Abstract
Manganese (Mn) is an essential micronutrient. However, exposure to high doses of Mn may lead to a neurological disease known as manganism, which is characterized by marked brain neuronal loss. K-homology splicing regulator protein (KHSRP) is a multifunctional RNA-binding protein and has been implicated in the regulation of multiple cellular signaling associated with neuronal apoptosis and survival, such as p38 mitogen-activated protein kinase (MAPK), nuclear factor kappaB (NF-κB), and Wnt/β-catenin pathways. In the present study, the role of KHSRP in Mn-induced neurotoxicity was investigated in vivo using a rat model of chronic Mn exposure and in vitro using differentiated PC12 cell cultures. Western blot and immunohistochemical analyses showed a significant upregulation of KHSRP in rat striatum following Mn exposure. Immunofluorescent labeling indicated that KHSRP was localized mainly in neurons. Terminal deoxynucleotidyl transferase-mediated biotinylated-dUTP nick end labeling (TUNEL) assay showed that KHSRP was mainly distributed in apoptotic neurons. Increased KHSRP expression was positively correlated with the upregulation of several apoptosis-related proteins, such as p53, bax, and active caspase-3. In addition, significant co-localization of KHSRP and active caspase-3 in neurons after Mn exposure was also observed, suggesting a potential involvement of KHSRP in the regulation of Mn-induced striatal neuronal apoptosis. Importantly, interference with KHSRP apparently decreased the level of p53 and attenuated Mn-induced neuronal apoptosis. Taken together, these results indicate that upregulation of KHSRP may be involved in the pathological process underlying Mn neurotoxicity via the modulation of p53 signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manganese (Mn) is a ubiquitous micronutrient required for normal growth, development, and cellular homeostasis (Aschner and Aschner 2005; Tkac et al. 2003). However, Mn is neurotoxic at high concentrations. Excessive Mn exposure may result in a neurodegenerative disease called manganism, which is characterized by symptoms that resemble idiopathic Parkinson’s disease (IPD) (Benedetto et al. 2009; Roth 2009). Mn overexposure can occur under multiple conditions, such as occupational, medical, and environmental exposure (Burton and Guilarte 2009). After uptake into the bodies, Mn can get through the blood–brain barrier by DMT-1-mediated and/or transferrin-mediated pathways and easily accumulate in brain striatum and globus pallidus (Dorman et al. 2006; Normandin et al. 2004; Roels et al. 1997). Data from nonhuman primates and humans demonstrate that striatal neurons are very vulnerable and undergo significant degeneration and loss after chronic exposure to excessive Mn (Yamada et al. 1986; Shinotoh et al. 1995; Shinotoh et al. 1997). However, the precise molecular mechanism underlying the process remains much unclear.
Neuronal apoptosis has been considered to be a crucial mechanism underlying Mn-induced neurotoxicity. Mn can trigger apoptosis in a majority of neurons (Chun et al. 2001; Hirata 2002; Malecki 2001; Seo et al. 2013). DNA fragmentation and caspase activation have been observed in Mn-exposed neurons (Latchoumycandane et al. 2005; Malecki 2001; Seo et al. 2013; Uchida et al. 2012). Further, Mn induces mitochondrial dysfunction (Gunter et al. 2009) and promotes mitochondrial cytochrome c release, caspase activation, and PARP-1 cleavage, eventually leading to neuronal apoptosis (Tamm et al. 2008; Afeseh Ngwa et al. 2011; Yoon et al. 2011). In addition, Mn could activate many signaling pathways associated with apoptosis beyond mitochondrial dysfunction, such as mitogen-activated protein kinase (MAPK) pathways, protein kinase C (PKC), and p53 (Guilarte et al. 2008; Jang 2009; Latchoumycandane et al. 2005; Zhang et al. 2013). Recent studies showed that p53 was a key determinant for the decision of neuronal survival and apoptosis (Costantini et al. 2005; Culmsee and Mattson 2005). It is well known that activated p53 translocates into the nucleus where it promotes the transcription of various target genes, such as the proapoptosis proteins Bax and Bim, triggering apoptosis (Martin et al. 2001).
K-homology splicing regulator protein (KHSRP), also known as FUSE-binding protein 2 (FBP2), is a multifunctional RNA-binding protein. KHSRP has been implicated in the regulation of various cellular processes, including RNA processing, trafficking, and degradation (Min et al. 1997; Snee et al. 2002; Gherzi et al. 2004). KHSRP can recruit RNA decay machinery to promote rapid decay of the selected messenger RNAs (mRNAs) (Chou et al. 2006; Ruggiero et al. 2007). KHSRP controls the intracellular distribution of microtubule-associated protein 2 (MAP2) and beta-actin mRNAs in neurons (Gu et al. 2002; Rehbein et al. 2002). KHSRP also modulates the biogenesis of a subset of miRNAs, which are potentially required for cell metabolism and survival (Trabucchi et al. 2009). Additionally, KHSRP could affect the expression of iNOS through posttranscriptional regulation, which in turn influences p38 MAPK and NF-κB signaling pathways (Fechir et al. 2005; Jayasooriya et al. 2014; Jeong et al. 2014; Linker et al. 2005). KHSRP has been also documented to regulate Wnt signaling through promoting the destabilization of β-catenin (Bikkavilli and Malbon 2010; Inestrosa and Arenas 2010; Kim et al. 2014; Zhang et al. 2008). Accordingly, these findings implied that KHSRP might be involved in the regulation of cell survival and apoptosis. However, whether KHSRP is implicated in Mn neurotoxicity remains virtually unknown.
In this study, we for the first time investigated the role of KHSRP in Mn-induced neurotoxicity. The expression profile of KHSRP was analyzed in vivo in a rat chronic Mn exposure model and in vitro using PC12 cell cultures. The correlation between aberrant expression of KHSRP and neuronal apoptosis was further evaluated. Our findings may provide novel insight into the mechanism of Mn-induced neurotoxicity.
