Abstract
We conducted a bibliographic survey, adding 69 taxa to a published list of 277 seaweeds, thereby updating the total worldwide list of non-native and cryptogenic seaweeds to 346. Polysiphonia Greville and Hypnea J.V. Lamouroux species were the most common taxa on this list, and the Mediterranean Sea and the NE Atlantic bioregions have received most of the 346 taxa. The most important vectors that carry non-native seaweeds are hull fouling and the transport of aquaculture products including ‘blind passengers’. Once a seaweed has arrived in a new location, it can establish a permanent population and spread through natural dispersal or human activity. Non-native seaweeds have negative impacts on native species through competition, habitat destruction and keystone competition, but also positive impacts through habitat formation, food provision and cascading habitat formation. Quantitative meta-analyses have shown that invasive seaweeds typically have a negative effect on local plants, but neutral or positive effects on animal communities. New meta-analyses presented here indicate that impacts increase with the abundance of non-native seaweeds and that non-native seaweeds may increase sample similarity in invaded plant communities, but not in animal communities. The literature on the impact of non-native seaweeds is extensive, but most studies have focused on a few high-profile species. Comprehensive analyses should be done for more species to allow for better predictions. We conclude that non-native seaweeds have altered shallow coastal communities in most biogeographical regions, and impacts will likely increase along with increases in human populations, transport and associated stressors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Invasion impact
- Invasion success
- Meta-analysis
- New invasions
- Density-dependent effects
- Trophic matching hypothesis
1 Introduction—Scope of Seaweed Invasions
Non-native seaweeds are marine macroalgae that have arrived at new locations aided by human activity, either deliberately or accidentally. Today, non-native seaweeds comprise hundreds of species distributed in the photic zone throughout the world’s major biogeographical regions. Many non-native seaweeds are ‘foundation species’ (Dayton 1972) and function as ‘ecosystem engineers’ (Jones et al. 1994), directly or indirectly affecting the availability of resources to other species by creating, modifying and maintaining habitats (Wallentinus and Nyberg 2007; Thomsen et al. 2010). Consequently, non-native seaweeds play key ecological and biogeochemical roles in their new marine ecosystems , sometimes controlling biodiversity , ecosystem functioning and energy flow (Williams and Smith 2007).
The success of a non-native seaweeds is typically considered a staged process that depends on uptake from a donor-region, transport, arrival and release to a receiver-region, local establishment and proliferation, population growth and regional spread and range expansion (Sakai et al. 2001; Bates et al. 2014). For each of these stages, a non-native seaweed has to overcome a series of physical, physiological, demographic and biological barriers and filters that act as a constant sink for new species and individuals. In this chapter, we first review which seaweed species have invaded various regions and then provide a brief overview of the two main approaches in invasion ecology, ‘success’ and ‘impact’ studies and their related frameworks and working hypotheses. We subsequently provide examples of impacts on local populations and evaluate existing quantitative syntheses of invasion impacts and identify current research gaps. We then address key research gaps using meta-analysis to test whether invasive seaweeds have density-dependent effects and decrease variability in community similarity. This ‘case study section’ is therefore more detailed than the rest of this review chapter. Finally, we provide a summary of the major findings from the chapter. Our focus is on non-native seaweeds , but we also comment on non-native seagrasses (marine or estuarine angiosperms) and terrestrial halophytes (salt marsh and mangrove angiosperms), since they interact with invasive seaweeds in intertidal or brackish habitats (e.g. Thomsen et al. 2012; Williams and Grosholz 2008).
We use the term ‘non-native’ (=alien, non-indigenous, exotic, introduced) to define species that live outside their native distributional range. We include species that have dispersed by their own means through man-made canals (e.g. Lessepsian migration where non-native species swim or drift through the Suez Canal) but not new species that arrived by their own dispersal across natural barriers, and only survive due to climate changes . We characterise non-native species that are ‘highly successful’ (e.g. spread rapidly and establish abundant populations) or have a ‘strong impact’ on resident populations or ecosystems as ‘invasive’, but acknowledge the ambiguities and lack of a clear definition for when or where non-native species are invasive or not (indeed, it is more appropriate to rank non-natives according to their ‘relative invasiveness’ within the success and impact criteria). We define cryptogenic species as species that are of uncertain origin: for these species, detailed taxonomic, biogeographical and molecular analyses are required to determine whether they are non-natives. Throughout this chapter, we describe non-native and invasive ‘species’, but we assert that data are always collected from specific populations (genotypes) that represent a limited subset of a species’ gene pool. Researchers should, of course, be cautious when success and impact of a non-native genotype is predicted from a study conducted on a different genotype.
2 Research on and Numbers of Non-native Seaweeds
Invasions by marine primary producers have been described by scientists for a long time. For example, the Danish oceanographer Carl Emil Hansen Ostenfeld described the invasion of the planktonic diatom Biddulphia ( Odontella) sinensis Greville into the North Sea in 1903, probably transported by ships (Ostenfeld 1908). Over 50 years ago, Charles Elton, in his seminal book on biological invasions, described dramatic ecosystem changes associated with Spartina von Schreber invasions, converting marine intertidal mudflats to salt marshes on massive scales (Elton 1958). Elton even applied invasion patterns to support the notion that ‘Falkenbergia rufolanosa’ was the tetrasporophytic phase of Asparagopsis armata Harvey, because these two seaweed morphologies showed simultaneous spread into the north-east Atlantic and Mediterranean Sea in the 1920s–1950s (originating from south-western Australia). However, research on seaweed invasions did not take off until the 1990s. A ‘topic’ search in the Web of Science using classic invasion terminology [TS = ((invasion* or exotic* or non-native* or alien*) and (seaweed* or macro-alga* or macroalga*))] revealed that the first scientific paper on the topic was published in 1973 (Wassman and Ramus 1973) followed by 2 papers in the 1980s (Russell 1981; Dale 1982), 37 from 1990 to 1999, 210 from 2000 to 2009 and 235 papers from 2010 to 2015. This search does not include all relevant references—and includes a few less relevant studies—but nevertheless documents that few studies predated the 1990s. The growing number of primary research studies on seaweed invasions has been reviewed in some detail (e.g. Thomsen et al. 2009b; Williams and Smith 2007; Schaffelke et al. 2006; Inderjit et al. 2006; Johnson and Chapman 2007). The most comprehensive of these reviews was by Williams and Smith (2007), describing 277 seaweed taxa introduced around the world including a few unidentified taxa within particular genera (e.g. Bryopsis J.V. Lamouroux., Ulva Linnaeus subspecies (e.g. Codium fragile ssp. scandinavicum P.C. Silva and tomentosoides (van Goor) P.C. Silva) and taxa of cryptogenic origin (including a few that probably are natives, e.g. Alaria esculenta (L.) Greville and Saccharina ochotensis (Miyabe) C.E. Lane, C. Mayes, Druehl and G.W. Saunders that are likely natives in Denmark).
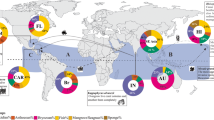
Williams and Smith’s (2007) tally is almost a decade old and we considered it timely to conduct a bibliographic survey to update their list. From this survey, we identified 69 taxa not included on the list (Table 6.1) expanding the number of non-native and cryptogenic seaweed taxa globally to 346. This list includes several cryptogenic taxa that future studies may reclassify as native, but we suggest that the 346 taxa still represent a conservative estimate because (1) our search may have missed studies, (2) seaweeds may have been introduced to new regions prior to modern science, (3) morphologically similar non-native and native species may co-occur and remain taxonomically cryptic , (4) under-studied regions can include non-native taxa that are (5) poorly described, (6) difficult to identify, (7) rare or (8) have invaded inaccessible areas and habitats. The 346 seaweeds are represented by 61 Chlorophycea, 77 Phaeophyceae and 208 Rhodophyceae derived from a global species pool of ca. 2400 Chlorophycea, 1800 Phaeophyceae and 6300 Rhodophyceae. The seaweed genera with most invasive taxa were Polysiphonia Greville (15 taxa), Hypnea J.V. Lamouroux (13), Codium Stackhouse and Caulerpa J.V. Lamouroux (11 each) and Gracilaria Greville (10) and the most invaded bioregions were the Mediterranean sea (160 taxa), followed by NE Atlantic (93), oceanic islands (73) Australasia (66), NW Atlantic (34) and the NE Pacific (33) (Fig. 6.1).
a The most invasive genera, compiled from our list of 346 non-native and cryptogenic seaweeds. b Regions invaded by most different seaweed taxa [combining our bibliography with that of Williams and Smith (2007)]. The same taxa can invade multiple bioregions and the total number of cases in plot b therefore exceeds 346
Finally, we note that in addition to the 346 seaweeds, at least four seagrasses ( Zostera japonica J.V. Lamouroux, Zostera tasmanica Martens ex Ascherson, Halophila stipulacea (Forsskål) Ascherson, H. Halophila decipiens Ostenfeld), 6 mangroves ( Lumnitzera racemosa Wild, Sonneratia caseolaris (Linnaeus), Sonneratia apetala Buch-Ham, Bruguiera gymnorrhiza (L.) Lam., Bruguiera sexangula (Lour.) Poiret, Rhizophora mangle Linnaeus) and 10 salt marsh taxa ( Spartina alterniflora Loiseleur-Deslongchamps., S. anglica C.E. Hubb, S. patens (Ait.) Muhl, S. densiflora Brögelmann., S alterniflora × foliosa von Trinius., S. densiflora × foliosa , Phragmites australis (Cav.) von Trinius ex von Steudel., Elymus athericus (Link) Kerguélen, Cotula coronopifolia Linnaeus, Ipomoea cairica (L.) Sweet) have invaded coastlines around the world (Williams 2007; Fourqurean et al. 2010; Chen et al. 2009; Ren et al. 2009; Krauss and Allen 2003; Adam 1990).
3 How Non-native Seaweeds Get Around
Non-native seaweeds arrive into new regions through intentional or unintentional vectors. Intentional releases are few, perhaps <3 % of reported new incursions (Hewitt et al. 2007), and are mainly associated with aquaculture or, in rare instances, with scientific experimentation (Pickering et al. 2007). New intentional introductions could become even rarer in future because the public, managers, politicians and scientists have all become increasingly aware of the potential impacts that might occur from intentional introductions. However, increased demand for food and seaweed-related products in a growing global population could supersede environmental concerns and lead to more introductions. Furthermore, some scientists advocate ‘assisted translocation’ (=‘intentional introduction’ in invasion research terminology) of species to new regions, to counter range contractions and extinctions in a warmer future (for discussions for and against, see Seddon 2010; Ricciardi and Simberloff 2009; Sandler 2010). Other researchers may focus on preserving and building ‘ecosystem services’ (Barbier et al. 2011) and therefore also suggest introductions to add or replace functional traits that are particularly valuable to humans, such as saltmarsh plants, mangroves and oysters that have been introduced to reduce erosion, build land and protect hinterland coastlines.
Most non-native seaweeds are, however, introduced accidentally rather than deliberately. The most important accidental vectors that have facilitated seaweed spread are the aquarium trade as ornamental plants, as ‘blind passengers’ associated with the intentional introductions, in ballast water and sea chests, attached to ship hulls, and infrastructure breaking down natural barriers such as canal building. Introductions associated with aquarium trade are particularly infamous due the release of Caulerpa taxifolia (M. Vahl) C. Agardh into the Mediterranean Sea (Meinesz 1999). However, the aquarium trade has become increasingly regulated with black-listed species, quarantine arrangements and stricter importation rules in many places (Hewitt et al. 2007; Padilla and Williams 2004). Still, non-native species are continually found in both commercial and personal aquaria and consequently pose a future invasion risk (Odom and Walters 2014; Williams et al. 2013). More seaweeds species (>25) have travelled through the Suez Canal, mainly from the Red Sea into the Mediterranean Sea (Williams and Smith 2007). However, there are few analogous marine examples around the world, suggesting that canal building, so far, has been a geographically isolated vector. Still, the future expansion of the Panama Canal could cause a similar spread of non-native species between Atlantic and Pacific bioregions (Ruiz et al. 2009).
Another vector is associated with ballast waters and sea chests (i.e. in waters and sediments held inside ships). Williams and Smith estimated about 25 known seaweed introductions through this vector, and although this vector is considered one of the most important for marine organisms generally, it is of comparatively little importance for seaweeds because of the adverse conditions for photosynthetic macrophytes (dark, nutrient poor, low water movements, high sediment loads) and because most seaweed propagules have relatively short lifespans (but see Flagella et al. (2007, 2010) for contrasting examples). Instead, ballast water is a much more important vector for invertebrates with pelagic larval stages, for sediment-associated fauna, for filter-feeding animals and for species with dormant resistant life stages such as many species of phytoplankton (Davidson and Simkanin 2012; Ibrahim and El-naggar 2012).
By far the most important vectors for seaweed introductions are associated with aquaculture, in particular shellfish transplants , and as hull-fouling organisms. Shellfish transplants have been particularly important in the past, with more than 70 species (mostly corticated rhodophyta) reported to be introduced by this vector (Mineur et al. 2007; Williams and Smith 2007). Oyster transplantations are likely responsible for several high-profile invasions, including those of Sargassum muticum (Yendo) Fensholt (Rueness 1989), Gracilaria vermiculophylla (Ohmi) Papenfuss (Thomsen et al. 2006a) and C. fragile (Suringar) Hariot (Trowbridge 1998). However, methods now exist to kill epibiotic organisms, including seaweeds, while ensuring that the shellfish survive, for example by using short-term bleaching before seeding (Fitridge et al. 2012). Furthermore, increasing use of shellfish cultured in local, land-based facilities, could also lower the importance of this vector in future.
