Abstract
Water pollution is one of the highly undesired drawbacks of the industrial development, and the discharge of wastewater in nature has attracted attention not only due to the short-term toxic effects, but especially to the negative effects on medium and long term. Adsorption is considered one of the most efficient methods to retain water pollutants due to its accessibility and flexibility. Lignocellulosic adsorbent materials based on agricultural waste represent a highly advantageous choice in terms of availability, low cost, biodiversity, as well as porous structure and the presence of reactive functional groups (such as carboxylic, hydroxylic, phenolic, and other oxygen-bearing moieties) able to bind organic (dyes, pesticides) and inorganic (heavy metal oxides and salts) pollutants. Some of the most recent data reported in the literature will be reviewed in the present chapter, highlighting a few factors that can influence the adsorption onto lignocellulosic adsorbents, and future directions of development will be presented as well.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Industrial water purification
- Lignocellulosic waste materials
- Waste biosorbents
- Adsorption
- Organic pollutants
- Dyes
- Pesticides
- Inorganic pollutants
- Heavy metals
- LCA
14.1 Introduction
In the last decades, the industry has recorded a rapid development which entailed increased amounts of wastewater discharged in nature, and this has become a challenge not only for the scientists, but for the entire society as well, in order to limit the environmental pollution and human health hazards. There are many conventional methods known and in use, such as biological treatments (Fazal et al. 2015), flocculation (Jawad et al. 2015), membrane processes, chemical precipitation, ion exchange (Rosales et al. 2017), etc. Retention onto activated carbon is considered efficient, reliable, but not cost-effective, so that the research for another approach was encouraged despite the advantages of this method (high capacity of retention, as related to the high surface area and surface reactivity, as well as mechanical strength) (Mashhadi et al. 2016).
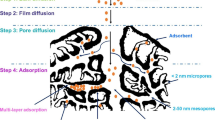
Basically, the mechanism of adsorption consists of a mass transfer with the accumulation at the interface of the two phases in direct contact. In the case of solid-liquid systems where adsorbed compounds are removed from their solution and linked onto the surface of the solid sorbent, both physisorption (weak and reversible) and chemisorption (strong binding through chemical bonds) occur and coexist rending stability to the process. Physisorption takes place at temperatures below or close to the critical temperature of the adsorbate and evolves with a decrease in free energy and entropy, and, thereby, the process is exothermic (Cooney 1999; De Gisi et al. 2016). On the other hand, the chemisorption occurs under the condition of a monolayer; hence the resulted bonds are hard to break. In time, a dynamic equilibrium is reached in relation to the adsorbate.
Adsorption onto lignocellulosic waste materials has been acknowledged as a viable alternative to the already existing technologies employed for the removal of various pollutants from the industrial wastewater. These biosorbents may come from agriculture and wood industry, and can originate in different parts of plants (bark, stem, leaves, roots, flowers, biomass, husk, hull, skin, shell, bran, kernel etc.), thus containing cellulose, hemicelluloses, and lignin in various ratios (Fig. 14.1) (Dai et al. 2018; Okoro and Okoro 2011).
Fiber composition (dry weight %) in correlation with their origin: A, rice straws; B, rice husk; C, wheat straws; D, corn stover; E, corncobs; F, rapeseed straws; G, sugarcane bagasse; H, sunflower stalks; I, sweet sorghum; J, palm kernel shell (redrawn after Dai et al. 2018)
Aside from the porosity of the materials, another characteristic of great relevance is the presence of reactive functional groups able to undergo reactions in solution and, therefore, bind pollutants such as heavy metal ions or organic small molecules by specific bonds (Nguyen et al. 2013; Chan et al. 2015; Heng et al. 2017; Zhang et al. 2016; Zhou et al. 2015). For example, most of the proton donor functional groups, namely, amine, amide or acetamide, carboxylic, phenolic, hydroxylic, etc., may interact with the metal ions in solution yielding in substitution compounds or even metallic complexes (Marin-Rangel et al. 2012; Saka et al. 2012). It has been shown that Cr(VI) was chemically bound to the adsorbent surface, and a certain amount of it was reduced to Cr(II) (Lin et al. 2018), as well as Cu, Ni, and Zn ions (Lee and Rowell 2004), while Pb(II) was retained by electrostatic attraction (Nguyen et al. 2013). As for the organic pollutants, adsorption was achieved through other types of bonds, such as hydrogen bonding (dyes), van der Waals bridging (pesticides), hydrophobic attraction (drugs), covalent bonds (oil), or even π-π stacking interactions (Zhou et al. 2015).
There are factors influencing the purification process onto lignocellulosic materials that have to be considered especially when it comes to the retention effectiveness. Thus, the pH and temperature of the wastewater, the initial concentration of the pollutants and the pollutant-to-adsorbent ratio, and even the ionic strength may affect the process.
Another issue associated with the industrial water purification by means of lignocellulosic waste materials is the so-called secondary pollution caused by the environmental accumulation of waste adsorbents which can become, in their turn, source of further pollution. This problem must be dealt with, and there are some methods commonly applied: solvent extraction (it employs an appropriate solvent for the adsorbate in order to remove it from the sorbent material); calcination (the high temperature treatment yields in organic or inorganic volatiles that are released from the support); and biologic regeneration (specific microorganisms are used to release the adsorbent) (Dai et al. 2018).
In the following, some of the pollutants most commonly found in the wastewater, organic and inorganic, as well as the corresponding lignocellulosic sorbents employed for their efficient removal, will be reviewed highlighting the mechanism of retention onto the surface of the sorbent and factors that may influence the yield of the process.
14.2 Organic Pollutants
In the last decades, the highly increasing societal demand for food and goods has entailed large-scale environmental problems. The water, air, and soil pollution caused by the improper disposal of various by-products and wastes from agriculture and industry, including oil spills, associated with the lack of effective appropriate policies in some countries and the use of outdated technologies, among other causes, generated dramatic climate changes, wildlife extinction, desertification, etc. (O’Donnel 2018).
Wastewater most commonly contains pesticides (such as 2,4-dichlorophenoxyacetic acid, metribuzin, and metalaxyl) and dyes (i.e., Congo red, rhodamine B, malachite green, methylene blue, crystal violet), but aromatic compounds (e.g., polycyclic aromatic hydrocarbons, phenol), oil, and drugs (such as tetracycline, caffeine, tylosin) were identified as well. Therefore, their removal was intensively studied, and various methods were developed in close correlation with specific pollutants: physical separation, sedimentation and filtration (Dai et al. 2018), flocculation (Guan and Tian 2017), membrane separation (Sarasidis et al. 2017), adsorption (Worch 2012), UV-vis treatment (Deng et al. 2017), and microwave-assisted purification (Gayathri et al. 2017); chemical, chemical, photochemical, and catalytic oxidation (Gayathri et al. 2017; Janssens et al. 2017); or even combinations of different methods (i.e., adsorption and photocatalysis on titanate nanotubes (Sandoval et al. 2017); membrane separation and oxidation, with simultaneous in situ regeneration of the sorbent) (Sarasidis et al. 2017), aiming at maximizing the yield of the process.
Unlike other methods, the adsorption onto lignocellulosic materials offers some specific advantages: simplicity, high removal rate, cost-effectiveness, an easy access to resources (agriculture and wood industry), and, of utmost importance, the presence of reactive functional groups able to bind specific organic pollutants – carboxyl (Bouguettoucha et al. 2016; Inam et al. 2015), carbonyl (Bouguettoucha et al. 2016; Zhu et al. 2016a, b, c, d), hydroxyl and amine (Inam et al. 2015; Shang et al. 2015), and amide (Sohrabi and Ameri 2015).
14.2.1 Pesticides
Pesticides are employed in agriculture and forestry in order to limit and prevent pests, fungi, and microorganism infestation, as well as weeds, and their extensive use affects the human health and environment.
Lignocellulosic waste sorbents proved to be highly effective for the retention of different pesticides (Fig. 14.2). Thus, the purification of wastewater containing 2,4-dichlorophenoxyacetic acid (a well-known systemic pesticide, selective, and used as herbicide and plant growth regulator) attracted the interest mainly after being reported in 1987 by the International Agency for Cancer Research as having carcinogenic potential, as well as mutagenic (International Agency for Research on Cancer (IARC) 1987).
Experiment conducted onto mustard plant ash (Trivedi et al. 2016) aimed at optimizing the purification process and factors affecting the adsorption, namely, the sorbent-to-sorbate ratio, initial pollutant concentration, contact time, and adsorption temperature, were investigated. Based on the experimental data, fitted to different kinetic and isothermal models, it was possible to indicate an optimum sorbent-to-pesticide ratio. The langsat empty fruits activated by phosphoric acid were also employed in the retention of 2,4-dichlorophenoxyacetic acid from wastewater (Njoku et al. 2015) with a reported efficiency of 261.1 mg/g, at 30 °C. The influence of the initial concentration of pollutant, shaking time, pH, and temperature was studied. It was also found the mechanism of sorption was the internal diffusion of particles. Reported data confirmed this material as an excellent sorbent. Another study employed fly ash originated from bagasse as a low-cost and effective sorbent (Deokar et al. 2016) using two different approaches: in-batch experiments and continuous packed-bed processes. The sorption process in batch depends on the initial amount of pesticide, contact time, pH, and temperature and the particle size of the sorbent, while the factors affecting the continuous packed-bed process are the concentration of the influent, flow rate, and bed height and model. It was thus possible to recommend optimal conditions for each study case. The sorbent particle size investigation showed the bigger the particles are, the higher is the amount of pollutant removed as a consequence of the greater BET surface.
