Abstract
Changes in macroalgal community structure caused by invasive seaweeds have strong impacts on the associated macrofauna due to the role of macroalgae as autogenic ecosystem engineers. This study investigates the effect of Undaria pinnatifida on the abundance and diversity of benthic macrofauna in Golfo Nuevo, northern Patagonia, using a manipulative experiment involving the systematic removal of Undaria microthalli from strips of hard bottom during its eighth-month growing period. Species richness and diversity were higher in plots covered by Undaria than when Undaria was removed. Also, the abundance of two species of crustaceans, one species of sea urchin, one species of nemertina and several species of polychaetes was higher. We attribute these effects to the provision of new habitat structure by Undaria, a larger and structurally more complex species than the local native seaweeds. These results support the hypothesis that complex habitats enhance abundance and species richness, by offering different shelter and foraging opportunities compared to morphologically simpler habitats. Based on a review of diet studies in the region, we speculate that Undaria could potentially produce a bottom-up effect on local food chains by increasing abundance of prey for a wide variety of predators, from invertebrates to marine mammals. While our study has a narrow temporal and spatial scale, we expect similar effects of Undaria on the macrofauna in other sites of the Argentine coast, especially those dominated by small native macroalgae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seaweed invasions and eutrophication are among the most common anthropogenic impacts on coastal systems around the world (Kraufvelin and Salovius 2004; Lotze et al. 2006). Invasive species in most cases dominate native algal communities, while eutrophication increases primary production, inducing blooms of opportunistic algae capable of rapidly exploiting the elevated amounts of nutrients (Duarte 1995; Johnson 2007; Johnson and Chapman 2007). These processes produce changes in the structure of algal communities, and consequently in the associated benthic fauna (Buschbaum et al. 2006). The strong influence of marine macroalgae on benthic faunal communities is due to their role as autogenic ecosystem engineers: they add spatial complexity to the substrate and further modify a wide range of physical and environmental factors at a local spatial scale (Bolam and Fernandes 2002; Bolam et al. 2000; Chemello and Milazzo 2002; Forrest and Taylor 2002; Gribben and Wright 2006; Gribben et al. 2009; Schaffelke and Hewitt 2007; Schmidt and Scheibling 2007; Stuart 2004).
Undaria pinnatifida is native of northeast Asia (Japan, Korea and China) (Akiyama and Kurogi 1982). In 1971 it was found in l`Etang de Thau (France) and since then it has spread throughout the Mediterranean coasts and to other temperate waters around the world, mainly by shipping (Curiel et al. 1994; Fletcher and Manfredi 1995; Hay 1990; Salinas et al. 1996; Stegenga 1999). Since its introduction in Puerto Madryn, Argentina, in 1992, it first spread all over Golfo Nuevo, and has now reached sites 700 km south of the gulf’s mouth on the outer coast, and recently managed to bypass (probably by shipping) the natural barrier represented by Península de Valdés, invading San José Gulf (Fig. 1) (Martin and Bastida 2008). With the exception of a few seasonal patches of Macrocystis pyrifera (Laminariales Laminariaceae) forests, most native species of macroalgae (e. g. Codium spp., Dictyota sp. and Ulva spp.) rarely exceed few centimetres in height (Eyras and Boraso 1994). The high densities of Undaria now observed along Golfo Nuevo have markedly changed the sea bottom landscape in waters between 0 and 15 m depth. Young Undaria sporophytes recruit in winter and grow rapidly to form dense forests between spring and early summer when plants reach a length of up to 2 m. The individuals begin to decompose in late spring, and their size progressively reduce until mid-autumn when hard bottoms become free of this algae (Casas et al. 2008). Golfo Nuevo belongs to the Peninsula Valdes area, listed as World Natural Heritage by the UNESCO in 1999. The conservation and management of this ecosystem are a big concern for both the public and private sectors. Yet, the impact of Undaria on local communities is unknown.
In this study we evaluate the effect of Undaria on the diversity and abundance of hard-bottom benthic macrofauna via a manipulative experiment in which we kept strips of sea bottom free of Undaria during its eighth-month growing period. In order to discuss potential bottom-up effects of the changes induced by Undaria we review regional diet studies to assess the relative importance of the taxa associated with Undaria as prey for other species.
