Abstract
Generalist herbivores in marine ecosystems are poorly examined for their potential to serve as a source of biotic resistance against algal invasion. We assessed how one of the main generalist herbivores in Mediterranean rocky reefs (the sea urchin Paracentrotus lividus) affects Lophocladia lallemandii and Caulerpa racemosa, two algal invaders with strong detrimental effects on native benthic communities. In a comparison of sea urchin gut contents to algal community composition, strong preferences were exhibited, leading to no relationship between consumption and availability. Both C. racemosa and L. lallemandi were abundant in algal assemblages (>60% occurrence), but C. racemosa (20% of diet) was consumed more than L. lallemandi (3.5%). Experimental enclosures of sea urchins (12 sea urchins * m−2) were carried out in locations where L. lallemandii was already established and C. racemosa was rare (new invasion) or abundant (established invasion). C. racemosa was negatively affected by sea urchins only when it was rare, and no effect was detected when the alga was already abundant. Results for L. lallemandi were exactly opposite: urchins limited seasonal increases in L. lallemandi in highly-invaded areas. Because of the small amount of direct consumption of L. lallemandi, its decrease in abundance may be related to the grazing of native algae where L. lallemandii is attached. Overall, our results show that high densities of native herbivores may reduce invasive algae at low densities, due to a combination of direct and indirect effects, but it has no significant effect in highly-invaded areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species interactions are a major driver of ecosystem dynamics (Sergio et al. 2006) and therefore also important in invasion biology and ecology (Colautti et al. 2004). Two main hypotheses have been advanced concerning the role of species interactions in invasion dynamics of exotic plants. The enemy release hypothesis (ERH) proposes that exotic species thrive because they lack coevolved enemies in their new ranges (Elton 1958; Keane and Crawley 2002). In the biotic resistance hypothesis (BRH), introduced species are limited in recipient communities by strong negative effects of native species (Elton 1958). An assumption behind both hypotheses is that specialist herbivores control plants in their native range, whereas generalist herbivores provide resistance to invasion in recipient communities (Maron and Vilà 2001; Parker et al. 2006). In this paper, we address biological interactions that affect the invasion of two exotic marine algae. Marine algae follow the general pattern of low establishment relative to introduction, and low rates of invasion relative to establishment (Williamson 1996; Boudouresque and Verlaque 2002), perhaps due to native species’ ability to resist damage from native herbivores and pathogens in the introduced range compared to exotic algae that are not invasive.
For marine macroalgae, vulnerability to native herbivores may be an important determinant of invasion success. In contrast to terrestrial systems, where many herbivores behave as specialists, most marine herbivores are generalists. While some studies reject the BRH for marine macroalgae (e.g. Sumi and Scheibling 2005; Gollan and Wright 2006), others provide evidence that invasive algae are actively grazed by native herbivores, which can control their populations (Scheibling et al. 2008; Strong et al. 2009). Sea urchins, as generalist herbivores with strong ecological roles in structuring benthic macrophyte communities (Lawrence 1975; Dayton 1985; Hay and Steinberg 1992), are good candidates to provide resistance to invasion.
The Mediterranean Sea harbours the largest number of introduced macrophytes in the world (Boudouresque and Verlaque 2002) due to the opening of the Suez Canal (Galil 2006), a long tradition in aquaculture (Ribera and Boudouresque 1995), and intensive marine traffic (Galil 2006). Two outstanding invasive algae in the Mediterranean are Caulerpa racemosa (Forsskål) J. Agardh var. cylindracea (Sonder) Verlaque, Huisman et Boudouresque (hereafter C. racemosa), and Lophocladia lallemandii (Montagne) F. Schmitz (hereafter L. lallemandii). Both have spread rapidly (Piazzi et al. 2005; Verlaque et al. 2004; Cebrian and Ballesteros 2007), with negative effects on native ecosystems (Piazzi et al. 2001, 2005; Balata et al. 2004; Ballesteros et al. 2007). Their success may in part be a function of high growth (Piazzi and Cinelli 1999), plasticity (Cebrian and Ballesteros 2009), and dispersal (Renoncourt and Meinesz 2001; Panayotidis and Zuljevic 2001) exhibited by C. racemosa, as well as the high reproductive output of L. lallemandii (Cebrian and Ballesteros 2010). In addition, sea urchins (Paracentrotus lividus) ingest C. racemosa (Ruitton et al. 2006; Tomas et al. in press) and influence local rates of C. racemosa expansion (Bulleri et al. 2009). However, sea urchin grazing pressure on L. lallemandii is unknown.
To examine the effects of sea urchins on the invasion of these two exotic algae, we first determined ingestion of algae by a comparison of sea-urchin gut contents with macrophytes available in the field. Second, we enclosed sea urchins in natural algal assemblages in the field and tested their effects on both native and exotic species.
