Abstract
Ultrasound promoted, cerium ammonium nitrate catalyzed sustainable synthesis of spiro[indoline3,4′-pyrano[2,3-c]pyrazole] derivatives (4a–l) is reported herein. The synthesized compounds were screened for their antioxidant activities as free radical scavenging effect on diphenylpicryl hydrazine (DPPH∙), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS∙+) and nitric oxide (NO) radicals. The screened compounds showed potent scavenging activities against DPPH∙, ABTS∙+ and NO radicals. In order to further extend on studies and to obtain a deep insight into structure activity relationship of this class of compounds, we designed N-substitution of indole moiety with the aim to study its antioxidant potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Macromolecules like proteins, lipids and DNA are major targets for free radical-induced damage. The oxidative damage to proteins has been found to be high in specific brain regions, and are elevated during ageing and in some types of neurodegenerative disorders (Foster et al., 1996; Floor and Wetzel, 1998). In particular, the damage to DNA is more erratic, and assail by free radicals can generate structural damage (i.e. strand breaks) and/or alteration of the bases. Unrepaired DNA lesions might impair transcription and protein synthesis (Hatahet et al., 1994).
Thus, an increasing interest in antioxidants, such as free radicals and reactive oxygen species (ROS), has engrossed substantial interest. The free radicals are also believed to be associated with carcinogenesis, mutagenesis, arthritis, diabetes, inflammation, cancer and genotoxicity (Kourounakis et al., 1999; Buyukokuroglu et al., 2001) due to oxidative stress, which arises as a result of imbalance between free radical generations.
Moreover, ROS are continuously generated in very low amounts in active cells of aerobic organisms as byproducts of metabolic processes, and they have found to be the key players in the pathophysiological mechanisms associated with various inflammatory disorders (Trouba et al., 2002). So the significance of ROS in the pathogenesis of multifarious diseases has attracted considerable attention. Disease progression may be retarded by administrating protective compounds, which can act in several different ways as inhibitors of ROS formation, free radical scavenging, chain breaking antioxidants or transition metal chelators, and therefore research on active antioxidant of natural or synthetic origin received a great attention (Delles et al., 2008).
Moreover, the development of hybrid molecules having different pharmacophores in one frame may lead to compounds with interesting pharmacological profiles. In this regards, substituted pyrano[2,3-c]pyrazoles are much sought after class of heterocycles exhibiting wide range of biological activities like antimicrobial (Mityurina et al., 1981; Lakshmi et al., 2010), anticancer (Wang et al., 2009), anti-inflammatory (Zaki et al., 2006), inhibitors of human Chk1 kinase (Foloppe et al. 2006) and also as biodegradable agrochemicals (Abdelrazek et al., 2007). Besides, indole derivatives constitute an important class of therapeutic agents in medicinal chemistry, including anticancer (Suzen and Buyukbingol, 2000; Lakshmi et al., 2010), antioxidant (Lakshmi et al., 2010), antirheumatoidal (Buyukbingol et al., 1994), anti- HIV (Suzen and Buyukbingol, 1998), and also play a vital role in the immune system (Lieberman et al., 1997; Page et al., 2007) and as potent scavengers of free radicals (Chyan et al., 1999).
Literature survey reveals that synthesis of pyran system has been accomplished by many workers (Saundane et al., 2013; Mandha et al., 2012) employing various catalysts as ammonium chloride (Dabiri et al., 2009), ethylenediamine diacetate (Lee and Hari, 2010), triethylbenzyl ammonium (TEBA) salt (Zhu et al., 2007), l-proline (Yuling et al., 2010), surfactant metal carboxylates (Wang et al., 2010), β-cyclodextrin (Sridhar et al., 2009), ionic liquids (Moghadam and Miri, 2011) and reaction conditions (Shanthi et al., 2007; Elinson et al., 2008). We have also reported the synthesis of spirooxindole derivatives by a multi-step process catalyzed by Et3N in ethanol under refluxing condition Dandia et al., (2003a, b; Joshi et al., 1989). Although most of the recent methods have their own merits, but some methods are weakened by at least one limitation such as low yield, (especially when bulky substituent on substrates lead to low solubility in water) complicated workup procedure, chromatographic separation and technical intricacy.
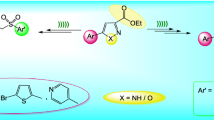
In the above regards and on the basis of pharmacological indications that show the existence of two or more different heterocyclic moieties in a single molecule often remarkably enhances the biocidal profile, we intended the synthesis of a series of spiro[indoline3,4′-pyrano[2,3-c]pyrazole] derivatives (4a–l) through a three component reaction of substituted isatin, active methylene reagent, and 3-methyl-1-phenyl-2-pyrazolin-5-one in the presence of catalytic amount of cerium ammonium nitrate as a green and efficient catalyst in water, thus utilizing the multipurpose promoter efficacy of CAN (Sridharan and Menendez, 2010).
Results and discussion
Chemistry
We approached the synthesis of a library of spiro[indoline3,4′-pyrano[2,3-c]pyrazole] derivatives (4a–l), bearing different substitutions and their evaluated for their antioxidant potential.
