Abstract
A biorefinery system scheme has developed great interest in researchers and industries to obtaining products. A biorefinery is based on efficient use of different types of raw material, such as biomass, for integrated or simultaneous obtaining of several kinds of value-added products. Among used biomass, it is highlighted the vegetable sources, which can come from agro-industrial crops or from waste of biomass processing. There is an extensive variety of biomass that implicates diverse conversion process. Lignocellulosic biomass, in particular, can be transformed into biobased products by different conversion stages. Biofuels, biopolymers, and others bioproducts come from several types of biomass and various processes. One of the most important phases in the transformation process is the hydrolysis, which can be performed by conventional (chemical) and non-conventional (biological) routes. Enzymatic hydrolysis process allows to breakdown chemical structures into smaller molecules, which can be took in advantage for generation of value-added products at subsequent stages in biorefineries.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction to a Biorefinery
In 2018, non-renewable fossil sources share 84.7% to sustain the world energy consumption matrix; among those, oil was the main contributor with 33.6% (British Petroleum 2019). Thanks to its chemical composition, oil is considered as a versatile raw material that allows to obtain different chemical products through refinery process (U.S. Energy Information Administration 2012). Despite this, crude oil systems for refining are great CO2 producers and are generally placed in water-stress sites (Guedes et al. 2019). Besides this, oil has serious problems related to its depletion, non-uniform distribution of reserves, price fluctuations and various negative impacts in human health and environment caused mostly by combustion of its derivatives (Hoegh-Guldberg et al. 2018; Statista 2020). According to this context, world and local researches have been developed to replace oil with renewable sources (biological material) that can be used in a similar process route than oil (refinery). Refinery operating with biological raw material (biomass) and basing on integrated or simultaneous generation of different types of products is named “biorefinery” (BRF).
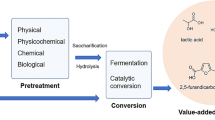
BRF system can be described through the basic process scheme shown in Fig. 9.1, where vegetal biomass (VB) is turned into value-added products by four main stages: pretreatment, hydrolysis, co-production process (conversion or transformation stage), and adjustment process (separation or purification stage). Raw VB generally is dried before get in BRF system in order to eliminate water content avoiding microorganism activity that causes modifications in physical-chemical properties and thus can be stored (Rentizelas 2016). In some cases, when VB is waste from any type of productive chain like fruits, flowers, or another type of vegetal species, an extraction step into a BRF can be considered important. Some fruit peels, barks, leaves, and others vegetal waste (petals, seeds, bagasse, etc.) have special substances in their chemical structure that can be extracted (McCabe et al. 2007; Seader et al. 2011). According to some researches, from fruit peels, vegetal barks, and leaves it is possible to obtain essential oils, antioxidants, or repellent substances, which have a high interest for some industrial sectors (Asha et al. 2015; Ortiz et al. 2017; Asadollahi et al. 2019).
After extracting stage, BRF process obtains its first value-added products (extracts in liquid fraction 1) and solid biomass, which can be either directly sent to transformation (third stage) or be transported to pretreatment process (first stage) where biomass chemical structure is modified. Pretreatment outflows are solid and liquid fractions of chemically modified biomass.
The main characteristic of pretreated biomass is that access to compounds of its chemical structure is enhanced, which is really helpful for subsequent procedures in BRF (Shimizu et al. 2020; Coral Medina et al. 2015). Liquid fraction generated after pretreatment (identified with number 2) incorporates soluble substances such as oligosaccharides and others that can be sent to the third stage for transformation (Silva et al. 2018). Pretreated biomass can be directly sent to conversion and obtaining of new products (biobased products 1) or be transported to hydrolysis process (second stage), where chemical structure is broken into smaller molecules such as monosaccharides (Loow et al. 2016). After hydrolysis, a hydrolyzed biomass (solid fraction 2) and hydrolysate (liquid fraction 3) are generated.
Depending on applied pretreatment and hydrolysis procedures, hydrolyzed solid fraction and liquid hydrolysate will have specific chemical compositions, which will determine the conversion route at co-production stage for product obtaining. From hydrolyzed solid fraction can be obtained products (biobased products 2) such as active carbon, lignin, biofilms, adsorption material, micro-fibrillated cellulose, biochar, bio-oil, electricity, etc. (Nanda et al. 2014; Coral Medina et al. 2015; Li and Kumar 2016; Vassileva et al. 2016; Virtanen et al. 2017; Rosales-Calderon and Arantes 2019). From liquid hydrolysate that contains soluble substances such as monosaccharides can be obtained a huge variety of products (biobased products 3); among them are bioethanol, furfural, hydroxymethylfurfural, carboxylic acids (itaconic, glutamic, lactic, and succinic), biopolymers, etc. (Sorokina et al. 2017; Steinbach et al. 2017; Rosales-Calderon and Arantes 2019; Dulie et al. 2020). After any kind of transformation process (chemical, physical, biological, or mixed) each crude outcome generally is sent to an adjustment stage, where separation operations occur for product quality improvement (Geankoplis 1993; Seader et al. 2011).
Aside to known processes involved in BRF, it is essential to find the best type of raw material (biomass) to employ in the process. Raw VB can come from agro-industrial crops or from waste of biomass processing; the last one usually is burnt in situ at rural fields as practice for solving accumulation problem (Almsatar 2020). This burning practice caused by human activities has generated significant concerns as a result of greenhouse gases and others chemical substances emitted into the atmosphere, both considered as pollutants (Zhang et al. 2016). Although burning raw VB waste solves accumulation problem in situ, this condition enlarges negative effects on environment (Onoja et al. 2018). To avoid this disadvantage, it is convenient to use mainly VB waste for transformation into value-added products by a BRF process.
9.2 Biomass Composition and Recalcitrance
Biomass is defined as any biological resource that can be turned into value-added products (including those energy producers). Biomass can be used in a direct way, when biological matter is consumed in its natural form; such as wood for firewood, and indirectly, when biological material undergoes some conversion process for product obtaining, such as biodiesel from oilseeds, carboxylic acids and biopolymers from lignocellulosic (LC), and bioethanol from starchy sources (Pervez et al. 2014; Sorokina et al. 2017; Rosales-Calderon and Arantes 2019). In addition, any kind of biomass is originated from spontaneous or induced biochemical processes occurring in biological sources that belong to vegetal, animal, and algae kingdoms (Allen et al. 2018; Jha and Kumar 2019; Sharma et al. 2019). Due to huge availability of biomass, chemical compositions are vast and different from one another, depending on source.
In the biosphere, VB is the largest biological biomass; around 450 gigatons of carbon are distributed over the Earth, meaning that 81.82% of the world biomass belongs to dominant kingdom of (mostly terrestrial) plants. Besides this, it is estimated that its renewal rate rises 3% per year. Bacteria biomass shares 12.73%, fungi 2.18%, archaea 1.27%, protists 0.73%, animal (predominantly oceanic) 0.36%, and others 0.90% (Gandini and Belgacem 2008; Bar-On et al. 2018). Thus, biomass from plants has caused great interest for scientific and industrial sectors. All plants have the common characteristic of being constituted by millions of cells, which are considered as the basic functional unit and the fundamental unit of life. These have enormous variety in shape, structure, and functions allowing existence of huge diversity of plant species (Revilla and Zarra 2013). Plants are multicellular organism constituted by eukaryotic cells, which have a nucleus, other organelles, a plasma membrane and a cell wall.
The plant cell wall encloses three main layers; the middle lamella, the primary wall, and the secondary wall (together with plasma membrane), respectively, from external to internal side. The first one is plentiful in pectic compounds and protein. The primary wall is subsequent to middle lamella and comprises cellulose microfibrils inserted in a matrix made of pectic compounds, hemicelluloses, and glucoproteins. The secondary wall is the thickest layer made of cellulose (CL), hemicelluloses (HC), and lignin (LG) (Manavalan et al. 2015). This three-layer structure is responsible for allowing the transit of benefit substances into cell and for protection against different kinds of chemical, thermal, physical, or biological attacks that can negatively affect the cell functions (Alberts et al. 2014). As the thickest layer makes up most of cell wall, then, the CL, HC, and LG contents are a determinant factor for using it as raw material in a BRF. Considering the latest, Table 9.1. shows different mass compositions (dry basis) of CL, HC, and LG of several VB.
The three-layer structure is rigid in order to act as protection system, which is very important when it comes to cell preserving; however, this cell wall characteristic is not useful when it is intended to use VB waste as raw material in a BRF (Bichot et al. 2018). This drawback is named recalcitrance, which is generated by complex interactions among LC fibers made of CL, HC, and LG. Besides, it is well-known that recalcitrance is a crucial issue when using plant biomass (preferably waste) in a BRF system (Holwerda et al. 2019). With the aim of overcoming the described impedement caused by reclacitrance, BRF has the pretreatment stage, which allows to disrupt rigid LC fibers into a looser material and to improve component (CL, HC, and LG) accessibility for its utilization in subsequent steps (Melati et al. 2019; Antunes et al. 2019).
9.3 Biomass Pretreatment to Improve Cellulose Accessibility
Many kinds of processes can be employed before pretreatments, such as milling, extraction, and drying. Material can be milled in order to reduce particle size and increase the exposed surface area, and thus raising effectiveness of pretreatment and subsequent enzymatic hydrolysis (Da Silva et al. 2013). The most commonly employed mills are ball mill (crushes material using spheres) (Lin et al. 2010) and discs mill (employs serrated plates to pulverize biomass) (Hideno et al. 2009). However, this milling step is expensive and hard to achieve in large scale. Some factors such as dry material and milling coupled with pretreatments affect hydrolysis performance. Milling process is pivotal to enable efficient pretreatments.
Pretreatments employing acid are known for solubilizing HC. These processes commonly use sulfuric acid at high temperature and pressure conditions (Brienzo et al. 2017). This pretreatment is sectioned roughly in these events (Herrera et al. 2003):
-
Proton scattering through damp LC material;
-
Protonation of ether-oxygen binds chaining monomeric sugars;
-
Rupture of glycosidic bond;
-
Generation of intermediate carbocation;
-
Solvation of carbocation in liquid;
-
Reconstruction of protons and sugars;
-
Distribution of products in liquid phase.
Looking at CL, this pretreatment can remove up to 20% of its content in biomass (Trajano and Wyman 2013). Acid process enhances acid hydrolysis accomplishment, but generates degradation products such as furfural and hydroxymethylfurfural (Alonso et al. 2013). Enzymatic hydrolysis is considerably influenced by HC and LG contents (Meng et al. 2017); lowering these contents is an essential step of biomass transformation. HC does not possess a linear structure, making it more exposed to acid pretreatments than CL. Therefore, HC content can be totally removed with mild pretreatments that will not degrade CL within biomass (Aguilar et al. 2002). Steam explosion pretreatment uses SO2 at high temperatures to reduce HC content in corn straw up to 65%, being an alternative to acid pretreatments (Öhgren et al. 2005). The kind of acid employed can also enhance HC removal. LC materials pretreated with sulfuric acid showed complete HC removal while steam explosion using SO2 showed minor promising results. Even though it removed most of HC, acid pretreatment produces more degradation products due to its severity, damaging obtained sugars and producing furfural (Martín et al. 2002). Sugars present in HC are solubilized into oligomers or monomers (influenced by reagent concentration, temperatures, and reaction times) (Trajano and Wyman 2013). Higher temperatures (Excoffier et al. 1991) and acid concentrations improve removal of HC content (Table 9.2) (Kumar et al. 2009).
Acid pretreatments are much less effective concerning LG removal regardless of acid employed (Kumar et al. 2009). Normally, severe conditions cause lower LG solubilization, resulting in condensation in fiber surface (Schmatz et al. 2020). During the process of LG removing (via acid route), usually occurs generation of aromatic molecules and different types of phenolic compounds (Du et al. 2010). Solid fraction microscopy shows considerable change in LG structure and content, presenting LG droplets within plant cells (Schmatz et al. 2020). These characteristics suggest a trend of rupture and aggregation that could cause less efficiency in LG content removal (Selig et al. 2007).
Many substances can be employed during alkaline pretreatments (NaOH, Ca(OH)2, KOH, etc.). During these pretreatments, solvation and saponification processes happen first, resulting in swelling and higher accessibility for enzymes to act within LC material. Aggressive alkaline pretreatments can cause dissolution, peeling, decomposition, and degradation of polysaccharides (Hendriks and Zeeman 2009). The focus of alkaline pretreatments is LG content reduction, clearing a path for enzymatic hydrolysis and enhancing polysaccharide reactivity (Table 9.2). Studies show that this trend is related to intermolecular saponification of ester links among HC and LG, increasing pore density (Da Silva et al. 2013). Peroxide pretreatments are also quite capable of HC and LG removal, generating a pretreated biomass with improved enzymatic digestibility (Monte et al. 2011). This pretreatment can successfully solubilize HC and leaves only residual LG (Brienzo et al. 2009).
9.4 Lignocellulosic Biomass Hydrolysis
Many variables are involved in LC biomass conversion into sugars through enzymatic hydrolysis. For example, accessibility issues and costs of employed technologies are obstacles often encountered. CL accessibility is crucial since it is closely tied to enzymatic hydrolysis (Crowe et al. 2017). Shortly, accessibility is a way to measure how much CL can be reached by enzymes during hydrolysis. Biomass on its own has an impact on accessibility since it is a heterogenic material, has variable crystallinity, polymerization degree, and HC and LG contents creating a physicochemical barrier. Actually, one of the hardest obstacles to jump is the difficulty to reach a large portion of CL covered by robust and organized microfibrils.
