Abstract
Persons with spinal cord injury (SCI) undergo immediate unloading of the skeleton and, as a result, have severe bone loss below the level of lesion associated with increased risk of long-bone fractures. The pattern of bone loss in individuals with SCI differs from other forms of secondary osteoporosis because the skeleton above the level of lesion remains unaffected, while marked bone loss occurs in the regions of neurological impairment. Striking demineralization of the trabecular epiphyses of the distal femur (supracondylar) and proximal tibia occurs, with the knee region being highly vulnerable to fracture because many accidents occur while sitting in a wheelchair, making the knee region the first point of contact to any applied force. To quantify bone mineral density (BMD) at the knee, dual energy x-ray absorptiometry (DXA) and/or computed tomography (CT) bone densitometry are routinely employed in the clinical and research settings. A detailed review of imaging methods to acquire and quantify BMD at the distal femur and proximal tibia has not been performed to date but, if available, would serve as a reference for clinicians and researchers. This article will discuss the risk of fracture at the knee in persons with SCI, imaging methods to acquire and quantify BMD at the distal femur and proximal tibia, and treatment options available for prophylaxis against or reversal of osteoporosis in individuals with SCI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
General considerations regarding bone loss in individuals with SCI and imaging methodologies
Histomorphometric studies of the sublesional skeleton early after spinal cord injury (SCI) reveal an increase in osteoblast and osteoclast activity that shifts fairly quickly to an increase in osteoclastic activity and a suppression of osteoblastic activity [1–3]. This uncoupling of osteoblast/osteoclast function is supported by the clinical findings of hypercalciuria and dramatic elevation of biochemical markers of bone resorption [4], leading to a rapid loss in bone mineral density (BMD) and deterioration of the trabecular lattice structure that is ultimately replaced by fatty marrow [5]. Contrary to that of normal skeleton remodeling, in which the quantity of bone resorbed is almost completely replaced by new bone formation, after SCI, the immediate unloading of the skeleton results in a pathophysiological scenario of the uncoupling of bone formation and resorption that rapidly results in severe bone loss of the sublesional skeleton. The most precipitous loss of bone occurs during the initial 12 to 24 months after acute SCI [6–8]. The loss of structure and strength in the lower extremity places a person with SCI at an increased risk of fracture. While it is appreciated that extreme demineralization occurs throughout the entire lower extremity, the distal femur (DF) epiphysis and proximal tibia (PT) epiphysis are the regions most vulnerable to fracture in persons with SCI; because a large fraction of accidents occur while sitting in a wheelchair, this makes the DF and PT region the first point of contact to any externally applied force. As a result of these anatomical considerations, the DF and PT are the most common sites for low-energy fracture in persons with SCI while performing activities of living [9]. In approximately 50 % of all cases, fractures result in contractures at the hip and knee, osteomyelitis, and concurrent pressure ulcers that may further serve to diminish mobility [10–12]. These outcomes adversely affect activities of daily living, interfere with the ability to maintain employment, and add significant medical costs to rehabilitative care [12, 13]. Several small cross-sectional and prospective cohort reports have quantified the loss of bone in persons with acute (<3 months from date of injury) [14] and chronic SCI (>1 year from date of injury) [15]. After acute SCI, an areal BMD (aBMD) loss of 27 % at 4 months and 32 % loss 14 months after initial assessment has been documented at the distal femur [16]. The reduction in BMD has been reported to approach a steady state for bone turnover approximately 3–8 years post-injury [17, 18]. Other investigations have observed a slower rate of bone loss that appears to continue into the chronic phase of injury [19, 20]. Compared to other well-recognized conditions of rapid bone loss, this incredibly high rate of bone loss after acute SCI is considerably higher than that observed in postmenopausal osteoporosis (3–5 % annually) [21], long-term bed rest (0.1 % per week) [22], and space flight (0.25 % per week) [23]. Dual energy X-ray absorptiometry (DXA), quantitative computed tomography (QCT), peripheral quantitative computed tomography (pQCT), and Magnetic resonance imaging (MRI) are commonly used imaging technologies to quantify BMD of the DF and PT, with the latter being used more frequently in the research setting [24, 25]. The rate of absolute BMD loss has been reported to be highly variable in persons with SCI when applying DXA or pQCT imaging methodologies [8, 26]. This observed variability is most likely the result of acquiring skeletal images with DXA and/or QCT/pQCT machines by different manufacturers and software applications or by acquiring similar, but inconsistent, parameters for the reported regions of interest (ROI) of the lower extremity that vary in the proportion of trabecular to cortical bone. A recent review article by Troy et al. [27] has recommended pQCT be considered as a first-line approach providing the primary outcomes that address skeletal changes in clinical trials in persons with SCI [27], a point that will be expanded upon in this review. An in-depth review of the two different imaging methodologies for acquiring BMD at the DF and PT in persons with SCI has yet to be reported. The objectives of this article are to review the considerations, experience, and use of DXA, QCT/pQCT, and MRI imaging methods to acquire BMD at regions representative of the DF and PT. To achieve this objective, a literature review was conducted in PubMed (MEDLINE), Cochrane, and CINAHL databases with the following bone loss-related keywords and Boolean operators: “Bone Mineral Density”[Mesh] OR “Bone Mineral Content” AND “DXA” OR “Dual Energy X-ray Absorptiometry” AND “Tomography Scanners, X-Ray Computed” [Mesh] OR “QCT” OR “Quantitative Computerized Tomography” OR “Peripheral Quantitative Computerized Tomography” OR “pQCT“ AND “Spinal Cord Injury” AND “Distal Femur” AND “Proximal Tibia” AND “Femur” AND “Tibia.” A secondary search of references from articles found in primary search and an electronic search of the Journal of Clinical Densitometry (all issues since the journal’s inception) on the International Society for Clinical Densitometry website (www.iscd.org). For the purposes of this review, the distal femur and proximal tibia are the regions constituting approximately 30 % of the distal region of the femur and 30 % of the proximal region of the tibia, with this 30 % area further stratified into the epiphyseal (0-10 %), metaphyseal (10-20 %), and diaphyseal (20-30 %) subregions (adapted from the study by Edwards et al. [28]; Fig. 1). Using these criteria, the authors incorporated only this information when summarizing the severity of bone loss that occurs at the DF and PT in persons with SCI (Tables 1 and 2), with all reports that addressed femoral and tibia shaft and distal tibia regions. To further limit this review, additional criteria used to exclude articles were as follows: DXA studies that measured the knee region using a custom ROI from a total body scan, studies performed in the pediatric SCI population, descriptive case reports and the baseline evaluations from pharmacological and mechanical interventions in persons with SCI. From this compilation of articles describing bone loss at the DF and PT, in cross-sectional and prospective investigations, a synthesis of the lower extremity bone loss that occurs in acute and chronic SCI as determined by DXA and QCT/pQCT is presented, along with a summary of fracture risk, as was determined by imaging methodologies. Finally, a brief review of conventional and emerging pharmacological and mechanical interventions to preserve bone mass at the DF and PT in persons with SCI has also been presented.
Illustration of the diaphysis (highlighted green), metaphysis (highlighted yellow), and epiphysis (highlighted blue) that comprises the distal area of the femur and proximal area of the tibia (adapted from previous work by Edwards et al. [28])
Lower extremity BMD with emphasis at the knee in individuals with SCI
DXA: areal BMD assessment
In cross-sectional investigations, DXA has been used as the primary imaging modality to acquire lower extremity BMD in persons with SCI, which is understandable because a predominance of studies was performed prior to the advent of QCT and pQCT technology. In one of the early cross-sectional studies of participants with SCI, Biering-Sorensen et al. [29] compared bone mineral content (BMC) of the PT in 26 SCI subjects 2 to 25 years after acute injury and observed that the PT was greater than 50 % lower than the values obtained in an able-bodied cohort. In agreement with these findings, Garland et al. [30] compared the combined BMD of the DF and PT in 28 individuals with chronic SCI (DOI 3–43 years) to 10 able-bodied controls; the mean BMD of the knee region was 50 % lower when compared to an age-matched able-bodied control group, with similar findings documented in a cross-sectional study of women with chronic SCI [31]. These cross-sectional findings support those of other investigators who compared lower extremity BMD values in those with SCI to that of healthy able-bodied controls [32] and a subgroup of persons with SCI with a history of fragility fracture [33].
Prospective studies using DXA have documented a loss in BMD over time in acute and chronic SCI populations (studies obtaining a baseline and at least one additional DXA scan). To evaluate loss of BMD shortly after SCI, Warden et al. [16] performed a prospective study in 15 SCI patients who had sublesional bone loss observed within 6 months of SCI and observed a rapid decrease in BMD of 5.3 % in the PT 6 weeks after the baseline measurement. In another study documenting the rapid loss of bone that occurs within the first 2 years after initial motor-complete SCI, Biering-Sorensen et al. [6] performed follow-up DXA scans on six men and two women with initial scans performed 9–167 days (median 43 days) after acute paralysis. Two years after the initial DXA evaluation, BMC at the PT was 40–50 % lower than it was at the baseline assessment. In a study documenting bone loss within the first 2 years after injury, Garland et al. [34] determined BMD of the DF and PT soon after injury (33.5 ± 10.8 days) with follow-up measurements at 523 ± 96.2 days post-injury in five patients with acute motor-complete SCI; results from the follow-up assessment revealed BMD at the DF and PT were reduced by 27 and 32 %, respectively. However, there has been only one longitudinal study using DXA imaging that has documented bone loss into the chronic phase of injury in men with SCI (n = 31) and a small cohort of women (n = 4) [35]. In this study of 31 men with chronic motor-complete SCI (DOI 14.6 ± 8.7 years; 13 with paraplegia and 14 with tetraplegia) and 4 women (DOI 19.8 ± 12.1 years; all with paraplegia), Garland and colleagues [35] demonstrated that not all persons with SCI had a continuous loss of bone into the chronic phase of SCI. Participants were stratified into participants who had increases and decreases in areal bone mineral density (aBMD) at the 5-year DXA follow-up measurement. In men, the mean aBMD at the DF decreased in slightly more than half of the participants and at the PT in two thirds of the participants, with an annual percent change in aBMD of −1.1 % at the DF and −1.5 % at the PT over a 5-year period. These findings demonstrate the overall loss of bone several years after acute immobilization. In women, the annual percent change in aBMD was −1.8 % at the DF with an increase at the PT of 1.0 %. These findings should be interpreted with a degree of caution considering the lack of studies reported in larger cohorts of men and women with SCI that incorporate advanced imaging techniques which would optimally be performed during the acute and then in the chronic phase of SCI. Thus, it is important for clinicians and investigators to understand that the bone loss which continues into the chronic phase of injury has not been thoroughly defined and that the time course to establish a relatively steady state for the sublesional skeleton in persons with SCI also needs to be further addressed. Because the majority of reports that documented bone loss in persons after SCI are of relatively small sample sizes, and participants had varying degree of motor function below the level of lesion, the rate and absolute loss in aBMD has varied considerably among studies to date. Furthermore, the possibility of obtaining less than optimum precision due to difficulty in proper positioning of the leg when scanning the knee due to increased spasticity, tone, and contractures makes it necessary to interpret the results with caution from relatively small changes that occur from clinical interventions. The use of DXA can be further confounded by the existence of heterotopic ossification at the knee region, spuriously elevating BMD results. In addition to the necessity of DXA precision assessment outlined by the ISCD guidelines [36], the International Osteoporosis Foundation (IOF) has published guidelines for treatment failure from changes in BMD at the individual level [37]. To account for the inter-participant variability in lower extremity bone loss and the differential effects of any treatment intervention, clinicians and researchers can compare the percent change in the aBMD of the DF and PT from a given intervention to the least significant change (LSC) (for the CI at 95 and 99 %) to determine true intervention-related change and actual efficacy the efficacy of an intervention. Despite the varying degrees of bone loss reported among these reports, it is essential for clinicians to understand the precipitous loss in the sublesional skeleton that results from the abrupt onset of paralysis, as well as the likely accelerated continued loss over a lifetime of immobilization, and to have an appreciation that the longevity of those with SCI approaces that of the able-bodied population. Thus, there exists the obvious need for safe and efficacious prescription of rehabilitative or pharmacological interventions once they become available to preserve or reverse osteoporosis in the SCI population. A comprehensive summary of descriptive studies that documented the bone loss at the DF and PT after SCI using DXA has been provided (Table 1).