Metribuzin is another pesticide of interest, as it is an herbicide used both pre- and post-emergence in crops (soy bean, potatoes, tomatoes, sugarcane, etc.), which acts by inhibiting photosynthesis of weeds. Since it was demonstrated that it contaminates the groundwater, its removal onto waste lignocellulosic materials was studied. A successful research reported on the reduction of water contamination by metribuzin using de-oiled two-phase olive mill waste (DW) as sorbent (Pena et al. 2016) in soil. The method consisted in preparing soils containing DW in various amounts and composted for different intervals. Thus, the study indicated that fresh DW retain lower amounts of metribuzin, as compared to aged DW. Therefore, the DW-composted soils may be a cost-effective solution for the sustainable decontamination of metribuzin-containing groundwater.
An interesting challenge was the removal of chiral pesticides such as metalaxyl , an acylalanine derivative (methyl N-(methoxyacetyl)-N-(2,6-xylyl)-DL-alaninate) fungicide with systemic function, and imazaquin, an imidazoline-based herbicide used to control a wide range of weed species. It was proven that adsorption onto lignocellulosic materials was applied only for metalaxyl (Gamiz et al. 2016) so far. The study evidenced that soils amended with composted olive mill waste retain lower amount of pesticide than soils containing the corresponding biochar. At the same time, the enantiomer R was preferentially retained in soils containing composted olive mill waste, but biochar-amended soils adsorbed both enantiomers and blocked their leaching almost completely.
Despite the progress reported by researchers on the removal of pesticides, further studies are required in order to develop new sorbents based on agricultural waste able to purify wastewater more efficiently.
14.2.2 Dyes
Dyes represent a broad class of organic pollutants largely found in industrial wastewater. Due to their chemical structure (high content in aromatics, halogens, metal ions, etc.), they can severely damage the environment when improperly discharged in natural streams as they cause a decrease in water transparency, and alter the photosynthesis and water oxygenation, as well as self-cleaning processes (Tomczak and Tosik 2017; Dardouri and Sghaier 2017). Basically, dyes can be divided into groups: acid dyes (Congo red, eosin, etc.), basic (cationic) dyes (methylene blue, basic fuchsin, crystal violet, etc.), direct (substantive) dyes, mordants, and vat, reactive, and non-ionic (disperse) dyes.
Simple, low-cost, and easy-to-access sorbent materials obtained from lignocellulosic waste were successfully employed in industrial water decontamination when dyes were the pollutant. Thus, such materials removed cationic dyes (Amela et al. 2012; Zhu et al. 2016b), azoic dyes (Tomczak and Tosik 2017; Chebli et al. 2015), direct dyes (Karthick et al. 2017), and reactive dyes (Hong and Wang 2017; Tunç et al. 2009; Suteu et al. 2011; Suteu et al. 2015) from wastewater.
14.2.2.1 Anionic Dyes
Anionic dyes display a wide structural variety. According to some literature data (Zhou et al. 2015), acidic dyes, direct dyes, and reactive dyes can be included in the same group of anionic dyes.
Acid dyes have been typically applied to textiles under low pH conditions and are used to dye wool, silk, or nylon, but not cotton fabrics. They contain different chromophores (azo groups, anthraquinone, naphthol, etc.) in their structure and hence the broad palette of colors. Information on this specific subject is scarce. Still, it was reported that Naphthol Blue Black, Basic Blue 41, and Reactive Black 5 dyes were removed from water solution using the avocado peel residue as agriculture waste (Palma et al. 2016), and rice husk was employed to retain methyl orange (Hosseinzadeh and Mohammadi 2016), while modified barley straws were used to remove Acid Blue 40 (Oei et al. 2009) and the powder of the jackfruit leafs for Amido Black 10B (Ojha and Bulasara 2015). Other studies focused on the adsorption of Synolon Black HWF-FS and Synolon Red 3HF onto mustard and linseed oil cakes (Safa 2016), when electrostatic interaction proved to be the sorption mechanism, and on the sorption of Acid Blue 113 onto the peel of overripe Cucumis sativus (Lee et al. 2015), which showed biosorption was endothermic, feasible, and spontaneous.
Direct dyes are water-soluble sodium salts of sulfonic acid bearing azoic groups as chromophores (some representative direct blue dyes are presented in Fig. 14.3). Despite the reports on the lignocellulosic materials originated from agricultural waste that can be used for the effective adsorption of direct dyes (Chebli et al. 2015), more research is still required.
Various lignocellulosic materials were tested for the sorption of direct dyes from industrial water. Thus, fibers of Stipa tenacissima were successfully used to remove the Congo red dye (Chebli et al. 2015) from aqueous solutions, mechanism of adsorption was identified (protonation, particle diffusion, electrostatic interactions), and an optimal set of parameters (pH = 4, temperature 25°) was recommended in order to reach the maximum of adsorption (7.93 mg/g). For the same dye, polyethyleneimine-modified wheat straws were employed (Shang et al. 2015), and it has been proven that the chemical modification of wheat straws enhanced their adsorption capacity. Another red dye, namely Direct Red 23, was effectively removed from wastewater using corn stover (Fathi et al. 2015).
Reactive dyes contain in their structure a chromophore bearing a substituent able to react with the substrate; thus, they bond it covalently during dyeing. The use of carbonaceous materials obtained from avocado skin for the removal of Reactive Black 5 was assessed (Palma et al. 2016) as related to a series of factors, such as carbonization parameters, and by comparison with other dyes. It has been shown that physical adsorption was the retention mechanism of Reactive Black 5 onto a new sorbent material based on banana pseudostem (Modenes et al. 2015). Modified barley straws turned out to be effective for the removal of reactive dyes (Oei et al. 2009) under certain conditions (sorbent-to-sorbate ratio, initial concentration of dye, solution pH, and temperature).
14.2.2.2 Cationic Dyes
Cationic dyes can dissociate and form positive ions in their corresponding aqueous solution and further interact with negatively charged groups on the fiber molecules. Representative dyes in this class are presented in Fig. 14.4.
Numerous and various recent reports have shown that agricultural waste can be turned into environmentally friendly adsorbent materials which can remove cationic dyes from industrial waters up to, or even beyond, satisfactory limits (Dai et al. 2018). Thus, Cucumis sativus was employed to remove such dyes , and the study on rhodamine B and crystal violet (Smitha et al. 2012) evidenced that the activation of the lignocellulosic waste using sulfuric acid enhanced the dye sorption, but the overall yield depended on the process parameters (dye concentration, sorbent-to-sorbate ratio, contact time, pH) as well.
For the sorption of methylene blue, different lignocellulosic materials were tested as sorbents. Melon peel (Djelloul and Hamdaoui 2015) was set in fixed-bed columns for a dynamic retention of dye (where column parameters were estimated depending on the flow rate and initial concentration of dye), and the maximum of the adsorption process has been found to correspond to simultaneous high bed height, low flow rate, and high initial concentration of dye. Sumac leaves (Gülen et al. 2015) were also tested to remove methylene blue, as well as waste tea leaves chemically modified under mild conditions with citric acid (Zhou et al. 2016), material which can be regenerated after exhaustion up to 98.90% by cold atmospheric plasma treatment.
Malachite green was successfully retained on stems and leaves of Solanum tuberosum as powder through physisorption (Gupta et al. 2016), method which was also effective for methylene blue sorption, but to a greater extent.
14.2.2.3 Non-ionic Dyes
The term refers to disperse dyes , the only water-insoluble dyes that are fit for dyeing polyester and acetate fibers. As compared with other dyes, disperse dyes have the smallest molecules. Structurally, their molecule is based on an azobenzene or anthraquinone unit bearing nitro-, amino, hydroxyl, etc. groups.
The information on this subject is scarce, although the dye removal from wastewater using agriculture waste is a subject of high and constant interest (Dai et al. 2018; Zhou et al. 2015; Yagub et al. 2014; Worch 2012; Demirbas 2009). Still, there are some studies reporting on the retention of disperse dyes onto lignocellulosic waste materials. Disperse blue and red dyes commercially available were treated with oil palm ash made of oil palm waste from an oil manufacture, in batch and continuous flow experiments (Isa et al. 2007). It has been shown that the optimum adsorption was reached for pH = 2 and agitation time 60 min, for both dyes. Activated carbon made of bamboo stalk was employed for the removal of Disperse Red 167 (Zhou et al. 2015).
As for the adsorption mechanisms involved in the process of removal of dyes from industrial water using lignocellulosic waste , some synthetic data are presented in Table 14.1.
Removal of various classes of dyes from industrial water using lignocellulosic waste from agriculture remains an active field of research, as low-cost adsorbents are promising in terms of benefits in the future. Still, further investigation is needed in order to optimize the process (sorbent-to-sorbate ratio, size of dye molecules, particle size of the sorbent, functional groups present on the surface of the adsorbent, initial pH of the solution, batch or column system, processing temperature, etc.). But new approaches are also emerging since the nanotechnology opens alternative directions even in this domain.
14.2.3 Pharmaceuticals
Pharmaceuticals are considered as environmental pollutants as they have been often detected in city wastewater and even in reclaimed water due to the inefficient purification of industrial water in wastewater treatment plants.
