Abstract
Manganese (Mn) is required for many essential biological processes as well as in the development and functioning of the brain. Extensive accumulation of Mn in the brain may cause central nervous system dysfunction known as manganism, a motor disorder associated with cognitive and neuropsychiatric deficits similar to parkinsonism. Vinpocetine, a synthetic derivative of the alkaloid vincamine, is used to improve the cognitive function in cerebrovascular diseases. It possesses antioxidant and antiinflammatory properties. The present work was designed to explore the potential neuroprotective mechanisms exerted by vinpocetine in the Mn-induced neurotoxicity in rats. Rats were allocated into four groups. First group was given saline. The other three groups were given MnCl2; two of them were treated with either l-dopa, the gold standard antiparkinsonian drug, or vinpocetine. Rats receiving MnCl2 exhibited lengthened catalepsy duration in the grid and bar tests, motor impairment in the open-field test and short-term memory deficit in the Y-maze test. Additionally, histological examination revealed structural alterations and degeneration in different brain regions. Besides, striatal monoamines and mitochondrial complex I contents were declined, apoptotic biomarker caspase-3 expression and acetylcholinesterase activity were elevated. Moreover, oxidative stress and inflammation were detected in the striata. l-dopa or vinpocetine exerted protective effects against MnCl2-induced neurotoxicity. It could be hypothesized that modulation of monoamines, upregulation of mitochondrial complex I, antioxidant, antiinflammatory, and antiapoptotic activities are significant mechanisms underlying the neuroprotective effect of vinpocetine in the Mn-induced neurotoxicity model in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manganese (Mn) is a fundamental and plenteous element essential for proper function (Kwakye et al. 2015). Despite its essentiality, high-dose Mn exposure like enormous and extended inhalation of Mn in mining, welding, and industries seriously leads to its accumulation in specific brain areas causing neurotoxicity and an extrapyramidal motor disorder, referred to as manganism (Perl and Olanow 2007). Manganism is characterized by behavioral changes, tremors, difficulty in walking and awkward movements. Additionally, pre-existing symptoms develop before advanced manganism, such as slowed hand movements, anxiety, hostility, and hallucinations. As in various neurodegenerative diseases, patients could be asymptomatic for months or even years following exposure (Peres et al. 2016a).
Mn-induced motor dysfunction and neuropsychiatric and cognitive deficits develop as a result of its accumulation in the basal ganglia which is implicated in the regulation of motor and non-motor functions and hence inducing progressive neuronal deterioration (Bakthavatsalam et al. 2014; Bouabid et al. 2014).
The molecular mechanisms by which Mn causes neurotoxicity are not clearly understood. Cellularly, Mn accumulates in mitochondria, where it inhibits the electron transport chain complexes (Zhang et al. 2004), altering oxidative phosphorylation (Gavin et al. 1992) and ATP production (Brouillet et al. 1993). The impaired energy production affects mitochondrial permeability transition, causing organelle swelling, disruption of the outer membrane, and subsequently the release of various apoptogenic factors into the cytosol, thereby promoting apoptosis (Milatovic et al. 2011). Depletion of high-energy phosphates is also accompanied with excessive generation of reactive oxygen species (ROS), which induces membrane polyunsaturated fatty acids oxidation, producing an array of lipid peroxidation products. Furthermore, production of ROS is accompanied with inflammatory responses and release of inflammatory mediators. It has been reported that inflammation is implicated in neuronal damage and death (Milatovic et al. 2009). These interrelated pathways of oxidative stress, inflammation, and apoptosis contribute to the pathophysiology of neurodegenerative diseases (Tansey et al. 2007).
Previous studies reported that neuroprotective agents which ameliorate cellular energy metabolism and/or possess antioxidative and antiinflammatory properties could be beneficial in modulating manganism (Milatovic et al. 2011; Santos et al. 2012a; Gawlik et al. 2017).
A synthetic derivative of the alkaloid vincamine, vinpocetine, has long been used as a nootropic agent enhancing the cognitive function of patients with cerebrovascular disease. It augments cerebral blood flow and glucose uptake (Vas et al. 2002). Also, it decreases the risk of transient ischemic attacks and strokes in chronic cerebrovascular insufficiency patients (Valikovics 2007). Vinpocetine is an efficient scavenger of free radicals and prevents lipid peroxidation (Zaitone et al. 2012). Furthermore, the drug displays memory-protective and memory-enhancing properties (DeNoble et al. 1986; Bhatti and Hindmarch 1987) and potent antiinflammatory effect (Jeon et al. 2010).
In addition, vinpocetine is a phosphodiesterase (PDE) 1 inhibitor (Van Staveren et al. 2001) and a blocker of voltage-gated Na+ channels (Sitges et al. 2005). Former in vitro studies confirmed that vinpocetine prevented the blockage of the mitochondrial complexes (II, III, IV) as well as entirely negated the deduction of pyruvate levels and the accumulation of ROS induced by toxic concentrations of Amyloid beta peptides in PC12 cells (Pereira et al. 2000).
The cognitive improvement properties and the antiinflammatory effect of vinpocetine make it a potential candidate for the treatment of various neurodegenerative diseases (Patyar et al. 2011).
According to the aforementioned data, the aim of this work was to test the neuroprotective role of the nootropic agent, vinpocetine, in improving the motor and cognitive functions of Mn-induced neurotoxicity in rats.
Materials and methods
Experimental rats
Thirty-two male Sprague–Dawley albino rats (200–250 g) purchased from The Nile Co., for Pharmaceuticals and Chemical Industries, Cairo, Egypt, were kept under the same adequate environmental conditions with 12/12-h light/dark cycle and provided with standard diet pellets that represent their daily dietary requirements and water was given ad libitum. The study complies with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publications No. 8023, revised 1978) and is approved by the Ethics Committee of Faculty of Pharmacy, Cairo University.
Drugs and chemicals
MnCl2.4H2O (MnCl2) and vinpocetine were bought from Sigma–Aldrich Chemical Co., USA. MnCl2 was freshly dissolved in saline. Vinpocetine was freshly suspended in saline with the addition of few drops of tween 80. l-dopa, which is used as a reference antiparkinsonian drug, was the commercially available Sinemet tablets (Global Napi Pharmaceuticals, Egypt). Each “Sinemet 25 mg/250 mg Tablet” has 27 mg carbidopa (equivalent to 25 mg of anhydrous carbidopa) and 250 mg l-dopa. Each tablet was freshly grinded and suspended in saline with the addition of few drops of tween 80. Highest purity and analytical grade chemicals were also utilized.
