Abstract
Peripheral quantitative computed tomography (pQCT) can be used to examine bone strength outcomes and muscle size and fatty infiltration. Our research team and others have used it to examine bone loss after spinal cord injury (SCI). However, the high prevalence of restricted lower extremity range of motion, spasticity, edema, excessive muscle atrophy, or severe osteoporosis necessitates changes to standard protocols for screening, positioning during scan acquisition, and analysis methods. This manuscript outlines the challenges that we experienced using pQCT in individuals with SCI, and provides solutions, ones that may also be applicable when using pQCT in individuals with other chronic conditions or in older adults. Suggestions for participant screening, positioning individuals for scanning while in a wheelchair, scan site selection, need for attendant assistance, and considerations in the presence of secondary complications, such as contracture, spasticity, and paralysis, are presented. In the presence of very low bone mineral density or severe muscle atrophy, the default analysis modes provided by the manufacturer may not provide valid estimates of bone or muscle indices; we propose alternates. We have used watershed segmentation methods to determine muscle size and density based on lower precision error compared to threshold-based edge-detection segmentation, particularly for adults with SCI, where more fatty infiltration was present. By presenting our “lessons learned,” we hope to reduce the learning curve for researchers using pQCT in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid alterations in bone strength and muscle cross-sectional area occur in conditions of neuromuscular or neurologic impairment-related paralysis, e.g., muscular dystrophy, stroke, and spinal cord injury (SCI). SCI during growth has been associated with reduced expansion of bone cortices [1], suggesting that axial loading, physical activity, or muscle activity contributes to cortical expansion during growth. Adult-onset SCI results in rapid muscle atrophy [2–4] and loss of bone mineral density (BMD) [5–23] (Table 1). Recent evidence highlights the inter-relationship between muscle and bone, and the need to study them in tandem [15, 24].
pQCT allows assessment of the bone (e.g., BMD, bone geometry) and muscle (e.g., muscle size and density) at the tibia, one of the most fracture-prone sites after SCI. A participant can be scanned while in a wheelchair, eliminating need for transfer. However, comorbid conditions or impairments (e.g., limited mobility, spasticity, edema) necessitate changes to protocols for image acquisition and image analysis (Table 2) [4–23]. Many of the protocol variations may also be applicable to individuals with impairments or conditions other than SCI (e.g., stroke, obesity, older adults). Herein, we describe our “lessons learned” about the challenges of using pQCT in a clinical population that presents challenges to standard operating procedures, namely individuals with SCI. We have categorized the challenges using the following subcategories: participant, rater (e.g., technician/researcher/clinician performing acquisition, analysis), and instrument (e.g., machine, software). We also conducted a literature search in PubMed (MEDLINE) using “peripheral quantitative computed tomography” and “spinal cord injury” as search terms to summarize findings related to bone and muscle outcomes and acquisition and analysis protocols from observational studies using pQCT in adults with SCI.
Participant
A majority of individuals ≥10 years post-SCI report a mean of seven comorbid conditions including obesity, diabetes, fragility fractures, urinary tract infections, pressure ulcers, autonomic dysfunction, impaired mobility or range of motion, pain, and spasticity. Unanticipated health complications and transportation barriers contribute to attrition and missed/late study visits, necessitating longer assessment windows. We add the following exclusion criteria to pQCT studies: bilateral metal implants at measurement site; calf circumference ≥439 mm (or ≥gantry diameter of 140 mm); spasticity sufficient to cause persistent movement artifact; or hip or knee flexion or ankle plantarflexion contractures limiting positioning.
Participant-related factors affecting prescreening
Prescreening for pQCT assessment should include inquiries about hand/foot dominance; presence of metal implants or prior fracture at measurement site of interest, and in females, the possibility of pregnancy. One must not assume that premenopausal women with SCI are not sexually active; screening for pregnancy must occur prior to each scan acquisition. Prior fracture at the measurement site of interest that has resulted in bony incongruities may warrant scanning the opposite side, or exclusion.
