen. The left button corresponded to an adjusting amount of money available immediately, and the right button corresponded to $100.00 after one of eight different delays (1 week, 2 weeks, 1 month, 4 months, 8 months, 1 year, 5 years, and 10 years) presented in random order. The algorithm described by Johnson and Bickel (2002) was used to determine the value of the immediate adjusting amount of money and the indifference point at each delay (see their appendix for details). The indifference point is the point at which the participant equally prefers the immediate amount of money to the delayed amount of money (i.e., indifferent between the two values). After every choice, a dialog box appeared requiring participants to confirm their choice. All choices during the discounting task were hypothetical in the sense that participants did not actually receive the amounts they chose. The only instructions for this task were located above the two response alternatives and said, “Please choose the option you prefer, based on how you feel right now”.
Finally, the laboratory model of abstinence reinforcement began. During this task, participants were seated in front of the computer and the interface consisted of a small yellow rectangular button in the center of the screen that said, “Take a puff.” To the left of the button was a black and white picture of a hand placing change into a piggy bank with the total accumulated amount of money displayed below the picture. The participant’s preferred brand of cigarettes, a lighter, and an ashtray were located next to the computer monitor and were available at all times during this portion of the session. When participants decided to take a puff, they placed a cigarette in the mouthpiece connected to Plowshare topography equipment, lit the cigarette, and then pressed the button labeled “Take a puff.” The button then turned green, became disabled, and was not re-enabled until the end of the 30 s interval. The 30-s interval was chosen because it approximates the average inter-puff interval found in a previous study on smoking topography (Lee et al. 2003). Thus, participants could take one puff per 30 s, and the puff could be taken at any point during the 30-s interval. At the end of each 30-s interval, a dollar sign appeared above the button with the amount of money earned during that interval. This amount was added to the total in the bank, and the button was re-enabled. After 20 intervals (i.e., 10 min), participants were required to take a 20-min break. During the break, they could watch television, talk on the phone, read, have a snack, or search the Internet. Participants were not allowed to smoke during the break.
The three different experimental sessions corresponded to three abstinence reinforcement conditions—control, low, and high. During the control condition, participants earned $0.16 for each 30 s interval regardless of whether they took a puff during that interval or not. Therefore, participants earned $12.80 for the four 10-min blocks (the approximate midpoint of the low and high amounts). During the low condition, participants earned $0.015 for the first puff-free interval, and for each subsequent puff-free interval, the amount increased by $0.005. If a puff was taken, no money was delivered at the end of that 30 s interval, and the amount of the next puff-free interval was reset to $0.015. At the beginning of each block, the value was reset to the starting value of $0.015. The maximum amount of money that could be earned during the low condition was $5.00 ($1.25 per block). The high condition was exactly the same as the low condition, except that all values were four times those used during the low condition (i.e., starting amount $0.06, increasing by $0.02). The maximum amount of money that could be earned during the high condition was $20.00 ($5.00 per block). Immediately before starting the abstinence reinforcement task, research assistants read aloud detailed instructions about how the participant could earn money during that portion of the session (e.g., Silverman et al. 1999). Part of the instructions consisted of showing graphs that illustrated how the amount of money increased with each puff-free interval and how the amount of money reset when a puff was taken.
At the end of the experiment, participants were asked the following question: “Do you think you were given (choose one answer), a) active nicotine patch, b) placebo patch, c) both (on different days), d) not sure.”
Data analysis
All statistical tests were considered significant at P < 0.05.
To assess whether the deprivation period produced the predicted decrease in CO and increase in craving, repeated measures analysis of variance (ANOVA) was conducted with time of day (pre- or postdeprivation) and patch assignment as factors. The sum of all four items from the Schuh–Stitzer was used in the analysis of craving. Bonferroni tests were used when significant main or interaction effects were found. All repeated measures data were adjusted for sphericity by Huynh–Feldt correction. Where appropriate and in separate analyses, session order was a factor.
The effects of the patch were assessed using independent samples t-tests for the self-report measures (craving, POMS). Data were collapsed across the three sessions for each group, as there were no significant effects of session order on these measures. An analysis of covariance (ANCOVA) was used to assess the effects of the patch on delay discounting. The screening discounting data was entered as a covariate.
