Abstract
Key message
The powdery mildew resistance locus was mapped to A. cristatum chromosome 6PL bin (0.27–0.51) and agronomic traits evaluation indicated that this locus has potential breeding application value.
Abstract
Agropyron cristatum (2n = 4x = 28, PPPP) is a wild relative of wheat with an abundance of biotic and abiotic stress resistance genes and is considered one of the best exogenous donor relatives for wheat breeding. A number of wheat-A. cristatum derived lines have been generated, including addition lines, translocation lines and deletion lines. In this study, the 6P disomic addition line 4844-12 (2n = 2x = 44) was confirmed to have genetic effects on powdery mildew resistance. Four 6P deletion lines (del16a, del19b, del21 and del27) and two translocation lines (WAT638a and WAT638b), derived from radiation treatment of 4844-12, were used to further assess the 6P powdery mildew resistance locus by powdery mildew resistance assessment, genomic in situ hybridization (GISH), fluorescence in situ hybridization (FISH) and 6P specific sequence-tagged-site (STS) markers. Collectively, the locus harboring the powdery mildew resistance gene was genetically mapped to a 6PL bin (0.27–0.51). The genetic effects of this chromosome segment on resistance to powdery mildew were further confirmed by del16a and del27 BC3F2 lines. Comprehensive evaluation of agronomic traits revealed that the powdery mildew resistance locus of 6PL (0.27–0.51) has potential application value in wheat breeding. A total of 22 resistant genes were annotated and 3 specific gene markers were developed for detecting chromatin of the resistant region based on genome re-sequencing. In summary, this study could broaden the powdery mildew resistance gene pool for wheat genetic improvements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum aestivum L.) is one of the staple crop species worldwide, but wheat diseases have long been threatening yield security (Dinu et al. 2018; Shewry 2009; Singh et al. 2016). Powdery mildew, caused by Blumeria graminis (DC.) Speer f. sp. tritici Em. Marchal (Bgt) (Jin et al. 2020), can infect various tissues of wheat, including stems, leaves and ears, affects yields by reducing the photosynthesis of leaves, and is a major destructive wheat disease worldwide. In addition, a large quantities of pesticides used to treat powdery mildew increased production costs and pollute the environment. (An et al. 2019; Wang et al. 2020b). Although 68 resistance genes of powdery mildew were identified from wheat and its relatives (He et al. 2021), most of these genes do not have broad-spectrum resistance; moreover, an increasing number of genes have lost their resistance to new races, and only a few of resistance genes are used in wheat breeding (Li et al. 2020; Zhang et al. 2021). Therefore, it is extremely important to explore new powdery mildew resistance genes.
Wild relatives of wheat provide a rich gene pool of resistance to powdery mildew, and approximately forty powdery mildew resistance genes have been reported. For instance, Pm7, Pm8 and Pm56 are derived from Secale cereale (Hao et al. 2018; McIntosh et al. 2011; Singh et al. 2018), Pm21, Pm55 and Pm62 are derived from Dasypyrum villosum (He et al. 2020; Zhang et al. 2018, 2016b). Similarly, Pm34, Pm35 and Pm58 are derived from Aegilops tauschii (Miranda et al. 2007, 2006; Wiersma et al. 2017), and Pm40 and Pm43 are derived from Thinopyrum intermedium (He et al. 2009; Luo et al. 2009). Notably, Pm21 is a cluster of genes that are widely used in wheat improvement and breeding, therefore, obtaining powdery mildew resistance genes especially broad-spectrum resistance genes from wheat relatives by distant hybridization is an effective method for wheat breeding (Cao et al. 2011; He et al. 2018; Wu et al. 2021).
Agropyron cristatum (2n = 4x = 28, PPPP) evolved under unfavorable environmental conditions and has strong tolerance to biotic and abiotic stresses (Dong et al. 1992; Wu et al. 2006). As expected, it carries many genes with excellent agronomic traits, such as many yield related genes and disease resistance genes, and is considered one of the best exogenous donor relatives for wheat breeding (Jiang et al. 2018; Li et al. 2017; Pan et al. 2017; Zhang et al. 2016a). In recent years, resistance genes on chromosome segments of 2P and 6P have been preliminarily mapped by deletion and translocation lines. For instance, stripe rust and leaf rust resistance genes have been mapped to 6PS (0.81–1.00) (Zhang et al. 2017b), and leaf rust and powdery mildew resistance genes have been mapped to 2PL (0.66–0.86) (Jiang et al. 2018; Li et al. 2017). Moreover, a novel broad-spectrum powdery mildew resistance gene from wheat-A. cristatum introgression line Pubing 74 was created, and could be detected by the 6PL specific sequence-tagged-site (STS) molecular marker Agc2970 (Lu et al. 2016). Copete and Cabrera (2017) used wheat conserved orthologous set (COS) markers to verify that A. cristatum exhibits resistance to powdery mildew via loci on chromosome arms 2PL and 6PL. Thus, genomic mapping of powdery mildew resistance loci from A. cristatum and the study of their association with crop yields are necessary and relevant tasks.