DXA software for the knee region
Previous investigations using DXA technology to quantify aBMD at the knee ROI have used software applications that were nonspecific to the region and were adapted from forearm or lumbar spine software packages to measure BMD [38–40]. The adaptation and application of nonspecific software programs of the DF and PT were performed out of necessity because validated software programs to measure aBMD of the knee ROI had not been commercially offered by the DXA manufacturers. Using nonspecific software (e.g., forearm software), McPherson et al. [41] reported in a cross-sectional validation study in 12 persons with acute and 34 persons with chronic SCI that the root mean square coefficient of variation percent (RMS-CV%) for the aBMD by DXA was more precise in the acute SCI cohort than in the chronic SCI cohort at the DF epiphyses (1.7 vs. 3.1 %), DF metaphysis (1.4 vs. 4.7 %), and PT epiphysis (1.7 vs. 3.4 %), respectively; all custom ROIs by DXA were highly correlated to these same image sites measured by QCT for volumetric BMD (vBMD) (r = 0.93). The DXA regions created corresponded to a method previously developed using QCT, as described by Edwards et al. [28] (Fig. 1), and also corresponded to pQCT-derived 4 % limb length image regions that define the DF and the 96 % limb length image region that defines the PT, as measured from distal to proximal. The intra- and inter-rater reliability estimates (two raters) were also determined in the groups with acute and chronic SCI, with high intra-class correlation coefficients (ICCs; 0.97–0.98). McPherson et al. concluded that the improved precision in the acute SCI cohort may be attributed to a higher mean absolute BMD than that in the chronic cohort, rather than a systematic difference in precision between the two groups.
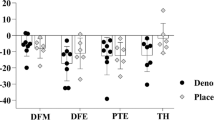
An orthopedic knee software program was developed to monitor BMD in patients after knee arthroplasty, and this software application has been recently approved by the Food and Drug Administration for commercial use; this software application is currently being used in rehabilitation [42] and pharmacological [43] clinical trials in persons with SCI. Bakkum et al. demonstrated excellent inter- and intra-rater reliability (ICCs between 0.97 and 0.98) using this software in an able-bodied cohort [44]. The precision of knee-specific software in 20 able-bodied adult patients who received unilateral knee prosthesis was provided by Gilchrist et al. [45]; DXA scans were performed on both knees and aBMD from seven custom ROIs obtained. The CV% at the knee with the implant ranged between 0.55 and 4.04 %, and no significant differences in precision were found in comparisons between the implanted and non-implanted knee [45]. In a cohort of subjects with chronic SCI, Morse et al. [46] tested the precision (e.g., RMS-CV%) of the orthopedic knee software using a custom ROI and found that the precision of the DF (3.01 %) had better reproducibility than that of the PT (5.91 %) (Fig. 2a). A precision error at the DF of 3.01 % and PT of 5.91 % would mean that a change in BMD of less than 6 % from a clinical intervention could not be detected. Furthermore, this study performed repeat scans on 20 SCI participants and not the 30 required by the ISCD guidelines to calculate LSC [36]. However, given the same precision error, if an additional 10 scans were completed, the LSC would have been 8.4 and 16.4 % [3.01 and 5.91 % (RMS-CV%) × 2.77 (multiplier at 95 % CI) = LSC], a margin of error that would not capture even a fairly robust effect of an efficacious clinical intervention. Because only a few studies in persons with SCI have reported the precision error (RMS-CV%) from repeat scans using different DXA acquisition and analysis protocols [41, 46–48], none to date have completed the 30 scans necessary to have the statistical power to report the LSC at a 95 % CI [37]. As previously stated, the decreased precision in persons with SCI can be partly attributed to increased spasticity and contractures that can limit the internal rotation needed to standardize the amount of overlap between the tibia and fibula and, ultimately, the location of the ROI box. The placement of the DF ROI can also be confounded by movement of the patella into the ROI area that was used at the baseline assessment, forcing a shift in the placement of the ROI box more distal along the femoral distal metaphysis when analyzing the follow-up DXA scan of the knee region.
Standard ROIs, regrettably, have not been uniformly developed and adopted by clinicians and investigators, which has created a variability and uncertainty in the effort to compare BMD findings at the knee among reports. Using non-knee-specific software, Shields et al. [32] developed a widely used method to isolate the DF and PT regions of interest, an approach which has been demonstrated to be highly reliable between multiple raters with ICC values for the DF of 0.98 and for the PT of 0.89 [32] (Fig. 2b); this methodology has been applied in subsequent studies of the knee in persons with SCI [47, 49]. However, this method obtains more of the metaphysis of the DF and PT, which does not correspond with the femoral 4 % (DF) and tibia 96 % (PT) regions captured when performing pQCT which have ROIs in the epiphysis, an important point to take into consideration if pQCT-derived images for regions of the DF and PT will be considered the reference in future validation studies. Slightly different custom ROIs that capture more of the epiphyseal region have been reported, and they have also proven to be highly reliable [41]. At the present time, there is no standardized method to capture the DXA-derived ROIs for the DF and PT and no study to date has prospectively compared the methods commonly reported in the literature. From the current body of literature, it would be logical for investigators to adopt the analysis method utilized by McPherson et al. as the best method to capture the DF and PT in prospective clinical trials [41] (Fig. 2c) because this method has been demonstrated to be both precise and accurate as determined by the strong relationship to measures of “true” vBMD, determined by CT technology. As previously mentioned, this method obtains vBMD of the distal femoral epiphysis, distal femoral metaphysis, and the proximal tibia epiphysis; epiphyseal ROIs that also correspond to commonly performed pQCT-derived images of the 4 % limb length reported as the DF and the 96 % limb length reported as the PT, as measured from distal to proximal. Future studies that perform repeat DXA scans of the knee using “on and off the table” and “different test days” methodologies, as well as validation studies comparing the different methodologies to capture QCT and pQCT, are necessary to understand the optimal method that should be utilized to perform DXA scans of the knee in persons with SCI.
Despite its obvious clinical feasibility, the use of DXA to evaluate BMD has appreciated drawbacks when applied to identify risk of fracture in the able-bodied population due to the confounding effects of bone size on aBMD [50], differences between manufacturer-derived algorithms to calculate aBMD [51], and DXA intra/inter-unit problems with accuracy and linearity [52]. Standardized scores for DXA (e.g., Z- and T-scores) at the knee have not yet been established. Thus, identification of a low bone mass that defines an increased risk for fracture at the knee is not currently available. As such, assessment of aBMD of the DF and PT alone without clinical risk factors for fracture is of limited use to guide exercise prescription and to reduce the possibility of fracture. At this time, the available evidence supports that the best use of knee software in clinical investigations is to monitor potential changes from mechanical and/or pharmacological interventions. Along with SCI-specific clinical risk factors [53], this information can guide clinical treatment options and/or research interventions associated with the application of substantial mechanical forces during supervised therapeutic sessions or exercise training routines. Because of the scarcity of literature that defines a given DXA-derived aBMD value, as well as the absence of a well-validated fracture threshold at the DF and PT, clinicians should consider collecting, if feasible, additional trabecular microarchitecture and bone geometry measures from CT or magnetic resonance imaging (MRI) to better identify persons who are at heightened risk of fragility fracture. Future research that establishes normative data generated from applying knee software from different manufacturers and ROIs to capture the DF and PT are needed to accurately and reproducibly identify the diagnostic threshold to predict increased risk of fracture at the DF and PT.
Advanced bone imaging: CT and MRI
Computed tomography (CT) is a photon absorptiometric technique similar to DXA with the unique ability to provide vBMD of a bone region. Quantitative CT (QCT) is a 3-D imaging technique that can acquire trabecular and cortical vBMD as well as geometry parameters, such as the polar stress strain index (SSIpol = surrogate measure of bone strength from the CSA) and the polar moment of inertia (IP = bone’s ability to resist torsional loads) but cannot obtain bone microarchitecture. The use of this methodology has direct relevance to the SCI population because there is evidence supporting severe trabecular vBMD loss at the DF and PT epiphysis in the period shortly after injury, with a corresponding thinning of the endocortical envelope at the diaphysis [18, 54]. The change in the endocortical envelope temporally occurred with no significant decrease in cortical vBMD. Thus, it would appear that loss of vBMD of the diaphysial endocortical envelope and cortical regions occurs at vastly different rates after acute SCI; however, a recent report suggests the deterioration of these two boney regions occurs at a similar rate and magnitude [55]. Thus, in contrast to DXA, where the specified DF and PT regions represent surrogate measures of trabecular bone, pQCT obtains actual trabecular and cortical vBMD of the epiphysis, metaphysis, and diaphysis at the DF and PT in persons with SCI (Fig. 1) [28]. Currently, multidetector CT (MDCT) devices produced by a number of manufacturers are readily available in most diagnostic imaging centers [56]. The advantage of this clinical technique is that central (spine and proximal femur) and peripheral (distal femur, proximal and distal tibia) skeletal sites and high-resolution microarchitecture parameters can be acquired. MDCT has shown to have moderate to strong correlations in vitro when compared to that of micro-CT (μCT) for vBMD and microarchitecture (app.BV.TV: r = 0.979, p < 0.005) [57], and it has recently been validated against DXA for all central aBMD sites as an accurate and reliable screening tool to diagnose osteoporosis in older men and women [58]. However, studies using MDCT are limited due to an effective radiation dose of approximately 3 mSv (approximately 1.5 years of background radiation). This is significantly higher than the effective radiation dose from DXA and pQCT, which ranges from 0.01 to 0.05 mSv (normal daily background radiation exposure). Bone imaging by pQCT also has the benefit of limiting direct radiation exposure to the extremities and does not expose radiosensitive organs to radiation [56].