Design of work and experimental groups
Rats were randomly allocated into four groups (8 animals/group) as follows; the first group was administered saline orally (1 ml/kg, p.o.) and intraperitoneally (1 ml/kg, i.p.) for 35 days representing control group. The second group was given (saline, p.o.) and MnCl2 (25 mg/kg, i.p.) daily for 35 days (Bouabid et al. 2014; Jiang et al. 2014). The third group was given l-dopa (25 mg/kg, p.o.) (Carvalho et al. 2013) and MnCl2 (25 mg/kg, i.p.) daily for 35 days. The fourth group received vinpocetine (6 mg/kg, p.o.) (Zaitone et al. 2012) and MnCl2 (25 mg/kg, i.p.) daily for 35 days. l-dopa and vinpocetine were given 1 h before MnCl2 administration. Concentration of the utilized drugs was selected as formerly reported and from our pilot experimental trials of the current study. Lastly, behavioral tests were performed, animals were sacrificed and the brain was removed, then striatum was dissected, rinsed with ice-cold saline, and stored instantly at − 80 °C for further biochemical investigation. Homogenization of striatal tissues was done in saline, and these homogenates were used to evaluate markers of oxidative stress (total antioxidant capacity (TAC), superoxide dismutase (SOD), and lipid peroxides), inflammatory cytokines (interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α)), and acetylcholinesterase (AChE) activity. Also, monoamines’ content (dopamine (DA), noradrenaline (NA), and serotonin (5-HT)), mitochondrial complex-1 content, and apoptotic biomarker Caspase-3 expression in striatum were assessed. Additionally, specimens from different brain areas of all treated groups were kept in 10% formaldehyde solution for histopathological examination using hematoxylin and eosin (H&E).
Measured parameters
Catalepsy test (grid test and bar test)
It is considered of great usefulness, because of its similarity to human symptoms of Parkinsonism, catatonic schizophrenia, and abnormal behavior resulting from brain damage to the basal ganglia (Sanberg et al. 1988). The first part of catalepsy test was the grid test which is a vertical grid (25.5 cm wide and 44 cm high with a space of 1 cm between each wire), on which the rat was hung by its paws and the time taken by the rat to move its paws or any kind of movement was detected. The second part was the bar test which is a horizontal bar (9 cm above and parallel from the base), on which the rat was placed with both forepaws and the time of paw removal was detected (Alam and Schmidt 2004; Abdin and Hamouda 2008).
Open–field test
It is the most widely used test to assess alterations of behavioral activities such as locomotor activity and exploratory behavior (Genaro and Schmidek 2000; Sedelis et al. 2001). The experiment was performed using a wooden box measuring 80 cm × 80 cm × 40 cm height (Cunha and Masur 1978), with white polished floor and red walls. The field floor was divided into 16 equal squares 4 × 4 (Vorhees 1974; Volosin et al. 1988). The latency to start the movement in the open-field is used to evaluate akinesia, while the decrease of locomotion (ambulation) and/or rearing indicates hypokinesia (Sedelis et al. 2001; Capitelli et al. 2008). Furthermore, dopaminergic mechanisms may be involved in the grooming behavior (Van Wimersma Greidanus et al. 1989).
Y-maze test
Spontaneous alternation behavior in the Y-maze task was used to evaluate short-term memory (Sarter et al. 1988). The used Y-maze was a wooden, black maze with three equal-sized arms labeled A, B, and C respectively. Each arm (12 cm wide, 40 cm long, 35 cm high) was oriented at an angle of 120° from the other two arms (Teixeira et al. 2013). Rats were located at the end of one arm and allowed to freely explore the maze during an 8-min session. Spontaneous alternation behavior was identified as entry into all three arms on successive choices. Percentage of spontaneous alternation was calculated according to Teixeira et al. (2013) and Aydin et al. (2016). The floor was cleaned with 10% ethanol and then dried with a clean cloth before the entry of the next rat.
Assessment of total protein
Bradford method was used to assess the protein content in striatal homogenates (Bradford 1976) with the use of bovine serum albumin as a standard.
Assessment of monoamines’ content
Monoamines’ content (DA, 5-HT, and NA) were evaluated in the striatal homogenates of all treated groups according to Ciarlone (1978). In this fluorometric assay, the obtained fluorescence is read at excitation 320 nm and emission 480 nm for DA, excitation 380 nm and emission 480 nm for NA, and excitation 355 nm and emission 470 nm for 5-HT using Hitachi (F3010 model) spectrophotofluorometer.
Assessment of oxidative stress biomarkers
Tissue levels of TAC and malondialdehyde (MDA) as well as SOD activity were estimated in all treated groups’ striatal homogenates. TAC was measured following the method of Koracevic et al. (2001). SOD activity in the samples was evaluated using the method described by Nishikimi et al. (1972). Estimation of thiobarbituric acid reactive substances (TBARS) level measured as MDA was used as an indicator for lipid peroxidation (Ohkawa et al. 1979). The assay for each oxidative stress biomarker was estimated using the Biodiagnostic colorimetric kit (Cairo, Egypt).
Assessment of inflammatory biomarkers
Both striatal IL-1β and TNF-α levels were quantified by the ELISA technique using commercial IL-1β and TNF-α ELISA Kits (R&D Systems, Inc., USA). The procedure was performed in accordance with the manufacturer’s instructions.
Assessment of caspase-3 protein expression using Western blotting
Lysis was done to the homogenized striatal tissues using ice-cold lysis buffer (10% glycerol, 2% SDS in 62 mM Tris-HCl, pH 6.8) containing a cocktail of protease inhibitors (Sigma–Aldrich, St. Louis, MO). Bradford method was used for determination of protein content in the collected protein lysates (Bradford 1976). Same amount of total protein (7.5 μg) was resolved under denaturing conditions by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto nitrocellulose membranes. After blocking with 6% non-fat dry milk in TBS-Tween buffer for 3 h at 4 °C. The nitrocellulose membranes were incubated with specific primary antibody against the detected protein (caspase-3) at 4 °C overnight. On the following day, β-actin monoclonal antibody was added and incubated for 1 h on a roller shaker at 4 °C. To remove unbound primary antibody, the membranes were washed five times, 5 min/each in TBS-Tween buffer, and incubated again with a proper HRP conjugated secondary antibody at 37 °C for 1 h. The membranes were rinsed with TBST buffer, scanning of the bands was done with ChemiDoc scanner, and then the densitometric intensity of each band was quantified.
Assessment of mitochondrial complex I content
This assay employs the quantitative sandwich ELISA technique. Striatal mitochondrial complex I was assessed by using commercial mitochondrial complex I ELISA Kit (Cusabio, Hubei, P.R. China) according to manufacturers’ prescripts.
Assessment of acetylcholinesterase activity
The assay of AChE is an optimized version of the Ellman method (Ellman et al. 1961). The kit used (Sigma–Aldrich Co., St Louis, MO, USA) has a linear range of 10–600 units/L of AChE activity.
Histopathological examination
Isolated brains were fixed in 10% formol saline for 24 h then rinsed with tap water. For light microscopy, brain sections were prepared and stained according to the method described by Bancroft and Gamble (2008). Dehydration was done using serial dilutions of different alcohols (methyl, ethyl, and absolute ethyl). Clearance of brain sections was done in xylene embedded in paraffin at 56 °C inside hot air oven for 24 h. Blocks of paraffin bees wax tissue were set for sectioning at 4-μm thickness by microtome. Sections of the obtained tissue were gathered on glass slides and deparaffinized. After that, staining with H&E was done for histological examination. Sections were examined by a skillful pathologist who was blinded to the investigational groups. Abnormal histopathological outcomes were assessed using a semi-quantitative method according to the following criteria; a scale of 0–4 in which 0 = no damage, 1 = up to 25% damage, 2 = 25%–50% damage, 3 = 50%–75% damage, and 4 = more than 75% damage (Behling et al. 2006).