Often, the nondominant hand or most affected limb is selected to scan. However, asymmetries in motor function (e.g., hemiplegia; diplegia) result in asymmetrical bone or muscle adaptations. Lower extremity motor or sensory scores from the International Standards for Neurological Classification of SCI can be used for site selection [http://www.asia-spinalinjury.org/elearning/ASIA_ISCOS_high.pdf]. In the absence of an ISNCSCI report, individuals can self-report neurological level and ASIA impairment scale with clinical prompts [25]. An a priori decision process for scan site(s) selection is needed. It is advisable to choose one side and only scan the alternate side when dictated by conditions precluding accurate regional BMD assessment (e.g., metal implants, spasticity, prior fracture). Alternatively, a hierarchical plan for selecting scan site can first determine if there are contraindications to scanning either side, or if motor or sensory impairments are greater on one side than the other, and if neither is present, then the nondominant side is chosen. The presence of a leg bag for urine collection that the participant declines to move may also inform selection. If the side to be scanned is determined in advance, participants who use a leg bag may be asked to secure it to the opposite leg for the day.
Spasticity is a an exaggerated stretch reflex secondary to hyperexcitability of spinal reflexes, with increased muscle response to applied stretch, positively correlated with lengthening rate [26]. Spasticity can contribute to involuntary movement and scan artifacts. Hip flexion, rapid knee extension, or plantar flexion of the foot required to thread the leg into the scanner can trigger local spasticity among individuals with extensor synergy at knee and ankle, which can cause tissue injury to the skin or heel if the leg hits the equipment with sufficient force. Prescreening should include an assessment of lower extremity range of motion and the presence and severity of spasticity, ideally using a validated tool (e.g., Penn spasm frequency scale) [27]. For individuals with high spasm frequency and severity scores, prescan administration of sublingual Ativan, prescribed by a physician, could be considered, a common practice in radiology [28]. Ativan administration requires an interprofessional team, standard operating procedure, and research ethics board approval. Spasticity and paralysis can contribute to the contracture development and reduced range of motion due to permanent shortening of muscles, ligaments, or tendons. Hip or knee flexion or ankle dorsiflexion or plantarflexion contractures may limit positioning or create obliquity in scanning.
Individuals who are overweight or with substantial peripheral edema may have a calf circumference that exceeds the pQCT gantry size (e.g., gantry diameter of 140 mm for XCT2000); calf circumference should be measured in prescreening if scans at widest calf cross section (66 %) are planned. Among 70 individuals with SCI in our prospective cohort study [13], three had calf circumferences exceeding 439 mm (or gantry diameter of 140 mm).
Rater
Issues specific to rater (e.g., individuals acquiring/analysing images) are as follows: training regarding safe transfers, availability of attendant services, knowledge of SCI impairments and secondary complications, and image quality.
Transfer training and attendant needs
The availability of trained personnel familiar with the needs of individuals with neurologic impairment should be considered and has implications for study budgets. If assistance in transferring from wheelchair to assessment chair is required, or the individual needs to be lifted and positioned closer to the scanner, attendants or technicians require transfer training. Further, once the individual is positioned close to the scanner, the leg needs positioning. Some individuals with SCI can use their hands or have sufficient motor function to lift the paralyzed leg, but most require one additional person present to lift and position the leg while the technician moves the scanner. Examples of professionals that could be contracted to perform the service include physical therapy assistants, personal support workers, or other health care professionals.
Knowledge of impairments and secondary complications
Individuals performing pQCT scanning or providing assistance require an understanding of the heterogeneity of motor and sensory impairments among individuals with SCI, and secondary complications (e.g., spasticity, edema, poor wound healing, and fracture risk). For example, the person positioning the leg needs to lift it slowly to avoid triggering a spasm, and be educated on how to minimize spasticity if a spasm occurs. Gentle range of motion exercise can be performed prescan to reduce the risk of regional spasticity. If a spasm occurs, the leg should be protected from forceful contact with the device or rigid objects, to avoid skin damage.
