Abstract
Two species of mangrove trees of Indo-Pacific origin have naturalized in tropical Atlantic mangrove forests in South Florida after they were planted and nurtured in botanic gardens. Two Bruguiera gymnorrhiza trees that were planted in the intertidal zone in 1940 have given rise to a population of at least 86 trees growing interspersed with native mangrove species Rhizophora mangle, Avicennia germinans and Laguncularia racemosa along 100 m of shoreline; the population is expanding at a rate of 5.6% year−1. Molecular genetic analyses confirm very low genetic diversity, as expected from a population founded by two individuals. The maximum number of alleles at any locus was three, and we measured reduced heterozygosity compared to native-range populations. Lumnitzera racemosa was introduced multiple times during the 1960s and 1970s, it has spread rapidly into a forest composed of native R. mangle, A. germinans, Laguncularia racemosa and Conocarpus erectus and now occupies 60,500 m2 of mangrove forest with stem densities of 24,735 ha−1. We estimate the population growth rate of Lumnitzera racemosa to be between 17 and 23% year−1. Populations of both species of naturalized mangroves are dominated by young individuals. Given the long life and water-dispersed nature of propagules of the two exotic species, it is likely that they have spread beyond our survey area. We argue that the species-depauperate nature of tropical Atlantic mangrove forests and close taxonomic relatives in the more species-rich Indo-Pacific region result in the susceptibility of tropical Atlantic mangrove forests to invasion by Indo-Pacific mangrove species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human-aided dispersal of organisms among habitats on Earth often has led to greatly altered community structure and ecosystem function (see Mack et al. 2000 for review). Some of the species that arrive in new habitats proliferate, spread and persist; such plants that do so can alter fire regimes, nutrient cycling, ecohydrology and primary productivity and can affect the abundance and threaten the survival of native species. Identifying the suite of life history characteristics that determine whether a species has the potential to become invasive in habitats outside its native range has so far been elusive, but species with close relatives in non-native ecosystems and those that have become invasive elsewhere seem likely candidates. Among the factors contributing to the invasibility of biological communities, Elton (1958) proposed that communities with low species richness should be vulnerable to invasion. Further, ecosystem disturbance may promote rapid proliferation and invasiveness of newly naturalized species (Harper 1965).
Mangrove forests dominate about 75% of the tropical coastlines in the world (Odum et al. 1982). They are valued for both the goods that can be extracted from the forests, the ecosystem services they provide (Costanza et al. 1997) and the support of coastal food webs by the detritus produced in the forests (Odum and Heald 1975; Meziane et al. 2006; Abrantes and Sheaves 2009). In South Florida, there are over 170,000 ha of mangrove forests (Odum et al. 1982). These forests are dominated by four tree species that thrive in the saline, flooded soils of the coastal zone: red mangrove (Rhizophora mangle L.), black mangrove (Avicennia germinans L.), white mangrove (Laguncularia racemosa (L.) C.F. Gaertn.) and buttonwood (Conocarpus erectus L.). The South Florida mangrove species have close relatives that occur in the mangrove forests of the Indo-Pacific: R. mangle has congeners including R. stylosa, R. mucronata and R. apiculata; and in the same family are the genera Bruguiera, Ceriops and Kandelia. A. germinans has the congeners A. marina and A. alba, among others. Laguncularia racemosa is in the same family (Combretaceae) as C. erectus and Lumnitzera, with two species in the Indo-Pacific.
The mangrove forests of the Indo-Pacific are much more diverse than those of the tropical Atlantic region (Duke 1992). The general pattern of higher diversity of mangroves, coral reefs and seagrasses in the tropical Indo-Pacific in comparison to those of the tropical Atlantic is likely the result of extinction events following the tectonic separation of the smaller tropical Atlantic region from the larger tropical Indo-Pacific region (McCoy and Heck 1976). Other mangrove tree species, notably Bruguiera spp., did occur in the Atlantic region in the past. Bruguiera-type pollen has been found in early Eocene (ca. 50 MYA) deposits in France and England as well as in Oligocene (ca. 30 MYA) sediments from England. Fossil hypocotyls resembling Bruguiera, as well as Ceriops, another genus not currently known from the tropical Atlantic, also have been found in the early Eocene London Clay flora of England (reviewed in Graham 2006). When these lineages were extirpated from the tropical Atlantic is unclear.
The structure of mangrove forests in South Florida, as in most other regions, is strongly controlled by disturbance (Smith et al. 1994, 2009). Small scale, frequent disturbances such as lightning strikes open up gaps in the canopy of forests dominated by tall, late-successional species like R. mangle, allowing for smaller species with high dispersal abilities, like Laguncularia racemosa, to persist in the forests. Larger, less frequent disturbances such as hurricanes can remove the canopy from thousands of hectares of mangrove forest in a single event, opening up space for new trees to colonize formerly dense, closed canopy forests. Mangrove forests also are disturbed by human land clearing, dredging and filling. The pervasiveness of disturbances in mangrove forests could allow newly established species to rapidly proliferate across the landscape.
Lugo (1998) suggests that established mangrove forests resist invasion by exotic species because of the challenging physical environment of mangrove forests—but this contention is based on the idea that invaders will be non-mangroves. In the modern era of human mediated long-distance plant dispersal, there is potential for mangroves from different ocean basins to invade forests following their introduction. We know of no reasons why tropical Atlantic mangrove forests should support fewer ecological niches than Indo-Pacific forests, so the current species-depauperate nature of tropical Atlantic mangrove forests may be a result of increased rate of extinction in the smaller area of the tropical Atlantic compared to the Indo-Pacific. It is reasonable to assume that, once freed from dispersal limitations by human activities, species of mangroves from the more species-rich Indo-Pacific region could survive quite well in the tropical Atlantic. There are close relatives of all of the tropical Atlantic mangrove species in the Indo-Pacific. Extinctions over the last 50 M years have led to low species richness and consequently unfilled or underutilized niches (sensu, Walker and Valentine 1984). Also, disturbances are prevalent structuring forces in mangrove forests. For these reasons, Atlantic mangrove forests may be ripe for invasion by new mangrove tree species.