Materials and methods
Study system
This study was carried out on shallow sublittoral rocky reefs in the Archipelago of Cabrera National Park (Western Mediterranean; 39° 12′ 21′′ N; 2° 58′ 44′′ E). C. racemosa was recorded in the archipelago for the first time in 2003 growing at 30–35 m depth, and rapidly spread to almost all benthic communities present between 0 and 65 m depth. L. lallemandii was also recorded for the first time in 2003 and is currently present in nearly all habitats between 5 and 45 m depth (authors’ pers. obs.). Depth in the experimental area ranges from 9 to 12 m and consists of gently sloping rocky platforms covered by dense stands of turf-forming algae (Corallina elongata, Sphacelaria cirrosa), small canopy algae (Stypocaulon scoparium, Dictyota dichotoma, Padina pavonica, Sargassum vulgare) and the encrusting coralline Neogoniolithon brassica-florida. The experimental area and time were chosen taking into account information from monitoring studies started in 2003 and conducted annually on the biology and phenology of C. racemosa and L. lallemandii in the Cabrera NP (Cebrian and Ballesteros 2009, 2010). The study area was first colonized by L. lallemandii in May 2003, and it now seasonally forms a homogeneous mat overgrowing native assemblages. L. lallemandi biomass is high from May to November, declines in winter, and rises again in summer (Cebrian and Ballesteros 2010) in the experimental area. There, L. lallemandii shows a seasonal trend, reaching the highest biomass in autumn, drastically declining in winter, and increasing again in summer (Cebrian and Ballesteros 2010). In contrast, C. racemosa shows a patchy distribution persisting all the year round with no evident seasonal trend (Cebrian and Ballesteros 2009).
The sea urchin P. lividus is present, but scarce, at the study area, hidden in crevices and in boulders (density < 0.1 ind m−2). It was absent on the rocky platforms where experiments were set up, and there was no evidence of grazing in this area.
P. lividus feeding patterns
To assess feeding preferences of sea urchins, we compared algal abundance in the habitat and in the gut contents by means of a multivariate rank-correlation on square root transformed Bray-Curtis similarity matrices (RELATE, Primer, 6.0).
Analysis of algal species composition of the algal assemblage
The experimental area consisted of a rocky platform of approximately 1,000 m2. Sampling was performed at the end of July 2007, ensuring high abundances of C. racemosa and L. lallemandii (Cebrian and Ballesteros 2009, 2010). Species composition of the algal assemblage was measured in six randomly-placed quadrats (0.25 × 0.25 m, divided into 25 subquadrats of 5 × 5 cm) (Sala and Ballesteros 1997; Cebrian et al. 2000; Cebrian and Ballesteros 2004). In each quadrat, species abundance was estimated as the percentage of subquadrats in which a species appeared. The total sampling area (total 6 × 0.25 × 0.25 m2) was selected to exceed the minimum sampling area necessary to properly characterise algal-dominated sublittoral Mediterranean assemblages (Coppejans 1980; Ballesteros 1992; Marti et al. 2005).
Analysis of gut content of sea urchins
Sea urchins were collected from the rocky platform at the end of July 2007. Since P. lividus food transit ranges between 20 h and 5 days (Lawrence et al. 1989) and their home range is between 50 and 302 cm (Hereu 2005), we can assume that all gut contents were coming from the rocky platform where algal assemblages were sampled. Gut contents of P. lividus (n = 16) were removed after collection and preserved in buffered 4% formaldehyde-seawater. Once at the laboratory, the gut contents were spread in a reticulated Petri dish, and algal species composition was determined under a dissecting microscope at 40 × with a transmitted light source. Algae were classified to species (or higher taxonomic levels when not possible). The abundance of each algal taxon present in each gut content was estimated as the mean percentage cover on the reticulated fields surveyed. Occasional presence of a species in a sample was given an arbitrary value of 0.1% relative abundance. Finally, the mean relative abundance of each algal species was calculated using the relative abundances in the gut contents of all sea urchins analyzed (n = 16; Lison de Loma and Ballesteros 2002; Lison de Loma et al. 2002).
Ivlev’s index was used as a measure of electivity (E) for invasive algae species: E = (di − ai)/(di + ai) where di = % of invasive algae in the gut content (use prevalence in diet), and ai = % of invasive algae available in the environment (prevalence in the environment). Values of Ivlev’s index (E) range from −1.00 (complete avoidance) to +1.00 (exclusive selection) and E > 0 means that food type is eaten more than its availability in the environment (selected). Algal abundances in the field were scaled to 100 as total cover usually exceeds this value (Sala and Ballesteros 1997).
As the cover of C. racemosa and L. lallemandii are strongly and significantly correlated with its biomass (r2 = 0.967; P < 0.0001 for C. racemosa and r2 = 0.892; P < 0.01 for L. lallemandii; see Cebrian and Ballesteros 2009, 2010), we assumed that the % of abundance measured as a percentage of cover by C. racemosa and L. lallemandii appropriately indicated algal availability for sea urchins.
