Abstract
During angiosperm male gametophyte development, the male germline is segregated by an asymmetric cell division of the haploid microspore. This review encompasses recent advances in understanding the genetic and molecular mechanisms involved in generating the male germline from this pluripotent germline initial and in specifying the production of the twin sperm cells required for double fertilization. Genetic studies and access to the transcriptome of isolated gametes have enabled remarkable progress in understanding some of the key regulators that control and integrate germ cell cycle progression with germline specification, and an emerging regulatory model is presented. Rapid advances have also been made in understanding epigenetic regulation and small RNA pathways in the male gametophyte and germline that impact on genome integrity and gamete development, traits that are shared with animal germlines. The review concludes with a perspective of the outstanding issues and directions of future research that will further our understanding of germline specification and the gametophytic control of pollen development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Germlines in plants: a challenging concept
In animals, cells destined to become germline cells are determined early in embryogenesis and remain as a distinct population of stem cells throughout life (reviewed in Hayashi and Surani 2009; Strome and Lehmann 2007). In contrast, flowering plants do not harbour a distinct germline in the sporophyte, but maintain populations of undifferentiated stem cells that continuously produce vegetative tissues and organs, before switching to the production of reproductive organs containing diploid sporogenous cells. These ‘mother’ cells serve as gametophyte initials that following meiosis give rise to the haploid microspores and megaspores. These spores develop, respectively, into the male (pollen) and female (embryo sac) gametophytes that produce the gametes and consist of only a few cells.
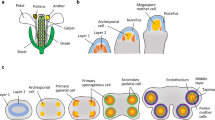
Cells that are programmed to produce the gametes constitute the germline; therefore, in plants, the germline must reside in the gametophyte. In multicellular volvocine algae, discussed in this issue (Hallmann 2011), and in early land plants, the germline is produced after multiple mitotic divisions in the gametophyte that include one or more asymmetric divisions. However, in flowering plants, the male gametophyte is so highly reduced that it is confined to just two divisions, during which the germline is segregated to produce the gametes. The first unequal division separates a vegetative cell from a unique male germ (generative) cell that divides once more to produce two sperm cells (Fig. 1a). The extreme reduction in the angiosperm male gametophyte provides a valuable model to dissect the mechanisms controlling germline specification and in the past 5 years remarkable progress has been achieved.
Schematic of male gametophyte development and mutants affecting cell division and patterning in Arabidopsis. a Illustration of the distinct cytological stages that accompany male gametophyte development. Individual haploid microspores released from tetrads formed after meiosis first grow by cell expansion, involving large vacuole formation and migration of the nucleus to the microspore wall. An asymmetric spindle is assembled, which leads to a highly asymmetric division producing a small germ cell that is subsequently engulfed by the vegetative cell. The germ cell elongates and divides to form twin sperm cells, whilst the vegetative cell differentiates with the capacity to develop a pollen tube following pollination. b Schematic illustrating the cell lineages and cell cycle progression in male gametophyte development in the context of gametophytic mutations that affect asymmetric division, cell cycle progression and patterning
Here, the key advances made in understanding male gametogenesis in flowering plants are reviewed with a focus on genetic studies of specification of the male germline and sperm cell differentiation. A model of the current regulatory networks governing male germline specification is presented, and emergent evidence for the role of epigenetic regulation and small RNA pathways in the male gametophyte and germline are discussed. The reader is referred to other recent reviews that collectively discuss male and female germline specification and gametophytic epigenetics (Berger and Twell 2011; Dickinson and Grant-Downton 2009; Grant-Downton 2010; Le Trionnaire and Twell 2010).
Specification of the male germline
According to the knowledge acquired from animal models, the definition of the germline requires two major steps (Strome and Lehmann 2007). The first comprises cytological events leading to a form of polarized division that sets apart the germ cell lineage. During this step, germ cell determinants are segregated and the germ cell transcriptome becomes distinct from the mother cell. Once segregation of the germline is achieved, a second step involves extensive reprogramming in the germline such that epigenetic modifications are passed on to the next generation. The germ cell genome must also be protected from invasion and reorganization by foreign elements (transposons and viruses). Although far from complete, knowledge of these different steps in male germline specification in angiosperms is discussed below.
The development of the male gametophyte in angiosperms takes place within the stamens, and the landmark events are outlined in Fig. 1a. Asymmetric division of the haploid microspore is a key event that produces the vegetative cell and the smaller generative (germ) cell that divides to produce the twin sperm cells required for double fertilization. The microspore is thus the pluripotent initial of the germline that establishes cells with two different identities. The larger vegetative cell exits the cell cycle in G1 phase and differentiates a terminal fate, its nucleus degenerating progressively during fertilization. In parallel, germline development involves the tight control of cell cycle progression to ensure twin sperm cell production and the cell cycle synchrony of male and female gametes at karyogamy (Friedman 1999). It also requires novel features such as adaptation to the unique ‘cell within a cell’ environment and dependence on the vegetative cell for survival and transport.
The role of asymmetric division
Division of the microspore is an example of an asymmetric cell division that relies on intrinsic factors (Horvitz and Herskowitz 1992) and results in the establishment of the male germline. This asymmetric division may be described as determinative in that daughter cells are immediately different (in size, transcriptional activity and chromatin structure) and that differential cell fate depends on division asymmetry.
Evidence that division asymmetry is determinative comes from experiments in which cultured microspores have been induced to divide symmetrically using microtubule inhibitors (Tanaka and Ito 1980, 1981; Zaki and Dickinson 1991), or by physical means such as centrifugation (Terasaka and Niitsu 1987). This results in the formation of two similar ‘vegetative’ cells that do not possess the condensed nuclear chromatin of the germ cell and adopt vegetative cell fate (Eady et al. 1995). The failure to clearly establish germ cell fate in gemini pollen 1 (gem1) (Park et al. 1998), gem2 (Park et al. 2004) and two-in-one (tio) mutants (Oh et al. 2005), which result from failure of cytokinesis, further indicates that the confinement of cell fate determinants by a new cell wall may be critical to ‘seal in’ germ cell fate. More recent genetic evidence has led to the identification of several essential regulators of male germ cell cycle progression and differentiation that are listed by their mutations (Fig. 1b) and discussed below (Brownfield et al. 2009a, b; Chen et al. 2009; Gusti et al. 2009; Kim et al. 2008).