Humans have a long history of importing new trees—including mangroves—for food, timber, and as ornamentals; these new species have the potential to naturalize and invade new regions. Mangrove trees flourish when deliberately introduced to tropical islands that had no native mangroves. Likely owing to isolation and the limited dispersal ability of mangrove trees, there were no native mangroves in the Hawaiian archipelago or in French Polynesia in the Pacific Ocean. In 1902, R. mangle from South Florida was introduced to the Hawaiian island of Moloka’i to stabilize the shoreline and for honey production (see citations in Chimner et al. 2006). It is likely that these mangroves spread from Moloka’i to other Hawaiian islands, as R. mangle was recorded on the neighboring island of Oahu as early as 1917. In 1922, several species of mangroves were introduced to Oahu (Chimner et al. 2006), including R. mangle and C. erectus from South Florida and Bruguiera sexangula, Bruguiera parvifolia, Ceriops tagal and Rhizophora mucronata from the Philippines (Allen 1998). Of the Philippine species, only B. sexangula is known to persist in Hawaii; it has spread very little from its original planting sites, being known from only four sites on Oahu (Allen 1998). On the other hand, the species introduced from South Florida have been very successful. C. erectus is commonly used as an ornamental in Hawaii and has escaped cultivation. R. mangle has spread throughout the archipelago and has now colonized all but two of the Hawaiian Islands. On Oahu, the range of mangroves continues to expand 90 years after their introduction. The expanding mangrove forests have negatively impacted at least one endangered species, the Hawaiian stilt, a mudflat-feeding bird (Allen 1998). Rhizophora stylosa was introduced to Moorea, French Polynesia in 1937, with the intention of providing oyster culture habitat on the prop roots. A few propagules were introduced to one bay, but mangroves are now established around Moorea and on some nearby islands. These mangroves have replaced salt grass (Paspalum vaginatum) marshes and colonized mud flats, leading to efforts to control their spread (Langer and Lipps 2006).
Humans have introduced non-native mangrove species to areas with native mangroves as well. Non-native Sonneratia caseolaris and S. apetala were introduced for timber production to a mangrove forest in Shenzen Bay, China in 1993. The species could sexually reproduce and individuals dispersed, but appeared to be inferior competitors to native species. As a result, Sonneratia spp. had limited impact on the structure of Shenzen Bay’s mangrove forests (Zan et al. 2003). In Bangladesh, Biswas et al. (2007) documented five non-native mangrove species that have naturalized in the Sundarbans mangrove forest. Lumnitzera racemosa, R. mangle, Bruguiera gymnorrhiza and Xylocarpus granatum have been planted in Tonga as part of land reclamation/stabilization efforts. Lumnitzera racemosa has been reported to be well-established there (Clarke and Thaman 1993). These examples show that mangrove forests can indeed be invaded by newly introduced tree species.
As with the cases in Hawaii, China, Bangladesh and Tonga, South Florida has also experienced its share of exotic mangrove introductions. We focus on the introductions of B. gymnorrhiza (L.) Savigny and Lumnitzera racemosa Willd. as case studies. Members of Bruguiera have showy, ornamental flowers. In Hawaii, flowers of the introduced Bruguiera sexangula are used in the production of flower leis (Allen 1998). B. gymnorrhiza, native to the region ranging from eastern Africa to SE Asia and the Pacific Ocean islands, has attractive, large (ca. 2.5 cm diameter) red flowers, making it a candidate for planting as an ornamental tree. Indeed, the early twentieth century plant explorer David Fairchild sought out specimens of B. gymnorrhiza from Indonesia to plant in the intertidal zone at his house in Miami, Florida, USA. He wrote in his memoirs that he was anxious for the two trees he planted in 1940 to be successful and to spread throughout the region: “If they fruit, perhaps some day they will brighten our coasts with their flowers” (Fairchild 1945, p 94). His Miami house, known as “The Kampong,” became a botanical garden in 1984, and as a result there is an historical record of the status of these introduced mangrove trees. Today, the progeny of the trees he planted have begun to expand and fulfill Fairchild’s vision of red-flowered mangroves in Biscayne Bay, Florida. Further, during the twentieth century, many more mangrove specimens from around the world, including other B. gymnorrhiza, were planted a few kilometers from The Kampong at Fairchild Tropical Botanic Garden (FTBG) in Coral Gables, Florida. Lumnitzera racemosa was planted as part of the living collections at FTBG in 1966 and 1971. The intentional introduction of these species, with the carefully collected records about their status through the years, has provided us with an opportunity to assess the likelihood that these species would spread further in the region and become invasive, and to ask whether these populations may have exchanged gametes or received gametes from another as yet unidentified source population.
Methods
Surveys at The Kampong, site of B. gymnorrhiza introduction
Between May 2008 and May 2009, we made approximately monthly visits to The Kampong (ca. 25.7147°N, 80.2495°W) in order to assess stand structure and flowering phenology in the mangrove stand where B. gymnorrhiza had been planted in 1940. All stems of B. gymnorrhiza (trees, saplings) were tagged with unique numbers and mapped (Smith 2004; Ward et al. 2006). However, because of the large area, native Florida mangroves (A. germinans, Laguncularia racemosa and R. mangle) were mapped only around the area of the densest B. gymnorrhiza stand. The resultant plot was 21 × 21 m in size (441 m2). A central position in the stand was determined and marked. The distance and bearing to all mangrove stems over 1.5 m in height was measured from that position. Stems were identified and measured for diameter at breast height (dbh, at ~1.5 m). Seedlings of B. gymnorrhiza (i.e. those individuals <1.5 m) were also mapped. However, because of their high densities, native mangrove seedlings were not mapped. Random plots were established (n = 6) and seedlings of all species were counted within a 1 m radius of the plot’s center (plot area = 3.14 m2). On May 13, June 16, August 1 and October 16, 2008, we assessed flowering/fruiting status of subsamples of the tagged B. gymnorrhiza individuals.
In August 2008, we arbitrarily sampled 33 individuals from the B. gymnorrhiza population at The Kampong in order to determine the level of genetic diversity of the population. We collected two unexpanded young leaves still enclosed in the stipule from a terminal branch of each tree. These leaves were preserved in silica gel and stored at 4°C prior to DNA extraction.
