Abstract
Purpose
Dynamic intraligamentary stabilization (DIS) is a primary repair technique for acute anterior cruciate ligament (ACL) tears. For internal bracing of the sutured ACL, a metal spring with 8 mm maximum length change is preloaded with 60–80 N and fixed to a high-strength polyethylene braid. The bulky tibial hardware results in bone loss and may cause local discomfort with the necessity of hardware removal. The technique has been previously investigated biomechanically; however, the amount of spring shortening during movement of the knee joint is unknown. Spring shortening is a crucial measure, because it defines the necessary dimensions of the spring and, therefore, the overall size of the implant.
Methods
Seven Thiel-fixated human cadaveric knee joints were subjected to passive range of motion (flexion/extension, internal/external rotation in 90° flexion, and varus/valgus stress in 0° and 20° flexion) and stability tests (Lachman/KT-1000 testing in 0°, 15°, 30°, 60°, and 90° flexion) in the ACL-intact, ACL-transected, and DIS-repaired state. Kinematic data of femur, tibia, and implant spring were recorded with an optical measurement system (Optotrak) and the positions of the bone tunnels were assessed by computed tomography. Length change of bone tunnel distance as a surrogate for spring shortening was then computed from kinematic data. Tunnel positioning in a circular zone with r = 5 mm was simulated to account for surgical precision and its influence on length change was assessed.
Results
Over all range of motion and stability tests, spring shortening was highest (5.0 ± 0.2 mm) during varus stress in 0° knee flexion. During flexion/extension, spring shortening was always highest in full extension (3.8 ± 0.3 mm) for all specimens and all simulations of bone tunnels. Tunnel distance shortening was highest (0.15 mm/°) for posterior femoral and posterior tibial tunnel positioning and lowest (0.03 mm/°) for anterior femoral and anterior tibial tunnel positioning.
Conclusion
During passive flexion/extension, the highest spring shortening was consistently measured in full extension with a continuous decrease towards flexion. If preloading of the spring is performed in extension, the spring can be downsized to incorporate a maximum length change of 5 mm resulting in a smaller implant with less bone sacrifice and, therefore, improved conditions in case of revision surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The current gold standard for treatment of anterior cruciate ligament (ACL) ruptures comprises arthroscopic resection of the ACL remnants and subsequent surgical reconstruction with the use of a tendon graft [4]. ACL repairs with static synthetic augmentations like bands made of Dacron, Gore-Tex or Trevira have led to poor clinical results in the past with high failure rates and high rates of non-infective synovitis [2, 7, 19]. More recent designs made of polyethylene terephthalate, specifically the Ligament Augmentation and Reconstruction System LARS, that includes some intrinsic elasticity due to its twisted design, showed lower failure rates of 2.6% and rates of non-infective synovitis of 0.2% over a mixed follow-up between 22 and 95 months [2, 18]. Recent studies also show that there is a potential for self-healing of a torn ACL if a beneficial healing environment is created [20, 24]. Dynamic intraligamentary stabilization (DIS) combines Steadman’s healing response, i.e. microfracturing at the femoral footprint of the ACL [24], with a fully dynamic augmentation that preserves primary ACL repair [23]. For internal bracing of the sutured ACL, a high-strength polyethylene braid with a pre-assembled button is anchored to the femur and clamped to a preloaded spring system in a screw (Ligamys), which is fixated into the tibial head (Fig. 1). In contrast to static augmentations, the Ligamys device compensates for non-isometric positioning of bone tunnels and therefore maintains anterior tibial translation (ATT) low during knee motion [11]. When a knee movement puts a stress on the repaired ACL, the polyethylene braid is put under tension and the spring is compressed. The current spring-screw device has a diameter of 10 mm and a length of 30 mm. This size is primarily dictated by the dimension of the spring (8 × 22 mm) that enables a total of 8 mm length change. Because of its relatively large size, the tibial hardware leads to a corresponding bone loss and results in frequent hardware removals due to local discomfort [15]. The technique has been previously biomechanically investigated [23]; however, the length change of the implant spring during motion of the knee joint is unknown. A smaller spring-screw device with less length change would reduce bone loss in the tibial head and potentially reduce the frequency of hardware removals.