It is safe to say that there is a close connection between internal surface area and enzymatic hydrolysis (Cosgrove 2005), being one of the obstacles in the way of biomass hydrolysis (Huang et al. 2010). Accessibility is also heavily tied to pore size distribution, significantly more than what is observed with external surface area (Wang et al. 2012). With that in mind, enzymes are able to reach internal surface through big enough pores (Harmoko et al. 2016). Pores smaller than 5.1 nm are not wide enough for enzymes to hydrolyze LC material. It is important to note that most studies use dried biomass, which shows lower digestibility due to shrinkage of pores (Luo and Zhu 2011).
LG and HC contents have a close relation to accessibility, influencing pore distribution, pretreatment efficiency, and enzymatic hydrolysis (Zhao et al. 2017). LC biomasses are heterogeneous, possessing its area sectioned into external and internal areas, presenting different characteristics. Regarding internal surface, accessibility can be inferred by looking at its rifts, voids, and openings generated by HC and LG removal by employing many kinds of pretreatments (steam explosion, peroxide, diluted acid, alkaline, and others) (Karimi and Taherzadeh 2016). It has been reported that HC removal could be a more important asset to accessibility than delignification (Leu and Zhu 2013). HC present barriers to enzymatic hydrolysis as it is placed between and around fibers within cell walls (Zhu et al. 2010).
LG inherently hinders enzymes by forming a physicochemical wall that adsorbs enzymes (Ximenes et al. 2011). By employing correct pretreatments, lower contents of LG and HC may diminish or stop enzymatic hydrolysis hindrance (Chen et al. 2015). While a complete removal of LG would be too expensive in large scale (Yang et al. 2015), mild delignification increases accessibility and can increase enzymatic hydrolysis yield dramatically (Shimizu et al. 2020).
Digestibility can also be influenced by particle size. Quantifying particle size is a problem since they have variable shapes and commonly agglomerate (Ek et al. 1994). Particle size can be visualized by employing microscopy, image analysis, or mechanized particle analyzers. On the down side, these techniques do not discriminate between material’s topology and ruptures that can increase exposed surface areas. However, it is expected that small particles provide higher digestibility by presenting a wider exposed surface area for enzyme action. In sum, HC and LG removal is related to accessibility just as much as physicochemical properties of biomass (Shimizu et al. 2020).
Lower degrees of polymerization present an increase in the number of linkage sites for enzymes, enhancing biomass hydrolysis. Regardless, CL needs to be exposed to be reached by enzymes. Altering of polymerization degree usually involves meddling with porosity and crystallinity, as the milling process rips fibers and increases pore size distribution (Zhao et al. 2012). Depolymerization happens when polysaccharides are broken into monomers (Goufo and Mugisha 2018). Depolymerization of CL chains within LC biomasses is a step of enzymatic hydrolysis process. For example, cellulases are capable of depolymerizing CL in order to obtain glucose (a specific monomer) (Yücel and Göycıncık 2015). Enzymes used and substrate employed are also important. The spread of enzymes through pores, the binding sites occupied during hydrolysis, inhibition products and other enzymes used to attack cell wall are properties related to accessibility (Zhao et al. 2017).
9.5 Enzymatic Hydrolysis
Cellulases are an enzymatic cocktail of various enzymes that depolymerize CL into sugars by breaking its glycosidic links. Cellulases are actually three enzymes: exo-β-1,4-glucanases, endo-β-1,4-glucanases, and β-glucosidases. These enzymes work in synergic manner, where their efficiency combined is higher than if they were used separately (Yücel and Göycıncık 2015). Among these enzymes, endoglucanases are capable of breaking chains through interchain, exo-β-1,4-glucanases attack free ends of chains created by endo-β-1,4-glucanases deploying water-soluble cellobiose, and β-glucosidases finish the process by hydrolyzing cellobiose into monomers. Difficulty is faced if LC materials possess HC and LG, which makes enzymes unable to reach and hydrolyze CL. Endo-β-1,4-glucanases role is most likely affected during hydrolysis since it starts the action on CL. The exposed surface area by pretreatments accessible to enzymes is closely related to glucose yield (Fig. 9.2) (Brienzo et al. 2017). Enzymatic hydrolysis of CL chain can be sectioned in these parts (Walker and Wilson 1991): Moving enzymes from liquid medium to LC material; Adsorption and generation of enzyme–substrate complex; Hydrolysis of CL chain; Moving of sugars obtained from biomass surface to the liquid medium; Hydrolysis of cellobiose and oligomers into monomers in liquid medium.
Many factors influence the enzymatic hydrolysis process, such as HC and LG contents present in LC material, generating a physicochemical barrier against enzymes (Fig. 9.3). Furthermore, during hydrolysis LG content increases, slowing down the process. While enzymatic hydrolysis process occurs, the material loses CL content, increasing LG/CL ratio and hindering hydrolysis (Wallace et al. 2016).
Mild delignification of VB enhances glucose yield, more so when LG content reaches 10% (Mooney et al. 1989). Other than that, it increases hydrolysis efficiency due to larger quantity of enzymes retrieved, caused by less unproductive binding to LG. High LG contents can cause redepositing, occupying pores that could be penetrated by enzymes. Enzymes are unproductively adsorbed by LG within biomass, severely hindering enzymatic hydrolysis (Schmatz et al. 2020). In addition, LG amounts in LC material can be used as a discrimination parameter for enzymatic hydrolysis (Brienzo et al. 2014).
9.5.1 Cellulases Performance
Enzymes that play a synergistic important role on CL depolymerization into cellobiose and glucose molecules at saccharification step are denominated cellulases or cellulolytic enzymes, which can be synthesized during growth of some microorganism (fungi or bacteria) in LC substrates through solid-state or submerged fermentations (Aruwajoye et al. 2020; Barbosa et al. 2020). CL hydrolysis requires participation of three types of cellulolytic enzymes, all of them characterized by cleaving glycosidic linkages β-(1→4).
Cellulases performance is synergistic, which means that if one of them does not act, then hydrolysis stage will have low yield and therefore BRF process will not be technically feasible. These drawbacks can occur by different factors such as temperature, pH, chemicals (that can come from pretreatment), or products generated by own cellulases action (Hsieh et al. 2014; Teixeira da Silva et al. 2016). Recent studies have confirmed inhibitory effects on cellulases by cellobiose and some monosaccharides like glucose, mannose, and galactose (Hsieh et al. 2014). Although inhibitory compounds concentration can vary, there is evidence of a generality; CMCases activity can be inhibited by cellobiose, CBHs can be deactivated by glucose, and β-glucosidases sensibility can be greater in glucose presence than mannose and galactose (Murphy et al. 2013; Hsieh et al. 2014; Vianna Bernardi et al. 2019). Nevertheless, one of the principal challenges is focus on researching minimum level of inhibitory concentration that can affect negatively the cellulases activities and that inhibitory effects can be reversible or irreversible.
In addition to mentioned inhibitors, others chemical compounds can affect cellulohydrolytic system performance, among them are ionic liquids, some salt and metal ions, phenolics and furans (Teixeira da Silva et al. 2016; Zhai et al. 2016; Summers et al. 2017).
9.5.2 Xylanases Performance
Because HC is a branched heteropolysaccharide constituted by several kinds of molecules, a multienzyme system that works synergistically is required for its depolymerization. These enzymes are denominated xylanases or xylanolytic enzymes, which can be synthesized by some microorganism (fungi, bacteria, yeast, or algae) using xylan-containing substrates through solid or submersed fermentations (Bhardwaj et al. 2019). Xylan hydrolysis needs contribution of various types of xylanolytic enzymes, which acted both on xylan main chain cleaving chemical bonds β-(1→4) and on main chain sides cleaving linkages α-(1→2) and α-(1→3) principally (Chakdar et al. 2016). In main chain, endoxylanases (EC 3.2.1.8) are responsible for depolymerizing the xylan backbone (β-(1→4) bonds) to obtain xylobiose and shorter xylooligomers. β-xylosidases (EC 3.2.1.37) breakdown xylobiose and other soluble xylooligomers into xylose molecules (Bosetto et al. 2016; Puchart et al. 2018). Accessory enzymes act on xylan side chains, among them are α-arabinofuranosidases (EC 3.2.1.55), α-glucuronidases (EC 3.2.1.131), β-galactosidases (EC 3.2.1.23), acetyl-xylan esterases (EC 3.1.1.6), ferulic acid esterases (EC 3.1.1.73), and p-coumaric acid esterases (EC 3.1.1.73) (Malgas et al. 2019).
The first one, also called α-arabinosidases, break α-(1→2), α-(1→3), and α-(1→5) bonds, and release arabinose from xylose–arabinose linkage (Terrone et al. 2020; Yeoman et al. 2010). α-glucuronidases disrupt α-(1→2) bonds and split off methyl glucuronic acid from acid-xylose linkages (Dashnyam et al. 2018). β-galactosidases are responsible to cleave β-(1→4) linkages, which allows galactose to unlink from galactose–xylose bonds (Khosravi et al. 2015). Acetyl-xylan esterases attack acetyl groups located at xylan branches, these type of enzymes release acetyl groups allowing endoxylanases to work on the main chain (Pawar et al. 2016; Razeq et al. 2018). Feruloyl and p-coumaroyl esterases are capable to cleave ester bonds in arabinose–ferulic acid linkages and in arabinose-p-coumaric acid linkages, respectively (Lopes et al. 2018).
The complex system of xylanolytic enzymes act to breakdown xylan (as is mentioned above), the process can develop some difficulties related to inhibitory effects in xylanases activities. Among factors that can negatively modify their activities are temperature, pH, chemicals (that can come from pretreatment), or products generated by own xylanases action (Bajpai 2014a, b). Although inhibitory substance concentration can differ, it exists an overview; endoxylanases activity can be susceptible to xylobiose and xylotriose presence, and β-xylosidases can be deactivated by xylose (Yeoman et al. 2010; Fu et al. 2019). It is necessary to expand and deepen studies of inhibitory effects on accessory enzymes; these could include analysis of dimmers and monomers produced in pretreatment stage.
Although xylanases and cellulases can be synthesized extracellularly by different fungi or bacteria species, few microorganisms are able to produce the whole set of hydrolytic enzymes. For this reason, it is important to continue with researches involving analysis of microorganisms, methods, and variables for hydrolytic enzymes production.
9.5.3 Cellulolytic Enzymes Synthesized by Fungi and Bacteria
CL is the main constituent of plant cell wall, in addition to being the most abundant natural polymer available (George and Sabapathi 2015). CL is chemically composed of linear chains of 7000 to 15,000 glucose residues joined by β-1,4 glucosidic bonds, without branches. β-type bond causes a 180° rotation alternating glucose unit, resulting in a linear chain. CL is hydrolyzed by the action of microbial enzymes, such as endoglucanases, exoglucanases, and β-glucosidases (Pino et al. 2018; Bagewadi et al. 2017; Sorensen et al. 2014).
Cellulases are produced by several species of fungi (Narra et al. 2014). Endoglucanases can be produced by Myceliophthora sp. (Zanelato et al. 2012), Streptomyces sp. (Chellapandi and Jani 2008), Trichoderma viride (Irfan et al. 2012), Trichoderma harzianum (Bagewadi et al. 2017), and Aspergillus oryzae (Kotaka et al. 2008). Exoglucanases, as well as endoglucanases, are also produced by a wide variety of fungal species, such as Rhizopus oryzae (Mukherjee et al. 2011), Aspergillus niger (Chandra et al. 2008), Aspergillus fumigatus (Mahmood et al. 2013), Trichoderma reesei (Elshafei et al. 2014), Trichoderma viride, and Ganoderma lucidum (Shahzadi et al. 2014).
In addition to endo and exoglucanases, β-glucosidases can be produced by Trichoderma reesei (Juhász et al. 2005), Penicillium funiculosum (Ramani et al. 2012), Aspergillus niger (García-Kirchner et al. 2005), Debaryomyces pseudopolymorphus (Barbosa et al. 2010), Pichia pastoris, (Batra et al. 2014) and Aspergillus saccharolyticus (Sorensen et al. 2014). Besides fungi, bacteria also produce cellulases of industrial importance. Endoglucanases can be produced by Bacillus agaradhaerens (Hirasawa et al. 2006), Clostridium thermocellum (Romaniec et al. 1992), Clostridium thermocellum (Fauth et al. 1991). Exoglucanases have also been identified in bacteria of species Clostridium stercorarium (Creuzet et al. 1983), Cellulomonas fimi (Duedu and French 2016), and Xanthomonas oryzae pv. oryzae (Tayi et al. 2018). Additionally, β-glucosidases can be produced by bacteria of species Dyella koreensis sp. nov. (An et al. 2005), Burkholderia ginsengisoli sp. nov. (Kim et al. 2006), Microbacterium ginsengisoli sp. nov. (Park et al. 2008), Lactobacillus kimchicus sp. nov. (Lian et al. 2011), Brevibacillus panacihumi (Kim et al. 2009), most of which are species that had never been described before.
Cellulases, in general, are enzymes that are of great use in several industrial sectors. Endoglucanases, exoglucanases, and β-glucosidases are enzymes widely used in bioethanol production (Kotaka et al. 2017; Singhania et al. 2017). Endoglucanases are used in textile and food industries (Narra et al. 2014). Exoglucanases are used mainly in textile and in pulp and paper industries (Castro and Pereira 2010).
Beside from cellulases, other enzymes play fundamental roles in the development of products with high added value, such as hemicellulases, which for industrial sector may be essential in obtaining medicines, packaging and products of relevance in the medical field. Hemicellulases, like cellulases, can also be produced by fungi (Liu et al. 2010) and bacteria (Walia et al. 2014).