In this study, a novel powdery mildew resistance locus was identified on the A. cristatum 6P chromosome. Various methods including disease evaluation, genomic in situ hybridization (GISH), fluorescence in situ hybridization (FISH), molecular markers analysis and genetic analysis, were employed to map the 6P chromosome segment where the resistance locus is located and to evaluate the application potential of this locus in wheat breeding. In addition, a total of 22 resistance genes have been annotated by re-sequencing, and 3 gene markers have been developed that could be used to detect chromatin fragments of resistance locus. This study provides an important insight into discovering disease resistance genes of A. cristatum in wheat and has important value for wheat breeding.
Materials and methods
Plant materials
A. cristatum (Z559; 2n = 4x = 28, PPPP) was collected from Xinjiang, China. The common wheat cultivar Fukuhokomugi (Fukuho; 2n = 6x = 42, AABBDD) from Japan was used as the current parent. The wheat-A. cristatum 6P disomic addition line 4844–12 (2n = 2x = 44) was generated by distant hybridization of Z559 and Fukuho (Wu et al. 2006). WAT638a, WAT638b del16a, del19b, del21 and del27 were created by irradiating 4844–12 with 60Co-γ rays, followed by pollination with Gaocheng8901 (Song et al. 2016). The resulting F1 progeny were backcrossed with Fukuho, obtained from the generation BC5F6. Zhongzuo9504 and Fukuho were used as the susceptible controls for powdery mildew resistance evaluation. The 6PL translocation line WAT638b was used to evaluate breeding potential. All the plant materials were preserved at the Center of Crop Germplasm Resources Research at the Institute of Crop Science, Chinese Academy of Agricultural Sciences, Beijing, China.
Assessment of powdery mildew resistance and agronomic traits
Bgt isolates E20 was provided by the Institute of Plant Protection, Chinese Academy of Agricultural Sciences. The powdery mildew resistance of the following lines was assessed during the adult stage in greenhouse in Beijing: homozygous 4844–12, del16a, del19b, del21, del27, WAT638a and WAT638b; the control parents Fukuho and Zhongzuo9504, 20 plants per material. In addition, 150 del16a and del27 plants from BC3F2 populations were planted in pots, with five plants per pot. The chi-square (χ2) test was used to test the population segregation ratios. Adult plant resistance was investigated three times after flowering and then scored when symptoms were severe on the susceptible line Zhongzuo9504 (Li et al. 2017). Powdery mildew resistance was evaluated by immunity type (IT) using a 0–9 scale. An IT of 0–4 indicated resistance, and at IT of 5–9 indicated susceptibility; specifically, an IT was 0 indicated immune; 0; nearly immune; 1–2, highly resistant; 3–4, moderately resistant; 5–6, moderately susceptible; 7–8, highly susceptible; and 9, extremely susceptible (Sheng and Duan 1991).
Agronomic traits of del16a, del19b, del21, del27 and the translocation line WAT638b were assessed in the field (2020–2021) in Henan via assessment of powdery mildew resistance (natural infection) and investigation of yield related traits including spike length, spikelet number per spike, kernel number per spikelet, grain number per spike, grain length and thousand grain weight. These traits were also used to evaluate their breeding potential.
Cytological identification
GISH was carried out to determine the karyotype of del16a, del19b, del21, del27, WAT638a, WAT638b and 4844–12 by genomic probe of Z559 to distinguish between Fukuho and A. cristatum chromosomes. FISH was used with oligonucleotide probes, including Oligo-pSc119.2–1 (6-FAM-5’) and Oligo-pTa535-1 (Tamra-5’) to detect chromosome variation in Fukuho, the probes were synthesized by Sangon Biotech (Shanghai) (Tang et al. 2014). Nitrous oxide gas and 90% acetic acid were used to treat the root tips of all the materials, similar processing procedures were performed as described by Wang et al. (2020b), in which a mixture of cellulase (R-10, Yakult Japan) and pectinase (Y-23, Yakult Japan) was used for cellenzymatic hydrolysis (Zhu et al. 2017). Root tip cells were exposed to ultraviolet light for 80 s at 125,000 μJ/cm2 and counterstained with 4’,6-Diamidino-2-phenylindole (DAPI). Fluorescent signals were observed via an Olympus AX80 fluorescence microscope (Olympus Corporation, Tokyo, Japan), imaged with a charge-coupled device (CCD) camera (Diagnostic Institute, Inc.Sterling Height, MI, USA) and modified with Photoshop CS6.