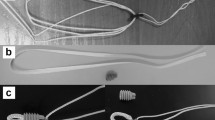
Principle of dynamic intraligamentary stabilization. A 10 × 30 mm spring-screw-device in the tibial head is fixed to a high-strength polyethylene braid [8]
Following previous investigators, knee joint kinematics, spring length, and tunnel distance as a surrogate for spring length were recorded with an optical measurement system in cadaveric knees [12, 14]. In a post-hoc computer simulation, the influence of bone tunnel positioning on spring length change was then analyzed. We hypothesized that less than 8 mm spring length change is required for unrestricted passive motion.
Materials and methods
Specimens and preparation
Four Thiel-fixated entire and intact human cadaveric bodies were obtained with informed consent from the Institute of Anatomy of the University of Bern (Table 1). Specimens were warmed up at room temperature overnight before testing. Femurs and tibiae of the specimens were marked with four fiducial screws each. The screws were inserted into the great trochanter, the femoral shaft, the medial and lateral epicondyle, the tibial tuberosity, the tibial shaft, and the medial and lateral malleolus. Then, a computed tomography (CT) scan of the whole lower body was acquired to ensure intra-osseous positioning of the screws and exclude specimens with signs of osteoarthritis or preexisting ACL ruptures. One knee joint was excluded because of a preexisting ACL rupture. Then, two 3.0 mm diameter Schanz screws were inserted into the diaphysis of the femur and tibia and the Schanz screws were subsequently connected to optical markers (Optorak Certus, NDI, Waterloo, Canada). The first ROM and stability test cycle described in section “test setup” was then initiated. After this, a medial arthrotomy was performed and the ACL was transected with a scalpel at its femoral insertion. The joint capsule was closed with a continuous suture and the second test cycle was initiated. The DIS procedure described in the section “surgical technique of Dynamic Intraligamentary Stabilization” was then performed and the third test cycle was started. Then, a second CT scan of the whole lower body was acquired.
Surgical technique of dynamic intraligamentary stabilization
Surgery was performed according to the manufacturer’s operational manual by two experienced surgeons. A small medial arthrotomy was performed and the tibial remnant of the ACL was sutured with three 2-0 PDS sutures. 2.4 mm femoral and tibial bone tunnels were drilled with K-wires through femoral and tibial footprints of the ACL. The tibial K-wire was overdrilled to a depth of 30 mm with a 10 mm cannulated drill bit and the Ligamys implant was screwed into the tibial head. A shuttle suture was pushed through the DIS implant into the knee and both, PDS and shuttle sutures, were transferred through the femoral tunnel. A polyethylene braid with a button on the femoral side was then pulled through the knee joint with the shuttle suture. Preloading of the polyethylene braid with 300 N was done with a tensioning device prior to fixation of the braid to the spring of the Ligamys implant at a preload of 80 N in 0° knee flexion.
Test setup
Cadavers were positioned supine on a testing bench. A custom-made test rig for instrumented Lachman/KT-1000 testing generated 134 N anteriorly directed force on the tibia. The rig was placed at the foot of the specimens’ bench. Two pulleys deflected the 134 N force generated by a weight (13.7 kg) to an aluminum hook (Fig. 2). One test cycle included six motions (flexion/extension, internal/external rotation in 90°, varus in 0° and 20°, and valgus in 0° and 20°) and Lachman/KT-1000 testing in 0°, 15°, 30°, 60°, and 90° flexion. Two examiners (JH and CK) each performed five repetitions of the 6 motions. Lachman/KT-1000 testing was performed by three examiners together, one examiner immobilizing the leg in the respective flexion angle and a second examiner positioning the hook of the test rig on the calf muscle at the height of the tibial tuberosity. A third person then connected the 13.7 kg weight to the rope.