Establishing the germline: microtubule-dependent polarity
Asymmetric division is preceded by the migration of the microspore nucleus to the future germ cell pole, and although the ontogeny of migration shows variation in different taxa, the end product is constant, a uninucleate polarized microspore (Twell et al. 1998). For example, in Arabidopsis, the centrally positioned microspore nucleus migrates to the pollen radial wall, whilst in tobacco, the nucleus further translocates along the radial wall to the future germ cell pole before spindle assembly (Fig. 2a, b). There is compelling evidence that microtubules contribute to polar migration of the microspore nucleus in different species (Eady et al. 1995; Terasaka and Niitsu 1987). Moreover, the absence of the pre-prophase band of microtubules in microspores highlights the role of other pre-mitotic microtubule structures in the determination of the division site and spindle orientation required to achieve asymmetric division (Brown and Lemmon 1991, 1992; Hause et al. 1991; Terasaka and Niitsu 1995; Zonia and Staiger 1999). Two functionally redundant γ-tubulin genes, TUBG1 and TUBG2, are required for spindle and phragmoplast organization in Arabidopsis microspores, further highlighting the importance of microtubule dynamics in establishing polar division of the microspore (Pastuglia et al. 2006). Recently developed microtubule reporters that allow live cell imaging have revealed unique gametophytic microtubule arrays in polarized tobacco microspores, including a cortical microtubule array associated with the nucleus at the germ cell pole (Oh et al. 2010). This basket-like nuclear cap of microtubules connects the nuclear envelope and cell cortex and could therefore have roles in generating cortical asymmetry as well as maintaining nuclear position prior to division (Fig. 2b).
Pre-mitotic microtubule arrays in polarized tobacco microspores labelled with a GFP fusion to Arabidopsis α-TUBULIN 6 (GFP::TUA6). a, b Reference microspores stained with DAPI to illustrate the position of the nuclei and plane of the following optical and confocal section images. a First nuclear position at the radial wall. b Second nuclear position at the germ cell pole prior to asymmetric division. a1, b1 Optical (left) and confocal (right) sections at planes indicated in panels a and b, respectively. GFP::TUA6 is excluded from the nucleus (n) and vacuole (v), which appear dark. a2, b2 Extended focus confocal image stacks showing GFP labelled cortical microtubules (a1) and a basket-like nuclear cap at the germ cell pole (b2)
In genetic studies, very few mutants have been identified that influence division asymmetry and/or formation of the germline. Most of the corresponding genes have functions associated with microtubule-dependent events. For example, Arabidopsis gem1 mutants disturb nuclear migration and/or cytokinesis (Park et al. 1998) and represent loss of function mutants in MOR1/GEM1, a plant orthologue of the conserved MAP215/Dis1 microtubule-associated protein family that promotes plus end growth (Twell et al. 2002). Microspore-targeted depletion of TMBP200, a tobacco homologue of MOR1/GEM1, induced similar division defects that were associated with altered spindle position and orientation (Oh et al. 2010). The observed uncoupling of nuclear migration and spindle axis determination indicates that these are successive events in microspore polarity, both dependent on microtubule growth mediated by TMBP200.
In contrast, several other mutants that fail to form the germline do not disturb polarity, but show specific defects in cytokinesis (Lee et al. 2007; Oh et al. 2005, 2008). The two-in-one (tio) microspores complete asymmetric nuclear division, but fail to complete cytokinesis resulting in binucleate pollen. TIO is the plant homologue of the Ser/Thr protein kinase FUSED and localizes to the phragmoplast midline where it has an essential role in cell plate expansion (Oh et al. 2005). Two functionally redundant microtubule motor kinesins, PAKRP1/Kinesin-12A and PAKRP1L/Kinesin-12B, also localize to the phragmoplast midline and double mutant microspores fail to form a well-organized antiparallel microtubule array between reforming nuclei (Lee et al. 2007). The signalling role of TIO could be linked with either Kinesin-12A/12-B or operate through the canonical NACK-PQR MAP kinase pathway at the cell plate, since HINKEL (AtNACK1) and TETRASPORE (AtNACK2) double mutant microspores show cell plate expansion and cytokinesis defects strikingly similar to those in tio microspores (Oh et al. 2005, 2008).
It is evident that microtubule-associated proteins and their regulators are important in establishing and maintaining the cell polarity required for asymmetric division. However, a major outstanding problem is how intrinsic or extracellular signals organize the polar microtubule arrays associated with nuclear positioning and control of the division plane. Since microspores are usually randomly orientated with regard to the anther locule, it is unlikely that polarity is determined by a directional external signal, (Park et al. 1998; Twell et al. 1998). Therefore, it is has been proposed that intrinsic factors localized to the future germ cell pole may act as polarity determinants (Twell et al. 1998). The activation of such a localized polarity determinant by a non-directional external signal in the anther locule could then initiate a signal transduction cascade leading to polarization of microtubule assembly and nuclear migration.
In comparison, the mechanisms that control migration and positioning of each nucleus in the female gametophyte remain largely unknown. Specific nuclear positioning acquired at the end of female gametogenesis indicates that most likely a gradient of factors such as RNAs and proteins is established in the embryo sac. What constitutes the gradient is unknown, but one report suggests that auxin plays a role in cell specification in the embryo and might participate in providing positional information (Pagnussat et al. 2009).
Differentiation of the male germline: molecular events
A series of studies have revealed distinctive transcriptome profiles in microspores, mature pollen and germline cells. The most complete datasets have been generated in the genetic model Arabidopsis, where transcriptome analyses have revealed developmental expression profiles from microspores to mature pollen (Honys and Twell 2004; Pina et al. 2005), the sperm cell transcriptome (Borges et al. 2008) and transcript changes associated with pollen germination and tube growth (Qin et al. 2009; Wang et al. 2008). Together with the recent discovery of several male gametophytic regulators of cell cycle progression and differentiation (see below), these datasets provide a strong molecular framework in which to decipher the regulatory networks governing male germline specification and gamete delivery.
The development of techniques for the isolation of generative and sperm cells has enabled transcriptome studies of the male germline. In several studies, the cDNA and EST sequences have been reported from generative cells of Lilium longiflorum (Okada et al. 2006a) and sperm cells from Nicotiana tabacum (Weterings et al. 1992), Zea mays (Engel et al. 2003) and Plumbago zeylanica (Gou et al. 2009). Commonly represented classes of genes are associated with general metabolism, cellular organization, DNA synthesis, chromatin structure and protein degradation. The most comprehensive datasets for Arabidopsis sperm cells also reveal enrichment for genes associated with cell cycle progression and DNA repair, reflecting the propagation and maintenance of germline genome integrity. Small non-coding RNA production and DNA methylation pathways are upregulated in sperm cells compared with vegetative cells (Borges et al. 2008). Proteins of the ubiquitin–proteasome system (UPS) are also highly represented in plant sperm cells, which parallel the essential role of the UPS in spermatogenesis and fertilization in metazoans (Baarends et al. 1999; Sakai et al. 2004). Genetic studies now provide unequivocal evidence for the role of the UPS in male germline cell cycle progression in Arabidopsis that includes F-box protein 17 discussed below (Gusti et al. 2009; Kim et al. 2008) and a pair of Ub-specific proteases (UBP3/UBP4) (Doelling et al. 2007).