Materials and methods
Study site
The field study was conducted close to Punta Cuevas, Golfo Nuevo (Fig. 1), on a relatively flat area occupied by limestone platforms and sandy patches. The hard bottoms (limestone platforms) are dominated by Codium spp., Dictyota sp, Ulva spp., the mussel Aulacomya atra atra and, since 2000, by dense seasonal forests of Undaria pinnatifida. Punta Cuevas was chosen because it is an accessible area.
Sampling design
An experimental grid consisting of 10 contiguous strips (2.25 m × 5 m) was set up in April 2008 on a limestone platform, at a depth that varied between 6 m at low tide and 11 m at high tide. Undaria sporophytes were systematically removed on every other strip starting in June 2008 when sporophytes begin to recruit. The final set up consisted of alternated strips of Undaria-removed and Undaria-intact treatments (Fig. 1), resulting in 5 replicates per treatment. On each removal event all microthalli (young sporophytes <15 cm) that had grown on the Undaria-removed strips were detached from the holdfast with a knife. After 8 months (December 2008), one 0.5-m2 quadrat was randomly placed within each strip (separated by more than 0.5 m from the borders) and all benthos was collected manually or with an iron spatula and stored in a mesh bag (0.5 mm). Undaria individuals with their associated fauna (from Undaria-intact samples) were collected in a separate mesh bag in order to distinguish the macrofauna directly associated to the plants from the rest. Samples were refrigerated and all macrofauna (defined as animals visible to the naked eye) were identified to the lowest possible taxonomic level in the laboratory. Finally, the surface area occupied by Undaria was estimated by measuring the holdfasts area in the laboratory.
Data analysis
To assess the effect of Undaria on macrofauna diversity we first calculated species richness S and the “Shannon-Wiener” diversity index H′ (Krebs 1989), according to:
S = number of taxa found
where p i = proportion of the total number of individuals in the sample belonging to i-th species.
The significance of the difference between samples with and without Undaria for each of the indices and for the abundance (=number of individuals) of each of the taxa was evaluated using a t-test, treating Undaria as fixed factor (Levels: removed and intact). Normal distribution and homogeneity were verified by homogeneity tests (Bartlet and Levene tests) and residual plots. All analyses were performed with the R software (R Development Core Team 2007).
To further illustrate differences in the diversity between treatments, we used rank-abundance plots, in which the logarithm (base 10) of each p i value was plotted against the species’ rank, from the most to the least abundant (Feisinger 2001).
Potential implications on local food webs
A bibliographic review of diet studies conducted in north and central Patagonia (from 42°S to 45°S) was used to evaluate the relative importance of the taxa found in the samples as prey of other species. Only taxa for which it was possible to compare abundance between treatments were considered (see “Results”). The occurrence of each taxa (% of analyzed stomachs or pellets in which the item was present) in the diet of each of the predators was classified into four arbitrary categories: x = 0–2.5, xx = 2.5–10, xxx = 10–25 and xxxx = 25–100.
Results
Samples with Undaria had an average of 2.4 ± 1 sporophytes (mean ± standard deviation), 1,308 ± 571 g of wet weight and a total holdfast area of 295 ± 107 cm2 covering 11% ± 4% of the total area of the samples (2,500 cm2). The total wet weight of native species of seaweeds (mostly Codium fragile, Dictyota dichotoma and Ulva spp.) was 120 ± 73 and 290 ± 120 g (mean ± standard deviation) in Undaria-intact and Undaria-removed samples, respectively.
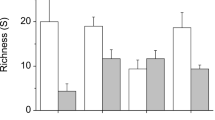
The presence of Undaria was associated with increased species richness and diversity. We found statistically significant differences for the species richness (S) and the Shannon-Wiener (H′) index (Table 1). Moreover, the rank-abundance graphs show that, despite the fact that both treatments are dominated by the same three taxa (Aulacomya atra atra, Tegula patagonica and polychaetes in the family Eunicidae), the dominance is lower in Undaria-intact samples. Furthermore, taxa of intermediate and low abundance had more evenness and richness on samples with Undaria (Fig. 2). We registered a total of 29 taxa, 26 in samples with Undaria-intact and 20 in samples with Undaria-removed. Nine taxa were found only in samples with Undaria and three were found only in samples without Undaria (Table 1). To our knowledge all taxa found are native species.