Motion tracking
A motion capture camera (Optorak Certus, NDI, Waterloo, Canada) recorded positions of active infrared markers at a frequency of 100 Hz. The markers were connected to Schanz screws in femur and tibia for ACL-intact and transected states. After DIS treatment, the tibial marker was removed and connected to the implant screw and one additional marker was connected to the implant spring (Fig. 3). Therefore, the Ligamys implants had been adapted to allow for a stable mechanical fixation of two markers: a longer threaded pin than originally used had been pre-assembled to the clamping cone of the Ligamys implant and the bush had been welded to a steel half-pipe (Fig. 4). The spatial error of the motion capture system is lower than 0.1 mm in the selected working volume. For a worst-case scenario, the error on knee translations is 0.2 mm. The 0.1 mm position error in a direction perpendicular to the axis of the tibia induces an angular error below or equal to 0.1° for flexion/extension, varus/valgus, and axial rotation.
Coordinate system
Origins for femoral and tibial anatomic coordinate systems were defined on CT scans along the mechanical axis (line connecting the femoral head and the center of the ankle) at the height of the intercondylar eminence. The X-axis was set medio-lateral connecting the medial and lateral epicondyle, the Y-axis was set antero-posterior and the Z-axis caudo-cranial along the mechanical axis.
Statistical analysis
Descriptive statistics were performed to calculate the mean and standard deviation values for spring shortening, tunnel shortening and anterior tibial translation (ATT) at the respective knee flexion angles. Statistical analysis was performed using Python 2.7 with SciPy stats and PumPy libraries (Oliphant, 2007). Data were screened for normality using the Shapiro–Wilk test. Student’s t test was used to identify significant differences in ATT between the different states. The level of significance was set at p = 0.05. Decrease of tunnel length is represented by positive tunnel shortening values and a decrease of spring length is represented by positive spring shortening values. Five cycles of each motion were performed, and the mean of the amplitudes was averaged. Initial tunnel lengths were defined in the CT image and transformed into the anatomical coordinate system. The positions of the 2.4 mm tunnels were read on CT scans and a circle with a radius of 5 mm was drawn around the tunnels in the transverse plane for the tibia and in the sagittal plane for the femur. All combinations between the four orthogonal positions of the tibial and femoral circle were calculated for flexion/extension. The highest and lowest length changes of tunnels are presented in this study.
Results
Figure 5 gives an overview of all ROM and stability tests that were performed. The figure illustrates also which data are shown in which figure. An additional figure shows that joint angles during all ROM tests (varus/valgus, flexion/extension and rotation in 90° flexion) were not affected by DIS ACL repair (see additional file 1).
Overview of ROM and stability tests with the respective kinematic data. The three drawings on the left represent the ACL-intact, ACL-transected, and DIS-repaired state. Recorded parameters during ROM testing: knee angles, spring and tunnel shortening. Recorded parameters during Lachman testing: anterior tibial translation (ATT), spring, and tunnel shortening
Tunnel positions within the seven specimens
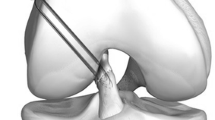
The positions of femoral and tibial tunnels were quantified according to the quadrant method described by Bernard and Hertel [3] (Fig. 6).
Positions of femoral and tibial bone tunnels according to the quadrant method described by Bernard and Hertel. The blue dots represent the bone tunnels of the seven specimens. The light blue dot represents the bone tunnel, which was used for the simulation of extreme positions within a radius of 5 mm. FA femoral distance perpendicular to Blumensaat line; FB femoral distance postero-anterior along Blumensaat line; TA tibial distance antero-posterior; TB tibial distance medio-lateral
Anterior tibial translation (ATT) during Lachman testing
ATT values after ACL transection increased significantly for all flexion angles and were highest in 15° and 30° with 9.0 ± 2.7 and 9.2 ± 3.4 mm, respectively (Fig. 7). ACL repair with DIS significantly reduced ATT for all flexion angles, except for 60°, where no significant differences in ATT between the ACL-intact and ACL-repaired states were observed. For all flexion angles, values were still significantly higher than the ACL-intact state.