Surveys of non-native mangroves at Fairchild Tropical Botanic Garden
We queried the plant records database at FTBG (ca. 25.6770°N, 80.2730°W) to determine the identity and planting dates of non-native mangrove tree species. We then conducted surveys to locate these individuals and determine if they had reproduced. Lumnitzera racemosa had spread; the area supporting individuals of this species was found to be quite extensive. We used Garmin GPS 60 units and ESRI ArcMap 9.3 to map the extent of the invasion. Twelve plots were sampled in the wetlands swale in and north of FTBG and extending into Matheson Hammock, a property managed by the Miami-Dade County Parks Department. The plots varied in size depending on densities of Lumnitzera racemosa encountered and ranged from 20 to 30 m2. All stems were identified to species and their dbh at ~1.4 m measured. The densest stands of Lumnitzera racemosa occurred in relatively narrow bands along shorelines or adjacent to mosquito control ditches; this precluded stem mapping. Because of the extent of the spread of this species and the proximity to other mangrove stands, an immediate eradication effort was begun.
Forest structure data analyses
The dbh data were used to construct size-frequency diagrams for all native and non-native mangrove species in the study. At both sites the data are on an areal basis (# ha−1). Differences in density of mangrove seedlings among mangrove species from The Kampong were analyzed as a simple one factor ANOVA.
Molecular genetic analysis of B. gymnorrhiza from The Kampong
Genomic DNA was extracted using a FastPrep FP120 tissue disrupter (Thermo Electron Corporation) and FastDNA spin kit (QBIOgene). Ten microsatellite regions previously designed for B. gymnorrhiza (Islam et al. 2006) were amplified via the polymerase chain reaction. PCR reactions were carried out in 25 μl volumes with ~50 ng genomic DNA, 2 mM MgCl2, each dNTP at 10 mM, 1× Promega GoTaq flexi buffer B, 0.75 units Promega Taq, and 1 μmole of each primer. PCR conditions included an initial denaturation step at 95°C for 5 min, and 36 cycles at 95°C for 30 s, 55°C for 45 s, and 72°C for 1 min. Microsatellite PCR products analyzed on an ABI Prism 3130 Genetic Analyzer using POP4 polymer, and fragment sizes were analyzed with GeneScan 3.7 or GeneMapper4 software (Applied Biosystems).
The data were analyzed using the programs GENALEX6 (Peakall and Smouse 2006) and GENEPOP 3.4 (Raymond and Rousset 1995) to test for Hardy–Weinberg equilibrium, to calculate observed and expected heterozygosities, and to characterize allele patterns.
Results
Stand structure at The Kampong
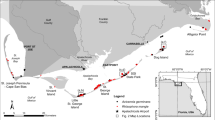
Two individuals of B. gymnorrhiza, originally collected from Dago Bay, Sangihe Island, Sulawesi, Indonesia (ca. 3.38°N, 125.55°E), were planted on the edge of a 100 m long dredged canal at The Kampong in 1940 (Fairchild 1945, p 94; Sweeney 1967). These prized plants were nurtured by gardening staff. A survey in 1971 showed that one of the original trees was still alive, and six saplings were growing near the original plantings (Gillis 1971). During surveys conducted in August 2008 we identified 86 individuals of B. gymnorrhiza growing along the canal at The Kampong where the original individuals were planted in 1940 (Fig. 1c). Assuming that we identified all of the individuals in the population, the three estimates of population size from 1940, 1971 and 2008 fit to an exponential population growth model (N t = N o e rt) yield a population growth rate (r) of 5.6% year−1 (R 2 = 0.98).
Study site locations. a Distribution of mangrove forests in southeastern North America and the Caribbean showing location of study area. b Location of study sites in reference to downtown Miami. c Mangrove forests at The Kampong, with distribution of Bruguiera gymnorrhiza indicated with black outlining. d Mangrove forests of Matheson Hammock Park, surrounding Fairchild Tropical Botanic Garden, with distribution of Lumnitzera racemosa indicated with black outlining
We counted 40 B. gymnorrhiza trees and saplings at The Kampong, of which 37 were in the core plot (Fig. 2). Two B. gymnorrhiza trees were growing at the mouth of the canal where it meets Biscayne Bay, some 65 m from the core plot and another tree was at the head of the canal, 40 m distant (Fig. 1c). There were 45 B. gymnorrhiza seedlings in the plot. The plot also contained 6 A. germinans, 27 Laguncularia racemosa and 115 R. mangle trees and saplings (Fig. 2). The majority of the B. gymnorrhiza adults and saplings were concentrated near the canal. B. gymnorrhiza seedlings, however, have spread well away from this core. Laguncularia racemosa was concentrated near the upland border of the plot whereas R. mangle was found throughout (Fig. 2). The few A. germinans were concentrated in the center of the plot along a small path that meanders through it.
Location of all trees and saplings (i.e., stems over 1.5 m tall) of native mangroves and the introduced species Bruguiera gymnorrhiza in the 441 m2 permanent plot at The Kampong (see Fig. 1c for location). The dashed line indicates the boundaries of the plot
Seedlings and saplings of B. gymnorrhiza and three of the native species (R. mangle, A. germinans and L. racemosa) were very abundant, with densities of 3,184 ha−1 for B. gymnorrhiza and >5,000 ha−1 for each of the native species (Fig. 3). B. gymnorrhiza saplings (≤2.5 cm dbh) were more numerous than saplings of the native species in our permanent plot (Fig. 3) whereas R. mangle had the highest density in the next four larger size classes. The largest B. gymnorrhiza stem we measured was 15.1 cm dbh. The two largest stems in the plot were A. germinans, each of which was >25.0 cm dbh (Fig. 3). Mangrove seedling densities were highly variable (Fig. 4) ranging between 0 and 9 m−2. There were no significant differences among species in density (F 3, 20 = 2.09, P = 0.10). Lowest seedling densities were recorded under the B. gymnorrhiza clump and only B. gymnorrhiza seedlings were found there.
