Abstract
In plants, embryogenesis generally occurs through the sexual process of double fertilization, which involves a haploid sperm cell fusing with a haploid egg cell to ultimately give rise to a diploid embryo. Embryogenesis can also occur asexually in the absence of fertilization, both in vitro and in vivo. Somatic or gametic cells are able to differentiate into embryos in vitro following the application of plant growth regulators or stress treatments. Asexual embryogenesis also occurs naturally in some plant species in vivo, from either ovule cells as part of a process defined as apomixis, or from somatic leaf tissue in other species. In both in vitro and in vivo asexual embryogenesis, the embryo precursor cells must attain an embryogenic fate without the act of fertilization. This review compares the processes of in vitro and in vivo asexual embryogenesis including what is known regarding the genetic and epigenetic regulation of each process, and considers how the precursor cells are able to change fate and adopt an embryogenic pathway.
Access provided by CONRICYT – Journals CONACYT. Download protocol PDF
Similar content being viewed by others
Key words
- Adventitious embryony
- Apomixis
- Cell fate
- Gametic embryogenesis
- Kalanchoë
- Parthenogenesis
- Somatic embryo genesis
1 Introduction
Embryogenesis describes the development of a single cell into an embryo. In plant embryogenesis there is no cell migration, so embryo pattern formation and cell type specification is interrelated with oriented cell division and expansion. Within sexual angiosperm plant species, embryogenesis usually occurs in vivo within floral organs during the events of seed formation. Formation of an embryo can also occur via asexual pathways in seeds, from somatic plant cells in vivo or be induced experimentally from somatic plant explants or gametes in vitro.
This review describes and compares the processes of in vivo and in vitro asexual embryogenesis including what is currently understood regarding the molecular mechanisms underlying each process.
2 Types of Embryogenesis
2.1 Zygotic (Sexual) Embryogenesis
The most prevalent form of embryogenesis in plants occurs following double fertilization in the female gametophyte (embryo sac) found in the ovule of the flower, which gives rise to the embryo and endosperm compartments of the seed (Table 1; Fig. 1a). Haploid male and female gametes form in the anther and ovule, respectively, via meiosis and subsequent mitosis [1, 2]. Double fertilization initiates when the male pollen tube containing two sperm cells enters the ovule. One haploid sperm cell fuses with the meiotically derived haploid egg cell in the female gametophyte to form the single-celled diploid zygote, which then undergoes cell division and pattern forming events to give rise to the diploid embryo [3]. The other haploid sperm cell fuses with the diploid central cell nucleus of the embryo sac, which initiates divisions to form triploid endosperm that provides resources to the developing embryo [4]. Ovule tissues that surround the embryo and endosperm contribute to the seed coat.
Asexual embryogenesis occurs in vivo and in vitro from different cell types. (a) Floral organs and leaves are some of the source plant tissue for inducing embryogenesis in vitro. Asexual embryos also form in ovules in vivo; (b) Parthenogenesis involves the development of a chromosomally reduced or unreduced egg cell (yellow) into an embryo without fertilization ; (c) Nucellar or integument cells (red) adjacent to an embryo sac within the ovule develop into embryos through adventitious embryony ; (d) In vivo somatic embryo genesis is known to occur in species such as Kalanchoë , where the embryos develop along leaf margins; (e) Gametic embryogenesis involves the experimental induction of embryogenesis from gametic cells such as microspores and ovules; (f) Embryogenesis can be induced in somatic cells following experimental treatment; (g) Embryos formed via asexual embryogenesis may or may not possess a suspensor . At a heart-shaped stage, the typical plant embryo contains precursor cells for the shoot apical meristem (blue cells), and the root apical meristem which consists of a quiescent center (orange cells) and columella stem cells (purple cells)
Evolutionary speaking, embryogenesis is a much older process than seed formation and initially resulted from the fusion of two homospores into the zygote, gradually evolving in present day heterospory [5, 6]. The zygote formed following fusion of parental gametes is the first cell evident during sexual reproduction with a competence for embryogenesis. In plants, an “embryogenic” state is not only restricted to the zygote and in the following sections, ways of attaining an embryogenic state other than via fertilization will be discussed (Table 1).
2.2 Asexual Embryogenesis in Seeds: Apomixis and Parthenogenesis in Cereals
Apomixis is a term describing a suite of developmental processes resulting in the formation of an asexual seed . Characteristic features of all apomicts include fertilization -independent formation of an egg cell or another somatic ovule cell into an embryo, and the development of functional endosperm in apomicts occurs either with or without fertilization [7, 8]. As a result, plants germinating from seeds derived via apomixis are genetically identical to the maternal parent.
Apomixis has evolved independently ac ross different angiosperm plant families and genera many times, and has been documented in more than 120 angiosperm genera that belong to approximately 40 families [9]. Apomixis is genetically controlled by dominant loci in studied species and is not prevalent in agronomically important plants [10]. Apomixis mechanisms are generally divided into two categories: gametophytic or sporophytic, based upon the location of the precursor cell which develops into the embryo. In gametophytic apomixis , the embryo develops without fertilization (termed parthenogenesis ) from an egg cell found inside an embryo sac that has formed mitotically without prior meiosis, and is thus chromosomally unreduced (Table 1; Fig. 1b). Two common mechanisms termed diplospory and apospory give rise to such embryo sacs. They are distinguished by whether the starting cell is a megaspore mother cell or another somatic cell in the ovule, respectively (see Hand and Koltunow [7] for further information). Gametophytic apomixis and parthenogenesis are found and studied in species including eudicots Taraxacum officinale (dandelion), Boechera spp., and Hieracium spp. and also in grasses Pennisetum squamulatum and Paspalum simplex among others [11–14].
During sporophytic apomixis , which is also called adventitious or nucellar embryony, embryos develop without fertilization directly from diploid somatic ovule cells surrounding an embryo sac (Table 1; Fig. 1c). Most commonly, the embryos arise from two different ovule tissues: the nucellus and the inner integument. Nucellar embryony is widespread among Citrus species [15, 16]. The embryo initial cells that give rise to the asexual embryos differentiate near the developing embryo sac [17] and they can be specified as early as the 2–4 nuclear stage of embryo sac formation [18, 19]. The embryo initial cells develop and form globular-shaped embryos that can only develop to maturity if the sexually derived embryo sac is fertilized, as the sexual and asexual embryos share the nutritive endosperm . The developing seed therefore consists of one sexual embryo and one or more asexual embryos and is termed polyembryonic. The sexually derived embryo may not develop or survive germination [17].
Asexual embryogenesis is evident within seeds of the “Salmon” system of wheat . In contrast to gametophytic apomixis , a chromosomally reduced embryo sac develops via the usual events of meiosis, spore selection, and mitosis evident in sexually reproducing angiosperms . However, salmon wheat lines are capable of up to 90 % parthenogenesis , whereby the egg is able to initiate embryogenesis without fertilization [20, 21]. Parthenogenesis capability results from translocation of the short arm of wheat chromosome 1B with the short arm of chromosome 1R of rye. This particular translocation results in the loss of two critical loci in wheat: Suppressor of parthenogenesis (Spg) and Restorer of fertility (Rfv1), along with the gain of a Parthenogenesis (Ptg) locus from rye. In addition to this translocation, parthenogenesis is dependent upon organellar DNA from Aegilops causdata or A. kotschyi, demonstrating the importance of cytoplasmic as well as nuclear factors in asexual embryogenesis in vivo [21]. The existence of fertilization-independent embryo development from different cell types in the ovules of apomicts suggests that multiple cells can acquire an embryogenic state. This contrasts with sexual reproduction where the embryogenic state is suppressed until fertilization and restricted to the egg cell within the female gametophyte. In parthenogenetic cereals the embryogenic state is attained by the egg in the absence of fertilization whilst embryogenic competency is suppressed in the remaining ovule cell types.
