Abstract
In apomictic Hieracium subgenus Pilosella species, embryo sacs develop in ovules without meiosis. Embryo and endosperm formation then occur without fertilization, producing seeds with a maternal genotype encased in a fruit (achene). Genetic analyses in H. praealtum indicate a dominant locus (LOA) controls meiotic avoidance, and another dominant locus (LOP) controls both fertilization-independent embryogenesis and endosperm formation. While cytologically examining developmental events in ovules of progeny from crosses between different wild-type and mutant Hieracium apomicts, and a sexual Hieracium species, we identified two plants, AutE196 and AutE24, which have lost the capacity for meiotic avoidance and fertilization-independent embryo formation. AutE196 and AutE24 exhibit autonomous endosperm formation and set parthenocarpic, seedless achenes at a penetrance of 18 %. Viable seed form after pollination. Cytological examination of 102 progeny from a backcross of AutE196 with sexual H. pilosella showed that autonomous endosperm formation is a heritable, dominant, qualitative trait, detected in 51 % of progeny. Variation in quantitative trait penetrance indicates other factors influence its expression. The correlation between autonomous endosperm development and mature parthenocarpic achene formation suggests the former is sufficient to trigger fruit maturation in Hieracium. The developmental component of autonomous endosperm formation is therefore genetically separable from those controlling meiotic avoidance and autonomous embryogenesis in Hieracium and has been denoted as AutE. We postulate that tight linkage of AutE and genes controlling autonomous embryogenesis at the LOP locus in H. praealtum may explain why inheritance of autonomous seed formation is typically observed as a single component.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apomixis is the mode of asexual reproduction by seed and is present in over 40 plant families and more than 400 genera including many Hieracium species (Fig. 1) (Carman 1997). Apomixis omits a number of the developmental steps required for sexual seed production. In sexual Hieracium species such as H. pilosella (P36; Fig. 1a, b), diploid male and female gamete precursor cells develop in the anther and ovule, respectively, and undergo meiosis to form reduced gametophytes. The reduced female gametophyte or embryo sac found in the Hieracium ovule is of the 8-nucleate Polygonum type, containing an egg, two central cell nuclei, two synergids, and three antipodals. Embryo and endosperm development occur when male sperm cells fuse with the egg and two central cell nuclei during double fertilization. The surrounding ovule structures contribute to the seed coat and concomitant fruit differentiation forms the achene which encases the seed. Members of Hieracium subgenus Pilosella are allopolyploids (Fehrer et al. 2007), and apomictic species such as H. praealtum (R35; Fig. 1c), H. piloselloides (D36; Fig. 1c), and H. aurantiacum (A35; Fig. 1d) undergo apospory. Initially, sexual female gametophyte development initiates with meiosis of the megaspore mother cell to form megaspores. However, in contrast to sexual development, somatic ovule cells near developing megaspores enlarge and differentiate to form aposporous initial (AI) cells. One cell undergoes mitotic embryo sac formation, and while modes may differ between species, one diploid aposporous embryo sac displaces the sexual cells and the sexual pathway terminates (Fig. 1c, d). Apomixis in Hieracium has three developmental components that differ from the sexual pathway, avoidance of meiosis during embryo sac formation, fertilization-independent embryo development, and the rare component of fertilization-independent endosperm formation (Koltunow et al. 1998, 2011; Koltunow and Grossniklaus 2003).
Capitulum development and reproductive modes in Hieracium plants used in this study. a Capitulum development, flowering and seed release in pollinated sexual H. pilosella (P36) through stages 4–14. Bar 1 cm. b–f Reproductive modes in the plants indicated. Refer text for details. mmc megaspore mother cell, ms megaspore, es embryo sac, a antipodals, ccn central cell nuclei, s synergids, ec egg cell, DF double fertilization, en endosperm, em embryo. Question mark indicates mechanism has not yet been determined. Yellow shading denotes sexual processes. Orange shading including striped regions denotes apomictic and autonomous processes
Genetic analyses of H. praealtum γ-deletion mutants have shown that apomixis in Hieracium is controlled by two dominant loci. The LOSS OF APOMEIOSIS (LOA) locus is required for meiotic avoidance and sexual suppression, while the LOSS OF PARTHENOGENESIS (LOP) locus is required for autonomous seed formation (Catanach et al. 2006; Koltunow et al. 2011). Molecular markers linked to LOA and highly repetitive sequence characteristic of the hemizygous chromosome carrying LOA are conserved between H. praealtum, H. piloselloides, and H. caespitosum (Fig. 1c) but are absent in H. aurantiacum, suggesting it may have a different locus for apospory (Fig. 1d) (Koltunow et al. 2011; Okada et al. 2011). Control of autonomous seed development in H. aurantiacum is also unclear (Fig. 1d). It has previously been established that the activation of the LOA locus in aposporous apomicts requires the initiation of sexual reproduction (Koltunow et al. 2011).
