Abstract
In order to better understand the developmental processes that govern the formation of somatic embryos in oil palm (Elaeis guineensis Jacq.), we investigated the transcription factor genes expressed during embryogenesis in this species. The AP2/EREBP transcription factor family includes the AP2 subgroup, which contains several proteins that play important roles in plant development. We identified and characterized EgAP2-1, which codes for a protein that contains two AP2 domains similar to those of the transcription factor BABYBOOM (BBM) and more generally AINTEGUMENTA-like (AIL) proteins of the AP2 subgroup. In a similar way to related genes from eudicots, ectopic expression of EgAP2-1 in transgenic Arabidopsis plants alters leaf morphology and enhances regeneration capacity. In oil palm, EgAP2-1 transcripts accumulate to the greatest extent in zygotic embryos. This expression pattern was investigated in more detail by in-situ hybridization, revealing that in both zygotic and somatic embryos, EgAP2-1 expression is concentrated in proliferating tissues associated with the early development of leaf primordia, root initials and provascular tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oil palm (Elaeis guineensis Jacq.) is a perennial monocotyledonous plant belonging to the Arecaceae family which recently became the world’s leading source of vegetable oil. Given its great economic importance and long life cycle, clonal propagation techniques for oil palm have been developed over a number of years as a way to disseminate high yielding hybrid plants selected by traditional breeding techniques. The lack of secondary shoot formation in this single stemmed species excludes the possibility of natural vegetative multiplication and necessitates the development of in vitro micropropagation processes that exploit the totipotency of the plant cells.
As is generally the case for monocotyledons, indirect embryogenesis through callus production is required to produce in vitro regenerated plants of oil palm (Pannetier et al. 1981). Protocols based on the use of embryogenic suspensions obtained from leaf or zygotic embryo-derived calli have been developed (de Touchet et al. 1991). Although cell suspension culture-based techniques are now used to propagate oil palm on a medium scale, some limitations still exist, the two most important being the production of embryogenic callus and the induction of shoots from somatic embryos, which is achieved with variable efficiency.
The understanding of the molecular mechanisms which control the process of somatic embryogenesis constitutes one of the most crucial questions currently faced in plant biology (Vogel 2005). Various transcription factors, when overexpressed, influence the transition from vegetative to embryonic growth (Lotan et al. 1998; Stone et al. 2001; Boutilier et al. 2002; Zuo et al. 2002; Harding et al. 2003) and the initiation of shoot regeneration (Banno et al. 2001; Daimon et al. 2003). Among these transcription factors, some members of the AP2/EREBP family (for APETALA2/Ethylene-responsive element binding protein) have been shown to promote embryo development or in vitro regeneration when overexpressed in Arabidopsis, notably those encoded by BABY BOOM (BBM), an AINTEGUMENTA-like (AIL) gene (Boutilier et al. 2002) or the ENHANCER OF SHOOT REGENERATION (ESR1) gene (Banno et al. 2001). AP2/EREBP genes form a large family and are involved in a number of key developmental processes throughout the plant’s life cycle (Riechmann and Meyerowitz 1998). Within the family of AP2 proteins, the AP2 subgroup, which contains a number of developmentally important members, is characterized by an AP2 domain with a variable number of residues (41–74; Feng et al. 2005). In Arabidopsis there are only 19 members, 13 of which are characterized by the presence of two AP2 domains in tandem (Feng et al. 2005). Members of the AP2 gene subgroup are expressed in multiple tissues and appear to be involved in a wide range of development processes like flowering, seed and root development (Riechmann and Meyerowitz 1998; Nole-Wilson et al. 2005). Based on genetic studies and expression pattern analysis in eudicot plants, at least four members of the AP2 subgroup potentially participate in embryo development: APETALA2, which regulates embryo size by negatively controlling cell proliferation during seed development (Ohto et al. 2005); AINTEGUMENTA (ANT), which is expressed in the primordia of cotyledons (Elliott et al. 1996); BBM, which may promote cell proliferation and morphogenesis during embryogenesis (Boutilier et al. 2002); and WRINKLED1 (WRl1), which is involved in the positive regulation of seed storage metabolism in Arabidopsis (Cernac and Benning 2004). To date, no members of the AP2 subfamily have been demonstrated to be involved in embryo development in monocotyledonous plants. Moreover, with the exception of BBM, no comparative studies of expression patterns during zygotic and somatic embryogenesis have been reported for genes of this group as a means of comparing the regulation processes involved.
The overall objectives of this work were to determine whether AP2 genes are specifically expressed during zygotic embryo development in oil palm and to investigate whether their regulation might be different during zygotic and somatic embryogenesis. We report here on the isolation and characterization of EgAP2-1, an AINTEGUMENTA-like (AIL) gene, expressed principally in the oil palm zygotic embryo. We compared the expression patterns observed for EgAP2-1 in differentiated zygotic and somatic embryos, as well as in different types of in vitro material such as embryogenic callus, non-embryogenic callus and the embryogenic cell suspension. Furthermore, we used transgenic Arabidopsis as an experimental system to investigate the effect of ectopic expression of EgAP2-1 on plant development and to compare it with data obtained for other AIL genes originating from eudicots. Our data support a role for EgAP2-1 in oil palm zygotic and somatic embryo development and show that heterologous EgAP2-1 expression enhances regeneration capacity in Arabidopsis.