Drugs , be they antibiotics or anti-inflammatory, painkillers or analgesics, psychotropics, antitumoral, or even disinfectants, as well as personal care products and steroids, may represent really hazardous agents when disposed of carelessly in the environment as they can degrade in water, air, and soil and, moreover, enter the metabolism of plants and animals.
Therefore, these organic compounds have to be removed from water in effective, harmless to the environment, ways. Adsorption onto lignocellulosic waste materials is one method that has been applied in this field in recent years with notable results (Blanch 2016; Portinho et al. 2017; Kyzas and Deliyanni 2015; de Andrade et al. 2018; Quesada et al. 2019).
Antibiotics are some of the most common organic pollutants, and their removal using agriculture waste was investigated. For the removal of tetracycline , sorbent derived from rice straws (Wang et al. 2017) and animal waste (Wang et al. 2018) were studied. Experimental data have shown that sorption process was spontaneous and endothermic, but the retention was more effective on the rice straw-derived biochar. Sugarcane bagasse was used as precursor in order to obtain magnetic carbon composites, prepared via mild HTC and simple heat treatment, as sorbent for tetracycline (Rattanachueskul et al. 2016). The composite materials adsorbed rapidly the antibiotic due to their high adsorption per unit surface area. A hybrid material made of maize straws and goethite (an iron-bearing hydroxide mineral of the diaspore group) was successfully tested for the retention of tylosin (Yin et al. 2016). The results have indicated that a high temperature could favor the sorption of the antibiotic on both sorbents, and pH and ionic strength of the solution can influence the process. The sorption mechanism onto maize straw mainly involves electrostatic interactions and hydrophobic interactions, while the modified maize straws retain the antibiotic by electrostatic interactions, H-bonding, hydrophobic interactions, and surface complexation.
The removal of anti-inflammatory drugs , such as diclofenac, ketoprofen, naproxen, nimesulide, ibuprofen, etc., was studied, and the corresponding retention mechanism was assessed (Zhou et al. 2015), as well as for other pharmaceuticals. For example, caffeine (a stimulant of the central nervous system, member of the methylxanthine class, considered the only psychoactive drug consumed worldwide) was retained from aqueous solution on sorbents based on grape stalks that were used in three different forms: raw, modified by phosphoric acid, and as activated carbon (Portinho et al. 2017). The best results have been obtained using the activated carbon material.
Pharmaceuticals, mainly antibiotics, and their use for the human health were a breakthrough in the history, but also entailed a series of risks which, without control, may lead to a global crisis. Therefore, their removal has to be of capital interest for academic and industrial R&D, and the recent experimental studies on various agricultural wastes as sorbents for drugs have indicated they are a viable option of great potential.
14.2.4 Oil and Other Organic Pollutants
Oil pollution came along with the industrial development, and it has been proven that oil spills are serious environmental concerns since they can easily spread onto water surface and form thin layers that hinder the oxygen transfer between air and water, thus severely affecting the marine biotope and the contiguous areas.
An increased attention is payed lately to specific sorbents made of lignocellulosic waste, such as barley straw (Ibrahim et al. 2010), coconut shells, garlic and onion peel (Zhou et al. 2015), and chemically modified sugarcane bagasse (Abdelwahab et al. 2017). In this latter case, the bagasse was modified by esterification with stearic acid and calcium oxide, and then coated with polyacrylonitrile, yielding in a hydrophobic adsorbent for the removal of diesel fuel from man-made seawater (mainly used in marine biology and in marine and reef aquaria). In the both stages of the chemical treatment, the resulted materials have acquired significantly increased sorption surface. The retention mechanism was chemisorption. It was also possible to assess the adsorption efficiency for different sorbates as follows: paraffin oil > vegetable oil > diesel oil > gasoline (Abdelwahab et al. 2017).
At the same time, the sorption of emulsified oil onto raw and modified bagasse and corn husk, by an MW-assisted procedure, has been also studied (Pachathu et al. 2016), when the maximum removal ratios were 98.07% (on modified bagasse) and 98.72% (on modified corn husk).
Other organic pollutants of high risk are the aromatic compounds, especially polycyclic aromatic ones, containing either separated or fused aromatic nuclei, as they have toxic, mutagenic, and carcinogenic potential. Despite the high interest of scientists on the removal of such pollutants from industrial water, mostly microbial, bacterial, and other biological approaches were investigated (Sherafatmand and Ng 2015; Kronenberg et al. 2017), while the information on sorption onto lignocellulosic materials is not so abundant (Zhou et al. 2015; De Gisi et al. 2016; Dai et al. 2018; Tran et al. 2015). Still, some research focused on obtaining activated carbon using waste banana peel (Gupta and Gupta 2015) when a sorption model has been established, and characteristic parameters of the adsorption isotherm were calculated.
The same situation is encountered for the retention of phenolic compounds using activated carbon from coconut shells (Karri et al. 2017), Typha orientalis (Feng et al. 2015), pine tree (Tonucci et al. 2015), and even zeolite-type materials made of bagasse fly ash (Shah et al. 2016). Nevertheless, these studies have indicated that the retention of phenolic compounds has been acquired by hydrogen bonding, hydrophobic and electrostatic interactions, π-π interactions, ion exchange, electronic interactions, diffusion at the interface, and internal diffusion of particles.
Further research is still needed in terms of expanding the range of lignocellulosic materials fit for the removal of organic pollutants from industrial water, increasing the volume of common agriculture waste used in such applications , and, last but not least, the possibility of using them in a less modified form.
14.3 Inorganic Pollutants
Most inorganic pollutants affecting the environment and, consequently, the wildlife and human health are heavy metals, and some phosphorus and nitrogen compounds, all resulted from industrial and agriculture activities. Industrial water containing such waste has to be specifically treated in order to remove these pollutants up to significant levels as they are toxic for the aquatic life and possess high mutagenic and carcinogenic character. Even more, once discharged in water, these pollutants enter the life cycle of plants and animals (fish included), which are subsequently used as food, thus generating a new cycle of pollution directly in the human body where they accumulate (entailing a toxic and inflammatory response) and cause genetic mutations.
Classic methods of removing heavy metals from wastewater are precipitation, flotation, ion exchange, and membrane processes, but all of them are energy intensive, use expensive equipment, require regeneration of the sorbents, and produce toxic sludge that is able to cause secondary pollution, and the level of retention is not as high as expected. Therefore, new solutions are required, and some of the most available are the lignocellulosic materials based on agriculture waste which have proved to be low-cost and non-aggressive toward the environment (Farhan et al. 2012; Du et al. 2016; Escudero-Oñate et al. 2017). The mechanisms involved in the retention of heavy metals from solution onto such adsorbent media include physisorption, chemisorption, ion exchange, membrane processes, particle diffusion, chelation, electrostatic interactions, surface complexation, ligand exchange, internal complexation, etc. (Nguyen et al. 2013; Agouborde and Navia 2009; Du et al. 2016). Some of them will be highlighted in the following. A schematic retention mechanism (ion exchange) is presented in Fig. 14.5.
There is a wide variety of reports on heavy metal retention on raw and modified agriculture waste, but only some of the most recent will be reviewed herein.
14.3.1 Lead
Lead (Pb) and its compounds (Pb(II) or Pb2+) are toxic to human health, and its rapid accumulation into the body gravely affects the nervous, cardiovascular, digestive, and endocrine system, as well as the hematopoietic function of the body.
Rice husk has been identified as an effective sorbent for Pb(II) (Amer et al. 2017) as the maximum removal of 94% was reached under optimal conditions , namely: contact time = 30 min, pH = 5.5, particle size = 75–150 μm, and initial concentration = 4 g/L. A multiple mechanism was considered: complexation reactions of carboxylic and hydroxylic reactive groups, ion exchange with Ca2+ and Mg2+, and physical attraction. It has been found that it is possible to regenerate the sorbent for further use. In a series of experiments on coconut shell, it was possible to prove that the raw sorbent retained a lower amount of Pb(II) than the corresponding modified coconut shell (activated with phosphoric acid), as the latter has a higher surface area and reactive functional groups (El-Deen and El-Deen 2016).
Other studies reported on the use of other lignocellulosic materials with different sorption capacity (mg/g): acid-modified rice straw, 18.98 (Guo et al. 2015); Acacia nilotica seed shell ash deposited onto magnetic nanoparticles , 37.6 (Omidvar-Hosseini and Moeinpour 2016); plum stone, 80.65 (Parlayıcı and Pehlivan 2017); Citrus reticulata waste biomass, 83.77 (Bhatti et al. 2010); and hazelnut husk-based activated carbon, 109.90 (Imamoglu et al. 2016).
14.3.2 Cadmium
Cadmium (Cd) is considered as extremely toxic (it is not biologic degradable) and can cause kidney failure upon accumulation into the human body. As pollutant, it may come from industry, and its accidental or careless disposal may entail the environmental accumulation and wildlife degradation due to its toxicity and mutagenic capacity.
The mechanism of removing Cd(II) by sorption onto raw and chemically modified walnut shells was the ion exchange, and it has been shown that the biosorption occurred spontaneously (Gondhalekar and Shukla 2015). By the chemical modification (alkaline treatment), the adsorption increased significantly for pH ranging from 2 to 6, and the desorption efficiency remained unmodified until the third cycle. The same mechanism was identified when succinyl-modified cellulosic biomass (Abelmoschus esculentus) was employed as sorbent for Cd(II) (Singha and Guleria 2014). The removal of Cd(II) from the aqueous solution (121.51 mg/g) depended on pH, contact time, temperature, and initial concentration of the metal ion. This sorbent was also effective in the case of other metal ions (adsorption capacity in mg/g): Cu2+, 72.72; Zn2+, 57.11; and Pb2+, 273.97.