Grazing control experiments
In order to test the possible control capacity of P. lividus on C. racemosa and L. lallemandii invasions, we caged sea urchins in field experiments set up in algal assemblages at three different stages of invasion: (i) area invaded by L. lallemandii; (ii) area invaded by C. racemosa; and (iii) area not invaded by C. racemosa, in order to test the possible control of sea urchins on new establishment C. racemosa. All experiments were situated near (<15 m) the rocky platform where the algal assemblage were characterized, and the C. racemosa non-invaded experiment occurred close enough to C. racemosa to allow colonisation (1–2 m).
Every experiment consisted of 12 plots of 1,600 cm2 each, located at random with three treatments of 4 plots (i.e. replicates) each. Because sea urchins were absent from the study area, the control treatment (CON) was a series of unmanipulated plots without sea urchins and experiencing low herbivory. Herbivory treatment (HERB) consisted of cages with 2 sea urchins, mimicking high densities that naturally occur (Guidetti et al. 2003; Tomas et al. 2004; Hereu 2006). Additional cages were also established without urchins to test for potential caging artefacts (cage control CAG-CON). Cages were roofless to prevent light-shading effects by fouling (Lewis 1986) and were 40 cm high, preventing sea urchins from escaping. Cages were cleaned of fouling at least once a month, when observations were taken inside and around the plots to ensure that there was no reinvasion in urchin-free treatments, or escape of sea urchins from inclusion treatments. We never observed sea urchins in urchin-free treatments and only rarely we had to replace sea urchins that had escaped.
Although increased densities of mesograzers are a potential confounding factor in the interpretation of experimental manipulations involving caging treatments (Dayton and Oliver 1980), the net mesh size used (1.5 cm) allowed free passage of gastropods, small crustaceans and juvenile echinoderms (0.2 to 1 cm) (Sala and Zabala 1996; Hereu et al. 2004; Tomas et al. 2004).
Experiments lasted 7 months, from May to November 2007, and the percent cover of Caulerpa racemosa, Lophocladia lallemandii and native algae was monitored in May, July and November by means of a photographic survey. Seaweed cover was calculated following the same method as used for the algal assemblage characterization. Each photograph was divided into 25 subquadrats of 5 × 5 cm. In each photograph, species abundance was the percentage of subquadrats in which a species appeared. Initial cover (May) of both native and invasive species for the three treatments (CON, HERB and CAG-CON) was first analysed by one-way analysis of variance (ANOVA) to ensure that plots were similar before treatments were allocated. Differences in native and invasive species cover among treatments were analysed by means of Repeated-Measures ANOVA containing two repeated measures (July and November). One-way ANOVAs were also used to analyse differences in cover between treatments at each observation time, with Tukey-tests for post-hoc comparisons. Prior to analysis, homogeneity of variances was tested by Cochran’s test. Analyses were carried out using Statistica 8.0.
At the end of the experiments we examined sea urchin gut contents as described above. Algal assemblage composition of gut contents of all the experimental sea urchins (in invaded and non-invaded plots) were analysed by non-metric multidimensional scaling (MDS Clarke and Warwick 1994), based on a Bray-Curtis similarity matrix of square root-transformed data. ANOSIM (Clarke and Warwick 1994) was applied to test the null hypothesis of no difference among the sea urchin diets in the C. racemosa invaded and non-invaded experiments. A t-test was used to compare C. racemosa abundance in sea urchin gut contents between the C. racemosa invaded and non-invaded experiments.
Results
P. lividus feeding patterns
Species composition of the algal assemblage and sea urchin gut contents are detailed in Table 1. No correlation was found between algal species composition of the habitat and gut contents (Spearman rank-correlation; Rho = 0.029; P > 0.5) (Fig. 1). Caulerpa racemosa, as well as other items (Haliptilon virgatum, Stypocaulon scoparium or Osmundaria volubilis), were consumed in proportion to their availability. Others, like Lophocladia lallemandii and Neogoniolithon brassica-florida were hardly found in gut contents even though they were amongst the most abundant algae present (66.6 and 99.6%, respectively). However, N. brassica-florida as well as other encrusting calcareous algae, is undistinguishable in gut contents and thus this result should be disregarded.
Scatterplot representation of the percentage of available algae in the environment against the percentage found in gut contents. X and Y error bars represent SE of the percentage cover in the environment and gut contents, respectively. Species codes are represented in Table 1
Ivlev’s Electivity Index for C. racemosa (E = 0.23) and for L. lallemandii (E = − 0.52) show that C. racemosa is consumed in proportion to its availability or preferred, whilst.
