Abstract
Purpose
There is a lack of objective evidence investigating how previous non-augmented ACL suture repair techniques and contemporary augmentation techniques in ACL suture repair restrain anterior tibial translation (ATT) across the arc of flexion, and after cyclic loading of the knee. The purpose of this work was to test the null hypotheses that there would be no statistically significant difference in ATT after non-, static- and dynamic-augmented ACL suture repair, and they will not restore ATT to normal values across the arc of flexion of the knee after cyclic loading.
Methods
Eleven human cadaveric knees were mounted in a test rig, and knee kinematics from 0° to 90° of flexion were recorded by use of an optical tracking system. Measurements were recorded without load and with 89-N tibial anterior force. The knees were tested in the following states: ACL-intact, ACL-deficient, non-augmented suture repair, static tape augmentation and dynamic augmentation after 10 and 300 loading cycles.
Results
Only static tape augmentation and dynamic augmentation restored ATT to values similar to the ACL-intact state directly postoperation, and maintained this after cyclic loading. However, contrary to dynamic augmentation, the ATT after static tape augmentation failed to remain statistically less than for the ACL-deficient state after cyclic loading. Moreover, after cyclic loading, ATT was significantly less with dynamic augmentation when compared to static tape augmentation.
Conclusion
In contrast to non-augmented ACL suture repair and static tape augmentation, only dynamic augmentation resulted in restoration of ATT values similar to the ACL-intact knee and decreased ATT values when compared to the ACL-deficient knee immediately post-operation and also after cyclic loading, across the arc of flexion, thus allowing the null hypotheses to be rejected. This may assist healing of the ruptured ACL. Therefore, this study would support further clinical evaluation of dynamic augmentation of ACL repair.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest in primary repair of acute ruptures of the ACL has reawakened in the last decade as more insights on biology and biomechanics of the ruptured ACL have emerged [6, 16, 29, 36, 37].
Contemporary ACL suture repair has yielded good histological and biomechanical results in porcine and ovine animal model studies [18, 31, 38] and promising short- to midterm results in prospective clinical series [1, 2, 4, 6, 13, 22, 23, 30, 33].
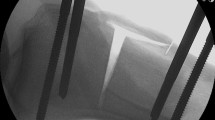
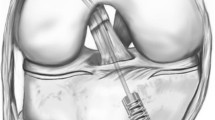
In contrast to previous procedures (Fig. 1), contemporary ACL suture repairs may be augmented with a suture or tape with bony fixation to approximate the ACL remnants, to help to maintain length, allow for early range of motion without compromising the repair site and promote healing [8,9,10, 17, 34] (Figs. 2 and 3).
However, in ‘static’ augmentation, where the suture or tape is fixed to both the tibial and the femoral bone directly, anisometric placement and cyclic loading could lead to elongation of the repair and increase of anterior tibial translation (ATT), and therefore isometric femoral and tibial tunnel position is important [10, 17]. Unfortunately, in practice, isometric tunnel placement can most likely not be achieved (Fig. 2) [17, 20, 40]. ‘Dynamic’ augmentation may address the problems associated with anisometric tunnel placement and cyclic loading by attaching the suture or tape to an additional elastic link (a spring-in-screw mechanism) on the tibial side [17, 34], to allow length changes to occur during knee motion while maintaining reduction of ATT (Fig. 3).
Although augmented suture repairs of the ruptured ACL are performed on patients today, there is a lack of objective evidence investigating how contemporary augmentation techniques in ACL suture repair affect ATT across the arc of flexion, and after cyclic loading of the knee (simulating postoperative rehabilitation), and how they relate to previous non-augmented repair techniques [1, 4, 6, 13, 19, 22, 23, 25, 28, 30, 33, 39].
Therefore, the purpose of this study was to gain insight into the biomechanical properties of contemporary static and dynamic augmentation techniques in ACL repair, and to put them in historical perspective by comparing them to a non-augmented ACL suture repair technique that was frequently used late in the last century, when suture repair of the ruptured ACL was abandoned in favour of ACL reconstruction. The aim was to examine the following null hypotheses: there would be no statistically significant difference in ATT after non-augmented sutured, static-augmented and dynamic-augmented ACL repair, and they will not restore ATT to normal values in all flexion angles of the knee after cyclic loading (simulating postoperative rehabilitation).
