Abstract
Invasive plants strongly affect physical and biotic environments of native ecosystems. Insects and other arthropods as one of the major components of many ecosystems are very sensitive to subtle changes in abiotic and biotic environments. We examined the effects of exotic Spartina alterniflora invasion on community structure and diets of arthropods in a saltmarsh previously dominated by native Phragmites australis in Yangtze River estuary through net sweeping and plant harvesting methods and stable isotope analysis. Our results showed that diversity indices were not significantly different between exotic and native plant communities, but the total abundance of insects estimated through plant harvesting method was found to be lower in Spartina monoculture than that in Phragmites monoculture. Community structure of insects in Spartina monoculture was dissimilar to that in Phragmites monoculture and Phragmites–Spartina mixture. Moreover, stable carbon isotope patterns of arthropods were significantly different between Phragmites and Spartina monocultures. Although some native arthropods (perhaps generalists) shifted their diets, many native taxa did prefer Phragmites to Spartina even in Spartina monoculture. Spartina invasions resulted in reduced abundances of some arthropds, and increased dominance of others feeding preferably on Spartina. This study provides evidence that invasive plants can change the community structure and diets of native arthropods, which will eventually alter the arthropod food web, and affect the integrity and functioning of native ecosystems within a nature reserve that has been set aside for conserving the native biodiversity and maintaining the ecosystem integrity. In this sense, Spartina invasions in the Yangtze River estuary need to be managed appropriately.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive plants seriously threaten native biotic communities and modify physical environments of the invaded systems (Chambers et al. 1999; Lodge et al. 2006; Levine 2008). They may competitively exclude native plants on which native animals (e.g. insects, benthos, birds) may depend (Benoit and Askins 1999; Gratton and Denno 2005), which may in turn influence the structure and functioning of native ecosystems (Vitousek et al. 1997; McKinney and Lockwood 1999; Dukes and Mooney 2004).
Insects are exceptionally diverse, and one of the major components of many ecosystems, which control key ecosystem processes, in particular, the transformation of plant materials to animal materials during primary consumption (Speight et al. 1999). Insects are also sensitive to the changes in both abiotic and biotic environments, so they can be envisaged as useful indicators of subtle environmental changes (Schowalter 2000). Plants are primary producers of ecosystems and provide consumers with both food and habitats. Therefore, any shift in species composition of plant communities may alter the resource availability and habitat properties for native consumers, and hence have profound influence on local fauna, particularly insects or arthropods in general (e.g., Tallamy 2004; Gratton and Denno 2005, 2006). In particular, invasions by exotic plants disrupt the ecological interactions between organisms at different trophic levels (Dukes and Mooney 2004), and thus affect the native arthropod communities (Carroll et al. 1997; Levine et al. 2003; Gratton and Denno 2005).
Many studies have examined the effects of invasive plants on native arthropod biodiversity, and the conclusions drawn from different studies appear to be mixed, depending on the study systems (e.g. Olckers and Hulley 1991; Agrawal and Kotanen 2003; Frenzel and Brandl 2003; Herrera and Dudley 2003; Gratton and Denno 2005, 2006). Moreover, most of the studies available have been conducted in typical terrestrial ecosystems. Although plant invasions are accelerating in many estuaries like San Francisco Bay (Cohen and Carlton 1998) and Yangtze River estuary (Li et al. 2008), few studies have been conducted to examine how arthropod communities in the estuarine wetlands respond to plant invasions. We here report a case study examining the effects of Spartina alterniflora (hereafter Spartina) invasions on arthropod communities in a saltmarsh in the Yangtze River estuary, China.
The Yangtze River estuary is an important ecoregion that has large area of estuarine wetlands, of which Dongtan on Chongming Island was recognized as the Wetland of International Importance, at which a nature reserve—Chongming Dongtan National Nature Reserve was established in 2005. However, Dongtan wetland set aside for conserving the native biodiversity and the ecosystem integrity is heavily infested with invasive exotic plant, Spartina, which is devaluing the wetland.
For the purposes of erosion control, soil amelioration and dike protection, Spartina was intentionally introduced from three sites in North America (North Carolina, Georgia and Florida) to China in 1979 (An et al. 2007). Spartina has a number of biological traits (e.g. fast growth, well-developed belowground structures, high salt tolerance, great reproductive capacity through both clonal growth and sexual reproduction), making it a suitable species for ecological restoration (Hinkle and Mitsch 2005). For this reason, it was widely introduced to the east coast of China (Chung 2006), and is widely distributed along the east coast of China, from Tianjin to Baihai in Guangxi (Wang et al. 2006a). The coverage of Spartina in China was approximately 260 ha in six counties by 1985 (Chung 1989) and increased to more than 112,000 ha by 2000 (An et al. 2007).
Spartina was first found in 1995 in Dongtan wetland on Chongming Island in the Yangtze River estuary, and is believed to have arrived there though natural dispersal by water flow from Qidong, Jiangsu Province. For rapid sediment accretion in mudflats in the estuary, Spartina was intentionally introduced to Dongtan wetland twice, in 2001 and 2003, leading to a rapid range expansion in the estuary. While the introductions of Spartina to the estuary have effectively promoted sedimentation, native plants in the Yangtze River estuary, such as Scirpus mariqueter (hereafter Scirpus; Chen et al. 2004) and Phragmites australis (hereafter Phragmites; Wang et al. 2006b) were rapidly replaced by the exotic plant as Spartina is much more competitive than the natives. The changes in physical environments caused by Spartina invasions have also contributed to such rapid species replacement. Spartina now occupies 49.5% of vegetated area at Dongtan, Chongming Island, and it has thus become the most abundant plant species in the saltmarshes just over 10 years since its first occurrence in the estuary (Wang 2007).
