Abstract
Main conclusion
Different types of P genome sequences and markers were developed, which could be used to analyze the evolution of P genome in Triticeae and identify precisely wheat- A. cristatum introgression lines.
P genome of Agropyron Gaertn. plays an important role in Triticeae and could provide many desirable genes conferring high yield, disease resistance, and stress tolerance for wheat genetic improvement. Therefore, it is significant to develop specific sequences and functional markers of P genome. In this study, 126 sequences were isolated from the degenerate oligonucleotide primed-polymerase chain reaction (DOP-PCR) products of microdissected chromosome 6PS. Forty-eight sequences were identified as P genome-specific sequences by dot-blot hybridization and DNA sequences analysis. Among these sequences, 22 displayed the characteristics of retrotransposons, nine and one displayed the characteristics of DNA transposons and tandem repetitive sequence, respectively. Fourteen of 48 sequences were determined to distribute on different regions of P genome chromosomes by fluorescence in situ hybridization, and the distributing regions were as following: all over P genome chromosomes, centromeres, pericentromeric regions, distal regions, and terminal regions. We compared the P genome sequences with other genome sequences of Triticeae and found that the similar sequences of the P genome sequences were widespread in Triticeae, but differentiation occurred to various extents. Additionally, thirty-four molecular markers were developed from the P genome sequences, which could be used for analyzing the evolutionary relationship among 16 genomes of 18 species in Triticeae and identifying P genome chromatin in wheat-A. cristatum introgression lines. These results will not only facilitate the study of structure and evolution of P genome chromosomes, but also provide a rapid detecting tool for effective utilization of desirable genes of P genome in wheat improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are abundant repetitive sequences in lots of plant genomes, which constitute the major component of these genomes (Flavell et al. 1974; Consortium 2012). Repeated DNA sequences could be categorized into two types: tandem repetitive DNA sequences and dispersed repetitive DNA sequences. Tandem repetitive sequences are mainly localized to specialized chromosomal regions, such as centromeres, telomeres, and heterochromatic regions (Gindullis et al. 2001; Plohl et al. 2008). The variations of tandem repetitive sequences could be used to study the evolution of plant genome as well as the evolutionary trends (Zhou et al. 2010). A majority of dispersed repetitive sequences had the characteristic of transposable elements (TEs) (Kubis et al. 1998), which were not only the main components of plant genomes, but also the major force in plant genome evolution (Fujimoto et al. 2008; Petit et al. 2010). TEs played a role in many processes of evolution, including insertion into protein-coding genes, illegitimate recombination and chromosome breakage (Slotkin and Martienssen 2007). To reveal the evolutionary dynamics, TEs were often used to study the different abundance of the same TE family among species and the copy number variation in the process of polyploidization (Ragupathy et al. 2010; Salina et al. 2011; Middleton et al. 2013; Yaakov et al. 2013). In recent years, some new dispersed repetitive DNA sequences have been isolated from plants, such as Revolver, Superior of rye (Tomita et al. 2008; Tomita 2010), C1-10, pDbC2 from Dasypyrum villosum (Li et al. 2012; Zhang et al. 2013b). These repetitive DNA sequences will be helpful to better understand the structure of retrotransposons and the evolution of plant genome.
It is an effective way to obtain repetitive sequences by means of chromosome microdissection. Some chromosome-specific DNA libraries of plant have been constructed through chromosome microdissection and microcloning which has been successful in wheat (Liu et al. 1997, 1999; Hu et al. 2004), barley (Schondelmaier et al. 1993), rye (Zhou et al. 1999), Haynaldia villosa (Kong et al. 2001), oat (Chen and Armstrong 1995), soybean (Zhou et al. 2001), and cotton (Peng et al. 2012). The chromosome-specific DNA library provides a basis for further screening repetitive sequences. Some repetitive sequences were isolated from DNA libraries, including pHvMWG2314 and pHvMWG2315 from chromosome 3HL of barley (Busch et al. 1995), pHVMK134 from 6VS of H. villosa (Kong et al. 2001), and some Revolver-related sequences from 1R of rye (Deng et al. 2014).
To broaden the genetic basis of wheat, an increasing number of desirable genes have been transferred from wild relatives into common wheat (Friebe et al. 1996; Gill et al. 2006). It is an important step to identify the alien chromatin or genes. Actually, the effective transfer and utilization of alien genes largely depended on the precise identification of alien chromatin (Zhang 1991). Genome-specific sequences, especially the genome-specific dispersed repetitive sequences, could be considered as genome-specific fluorescence in situ hybridization (FISH) probes to identify alien chromosomes. At present, these sequences have been isolated from wheat (Komuro et al. 2013), rye (Ko et al. 2002; Liu et al. 2008a; Jia et al. 2009), D. villosum (Zhang et al. 2013b), and A. cristatum (Han et al. 2015) in the tribe Triticeae. With the rapid development of genomics, molecular markers have been widely used in the identification of alien chromosomal segments in wide hybrids. Molecular markers designed on the basis of genome-specific sequences are useful and convenient tools for detecting alien chromatin incorporated into wheat genomes. The genome-specific markers obtained from RAPD analysis have been used to identify alien chromosomal segments (Guidet et al. 1991; Ko et al. 2002; Liu et al. 2008a; Jia et al. 2009; Wu et al. 2010; Zhang et al. 2013b).