Image quality
Images should be examined immediately for movement artifact, and the images discarded and scan acquisition repeated if present. A visual scale for assessment of movement artifact has been described [29]. It should be determined a priori how many attempts to scan will be made. In our cohort study of 70 individuals with SCI and three follow-up visits (210 images total), 12 images had movement artifact, with 7 individual datasets missing at least one image.
Environment and instrument
The lab needs a level entrance and adequate turning radius (60′ circle), or a T-shaped 60 × 60″ square with two 12 × 24″ sections removed, to ensure accessibility for both technologists and participants. The environment must be accessible (e.g., doors wide enough, no stairs), including an accessible restroom.
We use the Stratec (XCT-2000, Stratec Medizintechnik, Germany) for scanning the ultradistal tibia (4 %) and tibia shaft (38, 66 % sites) similar to other studies in the literature (Table 2). The 66 % site is used to estimate muscle size and muscle density (proxy for fatty infiltration of muscle). Our perspectives are based on one Stratec model (XCT-2000, Stratec Medizintechnik, Germany) and software (Stratec XCT-2000, version 6.00), and may not be applicable to other versions. We explored the potential for using HRpQCT (XtremeCT, Brüttisellen, Switzerland) in individuals with SCI. The narrow and shallow gantry of the first-generation scanner, combined with the inability to adjust the height of the opening limited the number of individuals that could be accommodated. Contractures, inability to transfer, variable wheelchair height, and spasticity are major concerns preventing safe and feasible HRpQCT image acquisition. Access to available participants is already limited. However, it may be feasible among other impairment groups who can transfer to a chair and do not have knee or ankle contractures.
Image acquisition
Some individuals with SCI have sufficient mobility to perform an independent level transfer from a wheelchair to sit in the scanning chair. pQCT scans can be performed while the individual remains in their wheelchair (Fig. 1a, b). The participant’s mobility (gait aid, manual versus power wheelchair), wheelchair height, trunk stability, and willingness or ability to transfer or lift leg into the scanner, should be recorded to inform the level of assistance required or attendant service needs, and scan setup (Fig. 1c). The wheelchair footplate (e.g., rigid or swing away) influences how close a wheelchair can be placed to the scanner (Fig. 1d).
Digital images of our protocol for peripheral quantitative computed tomography (pQCT) scan acquisition in an individual with spinal cord injury. The use of pQCT allows the scans to be performed while a person is in a wheelchair (a, b); note the position of the lower leg in the scanner and foot in the footplates (c, d)
We have performed the majority of our pQCT scans in individuals with SCI while they were seated in their wheelchairs. However, heights of wheelchairs vary (e.g., power wheelchairs may place the seated individual higher above the ground than manual wheelchairs). The pQCT scanner is on a pneumatic lift device with wheels, so we can adjust the scanner height (Fig. 2a–c). We have created metal stoppers of various sizes to secure the lift, in case the pneumatics fail (Fig. 2d); colleagues have reported one instance where this occurred, albeit not while the device was being used. We remove or swing away footplates. If the footplates are fixed, the individual will need to shift forward on their seat to ensure their limb can be inserted far enough into the scanner. Individuals with motor and sensory impairments of the trunk and upper extremities may need assistance shifting forward (e.g., lift them from behind, shift their hips or pull their pelvis forward). To reduce movement during scanning, we place a pillow behind the participant’s back if they are shifted forward on their seat.
Digital images of a pneumatic lift device with wheels as used in our peripheral quantitative computed tomography (pQCT) scanning protocol (a). The height of the pQCT scanner can be adjusted to the height of the chair (b, c). Metal stoppers of various sizes have been created to secure the lift device (d)
Clothing and assistive devices that may influence scan acquisition should be documented, ideally prior to arrival in case adaptations are needed. Adaptations should be performed consistently with repeat scans. For example, individuals who wear support stockings may not need to remove them, although it may influence palpation of surface anatomical landmarks or manual marking of the leg. We have applied strips of tape to make markings on. Obesity, swollen or edematous limbs also makes the palpation of surface anatomical landmarks difficult.
