Abstract
Increasing population, industrialization and urbanization has led to two most persistent problems for modern world, i.e. energy crisis and waste disposal. Microbial fuel cell (MFC) has emerged as a technique for the generation of electricity under the influence of the metabolic action of microbes. The technique is known since 100 years, but in the last two decades, the research group have shown keen interest in this technique as it is capable of solving energy crisis and waste management. Researchers have designed several types of MFCs which are capable of utilizing several waste materials such as lignocellulose biomass, toxic chemicals, polluted sediment soils, sewage sludge and petroleum hydrocarbons, etc. The substrate can be used under the influence of large group of microbes for generating energy which can be harnessed to meet the growing energy demand. In present the efforts of several research groups and technological advancement has made this technology affordable and cost-effective. This chapter focus on presenting recent technological developments in the MFC for concurrent bioelectricity generation and bioremediation with special focus on type of electrodes materials, substrates and various designs of MFC used for bioremediation. It also gives an insight into the economic feasibility of the technique for commercialization and future prospect of the technology.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
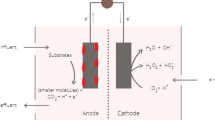
Industrialization and economic development of different countries can be measured in term of available energy source. For the past 200 years, fossil fuels were pillar of growth supporting the demand for energy, but each coin has two phases; therefore, along with advantages comes disadvantages. It damaged the environment leading to pollution, over-exploitation of natural resources and damaging the flora and fauna. These factors acted as promoter for different stakeholders to search for an affordable and environmental friendly alternative, such as biofuels and bioenergy. One such alternative was MFC; the concept came into existence in year 1910, when Michael C. Potter at University of Durham, UK, observed the ability of Escherichia coli to generate electricity (Potter 1911). It has emerged as a promising tool for bioenergy generation. MFC is a device that converts the chemical energy to electricity via biological pathways (Santoro et al. 2017; Zhang et al. 2016). The conventional chemical approaches are costly and require sophisticated infrastructure (Zaffar et al. 2016). MFC is a green tool for the treatment of different pollutants and simultaneous generation of electricity to meet the growing energy need of increasing human population. General schematic MFC found its application in wastewater treatment plants along with electricity and hydrogen generation (Wang et al. 2015), sediment bioremediation (Li and Yu 2015) and detoxification of polluted soil from toxic xenobiotic compounds (Rodrigo et al. 2014). The growing interests of scientific community in application of MFC and its further improvement can be easily observed through growing number of publication in this area (Zhang et al. 2016) which has gained impetus in the past 10 years (Fig. 11.1). This chapter gives an insight into the recent development in MFC technology as pollutant treatment units besides generating electricity, its limitations and economic feasibility for commercialization.
11.2 Improvement in the Microbial Fuel Cell Technology for Bioremediation
The development of MFC technology has come a long way since the discovery “animal electricity” by Luigi Galvani in year 1780. In the early eighteenth century, Volta’s experiment led to the invention of early battery, and in 1859 lead acid battery was invented by French physicist Gaston Plante. William Grove is considered as the father of fuel cell technology, and the concept acted as the theoretical base for the discovery of fuel cells in the future. Various vehicle manufacturers have shown keen interests in fuel cell technology which led to the discovery of different fuel cells such as soft oxide fuel cells (SOFCs) , molten carbonate fuel cells (MCFCs) and proton exchange membrane fuel cells (PEMFCs) . However these technologies had their own limitations such as high operating temperatures, slow start times, need of precious metals as catalysts, high temperature, high cost involved and highly corrosive media in some cases. The alternative to these technologies was MFC, an efficient alternative to the costly abiotic fuel cells, as it can be operated under ambient temperature and pressure. The MFCs technology also attracted the attention of NASA scientists in the year 1960s when they showed interests in turning of organic wastes into electricity during the space missions; however, that was a short-lived project. The MFC technology went on back seat until the work by Bennetto et al. (1983), where they reported the functioning of MFC with special focus on the use of mediators for electron transfer during electricity generation.
Since the advent of the twenty-first century, MFC attracted attentions of research groups around the world which is clearly visible from Fig. 11.1. Increase in number of publications of MFC with emphasis on bioremediation increased nearly sixty (60) times in year 2015–2016 as compared to year 2002–2003. It attracted attention of both developed and developing nation; China and the USA are in first place, and India is in third regarding MFC–bioremediation publications (Fig. 11.2).
In order to improve MFC, different strategies are used such as selection of electrodes, membranes materials, use of pollutants as substrates and designing new type of MFCs for specific applications which is explained later in the chapter.
11.3 Design of Microbial Fuel Cell
MFC can be divided into mainly types on the basis of design: single chambered and dual chambered. General schematic representation of MFC is described in Fig. 11.3 which contains separate cathodic and anodic chambers called as dual-chambered MFC whereas the single-chambered MFC contains both cathode and anode in a single chamber. Major components of MFC are cathode, anode and membrane.
The choice of material for each component is an important factor in energy output and economics of MFC as technology. Each component and advancement in the selection of materials for the cathode anode and membrane is described in the next section.
11.4 Electrode Materials
The performance of MFC for electricity generation and bioremediation is based on selection of proper anode and cathode materials. The basis for electrode selection usually depends on bacterial adhesion, electron transfer and electrochemical efficiency (Mustakeem 2015). In general practice the cost of materials used for electrode must be low and power densities maximized (Mustakeem 2015). The criteria for selecting the anodic and cathodic material are as follow:
-
A.
Electrical Conductivity: The electricity generation through MFC, an electron released from microbes, has to travel from the anode to the external circuit. The electrode material having less resistance will have higher conductivity for the electric current as it allow effortless flow of electron. The lower the resistance, the higher is the conductivity for the flow of electron and result generation of electricity. The low interfacial impedance also plays a key role in facilitating the electron transfer. In case of cathode, the higher ionic conductivity is required for facilitating the triple-phase boundary reaction (Mustakeem 2015; Natarajan and Van Nguyen 2004).
-
B.
Surface Area and Porosity: The surface area of electrode affects the final output power of MFCs. The loss of current is directly proportional to the electrode resistance. The efficiency of the MFC can be enhanced by decreasing the resistance of the electrode material that can be done by increasing the effective surface area whilst keeping the volume same. The larger surface area will provide large area for reactions that will enhance electrode kinetics (Wang et al. 2011; Rismani-Yazdi et al. 2008). Higher porosity allows bacteria to access and colonize more that help in biofilm formation.
-
C.
Stability and Durability: The material used for electrode must be capable of withstanding highly reducing and oxidizing environment in MFC that may lead to the swelling and decomposition of the materials. The electrode material must be highly durable which may be provided by increasing the surface roughness. However, excessive roughness may increase the probabilities of fouling that may decrease overall performance of the MFC in longer term. Therefore, a highly stable and durable electrode are required for overall enhanced performance of MFC (Santoro et al. 2017; Mustakeem 2015).
-
D.
Cost and Accessibility: It is necessary to use such material which is easily accessible to develop at lab scale and will help in impletion at large scale. The cost of the electrode material will influence the capital involved in construction of the MFC to a large extent. Thus, for implementation of the MFC at commercial scale, the material should be cheap, sustainable and easily available. For example, platinum is widely used; however, it is expensive. Carbonaceous and non-precious metal materials such as composites might be a substitute to precious metals in electrodes in the future (Santoro et al. 2017; Mustakeem 2015).
-
E.
Biocompatibility: It can be seen in term of compatibility of electrode surface for proper adhesion of the microbial biofilm. A highly biocompatible material will increase the bacterial adhesion and hence the life of the MFC and ease of current flow (Santoro et al. 2017; Mustakeem 2015).
11.4.1 Anode Materials
Anode is the electrode used in MFCs and is the site where electron donors undergo oxidization reactions (Zhang et al. 2016). The material used in anode preparation plays a significant role in the biofilm formation and the electron transfer between the microorganism and the electron acceptor. Various carbonaceous, metallic and composite materials are used for the construction of anode for increased power density and better energy output (Mustakeem 2015). List of different anode along with properties are tabulated in Table 11.1. The interaction between biofilm and anode electrode is affected by surface morphology and chemistry. The bacterial attachment with the electrode can be regulated by controlling the surface chemistry properties such as surface charge (Santoro et al. 2015), hydrophilicity/hydrophobicity (Du et al. 2017), oxygen/nitrogen functional groups and immobilized mediators (Santoro et al. 2017; Li et al. 2014). Further attachments can be regulated by surface morphology and can be controlled at nano- and micro-scale level using chemical treatments, surface coatings and electrochemical and thermal treatments. Recently lot of surface modifications such as 3D electrode are preferred over 2D electrode, as theoretically it is suggested that surface area is directly proportional to electricity generation. However, several studies have suggested that there are limitations for 3D surface such as pH gradients and diffusion transport phenomenon associated with product and reactant (Blanchet et al. 2016). Whilst designing a MFC, anode electrodes must be selected such as to avoid clogging and dead zone, as it will help in long-term operation (Santoro et al. 2017).