9.5.4 Hemicellulolytic Enzymes Synthesized by Fungi and Bacteria
Basically, xylan hydrolysis, the most abundant compound of HC in nature, occurs through endo-enzymes action, which act internally on main chain, and exo-enzymes or auxiliary enzymes that hydrolyze oligosaccharides and produce monosaccharides (Kalogeris et al. 2001). Some enzymes produced by microorganisms hydrolyze xylan in specific regions of its main chain and side chains, leading to monosaccharides production that are used by microorganisms themselves as carbon source for cellular metabolism.
Among microbial enzymes that hydrolyze xylan into monosaccharides, it can be highlighted endo-β-1,4-D-xylanases (EC 3.2.1.8), which acts on xylan main chain and generates low polymerization xylooligosaccharides that are substrates for exo-β1,4-xylanases (EC 3.2.1.37), that act through non-reducing terminals generating D-xylose (Beg et al. 2001; Wong et al. 1988).
In addition to the enzymes that hydrolyze xylan main chain, there are still some enzymes that hydrolyze the pendant-side groups. Among these accessory enzymes, α-arabinofuranosidases (EC 3.2.1.55) stands out, which removes arabinose, α-glucuronidases (EC 3.2.1.131) which removes glucuronic acids, acetyl-xylan esterases (EC 3.1.1.72), which removes acetyl groups (Juhász et al. 2005), and feruloyl esterases which remove ester bonds between hydroxycinnamic acids and sugars (Mathew and Abraham 2004). The consequent action of accessory enzymes produces debranched xylan, which is of great interest for industrial applications.
α-glucuronidases are classified into two families: GH 67, which cleaves uronic acids linked to non-reducing terminals of xylosyl residues of small xylooligosaccharides, and GH 115, which hydrolyze 4-O-D-methylgluvuronic acids linked to non-reducing terminals of xylopropanosyl residues and also those linked to internal xylosyl residues. They are produced by bacteria (about 70 kDa) and fungi (about 90 kDa) (Yeoman et al. 2010; Dimaragona and Topakas 2016).
Acetyl-xylan esterases are enzymes produced by bacteria and fungi. They are responsible for cleaving acetyl groups from heteroxylans. Microorganisms that produce these enzymes become capable of degrading acetylated xylans. Feruloyl esterases cleave bonds between esterified hydroxycinnamic acids for arabinoxylans. They are also produced by fungi and bacteria, and its production can be induced according to presence of certain substrates in microbial growth (Wong et al. 1988).
Complete hydrolysis of xylan requires joint action of enzymes that hydrolyze its main chain (xylanases and β-xylosidases) and accessory enzymes responsible for hydrolyzing its branches (α-arabinofuranosidases, acetyl-xylan esterases, α-glucuronosidases, and feruloyl esterases) (Yang et al. 2017). Several microorganisms have the capacity to produce xylanolytic enzymes, which in turn have great potential for industrial uses. Below, more details of some xylanase-producing microorganisms are presented.
Xylanolytic enzymes are usually best induced by xylan-containing substrate (Espinar et al. 1994). However, some authors show inducing effect with xylan fragments (Aro et al. 2005) and its repression by readily assimilable sugars such as glucose, lactose, and xylose (Gaspar et al. 1997). LC substrates are used to obtain xylanolytic enzymes on a large scale and at low cost in submerged or solid culture (Mishra et al. 1990).
Biochemical properties of endoxylanases from bacteria and fungi vary in terms of molecule size (8.5–85 KDa) and isoelectric point (4.0–10.3 pI) (Chakdar et al. 2016). Table 9.3 shows some xylanase-producing microorganisms found in specialized literature (Chakdar et al. 2016; Goswami and Pathak 2013; Walia et al. 2017), showing optimal conditions for action of such hemicellulases and then, its respective molecular sizes.
Among the products of high added value obtained by hemicellulases use, it can be highlighted: xylooligosaccharides (XOS), xylitol, hydrogel, dressings and substitutes for human skin, films for biodegradable packaging, medicine encapsulation, substitute for fat in cheeses, additives in paper production, substitute for gelatin and in food gums, additives in textile and cosmetic industries, production of new biomaterials such as glucagel, etc. (Ebringerová 2006).
The study of HC applications is constant, such as XOS with high purity (Reddy and Krishnan 2016), XOS for fermentation (Chen and Liu 2017), mucus adhesive film (Hanif and Zaman 2017), xylan-based thermoplastic-lactate copolymer (Zhang et al. 2017) and also desirable for biomedical applications (Jungles et al. 2017).
9.5.5 Enzymes Purification Systems
Over the past five decades, it has been a great development of techniques and methods for separation and purification of biological macromolecules such as enzymes. As a result, advances in biosciences and biotechnology have become increasingly important (Ersson et al. 2011; Yimer and Tilahun 2018).
In order to acquire knowledge about structural and functional properties of enzymes, the purification process is an essential step. Enzyme purification often requires the use of multiple purification steps applying different methods in suitable order to obtain the highest possible yield. However, it should be kept in mind that the more steps of purification, the lower the yield. Therefore, knowledge of enzyme’s properties is of upmost importance to reduce purification steps (General Electric Healthcare 2010).
The most common method for enzyme purification is chromatography (General Electric Healthcare 2010). Enzyme characteristics that should be taken into consideration when choosing a purification method are solubility, size, charge, and specific binding affinity (Berg et al. 2002; Yimer and Tilahun 2018).
Cellulases purification, an enzyme group that plays an important role in hydrolysis of renewable LC materials, can be carried out using different methods. Purification efficiency is generally analyzed in terms of yield and purification fold, as is presented in Table 9.4, along with purification methods used for cellulases reported in specialized literature.
Salting out and dialysis are methods that are usually used as first steps. Protein solubility usually decreases at high salt concentrations, causing its precipitation. Different proteins precipitate in different concentrations of salt; therefore, salting out can be used to fractionate proteins (Wingfield 2016; Duong-Ly and Gabelli 2014). Ammonium sulfate is commonly used as a precipitate agent to separate other proteins from cellulase (Farinas et al. 2011). Dialysis is usually used after salting out to remove salt or other small molecules through a semipermeable membrane, which can come in a variety of pore sizes (Berg et al. 2002; Yimer and Tilahun 2018).
Different types of chromatography can be used for cellulases purification. Ion exchange chromatography (Fig. 9.4) separates proteins based on their charge. Protein with positive charge will usually bind to a column negatively charged with sulfopropyl (SP) or carboxymethyl (CM). Likewise, negatively charged proteins will bind to a positively charged column containing quaternary amines (Q) or diethylaminoethyl (DEAE) (Berg et al. 2002). When above its isoelectric point (pI), an enzyme tends to bind to a positively charged anion exchanger, and when below its pI, a cation exchanger negatively charged matrix is where the enzyme will bind (Bauer and Schnapp 2007; General Electric Healthcare 2010).
Depending on pH, cellulases can be negatively or positively charged; however, several researches show that most of them present better purification when using anion exchange chromatography (Begun and Absar 2009; Genc et al. 2014; Gaur and Tiwari 2015; Megha et al. 2015; Goel et al. 2019). DEAE is a positively charged matrix commonly used as the stationary phase, where cellulases will bind if negatively charged. In order for cellulases to be eluted from the matrix, a linear sodium chloride gradient from zero to 1 mol/L is generally used. Chloride ions will compete with negatively charged groups on cellulases for binding to the column (Bauer and Schnapp 2007).
Size exclusion chromatography (Fig. 9.5) allows separations of substances with different molecular sizes. Large molecules that cannot enter into pores of the chromatography beads will elute first, while smaller molecules will penetrate the pores and therefore will take longer to elute (General Electric Healthcare 2010). Sephadex is the most popular matrix for gel exclusion chromatography. Molecular weight of cellulases produced by different microorganisms can vary from 12 to 126 kDa; therefore, column packing material with different beads sizes can be used for purification (Tao et al. 2010; Chandra and Madakka 2019).
Specific activity of cellulases is usually twofold lower than of other hydrolytic enzymes. For industrial purposes, large quantity of cellulases is needed and this production becomes a “metabolic burden” even for native cellulolytic organisms. Therefore, heterologous expression of cellulases in industrial microorganisms, such as E. coli, Pichia pastoris, and Saccharomyces cerevisiae, enables robust and cost-effective production (Vinuselvi and Lee 2012). Several researches with this goal have been carried out in the past years, mainly focusing on heterologous expression of cellulases produced by different microorganisms and termites in E. coli or other model microorganisms (Wei et al. 2015; Zeng et al. 2016; Chahed et al. 2018; Trollope et al. 2018; Zeeshan et al. 2018; Zhang et al. 2018; Raza et al. 2020).
Purification of recombinant proteins can be influenced by molecular biology of gene isolation and expression. For an economical production, efficient implementation of purification methods to maintain a high yield of the heterologous protein is of great importance. Affinity chromatography is one of the most efficient methods for heterologous proteins purification due to its high recovery yield and purity achieved (Evangelista and Suttnar 1997; Pina et al. 2014; Mahmoodi et al. 2019).
Affinity chromatography (Fig. 9.6) separates molecules based on a reversible interaction between the protein of interest and a specific ligand attached to a chromatography matrix. In this method, specific tag sequence is attached to the protein of interest at DNA level, generally fused to N- or C-terminal, which leads to expression of a tagged protein. Besides facilitating the purification process, theses tags also enhance protein stability and increase expression levels. Metal ions such as zinc, copper, and nickel have been found to bind favorably with histidine residues in proteins (Evangelista and Suttnar 1997; General Electric Healthcare 2010; Pina et al. 2014; Mahmoodi et al. 2019).
For purification of heterologous cellulases, the use of a nickel–nitrilotriacetic acid matrix, in which metal ions interact with histidine residues in the affinity tag, is commonly found in literature. Several methods can be used for cellulases elution from the matrix. High concentration of imidazole buffer, use of strong chelating agents such as ethylenediamine tetraacetic acid (EDTA) and lowering pH in order to protonate histidines are conditions that decrease cellulase affinity to the nickel resin, thus enabling enzyme recovery (Reverbel-Leroy et al. 1997; Campen et al. 2017; Ni et al. 2005; Shi et al. 2013; Zeng et al. 2016).
There are several strategies for purifying cellulases. It must be taken into consideration the best system to obtain the highest yield with the highest catalytic activity of the enzymes. Purity degree will depend on its end use; however, most of purification systems used in laboratory researches can be scaled up to industrial processes (Bajpai 2014a, b).
9.5.6 Interference Among Enzymes
Conversion process of polysaccharides on LC biomass into simple sugars through enzymatic hydrolysis is a crucial and limiting step for biofuel production (Sheldon 2014). Enzymatic hydrolysis is a heterogeneous process that involves close contact between enzyme and substrate, enzyme activity and reaction conditions such as temperature and pH (Saini et al. 2016).
Enzymatic hydrolysis of lignocellulose can be affected by several factors, mainly substrate-related since the presence of LG and HC in biomass can decrease accessible area to cellulases (Shimizu et al. 2020). Polymerization and crystallinity degrees of CL also play a role on enzymatic hydrolysis yield. Interaction between CL and cellulases can be inhibited by LG in different ways depending on its structural properties (Ying et al. 2018).
Inhibition of cellulases by LG can occur by three major factors: exposure to soluble lignin-derived compounds, non-productive binding of cellulase into LG, and physical barrier of cellulases progress (Fig. 9.7). Enzyme inhibition due to LG is a complex process and will depend on biomass botanical origin and pretreatments applied to it, since both affect localization and chemical properties of LG (Saini et al. 2016).
Phenolic compounds can cause loss of cellulases activity as the length of exposure to them increases. This occurs due to cellulases precipitation and can vary depending on microbial source of enzymes, type of cellulases, and type of phenolic compound (Saini et al. 2016). In addition to act as a shield preventing enzymes to reach CL, LG can also adsorb cellulases irreversibly by hydrophobic, electrostatic, or hydrogen-bonding interactions (Kumar et al. 2012).
All of these factors led to decrease of glucose release in enzymatic hydrolysis process. Therefore, a way to reduce cellulases–lignin interaction is widely studied. Some of the strategies used are chemical modification of LG or its complete removal, cellulases modification, and addition of LG blocking additives. Pretreatment of LC biomass can remove or modify LG and HC contents, which can raise accessibility for cellulases (Shimizu et al. 2020; Melati et al. 2019; Saini et al. 2016).
The rate of LC biomass enzymatic hydrolysis usually decreases during hydrolysis. Steam pretreated sugarcane bagasse was enzymatic hydrolyzed for 72 h, showing an initial phase with fast conversion of 61.7% glucose and a final conversion of 86%. As hydrolysis progressed, rate of conversion decreases as well as glucan content while LG, phenolic compounds, and ash increased. In addition, enzyme activity was lost during the first 24 h of hydrolysis. Recalcitrance increasing during hydrolysis and deactivation of enzymes due to thermal stability are factors that contribute for slowdown and incomplete hydrolysis (Wallace et al. 2016).
After different pretreatments, sugarcane biomass (external fraction, node, internode, and leaf) presented improve on CL conversion into glucose after enzymatic hydrolysis. A complete conversion of CL into glucose was possible after oxidative pretreatment of the internode fraction. LG removal of 15% and 10%, respectively, resulted in at least 60% of glucose yield (Shimizu et al. 2020). Addition of a lignin-blocking agent to sugarcane bagasse can increase hydrolysis yield by 40% or even 60%, depending on agent concentration. They prevent non-productive adsorption of cellulases by blocking exposed LG surfaces (Àzar et al. 2020).