Agropyron Gaertn. (P genome) is an important genus of wild relatives of wheat, which can provide many desirable genes for wheat improvement, such as resistance to certain diseases and abiotic stress, and high-yield characteristics of more fertile tillers and more florets (Dewey 1984; Dong et al. 1992). In our laboratory, a series of disomic addition lines has been obtained from the successful hybridization between common wheat Fukuho and A. cristatum (accession Z559) (Li et al. 1997, 1998). Wheat-A. cristatum 6P disomic addition lines contained desirable genes conferring high numbers of kernels per spike, and resistance to powdery mildew and leaf rust (Wu et al. 2006; Han et al. 2014; Song et al. 2016). Wheat-A. cristatum 2P addition lines showed high resistance to powdery mildew and leaf rust resistance, as well as narrow flag leaves (Li et al. 2016). Wheat-A. cristatum 7P addition lines conferred longer grain length and higher thousand-grain weigh (Lu et al. 2016). These wheat-A. cristatum addition lines were used as the fundamental materials to transfer useful genes from A. cristatum to wheat. There were some reports about repetitive sequences of Agropyron. Svitashev et al. (1998) developed two repeated sequences pAgc1 and pAgc30 from “Relic” DNA of A. cristatum, and used them in genome identification of Elymus. Wu et al. (2010) cloned three A. cristatum-specific sequences by RAPD and applied them to detect P genome chromosomes. Han et al. (2015) isolated a P genome-specific Gypsy-like sequence through chromosome microdissection and microcloning. The rDNA spacer sequence pAcR4 which located at NOR loci and the 5S DNA of A. cristatum were developed (Appels et al. 1986; Xin and Appels 1988). The A. cristatum repetitive sequences could not meet the needs of the study on structure and evolution of P genome chromosomes and the characterization of wheat-A. cristatum derivatives. Therefore, it is important to further isolate and characterize A. cristatum-specific sequences.
In this study, a lot of P genome-specific sequences were isolated from the microdissected chromosome 6PS which was successfully microdissected from a wheat-A. cristatum 6PS addition line in our previous study (Han et al. 2015), and their distribution patterns on P genome chromosomes were explored, then many molecular markers specific for A. cristatum were developed. The evolutionary relationships among 16 genomes of 18 species in Triticeae were analyzed by these markers. Meanwhile, P genome chromosomal fragments in wheat-A. cristatum derivatives were identified. These results will not only provide resources for the further study of structure and evolution of P genome chromosomes but also contribute to the identification and utilization of excellent wheat-A. cristatum derivatives.
Materials and methods
Plant materials
The detailed information of all the materials used in this study is shown in Table 1. The common wheat Fukuho was used as maternal parent in cross with A. cristatum accession Z559 (2n = 4x = 28, PPPP). All the fifteen different wheat-A. cristatum disomic addition lines (4844-12, 5113, 5114, II-26, II-29-2i, 5106, II-5-1, II-4-2, 5038, 5043, II-21-2, II-21-6, II-9-3, II-7-1, II-8-1) used contain 42 wheat chromosomes and a pair of alien A. cristatum chromosomes. Wheat-A. cristatum 6PL addition line D3-10 was obtained from the hybrids between wheat-A. cristatum 6P disomic addition line 4844-12 and T. aestivum cultivar Chinese Spring-Aegilops cylindrica Host 2C addition line. Wheat-A. cristatum 6PS addition line D14-19, deletion line D4804-3 (6PS-0.00-0.15), translocation line Pubing3035, and introgression line Pubing23667 were obtained from the irradiation of hybrids between 4844-12 and common wheat cultivar Gaocheng 8901. Eighteen species contain different genomes including P, PP, PPP, ABD, A, AB, AG, C, D, M, S, U, R, H, E, V, Ns, St, and Y genomes, with accessions Z1842, Z559, Z1750, Chinese Spring (CS), MO4, JingDR3, TI1, Ae14, Y93, Y258, Ae49, Y39, RM2161, Zhepi 1, PI547326, Z1731, R429, Z1365, and Z2192, respectively. Genome symbols were according to Wang et al. (1994).
Cloning of DOP-PCR products from microdissected 6PS
Chromosome 6PS were obtained by chromosome microdissection and the DNA from microdissected chromosome 6PS was subjected to two rounds of PCR amplification by DOP (Han et al. 2015). The first- and second-round DOP-PCR were performed in 50μL volume, and the second-round PCR used 5μL of the first-round DOP-PCR products as a template. In this study, four microlitres of the second-round DOP-PCR products were ligated into pMD19-T cloning vector (Code: D102A; TaKaRa Biotechnology, Dalian, China), which was used to transform competent cells of Escherichia coli strain DH5α. Then the DNA library of chromosome 6PS was constructed. The clones were detected with the universal primers M13 forward/reverse and checked on 1% agarose gels.
Dot-blot analysis
The positive clones were used as the DNA targets. The A. cristatum genomic DNA and Fukuho genomic DNA were used as probes, respectively. The dot-blot hybridization was performed as previously described by Malinen et al. (2003). The plasmids of which hybridization results varied between the two probes were selected. Subsequently, the selected plasmids were used for DNA sequencing (SinoGenoMax, Beijing, China) and FISH analysis.
Genomic in situ hybridization and FISH
Genomic in situ hybridization (GISH) was used to detect the P genome chromosomes in wheat-A. cristatum addition lines and introgression lines with A. cristatum and Fukuho genomic DNA as the probe and blocker, respectively. FISH using the DNA clones from DOP-PCR products as probes was performed to detect their distribution patterns on P genome chromosomes and wheat genome chromosomes. The procedures of GISH and FISH were followed the protocols described by Kato et al. (2004) and Han et al. (2006). The probes were labeled with Texas Red-5-dCTP or Fluorescein -12-dUTP (PerkinElmer, Boston, MA, USA). All the images were obtained using an Olympus AX80 (Olympus Corp., Tokyo, Japan) fluorescence microscope and processed with Photoshop CS 3.0 (Adobe, San Jose, CA, USA).
Sequence analysis and molecular marker analysis
Sequence analysis of isolated A. cristatum sequences was performed using BLASTn program in National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov), RepeatMaster online (http://www.repeatmasker.org/cgi-bin/WEBRepeatMasker), and Triticeae Repeat Sequence Database (TREP) (http://wheat.pw.usda.gov/GG2/blast.shtml).
The alignments were performed between isolated sequences and their corresponding sequences with high similarity selected through BLASTn program in T. aestivum twenty-one chromosomes sequence database, T. urartu genome sequence database, and Aegliops tauschii genome sequence database in URGI (https://urgi.versailles.inra.fr/blast/blast.php). Molecular markers were developed at differential regions or low-similarity regions using primer premier 5.0 (PREMIER Biosoft, Palo Alto, CA, USA). All the primers were named after DOP-PCR sequences without the first letter ‘p’. PCR was performed as previously described (Luan et al. 2010). The amplification products were separated on 1% agarose gels.