Studies of germline-expressed genes in Arabidopsis have focused on the characterization of genes homologous to those expressed in the germline of other species. A homologue of the lily gene GCS1 (also called HAP2) and three genes homologous to maize sperm cell expressed genes (GEX1, GEX2 and GEX3) are examples of Arabidopsis germline-expressed genes identified using this comparative approach (Alandete-Saez et al. 2008; Engel et al. 2005; Mori et al. 2006; von Besser et al. 2006). Although no direct homologues of two lily generative cell-specific H3 histones (gcH2A, gcH3) were identified in Arabidopsis, analysis of the histone family did reveal the HISTONE3 RELATED 10 variant (HTR10), also known as MALE GAMETE HISTONE 3 (MGH3), as a male gamete-specific histone (Ingouff et al. 2007; Okada et al. 2005, 2006a, b). The identification of several germline-specific promoters has provided valuable tools enabling the isolation of Arabidopsis sperm cells for transcriptomic studies (Borges et al. 2008) and the analysis of cell fate in various mutants affecting the germline (Brownfield et al. 2009a, b; Chen et al. 2008, 2009). Genetic analysis of mutants affecting germline division, discussed below, has also led to the identification of the male germline-specific R2R3 MYB transcription factor DUO1. The male germline-specific expression of DUO1 is illustrated in Fig. 3.
Evidence for co-expression of DUO1 and DUO3 in the male germline. The DUO3 promoter is active in both VC and GC and that the promoter is active in the germ cell during the period that the DUO1 promoter and DUO1 protein accumulate in the germ cell—consistent with their role in G2/M transition. Top row shows the germline-specific expression of a DUO1::mRFP1 fusion construct in Arabidopsis pollen. Bottom row shows the expression of the DUO3 promoter linked to H2B::GFP, which is first expressed in microspores and accumulates in nuclei of vegetative cells and in germ cells prior to division. A PromDUO3-DUO3::GFP construct is expressed at much lower levels and thus is only clearly detectable in the VC, although expression of DUO3 specifically in the germ cell is sufficient to complement rescue the division (Brownfield et al. 2009a, b).Thus, germline expression of DUO3 is normally required for germ cell division and specification. Stages are abbreviated as LMS (late microspore), EBC (early bicellular), LBC (late bicellular) and TC (tricellular)
Coordinating cell cycle progression with germline identity
The strict control of cell cycle progression in the vegetative and germ cells is an essential feature of germline specification and gametophyte patterning. Transcriptome analysis has shown that all major components of the core cell cycle machinery are expressed in microspores and their expression generally declines during pollen development (Honys and Twell 2004; Pina et al. 2005). This general decline is associated with the exit of the vegetative cell from the cell cycle and the limited germ cell contribution to the whole pollen transcriptome. For example, sperm cells isolated from Arabidopsis pollen at anthesis, which are in S-phase (Friedman 1999; Durbarry et al. 2005), show enhanced expression of S-phase transcripts (Borges et al. 2008).
Control of germ cell proliferation: genetic evidence
A number of mutants have been described in Arabidopsis that produce bicellular pollen harbouring a single germ cell due to failure of germ cell division. Collectively, the analysis of these mutants and others that cause cell cycle overproliferation has uncovered two parallel pathways that regulate (1) the maintenance of germ cell proliferation and mitotic arrest of the vegetative cell and (2) how germ cell differentiation is integrated with cell cycle progression.
Mutations in the A-type cyclin-dependent kinase (CDKA;1) result in retarded S-phase, and germ cell entry in mitosis is delayed until the pollen grain germinates (Iwakawa et al. 2006; Nowack et al. 2006), leading to germ cell division during pollen tube growth in the majority of pollen (Aw et al. 2010). Disruption of the F-box-Like 17 (FBL17) gene, which is only transiently expressed in the male germ cell was shown to phenocopy the cdka;1 germ cell defect (Gusti et al. 2009; Kim et al. 2008). FBL17 normally targets the CDK inhibitors KRP6 and KRP7 for proteasome-dependent degradation, enabling the germ cell to progress through S-phase (Kim et al. 2008). Conversely, the persistence of KRP6/7 in the vegetative cell is proposed to maintain inhibition of CDKA activity and vegetative cell cycle progression. Germline-specific expression of FBL17 thus enables differential control of the cell cycle in the germ and vegetative cells, effectively licensing germ cells for progression through S-phase (Fig. 4). Similarly, the proteasome-mediated degradation of KRP6 by two RING-finger E3 ligases, RHF1a and RHF2a is required for regular progression through both microspore and the subsequent germ cell mitotic cycle. RHF1a and RHF2a are proposed to reduce the level of KRP6 accumulated during meiosis, thereby preventing inhibition of CDK activity in microspores and germ cells (Liu et al. 2008).
A model of regulatory events that distinguish male germ cell and pollen vegetative cell fate. Following asymmetric division of the microspore, the CDK inhibitors KRP6 and KRP7 are present in both the germ cell and vegetative cell. Transient expression of FBL17 in the germ cell leads to their degradation allowing CDKA/CYCD to phosphorylate RBR. RBR-mediated repression of the E2F/DP pathway is relieved and allows progression of the germ cell through S-phase. FBL17 is not expressed in the vegetative cell and so CDKA inhibition by high levels of KRP6 and KRP7 may result in continued repression of the E2F/DP pathway. Together, these two pathways enforce vegetative cell fate by preventing cell cycle entry. Gamete specification is coincident with germ cell formation, where the expression of DUO1 and DUO3 leads to the activation of an overlapping set of germline differentiation genes. Following S-phase the DUO1-dependent activation of CYCLINB1;1 promotes entry into mitosis. DUO3 is also required for G2/M transition in the germ cell, but involves a CYCLINB1;1 independent pathway. DUO1 and DUO3 therefore integrate germline differentiation with cell division, and the cooperation of their regulatory networks is essential for the production of a pair of fully differentiated sperm cells
A phenotype opposite to that observed in cdka;1 mutant is caused by loss of function of the retinoblastoma-related (RBR) protein. Loss of RBR results in hyperproliferation of the vegetative cell and to a lesser extent the germline, leading to pollen with four sperm cells (Chen et al. 2009). Hyperproliferation in the absence of RBR is dependent on CDKA;1 activity since introduction of a cdka;1 mutant allele prevents cell proliferation in hypomorphic rbr pollen. This places RBR repression of the E2F-DP pathway downstream of KRP-dependent CDKA;1 inhibition, so that both mechanisms may cooperate to enforce cell cycle exit that is associated with commitment to vegetative cell fate.
Intriguingly, cell fate is also incorrectly specified in a proportion of rbr vegetative cells. These cells appear to retain the progenitor-like character of the microspore and attempt to imperfectly reiterate asymmetric division to produce an additional cell with a range of fate identities (Chen et al. 2009). Johnston et al. (2008), also observed patterning defects in rbr/RBR pollen and elevated expression of non-imprinted polycomb repressive complex (PRC2) components, SWN, FIE, CLF and MSI1 and the DNA methyl transferase (MET1), indicating that RBR is associated with the repression of PRC2 activity in the male gametophyte. The significance and cell specificity of PRC2 derepression in rbr mutant pollen remain unknown; however, the suppression of cell fate defects in rbr;cdka;1 double mutant pollen points to RBR primarily controlling cell cycle progression, whilst having a secondary impact on cell fate commitment (Chen et al. 2009).
