Abstract
Somatic embryogenesis in plants is a process by which embryos can be produced from somatic cells cultured under specific conditions. A key initial step is represented by the ability of some cells within the explants to dedifferentiate, i.e., reacquire a “young” or immature state, and then redirect their fate into an embryogenic pathway, demarked by precise changes in gene expression. While the initial morphological patterns of somatic embryo formation can be quite different and difficult to categorize, developing somatic embryos can be assigned similar stages ascribed to zygotic embryos. These similarities allow the utilization of somatic embryogenesis as a model system to investigate physiological and molecular events governing zygotic embryogenesis. The aim of this chapter is to provide a general overview of somatic embryogenesis, by describing and analyzing several in vitro embryogenic systems, and to decipher the molecular network responsible for the generation of somatic embryos.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Auxin
- Embryogenesis
- Microspore-derived embryos
- Somatic embryos
- Root apical meristem
- Shoot apical meristem
14.1 Introduction
In flowering plants, embryogenesis is demarked by the fusion of the haploid gametes, i.e., egg and sperm cells, which through a double fertilization process form a single-celled zygote and an endosperm cell. The subsequent development of the zygote is referred to as embryogenesis, during which the zygote forms a fully developed embryo through very precise apical-basal and radial cell division and differentiation patterns (De Smet et al. 2010). A fully developed embryo generally consists of one or more cotyledons surrounding a shoot apical meristem (SAM), an embryonic axis, and a root apical meristem (RAM).
Plant embryogenesis is characterized by three unique features that have immense implications on the elaboration of the different embryonic tissues and organs. Firstly, unlike animal cells, plant cells do not migrate during morphogenesis. Therefore, the final shape of the organism is the mere result of cell division and expansion. Secondly, the plant embryo is not a miniature plant, as it lacks many tissues and organs which are formed during postembryonic development. Thirdly, the final stage of embryogenesis is characterized by an imposed desiccation period required for the termination of the embryogenic program and the initiation of germination. The time and modality of the desiccation process is species specific and results in a drastic reprogramming of gene expression (Elhiti and Stasolla 2013).
Recapitulation of embryogenesis can also occur in the absence of fertilization through the generation of asexual embryos. Through this process, referred as apomixis, embryos can develop from unfertilized egg cells or cells of the maternal tissue (Nogler 1984). Formation of asexual embryos can also be achieved via in vitro culture through gametophytic or somatic embryogenesis. Somatic embryogenesis can be theoretically initiated from all cells within the sporophyte, except gametic cells, while gametophytic embryogenesis involves the formation of haploid embryos from either the male or female gametophyte (Bhojwani and Razdan 1996; Raghavan 2000).
As hinted above, somatic embryogenesis has acquired relevance in the study of plant embryogenesis for several reasons. Firstly, it allows the synchronous development of embryos which are exposed and easily accessible. This is in contrast to zygotic embryogenesis, where the embryos are encased in the maternal tissue and often impossible to excise. This characteristic becomes problematic especially for collecting a suitable number of zygotic embryos for physiological and/or molecular studies. In addition somatic embryos are similar to their zygotic counterparts, and therefore knowledge acquired in vivo can be transferred in vitro (Yeung and Meinke 1993). As a consequence, several studies dealing with somatic embryogenesis at cellular, tissue, and molecular levels are currently available (Willemsen and Scheres 2004). Finally, generation of embryos in culture allows the targeted manipulations of environmental and/or culture conditions which would be difficult, if not impossible to perform in vivo. The selective addition or removal of specific chemicals in the medium is often used as a strategy not only to optimize culture conditions but also especially to understand the nature of the environment inductive for the proper development of the embryos.
Despite the existence of many similarities between somatic and zygotic embryogenesis, it must be noted that the two processes are also characterized by substantial differences which must be considered in comparative studies. Unlike zygotic embryogenesis, formation of somatic embryos is dependent upon the competence that some somatic cells have to change their developmental fate. This change involves an extensive and poorly understood reprogramming of gene expression which is unique of in vitro systems (Feher et al. 2003). Another relevant consideration is the fact that in vitro conditions are not fully optimized and therefore the “embryonic environment” created in vitro is different from the seed environment. As such, differences in embryo physiology and storage product depositions are often observed between the two systems. Finally, there are instances of in vitro-produced embryos able to germinate without a dormancy period which is often needed in vivo (Elhiti and Stasolla 2013).
Taken together, these considerations suggest that in vitro embryogenesis can indeed be used as a model system to study plant embryogenesis, but with the due care of acknowledging potential differences with zygotic embryogenesis.
14.2 Plant In Vitro Embryogenesis Systems
Over the past years, in vitro embryogenic systems have been developed for many plant species, including Arabidopsis and Brassica napus. While not a relevant crop, Arabidopsis has been used quite extensively for in vitro studies due to the available genetic information which facilitates molecular and genetic analyses. Knowledge on Arabidopsis can also be transferred to Brassica napus, as the two species are related. The in vitro systems for the two species are very different, as somatic embryogenesis is used for Arabidopsis, while microspore-derived embryogenesis (androgenesis) is used for Brassica. It must also be mentioned that, unlike canola embryos which develop directly from immature microspores, somatic embryos in Arabidopsis arise from a callus derived from the explant. As such, this system is often referred as indirect somatic embryogenesis.
14.2.1 Arabidopsis Somatic Embryogenesis System
Although reports of Arabidopsis somatic embryogenesis from mature tissues are available, somatic embryos are more easily produced from immature explants, such as zygotic embryos (Mordhorst et al. 1998). Dissected zygotic embryos, preferably at the bent cotyledon stage of development, are cultured in a medium containing the auxin 2,4-dichlorophenoxyacetic acid (2,4-D), considered the inductive signal required for the dedifferentiation process of the somatic cells within the explants. Under these conditions, embryogenic callus is generated by the apical regions of the zygotic embryos and in particular from the adaxial side of the cotyledons. Removal of the auxin induces the formation of somatic embryos.