The relacement of the native plants by Spartina has profound effects on the native ecosystems in the Yangtze River estuary (see an overview by Li et al. 2008). Previous studies have examined the effects of Spartina invasions on biodiversity including native plants (Chen et al. 2004; Wang et al. 2006b), rhizosphere bacteria (Wang et al. 2007), benthos (Chen et al. 2005; Chen et al. 2007) and fish (Quan et al. 2007) and carbon and nitrogen processes in the estuary (Liao et al. 2007, 2008). Nevertheless, there is a lack of the data on how insects and other arthropods, as one of the major components of many ecosystems, are affected by Spartina invasions, which precludes us from making a convincing conclusion as to whether or not Spartina should be extirpated in the Yangtze River estuary. The major aim of the present study was to offer such information. In so doing, this study used net sweeping and plant harvesting methods to estimate the diversity of insects, and the natural abundances of stable isotopes to infer the reliance of arthropods on native Phragmites and exotic Spartina in terms of diet. The questions we asked are: (1) What effects do Spartina invasions have on the diversity of marsh insects in the Yangtze River estuary? (2) Can such effects be explained by the changes in arthropods’ diet caused by the replacement of Phragmites by Spartina?
Materials and methods
Study sites
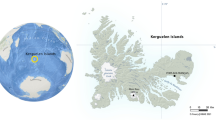
This study was conducted in Dongtan Wetland of International Importance on Chongming Island/Chongming Dongtan National Nature Reserve (31°25′–31°38′ N, 121°50′–122°05′ E) in Yangtze River estuary, China. The wetland consists three parts, Dongwangsha, Buyugang and Tuanjiesha (Fig. 1). Its annual precipitation is 1,022 mm; and mean temperature is 15.3°C, with monthly maximum of 27.5°C in July and minimum of 2.9°C in January. Total area of the vegetation varies from year to year due to rapid sedimentation, and was 3,822.57 hm2 in 2005. There are few native vascular plant species in the saltmarshes, and the dominant native species are Phragmites and Scirpus, both of which are C3 plants. An exotic C4 grass, Spartina, has invaded the saltmarshes since the mid 1990s, and has now become the most dominant plant species in Dongtan wetland. Native plant Phragmites is patchily distributed in the north and northeast of the island, and a large area of pure Phragmites stand unaffected by Spartina is restricted to Tuanjiesha, which is in the south of the island. Over the study area, Phragmites and Spartina form either monocultures or their mixtures, which exist in mosaics (Wang 2007).
Spatial distribution of plant communities in intertidal zones at Chongming Dongtan, in the Yangtze River estuary in 2005 (Wang 2007). Abbreviations of different plant communities are defined as PHM: Phragmites monoculture; PSM: Phragmites–Spartina mixture; SPM: Spartina monoculture; and RPM: reference Phragmites monoculture
Insect community structure
Sampling
To examine the effects of Spartina invasions on community structure of native insects in the saltmarshes, we sampled insects in three different habitat types (i.e., three types of plant communities): (1) Phragmites monoculture (in the north of Dongtan wetland, N 31°34.770′, E 121°54.077′); (2) Phragmites–Spartina mixture in which Phragmites was slightly taller than Spartina (in the north of Dongtan wetland, N 31°34.999′, E 121°54.392′); and (3) Spartina monoculture (in the northeast of Dongtan wetland, N 31°32.365′, E 121°58.082′). To make the results comparable among plant communities, all the three habitats were located between Dongwangsha and Buyugang (Fig. 1) in the north of Dongtan wetland, which were adjacent to each other. Phragmites–Spartina mixture represented a transitional type that Spartina was invading Phragmites community. Five plots (10 m × 10 m) in each habitat were randomly chosen as replicates. Those plots were at least 20 m away from each other to ensure sampling to be independent.
Because insects tend to move from one habitat to another, the changes of endophagous insects are more reliable to reflect alterations of plant communities than ectophagous insects (Bernay and Chapman 1994). In order to make our insect sampling effective, we employed both net sweeping (35-cm-diameter net) and plant harvesting methods, as practiced in a similar study system by Gratton and Denno (2005). With the net sweeping method, 200 sweeps were taken within each plot. In so doing, each plot was divided into four parallel transects which were apart at least 2 m from each other. We took 50 sweeps on each transect. Net contents of four 50-sweep samples were emptied into a sealable plastic bag and were combined to give a single sample for a plot. The same person sampled all sampling plots in order to avoid the differences in sampling effort caused by different persons. We used plant harvesting method to collect internal or concealed feeding insects. We randomly positioned five 0.25 m × 0.25 m quadrats in each plot, and harvested the aboveground parts of plants for each quadrat. All the materials were put into sealable plastic bags and were transported to the laboratory for inspection. Plant stems were dissected with a clipper, and all internally feeding insects were collected and counted. The insects of five quadrats were then combined to give a single sample. All insects were stored in refrigerator at −20°C till being processed. If the insects collected were larvae, they were fed in Petri dishes at room temperature until they were identifiable.
Because summer is the season of the greatest abundance and diversity of insects and other arthropods, sampling in the saltmarshes is suggested to be performed between June and September (Davis and Gray 1966; Gao et al. 2006). To make our single-season sampling meaningful, samples were collected from 28th June to 3rd July and from 18th to 22nd August, 2006 during the growing season of Phragmites and Spartina. Sampling time was between 10 AM and 3 PM on sunny days. The August sampling was supplementary to the June sampling. All insects were identified to family level or morphospecies, and their numbers were counted. Because of technical difficulties, spiders were not included in the analysis. The classification of insects followed Insect Morphology Taxonomy (Xin et al. 1985), Taxonomy of Insect Larva (Zhong 1990) and Insect Taxonomy (Zheng and Gui 1999).
Statistical analysis
Species/taxon richness (d), Simpson’s index (\( 1 - \lambda \)) and species density (Ds) were obtained from the collected data, whose changes were used to assess the effects of Spartina invasions on insect communities. Simpson’s index (1 − λ) measures the probability that two individuals randomly selected from a sample belong to different species (or taxon). The value of the index ranges from 0 to 1. Thus, the greater the value, the greater the sample diversity. These indices are calculated by:
where S is the total number of species per sampled plot, N is the total number of individuals per sampled plot, P i is the ratio of individuals of ith species in total individuals for a sampled plot, A is the area of a sampled plot.