Materials and methods
Whole plant material
Oil palm plant material was harvested from tenera palms at different locations: INRAB Pobé Station, Benin (seeds); FELDA Tun Razak plantation, Malaysia (immature inflorescences); and ASD Coto plantation, Costa Rica (15-month old shoot apex material). Root and young leaf samples were harvested from seed-derived greenhouse plants grown in Montpellier. Zygotic embryos were excised from dry seeds, previously imbibed for 5 days in water, and harvested after 2–16 days of in vitro culture on a germination medium as described by Aberlenc-Bertossi et al. (1999). Arabidopsis thaliana plants of the ecotype Columbia supplied by the Nottingham Arabidopsis Stock Center (NASC; http://www.nasc.nott.ac.uk) were used for plant transformation and in vitro regeneration tests.
In vitro plant material
Somatic embryos (SE) of palm were produced either from callus or from embryogenic suspension cells (SC) as described in Morcillo et al. (2006) and Aberlenc-Bertossi et al. (1999), respectively. In the former case, SE proliferate by secondary embryogenesis. In vitro induction of shoot regeneration from Arabidopsis thaliana was performed according to Vergunst’s protocol (Vergunst et al. 1998). Leaf, cotyledon, and root explants from 11-day-old seedlings of wild type plants and four independent transgenic lines were placed on either basal medium (BM) or media supplemented with plant growth regulators. In the experiments involving plant growth regulators, explants were first pre-cultured on callus-inducing medium (CIM, high auxin to cytokinin ratio) for 3 days before being transferred to shoot-inducing medium (SIM, high cytokinin to auxin ratio) for 20 days. Twenty organs were cultivated on each Petri dish, which was incubated under 16 h day length at 45 μmol m−2 s−1, 23°C and 60% humidity. This experiment was repeated four times.
Isolation and analysis of cDNAs
Total RNA was extracted from oil palm somatic embryos using the RNeasy™ Plant Kit (Qiagen). First strand cDNA was prepared using an oligo (dT) primer and 1 μg of RNA in conjunction with a SuperScript first strand kit (Invitrogen). Aliquots of 0.5 μl of reaction product were used as a substrate for PCR amplification using the specific primer pairs GRBNM3lkS/GRBNM3lkAS (CAGGTGGCTATGATAAGGAGGA/GGAAAGTTGGTTGTGGTGCT) corresponding to the AP2 domain of the rice BBM-related gene OsBNM3lk (accession Os01g67410) gene. PCR was performed in the presence of primers at 0.4 μM, dNTPs at 50 μM and 0.3 units of Taq polymerase (Promega), in the buffer supplied with the enzyme. Samples were amplified for 30 cycles under the following regime: denaturation at 94°C for 30 s, primer annealing for 30 s at 60°C and extension for 30 s at 72°C. From an aliquot of this first PCR product (1 μl of 1/50 diluted solution) a second run of PCR was performed using a slightly modified program (35 cycles, primer annealing at 55°C). PCR products were cloned into the pCRII-TOPO vector (Invitrogen) and sequenced (Genome Express, Meylan, France). A resulting cDNA fragment encoding an AP2 domain was used to screen a cDNA library, constructed from 14 to 24 week old zygotic embryos. Sequence similarities were studied using the BLASTX program (Altschul et al. 1990). For phylogenetic analyses, the data sets were compiled on the basis of BLASTP searches. Alignments of amino acid sequences were performed using the CLUSTALX program (Thompson et al. 1997). Phylogenetic trees were constructed using the neighbor-joining (NJ) method (Saitou and Nei 1987) in conjunction with CLUSTALX. The robustness of the nodes was assessed by bootstrap proportion (BP) analysis (Felsenstein 1985) computed from 100 replicates. The phylogenetic tree was edited using the TreeView program (http://www.taxonomy.zoology.gla.ac.uk/rod/treeview.html) and manually optimized for viewing clarity.