2.3 Somatic Embryogenesis In Vivo from Leaves
Somatic embryo genesis is known to occur in vivo in nature, where embryos develop on the surface of plant tissue (Fig. 1d) [22]. For example, plants of the genus Kalanchoë reproduce asexually through the ectopic formation of plantlets along their leaf margins [23]. The plantlets arise following proliferation of cells described as “dormant meristems” that are found in notches along the leaf margin [24, 25]. Some Kalanchoë species require stress to induce plantlet formation while others do not and constitutively form asexual plantlets. Because of this form of multiplication, Kalanchoë species are known as “mother of thousands.” The embryo resulting from somatic embryo genesis is diploid and genetically identical to the somatic precursor cells from which it was formed.
2.4 In Vitro Somatic and Gametic Embryogenesis
It is possible to induce asexual embryogenesis in vitro from gametic cells including male microspores (termed androgenesis ), and from egg cell s or the associated accessory cells found in the female gametophytes (termed gynogenesis) (Table 1; Fig. 1e). This process requires gametophytic cells to switch to a sporophytic embryo formation pathway. Application of various stress treatments such as cold/heat shock and starvation are applied to the anther, isolated microspores, cultured ovules, ovaries, or flower buds to induce the switch [26–28]. The resulting embryos are haploid , possessing either maternal or paternal chromosomes depending on the gametophytic precursor cell. The production of haploid plants through in vitro gametic embryogenesis is a powerful mechanism to generate homozygous lines much faster than using conventional breeding. Colchicine induced chromosome doubling of haploid embryos during, or just after, embryogenesis results in homozygous doubled-haploid plants which are useful tools in trait discovery and plant breeding applications [29]. Currently, microspore embryogenesis is favored over gynogenesis as a mode of gametic embryogenesis because of its higher efficiency [30].
In vitro somatic embryo genesis can also be induced in vegetative explants or cells following treatment with plant growth regulator s (PGR ) or stresses such as osmotic shock, dehydration, water stress , and alteration of pH (reviewed in [31]) (Fig. 1f). A few studies have addressed correspondences and differences between zygotic and somatic embryogenesis and suggest that the patterning and specification events are quite similar [32], with the exception of a lack of the suspensor and dormancy in in vitro cultured somatic embryos [33]. Therefore, the most important step in vegetative cells that undergo somatic embryogenesis must be to first gain the “embryogenic” state. Recent work suggests that a release in suppression of the embryogenic state is a plausible mechanism [6, 34].
3 Attaining an Embryogenic State
A prerequisite for embryogenesis in plants is that the precursor cell must attain an embryogenic state which provides the cellular competence for embryo formation. During gametic embryogenesis , and gametophytic apomixis , the developing gametophyte cells respond to induction signals that switch their fate from gametophytic to sporophytic. During zygotic embryo genesis , the zygote has acquired embryogenic competency following fertilization of the egg cell . In somatic embryo genesis in vitro, and adventitious embryony , the embryo precursor cells are somatic sporophytic cells which first must attain the embryogenic state. Changing the developmental fate of a cell is therefore an important component of both in vitro and in vivo asexual embryogenesis.
It has been proposed that somatic embryo genesis consists of two distinct phases which are independent of each other and are controlled by different factors [35]. The initial stage is induction, which involves the somatic cells attaining the embryogenic state usually by the exogenous application of PGR. The following stage is expression, where the newly differentiated embryonic cells develop into an embryo without any further exogenous signals. It is not yet known whether in vivo embryogenesis via adventitious embryony similarly consists of two separate independent phases. However, such a scenario could be envisaged where the sporophytic ovule cells also first acquire embryonic competence by a particular molecular signal, and then develop into an embryo without fertilization via a separate developmental program.
In the process of in vitro somatic embryo genesis, somatic cells attain the embryogenic state following the application of PGR . Auxin is most commonly used [36], although other PGR, including cytokinin and abscisic acid, have proven capable of inducing embryogenesis [37, 38]. Following treatment with PGR, the cells are cultured on a hormone-free medium. Auxin plays major roles in plant growth and morphogenesis including embryo sac development and embryo patterning [39, 40]. In addition to treatment with auxin, the frequency of somatic embryogenesis induction also depends on the species, genotype, tissue, stage of development, and endogenous hormone levels [35, 41]. Therefore although auxin is a universal induction molecule, other factors must be involved in the induction of embryonic competence. The role of cellular stress response s in the induction of somatic embryogenesis is increasingly being recognized. The process of culturing explants for somatic embryogenesis involves wounding, sterilization, and culturing of the explant, all which undoubtedly apply stress to the cells involved. Furthermore, exogenous stresses such as osmotic, heavy metal ion, temperature, and dehydration stresses can enhance somatic embryogenesis [42–46]. The induction of somatic embryogenesis through the application of auxin or stresses may imply an interaction between auxin and stress signaling . Auxin may therefore activate a stress signaling response, which is involved in inducing embryogenic competence . Many stress-related genes are up-regulated during the early phases of somatic embryogenesis, which supports this theory [47, 48].
Whether somatic cells in vitro and nucellar, integument cells and unreduced egg cell s in apomicts in vivo acquire an embryogenic state via the same mechanism is currently unknown. Unlike somatic embryo genesis, embryos formed through parthenogenesis and adventitious embryony in apomicts are subject to the developmental influences of the ovule which may produce alternate cues that induce an embryogenic state. Stress and alterations in ovule pattern formation lead to a deregulation of apomixis in Hieracium where embryos form ectopically in different ovule positions [49]. Although no genes have yet been identified that are responsible for inducing adventitious embryony, genes related to stress signaling have been implied in the process of nucellar embryony in Citrus. Kumar et al. [50] used suppression subtractive hybridization (SSH) and microarray to detect genes that were differentially expressed during asexual embryo initiation and discovered genes related to stress signaling, including heat shock proteins.
Some similarities exist in the morphology of the embryo precursor cell for in vitro somatic embryo genesis and in vivo adventitious embryony . In Citrus species that undergo adventitious embryony, those nucellar cells that ultimately differentiate into embryos are distinguished from surrounding nucellar cells by their large nuclei and dense cytoplasm [51]. These nucellar initial cells also have very thick callosic cell wall s and later become thinner walled, rounder, larger, and with a prominent nucleus prior to cell division [17]. Histological observations of embryonic somatic cells cultured in vitro from various species show that these embryonic cells are relatively small and also contain large nuclei and dense cytoplasm when compared to other somatic cells (reviewed in Namasivayam [52]). Large nuclei and dense cytoplasm are also characteristic of cells that are precursors of the female gametophyte, including the aposporous initial cell in aposporous apomictic plants, distinguishing them from surrounding somatic cells [1, 53].