Over the last decade, pQCT has been regularly used as a dedicated extremity imaging modality in the clinical research environment. This methodology is commonly used in clinical investigations to quantify bone changes in persons with SCI, with a recent review from an experienced research team detailing the challenges inherent when performing pQCT imaging in persons with SCI [59]. The two commercially available devices come with either a fixed gantry for the distal tibia (Xtreme CT; Scanco Medical AG, Brüttisellen, Switzerland) or with a sliding gantry that can obtain slices along the femur and tibia metaphysis and epiphysis, as well as at sites along the tibia diaphysis (XCT 2000/3000 scanner; Orthometrix/Stratec, White Plains, NY) [60]. The pQCT method has a relatively low resolution which is insufficient to obtain the trabecular microarchitecture, but it can acquire vBMD and geometry parameters of the cortical and trabecular regions. The Stratec pQCT scanner can obtain images at the 96 % tibia region (tibia epiphysis = PT) and the 4 % femoral region (femoral epiphyses = DF), with additional slices at the 38 % tibia region obtained for cortical bone density and thickness and the 66 % region to obtained muscle and fat volumes. A standardized protocol has been employed for both pQCT devices [59, 61, 62], with the starting point determined by a single scout projection required to assess the tomographic region of the talocrural joint to be able to place a reference line at the distal tibia plateau when using a sliding gantry (XCT 2000/3000 scanner). Using this reference line at the distal end of the tibia, the standard measurement site using pQCT is the distal tibia epiphysis measured at 4 % of the tibia length, moving distal to proximal. Using the fixed gantry scanner, high-resolution peripheral quantitative computed tomography (HR-pQCT) images can quantify the trabecular microarchitecture at the 4 % distal tibia region but not at the DF and PT epiphyseal sites [56]. HR-pQCT can be used to calculate trabecular microarchitecture parameters such as the bone volume/total volume ratio (BV/TV = ratio of the segmented bone volume to the total volume of the region of interest), trabecular number (Tb.N = measure of the average number of trabeculae per unit length), trabecular spacing (Tb.Sp = mean distance between trabeculae, assessed using direct 3-D methods), and trabecular thickness (Tb.Th = mean thickness of trabeculae, assessed using direct 3-D methods) [63, 64]. The 4 % distal tibia region has been widely studied in both the AB and SCI populations but may not have equivalent clinical utility in persons with SCI as that of the 96 % tibia region and the 4 % femoral region. To obtain an image of the proximal tibial epiphysis using this same reference line as the starting point, the scanner obtains an image at 96 % of the tibia length, moving distal to proximal. Similar to identification of the tibia region, the femoral epiphyseal region is palpated and a single scout projection is used to identify and place a reference line at the most distal point at the end of the lateral femoral condyle. Using this reference line at the distal femur, the scanner obtains an image at 4 % of the femur length, moving distal to proximal [56, 58, 65, 66].
While it is appreciated that bone loss occurs throughout the entire lower extremity, the trabecular area above the knee (DF) and below the knee (PT) is a ROI commonly studied because these areas have the highest prevalence of fracture [67], because wheelchair-related accidents are the most common events that make the DF and PT vulnerable to impact and fracture in person with SCI [13, 28, 33, 68]. Although DXA is the most affordable and accessible method to measure BMD at the DF and PT, pQCT is regarded as the more informative and sensitive method. In recent years, the general availability of pQCT has increased, permitting investigators to differentiate between trabecular and cortical bone compartments and changes in bone microarchitecture. As a result, there have been several cross-sectional reports comparing vBMD in persons with SCI to an age- and gender-matched able-bodied cohort. In 12 chronic SCI and 21 able-bodied participants, Giangregorio et al. [69] found total vBMD at the DF and PT was, as expected, significantly lower in the SCI group than that of able-bodied controls. In a cross-sectional study to assess bone loss in separate compartments of trabecular and cortical bone, Eser et al. [18] used pQCT to measure the epiphyses of the DF and PT in 89 men with motor-complete SCI (24 tetraplegia and 65 paraplegia with a duration of injury between 2 months and 50 years); the loss of bone mass in the epiphyses was ∼50 % in the DF and ∼60 % in the PT. In a cross-sectional study, Rittweger and colleagues [54] obtained serial slices of the tibia along its length in nine chronic SCI and nine able-bodied men and found those with SCI had 47 % lower BMC at the 95 % slice (PT). In addition to the cross-sectional reports described above, a few prospective studies have employed pQCT to capture the intra-patient loss of bone after SCI. A longitudinal study confirmed previous reports that a new steady-state for bone is reached in the paralyzed limbs several years after acute SCI. Frotzler et al. [17] used pQCT to measure vBMD at the DF at baseline and then at 15 and 30 months after the initial scan in 39 subjects with motor-complete acute and chronic SCI (duration of injury between 0.9 and 34 years) [18]. The authors observed a new steady state 3 to 8 years after SCI, with the onset of the steady-state condition dependent on the bone region and the specific pQCT densitometric variable; the femur reached steady-state more quickly than the tibia and the epiphyses more quickly than the diaphyses. In a report by Edwards et al. [28], QCT was used to quantify changes in vBMD and bone geometry and torsional stiffness at the trabecular and cortical region at the epiphyseal and metaphyseal region of the DF and PT in 13 SCI subjects soon after injury (mean measurement, 3.8 months after acute injury). The authors observed dramatic rates of monthly decline for BMC (−3 % to −4 %) and vBMD (−3 % to −5 %) at the epiphyseal trabecular region, as well as cortical diaphyseal BMC (−4 % to −5 %), with significantly smaller reductions in cortical vBMD of (−0.6 % to −0.8 %). The authors suggested that the loss of cortical bone was due to the thinning of the endocortical envelope. From these measurements, computation of bone strength and stiffness was performed and a two-fold greater reduction in these parameters was found when compared to reductions in BMD. These observations highlight the need for efficacious pharmacological or mechanical interventions shortly after injury to attenuate the skeletal losses in an effort to reduce the risk of fragility fracture [70]. Coupaud et al. [55] performed serial pQCT scans at the 96 % tibial region (PT) and 4 % femoral region (DF) at 4, 8, and 12 months after the initial scan on 26 acute subjects with SCI and noted a 20 % decrease in tibial and a 15 % decrease in femoral trabecular vBMD, with similar changes occurring at the tibial and femoral diaphysis cortical regions. This work suggested that absolute bone loss in the cortical diaphysis, where the predominant bone mass is located, occurs at approximately the same rate as trabecular bone in the epiphysis, findings that contradict previous reports that cortical vBMD is lost at a slower rate during this period. With the advent of clinical trials investigating advanced rehabilitation programs and new pharmacological treatments for bone loss after SCI, pQCT has become recognized as a method to monitor changes in trabecular and cortical vBMD compartments (Table 2) which DXA cannot capture.
MRI is an evolving high-resolution imaging methodology that is more readily available in the clinical environment than HR-pQCT. The use of MRI in the clinical setting has several benefits: it does not utilize ionizing radiation while maintaining excellent soft tissue contrast and yields geometry measures that are highly correlated with bone variables from μCT [71]. Contrary to HR-pQCT that can only obtain high-resolution measurements at the ultradistal tibia, MRI can acquire high-resolution images of cortical and trabecular bone and can acquire variables such as BV/TV, Tb.Th, Tb.Sp, and Tb.N [64] at the distal femur and proximal tibia. Improvements in MRI acquisition methods and image post-processing have made quantitative MRI a viable clinical option to differentiate small changes in trabecular microarchitecture after interventions to treat osteoporosis in able-bodied postmenopausal women [72, 73]. A small number of cross-sectional investigations have been performed using MRI to quantify changes in trabecular microarchitecture at the DF and PT in persons with SCI. In a study investigating the deterioration of bone microarchitecture by MRI at the DF and PT in 10 SCI men with chronic motor-complete SCI and 8 age-matched AB control participants, Modlesky et al. [20] found the DF in the SCI group had a 27 % lower apparent (app) BV/TV, 21 % lower app.Tb.N, and 44 % higher app.Tb.Sp, with an 8 % thinner app.Tb.Th. At the proximal tibia, the group with SCI had 20 % lower app.BV/TV and app.Tb.N, 33 % higher app.Tb.Sp, with no difference in app.Tb.Th between the groups. Assessment of aBMD of the PT by DXA revealed a significant positive correlation with app.BV/TV (r = 0.62) and app.Tb.N (r = 0.78) and an inverse correlation with app.Tb.Sp in both the SCI and AB control groups combined (r = 0.78). In another study by Slade et al. [74], the effect of menopause and immobilization from SCI on trabecular bone at the DF and PT was compared in pre- and postmenopausal ambulatory women, SCI younger (<30 years old) and SCI older (>35 years old) premenopausal women, and SCI postmenopausal women. Women with SCI had 30.9 % lower app.BV/TV, 61.7 % higher app.Tb.Sp, and app.Tb.Th that was 6.8 % thinner at the DF compared to the ambulatory group with no significant differences at the DF in any microarchitecture variable between the three groups of SCI women. At the PT, the SCI group had 23 % less app.BV/TV and 5.8 % lower app.TbTh compared to the ambulatory groups. Furthermore, an interaction effect was observed between app.Tb.Sp and ambulatory status with significantly greater spacing observed in the SCI groups compared to the ambulatory groups (Table 2). The degree of structural deterioration in these studies is similar to that reported when using pQCT. However, high-resolution microarchitecture parameters using pQCT are only possible at the ultradistal tibia; as such, direct comparison between pQCT and MRI of the DF and PT is not possible at this time. Furthermore, contrary to studies using pQCT, MRI cannot assess vBMD at the DF and PT, an essential component of fracture prediction and an essential variable to understand the effectiveness of any intervention. In the research setting, pQCT, HR-pQCT, and MRI imaging methodologies can be essential tools to assess changes in cortical and trabecular vBMD, bone geometry, and microarchitecture as a means to understand and test the effect of loading from advanced rehabilitation strategies and pharmacological interventions. In addition to serial measurements before and after intervention, additional descriptive studies are required to understand the change in the endocortical envelope during the acute stage of SCI with progression into the chronic stage of SCI, where measures of cortical porosity and thickness are essential outcome measurements [55]. This technology may provide researchers greater insight with regard to the efficacy of clinical interventions not possible using DXA alone with the anticipated increase in the availability of these measurements in the clinical environment.