Materials and methods
Specimen preparation, optical tracking, testing protocol and data analysis were performed as described extensively by Stephen et al. [35].
Specimen preparation
Fourteen fresh-frozen cadaveric knee specimens were obtained from a tissue bank. Two specimens were used to develop the testing protocol, and the data of one specimen were corrupted and could not be analysed. The remaining 11 knees were included for final data analysis (mean age 49 (range 28–59), 8 right sided, 3 left sided, 6 male, 5 female). The specimens were stored at − 20 °C and thawed for 24 h before use. After preparation of the specimens, leaving all soft tissues except the skin and subcutaneous layer intact, the femur and tibia were cut and cemented to axially aligned rods. After preparation, the femoral rod was secured in a rig allowing manual passive knee flexion–extension from 0° to 90° by moving the femur with the unconstrained tibia hanging vertically (Fig. 4). Anterior and posterior drawer forces without inducing rotational torque or inhibiting natural coupled tibial rotation, and rotational torques, could be imposed on the specimens as shown in Fig. 4. All surgical procedures and testing took place on the same day without removing the specimen from the test rig.

(reprinted with permission of Stephen et al. [35])
Test rig used for the study. The specimen position was adjusted to approximately align knee and rig flexion–extension axes. a Manual passive flexion–extension movements were applied to the femur; the motion of the hanging tibia (b) was otherwise unconstrained. The anterior (c) and posterior forces were applied with weights connected to the proximal tibia by cables passed over pulleys, via two semicircular hoops which were mounted on a Steinmann pin drilled mediolaterally across the tibia perpendicular to the shaft at the level of the tibial tuberosity. Internal and external rotation torques were applied with weights (d) connected via a pulley and string system to opposite poles of a 200-mm polyethylene disc secured at the end of the tibial intramedullary rod
Optical tracking
Tibiofemoral joint kinematics were measured by use of a Polaris optical tracking system (NDI—Northern Digital Inc.) with passive digitized sets of Brainlab reflective markers (Brainlab) mounted securely onto the tibia and femur. Kinematic data were processed by use of Visual3D (C-Motion Inc.). Zero degree knee flexion was defined when the tibial and femoral rods were parallel in the sagittal plane. Anterior–posterior translation was calculated as the perpendicular distance from the midpoint of the femoral epicondylar axis to the tibial coronal reference plane [3, 15, 21, 35]. The tracking system is known to have a translational accuracy of 0.1 mm, and this test method has been used previously [3, 15, 21, 35]. The intact knee at full extension (0° of flexion) was taken to be 0 mm translation and 0° rotation, and all measurements were normalized to this. The motions described are tibial motion in relation to the femur.
Surgical procedures
All surgical procedures were performed by the surgeon author (R. H.), who has considerable experience in ACL reconstruction surgery. After mounting the knee in the kinematic test rig, the integrity of the ligaments, menisci and joint surfaces was checked by manual testing of laxities, and with standard arthroscopy through anteromedial and anterolateral portals. Laxity tests were performed with the ACL-intact knee. Arthroscopically, the native ACL was transected close to the femoral attachment with a beaver knife, and left in situ. The laxity tests were repeated with the ACL-deficient knee.
The knee was prepared arthroscopically so three suture techniques could be performed.
To replicate in vivo circumstances, the femoral and tibial tunnels were created with the transected ACL in situ. Two 2.4 mm diameter femoral tunnels were created from the femoral ACL attachment to the lateral distal femoral cortex with a drill tip guide pin with eyelet, which was placed through an accessory anteromedial portal, just superior to the tibial plateau and medial meniscus and just anterior to the medial femoral condyle, with the knee in 120 degrees of flexion. One guide pin was positioned in the “isometric point” [11] in the “high” and “deep” part of the femoral anteromedial bundle attachment. Since this “isometric point” was not visible with the ruptured ACL in situ, an offset guide was used to replicate in vivo circumstances. The other guide pin was positioned in the femoral posterolateral bundle attachment freehand. An incision was made to expose the lateral femoral cortex in the trajectory of the guide pins, to allow cortical fixation of the buttons and sutures. Both guide pins were removed and shuttle wires were pulled through the tunnels.