We observed prolific flowering of B. gymnorrhiza, but no fruit set or new seedlings, over the period May–October 2008. On our first surveys in May 2008, there were many B. gymnorrhiza seedlings, some with as few as two pairs of leaves above still-visible hypocotyls, evidence of seedling establishment from the previous year’s reproduction, as well as numerous individuals with developing, unopened flowers. Seedlings as short as 1 m were observed with flower buds. By June 16, most of the trees in the population displayed crimson, open flowers; those flowers were concentrated on the portions of the trees receiving direct sunlight. On August 1, 2008, we carefully examined a subset of 44 individual saplings and trees for signs of flowering; 14 of the 44 examined individuals, as small as 1.4 m tall, were in flower, but we did not observe fruit or developing propagules. Flowering continued to be concentrated on trees and parts of trees exposed to direct sunlight. R. mangle individuals growing amongst the B. gymnorrhiza supported numerous 10 cm long propagules. By the time of our last survey on October 16, 2008, there were no more B. gymnorrhiza in flower, and we saw no evidence that there had been any fruit set during the entire reproductive season.
Mangroves at Fairchild Tropical Botanic Garden
Fairchild Tropical Botanic Garden (FTBG) is located near the coastline of Biscayne Bay (Fig. 1d) and the garden includes both intertidal wetland and upland plant communities. Records indicate that 129 individual members of 14 non-native species considered as true mangroves (sensu Tomlinson 1986) have been planted at FTBG since the garden opened to the public in 1938 (Table 1). Two of these taxa were only identified to the level of genus. Garden records indicate that during the last complete survey of mangrove collections in 1998, six of the original 14 taxa were still alive (Table 1). By 2008, surveys by the authors and FTBG staff revealed that five of the fourteen non-native mangrove taxa survived (Table 1). The B. gymnorrhiza individual died in the 1998–2008 interim, as evidenced by a single standing dead specimen.
Of the five non-native mangrove species that remain in the collections of FTBG, two apparently have not reproduced: Dolichandrone spathacea and Nypa fruticans. One adult specimen of R. stylosa had a dbh of 5.6 cm and had flower buds and immature fruit on the tree, while a specimen ~4 m from the flowering tree had a dbh of 5.1 cm and was not bearing fruit or flowers. We found no evidence of seedling or sapling R. stylosa near the two adult trees, and on a subsequent visit determined that no fruit had been produced by the one tree in flower. We found numerous seedlings under a Heritiera littoralis during our survey, but we did not observe seedlings of that species dispersing away from the adult, as the seedlings seemed to be kept in check by mowing of the lawn surrounding the parent tree.
In contrast to the other introduced species, Lumnitzera racemosa had reproduced repeatedly since introduction at FTBG. A total of fourteen Lumnitzera racemosa were planted in three different locations in FTBG’s lowlands in the late 1960s to early 1970s. By 2009, at least one of the original plants still remained. At each of the three planting locations, Lumnitzera racemosa naturalized and began expanding its range and was found in densities far greater than the native mangrove species (Fig. 5). The densities of Lumnitzera racemosa saplings and trees we found were extremely high for mangrove stands, with 24,735 ha−1. However, the weighted (by density) average stem diameter was quite small, only 1.63 cm. A few larger individuals (10–20 cm dbh) were encountered but not within our plots. These stems were located along an interior pond at FTBG and were eradicated before we could quantify densities. The size frequency distributions clearly show Lumnitzera racemosa dominating the mangrove forests at this location (Fig. 1d).
Size frequency distribution of saplings and trees (i.e., stems >1.5 m in height) for stands of Lumnitzera racemosa and native mangroves at FTBG and Matheson Hammock Park. See Fig. 1d for location
Assuming exponential growth of this population, we conservatively estimate the population growth rate (r) to be between 17 and 23% year−1. The population was in flower and fruit during our first survey in November 2008, and the preponderance of seedlings and saplings (Fig. 5) in the population suggest copious reproduction. The total geographic extent of the invasion from the original locations now reaches beyond FTBG’s borders into a neighboring county park. We estimate that within a span of 38–43 years, the population expanded to dominate about 60,500 m2 of mangrove forest.
Molecular genetics of B. gymnorrhiza population at The Kampong
We genotyped 33 individuals from the Kampong population for ten microsatellite loci. Three loci were monomorphic. The remaining loci were polymorphic, six with two alleles per locus, and one with three alleles (Table 2). Observed heterozygosity for the polymorphic loci ranged from 0.000 to 0.455. Three of the seven polymorphic loci exhibited significant departure from the Hardy–Weinberg expectation. In each case the departure involved significant heterozygote deficiency (Table 2). No pairs of loci showed evidence of significant linkage disequilibrium at the level of P < 0.01. These are characteristics of a genetically depauperate population.
Discussion
At least two species of Indo-Pacific mangroves (B. gymnorrhiza and Lumnitzera racemosa) have naturalized and spread in the mangrove forests of South Florida, showing that Atlantic mangrove forests are indeed susceptible to invasion. The two species we focus on were introduced and nurtured in botanic gardens, anthropogenically modified and disturbed environments in South Florida. These results emphasize the need for vigilance by those who may introduce and cultivate such species.
Bruguiera gymnorrhiza and Lumnitzera racemosa are likely to continue to invade mangrove forests if they are introduced to the tropical Atlantic region. In fact, both species have been recognized as having a high but unproven potential to become invasive (Allen and Duke 2006). The success of these particular species in South Florida, despite the presence of native mangrove competitors, is likely a consequence of the similar environments in tropical American and Indo-Pacific mangrove forests, the close taxonomic relationships between the invaders and native taxa, the species-depauperate flora of tropical American mangroves compared to the Indo-Pacific, and the prevalence of disturbance in the introduction sites. Further, given that these conditions are common to the entire tropical Atlantic coast, it is likely that the mangrove forests in the entire region are susceptible to invasion by Indo-Pacific mangroves.