14.2.2 Brassica napus Microspore-Derived Embryogenesis System
A key event during androgenesis in Brassica napus is the redifferentiation step in which the genetic program of the immature microspores is redirected toward the embryonic pathway. This redirection is triggered by several treatments including elevated temperatures (usually 32 °C; Keller and Armstrong 1979), colchicines (Zhao et al. 1996), gamma irradiation (Pechan and Keller 1989), ethanol (Pechan and Keller 1989), low temperatures (Kasha et al. 1995), change in pH (Barinova et al. 2004), and sucrose starvation (Touraev et al. 1996). The first sign of dedifferentiation of the microspores, as reviewed by Telmer et al. 1992, involves changes in cytoskeletal organization.
Simmonds and Keller (1999) observed that the pre-prophase band which is composed by arrays of microtubules tends to localize in the middle region of induced microspores. This positioning ensures the symmetric cell division of the microspore, which demarks the completion of the inductive events and the acquisition of the embryogenic competence (Yeung 2002). This is in contrast to the gametophytic developmental pathway which is initiated with an asymmetric mitotic division of the microspore. Of the two daughter cells originating from the microspore, one is committed to form the suspensor of the embryo, while the other, referred to as the pro-embryogenic cell, will generate the embryo proper. The whole process is accomplished within 3 days. Through a series of anticlinal and periclinal divisions, the pro-embryogenic cell gives rise to a cluster of cells demarking the globular stage of embryogenesis after 5 days in culture. Within 7–9 days in culture, a globular embryo is produced characterized by a well-developed protoderm, the precursor of the epidermis. During the following days, the growth of the embryo is characterized by the formation of two cotyledons and a morphologically visible SAM and RAM. A detailed description of the histodifferentiation events occurring during microspore-derived embryogenesis is available (Yeung et al. 1996).
14.3 Genetic Components of In Vitro Embryogenesis
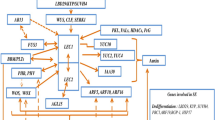
The mechanisms by which plant somatic embryogenesis is accomplished are quite complex but somehow conserved among plant species (Elhiti et al. 2013b). Simplified “molecular” steps of the in vitro embryogenic process have been reviewed by Elhiti et al. (2013a), and they are referred to as embryonic induction and development. The embryonic induction leads to the formation of embryogenic tissue and is further subdivided in dedifferentiation, acquisition of totipotency, and commitment. During the dedifferentiation step, cells within the explants must lose their pre-acquired fate; this is accompanied up by the acquisition of totipotency which enables the cells with the potential to differentiate into any cell type. The concept of totipotency is often associated to that of “stemness” as stem cells are indeed totipotent. The new developmental fate acquired by the totipotent cells is regulated by extrinsic factors, which in culture are often determined by the presence of plant growth regulators. During somatic embryogenesis, the fate of the totipotent cells is redirected or “committed” toward the embryogenic pathway.
The different phases of embryogenesis are accompanied by major “molecular” reprogramming. As described by Elhiti et al. (2013a), somatic embryogenesis encompasses two developmental stages: (1) embryonic induction and (2) development. The embryogenic induction stage is further subdivided into three main phases: (a) dedifferentiation, (b) expression of totipotency, and (c) commitment of induction phase. Hereafter we will describe the genetic networking during each stage of somatic embryogenesis. These initial phases are followed up by the “development” step which is characterized by the growth of the embryos which is often achieved in the absence of plant growth regulators (Sugiyama 1999; Elhiti 2010). The following sections will provide an updated description of the molecular events underlying the induction and development phases.
14.4 Genetic Network of Early Embryogenesis
14.4.1 Genetic Networking Regulating the Induction Phase
During this stage, the genetic program of the somatic cells under culture condition is reprogrammed by either applications of exogenous hormones or stresses (Feher et al. 2003). The induction stage of somatic embryogenesis is very difficult to study at molecular levels because of the lack of clear cytological markers permitting the identification of those clusters of somatic cells undergoing reprogramming in gene expression leading to the acquisition of the embryogenic fate. As such, gene network modeling and bioinformatic analyses are the only means to identify candidate genes required during the three different phases (dedifferentiation, acquisition of totipotency, and commitment) of the induction step (Elhiti et al. 2013a).
14.4.1.1 Dedifferentiation
The dedifferentiation of somatic cells, which in culture is often promoted by auxins, possibly involves a major reprogramming in gene expression. Microarray analyses in Arabidopsis have identified LATERAL ORGAN BOUNDARIES DOMAIN 29 (LBD29) as a key developmental gene controlling cell dedifferentiation processes both in vitro and in vivo (Liu et al. 2010). LBD29 has been identified as a downstream target of the auxin response factors ARF7 and ARF19 (Feng et al. 2012), and lbd29 cells show a reduced sensitivity to auxin and are unable to dedifferentiate. These observations suggest that the native function of this gene is necessary for dedifferentiation and reinforce the notion that auxin acts as the inductive signal (reviewed by Elhiti et al. 2013a). Other possible candidate genes participating in the dedifferentiation step are KRYPTONITE (KYP)/SUVH4, a gene encoding H3 lysine 9 methyltransferase, which, if mutated, reduces the formation of embryogenic tissue, and POLYCOMB REPRESSIVE COMPLEX 1 (PRC1) which has a repressive effect on the ability of cells to dedifferentiate upon the imposition of inductive signals (Bratzel et al. 2010).