Materials and Methods
Animals and Treatment
Adult male Sprague–Dawley (SD) rats (6 weeks old, weighing 180–210 g) were provided by the Experimental Animal Center of Nantong University. All animals were kept under a controlled environment (23 ± 1 °C, 50 ± 5 % humidity) on a 12-h light/dark cycle with free access to water and food. All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Chinese National Committee to Use of Experimental Animals for Medical Purposes, Jiangsu Branch. The animals were allowed to be acclimated for 3–5 days before the experiments commenced and then randomly divided into five groups with 9–11 animals in each group: control group and Mn-treated group (2, 5, 15, and 25 mg/kg body wt). All rats were treated for 30 consecutive days with one daily intraperitoneal (i.p.). Control group rats were injected with saline (NaCl, 0.9 %), and Mn-treated group rats were injected with MnCl2 (2, 5, 15, and 25 mg/kg body wt, diluted in saline). Rats (n = 3 per group) were sacrificed to measure the levels of Mn in striatal tissue 24 h after last injection. Rats (n = 3–4 per group) were sacrificed to extract the protein for Western blot analysis, and additional experimental rats (n = 3–4 per group) were also sacrificed for sections. All efforts were made to minimize the number of animals used and their suffering.
Cell Cultures and Stimulation
Nerve growth factor (NGF)-differentiated rat pheochromocytoma (PC12) cells, a dopaminergic neuronal cell line, were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma) supplemented with 10 % (v/v) fetal bovine serum (Hyclone), 5 % donor horse serum, and 1 % antibiotics at 37 °C under 5 % CO2 in humidified air. The cells were plated at a density of 5 × 106 cells/10-cm diameter dish, and the medium was replenished every 48 h. PC12 cells were treated with fresh medium or medium containing different concentrations of MnCl2 (0, 50, 100, 300, 500, and 1,000 μM) for 24 h or treated with or without medium containing 300 μM Mn for different time (1, 3, 6, 12, and 24 h). For all experiments, PC12 cells were grown to 70–80 % confluence and were subjected to no more than 20 cell passages. Protein samples were stored at −80 °C until use.
Determination of Mn Levels in Rat Brain Striatum
Mn levels in striatal tissue were measured by atomic absorption spectroscopy (AA-6800, ASA-2SP, Shimadzu Co., Japan). Briefly, rat striatum (80–120 mg) was dissected out and digested in 2.5 ml ultrapure nitric acid plus 0.5 ml perchloric acid for 48–72 h in a sand bath (60 °C). Next, the samples were collected and made up to a total volume of 30 ml using 2 % nitric acid. The mixture was then centrifuged, and the clear supernatant was used for determination. Bovine liver (NBS Standard Reference Material, USDC, Washington, DC, USA) (10 μg Mn/g) was digested in ultrapure nitric acid and used as standard control for the analysis. The data are expressed in microgram metal per gram tissue (mean ± SEM).
Western Blot Analysis
After given an overdose of sodium pentobarbital, all rats were sacrificed by decapitation. Briefly, striatum of rat was dissected out and stored at −80 °C until the following analysis. In order to prepare the lysates, frozen striatum tissue samples were weighed and minced on ice. Next, the samples were homogenized in a tissue lysis buffer (1 % NP-40, 100 mM Tris–HCl, 0.5 mM EDTA, 0.1 % sodium dodecyl sulfate (SDS), 10 μg/ml aprotinin, pH 7.5, 1 % sodium deoxycholate, 1 μg/ml leupeptin, 1 % Triton X-100, and 1 mM PMSF) (Sigma–Aldrich). The lysates were then centrifuged at 12,000 rpm and 4 °C for 20 min to collect the supernatant layer. PC12 cells were directly lysed with sodium lauryl sulfate loading buffer and stored at −80 °C until use. The protein concentration of the supernatant obtained was measured by the Bradford assay (Bio-Rad, Hercules, CA). For Western blot analysis, protein samples were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene diflouride filter (PVDF) membrane (Immobilon, Millipore). The membrane was blocked with 5 % nonfat powdered skim milk for 2 h at room temperature (RT), followed by incubation with the primary antibody against KHSRP (1:800; Cell Signaling), p53 (1:800; Santa Cruz), Bax (1:800; Cell Signaling), active caspase-3 (1:800; Cell Signaling), or GAPDH (1:1,000; Sigma) at 4 °C overnight. At last, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (1:2,000; SouthernBiotech) for 2 h and then visualized using an enhanced chemiluminescence system (Pierce Company, USA).
Sections and Immunohistochemistry
Rats were deeply anesthetized and perfused pericardially with sterile 0.9 % saline, followed by 4 % paraformaldehyde. After perfusion, the brains were removed and postfixed in the same fixative for 12 h and then replaced with 20 % sucrose for 2–3 days, followed by 30 % sucrose for 2–3 days. The tissues were then embedded in O.T.C. compound (Sakura) and 7-μm frozen cross sections prepared. All sections were stored at −20 °C before use. For KHSRP single immunostaining, sections were blocked with a solution consisting of 1 % (w/v) bovine serum albumin (BSA), 10 % donkey serum, 0.3 % Triton X-100, and 0.15 % Tween-20 for 2 h at RT and then incubated with anti-KHSRP antibody (1:100; Cell Signaling) overnight at 4 °C. Negative control sections were processed in parallel using a nonspecific immunoglobulin IgG (Sigma) at the same concentration as the primary antibody. Following incubation in the secondary antibody at 37 °C, the sections were color-reacted with the liquid mixture DAB (0.02 % diaminobenzidine tetrahydrochloride, 0.1 % PBS, and 3 % H2O2). Finally, slides were gradually dehydrated in ethanol, cleared in xylene, and covered with coverslips. The sections were visualized via Leica light microscope (Germany) at × 20 or × 40. Cells with strong or moderate brown staining were considered as positive, whereas cells with weak or no staining were counted as negative. Data were calculated as the average of assays from each group.