If both LOA and LOP functions are lost in R35, reversion to sexual reproduction occurs demonstrating that the LOA and LOP loci are not essential for sexual reproduction but enable a modified reproductive program in a specific cell lineage where meiosis and fertilization are bypassed (Koltunow et al. 2011). If LOP function is lost and LOA function is retained, unreduced aposporous embryo sacs form that require fertilization for seed and fruit development. If LOA function is lost and LOP function is retained as occurs in the R35 deletion mutant loa134 (Fig. 1e), AI cells do not form, the events of meiosis and sexual embryo sac formation occur to completion, and LOP segregates in the meiotically reduced gametophytes. Thus, 50 % of the eggs and central cells require fertilization for seed initiation. The other 50 % inherit LOP and undergo autonomous seed formation (Koltunow et al. 2011; Fig. 1e). The developmental components of autonomous endosperm formation (AutE) and autonomous embryogenesis (parthenogenesis) have not been genetically separated in R35 mutants that retain LOP but undergo meiotic recombination due to the absence of the LOA locus. The putative AutE and LOP components have not yet been separated in progeny from crosses between apomictic R35 and sexual tetraploid P36 (Catanach et al. 2006; Koltunow et al. 2011). The LOP locus also exhibits suppressed recombination (Catanach et al. 2006). It is currently unclear if one or more genes control the developmental components of autonomous embryogenesis and autonomous endosperm formation in R35 (Koltunow et al. 2011).
Here, we report the isolation of two independent Hieracium plants, AutE196 and AutE24, originating from crosses between P36 and D36, and loa134 and A35, respectively. AutE196 and AutE24 form reduced embryo sacs via the sexual pathway, exhibit autonomous endosperm formation and fruit development under non-pollinating conditions, and require fertilization for embryogenesis. Sexual backcrossing of AutE196 demonstrated that the autonomous endosperm formation trait was heritable and indicated AutE is an independent, dominant genetic component contributing to apomictic seed formation in Hieracium. The AutE196 and AutE24 plants provide novel material for the molecular characterization of the mechanism of autonomous endosperm development, and further understanding of parental genome dosage and ploidy relationships in viable seed formation.
Materials and methods
Hieracium subgenus Pilosella plants including AutE24 and AutE196 were maintained by vegetative micropropagation in culture and grown as described by Koltunow et al. (1998, 2000, 2011). Cross-pollinations, emasculations, seed germination, cytological analyses via ovule clearing, and plant ploidy determinations were carried out as described in Koltunow et al. (2011). DNA isolation, oligonucleotide sequences (Online Resource 1), PCR conditions, and sequencing procedures used to assay various LOA-linked and LOP-linked markers were as described by Catanach et al. (2006), Koltunow et al. (2011) and Okada et al. (2011).