Analysis of gene expression by RT-PCR
Total RNA was isolated from inflorescence and callus cultures as described previously (Morcillo et al. 2006). For zygotic embryos, total RNA was extracted using the lipid RNeasy™ Plant Kit (Qiagen) and for roots, leaves, shoot apex material, SC and SE total RNA was isolated with the RNeasy™ Plant Kit (Qiagen). For RT-PCR analysis, first strand cDNA was synthesized using an oligo (dT) primer and 1 μg RNA by means of a SuperScript first strand kit (Invitrogen). The primer pairs EgAP2S1/EgAP2AS1 (ATGTGGGGAGCTGGGTCACA/AGAAGTTGGGCGCATGACCA) and EgEF1-α1S2/EgEF1-α1AS2 (GGTGTGAAGCAGATGATTTGC/CCTGGATCATGTCAAGAGCC) were used for specific amplification of reverse transcripts of the oil palm EgAP2-1 and EgEF1-α1 (elongation factor alpha-1; accession AY550990) genes. PCR amplifications of EgEF1-α1 reverse transcripts were used as a control. The primers of the EgEF1-α1 were designed around an intron sequence to test for the presence of genomic DNA contamination in the reverse transcript sample. We verified that amplification was still in its linear range by monitoring amplification products after several different numbers of cycles. Amplified cDNAs were cloned and sequenced to check gene specificity.
Isolation of genomic DNA and Southern hybridization
Genomic DNA was isolated from inflorescence material as described by Rival et al. (1998) and 7 μg aliquots digested with the restriction enzymes BamHI, EcoRI and HindIII prior to agarose gel electrophoresis and Southern blotting. Hybridization was carried out at low and high stringency conditions with a 32P-radiolabelled EgAP2-1 DNA probe obtained by PCR amplification of the coding region outside the AP2 domain using the primer pair EgAP2S3/EgAP2AS3 (TCCAAAGATGGGCGTCTG/CCTTGTTTGCACTCCAAC).
RNA in-situ hybridization
PCR amplification was performed using the primer pair EgAP2-S1/EgAP2-T7AS1 the latter primer (GCGAAATTAATACGACTCACTATAGGGCGAAAGAAGTTGGGCGCATGACCA) containing the T7 RNA polymerase site (underlined). The resulting fragment was used as template for synthesizing antisense digoxigenin-labelled riboprobes with a UTP-DIG nucleotide mixture (Roche) in conjunction with a T7 Script kit (Ambion). The amplification product was tested by Southern blotting to evaluate the specificity of the probe. In-situ hybridation experiments were carried out essentially as described in Adam et al. (2007) on paraffin sections (15 μm) attached to precoated glass slides (Dako).
Generation of Arabidopsis thaliana transgenic lines overexpressing EgAP2-1
The complete open reading frame (ORF) of EgAP2-1 was amplified by PCR using the primer pair CegAP2S/CegAP2AS (CACCATGGACATGGACACTTCAC/CATCCATATCATTCCATTCC) to achieve positional cloning in pENTR. PCR fragments were cloned into the Gateway™-pENTR™ vector (Invitrogen) then placed adjacent to a dual 35S CaMV promoter in the destination vector pMDC32 (Curtis and Grossniklaus 2003) through BP/LR clonase™ reactions (Invitrogen). Arabidopsis thaliana plants were transformed using the floral dip method (Clough and Bent 1998) and the Agrobacterium tumefaciens C58C1 strain was used for the transformation. Transformed Arabidopsis that survived on the medium containing hygromycin were checked for the presence of the transgene by PCR and the number of transgene copies was evaluated by Southern analysis. The plants were grown at 22°C under 16 h day length. Only lines with either one or two copies of the transgene were used for further analysis.
Results
Isolation of an AP2 transcription factor cDNA from oil palm embryos: genomic complexity and sequence analysis
A 91 bp AP2-type cDNA fragment amplified by RT-PCR from somatic embryos was used to screen an oil palm zygotic embryo cDNA library from which a full-length cDNA of 1,786 bp named EgAP2-1 (accession AY691196) was isolated. Sequence analysis revealed that the deduced EgAP2-1 polypeptide contains two AP2/EREBP domains (residues 128–203 designated as repeat 1 and residues 230–298 designated as repeat 2) and a conserved linker region (residues 204–229). In each AP2 domain repeat, the highly conserved YRG element (residues 128–149 and 230–251 of repeats 1 and 2 respectively) and RAYD element (residues 161–203 and 255–298) are observed, as well as the specific central core of 18 amino acids within the RAYD element that is predicted to form an alpha helix (residues 169–186 and 264–282; Okamuro et al. 1997). The combined presence of two AP2 domains and the consensus sequences within the conserved YRG element indicates that EgAP2-1 is a transcription factor belonging to the AP2 subgroup of the AP2/EREBP family.
Southern hybridization was used to estimate the number of genes cross-hybridizing with the EgAP2-1 cDNA in oil palm. Using a 352 bp fragment from the coding region lacking the AP2 domains as probe, EcoRI digests produced one major and one minor hybridizing band, whereas HindIII and BamHI digests produced one major and two minor bands (Fig. 1a). These results suggest the existence one additional closely related gene.