We recently recruited 60 individuals with neurologic impairment to examine feasibility of routine pQCT scanning and encountered: one leg too large to fit; four expressed concerns about spasms; 1 expressed concern about the space available to place the nonscanned leg and risk of spasm; 1 where spasms prevented scanning; 12 that were difficult to position; 3 where ability to tilt pQCT would have assisted positioning; 3 where positioning was uncomfortable; and 1 where contractures prevented scanning. The most common positioning issues were wheelchair hardware preventing close enough positioning and difficulty positioning the leg due to limited gantry size.
Image analysis
Interpretation of any measure requires knowledge of its reproducibility, least significant change, and minimal clinically significant difference [30, 31], as well as the individuals’ impairments. CALCBD mode is used to calculate total, trabecular, and cortical-subcortical bone densities and areas at ultra-distal tibia (4 % of length moving distal to proximal), and requires the user to define a contour mode and a peel mode, and other inputs depending on modes chosen (e.g., thresholds, peel %). The default for CALCBD uses contour mode 1, with a user-defined threshold to separate soft tissue from the bone outer edge (default threshold = 280 mg/cm3). With contour mode 1, voxels within the cortex that have a lower attenuation coefficient than the threshold are removed, resulting in black lines through the representation of bone depicted on the computer screen (Fig. 3). Notably, the selection of pQCT analysis protocols varies across studies in individuals with SCI, and the reporting of analysis methods is often incomplete (e.g., lack of details on contour and peel modes) (Table 2).
Peripheral quantitative computed tomography image analysis of trabecular bone mineral density using CALCBD and default contour mode 1 procedures. Contour mode 1 applies a threshold of 280 mg/cm3 to separate soft tissue from the outer edge of the bone. Using contour mode 1, voxels within the cortex are removed if they have a lower attenuation coefficient than the threshold, resulting in black lines through the analyzed representation of the bone
Contour modes 2 and 3 use an iterative threshold-guided edge-detection algorithm to segment the bone edge, with default (169 mg/cm3), or user-defined thresholds, respectively. The threshold is used to guide the selection of a voxel on the bone edge, and then iteratively guide determination of adjacent voxels of equivalent or greater density to establish the bone contour. Pervasive low-density voxels on or near the bone edge can result in failure of the contour detection algorithm to iterate. In cases of low-density bone, a lower threshold than the default may be more appropriate. We did pilot work assessing the precision of pQCT-based bone measures [30], and a priori, we tested all three modes to inform the choice of analysis protocol; using the default analysis protocol for contour mode 1, we had several scans with “black lines” indicating the threshold was too high, and for contour mode 2 (threshold 169 mg/cm3), the contour detection algorithm failed to iterate in a number of scans.
Peel mode 1 concentrically “peels away” a user-defined percentage of the bone surface (default 55 %) starting from the periosteal edge, labeling it as the cortical-subcortical region, and what remains (default 45 %) is analyzed as trabecular bone. Peel mode 2 employs a user-defined threshold to separate cortical and trabecular bone. Other peel modes are available [32]. Peel mode 2 may be preferable when analyzing the bones of individuals with SCI, because bone area is reduced to variable degrees, so a standardized percent area to separate cortical and trabecular bone may not be appropriate. We have used contour mode 3 and peel mode 2 with an inner threshold of 130 mg/cm3 and an outer threshold of 400 mg/cm3 to avoid segmentation failures, consistent with Ashe et al. [32]. Several pQCT studies in the literature report the use of a standardized peel mode (default 45 %), which may be inappropriate given the variable bone loss across individuals with SCI (Table 2).
CORTBD mode is used to calculate total and cortical bone densities, bone areas, and bone geometry in scans taken of the bone shaft. Default analysis uses a 710 mg/cm3 threshold to define cortical bone. We have not experienced segmentation failures using the default. However, individuals with SCI experience substantial endocortical bone resorption, so a large portion of bone area is excluded in the CORTBD analysis because it does not meet the threshold. Therefore, cortical bone area, cortical thickness, and total bone density are typically much lower than would be expected in age- and gender-matched peers, but cortical BMD is normal or only slightly reduced. To obtain a true estimate of the cortical bone density including areas where bone resorption has created resorption bays, or “trabecularization” of cortical bone (Fig. 4), lower thresholds or alternative analysis methods are required, or total density or other variables should be considered in lieu of cortical density.