Low BMD and fragility fracture in SCI
In persons with SCI, the severity of demineralization that occurs during the first 2 years following acute injury makes absolute BMD the primary risk factor for the prediction of future fracture [75], with other non-modifiable clinical risk factors to improve the estimate of fracture risk [30, 76]. In a review of proposed paradigms to diagnose and treat sublesional osteoporosis, Craven et al. [53] summarized the risk factors associated with fragility fracture based on the available literature as follows: SCI occurred <16 years of age [77], paraplegia [78], excessive alcohol consumption after SCI (>5 servings per day) [9], completeness of injury (American Spinal Injury Association Impairment Scale (AIS) A and B) [11], family history of fragility fracture [76], female [30], previous fragility fracture [30], low BMI (<19 kg/m2) [79], and duration of injury ≥10 years [30]. While it is true that larger prospective cohort studies are needed to identify the extent to which these risk factors are independent of aBMD, physicians and therapists should utilize these SCI-specific clinical risk factors along with aBMD of the DF and PT to guide treatment throughout the chronic phase of injury. Contrary to the occurrence of fractures in postmenopausal osteoporosis, a condition in which fractures predominantly occur at the femoral neck, hip, lumbar spine, and distal ulnar/radius, in persons with chronic SCI, the epiphyses of the DF and PT are the regions that are most vulnerable to fracture [10, 11, 80]. Fragility fractures tend to occur after minor trauma, such as bending, transfers, and physical therapy exercises [81]. These fractures typically occur as a result of torsional stress on the bones of the lower extremity when transferring or when compressive forces occur from a low velocity fall with the load applied to the knee region. The studies documenting fracture in SCI are limited to small cross-sectional reports, with a few prospective cohort studies that have evaluated persons with SCI in close proximity to the time of fracture. As first described by Comarr et al. [82] in a cohort of 1363 SCI participants, fragility fractures were observed in 119 participants, of which 97 (82 %) occurred at the knee. The incidence of fracture increases with time after SCI, with a mean yearly fracture incidence of 1 % during the first year after SCI, with the incidence increasing to 4.6 % per year at 20–29 years after acute SCI [83]. More recently, in a retrospective study by Frotzler et al. [67], long-bone extremity fractures were assessed in 107 primarily motor-complete persons with SCI. Patients’ medical charts revealed a total of 156 long-bone fractures exclusive to the lower extremity with the highest prevalence in the femur (60.9 %) compared to that of the tibia and fibula (39.1 %). The authors found a significant difference in fracture location between the upper and lower femur with a higher prevalence of fracture occurring distally (44.7 %). At the tibia, the greatest prevalence of fracture was reported proximally (41.9 %), compared to the distal (38.7 %), diaphyseal (14.5 %), and malleolar (4.8 %) regions. In a recent retrospective cohort study in which the risk for fracture was determined, Gifre and colleagues [81] reviewed the medical records of 63 patients with traumatic SCI at the time of injury and at a follow-up visit 10 years following injury; a total of 18 fractures were found in 10 patients (25 %), a fracture rate of 2.9 fractures per 100 patient-years, and 80 % of these fractures were in persons with SCI with motor-complete injuries. In another prospective cohort study, Morse et al. [9] addressed if sociodemographic and health-related factors predict hospitalization due to low impact fracture in 328 veterans with SCI; the most common fracture that required hospitalization was a tibia/fibula fracture (47.5 %), followed by the distal femoral metaphysis (20 %), and then the proximal femur (15 %). A fall from a wheelchair was identified as the most common cause of fracture, followed by transfers, and striking the lower extremity during wheelchair propulsion [83]. In a recent retrospective cohort investigation [13], a medical record review was conducted in 7590 male veterans with chronic traumatic SCI to identify lower extremity fragility fracture. The authors found 155 fractures in 140 persons with SCI with the majority of fractures occurring at the femur (n = 52; 33 %) and tibia/fibula (n = 83; 54 %), with the primary cause related to activities performed from the wheelchair. A high prevalence of fractures at the knee has been reported by other investigators in cross-sectional and retrospective investigations, with the majority of fractures occurring at the supracondylar femur, femoral shaft, and tibia [1, 9–11, 33, 84] (Table 3).
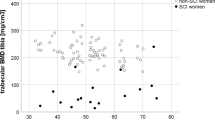
If fracture thresholds were defined and validated, they would be of great clinical relevance to guide therapeutic approaches. In a cohort of 70 SCI individuals, LaLa et al. [33] completed a retrospective analysis of baseline data from a 2-year prospective longitudinal study. The results of DXA and pQCT imaging (19 with history of fracture) found that participants who had a prior history of fragility fracture had significantly lower aBMD by DXA compared to individuals without a history of fracture; furthermore, there was an increasing risk of fracture for every unit of standard deviation decrease in aBMD by DXA or in vBMD by pQCT. In a study of 10 able-bodied controls, 18 SCI persons with SCI with recent LE fracture, and 10 randomly selected individuals with motor-complete SCI, Garland and colleagues [85] found the net mean aBMD at the knee (mean of the DF and PT) was 0.592 g/cm2 in participants with a recent history of LE fracture; the authors concluded the breakpoint for fracture at the knee was 0.6 g/cm2. In another observational study by this group [80], aBMD of the knee was assessed in 168 SCI participants with the cohort stratified into those with and without a history of fracture post-SCI (27 with LE fractures post-injury and 141 with no LE fracture). In those participants with no history of fracture, the mean aBMD was 0.6287 g/cm2 (95 % CI 0.5988 to 0.6586 g/cm2) and in those participants with a history of fracture the aBMD was 0.5279 g/cm2 (95 % CI 0.4802 to 0.5755 g/cm2), with the authors concluding that the findings from this larger cohort support the earlier finding that the breakpoint for fracture at the knee is 0.6 g/cm2. Using the methods to define the cutoff value for fracture in the AB population [86], data from this same cohort was reanalyzed in a later report; using this approach, the authors defined the fracture threshold as 0.78 g/cm2 and the fracture breakpoint as 0.49/cm2, which demonstrated... (the “2’s” should be superscripted, as they appear in the text) the variability in defining a fracture threshold solely dependent upon the method used to calculate the cutoff value [30]. Utilizing similar methodology, Eser et al. [87] found that determination of trabecular BMD by pQCT of the femur and tibia distal epiphyses was effective in identifying subjects with a history of fracture and that subjects with a history of fracture had trabecular BMD values that were <114 and <72 mg/cm3 for the DF and PT, respectively. The aforementioned studies by Garland et al. and Eser et al. have provided guidance on fracture threshold by DXA and pQCT in the SCI population but, to date, no longitudinal studies in persons with SCI have been performed that have acquired BMD of the DF and PT at or near the time of fracture occurrence. Thus, a fracture threshold has not been accepted for the knee by any imaging modality. It must also be recognized that the primary reason why a BMD fracture threshold cutoff value has not been established in persons with SCI is because BMD alone is a less than satisfactory predictor of fracture risk, accounting for approximately 50 % of the variance in strength, with measures of mechanical structure (bone geometry and microarchitecture) accounting for the remaining variance in this predictive model [60, 72]. Future prospective cohort studies in persons with SCI will be essential to determine a validated fracture threshold that will enable clinicians to diagnose osteoporosis at the knee and to safely prescribe conventional physical training and emerging technologies for rehabilitative care.
Treatment of lower extremity bone loss: rehabilitation and pharmacological interventions
The clinical aspiration is for rehabilitation professionals to have the ability to identify persons with SCI who have low BMD of the DF and PT and an increased risk of fracture from rehabilitation and/or exercise training modalities that stress tissues in the lower extremities, which currently includes robotic approaches for ambulation. The mechanostat theory, a derivative of Wolff’s law, states that there are strains within bone that are kept within certain limits by adding and removing bone tissue, resulting in improved bone strength according to the particular forces that are imposed [88]. However, if force is applied below a certain set point (i.e., due to immobilization secondary to paralysis), bone tissue will ultimately be lost [89]. Over the last two decades, several rehabilitation initiatives have implemented various types of ambulation and electrical stimulation (ES) and functional electrical stimulation (FES) programs in an attempt to improve bone mass and strength in persons with SCI [90–92]. In persons with SCI who have had severe bone loss at the DF and PT, there is an increased risk of fracture by participation in rehabilitation interventions, albeit this level of risk has not been described in the literature for specific interventions.
Locomotor training using body weight-supported treadmill training to activate the neuromuscular system below the level of lesion is a clinically available rehabilitation program. The primary purpose of the program is to optimize the recovery of function in persons with SCI with clinically motor-incomplete neurological injuries [93], with the potential endpoint of preservation of BMD in the lower extremities. While these programs have proven effective in strengthening the neural circuitry responsible for locomotion in those with motor-incomplete SCI, there is emerging evidence that this therapy alone is largely ineffective in attenuating the rapid bone loss at the DF and PT [94, 95]. There is considerable evidence that the use of cyclical muscle contraction induced by FES is at least partially effective in preserving BMD at the DF and PT soon after SCI, with FES cycle ergometry being the primary modality utilized to date. Preservation of site-specific bone tissue from FES cycling has also been documented in cohorts of individuals with chronic SCI. Using DXA as the imaging modality to assess aBMD of the DF and PT, Bloomfield and colleagues [96] conducted a 9-month FES cycling program in nine individuals with motor-complete SCI. The authors found that in a subset of subjects who achieved high power outputs (≥18 W) after 6 months of FES training, aBMD of the DF and PT was significantly increased [96]. In a longitudinal intervention using high volume FES cycling, Frotzler et al. [97] examined the change in vBMD at the DF and PT in 12 motor-complete individuals with chronic SCI who completed 180 min/week of FES cycling for 12 months. The final pQCT measurement revealed a significant increase in cross-sectional area (1.2 %), trabecular vBMD (14.4 %), and total vBMD (7.0 %) at the DF epiphyses, but no significant change was observed at the proximal tibia.