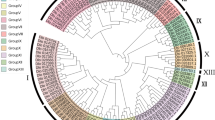
Southern hybridization and phylogenetic analysis of EgAP2-1 from oil palm. a DNA gel blot containing restriction digested oil palm genomic DNA hybridized with a 0.35 kb probe corresponding to the 3′ end of the EgAP2-1 coding region. Migration of DNA size markers (indicated in kilobases) are shown. H HindIII; B BamH1; E EcoRI. b Neighbor-joining tree obtained from the deduced amino acid sequences of the full AP2 domain of EgAP2-1 and various related proteins. The Chlamydomonas reinhardtii AV622151 sequence was used as outgroup. Bootstrap values are indicated for nodes supported by more than 50% of 100 bootstrap replicates. The scale bar corresponds to 0.1 amino acid substitutions per residue. Species names are shown next to each protein (or locus) name. The oil palm sequence is highlighted by a grey box. Grey star indicate the nodes of the clades. The grey circles indicate the node of the sub-clades. Accession numbers: Glossy 15 (AAV83488); PhAP2A (AF132001); LIPLESS1 (AA052746); PaAP2L1 (AAG32658); PtAP2L1 (BAD16603); Ids (AAC05206); PtANTlk (BAD16602); NtANTL (AAR22388); BnANT (ABA42146); MgBBM (AAW82334); BBM2 (AAM33801); BBM1 (AAM33802); BrAIL(AAZ66950); EgAP2-1 (AY691196); ZMMHCF1 (CAA87634)
To analyze sequence relationships between EgAP2-1 and other AP2 subgroup proteins from other species, a tree was constructed using the 171 amino acid sequence of the full AP2 domain including the repeat 1, linker and repeat 2 regions. Because of the large number of accessions present within the AP2 subgroup, a selected number of sequences which displayed 44–97% identities with the full AP2 domain of EgAP2-1 were used to produce a representative tree (Fig. 1b). Based on bootstrap values and irrespective of the origin of the sequences (monocots, eudicots or gymnosperms) the resulting phylogenetic tree illustrates that the AP2 subgroup consists of three major distinct clades. One clade corresponds to the APETALA2 (AP2) type proteins. A second clade corresponds to the WRINKLED1 (WRl1) type proteins. A third clade includes the AINTEGUMENTA-like (AIL) type proteins previously defined by Nole-Wilson et al. (2005). In the AIL clade, two subclades are resolved, I (ANT-related) and II (BBM/PLT-related). EgAP2-1 belongs to the second subclade (BBM/PLT-related) and within the studied region displays identities of up to 97% with other members of the group. EgAP2-1 does not show strong stretches of sequence similarity with these proteins outside the AP2 DNA binding domain region. However, Ser-rich (residues 313–324) and Gln-rich (residues 365–374) regions common to other proteins of subclade II and implicated in transcriptional activation were found to be present in the EgAP2-1 protein sequence, as observed for AIL5 (Ser-rich region) and AtBBM, PLT1 and AIL6 (Gln-rich region; Seipel et al. 1992; Gashler et al. 1993; Nole-Wilson et al. 2005).
Expression analysis of EgAP2-1 by RT-PCR and in-situ hybridization
An initial analysis of expression by northern hybridization using RNA extracted from zygotic embryos, flowers and leaves revealed a single band of 1,800 nt in zygotic embryos (data not shown). A more detailed study was then performed by semi-quantitative RT-PCR. EgAP2-1 transcripts were mainly detected in zygotic embryos and roots but not in leaves, nor in shoot apex-containing basal leaf segments nor in the immature inflorescence (Fig. 2a). EgAP2-1 transcripts were detected at all the stages of zygotic embryo development examined (Fig. 2b). Before 80 DAP it is difficult to isolate the zygotic embryo due to its small size, whereas 180 DAP corresponds to the mature fruit stage. A weaker signal was observed after 8 days of in vitro germination, and no transcripts were detected after 16 days (Fig. 2b). The same type of analysis conducted using in vitro material revealed the presence of EgAP2-1 transcripts in somatic embryos (SE) at different stages of development, in embryogenic cell suspensions, and in both embryogenic callus (EC) and non-embryogenic callus (NEC; Fig. 2a; Fig. S1).