Peripheral quantitative computed tomography image analyses of cortical bone mineral density using CORTBD default procedures. In cases of cortical bone loss where bone resorption bays and “trabecularization” of cortical bone are present, lower thresholds or alternative analysis methods are required. Total bone mineral density or other variables should be considered in lieu of cortical bone mineral density when assessing bone strength in individuals with neurologic impairment
The type and timing of impairment onset must be considered in the selection of variables to examine a priori. Previously, we reported that in 63 individuals with SCI, for each standard deviation decrease in polar moment of inertia at the 66 % site, there was an increased likelihood of having prevalent fractures (OR 3.2, 95 % CI 1.2–11.4) after controlling for presence of motor complete injury [13]. However, individuals who experience neurologic impairment at birth or during childhood develop normal total and cortical bone density, with little to no evidence of endocortical resorption; they have reduced periosteal expansion, and therefore have much lower total and cortical bone area, and lower moment of inertia compared to age- and gender-matched individuals [1].
Muscle analysis and data interpretation
pQCT has also been used to quantify muscle cross-sectional area (CSA) and muscle density, most commonly at the site of widest calf circumference (66 % tibia length). Stratec Analysis Software (V6.0, Orthometrix Inc., White Plains, NY, USA) provides instructions on how to adapt CALCBD analysis modes to estimate muscle CSA; total muscle + bone area is segmented from subcutaneous fat area, bone area is determined and subtracted from muscle + bone area to obtain muscle CSA. Muscle density can be derived by subtracting bone mass from bone + muscle mass to derive muscle mass, and dividing muscle mass by muscle CSA. However, one step in the muscle CSA analysis requires the scan to be analyzed using contour mode 3 with an edge detection algorithm (threshold 40 mg/cm3) to segment the outer border or fascia line of muscle CSA from subcutaneous fat. With muscle atrophy and fatty infiltration, the segmentation step may fail because of variation in linear attenuation values along the muscle border, preventing delineation of muscle-subcutaneous fat boundary. An additional step requires a threshold-based segmentation of bone from muscle using contour mode 1, peel mode 2, and a threshold of 280 mg/cm3. If voxels in the cortex have a density lower than 280 mg/cm3, they will be removed, resulting in a failed segmentation.
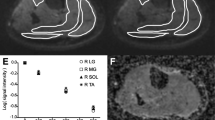
We have recently compared the manufacturer’s analysis method for muscle area and density to a method using watershed techniques (sliceOmatic software package v4.3, Tomovision, Magog, QC, Canada) [33]. A watershed algorithm creates what is akin to a topographical representation of the image, where the gray level of a voxel represents its “altitude,” and a relief is the difference in elevation from the lowest to highest point [34]. If a drop of water were to fall on the relief, it would flow toward a local low point. Where adjacent or nearby voxels have large differences in signal intensity, the difference in “elevation” would be high. The topographic representation can be used to define watershed lines and catchment basins (“pools”), such that watershed lines, or the edges around each “pool” are areas of large differences in signal intensity. The pools can then be “tagged” by tissue type and merged. The watershed segmentation method yielded lower precision error (1.18–2.01 %) and higher average muscle density (70.2 ± 9.2 mg/cm3) compared to threshold-based edge detection segmentation (1.77–4.06 % error, average muscle density 67.4 ± 10.3 mg/cm3), and for SCI, where more fatty infiltration was present, precision error improved by 1.56 and 2.64 % for muscle area and density, respectively. Notably, 20.3 % (35/172) of images failed step 1 segmentation using threshold-based edge detection. Among 35 images, 31.4 % (11/35) of the images failed step 2. In contrast, the entire muscle area was segmented for all images analyzed with the watershed algorithm. Therefore, the watershed-guided manual segmentation can be used to improve precision and reduce failed segmentations in images with muscle atrophy and fatty infiltration of muscle, if the software and expertise is available.