Results
Female gametophyte and seed development in unpollinated AutE196
Hieracium species are self-incompatible. Sexual species set seed following cross-pollination, and apomicts set seed in the absence of cross-pollination and also following emasculation of male floral organs in developing capitula. The tetraploid hybrid AutE196 was identified among 62 progeny from a cross between tetraploid sexual P36 as a female parent and the tetraploid apomict D36 as a male parent when developing capitula were examined in the absence of cross-pollination at stage 10 of capitulum development (Fig. 1a). Mature embryo sacs in the sexual P36 parent contained arrested egg and central cell nuclei, whereas the D36 apomict parent had formed embryos and endosperm (Fig. 2a, b; Table 1). AutE196 predominantly had developmentally arrested embryo sacs; however, 17 % of ovules had arrested eggs with proliferating nuclear and cellularized endosperm (Fig. 2c–e; Table 1). Complete cellularization occurred more frequently at later stages (Fig. 2f). The phenotype was not due to partial self-fertilization whereby the central cell and not the egg cell was fertilized, as both emasculation and self-pollination control experiments resulted in similar frequencies of endosperm formation, confirming AutE196 was self-incompatible and endosperm proliferation was autonomous (Table 2; Fig. 2g). Cytological analysis of female gametophyte and seed initiation at various developmental stages of AutE196 ovules indicated meiotically derived, reduced embryo sacs were present and aposporous embryo sacs were not formed (Table 1). Embryo development did not initiate at any stage in unpollinated florets. Endosperm proliferation initiated in a small fraction of ovules when 75 % of florets had opened (stage 8; Fig. 1a) and was observed at higher frequency at stage 10 (Table 1). Central cell nuclei fused prior to endosperm proliferation, and the pattern of development was the same as that seen in sexual and apomictic species with rapid growth in the number of free nuclei and cellularization occurring from peripheral walls of the developing seed (Fig. 2; Koltunow et al. 1998, 2000).
Embryo sac and seed phenotypes in AutE196, AutE24, P36, and D36. Flowers were fixed, ovules cleared and cytologically examined. Stage 10 embryo sacs are shown for a P36, b D36, c–g AutE196, and h AutE24. a–f, h Unpollinated flowers. g Emasculated flower. a, c Mature embryo sacs. b Embryogenesis and endosperm development. d Start of nuclear divisions in central cell. e, g, h Start of endosperm cellularization. f Cellularized endosperm. a antipodals, ccn central cell nuclei, ec egg cell, ne nuclear endosperm, em embryo, ce cellularizing endosperm, s synergid. Bar 100 μm
Consistent with these developmental phenotypes, AutE196 has not inherited core LOA markers associated with AI cell formation and sexual suppression from the apomictic D36 parent plant. Inheritance of the autonomous endosperm component is obviously attributed to the paternal D36 apomictic background and AutE196 has inherited the LOP278 marker (Table 3). Association of this LOP-linked marker with the AutE phenotype was examined further as described below.
Female gametophyte and seed development in unpollinated AutE24
Tetraploid AutE24 was identified among 20 plants phenotyped in a cross between the R35 γ-deletion mutant loa134 as a maternal parent and A35 as the pollen donor. loa134 is unable to undergo apospory and while it possesses many of the currently known LOA-linked markers, it is thought to have a small deletion in LOA (Koltunow et al. 2011). loa134 possesses a functional LOP locus (Fig. 1e) and the three examined LOP-linked markers (Table 3). During meiosis, LOP segregated among gametophytes resulting in autonomous seed formation (Table 4). Aposporous A35 lacks LOA-linked markers but possesses the central LOP93 marker (Table 3).
Like AutE196, AutE24 did not form aposporous embryo sacs or exhibit fertilization-independent embryogenesis, yet developed endosperm autonomously in 18 % of observed initiating seeds (Table 4). Endosperm proliferation began earlier in AutE24 in unopened florets at stage 6 and its frequency increased over stages 8 and 10 of capitulum development (Table 4). Endosperm pattern formation and cellularization resembled that of AutE196 and central cell nuclei fused prior to endosperm development (Fig. 2h). The frequency of autonomous endosperm proliferation did not change following either self-pollination or emasculation indicating that AutE24, like AutE196, is self-incompatible and the endosperm formation observed is fertilization-independent (Table 2).
AutE24 did not undergo apospory, yet possessed the LOA-linked markers indicating that it had inherited this chromosomal region from its maternal loa134 parent (Table 3). AutE24 did not possess the LOP93 marker present in A35 (Table 3). It is unclear whether the autonomous endosperm component observed in AutE24 had originated from the paternal A35 parent or from the R35 LOP locus in the maternal loa134 parent. We speculate that it may have originated from the A35 background due to the identification of AutE24 from a cross with a relatively small sampling of 20 progeny when compared with the inability to identify separation of autonomous embryo and endosperm components in progeny from crosses involving apomictic R35 and sexual P36 despite phenotyping over 300 progeny to date (Catanach et al. 2006; Johnson and Koltunow unpublished observations).