RT-PCR analysis of EgAP2-1 transcripts in oil palm tissues from the whole plant and in vitro materials. a RT-PCR analysis in palm tissues (R root; L young leaves; A vegetative shoot apex; F inflorescence; ZE zygotic embryo (120 DAP); SE somatic embryo; SC embryogenic suspension cells; EC embryogenic callus; NEC non-embryogenic callus). b RT-PCR analysis of EgAP2-1 during zygotic embryo maturation (80–150 DAP) and 2–16 days after germination in vitro
In-situ hybridization studies were performed on each type of material for which EgAP2-1 transcripts were observed by RT-PCR analysis (Fig. 3). In immature zygotic embryos, a strong signal was observed in young leaf primordia (Fig. 3a). In mature zygotic embryos (data not shown), as well as zygotic embryos after 10 days of in-vitro germination (Fig. 3b), a signal was also observed in the shoot apical dome of the meristem, the central region undergoing vascular tissue differentiation, and in the meristematic cells from which will originate the future root meristem. In-situ hybridization studies on material cultured in vitro showed that in embryogenic callus (Fig. 3c), EgAP2-1 transcripts were concentrated in the actively dividing inner tissues (e.g. tissues in box A) and in proembryogenic circular nodules, which will later form an embryo (Schwendiman et al. 1988; see e.g. tissues in box B). Within these proembryogenic circular nodules, which were surrounded by dead cells of small size lacking any cellular contents, a gradient of signal of increasing intensity towards the centre was observed, the layer of cells at the periphery of this circular nodule being only weakly stained compared with the more mitotically active centre of the proembryo (Fig. 3d). EgAP2-1 transcript accumulation was also observed in somatic embryos developing via secondary embryogenesis, as can be seen in Fig. 3e and f. In this type of proliferation, the embryos display a characteristic white protoderm, are at least partially individualized and depending on the stage of development, may exhibit root–shoot meristem differentiation (Pannetier et al. 1981; Schwendiman et al. 1988). In the section shown in Fig. 3e, two structures can be seen, one emerging from the periphery of the other (SEI and SEII). Although the root and shoot poles per se are not distinguishable at the stage shown, both structures display a protoderm and a specific polarization characterized by a meristematic pole at their base (consisting of small cells with low vacuole content), and a cotyledonary pole consisting of large vacuolised cells (Fig. 3e, f). In contrast with the proembryo (Fig. 3d), the staining has in this case become more localized. For both somatic embryos shown, no accumulation of EgAP2-1 transcripts was detected in the cotyledon parenchyma. In contrast, a specific signal can be observed at the base of SE (Fig. 3e, f) and in their provascular strands (SEI, Fig. 3e).
Comparison of spatial expression patterns of EgAP2-1 in vitro and in planta by in-situ hybridization. a Immature zygotic embryo harvested 83 DAP. b Zygotic embryo after 10 days of germination in vitro. c Embryogenic callus. d Detail of embryogenic callus (inset B in c). e, f Somatic embryos proliferating by secondary embryogenesis (inset A, actively dividing inner tissues; inset B, proembryogenic nodule; C cotyledon parenchyma; DV developing vascularization; SAM shoot apical meristem; SEI and SEII, primary and secondary somatic embryos generated by secondary somatic embryogenesis; LP leaf primordia; M meristematic tissues; PS provascular strands). Scale bar 500 μm
Over-expression of EgAP2-1 in transgenic Arabidopsis
To investigate the effects of heterologous expression of EgAP2-1 on plant development, Arabidopsis plants transformed with this gene under the control of the CaMV 35S promoter were generated. In the transformed plant which exhibited the highest levels of EgAP2-1 transcripts (Fig. S2a), pleiotropic phenotypes were observed. For example, a number of independent transgenic lines displayed slow growth and/or a rapid initiation of small, malformed leaves compared to the wild type (Fig. 4a, b); others displayed serrated leaves (Fig. 4c). For one transgenic line, some undifferentiated cell outgrowth structures emerging from the petiole of the cotyledon were also observed (Fig. 4d).
Examples of phenotypes observed in transgenic 35S::EgAP2-1 Arabidopsis plants. a Wild type leaf. b Transgenic line with slow growth and small, malformed leaves. c Transgenic line with serrated leaves. d Example of undifferentiated cell outgrowth (arrow) emerging from petiole of cotyledon in transgenic line. Scale bar 100 μm
Leaf, cotyledon and root explants from wild type and transgenic lines were placed on either basal tissue culture medium or media supplemented with plant growth regulators, so as to evaluate the effect of the EgAP2-1 transgene on regeneration in vitro. When cotyledon and leaf segments from the transgenic lines were precultured on callus-inducing medium (CIM) for 3 days before being transferred onto shoot-inducing medium (SIM) for 20 days, shoot and callus formation was observed on the surface of the explants, in contrast to wild type segments which responded to a much lesser extent (Fig. 5a, b). In the case of root material, 35S:EgAP2-1 explants displayed a greater number of shoots which were produced more rapidly compared with wild type in the presence of exogenous growth regulators (Fig. 5c; Fig. S2b).