Discussion and summary
Motor and sensory impairments and health complications present unique challenges to address during participant screening and pQCT image acquisition and analysis. The challenges described herein may be applicable in older adults or with other health conditions. For example, spasticity can be present with other neurologic impairments. Contractures can occur with neurologic impairment, arthritis, or joint replacement or injury. It is not uncommon to encounter adults whose calf circumference exceeds 439 mm. Lower extremity edema or obesity may also limit positioning or the location of bony landmarks. Day-to-day variability in edema may compromise reproducible landmarking.
Prior to initiating a pQCT study, accessibility of the instrument and environment, and availability of technicians or attendants with knowledge of the impairments and health complications of the population of interest should be considered in study planning. Older or obese adults with limited mobility or range of motion may also find it difficult to position the leg in the scanner independently and may require assistance. The main limitations that we encountered are gantry size and the limitations posed by some wheelchairs. We allow for extra time to “problem solve” positioning during the first study visit.
Default image analysis protocols may not work well in individuals with very low bone density, muscle atrophy, or fatty infiltration of muscle, such as older adults or individuals with diabetes [35, 36], and alternative image analysis protocols are available. The presence of low bone mineral density, muscle atrophy or fatty infiltration, positioning challenges, or movement artifact may influence the precision of bone and muscle indices. Precision in a given laboratory should be reported for the population of interest rather than a convenience sample. Reporting on feasibility aspects should become standard practice, such as the number excluded from the study or that could not be scanned and why, or modifications to image acquisition and analysis protocols. We outline challenges and solutions so that researchers and clinicians who use pQCT in future do not experience the learning curve that we have.
References
Giangregorio LM, McCartney N (2007) Reduced loading due to spinal-cord injury at birth results in “slender” bones: a case study. Osteoporos Int: A Journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 18(1):117–120. doi:10.1007/s00198-006-0201-3
Gorgey AS, Dudley GA (2007) Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 45(4):304–309. doi:10.1038/sj.sc.3101968
Shah PK, Stevens JE, Gregory CM, Pathare NC, Jayaraman A, Bickel SC, Bowden M, Behrman AL, Walter GA, Dudley GA, Vandenborne K (2006) Lower-extremity muscle cross-sectional area after incomplete spinal cord injury. Arch Phys Med Rehabil 87(6):772–778. doi:10.1016/j.apmr.2006.02.028
Moore CD, Craven BC, Thabane L, Laing AC, Frank-Wilson AW, Kontulainen SA, Papaioannou A, Adachi JD, Giangregorio LM (2015) Lower-extremity muscle atrophy and fat infiltration after chronic spinal cord injury. J Musculoskelet Neuronal Interact 15(1):32–41
Frey-Rindova P, de Bruin ED, Stussi E, Dambacher MA, Dietz V (2000) Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord 38(1):26–32
de Bruin ED, Vanwanseele B, Dambacher MA, Dietz V, Stussi E (2005) Long-term changes in the tibia and radius bone mineral density following spinal cord injury. Spinal Cord 43(2):96–101. doi:10.1038/sj.sc.3101685
de Bruin ED, Dietz V, Dambacher MA, Stussi E (2000) Longitudinal changes in bone in men with spinal cord injury. Clin Rehabil 14(2):145–152
de Bruin ED, Herzog R, Rozendal RH, Michel D, Stussi E (2000) Estimation of geometric properties of cortical bone in spinal cord injury. Arch Phys Med Rehabil 81(2):150–156