14.4.1.2 Totipotency
A key characteristic of plant cells is their inherent ability to retain all the genetic information required to alter their development fate even once fully differentiated (Birnbaum and Alvarado 2008). If expressed by appropriate environmental conditions, this ability, referred to as totipotency, allows the regeneration of the whole organism, as exemplified during somatic embryogenesis (Verdeil et al. 2007). Despite extensive efforts to identify key elements required for the expression of totipotency, our knowledge on the molecular regulation of this process is very scarce. Independent studies suggest that epigenetic changes play an important role in totipotency (Costa and Shaw 2007; Birnbaum and Alvarado 2008). Furthermore, Arabidopsis mutant analysis showed that the concomitant knockout of CURLY LEAF (CLF) and SWINGER (SWN), genes encoding two polycomb repressor protein 2 (PRC2) proteins, results in the spontaneous production of embryogenic callus in culture in the absence of plant growth regulators which are normally required for callus formation. Based on these observations, the involvement of PCR2 proteins in the manifestation of totipotency cannot be excluded (Chanvivattana et al. 2004). Two other genes possibly implicated with the manifestation of totipotency are PICKLE (PKL) and SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 (SERK1). It has been suggested that the function of PICKLE is to repress totipotency since embryogenic tissue and ultimately somatic embryos are produced spontaneously from Arabidopsis pkl roots in the absence of the inductive signals (Aichinger et al. 2009). As PKL encodes a putative CHD3 (chromatin helicase DNA binding protein 3), the authors suggest a possible implication of chromatin remodeling processes in totipotency. SERK1, which encodes a leucine-repeat receptor protein kinase, is highly expressed during early embryogenesis (Hecht et al. 2001). Ectopic expression of this gene favors the formation of embryogenic tissue and encourages somatic embryo production indicating an involvement in embryogenic competence (Hecht et al. 2001). Using gain-of-function screening approach Zuo et al. (2002) revealed that a shoot apical meristem-related gene, WUSHEL, is also expressed in Arabidopsis explants during the early inductive phases of somatic embryogenesis in specific domains giving rise to embryogenic cells. In the same study it was observed that overexpression of WUS in Arabidopsis roots, leaf petioles, stems, or leaves is sufficient to induce somatic embryogenesis. It must be noted, however, that the ability to produce somatic embryos is retained in WUS tissue (Zuo et al. 2002), thus suggesting the existence of complex and possible multiple pathways regulating embryogenesis in vitro.
Generation of Brassica microspore-derived embryos is dependent upon the ability of immature microspores to lose their gametophytic fate and acquire an embryogenic fate. Transcription studies have identified LEAFY COTYLEDON1 and LEAFY COTYLEDON2 (LEC1, LEC2) as potential candidates mediating this developmental switch and molecular markers for embryogenicity (Malik et al. 2007), a function which appears to be retained across species. A significant repression in Arabidopsis somatic embryo production was indeed observed in lec mutants (Harada 2001). It must be noted that during somatic embryogenesis, WUS, LEC1, and LEC2 share similar expression profiles (Elhiti and Stasolla 2011). Taken together the authors speculated that WUS and LEC genes may be involved in the acquisition of totipotency possibly through parallel mechanisms. Genetic studies, including the analyses of wus/lec1/lec2 triple mutants, might be needed to unravel the function of these genes in early embryogeny.
It is well known that LEC genes are required to promote the expression of AGAMOUS-LIKE 15 (AGL15), encoding a MADS-domain protein (Zheng et al. 2009). Induction of AGL15 strongly activates the gibberellin 2-oxidase GA2ox6 which represses gibberellic acid synthesis. Therefore it cannot be excluded that LECs operate through the inhibition of gibberellins, which have been shown to act in an antagonistic fashion to auxin, the signal promoting the dedifferentiation of somatic cells.
The participation of auxin during the early embryogenic phases was also demonstrated in Brassica napus using BABY BOOM1 (BBM1), an AP2/ERF transcription factor (Boutilier et al. 2002). Ectopic expression of BBM1 in Brassica seedlings results in the production of somatic embryos from leaf margins, while its overexpression in Arabidopsis produces cotyledon-like structures (Boutilier et al. 2002). Overall, the overexpression of BBM1 was associated to other changes in leaf and flower morphology as well as neoplastic growth. Furthermore, BBM1 overexpressing explants were able to regenerate through organogenesis and embryogenesis without applications of exogenous plant hormones, an observation suggesting that BBM1 may interfere with auxin sensitivity. The requirement of auxin signaling during early embryogeny was also demonstrated during Arabidopsis somatic embryogenesis. Elhiti et al. (2013b) demonstrated that the increased number of somatic embryos obtained by suppressing GLB2, a type 2 nonsymbiotic hemoglobin, was the result of elevated levels of auxins which accumulate at the sites of the explants where embryogenic tissue forms. The authors developed a model in which suppression of GLB2 results in an increase in nitric oxide which represses the transcription factor MYC2, a repressor of auxin synthesis. Collectively, these studies demonstrated a solid link between the acquisition and manifestation of totipotency to auxin.
14.4.1.3 Commitment
It is believed that once somatic cells express their totipotency, specific signal cascades must be activated to promote cell division and encourage the acquisition of meristematic identity. Both events are crucial for the proper development of the embryos. Overall, the genes involved in this phase of somatic embryo induction may be divided into three main categories: genes participating in cell cycle, genes required for meristematic cell formation and regulation, and genes involved in several signal transduction cascades.
14.4.1.3 Genes Participating in Cell Cycle
Cell division in plants is controlled by complicated mechanisms which are governed by the expression of cyclin-dependent kinases (CDKs). CDKs are proteins influencing the entry time into the different phases of the cell cycle (Elhiti et al. 2013a). According to their internal motives, CDKs are classified into eight groups, CDKA through CDKG and CDKL (Zhang et al. 2012). Functional genetic analyses revealed that only CDKA1 (also referred to as CDC2A) is involved in embryogenesis and its expression is induced by the plant growth regulators auxins and cytokinins (Nowack et al. 2006). In Arabidopsis, overexpression of CDC2A represses somatic embryogenesis, while a downregulation of the same gene enhances the number of somatic embryos produced (Hemerly et al. 2000). Another CDK possibly participating in embryogenesis is CDKA, the transcript levels of which increase during the early phases of somatic embryogenesis prior to declining as the embryos develop (Cortes et al. 2010).