Steam explosion and alkaline sulfate pretreatments on sugarcane bagasse resulted in more accessible substrate, with approximately 90% of CL hydrolyzed using high enzyme loadings. Besides, after pretreatment with sodium sulfate and sodium hydroxide hydrolysis using low loading of a modern enzyme preparation used in industrial processes, Cellic CTec3, resulted in 70% conversion into glucose (Siqueira et al. 2017). In contrast, the presence of vanillin, a lignin-derived phenolic, can cause cellulases deactivation and precipitation, resulting in a decrease of 20% in CL conversion with a concentration of 5 mg/mL vanillin and 50% with 10 mg/mL. (Qin et al. 2016).
The majority of β-glucosidases from commercial cellulases (Cellic CTec 2), derived from T. reesei, adsorb to LG, whereas the same enzyme derived from A. niger presents less inhibition. Therefore, engineering robust cellulases that are highly active and stable is a strategy to mitigate non-productive adsorption. Modification of cellulase from T. reesei surface charge by succinylation, converting positively charged primary amine groups to negatively charged acid and neutral acetyl groups, resulted in greater than twofold increase in Avicel conversion after 170 h (Nordwald et al. 2014).
Several techniques can be used to improve enzymatic hydrolysis of LC biomass; however, for economic feasible process a multi-disciplinary research involving areas of plant biotechnology, enzyme engineering, lignin-cellulase interactions, and process development is important (Sipponen et al. 2017).
9.5.7 Assistance Elements for Improving Enzyme Activity
For industrial applications, knowledge about enzymes activators and inhibitors is relevant. Many compounds can influence on enzymes activities and may be present in water and/other reagents employed in industrial processes or can be a result of equipment corrosion. Metal ions can activate or inhibit enzyme activity by interacting with amine or carboxylic acid group of amino acids. They can act like electron donors or acceptors by forming complexes with other molecules linked to enzymes, besides help reduce non-productive adsorption of enzymes. Ionic charge and ion radius size are properties that can influence activity and stability of enzymes (Pereira et al. 2017).
Effects of each metal ion can differ from cellulases, depending on its type and source. Addition of Cu2+, Fe2+, Co2+, and Mn2+ at 2 mmol/L in the reaction medium increased activity of endoglucanases from A. niger, especially manganese, which improved activity by 57%. However, β-glucosidases from the same microorganism showed increase of activity by 33% after Co2+ addition. Glucose releasing after enzymatic hydrolysis of acid pretreated bagasse increased 34% after addition of 10 mM Mn2+ (Vasconcellos et al. 2012).
Some metal ions can be cellulases inhibitors. Chelating agents such as EDTA (ethylene diamine tetra acetic acid) can be used to enhance cellulases activity since they act by sequestering inhibitors metal ions and as protease inhibitor. EDTA forms a complex with metal ions in the reaction medium, allowing the enzyme site to react with the substrate. After an addition of 2 mmol/L EDTA, endoglucanases from A. aculeatus presented increasing of 1.5-fold in its enzymatic activity and enzyme stability also increased (Naika and Tiku 2011).
The high crystallinity of CL structure makes cellulases penetration difficult. Surfactants tend to decrease surface tension of aqueous systems and their addition during enzymatic hydrolysis can modify cellulases surface and improve CL conversion. CL conversion increased 38.4% after addition of 0.5% (v/v) Triton X-100 and 60% after addition of azobenzene-based surfactant (Nurul Adela et al. 2015; Seidel and Lee 2020).
Although there are few studies about the effect of ultrasound on enzymes performance, the use of ultrasonic treatment at appropriate frequencies and intensity levels can increase enzymatic activity and favorably change enzymes without altering its structural integrity. After a 17.33 W/cm2 intensity and time of 30 min, ultrasonic treatment was able to increase cellulases activity by 25% (Subhedar and Gogate 2014).
Some HC, mostly xylan, can still remain in LC biomass after mild pretreatment conditions and this can influence cellulases accessibility. Xylanases supplementation along with cellulases can potentially improve glucose yield. The partial combination of cellulases with xylanases in enzymatic hydrolysis contribute to increase CL conversion to glucose from 70% to 86%. This improvement shows that there is a synergistic interaction between xylanases and cellulases, since xylanases can hydrolyze the remaining xylan improving CL accessibility (Hu et al. 2011).
9.6 Challenges on Industrial Scale of Cellulose Hydrolysis Via Enzymatic
Comparing biological hydrolysis with its chemical homologous process, it can be observed that the former presents more favorable characteristics, among them are: slight temperature conditions (up to 50–55 °C), possibility to raise sugar yields (especially when commercial enzymes are used), low operation costs, low energy supply, no use of corrosive chemicals (like inorganic acids), and no production of inhibitory substances (such as furfural and hydroxymethylfurfural principally) that contribute with conversion, low yields (Amezcua-Allieri et al. 2017; Jahnavi et al. 2017; Steinbach et al. 2017; Chen and Liu 2017). Nevertheless, enzyme hydrolysis has difficulties related to elevated process time, high cost of enzyme (in synthesis and purification, or purchasing), enzymatic loads, pH and temperature sensibility, mixing modes, stirring intensity, low degree of synergy, recovery system of used enzymes and some good experiences obtained in laboratory have not been tested in large scale (Balan 2014; Hu et al. 2016; Kadić 2017; Kumar et al. 2018; Singhvi and Gokhale 2019).
In the particular case of CL hydrolysis by biological route, it can be enlisted several strains found mainly in pilot-scale experiences. Elevated viscosity, unsuitable mass and heat transference, and significant inhibitory concentrations are reasons for decreasing in enzyme hydrolysis yields specially when high solid biomass loads are used (Agrawal et al. 2018). According to this, the research principle challenges are to focus on different solutions to design non-conventional units that let a continuous or fed-batch process; free-fall horizontal devices and helical impellers could be promising (Da Silva et al. 2020). Also regarding reactor design, it could be researched a reaction-separation system that allows a simultaneous extraction of intermediate-products while these are obtained, avoiding inhibitory effects (Kiss 2014). However, if conventional BRF process (hydrolysis and adjustment stages separately) is carried on, a recovery system of enzymes and how many times they could be used again as a recycling strategy into the BRF would be a great approach (Mesa et al. 2016).
On the other hand, physical issues associated with surface tension and viscosity are also treated. Currently, to correct surface tension problems in the system of solubilized enzymes in aqueous medium—pretreated biomass, different surfactant agents that do not destabilize enzymatic activity and reduce superficial tension in the aqueous medium can be added to improve enzymes access into biomass (Wang et al. 2018; Zhou et al. 2019; Yusuf and Kresnowati 2019). Taking viscosity into account, an intermittent or split feed of biomass and aqueous medium enzymes can improve hydrolysis yield since system viscosity decreases and enzyme contact to inhibition chemicals is reduced (Agrawal et al. 2018).
In addition to above, techno-economic studies are necessary to analyze the best configuration of BRF systems; considering variety, availability, and quality of raw material (biomass), desired outflows (biobased products), type and yield of pretreatments, and co-production and adjustment stage category (separation and purification process). Finally, it should reminded that enzymes are sensible to temperature and pH; therefore, it is suggested to conserve constant thermal and pH ranges in pretreatment and hydrolysis stages or to continue investigating thermal and pH tolerance in both processes working in sequential way.
9.7 Concluding Remarks
In each stage of proposed BRF, there is flexibility to use several types of processes (chemical, physical, biological, or combined). However, biological pretreatment and hydrolysis presents a special interest for new researches due to its mild conditions and lesser energy inputs compared with chemical route. These factors could be taken into account as potential for large-scale production. Different stages of configurations (extraction, pretreatment, hydrolysis, co-production, and adjustment) and types of processes (chemical, physical, biological, and combined) in the proposed BRF approach should be further researched and investigated in order to analyze limitations for BRF efficiency improvement.
Despite its recalcitrance behavior, LC biomass remains as a significant alternative to generate biobased products such as biofuels, biopolymers, and others biochemicals. Breaking CL and HC to obtain smaller molecules (monomers) is not a stress-free activity, the specific chemical bonds in both macromolecules and its curious configuration in the cell wall leads to continuous scientific developments in this area. The LC biomass is the most abundant resource in the world and is found in several categories; agro-industrial and industrial residues are attractive to take advantage and obtain different biobased products.
Cellulases purification can be carried out by different methods; however, all of them should aim for high yield and catalytic activity. Although addition of pure cellulases in LC biomass enzymatic hydrolysis process is an important factor to obtain great conversion to glucose, LG can negatively interfere in this process.
LG creates a barrier that undermines cellulases action by non-productive binding and its derived compounds can inhibit their activity; therefore, its removal increases enzymatic hydrolysis yield. Some other strategies such as cellulases modification and addition of LG blocking additives are also known to minimize this problem. On the other hand, some compounds can enhance cellulases activities such as few metal ions, surfactants, and auxiliary enzymes such as xylanases.
The co-work multisystem of hydrolytic enzymes in pilot-scale units for hydrolysis should be more explored in order to analyze how to increase mass and heat transfer efficiency, how to reduce viscosity effects (rheology), how to decrease surface tension effects and thus improve hydrolysis performance.
Abbreviations
- BRF:
-
Biorefinery
- CL:
-
Cellulose
- DEAE:
-
Diethylaminoethyl
- EDTA:
-
Ethylenediamine tetraacetic acid
- HC:
-
Hemicelluloses
- LC:
-
Lignocellulosic
- LG:
-
Lignin
- VB:
-
Vegetal biomass
- XOS:
-
Xylooligosaccharides
References
Agrawal R, Bhadana B, Mathur AS et al (2018) Improved enzymatic hydrolysis of pilot scale pretreated Rice straw at high Total solids loading. Front Energy Res 6:115. https://doi.org/10.3389/fenrg.2018.00115
Aguilar R, Ramirez JA, Garrote G, And Vazquez M (2002) Kinetic study of the acid hydrolysis of sugar cane bagasse. J Food Eng 55(4):309–318. https://doi.org/10.1016/S0260-8774(02)00106-1
Alberts B, Bray D, Hpkin K et al (2014) Essential Cell Biology, 4th edn. Garland Science, New York
Allen J, Unlu S, Demirel Y et al (2018) Integration of biology, ecology and engineering for sustainable algal-based biofuel and bioproduct biorefinery. Bioresour Bioprocess 5:1–28. https://doi.org/10.1186/s40643-018-0233-5
Almsatar T (2020) Environmental issues of biomass-burning in sub-Saharan African countries. In: Mammino L (ed) Biomass burning in sub-Saharan Africa. Springer, Dordrecht, pp 1–14
Alonso DM, Gallo JMR, Mellmer MA, Wettstein SG, Dumesic JA (2013) Direct conversion of cellulose to levulinic acid and gamma-valerolactone using solid acid catalysts. Cat Sci Technol 3(4):927–931. https://doi.org/10.1039/C2CY20689G
Amezcua-Allieri MA, Sánchez Durán T, Aburto J (2017) Study of chemical and enzymatic hydrolysis of cellulosic material to obtain fermentable sugars. J Chem 2017:1–9. https://doi.org/10.1155/2017/5680105
An DS, Im WT, Yang HC, Yang DC, Lee ST (2005) Dyella koreensis sp. nov., a β-glucosidase-producing bacterium. Int J Syst Evol Microbiol 55(4):1625–1628. https://doi.org/10.1099/ijs.0.63695-0