Initially, we tested the reaction of isatin, malanonitrile and 3-methyl-1-phenyl-2-pyrazolin-5-one as a simple model substrate under various reaction conditions (Scheme 1) for synthesis of spiro[indoline3,4′-pyrano[2,3-c]pyrazole] derivative (4a). The results are shown in Table 1. With reference to the crucial utility of solvent in chemical transformation, a variety of solvents were employed for the synthesis of spiro[indoline3,4′-pyrano[2,3-c]pyrazole] derivatives. As can be seen, that among all the solvents, water seems to be the solvent of choice both in terms of time and yields of the spiro[indoline3,4′-pyrano[2,3-c]pyrazole] derivatives (4a–l).
It was observed that when the reaction was carried out at room temperature stirring without any catalyst, the yield of product was very low (Table 1, 35 %, entry 1) even after prolong time. Increasing the temperature does not seem to affect the yields of the product. Afterwards, evaluation of various catalysts was carried out for the synthesis of spirooxindole derivatives in aqueous medium under ultrasonic irradiation (Table 1, entry 4–9). It can be seen that a mixture of isatin, ethylcyanoacetate and 3-methyl-1-phenyl-2-pyrazolin-5-one in the presence of catalytic amount of cerium ammonium nitrate afforded ethyl 6′-amino-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4a) in excellent yields (90 %), while with other catalysts, the product formed with yields ranging between 54 and 76 %. Although, ultrasound irradiation decreased the reaction time, but failed to increase the yield of the final product (Table 1, entry 3).
Further, the catalyst loading was optimized by using different concentration of CAN in the model reaction. It was found that with increasing the amount of CAN from 5 mol% to 10 and 20 mol%, the yields increased from 74 % to 90 and 92 %, respectively (Table 1, entries 10–12). Further increase in amount of catalyst does not seem to affect the overall yields of the product. 10 mol% CAN in water under ultrasonic irradiation, was found to the best combination for synthesis of spiro[indoline3,4′-pyrano[2,3-c]pyrazole] derivatives (4a–l) in excellent yields.
Under the optimized reaction conditions, a series of spiro[indoline3,4′-pyrano[2,3-c]pyrazole] derivatives (4a–l) were synthesized (Scheme 2). The results are summarized in Table 2.
The multicomponent synthesis of spiro[indoline3,4′-pyrano[2,3-c]pyrazole] derivatives can be explained by a plausible mechanism presented in Scheme 3. The ultrasonic cavitation induced shear forces and the jets produced near the surface of the vessel, and the catalyst may activate malanonitrile through sonolysis of the C–H bond. The reaction between the activated malanonitrile and the isatin (activated by CAN) facilitates the formations of the corresponding isatiylidene malanonitrile intermediate (c) under sonic condition, which in turn reacts with the active methylene site of 3-methyl-1-phenyl-2-pyrazolin-5-one. This C-4 alkylation of 3-methyl-1-phenyl-2-pyrazolin-5-one with electrophilic C=C of intermediate (c) followed by nucleophilic addition of the −OH group on the cyano moiety subsequently results in formation of the desired product (e).
Antioxidant activity
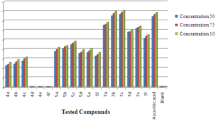
The antioxidant activities of spiroindolinones were determined as an index of pharmacological efficacy. Three model systems were used namely DPPH∙, ABTS∙+ and NO scavenging activity. In the assessment of antioxidant activity, only synthetic relevant free radicals were used. The synthetic nitrogen-centred DPPH∙, ABTS∙+ and NO radicals were used as indicator compounds in testing hydrogen transfer capacity that are related to the antioxidant activity. Antioxidant activities data were compared with standard drug ascorbic acid. In results, we have found correlation between substitution in indole ring and substitution of pyran ring at 5th position. Overall, all compounds exhibited good DPPH∙ and NO scavenging activity whereas, moderate ABTS∙+ scavenging activity. The antioxidant properties were expressed as EC50 values.
DPPH radical scavenging activity
Although the DPPH radical scavenging abilities of all the spiroindoline derivatives were moderately lower than those of ascorbic acid (409.75 ± 0.288) μg/ml, it was evident that they show reducing ability (possibly by hydrogen transfer) and could serve as free radical scavengers. It was anticipated that compounds possessing carboxylate group (an electron donating group) at 5th position of pyran ring showed higher activity except compound 4b and 4c. The combination of carboxylate group with N-benzyl substitution in indole ring (i.e. compound 4f) showed good activity. This revealed to be important for activity, since the N-benzyl substituted indole ring with carbonitrile group (i.e. compound 4l) showed decreased potency. The compound 4a, i.e. without any substitution in indole ring and carboxylate group at 5th position of pyran ring showed good activity, while compound 4g without any substitution in indole ring and carbonitrile group showed least activity. In compound 4d, with N-allyl substitution and carboxylate group showed moderate activity, while same indole ring with carbonitrile group, i.e. compound 4j, showed least activity. In compound 4i, incorporation of CH3 group at 5th position of isatin showed electron donating effect, but carbonitrile group with electron withdrawing effect turned it to give moderate activity, while in compound 4c two electron donating groups are present at 5th and 7th position of isatin with carboxylate group, but this compound is only moderately active whereas, compound 4b and 4h, i.e. Cl and Br substitution in indole ring, have not shown any effect and gives least activity. The N-ethyl substitution in indole ring with carboxylate group, i.e. compound 4e, showed good activity and with carbonitrile group showed least activity. The results are shown in Table 3 and Fig. 1. The results show that compound 4a and 4f showed highest activity. In compound 4a, there is presence of an electron donating groups (hydrogen in form of NH of indole ring and carboxylate group at 5th position of pyran ring), which stabilized the compound after donating the hydrogen to DPPH∙ radical. In compound 4f, there is presence of an electron donating groups (N-benzyl, a bulky group substitution on indole ring and carboxylate group at 5th position of pyran ring) which stabilized the compound after donating the hydrogen to DPPH∙ radical.