It has been reported that PROPORZ1 (PRZ1), a putative Arabidopsis transcriptional adaptor, mediates cell proliferation through auxin and cytokinin signaling (Sieberer et al. 2003). Compared to WT tissue, in which ectopic cell proliferation is observed in the presence of both auxin and cytokinins, prz1 tissue is able to produce callus when cultured with either auxin or cytokinin (Sieberer et al. 2003). Based on these observations, the authors suggested that PRZ1 mediates cell proliferation and differentiation by affecting the behavior of cell cycle regulators. Another possible component of the mitotic machinery with a possible involvement on somatic embryogenesis is histone H3-11, a mitosis-specific phosphorylation protein. Hendzel et al. (1997) suggested that histone H3-11 is particularly required during the inductive phases of embryogenesis, an observation consistent with the high levels of histone H3-11 transcripts measured in alfalfa tissue subjected to 2,4-D treatments which stimulate embryogenic tissue formation (Kapros et al. 1992).
14.4.1.3 Genes Involved in Meristematic Cell Formation
The competent cells formed in culture on the explants respond to an inductive signal, usually provided by specific culture addenda such as growth regulators, and become meristematic cells. Elhiti et al. (2010) proposed that meristematic cell formation in culture is regulated by similar mechanisms involved in the formation and maintenance of the meristematic cells within the SAM in vivo. Proper SAM homeostasis relies on two classes of genes: those promoting cell division and those favoring cell differentiation. Members of the former class are SHOOTMERISTEMLESS (STM) and WUSCHEL (WUS), while promoters of cell differentiation are CLAVATA1 and CLAVATA2 (CLV1 and CLV2). Using Brassica and Arabidopsis, it was demonstrated that while the constitutive expression of STM induces embryo formation in culture, overexpression of CLV1 represses the production of embryos (Elhiti et al. 2010). This contrasting behavior in vitro is analogous to that observed during the maintenance of the SAM in vivo.
A lot of attention has also been directed toward the interaction of WUS and CLV1, the function of which has been well documented. In the SAM the role of CLV1, a transmembrane receptor serine/threonine kinase with leucine-rich repeat (Clark et al. 1997), is to promote the differentiation of meristematic cells by repressing WUS expression through a complex signaling model involving other CLV proteins (Dodsworth 2009). In this model CLV3 produced by the apical cells of the SAM binds to CLV1/CLV2 receptor kinase complexes located in the subapical cells and through the activation of downstream signaling components downregulates WUS which is expressed in the “organizing center” (Dodsworth 2009). In Arabidopsis somatic embryogenesis system, the expression of WUS, induced by auxin, is first visible in those domains of the explants giving rise to the embryogenic tissue (Su and Zhang 2009). Chen et al. (2009) also demonstrated a cytokinin-mediated activation of WUS. The WUS-CLV interaction was shown to occur during M. truncatula somatic embryogenesis where the two genes competitively modulate the formation of embryogenic tissue formation (Chen et al. 2009). It must be noted, however, that all the SAM-related genes described above are not necessary for somatic embryo formation, as their respective Arabidopsis mutants are still able to produce somatic embryos in culture (Mordhorst et al. 1998).
It is well known that WUS acts as a transcription factor repressing A-type Arabidopsis response regulators, thereby activating cytokinin responses contributing to meristem maintenance (Leibfried et al. 2005). Several studies suggest that WUS activity in vivo requires the expression of the ARGONAUTE (AGO) protein ZWILLE/AGO10 (Tucker et al. 2008). AGO proteins are central elements of the RNA interference (RNAi) pathway and mediate the repression of target mRNA through mRNA degradation or translational inhibition (Mallory and Vaucheret 2010). Specifically, ZLL/AGO10 blocks the accumulation of microRNA165/microRNA166 in the stem cell niche of the SAM by sequestration mechanisms preventing the degradation of microRNA165/microRNA166 targets’ transcripts of HD-ZIPIII transcription factor (Knauer et al. 2013). Based on these observations, it might therefore be interesting to ascertain the participation of AGO proteins in the initial phases of embryogenesis.
14.4.1.3 Genes Involved in Signal Transduction Cascade
The CLV signaling described in the first section is modulated by downstream components, the function of which, although not tested during in vitro embryogenesis, might participate during in vitro embryogenesis. Two intermediary modulators of CLV signaling are a kinase-associated protein phosphatase (KAPP) and a rho-like GTPase (Rop) (Song et al. 2006). These two proteins interact directly with CLV1 forming a 450 kDa active signaling complex. KAPP functions in vivo as a negative regulator of the CLV signaling through direct dephosphorylation of CLV1, while Rop is assumed to transduce the CLV signal into the nucleus (reviewed by Elhiti et al. 2010). Future studies assessing the involvement of these two proteins during in vitro embryogenesis might further validate the notion that the formation of meristematic cells in vitro uses signaling systems governing SAM homeostasis.
Besides KAPP and Rop, SHEPHERD, a HSP90-like protein predicted to be required for correct folding of CLV complex (Ishiguro et al. 2002), and POLTERGEIST (POL), a nuclear-localized protein phosphatase 2C (PP2C) which acts downstream within the CLV transduction (Carles and Fletcher 2003), can be additional candidates to be tested during in vitro embryogenesis. These proposed studies would verify the proposed notion that meristematic cell formation in vitro relies on similar mechanisms governing SAM formation and maintenance in vivo.
14.5 Genes/Gene Homologues Influence Embryo Development
14.5.1 Genetic Networking Controlling Somatic Embryo Development
The developmental phase of in vitro embryogenesis culminates with the formation of fully developed embryos, the growth of which occurs along two axes: an apical-basal axis and a radial axis. While the apical-basal growth ensures the proper positioning of the cotyledons surrounding the SAM, a hypocotyl, and a RAM, the radial growth specifies concentric layers of tissues: the stele, cortex, and epidermis. Precise coordination of these events is paramount for the accurate establishment of the embryo body. The development of many Arabidopsis mutants, as well as high-resolution molecular techniques, has aided our understanding on the molecular networks coordinating apical-basal and radial growth.