The evidence previously presented which supports the use of FES in those with SCI should be balanced with a brief review of studies that have found FES exercise to have limited therapeutic value in the preservation of bone at the DF and PT. Of clinical relevance, preservation of bone after acute SCI [98, 99], or any reversal of bone after chronic SCI [100, 101], is rapidly lost once FES exercise is discontinued. Furthermore, a degree of controversy persists with regard to the ability of FES cycle training (30 min sessions performed three times a week) to attenuate vBMD loss at the tibial diaphysis in persons with SCI after acute traumatic motor-complete SCI [99]. In a study by Lai et al. [98], aBMD of the DF was assessed in 24 acutely injured (26-52 days post-injury) patients with motor-complete SCI to that of 12 participants receiving FES cycling and 12 age- and gender-matched participants in the control group; the rate of bone loss at the DF in the FES cycling group was significantly less than that of the control group, with the bone-sparing effect of FES completely lost once FES cycling was discontinued. These findings were supported in a study by Chen et al. [102] in which 15 participants with chronic SCI performed FES cycling 30 min a day, five times a week, for 6 months. At baseline, the DF and PT aBMD were 39 and 47 % lower compared to 15 age-matched able-bodied control participants. After 6 months of FES cycling, aBMD of the DF and PT increased by 11 and 13 %, respectively, with the aBMD of both regions decreasing significantly 6 months after FES was discontinued. Furthermore, in a study by Mohr et al. [103], 12 months of FES cycling on average 2.3 times a week increased aBMD of the PT by 10 %. However, a reduced training exercise prescription of 0.9 times per week was insufficient to maintain these positive changes in aBMD. In a larger cohort of acutely injured patients (4–5 weeks after SCI), 19 patients completed FES cycling three times a week for 6 months and 19 patients served as controls; a small, nonsignificant increase in tibia shaft vBMD in the FES group was observed compared to the control group, with the authors concluding that FES cycling immediately after SCI did not attenuate bone loss [99]. A possible explanation for these contradictory findings previous studies may have been that the mid-shaft of the tibia, which was the site imaged for changes in vBMD, is composed primarily of cortical bone, whereas in prior studies that showed FES to be effective, the DF and PT were the sites imaged, which are sites rich in trabecular bone. The negative findings should serve as an example that a given bone imaging modality, specific outcome variables [e.g., aBMD or vBMD, microarchitecture (Tb.N, Tb.Sp, Tb.Th), and/or geometry measures (CSA, PI, SSIpol)] should be sufficiently sensitive to detect a change in the primary objective of the intervention being tested.
In recent years, the effect of ES on sublesional bone in persons with SCI has been studied with increased load delivered to the bone, against resistance (e.g., resistance training), performed in the standing position, or a combination of these approaches applied to improve BMD. In a pilot study of four chronically injured persons with SCI, Shields et al. [104] studied the effect of 6 to 11 months of an FES training intervention on the soleus muscle using plantar flexion ES to deliver a mean estimated load to the tibia of 110 % of body weight to one leg while using the other leg as a control limb. The authors found that aBMD of the PT in three of the participants increased by 19 and 31 % at months 3 and 4, respectively, with no additional increases noted for two participants who continued 11 months into the training program. In addition to this early pilot work, in a study in 28 individuals with motor-complete SCI with varying durations of injury (0.2 to 24.3 years since date of injury), Dudley-Javoroski et al. [100] compared the effect of bone compressive loads using 0 % body weight (no standing), 40 % body weight (passive standing), and 150 % body weight (ES of the quadriceps delivered compressive loads) on the change in vBMD at the DF and PT; the slope of BMD loss in the high-dose group was three-times lower at the DF and 25.1 % lower at the PT than that of the low-dose or control groups. In addition to the DF and PT sites, generating 150 % of body weight using standing ES of the quadriceps increased vBMD at the 12 % femoral length region [105]. In persons with chronic SCI (mean duration of injury ∼10 years), Belanger et al. found a ∼30 % restoration in BMD of the DF and PT with stimulation of the quadriceps against an isokinetic load (1 h/day, 5 days a week for 24 weeks) compared to stimulation against gravity alone [101], with a report in preparation suggesting that ES of the upper and lower leg muscles combined with standing has a greater effect on aBMD of the DF and PT than did standing or ES alone [106]. This preliminary evidence supports the hypothesis that a combination of loading and intense cyclic muscle contractions against resistance in the upright position may be sufficient to elicit the minimum essential strain required to stimulate positive bone tissue adaptations at the DF and PT in persons with SCI, suggesting the value of the application of this approach in the clinic to ameliorate bone loss. Additional interventions have examined the effect of regular standing in a supportive frame, pulsed ultrasound at specific bone regions, and low-magnitude whole body vibration therapies on the preservation of BMD in the lower extremities [107–109], with none of these approaches demonstrating efficacy in the treatment of immobilization osteoporosis.
Bisphosphonates belong to a class of anti-resorptive compounds that are widely used in the treatment of postmenopausal, glucocorticoid-induced, and senile osteoporosis [110]. Investigators who have reported the administration of bisphosphonate preparations have had varying degrees of success in preventing sublesional bone loss in the SCI population [111–114]. The main confounding variable in these studies has been that persons with SCI had varying degrees of completeness of motor lesion and that the differences in weight-bearing activities/ambulation were not quantified over the course of the study. In a small randomized study investigating the effect of cyclical etidronate on preservation of bone within 6 weeks of acute SCI, Pearson et al. [112] found that persons with SCI who became ambulatory and received etidronate treatment (n = 2) had preservation of bone density compared to ambulatory SCI participants who did not receive drug (n = 3) and non-ambulatory SCI participants who received etidronate (n = 3), which supports the need for further work to evaluate the efficacy of bisphosphonates or other anti-resorptive agents to prevent bone loss in a larger sample of individuals who are capable of weight-bearing activities and/or ambulation after acute SCI. In a double-blinded, randomized, placebo-controlled study by Bauman et al., despite repeated administration of pamidronate (60 mg intravenously administered at baseline, 1, 3, 6, 9, and 12 months) after acute SCI, BMD was not significantly different at 1 year from the placebo group at the hip and knee [115]. The administration of zoledronic acid (ZA) shortly after SCI has been demonstrated to have a bone-sparing effect at the spine and hip regions in reports by Bubbear et al. and Shapiro et al. [116, 117], but in these reports, BMD at the DF and PT was not evaluated. Bubbear and colleagues [116] administered ZA in an open label study to 14 patients with acute SCI, 7 treated with ZA and 7 receiving placebo; these investigators noted significant benefit to BMD after 12 months at the lumbar spine (∼3 %), total hip (∼12 %), and greater trochanter (∼13 %), but no significant benefit was observed at the femoral neck. In 17 persons with acute SCI, Shapiro et al. [117] administered ZA and observed a benefit at 6 months post-therapy at the hip, a transient positive result which was almost totally lost by 12 months for BMD, cross-sectional area, and measures of predicted bone strength (i.e., section modulus and buckling ratio) by DXA. In a more recent report by Bauman and colleagues [43], ZA was administered in an open label study to 13 patients with SCI, 6 treated with ZA and 7 receiving no treatment. Compared to the treatment group, the control group lost a significantly greater percentage of BMD at the total hip at month 6 (−3.2 vs. −13.9 %) and at month 12 (−7.5 vs. −20.1 %). However, contrary to the findings at the hip, the treatment group had a greater loss in BMD compared to the control group at the DF and PT at month 6 (−7.9 vs. −2.7 % and −10.5 vs. −4.8 %) and at month 12 (−18.5 vs. −8.4 % and −20.4 vs. −7.9 %). The authors concluded that it would not be prudent at the present time to recommend the use of ZA in an effort to reduce bone loss after acute SCI due to this lack of efficacy at the knee region and the fact that patients with chronic SCI fracture more at the DF and PT than at the hip. Similar to these findings, Schnitzer et al. [48] administered ZA at baseline in a double-blinded, randomized, placebo-controlled trial to 12 patients with SCI, 6 treated with ZA and 6 receiving placebo, with 6 months being the primary time point for drug efficacy after drug administration. Compared to the treatment group, the placebo group lost a significantly greater percentage of aBMD at the total hip (right, −2.2 vs. −8.6 %; left, −3.7 vs. −12.3 %). However, contrary to the report by Bauman et al. [47], the placebo group had a greater percentage of aBMD loss at the DF, but this loss was not significant compared to the treatment group 6 months after BL drug administration. Until adequately powered, well-designed, randomized control trials have been completed, the safety and efficacy of prescribing ZA shortly after SCI remain questionable. A recent single arm investigation looking at the effectiveness of denosumab (60 mg every 6 months) to ameliorate bone loss in 14 patients with acute SCI found this treatment was highly effective at preventing aBMD loss at the lumbar spine and total hip, but aBMD of the DF and PT was not measured, as such, the efficacy of denosumab at these ROI, and comparison to trials using ZA cannot be made at this time [118]. Given the small size of the cohorts to date and the limitations inherent when performing a bone loss intervention clinical trial shortly after SCI [e.g., corticosteroid administration, individual variation in bone loss after SCI, and limited access to advanced imaging methods (CT and/or MRI)], future randomized control trials are warranted that compare multiple anti-resorptive agents with appropriate imaging modalities to more fully understand the drug with the greatest efficacy to preserve bone mass and architecture at the knee region.
Future directions
While the use of pQCT to obtain vBMD and microarchitecture of the DF and PT is well appreciated by clinicians and researchers, the fact remains that pQCT is not widely available and the possibility of third party reimbursement in the USA is, to date, not a tenable option when one considers that even reimbursement for DXA imaging has diminished to a great extent over the past decade [119, 120]. Over the past three decades, the success of bone densitometry to diagnose osteoporosis in the clinical environment is largely due to the accumulation of normative data at the hip, lumbar spine, and forearm regions. There is ample evidence that activities while seated in a wheelchair place a person with SCI at greatest risk for low impact fragility fracture at the DF and PT. The future of routine densitometry in the SCI population is dependent on the development of normative databases and improved cutoff values for fracture at the DF and PT using both DXA and advanced imaging methods. Furthermore, future prospective cohort studies are required that employ a multiple component model that accounts for BMD and bone geometry at the DF and PT, as well as risk factors for fracture [e.g., completeness of lesion (motor and/or sensory), age at injury (<18 years of age), duration of injury, etc.] to permit the development of SCI-appropriate algorithms validated in the clinical environment that predict fracture. The successful evaluation of any novel intervention to prevent sublesional osteoporosis is contingent upon obtaining accurate, reproducible, and high quality imaging of the DF and PT. This imaging technology is currently available to clinical investigators and should provide the ability to properly address questions of the efficacy of newer interventions to prevent bone loss at the knee.
Future clinical trials in persons with SCI that are designed to test emerging pharmacological therapies to more potently suppress osteoclast activity by RANKL inhibition [anti-RANKL antibody (denosumab)], to promote osteoblast activity by physical means or by the use of anabolic agents (e.g., testosterone, teriparatide, or its analogs), or to be both anti-resorptive and anabolic [e.g., rehabilitation interventions with or without the administration of anti-resorptive pharmacological therapies or, perhaps, anti-sclerostin antibody (romosozumab)] are needed to determine the efficacy of such therapeutic approaches at the knee in persons with SCI. Of great interest, preclinical studies from our group have shown the efficacy of sclerostin antagonism or SOST deletion in preventing loss of aBMD at the DF and PT by DXA and the relative preservation of vBMD and bone architecture by μCT after motor-complete SCI [121, 122]. This work suggests that pharmacological antagonism of inhibitors of the Wnt signaling pathway (e.g., sclerostin and/or DKK1), when these agents become commercially available, holds promise as a therapeutic approach to reduce the marked deterioration that occurs to the sublesional skeleton in individuals after the most neurologically complete forms of SCI and in other forms of extreme immobilization osteoporosis.