It has been noted that plants with broad natural ranges that include both Africa and Asia are much more likely to naturalize in new areas compared to species with more limited distributions (Pemberton and Liu 2009). Both B. gymnorrhiza and Lumnitzera racemosa have quite extensive native ranges, suggesting that they have broad environmental tolerances and great dispersal abilities. B. gymnorrhiza has the broadest natural range of all mangrove species, from East Africa to Polynesia and as far north as Ryukyu Island (ca. 26°N, Tomlinson 1986). Lumnitzera racemosa is found from East Africa to the western Pacific islands of Fiji and Tonga, tropical Australia, and Indo-China (Tomlinson 1986). The broad native ranges lend further support to the idea that these species will do well in the mangrove habitats of tropical America if individuals disperse from their current restricted distributions.
In addition to having broad ranges, both B. gymnorrhiza and Lumnitzera racemosa are capable of establishing and growing in a wide range of environmental conditions. For example Bruguiera sp. are routinely found in both non-tidal, freshwater Melaleuca swamps and Eleocharis marshes found upstream in estuaries in northeastern Queensland, Australia (TJS, personal observation). Presumably storm surges carried propagules into these locations, and once there, they survived, and it is possible that this species could become established in the Eleocharis marshes of the Everglades. We also found Lumnitzera racemosa thriving in a non-tidal freshwater pond at FTBG and in a high salinity swale north of the garden.
Populations of both introduced species have size-frequency distributions strongly skewed to very young individuals (Figs. 3, 5), consistent with rapidly expanding populations. The aggressiveness of the growth rate of the two exotic populations differed. The B. gymnorrhiza population was founded by only two individuals and therefore had very low genetic diversity. While we have not yet surveyed the genetic diversity of the Lumnitzera racemosa population, given that it was founded by many more individuals introduced over a 5 year period, it is likely to have greater genetic diversity than B. gymnorrhiza. Further, B. gymnorrhiza produces fewer, larger propagules per parent compared to Lumnitzera racemosa, which has more weedy life history characteristics.
Both Brugiuera gymnorrhiza and Lumnitzera racemosa have perfect flowers and are self-compatible (Tomlinson 1986), so that even a single individual of either species has the ability to produce seeds and fruit in isolation. The conspicuous red flowers of B. gymnorrhiza are pollinated mostly by birds in its native range (Tomlinson 1986). The lack of successful fruit set in B. gymnorrhiza during 2008 may have been due to lack of pollinators, or to some environmental condition not conducive to reproduction. The sporadic nature of successful reproduction in South Florida may have limited the rate of growth of this population. Seeds of B. gymnorrhiza, like those of the native R. mangle, germinate while still attached to the parent tree; these germinated propagules fall from the parent and are dispersed by water. The population of B. gymnorrhiza at The Kampong is adjacent to a short (100 m long) canal that opens into Biscayne Bay (Fig. 1c); we documented that B. gymnorrhiza seedlings dispersed ca. 50 m in either direction along the canal from the original planting location. We assume that propagules of this species have been released into the Bay for at least 50 years. Given that propagules of Bruguiera sp. are viable after floating for at least 60 days (Allen and Krauss 2006), it is very likely that B. gymnorrhiza is established in other mangrove stands in northern Biscayne Bay. In contrast to B. gymnorrhiza, the smaller white flowers of Lumnitzera racemosa are pollinated by a number of insects including wasps, bees, butterflies and moths (Tomlinson 1986). The smaller ungerminated seeds of Lumnitzera racemosa fall from the parent, and since they float, they can be dispersed by water (Tomlinson 1986). At FTBG, Lumnitzera racemosa was planted in areas with no regular connection to the open water of Biscayne Bay, and it appears that for now the expansion of Lumnitzera racemosa has been limited to the back mangrove environment of the network of mosquito control ditches surrounding FTBG (Fig. 1d). However, given the frequency of hurricanes in the region and importance of hurricanes in driving the dynamics of mangrove recruitment in South Florida (Smith et al. 1994, 2009), if left unchecked, it is likely that Lumnitzera racemosa seeds will soon spread beyond the back mangrove environment. And, given the small size of first reproduction and the numerous seeds produced by this species, its spread will likely be extremely rapid.
Both of the exotic mangrove populations we studied occur in areas supporting native mangrove species; the expansion of these populations suggests that the exotic mangroves can indeed compete with native species. Studies in Indo-Pacific countries where Bruguiera and Rhizophora co-occur suggest that species in these genera have similar environmental requirements, and that the competitive hierarchy between the genera changes depending on local conditions. In one case, Ye et al. (2004) found that B. gymnorrhiza seedlings grow faster in the lower intertidal than the high intertidal, but survivorship was lower in the lower intertidal because of physical disturbance. In another study, B. gymnorrhiza seedlings were found to have the lowest tolerance to flooding compared to three other mangrove species in China, which corresponds to the zonation of the mangrove forest there (He et al. 2007). In Hawaii, where both species are introduced, Bruguiera occurs higher in the intertidal than R. mangle, but the comparatively high shade tolerance of Bruguiera seedlings allows them to become established in R. mangle stands (Allen and Krauss 2006). Once established, R. mangle seedlings grow faster than Bruguiera sexangula seedlings under a wide variety of light and salinity conditions, but R. mangle is more tolerant of high salinity environments. Bruguiera seemed to have a slow-growth, tolerance strategy compared to R. mangle in Hawaii (Krauss and Allen 2003a).
The net effect of a change in disturbance regime on the success of B. gymnorrhiza populations is unclear. In a mixed-species mangrove forest in Micronesia, B. gymnorrhiza seedlings were more abundant than other species in both natural and anthropogenic light gaps as well as under the undisturbed canopy, despite the species composition of the canopy surrounding the gap, suggesting that species composition may shift towards B. gymnorrhiza if disturbance increases (Pinzon et al. 2003). Conversely, Imai et al. (2006) found that low levels of disturbance, and therefore low light levels reaching the forest floor, exclude seedlings of species that require high light levels, leading to dominance of the forest by B. gymnorrhiza, which has very shade-tolerant propagules.