Herbivory experiment
Initial coverage of native species in the 3 experiments averaged 100% cover across all treatments (Table 2). Where it had already invaded, C. racemosa occurred at 80.8% ± 4.8 cover in May (Table 2), and it was initially absent from plots in the un-invaded experiment. In the experiment in which L. lallemandii had already invaded, no invasive algae were present in May, consistent with seasonal phenology of L. lallemandii. These results demonstrate that initial conditions were similar among plots and posterior differences can be attributed to treatment effects.
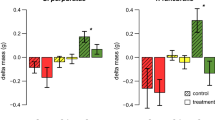
In the experiment within the area already colonized by Lophocladia lallemandii, grazing had a significant effect on the final coverage of L. lallemandii (Fig. 2a; Table 3). At the end of the experiment cover of L. lallemandii was significantly lower in plots with urchins compared with both control and control/caging treatment plots (Tukey-test P < 0.01).
Although C. racemosa was present in the gut contents of the sea urchins in the already invaded area, grazing had no effect on C. racemosa cover. However, C. racemosa cover decreased in all treatments from May to November (Fig. 2b; Table 3). In contrast, C. racemosa colonized control and cage-control plots in the un-invaded area and therefore increased in cover over time, whereas plots with urchins remained free of C. racemosa (Fig. 2c; Table 3). At the end of the experiment in the un-invaded area, C. racemosa cover was significantly lower with sea urchins (HERB) than without (CON and CAG-CON) (Tukey-test P < 0.001). Native algae showed similar patterns across all experiments, covering all available space in both May and July, but decreasing to about 70% cover in November. Also in November, plots that contained sea urchins had lower native algal cover, never exceeding 50% (Fig. 3; Table 3).
The percentage of L. lallemandii in gut contents (3.0%) was low and similar in all experiments (SE 0.28, N = 8). However, sea urchin diets differed across the three experiments primarily due to the relative abundance of C. racemosa. In the already-invaded area, C. racemosa made up 13.5% of the diet (SE = 5.4, N = 8), but only 6.1% in the un-invaded area (SE = 1.8, N = 8, T-test P < 0.01). The occurrence of C. racemosa in the guts of urchins in the un-invaded area suggests that the urchins were actively removing any C. racemosa that colonized, since none was present in the plots. If C. racemosa and non identified detritus are excluded from the analysis of sea urchin gut contents, native algal composition did not vary across the three experiments (ANOSIM; Rho = 0.089, P = 0.039) (Fig. 4). Diets of sea urchins collected for the comparative study and contained in enclosures included the seagrass Posidonia oceanica, which was not present at the rocky study site and probably consumed as drift (Table 1; Fig. 4).
Discussion
The importance of prey-predator interactions in determining the outcome of plant or algal invasions is a matter of debate, since the studies already performed provide mixed results with no general apparent trends (Agrawal et al. 2005 and references therein; Gollan and Wright 2006; Scheibling et al. 2008). However, although the biotic resistance hypothesis has not been widely-supported by empirical data, several cases certainly exist in which native herbivores reduce the abundance of an invader (Thibaut and Meinesz 2000; Scheibling and Anthony 2001). In our study, sea urchins (Paracentrotus lividus) were strong interactors, reducing cover of native and non-native species over 7 months. Until now, in the Mediterranean Sea, most studies dealing with herbivory on invasive species have been descriptive and only one reported the ingestion of C. racemosa by generalist herbivores (i.e. P. lividus and the fish Sarpa salpa) (Ruitton et al. 2006). However, present studies (Tomas et al. unpublished data) may suggest that high densities of the fish Sarpa salpa may also have a potential effect controlling C. racemosa invasion. Here, through a combined examination of sea urchin diets and experimental manipulations to measure interaction strength, we conclude that introduced C. racemosa is likely to be affected directly by consumption, whereas direct effects on introduced L. lallemandi are unlikely because it is avoided (see also Tomas et al. in press).
The lack of a relationship between available algae and algal ingestion (gut contents) indicates that P. lividus does not feed only according to algal availability, but that it has some preferences for algal consumption. L. lallemandii is highly abundant in the community while it is scarce in gut contents and therefore Ivlev’s index is negative. Sea urchins may avoid L. lallemandi because it contains lophocladines, alkaloids with cytotoxic effects (Gross et al. 2006), which are not present in any native algal species. In contrast, the high abundance of C. racemosa in the sea urchin gut contents and a positive Ivlev’s index indicate that this species is actively consumed. C. racemosa also contains an active anti-herbivore chemical, caulerpenyne, but at relatively low concentrations (Jung et al. 2002). In contrast, C. taxifolia, with 2 to 35–80 times more caulerpenyne (Dumay et al. 2002), is unpalatable, which may contribute to its invasion success (Gollan and Wright 2006). On the other hand, Cornell and Hawkins (2003) suggest that, when a secondary metabolite is already present in the new range, generalist herbivores are likely to possess the ability to detoxify algae defenses and feed on it. The presence of caulerpenyne in the Mediterranean before C. racemosa invasion (in the native Caulerpa prolifera) may explain that native generalist herbivores can feed on it. In contrast, the absence of lophocladines from the Mediterranean before L. lallemandii invasion may partially explain deterrence to generalist herbivores (Jogesh et al. 2008).