One 2.4 mm diameter tibial tunnel of at least 50 mm length was created with a drill tip guide pin using an aiming device from the anteromedial aspect of the tibial metaphysis to the “isometric point” in the anterior part of the tibial attachment of the remaining ACL stump [11]. A shuttle wire was pulled through the tibial tunnel.
Suture repair of the ruptured ACL
Four Ethibond-0 sutures (Ethicon, Somerville, NJ, USA) were passed through the distal part of the sectioned ACL, in an anterior to posterior direction, starting near the attached base and progressing toward the torn end (the Marshall technique) [26, 27, 32]. The suture ends were grouped together into two groups, keeping the anteriorly and posteriorly exiting sutures separate. The posterior suture group was pulled through the posterolateral femoral tunnel, and the anterior suture group was pulled through the “isometric” anteromedial femoral tunnel with shuttle wires. The knee was placed in 20 degrees of flexion, with a posterior translation force of 80-N imposed on the tibia by the kinematics test rig [34]. The individual suture ends were pulled tight to eliminate any slack, and the two groups of sutures were tied down as one unit over the cortical bone surface between the two femoral tunnels on the lateral aspect of the distal femur (Fig. 1). The laxity tests were repeated, and the ACL was resected and the Marshall sutures were removed.
Static tape augmentation of the ruptured ACL
New shuttle wires were placed in the tibial and “isometric” anteromedial femoral tunnels. A cortical button suspension with adjustable loop length (Tightrope™ RT, Arthrex, Naples, Florida, USA) was loaded with a double loop tape (FiberTape™ 2 mm, Arthrex, Naples, Florida, USA), and pulled through the tibial and femoral tunnel with shuttle wires, and it was verified that the button was fixed behind the lateral femoral cortex [12, 39]. The loop of the suspensory fixation was shortened until the tape was pulled approximately 20 mm inside the femoral tunnel. A 3.5-mm bone socket was created 10 mm distal to the tibial tunnel in the anteromedial aspect of the tibia. The socket was tapped to 4.75 mm. The tibial ends of the double loop tape were loaded into the eyelet at the tip of a screwdriver (SwiveLock™, Arthrex, Naples, Florida, USA). The knee was placed in zero degrees of flexion, with a posterior translation force of 80 N imposed on the proximal tibia by the kinematics test rig [25, 34]. While pulling the tape with manual tension in the direction of the tibial tunnel, the tip of the loaded screwdriver was placed in the opening of the tibial socket and the distal ends of the tape were pulled parallel to the screwdriver. The tape was marked at the level of the depth mark on the screwdriver. With the tip of the screwdriver repositioned to the level of the marking on the tape, the SwiveLock™ was pushed inside the 3.5 mm tibial socket manually until the depth mark on the screwdriver lined up with the tibial cortex. The tape was then secured in the tibial socket with the SwiveLock™ PEEK bone anchor interference screw (Fig. 2). The laxity tests were repeated. After the laxity tests, if this procedure was not randomized to be the last procedure, the tape was removed.
Dynamic augmentation of the ruptured ACL
A 2.4 mm guide pin was positioned in the 2.4 mm tibial tunnel. An outside-in tibial socket 30 mm long and 10 mm diameter was reamed over the guide pin with a cannulated drill with depth limitation. A Ligamys™ Monobloc second generation fixation device (Mathys, Betlach, Switzerland) was screwed inside the tibial bone tunnel over the guide pin, until it lined up with the tibial cortex. The guide pin was removed and a shuttle wire was led through the tibial and “isometric” anteromedial femoral tunnels. A Ligamys™ braid was pulled distally through the femoral and tibial tunnels with the shuttle wire, and it was verified that the proximal fixation button abutted the lateral femoral cortex. The knee was placed in 0 degrees of flexion [34]. With the tensioner, the braid was tensioned to maximal manual load and released, after which it was tensioned again to 80-N (Mathys Surgical Instructions) [34]. A clamping cone was fixed into the Ligamys™ Monobloc with a torque screwdriver (Fig. 3). The laxity tests were repeated.