The final major vector is hull fouling , which is responsible for more than 70 seaweed introductions (Hewitt et al. 2007; Williams and Smith 2007; Mineur et al. 2008a). Hull fouling is a particularly important vector for filamentous species (Williams and Smith 2007), although larger species, including high-profile invaders such as C. fragile and Undaria pinnatifida (Harvey) Suringar likely also have been introduced to many places through hull fouling. Virtually, all seaweeds are capable of hull fouling but survival on ship hulls can be constrained by vessel speed, changes to environmental conditions throughout the voyage (such as changing salinity and temperature), low nutrient levels in offshore waters and the smooth surface of many hulls combined with toxic antifouling paints and coatings (Mineur et al. 2007). Increasing boat activity, both of commercial and recreational vessels, travelled over long or short distances, will likely continue to make this vector important in transporting seaweeds around the globe. Finally, we note that vectors for most non-native seaweeds are poorly described in the scientific literature and even for the better known case studies, described vectors are more like plausible guesses than based on rigorous tests and data (Williams and Smith 2007).
4 Success and Impacts of Non-native Seaweeds
After uptake, transport to and release into a new location, the success of a non-native seaweed is measured by how well it survives, grows and reproduces and whether it disperses and expands to nearby habitats, sites and regions. In these ‘invasion success’ studies, invader attributes are considered dependent variables, thereby contrasting with ‘invasion impact’ studies where invader attributes are independent variables. This distinction between success and impact is straightforward when interpreting data from manipulative studies but can be blurred for mensurative experiments where key attributes are beyond scientific control. For example, if mensurative data show a negative relationship between Caulerpa and seagrass abundances, this can occur because seagrass has a negative effect on Caulerpa (a success interpretation), because Caulerpa has a negative effect on seagrass (an impact interpretation), or because the relationship may be a spurious effect caused by a third unmeasured factor (Bulleri et al. 2010; Glasby 2012; Klein and Verlaque 2008; Ceccherelli and Campo 2002; Jaubert et al. 1999).
Success studies Success of an invasive seaweed depends on attributes related to the non-native seaweed and the invaded system (Catford et al. 2009). System attributes are typically subdivided into attributes related to abiotic conditions (e.g. salinity, temperature, hydrodynamic forces and desiccation), resources (e.g. space, light, nitrogen and phosphorous) and resident community structures (e.g. the identity and abundance of competitors, facilitators, grazers and other organisms). Many hypotheses have been proposed to explain the invasion success (or lack of) non-native species including seaweeds (Mitchell et al. 2006; Catford et al. 2009; Valentine et al. 2007; Alpert 2006). At least 19 ‘core’ hypotheses are commonly cited, which can be grouped into three clusters that highlight different aspects of invasion success. We use Catford et al.’s hypothesis nomenclature (cf. Table 3 in Catford et al. 2009) but excluded hypotheses that combined multiple core hypotheses (‘Darwin’s naturalisation’, ‘Global competition’, ‘resource-enemy release’, ‘indirect effects’, ‘opportunity windows’), that just rebranded other core hypotheses (‘adaptation’ , ‘enemy reduction’) or that predicted success and failure at the same time and therefore are difficult to test (‘enemy inversion’, ‘dynamic equilibrium model’, ‘new associations’). We also added ‘facilitation- and habitat-cascades’ to the list (not included in Catford et al.’s review), because non-native seaweeds often are key players in these ecological interactions (Thomsen et al. 2010).
The first hypothesis cluster proposes that invasion success is determined by attributes associated with the non-native seaweed. This cluster includes the ‘ideal weed’ (Baker and Stebbens 1965), ‘propagule pressure’ (Lockwood et al. 2009) and ‘sampling’ (Crawley et al. 1999) hypotheses. The ideal weed hypothesis suggests that certain traits are universally important to successful invaders, particularly r-selected traits such as fast growth, high reproductive output and high dispersal capacity. However, there are few consistent patterns for the most successful invasive seaweeds, except perhaps for U. pinnatifida which has many weedy traits (Valentine et al. 2007). By comparison, propagule pressure and sampling hypotheses are ‘numbers game’ hypotheses that suggest that the more propagules (propagule pressure hypothesis, species traits are less important) or the more different species (sampling hypothesis, species traits are more important) that are introduced to a new region, the greater the chance that one of these propagules will establish a self-sustaining population (Britton‐Simmons and Abbott 2008; Vaz-Pinto et al. 2012).
The second cluster emphasises that attributes associated with the invaded system, such as resource levels and local abiotic conditions, can explain invasion success. These core hypotheses highlight that high ‘fluctuating resource availability’ (Davis et al. 2000), high ‘environmental heterogeneity’ (Melbourne et al. 2007), medium-to-high ‘disturbances’ (Sher and Hyatt 1999), availability of ‘empty niches’ (Hierro et al. 2005) and low ‘habitat filtering’ (Procheş et al. 2008) all increase the likelihood that non-native species can establish populations following arrival. These hypotheses suggest that almost any non-native seaweed can be successful in systems that have a plethora of microhabitats, are heterogeneous , are frequently disturbed and have unused resources in space and time. Several of these processes have been suggested to be important for high-profile seaweed invaders including U. pinnatifida, S. muticum and Caulerpa racemosa (Forsskål) J. Agardh (Valentine et al. 2007; Incera et al. 2010; Olabarria and Arenas 2014).
The final cluster emphasises that biological interactions, in particular lack of co-evolution with local species, can explain success of non-native species. In this cluster, ‘novel weapons’ (Callaway and Ridenour 2004), ‘enemy release’ (Keane and Crawley 2002), ‘evolution of increased competitive ability’ (Blossey and Notzold 1995), ‘enemy of my enemy’ (Eppinga et al. 2006), ‘limited similarity’ (Emery 2007), ‘specialist generalist’ (Sax et al. 2007), ‘invasional meltdown’ (Simberloff and Von Holle 1999) and ‘cascading habitat formation’ (Thomsen et al. 2010) highlight how non-native species become invasive, whereas ‘biotic resistance’ (Elton 1958), ‘missed mutualisms’ (Mitchell et al. 2006) and ‘increased susceptibility’ (Colautti et al. 2004) highlight why non-native species can fail to become invasive.
We are not aware of any research that has compared the relative merits of all these competing and overlapping core hypotheses for non-native seaweeds . However, we speculate that hypotheses assuming that seaweeds are limited by tight co-evolutionary processes at their place of origin (and lack of co-evolution at the new place) are less important for seaweeds, because seaweed–animal interactions are, compared to terrestrial systems, dominated by generalist-type interactions (Bell 1991; Enge et al. 2013; Hay and Steinberg 1992). Importantly, we hope future studies will test for interactions between multiple core hypotheses because we expect that many mechanisms exert selection pressure simultaneously and because mechanisms likely change during the life cycle of non-native seaweeds (Britton-Simmons 2006). For example, U. pinnatifida is well adapted to attach to ship hulls and spread in large numbers (~propagule pressure hypothesis) (Hay 1990) but has also many weedy traits, like fast growth and high reproductive output (~ideal weed hypothesis) (Dean and Hurd 2007; Schiel and Thompson 2012). U. pinnatifida is also successful in settling onto unoccupied microhabitat space and on biogenic substrata (~environmental heterogeneity hypothesis) (Schiel and Thompson 2012; Thompson and Schiel 2012), often becomes dominant following disturbances (~disturbance hypothesis) (Valentine and Johnson 2003, 2004) and is efficient in converting available resources into biomass (~fluctuating resource hypothesis) (Tait et al. 2015). Finally, U. pinnatifida is, of course, also constrained by physiological tolerance levels (~habitat-filtering hypothesis) (Morita et al. 2003; Peteiro and Sánchez 2012) even if Undaria colonises sites outside its native environmental range (James et al. 2014). In short, it is important that future studies test multiple core hypotheses simultaneously, rather than a single hypothesis at a time (Britton-Simmons 2006; Olabarria and Arenas 2014; Valentine et al. 2007; Williams and Smith 2007).
Impact studies In invasion impact studies, the non-native species is considered the causal agent of ecological change. Impact is therefore a synonym for ‘effect’, ‘consequence’ or ‘cause’. Impact studies focus on how invaders affect a particular property of a resident system, and the impact on this property can then be larger or smaller than a reference value (often the non-impacted reference value is defined as zero). Impact can therefore be positive (>0), neutral (0) or negative (<0), which is a statistical measure that differs from the anthropocentric value judgement of whether an impact is ‘good’ or ‘bad’. Impacts can be reported on cultural (economics, health, societal) or natural (biotic, abiotic) properties. Biotic properties can be divided into impacts reported on or above the species level; importantly, impacts reported above the species level can hide opposing effects if some species benefit and others are harmed. Invasion impacts, like successes, also depend on attributes of the non-native seaweeds and attributes associated with the biotic community, resource levels and abiotic conditions of the invaded system (Thomsen et al. 2011a). A highly cited impact formula suggests that the impact from a non-native seaweed is proportional to its abundance, its range (distribution) and its per capita effect (Parker et al. 1999), i.e. what it does to targeted response variables (in the context of its abundance and distribution). This framework highlights that processes which determine invasion success (cf. core hypotheses outlined in the previous section) also modify impact, partly by controlling the distribution and abundance of the non-native seaweed.
5 Common Types of Ecological Impacts
The impacts of invasive seaweeds have been reviewed in some detail (Williams 2007; Schaffelke and Hewitt 2007; Thomsen et al. 2009b; Williams and Smith 2007), concluding that impacts have been quantified from a fraction (<10 %) of known taxa, that impacts on native species can be strong and that underlying impact mechanisms are poorly known. Each of the 346 non-native and cryptogenic seaweeds have some level of impact on the invaded communities, contributing their biomass and adding new genotypes to the local species pools, modifying geochemical cycles through metabolic activities and by affecting other species in the community through ecological processes. However, impacts can be subtle or difficult to quantify, particularly if the non-native species is cryptic , if interactions with native species are few and weak, or, if strong interactions only occur in small areas, in short time windows, or in inaccessible habitats. Below we provide examples of ecological impacts grouped as ‘negative’ or ‘positive’, occurring through direct or indirect interactions (in indirect effects, an intermediate species is required for the impact to manifest).
Negative effects Many negative effects by non-native seaweeds occur through competition and habitat destruction (direct effects) or keystone competition and keystone habitat destruction (indirect effects). For example, non-native canopy-forming seaweeds, like S. muticum , U. pinnatifida and C. fragile sometimes have strong competitive effects on native seaweeds, competing for limiting resources such as nutrients, light and space (Staehr et al. 2000; Ambrose and Nelson 1982; Britton-Simmons 2004; Schmidt and Scheibling 2006; Levin et al. 2002; Casas et al. 2004). However, these negative effects appear to be weaker in intertidal areas (Sánchez and Fernández 2005; Forrest and Taylor 2003; South et al. 2015; Olabarria et al. 2009), perhaps because desiccation and super-saturation of light are strong stressors here. Furthermore, some of these canopy-forming non-native seaweeds shed much of their thalli (S. muticum and C. fragile) or the entire thallus (U. pinnatifida) (Schiel and Thompson 2012; Wernberg et al. 2001), and effects associated with a canopy cover therefore could only occur seasonally (South et al. 2015). Similar mechanisms have been observed in soft-bottom systems where invasive Caulerpa species can compete with native seagrasses (Ceccherelli and Cinelli 1997; de Villele and Verlaque 1995), although other studies suggest that competition from Caulerpa is relatively small (Thomsen et al. 2012; Jaubert et al. 1999, 2003; Ceccherelli and Sechi 2002). Non-native seaweeds can also have negative effects on native organisms through modification or destruction of habitats, for example if they convert non-vegetated mud or sand habitats to vegetated meadows. Well-documented examples include G. vermiculophylla (Byers et al. 2012; Thomsen et al. 2007, 2010), Caulerpa species (Gribben and Wright 2006; Wright and Gribben 2008; Gribben et al. 2009b; McKinnon et al. 2009; Byers et al. 2010; Pacciardi et al. 2011), S. muticum (Strong et al. 2006), and invasive seagrasses (Willette and Ambrose 2009, 2012; Baldwin and Lovvorn 1994; Posey 1988; Berkenbusch et al. 2007; Ruesink et al. 2010) and salt marsh plants and mangroves (Demopoulos and Smith 2010; Thomsen et al. 2009a; Wu et al. 2009; Neira et al. 2007). In these examples, local species that depend on sand and mud, such as many infaunal species and burrowing fish, may be negatively affected by the non-native macrophytes (Wright et al. 2007; Gribben et al. 2009b; Tsai et al. 2010), although in some cases, these organisms can also survive under, around or on the macrophytes (Gribben and Wright 2006; Wright and Gribben 2008; Gribben et al. 2009b; McKinnon et al. 2009; Byers et al. 2010; Klein and Verlaque 2011).