Statistical analysis
Results were presented as the mean ± S.E.M. and multiple comparisons were performed employing one-way analysis of variance (ANOVA) with Tukey’s multiple comparison post hoc test as appropriate to calculate significance of the difference between treatments. The probability level of 0.05 was utilized as the criterion for significance. GraphPad Prism (ISI®, USA) software (version 5) was used for statistical analysis and graphs sketching.
Results
Vinpocetine attenuated MnCl2-induced alterations in duration of catalepsy in the grid and bar tests
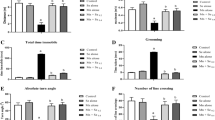
MnCl2 resulted in bradykinesia and rigidity manifested as lengthened catalepsy duration in the grid test (Fig. 1a) and the bar test (Fig. 1b) reaching 1202.74 and 1861.16% respectively as compared with the control group. l-dopa or vinpocetine obviously reduced the catalepsy duration to 20.20 and 19.42% respectively in the grid test as well as to 14.86 and 13.62% respectively in the bar test, as compared with MnCl2 treated rats. Intra-comparing the treatment regimens, the results indicated non marked alteration.
Vinpocetine attenuated MnCl2-induced alterations in duration of catalepsy in the grid (a) and bar (b) tests. All the values are expressed as the mean ± SEM of eight rats in each group. (a, b) Statistically significant from the control and the MnCl2 group, respectively, P < 0.001 using one-way ANOVA followed by Tukey as post hoc test
Vinpocetine attenuated MnCl2-induced alterations in rats’ motor performance in the open-field test
MnCl2 caused a significant deterioration in the motor performance and coordination of rats as compared with the control group exhibiting prolonged latency time and lowered ambulation, rearing and grooming frequencies (Fig. 2a–d). Either l-dopa or vinpocetine produced a distinct decrease in the latency time reaching 38.83 and 37.30%, respectively, along with reversing the decline in ambulation (156.67%, 152.22%), rearing (165.98%, 176.29%), and grooming (200%, 164.10%) frequencies respectively as compared with the MnCl2 group. Intra-comparing the treatment regimens, the results indicated non marked alteration.
Vinpocetine attenuated MnCl2-induced alterations in rats’ motor performance in the open-field test. All the values are expressed as the mean ± SEM of eight rats in each group. (a, b) Statistically significant from the control and the MnCl2 group, respectively, P < 0.001 using one-way ANOVA followed by Tukey as post hoc test. * and #: statistically significant from the control and the MnCl2 group, respectively, P < 0.01 using one-way ANOVA followed by Tukey as post hoc test. a Latency time. b Ambulation frequency. c Rearing frequency. d Grooming frequency
Vinpocetine attenuated MnCl2-induced alterations in rats’ working memory in the Y-maze test
Administration of MnCl2 revealed short-term memory deficit, indicated by marked drop in the percentage of spontaneous alternation recording approximately 0.6-fold as compared with the control rats (Fig. 3). Both l-dopa and vinpocetine significantly raised the spontaneous alternation to approximately 1.7-fold as compared with MnCl2 treated rats. Intra-comparing the treatment regimens, the results indicated non marked alteration.
Vinpocetine attenuated MnCl2-induced alterations in rats’ working memory in the Y-maze test. Spontaneous alternation (%) = \( \left(\frac{\mathrm{alternation}\ \mathrm{behavior}}{\mathrm{maximum}\ \mathrm{alternations}}\right)\times 100 \). All the values are expressed as the mean ± SEM of eight rats in each group. (a, b) Statistically significant from the control and the MnCl2 group, respectively, P < 0.001using one-way ANOVA followed by Tukey as post hoc test
Vinpocetine mitigated MnCl2-induced alterations in striatal monoamines’ content
MnCl2 significantly reduced the striatal levels of DA, 5-HT, and NA to 29.20, 35.33, and 34.97% respectively as compared with control animals (Fig. 4a–c). Interestingly, l-dopa and vinpocetine markedly reversed the striatal monoamines’ decline as compared with MnCl2 group. Intra-comparing the treatment regimens, the results indicated non marked alteration.
Vinpocetine mitigated MnCl2-induced alterations in striatal monoamines’ content. All the values are expressed as the mean ± SEM of six rats in each group. (a, b) Statistically significant from the control and the MnCl2 group, respectively, P < 0.001 using one-way ANOVA followed by Tukey as post hoc test. a Dopamine. b Serotonin. c Norepinephrine
Vinpocetine mitigated MnCl2-induced alterations in striatal oxidative stress biomarkers
MnCl2 produced a state of oxidative stress revealed by the significant elevation in striatal MDA level along with a significant decline in striatal TAC and striatal SOD activity as compared with the control animals (Fig. 5a–c). l-dopa or vinpocetine markedly decreased MDA level to 41.48 and 53.42%, respectively, while significantly raised the TAC (147.37%, 166.56%) and SOD activity (246.11%, 159.59%) respectively as compared with the MnCl2 group. Intra-comparing between the two treatment regimens, vinpocetine produced marked decrease in the SOD activity (64.84%) from l-dopa.
Vinpocetine mitigated MnCl2-induced alterations in striatal oxidative stress biomarkers. All the values are expressed as the mean ± SEM of six rats in each group. (a, b, c) Statistically significant from the control, the MnCl2, and the l-dopa group, respectively, P < 0.001 using one-way ANOVA followed by Tukey as post hoc test. #Statistically significant from the MnCl2 group, P < 0.01 using one-way ANOVA followed by Tukey as post hoc test. a Malondialdehyde. b Total antioxidant capacity. c Superoxide dismutase
Vinpocetine mitigated MnCl2-induced alterations in striatal inflammatory biomarkers
MnCl2 triggered inflammation via elevating the TNF-α and IL-1β content to 407 and 410.93% respectively in comparison with control animals (Fig. 6a, b). l-dopa or vinpocetine significantly reversed such increment in TNF-α and IL-1β content as compared with MnCl2 group. Intra-comparing the treatment regimens, the results indicated non marked alteration.
Vinpocetine mitigated MnCl2-induced alterations in striatal inflammatory biomarkers. All the values are expressed as the mean ± SEM of six rats in each group. (a, b) Statistically significant from the control and the MnCl2 group, respectively, P < 0.001 using one-way ANOVA followed by Tukey as post hoc test. a Tumor necrosis factor alpha. b Interleukin-1 beta
Vinpocetine downregulated MnCl2-induced alterations in striatal caspase-3 protein expression
MnCl2 dramatically upregulated striatal caspase-3 expression reaching approximately 11-fold as compared with the control rats (Fig. 7). Either l-dopa or vinpocetine downregulated the elevated caspase-3 expression to approximately 0.4-fold and 0.6-fold respectively as compared with MnCl2 treated rats. Intra-comparing the treatment regimens, the results indicated non marked alteration.