Differences in herbivory and seed predation among native and introduced species may partially regulate competition among the species and help explain the distribution of mangrove species in the intertidal zone (Smith 1987). Seed predation can be high in mangroves and suppress seedling establishment, but seedlings of B. gymnorrhiza were less heavily predated than other common mangrove species in forests in Northern Australia (Clarke and Kerrigan 2002). In contrast, Bosire et al. (2005) found that Bruguiera propagules on the floor of a replanted Rhizophora forest were preyed upon at a greater rate than the propagules of the dominant canopy species. Krauss and Allen (2003b) found that seedling success of B. gymnorrhiza was high under a broad range of tidal, light and salinity conditions and herbivory did not exert control over seedling survivorship in Kosrae, part of Micronesia. The importance of seed predation in controlling seedling success rate may be determined by whether the species are in their native ranges, with specialized seedling predators, or not. Predation on propagules of R. mangle is lower on Hawaii than in areas with native mangrove forests, and the lower predation has been hypothesized to be a result of the lack of non-indigenous propagule predators and a facilitator of rapid spread of this species in the Hawaiian Islands (Steele et al. 1999). Similarly, we expect that seedling predation should be more severe for R. mangle than for introduced B. gymnorrhiza in South Florida, but this hypothesis remains to be tested. Such a difference in seed predators may partially explain why B. gymnorrhiza has been able to colonize the mangrove forest at The Kampong.
Outside of their native ranges, some plant species develop unusual stand structure and density compared to their native ranges (as observed for Schinus terebinthifolius and Melaleuca quinquenervia in South Florida, Gordon 1998). In Hawaii, R. mangle has very high rates of net production and very high stem density compared to that measured in its native range. This is attributed to lack of competition with other woody plants and lack of herbivory on trees and propagules (Cox and Allen 1999). It is possible that either B. gymnorrhiza or Lumnitzera racemosa could behave similarly in the tropical Atlantic because of release from native competitors and predators.
Mangrove distributions tend to have pole-ward limits set by wintertime low temperatures, with varying degrees of cold tolerance among species. In South Florida, severe cold fronts can cause widespread mortality of native mangroves (Lugo and Zucca 1977). And, planting records from the Kampong and FTBG suggest that South Florida may be a marginal environment for B. gymnorrhiza, since the death of cultivated specimens was recorded following abnormal cold periods (Gillis 1971). It may be that the relatively pole-ward, subtropical climate of South Florida has limited the longevity of individual B. gymnorrhiza trees and therefore the rate of population growth. However, in the current situation of increasing global temperatures, the pole-ward extent of mangroves in general (Stevens et al. 2006), and B. gymnorrhiza in Florida in particular, is likely to increase.
Despite the very low genetic diversity of the B. gymnorrhiza population at The Kampong, the population has expanded since its planting in 1940 and we found no evidence of pollen exchange between the population at The Kampong and the specimens cultivated a few kilometers away at FTBG. Planting records indicate that two trees may have founded the population of B. gymnorrhiza at The Kampong almost 70 years ago. If this is the case, we would expect to see a limited number of alleles per locus (maximum of 4). We would also expect reduced heterozygosity relative to natural populations as a result of this bottleneck. This reduction in heterozygosity would be intensified by genetic drift in this small founding population over time, and by inbreeding. Results from our analyses of ten microsatellite markers are consistent with this historical record. A single locus has three alleles; all remaining loci possess 1 or 2 alleles. This is consistent with a population founded by as few as two individuals. Four of the loci that are variable in the native-range populations are fixed in The Kampong population, and four more have reduced heterozygosity compared to native-range populations. For the three loci that significantly deviate from the Hardy–Weinberg expectation, the deviation is a heterozygote deficit, again consistent with inbreeding and drift in a small population. It is also possible that some of the deviation from Hardy–Weinberg expectations may result from the presence of null alleles for these loci (Islam et al. 2006). Inbreeding and self fertilization have been previously reported in mangrove species (Maguire et al. 2000; Chen et al. 1996; Nunez-Farfan et al. 2002), so this may prove to be an adaptation of mangroves for colonizing new habitats.
Given the recognized importance of the mangroves of the tropical Atlantic to the functioning of the coastal seascape, the ecosystem functioning of the region’s mangrove forests may change as a consequence of invasive species. In the 1970s, Avicennia marina from the south Pacific was introduced into a salt marsh on Mission Bay, in San Diego, southern California, USA in order to provide specimens for plant physiology research (Jeff Crooks, personal communication, Tijuana River National Estuarine Research Reserve). Despite eradication attempts, this mangrove persists and its population is expanding. The invasion of the salt marsh by mangroves caused changes in nitrogen cycling in the sediments (Moseman et al. 2009). The cycling of the detrital material produced by mangrove forests, essential to coastal food webs in South Florida, may be altered by the presence of B. gymnorrhiza and Lumnitzera racemosa. An analysis of food web structure in the introduced R. mangle forest in Hawaii using stable isotopes suggests that mangrove detritus does not get assimilated in the food web, in contrast to a native R. mangle forest in Puerto Rico (Demopoulos et al. 2007). This suggests that the establishment of mangroves on the mudflats was not necessarily an enhancement of the ecosystem services provided by the previously existing mud flats of Hawaii, since the mangrove carbon was not efficiently taken up by Hawaiian marine food webs. Other introduced species in mangroves in South Florida have indeed caused changes in ecosystem functioning. S. terebinthifolius, the Brazilian pepper, was introduced to Florida in the 1840s, but it was not recognized as an aggressive invasive plant until surveys in the 1950s found it to be increasing in density in Everglades National Park. Today, it is found in both disturbed and undisturbed tropical hardwood forests, pine rocklands, sawgrass marshes and mangroves across South Florida (Jones and Doren 1997). S. terebinthifolius is not a true mangrove, but an opportunistic species that produces noxious secondary compounds that depress the growth rate of seedlings of R. mangle and A. germinans (Donnelly et al. 2008); it severely affects the habitat value of the systems it invades because of the dense tangle of vegetation and the toxic secondary compounds (Doren and Jones 1997, Gordon 1998).