Chromatin Assembly Factor-1 (CAF-1) pathway mutants (fas1, fas2, msi1) also cause cell cycle arrest, resulting in the production of a proportion of pollen with single germ cell able to fertilize either the egg or the central cell (Chen et al. 2008). This indicates a role for nucleosome and chromatin reassembly in germ cell cycle progression that is not essential for germline differentiation (Chen et al. 2008).
Regulators that integrate germline fate and cell cycle progression
Single germ cell phenotypes are characteristic of several duo pollen (duo) mutants that have been characterized. DUO1 encodes a novel conserved R2R3 MYB protein specifically expressed in the male germline (Rotman et al. 2005) (Fig. 3). Failure of mitotic entry in duo1 germ cells results, in part, from the lack of germline expression of the G2/M regulator CYCLIN B1;1 (Brownfield et al. 2009a). The duo1 germ cells are also incompletely differentiated and do not result in fertilization (Rotman et al. 2005), unlike cdka;1 mutant germ cells, which fertilize the egg cell or the central cell (Aw et al. 2010). The expression of several male germline-specific genes is suppressed in duo1 germ cells, including that of AtGCS1 (HAP2), which encodes an essential sperm cell surface protein required for efficient pollen tube guidance and gamete fusion (Mori et al. 2006; von Besser et al. 2006). Moreover, whilst germline differentiation genes are activated in response to ectopic DUO1 expression, this is not sufficient to induce CYCB1;1 transcripts (Brownfield et al. 2009a). DUO1-dependent expression of CYCB1;1 in the germline may operate through a novel germline-specific pathway or in association with potential regulators of CYCB1;1 that have been characterized in the sporophyte including MYB3R and TCP20 proteins (Li et al. 2005). Interestingly, a root-enhanced R2R3 MYB factor (AtMYB59) has been shown to be a direct regulator of the CYCB1;1 promoter that appears to regulate root growth by extending the metaphase of mitotic cells (Mu et al. 2009).
The recent identification of a novel nuclear protein DUO3, which is also required for male germ cell division and gamete specification in Arabidopsis, adds a regulatory node to the emergent male germline network (Brownfield et al. 2009b). DUO3 contains domains related to Gonadless-4 (GON-4), a cell lineage regulator of gonadogenesis in Caenorhabditis elegans (Friedman et al. 2000). Plant DUO3 proteins have conserved functions as DUO3 from moss can restore germ cell division and specification in Arabidopsis duo3 mutants. DUO3 may also have molecular functions related to those of GON4-like (GON4-L) proteins as there are phenotypic similarities between duo3 and gon4-l mutants, such as G2/M arrest and cell specification defects (Friedman et al. 2000; Liu et al. 2007).
DUO3 is required for G2/M transition and for the expression of a subset of DUO1 target genes. However, unlike DUO1, the requirement for DUO3 in the germline during G2/M transition acts independently of CYCB1;1 (Brownfield et al. 2009b). Although DUO3 is widely expressed, DUO1 and DUO3 are co-expressed in the germline and accumulate before division, consistent with their G2/M roles (Fig. 3). Although DUO1 is a direct regulator of a multiple germline-specific target genes (M. Borg et al., unpublished), the mechanism by which DUO3 influences the expression of a subset of DUO1 targets remains unknown. However, it has been proposed that DUO1 and DUO3 may cooperate in a transcriptional complex at common target promoters or that DUO3-dependent chromatin remodelling could promote DUO1 binding to MYB target sites (Brownfield et al. 2009b).
The discovery of DUO1 and DUO3 establishes a regulatory model in which these regulators integrate cell cycle progression and cell differentiation during male germline development that is illustrated in Fig. 4. Ongoing work is poised to uncover the diversity of target genes, their functions and the networks in which they operate (M. Borg et al., unpublished). However, an unresolved issue is how the expression of key germ cell determinants, including DUO1 is linked with asymmetric division of the microspore.
Here, the characterization of the lily Germline Restrictive Silencing Factor (GRSF) provides a potential derepression mechanism for the germline-specific expression of germ cell determinants (Haerizadeh et al. 2006). GRSF is a nuclear protein that is widely expressed but excluded from the male germline. GRSF binds to a silencer element in the lily germline-specific LGC1 promoter, and these silencer sequences are sufficient to repress the CaMV35S promoter in sporophytic cells in tobacco and Arabidopsis. Whether orthologues of GRSF in Arabidopsis function to repress germline genes remains to be established, since putative GRSF-binding sites in the DUO1 promoter do not function as sporophytic silencer elements (Brownfield et al. 2009a). Although a derepression mechanism cannot be ruled out for other germline genes, 5′ deletion analysis of the DUO1 promoter identified only positive regulatory elements (Brownfield et al. 2009a). This implicates upstream transcription factors and chromatin modifications as potential determinants restricting DUO1 function to the germline. Consequently, these germline determinants may be differentially distributed or post-translationally modified as a result of asymmetric division. It is also possible that microRNAs or epigenetic modifications could contribute to germline specification.
Epigenetic controls in the gametophyte and male germline
The striking difference in cell fate between the vegetative cell and germ cells is not only reflected in their distinctive patterns of cell cycle control and cellular differentiation but by their characteristic nuclear chromatin ‘states’. The vegetative cell has highly dispersed chromatin, whereas the germline chromatin is maintained in a highly condensed condition. Whether such distinct chromatin states participate in fate determination or accompany the unique differentiation programs of the vegetative cell and germline remains to be established. However, several lines of evidence demonstrate an important role for epigenetic regulation and small RNA pathways in the male gametophyte including genome integrity and germ cell functions.
Histone expression and modification
The angiosperm male germline is characterized by the expression of specific histone H3 variants. Purification of the generative cell from Lilium pollen grains led to the identification of gametic H2A, H2B and H3 histones specific to the germline that have divergent sequences compared to those expressed in vegetative tissues (Xu et al. 1999; Ueda et al. 2000; Okada et al. 2006a, b; Ueda and Tanaka 1995). Changes in global patterns of DNA histone acetylation have been reported after microspore division in lily, with the vegetative cell undergoing cycles of histone H4 de- and re-acetylation (Janousek et al. 2000). Also in lily, histone H3 methylation differs substantially between the vegetative and generative cells of mature pollen (Okada et al. 2006a, b), and histone H1 levels decrease in the vegetative nucleus (Tanaka et al. 1998). An important role for a SET-domain histone methyltransferase SDG4 that is highly expressed in pollen has been demonstrated. Histone methylation at H3K4 and H3K36 is significantly reduced in vegetative nuclei of pollen that lacks functional SDG4 and sdg4 mutants have reduced pollen tube growth (Cartagena et al. 2008).