Vinpocetine downregulated MnCl2-induced alterations in striatal caspase-3 protein expression. All the values are expressed as the mean ± SEM of six rats in each group. (a, b) Statistically significant from the control and the MnCl2 group, respectively, P < 0.001 using one-way ANOVA followed by Tukey as post hoc test
Vinpocetine downregulated MnCl2-induced alterations in striatal mitochondrial complex І content
Striatal mitochondrial complex І content was markedly decreased following the administration of MnCl2, reaching 56.55% as compared with the control animals (Fig. 8). Both l-dopa and vinpocetine significantly amended such alteration in mitochondrial complex І content recording 138.15 and 129.64% respectively as compared with MnCl2 group. Intra-comparing the treatment regimens, the results indicated non marked alteration.
Vinpocetine downregulated MnCl2-induced alterations in striatal mitochondrial complex І content. All the values are expressed as the mean ± SEM of six rats in each group. (a, b) Statistically significant from the control and the MnCl2 group, respectively, P < 0.001using one-way ANOVA followed by Tukey as post hoc test
Vinpocetine downregulated MnCl2-induced alterations in striatal acetylcholinesterase activity
MnCl2 significantly raised the AChE activity to 361.56% in comparison with control rats (Fig. 9). l-dopa or vinpocetine markedly suppressed the AChE activity to 58.84 and 53.55% respectively as compared with MnCl2 treated rats. Intra-comparing the treatment regimens, the results indicated non marked alteration.
Vinpocetine downregulated MnCl2-induced alterations in striatal acetylcholinesterase activity. All the values are expressed as the mean ± SEM of six rats in each group. (a, b) Statistically significant from the control and the MnCl2 group, respectively, P < 0.001 using one-way ANOVA followed by Tukey as post hoc test
Brain histopathology
The effect of treatments on different brain areas is shown in Figs. 10, 11, 12, 13, and 14 and the score for tissue damage is presented in Table 1.
Representative photomicrographs of cerebral cortex of experimental groups stained by H&E (magnification × 40): showing normal histological structure of the meninges (mn) and cerebral cortex (cc) of control rats (a), identifying mild congestion (cg) in cerebral cortical blood capillaries of MnCl2 group (b), normal histological structure of the cerebral cortex in l-dopa + MnCl2 group (c), and vinpocetine + MnCl2 group (d)
Representative photomicrographs of hippocampus of experimental groups stained by H&E (magnification × 40): showing normal histological structure of the hippocampus (hp) of control rats (a), detecting degeneration (dn) in the neurons with atrophy in subiculum (sb) and nuclear pyknosis (np) in most neurons of the fascia dentate (fs) in the hippocampus of MnCl2 group (b), normal histological structure of the hippocampus in l-dopa + MnCl2 group (c), and vinpocetine + MnCl2 group (d)
Representative photomicrographs of striatum of experimental groups stained by H&E (magnification × 40): showing normal histological structure of the striatum (st) of control rats (a), identifying focal gliosis (gl), focal eosinophilic plagues formation (pl) and congestion (cg) in the blood vessels in the striatum of MnCl2 group (b), congestion detected in the blood vessels of the striatum in l-dopa + MnCl2 group (c), and vinpocetine + MnCl2 group (d)
Representative photomicrographs of cerebellum of experimental groups stained by H&E (magnification × 40): showing normal histological structure of the cerebellum (cb) of control rats (a), identifying mild congestion (cg) in the blood vessels of the cerebellum of MnCl2 group (b), normal architecture of the cerebellum of l-dopa + MnCl2 group (c), and vinpocetine + MnCl2 group (d)
Control rats showed no histopathological alteration as well as normal cerebral cortex (Fig. 10a), hippocampus (subiculum and fascia dentate) (Fig. 11a), striatum (Fig. 12a), substantia nigra (Fig. 13a), and cerebellum (Fig. 14a). Rats injected with MnCl2 revealed moderate congestion in the cerebral cortical blood capillaries (Fig. 10b) whereas the cerebral cortex of l-dopa (Fig. 10c) and vinpocetine (Fig. 10d) treated rats exhibited no histopathological alteration. In MnCl2, the hippocampus showed degeneration in the neurons with atrophy in subiculum and nuclear pyknosis was also detected in most neurons of the fascia dentate (Fig. 11b). Interestingly, pretreatment with either l-dopa (Fig. 11c) or vinpocetine (Fig. 11d) significantly amended the pathological alterations induced by MnCl2 in the hippocampus. Following MnCl2 administration, the striatum showed focal gliosis, focal eosinophilic plagues formation, and congestion in the blood vessels (Fig. 12b) together with no alteration in the substantia nigra (Fig. 13b). Congestion was detected in the blood vessels of the striatum of both l-dopa (Fig. 12c) and vinpocetine (Fig. 12d) treated groups along with intact substantia nigra (Fig. 13c and Fig. 13d respectively). While MnCl2 induced mild congestion in the blood vessels of the cerebellum (Fig. 14b), l-dopa (Fig. 14c) and vinpocetine (Fig. 14d) restored its normal architecture.
Discussion
The present work pointed out the interrelation between behavioral changes, oxidative stress, inflammation, mitochondrial dysfunction, and apoptosis as well as histopathological changes due to exposure to MnCl2. We further probed the neuroprotective effect of vinpocetine on these interconnected pathways. To our knowledge, this is the first study to investigate the in vivo effect of vinpocetine on Mn-induced neurotoxicity in rats.
The present investigation showed that exposure of rats to MnCl2 caused a cataleptic behavior in the grid and bar tests, a result which reflects the development of bradykinesia and rigidity in rats, concomitantly with motor dysfunction in the open-field test and a marked decrease in striatal DA level. These results are in harmony with previous studies (Desole et al. 1994; Vidal et al. 2005; Vezér et al. 2007; Santos et al. 2012b, 2013).
Administration of vinpocetine attenuated the MnCl2-induced catalepsy as well as restored the impaired locomotor functions with subsequent restoration of striatal DA level. These effects were comparable with that of l-dopa, indicating the ability of vinpocetine to potentiate the dopaminergic transmission in the striatum. Former studies confirmed similar outcomes (Zaitone et al. 2012; Deshmukh and Sharma 2013; Sharma and Deshmukh 2015).
The current study showed that MnCl2 treatment caused a decline in cognitive functions in the Y-maze test, suggesting impaired spatial memory, together with raised striatal AChE activity. This result is in accordance with previous studies (Lai et al. 1992; Liapi et al. 2008; Hogas et al. 2011; Babadi et al. 2014). Cholinergic activity plays a significant role in the pathophysiology of Mn-induced neurotoxicity (Finkelstein et al. 2007). Mn effect on the cholinergic system may contribute to impairments in learning, memory, and locomotion (Peres et al. 2016b). With regard to the role of the cholinergic system in memory and learning functions, it could be hypothesized that the increase in AChE activity might be responsible for the decline in cognitive functions in Mn-treated rats.