The clamping cone was removed from the Monobloc to release the tension on the Ligamys™ braid, the same tensioning procedure was repeated with 60-N (to match the tension used in the other suturing methods) [34] and the laxity tests were repeated.
After the laxity tests, if this procedure was not randomized to be the last procedure, the Ligamys™ Monobloc was removed, a greased 2.4-mm drill pin was placed in the tibial tunnel, the tibial socket was filled with polyester car body filler and the drill pin was removed after the filler had hardened.
Testing protocol
The 6 degrees of freedom data of the position of the tibia with respect to the femur were recorded with no external loads applied to the tibia, only the weight of the hanging tibia and attached rod, which remained constant throughout testing. The kinematic data were also recorded with the following loads applied in randomized order: 89-N tibial anterior drawer force, 89-N tibial posterior drawer force, 5-Nm tibial internal rotation torque, 5-Nm tibial external rotation torque, and a combined 89-N tibial anterior drawer force and 5-Nm tibial internal rotation torque to simulate the pivot shift laxity [24].
This test protocol of six loading conditions was repeated with the knee in ten states: ACL-intact and ACL-sectioned state, as well as ACL suture repair, static tape augmentation and dynamic augmentation with 80-N and with 60-N pretension state after 10 and 300 cycles of flexion and extension between 0° and 90°.
During development of the protocol, testing of the non-augmented ACL repair led to pull-out of the sutures weakening the ACL stump. Therefore, after having tested the intact state and sectioned state, the repaired state of the ACL with the suture technique was tested first, after which the static tape augmentation and dynamic augmentation techniques were tested in randomized order per specimen. Testing of the dynamic augmentation with 80-N pretension always preceded testing with 60-N pretension.
During each test, three cycles of knee flexion–extension between 0° and 90° were repeated manually to gather the data.
The local Institutional Review Board (IRB: Imperial College Healthcare Tissue Bank, London, UK; IRB Nr. R17007) approved this study.
Statistical analysis
The mean tibial translations and rotations were calculated at 10° intervals from 0° to 90° of flexion. The coordinate system was defined so that ATT and external rotation were taken to be positive. Visual3D motion data were processed using custom-written Matlab scripts (The MathWorks Inc.).
A power calculation using G*Power software a priori [7], based on prior work that used the same optical tracking system [14] determined that a sample size of 11 would allow identification of changes of translation and rotation of 0.8 mm and 0.9°, respectively, with 80% power and 95% confidence. Dependent variables were anterior and posterior translation, internal and external rotation and combined anterior translation and internal rotation laxities.
Data were analysed in SPSS (version 22.0; IBM Corp). The primary factors investigated were the ten knee states and seven flexion angles (0°–10°–20°–30°–40°–60°–90°). A mixed-model analysis for repeated measures was performed to study both the effect of the different flexion arcs and the effect of the different knee states on the dependent variables. Post hoc SIDAK tests were applied when differences between knee states or flexion arcs were found to investigate which knee states or flexion arcs differed while controlling for multiple comparisons. Furthermore, the interaction effect of knee state and flexion arc on the dependent variables was studied. The level of significance was set at p < 0.05.
Results
Mean ATT across the arc of flexion for different states of the knee is presented in Table 1. Rather than present normal laxity data, Table 1 and the following sections display movements away from the free-hanging position of the tibia (neutral loading) when the ACL was intact, which has greater clarity regarding residual laxities after different stages of the experiment.
Anterior drawer
While applying 89-N of anterior force sectioning of the native ACL resulted in a significant increase of ATT (p = 0.000; Table 1).
Directly post-operation (10 cycles), with non-augmented suture repair of the ACL and static tape and dynamic augmentation (for both 80 and 60-N pretensioning) the ATT was not significantly different than in the intact knee. However, with non-augmented suture repair of the ACL the ATT was not significantly different than the ACL-deficient knee either, while with static tape (p = 0.011) and dynamic augmentation (for both 80 and 60-N pretensioning; p = 0.000) the ATT was significantly less than the ACL-deficient knee (Figs. 5, 6, 7, 8 respectively).