In Arabidopsis, the H3 variant HTR10 is specifically expressed in the germline and mature sperm (Ingouff et al. 2007; Okada et al. 2005) under the control of the DUO1 transcription factor (Brownfield et al. 2009a). In animals, H3 variants of the H3.3 type can be incorporated into chromatin in non-dividing cells (Corpet and Almouzni 2009). HTR10 carries a H3.3 signature and could be required to direct a germ cell-specific transcriptome or could be involved in sperm chromatin condensation. HTR10 is the major H3 expressed in the germline (Ingouff and Berger 2010), and it is completely removed from the zygote nucleus upon fertilization (Ingouff et al. 2007). A similar remodelling of the chromatin contributed by the sperm cell occurs in animals. The paternal chromatin is stripped of its H3 content and loaded with H3.3 variants provided by the female gamete (Ooi and Henikoff 2007). The impact of replacement of HTR10 by other H3.3 variants upon fertilization is not yet understood, but it may participate in global reprogramming events as suggested for dynamic replacement of H3.3 in the mammalian germline (Hajkova et al. 2008).
DNA methylation
The germline in animals is marked by epigenetic reprogramming, and DNA methylation is removed from the entire genome in primordial germ cells (Albert and Peters 2009; Hemberger et al. 2009; Sasaki and Matsui 2008). In Arabidopsis, the majority of DNA methylation occurs in CG context and depends on the maintenance DNA methyltransferase 1 (MET1) (Cokus et al. 2008; Zilberman 2008). Alterations to global DNA methylation have been reported in tobacco male gametophytes (Oakeley et al. 1997), as have cycles of DNA methylation and demethylation in vegetative cells of lily (Janousek et al. 2000). However, several lines of evidence indicate that major reprogramming of DNA methylation does not occur in the male gametes (Jullien and Berger 2010; Saze 2008). MET1 is expressed in male gametes (Jullien et al. 2008) and is required to maintain silencing at several loci (Jullien et al. 2006), whilst loss of MET1 in the male gametes causes a paternal effect on seed size (FitzGerald et al. 2008). Nevertheless, met1 mutant pollen grains do not show obvious defects in the vegetative cell or in germline function, indicating that CG methylation does not have an essential regulatory role in vegetative cell differentiation or germ cell division. DNA methylation does, however, play a major role in silencing transposable elements (Zilberman et al. 2007; Zilberman 2008). With established sperm cell isolation methods in Arabidopsis and rice, it is now within reach to determine the methylome of isolated gametes and vegetative cell nuclei that would provide unprecedented insight into the extent of epigenetic reprogramming that takes place in the vegetative cell and germline.
Small RNA pathways
Transcriptomic studies have detected the expression of various components of small RNA pathways in the male gametophyte. Transcriptomic profiling of genes involved in small RNA pathways (Honys and Twell 2004) indicated a general decline during pollen development (Pina et al. 2005), although many transcripts were shown to persist in mature pollen, results that were confirmed by RT-PCR analyses (Chambers and Shuai 2009; Grant-Downton et al. 2009a). Further analysis of isolated sperm cells showed strongly enhanced expression of small RNA pathway components, notably DCL1, RDR2, HEN1 and the dsRNA-binding protein DRB4 in sperm cells compared with pollen and seedlings (Borges et al. 2008).
Transcriptional silencing by RNA-directed DNA methylation
The animal germline is marked by the activity of small non-coding RNAs produced by the PIWI pathway (O’Donnell and Boeke 2007; Thomson and Lin 2009). Although this pathway may not be conserved in plants, two ARGONAUTE family members, AGO5 and AGO9, are highly enriched in sperm cells where they may participate in novel small RNA-mediated silencing pathways. Interestingly, the rice orthologue of AGO5, MEIOSIS ARRESTED AT LEPTOTENE1 (MEL1), is restricted to meiotic precursors and meiocytes, and mutations in MEL1 result in early meiotic arrest (Nonomura et al. 2007). In Arabidopsis, AGO9 preferentially interacts with 24-nucleotide sRNAs derived from transposable elements (TEs), and its activity is necessary to silence TEs in female gametes and their accessory cells (Olmedo-Monfil et al. 2010). AGO9 is expressed in sporophytic tissues surrounding the developing gametophyte and acts by repressing the ectopic differentiation of megaspore mother cells. Further investigation is required to establish whether AGO5 and AGO9 function in conventional or novel small RNA pathways, whether they have similar silencing roles in the male germline and whether they contribute to gamete specification.
A recent study has shown that the vegetative cell is characterized by increased activity of some classes of transposable elements. This is attributed to the absence of the nucleosome remodelling protein DECREASED DNAMETHYLATION1 (DDM1) and the hypomethylation of a class of retrotransposon sequences (Slotkin et al. 2009). It is suggested that siRNAs produced in the vegetative cell induce silencing of transposons in the sperm cells. This hypothesis requires unknown mechanisms that would allow siRNAs to cross the two membranes that separate the sperm nuclei from the cytoplasm of the vegetative cell and warrants further investigation.
In contrast with the hypomethylation of some TEs observed in pollen and their strong non-CG methylation in sperm cells (Slotkin et al. 2009), in a separate study, the loss of DDM1 from the vegetative cell has been linked to the hypermethylation of centromeric repeats and a class of associated retrotransposon sequences at non-CG sites (Schoft et al. 2009). These authors propose that down regulation of DDM1 leads to demethylation of H3K27me2 and disturbed centromeric heterochromatin, involving loss of the centromeric histone CenH3, allows siRNA directed DNA methylation (RdDM) at centromeric repeats. The apparent contradiction in methylation changes of transposon sequences in the DDM1-deficient vegetative cell in both studies may conceivably reflect the active targeting of DNA demethylases to non-centromeric TEs and the protection of centromeric TEs from the demethylation machinery through CenH3-dependent chromatin marks (Schoft et al. 2009). A more dynamic view of the distribution of siRNAs and DNA methylation during male gametophyte development will help to resolve the uncertainties regarding potential siRNA transport and the epigenetic control of different classes of transposon sequences.
Post-transcriptional regulation by small RNAs (miRNA, ta-siRNA and cis-nat-siRNAs)
Recent evidence has emerged for the expression and function of critical components of small RNA pathways in the developing male gametophyte (Ron et al. 2010; Schoft et al. 2009; Slotkin et al. 2009). Small RNA pathways, including miRNA and ta-siRNA pathways have been shown to be present and functional in mature pollen (Grant-Downton et al. 2009a, b, recently reviewed in Grant-Downton 2010; Le Trionnaire and Twell 2010).
A further system of siRNA biogenesis, the natural antisense (nat)-siRNA pathway has been recently shown to play an essential developmental role in the male gametophyte. Ron et al. (2010) demonstrate that the mis-regulation of a specific cis-nat-siRNA pair in Arabidopsis sperm cells resulted in single fertilization events of either the egg or the central cell resulting in seed abortion. They show that overlapping transcripts from the KOKOPELLI (KPL)/ARIADNE14 (ARI14) cis-NAT pair exhibit inverse expression patterns during pollen development. Analysis of kpl mutants confirmed the overexpression of ARI14 transcripts in pollen, which was correlated with impaired fertilization. They further demonstrated that a KPL/ARI14 nat-siRNA was generated only in sperm cells. This represents the first example of the functional role of a specific siRNA in pollen development and, intriguingly, it is likely to have had a recent evolutionary origin, with KPL restricted to the Brassicaceae (Ron et al. 2010).