Indirect negative effects from invasive seaweeds can occur through keystone competition where an invader reduces a resource that is important for other species within that community. For example, syngnathid and monacanthid fish were more abundant in native seagrass compared to invasive Caulerpa meadows (York et al. 2006), juvenile fish were more abundant in native seagrass beds than in the invasive seagrass H. stipulacea (Willette and Ambrose 2012) and gastropods and echinoderms were more abundant on native kelp compared to invasive C. fragile (Schmidt and Scheibling 2006). These fish, gastropods and echinoderms were probably less abundant around the non-native macrophytes (than around the native macrophytes), because these non-native species provide low-quality foraging grounds and poor protection against predators. In short, if these non-native macrophytes do indeed reduce the abundance of native seagrass and kelps, then they will have indirect negative effects on the same invertebrates and fish (=keystone competition).
Positive effects Invasive seaweeds can also have positive effects on local populations, directly through habitat formation and modification, and by being consumed by grazers, or indirectly through cascading habitat formation, consumption, competition or keystone consumption .
For example, seaweeds invading un-vegetated sediments provide habitat for epibenthic fauna (Thomsen and Wernberg 2015) that live among or on seaweeds, including C. racemosa (Klein and Verlaque 2011), C. Taxifolia (M. Vahl) C. Agardh (McKinnon et al. 2009) and G. vermiculophylla (Byers et al. 2012; Thomsen et al. 2010; Johnston and Lipcius 2012). Similar positive effects on fauna have also been shown for invasive seagrasses such as H. decipiens (Willette and Ambrose 2012) and Z. japonica Ascherson and Graebner in Engler (Berkenbusch and Rowden 2007; Berkenbusch et al. 2007; Posey 1988) when and where they colonise un-vegetated sediments. Many invasive seaweeds also add structure, biomass and productivity to vegetated rocky coasts (Thomsen et al. 2015). For example, the canopy-forming S. muticum and C. fragile provide additional habitat for epiphytic plants and invertebrates (Thomsen et al. 2006b; Jones and Thornber 2010; Schmidt and Scheibling 2006, 2007; Wernberg et al. 2004). Invasive seaweeds not only provide habitat, but can also be a food source, and indirectly fuel higher order consumers through trophic cascades. For example, juvenile invasive seaweeds (and seagrass) can provide a seasonal food supply (Sjotun et al. 2007; Thornber et al. 2004; Reynolds et al. 2012): Littorina Férussac snails consume C. fragile in tide pools (Scheibling et al. 2008), waterfowl graze invasive Z. japonica (Baldwin and Lovvorn 1994), and siphonalian seaweeds ( Codium and Caulerpa spp.) are likely to have increased food availability for specialist saccoglossan grazers (Harris and Jones 2005; Trowbridge 2002; Trowbridge and Todd 2001). Still, most invasive seaweeds are not considered preferred food for native larger generalist grazers and smaller meso-grazers and many produce grazer-deterring secondary metabolites, as shown for S. muticum (Britton-Simmons 2004; Engelen et al. 2011; Monteiro et al. 2009; Pedersen et al. 2005), C. fragile (Scheibling and Anthony 2001), Caulerpa species (Gollan and Wright 2006; Boudouresque et al. 1996), G. vermiculophylla (Nejrup and Pedersen 2010; Thomsen and McGlathery 2007; Nejrup et al. 2012; Rempt et al. 2012; Nylund et al. 2011) and Lophocladia lallemandii (Montagne) F. Schmitz and Womersleyella setacea (Hollenberg) R.E. Norris (Cebrian et al. 2011; Tomas et al. 2011a, b).
Invasive marine plants can also have positive indirect effects, for example through cascading habitat formation (Thomsen et al. 2010): invasive S. muticum , C. fragile and G. vermiculophylla provide primary habitat for sessile plants and animals, such as filamentous seaweeds and ascidians (Nyberg et al. 2009; Thomsen et al. 2006b, 2010; Engelen et al. 2013; Gestoso et al. 2012; Jones and Thornber 2010; Schmidt and Scheibling 2006, 2007; Wernberg et al. 2004). These epiphytic macro-organisms are likely to subsequently provide a secondary habitat for many other smaller organisms, such as hydrozoa, bryozoa and small mobile invertebrates. Invasive plants can also have indirect positive effects through cascading habitat modification. For example, C. taxifolia can reduce sediment redox potential, altering abiotic conditions and forcing infaunal bivalves to live at the sediment surface where they are exposed to sessile fouling organisms (Gribben et al. 2009a). Finally, positive effects of seaweed invasions can also be mediated through cascading consumption (trophic cascades), such as when invasive C. fragile is consumed by (invasive) Littorina snails (Scheibling et al. 2008), which are consumed by (invasive) crabs (Eastwood et al. 2007; Trussell et al. 2002, 2004), which are consumed by native crabs, seabirds and fish (de Rivera et al. 2005).
6 Impacts Reviewed Across Studies, Seaweeds and Habitats
The many case studies about impacts from invasive seaweeds (cf. last section) have stimulated quantitative meta-analyses that aim to identify impact generalities across different non-native seaweed species, invaded communities and environmental conditions. In these studies, documented effects are standardised, typically with the Hedges d effect size or a log-response ratio (Borenstein et al. 2009), so that effects can be compared between studies. Here, we summarise the main finding from meta-analyses that have included non-native seaweeds as a test factor.
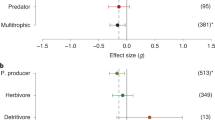
The first meta-analysis of seaweed invasion impacts reviewed field-based impact experiments and showed that invasive seaweeds generally had significant negative effects on local plant abundances, richness and diversity (Thomsen et al. 2009b). By contrast, there were no significant effects on seaweed ‘processes’ (e.g. growth, photosynthesis, respiration) or the abundance, richness and diversity of animal communities. However, the latter analysis was based on a small sample size and had large confidence limits. A follow-up analysis confirmed that non-native seaweeds, across the reviewed studies, have negative effects on diversity of native seaweeds (Thomsen et al. 2014). This study also tested whether trophic and functional ‘matching’ (i.e. pairing the trophic level or function of the invader and the impacted organism) could provide first-order predictions about impacts on diversity metrics. It was hypothesised that the net effect from non-native seaweeds on diversity would be negative within a trophic level but zero (or even positive) across trophic levels, based on the assumption that competition processes would dominate in the community within trophic levels (in plant–plant interactions) and habitat formation, habitat-modification and food-provisioning processes would dominate across trophic levels (in plant–animal interactions). The hypothesis was confirmed, showing negative effects of non-native seaweeds on the diversity of local seaweed communities, but with positive effects on local animal communities. Furthermore, a recent analysis reconfirmed that non-native seaweeds generally have negative effects on species within the same trophic levels (Maggi et al. 2015), although this study found no effects on species at higher trophic levels (effect size on animals was not statistically different from zero). The discrepancies between these meta-analyses (positive vs. no effect on animal communities) were likely due to there being different study-inclusion criteria. Maggi et al. (2015) included, in contrast to Thomsen et al. (2014), mensurative experiments, laboratory experiments and litter-bag experiments where the invader was dead and decomposing (Rodil et al. 2008; Taylor et al. 2010). These two analyses therefore highlight that readers should be careful when extrapolating meta-analytical results beyond the domain of reviewed primary studies.
Three other meta-analyses have examined aspects of seaweed invasions. An analysis of seaweed impacts on seagrasses , measured in field and laboratory experiments, showed that non-native seaweeds have a less negative impact than native seaweeds (Thomsen et al. 2012). However, this analysis was confounded by the taxonomic status and attachment type of the non-native seaweeds, which were dominated by studies on Caulerpa species, a special group of clonal seaweeds attached with rhizomes to sediments. These types of non-native seaweeds have few documented cases of negative effects on seagrasses compared to many native seaweed taxa that are found in seagrass beds entangled around stems or epiphytic on leaves.
More recently, Tamburello et al. (2015) used a subset of the data analysed in Maggi et al. (2015), to test whether impacts co-vary with Halpern’s (2008) ‘global cumulative human impact index’. In other words, they tested whether seaweed invasion impacts increase or decrease with impacts from human activities (Halpern’s index merges a range of human activities including pollution, fisheries, climate changes and eutrophication ). This analysis showed that impacts from non-native seaweeds on community biomass and abundance become less negative or even neutral when moving from relatively pristine to heavily impacted environments (but the opposite trend was found on community ‘evenness’). Thus, invasion impact appears to co-vary with other human stressors, being greater in stressed systems (presumably with lower resilience). Finally, Thomsen et al. (2015) conducted a meta-analysis to test whether invasion impacts on local plant communities differ when the invader itself was included as part of the total community. This analysis showed again that seaweed invaders have significant negative impacts on plant abundance and plant community richness, but also that these negative effects were cancelled out when the taxonomic status and abundance of the invader were included in the calculations of total community biomass and richness (i.e. the effect size was no longer significantly different from zero). Thus, non-native seaweeds appear, across species and invaded systems, to substitute, rather than increase or decrease, standing biomass and richness.
These meta-analyses all suggested that more impact studies are needed, especially on functional community responses, such as total system productivity, respiration, decomposition rates and nutrient uptake (Altieri et al. 2009; Green et al. 2012, 2013; Tait et al. 2015; Cacabelos et al. 2012; South et al. 2015). Furthermore, although the impact literature is extensive, the vast majority of case studies have tested for impacts of six high-profile invaders (S. muticum, U. pinnatifida , C. taxifolia , C. racemosa, C. fragile and G. vermiculophylla) likely biasing tests towards detecting strong impacts. It is critical that comprehensive impact analyses are conducted on more of the non-native seaweeds that have colonised coastlines around the world, to allow for better generalisations about seaweed invasions across habitats, bioregions and species assemblages.
7 New Meta-analysis; Impact Is Density-dependent and Non-native Seaweeds Affect Community Similarity
Two key aspects related to seaweed invasion impacts have not yet been tested with meta-analyses: density dependency and impacts on community structure measured by multivariate metrics. Below we address these research gaps.
7.1 New Meta-analysis 1: Density-dependent Effects
We tested whether impacts from non-native seaweeds are density dependent (density is defined loosely to include any abundance metric such as counts, length, coverage, biomass or volume); i.e. whether effects vary with the amount of an invasive seaweed found in a plot, site and region. We identified peer-reviewed papers describing manipulative field experiments in which invader abundance was controlled using addition or removal techniques with replicated treatments and controls. We included only experiments reporting impacts from at least three invasion densities (one of which could be a non-invaded control) so that paired effects within a study could be contrasted between low and high density treatments (Table 6.2).
We used Hedges effect size d, corrected for small sample sizes, to standardise effects between treatments, allowing us to include zero-value responses (Borenstein et al. 2009). An all-inclusive unbiased data selection criterion (Englund et al. 1999) was used to extract effect sizes for each paper, calculating d-values for all reported impacted resident organisms, test combinations (e.g. different depth levels Thomsen 2010) and quantified responses also on the same resident organism, such as seagrass leaf length, above ground biomass and below ground biomass (Drouin et al. 2012). One study presented data as x–y points on a graph of abundance of the seaweed versus fauna (because applied densities changed over time) (Byers et al. 2012). We extracted these x–y data and reclassified data into low (control), medium and high seaweed densities. To avoid problems associated with temporal autocorrelation, we included only the last data points from repeated measure experiments (Parker et al. 2006). Following the calculations of Hedges d between controls and invaded treatments, we calculated a ‘∆d’ for each paired response (responses are paired because the same un-invaded control data are used to calculate d for both the low and high density treatments) (Thomsen et al. 2011b). If more than three invasion densities were tested, we contrasted only the effects between the lowest and highest densities. Nested and orthogonal experiments within research papers were treated as independent studies. Two tests examined whether effect sizes depended on the density of the non-native seaweeds . In the first, we examined whether paired d-values were different, using the formula ∆d = ∣d high − d low∣. In the second, more conservative, test, we examined whether the high density d was numerically larger than its paired low density d, using the formula ∆d = ∣d high∣ − ∣d low∣. Analysing numerical ∆d-values is necessary to ensure that density-dependent facilitation is not cancelled out by density-dependent inhibition. Non-independent ∆d-values from a study were averaged to produce independent ∆d-values, using equal weight for each reported type of impact. Finally, un-weighted fixed effects analyses were made on the independent ∆d-values in Metawin 2.1, to calculate ∆d cumulative and 95 % bias-corrected confidence limits (CL) with 999 permutations (Rosenberg et al. 2000). ∆d cumulative was interpreted to be significantly different from zero if the 95 % CL did not overlap zero.
In the first test, overall heterogeneity of effect sizes was small (Q total = 1.49, df = 9, p = 0.99), indicating that effect sizes share a common value. The overall ∆d cumulative was 0.61 and the 95 % bias-corrected confidence limits did not bracket zero (0.40–0.84) indicating that d high was indeed different from d low. In the second test, overall heterogeneity of effect sizes was again small (Q total = 1.29, df = 9, p = 0.99). This ∆d cumulative was slightly lower (0.52) and the 95 % bias-corrected confidence limits again did not bracket zero (0.31–0.74) highlighting that d high had a larger magnitude than d low. We conclude, therefore, based on the few studies that have tested for density-dependent effects, that seaweed invasion impacts, as a general rule, are density dependent. However, more studies are needed to enable specific analyses, for example, to identify non-linear density dependency, changes from positive to negative effect sizes, to locate thresholds and to make regression models that can predict which non-native seaweeds have particular strong density-dependent impacts. This analysis also highlights that all seaweed invasion impact studies should report the abundance of the studied invader.