Spring and tunnel shortening during ROM testing
Spring length and tunnel distance for flexion/extension, internal/external rotation in 90° and varus/valgus in 0° and 20° are shown in Figs. 9 and 10. Over all ROM testing, spring shortening was highest (5.0 ± 0.2 mm) for varus testing in 0° (Fig. 8). For flexion/extension testing, spring shortening was highest in extension (3.8 ± 0.3 mm) for all specimens and then decreased continuously but variably with flexion reaching zero between 25° and 120° flexion (Fig. 9). In higher flexion, spring shortening remained zero, whereas tunnel shortening increased up to values of 7–14 mm, meaning that the spring was fully unloaded while the tunnels approached each other.
Spring and tunnel shortening during Lachman/KT-1000 testing
As for ROM testing, spring and tunnel shortening closely overlapped in the loaded region (Fig. 10). Lachman/KT-1000 testing in 0° resulted in the highest spring shortening (4.4 ± 0.3 mm). The higher the flexion of the knee joint, the higher was the spring length. In 60° knee flexion, mean values for spring shortening were 1.3 ± 0.9 mm during Lachman/KT 1000 testing and close to zero at rest and in 90° 0.7 ± 0.8 mm during Lachman/KT 1000 testing and zero at rest.
Simulation of tunnel positions within r = 5 mm range
Simulation of tunnel positions within a range r = 5 mm shows a high variation of length changes during flexion/extension with min and max tunnel shortenings of 4.0 and 20.9 mm, respectively (Fig. 11). However, tunnel distance was always highest in extension (zero position) and continuously decreased with flexion up to 11.0 ± 3.1 mm. In deep flexion > 120°, most specimens showed a slight increase in tunnel distance. Tunnel shortening between 0° and 120° was highest (0.15 mm/°) for posterior femoral and posterior tibial tunnel positioning and lowest (0.03 mm/°) for anterior femoral and anterior tibial tunnel positioning.
Simulation of tunnel positions within a range r = 5 mm. The dashed lines represent the seven specimens. The blue curve represents simulation of anterior femoral and anterior tibial tunnel positioning, whereas the red curve represents simulation of posterior femoral and posterior tibial tunnel positioning. The thick dashed curve represents the specimen that was used for simulation
Discussion
The most important findings of our study were that the integrity of the ACL only influenced anterior tibial translation (ATT) during Lachman test, whereas all range of motion tests (varus/valgus, flexion/extension, and rotation in 90° flexion) were not affected. This underlines the primary role of the ACL as a passive structure preventing anterior tibial subluxation but not interfering with normal passive motion. ACL repair with DIS successfully reduced knee laxity over all flexion angles to significantly lower values compared to the ACL-transected state, but values still differed significantly from the ACL-intact state. All ranges of motion tests were again not affected by the repair. Our data support the measurements made by Schliemann et al. who found that DIS with a preload of 80 N significantly reduced knee laxity in 15°–90° [23]. However, Schliemann et al. reported that DIS reduced ATT to the ACL-intact state, whereas our measurements still found significant differences between the transected and repaired state. This is most likely due to different preloading protocols: the group of Schliemann applied 80 N preload in 20°–30° flexion, whereas we consequently preloaded with 80 N in 0°, resulting in lower tension on the polyethylene braid and consequently lower knee stability. The clinical implication of this increased knee laxity with our preloading protocol should consequently be an elongation of the ACL repair. However, we did not find this effect in our clinical practice, where preloading is consequently performed in 0° and Lachman values measured 3 month post-surgery differed non-significantly by 0.8 mm on average from the contralateral healthy side [9]. This shows that biomechanical data of laxity measurements cannot be directly translated to clinical practice. The remaining laxity with DIS ACL repair might be a necessary stimulus for stable healing of the repair.