The egg and central cell are receptive to fertilization in AutE196 and AutE24 and produce viable seeds
Cross-pollinations of AutE196 and AutE24 with P36 and A35, respectively, were conducted to examine whether the egg and central cell were receptive to fertilization and developmentally functional (Table 2). The capitula were pollinated daily at stages 7–9 to coincide with sequential floral opening. Cytological examination of stage 10 ovules indicated that the pollination of AutE196 decreased the frequency of central and egg cells from 80 to 15 % and endosperm and embryo formation had increased from 0 to 77 % (Table 2). Similarly, cross-pollination of AutE24 had reduced the prevalence of central and egg cells from 65 to 19 %, and increased the formation of endosperm and embryos from 0 to 49 %. These frequencies were comparable to those observed in parental apomicts during the same stage of seed development (Tables 1, 4) and indicated that egg and central cells in plants capable of autonomous endosperm formation could be fertilized. Furthermore, plump seeds posited to contain embryos and endosperm arising from cross-pollination of AutE196 and AutE24 with P36 and A35, respectively, germinated at frequencies of 99 and 95 % demonstrating their viability. All resultant plants were clearly hybrids based on plant morphology.
The frequency of embryo sac abortion in AutE196 cross-pollinated with P36 was unchanged at 2 % while cross-pollination of AutE24 with A35 increased the prevalence of aborted embryo sacs from 7 to 17 % (Table 2). The relatively large number of aborted embryo sacs in AutE24xA35 would appear to largely account for the lower frequency of endosperm and embryo formation relative to AutE196xP36 ovules. The increased number of aborted embryo sacs in AutE24xA35 (17 %) relative to AutE196xP36 (2 %) can most likely be attributed to unknown factors within the different genetic backgrounds of these hybrids.
Cross-pollination resulted in similar decreases in the percentage of seed with autonomous endosperm and quiescent eggs from 17 to 6 % for AutE196 and from 22 to 9 % for AutE24 (Table 2). Autonomous endosperm development began in AutE196 at stage 8 and in AutE24 at stage 6 (Tables 1, 4). Pollination at stages 7–9 appeared to have partially reduced the observed frequency of seeds with only autonomous endosperm formed, presumably because double fertilization had occurred prior to the onset of autonomous development. We concluded that AutE196 and AutE24 produced meiotically reduced gametophytes with viable egg and central cells that could be fertilized. However, from the experimental data reported herein, it is unclear whether fertilization can occur once endosperm proliferation begins. Further analyses are required to examine the ploidy of the endosperm following cross-pollinations of AutE196 and AutE24 plants with other pollen donors.
Autonomous endosperm development is inherited as a dominant qualitative trait
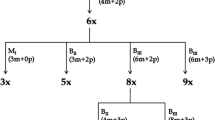
In order to examine whether autonomous endosperm formation was a genetically heritable trait in AutE196, we backcrossed it as the pollen donor to sexual P36 and cytologically examined 102 progeny for their capacity to form autonomous endosperm. A minimum of 100 ovules were examined per plant (Fig. 3; Online Resource 2). The plants were all hybrids with a high rate of phenotypic variation. Autonomous endosperm was observed in 52 out of the 102 lines (51 %), indicating dominant qualitative inheritance in the progeny if the locus is acting as a single allele in the allopolyploid exhibiting disomic inheritance (χ 2 = 0.039, 1 df, p = 0.84). In quantitative terms, the penetrance of autonomous endosperm formation ranged from 1 to 25 % of ovules in the capitulum (Fig. 3). This suggests that other loci probably influence the expression of autonomous endosperm development.
Genetic inheritance of autonomous endosperm phenotype in progeny from a P36xAutE196 backcross. The ovule phenotypes of 102 progeny from a P36xAutE196 backcross were cytologically examined. A minimum of 100 ovules were observed for each plant and the formation of autonomous endosperm was calculated as a percentage. The number of progeny with and without autonomous endosperm is summarized above the columns. The raw data are shown in Online Resource 2
Markers linked to AutE and autonomous embryogenesis
AutE196 contained the LOP278 marker (Table 3). To investigate whether LOP278 was linked to autonomous endosperm formation in AutE196, we used PCR to examine the presence or absence of this marker in 10 progeny arising from the backcross of AutE196 to sexual P36. Five of the plants possessed the autonomous endosperm phenotype and the remainder did not. All 10 of the plants possessed the marker (Online Resource 3), indicating it is not linked to the autonomous endosperm trait. The LOP93 marker may be linked to autonomous embryogenesis as it was present in apomictic D36, A35, and in the R35 loa134 mutant and absent in the AutE196 and AutE24 autonomous endosperm plants (Table 3). A wider assessment of the LOP93 marker in phenotyped apomictic Hieracium subgenus Pilosella germplasm is required to test linkage with autonomous endosperm formation.