Immunofluorescent Staining
The sections were blocked with a blocking buffer containing 0.1 % Triton X-100, 3 % (w/v) BSA, 10 % normal serum, and 0.05 % Tween-20 for 2 h at RT. Sections were then incubated with primary antibodies for KHSRP (1:100; Cell Signaling) and specific cell markers such as neuronal nuclei (NeuN) (1:200; Cell Signaling), glial fibrillary acidic protein (GFAP) (1:200; Sigma), and anti-active caspase-3 (1:200; Cell Signaling). Briefly, sections were incubated with both primary antibodies overnight at 4 °C, followed by a mixture of FITC- and TRITC-conjugated secondary antibodies for 2 h at 4 °C. The stained sections were examined under a Leica fluorescence microscope.
Terminal Deoxynucleotidyl Transferase-Mediated Biotinylated-dUTP Nick End Labeling (TUNEL) Assay
TUNEL assay was performed with an In Situ Cell Death Detection Kit (Roche Applied Science, Mannheim, Germany). Frozen tissue sections were rinsed with PBS and treated with 1 % Triton X-100 in PBS for 2 min on the ice. Slides were rinsed in PBS and incubated for 60 min at 37 °C with 50-μl TUNEL reaction mixture in a dark humidified chamber. For PC12 cells, after the given indicated treatments, cells were fixed with 4 % formaldehyde and incubated at RT for 40 min. It was followed by several rinses in PBS and permeabilization in 0.1 % Triton X-100 solution on ice for 5 min. Thereafter, 50-μl TUNEL reaction mixture was added on coverslips before being incubated for 60 min at 37 °C. Finally, the coverslips were incubated with DAPI for 20 min at RT. Apoptotic cells were detected as localized bright green cells (positive cells) with a Leica fluorescence microscope. Data were expressed as the ratio of apoptotic cells to total cells.
DAPI Staining
The fluorescent dye DAPI (Santa Cruz) was explored to detect nuclear fragmentation which is a characteristic of apoptotic cells. PC12 cells were given the indicated treatments for 24 h and then washed with PBS and fixed with 4 % paraformaldehyde for 40 min at RT. The fixed cells were then washed with PBS and stained with DAPI. After incubation for 20 min, the cells were again washed with PBS, and the plates were observed with a fluorescence microscope.
Cell Viability Assay
PC12 cells were plated at a density of 5 × 103 cells/well in 96-well plates, and the cell viability was measured by the Cell Counter Kit-8 (CCK-8) assay (Dojindo Laboratories, Japan) according to the manufacturer’s instruction. Briefly, the cells were incubated with the indicated treatments at 37 °C. After the treatment, 10 μl CCK-8 solution was added to each well of the plate, and the cells were incubated for 2 h at 37 °C. Finally, the absorbance was quantified on an automated reader (BioTek, VT USA).
KHSRP siRNA Vector Construction and Transfection
Double-stranded oligonucleotides corresponding to the target sequence for the KHSRP (Genbank accession no. NM 133602.1) gene were cloned into the pSilencer 4.1-CMV small interfering RNA (siRNA) plasmid (Invitrogen). Cells were transfected with the KHSRP siRNA plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Transfected cells were cultured for 48 h before subsequent experiments.
Quantitative Analysis
Cell quantification was performed in an unbiased manner in accordance with the principles described by Konigsmark and Murphy (1970). The number of KHSRP-positive cells in the striatum was counted at × 40. At least three separate regions in each section were examined. The cell counts in the three or four sections were then used to calculate the total number of KHSRP-positive cells per square millimeter (mm2). The number of cells double-labeled for KHSRP and other phenotypic markers like NeuN and GFAP applied in the experiment was quantified. To determine the proportion of NeuN-positive cells expressing KHSRP, all of the NeuN-positive cells (over 200 cells at least) were counted in each section. Then, double-labeled cells for KHSRP and NeuN were calculated and recorded. For all animal experiments, we counted and analyzed at least three nonadjacent sections per animal.
Statistical Analysis
All values are expressed as mean ± SEM for at least three independent experiments. One-way ANOVA followed by the Tukey’s post hoc multiple comparison tests was applied for statistical analysis. P values less than 0.05 was regarded as statistically significant. All data were analyzed with Stata 7.0 statistical software (Stata Corp., College Station, TX, USA).
Results
Behavioral Activity and Mn Accumulation
To explore whether KHSRP was involved in Mn neurotoxicity, a Mn-exposed rat model was established. After daily administration with different doses of Mn for 30 days, aberrant behaviors of rats were observed. We found that 15 and 25 mg/kg Mn-exposed rats displayed apparent behavioral abnormalities, such as sedateness, ataxia, rigidity, and bradykinesia, which were not observed in control rats. Mn concentrations in rat striatum were further analyzed. The level of Mn in the striatum of each group was determined by atomic absorption spectroscopy, and the data showed that Mn injections caused significant increase in Mn levels in rat striatum compared with the control group (P < 0.05), particularly in 25 mg/kg Mn-exposed rats (Table 1). These results were consistent with earlier studies (Cordova et al. 2013; Normandin et al. 2002; Roth 2009; Yamada et al. 1986). Thus, Mn exposure results in significant neurotoxic effects, which are correlated with marked Mn accumulation in the striatum of rats.
The Expression Profile of KHSRP in the Striatum of Rats Exposed to Mn
Western blot analysis was performed to investigate whether KHSRP level was altered in rat striatum after Mn exposure. As shown in Fig. 1a, the protein level of KHSRP was significantly elevated following treatment with 15 or 25 mg/kg Mn, compared with the saline-treated control group. To verify the changes, immunohistochemical staining was performed on transverse cryosections of the striatum. Consistent with Western blot results, the number of KHSRP-positive cells was obviously increased in 25 mg/kg Mn-treated group (Fig. 1b (c–d)), compared with the control group (Fig. 1b (a–b)). No staining was observed in the negative control sections (Fig. 1b (e)). The total number of KHSRP-positive cells in each group was counted and analyzed, which showed the increase in KHSRP-positive cells after Mn exposure was stastically significant (P < 0.05) (Fig. 1b (f)). These data suggest that Mn exposure remarkably increases the expression of KHSRP in rat striatum.