The observations we report here may be cause for concern, both for managers of coastal environments in the tropical Atlantic, as well as for directors and staff of botanical gardens. Species of mangroves from the Indo-Pacific region have become established in South Florida, and these established populations are producing propagules and seeds that are capable of being widely distributed by nearshore currents to large parts of the tropical Atlantic. The consequences of the invasion of tropical Atlantic ecosystems by these species are unclear, but a precautionary approach may be in order, in light of the myriad examples of ecosystem disruption by introduced plant species. To be cautious, managers of botanical gardens could periodically revisit their collection policies, especially when collecting and planting exotic species in a matrix of related native species. Further, care could be taken to communicate with the managers of neighboring natural areas and to prevent the spread of propagules outside the confines of the botanic garden, either by normal conditions or by extreme weather events. In order to limit the spread of such invasive mangrove species, it would be necessary to conduct broader surveys of areas that could have potentially been reached by water-dispersed propagules.
Plant explorers and botanic gardens have long been aware of the problem of weediness and the importance of quarantining candidate plants prior to their introduction to ensure the new plant would not become a pest in the new environment (Fairchild 1898). Yet, it was not until the 1990s when policies truly began to change. In the case studies we discuss, both gardens are taking action to address the spread of non-native mangroves. FTBG has already removed Lumnitzera racemosa from its collections and is currently cooperating with other land management agencies to eradicate the species from garden’s vicinity. Indeed, the Voluntary Codes of Conduct for Botanic Gardens and Arboreta (http://www.centerforplantconservation.org/invasives/gardensN.html), which both gardens have endorsed, lays out sound practices to help stem the spread of invasive plant species.
References
Abrantes K, Sheaves M (2009) Food web structure in a near-pristine mangrove areas of the Australian wet tropics. Estuar Coast Shelf Sci 82:597–607
Allen JA (1998) Mangroves as alien species: the case of Hawaii. Glob Ecol Biogeogr Lett 7:61–71
Allen JA, Duke NC (2006) Species profiles for Pacific Island Agroforestry. http://www.traditionaltree.org. Accessed 3 August 2009
Allen JA, Krauss KW (2006) Influence of propagule flotation longevity and light availability on establishment of introduced mangrove species in Hawai’i. Pac Sci 60:367–376
Biswas SR, Choudhury JK, Nishat A, Rahman MM (2007) Do invasive plants threaten the Sundarbans mangrove forest of Bangladesh? For Ecol Manag 245:1–9
Bosire JO, Kairo JG, Kazungu J, Koedam N, Dahdouh-Guebas F (2005) Predation on propagules regulates regeneration in a high-density reforested mangrove plantation. Mar Ecol Prog Ser 299:149–155
Chen X, Lin P, Lin Y (1996) Mating system and spontaneous mutation rates for chlorophyll deficiency in populations of the mangrove Kandelia candel. Hereditas 125:47–52
Chimner RA, Fry B, Kaneshiro MY, Cormier N (2006) Current extent and historical expansion of introduced mangroves on O’ahu, Hawai’i. Pac Sci 60:377–383
Clarke WC, Thaman RR eds (1993) Agroforestry in the Pacific Islands: systems for sustainability. United Nations University Press, Tokyo, 297 pp
Clarke PJ, Kerrigan RA (2002) The effects of seed predators on the recruitment of mangroves. J Ecol 90:728–736
Costanza R, d’Arge R, de Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260
Cox EF, Allen JA (1999) Stand structure and productivity of the introduced Rhizophora mangle in Hawaii. Estuaries 22:276–284
Demopoulos AWJ, Fry B, Smith CR (2007) Food web structure in exotic and native mangroves: a Hawaii—Puerto Rico comparison. Oecologia 153:675–686
Donnelly MJ, Green DM, Walters LJ (2008) Allelopathic effects of the fruits of the Brazillian perpper Schinus terebinthifolius on growth, leaf production and biomass of seedlings of the red mangrove Rhizophora mangle and the black mangrove Avicennia germinans. J Exp Mar Biol Ecol 357:149–156
Doren RF, Jones DT (1997) Management in Everglades National Park. In: Simberloff D, Schmitz DC, Brown TC (eds) Strangers in Paradise: impact and management of nonindigenous species in Florida. Island Press, Washington, DC, 467 pp
Duke NC (1992) Mangrove floristics and biogeography. In: Robertson AI, Alongi DM (eds) Tropical mangrove ecosystems. American Geophysical Union, Washington, DC, pp 63–100
Elton CS (1958) The ecology of invasions by animals and plants. Methuen, London
Fairchild D (1898) Systematic plant introduction: its purposes and methods. Bulletin #21, Division of Forestry, US Department of Agriculture, Government Printing Office, Washington, 21 pp
Fairchild D (1945) Garden Islands of the Great East. Charles Scribners, New York, 239 pp
Gillis WT (1971) A Bruguiera Party. Kampong Notes 6(3), 30 September 1971
Gordon DR (1998) Effects of invasive, non-indigenous plant species on ecosystem processes: lessons from Florida. Ecol Appl 8:975–989
Graham A (2006) Paleobotanical evidence and molecular data in reconstructing the historical phytogeography of the Rhizophoraceae. Ann Mo Bot Gard 93:325–334
Harper JL (1965) Establishment, aggression and cohabitation. In: Baker HG, Stebbins GL (eds) The genetics of colonizing species. Academic Press, New York, pp 243–265
He BY, Lai TH, Fan HQ, Wang WQ, Zheng HL (2007) Comparison of flooding-tolerance in four mangrove species in a diurnal tidal zone in the beibu Gulf. Estuar Coast Shelf Sci 74:254–262
Imai N, Takyu M, Nakamura Y, Nakamura T (2006) Gap formation and regeneration of tropical mangrove forests in Ranong, Thailand. Plant Ecol 186:37–46
Islam MS, Lian CL, Kameyama N, Wu B, Hogetsu T (2006) Development and characterization of ten new microsatellite markers in a mangrove tree species Bruguiera gymnorrhiza (L.). Mol Ecol Notes 6:30–32
Jones DT, Doren RF (1997) The distribution, biology and control of Schinus terebinthifolius in Southern Florida, with special reference to Everglades National Park. In: Brock JH, Wade M, Pysek P, Green D (eds) Plant invasions: studies from North America and Europe. Backhuys, Leiden, pp 81–93