When comparing our ATT values to literature data, we observed that our values are much closer to clinical measurements than previous biomechanical studies. Mean ATT values in 30° of ACL-transected knees in robotic setups range between 16.7 and 30.5 mm [10, 23], whereas clinical Rolimeter measurements in ACL-injured patients show values of 11.6 mm [1]. The ATT of 9.2 mm we measured is thus much closer to the clinical value than robotic measurements. This difference may be due to the setup and specimens we chose. We used intact whole cadaveric bodies, where the soft-tissue envelope around the knee joint further restrained laxity to lower ATT values. In addition, we directly measured translation in a setting that represents the clinical setup, whereas ATT measurements in robotic setups are dependent on specimen fixation to the socket and to the robotic arm.
Implant spring length and tunnel distance during passive ROM and Lachman/KT-1000 stability testing were further analyzed. Tunnel distance shortening was measured as a surrogate for spring shortening in positions, where the spring is not loaded and slack occurs in the polyethylene braid. Spring shortening is a crucial measure, because it defines how much maximum length change due to non-isometric bone tunnel positioning can be compensated by the DIS implant. For spring shortening, only positive values are clinically relevant. Negative values that were measured are due to the clamping element and the optical marker being pulled out of the bush by gravity. These are a consequence of the adaptations made to the bush and cannot happen in vivo where a mechanical stop hinders the clamping element from disintegration from the bush.
Spring shortening was highly consistent with tunnel distance shortening over all measurements. Spring length and tunnel distance measurements during Lachman/KT-1000 testing showed that the spring shortening was 4.1 ± 0.5 mm in 0° at rest, which is due to the preloading of the spring with 80 N during the DIS procedure. This value is only slightly exceeded in 0° when applying 134 N anteriorly directed force on the tibia (4.4 ± 0.3 mm). For all other flexion angles, spring shortening never exceeded 4.1 mm during Lachman/KT-1000 testing. During ROM testing, the highest spring shortening of 5.0 mm was found for varus stress in 0° i.e. on average 1.2 mm higher than during 80 N preloading. This is not surprising, as the ACL originates from the postero-lateral intercondylar notch and is attached to the tibia close to the anterior root of the lateral meniscus. During varus stress in extension, the lateral wall of the notch is lifted off the lateral tibial plateau, and therefore, the ACL is elongated, or in the case of ACL repair with DIS, tension is applied on the polyethylene braid with a corresponding spring shortening.
These results imply that a smaller spring with only 5 mm spring path would not restrict range of motion (max. 3.8 ± 0.3 mm) or ATT during Lachman/KT-1000 testing (max. 4.4 ± 0.3 mm) but varus in 0° (max. 5.0 ± 0.2 mm) in some cases. However, if we aim to reduce ATT closer to the ACL-intact state, a higher preload than 80 N should be applied in 0°, resulting in turn in the need for a longer spring.
Literature data on length change of the native ACL or ACL reconstructions for full flexion/extension of the knee joint range from 4.3 up to 13.1 mm [6, 13, 16]. Three computer simulation studies of human cadaveric knees reported mean length changes of 4.3 mm for the antero-medial and 7.1 mm for the postero-lateral bundle [13, 16, 25]. In a biomechanical study performed by Lubowitz, mean length change amounted to 6.7 mm for an anatomical femoral tunnel placement as the knee was moved from 0° to 120° [17]. The group of Robinson found length changes of 8.7 to 13.1 mm for postero-lateral bundle fibers [22]. The tunnel distance shortenings of 7 to 14 mm we measured in our study, therefore, coincide with postero-lateral bundle length changes in the previous studies. This is consistent with the surgical technique, where the tibial tunnel is intentionally positioned posterior to the anatomical footprint of the ACL in order not to harm the tibial remnant.