Autonomous endosperm development induces fruit development and maturation in Hieracium
In sexual Hieracium, ovules unfertilized by stage 14 when the capitulum opens (Fig. 1a) have arrested ovaries that are pale brown in color (Fig. 4a, panel i). Fruit or achene development is stimulated when double fertilization and seed formation occur. The differentiation and hardening of developing fruits result in a barb-like surface morphology and accumulation of pigment in the achenes. A fruit with a viable seed is plump and black (Fig. 4a, panels ii, vi). If seed initiation occurs and then aborts, a dark brown or black thin fruit will form depending on the stage of seed abortion (Koltunow et al. 1998, 2000). In open capitula of unpollinated AutE196 plants at stage 14, three “fruit types” were observed: brown, dark brown, and black (Fig. 4a, panels iii–v). The dark brown and black fruits were all significantly longer than the brown fruits (one-tailed t test, p > 0.01). The length of these dark brown and black fruits corresponded to fruit lengths obtained when AutE196 was cross-pollinated with P36 (Fig. 4b), indicating their phenotypic development as fruits rather than brown ovaries. The 17 % frequency of autonomous endosperm development in unpollinated AutE196 (Table 1) correlated with the combined 14 % frequency of the dark brown and black achenes (Fig. 4c; Online Resource 4). These data suggest either AutE196 has independent factors for both autonomous endosperm and fruit development, or autonomous endosperm development is sufficient to stimulate fruit formation.
Autonomous endosperm formation correlated with fruit development in AutE196 and the progeny of a P36xAutE196 backcross. a Fruit color in unpollinated P36, D36, and AutE196, and AutE196 cross-pollinated with P36. Fruits were collected at stage 14 from emasculated P36, D36, AutE196 flowers (i–v) or from AutE196 capitula after cross-pollination with P36 (vi). Fruit colors were categorized as either brown (Br), dark brown (Db), or black (Bl). The prevalence of fruit color in each plant is expressed as a percentage. The raw data are shown in Online Resource 4. Bar 1 mm. b Fruit length of P36, AutE196, and AutE196 cross-pollinated with P36. The fruit length of each color was measured and depicted as the mean ± SD, n = 10. An asterisk indicates a significant difference by one-tailed t test (p < 0.01). c Correlation between autonomous endosperm formation and fruit maturation in the progeny of a P36xAutE196 backcross. The frequency of autonomous endosperm and mature (dark brown or black) fruit formation was determined in capitula from each plant. The coefficient of determination (r 2) was determined for the P36xAutE196 backcross progeny. Symbols depict P36xAutE196 (black), P36 (blue) and AutE196 (red). Original data of autonomous endosperm and fruit are shown in Online Resource 5
To explore these possibilities, we examined whether there was any correlation between fruit phenotype and autonomous endosperm formation in the P36xAutE196 backcross progeny. The progeny that did not form autonomous endosperm developed only brown ovary structures as found in unpollinated P36 (Online Resource 5). By contrast, dark brown and black fruits did form in the progeny scored for autonomous endosperm formation. Positive correlation (r 2 = 0.91) was observed between the formation of autonomous endosperm and mature fruit (Fig. 4c). This result supports the latter notion that autonomous endosperm formation in the absence of embryogenesis provides sufficient developmental cues to stimulate achene development and maturation in Hieracium. However, we cannot discount the possibility that autonomous endosperm and fruit formation are independent factors which are linked and segregate as a single genetic component.