Another interesting study compared the retention capacity of sugarcane stalks over Phragmites australis (Farasati et al. 2015). The superior capacity of sorption of the sugarcane bagasse has been proven, and it was assessed that it is due to its extended contact surface. Activated carbons derived from waste of Typha angustifolia and Salix matsudana were prepared by phosphoric acid activation (Tang et al. 2017b) and then tested as sorbents for Cd(II) and Pb(II) in batch experiments. The adsorption process was spontaneous and endothermic and occurred through physical processes (intraparticle diffusion) and chemical reactions, both of them being limiting steps of the adsorption rate. At the same time, experimental data indicated that both sorbents can be satisfactorily regenerated and reused.
14.3.3 Copper
Copper and its alloys (brass and bronze) have been extensively used in a wide range of applications, ranging from common electric wiring to photovoltaic cells and phytotherapy. Cu(II) is a micronutrient, up to a certain concentration, necessary for the human and wildlife health. Its toxicity occurs upon accumulation in high amounts, and it affects mainly the liver and gall bladder. Retention of Cu(II) by adsorption is extensively used in wastewater treatment plants.
Considering the sorption of copper onto lignocellulosic materials, studies have shown that mechanisms involved were chemisorption, physisorption, electrostatic interactions, ion exchange, and particle diffusion (Dai et al. 2018). Thus, adsorption of Cu(II) onto potato peel was investigated in batch experiment and reached the maximum (84.74 mg/g) at 25 °C (Guechi and Hamdaoui 2015b), but depended on the initial concentration of ions.
Three different types of agriculture waste were studied as powders (banana stems, casuarina fruits, sorghum stems) (Mokkapati et al. 2016), and the corresponding adsorption capacity has been correlated with their microporous structure, given that all three sorbents have exhibited rough surface with some cavities. In the case of powders made of banana stems and casuarina fruits, the sorption mechanism was chemisorption, while for the sorghum stem powder, the mechanism occurred through physisorption and chemical attachment.
Raw pomegranate peel was employed for the adsorption of Cu(II) from its aqueous solutions (Ali et al. 2017). The highest sorption capacity was obtained at pH = 5.8, particle size = 630 μm, temperature = 40 °C, and contact time of 2 h, and by increasing the initial concentration of ion.
14.3.4 Nickel and Zinc
Nickel is essential for human life, but upon high concentrations (by absorption through the pores and sebaceous glands from the skin) it rouses an inflammatory response.
Different experimental studies have shown Ni(II) can be successfully retained onto sorbents made of lignocellulosic waste. For example, Citrus limetta peels (CLP) were employed for the sorption of Ni(II) ions from aqueous solution in batch system (Singh and Shukla 2017). The optimum values of process parameters were pH = 6, sorbent dose = 2 g/L, and contact time for equilibrium = 45 min. The maximum adsorption capacity was 27.78 mg/g. Experimental results have substantiated that the mechanism involved in sorption was the ion exchange, and by desorption, it was possible to reach almost complete recovery of Cu(II).
A complex study investigated the adsorbing capacity of different natural fibers (viz., spruce, coconut coir, sugarcane bagasse, kenaf bast, kenaf core, and cotton) as a function of their lignin content (Lee and Rowell 2004) in relation to copper, nickel, and zinc ions in aqueous solutions. The decreasing order of ion removal was as follows:
-
Cu(II): kenaf bast > spruce > coconut coir > kenaf core > bagasse > cotton
-
Zn(II): kenaf bast > kenaf core = spruce > coconut coir > bagasse > cotton
-
Ni(II): kenaf bast > spruce > kenaf core > coconut coir > bagasse > cotton
All the fibers tested were more efficient in the adsorption of Cu(II) and Zn(II) than of Ni(II). Employing the extraction of the fibers with various reagents (diethyl ether, ethyl alcohol, hot water, and 1% sodium hydroxide), different extractives and cell wall components were removed, but ion sorption did not significantly increase. By removing the cell wall constituents, more lignin becomes available to contact with ion solution; however the study has indicated no consistent correlation between the increasing amount of available lignin and metal ion sorption.
Interesting results were also obtained with chemically modified kapok fibers (silky fibers that enclose the seeds of kapok trees Ceiba pentandra) (Zheng et al. 2015). By combined processes (chlorite-periodate oxidation), these fibers have demonstrated elevated capacity to adsorb heavy metal ions (mg/g): Pb, 93.55%; Cu, 91.83%; Cd, 89.75%; and Zn, 92.85% (Chung et al. 2008). The chemically oxidized kapok fibers can be also used as excellent sorbents for heavy metals, and their enhanced sorption may be due to the formation of carboxylic functional groups able to react chemically with the sorbate. Kapok fibers can be functionalized with diethylenetriaminepentaacetic acid (DTPA) in order to obtain sorbents with fast adsorption for heavy metals (Duan et al. 2013). Prior to this reaction, kapok fibers were washed with dichloromethane for the removal of the natural wax, and by further treatment with NaOH solution, fibers with a hollow structure and open ends were obtained, hence a larger specific surface area available for the reaction with DTPA. Maximum adsorption capacity for thusly modified kapok fibers (mg/g) was: Pb2+, 310.6; Cd2+, 163.7; and Cu2+, 101.0. The sorption capacity of fibers remained high (over 90%) even after eight adsorption-desorption cycles.
Multi-ion sorbents were also investigated (Abdolali et al. 2014). A series of novel such multi-metal binding biosorbents (MMBB) was developed by combining in different formulations three lignocellulosic waste types (from agriculture and wood industry) for the effective removal of lead, cadmium, copper, and zinc from industrial water. The tested formulations ((1) tea waste, corncob, and sugarcane bagasse; (2) tea waste, corncob, and sawdust; (3) tea waste, corncob, and apple peel; (4) tea waste, corncob, and grape stalk) have proved to be more efficient for Cd(II), Cu(II), and Pb(II) than for Zn(II). The formulation 2 has maintained its excellent sorption capacity even after five sorption-desorption cycles.
14.3.5 Other Heavy Metals
Mercury (Hg(II)), chromium (Cr(VI)), and cobalt (Co(VI)) are highly toxic to human body; thus, their removal from industrial water is of utmost importance (Escudero-Oñate et al. 2017).
It has been found that rice husk and rice straw can be functionalized by the reaction with carbon disulfide (CS2) under alkaline conditions, when S-containing xanthogenate-type reactive groups resulted (–O–CS2]−Na+), and these materials can adsorb Hg(II) by the ion exchange mechanism (Song et al. 2015). The sorption has been proved to be an endothermic and spontaneous process, and the sorbents have proved to be selective toward Hg(II), as the maximum capacity of adsorption was 119 mg/g for rice husk and 92 mg/g for rice straw.
Cr(VI) can be retained onto rice husk in relevant amounts (18.20 mg/g) at room temperature and pH = 6 (Ding et al. 2016), but after hydrothermal carbonization, the amount of adsorbed Cr(VI) was 31.1 mg/g. Experimental data have shown that the adsorption was prevented and the reduction increased when the pH of the solution was lower than 3.
Aminated rice straw-grafted poly(vinyl alcohol) (A-RS/PVA) was also employed to remove Cr(VI) from its aqueous solutions in batch experiments (Lin et al. 2018). This new sorbent sorption capacity was up to 140.39 mg/g, at initial pH = 2.0 and temperature = 60 °C, almost three times larger than that of the initial rice straw (34.90 mg/g). The process was spontaneous and endothermic, and the mechanism of sorption is based on the reduction reaction of Cr(VI) to Cr(III) which is less toxic.
Magnetic biochar prepared from Melia azedarach wood was studied as a possible sorbent for the removal of Cr(VI) (Zhang et al. 2018). It has proved a higher removal efficiency (99.8%) as compared with the biochar under the same conditions (concentration = 5 g/L, pH = 3.0, initial concentration of Cr(VI) 10 mg/L). The experimental data have indicated that the removal process consisted of a sequence of adsorption-reduction-adsorption steps: first, Cr(VI) was adsorbed onto the surface of the magnetic biochar and, subsequently, reduced to Cr(III) which was further adsorbed on the surface.
Other studies indicated more raw and modified materials as sorbents for Cr(VI) and Cr(III) (Miretzky and Cirelli 2010): bagasse, bark, bran, cake, coir, husk and hull, sawdust, biomass, etc.
Another relevant heavy metal, cobalt Co(II), was subjected to adsorption onto lignocellulosic sorbents, namely, lemon peel, in batch experiments, in order to be removed from its solutions (Bhatnagar et al. 2010). The maximum adsorption capacity of lemon peel was 22 mg/g at 25 °C. The adsorption process has been found to be exothermic. The cost efficiency study has indicated lemon peel as the most advantageous sorbent, given that its production price is ten times lower than that of activated carbons.
14.3.6 Nitrogen and Phosphorus
Nitrogen and phosphorus anions (NO3−; PO43−; NH4+) become pollutants when various phytopharmaceuticals are excessively used. In principle, these anions are difficult to be retained onto the surface of common biochars due to their electroactive structure disparity.