The difference in anterior tibial translation (mean; mm) across the range of knee flexion (°) from the neutral position of the tibia in the intact knee under 89-N anterior translation, for the intact knee, after ACL transection, and for the ACL sutured state after 10 and 300 movement cycles (n = 11)
The difference in anterior tibial translation (mean; mm) across the range of knee flexion (°) from the neutral position of the tibia in the intact knee under 89-N anterior translation, for the intact knee, after ACL transection, and for the ACL with static tape augmentation after 10 and 300 movement cycles (n = 11)
The difference in anterior tibial translation (mean; mm) across the range of knee flexion (°) from the neutral position of the tibia in the intact knee under 89-N anterior translation, for the intact knee, after ACL transection, and for the ACL with the dynamic augmentation device set to 80-N after 10 and 300 movement cycles (n = 11)
The difference in anterior tibial translation (mean; mm) across the range of knee flexion (°) from the neutral position of the tibia in the intact knee under 89-N anterior translation, for the intact knee, after ACL transection, and for the ACL with the dynamic augmentation device set to 60-N after 10 and 300 movement cycles (n = 11)
After 300 movement cycles, with non-augmented suture repair of the ACL the ATT was significantly greater than the intact knee (p = 0.000) and was not significantly different to the ACL-deficient state. With static tape and dynamic augmentation, the ATT was not significantly greater than the intact knee. However, with static tape augmentation, the ATT was not significantly different to the ACL-deficient state either, while with dynamic augmentation the ATT remained significantly less than the ACL-deficient state (p = 0.000; Figs. 5, 6, 7, 8 respectively).
Although cyclic loading tended to cause the ATT to increase, it did not cause a significant increase in laxity for any of the repairs as compared to directly postoperation. When compared to non-augmented suture repair of the ACL, cyclic loading did not lead to greater reduction of ATT with static tape augmentation, whereas dynamic augmentation did lead to a significant greater reduction of ATT (p = 0.000; Fig. 9). Furthermore, when compared to static tape augmentation, cyclic loading did not result in a significant reduction of ATT with dynamic augmentation with 60-N pretensioning, whereas in contrast, dynamic augmentation with 80-N pretensioning did result in significant reduction of ATT (p = 0.028) (Fig. 9).
The difference in anterior tibial translation (mean; mm) across the range of knee flexion (°) from the neutral position of the tibia in the intact knee under 89-N anterior translation, for the intact knee, after ACL transection, and for the ACL sutured state, the ACL with static tape augmentation and the ACL with the dynamic augmentation device set to 80-N and 60-N after 300 movement cycles (n = 11)
Combined anterior drawer and internal rotation/posterior drawer/external rotation/internal rotation
The state of the knee had no significant effect on ATT and internal rotation laxity under combined 89-N anterior force and 5-Nm of internal rotational torque. Similarly, the state of the knee had no significant effect on posterior tibial translation, external or internal rotation laxity after application of a 89-N of posterior force and a 5-Nm of external or internal rotational torque, respectively.
Discussion
The most important finding of this study is that, across the arc of flexion of the knee, only dynamic augmentation was able to restore ATT to values similar to the ACL-intact state and decrease ATT significantly compared to the ACL-deficient state directly postoperation, and to maintain this after cyclic loading, thus allowing the null hypotheses to be rejected.
Several biomechanical studies have shown that previously used ACL suture repair techniques may lead to higher than normal forces in the repair tissue, which could lead to repair stretching and failure [5], and did not restore normal ATT compared to the ACL-intact state [9, 10, 32], which is line with this study.
Contemporary augmentation techniques use strong, small diameter, non-resorbable braid. These cause little disruption of the ACL attachment and ACL tissue, and leave room for formation of hypertrophic scar tissue [10]. Contrary to earlier findings [5], more recent biomechanical studies in porcine [10], caprine [8, 9] and human [17, 34] knee specimens using contemporary repair techniques have suggested that static tape and dynamic augmentation can restore ATT values to normal directly postoperation.
However, anisometric tunnel placement and cyclic loading may be of concern [10, 17]. In static tape augmentation, since there is no compensatory mechanism for length changes (other than limited elastic stretching/slackening of the tape), anisometric tunnel placement is associated with increased laxity both directly postoperation [10] and after cyclic loading [17], implying that anisometric tunnel placement can lead to elongation of a suture repair (and consequently, the ACL) during early postoperative mobilisation [10, 17].