Additionally, it is well accepted that the hippocampus is essential for spatial learning and memory performance (Martin and Clark 2007; Ryan et al. 2010). Also, Wang et al. (2014) reported that Mn caused hippocampal neurons’ injury in vitro. In line, our histological results indicated that Mn caused hippocampal damage and hence memory impairment which is in accordance with (Vezér et al. 2007).
Either l-dopa or vinpocetine improved the spatial working memory by attenuating the decline in cognitive functions in the Y-maze test, reduced the increase in AChE activity as well as ameliorated the hippocampal damage in MnCl2-treated rats. Enhancement in spatial memory demonstrated with vinpocetine might be due to amelioration of cholinergic functions, antioxidant activity, and inhibition of neuronal cell damage (Patyar et al. 2011). Moreover, the efficacy of vinpocetine in improving memory has been confirmed in cognitively healthy and compromised subjects (Subhan and Hindmarch 1985; Ogunrin 2014). Up till now, several mechanisms have been suggested for vinpocetine in improving memory deficits and blood flow; inhibition of PDE1 (Shang et al. 2016), as an antiinflammatory agent (Jeon et al. 2010; Zhang and Yang 2014); and increasing cerebral metabolism through enhancing cerebral blood flow, glucose and oxygen consumption in the brain, and ATP production (Shang et al. 2016).
MnCl2-treated rats exhibited decreased striatal DA reflecting deficits in the dopaminergic outcome and vulnerability of the dopaminergic system to Mn exposure. This is in line with the results of (Liccione and Maines 1988; Brouillet et al. 1993; Desole et al. 1994; Vidal et al. 2005). In addition, MnCl2 reduced 5-HT and NA contents, which is in line with Bouabid et al. (2014) and Beaudin et al. (2015). Anxiety and motor activity disorders associated with manganism may be due to dysregulation of the noradrenergic system. Also, modulation of the serotonergic neurons leads to depression, sleeplessness, and loss of memory, symptoms which are described as early symptoms of manganism (Bouabid et al. 2015).
The current work confirmed the ability of vinpocetine to attenuate the striatal monoaminergic hypofunction. In 1988, Kiss and Szporny stated the ability of vinpocetine to antagonize the decrease of monoamines induced by hypoxia. In line with our study, vinpocetine was reported to restore the DA level in both MPTP- and rotenone-treated rats (Zaitone et al. 2012; Sharma and Deshmukh 2015).
Oxidative damage has been considered as a cornerstone in Mn-induced neurotoxicity (Liu et al. 2013). The current data showed that MnCl2 decreased the TAC, elevated the MDA level and suppressed the SOD activity in the striatal tissues reflecting a state of oxidative stress. These results are in harmony with previous studies (Zhang and Huang 2008; Chtourou et al. 2010, 2012; Szpetnar et al. 2016).
In our study, concurrent treatment with either l-dopa or vinpocetine attenuated Mn-induced oxidative stress. Many previous studies reported the ability of vinpocetine to ameliorate the rise in ROS generation and the reduction in antioxidant levels either in vitro (Santos et al. 2000; Solanki et al. 2011; Herrera-Mundo and Sitges 2013) or in vivo (Deshmukh et al. 2009; Abdel-Salam et al. 2011).
Milatovic et al. (2009) showed that Mn exposure is accompanied by inflammatory responses and the release of inflammatory mediators. MnCl2 exposure increased the striatal pro-inflammatory cytokines TNF-α and IL-1β, indicating a pro-inflammatory activation. This result is in accordance with previous studies (Liu et al. 2009; Zhao et al. 2009).
The current study showed an inhibitory effect of l-dopa and vinpocetine on brain inflammation induced by MnCl2 through suppressing the TNF-α and IL-1β elevation. Former study reported that, in the central nervous system, PDE inhibitors such as vinpocetine downregulated inflammatory mediators like IL-1, IL-6, and TNF-α as well as upregulated inhibitory cytokines such as IL-10 (Yoshikawa et al. 1999).
Neuronal apoptosis has been implicated as a significant contributor to Mn-induced neurotoxicity. A growing evidence suggests that Mn triggers apoptosis in various cell types (Chun et al. 2001; Malecki 2001; Hirata 2002; Seo et al. 2013). In the current work, the level of caspase-3 was remarkably elevated in Mn-treated rats which is in accordance with previous studies (Jiang et al. 2014; Shi et al. 2015).
Administration of vinpocetine suppressed the striatal caspase-3 activity with similar effect as l-dopa. Consistent with the aforementioned vinpocetine’s antioxidant and antiinflammatory effects demonstrated in the current study, an antiapoptotic effect has been confirmed too. Our result is in harmony with previous in vitro studies (Erdö et al. 1990; Gabryel et al. 2002; Bora et al. 2016).
Taken together along with our results, we can propose that vinpocetine’s antiapoptotic action could serve as one of the pathways underlying its neuroprotective effect.
Intracellularly, Mn accumulates in mitochondria and inhibits the complexes of the electron transport chain (Zhang et al. 2004; Avila et al. 2010), thereby impairing oxidative phosphorylation (Gavin et al. 1992) and ATP production (Brouillet et al. 1993; Milatovic et al. 2007) and increasing the generation of ROS (Gunter et al. 2006; Milatovic et al. 2007). In the present study, treatment of rats with Mn downregulated the striatal complex I activity. This result clearly shows the ability of Mn to alter several pathways of the mitochondrial respiratory chain, especially at the complex I level. Our finding is in parallel with a recent study conducted in vivo by Apaydin et al. (2016). Also, previous in vitro studies are in agreement with our result (Galvani et al. 1995; Zhang et al. 2004; Zhang et al. 2008).
Vinpocetine reversed the decline of mitochondrial complex I activity similar to l-dopa. This reflects the ability of vinpocetine to ameliorate the mitochondrial dysfunction. It has been shown that vinpocetine significantly attenuated the diminished mitochondrial enzyme complexes I, II, and IV in the rat striatum in 3-nitropropionic acid-induced experimental Huntington’s disease (Gupta and Sharma 2014).
MnCl2 caused morphological changes and degeneration in different brain regions. These results are in harmony with previous literatures (Vezér et al. 2007; Wang et al. 2015; Bahar et al. 2017).
Microscopically, the current study revealed the neuroprotective effect of l-dopa and vinpocetine against the tissue damage induced by MnCl2.
The protective effect of vinpocetine might be attributed to its antiinflammatory, antioxidant, and antiapoptotic properties besides its inhibitory action on mitochondrial complex I, all of which will eventually target multiple factors underlying the pathogenesis of manganism thereby providing a better outcome.
Conclusion
The present study shows that vinpocetine could be recommended as a promising disease-modifying therapy to abate neurodegeneration and dementia when given early in the course of manganism, hence improving patients’ quality of life through alleviating both motor and non-motor symptoms besides being devoid of l-dopa’s undesirable adverse effects such as l-dopa-induced dyskinesias. The current work was able to draw a clearer image of the neuroprotective action of vinpocetine by evaluating its efficacy in Mn-induced neurotoxicity.