What is clear from a rapidly accumulating body of evidence is that non-coding small RNAs participate in multiple aspects of the male gametophytic phase of the life cycle, including the control of genome integrity and gamete function. The functional or regulatory roles for several different classes of small RNAs, however, remain to be identified, but it can be anticipated that novel small RNAs with significant roles in male gametophyte and germline development will be uncovered.
Perspectives
Examination of the recent progress in our knowledge of the mechanisms governing male germline specification in plants highlights common features that are shared with those in animals such as the role of asymmetric division and the importance of production of small non-coding RNAs preventing mutagenic activity of transposable elements. The establishment of the male germline and the male gametes involves the integration of progressive steps towards a differentiated state that involves the control of cell polarity and cell cycle progression. It is evident that microspore polarity is microtubule dependent, but the intrinsic organizers of polarity that orchestrate nuclear migration and asymmetric division remain to be identified. A further outstanding challenge is to discover the linked cell fate determinants responsible for specifying the differing fates of the vegetative and germ cells. On the other hand, knowledge of key transcription factors and cell cycle regulators together with methods for sperm cell isolation (Borges et al. 2008) now paves the way for rapid progress in the understanding of the regulatory networks involved in germline specification and the extent of epigenetic reprogramming that is involved in sperm cell production. Advances in transcriptomic analysis of female germ cells (Wuest et al. 2010) discussed in this issue (Sprunck and Groß-Hardt 2011) will allow a unique comparative view of germ cell specification in plants and further aid understanding of the maternal and paternal germline reprogramming that takes place once gametes unite.
References
Alandete-Saez M, Ron M, McCormick S (2008) GEX3, expressed in the male gametophyte and in the egg cell of Arabidopsis thaliana, is essential for micropylar pollen tube guidance and plays a role during early embryogenesis. Mol Plant 1:586–598
Albert M, Peters AH (2009) Genetic and epigenetic control of early mouse development. Curr Opin Genet Dev 19:113–121
Aw SJ, Hamamura Y, Chen Z, Schnittger A, Berger F (2010) Sperm entry is sufficient to trigger division of the central cell but the paternal genome is required for endosperm development in Arabidopsis. Development 137:2683–2690
Baarends WM, Hoogerbrugge JW, Roest HP, Ooms M, Vreeburg J, Hoeijmakers JH, Grootegoed JA (1999) Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev Biol 207:322–333
Berger F, Twell D (2011) Germline specification and function in plants. Annu Rev Plant Biol (in press)
Borges F, Gomes G, Gardner R, Moreno N, McCormick S, Feijo JA, Becker JD (2008) Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiol 148:1168–1181
Brown RC, Lemmon BE (1991) Pollen development in orchids. 3. A novel generative pole microtubule system predicts unequal pollen mitosis. J Cell Sci 99:273–281
Brown RC, Lemmon BE (1992) Pollen development in orchids. 4. Cytoskeleton and ultrastructure of the unequal pollen mitosis in Phalaenopsis. Protoplasma 167:183–192
Brownfield L, Hafidh S, Borg M, Sidorova A, Mori T, Twell D (2009a) A plant germline-specific integrator of sperm specification and cell cycle progression. PLoS Genet 5:e1000430
Brownfield L, Hafidh S, Durbarry A, Khatab H, Sidorova A, Doerner P, Twell D (2009b) Arabidopsis DUO POLLEN3 is a key regulator of male germline development and embryogenesis. Plant Cell 21:1940–1956
Cartagena JA, Matsunaga S, Seki M, Kurihara D, Yokoyama M, Shinozaki K, Fujimoto S, Azumi Y, Uchiyama S, Fukui K (2008) The Arabidopsis SDG4 contributes to the regulation of pollen tube growth by methylation of histone H3 lysines 4 and 36 in mature pollen. Dev Biol 315:355–368
Chambers C, Shuai B (2009) Profiling microRNA expression in Arabidopsis pollen using microRNA array and real-time PCR. BMC Plant Biol 9:87
Chen Z, Tan JLH, Ingouff M, Sundaresan V, Berger F (2008) Chromatin Assembly Factor 1 regulates the cell cycle but not cell fate during male gametogenesis in Arabidopsis thaliana. Development 135:65–73
Chen Z, Hafidh S, Poh SH, Twell D, Berger F (2009) Proliferation and cell fate establishment during Arabidopsis male gametogenesis depends on the retinoblastoma protein. Proc Natl Acad Sci USA 106:7257–7262
Cokus SJ, Feng S, Zhang X, Chen Z, Merriman B, Haudenschild CD, Pradhan S, Nelson SF, Pellegrini M, Jacobsen SE (2008) Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 452:215–219
Corpet A, Almouzni G (2009) Making copies of chromatin: the challenge of nucleosomal organization and epigenetic information. Trends Cell Biol 19:29–41
Dickinson HG, Grant-Downton R (2009) Bridging the generation gap: flowering plant gametophytes and animal germlines reveal unexpected similarities. Biol Rev Camb Philos Soc 84:589–615
Doelling JH, Phillips AR, Soyler-Ogretim G, Wise J, Chandler J, Callis J, Otegui MS, Vierstra RD (2007) The ubiquitin-specific protease subfamily UBP3/UBP4 is essential for pollen development and transmission in Arabidopsis. Plant Physiol 145:801–813
Durbarry A, Vizir I, Twell D (2005) Male germ line development in Arabidopsis. duo pollen mutants reveal gametophytic regulators of generative cell cycle progression. Plant Physiol 137:297–307
Eady C, Lindsey K, Twell D (1995) The significance of microspore division and division symmetry for vegetative cell-specific transcription and generative cell differentiation. Plant Cell 7:65–74
Engel ML, Chaboud A, Dumas C, McCormick S (2003) Sperm cells of Zea mays have a complex complement of mRNAs. Plant J 34:697–707
Engel ML, Holmes-Davis R, McCormick S (2005) Green sperm. Identification of male gamete promoters in Arabidopsis. Plant Physiol 138:2124–2133
FitzGerald J, Luo M, Chaudhury A, Berger F (2008) DNA methylation causes predominant maternal controls of plant embryo growth. PLoS One 3:e2298
Friedman WE (1999) Expression of the cell cycle in sperm of Arabidopsis: implications for understanding patterns of gametogenesis and fertilization in plants and other eukaryotes. Development 126:1065–1075
Friedman L, Santa Anna-Arriola S, Hodgkin J, Kimble J (2000) gon-4, a cell lineage regulator required for gonadogenesis in Caenorhabditis elegans. Dev Biol 228:350–362