Still, there are studies confirming that some lignocellulosic materials can retain certain anions (Dai et al. 2018). Thus, wood- and rice husk-based materials have proved to be able to adsorb ammonium ions in NH4Cl solution and slurry solutions (Kizito et al. 2015). The mechanisms involved were chemisorption and physisorption, and the amount of sorbate was satisfactory. Other data have shown that the activated carbons based on avocado seed can bind ammonium ions (5.40 mg/g), at 298 K and pH = 5.0, by an ion exchange mechanism (Zhu et al. 2016d).
Phosphate anions (PO43−) can be removed from polluted water using biochar made of lignocellulosic waste from different sources (oak, bamboo wood, maize, soybean, and peanut shell) (Jung et al. 2015).
It is generally accepted that there is, still, a great need for further research in this sensitive field, because heavy metal decontamination is a two-edge blade due to its high potential of secondary pollution, accumulation in living organisms, and cyclic contamination in an ascending spiral.
14.4 Concluding Remarks and Future Prospects
Purification of industrial water using lignocellulosic waste materials is a highly challenging theme of research.
On one hand, there is a significant amount of studies reporting on various materials and methods. On the other hand, most of the experimental data remain as literature because only very few of them are transferred to industry.
It is rather difficult to attempt to cover a wide range of inexpensive, locally available, and efficient materials as to be used instead of the commercially available activated carbons when it comes to removing various contaminants from industrial water. But little effort has been made to perform a cost comparison between these two classes of sorbents. This analysis is mainly needed in order to promote the large-scale use of lignocellulosic waste adsorbents so that their efficiency is maximized and the exploitation of local agriculture waste increases, as well as the technical feasibility, because, in the end, low-cost sorbents can provide promising benefits.
At this point, a few aspects of the ecological impact of the biobased materials should be mentioned. Concerns over global climate changes and the food insurance have been, at least partially, the driving force of the development of biobased materials. Still, this trend can be regarded as a challenge to academic and industry R&D scientists, and policy makers, as the manufacture of biobased materials requires land, sometimes the crops are used less for food, and it is associated with some adverse environmental effects. Recent data have indicated that one metric ton (t) of biobased materials may save, as compared to classic materials, 55 ± 34 GJ of primary energy and 3 ± 1 t carbon dioxide equivalents of greenhouse gases (Weiss et al. 2012). Still, the use of the biobased materials may also increase the eutrophication by 5 ± 7 kg phosphate equivalents/t and depletion of the ozone layer in the stratosphere by 1.9 ± 1.8 kg nitrous oxide equivalents/t. The additional land use may entail a partial loss of biodiversity, depletion of carbon in soil and soil erosion, possible deforestation, and greenhouse gas emission.
Nevertheless, future research must focus on:
-
identifying solutions for local pollution problems, because, in spite of the global character of some ideas, their application is intrinsically linked to and limited by the local availability of materials;
-
the design of new approaches by the use of combined methodologies (e.g., algae and microalgae, or combined bacterial and algal cultures applied directly in areas containing polluted water, followed by specific biologic decontamination and regeneration, if possible, in order to avoid secondary contamination);
-
creating hybrid methods (such as the direct transfer of the removed dyes to assigned facilities for further decomposition up to non-toxic or low toxic compounds) and materials (i.e., lignocellulosic fibers can be incorporated into biopolymeric thermoplastic matrices, as chitosan, starch, or even cellulose and lignin, and, at the same time, various natural nanofillers with sorbent properties, such as clays and zeolites, can be added to the formulations);
-
the research of new methods for the regeneration of sorbents without causing secondary pollution;
-
the development of highly effective green modifiers so that raw materials would be harmlessly and cost-effectively transformed as to achieve an improved adsorption.
And the list may continue as only the human intelligence and creativity can limit the development of this field of research.
References
Abdelwahab NA, Shukry N, El-kalyoubi SF (2017) Preparation and characterization of polymer coated partially esterified sugarcane bagasse for separation of oil from seawater. Environ Technol 38:1905–1914. https://doi.org/10.1080/09593330.2016.1240243
Abdolali A, Ngo HH, Guo WS, Lee DJ, Tung KL, Wang XC (2014) Development and evaluation of a new multi-metal binding biosorbent. Bioresour Technol 160:98–106. https://doi.org/10.1016/j.biortech.2013.12.038
Agouborde L, Navia R (2009) Heavy metals retention capacity of a non-conventional sorbent developed from a mixture of industrial and agricultural wastes. J Hazard Mater 167:536–544. https://doi.org/10.1016/j.jhazmat.2009.01.027
Ali SB, Jaouali I, Souissi-Najar S, Ouederni A (2017) Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J Clean Prod 142:3809–3821. https://doi.org/10.1016/j.jclepro.2016.10.081
Amela K, Hassen MA, Kerroum D (2012) Isotherm and kinetics study of biosorption of cationic dye onto banana peel. Energy Procedia 12:286–295. https://doi.org/10.1016/j.egypro.2012.05.208
Amer H, El-Gendy A, El-Haggar S (2017) Removal of lead (II) from aqueous solutions using rice straw. Water Sci Technol 76:1011–1021. https://doi.org/10.2166/wst.2017.249
Bhatnagar A, Minocha AK, Sillanpää M (2010) Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem Eng J 48:181–186. https://doi.org/10.1016/j.bej.2009.10.005
Bhatti HN, Bajwa II, Hanif MA, Bukhari IH (2010) Removal of lead and cobalt using lignocellulosic fiber derived from Citrus reticulata waste biomass. Korean J Chem Eng 27:218–227. https://doi.org/10.1007/s11814-009-0325-1
Blanch GL (2016) Removal of pharmaceuticals from WWTP streams by biological and physical processes. PhD Thesis, Universitat Autonome de Barcelona, Bellaterra, Spain. isbn: 9788449066733. https://www.tdx.cat/handle/10803/399517#page=1. Accessed 15 Aug 2019
Bouguettoucha A, Reffas A, Chebli D, Mekhalif T, Amrane A (2016) Novel activated carbon prepared from an agricultural waste, Stipa tenacissima, based on ZnCl2 activation—characterization and application to the removal of methylene blue. Desalin Water Treat 57:24056–24069. https://doi.org/10.1080/19443994.2015.1137231
Chan YH, Yusup S, Quitain AT, Tan RR, Sasaki M, Lam HL, Uemura Y (2015) Effect of process parameters on hydrothermal liquefaction of oil palm biomass for bio-oil production and its life cycle assessment. Energy Convers Manag 104:180–188. https://doi.org/10.1016/j.enconman.2015.03.075
Chebli D, Bouguettoucha A, Mekhalef T, Nacef S, Amrane A (2015) Valorization of an agricultural waste, Stipa tenassicima fibers, by biosorption of an anionic azo dye, Congo red. Desalin Water Treat 54:245–254. https://doi.org/10.1080/19443994.2014.880154
Chung BY, Cho JY, Lee MH, Wi SG, Kim JH, Kim JS, Kang PH, Nho YC (2008) Adsorption of heavy metal ions onto chemically oxidized Ceiba pentandra (L.) Gaertn. (kapok) fibers. J Appl Biol Chem 51:28–35. https://doi.org/10.3839/jabc.2008.006
Cooney DO (1999) Adsorption design for wastewater treatment. Lewis Publishers, CRC Press LLC, Boca Raton, p 182
Dai Y, Sun Q, Wang W, Lu L, Liu M, Li J, Yang S, Sun Y, Zhang K, Xu J, Zheng W, Hu Z, Yang Y, Gao Y, Chen Y, Zhang X, Gao F, Zhang Y (2018) Utilizations of agricultural waste as adsorbent for the removal of contaminants: a review. Chemosphere 211:235–253. https://doi.org/10.1016/j.chemosphere.2018.06.179
Dardouri S, Sghaier J (2017) A comparative study of adsorption and regeneration with different agricultural wastes as adsorbents for the removal of methylene blue from aqueous solution. Chin J Chem Eng 25:1282–1287. https://doi.org/10.1016/j.cjche.2017.01.012