A cluster analysis of 16 genomes was carried out to generate a dendrogram using the unweighted pair group method with arithmetic averages (UPGMA) in the SAS software package (V8.1, SAS Institute Inc., Cary, NC, USA).
Results
Preliminary analysis of DNA library of A. cristatum chromosome 6PS
A total of 1123 white colonies were recovered from the transformation of DOP-PCR products, and 918 colonies were positive detected by PCR amplification using M13 F/R primers (Suppl. Fig. S1). The size of the inserts was more than 200 bp. Nine hundred and eighteen plasmids were isolated from these positive colonies, and then screened by dot-blot hybridization. The plasmids which only hybridized with A. cristatum genomic DNA and the ones with stronger hybridization with A. cristatum genomic DNA than Fukuho genomic DNA were selected. Then 126 DNA clones were selected and sequenced according to this standard. But most of them contained the same or high similar sequences, and finally 48 different sequences were obtained. All the 48 sequences were registered in NCBI GenBank with the accession numbers KP231286 and KX390693-KX390739 (Suppl. data).
Characteristics analysis of 48 sequences
Various sequence characteristics were found in these 48 sequences by sequence analysis in NCBI, TREP, and RepeatMasker. The detailed information is shown in Suppl. Table S1. Twenty-two sequences displayed the characteristics of retrotransposons (45.8%), accounting for the highest percentage of all the sequences. Nine sequences displayed the characteristics of DNA transposons; one and three sequences displayed tandem repeat sequence and simple sequence repeat, respectively; however, the remaining 13 sequences did not show any typical characteristics. Eight of 48 sequences did not show any similarity with wheat genomic sequences. The other 40 sequences showed different similarity with species of Triticeae when performing BLASTn in NCBI, including T. aestivum, T. uraru, T. monococcum, T. turgidum, T. timopheevii, A. tauschii, A. speltoides, H. vulgare, S. cereale, Th. elongatum, Leymus arenarius. These results suggested that the A. cristatum sequences with the corresponding sequences of Triticeae might belong to the same family and these sequences were widespread in the tribe Triticeae. In addition, pAcCR1 had the characteristic of Gypsy retrotransposon through RepeatMasker, but its highly similar sequence could not be found. Tandem repeat pAcTRT1 showed 90% identity to the S. cereale tandem repeat pSc200, which suggested that pAcTRT1 might belong to 350-bp family same as pSc200. pAcP10 and pAcP40 had only one similar sequence in NCBI.
Distribution patterns of DNA clones on A. cristatum chromosomes
To explore distribution patterns of A. cristatum DNA clones on P chromosomes, FISH was performed on mitotic metaphase chromosomes of wheat-A. cristatum 6P addition line 4844-12 and diploid A. cristatum Z1842 using 48 A. cristatum DNA clones as probes, respectively. FISH results showed that only 14 DNA clones with the characteristics of retrotransposons and tandem repeat could generate fluorescence signals. According to the distribution patterns on P genome chromosomes, 14 DNA clones could be classified into five types (Table 2; Fig. 1). The first type consisted of eight DNA clones distributing all over A. cristatum chromosomes with relative weaker signals in the centromeric and telomeric regions. pAcCR1 was the only one classified in the second type, which was a centromeric sequence. The third and the fourth type comprised two clones distributing in the pericentromeric regions and distal regions, respectively; the fifth type (pAcTRT1) distributed in the terminals of chromosomes and the signals varied on different chromosomes. The remaining 34 DNA clones could not produce any hybridization signals on all the A. cristatum chromosomes. These results suggested that repetitive sequences distributed on certain regions of chromosomes, and some repetitive sequences differ strongly in their abundance in A. cristatum genome.
FISH patterns on mitotic chromosomes of wheat-A. cristatum 6P addition line 4844-12 (a, c, e, g, i) and diploid A. cristatum Z1842 (b, d, f, h, j) using five types of sequences specific to A. cristatum as probes. The probes used were pAcPR2, pAcCR1, pAcpCR1, pAcTR1 (a–h) and pAcTRT1 (i, j), respectively. Bars 10 μm
When all the types of DNA clones were hybridized to the chromosomes of 4844-12, the fourth type produced fluorescence signals on some wheat chromosomes besides the added A. cristatum chromosomes (Fig. 1g); other four types only produced fluorescence signals on two added A. cristatum chromosomes (Fig. 1a–f, i, j). These results suggested that these four types of sequences were P genome-specific sequences, which could be used as P genome-specific FISH markers. They could identify different regions of chromosomes according to their distribution patterns, and the origin of alien A. cristatum chromosomal segments in wheat-A. cristatum derivatives could be speculated. For example, pAcCR1 could identify centromeres of P genome chromosomes specifically. Type 1, 3, 5 sequences did not distribute on wheat chromosomes according to FISH results, but they showed similarity with wheat genome sequences (Suppl. Table S1). This suggested that repetitive sequences of the same family differed between A. cristatum and wheat. It might indicate that some transposons were genome-specific, and might promote genome evolution.
Development of P genome-specific molecular markers
Sixty-six primer pairs were designed based on 48 A. cristatum-specific sequences along with a P genome-specific sequence pAcPR1 published previously in Han et al. (2015). The molecular markers were used to analyze A. cristatum accession Z559, addition line 4844-12, and common wheat Fukuho. The number of markers specific to Z559 and 4844-12 with no bands in Fukuho was thirty-four (Suppl. Table S2), accounting for 51.5%.