Western blot and immunohistochemical analyses of KHSRP expression in the rat striatum following Mn exposure. a Western blot analysis was performed to determine the protein level of KHSRP in rat striatum after exposure to the indicated doses of Mn (a); quantification graphs represented band density ratio of KHSRP to GAPDH (b). The data are mean ± SEM (n = , *P < 0.05, significantly different from the control group). b Representative microphotographs of KHSRP immunohistochemical staining in control (a, b) and 25 mg/kg Mn-exposed (c, d) rat striatum. Negative control using normal IgG (e). Quantitative analysis of KHSRP-positive cells per square millimeter in the control and 25 mg/kg Mn-treated rat striatum (f). *P < 0.05, significantly different compared with the control group. Error bars represent SEM. Scale bar indicates 100 μm (a, c, e) and 50 μm (b, d)
Determination of KHSRP Expressing Cells in Rat Striatum After Mn Exposure
To further determine the distribution of KHSRP in different cell types in the striatum, double immunofluorescent labeling was performed to analyze the co-localization of KHSRP with different cell markers in transverse cryosections of the striatum, such as NeuN (neuron marker) and GFAP (astrocyte marker). As shown in Fig. 2, KHSRP was predominantly localized in neurons (Fig. 2a–f) and was rarely found in astrocytes (Fig. 2g–l). To determine the proportion of NeuN-positive cells expressing KHSRP, at least 200 phenotype-specific positive cells in each section were counted both in saline-treated and 25 mg/kg Mn-treated groups. Statistical data showed that 25 mg/kg Mn treatment significantly increased the number of KHSRP-positive neurons compared with the control group (P < 0.05) (Fig. 2o). These results implied that Mn exposure altered the expression of KHSRP mainly in neuronal cells.
Double immunofluorescent staining of KHSRP and different phenotype-specific markers in rat striatum. The cryosections from control and 25 mg/kg Mn-exposed rat striatum were immunostained. a–f The co-localization of neuron marker (green, a, d, NeuN) and KHSRP (red, b, e). g–l The co-localization of astrocyte marker (green, g, j, GFAP) and KHSRP (red, h, k). The yellow color visualized in the merged images represented co-localization of KHSRP with different cell markers (c, f, i, and l). m–n Negative control using normal IgG. o Quantitative analysis of NeuN-positive cells expressing KHSRP (%) in control and 25 mg/kg Mn-exposed groups. *P < 0.05, significantly different compared with control group. Error bars represent SEM. Scale bars indicate 20 μm (a–n) (color figure online)
Association Between Altered Expression of KHSRP with Mn-Induced Striatal Neuron Apoptosis
Previous studies have provided evidences that Mn triggered significant apoptosis of striatal neurons (Cordova et al. 2013; Jiang et al. 2014; Liu et al. 2006; Quintanar et al. 2012). Because Mn-induced alteration of KHSRP expression mainly occurred in neurons, we inferred that the altered expression of KHSRP might be related with Mn-induced striatal neuronal apoptosis. TUNEL staining was used to examine the involvement of KHSRP in neuronal apoptosis in the striatum. Representative images showed significant co-localizations of TUNEL with both NeuN and KHSRP (Fig. 3a), implying that KHSRP was associated with neuronal apoptosis. To further verify the relationship between KHSRP and neuronal apoptosis, we analyzed the expression profiles of several apoptosis-related proteins, such as p53, Bax, and active caspase-3 after Mn exposure using Western blot. Interestingly, the levels of these proteins were remarkably elevated, particularly in 15 or 25 mg/kg Mn-exposed group (Fig. 3b). More importantly, co-localizations of active caspase-3 with NeuN and KHSRP were markedly observed following Mn exposure (Fig. 3c). These findings together demonstrated that the upregulation of KHSRP might have an effect on provoking the activation of caspase-3 and the apoptosis of striatal neurons in the neuropathological process of manganism.
Association between KHSRP expression and striatal neuronal apoptosis after Mn expoure. a TUNEL staining showed that KHSRP was related with neuronal apoptosis following Mn administration. The cryosections from 25 mg/kg Mn-exposed rat striatum were used for TUNEL assay. The yellow color visualized in the merged images (c, f) represented co-localization of NeuN (red, a)/KHSRP (red, d) and TUNEL-positive cells (green, b, e). b Western blot analysis of the expression profiles of p53, Bax, and active caspase-3 in rat striatum after Mn administration (a); quantification graphs represented the band density ratio of p53, Bax, and active caspase-3 to GAPDH (b). The data are mean ± SEM (n = 3, *P < 0.05, significantly different from the control group). c Double immunofluorescent staining showed the co-localization of NeuN (red, a), KHSRP (red, d), and active caspase-3 (green, b, e) in rat striatum following 25 mg/kg Mn administration; merged images were shown in c and f. Scale bars indicate 20 μm (a, c) (color figure online)
Altered KHSRP Expression Is Associated with Mn-induced Apoptosis of PC12 Cells
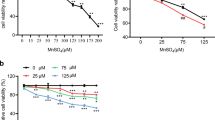
To verify the results above, we further investigated the effects of Mn-mediated toxicity on neurons by employing NGF-differentiated PC12 cells. The cytotoxicity induced by Mn was first measured. As shown in Fig. 4a, after 24 h of Mn treatment, Mn exposure led to a significant reduction in cell viability at a starting concentration of 100 μM. A concentration of 300 μM Mn was selected as the dose applied for further experiments. After treatment with 300 μM Mn for different times (0, 1, 3, 6, 12, or 24 h), the cell viability was reduced significantly at 12 or 24 h (P < 0.05) (Fig. 4b). These results show that the cell viability of the PC12 cells can be effectively inhibited by Mn in dose- and time-dependent manners. In addition, we observed significant increases in the levels of active caspase-3, p53, and Bax, particularly at 12 or 24 h following 300 μM Mn exposure (Fig. 4c, d), indicating that PC12 cells underwent significant apoptosis. Notably, Mn treatment also synchronously elevated the expression of KHSRP in PC12 cells (Fig. 4e, f). These data were consistent with the in vivo results. Therefore, our results demonstrated that KHSRP was upregulated in the process of Mn-induced p53 activation and neuronal apoptosis.