de Andrade JR, Oliveira MF, da Silva MGC, Vieira MGA (2018) Adsorption of pharmaceuticals from water and wastewater using nonconventional low-cost materials: a review. Ind Eng Chem Res 57:3103–3127. https://doi.org/10.1021/acs.iecr.7b05137
De Gisi S, Lofrano G, Grassi M, Notarnicola M (2016) Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: a review. Sustain Mater Technol 9:10–40. https://doi.org/10.1016/j.susmat.2016.06.002
Demirbas A (2009) Agricultural based activated carbons for the removal of dyes from aqueous solutions: a review. J Hazard Mater 167:1–9. https://doi.org/10.1016/j.jhazmat.2008.12.114
Deng F, Zhong F, Lin DC, Zhao LN, Liu YJ, Huang JH, Luo XB, Luo SL, Dionysiou DD (2017) One-step hydrothermal fabrication of visible-light responsive AgInS2/SnIn4S8 heterojunction for highly-efficient photocatalytic treatment of organic pollutants and real pharmaceutical industry wastewater. Appl Catal B Environ 219:163–172. https://doi.org/10.1016/j.apcatb.2017.07.051
Deokar SK, Mandavgane SA, Kulkarni BD (2016) Adsorptive removal of 2,4-dichlorophenoxyacetic acid from aqueous solution using bagasse fly ash as adsorbent in batch and packed-bed techniques. Clean Technol Environ 18:1971–1983. https://doi.org/10.1007/s10098-016-1124-0
Ding D, Ma X, Shi W, Lei Z, Zhang Z (2016) Insights into mechanisms of hexavalent chromium removal from aqueous solution by using rice husk pretreated with hydrothermal carbonization technology. RSC Adv 78:74675–74682. https://doi.org/10.1039/C6RA17707G
Djelloul C, Hamdaoui O (2015) Dynamic adsorption of methylene blue by melon peel in fixed-bed columns. Desalin Water Treat 56:2966–2975. https://doi.org/10.1080/19443994.2014.963158
Du ZL, Zheng T, Wang P, Hao LL, Wang YX (2016) Fast microwave-assisted preparation of a low-cost and recyclable carboxyl modified ligno-cellulose biomass jute fiber for enhanced heavy metal removal from water. Bioresour Technol 201:41–49. https://doi.org/10.1016/j.biortech.2015.11.009
Duan CT, Zhao N, Yu XL, Zhang XY, Xu J (2013) Chemically modified kapok fiber for fast adsorption of Pb2+, Cd2+, Cu2+ from aqueous solution. Cellulose 20:849–860. https://doi.org/10.1007/s10570-013-9875-9
El-Deen GES, El-Deen SEAS (2016) Kinetic and isotherm studies for adsorption of Pb(II) from aqueous solution onto coconut shell activated carbon. Desalin Water Treat 57:28910–28931. https://doi.org/10.1080/19443994.2016.1193825
Escudero-Oñate C, Fiol N, Poch J, Villaescusa I (2017) Chapter 17: valorisation of lignocellulosic biomass wastes for the removal of metal ions from aqueous streams: a review. In: Biomass volume estimation and valorization for energy. InTech Open, p 381–407. https://doi.org/10.5772/65958
Etim UJ, Umoren SA, Eduok UM (2016) Coconut coir dust as a low-cost adsorbent for the removal of cationic dye from aqueous solution. J Saudi Chem Soc 20:S67–S76. https://doi.org/10.1016/j.jscs.2012.09.014
Farasati M, Haghighi S, Boroun S (2015) Cd removal from aqueous solution using agricultural wastes. Desalin Water Treat 57:11164–11172. https://doi.org/10.1080/19443994.2015.1043588
Farhan AM, Salem NM, Al-Dujaili AH, Awwad AM (2012) Biosorption studies of Cr(VI) ions from electroplating wastewater by walnut shell powder. Am J Environ Eng 2:188–195. https://doi.org/10.5923/j.ajee.20120206.07
Fathi MR, Asfaram A, Farhangic A (2015) Removal of Direct Red 23 from aqueous solution using corn stalks: isotherms, kinetics and thermodynamic studies. Spectrochim Acta A 135:364–372. https://doi.org/10.1016/j.saa.2014.07.008
Fazal S, Zhang B, Mehmood Q (2015) Biological treatment of combined industrial wastewater. Ecol Eng 84:551–558. https://doi.org/10.1016/j.ecoleng.2015.09.014
Feng J, Qiao K, Pei LY, Lv JP, Xie SL (2015) Using activated carbon prepared from Typha orientalis Presl to remove phenol from aqueous solutions. Ecol Eng 84:209–217. https://doi.org/10.1016/j.ecoleng.2015.09.028
Gamiz B, Pignatello JJ, Cox L, Hermosín MC, Celis R (2016) Environmental fate of the fungicide metalaxyl in soil amended with composted olive-mill waste and its biochar: an enantioselective study. Sci Total Environ 541:776–783. https://doi.org/10.1016/j.scitotenv.2015.09.097
Gayathri PV, Yesodharan S, Yesodharan EP (2017) Purification of water contaminated with traces of rhodamine B dye by microwave-assisted, oxidant induced and zinc oxide catalyzed advanced oxidation process. Desalin Water Treat 85:161–174. https://doi.org/10.5004/dwt.2017.21286
Gondhalekar SC, Shukla SR (2015) Biosorption of cadmium metal ions on raw and chemically modified walnut shells. Environ Prog Sustain Energy 34:1613–1619. https://doi.org/10.1002/ep.12161
Guan W, Tian SC (2017) The modified chitosan for dyeing wastewater treatment via adsorption and flocculation. Sci Adv Mater 9:1603–1609. https://doi.org/10.1166/sam.2017.3156
Guechi EK, Hamdaoui O (2015a) Biosorption of methylene blue from aqueous solution by potato (Solanum tuberosum) peel: equilibrium modelling, kinetic, and thermodynamic studies. Desalin Water Treat 57:10270–10285. https://doi.org/10.1080/19443994.2015.1035338
Guechi EK, Hamdaoui O (2015b) Evaluation of potato peel as a novel adsorbent for the removal of Cu (II) from aqueous solutions: equilibrium, kinetic, and thermodynamic studies. Desalin Water Treat 57:10677–10688. https://doi.org/10.1080/19443994.2015.1038739
Gülen J, Akın B, Ozgür M (2015) Ultrasonic-assisted adsorption of methylene blue on sumac leaves. Desalin Water Treat 57:9286–9295. https://doi.org/10.1080/19443994.2015.1029002
Guo L, Liang LY, Wang YJ, Liu MD (2015) Biosorption of Pb2+ from aqueous solution by rice straw modified with citric acid. Environ Prog Sustain 35:359–367. https://doi.org/10.1002/ep.12225
Gupta H, Gupta B (2015) Adsorption of polycyclic aromatic hydrocarbons on banana peel activated carbon. Desalin Water Treat 57:9498–9509. https://doi.org/10.1080/19443994.2015.1029007
Gupta N, Kushwaha AK, Chattopadhyaya MC (2016) Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arab J Chem 9:S707–S716. https://doi.org/10.1016/j.arabjc.2011.07.021
Heng KS, Hatti-Kaul R, Adam F, Fukui T, Sudesh K (2017) Conversion of rice husks to polyhydroxyalkanoates (PHA) via a three-step process: optimized alkaline pretreatment, enzymatic hydrolysis, and biosynthesis by Burkholderia cepacia USM (JCM 15050). J Chem Technol Biotechnol 92(1):100–108. https://doi.org/10.1002/jctb.4993
Hong GB, Wang YK (2017) Synthesis of low-cost adsorbent from rice bran for the removal of reactive dye based on the response surface methodology. Appl Surf Sci 423:800–809. https://doi.org/10.1016/j.apsusc.2017.06.264
Hosseinzadeh H, Mohammadi S (2016) Biosorption of anionic dyes from aqueous solutions using a novel magnetic nanocomposite adsorbent based on rice husk ash. Sep Sci Technol 51:939–953. https://doi.org/10.1080/01496395.2016.1142564
Ibrahim S, Wang SB, Ang HM (2010) Removal of emulsified oil from oily wastewater using agricultural waste barley straw. Biochem Eng J 49:78–83. https://doi.org/10.1016/j.bej.2009.11.013
Imamoglu M, Şahin H, Aydın Ş, Tosunoglu F, Yılmaz H, Yıldız SZ (2016) Investigation of Pb(II) adsorption on a novel activated carbon prepared from hazelnut husk by K2CO3 activation. Desalin Water Treat 57:4587–4596. https://doi.org/10.1080/19443994.2014.995135
Inam EI, Etim UJ, Akpabio EG, Umoren SA (2015) Simultaneous adsorption of lead (II) and 3,7-bis(dimethylamino)-phenothiazin-5-ium chloride from aqueous solution by activated carbon prepared from plantain peels. Desalin Water Treat 57:6540–6553. https://doi.org/10.1080/19443994.2015.1010236
International Agency for Research on Cancer (IARC) (1987) Overall evaluations of carcinogenicity: An updating of IARC monographs volumes, vol 7. WHO, Lyon, pp 1–42. http://monographs.iarc.fr. Accessed 11 Aug 2019
Isa MH, Lang LS, Asaari FAH, Hamidi AA, Ramli NA, Dhas JPA (2007) Low cost removal of disperse dyes from aqueous solution using palm ash. Dyes Pigments 74:446–453. https://doi.org/10.1016/j.dyepig.2006.02.025
Janssens R, Mandal MK, Dubey KK, Luis P (2017) Slurry photocatalytic membrane reactor technology for removal of pharmaceutical compounds from wastewater: towards cytostatic drug elimination. Sci Total Environ 599–600:612–626. https://doi.org/10.1016/j.scitotenv.2017.03.253
Jawad AH, Rashid RA, Mahmuod RMA, Ishak MAM, Kasim NN, Ismail K (2015) Adsorption of methylene blue onto coconut (Cocos nucifera) leaf: optimization, isotherm and kinetic studies. Desalin Water Treat 57(19):8839–8853. https://doi.org/10.1080/19443994.2015.1026282