Evolutionary relationships between P genome and other genomes in Triticeae
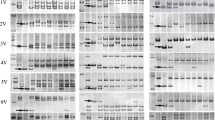
Thirty-four molecular markers were used to analyze 18 species containing 16 genomes (P, ABD, A, AB, AG, C, D, M, S, U, R, H, E, V, Ns, St and Y). The amplification results in these materials were good (Table 3). All the primers could be amplified in diploid, tetraploid, and hexaploid A. cristatum, except AcpCR2 and AcP6 which could not be amplified in hexaploid A. cristatum. Fourteen out of 34 primers were only amplified in P genome, such as AcP54 (Fig. 2a). The remaining 20 primers could be amplified in other genomes besides P genome, such as AcPR3 (Fig. 2b). These results suggested that these 20 primers contained lower specificity than those 14 primers. Cluster analysis were performed using UPGMA (SAS V8.1 software) based on the results of molecular markers amplified in 16 genomes (Fig. 3). The result of cluster analysis showed that P genome was closest to St genome, followed by Ns and E genomes.
PCR amplification profiles using primers AcP54 (a) and AcPR3 (b) in Triticeae plants. Lanes: M DNA ladder; 1 Z1842 (A. cristatum, 2×, P); 2 Z559 (A. cristatum, 4×, P); 3 Z1750 (A. cristatum, 6×, P); 4 CS (T. aestivum L., ABD); 5 Fukuho (T. aestivum L., ABD); 6 MO4 (T. monococcum L., A); 7 JingDR3 (T. durum Desf., AB); 8 TI1 (T. timopheevii Zhuk., AG); 9 Ae14 (Ae. caudata L., C); 10 Y93 (Ae. tauschii Cosson., D); 11 Y258 (Ae. comosa Sibth.et Sm., M); 12 Ae49 (Ae. speltoides Tausch., S); 13 Y39 (Ae. umbellulata Zhuk., U); 14 RM2161 (S. cereale L., R); 15 Zhepi 1 (H. vulgare L., H); 16 PI547326 (Th. elongatum, E); 17 R429 (Ps. juncea (Fisch.) Nevski, Ns); 18 Z1365 (P. strigosa A.Love, St); 19 Z1731 (Ha. villosa L., V); 20 Z2192 (R. kamoji ohwi, Y)
Application of P genome-specific markers in wheat-A. cristatum derivatives
The constitutions of six wheat-A. cristatum addition lines (4844-12, 5113, 5114, 5106, II-26, and II-29-2i) were identified to contain 42 wheat chromosomes and two 6P chromosomes in the previous study (Han et al. 2014). In this study, 5038, 5043, II-4-2, II-5-1, II-9-3, II-21-2, and II-21-6 were confirmed to be wheat-A. cristatum disomic addition lines by GISH, while II-7-1 and II-8-1 were wheat-A. cristatum ditelosomic addition lines (Suppl. Fig. S2). Thirty-four P genome markers were used to detect these addition lines as well as wheat-A. cristatum 6PS (D14-19) and 6PL (D3-10) addition lines. Twenty-six out of 34 markers produced P genome-specific bands in these wheat-A. cristatum addition lines (Fig. 4a; Suppl. Table S3), suggesting that these 26 markers could identify multiple P genome chromosomes in wheat background. Five markers including AcpCR2, AcP3, AcP4, AcP26, AcP54 could only produce P genome-specific bands in wheat-A. cristatum 6P addition lines 4844-12, 5113, 5114, 5106 and wheat-A. cristatum 6PS addition line D14-19 (Fig. 4b), suggesting that these five markers were 6PS-specific markers. Then the precise locations of these five markers were determined by deletion lines. AcpCR2 (Fig. 5a), AcP3 could generate specific bands in D4804-3 (6PS-0.00-0.15) and D14-19 (6PS-0.00-1.00), while AcP4, AcP26 (Fig. 5b), and AcP54 could only generate specific bands in D14-19. Thus, two markers (AcpCR2 and AcP3) were located to 6PS-0.00-0.15, and the other three markers (AcP4, AcP26, and AcP54) were located to 6PS-0.15-1.00 (Fig. 5c).
PCR amplification profiles using primers AcPR7 (a) and AcpCR2 (b) in the wheat-A. cristatum addition lines. Lanes: M DNA ladder; 1 Z559; 2 Fukuho; 3 4844-12; 4 5113; 5 5114; 6 II-26; 7 II-29-2i; 8 5106; 9 II-5-1; 10 II-4-2; 11 5038; 12 5043; 13 II-21-2; 14 II-21-6; 15 II-9-3; 16 II-7-1; 17 II-8-1; 18 D3-10; 19 D14-19
P genome-specific markers could be used to detect small alien P genome chromosomal segments in wheat-A. cristatum introgression lines. Pubing3035 was a wheat-A. cristatum translocation line, which could enhance thousand-grain weight and spike length (Zhang et al. 2015); Pubing23667 was a wheat-A. cristatum introgression line, which showed high numbers of kernels per spike (data not shown). GISH results revealed that Pubing3035 was an intercalary translocation line (Fig. 6a) and there was no visible signal in Pubing23667 (Fig. 6b). Sixteen P genome-specific markers could be amplified in Pubing3035 (Fig. 6c). However, one P genome-specific marker could be amplified in Pubing23667, suggesting that there were P chromatin in Pubing23667 but below the detection limit of GISH (Fig. 6d).
Discussion
High/medium copy sequences and low/single copy sequences were usually present in amplification products of microdissected chromosome. DNA library of microdissected chromosome 1HS of barley contained approximately 60% high/medium copy sequences (Schondelmaier et al. 1993). About 42% high/medium copy sequences were present in microclone library of 1R chromosome of rye (Zhou et al. 1999). The rate of the single and low copy sequences was over 47% in the library of chromosome 5 of Gossypium arboretum (Peng et al. 2012). Repetitive sequences were the focus of our study. Among the 48 isolated sequences, 32 sequences were determined to be high/medium copy sequences, including 22 retrotransposons partial sequences, 9 DNA transposons partial sequences, and one tandem repeat. In addition, the corresponding sequences of five markers specific to chromosome 6PS should be single or low copy sequences.