Abbreviations
- SCI:
-
Spinal cord injury
- DXA:
-
Dual energy x-ray absorptiometry
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- QCT:
-
Quantitative computed tomography
- MDCT:
-
Multidetector computed tomography
- pQCT:
-
Peripheral quantitative computerized tomography
- HR-pQCT:
-
High-resolution peripheral quantitative computed tomography
- mSv:
-
Millisievert
- DF:
-
Distal femur
- PT:
-
Proximal tibia
- LE:
-
Lower extremity
- ROI:
-
Region of interest
- RMS-CV%:
-
Root mean square coefficient of variation percent
- LSC:
-
Least significant change
- BMC:
-
Bone mineral content
- aBMD:
-
Areal bone mineral density
- vBMD:
-
Volumetric bone mineral density
- vBMDTb :
-
Trabecular volumetric bone mineral density
- vBMDCt :
-
Cortical volumetric bone mineral density
- App:
-
Apparent
- BV/TV:
-
Bone volume/tissue volume
- Tb.N:
-
Trabecular number
- Tb.Sp:
-
Trabecular spacing
- Tb.Th:
-
Trabecular thickness
- SSIpol :
-
Polar stress strain index
- PI:
-
Polar moment of inertia
- ES:
-
Electrical stimulation
- FES:
-
Functional electrical stimulation
- ZA:
-
Zoledronic acid
- IOF:
-
International Osteoporosis Foundation
- AIS:
-
American Spinal Injury Association Impairment Scale
References
Minaire P, Neunier P, Edouard C, Bernard J, Courpron P, Bourret J (1974) Quantitative histological data on disuse osteoporosis: comparison with biological data. Calcif Tissue Res 17(1):57–73
Bauman WA, Cardozo CP (2015) Osteoporosis in individuals with spinal cord injury. PM & R: the journal of injury, function, and rehabilitation 7(2):188–201 . doi:10.1016/j.pmrj.2014.08.948quiz 201
Chantraine A, Nusgens B, Lapiere CM (1986) Bone remodeling during the development of osteoporosis in paraplegia. Calcif Tissue Int 38(6):323–327
Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, McWhinney B, Hickman PE (1998) Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab 83(2):415–422. doi:10.1210/jcem.83.2.4581
Shields RK, Dudley-Javoroski S, Boaldin KM, Corey TA, Fog DB, Ruen JM (2006) Peripheral quantitative computed tomography: measurement sensitivity in persons with and without spinal cord injury. Arch Phys Med Rehabil 87(10):1376–1381. doi:10.1016/j.apmr.2006.07.257
Biering-Sorensen F, Bohr HH, Schaadt OP (1990) Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Investig 20(3):330–335
Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P (1995) Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia 33(11):674–677. doi:10.1038/sc.1995.141
Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF (2000) Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone 27(2):305–309
Morse LR, Battaglino RA, Stolzmann KL, Hallett LD, Waddimba A, Gagnon D, Lazzari AA, Garshick E (2009) Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 20(3):385–392. doi:10.1007/s00198-008-0671-6
Ingram RR, Suman RK, Freeman PA (1989) Lower limb fractures in the chronic spinal cord injured patient. Paraplegia 27(2):133–139. doi:10.1038/sc.1989.20
Ragnarsson KT, Sell GH (1981) Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil 62(9):418–423
Carbone LD, Chin AS, Burns SP, Svircev JN, Hoenig H, Heggeness M, Weaver F (2013) Morbidity following lower extremity fractures in men with spinal cord injury. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 24(8):2261–2267. doi:10.1007/s00198-013-2295-8
Akhigbe T, Chin AS, Svircev JN, Hoenig H, Burns SP, Weaver FM, Bailey L, Carbone L (2015) A retrospective review of lower extremity fracture care in patients with spinal cord injury. J Spinal cord Med 38(1):2–9. doi:10.1179/2045772313Y.0000000156
Frey-Rindova P, de Bruin ED, Stussi E, Dambacher MA, Dietz V (2000) Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord 38(1):26–32
de Bruin ED, Vanwanseele B, Dambacher MA, Dietz V, Stussi E (2005) Long-term changes in the tibia and radius bone mineral density following spinal cord injury. Spinal Cord 43(2):96–101. doi:10.1038/sj.sc.3101685
Warden SJ, Bennell KL, Matthews B, Brown DJ, McMeeken JM, Wark JD (2002) Quantitative ultrasound assessment of acute bone loss following spinal cord injury: a longitudinal pilot study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 13(7):586–592. doi:10.1007/s001980200077
Frotzler A, Berger M, Knecht H, Eser P (2008) Bone steady-state is established at reduced bone strength after spinal cord injury: a longitudinal study using peripheral quantitative computed tomography (pQCT). Bone 43(3):549–555. doi:10.1016/j.bone.2008.05.006
Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H (2004) Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 34(5):869–880. doi:10.1016/j.bone.2004.01.001
Bauman WA, Spungen AM, Wang J, Pierson RN Jr, Schwartz E (1999) Continuous loss of bone during chronic immobilization: a monozygotic twin study. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 10(2):123–127
Modlesky CM, Majumdar S, Narasimhan A, Dudley GA (2004) Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J Bone Miner Res Off J Am Soc Bone Miner Res 19(1):48–55. doi:10.1359/JBMR.0301208
Recker R, Lappe J, Davies K, Heaney R (2000) Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res Off J Am Soc Bone Miner Res 15(10):1965–1973. doi:10.1359/jbmr.2000.15.10.1965
Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM (1990) Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res Off J Am Soc Bone Miner Res 5(8):843–850. doi:10.1002/jbmr.5650050807
Vico L, Collet P, Guignandon A, Lafage-Proust MH, Thomas T, Rehaillia M, Alexandre C (2000) Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355(9215):1607–1611
Khoo BC, Brown K, Cann C, Zhu K, Henzell S, Low V, Gustafsson S, Price RI, Prince RL (2009) Comparison of QCT-derived and DXA-derived areal bone mineral density and T scores. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 20(9):1539–1545. doi:10.1007/s00198-008-0820-y
Li N, Li XM, Xu L, Sun WJ, Cheng XG, Tian W (2013) Comparison of QCT and DXA: osteoporosis detection rates in postmenopausal women. Int J Endocrinol 2013:895474. doi:10.1155/2013/895474
del Puente A, Pappone N, Mandes MG, Mantova D, Scarpa R, Oriente P (1996) Determinants of bone mineral density in immobilization: a study on hemiplegic patients. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 6(1):50–54
Troy KL, Morse LR (2015) Measurement of bone: diagnosis of SCI-induced osteoporosis and fracture risk prediction. Topics in spinal cord injury rehabilitation 21(4):267–274. doi:10.1310/sci2104-267
Edwards WB, Schnitzer TJ, Troy KL (2014) Bone mineral and stiffness loss at the distal femur and proximal tibia in acute spinal cord injury. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 25(3):1005–1015. doi:10.1007/s00198-013-2557-5
Biering-Sorensen F, Bohr H, Schaadt O (1988) Bone mineral content of the lumbar spine and lower extremities years after spinal cord lesion. Paraplegia 26(5):293–301
Garland DE, Adkins RH, Stewart CA (2005) Fracture threshold and risk for osteoporosis and pathologic fractures in individuals with spinal cord injury. Top Spinal Cord Inj 11(1):61–69
Garland DE, Adkins RH, Stewart CA, Ashford R, Vigil D (2001) Regional osteoporosis in women who have a complete spinal cord injury. J Bone Joint Surg Am 83-A(8):1195–1200
Shields RK, Schlechte J, Dudley-Javoroski S, Zwart BD, Clark SD, Grant SA, Mattiace VM (2005) Bone mineral density after spinal cord injury: a reliable method for knee measurement. Arch Phys Med Rehabil 86(10):1969–1973. doi:10.1016/j.apmr.2005.06.001
Lala D, Craven BC, Thabane L, Papaioannou A, Adachi JD, Popovic MR, Giangregorio LM (2014) Exploring the determinants of fracture risk among individuals with spinal cord injury. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 25(1):177–185. doi:10.1007/s00198-013-2419-1
Garland DE, Adkins RH, Scott M, Singh H, Massih M, Stewart C (2004) Bone loss at the os calcis compared with bone loss at the knee in individuals with spinal cord injury. J Spinal Cord Med 27(3):207–211
Garland DE, Adkins RH, Stewart CA (2008) Five-year longitudinal bone evaluations in individuals with chronic complete spinal cord injury. The journal of spinal cord medicine 31(5):543–550
Baim S, Wilson CR, Lewiecki EM, Luckey MM, Downs RW Jr, Lentle BC (2005) Precision assessment and radiation safety for dual-energy X-ray absorptiometry: position paper of the International Society for Clinical Densitometry. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry 8(4):371–378
Diez-Perez A, Adachi JD, Agnusdei D, Bilezikian JP, Compston JE, Cummings SR, Eastell R, Eriksen EF, Gonzalez-Macias J, Liberman UA, Wahl DA, Seeman E, Kanis JA, Cooper C, Group ICIRW (2012) Treatment failure in osteoporosis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 23(12):2769–2774. doi:10.1007/s00198-012-2093-8
Murphy E, Bresnihan B, FitzGerald O (2001) Validated measurement of periarticular bone mineral density at the knee joint by dual energy x ray absorptiometry. Ann Rheum Dis 60(1):8–13
Bohr HH, Schaadt O (1987) Mineral content of upper tibia assessed by dual photon densitometry. Acta Orthop Scand 58(5):557–559
Li MG, Nilsson KG, Nivbrant B (2004) Decreased precision for BMD measurements in the prosthetic knee using a non-knee-specific software. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry 7(3):319–325
McPherson JG, Edwards WB, Prasad A, Troy KL, Griffith JW, Schnitzer TJ (2014) Dual energy X-ray absorptiometry of the knee in spinal cord injury: methodology and correlation with quantitative computed tomography. Spinal Cord 52(11):821–825. doi:10.1038/sc.2014.122
Forrest G, Harkema S, Angeli C, Faghri P, Kirshblum S, Cirnigliaro C, Garbarinin E, Bauman W (2013) Preliminary results on the differential effect on bone of applying multi-muscle electrical stimulation to the leg while supine or standing in patients with SCI: the importance of combining a mechanical intervention with gravitational loading. J Spinal Cord Med Accepted for Publication
Bauman W, Cirnigliaro C, LaFountaine M, Martinez L, Kirshblum S, Spungen A (2014) Zoledronic acid administration failed to prevent bone loss at the knee in persons with acute spinal cord injury: an observational cohort study. J Bone Miner Metab Accepted for Publication