One-way ANOVA was used to examine diversity index (species richness and Simpson’s index) of insect communities and total density among habitats. Levene’s test was used to verify homogeneity of variance (diversity index and total density), among the sampled plots for each habitat type. The Tukey HSD tests were used to conduct the follow-up tests. If the variances were not homoscedastic, the data were then log transformed to meet the assumptions for statistics prior to analysis. Non-metric multidimensional scaling (MDS) was used to examine the similarities of insect communities among the three plant communities. All analyses were performed through using either STATISTICA 6.0 or PRIMER 5 (Clarke and Warwick 1994).
Arthropods’ diets
Sampling
Unlike the analysis of insect community structure, the samples of dietary analysis included spiders as top predators. For this reason, arthropod rather than insect is used in this section. The samples used for the above analysis of the community structure were also used for dietary analysis of arthropods using stable isotopes, which can ensure dietary analysis to be comparable to that of arthropod community structure. In order to make the comparisons more convincing, we also sampled arthropods from pure Phragmites stand at Tuanjiesha (see Fig. 1) which is here defined as reference Phragmites monoculture in further description, where Phragmites grew well isolated from Spartina, and thus should not have been affected by Spartina as the effects of Spartina are spatially local. The sampling procedure was the same as that used for insect community structure. In addition to herbivorous and predacious insects, spiders, as arthropod top predators in the saltmarsh, were also collected in our study.
Stable isotope analysis
Stable isotopes are the different forms of a given chemical element that have different atomic masses, but do not decay radioactively. They are increasingly used to reveal the trophic relationships between the biotic components of ecosystems based on energy flows. The stable isotopes of carbon include 13C and 12C (expressed as δ13C), which are relatively conserved as ‘carbon’ is transferred from resources (e.g. plants or prey) to consumers (e.g., herbivores or predators) through a food web (Gratton and Denno 2006). For this reason, the C isotope signatures exhibit little or no difference between trophic levels, and can be used to determine the sources of production for consumers (Vander Zanden et al. 1999). In contrast, stable isotopes of N have two forms (15N and 14N, expressed as δ15N), whose signatures become enriched by 3–4‰ between two consecutive trophic levels (Vander Zanden et al. 1999). Therefore, δ15N can be used to determine the consumer trophic position in a food web. In our study system, the dominant plants included a C3 plant (native Phragmites) and a C4 plant (exotic Spartina), which have distinctive δ13C isotope signatures due to their different photosynthetic pathways (Gratton and Denno 2006), allowing us to identify the food sources of the marsh arthropods through comparing their δ13C signatures with those of basal resources.
In order to meet the requirements for minimum amount of materials in stable isotope analysis (0.5–1.0 mg dry mass per sample), we only selected a limited number of taxa (8–16 species, see Table 4) that had enough materials for such analysis. Since body sizes vary among the taxa, varying numbers of individuals were used for different taxa in the analysis, i.e., 20–100 individuals for smaller taxa, 5 individuals for larger taxa. Leaf tissues of Phragmites or Spartina for isotope analysis were collected from 10 randomly selected plants from each sampled plot, immersed and rinsed with deionized water before use. Soil samples were collected on the soil surface in each habitat by soil core of 3 cm in diameter and 5 cm in depth (sampling depth), and also immersed and rinsed with deionized water, and then passed through a 0.15 mm sieve. All samples (including arthropods, plants and soil) were dried at 55°C for 48 h, and then ground to fine powder using mortar and pestle.
Carbon and nitrogen stable isotope ratios were analyzed on an isotope ratio mass spectrometer (Thermo Finnigen, Delta-Plus, Flash, EA, 1112 Series, USA) in the Stable Isotope Laboratory for Ecological and Environment Research, Institute of Botany, the Chinese Academy of Sciences, China. Urea and glycine were analyzed as accuracy and precision standards for isotopic ratios. Ratios of 13C/12C and 15N/14N are expressed in δ notation as ‰, which are consistent with international standards (Vienna Pee Dee Belemnite for carbon, and atmospheric for nitrogen). δ is calculated by the formula:
where X is 13C or 15N, R is the ratio of 13C/12C or 15N/14N. The analytical precisions of these measurements were 0.2‰ for δ13C and 0.3‰ for δ15N, respectively. We calculated the mean values of δ13C and δ15N for plants, soil and each taxon of arthropods selected.
To get an in-depth result, a two-source mixing model was used to quantify the contributions of food sources to arthropods’ diets. Mean δ13C values of two primary producers, insect consumers, and predator spiders were used in this equation. Isotopically feasible combination of source contributions summing to 100%, sources were aggregated into two groups, i.e. C3 vascular plant (Phragmites australis), C4 vascular plant (Spartina alterniflora). The respective percentage contributions from C3 plant and C4 plant are given by the following equations:
where P 3 and P 4 are respective percentage contributions of C 3 and C 4 plants; and δ 13C3 andδ13C4 are respective stable isotope values of C3 and C4 plants.
Results
Insect communities as affected by invasive Spartina
In total, 9960 individuals of insects were found from three plant communities, which belonged to 11 orders, 111 families and 212 species, respectively (Table 1, Appendix I). Both family and species numbers were the highest in Phragmites–Spartina mixture, and the lowest in Spartina monoculture. Diversity indices including species richness (F2,12 = 3.12, P = 0.08), Simpson’s index (F2,12 = 1.55, P = 0.25) and the total density of insects (F2,12 = 1.74, P = 0.22) by net sweeping method were not significantly different among the three plant communities (Table 2, Fig. 2). However, both species richness (F2,12 = 5.15 P < 0.05) and the total density of insects (F2,12 = 14.58 P < 0.01) estimated by the plant harvesting method were significantly different among the three plant communities although Simpson’s index was similar among the plant communities (F2,12 = 0.72 P = 0.51) (Table 2, Fig. 2). Only the total density of insects was found to be significantly different among the plant communities (F2,12 = 13.76 P < 0.01) when the collections by net sweeping and plant harvesting methods were combined (Table 2).