Distribution patterns vary among different repetitive sequences. In this study, five types of sequences with different distribution patterns were revealed by FISH. These kinds of sequences were also found to exist in many plant genomes. Lamb and Birchler (2006) published many transposons of maize, and their distribution patterns were similar to that of type 1 to type 4 sequences of this study. Some repetitive sequences were discovered to distribute in the pericentromeric and centromeric regions in alfalfa (Yu et al. 2013), but they belonged to microsatellite or tandem repeats different from the sequences in our study. There were many reports on genome-specific sequences distributing all over the chromosomes. Komuro et al. (2013) released many sequences only distributing along the A or B genome of wheat. In addition, similar sequences could also be found in rye and D. villosum (Liu et al. 2008a; Zhang et al. 2013b). Sequences located at telomere or subtelomere are mostly tandem repetitive sequences, which resembled pAcTRT1 mentioned in this study, pSc200 and pSc250 from rye (Vershinin et al. 1995), pAesKB52 from Ae. speltoides (Anamthawat-Jonsson and Heslop-Harrison 1993), and pHvMWG2315 isolated from barley by microdissection and microcloning (Busch et al. 1995). Sequences distributing in the pericentromeric regions have been reported in rye (Ko et al. 2002), but there are little reports on the sequences which resemble type 4 in the distal regions in Triticeae. Deng et al. (2014) thought that it was difficult to isolate centromeric sequences from DOP-PCR products of rye. Fortunately, we obtained a centromeric sequence pAcCR1 specific to P genome from DOP-PCR products of chromosome 6PS. Therefore, it is feasible and efficient to isolate repetitive sequences with different distribution patterns by microdissection and microcloning.
There are variations in the amount and copy number of TEs among different species in Triticeae (Middleton et al. 2013). Yaakov et al. (2013) assessed the relative copy numbers of 16 TE families in 22 Triticum and Aegilops species and revealed that high variations of TE were indeed present among these species. In this study, repetitive sequences of the same family differed between A. cristatum and wheat based on the results of dot blot, sequence analysis, and FISH. The different distributions of specific repetitive sequences in A. cristatum may indicate their different roles in the process of genome evolution, which would be helpful for understanding the evolution of repetitive sequences.
Dispersed repetitive sequences specific to P genome could be used as probes to identify alien A. cristatum chromosomes or chromosomal segments. The results of FISH using genome-specific repetitive sequences as probes are similar to that of GISH, because the efficacy of GISH is largely based on the genome-specific dispersed repetitive sequences (Kato et al. 2005). GISH using genomic DNA as probes generates signals in NOR regions sometimes. But it rarely occurs in FISH and there is no blocking DNA in FISH. The eight P genome-specific sequences dispersed all over the chromosomes of A. cristatum as well as pAcPR1 (Han et al. 2015) could be used as FISH probes to identify P genome chromosomal segments in wheat-A. cristatum derivatives. It was noted that we isolated centromeric and pericentromeric sequences specific to A. cristatum, which as probes could be helpful to deduce the region of P genome chromosomes of translocated chromosome roughly; just like Li et al. (2012), who isolated a centromeric sequence specific to D. villosum and used it to detect the centromere of D. villosum chromosomes.
Molecular markers based on P genome-specific dispersed repetitive sequences could realize efficient detection of wheat-A. cristatum introgression lines. The high content of dispersed repetitive sequences in genome determines their widespread distribution on chromosomes. Thus, these molecular markers could identify multiple sites of different A. cristatum chromosomes which were well demonstrated by the results in this study that one marker could identify multiple different wheat-A. cristatum addition lines. There was no lack of examples about identification of wheat-alien species chromosomes by genome-specific markers (Ko et al. 2002; Liu et al. 2008a; Jia et al. 2009; Li et al. 2012; Zhang et al. 2013b). Wu et al. (2010) obtained three SCAR markers specific to A. cristatum and used them to identify wheat-A. cristatum addition lines. More P genome-specific markers with repetitive sequences were developed in this study, which could identify small segment translocations and introgressions below the detection limit of GISH. Low copy sequences are usually chromosome-specific sequences, which can be used as chromosome-specific tags. Schondelmaier et al. (1993) found that low copy sequences from microdissected 1HS chromosome were all specific to chromosome 1HS. In this study, five markers were specific to chromosome 6PS and their amplified sequences should be chromosome 6PS-specific sequences. Liu et al. (1997) obtained four chromosome 5BL-specific sequences also by chromosome microdissection and DOP-PCR. Understanding of the homoeology between the alien and wheat chromosomes would facilitate utilization of the favorable genes on alien chromosomes in wheat improvement (McArthur et al. 2012). Thus, the five 6PS-specific markers could be used to judge whether the alien chromosomes belong to 6PS or not.
Repetitive sequences play an important role in genome evolution. As the dispersed repeats evolve faster than genes, they are the major force of genome evolution (Schwarzacher et al. 1989). Divergence of retroelements among the Zea and Tripsacum genera allowed to distinguish different genomes by FISH (Lamb and Birchler 2006). During the evolution of the genus Secale from wild to cultivated species, Revolver-related sequences displayed different variations (Tang et al. 2011). Cuadrado and Jouve (1997) classified the species of the genus Secale into two groups using repetitive sequences, and inferred that the cultivated ryes evolved from wild weedy species. In this study, cluster analysis indicated that the relationship between P and St genomes was closest. Wang (1992) also uncovered that P genome was closest to St genome according to chromosome pairing in diploid hybrids, and the relationships of P genome with other genomes were similar to that of this study. Translocations between St and P genomes were found in Kengyilia thoroldiana and K. hirsute (Wang et al. 2012; Dou et al. 2013). These results demonstrated that the relationship between P and St genomes was very close, and the determination of genome relationships based on repetitive sequences was reliable. In addition, cluster analysis showed that the relationships of P genome with genomes of Triticum were further than that with other seven genomes. However, sequence analysis indicated that P genome repetitive sequences showed similarity with genomes of genus Triticum. The reasons might be that the genome sequences of other genomes of Triticeae were limited.