Proportion of species in samples of Undaria-intact (A) and Undaria-removed (B) treatments. Species ordered from most to least abundant in the “x” axis discriminated by treatment: (1) Aulacomya atra atra, (2) Tegula patagonica, (3) Polichaeta (Nerididae), (4) Arbacia dufresnii, (5) Nauticaris magellanica, (6) Ophioplocus janarii, (7) Polichaeta (Eunicidae), (8) Nemertina (Hineidae, Neolineus), (9) Fissurella radiosa tixierae, (10) Coenophthalmus tridentatus, (11) Leucippa pentagona, (12) Polichaeta (Polinoydae), (13) Chaetopleura isabellei, (14) Halicarcinus planatus, (15) Trophon geversianus, (16) Plaxiphora aurata aurata, (17) Nemertina, (18) Polichaeta (Glyceridae), (19) Cnidaria, (20) Anasterias minuta, (21) Allotichaster capensis, (22) Epitonium georgettinae, (23) Aequipecten tehuelchus, (24) Musculus viator, (25) Platyhelminthes, (26) Urochordata (Ascidia), (27) Polichaeta (Flabelligeridae), (28) Tivela isabelleana, (29) Pareuthria powelli
On 16 of the 29 taxa abundance was high enough to allow comparisons between treatments: the crabs Coenophthalmus tridentatus and Leucippa pentagona, the shrimp Nauticaris magellanica, the sea urchin Arbacia dufresnii, the mussel Aulacomya atra atra, the chitons Chaetopleura isabellei and Plaxiphora aurata aurata, the lamp Fissurella radiosa tixierae, the snail Tegula patagonica, the ophiuroidea Ophioplocus janarii, the Nemertina (Hineidae Neolineus) and four polychaete families (Eunicidae, Nerididae, Glyceridae, Flabelligeridae and Polynoidae). Polychaetes were analyzed as a group as were chitons (Chaetopleura isabellei and Plaxiphora aurata aurata). In the case of the mussel Aulacomya atra atra, recruits (<8 mm) were discriminated from adults and analyzed separately.
Abundance was significantly higher in the samples with Undaria for the following taxa: the crustaceans Coenophthalmus tridentatus and Nauticaris magellanica, the sea urchin Arbacia dufresnii, the Nemertina (Hineidae Neolineus) and the polychaetes. The taxa that did not show significant differences were the crustacean Leucippa pentagona, the mussel Aulacomya atra atra (recruits and adults), the limpet Fissurella radiosa tixierae, the snail Tegula patagonica, the ophiuroidea Ophioplocus janarii and the chitons (Chaetopleura isabellei and Plaxiphora aurata aurata) (Fig. 3).
The shrimp N. magellanica was found only in samples with Undaria and directly associated to the holdfasts and sporophylls. In the case of the crab C. tridentatus, only one individual was directly associated to the Undaria structure, but all the others were found beneath Undaria fronds. The crab L. pentagona, the ophiuroidea O. janarii and the limpet F. radiosa tixierae were approximately twice more abundant in Undaria samples, but this difference was not statistically significant due its high variability. Larger samples or more replicates would be necessary for the assessment of these highly variable species.
All species of polychaete found in the samples were exclusively mobile species that depend on biogenic or abiotic refuges to live. 60% of the individuals collected in Undaria-intact samples were found inside the holdfasts.
The Nemertina showed contrasting abundance between treatments (n = 13 in Undaria-intact vs. 1 in Undaria-removed treatments), but only 6 of the 13 individuals were found directly associated to the algae.
Finally, the sea urchin Arbacia dufresnii showed markedly dissimilar abundances between treatments.