Repeated measures ANOVA was used to assess the number of puffs taken during the laboratory model, with patch assignment and condition (control, low, high) as factors. In addition, we used Cox regression to assess the latency to self-initiated smoking in the laboratory model of abstinence reinforcement. We compared abstinence survival rates between groups, and pair-wise comparisons were made for the three laboratory conditions (control, low, high).
Delay discounting was assessed in two ways. First, the area under the curve (AUC) was calculated. The AUC was used to permit inferential statistical analysis of the discounting data. The AUC yields a normally distributed measure of discounting (Myerson et al. 2001). To obtain the AUC, the delays and indifference points were normalized. The delay was expressed as a proportion of the maximum delay, and reward value (see Eq. 1) was expressed as a proportion of the undiscounted amount. Then, the AUC was calculated by summing the results of the following equation: \( x_{2} - x_{1} {\left[ {{{\left( {y_{1} + y_{2} } \right)}} \mathord{\left/ {\vphantom {{{\left( {y_{1} + y_{2} } \right)}} 2}} \right. \kern-\nulldelimiterspace} 2} \right]} \), where x 1 and x 2 are successive delays, and y 1 and y 2 are the present values associated with those delays. The AUC measure can thus range from 0 (maximum discounting) to 1 (no discounting).
A second quantitative method was used to assess the relation between discounting and smoking. Although the AUC provides a theoretically neutral index of discounting, we were also interested in the extent to which the data conformed to current theoretical accounts of discounting. Thus, Eq. (1) was fitted to the median indifference points for those who smoked and those who abstained during the abstinence reinforcement procedure. The equation was fitted by least-squares regression using Microsoft Excel’s Solver routine. Although the equation accounted for relatively high percentages of variance (i.e., R 2’s) in the indifference points, we found that the standardized residuals were significantly correlated with the predicted indifference points (Pearson’s product-moment correlation coefficients [r] ranged from −0.70 to −0.91). This means that the data systematically deviated from the model, and thus, the model does not describe the data (Motulsky and Christopoulos 2004). Myerson and Green (1995) suggested that a hyperbola-like model better described data obtained from humans, or:
where a is a nonlinear scaling parameter, which may reflect differences in sensitivity to the effects of delay. When a = 1, Eq. (2) reduces to Eq. (1). As reported below, we found that Eq. (2) described the data (i.e., high R 2’s and no significant correlations between the standardized residuals and predicted indifference points).
We compared the fits of Eq. (2) obtained from smokers and abstainers during the abstinence reinforcement procedure. The discounting data obtained before each respective abstinence reinforcement condition was used in the comparison. To evaluate whether two curves described the data from smokers and abstainers, we used Akaike’s information criterion (AIC; Motulsky and Christopoulos 2004). The AIC analysis indicates which of two models is more likely to be correct, and it takes into account goodness of fit and the relative complexity (i.e., number of parameters) in each model. The null hypothesis was that one curve could fit the data from smokers and abstainers. Thus, one curve (Eq. 2) was fitted to the data from both smokers and abstainers. The alternative hypothesis was that two curves describe the data from smokers and abstainers. Thus, two curves, one for smokers and one for abstainers, were fitted separately. AICs were computed for the one-curve and two-curve models. The model with the lower AIC is more likely to be correct, and the evidence ratio indicates how much more likely one model is correct relative to the other. The evidence ratio is defined by the equation: \( 1 \mathord{\left/ {\vphantom {1 {e^{{ - 0.5*\Delta AIC}} }}} \right. \kern-\nulldelimiterspace} {e^{{ - 0.5*\Delta AIC}} } \), where the change in AIC (ΔAIC) is simply the absolute difference between the two AICs (see Motulsky and Christopoulos 2004 for more details).
Results
Demographic characteristics
Two tailed t-tests revealed that participants assigned to the patch group showed higher baseline rates of delay discounting, measured as AUC, than participants in the placebo group, as assessed during the screening (see Table 1). There were no significant differences between the placebo and active patch groups in any other demographic characteristic.