Tunnel positioning in a range r = 5 mm showed that non-isometric tunnel positions led to a more pronounced shortening of the tunnel distance up to 0.15 mm/° between 0° and 120°, whereas nearly isometric positions led to only 0.03 mm/° shortening. Non-isometric tunnels, therefore, may result in early loss of tension at 25° flexion with the consequence that the ACL repair is no longer protected. Isometric positioning on the other hand sustains bracing of the ACL repair up to high flexion angles of 125°. This raises the question if isometric positioning should be strived for even though this leads to inferior rotational stability [5, 21]. Patients with DIS repair are not allowed to participate in pivoting sports during the first 6 months of rehabilitation, and therefore, there is no need to protect the ACL repair from excessive rotational strain. On the other hand, the ACL is especially prone to injury in 0°–30° because of the unfavorable lever arm of the hamstring muscles to restrain ATT. The most important range for DIS to protect the healing ACL from excess stress is, therefore, in 0°–30°. A near-isometric position of DIS might lead to an excellent protection of the repair up to deep flexion, however, with the drawback of inferior protection in 0° to 30°. Ultimately, what counts is the quality of the healing response and this can only be assessed in clinical trials. For future research, a randomized controlled trial should be undertaken to compare knee stability and clinical scores between non-isometric anatomical and near-isometric tunnel positions with DIS ACL repair.
This study shows that preloading and fixation of the spring in 30° instead of 0° can lead to high spring shortening in extension. In the case of non-isometric posterior femoral and posterior tibial bone tunnels, preloading with 80 N in 30° would lead to an extension deficit with the current implant, because the resulting spring shortening (3.8 + 30 × 0.15 = 8.3 mm) would exceed 8 mm. It is, therefore, recommended that the 0° position is respected during preloading of the device.
Most of the limitations of this study are similar to those inherent to cadaveric studies: degradation of the knee specimens was a concern; muscle tension was not present and therefore knee motion was uniquely passive. Kinematic data of femur, tibia, and the Ligamys spring system were measured with a highly precise instrument (Optotrak); however, the variability associated with the passive motion applied manually by the operators cannot be suppressed. A direct comparison of our data to the previous studies is associated with a significant limitation, namely, that the braid tension with DIS varies with knee flexion, whereas in other studies, a fixed tension was applied. Roof impingement of the ACL repair as a potential confounder for length change was not accounted for. Our test setup does not represent a fatigue test. The polyethylene braid as well as the specimens are, however, subjected to plastic deformation and wear under cyclic loading. It can be assumed that spring shortening will, therefore, decrease with time [8].
The main strength of this study is that it replicates the clinical situation much better than a simple ex-situ uniaxial quasi-static loading. Full cadaveric lower bodies with intact soft-tissue envelopes were used. The test condition represents the clinical setting well. Experienced knee surgeons (CK & JH) performed all Ligamys implantations. When the spring was loaded, values for spring and tunnel shortening closely overlapped, giving confidence in the measurements. In the “unloaded region”, tunnel shortening increased up to high values, whereas spring shortening remained at zero as would be expected with a slack in the polyethylene braid.
The impact of this work on the clinical routine is as follows: (1) always apply the pretension on the spring in full extension. This ensures that no extension deficit will be caused by implant tension. (2) If you wish to further decrease AP translation of the repaired knee, then increase the amount of pretension in full extension and do not change to flexion. (3) If these recommendations are followed, the implant can be downsized resulting in less bone loss and improved conditions in case of revision surgery.
Conclusion
During passive flexion/extension, the highest spring shortening was consistently measured in full extension with a continuous decrease towards flexion. If preloading of the spring is performed in extension, the spring can be downsized to incorporate a maximum length change of 5 mm resulting in a smaller implant with less bone consumption and, therefore, improved conditions in case of revision surgery.