ABTS radical scavenging activity
In ABTS assay, among the tested compounds, compound 4f with combination of carboxylate group at 5th position of pyran ring and N-benzyl substitution at indole ring, showed good activity which is equipotent to ascorbic acid, which we took as standard drug. Besides, with N-substitution and carboxylate group at 5th position of pyran ring (i.e. compound 4d) and with carbonitrile group (i.e. compounds 4j–l) showed least activity, but compound 4e, having N-ethyl substitution at indole ring and carboxylate group at 5th position of pyran ring showed moderate activity. Incorporation of Cl, H and Br (i.e. compound 4b, 4g and 4h) have not showed any effect and shows least activity. It was observed that unsubstituted in indole ring with carboxylate group (i.e. compound 4a) shown moderate activity. Whereas, incorporation of CH-3 group at 5th position in indole ring with carbonitrile group (i.e. compound 4i) also gave moderate activity, but the 5,7-diCH3 substitution with carboxylate group (i.e. compound 4c) displayed least activity (Table 3; Fig. 2). The results show that compound 4f showed highest activity. In compound 4f, there is presence of an electron donating groups (in form of benzyl group substituted on nitrogen of indole ring and carboxylate at 5th position of pyran ring) which stabilized the compound after donating the hydrogen or lone pair to ABTS∙+ radical cation.
Nitric oxide scavenging activity
In NO assay, among the tested compounds, compounds 4e and 4f having N-substituted indole ring with carboxylate group at 5th position of pyran ring, showed good activity. Other N-substituted indole with carboxylate group or with carbonitrile group (i.e. compounds 4d, 4j, 4k, 4l, respectively) showed moderate activity. Free NH functionality of indole ring with carboxylate group (i.e. compound 4a) showed moderate activity, while with carbonitirle group (i.e. compound 4g) showed least activity. The incorporation of CH-3 group at 5th position in indole ring with carbonitrile group (i.e. compound 4i) gives moderate activity, but the incorporation of 5,7-diCH3, Cl with carboxylate group (i.e. compounds 4b and 4c), and Br with carbonitrile group (i.e. compound 4h) have not shown any effect and gives least activity (Table 3; Fig. 3). The results show that compound 4f possess highest activity. In compound 4f, there is presence of an electron donating groups (in form of benzyl group substituted on nitrogen of indole ring and carboxylate at 5th position of pyran ring) which stabilized the compound after donating the hydrogen or lone pair to NO radical.
Conclusion
This work describes for the first time the in vitro antioxidant activities of spirooxindole derivatives, as a new class of potential antioxidants. The results showed that all the spirooxindole derivatives had demonstrated strong activity in DPPH∙ and NO scavenging method and moderate activity in ABTS∙+ scavenging procedure. The compound 4f (Ethyl6′-amino-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[1-benzylindoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate) has shown strong activity in DPPH∙, ABTS∙+ and NO scavenging screening. Overall, compound 4a having electron donating carboxylate group and compound 4f having both N-benzyl and carboxylate group having electron donating effect were found to be the most potent antioxidant described in this study.
Experimental
Chemistry
The melting points of all the compounds were determined on a Toshniwal apparatus. The purity of compounds was checked on thin layers of silica gel G-coated glass plates with n-hexane ethyl acetate (7:3) as eluent. Infrared (IR) spectra were recorded on a Shimadzu Fourier transform (FT)-IR 8400 S spectrophotometer using KBr pellets.
Sonication was carried out with the help of a standard ultrasonic irradiation instrument SonaprosPR-1000 MP (Oscar Ultrasonics Pvt. Ltd.) operating at 750 W and generating 23 KHz output frequency. It has the following characteristics: Standard Titanium horn with a diameter of 6 mm/12 mm, replaceable flat stain less steel tip and digital thermometer to determine temperature. The glass reactor was designed and made from borosil glass.
Synthesis of spiro[indoline3,4′-pyrano[2,3-c]pyrazole derivatives (4a–l)
An equimolar mixture of isatin (1 mmol, 0.147 g), ethylcyanoacetate (1 mmol, 0.113 g), 3-methyl-1-phenyl-2-pyrazolin-5-one (1 mmol, 0.174 g) and 10 mol% CAN in 5 ml water was introduced in a 20 mL, heavy-walled, pear-shaped, two-necked flask with non standard taper outer joint. The flask was attached to a 12 mm tip diameter probe, and the reaction mixture was sonicated at ambient temperature for the specified period at 50 % power of the processor and 230 W output in a 4 s pulse mode. At the end of the reaction period, thin-layer chromatography (TLC) was checked, and the flask was detached from the probe. The contents were transferred to a beaker. The formed solid was filtered off, washed thoroughly with warm water (2 × 20 ml), and then dried to obtain crude products which were purified by crystallization from mixture of methanol acetone (6:4) to give compound 4, giving satisfactory spectral and elemental analysis.