Molecular marker analysis
DNA of 4844–12, del16a, del19b, del21, del27, WAT638a and WAT638b was obtained by the modified cetyltrimethylammonium bromide (CTAB) method (Allen et al. 2006). Molecular markers were derived from the specific expressed-sequence-tags (ESTs) developed by the transcriptome sequencing of Z559 (Zhang et al. 2017a), and several EST markers were mapped to the 31 bins of chromosome 6P (Song et al. 2016). These 31 markers were used to determine the genotypes of the 4844–12 and their derived offspring. The genotypes of del27 and del16a BC3F2 population were determined via the Z559-specific repeat marker AcPR3 (Han et al. 2017). All the primers used were synthesized by Sangon Biotech (Shanghai). The PCR amplification system used consisted of 5.0 μl of 2 × Green Master Mix, 1.0 μl of DNA (50–100 ng/μl), 2.0 μl of a mixture of forward and reverse primers (2 μM) and up to 10.0 μl of ddH2O. The PCR products were detected on a 1% agarose gels and 8% nondenaturing polyacrylamide gels.
The disease resistance gene prediction and markers development
The del16a and del27 were re-sequenced to determine the physical interval of 6PL bin (0.27–0.51). The fresh leaves of seedlings were collected, mixed, and immediately frozen in liquid nitrogen for DNA extraction. The 350-bp paired-end (PE) libraries were prepared using the NEBNext Ultra DNA Library Prep Kit. Sequencing was performed through HiSeq 2500 (Illumina, San Diego, CA, USA) with a paired-end read length of 150 bp at Huazhi Biotech Co., Ltd (Changsha, China). About 5 × Illumina short reads were generated. Raw sequencing data were processed using ‘fastp’ for removing adapters, detecting poor quality reads and over-represented sequences (Van der Auwera et al. 2013; Winfield et al. 2012). The clean reads were mapped to the integrated reference sequences of A. cristatum Z1842 (unpublished) and Chinese Spring RefSeqv2.0 (https://urgi.versailles.inra.fr/download/iwgsc/IWGSC_RefSeq_Assemblies/v2.0/) using BWA-MEM (Oliva et al. 2021). PCR duplicates were detected and removed using Picard’s MarkDuplicates (https://broadinstitute.github.io/picard/). Finally, bedtools were used to calculate the sequencing coverage of reads to reference genome (Quinlan and Hall 2010). Introgression regions were identified by the distribution of coverage. The visualization of coverage was performed by ggplot2 package of R (Maag 2018). The disease resistance genes were obtained using a disease resistance gene analogue (RGA) prediction pipeline and homologous gene alignment with Arabidopsis thaliana and Oryza sativa. The gene markers were developed based on the sequence differences between orthologous genes of Chinese spring and A. cristatum.
Results
Powdery mildew resistance response of the A. cristatum 6P chromosome
At the adult stage, the following lines were inoculated with the Bgt races E20: the common wheat line Fukuho, Zhongzuo9504, and the wheat-A. cristatum 6P chromosome derived lines 4844–12, del16a, del19b, del21, del27, WAT638a and WAT638b with a genetic background of the common wheat cultivar Fukuho. Afterward, disease resistance was assessed once the susceptible control Zhongzuo9504 was thoroughly infected. The results showed that Zhongzuo9504 was extremely susceptible to powdery mildew, with an IT score of 9. The wheat-A.cristatum derived lines del21, del27, WAT638a and the recurrent parent Fukuho were highly susceptible to powdery mildew, each with an IT score of 7. In contrast, 4844–12, del19b, del16a and WAT638b were highly resistant to powdery mildew, each with an IT score of 2 (Fig. 1, Table 1). These results suggested that a resistance locus was present on the A. cristatum 6P chromosome.
Cytological characterization
To determine the chromosomal composition, 4844–12 and the derived lines del16a, del19b, del21, del27, WAT638a and WAT638b were further characterized by GISH and FISH.
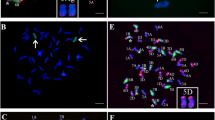
The results showed that the addition line 4844–12 has two chromosomes of Z559 and 42 chromosomes of Fukuho, with a clear green signal at the end of 6PL. It is a 6P chromosome additional line as previously reported (Wu et al. 2006) (Fig. 2a, 2b). WAT638a and WAT638b both have 42 chromosomes, and a full A. cristatum 6PS and 6PL chromosome segments were translocated onto wheat chromosomes as previously reported (Song et al. 2016) (Fig. 2c, 2d). Del16a, del19b, del21 and del27 all have 44 chromosomes with two obvious incomplete chromosomes from Z559, and the other 42 chromosomes are from wheat. No obvious abnormality of wheat chromosomes was observed among them (Fig. 2e, 2l). In summary, cytological identification revealed that del16a, del19b, del21 and del27 are wheat-A. cristatum 6P chromosomal deletion lines.