Tandem repeat sequences could identify plant chromosomes. Rye chromosomes could be identified by tandem repeat pSc119.2 (Mukai et al. 1992), and this sequence could also be used to distinguish B genome chromosomes of wheat (Mukai et al. 1993). The combination of tandem repeats pAs1 and GAA-satellite sequence pHvG38 allowed to discriminate the entire chromosomes of wheat (Pedersen and Langridge 1997). Busch et al. (1995) isolated a tandem repetitive sequence from microdissected 3HL chromosome and used it to discriminate barley chromosomes. Besides, Afa-family sequence pHvA14 could also be used to distinguish H genome chromosomes (Tsujimoto et al. 1997). In this study, we found that the distribution patterns as well as the signal intensity of pAcTRT1 varied among different A. cristatum chromosomes. Thus, A. cristatum chromosomes might be discriminated using these characteristics together with chromosome length and arm ratio. In addition, tandem repeats could be used to study the genetic rearrangements during the polyploidization (Han et al. 2005; Zhang et al. 2013a).
In plant centromeres, centromeric retrotransposons (CRs) and satellite DNA are the most abundant DNA elements and associated with the centromere-specific histone H3 variant (CENH3) (Nagaki et al. 2003, 2004). Although the functions of centromeres are conserved among all eukaryotes, there is no conservation of centromeric DNA sequences (Jiang et al. 2003). Wheat centromeres are mainly composed of centromeric retrotransposons (Liu et al. 2008b; Li et al. 2013). CCS1 and pSau3A9 are parts of Ty3/gypsy-type retrotransposons and conserved among cereal species (Aragón-Alcaide et al. 1996; Jiang et al. 1996). Besides the two highly conserved CRs, some genome-specific CR sequences were isolated in Triticeae (Francki 2001; Li et al. 2012). However, there was no report about this in Agropyron. In this study, a centromeric sequence pAcCR1 of Agropyron was obtained by chromosome microdissection and microcloning, and it was part of Ty3/gypsy-type retrotransposons and specific to P genome. The distribution patterns of pAcCR1 on tetraploid A. cristatum were similar to those on diploid A. cristatum (unpublished data), suggesting that pAcCR1 was conserved among different ploidy. Whether pAcCR1 associated with CENH3 will be studied in the further work. Therefore, it is significant to isolate a CR specific to A. cristatum for studying the structure, function, and evolution of centromeres of Agropyron chromosomes.
Author contribution statement
LHL and ZMH designed the research; HMH, WHL, YQL, JPZ, XMY, XQL conducted experiments; HMH, LHL, and ZMH analyzed the data; HMH and YQL wrote the manuscript.
Abbreviations
- DOP-PCR:
-
Degenerate oligonucleotide primed-polymerase chain reaction
- FISH:
-
Fluorescence in situ hybridization
- GISH:
-
Genomic in situ hybridization
References
Anamthawat-Jonsson K, Heslop-Harrison J (1993) Isolation and characterization of genome-specific DNA sequences in Triticeae species. Mol Gen Genet 240:151–158
Appels R, McIntyre C, Clarke B et al (1986) Alien chromatin in wheat: ribosomal DNA spacer probes for detecting specific nucleolar organizer region loci introduced into wheat. Can J Genet Cytol 28:665–672
Aragón-Alcaide L, Miller T, Schwarzacher T et al (1996) A cereal centromeric sequence. Chromosoma 105:261–268
Busch W, Martin R, Herrmann RG et al (1995) Repeated DNA sequences isolated by microdissection. I. Karyotyping of barley (Hordeum vulgare L.). Genome 38:1082–1090
Chen Q, Armstrong K (1995) Characterization of a library from a single microdissected oat (Avena sativa L.) chromosome. Genome 38:706–714
Consortium IBGS (2012) A physical, genetic and functional sequence assembly of the barley genome. Nature 491:711–716
Cuadrado A, Jouve N (1997) Distribution of highly repeated DNA sequences in species of the genus Secale. Genome 40:309–317
Deng CL, Bai LL, Shufen L et al (2014) DOP-PCR based painting of rye chromosomes in a wheat background. Genome 57:473–479. doi:10.1139/gen-2014-0110
Dewey DR (1984) The genomic system of classification as a guide to intergeneric hybridization with the perennial Triticeae. In: Gustafson JP (ed) Gene manipulation in plant improvement. In: Proceedings of 16th Stadler genetics symposium. Plenum Press, New York, pp 209–279
Dong YS, Zhou RH, Xu SJ et al (1992) Desirable characteristics in perennial Triticeae collected in China for wheat improvement. Hereditas 116:175–178
Dou Q, Wang RR, Lei Y et al (2013) Genome analysis of seven species of Kengyilia (Triticeae: Poaceae) with FISH and GISH. Genome 56:641–649
Flavell R, Bennett M, Smith J et al (1974) Genome size and the proportion of repeated nucleotide sequence DNA in plants. Biochem Genet 12:257–269
Francki MG (2001) Identification of Bilby, a diverged centromeric Ty1-copia retrotransposon family from cereal rye (Secale cereale L.). Genome 44:266–274
Friebe B, Jiang J, Raupp W et al (1996) Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91:59–87
Fujimoto R, Takuno S, Sasaki T et al (2008) The pattern of amplification and differentiation of Ty1-copia and Ty3-gypsy retrotransposons in Brassicaceae species. Genes Genet Syst 83:13–22
Gill BS, Friebe B, Raupp WJ et al (2006) Wheat genetics resource center: the first 25 years. Adv Agron 89:73–136
Gindullis F, Desel C, Galasso I et al (2001) The large-scale organization of the centromeric region in Beta species. Genome Res 11:253–265. doi:10.1101/gr.162301
Guidet F, Rogowsky P, Taylor C et al (1991) Cloning and characterisation of a new rye-specific repeated sequence. Genome 34:81–87
Han FP, Fedak G, Guo WL et al (2005) Rapid and repeatable elimination of a parental genome-specific DNA repeat (pGcIR-1a) in newly synthesized wheat allopolyploids. Genetics 170:1239–1245. doi:10.1534/genetics.104.039263