4 Embryo Morphology
Zygotic embryo genesis within angiosperms passes through a series of sequential stages to give rise to the mature differentiated structure. In Arabidopsis and some other angiosperms, the first division of the zygote produces an apical cell that continues to be embryogenic, while the second basal cell is no longer embryogenic and continues to form the multicelled suspensor . Further divisions of the apical cell produce a globular embryo, and differentiation and expansion of the cotyledons leads to heart and torpedo-shaped embryos [54]. Only the suspensor derived hypophyseal suspensor cell continues to form the quiescent center and the columella stem cells of the root meristem (Fig. 1g) [55]. Variation in early cell division patterning exists between different dicotyledonous species, although the typical globular, heart, and torpedo morphological stages still usually occur [54]. Zygotic embryogenesis in monocotyledonous species differs from dicots mostly with respect to planes of symmetry and the position of the shoot apical meristem [56]. Variation in embryo formation also exists between monocot species. The embryo is the only plant structure in which both the root and shoot apical meristem is formed simultaneously. This requires a highly complex series of pattern forming and specification events, including establishment of small populations of stem cells. These cells continue to support the formation and activity of meristems during the remainder of the plant life cycle (for a recent review see [57]). Extensive studies have revealed molecular details of the formation of the major tissue types as well as the meristems themselves during embryogenesis [6].
The processes of asexual embryogenesis, both in vivo and in vitro, often differ from the regular divisions and patterning events that define zygotic embryo genesis . Embryo pattern formation during apomictic embryogenesis (parthenogenesis ) can be irregular compared to zygotic embryogenesis in related sexual species. In aposporous Hieracium , for example, embryogenesis frequently commences earlier than in sexual plants as once the egg differentiates, it transits rapidly to embryogenesis, and in some cases altered division planes can result in a different embryo appearance. Multiple embryos can also form in aposporous Hieracium embryos in either the same or a secondary embryo sac [58]. Although most Hieracium parthenogenetic embryos resemble those formed by zygotic embryogenesis in sexual plants, embryos with one or three cotyledons have also been observed. Despite developmental alterations in the primary pattern of embryos formed in aposporous Hieracium species, the resulting germinated seedlings eventually exhibit normal plant growth when grown on hormone free media in vitro [58].
In vivo asexual embryogenesis in Kalanchoë species proceeds through the typical globular, heart and torpedo stages from meristematic cells along leaf margins [24]. However unlike zygotic embryo s, Kalanchoë asexual plantlets resemble shoots that then grow adventitious roots from a hypocotyl structure [23]. Once the root system has developed, Kalanchoë plantlets detach from the mother plant, fall to the ground and become new plants.
In vitro embryogenesis could also be described as heterogeneous, as multiple developmental pathways are possible which occur at varying frequencies within a single species and even the same culture [33, 59, 60]. Detailed characterization of in vitro embryogenesis pathways has been performed using time-lapse tracking from embryonic cell suspension s [33, 61]. Early development of most microspore derived embryos involves a globular embryo with little cellular organization that undergoes symmetrical division and does not resemble a typical zygotic embryo [54]. Other microspore-derived embryos appear to form via a developmental pathway that involves asymmetric division and consequently more closely resemble zygotic embryos. Recently, microspore embryogenesis systems have been developed that consistently produce such embryos [59, 62]. These systems involve a heat stress period that is either shorter or at a much lower temperature than is usually applied.
Early during zygotic embryo genesis , a region of the embryo differentiates to become a suspensor that functions to connect the embryo to surrounding tissues, thereby positioning the embryo inside the seed [63]. The suspensor also acts to transport nutrients and hormones to the embryo. When microspore--> embryogenesis more closely mimics zygotic embryogenesis, a recognizable suspensor is always present, which suggests the suspensor plays a role in supporting early patterning events [59, 62, 64]. A suspensor is also formed during in vivo asexual embryogenesis, although throughout Citrus nucellar embryony, the suspensor becomes evident at a much later stage of development than in zygotic embryos [15]. In aposporous Hieracium , embryos that develop in the micropylar end of the embryo sac always form a suspensor and embryos that develop within secondary chalazal embryo sacs may or may not form a suspensor and often arrest at the globular stage [65]. The development of suspensors in asexual embryogenesis suggests that fertilization is not required for formation of the suspensor.
Unlike asexual embryos formed in apomictic seeds which undergo desiccation and dormancy as part of seed maturation, embryos formed in vitro and in vivo in Kalanchoë develop directly into seedlings. Despite not developing within a seed, in vitro somatic embryo s also undergo some form of maturation and accumulate late embryogenesis abundant (LEA) proteins, although sometimes treatment with ABA is first required to induce maturation [66]. In vitro somatic embryos also accumulate seed storage proteins , which are recognized as important for the future development of in vitro somatic embryos into plants. Only those embryos that have accumulated enough storage proteins and have acquired desiccation tolerance will develop into normal plants [60]. A comparison between asexual in vivo and somatic in vitro embryogenesis processes was performed by measuring the accumulation of citrin seed storage proteins in polyembryonic seeds and in vitro cultured embryos in Citrus. This study revealed that in vitro embryos accumulate fewer citrins and at a later developmental stage than within the polyembryonic seed, suggesting that despite not being derived from fertilization events, the nucellar embryos are influenced by the seed environment [19].
Formation of endosperm is a crucial component of seed development which does not accompany in vitro embryogenesis. The precursor of the endosperm is the large diploid central cell of the embryo sac. During sexual seed formation, the endosperm will only develop following double fertilization , when one of the two sperm cells fuses with the two central cell nuclei to produce triploid endosperm. Formation of viable seed via apomixis also requires the formation of endosperm. The majority of apomictic species studied require fertilization to develop endosperm, a process which is termed pseudogamy. In some apomictic species, typically members of the Asteraceae, endosperm can develop without fertilization of the central cell. Maternal (m) and paternal (p) genome ratios in the endosperm are typically 2m:1p in sexual species and disturbance in this ratio may lead to seed abortion. Apomicts tend to tolerate variation in endosperm ploidy and maternal and paternal genome ratios which are not easily tolerated in sexually reproducing plants, and have developed various strategies to ensure seed viability [67].
Apomictic Hieracium species are able to form endosperm without fertilization . The polar nuclei fuse prior to the development of nuclear and then cellular endosperm, in the absence of fertilization and the resulting endosperm exhibits a 4m:0p genome ratio in aposporous Hieracium. The trait of autonomous endosperm (AutE) has recently been separated from fertilization-independent embryogenesis in Hieracium through two inter-specific crosses [68]. Two individuals were identified that form reduced embryo sacs containing meiotically derived eggs and central cells through the sexual pathway. However, egg cell s within these individuals are unable to commence embryogenesis without fertilization although in the absence of fertilization, the fused polar nuclei undergo proliferation and continue to develop cellular endosperm with a 2m:0p genome ratio. This indicates a paternal genome contribution is neither required for endosperm initiation, nor cellularization in both chromosomally reduced and unreduced embryo sacs. When egg cells from these individuals are fertilized, embryogenesis occurs to completion and viable seed is formed. It is currently unclear if the central cell is also able to be fertilized as this would result in a parental genome ratio of 2m:1p ratio as seen in sexual species [68].