Cytogenetic analysis by in situ hybridization. a GISH analysis of 4844–12; b FISH analysis of 4844–12; c GISH analysis of WAT638b; d GISH analysis of WAT638a; e GISH analysis of del16a; f FISH analysis of del16a; g GISH analysis of del27; h FISH analysis of del27; i GISH analysis of del21; j FISH analysis of del21; k GISH analysis of del19b; l FISH analysis of del19b; FISH probes Oligo-pTa535-1 (red), Oligo-pSc119.2–1(green) in (b, f, h, j,l), GISH probes (red) in (a, c, d, e, g, i, k). Chromosomes were counterstained with DAPI and visualized with blue fluorescence
Molecular marker analysis
In previous work, the 6P chromosome was divided into 31 segments: 14 segments of 6PS and 17 segments of 6PL (Song et al. 2016). To determine the segments of the A. cristatum 6P chromosome introduced to wheat-A.cristatum derived lines, a total of 31 representative 6P-specific molecular markers in each segment were screened within 4844–12, del16a, del19b, del21, del27, WAT638a and WAT638b. All 31 markers of 6P showed amplification with prominent bands specific to A. cristatumin 4844–12. However, these bands were absent in the recurrent parent Fukuho, indicating that 4844–12 is a wheat-A. cristatum addition line with a pair of complete 6P chromosomes. Fourteen markers belonging to 6PS were not amplified in del19b and WAT638b, indicating that the 6P chromosome in the dell9b and WAT638b genome are 6PL. Del21 and WAT638a, with a complete 6PS; del27, with complete 6PS and 6PL bin (0–0.27), and del16a, with 6PS and 6PL bin (0–0.51) were identified in the same way (Table 2). In addition, a specific marker of 6PL bin (0–0.51), Agc2970, on the 6P chromosome was detected in powdery mildew-resistant wheat-A. cristatum introgressive line Pubing74 (Fig. 3). The powdery mildew resistance gene in Pubing74 may be the same as the powdery mildew resistance genes in 6PL(0.27–0.51). Based on the above analysis of the cytology results and molecular markers and the assessment of powdery mildew, the powdery mildew resistance gene was located on 6PL (0.27–0.51) (Fig. 4).
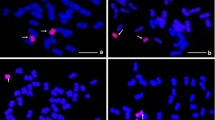
Genetic analysis of the powdery mildew resistance locus
To further determine the genetic effect of the powdery mildew resistance locus of 6PL (0.27–0.51), the del16a and del27 BC3F2 populations were inoculated with Bgt races E20 at the adult stage and then genotyped using the repeat sequence marker AcPR3a (Fig. 5). Both positive and negative del27 plants were highly susceptible to powdery mildew with IT score was 7. All 110 positive del16a plants were highly resistant to powdery mildew, with an IT score of 2, and all 36 negative plants were highly susceptible to powdery mildew, with an IT score of 7 (Fig. 6, Table 3). χ2 tests further confirmed that powdery mildew resistance was significantly affected by the 6PL (0.27–0.51) segment.
Evaluation of agronomic traits and breeding potential of the resistance locus
Exogenous resistance genes can be transferred into the common wheat through distant hybridization in the form of addition lines, substitution lines and translocation lines, but the large fragments of exogenous chromosomes introduced into wheat will produce negative agronomic traits, especially affect the development of wheat yield, resulting in resistance genes that are difficult to directly use for wheat improvement (Dai et al. 2020). To evaluate the breeding potential of the A.cristatum segments carrying this powdery mildew resistance locus, agronomic traits of all the wheat-A.cristatum derived lines (4844–12, del16a, del19b, del21, del27) and the 6PL translocation line WAT638b were evaluated. Compared to Fukuho, all lines, including 4844–12, del16a and WAT638b, had resistance to powdery mildew (spontaneously inoculated) (Fig. 7). Del19b, del16a and WAT638b carried 6PL (0.27–0.51) could increase spikelets number per spike (SNS) by 3.2, 5.0, 0.7, average kernel number per spikelet (KNS) could be increased by 1.5, 1.4, 1.0, and average grain number per spike (GNS) could be increased by 22.0, 19.0, 16.6 compared to Fukuho by Duncan’s multiple-range test, respectively, None of these materials had obvious negative response to other agronomic traits, such as thousand-grain weight. (Table 4). This shows that the 6PL resistance segment (0.27–0.51) also has the genetic effect of increasing the grain number per spike, which is consistent with the results of previous studies (Zhang et al. 2019). In summary, powdery mildew resistance locus 6PL (0.27–0.51) carried high grain number and disease resistance genes, no negative effect on thousand-grain weight, had potential application value for wheat breeding by creating small translocation lines in the future.