Gou X, Yuan T, Wei X, Russell SD (2009) Gene expression in the dimorphic sperm cells of Plumbago zeylanica: transcript profiling, diversity, and relationship to cell type. Plant J 60:33–47
Grant-Downton R (2010) Through a generation darkly: small RNAs in the gametophyte. Biochem Soc Trans 38:617–621
Grant-Downton R, Hafidh S, Twell D, Dickinson HG (2009a) Small RNA pathways are present and functional in the angiosperm male gametophyte. Mol Plant 2:500–512
Grant-Downton R, Le Trionnaire G, Schmid R, Rodriguez-Enriquez J, Hafidh S, Mehdi S, Twell D, Dickinson H (2009b) MicroRNA and tasiRNA diversity in mature pollen of Arabidopsis thaliana. BMC Genomics 10:643
Gusti A, Baumberger N, Nowack M, Pusch S, Eisler H, Potuschak T, De Veylder L, Schnittger A, Genschik P (2009) The Arabidopsis thaliana F-box protein FBL17 is essential for progression through the second mitosis during pollen development. PLoS One 4:e4780
Haerizadeh F, Singh MB, Bhalla PL (2006) Transcriptional repression distinguishes somatic from germ cell lineages in a plant. Science 313:496–499
Hajkova P, Ancelin K, Waldmann T, Lacoste N, Lange UC, Cesari F, Lee C, Almouzni G, Schneider R, Surani MA (2008) Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 452:877–881
Hallmann A (2011) Evolution of the germline in volvocine algae. Sex Plant Reprod (in press)
Hause G, Hause B, van Lammeren AAM (1991) Microtubular and actin-filament configurations during microspore and pollen development in Brassica napus L. cv. Topas. Can J Bot 70:1369–1376
Hayashi K, Surani MA (2009) Resetting the epigenome beyond pluripotency in the germline. Cell Stem Cell 4:493–498
Hemberger M, Dean W, Reik W (2009) Epigenetic dynamics of stem cells and cell lineage commitment: digging Waddington’s canal. Nat Rev Mol Cell Biol 10:526–537
Honys D, Twell D (2004) Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 5:R85
Horvitz HR, Herskowitz I (1992) Mechanisms of asymmetric cell division: two Bs or not two Bs, that is the question. Cell 68:237–255
Ingouff M, Berger F (2010) Histone3 variants in plants. Chromosoma 119:17–23
Ingouff M, Hamamura Y, Gourgues M, Higashiyama T, Berger F (2007) Distinct dynamics of HISTONE3 variants between the two fertilization products in plants. Curr Biol 17:1032–1037
Iwakawa H, Shinmyo A, Sekine M (2006) Arabidopsis CDKA;1, a cdc2 homologue, controls proliferation of generative cells in male gametogenesis. Plant J 45:819–831
Janousek B, Zluvova J, Vyscot B (2000) Histone H4 acetylation and DNA methylation dynamics during pollen development. Protoplasma 211:116–122
Johnston AJ, Matveeva E, Kirioukhova O, Grossniklaus U, Gruissem W (2008) A dynamic reciprocal RBR-PRC2 regulatory circuit controls Arabidopsis gametophyte development. Curr Biol 18:1680–1686
Jullien PE, Berger F (2010) DNA methylation reprogramming during plant sexual reproduction? Trends Genet (in press)
Jullien PE, Kinoshita T, Ohad N, Berger F (2006) Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell 18:1360–1372
Jullien PE, Mosquna A, Ingouff M, Sakata T, Ohad N, Berger F (2008) Retinoblastoma and its binding partner MSI1 control imprinting in Arabidopsis. PLoS Biol 6:e194
Kim HJ, Oh SA, Brownfield L, Hong SH, Ryu H, Hwang I, Twell D, Nam HG (2008) Control of plant germline proliferation by SCFFBL17 degradation of cell cycle inhibitors. Nature 455:1134–1137
Le Trionnaire G, Twell D (2010) Small RNAs in angiosperm gametophytes: from epigenetics to gamete development. Genes Dev 24:1081–1085
Lee YR, Li Y, Liu B (2007) Two Arabidopsis phragmoplast-associated kinesins play a critical role in cytokinesis during male gametogenesis. Plant Cell 19:2595–2605
Li C, Potuschak T, Colon-Carmona A, Gutierrez RA, Doerner P (2005) Arabidopsis TCP20 links regulation of growth and cell division control pathways. Proc Natl Acad Sci USA 102:12978–12983
Liu Y, Du L, Osato M, Teo EH, Qian F, Jin H, Zhen F, Xu J, Guo L, Huang H, Chen J, Geisler R, Jiang YJ, Peng J, Wen Z (2007) The zebrafish udu gene encodes a novel nuclear factor and is essential for primitive erythroid cell development. Blood 110:99–106
Liu J, Zhang Y, Qin G, Tsuge T, Sakaguchi N, Luo G, Sun K, Shi D, Aki S, Zheng N, Aoyama T, Oka A, Yang W, Umeda M, Xie Q, Gu H, Qu LJ (2008) Targeted degradation of the cyclin-dependent kinase inhibitor ICK4/KRP6 by RING-type E3 ligases is essential for mitotic cell cycle progression during Arabidopsis gametogenesis. Plant Cell 20:1538–1554
Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T (2006) GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol 8:64–71
Mu RL, Cao YR, Liu YF, Lei G, Zou HF, Liao Y, Wang HW, Zhang WK, Ma B, Du JZ, Yuan M, Zhang JS, Chen SY (2009) An R2R3-type transcription factor gene AtMYB59 regulates root growth and cell cycle progression in Arabidopsis. Cell Res 19:1291–1304
Nonomura K, Morohoshi A, Nakano M, Eiguchi M, Miyao A, Hirochika H, Kurata N (2007) A germ cell specific gene of the ARGONAUTE family is essential for the progression of premeiotic mitosis and meiosis during sporogenesis in rice. Plant Cell 19:2583–2594
Nowack MK, Grini PE, Jakoby MJ, Lafos M, Koncz C, Schnittger A (2006) A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat Genet 38:63–67
O’Donnell KA, Boeke JD (2007) Mighty Piwis defend the germline against genome intruders. Cell 129:37–44
Oakeley EJ, Podesta A, Jost J-P (1997) Developmental changes in DNA methylation of the two tobacco pollen nuclei during maturation. Proc Natl Acad Sci USA 94:11721–11725
Oh SA, Johnson A, Smertenko A, Rahman D, Park SK, Hussey PJ, Twell D (2005) A divergent cellular role for the FUSED kinase family in the plant-specific cytokinetic phragmoplast. Curr Biol 15:2107–2111
Oh SA, Bourdon V, Das ‘Pal M, Dickinson H, Twell D (2008) Arabidopsis kinesins HINKEL and TETRASPORE act redundantly to control cell plate expansion during cytokinesis in the male gametophyte. Mol Plant 1:794–799
Oh SA, Pal MD, Park SK, Johnson JA, Twell D (2010) The tobacco MAP215/Dis1-family protein TMBP200 is required for the functional organization of microtubule arrays during male germline establishment. J Exp Bot 61:969–981
Okada T, Endo M, Singh MB, Bhalla PL (2005) Analysis of the histone H3 gene family in Arabidopsis and identification of the male-gamete-specific variant AtMGH3. Plant J 44:557–568
Okada T, Bhalla PL, Singh MB (2006a) Expressed sequence tag analysis of Lilium longiflorum generative cells. Plant Cell Physiol 47:698–705