Despite the lack of information on diet of regional marine fauna, especially on fishes, we could list a wide range of predators for the macrofauna present in the samples, including species of high tropic level (Bigatti et al. MS-a, MS-b; Galván 2008; Galván et al. 2009; Gosztonyi and Kuba 1998; Herrera et al. 2005; Hidalgo et al. 2007; Ré et al. 2006; Sapoznikow 2006; Yorio and Bertellotti 2002): two species of gastropods, five of fishes, two of octopus, five sea birds and the sea lion Ottaria flavescens (Table 2). Furthermore, among the most commonly consumed species were those found in increased abundance in the presence of Undaria, as was the case of polychaetes and crustaceans. In addition, we found that some predators are important prey of other predators. For example, Octopus tehuelchus is an important prey of the cormorants (especially of Phalacrocorax magellanicus) while the two octopus species and reef fishes are common preys of Ottaria flavescens. The macrobenthic fauna from hard bottoms seems to play an important role in the diet of reef fishes, octopus species and cormorants: reef fishes and octopuses inhabit hard bottoms and cormorants are coastal divers which forage on subtidal hard bottoms. On the other hand, the sea lion Ottaria flavescens rarely prey on taxa analyzed in this study (only 1.6% of polychaetes occurrence was found on its diet), however octopuses and reef fishes are of main importance for the species.
Discussion
Worldwide, there has been a lack of information on the impact of alien macroalgae on native faunal communities (Wotton et al. 2004). Our work focuses on the effects of Undaria on the abundance and diversity of benthic macrofauna in Golfo Nuevo, northern Patagonia. We showed that the presence of Undaria is associated with increased species richness and diversity, and could potentially produce a bottom-up effect on local food chains by increasing the abundance of preys for a wide range of predators. Although we could not confirm that the increasingly abundant preys in Undaria-colonized habitats are available to predators during Undaria’s growing season, they certainly become available when sporophytes decompose during the summer.
Cryptic taxa of macrofauna (i.e. dependent on biogenic or abiotic refuges to live) collected in Undaria-intact samples were found mostly inside holdfast and sporophylls of Undaria (100% of the shrimps, 60% of the polychaetes and 47% of the nemertines). Considering that Undaria occupy on average 11% of the sampled area, we attribute the differences in abundance between treatments to an increase in the availability of refuges offered by Undaria. Furthermore, mobile taxa which depend on biogenic or abiotic refuges or substrates to live were also found mostly associated with Undaria (e.g. 85% of the crabs Leucipa pentagona, 50% of the lamps Fissurella tixierae, 40% of the sea urchin Arbacia dufresnii and 28% of the crabs Coenophthalmus tridentatus). In the case of sessile organisms (ascidians and cnidarians), they were found exclusively attached to Undaria fronds. These results support the hypothesis that the effects of Undaria on the native macrofauna assemblage are principally due to its character of bioengineer. Complex habitats provide different shelter and foraging opportunities than morphologically simpler habitats (Forrest and Taylor 2002; Schmidt and Scheibling 2007). The lack of effects of Undaria on mussels and chitons, which settle on hard substrates, reinforces this conclusion.
In addition to the habitat-forming effect of Undaria, this algae is a food resource for some species and could potentially enhance consumer populations. Recently, the green sea urchin and the snail tegula were identified as Undaria consumers. These herbivore species consume Undaria mainly during the senescent period (December to April), when the algae is palatable (Teso et al. 2009). Differences would be larger later in the season when high densities of T. patagonica can be observed covering the whole structure of Undaria. Other consumer species likely exist but have not been identified yet. For example, when Undaria is highly decomposed (during the summer) dense concentrations of small crustaceans (probably isopods and amphipods) are frequently observed associated with Undaria (personal observations).
Unfortunately, the logistic complications associated with maintaining the removal experiments given the prevailing rough weather and strong winds in Patagonia restricted the scale of the experiment. While we acknowledge that the narrow temporal and spatial scale of our study precludes generalizing our conclusions, we expect to observe similar effects of Undaria on the macrofauna in other sites of the Argentine coast, especially on sites dominated by small native macroalgae.