14.5.1.1 Establishment of the Apical-Basal Body Plan
The formation of apical-basal axis of a somatic embryo is responsible for the proper positioning of the SAM and RAM at the opposite regions of the hypocotyl. Among the processes ensuring this axis pattern are asymmetric cell division and preferential elongation along the desired axis (De Jong et al. 1993; Emons 1994). While asymmetric cell divisions are promoted by plant hormones that alter cell polarity by interfering with pH gradients or the electrical fields across membranes (Smith and Kirkorian 1990), cell expansion is associated with the composition of polysaccharides within the cell wall and specific hydrolytic enzymes (De Jong et al. 1993; Emons 1994; Fry 1995). The participation of asymmetric cell divisions and elongation for the establishment of the apical-basal axis during in vivo embryogenesis are manifested at the zygotic stage when the zygote elongates and undergoes an asymmetric division leading to the formation of small apical cells, precursors of the embryo proper, and larger basal cells forming the suspensor cell. The contribution of these two events is also crucial for the later stages of embryogenesis (Zhang and Laux 2011). Although the early phases of embryogenesis in vitro follow less precise patterns, the roles of asymmetric cell divisions and elongations are still apparent in some systems, including Brassica microspore-derived embryogenesis where the type of division observed in the microspore, i.e., symmetric or asymmetric, marks its developmental fate. Molecular analyses during the earliest phases of Brassica microspore-derived embryogenesis identified some potential genes possibly involved in this fate acquisition, including FUSCA3, LEAFY COTYLEDON1 (LEC1), LEC2, BABY BOOM (BBM), PINFORMED7 (PIN7), two WUSCHEL-related homeobox (WOX) genes, WOX2, WOX8 and WOX9, and ABSCISIC ACID INSENSITIVE3 (Joosen et al. 2007; Malik et al. 2007; Tsuwamoto et al. 2007). While the involvement of these genes in asymmetric cell division is known, more information is available for PIN7 and WOX2. During the asymmetric cell division of the zygote, PIN7 is preferentially localized in the basal cell, while expression of WOX2 is restricted in the apical cell. A mutation in either of the two genes compromises the ability of the zygote to divide properly (reviewed by Elhiti and Stasolla 2013).
The participation of auxin for the execution of asymmetric cell divisions is well established, and a precise distribution of this growth regulator is also required for the specification of somatic cells embarking in the embryogenic pathway. According to Su and Zhang (2009), the formation of an auxin gradient within the Arabidopsis embryogenic tissue is crucial for inducing the stem cell formation through the regulation of PIN1. This regulation would also mediate the expression of WUS and other WOX genes required for the establishment of the apical-basal axis. Of note, the observation that WOX8 and WOX9 are also expressed during conifer embryogenesis possibly through auxin-mediated mechanisms (Palovaara and Hakman 2009) raises the possibility of a more general involvement of these groups of genes in embryo patterning.
The apical domain of a fully developed embryo consists of cotyledons and a SAM. Independent studies have shown that the establishment of the apical embryonic domains in vivo is specified by GURKE and TOPLESS. GURKE encodes an acetyl-CoA carboxylase and, if mutated, precludes the formation of cotyledons and the SAM (Baud et al. 2003). Knockout of TOPLESS results in the formation of a root in the apical pole, thus indicating that the function of this gene is to abolish the manifestation of the basal patterning in the apical domains (Szemenyei et al. 2008).
The central embryonic domain consists of a hypocotyl, the specification of which is regulated by FACKEL, HYDRA1, and CEPHALOPOD (Willemsen and Scheres 2004). With mutations in these genes, embryos form without hypocotyls in which the apical domain is directly connected to the embryonic root (Lindsey et al. 2003). These genes participate in the biosynthesis of sterols, suggesting an involvement of these compounds in hypocotyl formation. Not surprisingly, auxin is also required for the development of a functional hypocotyl. Mutations of MONOPTEROS, a gene encoding an auxin responsive factor, produce embryos lacking a hypocotyl (Schruff et al. 2006). In the same study it is speculated that the specific function of these genes might be related to the formation of a functional stele, as this is the most affected tissue in the mutants.
Other genes interfering with auxin signaling: AUXIN-RESISTANT6 and BONDELOS are also required for the proper establishment of the central embryonic domain (Park and Harada 2008).
The embryonic basal domain includes the RAM which is composed of quiescent cells surrounded by the stem cells. During early phases of embryogenesis, expression of PINFORMED1, 4, and 7, all encoding auxin efflux carriers, are required for the formation of an auxin maximum at the basal domain, which is essential for the specification of the RAM (Willemsen and Scheres 2004). Mutations in auxin downstream components, such as PLETHORA, which is expressed in quiescent cells and encodes AP2 domain transcription factor, cause the mis-specification of quiescent cells and consequently the improper formation of the embryonic root (Aida et al. 2004). Analyses of these mutants showed that the effects of PLETHORA in the formation of embryonic root are mediated through interaction with SCARECROW and SHORTROOT (Aida et al. 2004). Furthermore, HOBBIT, a homologue of a subunit of the anaphase-promoting complex, is also required for proper localization of quiescent cells in embryonic root (Willemsen et al. 1998). Collectively, these studies show that the apical, central, and basal embryonic domains appear to be controlled by independent genetic mechanisms which are coordinated by a proper flow of auxin. The majority of these studies, however, have been conducted in vivo, and it is not clear whether similar mechanisms also operate in vitro, where tissue patterning is less organized and predictable.