Abbreviations
- ACL:
-
Anterior cruciate ligament
- ATT:
-
Anterior tibial translation
- CT:
-
Computed tomography
- DIS:
-
Dynamic intraligamentary stabilization
- ROM:
-
Range of motion
References
Balasch H, Schiller M, Friebel H, Hoffmann F (1999) Evaluation of anterior knee joint instability with the Rolimeter A test in comparison with manual assessment and measuring with the KT-1000 arthrometer. Knee Surg Sports Traumatol Arthrosc 7:204–208
Batty LM, Norsworthy CJ, Lash NJ, Wasiak J, Richmond AK, Feller JA (2015) Synthetic devices for reconstructive surgery of the cruciate ligaments: a systematic review. Arthroscopy 31:957–968
Bernard M, Hertel P, Hornung H, Cierpinski T (1997) Femoral insertion of the ACL. Radiographic quadrant method. Am J Knee Surg 10:14–21 (discussion 21–12)
Chambat P, Guier C, Sonnery-Cottet B, Fayard J-M, Thaunat M (2013) The evolution of ACL reconstruction over the last fifty years. Int Orthop 37:181–186
Driscoll MD, Isabell GP, Conditt MA, Ismaily SK, Jupiter DC, Noble PC et al (2012) Comparison of 2 femoral tunnel locations in anatomic single-bundle anterior cruciate ligament reconstruction: a biomechanical study. Arthroscopy 28:1481–1489
Ebersole GM, Eckerle P, Farrow LD, Cutuk A, Bledsoe G, Kaar S (2015) Anterior cruciate ligament graft isometry is affected by the orientation of the femoral tunnel. J Knee Surg. https://doi.org/10.1055/s-0035-1554926
Engebretsen L, Benum P, Fasting O, Mølster A, Strand T (1990) A prospective, randomized study of three surgical techniques for treatment of acute ruptures of the anterior cruciate ligament. Am J Sports Med 18:585–590
Haberli J, Henle P, Acklin YP, Zderic I, Gueorguiev B (2016) Knee joint kinematics with dynamic augmentation of primary anterior cruciate ligament repair—a biomechanical study. J Exp Orthop 3:29
Henle P, Roder C, Perler G, Heitkemper S, Eggli S (2015) Dynamic intraligamentary stabilization (DIS) for treatment of acute anterior cruciate ligament ruptures: case series experience of the first three years. BMC Musculoskelet Disord 16:27
Herbort M, Lenschow S, Fu FH, Petersen W, Zantop T (2010) ACL mismatch reconstructions: influence of different tunnel placement strategies in single-bundle ACL reconstructions on the knee kinematics. Knee Surg Sports Traumatol Arthrosc 18:1551–1558
Hoogeslag RAG, Brouwer RW, Huis In ‘t Veld R, Stephen JM, Amis AA (eds) (2018) Dynamic augmentation restores anterior tibial translation in ACL suture repair: a biomechanical comparison of non-, static and dynamic augmentation techniques. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-018-4848-z
Inderhaug E, Stephen JM, Williams A, Amis AA (2017) Biomechanical comparison of anterolateral procedures combined with anterior cruciate ligament reconstruction. Am J Sports Med 45:347–354
Kim HY, Seo YJ, Kim HJ, Nguyenn T, Shetty NS, Yoo YS (2011) Tension changes within the bundles of anatomic double-bundle anterior cruciate ligament reconstruction at different knee flexion angles: a study using a 3-dimensional finite element model. Arthroscopy 27:1400–1408
Kim Y, Lee BH, Mekuria K, Cho H, Park S, Wang JH et al (2017) Registration accuracy enhancement of a surgical navigation system for anterior cruciate ligament reconstruction: a phantom and cadaveric study. Knee 24:329–339
Krismer AM, Gousopoulos L, Kohl S, Ateschrang A, Kohlhof H, Ahmad SS (2017) Factors influencing the success of anterior cruciate ligament repair with dynamic intraligamentary stabilisation. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-017-4445-6
Lee JS, Kim TH, Kang SY, Lee SH, Jung YB, Koo S et al (2012) How isometric are the anatomic femoral tunnel and the anterior tibial tunnel for anterior cruciate ligament reconstruction? Arthroscopy 28:1504–1512 (1512 e1501–1502)