Dionyssiotis Y, Trovas G, Galanos A, Raptou P, Papaioannou N, Papagelopoulos P, Petropoulou K, Lyritis GP (2007) Bone loss and mechanical properties of tibia in spinal cord injured men. J Musculoskelet Neuronal Interact 7(1):62–68
Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H (2004) Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 34(5):869–880. doi:10.1016/j.bone.2004.01.001
Eser P, Frotzler A, Zehnder Y, Denoth J (2005) Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil 86(3):498–504. doi:10.1016/j.apmr.2004.09.006
Frotzler A, Berger M, Knecht H, Eser P (2008) Bone steady-state is established at reduced bone strength after spinal cord injury: a longitudinal study using peripheral quantitative computed tomography (pQCT). Bone 43(3):549–555. doi:10.1016/j.bone.2008.05.006
Lala D, Craven BC, Thabane L, Papaioannou A, Adachi JD, Popovic MR, Giangregorio LM (2014) Exploring the determinants of fracture risk among individuals with spinal cord injury. Osteoporos Int: A Journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 25(1):177–185. doi:10.1007/s00198-013-2419-1
McCarthy ID, Bloomer Z, Gall A, Keen R, Ferguson-Pell M (2012) Changes in the structural and material properties of the tibia in patients with spinal cord injury. Spinal Cord 50(4):333–337. doi:10.1038/sc.2011.143
Totosy de Zepetnek JO, Craven BC, Giangregorio LM (2012) An evaluation of the muscle-bone unit theory among individuals with chronic spinal cord injury. Spinal Cord 50(2):147–152. doi:10.1038/sc.2011.99
Coupaud S, McLean AN, Allan DB (2009) Role of peripheral quantitative computed tomography in identifying disuse osteoporosis in paraplegia. Skelet Radiol 38(10):989–995. doi:10.1007/s00256-009-0674-1
Coupaud S, McLean AN, Lloyd S, Allan DB (2012) Predicting patient-specific rates of bone loss at fracture-prone sites after spinal cord injury. Disabil Rehabil 34(26):2242–2250. doi:10.3109/09638288.2012.681831
Coupaud S, McLean AN, Purcell M, Fraser MH, Allan DB (2015) Decreases in bone mineral density at cortical and trabecular sites in the tibia and femur during the first year of spinal cord injury. Bone 74:69–75. doi:10.1016/j.bone.2015.01.005
Varzi D, Coupaud SA, Purcell M, Allan DB, Gregory JS, Barr RJ (2015) Bone morphology of the femur and tibia captured by statistical shape modelling predicts rapid bone loss in acute spinal cord injury patients. Bone 81:495–501. doi:10.1016/j.bone.2015.08.026
Dudley-Javoroski S, Shields RK (2010) Longitudinal changes in femur bone mineral density after spinal cord injury: effects of slice placement and peel method. Osteoporos Int: A Journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 21(6):985–995. doi:10.1007/s00198-009-1044-5
Dudley-Javoroski S, Shields RK (2012) Regional cortical and trabecular bone loss after spinal cord injury. J Rehabil Res Dev 49(9):1365–1376
Rittweger J, Goosey-Tolfrey VL, Cointry G, Ferretti JL (2010) Structural analysis of the human tibia in men with spinal cord injury by tomographic (pQCT) serial scans. Bone 47(3):511–518. doi:10.1016/j.bone.2010.05.025
Dionyssiotis Y, Stathopoulos K, Trovas G, Papaioannou N, Skarantavos G, Papagelopoulos P (2015) Impact on bone and muscle area after spinal cord injury. BoneKEy Rep 4:633. doi:10.1038/bonekey.2014.128
Gibbs JC, Craven BC, Moore C, Thabane L, Adachi JD, Giangregorio LM (2015) Muscle density and bone quality of the distal lower extremity among individuals with chronic spinal cord injury. Top Spinal Cord Inj Rehabil 21(4):282–293
Craven BC, Zeng L, Farahani F, Hitzig SL Is self-report of neurological impairment among persons living with chronic spinal cord injury sufficiently accurate for research studies? In: CAPM&R 62nd Annual Scientific Meeting, Sheraton Hotel Newfoundland, 2014. vol 10. Journal of Rehabilitation Medicine, St. John’s Newfoundland, p 1060