11.4.1.1 Role of Anode in Bioremediation
Most of the organic pollutants present in waste and wastewaters are in reduced form, and anode acts as a site for oxidation of electron donors (Zhang et al. 2016). The chemical oxygen demand (COD) is adopted as mean to quantify the oxidation power of the pollutants. In the anodic chamber, COD is converted to carbon dioxide and water (Zhang et al. 2016; Kim et al. 2015a, b). The recalcitrant pollutants such as azo dyes (Thung et al. 2015), polycyclic aromatic hydrocarbons (PAHs) (Sherafatmand and Ng 2015), benzene derivatives (Zhang et al. 2015), inorganic wastewaters containing sulphide (Raschitor et al. 2015), industrial wastewaters (Abbasi et al. 2016) and several organic wastes such as chicken feather (Chaturvedi and Verma 2014), poultry droppings and human excreta (Kretzschmar et al. 2017; Ieropoulos et al. 2013) are used. It has also been used for the treatment of various waste treatments such dairy manure, polluted soil sample and landfill leachate . However, solid waste digestion efficiency of the anode is low, but several modifications have been carried out to improve its efficiency, e.g. polluted sediments/soil/groundwater can also be remediated by embedded anodes in the polluted matrix with an external cathode exposed to the air.
11.4.2 Cathode Materials
The oxygen reduction reaction (ORR) takes place at the surface of cathode under three phasic interfaces, i.e. electrode (solid), electrolyte (liquid), air (gas), to form water. The ORR is the limiting reaction of the MFCs, and typical MFC cathode can be divided into three layers, i.e. diffusion layer, conducting support layer and catalyst. The materials used for the cathode (Table 11.2) must bear certain properties to act as robust cathode such as (a) high mechanical strength, (b) catalytic property and (c) high electronic and ionic conductivity (Santoro et al. 2017; Mustakeem 2015). Most of the materials used for anode can be used as cathode; however, carbon-based materials have poor catalytic activity, and an additional catalyst material is required to boost the reduction process. Different types of material used as cathode are tabulated in Table 11.2:
-
(a)
Cathode with Pt-based catalyst
-
(b)
Cathode with non-Pt-based catalyst
-
(c)
Cathode with metal-free catalyst
-
(d)
Biocathode
List of cathodic materials used in MFC along with their properties are tabulated in Table 11.2
11.4.2.1 Role of Cathode in Bioremediation
In MFC the electron acceptors undergo reduction reactions at cathode. The oxidized substrates are reduced at the cathode if the available electric potential exceeds the threshold of oxidized substances (Zhang et al. 2016; Nancharaiah et al. 2015). The environment of cathodic chamber is highly reducing; therefore it is widely used in waste (landfill leachate) and wastewater treatment (liquid fraction of pig slurry, swine wastewater), organic substances (chlorobenzene and trichloroethylene) heavy metals like (Cr6+, V5+) and inorganic substances like ammonia (Sotres et al. 2015), xenobiotic compounds, etc.
11.4.3 Membrane Material
The architecture, choice of material and overall arrangement of membrane in MFC affects the performance, cost and multi-level applications. A large array of materials have been tested for its application as membrane such as natural rubber (Rajan et al. 2006), laboratory gloves rubber (Winfield and Chambers 2014), j-cloth, nylon fibres (Zhang et al. 2010), glass fibres, biodegradable shopping bags and ceramics (Winfield et al. 2013). The cation-exchange membranes (CEM), e.g. Naflon, is the most commonly used membrane system (Santoro et al. 2017). Dual-purpose ion-exchange bridge, monolithic 3D printed materials and porous materials (sufficient strength, chemical inertness and longevity) can be employed as the membrane materials (Santoro et al. 2017; Philamore et al. 2015). Several microporous filtration membranes (Zhuang et al. 2009), nylon infused membrane (Hernández-Fernández et al. 2015), photocopy paper (Winfield et al. 2015), ceramics and terracotta materials (Winfield et al. 2016) have also been tested as membrane.
11.5 Types of Waste Materials Used as Substrates in MFC
MFCs are considered as an efficient technology which effectively utilizes wastewater for energy generation (Winfield et al. 2016), Various waste materials used as substrates in MFC are as follows:
-
1.
Lignocellulosic Biomass: Lignocellulosic materials are abundant and renewable natural resource. However it cannot be directly utilized as it has to be first converted to monosaccharides or low-molecular-weight compounds for the utilization by the microorganism (Huang et al. 2011; Ren et al. 2007) for electricity generation. Cellulose and chitin are cheap, renewable and readily available biopolymeric materials which can be used for electricity generation (Rezaei et al. 2009). For direct conversion of cellulose, the microorganism(s) must be able to hydrolyse cellulose anaerobically, utilizing anode as an electron acceptor as well as oxidizing metabolites obtained after cellulose hydrolysis. Thus, using a solid substrate such as cellulose or chitin, the power production is limited due to a low rate of hydrolysis of the particulate material. However few studies have been carried out using particulate substrates in MFC.
-
2.
Synthetic Wastewater: It has also been observed that synthetic or chemical wastewater having precise composition have also been used (Pant et al. 2010). However synthetic wastewater may contain redox mediators, such as cysteine and sulfur species (Aldrovandi et al. 2009). These redox mediators can act as abiotic electron donor and help in enhancing the production of electricity for a short while but it would not represent the true performance of the system (Aldrovandi et al. 2009) resulting in the ambiguity being generated in the results. Thus, this can be overcome by the use of minimal salt media with a single electron donor such as glucose or acetate.
-
3.
Brewery Wastewater: Breweries wastewater has been used in MFCs, primarily because of its low strength, suitable for electricity generation due to food-derived nature of organic matter and lack of high concentrations of inhibitory substances, e.g. ammonia in animal wastewaters (Feng et al. 2008). It can be an ideal substrate for MFCs due to its nature of high carbohydrate content and low ammonium nitrogen concentration (Pant et al. 2010).
-
4.
Starch Processing Wastewater: Starch processing wastewater (SPW) contains a relatively high content of carbohydrates sugars protein and starch which can be potentially converted to a wide variety of useful products (Jin et al. 1998). SPW was used as a fuel to enrich a microbial consortium generating electricity and current generation.
-
5.
Dye Wastewater: Azo dyes constitute the largest class of synthetic dyes and are extensively present in the effluent of dye and textile industries. The removal of toxic substances present in the effluents before discharge is of paramount importance as they are effecting the environment adversely. In aquatic system intense colour of dyes has led to obstruction of light and transfer of oxygen into the water bodies which is having detrimental effect on the aquatic flora and fauna (Pant et al. 2007). Very recently, efforts were made to utilize these dyes as substrate in MFC leading to detoxification and generating electricity. The concentration of glucose and dyes plays an important role in the current generation; however, the microorganism are unable to decolorize high concentrations of dye (Sun et al. 2009). Another method which can be used includes simultaneous treatment of azo dye-containing wastewater and readily biodegradable organic matter-containing wastewater. The addition of later could help in improving the dye degradation efficiency due to microbial consortium associated with it along with the presence of nutrient material, thus saving both cost and energy. However, the system still requires considerable improvements in terms of finding appropriate bacterial community that is capable of utilizing a mixture of dyes and other simple carbon sources in order to make MFCs a realistic solution for its extensive utility at large scale.
-
6.
Landfill Leachates: The use of landfill effluent in a biological fuel cell for COD removal was first reported by Habermann and Pommer (1991). Landfill leachates are heavily polluted landfill effluents with a complex composition containing four major groups of pollutants: dissolved organic matter, inorganic macro-components, heavy metals and xenobiotic organic compounds (Kjeldsen et al. 2002). Recently, Greenman et al. (2009) demonstrated that it is possible to generate electricity and simultaneously treat landfill leachate in MFC columns. Gálvez et al. (2009) operated three MFCs fluidically connected in series for simultaneous leachate treatment and electricity generation.
-
7.
Inorganic and Other Substrates: There are various substrates which have been used in the MFC as substrates such as paper-recycling plant wastewater (Huang and Logan 2008), unamended wastewater, phenol (Luo et al. 2009), carbon monoxide (Kim and Chang 2009), 1,2-dichloroethane (Pham et al. 2009), sulphate and thiosulphate (Zhao et al. 2009). MFC was used to remove the fermentation inhibitors in cellulosic biorefineries which included furfural, 5-hydroxymethylfurfural, vanillic acid, 4-hydroxybenzaldehyde and 4-hydroxyacetophenone with simultaneously generating electricity (Borole et al. 2009).
-
8.
Heavy Metals
-
Hexavalent Chromium: Chromium has various industrial applications such as leather tanning, metallurgy , electroplating and wood preservatives (Chaturvedi and Verma 2016). Cr(VI) is more hazardous due to its mutagenic and carcinogenic properties (Humphries et al. 2004); therefore, there is a need for detoxification of hexavalent chromium [Cr(VI)]. Chemical or electrochemical reduction into nontoxic chromium is the most preferred method; however, other approaches such as ion-exchange resins, filtration and direct chemical reduction have also been employed (Kurniawan et al. 2006). These technologies require high-energy inputs and produces by-products which itself are pollutants. Therefore, reduction of chromium coupled with electricity generation using MFC and can be applied for Cr(VI) treatment (Tandukar et al. 2009) at the cathode. Various microorganism used in the process include Trichococcus pasteurii and Pseudomonas aeruginosa.
-
Selenite: Selenium and its derivatives such as selenite (SeO3 −2) and selenate (SeO4 2) are widely used in industries, e.g. glass manufacturing and electronic industries. Selenium can enter in the environment through sewage sludge, fly ash from coal-fired power plants, oil refineries and mining of metal ores (Tandukar et al. 2009). Selenite is more toxic than selenate to aquatic invertebrates and fishes (Hamilton 2004; Lemly 1997), and it accumulates in aquatic plants thus causing bioaccumulation in higher organisms which can cause both acute and chronic toxicity in aquatic organisms (Rovira et al. 2008). The application of MFC technology in reduction of selenium and production of electricity by using selenium-containing waste was investigated by Catal et al. (2009).