Comparisons of insect communities collected from different plant communities through net sweeping (a, c, e) and plant harvesting methods (b, d, f). (a) and (b) Insect species richness; (c) and (d) Simpson’s index; and (e) and (f) total density of insects. The means (±standard errors) are presented for all the variables in the figure. PHM: Phragmites monoculture, PSM: Phragmites–Spartina mixture, SPM: Spartina monoculture. The same lower case letters indicate no significant difference among the plant communities, and the different letters significant differences (P < 0.05)
Similarity of insect communities inhabiting different plant communities
Insect communities inhabiting the three plant communities could be separated clearly by MDS ordination (Fig. 3, stress: 0.09). Results show that insect community in Phragmites monoculture was similar to that in Phragmites–Spartina mixture, implying that insect community in the mixture might be more affected by Phragmites than by Spartina. The insect community in Spartina monoculture appeared to be different from that in both Phragmites monoculture and Phragmites–Spartina mixture (Fig. 3).
Dietary partition through stable isotope analysis
Native Phragmites and exotic Spartina are respectively C3 and C4 plants. The δ13C values were –14.18‰ (±1.52‰) and −25.38‰ (±1.33‰) respectively for Spartina and Phragmites. The δ15N value of Phragmites was 5.58‰ (±1.16‰), being similar to that of Spartina (4.83‰±1.08‰). Soil δ13C value was −11.35‰ (±1.73‰); and soil δ15N value was 2.41‰ (±0.80‰) (Fig. 4).
Distribution of δ13C andδ15N isotopes for the major arthropod groups in four plant communities, collected by both net sweeping and plant harvesting methods. Base resources: Soil, soil; Pa, Phragmites; Sa, Spartina. Different symbols represent insect communities in different habitat types, i.e., ○: reference Phragmites monoculture; □: Phragmites monoculture; ◊: Phragmites–Spartina mixture; ∆: Spartina monoculture. 1: Coccinellidae; 2: Pedilidae; 3: Delphacidae; 4: Coccidae; 5: Lygaeidae; 6: Ichneumonidae; 7: Formicidae; 8: Tettigoniidae; 9: Gryllidae; 10: Caenagriidae; 11: Chrysopidae; 12: Leptophlebiidae; 13: Labiduridae; 14: Simuliidae; 15: Sarcophagidae; 16: Tachinidae; 17: Coenomyiidae; 18: Lauxaniidae; 19: Phoridae; 20: Pallopteridaec; 21: Spiders
Stable isotope patterns of arthropods in reference Phragemites monoculture were significantly different from those in other three plant communities (Fig. 4). The δ13C and δ15N values of most arthropod taxa were slightly lower in reference Phragmites monoculture than in other plant communities. The δ15N values of arthropods were higher in Phragmites monoculture and Phragmites–Spartina mixture than those in reference Phragmites and Spartina monocultures. Our results suggest that several taxa, including the generalist grasshoppers Tettigoniidae and Gryllidae, flies Tachinidae and Tachinidae, tended to select Spartina as their diet. However, some taxa, such as Coccinellidae and Ichneumonidae, still selected Phragmites as their diet. In general, the δ15N values of arthropods showed a slightly decreasing trend when Spartina invaded the Phragmites community. It is suggested that the food web in Phragmites monoculture and Phragmites–Spartina mixture was more complex than that in reference Phragmites and Spartina monocultures.
Surprisingly, spiders as the top arthropod predator did not have the highest δ15N value; and the δ15N values for the spiders were quite low, and varied among the plant communities except for reference Phragmites monoculture. The δ13C values of the spiders were lower than those of other taxa. In contrast, the δ15N values of some parasitic flies like Tachinidae and Saprophage like Lauxaniidae were higher than those of the spiders.
In order to better demonstrate the patterns given in Fig. 4, the mean distances between arthropods in a given habitat and the local dominant plant species/soil were calculated from the data given in Fig. 4 using the isotopic values, and presented in Table 3. Table 3 shows that arthropods were closer to Phragmites than Spartina and soil in relation to trophic relationship although those from Spartina monoculture were more similar to Spartina than others.
Phragmites and Spartina’s contributions to diets of arthropods
Although both Phragmites and Spartina were consumed by insects in the saltmarsh, their relative contributions to diets of insects and their predator spiders varied considerably among the arthropod taxa (Table 4). In reference Phragmites monoculture, insects mainly fed on Phragmites except for grasshopper (Tettigonidae). In Phragmites monocultures, there are members of a food web in which the dominant primary producer was Phragmites. The contributions of Phragmites to the diets of Diptera, Hymenoptera Ichneumonidae, Dermaptera Labiduridae and Neuroptera Chrysopidae in Phragmites–Sparitina mixture were lower than those in Phragmites monoculture. In Spartina monoculture, Spartina was the main food source for Diptera and the only food source for Orthoptera insects. Coleoptera insects just fed on Phragmites even in Spartina monoculture.
Discussion
Effects of Spartina invasion on insect community structure
Spartina is a global plant invader in coastal wetlands that can modify the abiotic and biotic environments through so-called ecosystem engineering processes (Crooks 2002). Our results obtained here show that Spartina invasion in saltmarshes altered native insect assemblages in the estuarine wetlands in the Yangtze River mouth. Although species richness, diversity and density of insects in Phragmites–Spartina mixture and Spartina monoculture obtained through using net sweeping method were not significantly different from those in Phragmites monoculture (Fig. 2), the number and structure of the native insect communities were considerably altered in response to Spartina invasion (Table 1, Fig. 3). It is highly likely that Spartina can modify physical environments of the wetlands (Lindsay and French 2006), and alters the quality of detritus and litter (Vince et al. 1981; Chen et al. 2007).