Jung KW, Hwang MJ, Ahn KH, Ok YS (2015) Kinetic study on phosphate removal from aqueous solution by biochar derived from peanut shell as renewable adsorptive media. Intl J Environ Sci Technol 12:3363–3372. https://doi.org/10.1007/s13762-015-0766-5
Karri RR, Jayakumar NS, Sahu JN (2017) Modelling of fluidized bed reactor by differential evolution optimization for phenol removal using coconut shells based activated carbon. J Mol Liq 231:249–262. https://doi.org/10.1016/j.molliq.2017.02.003
Karthick K, Namasivayam C, Pragasan LA (2017) Removal of direct red 12B from aqueous medium by ZnCl2 activated Jatropha husk carbon: adsorption dynamics and equilibrium studies. Indian J Chem Technol 24:73–81. http://nopr.niscair.res.in/handle/123456789/39755. Accessed 11 Aug 2019
Kizito S, Wu S, Kirui WK, Lei M, Lu Q, Bah H, Dong R (2015) Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci Total Environ 505:102–112. 10.1016/j.scitotenv.2014.09.096
Kronenberg M, Trably E, Bernet N, Patureau D (2017) Biodegradation of polycyclic aromatic hydrocarbons: using microbial bioelectrochemical systems to overcome an impasse. Environ Pollut 231:509–523. https://doi.org/10.1016/j.envpol.2017.08.048
Kyzas GZ, Deliyanni EA (2015) Modified activated carbons from potato peels as green environmental-friendly adsorbents for the treatment of pharmaceutical effluents. Chem Eng Res Des 97:135–144. https://doi.org/10.1016/j.cherd.2014.08.020
Lee BG, Rowell RM (2004) Removal of heavy metal ions from aqueous solutions using lignocellulosic fibers. J Nat Fibers 1:97–108. https://doi.org/10.1300/J395v01n01_07
Lee LY, Gan S, Tan MSY, Lim SS, Lee XJ, Lam YF (2015) Effective removal of acid blue 113 dye using overripe Cucumis sativus peel as an eco-friendly biosorbent from agricultural residue. J Clean Prod 113:194–203. https://doi.org/10.1016/j.jclepro.2015.11.016
Lin C, Luo W, Luo T, Zhou Q, Li H, Jing L (2018) A study on adsorption of Cr (VI) by modified rice straw: characteristics, performances and mechanism. J Clean Prod 196:626–634. https://doi.org/10.1016/j.jclepro.2018.05.279
Marin-Rangel VM, Cortes-Martines R, Villanueva RAC, Garnica-Romo MG, Martinez-Flores HE (2012) As(V) biosorption in an aqueous solution using chemically treated lemon (Citrus aurantifolia swingle) residues. J Food Sci 71:10–14. https://doi.org/10.1111/j.1750-3841.2011.02466.x
Mashhadi S, Javadian H, Ghasemi M, Saleh TA, Gupta VK (2016) Microwave induced H2SO4 activation of activated carbon derived from rice agricultural wastes for sorption of methylene blue from aqueous solution. Desalin Water Treat 57:21091–21104. https://doi.org/10.1080/19443994.2015.1119737
Miretzky P, Cirelli AF (2010) Cr (VI) and Cr (III) removal from aqueous solution by raw and modified lignocellulosic materials: a review. J Hazard Mater 180:1–19. https://doi.org/10.1016/j.jhazmat.2010.04.060
Modenes AN, Espinoza-Quinones FR, Geraldi CAQ, Manenti DR, Trigueros DEG, Oliveira APD, Borba CE, Kroumov AD (2015) Assessment of the banana pseudostem as a low-cost biosorbent for the removal of reactive blue 5G dye. Environ Technol 36:2892–2902. https://doi.org/10.1080/09593330.2015.1051591
Mokkapati RP, Mokkapati J, Ratnakaram VN (2016) Kinetic, isotherm and thermodynamics investigation on adsorption of divalent copper using agro-waste biomaterials, Musa acuminata, Casuarina equisetifolia L. and Sorghum bicolor. Pol J Chem Technol 18:68–77. https://doi.org/10.1515/pjct-2016-0031
Naushad M, Khan MA, Alothman ZA, Khan MR, Kumar M (2015) Adsorption of methylene blue on chemically modified pine nut shells in single and binary systems: isotherms, kinetics, and thermodynamic studies. Desalin Water Treat 57:15848–15861. https://doi.org/10.1080/19443994.2015.1074121
Nayak AK, Pal A (2017) Green and efficient biosorptive removal of methylene blue by Abelmoschus esculentus seed: process optimization and multi-variate modeling. J Environ Manag 200:145–159. https://doi.org/10.1016/j.jenvman.2017.05.045
Nguyen TAH, Ngo HH, Guo WS, Zhang J, Liang S, Yue QY, Li Q, Nguyen TV (2013) Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour Technol 148:574–585. https://doi.org/10.1016/j.biortech.2013.08.124
Njoku VO, Islam MA, Asif M, Hameed BH (2015) Adsorption of 2,4-dichlorophenoxyacetic acid by mesoporous activated carbon prepared from H3PO4-activated langsat empty fruit bunch. J Environ Manag 154:138–144. 10.1016/j.jenvman.2015.02.002
O’Donnel J (2018) Industrial pollution: causes and effects and biggest culprits of global warming. https://www.conservationinstitute.org/industrial-pollution. Accessed 11 Aug 2019
Oei BC, Ibrahim S, Wang SB, Ang HM (2009) Surfactant modified barley straw for removal of acid and reactive dyes from aqueous solution. Bioresour Technol 100:4292–4295. https://doi.org/10.1016/j.biortech.2009.03.063
Ojha AK, Bulasara VK (2015) Adsorption characteristics of jackfruit leaf powder for the removal of Amido Black 10B dye. Environ Prog Sustain 34:461–470. https://doi.org/10.1002/ep.12015
Okoro IA, Okoro SO (2011) Agricultural byproducts as green chemistry absorbents for the removal and recovery of metal ions from wastewater environment. Continental J Water Air Soil Pollut 2:15–22
Omidvar-Hosseini F, Moeinpour F (2016) Removal of Pb(II) from aqueous solutions using Acacia Nilotica seed shell ash supported Ni0.5Zn0.5Fe2O4 magnetic nanoparticles. J Water Reuse Desal 6:562–573. https://doi.org/10.2166/wrd.2016.073
Pachathu A, Ponnusamy K, Srinivasan SKVR (2016) Packed bed column studies on the removal of emulsified oil from water using raw and modified bagasse and corn husk. J Mol Liq 223:1256–1263. https://doi.org/10.1016/j.molliq.2016.09.048
Palma C, Lloret L, Puen A, Tobar M, Contreras E (2016) Production of carbonaceous material from avocado peel for its application as alternative adsorbent for dyes removal. Chin J Chem Eng 24:521–528. https://doi.org/10.1016/j.cjche.2015.11.029
Parlayıcı Ş, Pehlivan E (2017) Removal of metals by Fe3O4 loaded activated carbon prepared from plum stone (Prunus nigra): kinetics and modelling study. Powder Technol 317:23–30. https://doi.org/10.1016/j.powtec.2017.04.021
Pena D, Lopez-Pineiro A, Albarran A, Rato-Nunes JM, Sanchez-Llerena J, Becerra D, Ramírez M (2016) De-oiled two-phase olive mill waste may reduce water contamination by metribuzin. Sci Total Environ 541:638–645. https://doi.org/10.1016/j.scitotenv.2015.09.019
Portinho R, Zanella O, Feris LA (2017) Grape stalk application for caffeine removal through adsorption. J Environ Manag 202:178–187. https://doi.org/10.1016/j.jenvman.2017.07.033
Quesada HB, Takaoka Alves Baptista A, Cusioli LF, Seibert D, de Oliveira Bezerra C, Bergamasco R (2019) Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: a review. Chemosphere 222:766–780. https://doi.org/10.1016/j.chemosphere.2019.02.009
Rattanachueskul N, Saning A, Kaowphong S, Chumha N, Chuenchom L (2016) Magnetic carbon composites with a hierarchical structure for adsorption of tetracycline, prepared from sugarcane bagasse via hydrothermal carbonization coupled with simple heat treatment process. Bioresour Technol 226:164–172. https://doi.org/10.1016/j.biortech.2016.12.024
Rosales E, Meijide J, Pazos M, Sanroman MA (2017) Challenges and recent advances in biochar as low-cost biosorbent: from batch assays to continuous-flow systems. Bioresour Technol 246:176–192. https://doi.org/10.1016/j.biortech.2017.06.084
Safa Y (2016) Utilization of mustard and linseed oil cakes: novel biosorbents for removal of acid dyes. Desalin Water Treat 57:5914–5925. https://doi.org/10.1080/19443994.2015.1007087
Saka C, Sahin O, Kucuk MM (2012) Application on agricultural and forest waste adsorbents for the removal of Pb(II) from contaminated waters. Int J Environ Sci Technol 9:379–394. https://doi.org/10.1007/s13762-012-0041-y
Sandoval A, Hernandez-Ventura C, Klimova TE (2017) Titanate nanotubes for removal of methylene blue dye by combined adsorption and photocatalysis. Fuel 198:22–30. https://doi.org/10.1016/j.fuel.2016.11.007
Sarasidis VC, Plakas KV, Karabelas AJ (2017) Novel water-purification hybrid processes involving in situ regenerated activated carbon, membrane separation and advanced oxidation. Chem Eng J 328:1153–1163. https://doi.org/10.1016/j.cej.2017.07.084