-
9.
Nitrate: The use of nitrate -based fertilizers and animal waste has increased the presence of nitrate in water. Nitrate (nontoxic) can be transformed to nitrite (NO2−) after entering human body, which can cause “blue baby syndrome” normally observed in infants, or it can be converted to N-nitroso compounds which are carcinogenic into humans. Some of the treatment methods employed include electrochemical treatment, ion exchange (IE), reverse osmosis (RO), electrodialysis (ED) and heterogeneous catalysis (HC) (Park and Yoo 2009). As the methods employed are expensive, the use of MFC for removal of nitrate has gained importance due to the feasibility of the process. Few workers have employed a metal catalyst (Polatides and Kyriacou 2005) or microorganisms as catalysts on cathode electrode (He and Angenent 2006). In few studies, electrochemical denitrification process to remove nitrate ions was employed at cathode chamber of bio-electrochemical denitrification system (Kondaveeti and Min 2013).
-
10.
Marine Sediments Rich in Acetate: Marine sediments rich in acetate have been used as a substrate in MFC. Acetate presence helps in providing inertness towards alternative microbial conversions, fermentations and methanogenesis at room temperature (Sun et al. 2009; Aelterman 2009). Acetate can act as a carbon source to induce electroactive bacteria, and it is an end product in several metabolic pathways (including the Entner–Doudoroff pathway for glucose metabolism) for higher-order carbon sources (Biffinger et al. 2008; Bond and Lovley 2005). When Chae et al. 2009 compared the performance of four different substrates in terms of CE and power output, acetate-fed MFC showed the highest CE, followed by butyrate, propionate and glucose. According to Liu et al. (2009), acetate-based MFC achieved more electric power and external load resistance compared to those based on consortia induced by a protein-rich wastewater.
-
11.
Animal Wastes: Animal wastes such as poultry dropping, cow dung, human faeces and urine are reportedly used as substrate for the bioelectricity generation. Barbosa et al. (2017) and Jimenez et al. (2016) suggested that animal waste can be considered as potential substrate for generation of electricity due its high daily production, high COD, high nutrient (N and P) concentrations and high solution conductivity. Based on these observation, Ieropoulos et al. (2016) suggested that the treatment of urine can be performed in MFCs whilst generating electricity for low-power devices, such as light-emitting diode (LED) lights or sensors. Ieropoulos et al. (2016) ran field trials by connecting a stack of MFC to public urinals and used it for lighting a room. This was an attempt for taking the technology beyond laboratory, and it helped understand how fast the technology evolves and is being able to address the issue of public hygiene, sanitation, direct treatment of human waste and energy generation. Melhuish et al. (2006) designed a robot “EcoBot-II ”, which was powered by on-board MFC with oxygen cathodes, it simultaneously utilized unrefined insect biomass and converted into useful energy. Kretzschmar et al. (2017) provided an experimental proof of concept using human faeces as substrate in MFC for power generation.
-
12.
Petroleum Hydrocarbons: MFC can be a cost-effective and eco-friendly approach for bioremediation of petroleum hydrocarbons as these hydrocarbons act as substrate for microbes. The MFC technique was used for detoxification of soil decontaminated with petroleum at an increased rate and is energy sufficient. A U-tube MFC designed by where they observed enhanced degradation rate of petroleum hydrocarbons by 120% high charge output. Morris et al. (2009) used MFC for enhancing biodegradation of diesel. They demonstrated that in MFC diesel removal rate was increased by four times using MFC because the electrode served as an electron acceptor. MFC utilizing diesel (v/v 1%) as sole carbon source also resulted in high-power density and current density (Cheng et al. 2017). Therefore, MFC technology can be effectively used for detoxification and enhancing biodegradation of polluted soil/wastewater containing petroleum contaminants in anoxic environments, thus, eradicating the need to adjust terminal electron acceptors such as oxygen.
11.6 Types of Microbial Fuel Cell for Bioremediation of Pollutants
11.6.1 Anaerobic Microbial Fuel Cell (ANMFC)
ANMFC require oxygen that may cause loss of electrons and lead to increase in energy demand to carry out the process (Abbasi et al. 2016). Therefore, anaerobic MFC can be an environmental friendly and cost-effective alternative for simultaneous electricity generation and bioremediation. Wastewaters generated from brewery industries (Akman et al. 2013; Pant et al. 2010); distillery and domestic wastewater (Jiang et al. 2011); pharmaceutical industry (Velvizhi and Venkata Mohan 2012), petrochemical, vegetable oil, food industry and animal carcass wastewater (Li et al. 2013); textile (Solanki et al. 2013) swine wastewater (Zhuang et al. 2012); and municipal wastewater (Zhang et al. 2013) could be treated using anaerobic MFC. Abbasi et al. (2016) designed an anaerobic MFC where they utilized wastewater samples from vegetable oil industries, metal works, glass and marble industries as substrate. This process has significant effect on wastewater treatment efficiency for COD in range of 85–90% at 96 h of hydraulic retention time (HRT). The coulombic efficiency of 5184.7 C with maximum voltage of 890 mV was generated when vegetable oil industries discharge was treated in MFC. A positive significant co-relation was observed between COD concentration and voltage generated.
11.6.2 Sediment Microbial Fuel Cell (SMFC)
SMFC comprises of an anode buried in sediment and a cathode in an oxygen-rich water (Rezaei et al. 2007; Xia et al. 2015). The SMFC have been successfully tested in removal of persistent organic compounds such as polycyclic aromatic hydrocarbons (PAHs), xenobiotic compounds and pesticides, etc. along with electricity generation (Sherafatmand and Ng 2015). Similarly Yu et al. (2017) reported maximum power density together with removal of anthracene, phenanthrene and pyrene by a closed SMFC utilizing PAH-polluted soils. They further studied the influence of electrode interval and role of microbial community in electricity generation and removal of PAHs. The decrease in electrode interval resulted in efficient electricity generation and removal of PAHs. The SMFC was dominated by the genus of Geobacter and enriched in electrogenic bacteria at the anode surface. The growth of certain microbes (except electrogenic bacteria in the soil) was improved by electrical stimulation. Similarly Xu et al. (2017) found that genus Geobacter are predominately found in SMFC and more electrogenic bacteria are found attached in biofilm of anode. They also demonstrated that addition of Fe (III) oxide in SMFC enhances the removal efficiencies for organic pollutants. Xia et al. (2015) reported enhanced biodegradation of organic chemicals of high polarity by operating a SMFC in heavily contaminated sediment and analysing its global organic chemical degradation profile. The study showed that SMFC prefers to stimulate the degradation of organic chemicals with higher polarity. Cao et al. (2015) constructed a SMFC in the top soil contaminated with hexachlorobenzene (HCB), a toxic refractory organic pesticide. Under anaerobic condition in the soil MFC, HCB was degraded via the reductive dechlorination pathway and an existence of the anode promoted electrogenic bacteria provided more electrons that subsequently improved electricity generation.
11.6.3 Benthic Microbial Fuel Cells (BMFC)
BMFC consists of an anode present in anoxic benthic sediment and a cathode in oxic overlying water which is further connected using an external electric circuit. BMFC are different from SMFCs as the latter does not require in situ deployed in real water bodies (Li and Yu 2015; Holmes et al. 2004). BMFC has emerged as sustainable and efficient technology for cleaning up of contaminated sediments and simultaneous energy generation. The bioremediation of the contaminated sediments is performed by utilizing the natural metabolic activities of microbes in detoxification, decomposition or immobilization of environmental contaminants present in the sediments. BMFC is at initial stage but have shown many potential benefits such as accelerated decontamination, relatively easy deployment, self-sustained operation and control and environmental benignity. The relatively lower efficiency, limitations in respect of system design, electrode selection, microbial control and selection of deployment environment severely limit its application (Li and Yu 2015).
11.6.4 Enzyme-Based Microbial Fuel Cells (EBC)
The fuel cells are of two types first employing living cells known as microbial biofuel cells and the other utilizing enzymes and referred as enzymatic biofuel cells (EBC). The microbial biofuel cells have long lifetimes ~5 years (Moon et al. 2006; Kim et al. 2003) and can completely oxidize simple sugars to carbon dioxide (Bond et al. 2002). However they have low-power densities in W/cm2 per unit electrode surface area which is due to slow transport across cellular membranes (Palmore and Whitesides 1994). By contrast, enzymatic biofuel cells typically possess higher-power densities (although still lower than conventional fuel cells) and can partially oxidize the fuel and have limited lifetimes (typically 7–10 days) owing to the sensitive nature of the enzyme (Kim et al. 2006; Barton et al. 2004) and to eliminate the need for a membrane separator. One of the most significant advances in enzymatic biofuel cells is the development of biocathodes and bioanodes that employ direct electron transfer (DET) instead of mediated electron transfer (MET) . The DET is more preferred over the MET (Moore et al. 2004). The second method is the immobilization of the enzyme which has helped enzyme increase in the active lifetime of the enzymes (Topcagic et al. 2004). Recently, the active lifetimes have been extended beyond 1 year by encapsulation in micellar polymers (Akers et al. 2005; Moore et al. 2004; Topcagic et al. 2004). The EBC can be used extensively in biodegradation of toxic organic pollutant such as azo dyes, polyaromatic hydrocarbons, etc. The EBC have many disadvantages over traditional fuel cells and primary batteries; they remain limited by short lifetimes, catalytic inefficiencies, low fuel utilization and low-power densities (Minteer et al. 2007). However working solutions to short lifetimes and catalytic inefficiencies have been introduced, but advances in improved fuel utilization and power density are required.