5 Genes Implicated in In Vivo and In Vitro Asexual Embryogenesis
Similarities between asexual embryogenesis in vitro and in vivo raise questions regarding whether these processes are controlled by the same molecular mechanisms. Although no genes responsible for embryogenesis have yet been isolated from apomictic plants, a number of gene candidates have been identified through differential gene expression analysis, genetic mapping and study of sexual mutants with phenotypes that mimic asexual embryogenesis. Attempts to understand in vitro somatic and gametic embryogenesis have also resulted in a range of gene candidates that when expressed ectopically, result in embryo formation.
One of the first genes associated with somatic embryo genesis was SOMATIC EMBRYOGENESIS RECEPTOR KINASE ( SERK ) when its involvement was demonstrated in carrot cell cultures [69]. SERK was identified as a marker for cells transitioning from a somatic to an embryogenic state, due to its transient expression in established suspension cell cultures [69]. SERK is a leucine-rich repeat (LRR) receptor-like kinase that is also expressed in developing ovules and embryos in planta and may therefore influence somatic embryogenesis through the same mechanisms of the sexual pathway [69, 70]. Overexpression and downregulation of SERK increases and decreases the efficiency of somatic embryogenesis, respectively [70, 71]. Interestingly, a SERK gene has also been implicated in asexual reproduction within an apomictic grass, Poa pratensis. cDNA-AFLPs differentially expressed between apomictic and sexual lines of P. pratensis revealed a SERK gene that displays differential expression [72]. Apomixis in P. pratensis involves development of an embryo sac not from the megaspore mother cell (MMC) which is the typical precursor cell for the sexual pathway, but from a diploid somatic cell positioned nearby the MMC. These somatic precursor cells are found in the nucellar ovule tissue. Within P. pratensis, SERK is expressed in embryo sac precursor cells: the MMC in sexual plants, and somatic nucellar cells in apomictic plants [72]. The same expression profile was also observed in apomictic and sexual lines of Paspalum notatum [73]. SERK expression was also examined in apomictic Hieracium where it was detected throughout the ovule, and expression was not restricted to the nucellar region or MMC in Hieracium. SERK expression was also observed in developing Hieracium embryos [74]. SERK is therefore thought to play an important role in changing developmental fate of cells, both in stages of apomixis and in somatic embryogenesis. BABYBOOM (BBM) is another gene that has been associated with both in vitro and in vivo asexual embryogenesis. BBM is an APETELA2 (AP2) transcription factor that was originally identified following subtractive hybridization of cDNA from Brassica napus microspores undergoing embryogenesis [75]. Ectopic expression of BBM in Arabidopsis or B. napus induces somatic embryos, and constitutive expression of BBM gene s from other species also results in the emergence of ectopic embryos [75–77]. BBM expression was also observed in developing Arabidopsis zygotic embryo s [75]. These results suggest that BBM has a conserved role in the induction and/or maintenance of embryo development . BBM genes have also been identified within a genomic region essential for apomixis in the apomictic grass Pennisetum squamulatum [78]. The apospory-specific genomic region (ASGR) of Pennisetum was identified following marker analysis of a selection of apomictic and sexual plants, which revealed a set of apomixis-specific markers that define the ASGR [79]. Sequencing of BAC clones from within the ASGR revealed putative protein coding regions, including two of which had similarity to BBM of rice [78]. The ASGR is thought to contain genetic elements responsible for both the formation of a diploid embryo sac, and the process of parthenogenesis . The BBM genes within the ASGR are therefore candidate apomixis genes with strong potential to have a role in the induction or maintenance of asexual embryogenesis in Pennisetum apomicts. However, confirmation of a role for BBM in parthenogenesis has not yet been reported.
The involvement of common genes in zygotic and asexual embryogenesis implies that despite arising from different activation signals and different tissues, each embryogenesis process converges on a similar developmental pathway. Genes with a known involvement in zygotic embryo genesis have therefore been studied in asexual embryogenesis systems to understand whether such genes are also involved in asexual embryogenesis. The LEAFY COTYLEDON (LEC) family of transcription factor s is crucial for regular embryogenesis and is also implicated in somatic embryo genesis. Arabidopsis contains three LEC gene s : LEC1, LEC2, and FUSCA3 (FUS3), and each of these genes is expressed exclusively in the embryo [80–82]. Ectopic expression of each of the three LEC genes leads to vegetative cells adopting characteristics of maturation-phase embryos, and hence this gene family is associated with the process of somatic embryogenesis [80–82]. The LEC genes have been linked to auxin production, as LEC2 is known to activate the auxin biosynthesis genes YUCCA2 and YUCCA4 [83]. FUS3 expression also increases in response to auxin [84]. This interaction with auxin signaling is thought to be responsible for the ability of LEC gene expression to induce embryonic competence.
LEC1 has been studied in Kalanchoë species and is implicated in the process of asexual plantlet formation in these species. Compared to Arabidopsis, the LEC1 gene of Kalanchoë daigremontiana (KdLEC1) is truncated and does not rescue the Arabidopsis lec1 mutation, suggesting it functions differently to LEC1 in Arabidopsis [23]. A functional full length copy of LEC1 was created by replacing the deleted nucleotides in KdLEC1 with the corresponding nucleotides from Arabidopsis and transformation of Kalanchoë daigremontiana with this synthesized LEC1-LIKE gene results in disrupted asexual reproduction and in some instances abortion or absence of plantlet formation [85]. This study strongly supports the involvement of LEC1 in in vivo asexual embryogenesis in Kalanchoë and furthermore suggests that the switch from sexual to asexual propagation in the evolution of Kalanchoë was probably activated following truncation of the KdLEC1 gene [85].
Another gene that appears to be involved in the induction of an embryogenic state is the RWP-RK domain containing (RKD) transcription factor RKD2, which is preferentially expressed in the egg cell of Arabidopsis and wheat [86]. Ectopic expression of RKD2 results in ovule integument cells that become enlarged and densely cytoplasmic with prominent nuclei, suggesting these cells have become pluripotent [87]. Ectopic RKD2 expression also results in some integument cells adopting an egg cell identity, and a low frequency (ca. 0.1 %) of embryo-like structures also appear outside of the embryo sac [87]. This observation is reminiscent of adventitious embryony and may indicate that RKD2 is involved in the induction of embryogenesis from ovule tissue during adventitious embryony.
Additional genes including WUSCHEL and AGAMOUS-Like 15 (AGL15) are known to induce embryo formation from vegetative tissue when ectopically expressed, and have therefore been implicated in somatic embryo genesis [88, 89]. WUSCHEL is known to be involved in specifying and maintaining stem cells in the shoot and root meristem [90] while AGL15 is known to accumulate in developing embryos [91], therefore a role in embryogenesis is to be expected for both of these genes. However, with the exception of SERK , most of the genes shown to be involved in zygotic and asexual embryogenesis are not specifically expressed in the egg cell or the zygote. Therefore, whilst important for later stages of embryo development , these genes may not be involved in the process of embryo initiation which is possibly the most important aspect of asexual embryogenesis. It has been proposed that the observed ectopic embryo development associated with mis-expression of these genes, is a result of cellular stress, rather than a specific initiation signal expressed by the genes [92]. This hypothesis is consistent with embryonic competence being induced by stress factors, as discussed earlier.