Abbreviations
- AChE:
-
Acetylcholinesterase
- DA:
-
Dopamine
- H&E:
-
Hematoxylin and eosin
- IL-1β:
-
Interleukin-1 beta
- MDA:
-
Malondialdehyde
- Mn:
-
Manganese
- NA:
-
Noradrenaline
- PDE:
-
Phosphodiesterase
- ROS:
-
Reactive oxygen species
- 5-HT:
-
Serotonin
- SOD:
-
Superoxide dismutase
- TAC:
-
Total antioxidant capacity
- TNF-α:
-
Tumor necrosis factor-alpha
- α-Syn:
-
α-synuclein
References
Abdel-Salam OM, Khadrawy YA, Salem NA, Sleem AA (2011) Oxidative stress in a model of toxic demyelination in rat brain: the effect of piracetam and vinpocetine. Neurochem Res 36:1062–1072
Abdin AA, Hamouda HE (2008) Mechanism of the neuroprotective role of coenzyme Q10 with or without L-dopa in rotenone-induced parkinsonism. Neuropharmacology 55(8):1340–1346
Alam M, Schmidt WJ (2004) L-DOPA reverses the hypokinetic behaviour and rigidity in rotenone treated rats. Behav Brain Res 153(2):439–446
Apaydin M, Erbas O, Taskiran D (2016) Protection by Edaravone, a radical scavenger, against manganese-induced neurotoxicity in rats. J Biochem Mol Toxicol 30(5):217–223
Avila DS, Colle D, Gubert P, Palma AS, Puntel G, Manarin F, Noremberg S, Nascimento PC, Aschner M, Rocha JB, Soares FA (2010) A possible neuroprotective action of a vinylic telluride against Mn-induced neurotoxicity. Toxicol Sci 115(1):194–201
Aydin E, Hritcu L, Dogan G, Hayta S, Bagci E (2016) The effects of inhaled Pimpinella peregrina essential oil on scopolamine-induced memory impairment, anxiety, and depression in laboratory rats. Mol Neurobiol 53:6557–6567
Babadi VY, Sadeghi L, Shirani K, Malekirad AA, Rezaei M (2014) The toxic effect of manganese on the acetylcholinesterase activity in rat brains. J Toxicol 2014:946372
Bahar E, Lee GH, Bhattarai KR, Lee HY, Choi MK, Rashid HO, Kim JY, Chae HJ, Yoon H (2017) Polyphenolic extract of Euphorbia supina attenuates manganese-induced neurotoxicity by enhancing antioxidant activity through regulation of ER stress and ER stress-mediated apoptosis. Int J Mol Sci 18(2):300. https://doi.org/10.3390/ijms18020300
Bakthavatsalam S, Das Sharma S, Sonawane M, Thirumalai V, Datta AA (2014) Zebrafish model of manganism reveals reversible and treatable symptoms that are independent of neurotoxicity. Dis Model Mech 7(11):1239–1251
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques, 6th edn. Churchill Livingstone, London
Beaudin SA, Strupp BJ, Lasley SM, Fornal CA, Mandal S, Smith DR (2015) Oral methylphenidate alleviates the fine motor dysfunction caused by chronic postnatal manganese exposure in adult rats. Toxicol Sci 144(2):318–327
Behling EB, Sendão MC, Francescato HD, Antunes LM, Costa RS, Bianchi Mde L (2006) Comparative study of multiple dosage of quercetin against cisplatin-induced nephrotoxicity and oxidative stress in rat kidneys. Pharmacol Rep 58(4):526–532
Bhatti JZ, Hindmarch I (1987) Vinpocetine effects on cognitive impairments produced by flunitrazepam. Int Clin Psychopharmacol 2:325–331
Bora S, Erdogan MA, Armagan G, Sevgili E, Dagcı T (2016) Vinpocetine and vasoactive intestinal peptide attenuate manganese-induced toxicity in NE-4C cells. Biol Trace Elem Res 174(2):410–418
Bouabid S, Delaville C, de Deurwaerdère P, Lakhdar-Ghazal N, Benazzouz A (2014) Manganese-induced atypical parkinsonism is associated with altered basal ganglia activity and changes in tissue levels of monoamines in the rat. PLoS One 9(6):e98952
Bouabid S, Tinakoua A, Lakhdar-Ghazal N, Benazzouz A (2015) Manganese neurotoxicity: behavioral disorders associated with dysfunctions in the basal ganglia and neurochemical transmission. J Neurochem 136:677–691. https://doi.org/10.1111/jnc.13442
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF (1993) Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp Neurol 120(1):89–94
Capitelli C, Sereniki A, Lima MM, Reksidler AB, Tufik S, Vital MA (2008) Melatonin attenuates tyrosine hydroxylase loss and hypolocomotion in MPTP-lesioned rats. Eur J Pharmacol 594:101–108
Carvalho MM, Campos FL, Coimbra B, Pêgo JM, Rodrigues C, Lima R, Rodrigues AJ, Sousa N, Salgado AJ (2013) Behavioral characterization of the 6-hydroxidopamine model of Parkinson’s disease and pharmacological rescuing of non-motor deficits. Mol Neurodegener 8:14
Chtourou Y, Fetoui H, Sefi M, Trabelsi K, Barkallah M, Boudawara T, Kallel H, Zeghal N (2010) Silymarin, a natural antioxidant, protects cerebral cortex against manganese-induced neurotoxicity in adult rats. Biometals 23(6):985–996
Chtourou Y, Fetoui H, Garoui el M, Boudawara T, Zeghal N (2012) Improvement of cerebellum redox states and cholinergic functions contribute to the beneficial effects of silymarin against manganese-induced neurotoxicity. Neurochem Res 37(3):469–479
Chun HS, Lee H, Son JH (2001) Manganese induces endoplasmic reticulum (ER) stress and activates multiple caspases in nigral dopaminergic neuronal cells, SN4741. Neurosci Lett 316(1):5–8
Ciarlone AE (1978) Further modification of a flurometric method for analyzing brain amines. Microchem J 23:9–12
Cunha JM, Masur J (1978) Evaluation of psychotropic drugs with a modified open field test. Pharmacology 16(5):259–267
DeNoble VJ, Repetti SJ, Gelpke LW, Wood LM, Keim KL (1986) Vinpocetine: nootropic effects on scopolamine-induced and hypoxia-induced retrieval deficits of a step-through passive avoidance response in rats. Pharmacol Biochem Behav 24:1123–1128
Deshmukh R, Sharma PL (2013) Pharmacological inhibition of PDE1 by vinpocetine attenuates 3-nitropropionic acid-induced behavioral and biochemical abnormalities in rats. Innov Pharm Pharmacother (IPP) 1(2):145–161
Deshmukh R, Sharma V, Mehan S, Sharma N, Bedi KL (2009) Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine—a PDE1 inhibitor. Eur J Pharmacol 620(1–3):49–56
Desole MS, Miele M, Esposito G, Migheli R, Fresu L, De Natale G, Miele E (1994) Dopaminergic system activity and cellular defense mechanisms in the striatum and striatal synaptosomes of the rat subchronically exposed to manganese. Arch Toxicol 68(9):566–570
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Erdö SL, Cai NS, Wolff JR, Kiss B (1990) Vinpocetin protects against excitotoxic cell death in primary cultures of rat cerebral cortex. Eur J Pharmacol 187(3):551–553
Finkelstein Y, Milatovic D, Aschner M (2007) Modulation of cholinergic systems by manganese. Neurotoxicology 28(5):1003–1014