11.6.5 Air-Breathing Cathode-Based Microbial Fuel Cells (ABC-MFC)
An air-breathing cathode consists of electrode substrate, catalyst layer and air diffusion layer (Wang et al. 2017). Single-chamber, air-breathing MFC with a flexible graphite sheet as the anode was designed by Sonawane et al. (2017), in which they used landfill leachate as substrate. They obtained open-circuit voltage (OCV) of 1.29 V, which is the highest reported OCV in the literature till date by utilizing landfill leachate as substrate. The reactor also resulted in generation of maximum cathode area-specific power density of 1513 mW m−2. Jimenez et al. (2016) demonstrated the use of gas-diffusion air-breathing cathode-based MFC for generation of electricity utilizing urine as substrate. As discussed earlier cathode is site of ORR , and it is one of the limiting factor for MFC performance; therefore Kodali et al. (2017) used Mn-, Fe-, Co- and Ni-containing platinum group metal-free catalysts along with aminoantipyrine, AAPyr precursor for enhanced electricity generation. With increase in solution conductivity, it was observed that Fe-AAPyr was found to be the most suitable catalyst–precursor combination in air-breathing cathode-based MFC for enhanced electricity with high-power density.
11.6.6 Constructed Wetland Microbial Fuel Cells (CW-MFC)
Constructed wetlands (CWs) and MFCs are two different technologies, nevertheless very compatible technologies (Liu et al. 2013) . Both the techniques are dependent on the actions of bacteria to remove pollutants from wastewater, but MFC has added advantage being energy generator. Therefore, the two techniques are combined in such a way that anode is buried in the anaerobic condition of constructed wetland and the cathode exposed to oxygen in the plant rhizosphere and collectively called as CW-MFC. The low oxygen availability at anode and higher redox gradient are an essential feature for generating electric current in CW-MFCs. The upflow regime of CW-MFC is such that it reduces the availability of dissolved oxygen (DO) at the anode whilst ensuring its maximum availability in the cathode region and also provide sufficient redox profile for MFC integration (Corbella et al. 2014; Fang et al. 2013). This natural redox gradient comes at cost of ohmic resistance of 120–500 (Doherty et al. 2015b; Villaseñor et al. 2013). A multi-electrode MFC with a separator electrode assembly was suggested by Ahn and Logan (2012) that help in reducing the ohmic resistance to 33 Ω. Glass wool separators can be used in construction of CW-MFC in order to minimize electrode spacing. This technology is excessively used in wastewater treatment with the aim of improving the wastewater treatment capacity of wetlands whilst simultaneously producing electrical power (Doherty et al. 2015a).
11.6.7 Thermophilic Microbial Fuel Cells (TMFC)
MFC used for the generation of electricity usually operated at ambient or mesophilic temperatures. Carver et al. (2011) suggested that thermophilic systems have potential for increased microbial activity rates on the anode that can subsequently enhance electricity generation. Air-cathode single chambers and two-chamber designs are usually used as for electricity generation, but the real role is played by thermophilic microbes associated with anode under anaerobic condition. Carver et al. (2011) described a thermophilic MFC design maintained at 57 °C with an anaerobic and thermophilic consortium. This suggested design minimized evaporation and associated microbes respired with glucose to generate a power density of 375 mW m−2 after 590 h. Voltage data and polarization showed that the design can work in both batch and continuous mode. Dai et al. (2017) constructed a two-chamber TMFC and utilized ethanol as an electron donor. They obtained an open-circuit potential of approximately 650 mV, maximum voltage of 550 mV and maximum power density of 437 mW m−2, and the coulombic efficiency was 20.5 ± 6.0. They also analysed the microbial dynamics by high-throughput sequencing and 16S rRNA clone library sequencing; Firmicutes bacteria accounted for 90.9% of all bacteria associated with TMFC biofilm. The development of TMFC-involved biotechnologies will be beneficial for the wastewater treatment, production of valuable chemicals and generation of energy at the same time (Dai et al. 2017).
11.7 Commercial Application of MFC and Economic Feasibility
MFC technology has come a long way, and over the last decade, significant scientific and technological development had brought the MFC to the point of becoming commercialized technology. During commercialization of a technology, various market forces decide its success such as cheap, cost-effective, environmental friendly, larger consumer base and above all profitability. For assessing the profitability of the MFC and checking whether the technology is ready to enter market for commercial energy generation and waste treatment, Trapero et al. (2017) used classical evaluation criteria for investment decisions such as the net present value (NPV) and the internal rate of return (IRR). They have presented an economic assessment of a MFC in a juice processing plant where maximum power density of the cell using two different MFC cases, i.e. cathodes with and without Pt, was studied. The performance was then compared to the conventional activated sludge process. Three different scenarios, optimistic, pessimistic and most likely scenarios, were analysed. They also performed study to find the important factors and design influencing MFC performance. By a sensitivity analysis of the electrode area, and the annual growth rate of the electricity pricing, it was revealed that the electrode area parameter is the most influential factor. The results of the study clearly showed that the implementation of MFC is a favourable substitute to the use of classical aerated activated sludge, and it has potential economic benefits. Whilst designing for a current state-of-the-art MFC keeping in view the future challenges, research directions may be focused on selection of microbial consortium, along with operational consistency and stability of the developed system.
11.8 Future Prospects and Directions
MFC is a promising technique with great potential as it is an environment-friendly tool which does not involve the use of renewable natural resource and emission of pollution. In recent times substantial technological advancement and improvement are observed for waste/wastewater treatments in MFCs. MFCs have multiple applications which include energy generation, waste treatment and production of chemicals, sensors for pollution level in water/soil, etc. It can generate energy out of waste without any external/additional energy. This property will enable MFC technology to be used in remote areas for energy generation and waste treatment e.g., maintaining sanitation and hygiene, catering the need of poor people for affordable electricity which is of particular interest for countries and regions of the developing world. However, several aspects need to be addressed in order to improve the MFCs performance such as power output and efficiency, material costs for electrodes and separators, microbial consortium, suitable MFC design for treatment of specific waste and the optimization of the bio-electrochemical reactions. Keeping in mind the funding/development perspective, MFCs can be considered as nascent technology, but recent involvement of government laboratories, NGOs, philanthropist and business houses has provided helps and support for continuing research into MFC technology with a hope to find solutions to global environmental problems.
References
Abbasi U, Jin W, Pervez A, Ahmad Z, Tariq M, Shaheen S, Iqbal A, Mahmood Q (2016) Anaerobic microbial fuel cell treating combined industrial wastewater: correlation of electricity generation with pollutants. Bioresour Technol 200:1–7. https://doi.org/10.1016/j.biortech.2015.09.088
Aelterman P (2009) Microbial fuel cells for the treatment of waste streams with energy recovery. Dissertation, Ghent University, Faculty of Bioscience Engineering
Ahn Y, Logan BE (2012) A multi-electrode continuous flow microbial fuel cell with separator electrode assembly design. Appl Microbiol Biotechnol 93:2241–2248. https://doi.org/10.1007/s00253-012-3916-4
Akers NL, Moore CM, Minteer SD (2005) Development of alcohol/O2 biofuel cells using salt-extracted tetrabutylammonium bromide/Nafion membranes to immobilize dehydrogenase enzymes. Electrochim Acta 50:2521–2525. https://doi.org/10.1016/j.electacta.2004.10.080