Discussion
Asteraceae are among the few families that utilize the three apomictic components, meiotic avoidance, and fertilization-independent embryo and endosperm formation to form a viable seed and differentiated fruit via an asexual pathway. Factors leading to meiotic avoidance and the formation of an aposporous unreduced embryo sac and fertilization-independent seed formation have been genetically separated in H. praealtum. The two genomic regions associated with these components and linked markers have been identified and are under molecular investigation (Catanach et al. 2006; Koltunow et al. 2011; Okada et al. 2011). Here, we have identified two hybrid Hieracium plants, AutE196 and AutE24, of different genetic backgrounds where the component of autonomous endosperm formation has been genetically separated from the other two developmental components of apomixis in Hieracium. Autonomous endosperm development occurred in around 18 % of seeds that parthenocarpically form in unpollinated capitula, and subsequent pollination produced viable seeds. Autonomous endosperm formation was inherited as a dominant qualitative trait when AutE196 was backcrossed to sexual P36.
AutE196 has inherited autonomous endosperm formation from H. piloselloides (the paternal parent) which shares markers for meiotic avoidance and autonomous seed development with H. praealtum. AutE196 does not possess the examined subset of LOA-linked markers associated with meiotic avoidance, and the inherited LOP-linked marker is not linked to the autonomous endosperm phenotype. AutE24 may have inherited autonomous endosperm formation from either H. praealtum (the maternal parent) or H. aurantiacum. We suspect the latter because the separation of autonomous endosperm formation from autonomous embryogenesis in H. praealtum has not been achieved to date due to the suppression of recombination at the LOP locus (Catanach et al. 2006). The observation that autonomous endosperm is a genetically separable component in Hieracium suggests that the components of autonomous embryo and endosperm formation in H. praealtum may be encoded by separate elements tightly linked at the LOP locus. Marker analysis in AutE196, AutE24, and parental plants suggests that the LOP93 marker may be more closely associated with autonomous embryogenesis.
It is not known whether autonomous endosperm induction functions sporophytically or gametophytically in ovules of these plants. It is also unclear whether AutE196 and AutE24 possess the same or different loci for autonomous endosperm formation. We have recently determined that the two plants are cross-fertile and we have initiated the generation of reciprocal cross-populations to examine phenotypic effects on autonomous endosperm formation in the progeny and to ascertain whether the AutE loci evident in both plants function in the same pathway.
Erigeron annuus and Taraxacum officinale are apomictic Asteraceae family members which undergo a diplosporous mode of meiotic avoidance whereby the megaspore mother cell switches to a mitotic embryo sac formation pathway and then seed formation is fully autonomous. Genetic studies in these plants resolve meiotic avoidance and autonomous embryogenesis as genetically distinct components (Noyes and Rieseberg 2000; Tas and Van Dijk 1999; van Dijk and Bakx-Schotman 2004; van Dijk et al. 1999). No evidence for the independent control of autonomous embryo and endosperm formation has been observed in Erigeron where the process of autonomous seed development is considered to be possibly due to a single gene (Noyes et al. 2007). Two triploid plants have been recovered and cytologically characterized from crosses between sexual and apomictic Taraxacum that undergo diplosporous embryo sac formation and appear to produce autonomous endosperm while the egg remains undivided. This suggests that autonomous endosperm formation and autonomous embryogenesis may also be uncoupled in Taraxacum, although heritability has not been examined (van Dijk et al. 2003). Taraxacum plants solely exhibiting autonomous endosperm formation, as is the case for the AutE plants described in this study, have not been recovered in crosses between sexual and apomictic Taraxacum.
Although we have provided strong evidence for independent genetic control of autonomous endosperm formation in this study, the penetrance of the trait is relatively low in the AutE plants. The observed, variable penetrance in the examined backcross population indicates that other factors influence the expression of autonomous endosperm formation. The current model for the function of apomixis loci in Hieracium is that they are not required for sexual function, instead they moderate or heterochronically influence sexual developmental steps to enable apomixis to occur (Koltunow et al. 2011). AutE function induces cellular endosperm in the absence of fertilization; yet, it does not compromise fertilization in meiotically reduced embryo sacs as viable seed production is equivalent to sexual and apomictic plants. However, in AutE196 and AutE24, the autonomous endosperm formation factors influence central cell function in a haploid meiotically reduced embryo sac which contrasts with its typical role of influencing central cell function within or upon a diploid, unreduced aposporous embryo sac. Aposporous embryo sac development may result in the contribution of additional factors, absent in meiotically reduced embryo sacs which promote autonomous endosperm locus function. Alternatively, aposporous embryo sacs may lack or contain lower concentrations of repressive complexes such as the Polycomb repressive complex 2 (PRC2) which inhibits central cell nuclear proliferation in the absence of fertilization in Arabidopsis (Chaudhury et al. 1997; Ohad et al. 1999; Kiyosue et al. 1999; Köhler et al. 2003). The presence of the autonomous embryo formation locus may also be required for efficient AutE function in apomictic Hieracium and this component is absent in the AutE196 and AutE24 plants and progeny produced in the backcross. It is also possible that AutE196 and AutE24 contain only a portion of the entire locus required for efficient autonomous endosperm induction.