Food quality can influence feeding parameters and absorption efficiencies (Lawrence et al. 1989; Fernández and Boudouresque 2000), which might lead to misinterpretations of sea urchin preferences from their gut contents. In fact, calcareous algae are much less absorbed than those with fleshy tissue (Frantzis and Grémare 1992). However, our results show that even if the ingestion of fleshy seaweed was underestimated, C. racemosa is still more abundant than other algae less absorbable, such as the calcareous algae Haliptilon virgatum.
The experiment to assess the effect of P. lividus on L. lallemandii invasion indicated that, even though this species was hardly present in gut contents (3.0%), it displayed lower biomass in the grazed plots compared with the controls. It should be noted that although being lower, the coverage of L. lallemandii was still 40% in grazed plots, which considering the high reproductive output of this species (Cebrian and Ballesteros 2010), suggests that L. lallemandii expansion cannot be controlled by sea urchin grazing.
On the other hand, although C. racemosa is highly consumed by P. lividus, grazing had no effect on C. racemosa coverage in already invaded areas. The intrinsic features of C. racemosa biology, such as high plasticity (Verlaque et al. 2000) and vegetative and sexual reproduction capacity (Panayotidis and Zuljevic 2001; Ceccherelli and Piazzi 2001; Renoncourt and Meinesz 2001) might be more important than loss of biomass due to herbivory. Likewise, other such positive feedbacks of herbivory, such as the enhancement of overall plant production (Mitchell and Wass 1996), can also compensate for biomass loss. Bulleri et al. (2009) reported that particular densities of P. lividus may enhance the penetration of C. racemosa stolons, promoting algal turf assemblages, which may facilitate the spread of C. racemosa by enhancing the anchoring of stolons and/or trapping fragments. Similarly, P. lividus could also facilitate invasion or spread of C. racemosa by breaking its thalli into several fragments when grazing, which could promote the generation of new thalli (Klein and Verlaque 2008).
Although different herbivore pressure on C. racemosa and L. lallemandi would be predicted to generate different direct interactions, both of these introduced algae could experience similar indirect effects, mediated by the presence of native algae. Native algae coverage decreased with time in all experiments and treatments, following the typical seasonal pattern of shallow Mediterranean communities (Ballesteros 1990), but this decline was stronger in the presence of sea urchins. Loss of native algal cover is a common response in seaweed communities subjected to grazing (Hereu et al. 2008), potentially also affecting competition between invasive and native algae. However, because these exotic algae use native algae as an attachment site and effectively overgrow them, they are probably not space limited, and consumption of native algae would not release the exotics from competition and promote higher abundance (but see Bulleri et al. 2009). Instead, the epiphytic habit of L. lallemandi in particular may explain why its cover was lower with urchins even though it was not heavily consumed—sea urchins consumed algae overgrown by L. lallemandi or physically removed it when grazing adjacent algae.
Overall, effects of sea urchins were weak or negative on both native and exotic algae. Native algae were particularly affected during autumn, exceeding seasonal declines that occurred in the absence of urchins. Exotic algae were negatively affected when their initial densities were already low for C. racemosa, this occurred in the experiment set up in an un-invaded area, which was colonized during the experiment in the absence of urchins; for L. lallemandi, this occurred because the experiment was established prior to seasonal increases in abundance. However, in already invaded areas final abundance of exotic algae remained substantial (>40% appearance in subplots), so invader impacts may still be high.
Since eradication of C. racemosa is unlikely to be effective in already invaded areas (Klein and Verlaque 2008), and grazing by sea urchins does not exert any control when invasion is well-established, the observed effect of P. lividus grazing on limiting the new establishment of C. racemosa should be regarded as one of the main points of this study. In fact, across large scales, plant invasions are slowed down more by the removal of small emerging populations (nascent foci) than by a similar reduction in areas of well-established monocultures (Moody and Mack 1988; Cook et al. 1996).
Accordingly, Carlsson et al. (2009) already suggested that “partial biotic resistance” may be an important factor in reducing the population size and detrimental impacts of exotic species. Therefore, natural or human-driven declines in predator populations could lead to trophic cascades contributing in some way to biological invasions (Carlsson et al. 2009).
Because other studies have shown that sea urchins may enhance reproduction or spread of these invasive algae, the balance of positive and negative effects of P. lividus on C. racemosa and L. lallemandii are likely density dependent. Therefore, further research is necessary involving different sea urchin densities, greater areas, and longer time periods in order to acquire a better understanding of the potential role of native generalist herbivores on C. racemosa and L. lallemandii establishment and spread in Mediterranean rocky reefs.