Ethyl 6′-amino-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4a)
White crystalline solid; YIELD: 94 %; m.p. 238–240 °C; IR (KBr) : 3392, 3230, 3172, 1716, 1652, 1600, 1554, 1160 cm−1; 1H NMR (DMSO-d6, 300 MHz): δ 0.71 (t, 3H, CH3), 1.55 (s, 3H, CH3), 3.72 (q, J = 6.80 Hz, 2H, CH2), 6.84–6.93 (m, 3H), 7.18 (t, J = 5.40 Hz, 1H), 7.32 (t, J = 07.5 Hz, 1H), 7.49(t, J = 8.4 Hz, 2H), 7.75 (d, J = 7.5 Hz, 2H), 8.14 (brs, 2H, NH2, D2O exchangeable), 10.53(s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6, 75 MHz): δ 12.0, 13.4, 48.0, 59.6, 75.1, 98.7, 109.4, 120.5, 122.3, 123.5, 126.9, 128.3, 129.9, 136.1, 137.9, 142.4, 144.7, 161.7, 168.3, 179.9; MS (m/z): 416 (M+). Anal. calcd. for C23H20N4O4: C, 66.34, H, 4.84, N, 13.45. Found: C, 66.30, H, 4.81, N, 13.38.
Ethyl 6′-amino-5-chloro-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4b)
White crystalline solid; YIELD: 92 %; m.p. 246–248 °C; IR (KBr): 3396, 3228, 3172, 1698, 1644, 1608, 1566, 1160 cm−1; 1H NMR (DMSO-d6, 300 MHz) : δ 0.77 (t, 3H, CH3), 1.61 (s, 3H, CH3), 3.75 (q, J = 6.6 Hz, 2H, CH2), 6.89 (d, J = 8.4 Hz, 1H), 7.08 (s, 1H), 7.23 (d, J = 8.1 Hz, 1H), 7.33 (t, J = 7.2 Hz, 1H), 7.50 (t, J = 7.8 Hz, 2H), 7.80 (d, J = 8.1 Hz, 2H), 8.25 (brs, 2H, NH2, D2O exchangeable), 10.68 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6, 75 MHz): δ 11.8, 13.2, 47.8, 59.2, 74.1, 97.6, 110.4, 120.2, 123.5, 125.9, 126.5, 127.7, 129.5, 137.3, 137.9, 141.1, 144.1, 161.5, 167.8, 179.2; MS (m/z) : 450 (M+); Anal. calcd. for C23H19ClN4O4 : C, 61.27, H, 4.25, N, 12.43. Found : C, 61.30, H, 4.31, N, 12.42.
Ethyl 6′-amino-3′,5,7-trimethyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4c)
White crystalline solid; YIELD: 91 %; m.p. 260–262 °C; IR (KBr): 3398, 3228, 3170, 1702, 1648, 1611, 1570, 1168 cm−1; 1H NMR (DMSO-d6, 300 MHz) : δ 0.75 (t, 3H, CH3), 1.58 (s, 3H, CH3), 2.12 (s, 3H, CH3), 2.19 (s, 3H, CH3), 3.73 (q, J = 6.90 Hz, 2H, CH2), 6.57 (s, 1H), 6.78 (s, 1H), 7.32 (t, J = 7.2 Hz, 1H), 7.50 (t, J = 7.8 Hz, 2H), 7.80 (d, J = 7.8 Hz, 2H), 8.17 (brs, 2H, NH2, D2O exchangeable), 10.47 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6, 75 MHz): δ 11.8, 12.9, 16.3, 18.6, 20.6, 47.8, 56.2, 59.2, 75.0, 98.6, 117.9, 120.0, 121.2, 126.4, 129.4, 129.5, 130.6, 135.5, 137.4, 138.3, 144.0, 144.4, 161.3, 168.1, 179.9; MS (m/z) : 444 (M+); Anal. calcd. for C25H24N4O4 : C, 67.55, H, 5.44, N, 12.60. Found: C, 67.49, H, 5.39, N, 12.63.
Ethyl 6′-amino-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[1-allylindoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4d)
White crystalline solid; YIELD: 84 %; m.p. 182–184 °C; IR (KBr): 3398, 3228, 3170, 1702, 1648, 1611, 1570, 1168 cm−1; 1H NMR (DMSO-d6, 300 MHz) : δ 0.67 (t, 3H, CH3), 1.50 (s, 3H, CH3), 3.73 (q, J = 6.90 Hz, 2H, CH2), 4.29 (dd, 1H, CH2), 4.58 (dd, 1H, CH2), 5.25 (d, 1H, CH2), 5.43 (d, 1H, CH2), 5.84 (m, 1H, CH), 6.95–7.83 (m, 9H, ArH), 8.24 (brs, 2H, NH2, D2O exchangeable). 13C NMR (DMSO-d6, 75 MHz): δ 11.7, 13.4, 47.0, 58.9, 74.6, 97.6, 118.7, 119.8, 119.8, 122.2, 126.0, 127.4, 129.0, 131.4, 134.7, 137.4, 142.3, 143.9, 144.0, 167.6, 177.1; MS (m/z) : 457 (M+). Anal. calcd. for C26H24N4O4 : C, 68.40, H, 5.32, N, 12.10. Found: C, 68.49, H, 5.39, N, 12.13.