The functions of the members of the Arabidopsis PRC2 complex, which repress central cell proliferation in the absence of fertilization in Arabidopsis do not appear to be identical in Hieracium (Rodrigues et al. 2008, 2010). Down-regulation of the Hieracium PRC2 complex homologue of FERTILIZATION-INDEPENDENT ENDOSPERM (HFIE), a WD-40 protein whose Arabidopsis counterpart is a key complex linker protein, does not lead to autonomous endosperm or embryo proliferation in sexual Hieracium. Thus, it may not be part of a complex involved in repressing endosperm formation in the absence of fertilization in Hieracium. Post-fertilization seed development is compromised in sexual plants with down-regulated HFIE activity which corresponds to the secondary post-fertilization requirement for FIE in Arabidopsis. Down-regulation of HFIE in apomicts results in a decrease in the initiation frequency of autonomous seed formation and increased seed abortion. HFIE function is required for sexual and apomictic Hieracium seed formation (Rodrigues et al. 2008). HFIE is not linked to the LOP locus in H. praealtum (Koltunow et al. 2011). The Hieracium proteins interacting with HFIE and required for early embryo and endosperm formation remain unknown. AutE196 and AutE24 plants may prove useful for the further examination of the role of HFIE in endosperm formation in Hieracium.
We have established in this study that autonomous endosperm in Hieracium AutE196 plants is sufficient to trigger fruit development and maturation. This parallels prior observations in Arabidopsis where autonomous endosperm proliferation in PRC2 complex members triggers the maturation of the seed coat from the ovule integument and also fruit initiation (Chaudhury et al. 1997; Ohad et al. 1999). Interestingly, in Arabidopsis, cues from endosperm formation but not embryo development are required for seed coat maturation (Roszak and Köhler 2011).
Our future foci are to understand whether AutE loci are gametophytic or sporophytic in function, ascertain whether common loci are present in AutE24 and AutE196 plants and to resolve whether the AutE loci are linked to LOP in H. praealtum. Unpollinated AutE196 plants show a clear delay in timing between embryo sac maturity (stage 6) and the initiation of autonomous endosperm development (stage 8; Table 1). This makes it useful for transcriptomic analyses utilizing whole ovaries in addition to laser capture microdissection of embryo sacs, central cells, and developing endosperm to examine the pathways induced or altered by AutE compared with sexual and apomictic plants and mutants.