In this study, we sampled insects through using both net sweeping and plant harvesting methods, which are the two most appropriate ones. Other methods like color traps, sugar traps and pitfall traps are also available (Sutherland 2003), but could not be used in our study system that is greatly affected by tides. We found that the lowest density of insects in Spartina monoculture obtained through plant harvesting method. It is also suggested that fewer insects relied on Spartina as food and/or the habitats that Spartina created. Some insects we found through net sweeping might be opportunitists that might have randomly appeared in Spartina monoculture, which can be viewed as one of the limitations of net sweeping. For this reason, some of insect diversity indices obtained through net sweeping were not significantly different. In this sense, plant harvesting is better than net sweeping for estimating insect diversity indices, especially for determining the effects of plant invasions.
Our results support the view that most of native insects preferred native plants to exotic ones. It is suggested that Spartina would be little affected, at least less affected by native insects than Phragmites. It is highly possible that Spartina might have experienced, to a certain degree, natural enemy release in its non-native range, which has been confirmed in ‘young’ introduced Spartina alterniflora population from San Francisco, California (Daehler and Strong 1997). It might be also the enemy release that has facilitated rapid growth and spread of Spartina in the Yangtze River estuary. Although this enemy release hypothesis (ERH) has been tested for many species (see review by Keane and Crawley 2002), further studies are still needed to test for ERH in relation to fitness differences in Spartina
At Chongming Dongtan, Spartina and Phragmites form either their respective monocultures or mixtures in which Phragmites is generally taller than Spartina, which produces a mosaic of three types of plant communities in the saltmarshes. The greatest species number, diversity and density of native insects observed in Phragmtes–Spartina mixture suggest that plant communities with more species provided more heterogeneous habitats that can accommodate a greater array of insects (Denno et al. 2004; Langellotto and Denno 2004). However, another possibility might also exist that the exotic plant accumulates native insect pests that harm native plants. Although such effects have not yet been tested in relation to insects, a recent study has shown that exotic invasive plant can accumulate native soil pathogens which inhibit native plants, which facilitates the invasion of Chromolaena odorata in India (Mangla et al. 2008).
Diet choice of native arthropods in response to Spartina invasion
In the present study, the 13C and δ15N patterns suggest that arthropod food webs have shifted after Spartina invaded the saltmarshes in the Yangtze River estuary. Other studies have also reported similar results that invasive species alter food web structure (Spencer et al. 1999; Vander Zanden et al. 1999; Gratton and Denno 2005, 2006; Levin et al. 2006). Our dietary analysis revealed that some native insects such as generalist grasshoppers changed their diet when they inhabited Spartina monoculture. Furthermore, our study shows that the insects like Coccinellidae still fed only on native plant Phragmites, even in Spartina monoculture. Therefore, those feeding only on Phragmites may be threatened after Spartina further invades the Dongtan wetland on Chongming Island. The δ13C values of some insects stayed between C3 (Phragmites) and C4 plants (Spartina), which reflected the fact that these insects moved between these two plants, and fed on both of them. However, most insects in Spartina monoculture still used Phragmites as their basal food resource. Therefore, if exotic Spartina replaced the native plants, and occupied the whole area, the abundances of the insects relying mainly on Phragmites would be reduced, and those of the insect feeding mainly on Spartina would become the dominant species in arthropod community.
The δ15N values for some insects like flies (Phoridae) and grasshoppers in Spartina monoculture were lower than those in Phragmites monoculture and Phragmites–Spartina mixture, indicating that the position of these insects in food web was lower in Spartina monoculture than that in other plant communities. The δ13C values of spiders were close to those of Spartina, and their δ15N values were lower in Spartina monoculture than in other communities. It implies that spiders as a top predator in the arthropod food-web were also altered after Spartina invasion, and that Spartina invasion might have simplified the arthropod food web. In addition, the δ13C values of some insect taxa (e.g., Pedilidae, Caenagriidae, Leptophlebiidae, Simuliidae) in the reference Phragmites monoculture were lower than those of Phragmites, reflecting that these taxa might have fed on other food sources with lower δ13C values. Detritus or algae can also be the main food source for some insects in saltmarshes (Gratton and Denno 2006). Stable isotope analysis of soil samples is an indirect method to determine whether detritus or algae are the food source of insects. Our results show that the δ13C value of soil was much higher than that of Phragmites and Spartina. The δ13C values of all arthropods were much lower than that of soil, being between those of Phragmites and Spartina. The possibility might be low that detritus or algae were the basal food sources of arthropods.
Exotic Spartina locally replaced native Phragmites in the Yangtze River estuary, which is a threat to the native insects feeding on native Phragmites. Most of those insects would decrease in abundance due to the lack of food sources and habitats, whereas other insects that feed mainly on exotic Spartina would expand rapidly due to the release of opportunity niches. There might be a possibility that just a few dominate the arthropod communities in the saltmarshes in the Yangtze River estuary with further expansion of Spartina. Therefore, it is necessary to mange Spartina invasions appropriately so as to maintain the native biodiversity and the integrity of the saltmarshes in the Yangtze River estuary.
In this study, arthropod communities were investigated only in a growing season (June and August), albeit in a season when arthropod diversity may be the highest. We made the assumptions: I) that the arthropod diversity observed in growing season can basically reflect that for the whole year, II) that the isotopic values of the taxa sampled reflect the resources they have been consuming over the growing season (Gratton and Denno 2006). However, it is well known that species richness and abundance are seasonally and inter-annually variable, which may make assumption I invalid. For this reason, it is likely to miss certain species that may be actually abundant in our study area. A single-season dataset obviously prevents us from obtaining a general picture of the effects of Spartina invasions on arthropod communities although it is probably valid only for the comparative purpose. For assumption II, it can be valid for long-lived arthropods like spiders and for host-specific herbivores that do not change their plants hosts (Gratton and Denno 2006), but what remains unknown is whether assumption II holds valid for other organisms in our study. Furthermore, it is also uncertain whether the differences in arthropod communities between plant communities and the patterns of C and N isotopes would hold true at other times of the year or in different years. It is not possible for us to exclude the possibility that Spartina supports other arthropod species at other times of the year or in different years.