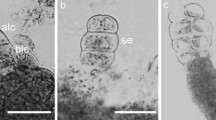
To understand the genetic elements responsible for inducing embryonic competence in both in vitro and in vivo asexual embryogenesis, future experiments will likely focus on comparison of gene expression from embryo precursor cells directly before and after the initiation of embryogenesis. Genetic mapping of apomixis loci may also reveal which genes are responsible for the initiation of asexual embryogenesis. Genetic analyses of apomicts have shown that gametophytic apomixis is inherited as a dominant trait. In many apomictic species, developmental components of apomixis (meiotic avoidance and parthenogenesis ) are controlled by independent loci and further research is underway to isolate the causal sequences that underlie these loci. For example, characterized deletion mutants developed in apomictic Hieracium praealtum revealed a genomic region responsible for fertilization-independent embryogenesis and endosperm formation, named LOSS OF PARTHENOGENESIS (LOP) [93]. Deletion of LOP sees the plant become dependent upon fertilization for both embryo and endosperm development (Fig. 2) [13]. Genetic mapping of LOP and AutE is the focus of current work that may lead to isolation of the causal sequences for both traits.
Cleared ovules from wildtype apomict Hieracium praealtum (a), and a H. praealtum lop mutant (b) that has lost the capacity to undergo parthenogenesis and autonomous endosperm development. Within apomictic H. praealtum ovules, embryo and endosperm develop from the egg and central cell, respectively, without fertilization (a). H. praealtum lop deletion mutant m179 (b) has lost this capacity and the egg and central cells do not develop further without fertilization. Scale bars = 50 μm. em embryo, e egg, cc central cell, ne nuclear endosperm. Ovules were collected at stages 6 (a) and 10 (b) of capitulum development according to Koltunow et al. [65]
A genomic locus strongly associated with adventitious embryony in Citrus has also been identified [94]. Further characterization of this locus may clarify the mechanism of adventitious embryony and identify the genetic element responsible for inducing asexual embryogenesis in planta. Similarly, the search for genes within the controlling parthenogenesis loci of the salmon wheat system may reveal those genes that are responsible for parthenogenesis in this system. Although controlling genes are currently unknown for the salmon wheat system, it is likely that they have lost those genes required for repressing fertilization independent embryogenesis in sexual plant species.
6 Epigenetic Influence on Asexual Embryogenesis
Various epigenetic marks and pathways have been associated with both sexual and asexual embryogenesis processes, suggesting that the induction and regulation of asexual embryogenesis may involve epigenetic components. For instance, the application of exogenous auxin during somatic embryo induction results in DNA hypermethylation [95], and inhibition of DNA methylation suppresses the formation of embryogenic cells from cultured carrot epidermal cells [96]. Auxin could therefore possibly reprogram gene expression through DNA methylation, leading to the induction of embryogenesis pathways within somatic cells.
Genes within epigenetic pathways have also been implicated in both in vitro and in vivo asexual embryogenesis. One such epigenetic factor implicated in asexual embryogenesis is PICKLE (PKL), a chromatin remodeling protein [97]. PKL is responsible for repressing the LEC family of transcription factor s, as pkl mutants display overexpression of LEC1, LEC2 and FUS3, and display a phenotype similar to that seen from LEC1 overexpression [97, 98]. PKL activity is therefore acknowledged as an important regulatory mechanism for repressing embryonic identity throughout seedling growth, by suppressing the embryogenic program in somatic cells [99]. For this reason, PKL is also a candidate for the induction of asexual embryogenesis. Deregulation of PKL in somatic cells or within the egg cell would permit expression of embryogenic genes that are generally only expressed by the developing embryo following fertilization . However to date, PKL has not been specifically associated with asexual embryogenesis in any natural apomictic plant.
Strong evidence exists suggesting that epigenetic pathways play a crucial role in asexual embryo and endosperm development during apomixis . Mutants of the Polycomb -Group (PcG) chromatin modeling complex show phenotypes reminiscent of fertilization independent embryogenesis and endosperm formation seen in gametophytic apomixis. In particular, the Polycomb Repressive Complex 2 (PRC2) is known to be involved in the suppression of seed development in the absence of fertilization. The PRC2 is conserved between plants and animals and represses gene expression via trimethylation of histone H3 at lysine 27 (H3K27me3). Phenotypes of asexual embryo and endosperm development have been observed when core PRC2 genes are mutated in Arabidopsis. For instance, the fertilization-independent seed (FIS) PRC2 complex (FIS-PRC2) consists of the genes MEDEA (MEA), FIS2, FERTILIZATION-INDEPENDENT ENDOSPERM (FIE), and MULTICOPY SUPPRESSOR OF IRA1 (MSI1). Loss of function of any of these genes results in endosperm initiation and proliferation without fertilization. However, the endosperm does not celluarize [100–102]. The role of the FIS-PRC2 complex is therefore considered to inhibit central cell proliferation. In the case of MSI1 mutants, low levels of parthenogenetic embryo initiation are observed, followed by embryo arrest, so that viable seeds are not formed [103]. The role of some of the FIS-PRC2 genes has been investigated during seed initiation in Hieracium spp., one of the few groups of apomicts that develop endosperm without fertilization. Downregulation of Hieracium FIE (HFIE), a protein linking multiple PRC2 components inhibiting fertilization-independent endosperm proliferation in Arabidopsis does not result in fertilization-independent endosperm proliferation in sexual plants. HFIE function is required for completion of both sexual and asexual embryo and endosperm development in examined Hieracium species [104]. These results demonstrate that the capacity for embryogenic competence and endosperm formation in apomicts may function via deregulation of other PRC2 complex family members and that additional factors are required to produce viable asexual embryos and endosperm. The identified Hieracium AutE plants that form endosperm, but not embryos, without fertilization may help identify and define the roles of genes that regulate the autonomous endosperm mechanism.
The PRC2 complex interacts with other genes implicated in asexual embryogenesis, including the LEC gene family. LEC1, LEC2, and FUS3 are all overexpressed in CURLY LEAF (CLF) and SWINGER (SWN) double mutants, which are PcG gene homologues of the PRC2 gene MEA [105]. A cis regulatory element has been identified within the LEC2 promoter which is responsible for recruiting the PRC2 complex [106]. These results suggest that the PcG acts to repress embryonic gene expression by histone methylation. Histone acetylation is another epigenetic mark that in contrast to histone methylation, is generally associated with transcriptional activation. Removal of the acetylation is performed by histone deacetylase (HDAC), which consequently results in transcriptional repression. Interestingly, two histone deacetylase genes (HDA6 and HDA19) are partly responsible for repressing the embryonic program during Arabidopsis germination [107]. Another HDAC gene (HDA7) in Arabidopsis is known to be important for normal embryo development [108]. Inefficient or defective histone deacetylation of key embryonic genes may therefore be a candidate mechanism for inducing asexual embryogenesis.