References
Akiyama K, Kurogi M (1982) Cultivation of Undaria pinnatifida (Harvey) Suringar, the decrease in crops from natural plants following crop increase from cultivation. Tohoku Reg Fish Res Lab Bull 44:91–100
Bigatti G, Sacristán S, Rodríguez MC, Stortz CA, Penchaszadeh PA (MS-a) Diet, prey narcotization and biochemical composition of salivary glands of the volutid snail Odontocymbiola magellanica
Bigatti G, Sanchez Antelo CJM, Miloslavich P, Penchaszadeh PE (MS-b) Feeding behavior of Adelomelon ancilla: a predatory neogastropod in Patagonian benthic communities
Bolam SG, Fernandes TF (2002) The effects of macroalgal cover on the spatial distribution of macrobenthic invertebrates: the effect of macroalgal morphology. Hydrobiologia 475(476):437–448
Bolam SG, Fernandes TF, Read P, Raffaelli D (2000) Effects of macroalgal mats on intertidal sandflats: an experimental study. J Exp Mar Biol Ecol 249:123–137
Buschbaum C, Chapman AS, Saier B (2006) How an introduced seaweed can affect epibiota diversity in different coastal systems. Mar Biol 148:743–754
Casas GN, Piriz ML, Parodi EL (2008) Population features of the invasive kelp Undaria pinnatifida (Phaeophyceae:Laminariales) in Nuevo Gulf (Patagonia, Argentina). J Mar Biol Assoc UK 88(1):21–28
Chemello R, Milazzo M (2002) Effect of algal architecture on associated fauna: some evidence from phytal molluscs. Mar Biol 140:981–990
Curiel D, Rismondo D, Marzocchi M, Solazzi A (1994) Distribuzione di Undaria pinnatifida (Harvey) Suringar (Laminariales, Phaeophyta) nella laguna di venezia. Soc veneziana Sci Nat 19:121–126
Development Core Team R (2007) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Duarte CM (1995) Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia 41:87–112
Eyras MC, Boraso AL (1994) Aspectos de la estrategia reproductiva de Macrocystis Pyrifera (Phaeophyta, Laminariales) en poblaciones de la costa Argentina. Nat patagónica 2:33–47
Feisinger P (2001) Designing field studies for biodiversity conservation. Island Press. Washington, Covelo, London
Fletcher RL, Manfredi C (1995) The occurrence of Undaria pinnatifida (Phaeficeae, Laminariales) on the South coast of England. Botanica Marina 38:355–358
Forrest BM, Taylor MD (2002) Assessing invasion impact: survey design considerations and implications for management of an invasive marine plant. Biol Invasions 4:375–386
Galván DE (2008) Ensambles de peces en los arrecifes norpatagónicos: diversidad. abundancia y relaciones tróficas y con el hábitat, Doctoral tesis, Universidad Nacional Del Comahue
Galván DE, Botto F, Parma A, Bandieri L, Mohamed N, Iribarne OO (2009) Food partitioning and spatial subsidy in shelter-limited fishes inhabiting patchy reefs of Patagonia. J Fish Biol 75:2585–2605
Gosztonyi AE, Kuba L (1998) Fishes in the diet of the imperial cormorant Phalacrocorax atriceps at Punta Lobería, Chubut, Argentina. Mar Ornithol 26:59–61
Gribben PE, Wright JT (2006) Invasive seaweed enhances recruitment of a native bivalve: roles of refuge from predation and the habitat choice of recruits. Mar Ecol Prog Ser 318:177–185
Gribben PE, Wright JT, O’Connor WA, Doblin MA, Eyre B, Steinberg PD (2009) Reduced performance of native infauna following recruitment to a habitat-forming invasive marine alga. Glob Chang Ecol 158:733–745
Hay HC (1990) The dispersal of sporophytes of Undaria pinnatifida by coastal shipping in New Zealand, and implications for further dispersal of Undaria in France. Br Phycol 25:301–313
Herrera G, Punta G, Yorio P (2005) Diet specialization of Olrog’s Gull Larus atlanticus during the breeding season at Golfo San Jorge, Argentina. Bird Conserv Int 15:89–97