References
Carman JG (1997) Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol J Linn Soc 61:51–94
Catanach AS, Erasmuson SK, Podivinsky E, Jordan BR, Bicknell R (2006) Deletion mapping of genetic regions associated with apomixis in Hieracium. PNAS 103:18650–18655
Chaudhury AM, Ming L, Miller C, Craig S, Dennis ES, Peacock WJ (1997) Fertilization-independent seed development in Arabidopsis thaliana. PNAS 94:4223–4228
Fehrer J, Krahulcová A, Krahulec F, Chrtek J, Rosenbaumovaá R, Bräutigam S (2007) Evolutionary aspects in Hieracium subgenus Pilosella. In: Hörandl E, Grossniklaus U, van Dijk PJ, Sharbel T (eds) Apomixis: evolution, mechanisms and perspectives. Königstein, Koeltz, pp 359–390
Kiyosue T, Ohad N, Yadegari R, Hannon M, Dinneny J, Wells D, Katz A, Margossian L, Harada JJ, Goldberg RB, Fischer RL (1999) Control of fertilization-independent endosperm development by the MEDEA polycomb gene in Arabidopsis. PNAS 96:4186–4191
Köhler C, Hennig L, Spillane C, Pien S, Gruissem W, Grossniklaus U (2003) The Polycomb-group protein MEDEA regulates seed development by controlling expression of the MADS-box gene PHERES1. Genes Dev 17:1540–1553
Koltunow AM, Grossniklaus U (2003) Apomixis: a developmental perspective. Annu Rev Plant Biol 54:547–574
Koltunow AM, Johnson SD, Bicknell RA (1998) Sexual and apomictic development in Hieracium. Sex Plant Reprod 11:213–230
Koltunow AM, Johnson SD, Bicknell RA (2000) Apomixis is not developmentally conserved in related, genetically characterized Hieracium plants of varying ploidy. Sex Plant Reprod 12:253–266
Koltunow AM, Johnson SD, Rodrigues JC, Okada T, Hu Y, Tsuchiya T, Wilson S, Fletcher P, Ito K, Suzuki G, Mukai Y, Fehrer J, Bicknell RA (2011) Sexual reproduction is the default mode in apomictic Hieracium subgenus Pilosella, in which two dominant loci function to enable apomixis. Plant J 66:890–902
Noyes RD, Rieseberg LH (2000) Two independent loci control agamospermy (Apomixis) in the triploid flowering plant Erigeron annuus. Genetics 155:379–390
Noyes RD, Baker R, Mai B (2007) Mendelian segregation for two-factor apomixis in Erigeron annuus (Asteraceae). Heredity 98:92–98
Ohad N, Yadegari R, Margossian L, Hannon M, Michaeli D, Harada JJ, Goldberg RB, Fischer RL (1999) Mutations in FIE, a WD polycomb group gene, allow endosperm development without fertilization. Plant Cell 11:407–416
Okada T, Ito K, Johnson SD, Oelkers K, Suzuki G, Houben A, Mukai Y, Koltunow AM (2011) Chromosomes carrying meiotic avoidance loci in three apomictic eudicot Hieracium subgenus Pilosella species share structural features with two monocot apomicts. Plant Physiol 157:1327–1341
Rodrigues JC, Tucker MR, Johnson SD, Hrmova M, Koltunow AM (2008) Sexual and apomictic seed formation in Hieracium requires the plant polycomb-group gene FERTILIZATION INDEPENDENT ENDOSPERM. Plant Cell 20:2372–2386
Rodrigues JC, Luo M, Berger F, Koltunow AM (2010) Polycomb group gene function in sexual and asexual seed development in angiosperms. Sex Plant Reprod 23:123–133
Roszak P, Köhler C (2011) Polycomb group proteins are required to couple seed coat initiation to fertilization. PNAS 108:20826–20831
Tas IC, van Dijk PJ (1999) Crosses between sexual and apomictic dandelions (Taraxacum). I. The inheritance of apomixis. Heredity 83:707–714
van Dijk PJ, Bakx-Schotman JM (2004) Formation of unreduced megaspores (diplospory) in apomictic dandelions (Taraxacum officinale, s.l.) is controlled by a sex-specific dominant locus. Genetics 166:483–492
van Dijk PJ, Tas IC, Falque M, Bakx-Schotman T (1999) Crosses between sexual and apomictic dandelions (Taraxacum). II. The breakdown of apomixis. Heredity 83:715–721
van Dijk PJ, van Baarlen P, de Jong JH (2003) The occurrence of phenotypically complementary apomixis-recombinants in crosses between sexual and apomictic dandelions (Taraxacum officinale). Sex Plant Reprod 16:71–76
Acknowledgments
We thank John Carman for discussions on potential functions of AutE. The research was supported by a Science and Industry Endowment Foundation grant to A.M.K. D.O. was supported by a fellowship from the Japanese Society for the Promotion of Science (JSPS). We thank Takashi Okada for assisting D.O. in the JSPS Fellowship application.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Claudia Köhler.
Daisuke Ogawa and Susan D. Johnson contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ogawa, D., Johnson, S.D., Henderson, S.T. et al. Genetic separation of autonomous endosperm formation (AutE) from the two other components of apomixis in Hieracium . Plant Reprod 26, 113–123 (2013). https://doi.org/10.1007/s00497-013-0214-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-013-0214-y