Dynamic augmentation addresses the concern about anisometric tunnel placement and cyclic loading in augmented ACL suture repair [17, 34]. It has been reported that dynamic augmentation with 85-N pretensioning restored laxity to normal directly postoperation and after cyclic loading, when tested in one flexion angle [17]. A comparison of dynamic stabilisation with 80-N versus 60-N pretensioning reported that 80-N pretensioning could restore ATT to normal across the arc of flexion directly postoperation [34].
This study supports these findings and found that dynamic augmentation restored ATT to normal values compared to the ACL-intact state, and decreased ATT significantly compared to the ACL-deficient state directly postoperation, and maintained that difference after cyclic loading, across the arc of flexion.
Although anisometric tunnel placement is addressed by dynamic augmentation, it does raise the concern of overconstraint or residual laxity depending on the amount of pretensioning. Although no statistical analysis was described, one biomechanical study seems to show overconstraint of the knee specimens compared to the ACL-intact state (mean − 4.6 mm) after 85-N pretensioning [17], while another reported normal ATT values with 80-N pretensioning and significant residual ATT with 60-N pretensioning [34]. Therefore, in this study, after dynamic augmentation, ATT was evaluated with 80-N as well as 60-N pretensioning. In contrast, this study found that dynamic augmentation yielded similar results with 60-N and 80-N pretensioning. The differences in findings between studies may partly have resulted from the force used during ATT tests: Schliemann et al. [34] used 134 N, Kohl et al. [17] used 100 N, and the present study used 89 N.
Besides the limitations that are inherent to all ex vivo testing some specific limitations apply to this study. The mean age of the specimens tested was higher than the typical age of the patient with this type of injury, despite efforts to source younger specimens. The results are only valid close to time zero, and it is not known how biological healing affects the repair over time, requiring further in vivo studies. Biomechanical testing may degrade the biomechanical properties of the ACL stump. Therefore, the non-augmented ACL suture repair was performed first, and the order of testing was thereafter randomized between static and dynamic augmentation.
It should be noted that the non-augmented ACL suture repair did reduce ATT so that it was not significantly increased compared to the ACL-intact state directly postoperative, although there was a significant increase in ATT after cyclic loading. Therefore, although this was not evaluated in this study, adding an ACL suture repair might improve the results of static tape augmentation and might also benefit a dynamic augmentation by helping to maintain apposition of the healing tissue. To establish healing of the ruptured ACL in vivo, adding suture repair to static or dynamic augmentation seems warranted.
The clinical relevance of this study is that it suggests that dynamic augmentation (Ligamys™) with 80-N pretensioning can control ATT laxity directly postoperation and can maintain this after short-term cyclic loading, which may assist healing of the ruptured ACL. Therefore, this study would support further clinical evaluation of dynamic augmentation of ACL repair.
Conclusion
The results of this cadaveric study have shown that, in contrast to non-augmented ACL suture repair and static tape augmentation, dynamic augmentation with 80-N pretensioning resulted in restoration of ATT values similar to the ACL-intact knee and decreased ATT values when compared to the ACL-deficient knee immediately post-operation and also after cyclic loading, across the arc of flexion.