Gabryel B, Adamek M, Pudełko A, Małecki A, Trzeciak HI (2002) Piracetam and vinpocetine exert cytoprotective activity and prevent apoptosis of astrocytes in vitro in hypoxia and reoxygenation. Neurotoxicology 23(1):19–31
Galvani P, Fumagalli P, Santagostino A (1995) Vulnerability of mitochondrial complex I in PC12 cells exposed to manganese. Eur J Pharmacol 293(4):377–383
Gavin CE, Gunter KK, Gunter TE (1992) Mn2+ sequestration by mitochondria and inhibition of oxidative phosphorylation. Toxicol Appl Pharmacol 115(1):1–5
Gawlik M, Gawlik MB, Smaga I, Filip M (2017) Manganese neurotoxicity and protective effects of resveratrol and quercetin in preclinical research. Pharmacol Rep 69(2):322–330
Genaro G, Schmidek WR (2000) Exploratory activity of rats in three different environments. Ethology 106:849–859
Gunter TE, Gavin CE, Aschner M, Gunter KK (2006) Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. Neurotoxicology 27(5):765–776
Gupta S, Sharma B (2014) Protective effects of phosphodiesterase-1 (PDE1) and ATP sensitive potassium (KATP) channel modulators against 3-nitropropionic acid induced behavioral and biochemical toxicities in experimental Huntington′s disease. Eur J Pharmacol 732:111–122
Herrera-Mundo N, Sitges M (2013) Vinpocetine and α-tocopherol prevent the increase in DA and oxidative stress induced by 3-NPA in striatum isolated nerve endings. J Neurochem 124(2):233–240
Hirata (2002) Manganese-induced apoptosis in PC12 cells. Neurotoxicol Teratol 24(5):639–653
Hogas M, Ciobica A, Hogas S, Bild V, Hritcu L (2011) The effects of the administration of two different doses of manganese on short-term spatial memory and anxiety-like behavior in rats. Arch Biol Sci, Belgrade 63(4):1031–1036
Jeon KI, XiangbinXu X, Aizawa T, Lim JH, Jono H, Kwon DS, Abe J, Berk BC, Jian-Dong Li JD, Chen Yan C (2010) Vinpocetine inhibits NF-κB–dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proc Natl Acad Sci U S A 107(21):9795–9800
Jiang J, Shi S, Zhou Q, Ma X, Nie X, Yang L, Han J, Xu G, Wan C (2014) Downregulation of the Wnt/β-catenin signaling pathway is involved in manganese-induced neurotoxicity in rat striatum and PC12 cells. J Neurosci Res 92(6):783–794
Kiss B, Szporny L (1988) On the possible role of central monoaminergic systems in the central nervous system actions of vinpocetine. Drug Dev Res 14:263–279
Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V (2001) Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54:356–361
Kwakye GF, Paoliello MM, Mukhopadhyay S, Bowman AB, Aschner M (2015) Manganese-induced parkinsonism and Parkinson’s disease: shared and distinguishable features. Int J Environ Res Public Health 12(7):7519–7540
Lai JC, Chan AW, Leung TK, Minski MJ, Lim L (1992) Neurochemical changes in rats chronically treated with a high concentration of manganese chloride. Neurochem Res 17(9):841–847
Liapi C, Zarros A, Galanopoulou P, Theocharis S, Skandali N, Al-Humadi H, Anifantaki F, Gkrouzman E, Mellios Z, Tsakiris S (2008) Effects of short-term exposure to manganese on the adult rat brain antioxidant status and the activities of acetylcholinesterase, (Na,K)-ATPase and Mg-ATPase: modulation by L-cysteine. Basic Clin Pharmacol Toxicol 103(2):171–175
Liccione JJ, Maines MD (1988) Selective vulnerability of glutathione metabolism and cellular defense mechanisms in rat striatum to manganese. J Pharmacol Exp Ther 247(1):156–161
Liu M, Cai T, Zhao F, Zheng G, Wang Q, Chen Y, Huang C, Luo W, Chen J (2009) Effect of microglia activation on dopaminergic neuronal injury induced by manganese, and its possible mechanism. Neurotox Res 16(1):42–49
Liu Y, Barber DS, Zhang P, Liu B (2013) Complex II of the mitochondrial respiratory chain is the key mediator of divalent manganese-induced hydrogen peroxide production in microglia. Toxicol Sci 132(2):298–306
Malecki EA (2001) Manganese toxicity is associated with mitochondrial dysfunction and DNA fragmentation in rat primary striatal neurons. Brain Res Bull 55(2):225–228
Martin SJ, Clark RE (2007) The rodent hippocampus and spatial memory: from synapses to systems. Cell Mol Life Sci 64(4):401–431
Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M (2007) Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol Sci 98(1):198–205
Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M (2009) Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol Appl Pharmacol 240(2):219–225
Milatovic D, Gupta RC, Yu Y, Zaja-Milatovic S, Aschner M (2011) Protective effects of antioxidants and anti-inflammatory agents against manganese-induced oxidative damage and neuronal injury. Toxicol Appl Pharmacol 256(3):219–226
Nishikimi M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun 46:849–864
Ogunrin A (2014) Effect of vinpocetine (cognitol™) on cognitive performances of a nigerian population. Ann Med Health Sci Res 4(4):654–661
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Patyar S, Prakash A, Modi M, Medhi B (2011) Role of vinpocetine in cerebrovascular diseases. Pharmacol Rep 63:618–628
Pereira C, Agostinho P, Oliveira CR (2000) Vinpocetine attenuates the metabolic dysfunction induced by amyloid beta-peptides in PC12 cells. Free Radic Res 33(5):497–506
Peres TV, Parmalee NL, Martinez-Finley EJ, Aschner M (2016a) Untangling the manganese-α-Synuclein web. Front Neurosci 10:364
Peres TV, Schettinger MR, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M (2016b) Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol Toxicol 17(1):57
Perl DP, Olanow CW (2007) The neuropathology of manganese-induced parkinsonism. J Neuropathol Exp Neurol 66:675–682
Ryan L, Lin CY, Ketcham K, Nadel L (2010) The role of medial temporal lobe in retrieving spatial and nonspatial relations from episodic and semantic memory. Hippocampus 20(1):11–18
Sanberg PR, Bunsey MD, Giordano M, Norman AB (1988) The catalepsy test: its ups and downs. Behav Neurosci 102(5):748–759
Santos MS, Duarte AI, Moreira PI, Oliveira CR (2000) Synaptosomal response to oxidative stress: effect of vinpocetine. Free Radic Res 32(1):57–66
Santos AP, Lucas RL, Andrade V, Mateus ML, Milatovic D, Aschner M, Batoreu MC (2012a) Protective effects of ebselen (Ebs) and para-aminosalicylic acid (PAS) against manganese (Mn)-induced neurotoxicity. Toxicol Appl Pharmacol 258(3):394–402