In conclusion, like the replacement of Spartina by Phragmites in the saltmarshes in the USA (Gratton and Denno 2005, 2006), Spartina invasion into Phragmites communities in the Yangtze River estuary altered the arthropod assemblages, especially led to reduced abundance of concealed feeding insects. A stable isotope analysis of the arthropod assemblages showed that although most of native arthropods of the saltmarshes in the Yangtze River estuary did prefer Phragmites to Spartina even in Spartina monoculture, some of native arthropods (perhaps generalists) shifted their diets to Spartina. With further invasions in the Yangtze River estuary, these arthropods with dietary shift will become more abundant, which will eventually alter arthropod food web, and affect integrity and functioning of native ecosystems. Considering that Spartina invasions affect other aspects of biodiversity and ecosystem processes in the estuarine wetlands (Li et al. 2008), and that Chongming Dongtan has been set aside for conserving the native biodiversity and maintaining the ecosystem integrity, Spartina invasions in the Yangtze River estuary need to be managed appropriately.
References
Agrawal AA, Kotanen PM (2003) Herbivores and the success of exotic plants: a phylogenetically controlled experiment. Ecol Lett 6:712–715
An SQ, Gu BH, Zhou CF et al (2007) Spartina invasion in China: implications for invasive species management and future research. Weed Res 47:183–191
Benoit LK, Askins RA (1999) Impact of the spread of Phragmites on the distribution of birds in Connecticut tidal marshes. Wetlands 19:194–208
Bernay EA, Chapman RF (1994) Host-plant selection by phytophagous insects. Chapman and Hall, New York
Carroll SP, Dingle H, Klassen SP (1997) Genetic differentiation of fitness-associated traits among rapidly evolving populations of the soapberry bug. Evolution 51:1182–1188
Chambers RM, Meyerson LA, Saltonstall K (1999) Expansion of Phragmites australis into tidal wetlands of North America. Aquat Bot 64:261–273
Chen ZY, Li B, Chen JK (2004) Ecological consequences and management of Spartina spp. invasions in coastal ecosystems. Biodivers Sci 12:280–289 (in Chinese with English abstract)
Chen ZY, Fu CZ, Wang HY et al (2005) Effects of Spartina alterniflora invasive on the benthic macro-onvertebrates community at Dongtan of Chongming salt marsh, the Yangtze river estuary. Wetland Sci 1:1–7 (in Chinese with English abstract)
Chen HL, Li B, Fang CM et al (2007) Exotic plant influences soil nematode communities through litter input. Soil Biol Biochem Soil 39:1782–1793
Chung CH (1989) Ecological engineering of coastline with salt marsh plantations. In: Mitsch WJ, Jorgensen SA (eds) Ecological engineering: an introduction to eco-technology. Wiley, New York, pp 255–289
Chung CH (2006) Forty years of ecological engineering with Spartina plantations in China. Ecol Eng 27:49–57
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth, Plymouth
Cohen AN, Carlton JT (1998) Accelerating invasion rate in a highly invaded estuary. Science 279:555–558
Crooks JA (2002) Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97:153–166
Daehler CC, Strong DR (1997) Reduced herbivore resistance in introduced smooth cordgrass (Spartina alterniflora) after a century of herbivore-free growth. Oecologia 110:99–108
Davis LV, Gray IE (1966) Zonal and seasonal distribution of insects in North Carolina salt marshes. Ecol Monogr 36:275–295
Denno RF, Mitter MS, Langellotto GA et al (2004) Interactions between a hunting spider and a web-builder: consequences of intraguild predation and cannibalism for prey suppression. Ecol Entomol 29:566–577
Dukes JS, Mooney HA (2004) Disruption of ecosystem processes in western North America by invasive species. Rev Chil Hist Nat 77:411–437
Frenzel M, Brandl R (2003) Diversity and abundance patterns of phytophagous insect communities on alien and native host plants in the Brassicaceae. Ecography 26:723–730
Gao H, Peng XW, Li B et al (2006) Effects of the invasive plant Spartina alterniflora on insect diversity in Jiuduansha wetlands in the Yangtze River Estuary. Biodiversity Science 14:400–409 (in Chinese with English abstract)
Gratton C, Denno RF (2005) Restoration of arthropod assemblages in a Spartina salt marsh following removal of the invasive plant Phragmites australis. Restor Ecol 13:358–372
Gratton C, Denno RF (2006) Arthropod food web restoration following removal of an invasive wetland plant. Ecol Appl 16:622–631
Herrera AM, Dudley TL (2003) Reduction of riparian arthropod abundance and diversity as a consequence of giant reed (Arundo donax) invasion. Biol Invasions 5:167–177
Hinkle RL, Mitsch WJ (2005) Salt marsh vegetation recovery at salt hay farm wetland restoration sites on Delaware Bay. Ecol Eng 25:240–251