References
Agrawal A, Kotanen PM, Mitchell CE, Power AG, Godsoe WJ (2005) Enemy Release? An experiment with congeneric plant pairs and diverse above- and belowground enemies. Ecology 86:2979–2989
Balata D, Piazzi L, Cinelli F (2004) A comparison among assemblages in areas invaded by Caulerpa taxifolia and C. racemosa on a subtidal Mediterranean rocky bottom. Mar Ecol 25:1–13
Ballesteros E (1990) Structure and dynamics of Cystoseira caespitosa Sauvageau (Fucales, Phaeophyceae) community in the north-western Mediterranean. Sci Mar 54:155–168
Ballesteros E (1992) Els vegetals i la zonació litoral: espècies, comunitats i factors que influeixen en la seva distribució. Arx Secc Ciènc Inst Est Cat 101:1–616
Ballesteros E, Cebrian E, Alcoverro T (2007) Mortality of shoots of Posidonia oceanica following meadow invasion by the red alga Lophocladia lallemandii. Bot Mar 50:8–13
Boudouresque CF, Verlaque M (2002) Biological pollution in the Mediterranean Sea: invasive versus introduced macrophytes. Mar Pollut Bull 44:32–38
Bulleri F, Tamburello L, Benedettti-Cecchi L (2009) Loss of consumers alters the effects of resident assemblages on the local spread of an introduced macroalga. Oikos 118:269–279
Carlsson NO, Sarnelle O, Strayer D (2009) Native predators and exotic prey–an acquired taste? Front Ecol Environ 7:525–532
Cebrian E, Ballesteros E (2004) Zonation patterns of benthic communities in La Herradura (Alboran Sea, Western Mediterranean). Sci Mar 68:69–84
Cebrian E, Ballesteros E (2007) Invasion of the alien species Lophocladia lallemandii in Eivissa-Formentera (Balearic Islands). In: Pergent Martini C, El Asmi S (eds) Proceedings 3rd Mediterranean Symposium on Marine Vegetation. C. Le Ravallec, Marseilles, France. RAC/SPA publ, Tunis, pp 34–41
Cebrian E, Ballesteros E (2009) Temporal and spatial variability in shallow- and deep-water population of the invasive Caulerpa racemosa var. cylindracea in the Western Mediterranean. Estuar Coast Shelf Sci 83:469–474
Cebrian E, Ballesteros E (2010) Invasion of Mediterranean benthic assemblages by red alga Lophocladia lallemandii (Montagne) F. Schmitz: depth-related temporal variability in biomass and phenology. Aquat Bot 92:81–85
Cebrian E, Ballesteros E, Canals M (2000) Shallow rocky bottom benthic assemblages as calcium carbonate producers in the Alboran Sea (southwestern Mediterranean). Oceanol Acta 23:311–322
Ceccherelli G, Piazzi L (2001) Dispersal of Caulerpa racemosa fragments in the Mediterranean: lack of detachment time effect on establisment. Bot Mar 44:209–213
Clarke KR, Warwick RM (1994) Similarity-based testing for community pattern: the 2-way layout with no replication. Mar Biol 118:167–176
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by enemy release hypothesis? Ecol Lett 7:721–733
Cook GD, Setterfield SA, Maddison JP (1996) Shrub invasion of a tropical wetland: implications for weed management. Ecol Appl 6:513–537
Coppejans E (1980) Phytosociological studies on Mediterranean algal vegetation: rocky surfaces of the photophilic infralittoral zone. In: Price JH, Irvine DEG, Farnham WF (eds) Ecosystems: the shore environment, vol 2. Academic, London, pp 371–393
Cornell HV, Hawkins BA (2003) Herbivore responses to plant secondary compounds: a test of phytochemical coevolution theory. Am Nat 161:507–522
Dayton PK (1985) Ecology of kelp communities. Ann Rev Ecol Syst 16:215–245
Dayton PK, Oliver JS (1980) An evaluation of experimental analyses of populations and community patterns in benthic marine environments. In: Tenore KR, Coull BC (eds) Marine benthic dynamics. University of South California Press, Georgetown, pp 93–120
Dumay O, Pergent G, Pergent-Martini C, Amade P (2002) Variations in caulerpenyne contents in Caulerpa taxifolia and Caulerpa racemosa. J Chem Ecol 28:343–352
Elton CS (1958) The ecology of invasions by animals and plants. University of Chicago Press, Chicago
Fernández C, Boudouresque CF (2000) Nutrition of the sea urchin Paracentrotus lividus (Echinodermata: Echinoidea) fed different artificial food. Mar Ecol Prog Ser 204:131–141
Frantzis A, Grémare A (1992) Ingestion, absorption, and growth rates of Paracentrotus lividus (Echinodermata: Echinoidea) fed different macrophytes. Mar Ecol Prog Ser 95:169–183