Han F, Lamb JC, Birchler JA (2006) High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc Natl Acad Sci USA 103:3238–3243
Han H, Bai L, Su J et al (2014) Genetic rearrangements of six wheat-Agropyron cristatum 6P addition lines revealed by molecular markers. PLoS One 9:e91066
Han H, Zhang Y, Liu W et al (2015) Degenerate oligonucleotide primed-polymerase chain reaction-based chromosome painting of P genome chromosomes in Agropyron cristatum and wheat-A. cristatum addition lines. Crop Sci 55:2798–2805
Hu ZM, Wang H, Shi R et al (2004) Microdissection and construction of region-specific DNA libraries of wheat chromosome 6B. Acta Bot Sin 46:1357–1365
Jia J, Yang Z, Li G et al (2009) Isolation and chromosomal distribution of a novel Ty1-copia-like sequence from Secale, which enables identification of wheat-Secale africanum introgression lines. J Appl Genet 50:25–28
Jiang J, Nasuda S, Dong F et al (1996) A conserved repetitive DNA element located in the centromeres of cereal chromosomes. Proc Natl Acad Sci USA 93:14210–14213
Jiang J, Birchler JA, Parrott WA et al (2003) A molecular view of plant centromeres. Trends Plant Sci 8:570–575
Kato A, Lamb JC, Birchler JA (2004) Chromosome painting using repetitive DNA sequences as probes for somatic chromosome identification in maize. Proc Natl Acad Sci USA 101:13554–13559
Kato A, Vega JM, Han F et al (2005) Advances in plant chromosome identification and cytogenetic techniques. Curr Opin Plant Biol 8:148–154
Ko J-M, Do G-S, Suh D-Y et al (2002) Identification and chromosomal organization of two rye genome-specific RAPD products useful as introgression markers in wheat. Genome 45:157–164
Komuro S, Endo R, Shikata K et al (2013) Genomic and chromosomal distribution patterns of various repeated DNA sequences in wheat revealed by a fluorescence in situ hybridization procedure. Genome 56:131–137
Kong F, Chen X, Ma Y et al (2001) Microdissection of Haynaldia villosa Telosome 6VS and cloning of species-specific DNA sequences. Acta Bot Sin 44:307–313
Kubis S, Schmidt T, Heslop-Harrison JSP (1998) Repetitive DNA elements as a major component of plant genomes. Ann Bot 82:45–55
Lamb JC, Birchler JA (2006) Retroelement genome painting: cytological visualization of retroelement expansions in the genera Zea and Tripsacum. Genetics 173:1007–1021
Li L, Li X, Li P et al (1997) Establishment of wheat-Agropyron cristatum alien addition lines. I. Cytology of F3, F2BC1, BC4, and BC3F1 progenies. Acta Genet Sin 24:154–159
Li L, Yang X, Zhou R et al (1998) Establishment of wheat-Agropyron cristatum alien addition lines II. Identification of alien chromosomes and analysis of development approaches. Acta GenetSin 25:538–544
Li G, Liu C, Wei P et al (2012) Chromosomal distribution of a new centromeric Ty3-gypsy retrotransposon sequence in Dasypyrum and related Triticeae species. J Genet 91:343–348
Li B, Choulet F, Heng Y et al (2013) Wheat centromeric retrotransposons: the new ones take a major role in centromeric structure. Plant J 73:952–965
Li H, Lv M, Song L et al (2016) Production and identification of wheat-Agropyron cristatum 2P translocation lines. PLoS One 1:e0145928. doi:10.1371/journal.pone.0145928
Liu B, Segal G, Vega JM et al (1997) Isolation and characterization of chromosome-specific DNA sequences from a chromosome arm genomic library of common wheat. Plant J 11:959–965
Liu B, Rong J, Dong Y et al (1999) Microdissection of chromosome 7B of common wheat and cloning of low-copy specific DNA sequences. Chin Sci Bull 44:632–636
Liu C, Yang Z, Li G et al (2008a) Isolation of a new repetitive DNA sequence from Secale africanum enables targeting of Secale chromatin in wheat background. Euphytica 159:249–258
Liu Z, Yue W, Li D et al (2008b) Structure and dynamics of retrotransposons at wheat centromeres and pericentromeres. Chromosoma 117:445–456
Lu M, Lu Y, Li H et al (2016) Transferring desirable genes from Agropyron cristatum 7P chromosome into common wheat. PLoS One 11(7):e0159577. doi:10.1371/journal.pone.0159577
Luan Y, Wang X, Liu W et al (2010) Production and identification of wheat-Agropyron cristatum 6P translocation lines. Planta 232:501–510
Malinen E, Kassinen A, Rinttilä T et al (2003) Comparison of real-time PCR with SYBR Green I or 5′-nuclease assays and dot-blot hybridization with rDNA-targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology 149:269–277
McArthur RI, Zhu X, Oliver R et al (2012) Homoeology of Thinopyrum junceum and Elymus rectisetus chromosomes to wheat and disease resistance conferred by the Thinopyrum and Elymus chromosomes in wheat. Chromosome Res 20:699–715
Middleton CP, Stein N, Keller B et al (2013) Comparative analysis of genome composition in Triticeae reveals strong variation in transposable element dynamics and nucleotide diversity. Plant J 73:347–356
Mukai Y, Friebe B, Gill BS (1992) Comparison of C-banding patterns and in situ hybridization sites using highly repetitive and total genomic rye DNA probes of ‘Imperial’ rye chromosomes added to ‘Chinese Spring’ wheat. Jpn J Genet 67:71–83
Mukai Y, Nakahara Y, Yamamoto M (1993) Simultaneous discrimination of the three genomes in hexaploid wheat by multicolor fluorescence in situ hybridization using total genomic and highly repeated DNA probes. Genome 36:489–494
Nagaki K, Talbert PB, Zhong CX et al (2003) Chromatin immunoprecipitation reveals that the 180-bp satellite repeat is the key functional DNA element of Arabidopsis thaliana centromeres. Genetics 163:1221–1225
Nagaki K, Cheng Z, Ouyang S et al (2004) Sequencing of a rice centromere uncovers active genes. Nat Genet 36:138–145