Effects of ectopic overexpression of EgAP2-1 on shoot production. Cotyledon (a), leaf (b), and root (c) segments from 11-day-old seedlings of wild type plants and four independent transgenic lines were precultured on callus-inducing medium (CIM) supplemented with 2.2 μM 2,4-D and 0.2 μM kinetin for 3 days before being transferred onto shoot-inducing medium (SIM) supplemented with 0.9 μM indole-3-acetic acid and 5 μM 2-isopentenyladenine for 20 days
Discussion
EgAP2-1 codes for an AINTEGUMENTA-like protein with functional similarities to eudicot relatives
In this study we characterized an oil palm gene encoding a transcription factor belonging to the AP2 subgroup of the EREBP/AP2 family and more specifically to the AINTEGUMENTA-like (AIL) clade previously defined by Nole-Wilson et al. (2005). The AIL clade includes proteins from monocots, eudicots and gymnosperms, suggesting that a duplication giving rise to the ancestral AIL gene occurred relatively early in plant evolution before the appearance of angiosperms. With respect to the two distinct sub-clades of the AIL group, EgAP2-1 branches with clade II, which also includes the Arabidopsis proteins BBM, PLT and AIL5/6/7. The genes encoding these proteins are known to be expressed in different tissues of the plant such as seeds, roots and flowers respectively, indicating that within the same species, widely contrasting expression patterns exist amongst paralogues of this clade (Boutilier et al. 2002; Aida et al. 2004; Nole-Wilson et al. 2005). EgAP2-1 shows only low similarity with these proteins outside the AP2 DNA binding domain, illustrating the high species-specificity of protein sequences in these regions. While all of the eudicot AIL genes mentioned have been functionally characterized, no functional information has been available from monocotyledons until now.
Plants overexpressing EgAP2-1 display leaf morphological abnormalities. Similar effects have been observed with other members of the AP2/EREBP family, all from eudicots, when overexpressed in Arabidopsis. For example, leaf morphological abnormalities have been reported for LEP (LEAFY PETIOLE), BBM and PLT (Van der Graaff et al. 2002; Boutilier et al. 2002; Aida et al. 2004). In addition to its phenotypic effects in planta, the ectopic expression of EgAP2-1 enhances in vitro regenerative capacity by increasing cell proliferation and organogenesis on shoot-inducing medium. In a similar manner, shoot regeneration and callus production in vitro were observed to be enhanced by the transgenic expression of ESR1 or BBM, although in these two cases the phenomenon is observed in the absence of hormones (Banno et al. 2001; Boutilier et al. 2002). Similarities in phenotypic effects induced by transgenic expression suggest a conservation of biochemical properties and similar targets for the proteins encoded by EgAP2-1 and other AP2 members.
EgAP2-1 is expressed in meristematic or proliferating tissues of the oil palm zygotic embryo
Although the closest characterised relative of EgAP2-1 is the Arabidopsis gene AIL5, which is expressed in most organs of the plant, EgAP2-1 mRNAs accumulate in planta mainly in the developing zygotic embryo. A weaker signal was observed after 8 days of in vitro germination, and no EgAP2-1 transcripts were detected after 16 days. At both of these developmental stages, embryo elongation and storage protein hydrolysis occur, which is characteristic of germinative growth (Bewley 1997). Collectively these gene expression data suggest a role for EgAP2-1 principally during embryonic development, up to and including the transition phase to germinative growth. An expression pattern mainly in embryonic tissues has been observed for at least one other member of the AIL subgroup, namely BBM. The expression of BBM is induced in Brassica napus microspores during the acquisition of embryogenic competence (Boutilier et al. 2002); however, the exact function of BBM during embryo development is still not clear. Ectopic BBM expression results in the formation of somatic embryos at the cotyledon margins in Arabidopsis seedlings (Boutilier et al. 2002). In contrast, we did not observe ectopic embryo formation when EgAP2-1 was constitutively expressed in Arabidopsis. Our data do not provide evidence of a role for EgAP2-1 in the transition of somatic cell fate towards embryogenic competence. Likewise, a recent report showed that the ectopic expression of BBM gene in tobacco (Nicotiana tabacum L.) does not lead to an induction of spontaneous somatic embryo formation (Srinivasan et al. 2007). One explanation for these results could be the requirement of specific protein interactions that may not be possible in heterologous expression studies with diverse species such as oil palm, tobacco and Brassicaceae.
In zygotic embryos, EgAP2-1 expression is concentrated in proliferating tissues associated with the early development of leaf primordia, root initials and provascular tissues. This general expression pattern in actively dividing young tissues of the plant is characteristic of various different AIL genes (Mizukami and Fisher 2000; Nole-Wilson et al. 2005). Our data support a role for EgAP2-1 in the activity or maintenance of meristematic/proliferating tissues which give rise to young organs and tissues, as proposed for other eudicot relatives (Nole-Wilson et al. 2005). Although a weak signal was observed in roots (a common feature amongst known AIL genes; Feng et al. 2005; Nawy et al. 2005; Nole-Wilson et al. 2005), EgAP2-1 transcripts were undetected in young leaves, inflorescences at different stages of development (data not shown) and the vegetative shoot apex, suggesting that down-regulation occurs during post-embryonic development. Other transcription factor genes have been shown to display down-regulation during post embryonic development, including embryo-specific genes as LEC1 and LEC2 for which transcripts were not detected in seedlings or vegetative tissues of mature plants (Lotan et al. 1998; Stone et al. 2001). It is of great importance to establish the identity of regulatory factors acting upstream of this gene in order to understand how its embryo related expression is controlled.