Validation of the deprivation protocol
There were no significant differences between active and placebo groups in CO when it was measured before (placebo = 26.6 ppm, SEM = 1.7, active = 30.0 ppm, SEM = 2) or after (placebo = 14.4 ppm, SEM = 1.6; active = 15.1 ppm, SEM = 2.1) the deprivation period. There were also no significant differences between active and placebo groups in craving measures when it was measured before (placebo = 80.2 ppm, SEM = 20.7; active = 60.2 ppm, SEM = 16.9) or after (placebo = 253.6 ppm, SEM = 29.7; active = 248.7 ppm, SEM = 23.2) the deprivation period. We should also note that there were no significant differences when these group comparisons were made on a condition by condition basis.
The top panel in Fig. 2 presents the average decrease in CO from the predeprivation period to the postdeprivation period for each condition. There was a significant decrease in CO from pre- to postdeprivation [F(1,28) = 167.9, P < 0.05]. (The difference in CO under the high magnitude condition was not statistically significant even when analyzed separately using a t-test.) The bottom panel in Fig. 2 shows that craving was significantly elevated during the postdeprivation period relative to the predeprivation period [F(1,28) = 60.0, P < 0.05].
Integrity of the blinding procedures
On the exit survey, 6.7% of placebo patch participants accurately identified their group assignment, 6.7% selected the wrong group assignment, 66.7% selected that they were given both active and placebo on different days, and 20% stated that they were not sure whether they were given the active or placebo patch. Among active patch participants, 26.7% accurately identified their group assignment, 0% selected the wrong group assignment, 60% selected that they were given both active and placebo on different days, and 13.3% stated that they were not sure whether they were given the active or placebo patch.
Effects of the nicotine patch on self-report measures, delay discounting, and smoking
The active patch significantly decreased negative affect [t(88) = 3.532, P < 0.05], which was a composite of the anger, depression, and anxiety factors of the POMS. As illustrated in Fig. 2, there were no significant differences in craving between the active and placebo patch groups.
ANCOVA indicated that there were no significant differences between active and placebo groups in delay discounting, as measured by AUC, when baseline delay discounting was entered as a covariate.
There were no significant differences between active and placebo patch participants on latency or amount of smoking during the laboratory model of abstinence reinforcement. Finally, there were no statistically significant differences between groups in puff volume, puff duration, or maximum flow.
Laboratory model of abstinence reinforcement with and without the nicotine patch
Figure 3 is a survival plot showing the cumulative number of abstinent participants as a function of minutes into the session. The latency to take the first puff of the session increased for both placebo and active patch participants when the contingencies for not smoking were implemented (low vs control: hazard ratio = 2.63 [CI = 1.435−4.820], P < 0.05; high vs control: hazard ratio = 5.125 [CI = 2.551−10.295], P < 0.05). Although the figure indicates some differences in the latency to the first puff between the low and high conditions, these differences did not meet conventional levels of significance.
Survival plots showing the cumulative proportion of abstinent participants as a function of minutes into the abstinence reinforcement procedure. Placebo participants are shown in the top panel, and patch participants are shown in the bottom panel. During the control condition, participants received monetary consequences every 30 s, whereas during the low ($5.00 available) and high ($20.00 available) magnitude conditions, participants could receive monetary consequences for not taking a puff during 30 s intervals
Figure 4 shows the total number of puffs smoked during each of the three conditions for active and placebo patch participants. There was a significant effect of condition [F(2,28) = 63.5, P < 0.05]. Bonferroni analyses showed that participants took significantly more puffs during the control condition compared to the low and high conditions; however, there was no significant difference between the low and high conditions. Furthermore, there was no significant interaction between condition and patch assignment.
When each of the four blocks were analyzed separately, the same condition effect was replicated during each of the four blocks [Block 1: F(2,28) = 46.7, P < 0.05; Block 2: F(2,28) = 28.1, P < 0.05; Block 3: F(2,28) = 33.1, P < 0.05; Block 4: F(2,28) = 39.4, P < 0.05].
There were no statistically significant differences across conditions in puff volume, puff duration, or maximum flow.
Delay discounting and smoking
The AUC remained stable across all three sessions as indicated by Pearson correlation coefficients (Session 1 vs 2: r = 0.97; 1 vs 3: r = 0.96; 2 vs 3: r = 0.98). All correlations were statistically significant. There was no significant effect of session order on AUC.