Shah BA, Pandya DD, Shah HA (2016) Impounding of ortho-chlorophenol by zeolitic materials adapted from bagasse fly ash: four factor three level Box-Behnken design modelling and optimization. Arab J Sci Eng 42:241–260. https://doi.org/10.1007/s13369-016-2294-0
Shang Y, Zhang JH, Wang X, Zhang RD, Xiao W, Zhang SS, Han RP (2015) Use of polyethyleneimine-modified wheat straw for adsorption of Congo red from solution in batch mode. Desalin Water Treat 57:8872–8883. https://doi.org/10.1080/19443994.2015.1027280
Sharma S, Tiwari DP (2016) Model-fitting approach for methylene blue dye adsorption on Camelina and Sapindus seeds-derived adsorbents. Adsorpt Sci Technol 34:565–580. https://doi.org/10.1177/0263617416674949
Sherafatmand M, Ng HY (2015) Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresour Technol 195:122–130. https://doi.org/10.1016/j.biortech.2015.06.002
Singh S, Shukla SR (2017) Theoretical studies on adsorption of Ni (II) from aqueous solution using Citrus limetta peels. Environ Prog Sustain Energy 36:864–872. https://doi.org/10.1002/ep.12526
Singha AS, Guleria A (2014) Utility of chemically modified agricultural waste okra biomass for removal of toxic heavy metal ions from aqueous solution. Eng Agr Environ Food 8:52–60. https://doi.org/10.1016/j.eaef.2014.08.001
Smitha T, Santhi T, Prasad AL, Manonman S (2012) Cucumis sativus used as adsorbent for the removal of dyes from aqueous solution. Arab J Chem 10:S244–S251. https://doi.org/10.1016/j.arabjc.2012.07.030
Sohrabi H, Ameri E (2015) Adsorption equilibrium, kinetics, and thermodynamics assessment of the removal of the reactive red 141 dye using sesame waste. Desalin Water Treat 57:18087–18098. https://doi.org/10.1080/19443994.2015.1087345
Song ST, Saman N, Johari K, Mat H (2015) Biosorption of mercury from aqueous solution and oilfield produced water by pristine and sulfur functionalized rice residues. Environ Prog Sustain Energy 34:1298–1310. https://doi.org/10.1002/ep.12116
Subbaiah MV, Kim DS (2016) Adsorption of methyl orange from aqueous solution by aminated pumpkin seed powder: kinetics, isotherms, and thermodynamic studies. Ecotox Environ Safe 128:109–117. https://doi.org/10.1016/j.ecoenv.2016.02.016
Suteu D, Malutan T, Bilba D (2011) Agricultural waste corn cob as a sorbent for removing reactive dye Orange 16: equilibrium and kinetic study. Cellulose Chem Technol 45:413–420. http://www.cellulosechemtechnol.ro. Accessed 11 Aug 2019
Suteu D, Biliuta G, Rusu L, Coseri S, Nacu G (2015) Cellulose cellets as new type of adsorbent for the removal of dyes from aqueous media. Environ Eng Manag J 14:525–532. http://www.eemj.icpm.tuiasi.ro/pdfs/vol14/no3/4_998_Suteu_14.pdf. Accessed 13 Aug 2019
Tang RX, Dai C, Li C, Liu WH, Gao ST, Wang C (2017a) Removal of methylene blue from aqueous solution using agricultural residue walnut shell: equilibrium, kinetic, and thermodynamic studies. J Chem 2017, 10 pages. https://doi.org/10.1155/2017/84049651-10:8404965
Tang CF, Shu Y, Zhang RQ, Li X, Song JF, Li B, Zhang YT, Ou DL (2017b) Comparison of the removal and adsorption mechanisms of cadmium and lead from aqueous solution by activated carbons prepared from Typha angustifolia and Salix matsudana. RSC Adv 26:16092–16103. https://doi.org/10.1039/C6RA28035H
Tomczak E, Tosik P (2017) Waste plant material as a potential adsorbent of a selected azo dye. Chem Process Eng 38:283–294. https://doi.org/10.1515/cpe-2017-0021
Tonucci MC, Gurgel LVA, Aquino SFD (2015) Activated carbons from agricultural byproducts (pine tree and coconut shell), coal, and carbon nanotubes as adsorbents for removal of sulfamethoxazole from spiked aqueous solutions: kinetic and thermodynamic studies. Ind Crop Prod 74:111–121. https://doi.org/10.1016/j.indcrop.2015.05.003
Toumi LB, Hamdi L, Salem Z, Allia K (2015) Batch adsorption of methylene blue from aqueous solutions by untreated Alfa grass. Desalin Water Treat 53:806–817. https://doi.org/10.1080/19443994.2013.846236
Tran VS, Ngo HH, Guo WS, Zhang J, Liang S, Ton-That C, Zhang XB (2015) Typical low cost biosorbents for adsorptive removal of specific organic pollutants from water. Bioresour Technol 182:353–363. https://doi.org/10.1016/j.biortech.2015.02.003
Trivedi NS, Mandavgane SA, Kulkarn BD (2016) Mustard plant ash: a source of micronutrient and an adsorbent for removal of 2,4-dichlorophenoxyacetic acid. Environ Sci Pollut Res 23(20):20087–20099. https://doi.org/10.1007/s11356-016-6202-7
Tunç O, Tanaci H, Aksu Z (2009) Potential use of cotton plant wastes for the removal of Remazol Black B reactive dye. J Hazard Mater 163:187–198. https://doi.org/10.1016/j.jhazmat.2008.06.078
Wang P, Ma QY, Hu DY, Wang LJ (2015) Adsorption of methylene blue by a low-cost biosorbent: citric acid modified peanut shell. Desalin Water Treat 57:10261–10269. https://doi.org/10.1080/19443994.2015.1033651
Wang H, Chu Y, Fang C, Huang F, Song Y, Xue X (2017) Sorption of tetracycline on biochar derived from rice straw under different temperatures. PLoS One 12:e0182776. https://doi.org/10.1371/journal.pone.0182776
Wang H, Fang C, Wang Q, Chu Y, Song Y, Chen Y, Xue X (2018) Sorption of tetracycline on biochar derived from rice straw and swine manure. RSC Adv 8:16260–16268. https://doi.org/10.1039/C8RA01454J
Weiss M, Haufe J, Carus M, Brandão M, Bringezu S, Hermann B, Patel MK (2012) A review of the environmental impacts of biobased materials. J Ind Ecol 16:S169–S181. https://doi.org/10.1111/j.1530-9290.2012.00468.x
Worch E (2012) Adsorption technology in water treatment: fundamentals, processes, and modeling. Walter de Gruyter, Berlin/Boston, p 6–10. isbn: 978-3-11-024022-1, e-isbn: 978-3-11-024023-8
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184. https://doi.org/10.1016/j.cis.2014.04.002
Yin YY, Guo XT, Yang C, Gao LM, Hu YB (2016) An efficient method for tylosin removal from an aqueous solution by goethite modified straw mass. RSC Adv 98:95425–95434. https://doi.org/10.1039/C6RA19172J
Zhang J, Chen H, Chen Z, He J, Shi W, Liu D, Chi H, Fuyi C, Wang W (2016) Microstructured macroporous adsorbent composed of polypyrrole modified natural corn cob-core sponge for Cr (VI) removal. RSC Adv 64:59292–59298. https://doi.org/10.1039/C6RA07687D
Zhang X, Lv L, Qin Y, Xu M, Jia X, Chen Z (2018) Removal of aqueous Cr (VI) by a magnetic biochar derived from Melia azedarach wood. Bioresour Technol 256:1–10. https://doi.org/10.1016/j.biortech.2018.01.145
Zheng Y, Wang J, Zhu Y, Wang A (2015) Research and application of kapok fiber as an absorbing material: a mini review. J Environ Sci 27:21–32. https://doi.org/10.1016/j.jes.2014.09.026
Zhou Y, Zhang L, Cheng ZJ (2015) Removal of organic pollutants from aqueous solution using agricultural wastes: a review. J Mol Liq 212:739–762. https://doi.org/10.1016/j.molliq.2015.10.023
Zhou RS, Zhou RW, Zhang XH, Tu S, Yin YW, Yang SZ, Ye LY (2016) An efficient bio-adsorbent for the removal of dye: adsorption studies and cold atmospheric plasma regeneration. J Taiwan Inst Chem Eng 68:372–378. https://doi.org/10.1016/j.jtice.2016.09.030
Zhu C, Duan YF, Wu CY, Zhou Q, She M, Yao T, Zhang J (2016a) Mercury removal and synergistic capture of SO2/NO by ammonium halides modified rice husk char. Fuel 172:160–169. https://doi.org/10.1016/j.fuel.2015.12.061
Zhu L, Wang Y, He TT, You LJ, Shen XQ (2016b) Assessment of potential capability of water bamboo leaves on the adsorption removal efficiency of cationic dye from aqueous solutions. J Polym Environ 24:148–158. https://doi.org/10.1007/s10924-016-0757-8
Zhu NY, Yan TM, Qiao J, Cao HL (2016c) Adsorption of arsenic, phosphorus and chromium by bismuth impregnated biochar: adsorption mechanism and depleted adsorbent utilization. Chemosphere 164:32–40. https://doi.org/10.1016/j.chemosphere.2016.08.036
Zhu YY, Kolar P, Shah SB, Cheng JJ, Lim PK (2016d) Avocado seed-derived activated carbon for mitigation of aqueous ammonium. Ind Crops Prod 92:34–41. https://doi.org/10.1016/j.indcrop.2016.07.016
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tanasă, F., Teacă, CA., Nechifor, M. (2020). Lignocellulosic Waste Materials for Industrial Water Purification. In: Inamuddin, Asiri, A. (eds) Sustainable Green Chemical Processes and their Allied Applications. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-42284-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-42284-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42283-7
Online ISBN: 978-3-030-42284-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)