Okada T, Singh MB, Bhalla PL (2006b) Histone H3 variants in male gametic cells of lily and H3 methylation in mature pollen. Plant Mol Biol 62:503–512
Olmedo-Monfil V, Durán-Figueroa N, Arteaga-Vázquez M, Demesa-Arévalo E, Autran D, Grimanelli D, Slotkin RK, Martienssen RA, Vielle-Calzada JP (2010) Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature 464:628–632
Ooi SL, Henikoff S (2007) Germline histone dynamics and epigenetics. Curr Opin Cell Biol 19:257–265
Pagnussat GC, Alandete-Saez M, Bowman JL, Sundaresan V (2009) Auxin-dependent patterning and gamete specification in the Arabidopsis female gametophyte. Science 324:1684–1689
Park SK, Howden R, Twell D (1998) The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development 125:3789–3799
Park SK, Rahman D, Oh SA, Twell D (2004) gemini pollen 2, a male and female gametophytic cytokinesis defective mutation. Sex Plant Reprod 17:63–70
Pastuglia M, Azimzadeh J, Goussot M, Camilleri C, Belcram K, Evrard JL, Schmit AC, Guerche P, Bouchez D (2006) Gamma-tubulin is essential for microtubule organization and development in Arabidopsis. Plant Cell 18:1412–1425
Pina C, Pinto F, Feijo JA, Becker JD (2005) Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiol 138:744–756
Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R (2009) Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet 5:e1000621
Ron M, Saez MA, Williams LE, Fletcher JC, McCormick S (2010) Proper regulation of a sperm-specific cis-nat-siRNA is essential for double fertilization in Arabidopsis. Genes Dev 24:1010–1021
Rotman N, Durbarry A, Wardle A, Yang WC, Chaboud A, Faure JE, Berger F, Twell D (2005) A novel class of MYB factors controls sperm-cell formation in plants. Curr Biol 15:244–248
Sakai N, Sawada MT, Sawada H (2004) Non-traditional roles of ubiquitin–proteasome system in fertilization and gametogenesis. Int J Biochem Cell Biol 36:776–784
Sasaki H, Matsui Y (2008) Epigenetic events in mammalian germ-cell development: reprogramming and beyond. Nat Rev Genet 9:129–140
Saze H (2008) Epigenetic memory transmission through mitosis and meiosis in plants. Semin Cell Dev Biol 19:527–536
Schoft VK, Chumak N, Mosiolek M, Slusarz L, Komnenovic V, Brownfield L, Twell D, Kakutani T, Tamaru H (2009) Induction of RNA-directed DNA methylation upon decondensation of constitutive heterochromatin. EMBO Rep 10:1015–1021
Slotkin RK, Vaughn M, Borges F, Tanurdzic M, Becker JD, Feijó JA, Martienssen RA (2009) Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136:461–472
Sprunck S, Groß-Hardt R (2011) Nuclear behavior, cell polarity and cell specification in the female gametophyte. Sex Plant Reprod 24 (this issue)
Strome S, Lehmann R (2007) Germ versus soma decisions: lessons from flies and worms. Science 316:392–393
Tanaka I, Ito M (1980) Induction of typical cell division in isolated microspores of Lilium longiflorum and Tulipa gesneriana. Plant Sci Lett 17:279–285
Tanaka I, Ito M (1981) Control of division patterns in explanted microspores of Tulipa gesneriana. Protoplasma 108:329–340
Tanaka I, Ono K, Fukuda T (1998) The developmental fate of angiosperm pollen is associated with a preferential decrease in the level of histone H1 in the vegetative nucleus. Planta 206:561–569
Terasaka O, Niitsu T (1987) Unequal cell division and chromatin differentiation in pollen grain cells. I. Centrifugal, cold and caffeine treatments. Bot Mag Tokyo 100:205–216
Terasaka O, Niitsu T (1995) The mitotic apparatus during microspore division observed by a confocal laser scanning microscope. Protoplasma 189:187–193
Thomson T, Lin H (2009) The biogenesis and function of PIWI proteins and piRNAs: progress and prospect. Annu Rev Cell Dev Biol 25:355–376
Twell D, Park SK, Lalanne E (1998) Asymmetric division and cell-fate determination in developing pollen. Trends Plant Sci 3:305–310
Twell D, Park SK, Hawkins TJ, Schubert D, Schmidt R, Smertenko A, Hussey PJ (2002) MOR1/GEM1 has an essential role in the plant-specific cytokinetic phragmoplast. Nat Cell Biol 4:711–714
Ueda K, Tanaka I (1995) The appearance of male gamete-specific histones gH2B and gH3 during pollen development in Lilium longiflorum. Dev Biol 169:210–217
Ueda K, Kinoshita Y, Xu ZJ, Ide N, Ono M, Akahori Y, Tanaka I, Inoue M (2000) Unusual core histones specifically expressed in male gametic cells of Lilium longiflorum. Chromosoma 108:491–500
von Besser K, Frank AC, Johnson MA, Preuss D (2006) Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 133:4761–4769
Wang Y, Zhang WZ, Song LF, Zou JJ, Su Z, Wu WH (2008) Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiol 148:1201–1211
Weterings K, Reijnen W, van Aarssen R, Kortstee A, Spijkers J, van Herpen M, Schrauwen J, Wullems G (1992) Characterization of a pollen-specific cDNA clone from Nicotiana tabacum expressed during microgametogenesis and germination. Plant Mol Biol 18:1101–1111
Wuest SE, Vijverberg K, Schmidt A, Weiss M, Gheyselinck J, Lohr M, Wellmer F, Rahnenfuhrer J, von Mering C, Grossniklaus U (2010) Arabidopsis female gametophyte gene expression map reveals similarities between plant and animal gametes. Curr Biol 20:506–512
Xu H, Swoboda I, Bhalla PL, Singh MB (1999) Male gametic cell-specific expression of H2A and H3 histone genes. Plant Mol Biol 39:607–614
Zaki MAM, Dickinson HG (1991) Microspore-derived embryos in Brassica: the significance of division asymmetry in pollen mitosis I to embryogenic development. Sex Plant Reprod 4:48–55
Zilberman D (2008) The evolving functions of DNA methylation. Curr Opin Plant Biol 11:554–559
Zilberman D, Gehring M, Tran RK, Ballinger T, Henikoff S (2007) Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet 39:61–69
Zonia LTJ, Staiger CJ (1999) Unique actin and microtubule arrays co-ordinate the differentiation of microspores to mature pollen in Nicotiana tabacum. J Exp Bot 50:581–594
Acknowledgments
I thank Twell lab members and Fred Berger, Hugh Dickinson and Robert Grant-Downton for their support and valued discussions on the topics reviewed, and two anonymous reviewers for helpful suggestions. DT is funded by the UK Biotechnology and Biological Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Thomas Dresselhaus.
Rights and permissions
About this article
Cite this article
Twell, D. Male gametogenesis and germline specification in flowering plants. Sex Plant Reprod 24, 149–160 (2011). https://doi.org/10.1007/s00497-010-0157-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00497-010-0157-5