7.2 New Meta-analysis 2: Effects on Community Structures
Non-native seaweeds have strong effects on population abundances, growth, survival and community diversity and richness (all univariate metrics, see previous sections). However, non-native seaweeds can also affect multivariate community structure, often assessed through multivariate analysis of variance (Manova, Permanova) and multivariate regressions , and visualised on dimensional reduction methods such as principal coordinates analysis (PCA , PCO) or multidimensional scaling (MDS) (Viejo 1999; Staehr et al. 2000; Piazzi and Balata 2008). These methods typically test whether multivariate ‘centroids’ or ‘dispersion’ differ between invaded and non-invaded communities. The ‘centroid-analysis’ is non-directional in that it tests whether the ‘mean’ invaded community is different from the ‘mean’ non-invaded community. However, the ‘dispersion-analysis’ is directional, with several well-known examples suggesting that invaded communities are less dispersed in multivariate space (i.e. are more ‘homogenous’) than non-invaded communities (Staehr et al. 2000; Piazzi and Balata 2008). Unfortunately, invasion studies do not usually publish the entire species-sample data matrix or the associated sample similarity matrix from which multivariate dispersions can be calculated. Instead, multivariate invasion impact is typically visualised with 2D plots, where each x–y point represents a sample that can contain many species. The spatial distances between these sample points correlate with how similar the communities are, and invaded samples will be clustered compared to non-invaded samples if the invader increases community similarity in space (e.g. Fig. 2, Piazzi and Balata 2008) or over time (e.g. Fig. 5b, Staehr et al. 2000).
Here, we develop a method to test whether non-native seaweeds increase sample similarity across studies, by extracting x–y coordinates from published MDS plots. From these coordinates, sample matrices can be reconstructed, composed of two universal ‘pseudo-species’ from which the (imperfect) sample similarity matrices can be calculated. Reconstructed similarity matrices were then used to calculate Multivariate Dispersion Index values to test whether invasive seaweeds , across studies, increased between-sample similarities. These ‘MDI’ values have a minimum of −1 (dissimilarities among invaded samples are lower than any dissimilarities among non-invaded samples = non-native seaweeds ‘homogenise’ local communities) and a maximum of 1 (the opposite case = non-native seaweeds make local communities more heterogeneous (Warwick and Clarke 1993).
We located peer-reviewed studies that compared samples from invaded and non-invaded communities in 2D MDS plots (Table 6.2) by searching Google Scholar and by back-tracking references in past reviews (Williams 2007; Thomsen et al. 2009b, 2011b, 2014; Schaffelke and Hewitt 2007; Maggi et al. 2015; Williams and Smith 2007). We included a few studies that did not show MDS plots for which we had access to raw data and therefore could calculate the sample similarity matrices (using common methods; Bray–Curtis similarity coefficient, square root transformed data and excluding the abundance of the invasive species) (Thomsen et al. 2010; Thomsen and McGlathery 2006; South et al. 2015). MDS plots were imported into TechDig and rescaled from 0 to 1 for the longest axis. Each sample was classified as invaded or non-invaded and its x–y coordinates extracted. A few samples overlapped on the plots, making it difficult to extract all samples (but >90 % of all samples were extracted from each plot). Extracted x–y coordinates represent the relative abundance of two ‘universal pseudo-species’ in the invaded versus non-invaded samples. Paired similarity matrices between the invaded and non-invaded samples were reconstructed from the two-species communities using Euclidean distances for untransformed data. This method provides a perfect spatial fit between the abundance of the pseudo-species and the published MDS plots they were derived from (see Fig. 6.2 for examples). For each plot, we also extracted data to test whether MDI values depended on whether (1) the impacted community was composed of plants, animals or both (Thomsen et al. 2014), (2) the impacted community was composed of sessile, mobile organisms or both (Thomsen et al. 2014), (3) the field method was based on a manipulative or mensurative experiment (Maggi et al. 2015) and (4) analysis included (Staehr et al. 2000; Klein and Verlaque 2011) or excluded (Balata et al. 2004) the abundance of the invader in the similarity matrix. We finally calculated a MDI value for each reconstructed paired invaded versus non-invaded similarity matrix (Warwick and Clarke 1993).
Relative abundance of universal ‘pseudo-species’ 1 and 2 in invaded (black squares) and non-invaded (white circles) samples, reconstructed from two MDS plots; Fig. 5 in Staehr et al. (2000) and Fig. 2 in Piazzi and Balata (2008). The MDS plots were imported into TechDig and rescaled from 0 to 1 for the longest axis. Each sample was classified as invaded or non-invaded and its coordinates extracted (representing the relative abundance of universal ‘pseudo-species 1 and 2’). Similarity matrices were calculated for invaded and non-invaded samples using Euclidean distances based on the relative abundances of the two pseudo-species. Finally, MDI values were calculated for each set of paired similarity matrices (Warwick and Clarke 1993). The negative MDI values shown above each plot indicate invasion-driven ‘homogenisation’, i.e. invaded samples are spatially clustered both in time (a before vs. after invasion) and space (b at 5 m depth, c at 25 m depth)
Meta-analytical methods followed the previous density analysis; factorial and nested experiments were treated as independent data, as were effects from the same invasive species reported in different studies. Only the last data point was used from repeated measure data and only effects that compared the highest invader density to non-invaded samples were used from multidensity experiments. Invasion effects reported on different communities within a single study (e.g. on endobenthic and epibenthic communities, Lang and Buschbaum 2010) were also treated as independent effects. Cumulative effect sizes (MDIcummulative) with 95 % bias-corrected CL were calculated in Metawin 2.1 with 999 permutations (Rosenberg et al. 2000). MDIcummulative was interpreted to be significantly different from zero or another MDIcummulative if the 95 % CL did not overlap zero or each other, respectively.
We found no significant effects, i.e. treatments were not significantly different from zero or each other (Fig. 6.3a–d). Still, there were several interesting (non-significant) trends, indicating that effect sizes were larger and more negative within rather than between trophic or functional groups (Fig. 6.3a, b). Perhaps with more studies, and smaller confidence limits, it will become clearer whether non-native seaweeds , as a general rule, homogenise ‘similar’ invaded plant communities but not ‘different’ invaded animal communities. Our results also highlight that conclusions based on a few well-cited studies (Staehr et al. 2000; Piazzi and Balata 2008) should only cautiously be interpreted out of the context of the methods, invasive species, invaded community and surrounding abiotic environment. We expect analogous multivariate directional tests in future will have stronger predictive power, when (1) more studies report impacts on different types of communities, (2) more test factors and levels within factors are included in tests (e.g. on form-groups such as epiphytes , canopies and understory communities) and (3) tests also include interactions between factors (e.g. between form-groups and field and data collection methods). Finally, future studies should also test how robust our ‘dispersion meta-analysis’ is by modelling how much dispersion values can vary in direction and magnitude depending on MDS stress values, chosen dispersion index (e.g. Permdisp vs. MDI), plot type (e.g. PCO vs. MDS), distance metric (e.g. Bray–Curtis vs. Gower), measurements variables (e.g. cover vs. biomass data) and transformation methods (e.g. untransformed vs. log-transformed).
Meta-analysis of 243 MDI values extracted from MDS plots that contrast invaded and non-invaded community samples. Negative values correspond to samples being more similar (‘homogenisation’) and numbers in brackets correspond to replication levels. Impacts of invasive seaweeds were evaluated on (a) plant (Pla), animal (Ani) or combined (Pla + Ani) communities, (b) sessile (Ses), mobile (Mob) or combined (Ses + Mob) communities, (c) communities measured in mensurative (Mens) or manipulative (Manip) field experiments and (d) communities where the invader itself was included or excluded. For plot d, MDI values were calculated for 5 types of reported similarity matrices; (1) excluding the invader (Excluded), (2) including the invader’s new recruits (Rec), (3) including both the new recruits and the abundance of the invader itself (Rec + Adult), (4) studies where it could not be determined (Unknown) and (5) irrelevant studies (Not Rel), i.e. where the invaded community is different from the invasive species (here animal communities)
8 Summary
We conducted a bibliographic survey, adding 69 taxa to a published list of 277 seaweeds, thereby updating the total worldwide list of non-native and cryptogenic seaweeds to 346. None of these new non-native seaweeds have received much scientific scrutiny, but several may over time become abundant in invaded regions. The seaweed genera with most non-native taxa are Polysiphonia , Hypnea , Codium , Gracilaria and Caulerpa . Bioregions that have received most non-native seaweeds are the Mediterranean Sea, followed by NE Atlantic, Australasia, NE Pacific and the NW Atlantic. Our tally is most likely an underestimate because (1) seaweeds may have been introduced to new regions prior to modern science, (2) morphologically similar non-native and native species may co-occur and remain taxonomically cryptic, (3) under-studied regions can include non-native taxa that are (4) poorly described, (5) difficult to identify, (6) rare or (7) have invaded inaccessible areas and habitats. The most important vectors that carry seaweeds around the world are hull fouling and as ‘blind passengers’ associated with aquaculture (in particular seaweeds attached to shell transplants), together accounting for >75 % of known seaweed introductions . These vectors are likely to continue to spread seaweeds around the world.
Once a non-native seaweed has arrived in a new location, it can establish a permanent population, spread (secondarily) through natural dispersal or associated with human vectors, increase in abundance and affect local species and ecosystem properties. Establishment, increase in abundances and secondary spread is quantified in ‘success studies’, whereas the effects on the invaded communities are quantified in ‘impact studies’. The success of non-native seaweeds depends on attributes of the non-native seaweeds and attributes associated with the invaded system, including the biotic community, resource levels and abiotic conditions. Many ‘core hypotheses’ have been suggested to explain why some non-native species (seaweeds included) become invasive or fail, of which the ‘ideal weed’, ‘propagule pressure’, ‘sampling’, ‘fluctuating resource availability’, ‘environmental heterogeneity’ , ‘disturbances’, ‘empty niches ‘, ‘habitat filtering’, ‘limited similarity’ and ‘specialist-generalist matching’ are important to understand. Impact framework also suggests the direction and magnitude of impacts depends on attributes of the non-native seaweeds and attributes associated with the biotic community, resource levels and abiotic conditions of the invaded system. Thus, any process that increases success (cf. core hypotheses outlined above) should modify impact, by controlling the distribution and abundance of the non-native seaweed. Impacts can, like success, be difficult to quantify, if the non-native seaweed is inconspicuous or difficult to identify or find, if interactions with native species are few and weak, or if strong interactions occur only in small areas, short time windows and in inaccessible habitats. Case studies have shown that many negative effects by non-native seaweeds occur though competition and habitat destruction (direct effects) or keystone competition and keystone habitat destruction (indirect effects). However, invasive seaweeds can also have positive effects on local populations, directly through habitat formation and modification and by being consumed, or indirectly through cascading habitat formation, consumption, competition or keystone consumption .
Case studies documenting seaweed invasion impacts have stimulated meta-analytical synthesis that aims to identify impact generalities across different invasive seaweeds, invaded communities and environmental conditions. Meta-analyses have shown that invasive seaweeds typically have a negative effect on local plant abundances, richness and diversity but positive or neutral effects on animal communities. Furthermore, negative effects reported on local plants can become less negative or neutral when moving from pristine to heavily human-impacted environments. Finally, our new meta-analyses presented in this chapter indicate that impacts increase with the abundance of non-native seaweeds and that non-native seaweeds may increase sample similarity in invaded plant communities, but not in animal communities (but these findings were based on a non-significant trend). Although the impact literature is extensive, most studies have tested for impacts of only 6 high-profile invaders (S. muticum , U. pinnatifida , C. taxifolia , C. racemosa, C. fragile and G. vermiculophylla) . It is therefore important that similar comprehensive analyses are conducted on non-native seaweeds to allow for better generalisations and predictions about seaweed invasion impacts.
In conclusion, at least 346 non-native and cryptogenic seaweeds have altered local shallow water coastal communities in most major biogeographical regions around the world, and this pattern is likely to increase as human populations, transport and other co-occurring stressors continue to increase.
References
Adam P. Saltmarsh ecology. Cambridge: Cambridge University Press; 1990. p. 461.
Alpert P. The advantages and disadvantages of being introduced. Biol Invasions. 2006;8(7):1523–34. doi:10.1007/s10530-005-5844-z.
Altieri A, Trussell G, Ewanchuck P, Bernatchez G. Consumers control diversity and functioning of a natural marine ecosystem. PLoS Biol. 2009;4:e5291.
Ambrose WG, Nelson BW. Inhibition of giant kelp recruitment by an introduced brown alga. Bot Mar. 1982;25:265–7.
Baker HG, Stebbens GL. The genetics of colonizing species. New York: Academic Press; 1965.