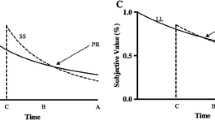
Figure 5 shows median indifference points from the delay discounting task for those who smoked (open circles) and those who abstained (closed circles). Because there were no significant differences in smoking or discounting between the patch and placebo groups, these groups were collapsed into a single group in the current analysis. Curves represent the fits of Eq. (2) to the data. Only data from the low (left panel) and high (right panel) magnitude condition are shown, as all but one participant smoked in the control condition. Residual analysis indicated no significant correlations between the standardized residuals and the predicted indifference points. For the low condition, the evidence ratio indicated that the two-curve model was 8,198 times more likely to be correct compared to the one-curve model. For the high condition, the evidence ratio indicated that the two-curve model was 100 times more likely to be correct. These evidence ratios provide overwhelming support for the two-curve models (see Motulsky and Christopoulos 2004 for more information about evaluating evidence ratios). In other words, it is extremely likely that different discounting curves describe the data from smokers and abstainers. The estimated parameters and R 2’s are shown in Table 2. As reflected in Fig. 5, the ks differed depending on smoking status. However, the sensitivity parameter, a, remained fairly constant across condition and smoking status.
Delay discounting functions for participants who smoked during the session (open circles) and for those who abstained during the session (closed circles). Points are median indifference points. Curves represent the hyperbolic-like function, Eq. (2), fitted to the data for each group
As previously noted, all but one participant smoked during the control condition. Interestingly, this abstainer during the control session also abstained during the low and high conditions and consistently showed essentially no discounting of delayed rewards. That is, $100 lost very little value as delay increased, and thus the indifference points did not show enough variability to fit the hyperbolic-like function for any session.
Also indicated in Table 2 is the number of participants who smoked or abstained in each condition. We analyzed further delay discounting for those participants who “defected” from being a smoker during the low condition to being an abstainer during the high condition. Twenty participants smoked during the low condition. Thirteen of these smokers also smoked during the high condition. Seven participants defected to abstinence in the high condition. (One participant defected from smoking during the high to abstinence during the low condition; data not shown.) These seven “defectors” showed lower rates of discounting, in both the low and high conditions, compared to the subset of 13 individuals who smoked during both conditions. Table 3 shows the results of the fits of Eq. (2) to the discounting data. The AIC analysis indicated that the two-curve model (separate fits of Eq. (2)) was 38 times more likely to be correct compared to the one-curve model in the low condition but no more likely to be correct for the high condition (AIC < 1.0).
Discussion
Higher rates of delay discounting were related to the decision to smoke in a laboratory model of abstinence reinforcement. Delay discounting was assessed by fitting Eq. (2) to the median indifference points obtained from smokers and abstainers and statistically estimating two parameters, k and a. The fits of Eq. (2) to the delay discounting data were excellent (see Table 2). Individuals who smoked showed higher ks, or rates at which delayed rewards lost value, than individuals who abstained. It is interesting that only k differed depending on smoking status. In contrast, the exponent a remained fairly constant, which implies that sensitivity to the effects of delay did not differ across condition or smoking status (Myerson and Green 1995). In other words, only the rate of discounting, and not sensitivity to delay, differed depending on smoking status. (There was one exception to this general finding, see Table 3. The reason for the elevated a parameter for the defectors in the low condition is unclear.) Furthermore, the group of seven defectors, those who smoked during the low condition and then abstained during the high condition, showed lower rates of discounting compared to the 13 individuals who smoked in both sessions. These results should be interpreted with caution, however, as the difference was likely only during the low condition and not during the high condition.
These results suggest that differences in delay discounting may be important predictors of treatment success, or failure, under outpatient reinforcement interventions. After all, the choice to resume smoking and forgo the delayed positive consequences of abstinence is by definition an impulsive choice. For those who show high rates of discounting, it may be that more potent alternative rewards are necessary for these individuals to forgo smoking. A wealth of research has already demonstrated that delay discounting is associated with substance abuse (Bickel et al. 1999; Kirby et al. 1999; Madden et al. 1997, 1999; Mitchell 1999; Vuchinich and Simpson 1998). In the animal laboratory, delay discounting has been found to predict cocaine self-administration (Perry et al. 2005). The current results extend these findings by suggesting that discounting may be related to treatment success, or failure, in a model of abstinence reinforcement treatment.