References
Ateschrang A, Ahmad SS, Stockle U, Schroeter S, Schenk W, Ahrend MD (2017) Recovery of ACL function after dynamic intraligamentary stabilization is resultant to restoration of ACL integrity and scar tissue formation. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-017-4656-x
Buchler L, Regli D, Evangelopoulos DS, Bieri K, Ahmad SS, Krismer A et al (2016) Functional recovery following primary ACL repair with dynamic intraligamentary stabilization. Knee 23:549–553
Cuomo P, Rama KR, Bull AM, Amis AA (2007) The effects of different tensioning strategies on knee laxity and graft tension after double-bundle anterior cruciate ligament reconstruction. Am J Sports Med 35:2083–2090
Eggli S, Roder C, Perler G, Henle P (2016) Five year results of the first ten ACL patients treated with dynamic intraligamentary stabilisation. BMC Musculoskelet Disord 17:105
Engebretsen L, Lew WD, Lewis JL, Hunter RE (1989) Knee mechanics after repair of the anterior cruciate ligament. A cadaver study of ligament augmentation. Acta Orthop Scand 60:703–709
Evangelopoulos DS, Kohl S, Schwienbacher S, Gantenbein B, Exadaktylos A, Ahmad SS (2015) Collagen application reduces complication rates of mid-substance ACL tears treated with dynamic intraligamentary stabilization. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-015-3838-7
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Fisher MB, Jung HJ, McMahon PJ, Woo SL (2010) Evaluation of bone tunnel placement for suture augmentation of an injured anterior cruciate ligament: effects on joint stability in a goat model. J Orthop Res 28:1373–1379
Fisher MB, Jung HJ, McMahon PJ, Woo SL (2011) Suture augmentation following ACL injury to restore the function of the ACL, MCL, and medial meniscus in the goat stifle joint. J Biomech 44:1530–1535
Fleming BC, Carey JL, Spindler KP, Murray MM (2008) Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res 26:1500–1505
Friederich NF, Muller W, O’Brien WR (1992) [Clinical application of biomechanic and functional anatomical findings of the knee joint]. Orthopade 21:41–50
Heitmann M, Dratzidis A, Jagodzinski M, Wohlmuth P, Hurschler C, Puschel K et al (2014) Ligament bracing-augmented cruciate ligament sutures: biomechanical studies of a new treatment concept. Unfallchirurg 117:650–657
Henle P, Roder C, Perler G, Heitkemper S, Eggli S (2015) Dynamic Intraligamentary Stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord 16:27
Inderhaug E, Stephen JM, Williams A, Amis AA (2017) Biomechanical comparison of anterolateral procedures combined with anterior cruciate ligament reconstruction. Am J Sports Med 45:347–354
Khadem R, Yeh CC, Sadeghi-Tehrani M, Bax MR, Johnson JA, Welch JN et al (2000) Comparative tracking error analysis of five different optical tracking systems. Comput Aid Surg 5:98–107
Kiapour AM, Murray MM (2014) Basic science of anterior cruciate ligament injury and repair. Bone Joint Res 3:20–31
Kohl S, Evangelopoulos DS, Ahmad SS, Kohlhof H, Herrmann G, Bonel H et al (2014) A novel technique, dynamic intraligamentary stabilization creates optimal conditions for primary ACL healing: a preliminary biomechanical study. Knee 21:477–480
Kohl S, Evangelopoulos DS, Kohlhof H, Hartel M, Bonel H, Henle P et al (2013) Anterior crucial ligament rupture: self-healing through dynamic intraligamentary stabilization technique. Knee Surg Sports Traumatol Arthrosc 21:599–605
Kohl S, Evangelopoulos DS, Schar MO, Bieri K, Muller T, Ahmad SS (2016) Dynamic intraligamentary stabilisation: initial experience with treatment of acute ACL ruptures. Bone Joint J 98-B:793–798
Kohn D, Busche T, Carls J (1998) Drill hole position in endoscopic anterior cruciate ligament reconstruction. Results of an advanced arthroscopy course. Knee Surg Sports Traumatol Arthrosc 6(Suppl 1):S13–15
Kondo E, Yasuda K, Azuma H, Tanabe Y, Yagi T (2008) Prospective clinical comparisons of anatomic double-bundle versus single-bundle anterior cruciate ligament reconstruction procedures in 328 consecutive patients. Am J Sports Med 36:1675–1687
Kosters C, Herbort M, Schliemann B, Raschke MJ, Lenschow S (2015) Dynamic intraligamentary stabilization of the anterior cruciate ligament. Operative technique and short-term clinical results. Unfallchirurg 118:364–371
Krismer AM, Gousopoulos L, Kohl S, Ateschrang A, Kohlhof H, Ahmad SS (2017) Factors influencing the success of anterior cruciate ligament repair with dynamic intraligamentary stabilisation. Knee Surg Sports Traumatol Arthrosc 25:3923–3928