Balata D, Piazzi L, Cinelli F. A comparison among assemblages in areas invaded by Caulerpa taxifolia and C. racemosa on a subtidal Mediterranean rocky bottom. Marine Ecology-Pubblicazioni Della Stazione Zoologica Di Napoli I. 2004;25(1):1–13. doi:10.1111/j.1439-0485.2004.00013.x
Baldwin JR, Lovvorn JR. Expansion of seagrass habitat by the exotic Zostera japonica, and its use by dabbling ducks and brant in Boundary Bay, British Colombia. Mar Ecol Prog Ser. 1994;103:119–27.
Barbier EB, Hacker SD, Kennedy CJ, Koch EW, Stier AC, Silliman BR. The value of estuarine and coastal ecosystem services. Ecol Monogr. 2011;81:169–93.
Bates AE, Pecl GT, Frusher S, Hobday AJ, Wernberg T, Smale DA, Sunday JM, Hill NA, Dulvy NK, Colwell RK. Defining and observing stages of climate-mediated range shifts in marine systems. Glob Environ Change. 2014;26:27–38.
Bedini R, Bonechi L, Piazzi L. Mobile epifaunal assemblages associated with Cystoseira beds: comparison between areas invaded and not invaded by Lophocladia lallemandii. Scientia Marina. 2014;78(3):425–32. doi:10.3989/scimar.03995.28B.
Bell SS. Amphipods as insect equivalents? An alternative view. Ecology. 1991;72:350–4.
Berkenbusch K, Rowden AA. An examination of the spatial and temporal generality of the influence of ecosystem engineers on the composition of associated assemblages. Aquat Ecol. 2007;41:129–47.
Berkenbusch K, Rowden AA, Myers TE. Interactions between seagrasses and burrowing ghost shrimps and their influence on infaunal assemblages. J Exp Mar Biol Ecol. 2007;341:70–84.
Blossey B, Notzold R. Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J Ecol. 1995;83(5):887–9. doi:10.2307/2261425.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. West Sussex, United Kingdom: Wiley; 2009. p. 421.
Boudouresque CF, Lemée R, Mari X, Meinesz A. The invasive alga Caulerpa taxifolia is not a suitable diet for the sea urchin Paracentrotus lividus. Aquat Bot. 1996;53:245–50.
Box A, Martin D, Deudero S. Changes in seagrass polychaete assemblages after invasion by Caulerpa racemosa var. cylindracea (Chlorophyta: Caulerpales): community structure, trophic guilds and taxonomic distinctness. Scientia Marina. 2010;74(2):317–29.
Britton-Simmons KH. Direct and indirect effects of the introduced alga Sargassum muticum on benthic, subtidal communities of Washinton State, USA. Mar Ecol Prog Ser. 2004;277:61–78.
Britton-Simmons KH. Functional group diversity, resource preemption and the genesis of invasion resistance in a community of marine algae. Oikos. 2006;113(3):395–401.
Britton-Simmons KH, Abbott KC. Short-and long-term effects of disturbance and propagule pressure on a biological invasion. J Ecol. 2008;96(1):68–77.
Bulleri F, Balata D, Bertocci I, Tamburello L, Benedetti-Cecchi L. The seaweed Caulerpa racemosa on Mediterranean rocky reefs: from passenger to driver of ecological change. Ecology. 2010;91(8):2205–12. doi:10.1890/09-1857.1.
Buschbaum C, Chapman AS, Saier B. How an introduced seaweed can affect epibiota diversity in different coastal systems. Mar Biol. 2006;148(4):743–54. doi:10.1007/s00227-005-0128-9.
Byers J, Wright JT, Gribben PE. Variable direct and indirect effects of a habitat-modifying invasive species on mortality of native fauna. Ecology. 2010;91:1787–98.
Byers JE, Gribben PE, Yeager C, Sotka EE. Impacts of an abundant introduced ecosystem engineer within mudflats of the southeastern US coast. Biol Invasions. 2012;14(12):2587–600. doi:10.1007/s10530-012-0254-5.
Cacabelos E, Olabarria C, Incera M, Troncoso JS. Effects of habitat structure and tidal height on epifaunal assemblages associated with macroalgae. Estuar Coast Shelf Sci. 2010;89(1):43–52. doi:10.1016/j.ecss.2010.05.012.
Cacabelos E, Engelen AH, Mejia A, Arenas F. Comparison of the assemblage functioning of estuary systems dominated by the seagrass Nanozostera noltii versus the invasive drift seaweed Gracilaria vermiculophylla. J Sea Res. 2012;72:99–105. doi:10.1016/j.seares.2012.02.003.
Callaway RM, Ridenour WM. Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ. 2004;2:436–43.
Carlton JT, Eldredge LG. Update and revisions of the marine bioinvasions of Hawai‘i: the introduced and cryptogenic marine and estuarine animals and plants of the Hawaiian Archipelago. In: Lucius G Eldredge III Memorial Volume: Tribute to a Polymath. 2015. p. 25.
Casas G, Scrosati R, Piriz ML. The invasive kelp Undaria pinnatifida (Phaeophyceae, Laminariales) reduces native seaweed diversity in Nuevo Gulf (Patagonia, Argentina). Biol Invasions. 2004;6(4):411–6. doi:10.1023/b:binv.0000041555.29305.41.
Catford JA, Jansson R, Nilsson C. Reducing redundancy in invasion ecology by integrating hypothesis into a single theoretical framework. Divers Distrib. 2009;15:22–40.
Cebrian E, Ballesteros E, Linares C, Tomas F. Do native herbivores provide resistance to Mediterranean marine bioinvasions? A seaweed example. Biol Invasions. 2011;13(6):1397–408. doi:10.1007/s10530-010-9898-1.
Ceccherelli G, Campo D. Different effects of Caulerpa racemosa on two co-occuring seagrasses in the Mediterranean. Bot Mar. 2002;45:71–6.
Ceccherelli G, Cinelli F. Short-term effects of nutrient enrichment of the sediment and interactions between the seagrass Cymodocea nodosa and the introduced green alga Caulerpa taxifolia in a Mediterranean bay. J Exp Mar Biol Ecol. 1997;217:165–77.
Ceccherelli G, Sechi N. Nutrient availability in the sediment and the reciprocal effects between the native seagrass Cymodocea nodosa and the introduced green alga Caulerpa taxifolia in a Mediterranean bay. Hydrobiologia. 2002;474:57–66.
Chen L, Zan Q, Li M, Shen J, Liao W. Litter dynamics and forest structure of the introduced Sonneratia caseolaris mangrove forest in Shenzhen, China. Estuar Coast Shelf Sci. 2009;85(2):241–6.
Colautti RI, Ricciardi A, Grigorovich LA, MacIsaac HJ. Is invasion success explained by the enemy release hypothesis? Ecol Lett. 2004;7:721–33.
Conklin KY, O’Doherty DC, Sherwood AR. Hydropuntia perplexa, n. comb. (Gracilariaceae, Rhodophyta), first record of the genus in Hawai’i. Pac Sci. 2014;68(3):421–34. doi:10.2984/68.3.9.
Crawley MJ, Brown SL, Heard MS, Edwards GR. Invasion-resistance in experimental grassland communities: species richness or species identity? Ecol Lett. 1999;2(3):140–8.
Dale M. Phytosociological structure of seaweed communities and the invasion of Fucus serratus in Nova Scotia. Can J Bot. 1982;60(12):2652–8.
Davidson IC, Simkanin C. The biology of ballast water 25 years later. Biol Invasions. 2012;14:9–13.
Davis MA, Grime JP, Thompsen K. Fluctuating resources in plant communities: a general theory of invasibility. J Ecol. 2000;88:528–34.
Dayton PK. Towards an understanding of community resilience and the potential effects of enrichment to the benthos of McMurdo Sound, Antarctica. In: Proceedings of the colloquium on conservation problems in Antartica. 1972. pp. 81–96.
Dean PR, Hurd CL. Seasonal growth, erosion rates, and nitrogen and photosynthetic ecophysiology of Undaria pinnatifida (Heterokontophyta) in southern New Zealand1. J Phycol. 2007;43(6):1138–48.
Demopoulos AWJ, Smith CR. Invasive mangroves alter macrofaunal community structure and facilitate opportunistic exotics. Mar Ecol Prog Ser. 2010;404:51–67. doi:10.3354/meps08483.
de Jesus PB, Silva MS, de Mattos Lyra G, de Castro Nunes JM, Schnadelbach AS. Extension of the distribution range of Hypnea stellulifera (Cystocloniaceae, Rhodophyta) to the South Atlantic: morphological and molecular evidence. Aquat Bot. 2014;123:26–36.
de Rivera CE, Ruiz GM, Hines AH, Jivoff P. Biotic resistance to invasion: native predator limits abundance and distribution of an introduced crab. Ecology. 2005;86(12):3364–76. doi:10.1890/05-0479.
de Villele X, Verlaque M. Changes and degradation in a Posidonia oceanica bed invaded by the introduced tropical alga Caulerpa taxifolia in the North Western Mediterranean. Bot Mar. 1995;38(1):79–87.
Díaz-Tapia P, Sook Kim M, Secilla A, Bárbara I, Cremades J. Taxonomic reassessment of Polysiphonia foetidissima (Rhodomelaceae, Rhodophyta) and similar species, including P. schneideri, a newly introduced species in Europe. Eur J Phycol. 2013;48(4):345–62. doi:10.1080/09670262.2013.842655
Dijoux L, Viard F, Payri C. The more we search, the more we find: discovery of a new lineage and a new species complex in the Genus Asparagopsis. PLoS ONE. 2014;9(7):e103826.
Drouin A, McKindsey CW, Johnson LE. Detecting the impacts of notorious invaders: experiments versus observations in the invasion of eelgrass meadows by the green seaweed Codium fragile. Oecologia. 2012;168(2):491–502. doi:10.1007/s00442-011-2086-x.
Eastwood MM, Donahue MJ, Fowler AE. Reconstructing past biological invasions: niche shifts in response to invasive predators and competitors. Biol Invasions. 2007;9:397–407.
Eklöf JS, de la Torre Castro M, Adelsköld L, Jiddawi NS, Kautsky N. Differences in macrofaunal and seagrass assemblages in seagrass beds with and without seaweed farms. Estuar Coast Shelf Sci. 2005;63(3):385–96.
Elton CS (1958) The ecology of invasions by animals and plants. London: Mathuess; 1996.
Emery SM. Limiting similarity between invaders and dominant species in herbaceous plant communities? J Ecol. 2007;95(5):1027–35. doi:10.1111/j.1365-2745.2007.01274.x.
Enge S, Nylund GM, Pavia H. Native generalist herbivores promote invasion of a chemically defended seaweed via refuge-mediated apparent competition. Ecol Lett. 2013;16(4):487–92.
Engelen A, Henriques N, Monteiro C, Santos R. Mesograzers prefer mostly native seaweeds over the invasive brown seaweed Sargassum muticum. Hydrobiologia. 2011;669(1):157–65. doi:10.1007/s10750-011-0680-x.
Engelen A, Primo A, Cruz T, Santos R. Faunal differences between the invasive brown macroalga Sargassum muticum and competing native macroalgae. Biol Invasions. 2013;15(1):171–83. doi:10.1007/s10530-012-0276-z.
Englund G, Sarnelle O, Cooper SD. The importance of data-selection criteria: meta-analysis of stream predation experiments. Ecology. 1999;80:1132–41.
Eppinga MB, Rietkerk M, Dekker SC, De Ruiter PC, Van der Putten WH. Accumulation of local pathogens: a new hypothesis to explain exotic plant invasions. Oikos. 2006;114(1):168–76. doi:10.1111/j.2006.0030-1299.14625.x.
Falcao C, de Szechy MTM. Changes in shallow phytobenthic assemblages in southeastern Brazil, following the replacement of Sargassum vulgare (Phaeophyta) by Caulerpa scalpelliformis (Chlorophyta). Bot Mar. 2005;48:208–17.
Fitridge I, Dempster T, Guenther J, de Nys R. The impact and control of biofouling in marine aquaculture: a review. Biofouling. 2012;28(7):649–69.
Flagella MM, Verlaque M, Soria A, Buia MC. Macroalgal survival in ballast water tanks. Mar Pollut Bull. 2007;54(9):1395–401. doi:10.1016/j.marpolbul.2007.05.015.
Flagella MM, Andreakis N, Hiraoka M, Verlaque M, Buia MC. Identification of cryptic Ulva species (Chlorophyta, Ulvales) transported by ballast water. J Biol Res Thessaloniki. 2010;13:47–57.
Forrest BM, Taylor MD. Assessing invasion impact: survey design considerations and implications for management of an invasive marine plant. Biol Invasions. 2003;4:375–86.
Fourqurean JW, Smith TJ III, Possley J, Collins TM, Lee D, Namoff S. Are mangroves in the tropical Atlantic ripe for invasion? Exotic mangrove trees in the forests of South Florida. Biol Invasions. 2010;12(8):2509–22.
Fukunaga A, Peyton KA, Thomas FIM. Epifaunal community structure and ammonium uptake compared for the invasive algae, Gracilaria salicornia and Acanthophora specifera, and the native alga, Padina thivyi. J Exp Mar Biol Ecol. 2014;456:78–86. doi:10.1016/j.jembe.2014.03.013.
Gallucci F, Hutchings P, Gribben P, Fonseca G. Habitat alteration and community-level effects of an invasive ecosystem engineer: a case study along the coast of NSW, Australia. Mar Ecol Prog Ser. 2012;449:95–108.