14.5.1.2 Establishment of Embryonic Shoot Apical Meristem (SAM)
The establishment of the SAM is considered a key event during embryogenesis and encompasses three phases: the specification of apical domain (discussed in the previous sections), the formation of the stem cell niche, and the separation of the central and peripheral domains. The transcription factor WUS defines the organizing center of the meristem and is considered the initial marker for the specification of the stem cell niche. Localization studies in Arabidopsis demonstrate that WUS transcripts appear very early during somatic embryogenesis (Su and Zhang 2009). The main function of WUS is to maintain the stem cells in an undifferentiated state, thereby ensuring the proper maintenance of the apical region. As previously described, WUS is regulated by CLV feedback mechanisms through the interaction of CLV1-3. Another marker of the initial formation of the SAM is the homeodomain transcription factor STM which is also expressed in somatic embryos starting from the globular stage of development (Elhiti 2010). Downregulation of STM results in fusion in the embryonic cotyledons resulting in the production of trumpet-shaped embryos (Elhiti et al. 2010). The demarcation between the central and peripheral domains of the SAM is necessary for the proper positioning of the cotyledons relative to the SAM (reviewed by Elhiti and Stasolla 2013). This process is mediated by CUP-SHAPED COTYLEDON (CUC1, 2, 3), expressed at the boundary between the cotyledons and the SAM (Aida et al. 1999). Knockout of CUC phenocopies the stm phenotype (trumpet-shaped embryo), suggesting that STM and CUC may share the same pathway. It has been observed that accumulation of CUC transcripts is regulated by microRNA164 (Zhang et al. 2006).
14.5.1.3 Establishment of the Embryonic Radial Pattern
Radial patterning results in the proper specification of the epidermis, cortical tissue, and vascular tissues. The first hint of radial pattern formation during in vivo and in vitro embryogenesis corresponds with the separation of the protoderm from the inner cells (Elhiti and Stasolla 2013). Expression analyses in Arabidopsis indicate that ARABIDOPSIS THALIANA MERISTEM LAYER1 and PROTODERMAL FACTOR2, encoding transcription factors containing the START domain, are implicated in the radial specification of the protodermal layer (Abe et al. 2003). Other genes involved in radial patterning are KEULE and KNOLLE, as a radial axis is never initiated in the two mutants. While their function is not fully clear, it has been shown that KNOLLE encodes a syntaxin-like protein involved in secretary processes (Song et al. 2000). A mutation in this gene results in abnormal cytokinesis due to incomplete formation of the cell wall separating the two daughter cells (Song et al. 2000).
SHORT ROOT (SHR) and SCARECROW (SCW), encoding transcription factors of the GRAS family, are required for the proper specification of endodermal and cortical layers. Knockout of SHR results in absence of the endodermis, while scw mutants have a single file of cells in place of cortex and endodermis (Di Laurenzio et al. 1996). Localization studies indicated that SHR is expressed in the vascular tissue and translocated into the endodermal layer where SCW is expressed (Di Laurenzio et al. 1996).
14.6 Conclusions
Embryo formation in vivo is initiated with the fusion of the gametes, i.e., sperm and egg, resulting in the formation of the zygote. Through precise cell division and differentiation processes, the zygote produces a fully develop embryo, composed of an apical, a central, and a basal domain. Recapitulation of embryogenesis can also occur in vitro through somatic and gametophytic embryogenesis. Formation of in vitro embryos relies on similar genetic mechanisms operating during in vivo embryogenesis although the culture conditions are less stable and often not optimized. As a result, the molecular events controlling in vitro embryogenesis are less defined. Overall, somatic embryogenesis can be divided in two distinct phases: induction and development. The first phase requires the dedifferentiation of the somatic cells, the acquisition of totipotency, and the commitment to embark an embryogenic fate. These events, critical for the overall embryogenesis, do not occur in vivo and are therefore specific to culture systems. Independent studies have demonstrated the relevance of auxin for the inductive step and the participation of genes regulating SAM formation and maintenance. Removal of plant regulators is often required to initiate the development of the somatic embryos, and during this event, the embryo body is elaborated. Like the in vivo system, the tissue patterning of in vitro-produced embryos occurs through an apical-basal and a radial axis. Growth along the two axes is mediated by distinct genetic networks, although auxin seems to be implicated with both. As the developmental phases of in vitro embryogenesis are very similar to those observed in zygotic embryos, knowledge on the molecular mechanisms operating in the latter system are often transferred to the former.
References
Abe M, Katsumata H, Komeda Y, Takahashi T (2003) Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 130:635–643
Aichinger E, Villar CBR, Farrona S, Reyes JC, Hennig L, Kohle C (2009) CHD3 proteins and polycomb group proteins antagonistically determine cell identity in Arabidopsis. PLoS Genet 5, e1000605
Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B (2004) The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119:109–120
Aida M, Ishida T, Tasaka M (1999) Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126:1563–1570
Barinova I, Clement C, Martiny L, Baillieul F, Soukupova H, Heberle-Bors E, Touraev A (2004) Regulation of developmental pathways in cultured microspores of tobacco and snapdragon by medium pH. Planta 219:141–146