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Langellotto GA, Denno RF (2004) Responses of invertebrate natural enemies to complex-structured habitats: a meta-analytical synthesis. Oecologia 139:1–10
Levine JM (2008) Biological invasions. Curr Biol 18:R57–R60
Levine JM, Vila M, D’Antonio CM et al (2003) Mechanisms underlying the impacts of exotic plant invasions. Proc R Soc Lond B 270:775–781
Levin LA, Neira C, Grosholz ED (2006) Invasive cordgrass modifies wetland trophic function. Ecology 87:419–432
Li B, Liao CZ, Zhang XD et al (2008) Spartina alterniflora invasions in the Yangtze River estuary, China: an overview of current status and ecosystem effects. Ecol Eng (in press)
Liao CZ, Luo YQ, Jiang LF et al (2007) Invasion of Spartina alterniflora enhanced ecosystem carbon and nitrogen stocks in the Yangtze Estuary, China. Ecosystems 10:1351–1361
Liao CZ, Luo YQ, Fang CM et al (2008) Litter pool sizes, decomposition, and nitrogen dynamics in Spartina alterniflora-invaded and native coastal marshlands of the Yangtze Estuary. Oecologia 156:589–600
Lindsay EA, French K (2006) The impact of the weed Chrysanthemoides monilifera ssp. rotundata on coastal leaf litter invertebrates. Biol Invasions 8:177–192
Lodge DM, Williams S, MacIsaac HJ et al (2006) Biological invasions: recommendations for US policy and management. Ecol Appl 16:2035–2054
Mangla S, Inderjit, Callaway RM (2008) Exotic invasive plant accumulates native soil pathogens which inhibit native plants. J Ecol 96:58–67
McKinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14:450–453
Olckers T, Hulley PE (1991) Impoverished insect herbivore faunas on the exotic bugweed Solanum mauritianum Scop relative to indigenous Solanum species in Natal Kwazulu and the Transkei. J Entomol Soc South Afr 54:39–50
Quan WM, Fu CZ, Jin BS et al (2007) Tidal marshes as energy sources for commercially important nektonic organisms: stable isotope analysis. Mar Ecol Prog Ser 352:89–99
Schowalter TD (2000) Insect ecology, an ecosystem approach. Academic Press, San Diego
Spencer CN, Potter DS, Bukantis RT et al (1999) Impact of predation by Mysis relicta on zooplankton in Flathead Lake, Montana, USA. J Plankton Res 21:51–64
Speight MR, Hunter MD, Watt AD (1999) Ecology of insects: concepts and applications. Blackwell Science, Oxford
Sutherland WJ (2003) Ecological census techniques: a handbook. Cambridge University Press, Cambridge
Tallamy DW (2004) Do alien plants reduce insect biomass? Conserv Biol 18:1689–1692
Vander Zanden MJ, Casselman JM, Rasmussen JB (1999) Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 401:464–467
Vince SW, Valiea I, Teal JM (1981) An experimental study of the structure of herbivorous insect communities in a salt marsh. Ecology 62:1662–1678
Vitousek PM, Mooney HA, Lubchenco J et al (1997) Human domination of earth’s ecosystems. Science 277:494–499
Wang Q (2007) The dynamics of plant community distribution of the salt marshes in the Yangtze River estuary as influenced by Spartina alterniflora invasions. Dissertation. Fudan University, Shanghai (in Chinese with English abstract)
Wang Q, An SQ, Ma ZJ et al (2006a) Invasive Spartina alterniflora-biology, ecology and management. Acta Phytotaxon Sin 44:559–588 (in Chinese with English abstract)
Wang Q, Wang CH, Zhao B et al (2006b) Effects of growing conditions on the growth of and interactions between salt marsh plants: implications for invasibility of habitats. Biol Invasions 8:1547–1560
Wang M, Chen JK, Li B (2007) Characterization of bacterial community structure and diversity in rhizosphere soils of three plants in rapidly changing salt marshes using 16S rDNA. Pedosphere 17:545–556
Xin JL, Yang QS, Hu CY (1985) Insect morphology taxonomy. Fudan University Press, Shanghai (in Chinese)
Zheng LY, Gui H (1999) Insect taxonomy. Nanjing Normal University Press, Nanjing (in Chinese)
Zhong JM (1990) Taxonomy of insect larva. Agriculture Press, Beijing (in Chinese)
Acknowledgements
We thank the students in the Lab of Biological Invasions at Fudan University for their assistance with field work, and the Stable Isotope Laboratory for Ecological and Environment Research, Institute of Botany, the Chinese Academy of Sciences for stable isotope analysis. This work was financially supported by National Basic Research Program of China (grant no. 2006CB403305), Natural Science Foundation of China (Grant No.: 30670330 and 30370235), and the Ministry of Education of China (Grant No.: 105063) to Bo Li. Finally but not least, we thank one of journal editors and two anonymous referees for their constructive comments on the early version of this manuscript, which greatly improved the quality of our paper.
Author information
Authors and Affiliations