Identification of candidate genes and development of markers
To determine the accuracy of the 6PL powdery mildew resistance segment (0.27–0.51) genomic region, the del16a and del27 were re-sequenced and mapped on A. cristatum Z1842 genome (Unpublished). By calculating the chromosome coverage, we determined that del27 carried ~ 387.02Mb of 6P, while del16a carried ~ 500.13Mb of 6P (Fig. 8). Therefore, the powdery mildew resistance gene(s) was located in the 387.02Mb ~ 500.13Mb of 6P chromosome. According to the gene annotation, a total of 813 genes (Acristatum06G039150 ~ Acristatum06G047420) were deposited in this interval. Among these genes, 17 resistance genes were predicted to be disease resistance genes by the RGAugury pipeline and 5 resistant genes were obtained homologous gene alignment with Arabidopsis thaliana and Oryza sativa (Table 5). Through sequence alignment with orthologous genes in Chinese Spring, we developed 3 A. cristatum specific gene markers (Ac41770-2, Ac41790-2 and Ac44250-2 correspond to gene Acristatum06G041770, Acristatum06G041790 and Acristatum06G044250, respectively) (Fig. 9, Table 6). It indicated that these markers could detect chromatin of resistant regions in wheat-A.cristatum derived lines and beneficial to use molecular marker-assisted selection breeding in wheat.
Discussion
Many powdery mildew resistance genes in wheat have lost their original resistance to new races. In this case, transferring genes from wild relatives to common wheat is an effective approach to expand genetic resources. At present, approximately 40 powdery mildew resistance genes have been discovered from wheat relatives. As an excellent gene donor parent for wheat breeding, A. cristatum has many resistance genes. A broad-spectrum powdery mildew resistance-associated segment located in 2PL bin 0.66–0.86 and the Pmpb74 gene from the wheat-A.cristatum introgressive line Pubing74 have been reported (Li et al. 2017; Lu et al. 2016). In the present study, a new 6P powdery mildew resistance gene whose effects are apparent at the adult stage was identified. Hence, our study could broaden the pool of resistance genes for wheat breeding.
It is feasible method to use deletion lines and translocation lines carried different segments to divide chromosomal regions to ultimately locate foreign genes. In recent years, genes with providing broad-spectrum resistance to powdery mildew and leaf rust were located on 2PL (0.66–0.86), and stripe rust resistance genes and leaf rust resistance genes were located on 6PS (0.81–1.00) by mapping deletion lines and translocation lines (Jiang et al. 2018; Li et al. 2017; Song et al. 2016; Zhang et al. 2017b). In addition, the blue-grained gene from Thinopyrum ponticum chromosome 4Ag was successfully located by the use of a similar method (Liu et al. 2018). In our study, four deletion lines (del16a, del19b, del21, del27) and two translocation lines (WAT638a, WAT638b) were used to locate powdery mildew resistance genes, and their location was narrowed to 6PL (0.27–0.51). On the other hand, developing a large number of specific molecular markers of wheat relatives is a convenient way to determine the segments of derived lines. Thirty-one expressed sequence tag-sequence-tagged site (EST-STS) markers were designed according to transcriptome sequencing data, and the loci of the deletion lines del16a, del19b, del21 and del27 were accurately determined. Based on the high similarity of the transcriptome sequences between A. cristatum and wheat (Chinese Spring), Ma et al. (2019) developed specific single-nucleotide polymorphism (SNP) markers for the P genome of A. cristatum under the background of multiple wheat varieties on a large scale. Moreover, using a wheat 660 K SNP array, Zhou et al. (2018) constructed high-density genetic linkage maps of Agropyron Gaertn. In this study, we developed 3 molecular markers, which could effectively detect chromatin of resistant locus in wheat-A. cristatum derived lines. Through the process of distant hybridization, a growing number of addition lines and translocation lines have been generated. Although many derived lines carry excellent exogenous resistance genes, most of these genes cannot be directly used in wheat breeding due to genetic linkage drag, and only a few excellent gene clusters are used in wheat breeding, for example, Lr26/Sr31/Yr9/Pm8 resistance gene clusters of the 1B/1R translocation line from Secale cereale (Lazaridou et al. 2017; Mater et al. 2004); and Lr24/Sr24 resistance gene clusters from Thinopyrum elongatum (Rai et al. 2021). These gene clusters provide stable broad-spectrum resistance and have almost no negative effects on agronomic traits. Thus, some materials such as WAT638b and del16a, have 6PL (0.27–0.51) segments, maybe carries genes clusters for resistance to powdery mildew and increasing grain numbers and spikelet numbers per spike. Therefore, create the wheat-A.cristatum translocation line of small fragment with carrying 6PL (0.27–0.51) have great application potential for wheat resistance breeding. At the same time, we are working on transferring 6PL (0.27–0.51) to some main varieties, which maybe play an important role in disease resistance breeding in the future.
With the development of sequencing technology and the reduction of sequencing cost, more exogenous relatives of wheat have been sequenced, which speeds up the cloning foreign genes in the wheat, such as Fhb7 has been cloned from Thinopyrum elongatum (Wang et al. 2020a). Genome resequencing is an effective technology to quickly determine the physical segments and discovering candidate genes of the exogenous fragment in translocation lines and deletion lines. In this study, del16a and del27 were re-sequenced to determine powdery mildew resistance locus about 113.11Mb from 387.02Mb to 500.13Mb on chromosome 6P. A total of 22 resistance genes have been annotated by RGAugury pipeline and homologous annotation with Arabidopsis thaliana and Oryza sativa, and 3 gene markers have been developed, it is benefit to molecular marker-assisted selection breeding. In summary, this study provides a basic to clone powdery mildew resistance genes in the future.