To our knowledge, the present study is the first to demonstrate a relation between discounting and the choice to smoke in the human laboratory. We should note that the smokers in the present study had no intention to quit, thus it is unknown whether these findings would generalize to treatment-seeking smokers. In an outpatient setting, however, Doran et al. (2004) found that a trait measure of impulsivity predicted more rapid relapse in a sample of 45 smokers. The authors found that impulsivity accounted for 14.7% of the variance in time to relapse, after controlling for several covariates (treatment condition, baseline nicotine dependence, and age) in a regression analysis. Relative to the current study, it is likely that the trait questionnaire assessed dimensions of impulsivity that were not captured by the delay discounting task. That is, the questionnaire assessed tendencies other than the propensity to choose smaller, sooner rewards over larger, later rewards. Indeed, Mitchell (1999) found that correlations between trait measures and delay discounting measures of impulsivity were rare, and when significant, the correlations were small. Similarly, Acheson et al. (2006) found that diazepam impaired behavior on a behavioral inhibition task but had no effect on delay discounting tasks. In concert, these results suggest that some dimensions of impulsivity may be more predictive of, and affected by, drug use. As such, it would be useful to clarify further the dimensions of impulsive behavior (e.g., delay discounting, response inhibition) that also increase the risk of relapse, as novel or more intensive treatments may be necessary for individuals who demonstrate these risk factors.
Deprivation from nicotine has been shown to increase impulsive choice (Field et al. 2006). We were interested in whether a nicotine patch could reverse these deprivation-induced increases in discounting. We attempted to equate baseline levels of nicotine as much as possible by requiring a presession cigarette. There was no significant difference in CO between the nicotine and placebo patch groups before the patches were adhered. Given that breath CO is highly correlated with plasma nicotine levels (Jarvik et al. 2000), we can infer that baseline nicotine blood levels were approximately equivalent between the two groups before the abstinence period. Given the relatively short half-life of nicotine of about 2 h (Jarvik et al. 2000) and an exponential elimination rate, we can also infer that nicotine levels in the placebo group after the 3 h abstinence period were at least half of what they were at baseline during each session. For example, if a participant’s plasma level was 30 ng/ml at baseline—a reasonable estimate for participants in the current study based on baseline CO—then after 3 h, the level should be approximately 5–10 ng/ml (Jarvik et al. 2000). We chose the 14-mg Nicoderm patch because it has been shown to produce peak plasma levels of about 15 ng/ml after approximately 3–4 h (Palmer et al. 1992; Perkins et al. 2004). Therefore, if nicotine deprivation per se were mediating the increases in impulsive choice observed in the Field et al. 2006 study, we expected to see at least a partial attenuation of these effects after patch administration in the current study.
Contrary to our expectations, a 14-mg dose of the transdermal nicotine patch did not significantly affect delay discounting relative to the placebo control group. Similarly, the patch did not produce significant decreases in craving (Fig. 2). The finding that the patch did not alter craving ratings is consistent with other studies that have used similar and higher doses of the nicotine patch (e.g., Pickworth et al. 1996). Shiffman et al. (2006) found that much higher doses than the one we used can produce decreases in craving. Furthermore, the nicotine patch produced small but insignificant decreases in smoking relative to the placebo patch group, and there was little evidence that the patch enhanced the effectiveness of the contingencies for abstinence. The only evidence that the patch produced pharmacological effects was that the active patch group reported lower ratings of negative affect relative to the placebo patch group. However, because baseline, pregroup assignment measures of negative affect were not obtained, it is unknown whether these differences were, in fact, the result of the patch or whether they were preexisting. Regardless, a higher dose of the nicotine patch may produce decreases in craving and impulsive choice and also subsequent decreases in smoking.
One feature of most delay discounting tasks that may limit their sensitivity to pharmacological manipulations is that choices between immediate and delayed outcomes are hypothetical. Participants do not actually experience the delays associated with their choices because the consequences are never delivered. Several studies, however, have found no meaningful differences in the pattern or rate of discounting between hypothetical and real outcomes (Johnson and Bickel 2002; Lagorio and Madden 2005; Madden et al. 2003, 2004). Nevertheless, it is plausible that verbally-mediated hypothetical choices are less sensitive to pharmacological variables than behavior-based choices, where behavior contacts the actual delays and consequences for each choice. Thus, future studies on pharmacological influences in impulsive choice may benefit from incorporating behavior-based measures of impulsive choice (e.g., Cherek and Lane 2001).