Similarities between EgAP2-1 expression patterns during the differentiation of zygotic and somatic embryos
In both zygotic and somatic embryos, EgAP2-1 transcripts accumulate in the vascular system and in the basal meristematic tissues that give rise to the root meristem. Although zygotic embryos and somatic embryos are initiated differently, the two processes share common morphological, physiological and molecular characteristics (Zimmerman 1993). For example, the LEC1-like gene HaL1 of Helianthus annuus has been observed to be highly expressed in both zygotic and somatic embryogenesis (Fambrini et al. 2006). The spatial expression pattern of knotted1 is also similar during somatic and zygotic embryogenesis in Zea mays (Zhang et al. 2002).
In general, EgAP2-1 is expressed in all cells undergoing proliferation during in vitro culture, including embryonic, embryogenic and non-embryogenic tissues. In contrast, the in-planta expression of EgAP2-1 is more spatially restricted than in vitro and is mainly associated with embryonic meristematic/proliferating tissues. Interestingly, gene expression analysis in Arabidopsis by massively parallel signature sequencing (MPSS) revealed that six of the seven AIL genes in this species exhibit their strongest expression in callus despite having very marked tissue-specific patterns in planta (Nole-Wilson et al. 2005).
In conclusion, we present here the first report of a monocot AIL gene, EgAP2-1, from oil palm. Our results indicate that the expression of this gene is associated with embryo development. In tissues cultured in vitro, the expression of this gene is not restricted to embryonic tissues. More specifically the expression of EgAP2-1 is associated with tissues undergoing organ and vascular tissue differentiation during embryo morphogenesis. Since heterologous expression of this gene in Arabidopsis enhances hormone-induced shoot regeneration, it might have uses for the improvent of in vitro regeneration in oil palm. It will be of interest to study the interactions of EgAP2-1 with other genes and to identify targets of the EgAP2-1 protein which might be involved in somatic and zygotic embryogenesis. Further studies on functional diversity within the AIL group in oil palm and other species will contribute to a greater understanding of the role of these genes in higher plants.
Abbreviations
- AIL:
-
AINTEGUMENTA-like
- AP2/EREBP:
-
APETALA2/Ethylene-responsive element binding protein
- BM:
-
Basal medium
- CIM:
-
Callus-inducing medium
- DAP:
-
Days after pollination
- EC:
-
Embryogenic callus
- NEC:
-
Non-embryogenic callus
- SIM:
-
Shoot-inducing medium
- SC:
-
Embryogenic suspension cells
- SE:
-
Somatic embryos
References
Aberlenc-Bertossi F, Noirot M, Duval Y (1999) BA enhances the germination of oil palm somatic embryos derived from embryogenic suspension cultures. Plant Cell Tissue Organ 56:53–57
Adam H, Jouannic S, Orieux Y, Morcillo F, Richaud F, Duval Y, Tregear JW (2007) Functional characterization of MADS box genes involved in the determination of oil palm flower structure. J Exp Bot 58:1245–1259
Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B (2004) The PLETHORA genes mediates patterning of the Arabidopsis root stem cell niche. Cell 119:109–120
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Banno H, Ikeda Y, Niu QW, Chua NH (2001) Overexpression of Arabidopsis ESR1 induces initiation of shoot regeneration. Plant Cell 13:2609–2618
Bewley JD (1997) Seed germination and dormancy. Plant Cell 9:1055–1066
Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu C-M, Van Lammeren AAM, Miki BLA, Custers JBM, Van Lookeren Campagne MM (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14:1737–1749
Cernac A, Benning C (2004) WRINKLED encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J 40:575–585
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133:462–469
Daimon Y, Takabe K, Tasaka M (2003) The CUP-SHAPED COTYLEDON genes promote adventitious shoot formation on calli. Plant Cell Physiol 44:113–121
Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQJ, Gerentes D, Perez P, Smyth DR (1996) AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8:155–168
Fambrini M, Durante C, Cionini G, Geri C, Giorgetti L, Michelotti V, Salvini M, Pugliesi C (2006) Characterization of LEAFY COTYLEDON1-LIKE gene in Helianthus annuus and its relationship with zygotic and somatic embryogenesis. Dev Genes Evol 216:253–264
Felsenstein J (1985) Confidence limits on phylogenies: an approach using bootstrap. Evolution 39:783–791
Feng JX, Liu D, Pan Y, Gong W, Ma LG, Luo JC, Deng XW, Zhu YX (2005) An annotation update via cDNA sequence analysis and comprehensive profiling of developmental, hormonal or environmental responsiveness of the Arabidopsis AP2/EREBP transcription factor gene family. Plant Mol Biol 59:853–868