References
Allen GC, Flores-Vergara MA, Krasynanski S, Kumar S, Thompson WF (2006) A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammonium bromide. Nat Protoc 1:2320–2325
An D, Ma P, Zheng Q, Fu S, Li L, Han F, Han G, Wang J, Xu Y, Jin Y, Luo Q, Zhang X (2019) Development and molecular cytogenetic identification of a new wheat-rye 4R chromosome disomic addition line with resistances to powdery mildew, stripe rust and sharp eyespot. Theor Appl Genet 132:257–272
Cao A, Xing L, Wang X, Yang X, Wang W, Sun Y, Qian C, Ni J, Chen Y, Liu D, Wang X, Chen P (2011) Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc Natl Acad Sci U S A 108:7727–7732
Copete A, Cabrera A (2017) Chromosomal location of genes for resistance to powdery mildew in Agropyron cristatum and mapping of conserved orthologous set molecular markers. Euphytica 213:1–9
Dai K, Zhao R, Shi M, Xiao J, Yu Z, Jia Q, Wang Z, Yuan C, Sun H, Cao A, Zhang R, Chen P, Li Y, Wang H, Wang X (2020) Dissection and cytological mapping of chromosome arm 4VS by the development of wheat-Haynaldia villosa structural aberration library. Theor Appl Genet 133:217–226
Dinu M, Whittaker A, Pagliai G, Benedettelli S, Sofi F (2018) Ancient wheat species and human health: biochemical and clinical implications. J Nutr Biochem 52:1–9
Dong Y, Zhou R, Xu S, Li L, Cauderon Y, Wang R (1992) Desirable characteristics in perennial Triticeae collected in China for wheat improvement. Hereditas 116:175–178
Han H, Liu W, Lu Y, Zhang J, Yang X, Li X, Hu Z, Li L (2017) Isolation and application of P genome-specific DNA sequences of Agropyron Gaertn. in Triticeae. Planta 245:425–437
Hao M, Liu M, Luo J, Fan C, Yi Y, Zhang L, Yuan Z, Ning S, Zheng Y, Liu D (2018) Introgression of powdery mildew resistance gene Pm56 on rye chromosome arm 6RS into wheat. Front Plant Sci 9:1040
He R, Chang Z, Yang Z, Yuan Z, Zhan H, Zhang X, Liu J (2009) Inheritance and mapping of powdery mildew resistance gene Pm43 introgressed from Thinopyrum intermedium into wheat. Theor Appl Genet 118:1173–1180
He H, Zhu S, Zhao R, Jiang Z, Ji Y, Ji J, Qiu D, Li H, Bie T (2018) Pm21, encoding a typical CC-NBS-LRR protein, confers broad-spectrum resistance to wheat powdery mildew disease. Mol Plant 11:879–882
He H, Ji J, Li H, Tong J, Feng Y, Wang X, Han R, Bie T, Liu C, Zhu S (2020) Genetic diversity and evolutionary analyses reveal the powdery mildew resistance gene Pm21 undergoing diversifying selection. Front Genet 11:489
He H, Liu R, Ma P, Du H, Zhang H, Wu Q, Yang L, Gong S, Liu T, Huo N, Gu YQ, Zhu S (2021) Characterization of Pm68, a new powdery mildew resistance gene on chromosome 2BS of Greek durum wheat TRI 1796. Theor Appl Genet 134:53–62
Jiang B, Liu T, Li H, Han H, Li L, Zhang J, Yang X, Zhou S, Li X, Liu W (2018) Physical mapping of a novel locus conferring leaf rust resistance on the long arm of Agropyron cristatum chromosome 2P. Front Plant Sci 9:817
Jin Y, Xue F, Zhou Y, Duan X, Hu J, Li Y, Zhu H, Sun J (2020) Fine-mapping of the powdery mildew resistance gene mlxbd in the common wheat landrace xiaobaidong. Plant Dis 104:1231–1238
Lazaridou TB, Pankou CI, Xynias IN, Roupakias DG (2017) Effect of the 1BL.1RS wheat-rye translocation on the androgenic response in spring bread wheat. Cytol Genet 51:485–490
Li H, Jiang B, Wang J, Lu Y, Zhang J, Pan C, Yang X, Li X, Liu W, Li L (2017) Mapping of novel powdery mildew resistance gene(s) from Agropyron cristatum chromosome 2P. Theor Appl Genet 130:109–121
Li H, Dong Z, Ma C, Xia Q, Tian X, Sehgal S, Koo DH, Friebe B, Ma P, Liu W (2020) A spontaneous wheat-Aegilops longissima translocation carrying Pm66 confers resistance to powdery mildew. Theor Appl Genet 133:1149–1159
Liu L, Luo Q, Li H, Li B, Li Z, Zheng Q (2018) Physical mapping of the blue-grained gene from Thinopyrum ponticum chromosome 4Ag and development of blue-grain-related molecular markers and a FISH probe based on SLAF-seq technology. Theor Appl Genet 131:2359–2370