Corresponding author
Appendix I
Appendix I
Relative abundance of arthropod taxa in three plant communities in Dongtan saltmarsh of the Yangtze River estuary, expressed as %
Order/Family | Relative abundance (%) | ||
|---|---|---|---|
Phragmites monoculture | Phragmites–Spartina mixture | Spartina monoculture | |
Coleoptera | |||
Aglycyderidae | 0 | 0 | <1 (S) |
Bostrychidae | 0 | 0 | <1(S) |
Carabidae | 0 | <1 (C) | 0 |
Chrysomelidae | 0 | 0 | <1 (H) |
Coccinellidae | 15 (C) | 10 (C) | 5 (S) |
Dermestidae | <1 (S) | 1 (C) | <1 (S) |
Elateridae | <1 (S) | 0 | 0 |
Lyctidae | <1 (H) | <1 C | 0 |
Nitidulidae | <1 (S) | <1 (H) | 0 |
Oedemeridae | <1 (H) | 0 | 0 |
Pedilidae | <1 (C) | <1 (S) | 0 |
Sarothriidae | 0 | <1 (C) | 0 |
Scaphidiidae | 1 (C) | 1 (C) | <1 (S) |
Staphylinidae | <1 (H) | <1 (S) | 0 |
Tenebrionidae | 1 (S) | 1 (C) | <1 (S) |
Xylophilidae | <1 (S) | 0 | 0 |
Dermaptera | |||
Labiduridae | <1 (S) | <1 (C) | 0 |
Diptera | |||
Anthomyiidae | <1 (S) | 0 | 0 |
Bibionidae | <1 (S) | <1 (C) | <1 (S) |
Calliphoridae | <1 (S) | 0 | 0 |
Canaceidae | 0 | <1 (S) | <1 (S) |
Cecidomyiidae | <1 (C) | 1 (C) | 1 (C) |
Chironomidae | <1 (S) | <1 (S) | 5 (S) |
Chloropidae | 0 | 0 | 1 (S) |
Clusiidae | 0 | <1 (S) | 0 |
Coelopidae | 0 | 0 C | <1 (C) |
Coenomyiidae | <1 (S) | 1 (S) | 0 |
Cordyluridae | 0 | 0 | <1 (S) |
Culicidae | 0 | <1 (S) | <1 (S) |
Dixidae | 0 | 0 | <1 (S) |
Dolichopodidae | <1 (S) | 0 | 0 |
Empididae | <1 (S) | <1 (S) | <1 (C) |
Gastrophilidae | 1 (S) | <1 (S) | <1 (S) |
Hypodermatidae | <1 (S) | <1 (S) | 0 |
Lauxaniidae | <1 (S) | 2 (S) | 1 (C) |
Lonchaeidae | <1 (S) | 0 | <1 (S) |
Muscidae | 0 | <1 (S) | 0 |
Mycetophilidae | <1 (S) | 0 | <1 (S) |
Ochthiphilidae | <1 (S) | <1 (C) | <1 (S) |
Oestridae | 0 | <1 (S) | 0 |
Otitidae | 1 (C) | <1 (S) | <1 (S) |
Pallopteridae | 5 (C) | 17 (S) | 21 (S) |
Platypezidae | 0 | <1 (S) | 0 |
Rhinophoridae | 0 | 0 | <1 (S) |
Ropalomeridae | <1 (S) | 0 | <1 (S) |
Sarcophagidae | 0 | <1 (S) | 0 |
Scastopsidae | <1 (S) | <1 (S) | 0 |
Scatopsidae | 0 | <1 (S) | 1 (S) |
Sciomyzidae | <1 (S) | 0 | 0 |
Simuliidae | <1 (S) | <1 (C) | <1 (S) |
Stratiomyiidae | <1 (S) | <1 (S) | 0 |
Synneuridae | <1 (S) | 0 | 0 |
Syrphidae | 2 (S) | <1 (C) | <1 (S) |
Thaumaleidae | 0 | <1 (C) | 0 |
Therevidae | <1 (S) | <1 (S) | 0 |
Tipulidae | <1 (S) | <1 (S) | 2 (S) |
Trichoceridae | 0 | <1 (S) | <1 (S) |
Trypetidae | 0 | <1 (S) | <1 (S) |
Hemiptera | |||
Corixidae | <1 (S) | 0 | 0 |
Isometopidae | <1 (C) | <1 (S) | <1 (S) |
Lygaeidae | 3 (C) | 15 (C) | <1 (S) |
Miridae | 0 | <1 (S) | <1 (S) |
Nabidae | 0 | 0 | <1 (S) |
Urostylidae | <1 (S) | 0 | 0 |
Homoptera | |||
Achilidae | <1 (S) | <1 (S) | <1 (S) |
Aphididae | <1 (S) | <1 (S) | <1 (S) |
Coccidae | 41 (C) | 12 (C) | 0 |
Delphacidae | 11 (C) | 5 (C) | 2 (S) |
Fulgoridae | 0 | <1 (C) | 0 |
Gyponidae | 0 | <1 (S) | 0 |
Phylloxeridae | <1 (S) | 0 | 0 |
Hymenoptera | |||
Aphelinidae | <1 (C) | <1 (C) | 0 |
Braconidae | <1 (S) | 0 | 0 |
Chalcidae | <1 (S) | <1 (H) | 0 |
Cleonymidae | <1 (S) | <1 (S) | <1 (S) |
Cynipidae | 0 | <1 (S) | 0 |
Dryinidae | <1 (C) | 0 | 0 |
Elasmidae | <1 (S) | <1 C | 0 |
Encyrtidae | <1 (S) | 0 | 0 |
Eucharitidae | <1 (S) | 0 | 0 |
Eucoilidae | <1 (S) | <1 (S) | 0 |
Eulophidae | <1 (C) | 1 (C) | <1 (S) |
Eupelmidae | 0 | <1 (H) | 0 |
Figitidae | <1 (C) | <1 (S) | <1 (S) |
Formicidae | <1 (S) | 5 (C) | <1 (S) |
Ibaliidae | <1 (S) | 0 | 0 |
Ichneumonidae | 3 (C) | 3 (C) | <1 (C) |
Mymaridae | 0 | <1 (S) | <1 (S) |
Perilampidae | <1 (S) | <1 (S) | 0 |
Platygasteridae | <1 (S) | <1 (H) | 0 |
Pteromalidae | <1 (S) | <1 (S) | <1 (S) |
Scelionidae | 0 | <1 (S) | 0 |
Tetracampidae | <1 (S) | <1 (S) | <1 (S) |
Thysanidae | 0 | <1 (S) | 0 |
Trigonalidae | 0 | <1 (S) | 0 |
Lepidoptera | |||
Geometridae | <1 (S) | 0 | <1(S) |
Gracilariidae | 0 | 0 | <1 (H) |
Tineidae | 0 | <1 (S) | 0 |
Neuroptera | |||
Chrysopidae | <1 (S) | <1 (C) | <1 (S) |
Hemerobiidae | 0 | <1 (S) | 0 |
Sisyridae | <1 (S) | 0 | 0 |
Odonata | |||
Caenagriidae | <1 (S) | <1 (S) | <1 (S) |
Orthoptera | |||
Acridiidae | 0 | 0 | <1 (S) |
Gryllidae | 0 | <1 (S) | 1 (S) |
Tettigoniidae | 0 | <1 (S) | <1 (S) |
Psocptera | |||
Psyllipsocidae | <1 (C) | <1 (S) | 3 (C) |
Thysanoptera | |||
Phloeotripidae | <1 (C) | 1 (C) | 9 (S) |
Spider | <1 (S) | <1 (S) | <1 (S) |
Rights and permissions
About this article
Cite this article
Wu, YT., Wang, CH., Zhang, XD. et al. Effects of saltmarsh invasion by Spartina alterniflora on arthropod community structure and diets. Biol Invasions 11, 635–649 (2009). https://doi.org/10.1007/s10530-008-9279-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-008-9279-1







 : Phragmites–Spartina mixture; □: Spartina monoculture
: Phragmites–Spartina mixture; □: Spartina monoculture