Akman D, Cirik K, Ozdemir S, Ozkaya B, Cinar O (2013) Bioelectricity generation in continuously – fed microbial fuel cell: effects of anode electrode material and hydraulic retention time. Bioresour Technol 149:459–464. https://doi.org/10.1016/j.biortech.2013.09.102
Aldrovandi A, Marsili E, Stante L, Paganin P, Tabacchioni S, Giordano A (2009) Sustainable power production in a membrane-less and mediator-less synthetic wastewater microbial fuel cell. Bioresour Technol 100:3252–3260. https://doi.org/10.1016/j.biortech.2009.01.041
Amade R, Vila-Costa M, Hussain S, Casamayor EO, Bertran E (2015) Vertically aligned carbon nanotubes coated with manganese dioxide as cathode material for microbial fuel cells. J Mater Sci 50:1214–1220. https://doi.org/10.1007/s10853-014-8677-2
Artyushkova K, Roizman D, Santoro C, Doyle LE, Fatima Mohidin A, Atanassov P, Marsili E (2016) Anodic biofilms as the interphase for electroactive bacterial growth on carbon veil. Biointerphases 11:31013. https://doi.org/10.1116/1.4962264
Barbosa SG, Peixoto L, Ter Heijne A, Kuntke P, Alves MM, Pereira MA (2017) Investigating bacterial community changes and organic substrate degradation in microbial fuel cells operating on real human urine. Environ Sci Water Res Technol 3:897–904. https://doi.org/10.1039/C7EW00087A
Barton SC, Gallaway J, Atanassov P (2004) Enzymatic biofuel cells for implantable and microscale devices. Chem Rev 104:4867–4886
Baudler A, Langner M, Rohr C, Greiner A, Schröder U (2017) Metal–polymer hybrid architectures as novel anode platform for microbial electrochemical technologies. ChemSusChem 10:253–257. https://doi.org/10.1002/cssc.201600814
Bennetto HP, Stirling JL, Tanaka K, Vega CA (1983) Anodic reactions in microbial fuel cells. Biotechnol Bioeng 25:559–568. https://doi.org/10.1002/bit.260250219
Biffinger JC, Byrd JN, Dudley BL, Ringeisen BR (2008) Oxygen exposure promotes fuel diversity for Shewanella oneidensis microbial fuel cells. Biosens Bioelectron 23:820–826. https://doi.org/10.1016/j.bios.2007.08.021
Blanchet E, Erable B, De Solan ML, Bergel A (2016) Two-dimensional carbon cloth and three-dimensional carbon felt perform similarly to form bioanode fed with food waste. Electrochem Commun 66:38–41. https://doi.org/10.1016/j.elecom.2016.02.017
Boghani HC, Papaharalabos G, Michie I, Fradler KR, Dinsdale RM, Guwy AJ, Ieropoulos I, Greenman J, Premier GC (2014) Controlling for peak power extraction from microbial fuel cells can increase stack voltage and avoid cell reversal. J Power Sources 269:363–369. https://doi.org/10.1016/j.jpowsour.2014.06.059
Bond DR, Lovley DR (2005) Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl Environ Microbiol 71:2186–2189. https://doi.org/10.1128/AEM.71.4.2186
Bond DR, Holmes DE, Tender LM, Lovley DR (2002) Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483–485
Borole AP, Mielenz JR, Vishnivetskaya TA, Hamilton CY (2009) Controlling accumulation of fermentation inhibitors in biorefinery recycle water using microbial fuel cells. Biotechnol Biofuels 2:7. https://doi.org/10.1186/1754-6834-2-7
Calignano F, Tommasi T, Manfredi D, Chiolerio A (2015) Additive manufacturing of a microbial fuel cell—a detailed study. Sci Rep 5:17373. https://doi.org/10.1038/srep17373
Cao YL, Yang HX, Ai XP, Xiao LF (2003) The mechanism of oxygen reduction on MnO2-catalyzed air cathode in alkaline solution. J Electroanal Chem 557:127–134. https://doi.org/10.1016/S0022-0728(03)00355-3
Cao X, Song H, Yu C, Li X (2015) Simultaneous degradation of toxic refractory organic pesticide and bioelectricity generation using a soil microbial fuel cell. Bioresour Technol 189:87–93. https://doi.org/10.1016/j.biortech.2015.03.148
Carver SM, Vuoriranta P, Tuovinen OH (2011) A thermophilic microbial fuel cell design. J Power Sources 196:3757–3760. https://doi.org/10.1016/j.jpowsour.2010.12.088
Catal T, Bermek H, Liu H (2009) Removal of selenite from wastewater using microbial fuel cells. Biotechnol Lett 31(8):1211–1216
Chae KJ, Choi MJ, Lee JW, Kim KY, Kim IS (2009) Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour Technol 100:3518–3525. https://doi.org/10.1016/j.biortech.2009.02.065
Chaturvedi V, Verma P (2014) Metabolism of chicken feathers and concomitant electricity generation by Pseudomonas aeruginosa by employing microbial fuel cell (MFC). J Waste Manag 2014:9. https://doi.org/10.1155/2014/928618
Chaturvedi V, Verma P (2016) Microbial fuel cell: a green approach for the utilization of waste for the generation of bioelectricity. Bioresour Bioprocess 3:38. https://doi.org/10.1186/s40643-016-0116-6
Chen S, He G, Carmona-Martinez AA, Agarwal S, Greiner A, Hou H, Schröder U (2011) Electrospun carbon fiber mat with layered architecture for anode in microbial fuel cells. Electrochem Commun 13:1026–1029. https://doi.org/10.1016/j.elecom.2011.06.009
Chen S, He G, Liu Q, Harnisch F, Zhou Y, Chen Y, Hanif M, Wang S, Peng X, Hou H, Schroder U (2012a) Layered corrugated electrode macrostructures boost microbial bioelectrocatalysis. Energy Environ Sci 5:9769–9772. https://doi.org/10.1039/C2EE23344D
Chen S, He G, Hu X, Xie M, Wang S, Zeng D, Hou H, Schröder U (2012b) A three-dimensionally ordered macroporous carbon derived from a natural resource as anode for microbial bioelectrochemical systems. ChemSusChem 5:1059–1063. https://doi.org/10.1002/cssc.201100783
Cheng S, Logan BE (2007) Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem Commun 9:492–496 https://doi.org/10.1016/j.elecom.2006.10.023
Cheng S, Liu H, Logan BE (2006a) Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ Sci Technol 40:2426–2432. https://doi.org/10.1021/es051652w
Cheng S, Liu H, Logan BE (2006b) Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (Nafion and PTFE) in single chamber microbial fuel cells. Environ Sci Technol 40:364–369. https://doi.org/10.1021/es0512071
Cheng Y, Wang L, Faustorilla V, Megharaj M (2017) Chemosphere integrated electrochemical treatment systems for facilitating the bioremediation of oil spill contaminated soil. Chemosphere 175:294–299. https://doi.org/10.1016/j.chemosphere.2017.02.079
Ci S, Wu Y, Zou J, Tang L, Luo S, Li J, Wen Z (2012) Nitrogen-doped graphene nanosheets as high efficient catalysts for oxygen reduction reaction. Chinese Sci Bull 57:3065–3070. https://doi.org/10.1007/s11434-012-5253-5
Corbella C, Garfí M, Puigagut J (2014) Vertical redox profiles in treatment wetlands as function of hydraulic regime and macrophytes presence: surveying the optimal scenario for microbial fuel cell implementation. Sci Total Environ 470–471:754–758. https://doi.org/10.1016/j.scitotenv.2013.09.068
Dai K, Wen J-L, Zhang F, Ma X-W, Cui X-Y, Zhang Q, Zhao T-J, Zeng RJ (2017) Electricity production and microbial characterization of thermophilic microbial fuel cells. Bioresour Technol 243:512–519. https://doi.org/10.1016/j.biortech.2017.06.167
Delord B, Neri W, Bertaux K, Derre A, Ly I, Mano N, Poulin P (2017) Carbon nanotube fiber mats for microbial fuel cell electrodes. Bioresour Technol 243:1227–1231. https://doi.org/10.1016/j.biortech.2017.06.170
Dewan A, Beyenal H, Lewandowski Z (2008) Scaling up microbial fuel cells. Environ Sci Technol 42:7643–7648. https://doi.org/10.1021/es800775d
Doherty L, Zhao Y, Zhao X, Hu Y, Hao X (2015a) A review of a recently emerged technology: constructed wetland microbial fuel cells. Water Res 85:38–45. https://doi.org/10.1016/j.watres.2015.08.016
Doherty L, Zhao Y, Zhao X, Wang W (2015b) Nutrient and organics removal from swine slurry with simultaneous electricity generation in an alum sludge-based constructed wetland incorporating microbial fuel cell technology. Chem Eng J 266:74–81. https://doi.org/10.1016/j.cej.2014.12.063
Du Q, An J, Li J, Zhou L, Li N, Wang X (2017) Polydopamine as a new modification material to accelerate startup and promote anode performance in microbial fuel cells. J Power Sources 343:477–482. https://doi.org/10.1016/j.jpowsour.2017.01.093
Fang Z, Song HL, Cang N, Li XN (2013) Performance of microbial fuel cell coupled constructed wetland system for decolorization of azo dye and bioelectricity generation. Bioresour Technol 144:165–171. https://doi.org/10.1016/j.biortech.2013.06.073
Feng Y, Wang X, Logan BE, Lee H (2008) Brewery wastewater treatment using air-cathode microbial fuel cells. Appl Microbiol Biotechnol 78:873–880. https://doi.org/10.1007/s00253-008-1360-2
Feng Y, Lee H, Wang X, Liu Y, He W (2010) Continuous electricity generation by a graphite granule baffled air-cathode microbial fuel cell. Bioresour Technol 101:632–638. https://doi.org/10.1016/j.biortech.2009.08.046
Gálvez A, Greenman J, Ieropoulos I (2009) Landfill leachate treatment with microbial fuel cells; scale-up through plurality. Bioresour Technol 100:5085–5091. https://doi.org/10.1016/j.biortech.2009.05.061