Altered KHSRP expression is associated with Mn-induced apoptosis of PC12 cells. Cell viability was determined by CCK-8 assay. a PC12 cells were treated with Mn of various concentrations (0, 50, 100, 300, 500, and 1,000 μM) for 24 h. b PC12 cells were treated with 300 μM Mn for the indicated periods of time, and cell viability was assessed at each time point. The data are mean ± SEM (n = 3, *P < 0.05, significantly different from the control group). c Western blot analysis of the levels of active caspase-3, p53, and Bax in PC12 cells after treatment with 300 μM Mn for the indicated periods of time. d Quantification graphs represented the band density ratios of active caspase-3, p53, and Bax to GAPDH; the data are mean ± SEM (n = 3, *P < 0.05, significantly different from the control group). e Western blot analysis of KHSRP level in Mn-treated PC12 cells. f Quantification graphs represented the band density ratio of KHSRP to GAPDH. The data are mean ± SEM (n = 3, *P < 0.05, significantly different from the control group)
Interference with KHSRP-Suppressed Mn-Induced Upregulation of p53 Level in PC12 Cells
To further explore whether KHSRP was involved in the regulation of p53 signaling in neuronal cells, an RNA interference assay was performed to knock down KHSRP expression in PC12 cells. As shown in Fig. 5a, b, the protein level of KHSRP was significantly reduced after transfection with KHSRP-targeting siRNA. In line with our hypothesis, we found that interference with KHSRP significantly attenuated Mn-induced upregulation of p53 level, compared with Mn treatment only in PC12 cells (Fig. 5c, d). These findings suggested that KHSRP might regulate the expression of p53, which in turn promotes subsequent neuronal apoptosis.
Interference with KHSRP-suppressed Mn-induced upregulation of p53 level in PC12 cells. a Western blot showed the interfering efficiency of KHSRP siRNA oligos in PC12 cells at 48 h after transfection. b Quantification graphs represented the band density ratio of KHSRP to GAPDH. The data are mean ± SEM (n = 3, *P < 0.05, significantly different from the nonspecific siRNA group). c PC12 cells transfected with nonspecific siRNA or KHSRP-targeting siRNA oligos were treated with 300 μM Mn for 24 h and subjected to Western blot analysis to determine the cellular level of p53. d Quantification graphs represented the band density ratios of KHSRP and p53 to GAPDH. The data are mean ± SEM (n = 3, *P < 0.05, significantly different from the control group; #P < 0.05, significantly different from the Mn-only group)
Interference with KHSRP Conferred Resistance to Mn-Induced Apoptosis and Cytotoxicity in PC12 Cells
Next, we further investigated the role of KHSRP in Mn-induced neuronal apoptosis. Aberrant nuclear morphology was observed at 24 h following exposure after the indicated treatments in PC12 cells. The DAPI staining showed that cells exposed to 300 μM Mn exhibited apparent chromatin condensation and the presence of apoptotic bodies (Fig. 6a). In addition, TUNEL assay revealed that Mn exposure obviously increased the number of TUNEL-positive cells compared with the control group, while interference with KHSRP markedly attenuated Mn-induced apoptosis of PC12 cells (Fig. 6a, b). Finally, the CCK-8 assay indicated that knockdown of KHSRP significantly increased the cell viability compared with Mn treatment only (Fig. 6c). Therefore, we speculated that KHSRP might participate in Mn-induced neuronal apoptosis, probably through modulating p53 signaling.
Interference with KHSRP attenuated Mn-induced apoptosis and cytotoxicity in PC12 cells. a Interference with KHSRP impaired the number of TUNEL-positive cells following Mn exposure. After exposure to 300 μM Mn for 24 h, PC12 cells were analyzed using DAPI staining (blue), and TUNEL staining (green) to determine the proportion of apoptotic cells. b Statistical analysis of the percentage of TUNEL-positive cells in each group of PC12 cells. The data are mean ± SEM (n = 3, *P < 0.05, significantly different from the control group; #P < 0.05, significantly different from the Mn-only group). c Determination of PC12 cell viability after the indicated treatments using CCK-8 assay. The data are mean ± SEM (n = 3, *P < 0.05, significantly different from the control group; #P < 0.05, significantly different from the Mn-only group) (color figure online)
Discussion
Manganese (Mn) is an essential nutrient and plays an important role in the regulation of metabolism (Aschner and Aschner 2005). However, exposure to excessive Mn may lead to irreversible CNS damage (Lazrishvili et al. 2009), which is characterized by neuronal loss and marked astrocytosis, particularly in the striatum, globus pallidus, and substantia nigra (Normandin et al. 2002; Roth 2009). In the present study, we established the models of manganism in vivo and in vitro, respectively, and investigated the role of KHSRP in Mn-induced neurotoxicity. We found that Mn exposure significantly elevated the expression of KHSRP, which was correlated with the activation of cellular p53 signaling and apoptotic response. Most importantly, interference with KHSRP attenuated Mn-induced upregulation of p53 level and subsequent apoptosis. Taken together, our results indicate that KHSRP participates in the pathological process of Mn neurotoxicity, probably through modulating p53 signaling.