Bakkum AJ, Janssen TW, Rolf MP, Roos JC, Burcksen J, Knol DL, de Groot S (2014) A reliable method for measuring proximal tibia and distal femur bone mineral density using dual-energy X-ray absorptiometry. Med Eng Phys 36(3):387–390. doi:10.1016/j.medengphy.2013.08.010
Gilchrist N, Hooper G, Frampton C, Maguire P, Heard A, March RL, Maxwell R, Penny I (2013) Measurement of bone density around the Oxford medial compartment knee replacement using iDXA. A precision study. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry 16(2):178–182. doi:10.1016/j.jocd.2012.02.015
Morse LR, Lazzari AA, Battaglino R, Stolzmann KL, Matthess KR, Gagnon DR, Davis SA, Garshick E (2009) Dual energy x-ray absorptiometry of the distal femur may be more reliable than the proximal tibia in spinal cord injury. Arch Phys Med Rehabil 90(5):827–831. doi:10.1016/j.apmr.2008.12.004
Bauman WA, Cirnigliaro CM, La Fountaine MF, Martinez L, Kirshblum SC, Spungen AM (2014) Zoledronic acid administration failed to prevent bone loss at the knee in persons with acute spinal cord injury: an observational cohort study. J Bone Miner Metab. doi:10.1007/s00774-014-0602-x
Schnitzer TJ, Kim K, Marks J, Yeasted R, Simonian N, Chen D (2016) Zoledronic acid treatment after acute spinal cord injury: results of a randomized, placebo-controlled pilot trial. PM & R: the journal of injury, function, and rehabilitation. doi:10.1016/j.pmrj.2016.01.012
Lauer R, Johnston TE, Smith BT, Mulcahey MJ, Betz RR, Maurer AH (2007) Bone mineral density of the hip and knee in children with spinal cord injury. J Spinal cord Med 30(Suppl 1):S10–S14
Prentice A, Parsons TJ, Cole TJ (1994) Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr 60(6):837–842
Tothill P, Avenell A, Reid DM (1994) Precision and accuracy of measurements of whole-body bone mineral: comparisons between Hologic, Lunar and Norland dual-energy X-ray absorptiometers. Br J Radiol 67(804):1210–1217. doi:10.1259/0007-1285-67-804-1210
Pors Nielsen S, Barenholdt O, Diessel E, Armbrust S, Felsenberg D (1998) Linearity and accuracy errors in bone densitometry. Br J Radiol 71(850):1062–1068. doi:10.1259/bjr.71.850.10211067
Craven R, McGillivray A (2009) Detection and treatment of sublesional osteoporosis among patients with chronic spinal cord injury. Topics in spinal cord injury rehabilitation 14(4):1–22. doi:10.1310/sci1404-1
Rittweger J, Goosey-Tolfrey VL, Cointry G, Ferretti JL (2010) Structural analysis of the human tibia in men with spinal cord injury by tomographic (pQCT) serial scans. Bone 47(3):511–518. doi:10.1016/j.bone.2010.05.025
Coupaud S, McLean AN, Purcell M, Fraser MH, Allan DB (2015) Decreases in bone mineral density at cortical and trabecular sites in the tibia and femur during the first year of spinal cord injury. Bone 74:69–75. doi:10.1016/j.bone.2015.01.005
Burghardt AJ, Link TM, Majumdar S (2011) High-resolution computed tomography for clinical imaging of bone microarchitecture. Clin Orthop Relat Res 469(8):2179–2193. doi:10.1007/s11999-010-1766-x
Ito M, Ikeda K, Nishiguchi M, Shindo H, Uetani M, Hosoi T, Orimo H (2005) Multi-detector row CT imaging of vertebral microstructure for evaluation of fracture risk. J Bone Miner Res Off J Am Soc Bone Miner Res 20(10):1828–1836. doi:10.1359/JBMR.050610
Lee SY, Kwon SS, Kim HS, Yoo JH, Kim J, Kim JY, Min BC, Moon SJ, Sung KH (2015) Reliability and validity of lower extremity computed tomography as a screening tool for osteoporosis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 26(4):1387–1394. doi:10.1007/s00198-014-3013-x
Giangregorio LM, Gibbs JC, Craven BC (2016) Measuring muscle and bone in individuals with neurologic impairment; lessons learned about participant selection and pQCT scan acquisition and analysis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 27(8):2433–2446. doi:10.1007/s00198-016-3572-0
Engelke K, Lang T, Khosla S, Qin L, Zysset P, Leslie WD, Shepherd JA, Schousboe JT (2015) Clinical use of quantitative computed tomography (QCT) of the hip in the management of osteoporosis in adults: the 2015 ISCD official positions—part I. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry 18(3):338–358. doi:10.1016/j.jocd.2015.06.012
Cervinka T, Sievanen H, Hyttinen J, Rittweger J (2014) Bone loss patterns in cortical, subcortical, and trabecular compartments during simulated microgravity. J Appl Physiol 117(1):80–88. doi:10.1152/japplphysiol.00021.2014
Dudley-Javoroski S, Amelon R, Liu Y, Saha PK, Shields RK (2014) High bone density masks architectural deficiencies in an individual with spinal cord injury. J Spinal cord Med 37(3):349–354. doi:10.1179/2045772313Y.0000000166
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res Off J Am Soc Bone Miner Res 25(7):1468–1486. doi:10.1002/jbmr.141
Krug R, Burghardt AJ, Majumdar S, Link TM (2010) High-resolution imaging techniques for the assessment of osteoporosis. Radiol Clin N Am 48(3):601–621. doi:10.1016/j.rcl.2010.02.015
Dudley-Javoroski S, Shields RK (2012) Regional cortical and trabecular bone loss after spinal cord injury. J Rehabil Res Dev 49(9):1365–1376
Coupaud S, McLean AN, Allan DB (2009) Role of peripheral quantitative computed tomography in identifying disuse osteoporosis in paraplegia. Skelet Radiol 38(10):989–995. doi:10.1007/s00256-009-0674-1
Frotzler A, Cheikh-Sarraf B, Pourtehrani M, Krebs J, Lippuner K (2015) Long-bone fractures in persons with spinal cord injury. Spinal Cord 53(9):701–704. doi:10.1038/sc.2015.74
Pop LC, Sukumar D, Tomaino K, Schlussel Y, Schneider SH, Gordon CL, Wang X, Shapses SA (2015) Moderate weight loss in obese and overweight men preserves bone quality. Am J Clin Nutr 101(3):659–667. doi:10.3945/ajcn.114.088534
Giangregorio L, Lala D, Hummel K, Gordon C, Craven BC (2013) Measuring apparent trabecular density and bone structure using peripheral quantitative computed tomography at the tibia: precision in participants with and without spinal cord injury. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry 16(2):139–146. doi:10.1016/j.jocd.2012.02.003
Edwards WB, Schnitzer TJ, Troy KL (2014) The mechanical consequence of actual bone loss and simulated bone recovery in acute spinal cord injury. Bone 60:141–147. doi:10.1016/j.bone.2013.12.012
Rajapakse CS, Magland J, Zhang XH, Liu XS, Wehrli SL, Guo XE, Wehrli FW (2009) Implications of noise and resolution on mechanical properties of trabecular bone estimated by image-based finite-element analysis. Journal of orthopaedic research: official publication of the Orthopaedic Research Society 27(10):1263–1271. doi:10.1002/jor.20877
Wehrli FW, Gomberg BR, Saha PK, Song HK, Hwang SN, Snyder PJ (2001) Digital topological analysis of in vivo magnetic resonance microimages of trabecular bone reveals structural implications of osteoporosis. J Bone Miner Res Off J Am Soc Bone Miner Res 16(8):1520–1531. doi:10.1359/jbmr.2001.16.8.1520
Chesnut CH 3rd, Majumdar S, Newitt DC, Shields A, Van Pelt J, Laschansky E, Azria M, Kriegman A, Olson M, Eriksen EF, Mindeholm L (2005) Effects of salmon calcitonin on trabecular microarchitecture as determined by magnetic resonance imaging: results from the QUEST study. J Bone Miner Res Off J Am Soc Bone Miner Res 20(9):1548–1561. doi:10.1359/JBMR.050411
Slade JM, Bickel CS, Modlesky CM, Majumdar S, Dudley GA (2005) Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free postmenopausal women. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 16(3):263–272. doi:10.1007/s00198-004-1665-7
Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M (2001) Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord 39(4):208–214. doi:10.1038/sj.sc.3101139
Vestergaard P, Krogh K, Rejnmark L, Mosekilde L (1998) Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord 36(11):790–796
Parsons KC, Lammertse DP (1991) Rehabilitation in spinal cord disorders. 1. Epidemiology, prevention, and system of care of spinal cord disorders. Arch Phys Med Rehabil 72(4-S):S293–S294
Freehafer AA (1995) Limb fractures in patients with spinal cord injury. Arch Phys Med Rehabil 76(9):823–827
Garland DE, Adkins RH, Kushwaha V, Stewart C (2004) Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med 27(3):202–206
Martinez A, Cuenca J, Herrera A, Domingo J (2002) Late lower extremity fractures in patients with paraplegia. Injury 33(7):583–586
Gifre L, Vidal J, Carrasco J, Portell E, Puig J, Monegal A, Guanabens N, Peris P (2014) Incidence of skeletal fractures after traumatic spinal cord injury: a 10-year follow-up study. Clin Rehabil 28(4):361–369. doi:10.1177/0269215513501905
Comarr AE, Hutchinson RH, Bors E (1962) Extremity fractures of patients with spinal cord injuries. Am J Surg 103:732–739
Zehnder Y, Luthi M, Michel D, Knecht H, Perrelet R, Neto I, Kraenzlin M, Zach G, Lippuner K (2004) Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 15(3):180–189. doi:10.1007/s00198-003-1529-6
Freehafer AA, Mast WA (1965) Lower extremity fractures in patients with spinal-cord injury. J Bone Joint Surg Am 47:683–694
Garland DE, Maric Z, Adkins RH, Stewart CA (1993) Bone mineral density about the knee in spinal cord injured patients with pathologic fractures. Contemp Orthop 26:375–379
Mazess RB (1990) Bone densitometry of the axial skeleton. Orthopedic Clinic North Am 21(1):51–63
Eser P, Frotzler A, Zehnder Y, Denoth J (2005) Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil 86(3):498–504. doi:10.1016/j.apmr.2004.09.006
Frost HM (1987) The mechanostat: a proposed pathogenic mechanism of osteoporosis and the bone mass effects of mechanical and nonmechanical agents. Bone Min 2(2):73–85
Isaacson J, Brotto M (2014) Physiology of mechanotransduction: how do muscle and bone “talk” to one another? Clin Rev Bone Min metab 12(2):77–85. doi:10.1007/s12018-013-9152-3
Dolbow DR, Gorgey AS, Gater DR, Moore JR (2014) Body composition changes after 12 months of FES cycling: case report of a 60-year-old female with paraplegia. Spinal Cord 52(Suppl 1):S3–S4. doi:10.1038/sc.2014.40