Krauss KW, Allen JA (2003a) Influences of salinity and shade on seedling photosynthesis and growth of two mangrove species, Rhizophora mangle and Bruguiera sexangula, introduced to Hawaii. Aquat Bot 77:311–324
Krauss KW, Allen JA (2003b) Factors influencing the regeneration of the mangrove Bruguiera gymnorrhiza (L.) Lamk. on a tropical Pacific Island. For Ecol Manag 176:49–60
Langer MR, Lipps JH (2006) Assembly and persistence of foraminifera in introduced mangroves on Moorea, French Polynesia. Micropaleontology 52:343–355
Lugo AE (1998) Mangrove forests: a tough system to invade but an easy one to rehabilitate. Mar Pollut Bull 37:427–430
Lugo AE, Zucca CP (1977) The impact of low temperature stress on mangrove structure and growth. Trop Ecol 18:149–161
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Maguire TL, Saenger P, Baverstock P, Henry R (2000) Microsatellite analysis of genetic structure in the mangrove species Avicennia marina (Forsk.) Viehr. (Avicenniaceae). Mol Ecol 9:1853–1862
McCoy ED, Heck KL Jr (1976) Biogeography of corals, seagrasses, and mangroves: an alternative to the center of origin concept. Syst Zool 25:201–210
Meziane T, d’Agata F, Lee SY (2006) Fate of mangrove organic matter along a subtropical estuary: small-scale exportation and contribution to the food of mangrove crab communities. Mar Ecol Prog Ser 312:15–27
Moseman SM, Zhang R, Qian PY, Levin LA (2009) Diversity and functional responses of nitrogen-fixing microbes to three wetland invasions. Biol Invasions 11:225–239
Nunez-Farfan J, Dominguez CA, Eguiarte LE, Cornejo A, Quijano M, Vargas J, Dirzo R (2002) Genetic divergence among Mexican populations of red mangrove (Rhizophora mangle): geographic and historic effects. Evol Ecol Res 4:1049–1064
Odum WE, Heald EJ (1975) The detritus-based food web of an estuarine mangrove community. In: Cronin LE (ed) Estuarine research. Academic Press, New York, pp 265–286
Odum WE, McIvor CC, Smith TJ III (1982) The ecology of the mangroves of South Florida: a community profile. FWS/OBS-81/24. US Fish and Wildlife Service, Office of Biological Services, Washington, DC
Peakall R, Smouse P (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Pemberton RE, Liu H (2009) Marketing time predicts naturalization of horticultural plants. Ecology 90(1):69–80
Pinzon ZS, Ewel KC, Putz FE (2003) Gap formation and forest regeneration in a Micronesian mangrove forest. J Trop Ecol 19:143–153
Raymond M, Rousset F (1995) GENEPOP (version 3.4): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Smith TJ III (1987) Seed predation in relation to tree dominance and distribution in mangrove forests. Ecology 68:266–273
Smith TJ III (2004) Development of a long-term sampling network to monitor restoration success in the southwest coast everglades: vegetation, hydrology, and sediments. USGS Fact Sheet FS-2004-3015. http://sofia.usgs.gov/publications/fs/2004-3015/
Smith TJ III, Robblee MB, Wanless HR, Doyle TW (1994) Mangroves, hurricanes and lightning strikes. Bioscience 44:256–262
Smith TJ III, Anderson GH, Balentine K, Tiling G, Ward GA, Whelan KRT (2009) Cumulative impacts of hurricanes on Florida mangrove ecosystems: sediment deposition, storm surges and vegetation. Wetlands 29:24–34
Steele OC, Ewel KC, Goldstein G (1999) The importance of propagule predation in a forest of non-indigenous mangrove trees. Wetlands 19:705–708
Stevens PW, Fox SL, Montague CL (2006) The interplay between mangroves and saltmarhes at the transition between temperate and subtropical climate in Florida. Wetlands Ecol Manage 14:435–444
Sweeney K (1967) Kampong Notes 2(6), March 1967
Tomlinson B (1986) The botany of mangroves. Cambridge University Press, Cambridge 419 pp
Walker TD, Valentine JW (1984) Equilibrium models of evolutionary species diversity and the number of empty niches. Am Nat 124:887–889
Ward GA, Smith TJ III, Whelan KRT, Doyle TW (2006) Regional processes in mangrove ecosystems: spatial scaling relationships, biomass, and turnover rates following catastrophic disturbance. Hydrobiologia 569:517–527
Ye Y, Tam NFY, Wong YS, Lu CY (2004) Does sea level rise influence propagule establishment, early growth and physiology of Kandelia candel and Bruguiera gymnorrhiza? J Exp Mar Biol Ecol 306:197–215
Zan QJ, Wang BS, Wang YJ, Li MG (2003) Ecological assessment on the introduced Sonneratia caseolaris and S-apetala at the mangrove forest of Shenzen Bay, China. Acta Bot Sin 45:544–551
Acknowledgments
David T. Jones and the staff at the Kampong of the National Tropical Botanic Garden provided unfettered access to their grounds and helped to document the history of the introduction of B. gymnorrhiza there. Our work would not have been possible without their full cooperation and assistance. We thank Mary Collins for help in obtaining information on non-native mangrove species at FTBG, and a cooperative agreement between FTBG and Miami-Dade County Department of Environmental Resources Management, Environmentally Endangered Lands Program. This study was partially supported by research funds of FTBG allocated to the Plant Molecular Systematics and Conservation Genetics lab jointly operated by FTBG and FIU. The help of K. Balentine, D. Broeksteeg, J. Eells, M. Eygenraam, H. de Groot, F. Scheibler and G. Tiling during field sampling is gratefully acknowledged. This manuscript was improved by the input of four anonymous reviewers. T. J. Smith received financial support from the Terrestrial, Freshwater and Marine Ecosystem Program of the US Geological Survey. This is contribution # 461 of the Southeast Environmental Research Center and contribution # 176 of the Tropical Biology Program at Florida International University. Mention of product and/or trade names does not imply endorsement on the part of the US Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fourqurean, J.W., Smith, T.J., Possley, J. et al. Are mangroves in the tropical Atlantic ripe for invasion? Exotic mangrove trees in the forests of South Florida. Biol Invasions 12, 2509–2522 (2010). https://doi.org/10.1007/s10530-009-9660-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9660-8