It has been well-documented that Mn exposure triggered neuronal apoptosis through complex molecular mechanism (Chun et al. 2001; Hirata 2002; Malecki 2001; Seo et al. 2013). Mitochondrial dysfunction has been considered to play an integral role in Mn-induced neuronal apoptosis (Gunter et al. 2009; Tamm et al. 2008). Mn can directly accumulate in mitochondria and cause a wide range of deficits in mitochondrial function after its entrance into neuronal cells (Gunter et al. 2009). Following mitochondrial dysfunction, a variety of cellular events coupled with cell apoptosis may occur and confer neuronal apoptosis, including p53 accumulation, oxidative stress, endoplasmic reticulum stress, PKC activation, and the activation of the mitogen-activated protein kinases (Avila et al. 2008; Cai et al. 2012; Cordova et al. 2013; Guilarte et al. 2008; Hirata et al. 2004; Ito et al. 2006; Latchoumycandane et al. 2005; Tamm et al. 2008; Xu et al. 2013; Zhang et al. 2013). In these cellular events, p53 signaling is of particular importance in the modulation of neuronal apoptosis (Culmsee and Mattson 2005; Guilarte et al. 2008; Zhang et al. 2013). p53 could be rapidly activated under stress conditions and facilitate apoptosis through promoting the transcription of its downstream proapoptotic target genes like p21 and Bax (Culmsee and Mattson 2005; Kim et al. 2014). Bax is a member of the Bcl-2 family and has been confirmed to regulate mitochondrial membrane permeability and the release of cytochrome c. Our study showed that Mn expoure parallelly increased the expression of p53 and Bax, which were correlated with the upregulation of KHSRP expression. The regulation of p53 signaling by KHSRP was further confirmed by interfering the expression of KHSRP in PC12 cells, suggesting that the upregulation of KHSRP could promote the activation of p53 signaling. Importantly, interference of KHSRP expression attenuated Mn-induced apoptosis and improved cell viability in Mn-treated PC12 cells. Therefore, our results demonstrate that upregulation of KHSRP may contribute to Mn-induced neuronal apoptosis via activation of p53 signaling.
KHSRP is a multiple functional protein and has been associated with various cellular processes. KHSRP can recruit degradation machinery to promote mRNA turnover (Gherzi et al. 2004). KHSRP has been shown to function as a transcriptional regulator and is involved in neuro-specific alternative splicing (Min et al. 1997). KHSRP can also bind to the terminal loop of the target miRNA precursors and then control their biogenesis by associating with both the Drosha and Dicer multiprotein complexes (Trabucchi et al. 2009). KHSRP also participates in multiple cellular signaling associated with survival and apoptosis, such as p38 MAPK and NF-κB (Fechir et al. 2005; Jayasooriya et al. 2014; Jeong et al. 2014; Linker et al. 2005; Briata et al. 2005). In this way, KHSRP may participate in the regulation of various cellular processes, including proliferation, apoptosis, and differentiation (Min et al. 1997). Previous studies have demonstrated that KHSRP was abundantly expressed in both neural and nonneural cells (Hall et al. 2004; Min et al. 1997). The present study showed that excessive level of KHSRP contributed to Mn-induced neuronal apoptosis. All these findings implicated that KHSRP might play an important role in the regulation of neuronal physiology and stress responses, while its misregulation may cause aberrant neuronal physiology and even apoptosis. In the present study, we found that interference of KHSRP expression could downregulate the p53 signaling and mildly attenuate Mn-induced apoptosis of PC12 cells. However, because Mn exposure could trigger complex molecular alterations beyond p53 signaling, we speculated that other pathways, such as JNK, PKC, and NF-κB pathways, might be critically involved in Mn-induced neurotoxicity completely. Our present studies indicated that upregulated KHSRP expression might contribute to p53 activation, which partially accounted for Mn-induced neuronal apoptosis. However, the precise role of KHSRP in neuronal cells remains much elusive and needs further investigation.
The mechanism of KHSRP in regulating the cellular p53 level remains virtually unknown. KHSRP was reported to regulate Wnt signaling through promoting the destabilization of β-catenin (Bikkavilli and Malbon 2010; Gherzi et al. 2006). β-Catenin plays a crucial role in various cellular processes, including cell proliferation, differentiation, motility, and survival (Inestrosa and Arenas 2010; Jiang et al. 2014). Reduced level of β-catenin could directly decrease the expression of downstream prosurvival Wnt target genes, such as c-myc and survivin (Jiang et al. 2014; Zhang et al. 2008). Recent studies reported that β-catenin could negatively regulate the activity of p53, leading to subsequent apoptosis (Kim et al. 2014). As such, we speculated that the regulation of p53 signaling by KHSRP might be mediated by some important molecules related with cell survival or apoptosis, such as β-catenin. Further investigation is required to clarify the detailed mechanism in this regard.
In conclusion, the present study found that Mn exposure significantly increased the level of KHSRP in rat striatum and PC12 cells. Moreover, abarrent expression of KHSRP was associated with aberrant activation of p53 signaling and neuronal apoptosis following Mn exposure. Thus, we proposed that the upregulation of KHSRP might play a vital role in Mn-induced neurotoxicity. However, the precise role of KHSRP underlying Mn neurotoxicity remains much unclear and needs more extensive studies.