Ethyl 6′-amino-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[1-ethylindoline-3,4′-pyrano[2, 3-c]pyrazole]-5′-carboxylate (4e)
White crystalline solid; YIELD: 92 %; m.p. 208–210 °C; IR (KBr): 3398, 3228, 3170, 1702, 1648, 1611, 1570, 1168 cm−1; 1H NMR (DMSO-d6, 300 MHz) : δ 0.63 (t, 3H, CH3), 1.33 (t, 3H, J = 6.8 Hz), 1.50 (s, 3H, CH3), 3.73 (q, 2H, J = 6.8 Hz), 6.39–7.24 (m, 9H, ArH), 8.29 (brs, 2H, NH2, D2O exchangeable); 13C NMR (DMSO-d6, 75 MHz): δ 10.2, 11.7, 47.5, 58.7, 72.6, 95.6, 114.2, 117.9, 118.2, 120.0, 121.2, 124.4, 127.4, 129.5, 130.6, 134.3, 137.4, 142.5, 143.3, 144.0, 166.1, 176.9; MS (m/z) : 445 (M+); Anal. calcd. For C25H24N4O4 : C, 67.50, H, 5.41, N, 12.57. Found: C, 67.55, H, 5.44, N, 12.60.
Ethyl 6′-amino-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[1-benzylindoline-3,4′-pyrano[2, 3-c]pyrazole]-5′-carboxylate (4f)
White crystalline solid; YIELD: 97 %; m.p. 244–246 °C; IR (KBr): 3398, 3228, 3170, 1702, 1648, 1611, 1570, 1168 cm−1. 1H NMR (DMSO-d6, 300 MHz) : δ 0.63 (t, 3H, CH3), 1.50 (s, 3H, CH3), 3.73 (q, J = 6.90 Hz, 2H, CH2), 4.91 and 5.13 (2H, AB system J = 15.5 Hz), 6.57–7.89 (m, 14H, ArH), 8.20 (brs, 2H, NH2, D2O exchangeable); 13C NMR (DMSO-d6, 75 MHz): δ 11.5, 13.6, 46.9, 58.9, 73.6, 96.6, 117.9, 118.2, 120.0, 122.2, 126.4, 127.4, 129.5, 131.6, 134.3, 137.4, 142.3, 143.8, 144.0, 161.6, 165.5, 176.9; MS (m/z) : 507 (M+); Anal. calcd. for C30H26N4O4 : C, 71.04, H, 5.13, N, 11.01. Found: C, 71.13, H, 5.17, N, 11.06.
6′-amino-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carboxylate (4g)
White crystalline solid; YIELD: 95 %; m.p. 237–238 °C; IR (KBr): 3412, 3280, 3174, 2200, 1692, 1650, 1526, 1132 cm−1; 1H NMR (DMSO-d6, 300 MHz) : δ 1.55 (s, 3H, CH3), 6.94 (d, J = 7.4 Hz, 1H), 7.03 (t, J = 7.6 Hz, 1H), 7.18 (d, J = 7.2 Hz, 1H), 7.28 (t, J = 7.5 Hz, 1H), 7.36 (t, J = 7.6 Hz, 1H), 7.52 (t, J = 7.8 Hz, 2H), 7.58 (brs, 2H, NH2, D2O exchangeable), 7.79 (d, J = 7.9 Hz, 2H), 10.76 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6, 75 MHz): δ 12.3, 48.3, 56.6, 96.9, 110.4, 118.6, 120.7, 123.2, 125.4, 127.1, 129.8, 130.8, 132.6, 138.2, 142.1, 144.5, 145.5, 162.3, 178.8; MS (m/z) : 369 (M+); Anal. calcd. for C21H15N5O2 : C, 68.28, H, 4.09, N, 18.96. Found: C, 68.26, H, 4.04, N, 18.88.
6′-Amino-5-bromo-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile (4h)
White crystalline solid; YIELD: 92 %; m.p. 242–244 °C; IR (KBr): 3436, 3269, 3168, 2198, 1706, 1650, 1576, 1168 cm−1; 1H NMR (DMSO-d6, 300 MHz): δ 1.58 (s, 3H, CH3), 6.91 (d, J = 7.6 Hz, 1H), 7.42–7.54(4H, m), 7.28 (t, J = 7.9 Hz, 1H), 7.64 (brs, 2H, NH2, D2O exchangeable), 7.79 (d, J = 7.8 Hz, 2H), 10.90 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6, 75 MHz): δ 12.1, 47.9, 56.4, 96.7, 110.6, 118.2, 120.9, 125.8, 126.6, 129.5, 129.6, 131.7, 132.3, 137.3, 139.2, 144.1, 145.0, 161.0, 178.5; MS (m/z): 447(M+); Anal. calcd. for C21H14BrN5O2: C, 56.27, H, 3.15, N, 15.62. Found: C, 56.17, H, 3.21, N, 15.60.