In contrast to the relatively small changes in smoking produced by the nicotine patch, the presence of contingencies for abstinence produced pronounced decreases in smoking relative to control conditions. These findings parallel results found in outpatient abstinence reinforcement studies in two ways. First, abstinence reinforcement produces fairly large decreases in smoking relative to control conditions (Dallery et al. in press; Higgins et al. 2004). For example, Higgins et al. (2004) found that voucher-based abstinence reinforcement produced higher rates of abstinence in pregnant women relative to a control group that received vouchers regardless of smoking status. Second, adding a patch to abstinence reinforcement has not produced greater decreases in smoking relative to abstinence reinforcement alone (Tidey et al. 2002; Wiseman et al. 2005).
There is another interesting parallel between the resumption of smoking in outpatient, clinical settings and smoking in the laboratory model. That is, most smokers who resume smoking do so very early in treatment, and participants who smoked in the laboratory model did so early in the session (Fig. 3). In clinical settings, at least 75% of cigarette smokers relapse within 2–3 days after a quit attempt (Hughes 1992), and abstinence during the first 2 weeks of a quit attempt is strongly predictive of long-term treatment success (Garvey et al. 1992; Gourlay et al. 1994; Kenford et al. 1994). These findings suggest that there may be a “critical period” to initiate and sustain abstinence during a quit attempt whether modeled in the human laboratory or observed in a clinical setting. It would be useful to explore whether methods to promote abstinence during this critical period in the human laboratory translate into clinical settings.
The modest outcomes of the magnitude manipulation, however, are less consistent with several outpatient studies of abstinence reinforcement that have shown magnitude effects (Dallery et al. 2001; Silverman et al. 1999). Although Figs. 3 and 4 suggest some differences between the low and high magnitude conditions, these differences did not reach conventional levels of significance. There are several potential explanations for the discrepancy. First, in the current study the high magnitude was four times the value of the low magnitude. In several outpatient studies, the high magnitude intervention was approximately nine times the value of the low magnitude intervention (e.g., Dallery et al. 2001; Silverman et al. 1999). Thus, the relative difference between the magnitudes used in the present study may have been too small. Second, the function form describing the relation between nominal amounts of money and their perceived values may be nonlinear. The amounts of money in the low magnitude and high magnitude condition may have been equally motivating. We simply have no way of knowing where the values we used are on the function relating nominal amounts of money to their perceived values and thus their ability to motivate abstinence.
To the extent the present results parallel results obtained in outpatient settings, the laboratory model of abstinence reinforcement has good predictive validity. Katz and Higgins (2003) recommended DRO procedures in laboratory settings on the basis that they resemble the “complex interplay of social contingencies involving alternative reinforcers scheduled to be incompatible with...drug use” (p. 28) in clinical settings and because they represent the controlling variable in highly successful contingency management interventions for drug use. In addition to their face and predictive validity, such procedures may be an ideal way to isolate individual differences responsible for treatment failure and to test environmental and pharmacological treatments that may mitigate these risk factors. The present study demonstrated that higher rates of delay discounting were associated with smoking in the laboratory. Future research should evaluate the predictive validity of this relation.