Gennaro P, Piazzi L. Synergism between two anthropic impacts: Caulerpa racemosa var. cylindracea invasion and seawater nutrient enrichment. Mar Ecol Prog Ser. 2011;427:59–70.
Gestoso I, Olabarria C, Troncoso JS. Variability of epifaunal assemblages associated with native and invasive macroalgae. Mar Freshw Res. 2010;61(6):724–31. doi:10.1071/mf09251.
Gestoso I, Olabarria C, Troncoso J. Effects of macroalgal identity on epifaunal assemblages: native species vs the invasive species Sargassum muticum. Helgol Mar Res. 2012;66(2):159–66. doi:10.1007/s10152-011-0257-0.
Glasby T. Caulerpa taxifolia in seagrass meadows: killer or opportunistic weed? Biol Invasions. 2012;1–19. doi:10.1007/s10530-012-0347-1
Gollan JR, Wright JT. Limited grazing pressure by native herbivores on the invasive seaweed Caulerpa taxifolia in a temperate Australian estuary. Mar Freshw Res. 2006;57:685–94.
Green DS, Boots B, Crowe TP. Effects of non-indigenous oysters on microbial diversity and ecosystem functioning. PLoS ONE. 2012;7(10):e48410.
Green D, Rocha C, Crowe T. Effects of non-indigenous oysters on ecosystem processes vary with abundance and context. Ecosystems. 2013;16:881–93. doi:10.1007/s10021-013-9659-y.
Gribben PE, Wright JT. Sublethal effects on reproduction in native fauna: are females more vulnerable to biological invasion? Oecologia. 2006;149:352–61.
Gribben PE, Byers J, Clements M, McKenzie LA, Steinberg PD, Wright JT. Behavioural interactions between ecosystem engineers control community species richness. Ecol Lett. 2009a;12:1127–36.
Gribben PE, Wright JT, O’Connor WA, Doblin MA, Eyre B, Steinberg PD. Reduced performance of native infauna following recruitment to a habitat-forming invasive marine alga. Oecologia. 2009b;158:733–45.
Gribben PE, Byers JE, Wright JT, Glasby TM. Positive versus negative effects of an invasive ecosystem engineer on different components of a marine ecosystem. Oikos. 2013;122(6):816–24. doi:10.1111/j.1600-0706.2012.20868.x.
Guerra-García JM, Ros M, Izquierdo D, Soler-Hurtado MM. The invasive Asparagopsis armata versus the native Corallina elongata: differences in associated peracarid assemblages. J Exp Mar Biol Ecol. 2012;416(417):121–8.
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, Agrosa C. A global map of human impact on marine ecosystems. Science. 2008;319:948–52.
Hammann M, Buchholz B, Karez R, Weinberger F. Direct and indirect effects of Gracilaria vermiculophylla on native Fucus vesiculosus. Aquat Invasions. 2013;8(2):121–32. doi:10.3391/ai.2013.8.2.01.
Harries DB, Harrow S, Wilson JR, Mair JM, Donnan DW. The establishment of the invasive alga Sargassum muticum on the west coast of Scotland: a preliminary assessment of community effects. J Mar Biol Ass UK. 2007;87:1057–67.
Harris LG, Jones AC. Temperature, herbivory and epibiont acquisition as factors controlling the distribution and ecological role of an invasive seaweed. Biol Invasions. 2005;7:913–24.
Hay CH. The dispersal of sporophytes of Undaria pinnatifida by coastal shipping in New Zealand, and implications for further dispersal of Undaria in France. Brit Phycol J. 1990;25(4):301–13.
Hay M, Steinberg P. The chemical ecology of plant-herbivore interactions in marine versus terrestrial communities. Herbivores Interact Secondary Plant Metabolites. 1992;2:371–413.
Hewitt CL, Campbell ML, Schaffelke B. Introductions of seaweeds: accidental transfer pathways and mechanisms. Bot Mar. 2007;50(5–6):326–37. doi:10.1515/bot.2007.038.
Hierro JL, Maron JL, Callaway RM. A biogeographical approach to plant invasions: the importance of studying exotics in their introduced and native range. J Ecol. 2005;93:5–15.
Hoffman R, Shemesh E, Ramot M, Dubinsky Z, Pinchasov-Grinblat Y, Iluz D. First record of the Indo-Pacific seaweed Codium arabicum Kutz. (Bryopsidales, Chlorophyta) in the Mediterranean Sea. Bot Mar. 2011;54(5):487–495. doi:10.1515/bot.2011.056.
Hoffman R, Sternberg M, Serio D. First report of Laurencia chondrioides (Ceramiales, Rhodophyta) and its potential to be an invasive in the eastern Mediterranean Sea. Bot Mar. 2014;57(6):449–57. doi:10.1515/bot-2014-0053.
Ibrahim AM, El-naggar MM. Ballast water review: impacts, treatments and management. Middle-East J Sci Res. 2012;12(7):976–84.
Incera M, Bertocci I, Benedetti-Cecchi L. Effects of mean intensity and temporal variability of disturbance on the invasion of Caulerpa racemosa var. cylindracea (Caulerpales) in rock pools. Biol Invasions. 2010;12(3):501–14.
Inderjit Chapman D, Ranelletti M, Kaushik S. Invasive marine algae: an ecological perspective. Bot Rev. 2006;72(2):153–78. doi:10.1663/0006-8101(2006)72[153:imaaep]2.0.co;2.
Irigoyen AJ, Trobbiani G, Sgarlatta MP, Raffo MP. Effects of the alien algae Undaria pinnatifida (Phaeophyceae, Laminariales) on the diversity and abundance of benthic macrofauna in Golfo Nuevo (Patagonia, Argentina): potential implications for local food webs. Biol Invasions. 2011;13(7):1521–32. doi:10.1007/s10530-010-9910-9.
James K, Middleton I, Middleton C, Shears NT. Discovery of Undaria pinnatifida (Harvey) Suringar, 1873 in northern New Zealand indicates increased invasion threat in subtropical regions. BioInvasions Rec. 2014;3(1):21–4.
Jaubert JM, Chisholm JRM, Ducrot D, Ripley HT, Roy L, Passeron GS. No deleterious alterations in Posidonia beds in the Bay of Menton (France) eight years after Caulerpa taxifolia colonization. J Phycol. 1999;35:1113–9.
Jaubert JM, Chisholm JRM, Minghelli-Roman A, Marchioretti M, Morrow JH, Ripley HT. Re-evaluation of the extent of Caulerpa taxifolia development in the northern Mediterranean using airborne spectrographic sensing. Mar Ecol Prog Ser. 2003;263:75–82.
Johnson CR, Chapman ARO. Seaweed invasions: introduction and scope. Bot Mar. 2007;50(5–6):321–5. doi:10.1515/bot.2007.037.
Johnston CA, Lipcius RN. Exotic macroalga Gracilaria vermiculophylla provides superior nursery habitat for native blue crab in Chesapeake Bay. Mar Ecol Prog Ser. 2012;467:137–46. doi:10.3354/meps09935.
Jones E, Thornber CS. Effects of habitat-modifying invasive macroalgae on epiphytic algal communities. Mar Ecol Prog Ser. 2010;400:87–100.
Jones CG, Lawton JH, Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69:373–86.
Jongma DN, Campo D, Dattolo E, D’Esposito D, Duchi A, Grewe P, Huisman J, Verlaque M, Yokes MB, Procaccini G. Identity and origin of a slender Caulerpa taxifolia strain introduced into the Mediterranean Sea. Bot Mar. 2013;56(1):27–39. doi:10.1515/bot-2012-0175.
Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. TREE. 2002;17:164–70.
Klein J, Verlaque M. The Caulerpa racemosa invasion: a critical review. Mar Pollut Bull. 2008;56(2):205–25. doi:10.1016/j.marpolbul.2007.09.043.
Klein JC, Verlaque M. Experimental removal of the invasive Caulerpa racemosa triggers partial assemblage recovery. J Mar Biol Assoc UK. 2011;91(1):117–25. doi:10.1017/s0025315410000792.
Krauss KW, Allen JA. Influences of salinity and shade on seedling photosynthesis and growth of two mangrove species, Rhizophora mangle and Bruguiera sexangula, introduced to Hawaii. Aquat Bot. 2003;77(4):311–24.
Lang AC, Buschbaum C. Facilitative effects of introduced Pacific oysters on native macroalgae are limited by a secondary invader, the seaweed Sargassum muticum. J Sea Res. 2010;63(2):119–28. doi:10.1016/j.seares.2009.11.002.
Levin PS, Coyer JA, Petrik R, Good TP. Community-wide effects of nonindigenous species on temperate rocky reefs. Ecology. 2002;83(11):3182–93. doi:10.2307/3071852.
Lockwood JL, Cassey P, Blackburn T. The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Divers Distrib. 2009;15:904–10.
Lutz M, Davis A, Minchinton T. Non-indigenous macroalga hosts different epiphytic assemblages to conspecific natives in southeast Australia. Mar Biol. 2010;157(5):1095–03. doi:10.1007/s00227-010-1391-y.
Maggi E, Benedetti-Cecchi L, Castelli A, Chatzinikolaou E, Crowe TP, Ghedini G, Kotta J, Lyons DA, Ravaglioli C, Rilov G, Rindi L, Bulleri F. Ecological impacts of invading seaweeds: a meta-analysis of their effects at different trophic levels. Divers Distrib. 2015;21(1):1–12. doi:10.1111/ddi.12264.
Maggs CA, Gall LL, Mineur F, Provan J, Saunders GW. Fredericqia deveauniensis, gen. et sp. nov. (Phyllophoraceae, Rhodophyta), a new cryptogenic species. Cryptogam Algologie. 2013;34(3):273–296.
Mateu-Vicens G, Box A, Deudero S, Rodriguez B. Comparative analysis of epiphytic foraminifera in sediments colonized by seagrass Posidonia oceanica and invasive macroalgae Caulerpa spp. J Foramin Res. 2010;40(2):134–47.
Mazariegos-Villareal A, Riosmena-Rodríguez R, Rosa Rivera-Camacho A, Serviere-Zaragoza E. First report of Cladostephus spongiosus (Sphacelariales: Phaeophyta) from the Pacific coast of Mexico. Bot Mar. 2010;53(2):153–7.
McKinnon JG, Gribben PE, Davis AR, Jolley DF, Wright JT. Differences in soft-sediment macrobenthic assemblages invaded by Caulerpa taxifolia compared to uninvaded habitats. Mar Ecol Prog Ser. 2009;380:59–71. doi:10.3354/meps07926.
Meinesz A. Killer algae—the true tale of a biological invasion. Chicago: The University of Chicago Press; 1999. p. 360.
Melbourne BA, Cornell HV, Davies KF, Dugaw CJ, Elmendorf S, Freestone AL, Hall RJ, Harrison S, Hastings A, Holland M, Holyoak M, Lambrinos L, Moore K, Yokomizo H. Invasion in a heterogeneous world: resistance, coexistence or hostile takeover? Ecol Lett. 2007;10:77–97.
Micael J, Parente MI, Costa AC. Tracking macroalgae introductions in North Atlantic oceanic Islands. Helgol Mar Res. 2014;68(2):209–19. doi:10.1007/s10152-014-0382-7.
Mineur F, Johnson MP, Maggs CA, Stegenga H. Hull fouling on commercial ships as a vector of macroalgal introduction. Mar Biol. 2007;151(4):1299–307.
Mineur F, Johnson MP, Maggs CA. Macroalgal introductions by hull fouling on recreational vessels: seaweeds and sailors. Environ Manage. 2008a;42(4):667–76. doi:10.1007/s00267-008-9185-4.
Mineur F, Johnson MP, Maggs CA. Non-indigenous marine macroalgae in native communities: a case study in the British Isles. J Mar Biol Assoc UK. 2008b;88(4):693–8. doi:10.1017/s0025315408001409.
Mineur F, De Clerck O, Le Roux A, Maggs CA, Verlaque M. Polyopes lancifolius (Halymeniales, Rhodophyta), a new component of the Japanese marine flora introduced to Europe. Phycologia. 2009;49(1):86–96. doi:10.2216/09-45.1.
Mineur F, Le Roux A, Stegenga H, Verlaque M, Maggs CA. Four new exotic red seaweeds on European shores. Biol Invasions. 2012;14(8):1635–41. doi:10.1007/s10530-012-0186-0.
Mitchell CE, Agrawal AA, Bever JD, Gilbert GS, Hufbauer RA, Klironomos JN, Maron JL, Morris WF, Parker IM, Power AG, Seabloom EW, Torchin ME, Vazquez DP. Biotic interactions and plant invasions. Ecol Lett. 2006;9:726–40.
Monteiro C, Engelen AH, Santos RO. Macro- and mesoherbivores prefer native seaweeds over the invasive brown seaweed Sargassum muticum: a potential regulating role on invasions. Mar Biol. 2009;156:2505–15.
Morita T, Kurashima A, Maegawa M. Temperature requirements for the growth and maturation of the gametophytes of Undaria pinnatifida and U. undarioides (Laminariales, Phaeophyceae). Phycol Res. 2003;51(3):154–60.