Lubowitz JH (2014) Anatomic ACL reconstruction produces greater graft length change during knee range-of-motion than transtibial technique. Knee Surg Sports Traumatol Arthrosc 22:1190–1195
Machotka Z, Scarborough I, Duncan W, Kumar S, Perraton L (2010) Anterior cruciate ligament repair with LARS (ligament advanced reinforcement system): a systematic review. BMC Sports Sci Med Rehabil 2:29
Maletius W, Gillquist J (1997) Long-term results of anterior cruciate ligament reconstruction with a Dacron prosthesis: the frequency of osteoarthritis after seven to eleven years. Am J Sports Med 25:288–293
Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB (2007) Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res 25:1007–1017
Musahl V, Plakseychuk A, VanScyoc A, Sasaki T, Debski RE, Mcmahon PJ et al (2005) Varying femoral tunnels between the anatomical footprint and isometric positions: effect on kinematics of the anterior cruciate ligament-reconstructed knee. Am J Sports Med 33:712–718
Robinson J, Stanford FC, Kendoff D, Stuber V, Pearle AD (2009) Replication of the range of native anterior cruciate ligament fiber length change behavior achieved by different grafts: measurement using computer-assisted navigation. Am J Sports Med 37:1406–1411
Schliemann B, Lenschow S, Domnick C, Herbort M, Haberli J, Schulze M et al. (2015) Knee joint kinematics after dynamic intraligamentary stabilization: cadaveric study on a novel anterior cruciate ligament repair technique. Knee Surg Sports Traumatol Arthrosc. https://doi.org/10.1007/s00167-015-3735-0
Steadman J, Matheny L, Briggs K, Rodkey W, Carreira D (2012) Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg 25:255–260
Yoo YS, Jeong WS, Shetty NS, Ingham SJ, Smolinski P, Fu F (2010) Changes in ACL length at different knee flexion angles: an in vivo biomechanical study. Knee Surg Sports Traumatol Arthrosc 18:292–297
Acknowledgements
Alexander Bürki is acknowledged for his excellent support during biomechanical testing.
Funding
The study was funded and implants were provided by Mathys Ltd., Bettlach, Switzerland.
Author information
Authors and Affiliations
Contributions
JH co-designed the study, composed the manuscript concept, and wrote the manuscript. BV conducted the biomechanical tests, performed all statistical analyses, and edited the complete manuscript. CK and JH operated all cases and helped editing the final draft version of the manuscript. DD supervised the study and helped editing the final draft version of the manuscript. PhZ co-designed the study, supervised it, and edited the complete manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
DD is a member of Mathys company. All other authors declare that they have no conflicts of interest.
Ethical approval and Informed consent
Four Thiel-fixated entire and intact human cadaveric bodies were obtained with informed consent from the Institute of Anatomy of the University of Bern.
Electronic supplementary material
Below is the link to the electronic supplementary material.
167_2018_5002_MOESM1_ESM.png
Additional file 1: Records of knee joint angles during all ROM tests (flexion/extension, internal/external rotation in 90° and varus/valgus in 0° and 20°). The two curves represent the two different examiners. The red curve represents JH and the blue curve CK (PNG 4250 KB)
Rights and permissions
About this article
Cite this article
Häberli, J., Voumard, B., Kösters, C. et al. Implant preloading in extension reduces spring length change in dynamic intraligamentary stabilization: a biomechanical study on passive kinematics of the knee. Knee Surg Sports Traumatol Arthrosc 26, 3582–3592 (2018). https://doi.org/10.1007/s00167-018-5002-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-5002-7