References
Acheson A, Reynolds B, Richards JB, de Wit H (2006) Diazepam impairs behavioral inhibition but not delay discounting or risk taking in healthy adults. Exp Clin Psychopharmacol 14:190–198
Bickel WK, Odum AL, Madden GJ (1999) Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology 146:447–454
Cherek DR, Lane SD (2001) Acute effects of d-fenfluramine on simultaneous measures of aggressive escape and impulsive responses of adult males with and without a history of conduct disorder. Psychopharmacology 157:221–227
Critchfield TS, Kollins SH (2001) Temporal discounting: Basic research and the analysis of socially important behavior. J Appl Behav Anal 34:101–122
Dallery J, Glenn IM (2005) Effects of an Internet-based voucher reinforcement program for smoking abstinence: a feasibility study. J Appl Behav Anal 38:349–357
Dallery J, Silverman K, Chutuape M, Bigelow GE, Stitzer ME (2001) Voucher-based reinforcement of opiate plus cocaine abstinence in treatment-resistant methadone patients: effects of reinforcer magnitude. Exp Clin Psychopharmacol 9:317–325
Dallery J, Glenn IM, Raiff BR (in press) An Internet-based abstinence reinforcement treatment for cigarette smoking. Drug Alcohol Depend
Doran N, Spring B, McChargue D, Pergadia M, Richmond M (2004) Impulsivity and smoking relapse. Nicotine Tob Res 6:641–647
Fagerström K, Schneider NG (1989) Measuring nicotine dependence: a review of the Fagerström Tolerance Questionnaire. J Behav Med 12:159–182
Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J (2006) Delay discounting and the behavioral economics of cigarette purchases in smokers: the effects of nicotine deprivation. Psychopharmacology 186:255–263
Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B (1992) Predictors of smoking relapse among self-quitters: a report from the normative aging study. Addict Behav 17:367–377
Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ (2002) Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology 163:174–182
Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ (1994) Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. Br Med J 309:842–846
Heather N (1998) Conceptual framework for explaining drug addiction. J Psychopharmacol 12:3–7
Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO (1991) The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict 86:1119–1127
Herrnstein RJ, Prelec D (1992) A theory of addiction. In: Loewenstein G, Elster J (eds) Choice over time. Russell Sage, New York, pp. 235–264
Higgins ST, Budney AJ, Bickel WK, Foerg F, Donham R, Badger MS (1994) Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry 51:568–576
Higgins ST, Heil SH, Solomon, LJ Lussier JP, Abel RL, Lynch ME, Badger GJ (2004) A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res 6:1015–1020
Hughes JR (1992) Tobacco withdrawal in self-quitters. J Consult Clin Psychol 60:689–697
Jarvik ME, Madsen DC, Olmstead RE, Owamoto-Schaap PN, Elins JL, Benowitz NL (2000) Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav 66:553–558
Johnson MW, Bickel WK (2002) Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav 77:129–146
Katz J, Higgins ST (2003) The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology 168:21–30
Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB (1994) Predicting smoking cessation, who will quit with and without the nicotine patch. J Am Med Assoc 271:589–594
Kirby KN, Petry NM, Bickel WK (1999) Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen 128:78–87
Lagorio CH, Madden GJ (2005) Delay discounting of real and hypothetical rewards III: steady-state assessments, forced-choice trials, and all real rewards. Behav Processes 69:173–187
Lee EM, Malson JL, Waters AJ, Moolchan ET, Pickworth WB (2003) Smoking topography: reliability and validity in dependent smokers. Nicotine Tob Res 5:673–679
Lussier JP, Higgins ST, Badger GJ (2005) Influence of the duration of abstinence on the relative reinforcing effects of cigarette smoking. Psychopharmacology 181:486–495
Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST (2006) A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 101:192–203
Madden GJ, Petry NM, Badger GJ, Bickel WK (1997) Impulsive and self-control choices in opioid-dependent patients and non-drug-using controls participants: drug and monetary rewards. Exp Clin Psychopharmacol 5:256–262
Madden GJ, Bickel WK, Jacobs EA (1999) Discounting of delayed rewards in opioid-dependent outpatients: exponential or hyperbolic discounting functions. Exp Clin Psychopharmacol 7:284–293
Madden GJ, Begotka AM, Raiff BR, Kastern LL (2003) Delay discounting of real and hypothetical rewards. Exp Clin Psychopharmacol 11:139–145