Ghasemi M, Shahgaldi S, Ismail M, Kim BH, Yaakob Z, Wan Daud WR (2011) Activated carbon nanofibers as an alternative cathode catalyst to platinum in a two-chamber microbial fuel cell. Int J Hydrogen Energy 36:13746–13752 https://doi.org/10.1016/j.ijhydene.2011.07.118
Gong K, Du F, Xia Z, Durstock M, Dai L (2009) Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 323:760–764
Greenman J, Gálvez A, Giusti L, Ieropoulos I (2009) Electricity from landfill leachate using microbial fuel cells: comparison with a biological aerated filter. Enzyme Microb Technol 44:112–119. https://doi.org/10.1016/j.enzmictec.2008.09.012
Guerrini E, Grattieri M, Trasatti SP, Bestetti M, Cristiani P (2014) Performance explorations of single chamber microbial fuel cells by using various microelectrodes applied to biocathodes. Int J Hydrogen Energy 39:21837–21846. https://doi.org/10.1016/j.ijhydene.2014.06.132
Guo K, Hidalgo D, Tommasi T, Rabaey K (2016) Pyrolytic carbon-coated stainless steel felt as a high-performance anode for bioelectrochemical systems. Bioresour Technol 211:664–668. https://doi.org/10.1016/j.biortech.2016.03.161
Habermann W, Pommer EH (1991) Biological fuel cells with sulphide storage capacity. Appl Microbiol Biotechnol 35:128–133. https://doi.org/10.1007/BF00180650
Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Sci Total Environ 326:1–31. https://doi.org/10.1016/j.scitotenv.2004.01.019
He Z, Angenent LT (2006) Application of bacterial biocathodes in microbial fuel cells. Electroanalysis 18:2009–2015. https://doi.org/10.1002/elan.200603628
He G, Gu Y, He S, Schröder U, Chen S, Hou H (2011) Effect of fiber diameter on the behavior of biofilm and anodic performance of fiber electrodes in microbial fuel cells. Bioresour Technol 102:10763–10766. https://doi.org/10.1016/j.biortech.2011.09.006
Heijne A, Hamelers HVM, Saakes M, Buisman CJN (2008) Performance of non-porous graphite and titanium-based anodes in microbial fuel cells. Electrochim Acta 53:5697–5703. https://doi.org/10.1016/j.electacta.2008.03.032
Hernández-Fernández FJ, Pérez de los Ríos A, Mateo-Ramírez F, Godínez C, Lozano-Blanco LJ, Moreno JI, Tomás-Alonso F (2015) New application of supported ionic liquids membranes as proton exchange membranes in microbial fuel cell for waste water treatment. Chem Eng J 279:115–119. https://doi.org/10.1016/j.cej.2015.04.036
Holmes DE, Bond DR, O’Neil RA, Reimers CE, Tender LR, Lovley DR (2004) Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb Ecol 48:178–190. https://doi.org/10.1007/s00248-003-0004-4
Huang L, Logan BE (2008) Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Appl Microbiol Biotechnol 80:349–355. https://doi.org/10.1007/s00253-008-1546-7
Huang YX, Liu XW, Sun XF, Sheng GP, Zhang YY, Yan GM, Wang SG, Xu AW, Yu HQ (2011) A new cathodic electrode deposit with palladium nanoparticles for cost-effective hydrogen production in a microbial electrolysis cell. Int J Hydrogen Energy 36:2773–2776. https://doi.org/10.1016/j.ijhydene.2010.11.114
Humphries AC, Nott KP, Hall LD, Macaskie LE (2004) Continuous removal of Cr (VI) from aqueous solution catalysed by palladised biomass of Desulfovibrio vulgaris. Biotechnol Lett 26:1529–1532
Ieropoulos IA, Ledezma P, Stinchcombe A, Papaharalabos G, Melhuish C, Greenman J (2013) Waste to real energy: the first MFC powered mobile phone. Phys Chem Chem Phys 15:15312. https://doi.org/10.1039/c3cp52889h
Ieropoulos IA, Stinchcombe A, Gajda I, Forbes S, Merino-Jimenez I, Pasternak G, Sanchez-Herranz D, Greenman J (2016) Pee power urinal – microbial fuel cell technology field trials in the context of sanitation. Environ Sci Water Res Technol 2:336–343. https://doi.org/10.1039/C5EW00270B
Jiang D, Li B (2009) Granular activated carbon single-chamber microbial fuel cells (GAC-SCMFCs): a design suitable for large-scale wastewater treatment processes. Biochem Eng J 47:31–37. https://doi.org/10.1016/j.bej.2009.06.013
Jiang D, Curtis M, Troop E, Scheible K, McGrath J, Hu B, Suib S, Raymond D, Li B (2011) A pilot-scale study on utilizing multi-anode/cathode microbial fuel cells (MAC MFCs) to enhance the power production in wastewater treatment. Int J Hydrogen Energy 36:876–884. https://doi.org/10.1016/j.ijhydene.2010.08.074
Jimenez IM, Santoro C, Carbonell SR, Greenman J, Ieropoulos I, Atanassov P (2016) Carbon-based air-breathing cathodes for microbial fuel cells. Catalyst 6:1–13. https://doi.org/10.3390/catal6090127
Jin B, van Leeuwen HJ, Patel B, Yu Q (1998) Utilisation of starch processing wastewater from production of microbial biomass protein and fungal α-amylase by Aspergillus oryzae. Bioresour Technol 66:201–206
Karra U, Manickam SS, Mccutcheon JR, Patel N, Li B (2012) Power generation and organics removal from wastewater using activated carbon nanofiber (ACNF) microbial fuel cells (MFCs). Int J Hydrogen Energy 38:1588–1597. https://doi.org/10.1016/j.ijhydene.2012.11.005
Karthikeyan R, Wang B, Xuan J, Wong JWC, Lee PKH, Leung MKH (2015) Interfacial electron transfer and bioelectrocatalysis of carbonized plant material as effective anode of microbial fuel cell. Electrochim Acta 157:314–323. https://doi.org/10.1016/j.electacta.2015.01.029
Kim D, Chang IS (2009) Electricity generation from synthesis gas by microbial processes: CO fermentation and microbial fuel cell technology. Bioresour Technol 100:4527–4530. https://doi.org/10.1016/j.biortech.2009.04.017
Kim BH, Chang IS, Gil GC, Park HS, Kim HJ (2003) Novel BOD (biological oxygen demand) sensor using mediator-less microbial fuel cell. Biotechnol Lett 25:541–545
Kim J, Jia H, Wang P (2006) Challenges in biocatalysis for enzyme-based biofuel cells. Biotechnol Adv 24:296–308. https://doi.org/10.1016/j.biotechadv.2005.11.006
Kim B, An J, Fapyane D, Chang IS (2015a) Bioelectronic platforms for optimal bio-anode of bio-electrochemical systems: from nano-to macro scopes. Bioresour technol 195:2–13
Kim KY, Yang W, Logan BE (2015b) Impact of electrode configurations on retention time and domestic wastewater treatment efficiency using microbial fuel cells. Water Res 80:41–46
Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, Christensen TH (2002) Present and long-term composition of MSW landfill leachate: a review. Crit Rev Environ Sci Technol 32:297–336. https://doi.org/10.1080/10643380290813462
Kodali M, Santoro C, Serov A, Kabir S, Artyushkova K, Matanovic I, Atanassov P (2017) Air breathing cathodes for microbial fuel cell using Mn-, Fe-, Co- and Ni-containing platinum group metal-free catalysts. Electrochim Acta 231:115–124. https://doi.org/10.1016/j.electacta.2017.02.033
Kondaveeti S, Min B (2013) Nitrate reduction with biotic and abiotic cathodes at various cell voltages in bioelectrochemical denitrification system. Bioprocess Biosyst Eng 36:231–238. https://doi.org/10.1007/s00449-012-0779-0
Kretzschmar J, Riedl S, Brown RK, Schröder U, Harnisch F (2017) eLatrine: lessons learned from the development of a low-tech MFC based on cardboard electrodes for the treatment of human feces. J Electrochem Soc 164:H3065–H3072. https://doi.org/10.1149/2.0121703jes
Kurniawan TA, Chan GYS, Lo W-H, Babel S (2006) Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci Total Environ 366:409–426. https://doi.org/10.1016/j.scitotenv.2005.10.001
Lemly AD (1997) Ecosystem recovery following selenium contamination in a freshwater reservoir. Ecotoxicol Environ Saf 36:275–281. https://doi.org/10.1006/eesa.1996.1515
Lepage G, Albernaz FO, Perrier G, Merlin G (2012) Characterization of a microbial fuel cell with reticulated carbon foam electrodes. Bioresour Technol 124:199–207. https://doi.org/10.1016/j.biortech.2012.07.067
Li W, Yu H (2015) Stimulating sediment bioremediation with benthic microbial fuel cells. Biotechnol Adv 33:1–12. https://doi.org/10.1016/j.biotechadv.2014.12.011
Li X, Zhu N, Wang Y, Li P, Wu P, Wu J (2013) Animal carcass wastewater treatment and bioelectricity generation in up-flow tubular microbial fuel cells: effects of HRT and non-precious metallic catalyst. Bioresour Technol 128:454–460. https://doi.org/10.1016/j.biortech.2012.10.053
Li B, Zhou J, Zhou X, Wang X, Li B, Santoro C, Grattieri M, Babanova S, Artyushkova K, Atanassov P, Schuler AJ (2014) Surface modification of microbial fuel cells anodes: approaches to practical design. Electrochim Acta 134:116–126. https://doi.org/10.1016/j.electacta.2014.04.136
Liao Q, Zhang J, Li J, Ye D, Zhu X, Zhang B (2015) Increased performance of a tubular microbial fuel cell with a rotating carbon-brush anode. Biosens Bioelectron 63:558–561. https://doi.org/10.1016/j.bios.2014.08.014
Lim DH, Wilcox J (2012) Mechanisms of the oxygen reduction reaction on defective graphene-supported Pt nanoparticles from first-principles. J Phys Chem C 116:3653–3660. https://doi.org/10.1021/jp210796e