7 Conclusions
While the pathways involved in developing the embryo itself appear common between the various modes of embryogenesis described here, many differences exist between the initiation processes of asexual embryogenesis in vitro and in vivo. Unlike somatic or gametic embryogenesis , apomixis -associated embryogenesis occurs near maternal reproductive tissue, and develops within a seed structure. Despite these differences, in vitro and in vivo asexual embryogenesis share some common factors: a change in the developmental fate of embryogenic precursor cells; and expression of an embryonic pathway in such cells without fertilization . Identifying molecular mechanisms that underlie these processes within in vitro systems may help to understand pathways that lead to apomixis. While some candidate genes for both in vitro and in vivo asexual embryogenesis have been identified, a role in apomixis has not yet been confirmed for any of these genes. One possibility is that embryogenesis related genes are deregulated by epigenetic factors during asexual embryogenesis. Continued research into asexual embryogenesis will yield important findings related to plant cell fate specification and the molecular regulation of embryogenesis.
References
Drews G, Koltunow AM (2011) The female gametophyte. Arabidopsis Book 9:e0155
Twell D (2011) Male gametogenesis and germline specification in flowering plants. Sex Plant Reprod 24:149–160
Capron A, Chatfield S, Provart N, Berleth T (2009) Embryogenesis: pattern formation from a single cell. Arabidopsis Book 7:e0126
Berger F, Hamamura Y, Ingouff M, Higashiyama T (2008) Double fertilization: caught in the act. Trends Plant Sci 13:437–443
Taylor TN, Taylor EL, Krings M (2008) Paleobotany. The biology and evolution of fossil plants, 2nd edn. Academic Press, New York
Radoeva T, Weijers D (2014) A roadmap to embryo identity in plants. Trends Plant Sci 19(11):709–716
Hand ML, Koltunow A (2014) The genetic control of apomixis: asexual seed formation. Genetics 197:441–450
Barcaccia G, Albertini E (2013) Apomixis in plant reproduction: a novel perspective on an old dilemma. Plant Reprod 26:159–179
Carman JG (1997) Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol J Linn Soc 61:51–94
Koltunow AM, Ozias-Akins P, Siddiqi I (2013) Apomixis. In: Becraft PW (ed) Seed genomics. Wiley, New York, pp 83–110
Vijverberg K, Milanovic-Ivanovic S, Bakx-Schotman T, van Dijk PJ (2010) Genetic fine-mapping of DIPLOSPOROUS in Taraxacum (dandelion; Asteraceae) indicates a duplicated DIP-gene. BMC Plant Biol 10
Ortiz JPA, Quarin CL, Pessino SC, Acuña C, Martínez EJ, Espinoza F et al (2013) Harnessing apomictic reproduction in grasses: what we have learned from Paspalum. Ann Bot 112:767–787
Koltunow AMG, Johnson SD, Rodrigues JCM, Okada T, Hu Y, Tsuchiya T et al (2011) Sexual reproduction is the default mode in apomictic Hieracium subgenus Pilosella, in which two dominant loci function to enable apomixis. Plant J 66:890–902
Akiyama Y, Conner JA, Goel S, Morishige DT, Mullet JE, Hanna WW et al (2004) High-resolution physical mapping in Pennisetum squamulatum reveals extensive chromosomal heteromorphism of the genomic region associated with apomixis. Plant Physiol 134:1733–1741
Wakana A, Uemoto S (1988) Adventive embryogenesis in Citrus (Rutaceae). II. Postfertilization development. Am J Bot 75:1033–1047
Wakana A, Uemoto S (1987) Adventive embryogenesis in Citrus. I. The occurrence of adventive embryos without pollination or fertilization. Am J Bot 74:517–530
Koltunow AM, Soltys K, Nito N, McClure S (1995) Anther, ovule, seed, and nucellar embryo development in Citrus sinensis cv. Valencia. Can J Bot 73:1567–1582
Koltunow AM (1993) Apomixis: embryo sacs and embryos formed without meiosis or fertilization in ovules. Plant Cell 5:1425–1437
Koltunow AM, Hidaka T, Robinson SP (1996) Polyembryony in Citrus: accumulation of seed storage proteins in seeds and in embryos cultured in vitro. Plant Physiol 110:599–609
Matzk F (1996) The Salmon system of wheat: a suitable model for apomixis research. Hereditas 125:299–301
Tsunewaki K, Mukai Y (1990) Wheat haploids through the Salmon method. In: Bajaj YPS (ed) Wheat, biotechnology in agriculture and forestry 13. Springer, Berlin/Heidelberg/New York, pp 460–478
Williams EG, Maheswaran G (1986) Somatic embryogenesis: factors influencing coordinated behaviour of cells as an embryogenic group. Ann Bot 57:443–462
Garcês HMP, Champagne CEM, Townsley BT, Park S, Malhó R, Pedroso MC et al (2007) Evolution of asexual reproduction in leaves of the genus Kalanchoë. Proc Natl Acad Sci U S A 104:15578–15583
Batygina TB, Bragina EA, Titova GE (1996) Morphogenesis of propagules in viviparous species Bryophyllum daigremontianum and B. calycinum. Acta Soc Bot Pol 65:127–133
Yarbrough JA (1932) Anatomical and developmental studies of the foliar embryos of Bryophyllum calycinum. Am J Bot 19:443–453
Sibi ML, Kobaissi A, Shekafandeh A (2001) Green haploid plants from unpollinated ovary culture in tetraploid wheat (Triticum durum Defs.). Euphytica 122:351–359
Gémes-Juhász A, Balogh P, Ferenczy A, Kristóf Z (2002) Effect of optimal stage of female gametophyte and heat treatment on in vitro gynogenesis induction in cucumber (Cucumis sativus L.). Plant Cell Rep 21:105–111
Islam SMS, Tuteja N (2012) Enhancement of androgenesis by abiotic stress and other pretreatments in major crop species. Plant Sci 182:134–144
Forster BP, Heberle-Bors E, Kasha KJ, Touraev A (2007) The resurgence of haploids in higher plants. Trends Plant Sci 12:368–375
Germanà MA (2011) Gametic embryogenesis and haploid technology as valuable support to plant breeding. Plant Cell Rep 30:839–857
Zavattieri MA, Frederico AM, Lima M, Sabino R, Arnholdt-Schmitt B (2010) Induction of somatic embryogenesis as an example of stress-related plant reactions. Electronic Journal of Biotechnology 13(1)
Mordhorst AP, Hartog MV, El Tamer MK, Laux T, de Vries SC (2002) Somatic embryogenesis from Arabidopsis shoot apical meristem mutants. Planta 214:829–836
Toonen MAJ, Hendriks T, Schmidt EDL, Verhoeven HA, Vankammen A, Devries SC (1994) Description of somatic-smbryo-forming single cells in carrot suspension-cultures employing video cell tracking. Planta 194:565–572
Rademacher EH, Lokerse AS, Schlereth A, Llavata-Peris CI, Bayer M, Kientz M et al (2012) Different auxin response machineries control distinct cell fates in the early plant embryo. Dev Cell 22:211–222
Jiménez VM (2001) Regulation of in vitro somatic embryogenesis with emphasis on the role of endogenous hormones. Rev Bras Fisiol Veg 13:196–223