Baud S, Guyon V, Kronenberger J, Wuillème S, Miquel M, Caboche M, Lepiniec L, Rochat C (2003) Multifunctional acetyl‐CoA carboxylase 1 is essential for very long chain fatty acid elongation and embryo development in Arabidopsis. Plant J 33:75–86
Bhojwani S, Razdan M (1996) Plant tissue culture: theory and practice. Elsevier, Amsterdam, pp 125–166
Birnbaum KD, Alvarado AS (2008) Slicing across kingdoms: regeneration in plants and animals. Cell 132:697–710
Boutilier K, Offringa R, Sharma V, Kieft H, Ouellet T, Zhang L, Hattori J, Lui C, van Lammeren A, Miki B (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737
Bratzel F, Lopez-Torrejon G, Koch M, Del Pozo JC, Calonje M (2010) Keeping cell identity in Arabidopsis requires PRC1 RING-finger homologs that catalyze H2A monoubiquitination. Curr Biol 20:1853–1859
Carles CC, Fletcher JC (2003) Shoot apical meristem maintenance: the art of a dynamic balance. Trends Plant Sci 8:394–401
Chanvivattana Y, Bishopp A, Schubert A, Stock C, Moon YH, Sung ZR, Goodrich J (2004) Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development 131:5263–5276
Chen SK, Kurdyukov S, Kereszt A, Wang XD, Gresshoff PM, Rose RJ (2009) The association of homeobox gene expression with stem cell formation and morphogenesis in cultured Medicago truncatula. Planta 230:827–840
Clark S, Williams R, Meyerowitz E (1997) The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89:575–585
Cortes MM, Paredes FR, Burgeff C, Nunez TP, Cordova I, Oropeza C, Verdeil JL, Saenz L (2010) Characterisation of a cyclin-dependent kinase (CDKA) gene expressed during somatic embryogenesis of coconut palm. Plant Cell Tiss Org Cult 102:251–258
Costa S, Shaw P (2007) ‘Open minded’ cells: how cells can change fate. Trends Cell Biol 17:101–106
De Jong AJ, Heidstra R, Spaink HP, Hartog MV, Meijer EA, Hendriks T, Lo Shiavo F, Terzi M, Bisseling T, van Kammen A, de Vries SC (1993) Rhizobium lipo-oligosacharides rescue a Daucus carota somatic embryo variant. Plant Cell 5:615–620
De Smet I, Lau S, Mayer U, Jürgens G (2010) Embryogenesis – the humble beginnings of plant life. Plant J 61:959–970
Di Laurenzio L, Wysocka-Diller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey FN (1996) The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 8:423–433
Dodsworth S (2009) A diverse and intricate signalling network regulates stem cell fate in the shoot apical meristem. Dev Biol 336:1–9
Elhiti M, Hebelstrup KH, Wang A, Li C, Cui Y, Hill RD, Stasolla C (2013a) Function of the type-2 Arabidopsis hemoglobin in the auxin-mediated formation of embryogenic cells during morphogenesis.The. Plant J 74:946–958
Elhiti M, Stasolla C (2011) Ectopic expression of the Brassica SHOOTMERISTEMLESS attenuates the deleterious effects of the auxin transport inhibitor TIBA on somatic embryo number and morphology. Plant Sci 180:383–390
Elhiti M, Stasolla C, Wang A (2013b) Molecular regulation of plant somatic embryogenesis. In Vitro Cell Dev Biol Plant 49:631–642
Elhiti M, Stasolla S (2013) Genetic of embryogenesis. Brenner’s encyclopedia of genetics (2nd edition) 5:343–345
Elhiti M, Tahir M, Gulden RH, Khamiss K, Stasolla C (2010) Modulation of embryo-forming capacity in culture through the expression of Brassica genes involved in the regulation of the shoot apical meristem. J Exp Bot 61:4069–4085
Elhiti MA (2010) Molecular characterization of several brassica shoot apical meristem genes and the effect of their altered expression during in vitro morphogenesis. Ph.D. thesis, Faculty of Graduate Studies, University of Manitoba
Emons AMC (1994) Somatic embryogenesis: cell biological aspects. Acta Bot Neerl 43:1–14
Feher A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Org Cult 74:201–228
Feng Z, Sun X, Wang G, Liu H, Zhu J (2012) LBD29 regulates the cell cycle progression in response to auxin during lateral root formation in Arabidopsis thaliana. Ann Bot 160:2–10
Fry SC (1995) Polysaccharide-modifying enzymes in the plant-cell wall. Annu Rev Plant Physiol Plant Mol Biol 46:497–520
Harada JJ (2001) Role of Arabidopsis LEAFY COTYLEDON genes in seed development. J Plant Physiol 158:405–409
Hecht V, Vielle-Calzada JP, Hartog MV, Schmidt EDL, Boutilier K, Grossniklaus U, De Vries SC (2001) The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. J Plant Physiol 127:803–816
Hemerly AS, Ferreira PC, Van Montagu M, Engler G, Inze D (2000) Cell division events are essential for embryo patterning and morphogenesis: studies on dominant negative cdc2aAt mutants of Arabidopsis. Plant J 23:123–130
Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD (1997) Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106:348–360
Ishiguro S, Watanabe Y, Ito N, Nonaka H, Takeda N, Sakai T, Kanaya H, Okada K (2002) SHEPHERD is the Arabidopsis GRP94 responsible for the formation of functional CLAVATA proteins. EMBO J 21:898–908
Joosen R, Cordewener J, Supena EDJ, Vorst O, Lammers M, Maliepaard C, Zeilmaker T, Miki B, America T, Custers J, Boutilier K (2007) Combined transcriptome and proteome analysis identifies pathways and markers associated with the establishment of rapeseed microspore-derived embryo development. Plant Physiol 144:155–172
Kapros T, Bogre L, Nemeth K, LaszBako L, Gyorgyey J, Wu SC, Dudits D (1992) Differential expression of histone H3 gene variants during cell cycle and somatic embryogenesis in alfalfa. Plant Physiol 98:621–625
Kasha K, Yao Q, Simon E, Oro R, Hu T (1995) Production and application of double haploids in crops. University of Vienna Pusblisher, IAEA
Keller W, Armstrong K (1979) Stimulation of embryogenesis and haploid production in Brassica campestris anther culture by elevated temperature treatments. Theor Appl Genet 55:65–67
Knauer S, Holt AL, Rubi-Somoza I, Tucker EJ, Hinze A, Pisch M, Javelle M, Timmermans MC, Tucker M, Laux T (2013) A protodermal miR394 signal defines a region of stem cell competence in the Arabidopsis shoot meristem. Dev Cell 24:125–132
Leibfried A, To J, Busch W, Stehling S, Kehle A, Demar M, Kieber J, Lohmann J (2005) WUSCHEL controls meristem function by direct regulation of cytokinin inducible response regulators. Nature 438:1172–1175