6′-Amino-3′,5-dimethyl-2-oxo-1′-phenyl-1′H-spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile (4i)
White crystalline solid; YIELD: 98 %; m.p. 288–290 °C; IR (KBr): 3426, 3269, 3178, 2192, 1696, 1650, 1576, 1174 cm−1; 1H NMR (DMSO-d6, 300 MHz): δ 1.56 (s, 3H, CH3), 2.26 (s, 3H, CH3),6.84 (d, J = 7.8 Hz, 1H), 7.08 (s, 1H), 7.12 (d, J = 7.6 Hz, 1H), 7.35 (t, J = 8 Hz, 1H), 7.52 (t, J = 8.4 Hz, 2H), 7.55 (brs, 2H, NH2, D2O exchangeable), 7.78 (d, J = 8.3 Hz, 2H), 10.64 (s, 1H, NH, D2O exchangeable); 13C NMR (DMSO-d6,75 MHz): 11.9, 20.4, 48.2, 56.7, 96.6, 110.1, 118.4, 119.9, 125.3, 126.8, 129.9, 130.5, 131.9, 132.6, 137.4, 139.8, 144.3, 145.7, 161.2, 177; MS(m/z):383(M+); Anal. calcd. for C22H17N5O2: C, 68.92, H, 4.47, N, 18.27. Found: C, 68.82, H, 4.51, N, 18.11.
6′-amino-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[1-allylindoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile (4j)
White crystalline solid; YIELD: 90 %; m.p. 218–220 °C; IR (KBr): 3408, 1708, 1660, 1605, 1540, 1166 cm−1; 1H NMR (DMSO-d6, 300 MHz): 1.42 (s, 3H, CH3), 4.29 (dd, 1H, CH2), 4.58 (dd, 1H, CH2), 5.25 (d, 1H, CH), 5.43 (d, 1H, CH), 5.84 (m, 1H, CH), 6.94–7.50 (m, ArH, 14H), 8.28 (brs, 2H, NH2, D2O exchangeable); 13C NMR (DMSO-d6, 75 MHz): 11.7, 43.4, 55.9, 109.2, 117.8, 120.0, 123.2, 124.5, 126.2, 127.3, 128.3, 129.0, 131.2, 135.5, 137.2, 141.9, 143.8, 144.9, 161.1, 176.0; MS (m/z): 410 (M+); Anal. calcd. for C24H19N5O2 : C, 70.35, H, 4.59, N, 17.15. Found: C, 70.40, H, 4.68, N, 17.10.
6′-amino-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[1-ethylindoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile (4k)
White crystalline solid; YIELD: 84 %; m.p. 210–212 °C; IR (KBr): 3406, 1705, 1656, 1608, 1542, 1162 cm−1; 1H NMR (DMSO-d6, 300 MHz): δ 1.33 (t, 3H, J = 6.8 Hz), 1.42 (s, 3H, CH3), 3.85 (q, 2H, J = 6.8 Hz), 6.92–7.52 (m, ArH, 14H), 8.23 (brs, 2H, NH2, D2O exchangeable); 13C NMR (DMSO-d6, 75 MHz): 11.7, 43.4, 55.9, 109.2, 117.8, 120.0, 123.2, 124.5, 126.2, 127.3, 128.3, 129.0, 131.2, 135.5, 137.2, 141.9, 143.8, 144.9, 161.1, 176.0; MS (m/z): 398 (M+); Anal. calcd. for C23H19N5O2 : C, 73.29, H, 4.67, N, 15.27. Found: C, 73.19, H, 4.61, N, 15.24.
6′-amino-3′-methyl-2-oxo-1′-phenyl-1′H-spiro[1-benzylindoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitrile (4l)
White crystalline solid; YIELD: 97 %; m.p. 232–234 °C; IR (KBr): 3406, 1705, 1656, 1608, 1542, 1162 cm−1; 1H NMR (DMSO-d6, 300 MHz): δ 1.42 (s, 3H, CH3), 4.89 (d, 1H, J = 15.6 Hz, benzylic proton), 5.09 (d, 1H, J = 15.6 Hz, benzylic proton), 6.97–7.42 (m, ArH, 14H), 8.13 (brs, 2H, NH2, D2O exchangeable); 13C NMR (DMSO-d6, 75 MHz): 11.7, 43.4, 55.9, 109.2, 117.8, 120.0, 123.2, 124.5, 126.2, 127.3, 128.3, 129.0, 131.2, 135.5, 137.2, 141.9, 143.8, 144.9, 161.1, 176.0; MS (m/z): 460 (M+); Anal. calcd. for C28H21N5O2 : C, 68.92, H, 4.47, N, 18.27. Found: C, 68.82, H, 4.51, N, 18.11.
Antioxidant activity
Antioxidant activities of test compounds were measured by estimating DPPH∙, ABTS∙+ and NO scavenging activity in vitro using ascorbic acid as standard drug.