Feher A, Pasternak T, Otvos K, Miskolczi P, Dudits D (2002) Induction of embryogenic competence in somatic plant cells: a review. Biologia 57:5–12
Sagare AP, Lee YL, Lin TC, Chen CC, Tsay HS (2000) Cytokinin-induced somatic embryogenesis and plant regeneration in Corydalis yanhusuo (Fumariaceae)—a medicinal plant. Plant Sci 160:139–147
Nishiwaki M, Fujino K, Koda Y, Masuda K, Kikuta Y (2000) Somatic embryogenesis induced by the simple application of abscisic acid to carrot (Daucus carota L.) seedlings in culture. Planta 211:756–759
Weijers D, Schlereth A, Ehrismann JS, Schwank G, Kientz M, Jurgens G (2006) Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev Cell 10:265–270
Ceccato L, Masiero S, Sinha Roy D, Bencivenga S, Roig-Villanova I, Ditengou FA et al (2013) Maternal control of PIN1 is required for female gametophyte development in Arabidopsis. PLoS One 8:e66148
Carman JG (1990) Embryogenic cells in plant-tissue cultures: occurrence and behavior. In Vitro Cell Dev Biol 26:746–753
Ikeda-Iwai M, Umehara M, Satoh S, Kamada H (2003) Stress-induced somatic embryogenesis in vegetative tissues of Arabidopsis thaliana. Plant J 34:107–114
Karami O, Deljou A, Esna-Ashari M, Ostad-Ahmadi P (2006) Effect of sucrose concentrations on somatic embryogenesis in carnation (Dianthus caryophyllus L.). Sci Hortic 110:340–344
Kumria R, Sunnichan VG, Das DK, Gupta SK, Reddy VS, Bhatnagar RK et al (2003) High-frequency somatic embryo production and maturation into normal plants in cotton (Gossypium hirsutum) through metabolic stress. Plant Cell Rep 21:635–639
Patnaik D, Mahalakshmi A, Khurana P (2005) Effect of water stress and heavy metals on induction of somatic embryogenesis in wheat leaf base cultures. Indian J Exp Biol 43:740–745
Santarem ER, Pelissier B, Finer JJ (1997) Effect of explant orientation, pH, solidifying agent and wounding on initiation of soybean somatic embryos. In Vitro Cell Dev Biol—Plant 33:13–19
Jin F, Hu L, Yuan D, Xu J, Gao W, He L et al (2014) Comparative transcriptome analysis between somatic embryos (SEs) and zygotic embryos in cotton: evidence for stress response functions in SE development. Plant Biotechnol J 12:161–173
Karami O, Saidi A (2010) The molecular basis for stress-induced acquisition of somatic embryogenesis. Mol Biol Rep 37:2493–2507
Tucker MR, Okada T, Johnson SD, Takaiwa F, Koltunow AMG (2012) Sporophytic ovule tissues modulate the initiation and progression of apomixis in Hieracium. J Exp Bot 63:3229–3241
Kumar V, Malik SK, Pal D, Srinivasan R, Bhat SR (2014) Comparative transcriptome analysis of ovules reveals stress related genes associated with nucellar polyembryony in citrus. Tree Genet Genomes 1–16
Wilms HJ, van Went JL, Cresti M, Ciampolini F (1983) Adventive embryogenesis in citrus. Caryologia 36:65–78
Namasivayam P (2007) Acquisition of embryogenic competence during somatic embryogenesis. Plant Cell Tiss Org Cult 90:1–8
Zhang B, Wang ZJ, Jin SH, Xia GH, Huang YJ, Huang JQ (2012) A pattern of unique embryogenesis occurring via apomixis in Carya cathayensis. Biologia Plantarum 56:620–627
Mordhorst AP, Toonen MAJ, De Vries SC (1997) Plant embryogenesis. Crit Rev Plant Sci 16:535–576
Scheres B, Wolkenfelt H, Viola W, Terlouw M, Lawson E, Dean C et al (1994) Embryonic origin of the Arabidopsis primary root and root meristem initials. Development 120:2475–2487
Chandler J, Nardmann J, Werr W (2008) Plant development revolves around axes. Trends Plant Sci 13:78–84
Wendrich JR, Weijers D (2013) The arabidopsis embryo as a miniature morphogenesis model. New Phytol 199:14–25
Koltunow AM, Johnson SD, Bicknell RA (2000) Apomixis is not developmentally conserved in related, genetically characterized Hieracium plants of varying ploidy. Sex Plant Reprod 12:253–266
Prem D, Solis MT, Barany I, Rodriguez-Sanz H, Risueno MC, Testillano PS (2012) A new microspore embryogenesis system under low temperature which mimics zygotic embryogenesis initials, expresses auxin and efficiently regenerates doubled-haploid plants in Brassica napus. BMC Plant Biol 12
Von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tiss Org Cult 69:233–249
Filonova LH, Bozhkov PV, Von Arnold S (2000) Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking. J Exp Bot 51:249–264
Supena EDJ, Winarto B, Riksen T, Dubas E, Van Lammeren A, Offringa R et al (2008) Regeneration of zygotic-like microspore-derived embryos suggests an important role for the suspensor in early embryo patterning. J Exp Bot 59:803–814
Yeung EC, Meinke DW (1993) Embryogenesis in angiosperms: development of the suspensor. Plant Cell 5:1371–1381
Joosen R, Cordewener J, Supena EDJ, Vorst O, Lammers M, Maliepaard C et al (2007) Combined transcriptome and proteome analysis identifies pathways and markers associated with the establishment of rapeseed microspore-derived embryo development. Plant Physiol 144:155–172
Koltunow AM, Johnson SD, Bicknell RA (1998) Sexual and apomictic development in Hieracium. Sex Plant Reprod 11:213–230
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48:1493–1509
Koltunow AM, Grossniklaus U (2003) Apomixis: a developmental perspective. Annu Rev Plant Biol 54:547–574
Ogawa D, Johnson SD, Henderson ST, Koltunow AM (2013) Genetic separation of autonomous endosperm formation (AutE) from two other components of apomixis in Hieracium. Plant Reprod 26:113–123
Schmidt EDL, Guzzo F, Toonen MAJ, De Vries SC (1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124:2049–2062
Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt EDL, Boutilier K, Grossniklaus U et al (2001) The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127:803–816
Hu H, Xiong L, Yang Y (2005) Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta 222:107–117
Albertini E, Marconi G, Reale L, Barcaccia G, Porceddu A, Ferranti F et al (2005) SERK and APOSTART. Candidate genes for apomixis in Poa pratensis. Plant Physiol 138:2185–2199
Podio M, Felitti SA, Siena LA, Delgado L, Mancini M, Seijo JG et al (2014) Characterization and expression analysis of SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) genes in sexual and apomictic Paspalum notatum. Plant Mol Biol 84:479–495
Tucker MR, Araujo ACG, Paech NA, Hecht V, Schmidt EDL, Rossell JB et al (2003) Sexual and apomictic reproduction in Hieracium subgenus Pilosella are closely interrelated developmental pathways. Plant Cell 15:1524–1537
Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L et al (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737–1749
Morcillo F, Gallard A, Pillot M, Jouannic S, Aberlenc-Bertossi F, Collin M et al (2007) EgAP2-1, an AINTEGUMENTA-like (AIL) gene expressed in meristematic and proliferating tissues of embryos in oil palm. Planta 226:1353–1362