Annamalai N, Sivakumar N (2016) Production of polyhydroxybutyrate from wheat bran hydrolysate using Ralstonia eutropha through microbial fermentation. J Biotechnol 237:13–17. https://doi.org/10.1016/j.jbiotec.2016.09.001
Antunes FAF, Chandel AK, Terán-Hilares R et al (2019) Overcoming challenges in lignocellulosic biomass pretreatment for second-generation (2G) sugar production: emerging role of nano, biotechnological and promising approaches. 3 Biotech 9:1–17. https://doi.org/10.1007/s13205-019-1761-1
Aro N, Pakula T, Pentillä M (2005) Transcriptional regulation of plant cell wall degradation by filamentous fungi. FMES Microbiol Rev 29(4):719–739. https://doi.org/10.1016/j.femsre.2004.11.006
Aruwajoye GS, Faloye FD, Kana EG (2020) Process optimisation of enzymatic Saccharification of soaking assisted and thermal pretreated cassava peels waste for bioethanol production. Waste and Biomass Valorization 11:2409–2420. https://doi.org/10.1007/s12649-018-00562-0
Asadollahi A, Khoobdel M, Zahraei-Ramazani A et al (2019) Effectiveness of plant-based repellents against different Anopheles species: a systematic review. Malar J 18:436. https://doi.org/10.1186/s12936-019-3064-8
Asha A, Manjunatha M, Rekha RM et al (2015) Antioxidant activities of orange peel extract in ghee (butter oil) stored at different storage temperatures. J Food Sci Technol 52:8220–8227. https://doi.org/10.1007/s13197-015-1911-3
Àzar R et al (2020) Effect of lignin content on cellulolytic saccharification of liquid hot water pretreated sugarcane bagasse. Molecules 25(3):1–12. https://doi.org/10.3390/molecules25030623
Bagewadi ZK, Mulla SI, Ninnekar HZ (2017) Optimization of endoglucanase production from Trichoderma harzianum strain HZN11 by central composite design under response surface methodology. Biomass Conv Bioref 8:305–316. https://doi.org/10.1007/s13399-017-0285-3
Bajpai P (2014a) Chapter 3 - microbial Xylanolytic systems and their properties. In: Bajpai P (ed) Xylanolytic enzymes. Academic Press, Amsterdam, pp 19–36
Bajpai P (2014b) Chapter 6 - purification of Xyanases. In: Bajpai P (ed) Xylanolytic enzymes. Academic Press, Amsterdam, pp 53–61
Balan V (2014) Current challenges in commercially producing biofuels from Lignocellulosic biomass. ISRN Biotechnol 2014:1–31. https://doi.org/10.1155/2014/463074
Barbosa AM, Giese EC, Dekker RFH, Borsato D, Pérez AIB, Iranzo JFU (2010) Extracellular β-glucosidase production by the yeast Debaryomyces pseudopolymorphus UCLM-NS7A: optimization using response surface methodology. New Biotechnol 27(4):374–381. https://doi.org/10.1016/j.nbt.2010.05.013
Barbosa FC, Silvello MA, Goldbeck R (2020) Cellulase and oxidative enzymes: new approaches, challenges and perspectives on cellulose degradation for bioethanol production. Biotechnol Lett 42:875–884. https://doi.org/10.1007/s10529-020-02875-4
Bar-On YM, Phillips R, Milo R (2018) The biomass distribution on earth. Proc Natl Acad Sci 115:6506–6511. https://doi.org/10.1073/pnas.1711842115
Batra J, Beri D, Mishra S (2014) Response surface methodology based optimization of β-Glucosidase production from Pichia pastoris. App Biochem Biotechnol 172:380–393. https://doi.org/10.1007/s12010-013-0519-1
Bauer MMT, Schnapp G (2007) Protein production for three-dimensional structural analysis. In: Taylor JB, Triggle DJ (eds) Comprehensive medicinal chemistry II, 2nd edn. Elsevier, Amsterdam, pp 411–432
Beg Q, Kapoor M, Mahajan L, Hoondal G (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338. https://doi.org/10.1007/s002530100704
Begun F, Absar N (2009) Purification and characterization of intracellular cellulase from Aspergillus oryzae ITCC-4857.01. Mycobiology 37(2):121–127. https://doi.org/10.4489/MYCO.2009.37.2.121
Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry. W H Freeman, New York
Bhardwaj N, Kumar B, Verma P (2019) A detailed overview of xylanases: an emerging biomolecule for current and future prospective. Bioresour Bioprocess 6:1–36. https://doi.org/10.1186/s40643-019-0276-2
Bichot A, Delgenès J-P, Méchin V et al (2018) Understanding biomass recalcitrance in grasses for their efficient utilization as biorefinery feedstock. Rev Environ Sci Bio/Technology 17:707–748. https://doi.org/10.1007/s11157-018-9485-y
Bosetto A, Justo PI, Zanardi B et al (2016) Research Progress concerning fungal and bacterial β-Xylosidases. Appl Biochem Biotechnol 178:766–795. https://doi.org/10.1007/s12010-015-1908-4
Brienzo M, Siqueira AF, Milagres AMF (2009) Search for optimum conditions of sugarcane bagasse hemicellulose extraction. Biochem Eng J 46(2):199–204. https://doi.org/10.1016/j.bej.2009.05.012
Brienzo M, Ferreira S, Vicentim MP, De Souza W, Sant’anna C (2014) Comparison study on the biomass recalcitrance of different tissue fractions of sugarcane culm. BioEnergy Res 7(4):1454–1465. https://doi.org/10.1007/s12155-014-9487-8
Brienzo M, Fikizolo S, Benjamin Y, Tyhoda L, Görgens J (2017) Influence of pretreatment severity on structural changes, lignin content and enzymatic hydrolysis of sugarcane bagasse samples. Renew Energy 104:271–280. https://doi.org/10.1016/j.renene.2016.12.037
British Petroleum (2019) BP statistical review of world energy. Pureprint Group Limited, London
Campen SA et al (2017) Expression of naturally ionic liquid-tolerant thermophilic cellulases in Aspergillus Niger. PLoS One 12(12):1–15. https://doi.org/10.1371/journal.pone.0189604
Castro AM, Pereira N (2010) Production, properties and application of cellulases in the hydrolysis of agroindustrial residues. Quím Nova 33(1):181–188. https://doi.org/10.1590/S0100-40422010000100031
Chahed H et al (2018) Heterologous expression and biochemical characterization of a novel thermostable Sclerotinia sclerotiorum GH45 endoglucanase in Pichia pastoris. Int J Biol Macromol 106:629–635. https://doi.org/10.1016/j.ijbiomac.2017.08.062
Chakdar H, Kumar M, Pandiyan K et al (2016) Bacterial xylanases: biology to biotechnology. 3 Biotech 6:1–15. https://doi.org/10.1007/s13205-016-0457-z
Chandra M, Madakka M (2019) Comparative biochemistry and kinetics of microbial lignocellulolytic enzymes. In: Buddolla V (ed) Recent developments in applied microbiology and Biochemistry. Academic Press, Amsterdam, pp 147–159
Chandra RP, Esteghlalian AR, Saddler JN (2008) Assessing substrate accessibility to enzymatic hydrolysis by cellulases. In: Characterization of lignocellulosic materials. Wiley, Hoboken, pp 60–80
Chellapandi P, Jani HM (2008) Production of endoglucanase by the native strains of Streptomyces isolates in submerged fermentation. Brazilian J Microb 39(1):122–127. https://doi.org/10.1590/S1517-83822008000100026
Chen H-Z, Liu Z-H (2017) Enzymatic hydrolysis of lignocellulosic biomass from low to high solids loading. Eng Life Sci 17:489–499. https://doi.org/10.1002/elsc.201600102
Chen JH, Wang K, Xu F, Sun RC (2015) Effect of hemicellulose removal on the structural and mechanical properties of regenerated fibers from bamboo. Cellulose 22(1):63–72. https://doi.org/10.1007/s10570-014-0488-8
Chen B, Luo Z, Chen H et al (2020) Wood plastic composites from the waste Lignocellulosic biomass fibers of bio-fuels processes: a comparative study on mechanical properties and weathering effects. Waste and Biomass Valorization 11:1701–1710. https://doi.org/10.1007/s12649-018-0413-8
Coral Medina JD, Woiciechowski A, Zandona Filho A et al (2015) Lignin preparation from oil palm empty fruit bunches by sequential acid/alkaline treatment – a biorefinery approach. Bioresour Technol 194:172–178. https://doi.org/10.1016/J.BIORTECH.2015.07.018
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6(11):850–861. https://doi.org/10.1038/nrm1746
Creuzet N, Berenger JF, Frixon C (1983) Characterization of exoglucanase and synergistic hydrolysis of cellulose in Clostridium stercorarium. FEMS Microbiol Lett 20(3):347–350. https://doi.org/10.1111/j.1574-6968.1983.tb00145.x
Crowe JD, Zarger RA, Hodge DB (2017) Relating nanoscale accessibility within plant cell walls to improved enzyme hydrolysis yields in corn Stover subjected to diverse pretreatments. J Agric Food Chem 65(39):8652–8662. https://doi.org/10.1021/acs.jafc.7b03240
Cypriano ZD, Da Silva LL, Bohórquez AM, Ljubica T (2017) A Biomassa da Laranja e seus Subprodutos. Rev Virtual Química 9:176–191. https://doi.org/10.21577/1984-6835.20170014
Da Silva ASA, Teixeira RSS, De Oliveira Moutta R, Ferreira-Leitão VS, De Barros RD, Ferrara MA, Da Silva Bom EP (2013) Sugarcane and woody biomass pretreatments for ethanol production. In: Sustainable degradation of lignocellulosic biomass-techniques, applications and commercialization. Intechopen, London, pp 47–88
Da Silva AS, Espinheira RP, Teixeira RSS et al (2020) Constraints and advances in high-solids enzymatic hydrolysis of lignocellulosic biomass: a critical review. Biotechnol Biofuels 13:1–28. https://doi.org/10.1186/s13068-020-01697-w
Dashnyam P, Mudududdla R, Hsieh T-J et al (2018) β-Glucuronidases of opportunistic bacteria are the major contributors to xenobiotic-induced toxicity in the gut. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-34678-z
Dheeran P, Nandhagopal N, Kumar S, Jaiswal YK, Adhikari DK (2012) A novel thermostable xylanase of Paenibacillus macerans IIPSP3 isolated from the termite gut. J Ind Microbiol Biotechnol 39(6):851–860. https://doi.org/10.1007/s10295-012-1093-1
Dimaragona M, Topakas E (2016) Regulation and heterologous expression of lignocellulosic enzymes in Aspergillus. In: New and future developments in microbial biotechnology and bioengineering. Elsevier, pp 171–190
Du B, Sharma LN, Becker C, Chen SF, Mowery RA, Van Walsum GP, Chambliss CK (2010) Effect of varying feedstock–pretreatment chemistry combinations on the formation and accumulation of potentially inhibitory degradation products in biomass hydrolysates. Biotechnol Bioeng 107(3):430–440. https://doi.org/10.1002/bit.22829
Duedu KO, French CE (2016) Characterization of a Cellulomonas fimi exoglucanase/xylanase-endoglucanase gene fusion which improves microbial degradation of cellulosic biomass. Enz Microb Technol 93–94:113–121. https://doi.org/10.1016/j.enzmictec.2016.08.005
Dulie NW, Woldeyes B, Demsash HD, Jabasingh AS (2020) An insight into the valorization of hemicellulose fraction of biomass into furfural: catalytic conversion and product separation. Waste Biomass Valoriz. https://doi.org/10.1007/s12649-020-00946-1
Duong-Ly KC, Gabelli SB (2014) Salting out of proteins using ammonium sulfate precipitation. Methods Enzymol 541:85–94. https://doi.org/10.1016/B978-0-12-420119-4.00007-0
Ebringerová A (2006) Structural diversity and application potential of hemicelluloses. Macromol Symp 232:1–12. https://doi.org/10.1002/masy.200551401
Ek R, Alderborn G, Nyström C (1994) Particle analysis of microcrystalline cellulose: differentiation between individual particles and their agglomerates. Int J Pharm 111(1):43–50. https://doi.org/10.1016/0378-5173(94)90400-6
Elshafei AM, Hussein F, El-Mahalawy A, Kahil T, Mostafa EM, Abd-Eltawab B (2014) Upgrading of Exoglucanase production by Trichoderma reesei NRC 210. Int J Eng Res Develop 10(8):68–73
Ersson B, Rydén L, Janson JC (2011) Introduction to protein purification. In: Janson JC (ed) Protein purification: principles, high resolution methods and applications, 3rd edn. Wiley, Hoboken, pp 3–22
Espinar MF, Piñaga F, Graaff L, Visser J, Ramón D, Vallés S (1994) Purification, characterization and regulation of the synthesis of an Aspergillus nidulans acidic xylanase. Biochem Eng 42:555–562. https://doi.org/10.1007/BF00173920
Evangelista D, Suttnar J (1997) Separation used for purification of recombinant proteins. J Chromatogr B Biomed Sci Appl 699(1–2):383–401. https://doi.org/10.1016/s0378-4347(97)00201-6
Excoffier G, Toussaint B, Vignon MR (1991) Saccharification of steam-exploded poplar wood. Biotechnol Bioeng 38(11):1308–1317. https://doi.org/10.1002/bit.260381108
Farinas CS et al (2011) Evaluation of operational parameters on the precipitation of endoglucanase and xylanase produced by solid state fermentation of Aspergillus Niger. Braz J Chem Eng 28(1):17–26. https://doi.org/10.1590/S0104-66322011000100003
Fauth U, Romaniec MPM, Kobayashi T, Demain AL (1991) Purification and characterization of endoglucanase Ss from Clostridium thermocellum. The Biochem J 279(1):67–73. https://doi.org/10.1042/bj2790067