DPPH∙ scavenging activity
Ability of synthesized compounds to scavenge the stable free radical, DPPH∙ is measured by the method, appeared in the literature (Mensor et al., 2001). To 1 ml methanolic solution of DPPH∙ (0.25 mM), 1 ml of ethanolic solution of synthesized compounds was added. To prepare control, 1 ml of methanol was added to the 1 ml methanolic solution of DPPH∙ (0.25 mM). After 20 min, absorbance was recorded at 517 nm in a UV–Vis double beam spectrophotometer. The inhibition (%) of free radicals was calculated by using the following formula:
where AC absorbance of control and AA absorbance of tested compounds.
ABTS∙+ scavenging activity
ABTS∙+ scavenging activity of synthesized compounds were measured by the method, appeared in the literature (Re et al., 1999). First, ABTS∙+ free radicals were generated through the oxidation of ABTS with potassium persulphate. For this purpose, ABTS was dissolved in deionized water to 7 mM concentration, and potassium persulphate was added to a concentration of 2.45 mM. The reaction mixture was kept in dark at room temperature for 12–16 h before final use. Lastly, the ABTS∙+ solution was diluted with absolute ethanol till the absorbance was read 0.700 ± 0.020 at 734 nm. Synthesized compounds in ethanol were added to 3 ml of ABTS∙+ solution and the absorbance was read after 6 min.
Nitric oxide scavenging activity
The interaction of synthesized compounds with nitric oxide (NO) was assessed by the nitrate ion detection method (Sreejayan, 1997). Sodium nitropruside (5 mM) in phosphate buffer spontaneously generates NO in an aqueous solution. NO interacts with oxygen and produces nitrate ions, which can be estimated by the use of Greiss reagent (1 % sulphanilamide, 2 % H3PO4 and 0.1 % napthylethylene diamine dihydrochloride). Sodium nitroprusside (5 mM) in phosphate buffer was mixed with synthesized compounds and incubated at 25 °C for 150 min. Prepared samples were allowed to react with Greiss reagent. The absorbance of chromophore formed during the diazotization of nitrite with sulphanilamide and subsequent coupling with napthylethylene diamine was read at 546 nm. The same reaction mixture without the synthesized compound, but with equal amount of distilled water served as control.
References
Abdelrazek FM, Metz P, Kataeva O, Jaeger A, El-Mahrouky SF (2007) Synthesis and molluscicidal activity of new chromene and pyrano[2,3-c]pyrazole derivatives. Arch Pharm 340:543–548
Buyukbingol E, Suzen S, Klopman G (1994) Studies on the synthesis and structure-activity relationships of 5-(3′-indolal)-2-thiohydantoin derivatives as aldose reductase enzyme inhibitors. Il Farmaco 49:443–447
Buyukokuroglu ME, Gulcin I, Oktay M, Kufrevioglu OI (2001) In-vitro antioxidant properties of dantrolene sodium. Pharm Res 44:491–494
Chyan YJ, Poeggler B, Omar RA, Chain DG, Frangione B, Ghiso J, Pappolla MA (1999) Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid. J Biol Chem 274:21937–21942
Dabiri M, Bahramnnejad M, Baghbanzadeh M (2009) Ammonium salt catalyzed multicomponent transformation: simple route to functionalized spirochromenes and spiroacridines. Tetrahedron 65:9443–9447
Dandia A, Arya K, Sati M, Sharma R (2003a) Facile microwave-assisted one-pot solid phase synthesis of spiro[3H-indole-3,4′-pyrazolo[3,4-b] pyridines]. Heterocycl Commun 9:415–420
Dandia A, Singh R, Sachdeva H, Gupta R, Paul S (2003b) Microwave promoted and improved thermal synthesis of spiro[indole-pyranobenzopyrans] and spiro[indole-pyranoimidazoles]. J Chin Chem Soc 50:273–278
Delles C, Miller WH, Dominiczak AF (2008) Targeting reactive oxygen species in hypertension. Antioxid Redox Signal 10:1061–1077
Elinson MN, Dorofeev AS, Miloserdov FM, Nikishin GI (2008) The electrocatalytic multicomponent assemling of isatins, 3-methyl-2-pyrazolin-5-ones and malononitrile: facile and convenient way to functionalized spirocyclic [indole-3,4′-pyrano[2,3-c]pyrazole]system. Mol Divers 13:47–52
Floor E, Wetzel MGW (1998) Increased protein oxidation in human substantia nigra par compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem 70:268–275
Foloppe N, Fisher LM, Howes R, Potter A, Robertson AGS, Surgenor AE (2006) Identification of chemically diverse Chk1 inhibitors by receptor-based virtual screening. Bioorg Med Chem 14:4792–4802
Foster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS (1996) Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci USA 93:4765–4769
Hatahet Z, Purmal AA, Wallace SS (1994) Oxidative DNA lesions as blocks to in vitro transcription by phage T7 RNA polymerase. Ann N Y Acad Sci 726:346–348