References
Afeseh Ngwa H, Kanthasamy A, Gu Y, Fang N, Anantharam V, Kanthasamy AG (2011) Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol Appl Pharmacol 256:227–240
Aschner JL, Aschner M (2005) Nutritional aspects of manganese homeostasis. Mol Aspects Med 26:353–362
Avila DS, Gubert P, Fachinetto R, Wagner C, Aschner M, Rocha JB, Soares FA (2008) Involvement of striatal lipid peroxidation and inhibition of calcium influx into brain slices in neurobehavioral alterations in a rat model of short-term oral exposure to manganese. Neurotoxicology 29:1062–1068
Benedetto A, Au C, Aschner M (2009) Manganese-induced dopaminergic neurodegeneration: insights into mechanisms and genetics shared with Parkinson’s disease. Chem Rev 109:4862–4884
Bikkavilli RK, Malbon CC (2010) Dishevelled-KSRP complex regulates Wnt signaling through post-transcriptional stabilization of beta-catenin mRNA. J Cell Sci 123:1352–1362
Briata P, Forcales SV, Ponassi M, Corte G, Chen CY, Karin M, Puri PL, Gherzi R (2005) p38-dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol Cell 20:891–903
Burton NC, Guilarte TR (2009) Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates. Environ Health Perspect 117:325–332
Cai TJ, Che HL, Yao T, Chen YM, Huang CS, Zhang WB, Du KJ, Zhang JB, Cao YX, Chen JY, Luo WJ (2012) Manganese induces tau hyperphosphorylation through the activation of ERK MAPK pathway in PC12 cells (vol 119, pg 169, 2011). Toxicol Sci 127:620–620
Chou CF, Mulky A, Maitra S, Lin WJ, Gherzi R, Kappes J, Chen CY (2006) Tethering KSRP, a decay-promoting AU-rich element-binding protein, to mRNAs elicits mRNA decay. Mol Cell Biol 26:3695–3706
Chun HS, Lee H, Son JH (2001) Manganese induces endoplasmic reticulum (ER) stress and activates multiple caspases in nigral dopaminergic neuronal cells, SN4741. Neurosci Lett 316:5–8
Cordova FM, Aguiar AS Jr, Peres TV, Lopes MW, Goncalves FM, Pedro DZ, Lopes SC, Pilati C, Prediger RD, Farina M, Erikson KM, Aschner M, Leal RB (2013) Manganese-exposed developing rats display motor deficits and striatal oxidative stress that are reversed by Trolox. Arch Toxicol 87:1231–1244
Costantini C, Rossi F, Formaggio E, Bernardoni R, Cecconi D, Della-Bianca V (2005) Characterization of the signaling pathway downstream p75 neurotrophin receptor involved in beta-amyloid peptide-dependent cell death. J Mol Neurosci 25:141–156
Culmsee C, Mattson MP (2005) p53 in neuronal apoptosis. Biochem Biophys Res Commun 331:761–777
Dorman DC, Struve MF, Marshall MW, Parkinson CU, James RA, Wong BA (2006) Tissue manganese concentrations in young male rhesus monkeys following subchronic manganese sulfate inhalation. Toxicol Sci 92:201–210
Fechir M, Linker K, Pautz A, Hubrich T, Forstermann U, Rodriguez-Pascual F, Kleinert H (2005) Tristetraprolin regulates the expression of the human inducible nitric-oxide synthase gene. Mol Pharmacol 67:2148–2161
Gherzi R, Lee KY, Briata P, Wegmuller D, Moroni C, Karin M, Chen CY (2004) A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol Cell 14:571–583
Gherzi R, Trabucchi M, Ponassi M, Ruggiero T, Corte G, Moroni C, Chen CY, Khabar KS, Andersen JS, Briata P (2006) The RNA-binding protein KSRP promotes decay of beta-catenin mRNA and is inactivated by PI3K-AKT signaling. PLoS Biol 5:e5
Gu W, Pan F, Zhang H, Bassell GJ, Singer RH (2002) A predominantly nuclear protein affecting cytoplasmic localization of beta-actin mRNA in fibroblasts and neurons. J Cell Biol 156:41–51
Guilarte TR, Burton NC, Verina T, Prabhu VV, Becker KG, Syversen T, Schneider JS (2008) Increased APLP1 expression and neurodegeneration in the frontal cortex of manganese-exposed non-human primates. J Neurochem 105:1948–1959
Gunter TE, Gavin CE, Gunter KK (2009) The case for manganese interaction with mitochondria. Neurotoxicology 30:727–729
Hall MP, Huang S, Black DL (2004) Differentiation-induced colocalization of the KH-type splicing regulatory protein with polypyrimidine tract binding protein and the c-src pre-mRNA. Mol Biol Cell 15:774–786
Hirata Y (2002) Manganese-induced apoptosis in PC12 cells. Neurotoxicol Teratol 24:639–653
Hirata Y, Furuta K, Miyazaki S, Suzuki M, Kiuchi K (2004) Anti-apoptotic and pro-apoptotic effect of NEPP11 on manganese-induced apoptosis and JNK pathway activation in PC12 cells. Brain Res 1021:241–247
Inestrosa NC, Arenas E (2010) Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci 11:77–86
Ito Y, Oh-Hashi K, Kiuchi K, Hirata Y (2006) p44/42 MAP kinase and c-Jun N-terminal kinase contribute to the up-regulation of caspase-3 in manganese-induced apoptosis in PC12 cells. Brain Res 1099:1–7
Jang BC (2009) Induction of COX-2 in human airway cells by manganese: role of PI3K/PKB, p38 MAPK, PKCs, Src, and glutathione depletion. Toxicol In Vitro 23:120–126
Jayasooriya RG, Lee KT, Lee HJ, Choi YH, Jeong JW, Kim GY (2014) Anti-inflammatory effects of beta-hydroxyisovalerylshikonin in BV2 microglia are mediated through suppression of the PI3K/Akt/NF-kB pathway and activation of the Nrf2/HO-1 pathway. Food Chem Toxicol 65:82–89
Jeong YH, Kim Y, Song H, Chung YS, Park SB, Kim HS (2014) Anti-inflammatory effects of alpha-galactosylceramide analogs in activated microglia: involvement of the p38 MAPK signaling pathway. PLoS One 9:e87030
Jiang J, Shi S, Zhou Q, Ma X, Nie X, Yang L, Han J, Xu G, Wan C (2014) Downregulation of the Wnt/beta-catenin signaling pathway is involved in manganese-induced neurotoxicity in rat striatum and PC12 cells. J Neurosci Res
Kim SJ, Lim JY, Lee JN, Choe SK, Kim YI, Song SR, Cho M, So HS, Park R (2014) Activation of beta-catenin by inhibitors of glycogen synthase kinase-3 ameliorates cisplatin-induced cytotoxicity and pro-inflammatory cytokine expression in HEI-OC1 cells. Toxicology. doi:10.1016/j.tox.2014.01.013. Epub 2014 Feb 19