Santos D, Milatovic D, Andrade V, Batoreu MC, Aschner M, Marreilha dos Santos AP (2012b) The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain. Toxicology 292(2–3):90–98
Santos D, Batoréu MC, Tavares de Almeida I, Davis Randall L, Mateus ML, Andrade V, Ramos R, Torres E, Aschner M, Marreilha dos Santos AP (2013) Evaluation of neurobehavioral and neuroinflammatory end-points in the post-exposure period in rats sub-acutely exposed to manganese. Toxicology 314(1):95–99
Sarter M, Bodewitz G, Stephens DN (1988) Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology 94(4):491–495
Sedelis M, Schwarting RK, Huston JP (2001) Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav Brain Res 125:109–125
Seo YA, Li Y, Wessling-Resnick M (2013) Iron depletion increases manganese uptake and potentiates apoptosis through ER stress. Neurotoxicology 38:67–73
Shang Y, Wang L, Li Y, Gu PF (2016) Vinpocetine improves scopolamine induced learning and memory dysfunction in C57 BL/6J mice. Biol Pharm Bull 39(9):1412–1418
Sharma S, Deshmukh R (2015) Vinpocetine attenuates MPTP-induced motor deficit and biochemical abnormalities in Wistar rats. Neuroscience 286:393–403
Shi S, Zhao J, Yang L, Nie X, Han J, Ma X, Wan C, Jiang J (2015) KHSRP participates in manganese-induced neurotoxicity in rat striatum and PC12 cells. J Mol Neurosci 55(2):454–465
Sitges M, Galvan E, Nekrassov V (2005) Vinpocetine blockade of sodium channels inhibits the rise in sodium and calcium induced by 4 aminopyridine in synaptosomes. Neurochem Int 46:533–540
Solanki P, Prasad D, Muthuraju S, Sharma AK, Singh SB, Ilavzhagan G (2011) Preventive effect of piracetam and vinpocetine on hypoxia-reoxygenation induced injury in primary hippocampal culture. Food Chem Toxicol 49(4):917–922
Subhan Z, Hindmarch I (1985) Psychopharmacological effects of vinpocetine in normal healthy volunteers. Eur J Clin Pharmacol 28(5):567–571
Szpetnar M, Luchowska-Kocot D, Boguszewska-Czubara A, Kurzepa J (2016) The influence of manganese and glutamine intake on antioxidants and neurotransmitter amino acids levels in rats’ brain. Neurochem Res 41(8):2129–2139
Tansey MG, McCoy MK, Frank-Cannon TC (2007) Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol 208(1):1–25
Teixeira MD, Souza CM, Menezes AP, Carmo MR, Fonteles AA, Gurgel JP, Lima FA, Viana GS, Andrade GM (2013) Catechin attenuates behavioral neurotoxicity induced by 6-OHDA in rats. Pharmacol Biochem Behav 110:1–7
Valikovics A (2007) Investigation of the effect of vinpocetine on cerebral blood flow and cognitive functions. Ideggyogy Sz 60:301–310
Van Staveren WCG, Markerink-van Ittersum M, Steinbusch HW, de Vente J (2001) The effects of phosphodiesterase inhibition on cyclic GMP and cyclic AMP accumulation in the hippocampus of the rat. Brain Res 888:275–286
Van Wimersma Greidanus TB, Maigret C, Torn M, Ronner E, Van der Kracht S, Van der Wee NJ, Versteeg DH (1989) Dopamine D-1 and D-2 receptor agonists and antagonists and neuropeptide-induced excessive grooming. Eur J Pharmacol 173:227–231
Vas A, Gulyas B, Szabo Z, Bonoczk P, Csiba L, Kiss B et al (2002) Clinical and non-clinical investigations using positron emission tomography, near infrared spectroscopy and transcranial Doppler methods on the neuroprotective drug vinpocetine: a summary of evidences. J Neurol Sci 203-204:259–262
Vezér T, Kurunczi A, Náray M, Papp A, Nagymajtényi L (2007) Behavioral effects of subchronic inorganic manganese exposure in rats. Am J Ind Med 50(11):841–852
Vidal L, Alfonso M, Campos F, Faro LR, Cervantes RC, Durán R (2005) Effects of manganese on extracellular levels of dopamine in rat striatum: an analysis in vivo by brain microdialysis. Neurochem Res 30(9):1147–1154
Volosin M, Cancela L, Molina V (1988) Influence of adrenocorticotrophic hormone on the behaviour in the swim test of rats treated chronically with desipramine. J Pharm Pharmacol 40(1):74–76
Vorhees CV (1974) Some behavioral effects of maternal hypervitaminosis A in rats. Teratology 10(3):269–273
Wang F, Wang C, Jiang Y, Deng X, Lu J, Ou S (2014) Protective role of sodium para-amino salicylic acid against manganese-induced hippocampal neurons damage. Environ Toxicol Pharmacol 37(3):1071–1078
Wang T, Li X, Yang D, Zhang H, Zhao P, Fu J, Yao B, Zhou Z (2015) ER stress and ER stress-mediated apoptosis are involved in manganese-induced neurotoxicity in the rat striatum in vivo. Neurotoxicology 48:109–119
Yoshikawa M, Suzumura A, Tamaru T, Takayanagi T, Sawada M (1999) Effects of phosphodiesterase inhibitors on cytokine production by microglia. Mult Scler 5(2):126–133
Zaitone SA, Abo-Elmatty DM, Elshazly SM (2012) Piracetam and vinpocetine ameliorate rotenone-induced parkinsonism in rats. Indian J Pharmacol 44(6):774–779
Zhang ZM, Huang SW (2008) Intervention effect of taurine on neurotoxicity of manganese in rat’s prefrontal cortex. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 26(10):601–604
Zhang L, Yang L (2014) Anti-inflammatory effects of vinpocetine in atherosclerosis and ischemic stroke: a review of the literature. Molecules 20(1):335–347
Zhang S, Fu J, Zhou Z (2004) In vitro effect of manganese chloride exposure on reactive oxygen species generation and respiratory chain complexes activities of mitochondria isolated from rat brain. Toxicol in Vitro 18(1):71–77
Zhang F, Xu Z, Gao J, Xu B, Deng Y (2008) In vitro effect of manganese chloride exposure on energy metabolism and oxidative damage of mitochondria isolated from rat brain. Environ Toxicol Pharmacol 26(2):232–236
Zhao F, Cai T, Liu M, Zheng G, Luo W, Chen J (2009) Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol Sci 107(1):156–164
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study complies with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publications No. 8023, revised 1978) and is approved by the Ethics Committee of Faculty of Pharmacy, Cairo University.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nadeem, R.I., Ahmed, H.I. & El-Sayeh, B.M. Protective effect of vinpocetine against neurotoxicity of manganese in adult male rats. Naunyn-Schmiedeberg's Arch Pharmacol 391, 729–742 (2018). https://doi.org/10.1007/s00210-018-1498-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-018-1498-0