Pacy PJ, Hesp R, Halliday DA, Katz D, Cameron G, Reeve J (1988) Muscle and bone in paraplegic patients, and the effect of functional electrical stimulation. Clin Sci 75(5):481–487
Biering-Sorensen F, Hansen B, Lee BS (2009) Non-pharmacological treatment and prevention of bone loss after spinal cord injury: a systematic review. Spinal Cord 47(7):508–518. doi:10.1038/sc.2008.177
Guertin PA (2009) Recovery of locomotor function with combinatory drug treatments designed to synergistically activate specific neuronal networks. Curr Med Chem 16(11):1366–1371
de Bruin ED, Frey-Rindova P, Herzog RE, Dietz V, Dambacher MA, Stussi E (1999) Changes of tibia bone properties after spinal cord injury: effects of early intervention. Arch Phys Med Rehabil 80(2):214–220
Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, McCartney N (2005) Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord 43(11):649–657. doi:10.1038/sj.sc.3101774
Bloomfield SA, Mysiw WJ, Jackson RD (1996) Bone mass and endocrine adaptations to training in spinal cord injured individuals. Bone 19(1):61–68
Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, Donaldson Nde N, Eser P (2008) High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone 43(1):169–176. doi:10.1016/j.bone.2008.03.004
Lai CH, Chang WH, Chan WP, Peng CW, Shen LK, Chen JJ, Chen SC (2010) Effects of functional electrical stimulation cycling exercise on bone mineral density loss in the early stages of spinal cord injury. J Rehabil Med 42(2):150–154. doi:10.2340/16501977-0499
Eser P, de Bruin ED, Telley I, Lechner HE, Knecht H, Stussi E (2003) Effect of electrical stimulation-induced cycling on bone mineral density in spinal cord-injured patients. Eur J Clin Investig 33(5):412–419
Dudley-Javoroski S, Saha PK, Liang G, Li C, Gao Z, Shields RK (2012) High dose compressive loads attenuate bone mineral loss in humans with spinal cord injury. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 23(9):2335–2346. doi:10.1007/s00198-011-1879-4
Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B (2000) Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil 81(8):1090–1098
Chen SC, Lai CH, Chan WP, Huang MH, Tsai HW, Chen JJ (2005) Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil Rehabil 27(22):1337–1341. doi:10.1080/09638280500164032
Mohr T, Podenphant J, Biering-Sorensen F, Galbo H, Thamsborg G, Kjaer M (1997) Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int 61(1):22–25
Shields RK, Dudley-Javoroski S (2007) Musculoskeletal adaptations in chronic spinal cord injury: effects of long-term soleus electrical stimulation training. Neurorehabil Neural Repair 21(2):169–179. doi:10.1177/1545968306293447
Dudley-Javoroski S, Shields RK (2013) Active-resisted stance modulates regional bone mineral density in humans with spinal cord injury. J spinal cord Med 36(3):191–199. doi:10.1179/2045772313Y.0000000092
Forrest G, Harkema SJ, Angeli CA, Faghri PD, Kirshblum SC, Cirnigliaro CM, LaFountaine, Garbarini E, Bauman WA. (2014) Preliminary results on the differential effect on bone of applying multi-muscle electrical stimulation to the leg while supine or standing in patients with SCI: the importance of combining a mechanical intervention with gravitational loading. The journal of spinal cord medicine In Preparation
Ben M, Harvey L, Denis S, Glinsky J, Goehl G, Chee S, Herbert RD (2005) Does 12 weeks of regular standing prevent loss of ankle mobility and bone mineral density in people with recent spinal cord injuries? Australian J physiother 51(4):251–256
Warden SJ, Bennell KL, Matthews B, Brown DJ, McMeeken JM, Wark JD (2001) Efficacy of low-intensity pulsed ultrasound in the prevention of osteoporosis following spinal cord injury. Bone 29(5):431–436
Wuermser LA, Beck LA, Lamb JL, Atkinson EJ, Amin S (2014) The effect of low-magnitude whole body vibration on bone density and microstructure in men and women with chronic motor complete paraplegia. J Spinal cord Med. doi:10.1179/2045772313Y.0000000191
Bultink IE, Baden M, Lems WF (2013) Glucocorticoid-induced osteoporosis: an update on current pharmacotherapy and future directions. Expert Opin Pharmacother 14(2):185–197. doi:10.1517/14656566.2013.761975
Chappard D, Minaire P, Privat C, Berard E, Mendoza-Sarmiento J, Tournebise H, Basle MF, Audran M, Rebel A, Picot C et al (1995) Effects of tiludronate on bone loss in paraplegic patients. J Bone Miner Res Off J Am Soc Bone Miner Res 10(1):112–118. doi:10.1002/jbmr.5650100116
Pearson EG, Nance PW, Leslie WD, Ludwig S (1997) Cyclical etidronate: its effect on bone density in patients with acute spinal cord injury. Arch Phys Med Rehabil 78(3):269–272
Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D (1999) Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil 80(3):243–251
Gilchrist NL, Frampton CM, Acland RH, Nicholls MG, March RL, Maguire P, Heard A, Reilly P, Marshall K (2007) Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 92(4):1385–1390. doi:10.1210/jc.2006-2013
Bauman WA, Wecht JM, Kirshblum S, Spungen AM, Morrison N, Cirnigliaro C, Schwartz E (2005) Effect of pamidronate administration on bone in patients with acute spinal cord injury. J Rehabil Res Dev 42(3):305–313
Bubbear JS, Gall A, Middleton FR, Ferguson-Pell M, Swaminathan R, Keen RW (2011) Early treatment with zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 22(1):271–279. doi:10.1007/s00198-010-1221-6
Shapiro J, Smith B, Beck T, Ballard P, Dapthary M, BrintzenhofeSzoc K, Caminis J (2007) Treatment with zoledronic acid ameliorates negative geometric changes in the proximal femur following acute spinal cord injury. Calcif Tissue Int 80(5):316–322. doi:10.1007/s00223-007-9012-6
Gifre L, Vidal J, Carrasco JL, Muxi A, Portell E, Monegal A, Guanabens N, Peris P (2016) Denosumab increases sublesional bone mass in osteoporotic individuals with recent spinal cord injury. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 27(1):405–410. doi:10.1007/s00198-015-3333-5
Schousboe JT (2014) ISCD in 2014: state of the society. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry 17(3):328–329. doi:10.1016/j.jocd.2014.04.118
Krueger D (2015) ISCD in 2015: state of the society. Journal of clinical densitometry: the official journal of the International Society for Clinical Densitometry 18(3):445–446. doi:10.1016/j.jocd.2015.06.004
Qin W, Li X, Peng Y, Harlow LM, Ren Y, Wu Y, Li J, Qin Y, Sun J, Zheng S, Brown T, Feng JQ, Ke HZ, Bauman WA, Cardozo CP (2015) Sclerostin Antibody Preserves the Morphology and Structure of Osteocytes and Blocks the Severe Skeletal Deterioration After Motor-Complete Spinal Cord Injury in Rats. J Bone Miner Res 30(11):1994–2004
Qin W, Zhao W, Li X, Peng Y, Harlow LM, Li J, Qin Y, Pan J, Wu Y, Ran L, Ke HZ, Cardozo CP, Bauman WA. Mice with sclerostin gene deletion are resistant to the severe sublesional bone loss induced by spinal cord injury. Osteoporos Int. 2016 20. [Epub ahead of print])
Battaglino RA, Sudhakar S, Lazzari AA, Garshick E, Zafonte R, Morse LR (2012) Circulating sclerostin is elevated in short-term and reduced in long-term SCI. Bone 51(3):600–605. doi:10.1016/j.bone.2012.04.019
Doherty AL, Battaglino RA, Donovan J, Gagnon D, Lazzari AA, Garshick E, Zafonte R, Morse LR (2014) Adiponectin is a candidate biomarker of lower extremity bone density in men with chronic spinal cord injury. J Bone Miner Res Off J Am Soc Bone Miner Res 29(1):251–259. doi:10.1002/jbmr.2020
Moreno C (2001) Protocol for using dual photon absorptiometry software to measure BMD of distal femur and proximal tibia. Master’s thesis, McMaster University, Hamilton
Morse LR, Sudhakar S, Danilack V, Tun C, Lazzari A, Gagnon DR, Garshick E, Battaglino RA (2012) Association between sclerostin and bone density in chronic spinal cord injury. J Bone Miner Res Off J Am Soc Bone Miner Res 27(2):352–359. doi:10.1002/jbmr.546
Morse LR, Sudhakar S, Lazzari AA, Tun C, Garshick E, Zafonte R, Battaglino RA (2013) Sclerostin: a candidate biomarker of SCI-induced osteoporosis. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 24(3):961–968. doi:10.1007/s00198-012-2072-0
McCarthy ID, Bloomer Z, Gall A, Keen R, Ferguson-Pell M (2012) Changes in the structural and material properties of the tibia in patients with spinal cord injury. Spinal Cord 50(4):333–337. doi:10.1038/sc.2011.143
Tan CO, Battaglino RA, Doherty AL, Gupta R, Lazzari AA, Garshick E, Zafonte R, Morse LR (2014) Adiponectin is associated with bone strength and fracture history in paralyzed men with spinal cord injury. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 25(11):2599–2607. doi:10.1007/s00198-014-2786-2
Dudley-Javoroski S, Shields RK (2010) Longitudinal changes in femur bone mineral density after spinal cord injury: effects of slice placement and peel method. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 21(6):985–995. doi:10.1007/s00198-009-1044-5
Acknowledgments
The authors would like to thank the James J. Peters Veterans Affairs Medical Center, Bronx, NY, the Department of Veterans Affairs Rehabilitation Research & Development Service, and the Kessler Institute for Rehabilitation, West Orange, NJ, for their support to perform this work. The authors would also like to thank the Department of Physical Therapy, School of Health Related Professions, Rutgers New Jersey Medical School, Newark, NJ, USA and Alex T. Lombard for his assistance completing the comprehensive literature review necessary to complete this review article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Grant sources
Veterans Affairs Rehabilitation Research and Development Service (#B9212-C, B2020-C) and the James J. Peters VA Medical Center.
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Cirnigliaro, C.M., Myslinski, M.J., La Fountaine, M.F. et al. Bone loss at the distal femur and proximal tibia in persons with spinal cord injury: imaging approaches, risk of fracture, and potential treatment options. Osteoporos Int 28, 747–765 (2017). https://doi.org/10.1007/s00198-016-3798-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3798-x