Fu L-H, Jiang N, Li C-X et al (2019) Purification and characterization of an endo-xylanase from Trichoderma sp., with xylobiose as the main product from xylan hydrolysis. World J Microbiol Biotechnol 35:171. https://doi.org/10.1007/s11274-019-2747-1
Gandini A, Belgacem MN (2008) Chapter 1 - the state of the art. In: Belgacem MN, Gandini Polymers and Composites from Renewable Resources ABT-M (eds) Monomers, polymers and composites from renewable resources. Elsevier, Amsterdam, pp 1–16
Gaspar A, Cosson T, Roque C, Thonart P (1997) Study on the production of a xylanolytic complex from Penicillium canescens 10-10c. Appl Biochem Biotechnol 67:45–67. https://doi.org/10.1007/BF02787840
Gaur R, Tiwari S (2015) Isolation, production, purification and characterization of an organic-solvent-thermostable alkalophilic cellulase from Bacillus vallismortis RG-07. BMC Biotechnol 15(19):1–12. https://doi.org/10.1186/s12896-015-0129-9
Geankoplis C (1993) Transport processes and unit operations, 3rd edn. Prentice-Hall International, Englewood Cliffs
Genc B et al (2014) Purification and characterization of an extracellular cellulase from Anoxybacillus gonensis O9 isolated from geothermal area. J Environ Biol 36(6):1319–1324
General Electric Healthcare (2010) Strategies for protein purification handbook, 1st edn. General Electric Healthcare, Uppsala
George J, Sabapathi SN (2015) Cellulose nanocrystals: synthesis, functional properties, and applications. Nanotechnol Sci Appl 8:45–54. https://doi.org/10.2147/NSA.S64386
Goel N et al (2019) Purification and characterization of cellulase from Pseudomonas sp. isolated from waste dumping site soil. Journal of Applied Biotechnol Bioeng 6(3):118–124
Goswami GK, Pathak RR (2013) Microbial xylanases and their biomedical applications: a review. Int J Basic Clin Pharmacol 2(3):237–246. https://doi.org/10.5455/2319-2003.ijbcp20130602
Goufo ED, Mugisha S (2018) Complex harmonic poles in the evolution of macromolecules depolymerization. J Comput Anal Appl 25(8):1490–1503. https://bit.ly/3ebUGnK
Guedes F, Szklo A, Rochedo P et al (2019) Climate-energy-water nexus in Brazilian oil refineries. Int J Greenh Gas Control 90:1–11. https://doi.org/10.1016/j.ijggc.2019.102815
Hamdan NT, Jasim HM (2018) Purification and characterization of cellulase enzyme from Trichoderma longibrachiatum isolated in Iraqi soil. J Biotechnol Biochem 4(1):32–41. https://doi.org/10.1080/17597269.2017.1345357
Hanif M, Zaman M (2017) Thiolation of arabinoxylan and its application in the fabrication of controlled release mucoadhesive oral films. DARU J Pharm Sci 25(6):1–13. https://doi.org/10.1186/s40199-017-0172-2
Harmoko C, Sucipto KI, Ery Retnoningtyas S, Hartono SB (2016) Vinyl functionalized cubic mesoporous silica nanoparticles as supporting material to enhance cellulase enzyme stability. ARPN Journal of Engineering and Applied Sciences 11(5):2981–2992. http://www.arpnjournals.org/jeas/research_papers/rp_2016/jeas_0316_3747.pdf
Hendriks AT, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100(1):10–18. https://doi.org/10.1016/j.biortech.2008.05.027
Herrera A, Téllez-Luis SJ, Ramirez JA, Vázquez M (2003) Production of xylose from sorghum straw using hydrochloric acid. J Cereal Sci 37(3):267–274. https://doi.org/10.1006/jcrs.2002.0510
Hideno A, Inoue H, Tsukahara K, Fujimoto S, Minowa T, Inoue S, Sawayama S (2009) Wet disk milling pretreatment without sulfuric acid for enzymatic hydrolysis of rice straw. Bioresour Technol 100(10):2706–2711. https://doi.org/10.1016/j.biortech.2008.12.057
Hirasawa K, Uchimura K, Kashiwa M, Grant WD, Ito S, Kobayashi T (2006) Salt-activated endoglucanase of a strain of alkaliphilic Bacillus agaradhaerens. Antonie Van Leeuwenhoek 89:211–219. https://doi.org/10.1007/s10482-005-9023-0
Hirunsupachote S, Chavalparit O (2019) Predicting the Biomethanation potential of some Lignocellulosic Feedstocks using linear regression models: the effect of pretreatment. KSCE J Civ Eng 23:1501–1512. https://doi.org/10.1007/s12205-019-1589-6
Hoegh-Guldberg O, Jacob D, Taylor M (2018) Impacts of 1.5°C of global warming on natural and human systems. In: Marengo JA, Pereira J, Sherstyukov B (eds) Global warming of 1.5°C. United Nations Environment Programme and World Meteorological Organization, Geneva, pp 175–311
Holwerda EK, Worthen RS, Kothari N et al (2019) Multiple levers for overcoming the recalcitrance of lignocellulosic biomass. Biotechnol Biofuels 12:15. https://doi.org/10.1186/s13068-019-1353-7
Hsieh CC, Cannella D, Jørgensen H et al (2014) Cellulase inhibition by high concentrations of Monosaccharides. J Agric Food Chem 62:3800–3805. https://doi.org/10.1021/jf5012962
Hu J, Arantes V, Saddler JN (2011) The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanases? Is it an additive or synergistic effect? Biotechnol Biofuels 4(36):1–13. https://doi.org/10.1186/1754-6834-4-36
Hu B, Zhu S, Fang S et al (2016) Optimization and scale-up of enzymatic hydrolysis of wood pulp for cellulosic sugar production. Bioresources 11:7242–7257. https://doi.org/10.15376/biores.11.3.7242-7257
Huang R, Su R, Qi W, He Z (2010) Understanding the key factors for enzymatic conversion of pretreated lignocellulose by partial least square analysis. Biotechnol Prog 26(2):384–392. https://doi.org/10.1002/btpr.324
Irfan M, Nadeem M, Syed Q (2012) Influence of nutritional conditions for Endoglucanase production by Trichoderma viride in SSF. Global J Biotech Biochem 7(1):7–12. https://doi.org/10.5829/idosi.gjbb.2012.7.1.6210
Islam F, Roy N (2018) Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC Res Notes 11:445): 1–445): 6. https://doi.org/10.1186/s13104-018-3558-4
Jahnavi G, Prashanthi GS, Sravanthi K, Rao LV (2017) Status of availability of lignocellulosic feed stocks in India: biotechnological strategies involved in the production of bioethanol. Renew Sust Energ Rev 73:798–820. https://doi.org/10.1016/j.rser.2017.02.018
Jha A, Kumar A (2019) Biobased technologies for the efficient extraction of biopolymers from waste biomass. Bioprocess Biosyst Eng 42:1893–1901. https://doi.org/10.1007/s00449-019-02199-2
Juhász T, Egyházi A, Réczey K (2005) β-Glucosidase production by Trichoderma reesei. In: Davidson B (ed) Applied biochemical and biotechnology. Springer, Berlin, pp 243–254
Jungles TMC, Iacomini M, Cipriani TR, Cordeiro LMC (2017) Isolation and characterization of a xylan with industrial and biomedical applications from edible açaí berries (Euterpe oleraceae). Food Chem 221:1595–1597. https://doi.org/10.1016/j.foodchem.2016.10.133
Kadić A (2017) The effects of mixing on the enzymatic hydrolysis of lignocellulosic biomass. Lund University, Lund
Kalogeris E, Christakopoulos P, Vršanská M, Biely P, Macris BJ (2001) Catalytic properties of the endoxylanase I from Thermoascus aurantiacus. J Mol Catal B Enzym 11(4–6):491–501. https://doi.org/10.1016/S1381-1177(00)00178-8
Karimi K, Taherzadeh MJ (2016) A critical review on analysis in pretreatment of lignocelluloses: degree of polymerization, adsorption/desorption, and accessibility. Bioresour Technol 203:348–356. https://doi.org/10.1016/j.biortech.2015.12.035
Khosravi C, Benocci T, Battaglia E et al (2015) Chapter one - sugar catabolism in Aspergillus and other Fungi related to the utilization of plant biomass. In: Sariaslani S, Gadd G (eds) Advances in applied microbiology. Academic Press, Amsterdam, pp 1–28
Kim HB, Park MJ, Yanh HC, An DS, Jin HZ, Yang DC (2006) Burkholderia ginsengisoli sp. nov., a β-glucosidase-producing bacterium isolated from soil of a ginseng field. Int J Syst Evolut Microb 56(11):2529–2533. https://doi.org/10.1099/ijs.0.64387-0
Kim MK, Sathiyaraj S, Pulla RK, Yang DC (2009) Brevibacillus panacihumi sp. nov., a β-glucosidase-producing bacterium. Int J Syst Evol Microbiol 59(5):1227–1231. https://doi.org/10.1099/ijs.0.001248-0
Kim HS, Oh YH, Jang Y-A et al (2016) Recombinant Ralstonia eutropha engineered to utilize xylose and its use for the production of poly(3-hydroxybutyrate) from sunflower stalk hydrolysate solution. Microb Cell Factories 15:95. https://doi.org/10.1186/s12934-016-0495-6
Kirchner OG, Granados MS, Pascual PS (2005) Effect of media composition and growth conditions on production of β-Glucosidase by Aspergillus niger C-6. In: Davinson B (ed) Applied biochemistry and biotechnology. Springer, Berlin, pp 347–359
Kiss AA (2014) Reactive separation processes BT - process intensification Technologies for Biodiesel Production: reactive separation processes. In: Kiss AA (ed) Process intensification Technologies for Biodiesel Production. Springer, Cham, pp 25–33
Kotaka A, Bando H, Kaya M, Murai MK, Kuroda K, Sahara H, Hata Y, Kondo A, Ueda M (2008) Direct ethanol production from barley β-glucan by sake yeast displaying Aspergillus oryzae β-glucosidase and endoglucanase. J Biosci Bioeng 105(6):622–627. https://doi.org/10.1263/jbb.105.622
Kucharska K, Słupek E, Cieśliński H, Kamiński M (2020) Advantageous conditions of saccharification of lignocellulosic biomass for biofuels generation via fermentation processes. Chem Pap 74:1199–1209. https://doi.org/10.1007/s11696-019-00960-1
Kumar R, Mago G, Balan V, Wyman CE (2009) Physical and chemical characterizations of corn Stover and poplar solids resulting from leading pretreatment technologies. Bioresour Technol 100(17):3948–3962. https://doi.org/10.1016/j.biortech.2009.01.075
Kumar L et al (2012) The lignin present in steam pretreated softwood binds enzymes and limits cellulose accessibility. Bioresour Technol 103(1):201–208. https://doi.org/10.1016/j.biortech.2011.09.091
Kumar V, Satyanarayana T (2013) Biochemical and thermodynamic characteristics of thermoalkali-stable xylanase from a novel polyextremophilic Bacillus halodurans TSEV1. Extremophiles 17(5):797–808. https://doi.org/10.1007/s10529-011-0698-1
Kumar A, Nakachew M, Yilkal B, Yadav M (2018) A review of factors affecting enzymatic hydrolysis of pretreated lignocellulosic biomass. Res J Chem Environ 22:62–67
Leu SY, Zhu JY (2013) Substrate-related factors affecting enzymatic saccharification of lignocelluloses: our recent understanding. BioEnergy Res 6(2):405–415. https://doi.org/10.1007/s12155-012-9276-1
Li C, Kumar S (2016) Preparation of activated carbon from un-hydrolyzed biomass residue. Biomass Convers Biorefinery 6:407–419. https://doi.org/10.1007/s13399-016-0197-7
Lian ZQ, Srinivasan S, Kim YJ, Kim HB, Wang HT, Yang DC (2011) Lactobacillus kimchicus sp. nov., a β-glucosidase-producing bacterium isolated from kimchi. Int J Syst Evolut Microb 61(4):894–897. https://doi.org/10.1099/ijs.0.017418-0
Lin Z, Huang H, Zhang H, Zhang L, Yan L, Chen J (2010) Ball milling pretreatment of corn Stover for enhancing the efficiency of enzymatic hydrolysis. Appl Biochem Biotechnol 162(7):1872–1880. https://doi.org/10.1007/s12010-010-8965-5
Liu W, Shi P, Chen Q, Yang P, Wang G, Wang Y, Luo H, Yao B (2010) Gene cloning, overexpression, and characterization of a xylanase from Penicillium sp. CGMCC 1669. Appl Biochem Biotechnol 162(1):1–12. https://doi.org/10.1007/s12010-009-8719-4
Loow Y-L, Wu TY, Jahim JM et al (2016) Typical conversion of lignocellulosic biomass into reducing sugars using dilute acid hydrolysis and alkaline pretreatment. Cellulose 23:1491–1520. https://doi.org/10.1007/s10570-016-0936-8
Lopes AM, Ferreira Filho EX, Moreira LRS (2018) An update on enzymatic cocktails for lignocellulose breakdown. J Appl Microbiol 125:632–645. https://doi.org/10.1111/jam.13923
Luo X, Zhu JY (2011) Effects of drying-induced fiber hornification on enzymatic saccharification of lignocelluloses. Enzym Microb Technol 48(1):92–99. https://doi.org/10.1016/j.enzmictec.2010.09.014
Mahmood RT, Asad MJ, Mehboob N, Mushtaq M, Gulfraz M, Asgher M, Minhas MN, Hadri SH (2013) Production, purification, and characterization of exoglucanase by Aspergillus fumigatus. Appl Biochem Biotechnol 170:895–908. https://doi.org/10.1007/s12010-013-0227-x
Mahmoodi S et al (2019) Current affinity approaches for purification of recombinant proteins. Cog Biol 5(1):1–18. https://doi.org/10.1080/23312025.2019.1665406
Malgas S, Mafa MS, Mkabayi L, Pletschke BI (2019) A mini review of xylanolytic enzymes with regards to their synergistic interactions during hetero-xylan degradation. World J Microbiol Biotechnol 35:187. https://doi.org/10.1007/s11274-019-2765-z
Manavalan T, Manavalan A, Heese K (2015) Characterization of Lignocellulolytic enzymes from white-rot Fungi. Curr Microbiol 70:485–498. https://doi.org/10.1007/s00284-014-0743-0