Galil BS (2006) Shipwrecked: shipping impacts on the biota of the Mediterranean Sea. In: Davenport JL, Davenport J (eds) The ecology of transportation: managing mobility for the environment. Environ Pollut 10:39–69
Gollan JR, Wright JT (2006) Limited grazing pressure by native herbivores on the invasive seaweed Caulerpa taxifolia in a temperate Australian estuary. Mar Freshw Res 57:685–694
Gross H, Goeger DE, Hills P, Mooberry SL, Ballantine DL, Murray TF, Valeriote FA, Gerwick WH (2006) Lophocladines, bioactive alkaloids from the red alga Lophocladia sp. J Nat Prod 69:640–644
Guidetti P, Fraschetti S, Terlizzi A, Boero F (2003) Distribution patterns of sea urchins and barrens in shallow Mediterranean rocky reefs impacted by the illegal fishery of the rock-boring mollusc Lithophaga lithophaga. Mar Biol 143:1135–1142
Hay ME, Steinberg PD (1992) The chemical ecology of plant-herbivore interactions in marine versus terrestrial communities. In: Rosenthal JA, Berenbaum MR (eds) Herbivores: their interaction with secondary metabolites, evolutionary and ecological processes. Academic Press, San Diego, pp 371–413
Hereu B (2005) Movements pattern of the sea urchin Paracentrotus lividus in the NW Mediterranean. Mar Ecol 26:54–62
Hereu B (2006) Depletion of palatable algae by sea urchins and fishes in a Mediterranean subtidal community. Mar Ecol Prog Ser 113:95–103
Hereu B, Zabala M, Linares C, Sala E (2004) Temporal and spatial variability in settlement of the sea urchin Paracentrotus lividus in the NW Mediterranean. Mar Biol 144:1011–1018
Hereu B, Zabala M, Sala E (2008) Multiple controls of community structure and dynamics in a subtidal marine environment. Ecology 89:3423–3435
Jogesh T, Carpenter D, Cappuccino N (2008) Herbivory on invasive exotic plants and their non-invasive relatives. Biol Invasions 10:797–804
Jung V, Thibaut T, Meinesz A, Pohnert G (2002) Comparison of the wound-activated transformation of caulerpenyne by invasive and noninvasive Caulerpa species of the Mediterranean. J Chem Ecol 28:2091–2105
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–169
Klein J, Verlaque M (2008) The Caulerpa racemosa invasion: a critical review. Mar Pollut Bull 56:205–225
Lawrence JM (1975) On the relationships between marine plants and sea urchins. Oceanogr Mar Biol Ann Rev 13:213–286
Lawrence JM, Regis MB, Delmas P, Gras G, Klinger T (1989) The effect of quality of food on feeding and digestion in Paracentrotus lividus (Lamarck) (Echinodermata: Echinoidea). Mar Behav Physiol 15:137–144
Lewis SM (1986) The role of fishes on the organization of a Caribbean reef community. Ecol Monogr 56:183–200
Lison de Loma T, Ballesteros E (2002) Microspatial variability inside epilithic algal communities within territories of the damselfish Stegastes nigricans at La Réunion (Indian Ocean). Bot Mar 45:316–323
Lison de Loma T, Conand C, Harmelin-Vivien M, Ballesteros E (2002) Food selectivity of Tripneustes gratilla (L.) (Echinodermata: Echinoidea) in oligotrophic and nutrient-enriched coral reefs at La Réunion (Indian Ocean). Bull Mar Sci 70:927–938
Maron JL, Vilà M (2001) When do herbivores affect plant invasion? Evidence of the natural enemies and biotic resistance hypothesis. Oikos 95:361–373
Marti R, Uriz MJ, Ballesteros E, Turon X (2005) Seasonal variation in the structure of three Mediterranean algal communities in various light conditions. Estuar Coast Shelf Sci 64:613–622
Mitchell SF, Wass RT (1996) Quantifying herbivory: grazing consumption and interaction strength. Oikos 76:125–129
Moody ME, Mack RN (1988) Controlling the spread of plant invasions: the importance of nascent foci. J Appl Ecol 25:1009–1021
Panayotidis P, Zuljevic A (2001) Sexual reproduction of the invasive green alga Caulerpa racemosa var. occidentalis in the Mediterranean Sea. Oceanol Acta 24:199–203
Parker DJ, Burkepile DE, Hay ME (2006) Opposing effects of native and exotic herbivores on plant invasion. Science 311:1459–1461
Piazzi L, Cinelli F (1999) Développement et dynamique saisonnière d’un peuplement méditerranéen de l’algue tropicale Caulerpa racemosa (Forsskal) J. Agardh. Cryptogam Algol 20:295–300
Piazzi L, Ceccherelli G, Cinelli F (2001) Threat to macroalgal diversity: effects of the introduced green alga Caulerpa racemosa in the Mediterranean. Mar Ecol Prog Ser 210:149–159