Gashler AL, Swaminathan S, Sukhatme VP (1993) A novel repression module, an extensive activation domain, and a bipartite nuclear localization signal defined in the immediate-early transcription factor Egr-1. Mol Cell Biol 13:4556–4571
Harding WE, Weining T, Nichols KW, Fernandez, Perry SE (2003) Expression and maintenance of embryogenic potential is enhanced through constitutive expression of AGAMOUS-like 15. Plant Physiol 133:653–663
Lotan T, Ohto M, Yee K.M, Lo R, Kwong L, Yamagashi K, Fischer R, Goldberg B, Harada J (1998) Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93:1195–1205
Morcillo F, Gagneur C, Adam H, Richaud F, Singh R, Cheah SC, Rival A, Duval Y, Tregear JW (2006) Somaclonal variation in micropropagated oil palm. Charaterization of two novel genes with enhanced expression in epigenetically abnormal cell lines and in response to auxin. Tree Physiol 26:585–594
Mizukami Y, Fisher RL (2000) Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc Natl Acad Sci USA 97:942–947
Nawy T, Lee JY, Colinas J, Wang JY, Thongrod SC, Malamy JE, Birnbaum K, Benfey PN (2005) Transcriptional profile of the Arabidopsis root quiescent center. Plant Cell 17:1908–1925
Nole-Wilson S, Tranby TL, Krizek BA (2005) AINTEGUMENTA-like (AIL) genes are expressed in young tissues and may specify meristematic or division-competent states. Plant Mol Biol 57:613–628
Okamuro JK, Caster B, Villarroel R, Van Montagu M, Jofuku KD (1997) The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc Natl Acad Sci USA 94:7076–7081
Ohto MA, Fischer RL, Goldberg RB, Nakamura K, Harada JJ (2005) Control of seed mass by APETALA2. Proc Natl Acad Sci USA 102:3123–3128
Pannetier C, Arthuis P, Lievoux D (1981) Néoformation de jeunes plantes d’Elaeis guineensis à partir de cals primaires obtenus sur fragments foliaires cultivés in vitro. Oléagineux 36:119–122
Riechmann JL, Meyerowitz EM (1998) The AP2/EREBP family of plant transcription factors. Biol Chem 379:633–646
Rival A, Bertrand L, Beule T, Combes MC, Trouslot P, Lashermes P (1998) Suitability of RAPD analysis for the detection of somaclonal variants in oil palm. Plant Breeding 117:73–76
Saitou N, Nei M (1987) The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schwendiman J, Pannetier C, Michaux-Ferriere N (1988) Histology of somatic embryogenesis from leaf explants of the oil palm Elaeis guineensis. Ann Bot 62:43–52
Seipel K, Georgiev O, Schaffmer W (1992) Different activation domains stimulate transcription from remote (‘enhancer’) and proximal (‘promoter’) positions. EMBO J 11:4961–4968
Srinivasan C, Liu Z, Heidmann I, Supena EDJ, Fukuoka H, Joosen R, Lambalk J, Angenent G, Scorza R, Custers JBM, Boutilier K (2007) Heterologous expression of the BABY BOOM AP2/ERF transcription factor enhances the regeneration capacity of tobacco (Nicotiana tabacum L.). Planta 225:341–351
Stone S, Kwong L, Yee K.M, Pelletier J, Lepiniec L, Fischer R, Goldberg B, Harada J (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci USA 98:11806–11811
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The clustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res 24:4876–4882
de Touchet B, Duval Y, Pannetier C (1991) Plant regeneration from embryogenic suspension culture of oil palm (Elaeis guineensis Jacq.). Plant Cell Rep 10:529–532
Van der graaff E, Dulk-Ras AD, Hooykaas PJ, Keller B (2002) Activation tagging of the LEAFY PETIOLE gene affects leaf petiole development in Arabidopsis thaliana. Development 22:4971–4980
Vergunst AC, de Waal EC, Hooykaas PJJ (1998) Root transformation by Agrobacterium tumefaciens. In: Martinez-Zapater JM, Salinas J (eds) Arabidopsis protocols. Humana Press, Totowa, pp 227–244
Vogel G (2005) How does a single somatic cell become a whole plant? Science 309:86
Zhang S, Wong L, Meng L, LemauxPG (2002) Similarity of expression patterns of knotted1 and ZmLEC1 during somatic and zygotic embryogenesis in maize (Zea mays L.). Planta 215:191–194
Zimmerman JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1411–1423
Zuo J, Niu Q, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative to embryonic transition in Arabidopsis. Plant J 30:349–359
Acknowledgments
We thank Frédérique Richaud for help in cDNA library screening. The authors acknowledge the generous support of colleagues at INRAB Pobé Experimental Station in Benin, FELDA Agriculture Services in Malaysia and ASD Coto in Costa Rica for providing plant material. We are grateful to Timothy Tranbarger for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morcillo, F., Gallard, A., Pillot, M. et al. EgAP2-1, an AINTEGUMENTA-like (AIL) gene expressed in meristematic and proliferating tissues of embryos in oil palm. Planta 226, 1353–1362 (2007). https://doi.org/10.1007/s00425-007-0574-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0574-3