Martín C, Galbe M, Nilvebrant NO, Jönsson LJ (2002) Comparison of the fermentability of enzymatic hydrolyzates of sugarcane bagasse pretreated by steam explosion using different impregnating agents. Appl Biochem Biotechnol 98(100):699–716. https://doi.org/10.1385/abab:98-100:1-9:699
Mathew S, Abraham TE (2004) Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit Rev Biotechnol 24:59–83. https://doi.org/10.1080/07388550490491467
McCabe W, Smith J, Harriot P (2007) Operaciones Unitarias en Ingeniería Química, 7th edn. McGraw-Hill, Mexico City
Medina JDC, Woiciechowski A, Filho AZ et al (2016) Steam explosion pretreatment of oil palm empty fruit bunches (EFB) using autocatalytic hydrolysis: a biorefinery approach. Bioresour Technol 199:173–180. https://doi.org/10.1016/j.biortech.2015.08.126
Megha SV et al (2015) Isolation and purification of cellulase. Int J of Sci Nat 6(3):474–479
Melati RB, Shimizu FL, Oliveira G, Pagnocca FC, De Souza W, Sant’Anna C, Brienzo M (2019) Key factors affecting the recalcitrance and conversion process of biomass. BioEnergy Res 12:1–20. https://doi.org/10.1007/s12155-018-9941-0
Meng X, Pu Y, Yoo CG, Li M, Bali G, Park DY, Tschaplinski TJ (2017) An in-depth understanding of biomass recalcitrance using natural poplar variants as the feedstock. ChemSusChem 10(1):139–150. https://doi.org/10.1002/cssc.201601303
Mishra C, Forrester IT, Kelley BD, Burguess RR, Leatham GF (1990) Characterization of a major xylanase purified from Lentinula edodes cultures grown on a commercial solid lignocellulosic substrate. Appl Microbiol Biotechnol 33(2):226–232. https://doi.org/10.1007/BF00176530
Mesa L, González E, Cara C et al (2016) An approach to cellulase recovery from enzymatic hydrolysis of pretreated sugarcane bagasse with high lignin content. Biocatal Biotransformation 33:287–297. https://doi.org/10.3109/10242422.2016.1168816
Monte JR, Brienzo M, Milagres AMF (2011) Utilization of pineapple stem juice to enhance enzyme-hydrolytic efficiency for sugarcane bagasse after an optimized pre-treatment with alkaline peroxide. Appl Energy 88(1):403–408. https://doi.org/10.1016/j.apenergy.2010.08.009
Mooney CA, Mansfield SD, Touhy MG, Saddler JN (1989) The effect of initial pore volume and lignin content on the enzymatic hydrolysis of softwoods. Bioresour Technol 64(2):113–119. https://doi.org/10.1016/S0960-8524(97)00181-8
Mukherjee S, Karmakar M, Ray RR (2011) Production of extra cellular Exoglucanase by Rhizopus oryzae from submerged fermentation of agro wastes. Rec Res Sci Technol 3(3):69–75
Murphy L, Bohlin C, Baumann MJ et al (2013) Product inhibition of five Hypocrea jecorina cellulases. Enzym Microb Technol 52:163–169. https://doi.org/10.1016/j.enzmictec.2013.01.002
Naika GS, Tiku PK (2011) Influence of ethyleneaminetetraacetic acid (EDTA) on the structural stability of endoglucanase from Aspergillus aculeatus. J Agric Food Chem 59:7341–7345. https://doi.org/10.1021/jf103889m
Nanda S, Azargohar R, Kozinski JA, Dalai AK (2014) Characteristic studies on the pyrolysis products from hydrolyzed Canadian Lignocellulosic Feedstocks. BioEnergy Res 7:174–191. https://doi.org/10.1007/s12155-013-9359-7
Narra M, Dixit G, Divecha J, Kumar K, Madamwar D, Shah AR (2014) Production, purification and characterization of a novel GH 12 family endoglucanase from Aspergillus terreus and its application in enzymatic degradation of delignified rice straw. Internat Biodeter Biodegrad 88:150–161. https://doi.org/10.1016/j.ibiod.2013.12.016
Ni J, Takehara M, Watanabe H (2005) Heterologous overexpression of a mutant termite cellulase gene in Escherichia coli by DNA shuffling of four orthologous parental cDNAs. Biosci, Biotech and Bioch 69(9):1711–1720. https://doi.org/10.1271/bbb.69.1711
Nordwald E et al (2014) Charge engineering of cellulases improves ionic liquid tolerance and reduces lignin inhibition. Biotechnol Bioeng 111(8):1541–1549. https://doi.org/10.1002/bit.25216
Nurul Adela B, Loh SK, Nasrin AB (2015) The improvement of enzymatic hydrolysis of oil palm (Elaeis guineensis) empty fruit bunch lignocellulose. Malays App Biol 44(1):95–100
Öhgren K, Galbe M, Zacchi G (2005) Optimization of steam pretreatment of SO2-impregnated corn Stover for fuel ethanol production. In: Twenty-sixth symposium on biotechnology for fuels and chemicals. Humana Press, Totowa, pp 1055–1067
Okafor UA, Okochi VI, Okerenta OBM, Chinedu NS (2007) Xylanase production by Aspergillus niger ANL 301 using agro-wastes. Afr J Biotechnol 6(14):1710–1714
Onoja E, Chandren S, Abdul Razak FI et al (2018) Oil palm (Elaeis guineensis) biomass in Malaysia: the present and future prospects. Waste and Biomass Valorization 10:2099–2117. https://doi.org/10.1007/s12649-018-0258-1
Ortiz L, Dorta E, Gloria Lobo M et al (2017) Use of Banana (Musa acuminata Colla AAA) Peel extract as an antioxidant source in Orange juices. Plant Foods Hum Nutr 72:60–66. https://doi.org/10.1007/s11130-016-0591-0
Özsin G, Pütün AE, Pütün E (2019) Investigating the interactions between lignocellulosic biomass and synthetic polymers during co-pyrolysis by simultaneous thermal and spectroscopic methods. Biomass Convers Biorefinery 9:593–608. https://doi.org/10.1007/s13399-019-00390-9
Park MJ, Kim MK, Kim HB, Im WT, Yi TH, Kim SY, SOung NK, Yang DC (2008) Microbacterium ginsengisoli sp. nov., a β-glucosidase-producing bacterium isolated from soil of a ginseng field. Int J Syst Evolut Microb 58(2):429–433. https://doi.org/10.1099/ijs.0.65226-0
Pawar PM-A, Derba-Maceluch M, Chong S-L et al (2016) Expression of fungal acetyl xylan esterase in Arabidopsis thaliana improves saccharification of stem lignocellulose. Plant Biotechnol J 14:387–397. https://doi.org/10.1111/pbi.12393
Pereira JC et al (2017) Effect of metal ions, chemical agents and organic compounds on lignocellulolytic enzymes activities. In: Senturk M (ed) Enzyme inhibitors and activators. Intechopen, London, pp 139–164
Pervez S, Aman A, Iqbal S et al (2014) Saccharification and liquefaction of cassava starch: an alternative source for the production of bioethanol using amylolytic enzymes by double fermentation process. BMC Biotechnol 14:1–10. https://doi.org/10.1186/1472-6750-14-49
Pina AS, Lowe CR, Roque AC (2014) Challenges and opportunities in the purification of recombinant tagged proteins. Biotechnol Adv 32(2):366–381. https://doi.org/10.1016/j.biotechadv.2013.12.001
Pino M, Rodríguez-Jasso RM, Michelin M, Flores-Gallegos AC, Morales-Rodriguez R, Teixeira JA, Ruiz HA (2018) Bioreactor design for enzymatic hydrolysis of biomass under the biorefinery concept. Chem Eng J 347:119–136. https://doi.org/10.1016/j.cej.2018.04.057
Pocan P, Bahcegul E, Oztop MH, Hamamci H (2018) Enzymatic hydrolysis of fruit peels and other Lignocellulosic biomass as a source of sugar. Waste and Biomass Valorization 9:929–937. https://doi.org/10.1007/s12649-017-9875-3
Puchart V, Fraňová L, Mørkeberg Krogh KBR et al (2018) Action of different types of endoxylanases on eucalyptus xylan in situ. Appl Microbiol Biotechnol 102:1725–1736. https://doi.org/10.1007/s00253-017-8722-6
Qin L et al (2016) Inhibition of lignin-derived phenolic compounds to cellulase. Biotechnol Biofuels 9(1):1–10. https://doi.org/10.1186/s13068-016-0485-2
Radionova NA, Dubovaya NV, Eneiskaya EV, Martinovich LI, Gracheva IM, Bezborodov AM (2000) Purification and characteristic of endo-(1-4)-β-xylanase from Geotrichum candidum 3C. Appl Biochem Microbiol 36(5):460–465
Ramani G, Meera B, Vanitha C, Rao M, Gunasekaran P (2012) Production, purification, and characterization of a β-Glucosidase of Penicillium funiculosum NCL1. App Biochem Biotechnol 167:959–972. https://doi.org/10.1007/s12010-012-9645-4
Raza A et al (2020) Identification and functional characterization of a β-glucosidase from Bacillus tequelensis BD69 expressed in bacterial and yeast heterologous systems. PeerJ 8:e8792. https://doi.org/10.7717/peerj.8792
Razeq FM, Jurak E, Stogios PJ et al (2018) A novel acetyl xylan esterase enabling complete deacetylation of substituted xylans. Biotechnol Biofuels 11:1–12. https://doi.org/10.1186/s13068-018-1074-3
Reddy SS, Krishnan C (2016) Production of high-pure xylooligosaccharides from sugarcane bagasse using crude β-xylosidase-free xylanase of Bacillus subtilis KCX006 and their bifidogenic function. LWT Food Sci Technol 65:237–245. https://doi.org/10.1016/j.lwt.2015.08.013
Rentizelas AA (2016) 6 - biomass storage. In: Holm-Nielsen JB, Ehimen EA (eds) Biomass supply chains for bioenergy and biorefining. Woodhead Publishing, Amsterdam, pp 127–146
Reverbel-Leroy C et al (1997) The processive endocellulase CelF, a major component of the Clostridium cellulolyticum cellulosome: purification and characterization of the recombinant form. J Bacteriol 179(1):46–52. https://doi.org/10.1128/jb.179.1.46-52.1997
Revilla G, Zarra I (2013) Fisiología vegetal. Introducción a las células de las plantas: membranas y pared. In: Azcón-Bieto J, Talón M (eds) Fundamentos de Fisiología Vegetal, 2nd edn. McGraw-Hill, Madrid, p 651
Romaniec MP, Fauth U, Kobayashi T, Huskisson NS, Barker PJ, Demain AJ (1992) Purification and characterization of a new endoglucanase from Clostridium thermocellum. Biochem J 283(1):69–73. https://doi.org/10.1042/bj2830069
Rosales-Calderon O, Arantes V (2019) A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol Biofuels 12:240. https://doi.org/10.1186/s13068-019-1529-1
Rosen Y, Mamane H, Gerchman Y (2019) Short ozonation of Lignocellulosic waste as energetically favorable pretreatment. BioEnergy Res 12:292–301. https://doi.org/10.1007/s12155-019-9962-3
Ryan SE, Nolan K, Thompson R, Gubitz GM, Savage AV, Tuohya MG (2003) Purification and characterization of a new low molecular weight endoxylanase from Penicillium capsulatum. Enzym Microb Technol 33(6):775–785. https://doi.org/10.1016/S0141-0229(03)00176-5
Saini JK et al (2016) Cellulase adsorption on lignin: a roadblock for economic hydrolysis of biomass. Renew Energy 98:29–42. https://doi.org/10.1016/j.renene.2016.03.089
Schmatz AA, Tyhoda L, Brienzo M (2020) Sugarcane biomass conversion influenced by lignin. Biofuels Bioprod Biorefin 14(2):469–480. https://doi.org/10.1002/bbb.2070
Seader JD, Henley E, Roper K (2011) Separation process principles chemical and biochemical operations, 3rd edn. Wiley, Hoboken
Seidel ZP, Lee CT (2020) Enhanced activity of the cellulase enzyme β-glucosidase upon addition of an azobenzene-based surfactant. ACS Sustain Chem Eng 8(4):1751–1761. https://doi.org/10.1021/acssuschemeng.9b05240
Selig MJ, Viamajala S, Decker SR, Tucker MP, Himmel ME, Vinzant TB (2007) Deposition of lignin droplets produced during dilute acid pretreatment of maize stems retards enzymatic hydrolysis of cellulose. Biotechnol Prog 23(6):1333–1339. https://doi.org/10.1021/bp0702018
Shahzadi T, Anwar Z, Iqbal Z, Anjum A, Aqil T, Bakhtawar AA, Kamran M, Mehmood S, Irshad M (2014) Induced production of Exoglucanase, and β-Glucosidase from fungal co-culture of T. viride and G. lucidum. Adv Biosci Biotechnol 5(5):426–433. https://doi.org/10.4236/abb.2014.55051
Sharma HK, Xu C, Qin W (2019) Biological pretreatment of Lignocellulosic biomass for biofuels and bioproducts: An overview. Waste and Biomass Valorization 10:235–251. https://doi.org/10.1007/s12649-017-0059-y
Sheldon R (2014) Green and sustainable manufacture of chemicals from biomass: state of the art. Green Chem 16(3):950–963. https://doi.org/10.1039/C3GC41935E
Shi R et al (2013) Heterologous expression and characterization of a novel thermo-halotolerant endoglucanase Cel 5H from Dictyoglomus thermophilum. Bioresour Technol 142:338–344. https://doi.org/10.1016/j.biortech.2013.05.037
Shimizu FL, de Azevedo GO, Coelho LF, Pagnocca FC, Brienzo M (2020) Minimum lignin and xylan removal to improve cellulose accessibility. BioEnergy Res 13:775–785. https://doi.org/10.1007/s12155-020-10120-z
Silva TAL, Zamora HDZ, Varão LHR et al (2018) Effect of steam explosion pretreatment catalysed by organic acid and alkali on chemical and structural properties and enzymatic hydrolysis of sugarcane bagasse. Waste and Biomass Valorization 9:2191–2201. https://doi.org/10.1007/s12649-017-9989-7
Singhania A, Patel AK, Pandey A, Ganansounou E (2017) Genetic modification: a tool for enhancing beta-glucosidase production for biofuel application. Bioresour Technol 245(B):1352–1361. https://doi.org/10.1016/j.biortech.2017.05.126
Singhvi MS, Gokhale DV (2019) Lignocellulosic biomass: hurdles and challenges in its valorization. Appl Microbiol Biotechnol 103:9305–9320. https://doi.org/10.1007/s00253-019-10212-7