Konigsmark BW, Murphy EA (1970) Neuronal populations in the human brain. Nature 228:1335–1336
Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG (2005) Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J Pharmacol Exp Ther 313:46–55
Lazrishvili IL, Shukakidze AA, Chkhartishvili NN, Bikashvili TZ (2009) Morphological changes and manganese content in the brains of rat pups subjected to subchronic poisoning with manganese chloride. Neurosci Behav Physiol 39:7–12
Linker K, Pautz A, Fechir M, Hubrich T, Greeve J, Kleinert H (2005) Involvement of KSRP in the post-transcriptional regulation of human iNOS expression-complex interplay of KSRP with TTP and HuR. Nucleic Acids Res 33:4813–4827
Liu X, Sullivan KA, Madl JE, Legare M, Tjalkens RB (2006) Manganese-induced neurotoxicity: the role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol Sci 91:521–531
Malecki EA (2001) Manganese toxicity is associated with mitochondrial dysfunction and DNA fragmentation in rat primary striatal neurons. Brain Res Bull 55:225–228
Martin LJ, Kaiser A, Yu JW, Natale JE, Al-Abdulla NA (2001) Injury-induced apoptosis of neurons in adult brain is mediated by p53-dependent and p53-independent pathways and requires Bax. J Comp Neurol 433:299–311
Min H, Turck CW, Nikolic JM, Black DL (1997) A new regulatory protein, KSRP, mediates exon inclusion through an intronic splicing enhancer. Genes Dev 11:1023–1036
Normandin L, Carrier G, Gardiner PF, Kennedy G, Hazell AS, Mergler D, Butterworth RF, Philippe S, Zayed J (2002) Assessment of bioaccumulation, neuropathology, and neurobehavior following subchronic (90 days) inhalation in Sprague–Dawley rats exposed to manganese phosphate. Toxicol Appl Pharmacol 183:135–145
Normandin L, Ann Beaupre L, Salehi F, St-Pierre A, Kennedy G, Mergler D, Butterworth RF, Philippe S, Zayed J (2004) Manganese distribution in the brain and neurobehavioral changes following inhalation exposure of rats to three chemical forms of manganese. Neurotoxicology 25:433–441
Quintanar L, Montiel T, Marquez M, Gonzalez A, Massieu L (2012) Calpain activation is involved in acute manganese neurotoxicity in the rat striatum in vivo. Exp Neurol 233:182–192
Rehbein M, Wege K, Buck F, Schweizer M, Richter D, Kindler S (2002) Molecular characterization of MARTA1, a protein interacting with the dendritic targeting element of MAP2 mRNAs. J Neurochem 82:1039–1046
Roels H, Meiers G, Delos M, Ortega I, Lauwerys R, Buchet JP, Lison D (1997) Influence of the route of administration and the chemical form (MnCl2, MnO2) on the absorption and cerebral distribution of manganese in rats. Arch Toxicol 71:223–230
Roth JA (2009) Are there common biochemical and molecular mechanisms controlling manganism and parkisonism. Neuromol Med 11:281–296
Ruggiero T, Trabucchi M, Ponassi M, Corte G, Chen CY, al-Haj L, Khabar KS, Briata P, Gherzi R (2007) Identification of a set of KSRP target transcripts upregulated by PI3K-AKT signaling. BMC Mol Biol 8:28
Seo YA, Li Y, Wessling-Resnick M (2013) Iron depletion increases manganese uptake and potentiates apoptosis through ER stress. Neurotoxicology 38:67–73
Shinotoh H, Snow BJ, Hewitt KA, Pate BD, Doudet D, Nugent R, Perl DP, Olanow W, Calne DB (1995) MRI and PET studies of manganese-intoxicated monkeys. Neurology 45:1199–1204
Shinotoh H, Snow BJ, Chu NS, Huang CC, Lu CS, Lee C, Takahashi H, Calne DB (1997) Presynaptic and postsynaptic striatal dopaminergic function in patients with manganese intoxication: a positron emission tomography study. Neurology 48:1053–1056
Snee M, Kidd GJ, Munro TP, Smith R (2002) RNA trafficking and stabilization elements associate with multiple brain proteins. J Cell Sci 115:4661–4669
Tamm C, Sabri F, Ceccatelli S (2008) Mitochondrial-mediated apoptosis in neural stem cells exposed to manganese. Toxicol Sci 101:310–320
Tkac I, Rao R, Georgieff MK, Gruetter R (2003) Developmental and regional changes in the neurochemical profile of the rat brain determined by in vivo 1H NMR spectroscopy. Magn Reson Med 50:24–32
Trabucchi M, Briata P, Garcia-Mayoral M, Haase AD, Filipowicz W, Ramos A, Gherzi R, Rosenfeld MG (2009) The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature 459:1010–1014
Uchida A, Oh-hashi K, Kiuchi K, Hirata Y (2012) Manganese regulates caspase-3 gene promoter activity by inducing Sp1 phosphorylation in PC12 cells. Toxicology 302:292–298
Xu B, Shan M, Wang F, Deng Y, Liu W, Feng S, Yang TY, Xu ZF (2013) Endoplasmic reticulum stress signaling involvement in manganese-induced nerve cell damage in organotypic brain slice cultures. Toxicol Lett 222:239–246
Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H (1986) Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol 70:273–278
Yoon H, Kim DS, Lee GH, Kim KW, Kim HR, Chae HJ (2011) Apoptosis induced by manganese on neuronal SK-N-MC cell line: endoplasmic reticulum (ER) stress and mitochondria dysfunction. Environ Health Toxicol 26:e2011017
Zhang QG, Wang R, Khan M, Mahesh V, Brann DW (2008) Role of Dickkopf-1, an antagonist of the Wnt/beta-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J Neurosci 28:8430–8441
Zhang L, Sang H, Liu Y, Li J (2013) Manganese activates caspase-9-dependent apoptosis in human bronchial epithelial cells. Hum Exp Toxicol 32:1155–1163
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81300720).
Conflict of Interest
The authors declare no conflicting interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Shangshi Shi and Jianya Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shi, S., Zhao, J., Yang, L. et al. KHSRP Participates in Manganese-Induced Neurotoxicity in Rat Striatum and PC12 Cells. J Mol Neurosci 55, 454–465 (2015). https://doi.org/10.1007/s12031-014-0367-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0367-7