Neira C, Levin LA, Grosholz ED, Mendoza G. Influence of invasive Spartina growth stages on associated macrofaunal communities. Biol Invasions. 2007;9(8):975–93. doi:10.1007/s10530-007-9097-x.
Nejrup LB, Pedersen MF. Growth and biomass development of the introduced red alga Gracilaria vermiculophylla is unaffected by nutrient limitation and grazing. Aquat Biol. 2010;10(3):249–59. doi:10.3354/ab00281.
Nejrup L, Pedersen M, Vinzent J. Grazer avoidance may explain the invasiveness of the red alga Gracilaria vermiculophylla in Scandinavian waters. Mar Biol. 2012;159(8):1703–12. doi:10.1007/s00227-012-1959-9.
Nyberg CD, Thomsen MS, Wallentinus I. Flora and fauna associated with the introduced red alga Gracilaria vermiculophylla. Eur J Phycol. 2009;44(3):395–403. doi:10.1080/09670260802592808.
Nylund GM, Weinberger F, Rempt M, Pohnert G. Metabolomic assessment of induced and activated chemical defence in the invasive red alga Gracilaria vermiculophylla. PLoS ONE. 2011;6(12):e29359.
Odom RL, Walters LJ. A safe alternative to invasive Caulerpa taxifolia (Chlorophtya)? Assessing aquarium-release invasion potential of aquarium strains of the macroalgal genus Chaetomorpha (Chlorophyta). Biol Invasions. 2014;16(8):1589–97.
Olabarria C, Arenas F. Understanding biological invasions by seaweeds. Mar Algae Biodiver Taxonomy Environ Assess Biotechnol. 2014;140.
Olabarria C, Rodil IF, Incera M, Troncoso JS. Limited impact of Sargassum muticum on native algal assemblages from rocky intertidal shores. Mar Environ Res. 2009;67(3):153–8. doi:10.1016/j.marenvres.2008.12.007.
Ostenfeld CH. On the immigration of Biddulphia sinensis Grev. and its occurrence in the North Sea during 1903–1907 and on its use for the study of the direction and rate of flow of the currents. Meddelelser fra Kommissionen for Danmarks Fiskeri- og Havundersøgelser: Serie Plankton. 1908;6:1–44.
Pacciardi L, De Biasi AM, Piazzi L. Effects of Caulerpa racemosa invasion on soft-bottom assemblages in the Western Mediterranean Sea. Biol Invasions. 2011;13(12):2677–90. doi:10.1007/s10530-011-9938-5.
Padilla DK, Williams SL. Beyond ballast water: aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Front Ecol Environ. 2004;2(3):131–8.
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham MJ, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JL, Goldwasser L. Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions. 1999;1:3–19.
Parker DJ, Burkepile DE, Hay ME. Opposing effects of native and exotic herbivores on plant invasion. Science. 2006;311:1459–61.
Pedersen MF, Stæhr PA, Wernberg T, Thomsen M. Biomass dynamics of exotic Sargassum muticum and native Halidrys siliquosa in Limfjorden, Denmark—implications of species replacements on turnover rates. Aquat Bot. 2005;83:31–47.
Peteiro C, Sánchez N. Comparing salinity tolerance in early stages of the sporophytes of a non-indigenous kelp (Undaria pinnatifida) and a native kelp (Saccharina latissima). Russ J Mar Biol. 2012;38(2):197–200.
Piazzi L, Balata D. The spread of Caulerpa racemosa var. cylindracea in the Mediterranean Sea: an example of how biological invasions can influence beta diversity. Mar Environ Res. 2008;65(1):50–61.
Piazzi L, Balata D. Invasion of alien macroalgae in different Mediterranean habitats. Biol Invasions. 2009;11(2):193–204. doi:10.1007/s10530-008-9224-3.
Piazzi L, Ceccherelli G. Persistence of biological invasion effects: recovery of macroalgal assemblages after removal of Caulerpa racemosa var. cylindracea. Estuar Coast Shelf Sci. 2006;68(3–4):455–61. doi:10.1016/j.ecss.2006.02.011.
Piazzi L, Cinelli F. Evaluation of benthic macroalgal invasion in a harbour area of the western Mediterranean Sea. Eur J Phycol. 2003;38(3):223–31. doi:10.1080/1364253031000136358.
Piazzi L, Balata D, Cinelli F. Epiphytic macroalgal assemblages of Posidonia oceanica rhizomes in the western Mediterranean. Eur J Phycol. 2002;37(1):69–76. doi:10.1017/s0967026201003432.
Piazzi L, Balata D, Cecchi E, Cinelli F. Co-occurrence of Caulerpa taxifolia and C. racemosa in the Mediterranean Sea: interspecific interactions and influence on native macroalgal assemblages. Cryptogamie Algologie. 2003;24(3):233–43.
Piazzi L, Balata D, Ceccherelli G, Cinellia F. Interactive effect of sedimentation and Caulerpa racemosa var. cylindracea invasion on macroalgal assemblages in the Mediterranean Sea. Estuar Coast Shelf Sci. 2005;64:467–74.
Pickering TD, Skelton P, Sulu RJ. Intentional introductions of commercially harvested alien seaweeds. Bot Mar. 2007;50(5–6):338–50. doi:10.1515/bot.2007.039.
Pochon X, Atalah J, Wood SA, Hopkins GA, Watts A, Boedeker C. Cladophora ruchingeri (C. Agardh) Kützing, 1845 (Cladophorales, Chlorophyta): a new biofouling pest of green-lipped mussel Perna canaliculus (Gmelin, 1791) farms in New Zealand; 2015.
Posey MH. Community changes associated with the spread of an introduced seagrass, Zostera Japonica. Ecology. 1988;69:974–83.
Procheş Ş, Wilson JRU, Richardson DM, Rejmánek M. Searching for phylogenetic pattern in biological invasions. Glob Ecol Biogeogr. 2008;17(1):5–10. doi:10.1111/j.1466-8238.2007.00333.x.
Rempt M, Weinberger F, Grosser K, Pohnert G. Conserved and species-specific oxylipin pathways in the wound-activated chemical defense of the noninvasive red alga Gracilaria chilensis and the invasive Gracilaria vermiculophylla. Beilstein J Org Chem. 2012;8(1):283–9.
Ren H, Lu H, Shen W, Huang C, Guo Q, Za Li, Jian S. Sonneratia apetala Buch. Ham in the mangrove ecosystems of China: an invasive species or restoration species? Ecol Eng. 2009;35(8):1243–8.
Reynolds LK, Carr LA, Boyer KE. A non-native amphipod consumes eelgrass inflorescences in San Francisco Bay. Mar Ecol Prog Ser. 2012;451:107–18. doi:10.3354/meps09569.
Ricciardi A, Simberloff D. Assisted colonization is not a viable conservation strategy. Trends Ecol Evol. 2009;24(5):248–53.
Rodil IF, Olabarria C, Lastra M, Lopez J. Differential effects of native and invasive algal wrack on macrofaunal assemblages inhabiting exposed sandy beaches. J Exp Mar Biol Ecol. 2008;358(1):1–13. doi:10.1016/j.jembe.2007.12.030.
Rosenberg MS, Adams DC, Gurevitch J. Metawin: statistical software for meta-analysis, vol Version 2. Massachusetts: Sinauer Associates; 2000.
Rueness J. Sargassum muticum and other introduced Japanese macroalgae: biological pollution of European coasts. Mar Pollut Bull. 1989;20:173–6.
Ruesink JL, Hong JS, Wisehart L, Hacker SD, Dumbauld BR, Hessing-Lewis M, Trimble AC. Congener comparison of native (Zostera marina) and introduced (Z. japonica) eelgrass at multiple scales within a Pacific Northwest estuary. Biol Invasions. 2010;12(6):1773–89. doi:10.1007/s10530-009-9588-z.
Ruiz GM, Torchin ME, Grant K. Using the Panama Canal to test predictions about tropical marine invasions. In: Proceedings of the Smithsonian Marine Science Symposium; 2009. pp. 291–299.
Russell DJ. Introduction of alien seaweeds to Hawaii. Phycologia. 1981;20(2):112.
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand N, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG. The population biology of invasive species. Annu Rev Ecol Syst. 2001;32:305–32.
Sánchez I, Fernández C. Impact of the invasive seaweed Sargassum muticum (Phaeophyta) on an intertidal macroalgal assemblage. J Phycol. 2005;41:923–30.
Sánchez I, Fernández C, Arrontes J. Long-term changes in the structure of intertidal assemblages after invasion by Sargassum muticum (Phaeophyta). J Phycol. 2005;41:942–9.
Sandler R. The value of species and the ethical foundations of assisted colonization. Conserv Biol. 2010;24(2):424–31. doi:10.1111/j.1523-1739.2009.01351.x.
Sax DF, Stachowicz JJ, Brown JH, Bruno JF, Dawson MN, Gaines SD, Grosberg RK, Hastings H, Holt RD, Mayfield MM, O’Connor MI, Rice WR. Ecological and evolutionary insights from species invasions. Trends Ecol Evol. 2007;22:465–71.
Schaffelke B, Hewitt CL. Impacts of introduced seaweeds. Bot Mar. 2007;50:397–417.
Schaffelke B, Smith JE, Hewitt CL. Introduced macroalgae—a growing concern. J Appl Phycol. 2006;18(3–5):529–41. doi:10.1007/s10811-006-9074-2.
Scheibling RE, Anthony SX. Feeding, growth and reproduction of sea urchins (Strongylocentrotus droebachiensis) on single and mixed diets of kelp (Laminaria spp.) and the invasive alga Codium fragile spp. tometosoides. Mar Biol. 2001;139:139–46.
Scheibling RE, Lyons DA, Sumi CBT. Grazing of the invasive alga Codium fragile ssp. tomentosoides by the common periwinkle Littorina littorea: effects of thallus size, age and condition. J Exp Mar Biol Ecol. 2008;355(2):103–13. doi:10.1016/j.jembe.2007.12.002.
Schiel DR, Thompson GA. Demography and population biology of the invasive kelp Undaria pinnatifida on shallow reefs in southern New Zealand. J Exp Mar Biol Ecol. 2012;434:25–33.
Schmidt AL, Scheibling RE. A comparison of epifauna and epiphytes on native kelps (Laminaria species) and an invasive alga (Codium fragile ssp. tomentosoides) in Nova Scotia, Canada. Bot Mar. 2006;49:315–330.
Schmidt AL, Scheibling RE. Effects of native and invasive macroalgal canopies on composition and abundance of mobile benthic macrofauna and turf-forming algae. J Exp Mar Biol Ecol. 2007;341:110–30.
Seddon PJ. From reintroduction to assisted colonization: moving along the conservation translocation spectrum. Restor Ecol. 2010;18(6):796–802. doi:10.1111/j.1526-100X.2010.00724.x.
Sher AA, Hyatt LA. The disturbed resource-flux invasion matrix: a new framework for patterns of plant invasions. Biol Invasions. 1999;1:107–14.
Simberloff D, Von Holle B. Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions. 1999;1:21–32.
Sissini MN, Longo GO, Martins CDL, Floeter SR, Pereira SB, Horta PA. First record of the green alga Halimeda (Bryopsidales: Chlorophyta) at Rocas Atoll—natural dispersion or anthropogenic causes? Mar Biodivers Rec. 2014;7:e104.
Sjotun K, Eggereide SF, Hoisaeter T. Grazer-controlled recruitment of the introduced Sargassum muticum (Phaeophycae, Fucales) in Northern Europe. Mar Ecol Prog Ser. 2007;342:127–38.
Smith JR, Vogt SC, Creedon F, Lucas BJ, Eernisse DJ. The non-native turf-forming alga Caulacanthus ustulatus displaces space-occupants but increases diversity. Biol Invasions. 2014;16(10):2195–208. doi:10.1007/s10530-014-0658-5.
South PM, Lilley S, Tait LW, Alestra T, Hickford MJH, Thomsen MS, Schiel DR. Transient effects of an invasive kelp on the community structure and primary productivity of an intertidal assemblage. Marine and Freshwater Research MF14152. 2015. Accepted 14 Aug 2014.
Staehr PA, Pedersen MF, Thomsen MS, Wernberg T, Krause-Jensen D. Invasion of Sargassum muticum in Limfjorden (Denmark) and its possible impact on the indigenous macroalgal community. Mar Ecol Prog Ser. 2000;207:79–88.
Strong JA, Dring MJ, Maggs CA. Colonisation and modification of soft substratum habitats by the invasive macroalga Sargassum muticum. Mar Ecol Prog Ser. 2006;321:87–97.
Tait LW, South PM, Lilley SA, Thomsen MS, Schiel DR. Assemblage and understory carbon production of native and invasive canopy-forming macroalgae. J Exp Mar Biol Ecol. 2015;469:10–7. doi:10.1016/j.jembe.2015.04.007.
Tamburello L, Maggi E, Benedetti-Cecchi L, Bellistri G, Rattray AJ, Ravaglioli C, Rindi L, Roberts J, Bulleri F. Variation in the impact of non-native seaweeds along gradients of habitat degradation: a meta-analysis and an experimental test. 2015. Oikos:n/a-n/a. doi:10.1111/oik.02197
Taylor SL, Bishop MJ, Kelaher BP, Glasby TM. Impacts of detritus from the invasive alga Caulerpa taxifolia on a soft sediment community. Mar Ecol Prog Ser. 2010;420:73–81.