Joshi KC, Dandia A, Baweja S, Joshi A (1989) Studies in spiroheterocycles. Part XVII. synthesis of novel fluorine containing spiro[indole-pyranobenzopyran] and spiro[indenopyran-indole] derivatives. J Heterocycl Chem 26:1097–1099
Kourounakis AP, Galanakis D, Tsiakitzis K (1999) Synthesis and pharmacological evaluation of novel derivatives of anti-inflammatory drugs with increased antioxidant and anti-inflammatory activities. Drug Dev Res 47:9–16
Lakshmi NV, Thirumurugan P, Noorulla KM, Perumal PT (2010) InCl3 mediated one-pot multicomponent synthesis, anti-microbial, antioxidant and anticancer evaluation of 3-pyranyl indole derivatives. Bioorg Med Chem Lett 20:5054–5061
Lee YR, Hari GS (2010) Efficient one-pot synthesis of spirooxindole derivatives by ethylenediamine diacetate catalyzed reactions in water. Synthesis 3:453–464
Lieberman PM, Wolfler A, Felsner P, Hofer D, Schauenstien K (1997) Melatonin and the immune system. Int Arch Allergy Immunol 112:203–211
Mandha SR, Siliveri S, Alla M, Bommena VR, Bommineni MR, Balasubramanian S (2012) Eco-friendly synthesis and biological evaluation of substituted pyrano[2,3-c]pyrazoles. Bioorg Med Chem Lett 22:5272–5278
Mensor LL, Menezes FS, Leitao GG, Reis AS, Dos Santos TC, Coube CS, Leitao SG (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15:127–130
Mityurina KV, Kulikova LK, Krasheninnikova MK, Kharchenko VG (1981) Synthesis and antimicrobial activity of substituted 4H-pyrano- and dihydropyrano[3,2-d]pyrazoles. Pharm Chem J 15:861–863
Moghadam KR, Miri LY (2011) Ambient synthesis of spiro[4H-pyran-oxindole] derivatives under [BMIm]BF4 catalysis. Tetrahedron 67:5693–5699
Page D, Yang H, Brown W, Walpole C, Fleurent M, Fyfe M, Gaudreault F, Onge SS (2007) New 1,2,3,4-tetrahydropyrrolo[3,4-b]indole derivatives as selective CB2 receptor agonists. Bioorg Med Chem Lett 22:6183–6187
Re R, Pellegrini N, Proteggente A, Pannal A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med 26:1231–1237
Saundane AR, Vijaykumar K, Vaijinath AV (2013) Synthesis of novel 2-amino-4-(5′-substituted 2′-phenyl-1H-indol-3′-yl)-6-aryl-4H-pyran-3-carbonitrile derivatives as antimicrobial and antioxidant agents. Bioorg Med Chem Lett, in press, accepted manuscript
Shanthi G, Subbulakshmi G, Perumal PT (2007) A new InCl3-catalyzed, facile and efficient method for the synthesis of spirooxindoles under conventional and solvent-free microwave conditions. Tetrahedron 63:2057–2063
Sreejayan Rao MNA (1997) Nitric oxide scavenging by curcuminoids. J Pharma Pharmacol 49:105–107
Sridhar R, Srinivas B, Madhav B, Reddy VP, Nageswar YVD, Rao KR (2009) Multi-component supramolecular synthesis of spirooxindoles catalyzed by β-cyclodextrin in water. Can J Chem 87:1704–1707
Sridharan V, Menendez JC (2010) Cerium(IV) ammonium nitrate as a catalyst in organic synthesis. Chem Rev 110:3805–3849
Suzen S, Buyukbingol E (1998) Evaluation of anti-HIV activity of 5-(2-phenyl-3′-indolal)-2-thiohydantoin. Farmaco 53:525–527
Suzen S, Buyukbingol E (2000) Anti-cancer activity studies of indolalthiohydantoin (PIT) on certain cancer cell lines. Farmaco 55:246–248
Trouba KJ, Hamadeh HK, Amin RP, Germolec DR (2002) Oxidative stress and its role in skin disease. Antioxid Redox Signal 4:665–673
Wang JL, Liu D, Zheng ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z (2009) Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA 97:7124–7129
Wang LM, Jiao N, Qiu J, Yu JJ, Liu JQ, Guo FL, Liu Y (2010) Sodium stearate-catalyzed multicomponent reactions for efficient synthesis of spirooxindoles in aqueous micellar media. Tetrahedron 66:339–343
Yuling L, Hui C, Chunling S, Daqing S, Shunjun J (2010) Efficient one-pot synthesis of spirooxindole derivatives catalyzed by l-proline in aqueous medium. J Comb Chem 12:231–237
Zaki MEA, Saliman HA, Hickal OA, Rashad AE (2006) Pyrazolopyranopyrimidines as a class of anti-inflammatory agents. Z Naturforsch C 61:1–5
Zhu S-L, Ji S-J, Zhang Y (2007) A simple and clean procedure for three-component synthesis of spirooxindoles in aqueous medium. Tetrahedron 63:9365–9372
Acknowledgments
Regional Sophisticated Instrumentation Centre, Chandigarh and Central Drug Research Institute, Lucknow for the elemental and spectral analyses. Department of Zoology, University of Rajasthan for their help in biological studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dandia, A., Saini, D., Bhaskaran, S. et al. Ultrasound promoted green synthesis of spiro[pyrano[2,3-c]pyrazoles] as antioxidant agents. Med Chem Res 23, 725–734 (2014). https://doi.org/10.1007/s00044-013-0671-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0671-8