Piazzi L, Meinesz A, Verlaque M, Akçali B, Antolič B, Argyrou M, Balata D, Ballesteros E, Calvo S, Cinelli F, Cirik S, Cossu A, D’Archino R, Djellouli SA, Javel F, Lanfranco E, Mifsud C, Pala D, Panayotidis P, Peirano A, Pergent G, Petrocelli A, Ruitton S, Žuljević A, Ceccherelli G (2005) Invasion of Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) in the Mediterranean Sea: an assessment of the spread. Cryptogam Algol 26:189–202
Renoncourt L, Meinesz A (2001) Formation of propagules on an invasive strain of Caulerpa racemosa (Chlorophyta) in the Mediterranean Sea. Phycologia 41:533–535
Ribera MA, Boudouresque CF (1995) Introduced marine plants, with special reference to macroalgae: mechanisms and impact. Prog Phycol Res 11:187–268
Ruitton S, Verlaque M, Aaubin G, Boudouresque CF (2006) Grazing on Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) in the Mediterranean Sea by herbivorous fishes and sea urchins. Vie Milieu 56:33–41
Sala E, Ballesteros E (1997) Partitioning of space and food resources by three fish of the genus Diplodus (Sparidae) in a Mediterranean rocky infralittoral ecosystem. Mar Ecol Prog Ser 152:273–283
Sala E, Zabala M (1996) Fish predation and the structure of the sea urchin Paracentrotus lividus populations in the NW Mediterranean. Mar Ecol Prog Ser 140:71–81
Scheibling RE, Anthony SX (2001) Feeding, growth and reproduction of sea urchins (Strongylocentrotus droebachiensis) on single and mixed diets of kelp (Laminaria spp.) and the invasive alga Codium fragile ssp. tomentosoides. Mar Biol 139:139–146
Scheibling RE, Lyons DA, Sumi CBT (2008) Grazing of the invasive alga Codium fragile ssp. tomentosoides by the common periwinkle Littorina littorea: effects of thallus size, age and condition. J Exp Mar Biol Ecol 355:103–113
Sergio F, Newton I, Marchesi L, Pedrini P (2006) Ecologically justified charisma: preservation of top predators delivers biodiversity conservation. J Appl Ecol 43:1049–1055
Strong JA, Maggs CA, Johnson MP (2009) The extent of grazing release from epiphytism for Sargassum muticum (Phaeophyceae) within the invaded range. J Mar Biol Assoc UK 89:303–314
Sumi CBT, Scheibling RE (2005) Potential role of grazing by sea urchins (Strongylocentrotus droebachiensis) in regulating the invasive alga Codium fragile ssp. tomentosoides in Nova Scotia. Mar Ecol Prog Ser 292:203–212
Thibaut T, Meinesz A (2000) Are Mediterranean ascoglossum molluscs Oxynoe olivacea and Lobiger serradifalci suitable agents for the biological control against the invading algae Caulerpa taxifolia? Comp Rend Acad Sci Paris 323:477–488
Tomas F, Romero J, Turon X (2004) Settlement and recruitment of the sea urchin Paracentrotus lividus in two contrasting habitats in the Mediterranean. Mar Ecol Prog Ser 282:173–184
Tomas F, Box A, Terrados J (in press) Effects of invasive seaweeds on feeding preference and performance of a keystone Mediterranean herbivore. Biol Inv
Verlaque M, Afonso-Carrillo J, Gil-Rodríguez MC, Durand C, Boudouresque CF, Le Parco Y (2004) Blitzkrieg in a marine invasion: Caulerpa racemosa var. cylindracea (Bryopsidales, Chlorophyta) reaches the Canary Islands (north-east Atlantic). Biol Invasions 6:269–281
Verlaque M, Boudouresque CF, Meinesz A, Gravez V (2000) The Caulerpa racemosa complex (Caulerpales, Ulvophyceae) in the Mediterranean Sea. Bot Mar 43:49–68
Williamson M (1996) Biological invasions. Chapman and Hall, London
Acknowledgments
Financial support was provided by Fundación Biodiversidad and Grant CTM2005-01434/MAR and GRACCIE project C5D2007–00067 from the Spanish Ministry of Science and Innovation. EC was funded via a EU Marie Curie Fellowship (MEIF-2006-041315 and ERG-2009-248252). FT was funded by Juan de la Cierva Postdoctoral Fellowship. CL was funded by a Spanish Postdoctoral Fellowship. We are particularly grateful to D. Diaz for his valuable fieldwork assistance and to M. Sales, J Ruesink and two anonymous reviewers for insightful comments on previous drafts of the paper. We would like to thank the Director and Staff of the Archipelago of Cabrera National Park for permissions and facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cebrian, E., Ballesteros, E., Linares, C. et al. Do native herbivores provide resistance to Mediterranean marine bioinvasions? A seaweed example. Biol Invasions 13, 1397–1408 (2011). https://doi.org/10.1007/s10530-010-9898-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9898-1