Lindsey K, Pullen ML, Topping JF (2003) Importance of plant sterols in pattern formation and hormone signalling. Trends Plant Sci 8:521–525
Liu HI, Wang GC, Feng Z, Zhu J (2010) Screening of genes associated with dedifferentiation and effect of LBD29 on pericycle cells in Arabidopsis thaliana. Plant Growth Regul 62:127–136
Malik M, Wang F, Dirpaul J, Zhou N, Polowick P, Ferrie A, Krochko J (2007) Transcript profiling and identification of molecular markers for early microspore embryogenesis in Brassica napus. Plant Physiol 144:134–154
Mallory A, Vaucheret H (2010) Form, function, and regulation of ARGONAUTE proteins. Plant Cell 22:3879–3889
Mordhorst AP, Voerman KJ, Hartog MV, Meijer EA, van Went J, Koornneef M, de Vries SC (1998) Somatic embryogenesis in Arabidopsis thaliana is facilitated by mutations in genes repressing meristematic cell divisions. Genetics 149:549–563
Nogler G (1984) Gametophytic apomixis. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin, pp 475–518
Nowack MK, Grini PE, Jakoby MJ, Lafos M, Koncz C, Schnittger A (2006) A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat Genet 38:63–67
Palovaara J, Hakman I (2009) WOX2 and polar auxin transport during spruce embryo pattern formation. Plant Signal Behav 4:153–155
Park S, Harada JJ (2008) Arabidopsis embryogenesis. In: Plant embryogenesis, Humana Press, Totowa, pp 3–16
Pechan P, Keller W (1989) Induction of microspore embryogenesis in Brassica napus L. by gamma irradiation and ethanol stress. In vitro Cell Dev Biol Plant 25:1073–1074
Raghavan V (2000) Developmental biology of flowering plants. Springer, New York
Schruff MC, Spielman M, Tiwari S, Adams S, Fenby N, Scott RJ (2006) The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs. Development 133:251–261
Sieberer T, Hauser MT, Seifert GJ, Luschnig C (2003) PROPORZ1, a putative Arabidopsis transcriptional adaptor protein, mediates auxin and cytokinin signals in the control of cell proliferation. Curr Biol 13:837–842
Simmonds D, Keller W (1999) Significance of preprophase bands of microtubules in the induction of microspore embryogenesis of Brassica napus. Planta 208:383–391
Smith DL, Kirkorian AD (1990) Somatic proembryo production from excised, wounded zygotic carrot embryos on hormone-free medium: evaluation of the effects of pH, ethylene and activated charcoal. Plant Cell Rep 9:468–470
Song JY, Leung T, Ehler LK, Wang C, Liu Z (2000) Regulation of meristem organization and cell division by TSO1, an Arabidopsis gene with cysteine-rich repeats. Development 127:2207–2217
Song S, Lee M, Clark S (2006) POL and PLL1 phosphatases are CLAVATA1 signaling intermediates required for Arabidopsis shoot and floral stem cells. Development 133:4691–4698
Su YH, Zhang XS (2009) Auxin gradients trigger de novo formation of stem cells during somatic embryogenesis. Plant Signal Behav 4:574–576
Sugiyama M (1999) Organogenesis in vitro. Curr Opin Plant Biol 2:61–64
Szemenyei H, Hannon M, Long JA (2008) TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319:1384–1386
Telmer C, Simmond D, Newcomb W (1992) Determination of developmental stage to obtain high frequencies of embryogenic microspores in Brassica napus. Physiol Plant 84:417–424
Touraev A, Pfosser M, Vicente O, Heberle-Bors E (1996) Stress as the major signal controlling the developmental fate of tobacco microspores: towards a unified model of induction of microspore/pollen embryogenesis. Planta 200:144–152
Tsuwamoto R, Fukuoka H, Takahata Y (2007) Identification and characterization of genes expressed in early embryogenesis from microspores of Brassica napus. Planta 225:641–652
Tucker M, Hinze A, Tucker E, Takada S, Jurgens G, Laux T (2008) Vascular signaling mediated by ZWILLE potentiates WUSCHEL function during shoot meristem stem cell development in the Arabidopsis embryo. Development 135:28–39
Verdeil JL, Alemanno L, NiemenakN TTJ (2007) Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends Plant Sci 12:245–252
Willemsen V, Scheres B (2004) Mechanisms of pattern formation in plant embryogenesis. Annu Rev Genet 38:587–614
Willemsen V, Wolkenfelt H, de Vrieze G, Weisbeek P, Scheres B (1998) The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development 125:521–531
Yeung E (2002) The canola microspore-derived embryo as a model system to study developmental process in plants. J Plant Biol 45:119–133
Yeung E, Rahman M, Thorpe T (1996) Comparative development of zygotic and microspore-derived embryos in Brassica napus L. cv Topas. I. Histodifferentiation. Int J Plant Sci 157:27–39
Yeung EC, Meinke DW (1993) Embryogenesis in angiosperms: development of the suspensor. Plant Cell 5:1371–1381
Zhang B, Pan X, Cannon CH, Cobb GP, Anderson TA (2006) Conservation and divergence of plant microRNA genes. Plant J 46:243–259
Zhang G, Song C, Zhao MM, Li B, Guo SX (2012) Characterization of an A-type cyclin-dependent kinase gene from Dendrobium candidum. Biologia 67:360–368
Zhang Z, Laux T (2011) The asymmetric division of the Arabidopsis zygote: from cell polarity to an embryo axis. Sex Plant Reprod 24:161–169
Zhao J, Simmonds D, Newcomb W (1996) Induction of embryogenesis with colchicines instead of heat in microspores of Brassica napus L.c. Topaz. Planta 198:433–439
Zheng Y, Ren N, Wang H, Stromberg AJ, Perrya SE (2009) Global identification of targets of the Arabidopsis MADS domain protein AGAMOUS-Like15. Plant Cell 21:2563–2577
Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30:349–359
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer India
About this chapter
Cite this chapter
Elhiti, M., Stasolla, C. (2016). Somatic Embryogenesis: The Molecular Network Regulating Embryo Formation. In: Mujib, A. (eds) Somatic Embryogenesis in Ornamentals and Its Applications. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2683-3_14
Download citation
DOI: https://doi.org/10.1007/978-81-322-2683-3_14
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2681-9
Online ISBN: 978-81-322-2683-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)