Pandyan AD, Gregoric M, Barnes MP, Wood D, Van Wijck F, Burridge J, Hermens H, Johnson GR (2005) Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil 27(1–2):2–6
Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, Kroin JS (1989) Intrathecal baclofen for severe spinal spasticity. N Engl J Med 320(23):1517–1521. doi:10.1056/NEJM198906083202303
Royal College of Radiologists (2003) Safe sedation, analgesia, and anaesthesia within. Radiology Departments, London
Blew RM, Lee VR, Farr JN, Schiferl DJ, Going SB (2014) Standardizing evaluation of pQCT image quality in the presence of subject movement: qualitative versus quantitative assessment. Calcif Tissue Int 94(2):202–211. doi:10.1007/s00223-013-9803-x
Giangregorio L, Lala D, Hummel K, Gordon C, Craven BC (2013) Measuring apparent trabecular density and bone structure using peripheral quantitative computed tomography at the tibia: precision in participants with and without spinal cord injury. J Clin Densitom: Off J Int Soc Clin Densitom 16(2):139–146. doi:10.1016/j.jocd.2012.02.003
Shepherd JA, Schousboe JT, Broy SB, Engelke K, Leslie WD (2015) Executive summary of the 2015 ISCD position development conference on advanced measures from DXA and QCT: fracture prediction beyond BMD. J Clin Densitom: Off J Int Soc Clin Densitom 18(3):274–286. doi:10.1016/j.jocd.2015.06.013
Ashe MC, Khan KM, Kontulainen SA, Guy P, Liu D, Beck TJ, McKay HA (2006) Accuracy of pQCT for evaluating the aged human radius: an ashing, histomorphometry and failure load investigation. Osteoporos Int: A Journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 17(8):1241–1251. doi:10.1007/s00198-006-0110-5
Wong AK, Hummel K, Moore C, Beattie KA, Shaker S, Craven BC, Adachi JD, Papaioannou A, Giangregorio L (2015) Improving reliability of pQCT-derived muscle area and density measures using a watershed algorithm for muscle and fat segmentation. J Clin Densitom: Off J Int Soc Clin Densitom. doi:10.1016/j.jocd.2014.04.124
Fu G, Hojjat SA, Colchester AC (2004) Integrating watersheds and critical point analysis for object detection in discrete 2D images. Med Image Anal 8(3):177–185. doi:10.1016/j.media.2004.06.002
Miljkovic-Gacic I, Gordon CL, Goodpaster BH, Bunker CH, Patrick AL, Kuller LH, Wheeler VW, Evans RW, Zmuda JM (2008) Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am J Clin Nutr 87(6):1590–1595
Butner KL, Creamer KW, Nickols-Richardson SM, Clark SF, Ramp WK, Herbert WG (2012) Fat and muscle indices assessed by pQCT: relationships with physical activity and type 2 diabetes risk. J Clin Densitom: Off J Int Soc Clin Densitom 15(3):355–361. doi:10.1016/j.jocd.2012.01.012
Acknowledgments
The present work was prepared independent of any input from the manufacturer of the pQCT device described here. The authors acknowledge the support from the Ontario Neurotrauma Foundation (grant no. 2009-SC-MA-684), the Canadian Institutes of Health Research (CIHR, grant no. 86521), the Spinal Cord Injury Solutions Network (RHI; grant no. 2010-43), the Canadian Foundation for Innovation, University Health Network Rehabilitation Medicine Associates and Ontario Research Fund. Dr. Giangregorio is a CIHR New Investigator, recipient of an Early Researcher Award from the Ontario Ministry of Research and Innovation, and recipient of the Bloomberg Manulife Prize for the Promotion of Active Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Giangregorio has received research funding from Merck Canada and ICON, unrelated to the work presented here.
Rights and permissions
About this article
Cite this article
Giangregorio, L.M., Gibbs, J.C. & Craven, B.C. Measuring muscle and bone in individuals with neurologic impairment; lessons learned about participant selection and pQCT scan acquisition and analysis. Osteoporos Int 27, 2433–2446 (2016). https://doi.org/10.1007/s00198-016-3572-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3572-0