Lu Y, Yao M, Zhang J, Song L, Liu W, Yang X, Li X, Li L (2016) Genetic analysis of a novel broad-spectrum powdery mildew resistance gene from the wheat-Agropyron cristatum introgression line Pubing 74. Planta 244:713–723
Luo PG, Luo HY, Chang ZJ, Zhang HY, Zhang M, Ren ZL (2009) Characterization and chromosomal location of Pm40 in common wheat: a new gene for resistance to powdery mildew derived from Elytrigia intermedium. Theor Appl Genet 118:1059–1064
Maag JLV (2018) gganatogram: an R package for modular visualisation of anatograms and tissues based on ggplot2. F1000 Res 7:1576
Mater Y, Baenziger S, Gill K, Graybosch R, Whitcher L, Baker C, Specht J, Dweikat I (2004) Linkage mapping of powdery mildew and greenbug resistance genes on recombinant 1RS from “Amigo” and “Kavkaz” wheat-rye translocations of chromosome 1RS.1AL. Genome 47:292–298
McIntosh RA, Zhang P, Cowger C, Parks R, Lagudah ES, Hoxha S (2011) Rye-derived powdery mildew resistance gene Pm3 in wheat is suppressed by the Pm3 locus. Theor Appl Genet 123:359–367
Miranda LM, Murphy JP, Marshall D, Leath S (2006) Pm34: a new powdery mildew resistance gene transferred from Aegilops tauschii coss. to common wheat (Triticum aestivum L.). Theor Appl Genet 113:1497–1504
Miranda LM, Murphy JP, Marshall D, Cowger C, Leath S (2007) Chromosomal location of Pm35, a novel Aegilops tauschii derived powdery mildew resistance gene introgressed into common wheat (Triticum aestivum L.). Theor Appl Genet 114:1451–1456
Oliva A, Tobler R, Llamas B, Souilmi Y (2021) Additional evaluations show that specific BWA-aln settings still outperform BWA-mem for ancient DNA data alignment. Ecol Evol 11:18743–18748
Pan C, Li Q, Lu Y, Zhang J, Yang X, Li X, Li L, Liu W (2017) Chromosomal localization of genes conferring desirable agronomic traits from Agropyron cristatum chromosome 1P. PLoS ONE 12:e0175265
Quinlan AR, Hall IM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842
Rai A, Ahlawat AK, Shukla RB, Jain N, Kumar RR, Mahendru-Singh A (2021) Quality evaluation of near-isogenic line of the wheat variety HD2733 carrying the Lr24/Sr24 genomic region. 3 Biotech 11:130
Sheng BQ, Duan XY (1991) Modification on the evaluation methods of 0–9 level of powdery mildew infection on wheat. Biotech J Agric Sci 9:37–39
Shewry PR (2009) Wheat. J Exp Bot 60:1537–1553
Singh RP, Singh PK, Rutkoski J, Hodson DP, He X, Jorgensen LN, Hovmoller MS, Huerta-Espino J (2016) Disease impact on wheat yield potential and prospects of genetic control. Annu Rev Phytopathol 54:303–322
Singh SP, Hurni S, Ruinelli M, Brunner S, Sanchez-Martin J, Krukowski P, Peditto D, Buchmann G, Zbinden H, Keller B (2018) Evolutionary divergence of the rye Pm17 and Pm8 resistance genes reveals ancient diversity. Plant Mol Biol 98:249–260
Song L, Lu Y, Zhang J, Pan C, Yang X, Li X, Liu W, Li L (2016) Physical mapping of Agropyron cristatum chromosome 6P using deletion lines in common wheat background. Theor Appl Genet 129:1023–1034
Tang Z, Yang Z, Fu S (2014) Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa71, CCS1, and pAWRC.1 for FISH analysis. J Appl Genet 55:313–318
Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J, Banks E, Garimella KV, Altshuler D, Gabriel S, DePristo MA (2013) From fastq data to high confidence variant calls: the genome analysis toolkit best practices pipeline. Curr Protoc Bioinform 43:11–10
Wang H, Sun S, Ge W, Zhao L, Hou B, Wang K, Lyu Z, Chen L, Xu S, Guo J, Li M, Su P, Li X, Wang G, Bo C, Fang X, Zhuang W, Cheng X, Wu J, Dong L, Chen W, Li W, Xiao G, Zhao J, Hao Y, Xu Y, Gao Y, Liu W, Liu Y, Yin H, Li J, Li X, Zhao Y, Wang X, Ni F, Ma X, Li A, Xu SS, Bai G, Nevo E, Gao C, Ohm H, Kong L (2020) Horizontal gene transfer of Fhb7 from fungus underlies fusarium head blight resistance in wheat. Science 368:eaba5435
Wang Y, Long D, Wang Y, Wang C, Liu X, Zhang H, Tian Z, Chen C, Ji W (2020) Characterization and evaluation of resistance to powdery mildew of wheat-Aegilops geniculata Roth 7M(g) (7A) alien disomic substitution line W16998. Int J Mol Sci 21:1861