Ouakfaoui SE, Schnell J, Abdeen A, Colville A, Labbé H, Han S et al (2010) Control of somatic embryogenesis and embryo development by AP2 transcription factors. Plant Mol Biol 74:313–326
Conner JA, Goel S, Gunawan G, Cordonnier-Pratt MM, Johnson VE, Liang C et al (2008) Sequence analysis of bacterial artificial chromosome clones from the apospory-specific genomic region of Pennisetum and Cenchrus. Plant Physiol 147:1396–1411
Ozias-Akins P, Roche D, Hanna WW (1998) Tight clustering and hemizygosity of apomixis-linked molecular markers in Pennisetum squamulatum implies genetic control of apospory by a divergent locus that may have no allelic form in sexual genotypes. Proc Natl Acad Sci U S A 95:5127–5132
Lotan T, Ohto MA, Matsudaira Yee K, West MAL, Lo R, Kwong RW et al (1998) Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93:1196–1205
Luerssen K, Kirik V, Herrmann P, Misera S (1998) FUSCA3 encodes a protein with a conserved VP1/ABI3-like B3 domain which is of functional importance for the regulation of seed maturation in Arabidopsis thaliana. Plant J 15:755–764
Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL et al (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci U S A 98:11806–11811
Stone SL, Braybrook SA, Paula SL, Kwong LW, Meuser J, Pelletier J et al (2008) Arabidopsis LEAFY COTYLEDON2 induces maturation traits and auxin activity: Implications for somatic embryogenesis. Proc Natl Acad Sci 105:3151–3156
Gazzarrini S, Tsuchiya Y, Lumba S, Okamoto M, McCourt P (2004) The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Dev Cell 7:373–385
Garcês HMP, Koenig D, Townsley BT, Kim M, Sinha NR (2014) Truncation of LEAFY COTYLEDON1 protein is required for asexual reproduction in Kalanchoë daigremontiana. Plant Physiol 165:196–206
Kőszegi D, Johnston AJ, Rutten T, Czihal A, Altschmied L, Kumlehn J et al (2011) Members of the RKD transcription factor family induce an egg cell-like gene expression program. Plant J 67:280–291
Lawit S, Chamberlin M, Agee A, Caswell E, Albertsen M (2013) Transgenic manipulation of plant embryo sacs tracked through cell-type specific fluorescent markers: cell labelling, cell ablation and adventitious embryos. Plant Reprod 26:125–137
Harding EW, Tang W, Nichols KW, Fernandez DE, Perry SE (2003) Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-Like 15. Plant Physiol 133:653–663
Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30:349–359
Laux T, Mayer KFX, Berger J, Jurgens G (1996) The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122:87–96
Heck GR, Perry SE, Nichols KW, Fernandez DE (1995) AGL15, a MADS domain protein expressed in developing embryos. Plant Cell 7:1271–1282
Curtis MD, Grossniklaus U (2008) Molecular control of autonomous embryo and endosperm development. Sex Plant Reprod 21:79–88
Catanach AS, Erasmuson SK, Podivinsky E, Jordan BR, Bicknell R (2006) Deletion mapping of genetic regions associated with apomixis in Hieracium. Proc Natl Acad Sci U S A 103:18650–18655
Nakano M, Shimada T, Endo T, Fujii H, Nesumi H, Kita M et al (2012) Characterization of genomic sequence showing strong association with polyembryony among diverse Citrus species and cultivars, and its synteny with Vitis and Populus. Plant Sci 183:131–142
Leljak-Levanić D, Bauer N, Mihaljević S, Jelaska S (2004) Changes in DNA methylation during somatic embryogenesis in Cucurbita pepo L. Plant Cell Rep 23:120–127
Yamamoto N, Kobayashi H, Togashi T, Mori Y, Kikuchi K, Kuriyama K et al (2005) Formation of embryogenic cell clumps from carrot epidermal cells is suppressed by 5-azacytidine, a DNA methylation inhibitor. J Plant Physiol 162:47–54
Ogas J, Kaufmann S, Henderson J, Somerville C (1999) PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proc Natl Acad Sci U S A 96:13839–13844
Dean Rider Jr SD, Henderson JT, Jerome RE, Edenberg HJ, Romero-Severson J, Ogas J (2003) Coordinate repression of regulators of embryonic identity by PICKLE during germination in Arabidopsis. Plant J 35:33–43
Henderson JT, Li HC, Rider SD, Mordhorst AP, Romero-Severson J, Cheng JC et al (2004) PICKLE acts throughout the plant to repress expression of embryonic traits and may play a role in gibberellin-dependent responses. Plant Physiol 134:995–1005
Chaudhury AM, Ming L, Miller C, Craig S, Dennis ES, Peacock WJ (1997) Fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci U S A 94:4223–4228
Ohad N, Yadegari R, Margossian L, Hannon M, Michaeli D, Harada JJ et al (1999) Mutations in FIE, a WD polycomb group gene, allow endosperm development without fertilization. Plant Cell 11:407–415
Schmidt A, Wöhrmann HJP, Raissig MT, Arand J, Gheyselinck J, Gagliardini V et al (2013) The Polycomb group protein MEDEA and the DNA methyltransferase MET1 interact to repress autonomous endosperm development in Arabidopsis. Plant J 73:776–787
Guitton AE, Berger F (2005) Loss of function of MULTICOPY SUPPRESSOR of IRA 1 produces nonviable parthenogenetic embryos in Arabidopsis. Curr Biol 15:750–754
Rodrigues JCM, Tucker MR, Johnson SD, Hrmova M, Koltunow AMG (2008) Sexual and apomictic seed formation in Hieracium requires the plant polycomb-group gene FERTILIZATION INDEPENDENT ENDOSPERM. Plant Cell 20:2372–2386
Makarevich G, Leroy O, Akinci U, Schubert D, Clarenz O, Goodrich J et al (2006) Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep 7:947–952
Berger N, Dubreucq B, Roudier F, Dubos C, Lepiniec L (2011) Transcriptional regulation of Arabidopsis LEAFY COTYLEDON2 involves RLE, a cis-element that regulates trimethylation of histone H3 at lysine-27. Plant Cell 23:4065–4078
Tanaka M, Kikuchi A, Kamada H (2008) The Arabidopsis histone deacetylases HDA6 and HDA19 contribute to the repression of embryonic properties after germination. Plant Physiol 146:149–161
Cigliano RA, Cremona G, Paparo R, Termolino P, Perrella G, Gutzat R et al (2013) Histone deacetylase AtHDA7 is required for female gametophyte and embryo development in Arabidopsis. Plant Physiol 163:431–440
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Hand, M.L., de Vries, S., Koltunow, A.M.G. (2016). A Comparison of In Vitro and In Vivo Asexual Embryogenesis. In: Germana, M., Lambardi, M. (eds) In Vitro Embryogenesis in Higher Plants. Methods in Molecular Biology, vol 1359. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3061-6_1
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3061-6_1
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3060-9
Online ISBN: 978-1-4939-3061-6
eBook Packages: Springer Protocols