Liu H, Ramnarayanan R, Logan BE (2004) Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ Sci Technol 38:2281–2285. https://doi.org/10.1021/es034923g
Liu Z, Liu J, Zhang S, Su Z (2009) Study of operational performance and electrical response on mediator-less microbial fuel cells fed with carbon- and protein-rich substrates. Biochem Eng J 45:185–191. https://doi.org/10.1016/j.bej.2009.03.011
Liu S, Song H, Li X, Yang F (2013) Power generation enhancement by utilizing plant photosynthate in microbial fuel cell coupled constructed wetland system. Int J Photoenergy 2013:10. https://doi.org/10.1155/2013/172010
Lowy DA, Tender LM, Zeikus JG, Park DH, Lovley DR (2006) Harvesting energy from the marine sediment–water interface II: Kinetic activity of anode materials. Biosens Bioelectron 21:2058–2063 https://doi.org/10.1016/j.bios.2006.01.033
Lu Z-J, Bao S-J, Gou Y-T, Cai C-J, Ji C-C, Xu M-W, Song J, Wang R (2013) Nitrogen-doped reduced-graphene oxide as an efficient metal-free electrocatalyst for oxygen reduction in fuel cells. RSC Adv 3:3990. https://doi.org/10.1039/c3ra22161j
Luo H, Liu G, Zhang R, Jin S (2009) Phenol degradation in microbial fuel cells. Chem Eng J 147:259–264. https://doi.org/10.1016/j.cej.2008.07.011
Matter PH, Zhang L, Ozkan US (2006) The role of nanostructure in nitrogen-containing carbon catalysts for the oxygen reduction reaction. J Catal 239:83–96 https://doi.org/10.1016/j.jcat.2006.01.022
Melhuish C, Ieropoulos I, Greenman J, Horsfield I (2006) Energetically autonomous robots: food for thought. Auton Robots 21:187–198. https://doi.org/10.1007/s10514-006-6574-5
Minteer SD, Liaw BY, Cooney MJ (2007) Enzyme-based biofuel cells. Curr Opin Biotechnol 18(3):228–234
Moon H, Chang IS, Kim BH (2006) Continuous electricity production from artificial wastewater using a mediator-less microbial fuel cell. Bioresour Technol 97:621–627. https://doi.org/10.1016/j.biortech.2005.03.027
Moore CM, Akers NL, Hill AD, Johnson ZC, Minteer SD (2004) Improving the environment for immobilized dehydrogenase enzymes by modifying Nafion with tetraalkylammonium bromides. Biomacromolecules 5(4):1241–1247
Morris JM, Jin S, Wang J, Zhu C, Urynowicz MA (2007) Lead dioxide as an alternative catalyst to platinum in microbial fuel cells. Electrochem Commun 9:1730–1734. https://doi.org/10.1016/j.elecom.2007.03.028
Morris JM, Jin S, Crimi B, Pruden A (2009) Microbial fuel cell in enhancing anaerobic biodegradation of diesel. Chem Eng J 146:161–167. https://doi.org/10.1016/j.cej.2008.05.028
Mustakeem (2015) Electrode materials for microbial fuel cells: nanomaterial approach. Mater Renew Sustain Energy 4:1–11. https://doi.org/10.1007/s40243-015-0063-8
Nancharaiah YV, Mohan SV, Lens PNL (2015) Metals removal and recovery in bioelectrochemical systems: a review. Bioresour Technol 195:102–114
Natarajan D, Van Nguyen T (2004) Effect of electrode configuration and electronic conductivity on current density distribution measurements in PEM fuel cells. J Power Sources 135:95–109. https://doi.org/10.1016/j.jpowsour.2004.03.063
Palmore GTR, Whitesides GM (1994) Microbial and enzymatic biofuel cells. In: Enzymatic conversion of biomass for fuels production. ACS Publications, Washington, DC, pp 271–290
Pant D, Singh A, Satyawali Y, Gupta RK (2007) Effect of carbon and nitrogen source amendment on synthetic dyes decolourizing efficiency of white-rot fungus, Phanerochaete chrysosporium. J Environ Biol 29:79
Pant D, Van Bogaert G, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol 101:1533–1543. https://doi.org/10.1016/j.biortech.2009.10.017
Park JY, Yoo YJ (2009) Biological nitrate removal in industrial wastewater treatment: which electron donor we can choose. Appl Microbiol Biotechnol 82:415–429. https://doi.org/10.1007/s00253-008-1799-1
Park DH, Zeikus JG (2003) Improved fuel cell and electrode designs for producing electricity from microbial degradation. Biotechnol Bioeng 81:348–355
Pham H, Boon N, Marzorati M, Verstraete W (2009) Enhanced removal of 1,2-dichloroethane by anodophilic microbial consortia. Water Res 43:2936–2946. https://doi.org/10.1016/j.watres.2009.04.004
Philamore H, Rossiter J, Walters P, Winfield J, Ieropoulos I (2015) Cast and 3D printed ion exchange membranes for monolithic microbial fuel cell fabrication. J Power Sources 289:91–99. https://doi.org/10.1016/j.jpowsour.2015.04.113
Polatides C, Kyriacou G (2005) Electrochemical reduction of nitrate ion on various cathodes – reaction kinetics on bronze cathode. J Appl Electrochem 35:421–427. https://doi.org/10.1007/s10800-004-8349-z
Potter MC (1911) Electrical effects accompanying the decomposition of organic compounds. Proc R Soc B Biol Sci 84:260–276. https://doi.org/10.1098/rspb.1911.0073
Rabaey K, Rozendal RA (2010) Microbial electrosynthesis revisiting the electrical route for microbial production. Nat Rev Microbiol 8:706–716
Rajan VV, Dierkes WK, Joseph R, Noordermeer JWM (2006) Science and technology of rubber reclamation with special attention to NR-based waste latex products. Prog Polym Sci 31:811–834. https://doi.org/10.1016/j.progpolymsci.2006.08.003
Raschitor A, Soreanu G, Fernandez-Marchante CM, Lobato J, Cañizares P, Cretescu I, Rodrigo MA (2015) Bioelectro-claus processes using MFC technology: influence of co-substrate. Bioresour Technol 189:94–98. https://doi.org/10.1016/j.biortech.2015.03.115
Ren Z, Ward TE, Regan JM (2007) Electricity production from cellulose in a microbial fuel cell using a defined binary culture. Environ Sci Technol 41:4781–4786. https://doi.org/10.1021/es070577h
Rezaei F, Richard TL, Brennan RA, Logan BE (2007) Substrate-enhanced microbial fuel cells for improved remote power generation from sediment-based systems. Environ Sci Technol 41:4053–4058. https://doi.org/10.1021/es070426e
Rezaei F, Richard TL, Logan BE (2009) Analysis of chitin particle size on maximum power generation, power longevity, and Coulombic efficiency in solid-substrate microbial fuel cells. J Power Sources 192:304–309. https://doi.org/10.1016/j.jpowsour.2009.03.023
Rismani-Yazdi H, Carver SM, Christy AD, Tuovinen OH (2008) Cathodic limitations in microbial fuel cells: an overview. J Power Sources 180:683–694. https://doi.org/10.1016/j.jpowsour.2008.02.074
Rodrigo J, Boltes K, Esteve-nuñez A (2014) Microbial-electrochemical bioremediation and detoxification of dibenzothiophene-polluted soil. Chemosphere 101:61–65. https://doi.org/10.1016/j.chemosphere.2013.11.060
Rovira M, Gim J, Mart X, de Pablo J, Mart V, Duro L (2008) Sorption of selenium (IV) and selenium (VI) onto natural iron oxides: goethite and hematite. J Hazard Mater 150:279–284. https://doi.org/10.1016/j.jhazmat.2007.04.098
Roy JN, Babanova S, Garcia KE, Cornejo J, Ista LK, Atanassov P (2014) Catalytic biofilm formation by Shewanella oneidensis MR-1 and anode characterization by expanded uncertainty. Electrochim Acta 126:3–10. https://doi.org/10.1016/j.electacta.2013.07.075
Santoro C, Li B, Cristiani P, Squadrito G (2013) Power generation of microbial fuel cells (MFCs) with low cathodic platinum loading. Int J Hydrogen Energy 38:692–700. https://doi.org/10.1016/j.ijhydene.2012.05.104
Santoro C, Guilizzoni M, Correa Baena JP, Pasaogullari U, Casalegno A, Li B, Babanova S, Artyushkova K, Atanassov P (2014) The effects of carbon electrode surface properties on bacteria attachment and start up time of microbial fuel cells. Carbon N Y 67:128–139 https://doi.org/10.1016/j.carbon.2013.09.071
Santoro C, Artyushkova K, Cornejo JA, Ista L, Bretschger O, Marsili E, Atanassov P, Schuler AJ (2015) Influence of anode surface chemistry on microbial fuel cell operation. Bioelectrochemistry 106:141–149
Santoro C, Arbizzani C, Erable B, Ieropoulos I (2017) Microbial fuel cells: from fundamentals to applications. A review. J Power Sources 356:225–244. https://doi.org/10.1016/j.jpowsour.2017.03.109
Seviour T, Doyle LE, Lauw SJL, Hinks J, Rice SA, Nesatyy VJ, Webster RD, Kjelleberg S, Marsili E (2015) Voltammetric profiling of redox-active metabolites expressed by Pseudomonas aeruginosa for diagnostic purposes. Chem Commun 51:3789–3792. https://doi.org/10.1039/C4CC08590F
Sharma T, Mohana AL, Chandra TS, Ramaprabhu S (2008) Development of carbon nanotubes and nanofluids based microbial fuel cell. Int J Hydrogen Energy 33:6749–6754. https://doi.org/10.1016/j.ijhydene.2008.05.112
Sherafatmand M, Ng HY (2015) Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresour Technol 195:122–130. https://doi.org/10.1016/j.biortech.2015.06.002
Solanki K, Subramanian S, Basu S (2013) Microbial fuel cells for azo dye treatment with electricity generation: a review. Bioresour Technol 131:564–571. https://doi.org/10.1016/j.biortech.2012.12.063