Wiersma AT, Pulman JA, Brown LK, Cowger C, Olson EL (2017) Identification of Pm58 from Aegilops tauschii. Theor Appl Genet 130:1123–1133
Winfield MO, Wilkinson PA, Allen AM, Barker GL, Coghill JA, Burridge A, Hall A, Brenchley RC, D’Amore R, Hall N, Bevan MW, Richmond T, Gerhardt DJ, Jeddeloh JA, Edwards KJ (2012) Targeted re-sequencing of the allohexaploid wheat exome. Plant Biotechnol J 10:733–742
Wu J, Yang X, Wang H, Li H, Li L, Li X, Liu W (2006) The introgression of chromosome 6P specifying for increased numbers of florets and kernels from Agropyron cristatum into wheat. Theor Appl Genet 114:13–20
Wu X, Bian Q, Gao Y, Ni X, Sun Y, Xuan Y, Cao Y, Li T (2021) Evaluation of resistance to powdery mildew and identification of resistance genes in wheat cultivars. PeerJ 9:e10425
Zhang J, Zhang J, Liu W, Wu X, Yang X, Li X, Lu Y, Li L (2016a) An intercalary translocation from Agropyron cristatum 6P chromosome into common wheat confers enhanced kernel number per spike. Planta 244:853–864
Zhang R, Sun B, Chen J, Cao A, Xing L, Feng Y, Lan C, Chen P (2016b) Pm55, a developmental-stage and tissue-specific powdery mildew resistance gene introgressed from Dasypyrum villosum into common wheat. Theor Appl Genet 129:1975–1984
Zhang J, Liu W, Lu Y, Liu Q, Yang X, Li X, Li L (2017a) A resource of large-scale molecular markers for monitoring Agropyron cristatum chromatin introgression in wheat background based on transcriptome sequences. Sci Rep 7:11942
Zhang Z, Song L, Han H, Zhou S, Zhang J, Yang X, Li X, Liu W, Li L (2017b) Physical localization of a locus from Agropyron cristatum conferring resistance to stripe rust in common wheat. Int J Mol Sci 18:2403
Zhang R, Fan Y, Kong L, Wang Z, Wu J, Xing L, Cao A, Feng Y (2018) Pm62, an adult-plant powdery mildew resistance gene introgressed from Dasypyrum villosum chromosome arm 2VL into wheat. Theor Appl Genet 131:2613–2620
Zhang Z, Han H, Liu W, Song L, Zhang J, Zhou S, Yang X, Li X, Li L (2019) Deletion mapping and verification of an enhanced-grain number per spike locus from the 6PL chromosome arm of Agropyron cristatum in common wheat. Theor Appl Genet 132:2815–2827
Zhang R, Xiong C, Mu H, Yao R, Meng X, Kong L, Xing L, Wu J, Feng Y, Cao A (2021) Pm67, a new powdery mildew resistance gene transferred from Dasypyrum villosum chromosome 1V to common wheat (Triticum aestivum L.). Crop J 9:882–888
Zhu C, Wang Y, Chen C, Wang C, Zhang A, Peng N, Wang Y, Zhang H, Liu X, Ji W (2017) Molecular cytogenetic identification of a wheat-Thinopyrum ponticum substitution line with stripe rust resistance. Genome 60:860–867
Acknowledgements
Thanks to Wheat Resource Center, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences.
Funding
This research was funded by National Science Foundation of China (31801359) and the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2021-ICS). National Aerospace Science Foundation of China, 31801359, Shenghui Zhou.
Author information
Authors and Affiliations
Contributions
LHL conceived the research. YDL performed the research. YDL and SHZ wrote the paper. BH and XZL participated in part of cytology work. BJG, HMH, JPZ, YQL, ZZ, XMY, XQL and WHL participated in the preparation of the reagents and materials used in this study.
Corresponding author
Ethics declarations
Competing interest
The authors declare no conflict of interest.
Available of data and material
Data supporting the current study can be obtained by contacting the corresponding authors.
Code availability
Not applicable.
Additional information
Communicated by Reem Aboukhaddour.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yida Lin and Shenghui Zhou have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lin, Y., Zhou, S., Liang, X. et al. Chromosomal mapping of a locus associated with adult-stage resistance to powdery mildew from Agropyron cristatum chromosome 6PL in wheat. Theor Appl Genet 135, 2861–2873 (2022). https://doi.org/10.1007/s00122-022-04155-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-022-04155-3