Pedersen C, Langridge P (1997) Identification of the entire chromosome complement of bread wheat by two-colour FISH. Genome 40:589–593
Peng RH, Liu F, Hu X et al (2012) Microdissection and microcloning of chromosome 5 in Gossypium arboreum. Plant Mol Biol Rep 30:1218–1228. doi:10.1007/s11105-012-0438-2
Petit M, Guidat C, Daniel J et al (2010) Mobilization of retrotransposons in synthetic allotetraploid tobacco. New Phytol 186:135–147
Plohl M, Luchetti A, Meštrović N et al (2008) Satellite DNAs between selfishness and functionality: structure, genomics and evolution of tandem repeats in centromeric (hetero) chromatin. Gene 409:72–82
Ragupathy R, Banks T, Cloutier S (2010) Molecular characterization of the Sasanda LTR copia retrotransposon family uncovers their recent amplification in Triticum aestivum (L.) genome. Mol Genet Genomics 283:255–271
Salina EA, Sergeeva EM, Adonina IG et al (2011) The impact of Ty3-gypsy group LTR retrotransposons Fatima on B-genome specificity of polyploid wheats. BMC Plant Biol 11:99
Schondelmaier J, Martin R, Jahoor A et al (1993) Microdissection and microcloning of the barley (Hordeum vulgare L.) chromosome 1HS. Theor Appl Genet 86:629–636
Schwarzacher T, Leitch A, Bennett M et al (1989) In situ localization of parental genomes in a wide hybrid. Ann Bot 64:315–324
Slotkin RK, Martienssen R (2007) Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet 8:272–285
Song L, Lu Y, Zhang J et al (2016) Physical mapping of Agropyron cristatum chromosome 6P using deletion lines in common wheat background. Theor Appl Genet 129:1023–1034
Svitashev S, Bryngelsson T, Li X et al (1998) Genome-specific repetitive DNA and RAPD markers for genome identification in Elymus and Hordelymus. Genome 41:120–128
Tang Z, Fu S, Ren Z et al (2011) Diversity and evolution of four dispersed repetitive DNA sequences in the genus Secale. Genome 54:285–300
Tomita M (2010) Revolver and Superior: novel transposon-like gene families of the plant kingdom. Curr Genomics 11:62
Tomita M, Shinohara K, Morimoto M (2008) Revolver is a new class of transposon-like gene composing the Triticeae genome. DNA Res 15:49–62
Tsujimoto H, Mukai Y, Akagawa K et al (1997) Identification of individual barley chromosomes based on repetitive sequences: conservative distribution of Afa-family repetitive sequences on the chromosomes of barley and wheat. Genes Genet Syst 72:303–309
Vershinin AV, Schwarzacher T, Heslop-Harrison JS (1995) The large-scale genomic organization of repetitive DNA families at the telomeres of rye chromosomes. Plant Cell 7:1823–1833
Wang RRC (1992) Genome relationships in the perennial Triticeae based on diploid hybrids and beyond. Hereditas 116:133–136
Wang R, Von Bothmer R, Dvorak J et al (1994) Genome symbols in the Triticeae (Poaceae). In: Proceedings of the 2nd international Triticeae symposium. Utah State University Publication Design and Production, Logan, pp 29–34
Wang Q, Liu H, Gao A et al (2012) Intergenomic rearrangements after polyploidization of Kengyilia thoroldiana (Poaceae: Triticeae) affected by environmental factors. PLoS One 7:e31033
Wu J, Yang X, Wang H et al (2006) The introgression of chromosome 6P specifying for increased numbers of florets and kernels from Agropyron cristatum into wheat. Theor Appl Genet 114:13–20
Wu M, Zhang J, Wang J et al (2010) Cloning and characterization of repetitive sequences and development of SCAR markers specific for the P genome of Agropyron cristatum. Euphytica 172:363–372
Xin Z-Y, Appels R (1988) Occurrence of rye (Secale cereale) 350-family DNA sequences in Agropyron and other Triticeae. Plant Syst Evol 160:65–76
Yaakov B, Meyer K, Ben-David S et al (2013) Copy number variation of transposable elements in Triticum-Aegilops genus suggests evolutionary and revolutionary dynamics following allopolyploidization. Plant Cell Rep 32:1615–1624
Yu F, Lei Y, Li Y et al (2013) Cloning and characterization of chromosomal markers in alfalfa (Medicago sativa L.). Theor Appl Genet 126:1885–1896
Zhang X (1991) Production and utilization of alien translocation lines of common wheat. Hereditas 13:39–44
Zhang HK, Bian Y, Gou X et al (2013a) Intrinsic karyotype stability and gene copy number variations may have laid the foundation for tetraploid wheat formation. Proc Natl Acad Sci USA 110:19466–19471. doi:10.1073/pnas.1319598110
Zhang J, Long H, Pan Z et al (2013b) Characterization of a genome-specific Gypsy-like retrotransposon sequence and development of a molecular marker specific for Dasypyrum villosum (L.). J Genet 92:103
Zhang J, Zhang J, Liu W et al (2015) Introgression of Agropyron cristatum 6P chromosome segment into common wheat for enhanced thousand-grain weight and spike length. Theor Appl Genet 128:1827–1837
Zhou YH, Hu ZM, Dang BY et al (1999) Microdissection and microcloning of rye (Secale cereale L.) chromosome 1R. Chromosoma 108:250–255. doi:10.1007/s004120050375
Zhou Y, Dang B, Wang H et al (2001) Microdissection of a single chromosome and construction of the microclone library from soybean. Euphytica 121:129–135
Zhou J, Yang Z, Li G et al (2010) Diversified chromosomal distribution of tandemly repeated sequences revealed evolutionary trends in Secale (Poaceae). Plant Syst Evol 287:49–56
Acknowledgements
This research is supported by grants from the National Science and Technology Support Program of China (2013BAD01B00) and the National Natural Science Foundation of China (No. 31271714).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Han, H., Liu, W., Lu, Y. et al. Isolation and application of P genome-specific DNA sequences of Agropyron Gaertn. in Triticeae. Planta 245, 425–437 (2017). https://doi.org/10.1007/s00425-016-2616-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2616-1