Madden GJ, Raiff BR, Lagorio CH, Begotka AM, Mueller AM, Hehli DJ, Wegener AA (2004) Delay discounting of potentially real and hypothetical rewards II: between-and within-subject comparisons. Exp Clin Psychopharmacol 12:251–261
Mazur JE (1987) An adjusting amount procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H (eds) Quantitative analyses of behavior: the effects of delay and of intervening events on reinforcement value. Erlbaum, Hillsdale, NJ, pp 55–73
McNair DM, Lohr M, Doppleman LF (1971) Profile of mood states (manual). Education and Industrial Testing Service, San Diego
Middleton ET, Morice AH (2000) Breath carbon monoxide as an indication of smoking habit. Chest 117:163–758
Mitchell SH (1999) Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology 146:455–464
Mitchell SH (2004) Effects of short-term nicotine deprivation on decision-making: delay, uncertainty and effort discounting. Nicotine Tob Res 6:819–828
Motulsky H, Christopoulos A (2004) Fitting Models to Biological data using linear and nonlinear regression: a practical guide to curve fitting. Oxford University Press, Oxford
Myerson J, Green L (1995) Discounting of delayed rewards: models of individual choice. J Exp Anal Behav 64:263–276
Myerson J, Green L, Warusawitharana M (2001) Area under the curve as a measure of discounting. J Exp Anal Behav 76:235–243
Ohmura Y, Takahashi T, Kitamura N (2005) Discounting delayed and probabilistic monetary gains and losses by smokers of cigarettes. Psychopharmacology (Berl) 182:508–515
Palmer KJ, Buckley MM, Faulds D (1992) Transdermal nicotine. Drugs 44:498–529
Perkins KA, Lerman C, Keenan J, Fonte C, Coddington S (2004) Rate of nicotine onset from nicotine replacement therapy and acute responses in smokers. Nicotine Tob Res 6:501–507
Perry JL, Larson EB, German JP, Madden GJ, Carroll ME (2005) Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology 178:193–201
Pickworth WB, Fant RV, Butschky MF, Henningfield JE (1996) Effects of transdermal nicotine delivery on measures of acute nicotine withdrawal. J Pharmacol Exp Ther 279:450–456
Prochaska J, DiClemente C (1983) Stages and process of self-change of smoking: toward and integrative model of change. J Consult Clin Psychol 51:390–395
Rachlin H (1997) Four teleological theories of addiction. Psychon Bull Rev 4:462–473
Reynolds B (2006) The experiential discounting task is sensitive to cigarette-smoking status and correlates with a measure of delay discounting. Behav Pharmacol 17:133–142
Roll JM, Higgins ST (2000) A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug Alcohol Depend 58:103–109
Roll JM, Reilly MP, Johanson CE (2000) The influence of exchange delays on cigarette versus money choice: a laboratory analog of voucher-based reinforcement therapy. Exp Clin Psychopharmacol 8:366–370
Roll JM, Huber A, Sodano R, Chudzynski JE, Moynier E, Shoptaw S (2006) A comparison of five reinforcement schedules for use in contingency management-based treatment of methamphetamine abuse. Psychol Rec 56:67–81
Schuh KJ, Stitzer ML (1995) Desire to smoke during spaced smoking intervals. Psychopharmacology 120:289–295
Shiffman SM, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG (2006) Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology 184:637–644
Silverman K, Chutuape MA, Bigelow GE, Stitzer ML (1999) Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone patients: effects of reinforcement magnitude. Psychopharmacology 146:128–138
Stitzer ML, Bigelow G, Lawrence C, Cohen J, D’Lugoff B, Hawthorne J (1977) Medication take-home as a reinforcer in a methadone maintenance program. Addict Behav 2:9–14
Tidey JW, O’Neill SC, Higgins ST (2002) Contingent monetary reinforcement of smoking reductions, with and without transdermal nicotine, in outpatients with schizophrenia. Exp Clin Psychopharmacol 10:241–247
Vuchinich RE, Simpson CA (1998) Hyperbolic temporal discounting in social drinkers and problem drinkers. Exp Clin Psychopharmacol 6:292–305
Wiseman EJ, Williams DK, McMillan DE (2005) Effectiveness of payment for reduced carbon monoxide levels and noncontingent payments on smoking behaviors in cocaine-abusing outpatients wearing nicotine or placebo patches. Exp Clin Psychopharmacol 13:102–110
Acknowledgment
We thank Jeanne Donaldson, Renee Saulnier, and John Chesley Wilson for their help with data collection and Matthew Locey and Paul Soto for their help with computer programming. This research was supported by US Public Health grant R03DA015373.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dallery, J., Raiff, B.R. Delay discounting predicts cigarette smoking in a laboratory model of abstinence reinforcement. Psychopharmacology 190, 485–496 (2007). https://doi.org/10.1007/s00213-006-0627-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0627-5