Lewis JL, Lew WD, Schmidt J (1988) Description and error evaluation of an in vitro knee joint testing system. J Biomech Eng 110:238–248
Mackay GM, Blyth MJ, Anthony I, Hopper GP, Ribbans WJ (2015) A review of ligament augmentation with the InternalBrace: the surgical principle is described for the lateral ankle ligament and ACL repair in particular, and a comprehensive review of other surgical applications and techniques is presented. Surg Technol Int 26:239–255
Marshall JL, Rubin RM (1977) Knee ligament injuries—a diagnostic and therapeutic approach. Orthop Clin North Am 8:641–668
Marshall JL, Warren RF, Wickiewicz TL, Reider B (1979) The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res 97–106
Meister M, Koch J, Amsler F, Arnold MP, Hirschmann MT (2017) ACL suturing using dynamic intraligamentary stabilisation showing good clinical outcome but a high reoperation rate: a retrospective independent study. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-017-4726-0
Murray MM, Fleming BC (2013) Biology of anterior cruciate ligament injury and repair: Kappa delta ann doner vaughn award paper 2013. J Orthop Res 31:1501–1506
Murray MM, Flutie BM, Kalish LA, Ecklund K, Fleming BC, Proffen BL et al (2016) The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med 4:2325967116672176
Murray MM, Spindler KP, Abreu E, Muller JA, Nedder A, Kelly M et al (2007) Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res 25:81–91
Radford WJ, Amis AA, Heatley FW (1994) Immediate strength after suture of a torn anterior cruciate ligament. J Bone Joint Surg Br 76:480–484
Schliemann B, Glasbrenner J, Rosenbaum D, Lammers K, Herbort M, Domnick C et al (2017) Changes in gait pattern and early functional results after ACL repair are comparable to those of ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-017-4618-3
Schliemann B, Lenschow S, Domnick C, Herbort M, Haberli J, Schulze M et al (2015) Knee joint kinematics after dynamic intraligamentary stabilization: cadaveric study on a novel anterior cruciate ligament repair technique. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-015-3735-0
Stephen JM, Halewood C, Kittl C, Bollen SR, Williams A, Amis AA (2016) Posteromedial meniscocapsular lesions increase tibiofemoral joint laxity with anterior cruciate ligament deficiency, and their repair reduces laxity. Am J Sports Med 44:400–408
van der List JP, DiFelice GS (2017) Role of tear location on outcomes of open primary repair of the anterior cruciate ligament: a systematic review of historical studies. Knee 24:898–908
van Eck CF, Limpisvasti O, ElAttrache NS (2017) Is there a role for internal bracing and repair of the anterior cruciate ligament? A systematic literature review. Am J Sports Med. https://doi.org/10.1177/0363546517717956363546517717956
Vavken P, Fleming BC, Mastrangelo AN, Machan JT, Murray MM (2012) Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy 28:672–680
Wilson WT, Hopper GP, Byrne PA, MacKay GM (2016) Anterior cruciate ligament repair with internal brace ligament augmentation. Surg Technol Int XXIX:273–278
Zavras TD, Race A, Bull AM, Amis AA (2001) A comparative study of ‘isometric’ points for anterior cruciate ligament graft attachment. Knee Surg Sports Traumatol Arthrosc 9:28–33
Funding
No outside funding was received for the conduct of this study.
Author information
Authors and Affiliations
Contributions
RAGH, RW and AAA have made substantial contributions to conception and design of the study. RAGH, RW and JMS have made substantial contributions to acquisition of data. All authors have made substantial contributions to analysis and interpretation of data, and have been involved in drafting the manuscript and revising it critically for important intellectual content. All authors have given final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The local Institutional Review Board (IRB: Imperial College Healthcare Tissue Bank, London, UK; IRB Nr. R17007) approved this study.
Informed consent
No informed consent statements were applicable.
Rights and permissions
About this article
Cite this article
Hoogeslag, R.A.G., Brouwer, R.W., Huis in ‘t Veld, R. et al. Dynamic augmentation restores anterior tibial translation in ACL suture repair: a biomechanical comparison of non-, static and dynamic augmentation techniques. Knee Surg Sports Traumatol Arthrosc 26, 2986–2996 (2018). https://doi.org/10.1007/s00167-018-4848-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-4848-z