Thomsen MS. Experimental evidence for positive effects of invasive seaweed on native invertebrates via habitat-formation in a seagrass bed. Aquat Invasions. 2010;5(4):341–6. doi:10.3391/ai.2010.5.4.02.
Thomsen MS, McGlathery K. Effects of accumulations of sediments and drift algae on recruitment of sessile organisms associated with oyster reefs. J Exp Mar Biol Ecol. 2006;328(1):22–34. doi:10.1016/j.jembe.2005.06.016.
Thomsen MS, McGlathery KJ. Stress tolerance of the invasive macroalgae Codium fragile and Gracilaria vermiculophylla in a soft-bottom turbid lagoon. Biol Invasions. 2007;9(5):499–513. doi:10.1007/s10530-006-9043-3.
Thompson GA, Schiel DR. Resistance and facilitation by native algal communities in the invasion success of Undaria pinnatifida. Mar Ecol Prog Ser. 2012;468:95.
Thomsen MS, Wernberg T. The devil in the detail: harmful seaweeds are not harmful to everyone. Global Change Biology. 2015;21(4):1381–2.
Thomsen MS, Gurgel CFD, Fredericq S, McGlathery KJ. Gracilaria vermiculophylla (Rhodophyta, Gracilariales) in Hog Island Bay, Virginia: a cryptic alien and invasive macroalga and taxonomic correction. J Phycol. 2006a;42(1):139–41. doi:10.1111/j.1529-8817.2005.00160.x.
Thomsen MS, Wernberg T, Stæhr PA, Pedersen MF. Spatio-temporal distribution patterns of the invasive macroalga Sargassum muticum within a Danish Sargassum-bed. Helgol Mar Res. 2006b;60:50–8.
Thomsen MS, Stæhr P, Nyberg CD, Krause-Jensen D, Schwærter S, Silliman B. Gracilaria vermiculophylla in Northern Europe, with focus on Denmark, and what to expect in the future. Aquat Invasions. 2007;2:83–94.
Thomsen MS, Adam P, Silliman B. Anthropogenic threats to Australasian coastal salt marshes. In: Silliman BR, Bertness MD, Strong D, editors. Anthropogenic modification of North American salt marshes. California: University of California Press; 2009a. pp. 361–390.
Thomsen MS, Wernberg T, Tuya F, Silliman BR. Evidence for impacts of non-indigenous macroalgae: a meta-analysis of experimental field studies. J Phycol. 2009b;45:812–9.
Thomsen MS, Wernberg T, Altieri AH, Tuya F, Gulbransen D, McGlathery KJ, Holmer M, Silliman BR. Habitat cascades: the conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integr Comp Biol. 2010;50(2):158–75. doi:10.1093/icb/icq042.
Thomsen MS, Olden JD, Wernberg T, Griffin JN, Silliman BR. A broad framework to organize and compare ecological invasion impacts. Environ Res. 2011a;111:899–908.
Thomsen MS, Wernberg T, Olden JD, Griffin JN, Silliman BR. A framework to study the context-dependent impacts of marine invasions. J Exp Mar Biol Ecol. 2011b;400(1–2):322–7. doi:10.1016/j.jembe.2011.02.033.
Thomsen MS, Wernberg T, Engelen AH, Tuya F, Vanderklift MA, Holmer M, McGlathery KJ, Arenas F, Kotta J, Silliman BR. A meta-analysis of seaweed impacts on seagrasses: generalities and knowledge gaps. PLoS ONE. 2012;7(1):e28595.
Thomsen MS, Byers JE, Schiel DR, Bruno JF, Olden JD, Wernberg T, Silliman BR. Impacts of marine invaders on biodiversity depend on trophic position and functional similarity. Mar Ecol Prog Ser. 2014;495:39–47. doi:10.3354/meps10566.
Thomsen MS, Wernberg T, Schiel DR. Invasions by non-indigenous species. In: Crowe TP, Frid CLJ, editors. Marine ecosystems: human impacts on biodiversity, functioning and services. Cambridge: Cambridge University Press; 2015. p. 274–332.
Thornber CS, Kinlan BP, Graham MH, Stachowicz JJ. Population ecology of the invasive kelp Undaria pinnatifida in California: environmental and biological controls on demography. Mar Ecol Prog Ser. 2004;268:69–80.
Tomas F, Box A, Terrados J. Effects of invasive seaweeds on feeding preference and performance of a keystone Mediterranean herbivore. Biol Invasions. 2011a;13(7):1559–70. doi:10.1007/s10530-010-9913-6.
Tomas F, Cebrian E, Ballesteros E. Differential herbivory of invasive algae by native fish in the Mediterranean Sea. Estuar Coast Shelf Sci. 2011b;92(1):27–34.
Trowbridge CD. Ecology of the green macroalga Codium fragile (Suringar) Hariot 1889: invasive and non-invasive subspecies. Oceanogr Mar Biol. 1998;36:1–64.
Trowbridge CD. Local elimination of Codium fragile ssp. tomentosoides: indirect evidence of sacoglossan herbivory. J Mar Biol Ass UK. 2002;82:1029–30.
Trowbridge CD, Todd CD. Host-plant change in marine specialist herbivores: ascoglossan sea slugs on introduced Macroalgae. Ecol Monogr. 2001;71:219–43.
Trussell G, Ewanchuck P, Bertness MD. Field evidence of trait-mediated indirect interactions in a rocky intertidal food web. Ecol Lett. 2002;5:241–5.
Trussell G, Ewanchuk P, Bertness MD, Silliman BR. Trophic cascades in rocky shore tide pools: distinguishing lethal and non-lethal effects. Oecologia. 2004;139:427–32.
Tsai C, Yang S, Trimble AC, Ruesink JL. Interactions betweem two introduced species: Zostera japonica (dwarf eelgrass) facilitates itself and reduces condition of Ruditapes philippinarum (Manila clam) on intertidal mudflats. Mar Biol. 2010;157:1929–36.
Tsiamis K, Verlaque M. A new contribution to the alien red macroalgal flora of Greece (Eastern Mediterranean) with emphasis on Hypnea species. Cryptogamie Algologie. 2011;32(4):393–410.
Valentine JP, Johnson CR. Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. J Exp Mar Biol Ecol. 2003;295(1):63–90. doi:10.1016/s0022-0981(03)00272-7.
Valentine JP, Johnson CR. Establishment of the introduced kelp Undaria pinnatifida following dieback of the native macroalga Phyllospora comosa in Tasmania, Australia. Mar Freshw Res. 2004;55(3):223–30. doi:10.1071/mf03048.
Valentine JP, Magierowski RH, Johnson CR. Mechanisms of invasion: establishment, spread and persistence of introduced seaweed populations. Bot Mar. 2007;50(5–6):351–60. doi:10.1515/bot.2007.040.
Vaz-Pinto F, Olabarria C, Arenas F. Propagule pressure and functional diversity: interactive effects on a macroalgal invasion process. Mar Ecol Prog Ser. 2012;471:51–60. doi:10.3354/meps10024.
Vázquez-Luis M, Borg JA, Sanchez-Jerez P, Bayle-Sempere JT. Habitat colonisation by amphipods: comparison between native and alien algae. J Exp Mar Biol Ecol. 2012;432–433:162–70.
Verges A, Sanchez N, Peteiro C, Polo L, Brodie J. Pyropia suborbiculata (Bangiales, Rhodophyta): first records from the northeastern Atlantic and Mediterranean of this North Pacific species. Phycologia. 2013;52(2):121–9. doi:10.2216/12-003.1.
Verlaque M, Steen F, De Clerck O. Rugulopteryx (Dictyotales, Phaeophyceae), a genus recently introduced to the Mediterranean. Phycologia. 2009;48(6):536–42.
Viejo RM. Mobile epifauna inhabiting the invasive Sargassum muticum and two local seaweeds in northern Spain. Aquat Bot. 1999;64:131–49.
Wallentinus I, Nyberg CD. Introduced marine organisms as habitat modifiers. Mar Pollut Bull. 2007;55:323–32.
Warwick RM, Clarke KR. Increased variability as a symptom of stress in marine communities. J Exp Mar Biol Ecol. 1993;172:215–26.
Wassman R, Ramus J. Seaweed invasion. Nat Hist. 1973;82(10):25.
Wernberg T, Thomsen MS, Stæhr PA, Pedersen MF. Comparative phenology of Sargassum muticum and Halidrys siliquosa (Phaeophyceae: Fucales) in Limfjorden, Denmark. Bot Mar. 2001;44:31–9. doi:10.1515/BOT.2001.005.
Wernberg T, Thomsen MS, Staerh PA, Pedersen MF. Epibiota communities of the introduced and indigenous macroalgal relatives Sargassum muticum and Halidrys siliquosa in Limfjorden (Denmark). Helgol Mar Res. 2004;58:154–61.
White LF, Shurin JB. Density dependent effects of an exotic marine macroalga on native community structure. J Exp Mar Biol Ecol. 2011;405:111–9.
Willette DA, Ambrose RF. The distribution and expansion of the invasive seagrass Halophila stipulacea in Dominica, West Indies, with a preliminary report from St.Lucia. Aquat Bot. 2009;91(3):137–42.
Willette DA, Ambrose RF. Effects of the invasive seagrass Halophila stipulacea on the native seagrass, Syringodium filiforme, and associated fish and epibiota communities in the Eastern Caribbean. Aquat Bot. 2012;103:74–82.
Williams SL. Introduced species in seagrass ecosystems: status and concerns. J Exp Mar Biol Ecol. 2007;350(1–2):89–110. doi:10.1016/j.jembe.2007.05.032.
Williams SL, Grosholz ED. The invasive species challenge in estuarine and coastal environments: marrying management and science. Estuaries Coasts. 2008;31(1):3–20.
Williams SL, Smith JE. A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annu Rev Ecol Evol Syst. 2007;38:327–59. doi:10.1146/annurev.ecolsys.38.091206.095543
Williams SL, Davidson IC, Pasari JR, Ashton GV, Carlton JT, Crafton RE, Fontana RE, Grosholz ED, Miller AW, Ruiz GM. Managing multiple vectors for marine invasions in an increasingly connected world. Bioscience. 2013;63(12):952–66.
Wolf MA, Sfriso A, Andreoli C, Moro I. The presence of exotic Hypnea flexicaulis (Rhodophyta) in the Mediterranean Sea as indicated by morphology, rbcL and cox1 analyses. Aquat Bot. 2011;95(1):55–8. doi:10.1016/j.aquabot.2011.02.009.
Wolf MA, Sfriso A, Moro I. Thermal pollution and settlement of new tropical alien species: the case of Grateloupia yinggehaiensis (Rhodophyta) in the Venice Lagoon. Estuar Coast Shelf Sci. 2014;147:11–6. doi:10.1016/j.ecss.2014.05.020.
Wright JT, Gribben PE. Predicting the impact of an invasive seaweed on the fitness of native fauna. J Appl Ecol. 2008;45:1540–9.
Wright JT, McKenzie LA, Gribben PE. A decline in the abundance and condition of a native bivalve associated with Caulerpa taxifolia invasion. Mar Freshw Res. 2007;58:263–72.
Wu YT, Wang CH, Zhang XD, Zhao B, Jiang LF, Chen JK, Li B. Effects of saltmarsh invasion by Spartina alterniflora on arthropod community structure and diets. Biol Invasions. 2009;11(3):635–49. doi:10.1007/s10530-008-9279-1.
York PH, Booth DJ, Glasby TM, Pease BC. Fish assemblages in habitats dominated by Caulerpa taxifolia and native seagrasses in south-eastern Australia. Mar Ecol Prog Ser. 2006;312:223–34.
Zenetos A, Gofas S, Verlaque M, Çinar ME, Garcia Raso J, Bianchi C, Morri C, Azzurro E, Bilecenoglu M, Froglia C. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution. Mediterr Mar Sci. 2010;11:381–493.
Zenetos A, Gofas S, Morri C, Rosso A, Violanti D, Raso JEG, Cinar ME, Almogi-Labin A, Ates AS, Azzurro E, Ballesteros E, Bianchi CN, Bilecenoglu M, Gambi MC, Giangrande A, Gravili C, Hyams-Kaphzan O, Karachle PK, Katsanevakis S, Lipej L, Mastrototaro F, Mineur F, Pancucci-Papadopoulou MA, Espla AR, Salas C, San Martin G, Sfriso A, Streftaris N, Verlaque M. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr Mar Sci. 2012;13(2):328–52.
Acknowledgements
MST was supported by the Marsden Fund Council from Government funding, administered by the Royal Society of New Zealand. TW was supported by a Future Fellows grant from the Australian Research Council. DRS gratefully acknowledges the continued support by the New Zealand Ministry of Science and Innovation and the National Institute of Water and Atmospheric Research (contract C01X0501).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Thomsen, M.S., Wernberg, T., South, P.M., Schiel, D.R. (2016). Non-native Seaweeds Drive Changes in Marine Coastal Communities Around the World. In: Hu, ZM., Fraser, C. (eds) Seaweed Phylogeography. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7534-2_6
Download citation
DOI: https://doi.org/10.1007/978-94-017-7534-2_6
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7532-8
Online ISBN: 978-94-017-7534-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)