Hidalgo FJ, Silliman BR, Bazterrica MC, Bertness MD (2007) Predation on the Rocky Shores of Patagonia, Argentina. Estuar and Coasts 30:886–894
Johnson CR (2007) Seaweed invasions: conclusions and future directions. Botanica Marina 50:451–457
Johnson CR, Chapman ARO (2007) Seaweed invasions: introduction and scope. Botanica Marina 50:321–325
Kraufvelin P, Salovius S (2004) Animal diversity in Baltic rocky shore macroalgae: can Cladophora glomerata compensate for lost Fucus vesiculosus? Estuarine. Coast Shelf Sci 61:369–378
Krebs CJ (1989) Ecological methodology. Harper Collins Publishers, New York
Lotze HK, Lenihan HS, Bourque BJ, Bradbury RH, Cooke RG, Kay MC, Kidwell SM, Kirby MX, Peterson CH, Jackson GBC (2006) Depletion, degradation, and recovery potential of Estuaries and Coastal Seas. Science 312:1806–1809
Martin JP, Bastida R (2008) El alga invasora Undaria pinnatifida (Harvey) Suringar en la Ría Deseado (Patagonia austral, Argentina): ciclo del esporofito y factores ambientales determinantes de su distribución. Rev Biol Marina Oceanogr 43(2):335–344
Ré ME, Kuba L, Márquez F, Hermosilla C (2006) Dieta del pulpo colorado (Enteroctopus megalocyathus) en la costa patagónica argentina. Argentina, VI Jornadas Nacionales de Ciencias del Mar, Puerto Madryn
Salinas JM, Llera EM, Fuertes C (1996) Nota sobre la prescencia de Undaria pinnatifida (Harvey) Suringar (Laminariales, Paheophyta) en Asturias (mar cantábrico). Inst Esp Oceanogr Bol 12:77–79
Sapoznikow A (2006) Ecología reproductiva y trófica del cormorán cuello negro (Phalacrocorax magellanicus) en relación a las características de su fuente de alimento. Doctoral tesis, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires
Schaffelke B, Hewitt CL (2007) Impacts of introduced seaweeds. Botanica Marina 50:397–417
Schmidt AL, Scheibling RE (2007) Effects of native and invasive macroalgal canopies on composition and abundance of mobile benthic macrofauna and turf-forming algae. J Exp Mar Biol Ecol 341:110–130
Stegenga H (1999) Undaria pinnatifida in Nederland gearriveerd. Het Zeepaard 59:71–73
Stuart MD (2004) Review of research on Undaria pinnatifida in New Zealand and its potential impacts on the eastern coast of the South Island. Department of Conservation, Wellington, New Zealand
Teso SV, Bigatti G, Casas GN, Piriz ML, Penchaszadeh PE (2009) Do native grazers from Patagonia, Argentina consume the invasive kelp Undaria pinnatifida? Rev Mus Argent de Cienc Nat 11(1):7–14
Wotton DM, O`Brien C, Fergus JD, Stuart MD (2004) Eradication success down under: heat treatment of a sunken trawler to kill the invasive seaweed Undaria pinnatifida. Mar Pollut Bull 49:844–849
Yorio P, Bertellotti NM (2002) Espectro trófico de la gaviota cocinera (Larus dominicanus) en tres áreas protegidas de chubut, Argentina. Hornero 17(2):91–95
Acknowledgments
We thank Mariano Cuestas, Martin de Francesco, Andrea Marino, Pedro Fiorda and Fabian Quiroga for their disinterested support in the field and Emilia Diez for the identification of polychaetes. We are particularly grateful to Enrique Amoroso and the interdisciplinary group “We review your paper for a large meal” for their comments. Field work was financed by PADI Foundation. AJI was supported by the ‘Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET)’ of Argentina.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Irigoyen, A.J., Trobbiani, G., Sgarlatta, M.P. et al. Effects of the alien algae Undaria pinnatifida (Phaeophyceae, Laminariales) on the diversity and abundance of benthic macrofauna in Golfo Nuevo (Patagonia, Argentina): potential implications for local food webs. Biol Invasions 13, 1521–1532 (2011). https://doi.org/10.1007/s10530-010-9910-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9910-9