Sonawane JM, Adeloju SB, Ghosh PC (2017) Landfill leachate: a promising substrate for microbial fuel cells. Int J Hydrogen Energy 42:23794–23798. https://doi.org/10.1016/j.ijhydene.2017.03.137
Sotres A, Cerrillo M, Vias M, Bonmat A (2015) Nitrogen recovery from pig slurry in a two-chambered bioelectrochemical system. Bioresour Technol 194:373–382. https://doi.org/10.1016/j.biortech.2015.07.036
Srikanth S, Marsili E, Flickinger MC, Bond DR (2008) Electrochemical characterization of Geobacter sulfurreducens cells immobilized on graphite paper electrodes. Biotechnol Bioeng 99:1065–1073. https://doi.org/10.1002/bit.21671
Su DS, Zhang J, Frank B, Thomas A, Wang X, Paraknowitsch J, Schlögl R (2010) Metal-free heterogeneous catalysis for sustainable chemistry. ChemSusChem 3:169–180
Sun J, Hu Y-Y, Bi Z, Cao Y-Q (2009) Simultaneous decolorization of azo dye and bioelectricity generation using a microfiltration membrane air-cathode single-chamber microbial fuel cell. Bioresour Technol 100:3185–3192. https://doi.org/10.1016/j.biortech.2009.02.002
Tandukar M, Huber SJ, Onodera T, Pavlostathis SG (2009) Biological chromium(VI) reduction in the cathode of a microbial fuel cell. Environ Sci Technol 43:8159–8165. https://doi.org/10.1021/es9014184
Thung WE, Ong SA, Ho LN, Wong YS, Ridwan F, Oon YL, Oon YS, Lehl HK (2015) A highly efficient single chambered up-flow membrane-less microbial fuel cell for treatment of azo dye Acid Orange 7-containing wastewater. Bioresour technol 197:284–288
Topcagic S, Treu BL, Minteer SD (2004) Characterization/optiminization of oxygen biocathodes for membraneless biofuel cells. In: 206th ECS meeting, Honolulu, HI, 3–8 October 2004
Trapero JR, Horcajada L, Linares JJ, Lobato J (2017) Is microbial fuel cell technology ready ? An economic answer towards industrial commercialization. Appl Energy 185:698–707. https://doi.org/10.1016/j.apenergy.2016.10.109
Velvizhi G, Venkata Mohan S (2012) Electrogenic activity and electron losses under increasing organic load of recalcitrant pharmaceutical wastewater. Int J Hydrogen Energy 37:5969–5978. https://doi.org/10.1016/j.ijhydene.2011.12.112
Villaseñor J, Capilla P, Rodrigo MA, Cañizares P, Fernández FJ (2013) Operation of a horizontal subsurface flow constructed wetland – microbial fuel cell treating wastewater under different organic loading rates. Water Res 47:6731–6738. https://doi.org/10.1016/j.watres.2013.09.005
Wang X, Cheng S, Feng Y, Merrill MD, Saito T, Logan BE (2009) Use of carbon mesh anodes and the effect of different pretreatment methods on power production in microbial fuel cells. Environ Sci Technol 43:6870–6874
Wang HY, Bernarda A, Huang CY, Lee DJ, Chang JS (2011) Micro-sized microbial fuel cell: a mini-review. Bioresour Technol 102:235–243. https://doi.org/10.1016/j.biortech.2010.07.007
Wang D, Li Y, Li G, Wang C, Wang P, Zhang W, Wang Q (2015) Dye-sensitized photoelectrochemical cell on plasmonic Ag/AgCl @ chiral TiO2 nanofibers for treatment of urban wastewater effluents, with simultaneous production of hydrogen and electricity. Appl Catal B 169:25–32. https://doi.org/10.1016/j.apcatb.2014.11.012
Wang Z, Dummi G, Wu Y, Zhao F (2017) Progress of air-breathing cathode in microbial fuel cells. J Power Sources 356:245–255. https://doi.org/10.1016/j.jpowsour.2017.02.004
Watanabe K (2008) Recent developments in microbial fuel cell technologies for sustainable bioenergy. J Biosci Bioeng 106:528–536 https://doi.org/10.1263/jbb.106.528
Winfield J, Chambers L (2014) Towards disposable microbial fuel cells: natural rubber glove membranes. Int J Hydrogen Energy 39:21803–21810
Winfield J, Chambers LD, Rossiter J, Ieropoulos I (2013) Comparing the short and long term stability of biodegradable, ceramic and cation exchange membranes in microbial fuel cells. Bioresour Technol 148:480–486. https://doi.org/10.1016/j.biortech.2013.08.163
Winfield J, Chambers LD, Stinchcombe A, Rossiter J, Ieropoulos I (2014) The power of glove: soft microbial fuel cell for low-power electronics. J Power Sources 249:327–332 https://doi.org/10.1016/j.jpowsour.2013.10.096
Winfield J, Chambers LD, Rossiter J, Greenman J, Ieropoulos I (2015) Urine-activated origami microbial fuel cells to signal proof of life. J Mater Chem A 3:7058–7065. https://doi.org/10.1039/C5TA00687B
Winfield J, Gajda I, Greenman J, Ieropoulos I (2016) A review into the use of ceramics in microbial fuel cells. Bioresour Technol 215:296–303. https://doi.org/10.1016/j.biortech.2016.03.135
Wu S, He W, Yang W, Ye Y, Huang X, Logan BE (2017) Combined carbon mesh and small graphite fiber brush anodes to enhance and stabilize power generation in microbial fuel cells treating domestic wastewater. J Power Sources 356:348–355. https://doi.org/10.1016/j.jpowsour.2017.01.041
Xia C, Xu M, Liu J, Guo J, Yang Y (2015) Sediment microbial fuel cell prefers to degrade organic chemicals with higher polarity. Bioresour Technol 190:420–423. https://doi.org/10.1016/j.biortech.2015.04.072
Xu X, Zhao Q, Wu M, Ding J, Zhang W (2017) Biodegradation of organic matter and anodic microbial communities analysis in sediment microbial fuel cells with/without Fe (III) oxide addition. Bioresour Technol 225:402–408. https://doi.org/10.1016/j.biortech.2016.11.126
Yasri NG, Nakhla G (2017) The performance of 3-D graphite doped anodes in microbial electrolysis cells. J Power Sources 342:579–588. https://doi.org/10.1016/j.jpowsour.2016.12.081
Yu B, Tian J, Feng L (2017) Remediation of PAH polluted soils using a soil microbial fuel cell: Influence of electrode interval and role of microbial community. J Hazard Mater 336:110–118
Yuan Y, Zhao B, Jeon Y, Zhong S, Zhou S, Kim S (2011) Iron phthalocyanine supported on amino-functionalized multi-walled carbon nanotube as an alternative cathodic oxygen catalyst in microbial fuel cells. Bioresour Technol 102:5849–5854. https://doi.org/10.1016/j.biortech.2011.02.115
Zaffar H, Irshad U, Pervez A, Naqvi TA (2016) Mode of action, toxicity and biodegradation of organochlorinated pesticides: a mini review. J Appl Environ Biol Sci 6:1–7
Zhang X, Cheng S, Huang X, Logan BE (2010) The use of nylon and glass fiber filter separators with different pore sizes in air-cathode single-chamber microbial fuel cells. Energy Environ Sci 3:659–664. https://doi.org/10.1039/B927151A
Zhang F, Ge Z, Grimaud J, Hurst J, He Z (2013) Long-term performance of liter-scale microbial fuel cells treating primary effluent installed in a municipal wastewater treatment facility. Environ Sci Technol 47:4941–4948. https://doi.org/10.1021/es400631r
Zhang B, Wang Z, Zhou X, Shi C, Guo H, Feng C (2015) Electrochemical decolorization of methyl orange powered by bioelectricity from single-chamber microbial fuel cells. Bioresour Technol 181:360–362. https://doi.org/10.1016/j.biortech.2015.01.076
Zhang Q, Hu J, Lee D (2016) Microbial fuel cells as pollutant treatment units: research updates. Bioresour Technol 217:121–128. https://doi.org/10.1016/j.biortech.2016.02.006
Zhao F, Rahunen N, Varcoe JR, Roberts AJ, Avignone-Rossa C, Thumser AE, Slade RCT (2009) Factors affecting the performance of microbial fuel cells for sulfur pollutants removal. Biosens Bioelectron 24:1931–1936. https://doi.org/10.1016/j.bios.2008.09.030
Zhao L, Li J, Battaglia F, He Z (2016) Computational investigation of the flow field contribution to improve electricity generation in granular activated carbon-assisted microbial fuel cells. J Power Sources 333:83–87. https://doi.org/10.1016/j.jpowsour.2016.09.113
Zhuang L, Zhou S, Wang Y, Liu C, Geng S (2009) Membrane-less cloth cathode assembly (CCA) for scalable microbial fuel cells. Biosens Bioelectron 24:3652–3656. https://doi.org/10.1016/j.bios.2009.05.032
Zhuang L, Zheng Y, Zhou S, Yuan Y, Yuan H, Chen Y (2012) Scalable microbial fuel cell (MFC) stack for continuous real wastewater treatment. Bioresour Technol 106:82–88. https://doi.org/10.1016/j.biortech.2011.11.019
Acknowledgement
The authors acknowledge the financial support provided by Central University of Rajasthan, Ajmer, India.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Kumar, B., Agrawal, K., Bhardwaj, N., Chaturvedi, V., Verma, P. (2018). Advances in Concurrent Bioelectricity Generation and Bioremediation Through Microbial Fuel Cells. In: Sivasankar, V., Mylsamy, P., Omine, K. (eds) Microbial Fuel Cell Technology for Bioelectricity. Springer, Cham. https://doi.org/10.1007/978-3-319-92904-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-92904-0_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92903-3
Online ISBN: 978-3-319-92904-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)