Sipponen MH et al (2017) Structural changes of lignin in biorefinery pretreatments and consequences to enzyme-lignin interactions. Nord Pulp Pap Res J 32(4):550–571. https://doi.org/10.3183/npprj-2017-32-04_p550-571_sipponen
Siqueira G et al (2017) Limitation of cellulose accessibility and unproductive binding of cellulases by pretreated sugarcane bagasse lignin. Biotechnol Biofuels 10(176):1–12. https://doi.org/10.1186/s13068-017-0860-7
Sorensen A, Andersen JJ, Ahring BK, Teller PJ, Lübeck M (2014) Screening of carbon sources for beta-glucosidase production by Aspergillus saccharolyticus. Int Biodet Biodegrad 93:78–83. https://doi.org/10.1016/j.ibiod.2014.05.011
Sorokina KN, Samoylova YV, Piligaev AV et al (2017) New methods for the one-pot processing of polysaccharide components (cellulose and hemicelluloses) of lignocellulose biomass into valuable products. Part 3: products synthesized via the biotechnological conversion of poly- and monosaccharides of biomass. Catal Ind 9:270–276. https://doi.org/10.1134/S2070050417030138
Statista (2020) Average annual Brent crude oil price from 1976 to 2020. https://www.statista.com/statistics/262860/uk-brent-crude-oil-price-changes-since-1976/. Accessed 1 May 2020
Steinbach D, Kruse A, Sauer J (2017) Pretreatment technologies of lignocellulosic biomass in water in view of furfural and 5-hydroxymethylfurfural production- a review. Biomass Convers Biorefinery 7:247–274. https://doi.org/10.1007/s13399-017-0243-0
Subhedar PB, Gogate PR (2014) Enhancing the activity of cellulase enzyme using ultrasonic irradiations. J Mol Catal B Enzym 101:108–114. https://doi.org/10.1016/j.molcatb.2014.01.002
Subramaniyan S (2012) Isolation, purification and characterisation of low molecular weight xylanase from Bacillus pumilus SSP-34. Appl Biochem Biotechnol 166:1831–1842. https://doi.org/10.1007/s12010-012-9600-4
Summers SR, Sprenger KG, Pfaendtner J et al (2017) Mechanism of competitive inhibition and destabilization of Acidothermus cellulolyticus Endoglucanase 1 by ionic liquids. J Phys Chem B 121:10793–10803. https://doi.org/10.1021/acs.jpcb.7b08435
Tao Y et al (2010) Purification and properties of endoglucanase from a sugar cane bagasse hydrolyzing strain, Aspergillus glaucus XC9. J Agric Food Chem 58(10):6126–6130. https://doi.org/10.1021/jf1003896
Tayi L, Kumar S, Nathawat R, Haque AS, Maku RV, Patel HK, Sankaranarayanan R, Sonti RV (2018) A mutation in an exoglucanase of Xanthomonas oryzae pv. oryzae, which confers an endo mode of activity, affects bacterial virulence, but not the induction of immune responses, in rice. Mol Plant Pathol 19(6):1364–1376. https://doi.org/10.1111/mpp.12620
Teixeira da Silva VDC, de Souza Coto AL, de Carvalho Souza R et al (2016) Effect of pH, temperature, and chemicals on the Endoglucanases and β-Glucosidases from the Thermophilic fungus Myceliophthora heterothallica F.2.1.4. Obtained by solid-state and submerged cultivation. Biochem Res Int 2016:1–9. https://doi.org/10.1155/2016/9781216
Tenkanen M, Puls J, Poutanen K (1992) Two major xylanases of Trichoderma reesei. Enzym Microb Technol 14(7):566–574. https://doi.org/10.1016/0141-0229(92)90128-B
Terrone C et al (2020) Salt-tolerant α-arabinofuranosidase from a new specie Aspergillus hortai CRM 1919: production in acid conditions, purification, characterization and application on xylan hydrolysis. Biocat Agric Biotech 23:1–18. https://doi.org/10.1016/j.bcab.2019.101460
Trajano HL, Wyman CE (2013) Fundamentals of biomass pretreatment at low pH. In: Aqueous pretreatment of plant biomass for biological and chemical conversion to fuels and chemicals. Wiley, Hoboken, pp 103–128
Trollope K, Nel DW, Volschenk H (2018) The heterologous expression potential of an acid-tolerant Talaromyces pinophilus β-glucosidase in Saccharomyces cerevisiae. Folia Microbiol 63:725–734. https://doi.org/10.1007/s12223-018-0613-4
U.S. Energy Information Administration (2012) Crude oil distillation and the definition of refinery capacity. U.S. EIA, Washington, DC
Vasconcellos VM et al (2012) Addition of metal ions to a (hemi)cellulolytic enzymatic cocktail produced in-house improves its activity, thermostability, and efficiency in the saccharification of pretreated sugarcane bagasse. New Biotechnol 33(3):331–337. https://doi.org/10.1016/j.nbt.2015.12.002
Vassileva PS, Radoykova TH, Detcheva AK et al (2016) Adsorption of Ag+ ions on hydrolyzed lignocellulosic materials based on willow, paulownia, wheat straw and maize stalks. Int J Environ Sci Technol 13:1319–1328. https://doi.org/10.1007/s13762-016-0970-y
Vianna Bernardi A, Kimie Yonamine D, Akira Uyemura S, Magnani Dinamarco T (2019) A Thermostable Aspergillus fumigatus GH7 Endoglucanase over-expressed in Pichia pastoris stimulates Lignocellulosic biomass hydrolysis. Int J Mol Sci 20:2261. https://doi.org/10.3390/ijms20092261
Vinuselvi P, Lee SK (2012) Heterologous expressions and extracellular secretion of cellulases in recombinant microbes. In: Bioethanol. Intechopen, London, pp 239–252
Virtanen S, Chowreddy RR, Irmak S et al (2017) Food industry co-streams: potential raw materials for biodegradable mulch film applications. J Polym Environ 25:1110–1130. https://doi.org/10.1007/s10924-016-0888-y
Walia A, Guleria S, Mehta P, Chauhan A, Kulshrestha S, Shirkot CK (2014) Purification and characterization of cellulase-free low molecular weight endo b-1, 4 xylanase from an alkalophilic Cellulosimicrobium cellulans CKMX1 isolated from mushroom compost. World J Microbiol Biotechnol 30(10):2597–2608. https://doi.org/10.1007/s11274-014-1683-3
Walia A, Guleria S, Mehta P, Chauhan A, Parkash J (2017) Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. Biotech 7(11):1–12. https://doi.org/10.1007/s13205-016-0584-6
Walker LP, Wilson DB (1991) Enzymatic hydrolysis of cellulose: an overview. Bioresour Technol 36(1):3–14. https://doi.org/10.1016/0960-8524(91)90095-2
Wallace J, Brienzo M, García-Aparicio MP, Görgens JF (2016) Lignin enrichment and enzyme deactivation as the root cause of enzymatic hydrolysis slowdown of steam pretreated sugarcane bagasse. New Biotechnol 33(3):361–371. https://doi.org/10.1016/j.nbt.2016.01.004
Wang QQ, He Z, Zhu Z, Zhang YH, Ni Y, Luo XL, Zhu JY (2012) Evaluations of cellulose accessibilities of lignocelluloses by solute exclusion and protein adsorption techniques. Biotechnol Bioeng 109(2):381–389. https://doi.org/10.1002/bit.23330
Wang W, Zhuang X, Tan X et al (2018) Dual effect of nonionic surfactants on improving the enzymatic hydrolysis of lignocellulose. Energy Fuel 32:5951–5959. https://doi.org/10.1021/acs.energyfuels.8b00225
Wei K et al (2015) Cloning, expression and characterization of the endoglucanase gene from Bacillus subtilis UMC7 isolated from the gut of the indigenous termite Macrotermes malaccensis in Escherichia coli. Electron J Biotechnol 18(2):103–109. https://doi.org/10.1016/j.ejbt.2014.12.007
Wingfield P (2016) Protein precipitation using ammonium sulfate. Curr Protoc Protein Sci 84(1):A3F1–A3F9. https://doi.org/10.1002/0471140864.psa03fs84
Wong KK, Deverell KF, Mackie KL, Clark TA, Donaldson LA (1988) The relationship between fiber-porosity and cellulose digestibility in steam-exploded Pinus radiata. Biotechnol Bioeng 31(5):447–456. https://doi.org/10.1002/bit.260310509
Ximenes E, Kim Y, Mosier N, Dien B, Ladisch M (2011) Deactivation of cellulases by phenols. Enzym Microb Technol 48(1):54–60. https://doi.org/10.1016/j.enzmictec.2010.09.006
Yang L, Xu F, Ge X, Li Y (2015) Challenges and strategies for solid-state anaerobic digestion of lignocellulosic biomass. Renew Sust Energ Rev 44:824–834. https://doi.org/10.1016/j.rser.2015.01.002
Yang Y, Zhu N, Yang J, Lin Y, Liu J, Wang R, Wang F, Yuan H (2017) A novel bifunctional acetyl xylan esterase/arabinofuranosidase from Penicillium chrysogenum P33 enhances enzymatic hydrolysis of lignocellulose. Microb Cell Fact 16(166):1–12. https://doi.org/10.1186/s12934-017-0777-7
Yeoman CJ, Han Y, Dodd D et al (2010) Chapter 1 - Thermostable enzymes as biocatalysts in the biofuel industry. In: Laskin AI, Sariaslani S, Gadd GM (eds) Advances in applied microbiology. Academic Press, Berlin, pp 1–55
Yimer D, Tilahun A (2018) Microbial biotechnology review in microbial enzyme production methods, assay techniques and protein separation and purifications. J Nutr Health Food Eng 8(1):1–7. https://doi.org/10.15406/jnhfe.2018.08.00249
Ying W et al (2018) Effect of alkaline lignin modification on cellulase-lignin interactions and enzymatic saccharification yield. Biotechnol Biofuels 11:1–13. https://doi.org/10.1186/s13068-018-1217-6
Yücel Y, Göycıncık S (2015) Optimization and Modelling of process conditions using response surface methodology (RSM) for enzymatic Saccharification of spent tea waste (STW). Waste and Biomass Valorization 6:1077–1084. https://doi.org/10.1007/s12649-015-9395-y
Yusuf AAIS, Kresnowati MTAP (2019) Evaluation of surfactant addition to enzymatic hydrolysis of oil palm empty fruit bunch (OPEFB). AIP Conf Proc 2085:1–8. https://doi.org/10.1063/1.5094988
Zanelato AI, Shiota VM, Gomes E, Silva R, Thoméo JC (2012) Endoglucanase production with the newly isolated Myceliophtora sp. i-1d3b in a packed bed solid state fermentor. Brazilian J Microb 43(4):1536–1544. https://doi.org/10.1590/S1517-83822012000400038
Zeeshan N et al (2018) Heterologous expression and enhanced production of β-1,4-glucanase of Bacillus halodurans C-125 in Escherichia coli. Electron J Biotechnol 34:29–36. https://doi.org/10.1016/j.ejbt.2018.05.001
Zeng R et al (2016) Cloning a novel endo-1,4-β-d-glucanase gene from Trichoderma virens and heterologous expression in E. coli. AMB Express 6(1):1–7. https://doi.org/10.1186/s13568-016-0282-0
Zhai R, Hu J, Saddler JN (2016) What are the major components in steam pretreated Lignocellulosic biomass that inhibit the efficacy of Cellulase enzyme mixtures? ACS Sustain Chem Eng 4:3429–3436. https://doi.org/10.1021/acssuschemeng.6b00481
Zhang M, Buekens A, Li X (2016) Dioxins from biomass combustion: An overview. Waste and Biomass Valorization 8:1–20. https://doi.org/10.1007/s12649-016-9744-5
Zhang X, Wang H, Liu C, Zhang A, Ren J (2017) Synthesis of thermoplastic XylanLactide copolymer with amidine mediated organocatalyst in ionic liquid. Sci Rep 7(551):1–10. https://doi.org/10.1038/s41598-017-00464-6
Zhang P et al (2018) Heterologous expression and biochemical characterization of a GHF9 endoglucanase from the termite Reticulitermes speratus in Pichia pastoris. BMC Biotechnol 18(1):1–9. https://doi.org/10.1186/s12896-018-0432-3
Zhao X, Zhang L, Liu D (2012) Biomass recalcitrance. Part I: the chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod Biorefin 6(4):465–482. https://doi.org/10.1002/bbb.1331
Zhao X, Li S, Wu R, Liu D (2017) Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: chemistry, kinetics, and substrate structures. Biofuels Bioprod Biorefin 11:567–590. https://doi.org/10.1002/bbb.1768
Zheng H, Zhe MS, Cai LM, Sheng HP, Qing XZ, Zheng Y, Wenhui Z, Zhang J, Hu J, Lu F, Sun J (2014) Purification and characterization of a thermostable xylanase from Paenibacillus sp. NF1 and its application in xylooligosaccharides production. J Microb Biotechnol 24(4):489–496. https://doi.org/10.4014/jmb.1312.12072
Zhou Y, Yang J, Luo C et al (2019) Effect of metal ions and surfactants on the enzymatic hydrolysis of pretreated lignocellulose. Bioresources 14:1653–1667. https://doi.org/10.15376/biores.14.1.1653-1667
Zhu JY, Pan X, Zalesny RS (2010) Pretreatment of woody biomass for biofuel production: energy efficiency, technologies, and recalcitrance. Appl Microbiol Biotechnol 87(3):847–857. https://doi.org/10.1007/s00253-010-2654-8
Acknowledgement
The authors are thankful for the support of Brazilian Council for Research and Development—CNPq (grant 401900/2016-9), and São Paulo Research Foundation (FAPESP, grant 2017/11345-0 and 2017/22401-8).
Competing Interests
All the authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Zamora Zamora, H.D., de Freitas, C., Bueno, D., Shimizu, F.L., Contiero, J., Brienzo, M. (2020). Biomass Fractionation Based on Enzymatic Hydrolysis for Biorefinery Systems. In: Verma, P. (eds) Biorefineries: A Step Towards Renewable and Clean Energy. Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-15-9593-6_9
Download citation
DOI: https://doi.org/10.1007/978-981-15-9593-6_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-9592-9
Online ISBN: 978-981-15-9593-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)