Abstract
The increasing demands of efficient and sustainable energy generation methods from waste products have taken a giant leap in the last century, and especially in the previous two decades. Wastewater treatment has also been a much-researched topic in recent years owing to the exponential increase in effluent-laden wastewater from industries, the agricultural sector and food sector, and its effects on the environment. There have been plenty of wastewater treatment techniques over the years, but most of them lack in terms of cost-effectiveness, durability, and energy recovery rates. Microbial fuel cells can prove to be of great use to tackle both of these issues in one go, as they perform bioelectrochemical processes on organic biodegradable compounds to oxidize them to generate power which can be harnessed by various means. This article explains the aim, construction, mechanism, and application of microbial fuel cells; the economic and scientific challenges that they face in the future; and microbial fuel cell (MFC) hybrid systems which make use of MFCs combined with other useful technologies for greater aims and better efficiencies. It overall discusses the various ways in which MFCs outperform other wastewater treatment technologies by significantly decreasing sludge production and being environment-friendly, and also some limitations and drawbacks that MFCs face owing to the fact that they are relatively newer technologies and still require decades of modifications until they reach excellent output rates. MFCs are known not only for wastewater treatment but also for contaminant removal, heavy metal removal, biohydrogen production, applications in biosensors, etc., as also discussed in this article.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Energy crises and environmental degradation have adverse impacts on ecosystems and human health. With the growing world population, energy and water resources are becoming limited. Other challenges associated with the increasing population are the rise in wastewater generation and environmental contamination [1]. Thus, wastewater/sludge management should be aimed not only at reducing environmental and health risks but also at improving sustainability by recovering resources [2]. As a green energy option, owing to the lack of fossil fuels and the effects of global warming, readily available biomass has gained a lot of attention. A widely used process for the treatment of organic waste is anaerobic bacterial digestion. In the last few decades, due to their mild operating conditions, where biodegradable substrates can be used as a fuel, great attention has been paid to microbial fuel cells (MFCs). More recently, researchers have shown that this is a technology framework, which basically means that MFCs can have many applications [3]. MFCs can be used to break down organic matter to produce energy in wastewater treatment plants, and they have also been analyzed for various applications, such as biosensors and biohydrogen processing. MFCs have been recognized as an exciting and demanding tool in parallel wastewater treatment to save energy and address environmental issues [4]. MFCs have been shown to be a feasible approach to the elimination of chemical oxygen demand with simultaneous energy production [5].
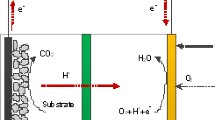
MFCs are a significant and continually evolving field of science and technology that combines biological redox behavior with classic abiotic electrochemical reactions and physics [6]. The inclusion of microorganisms responsible for catalyzing electrochemical reactions gives these cells a degree of complexity that can exceed that of complex electrochemical systems that are already in operation (e.g., batteries, fuel cells, and supercapacitors). Two chambers, the anodic chamber and the cathodic chamber, each of which holds an electrode, divided by a cation permeable membrane, form a standard MFC (shown in Fig. 1) [7]. Respiratory bacteria oxidize the organic substrates provided for generating protons and electrons in anode chamber [8]. Using an external circuit, the electrons are transferred to the cathodic chamber and the protons travel directly to the cathodic chamber via the proton exchange membrane (PEM), where they react with oxygen to create water [9]. MFC technology exhibits the possibility of reaching conversion efficiencies of up to 50% in theory [10], and can oxidize simple carbonates to carbon dioxide (CO2), and also allow biochemical reduction to transport electrons. A study [11] explained that, when applied to wastewater treatment, there are several reasons why MFCs are more sustainable than other technologies. Their ability to directly transform substrate energy into electricity is the first benefit. Secondly, relative to anaerobic digestion, the use of MFCs helps wastewater processes to decrease activated sludge. Its insensitivity to the operating environment is the third gain. The fourth is that, due to recycling and conversion, MFCs do not need any gas treatment, so energy can conserve much more energy without any energy input required for aeration. Fifth and last, in areas where there is inadequate electrical infrastructure, MFCs can be used widely. Despite such benefits, numerous challenges for effective deployment in real environments remain unanswered in the MFC sector. Real-world applications face many challenges, such as pH imbalances, anodic chamber electron transfer mechanism issues, effects of temperature changes, terminal electron acceptors, substrate, membrane, and electrode materials, all of which can affect the output of MFCs individually and collectively [12, 13]. The scaling up of MFCs is correlated with many limitations. Firstly, as the volume of a MFCs increases, volumetric power production decreases proportionally. A higher volume of bioreactors is needed for the scaling up of an MFC. Due to multiple electrochemical losses, the voltage actually produced is often lower than its ideal value: loss of the activation barrier, ohmic loss, and loss of mass transfer. Secondly, when multiple MFCs are connected electrically to increase voltage, electrochemical degradation occurs. This phenomenon is known as voltage reversal phenomenon [12, 14].
The goal of this review is to illustrate the current understanding of MFCs while offering a detailed overview of the technology. The fundamentals of MFC technologies, electrode materials, electron transport mechanisms, and field standardization are given specific importance. In addition, this analysis focuses primarily on improving the efficiency of MFCs by optimizing particular parameters in order to highlight the key limiting factors and bring them to the forefront of future research. Emphasis on innovations and advancements of electrode and membrane materials, to enhance MFC output, has been made. Various sorts of electrodes have been addressed and reviewed, i.e., anode and cathode, materials with different structural compositions and implementations of other technologies with MFCs. In addition, biocompatible, cost-effective, and highly stable electrode materials with improved microbial fuel cell performances are also included. In addition, the opportunities and challenges faced are also addressed for scaling up and future applications.
2 Aim of microbial fuel cells
Owing to the increasing global demand for the finite oil and gas supplies, and the need to simultaneously reduce greenhouse gas emissions, the race of the search for alternatives to fossil fuels has accelerated [15, 16].
Although wastewater is considered to be a profitable source of energy, pure water and fertilizers, its treatment technologies have many output-related constraints [13]. Carbon neutral and renewable energy technologies are the present needs of our times.
Microbial fuel cells (MFCs), which convert biochemical energy (through microbes-assisted bio-oxidation) to electrical energy, could potentially be a big part of the picture as they provide various ways for the sustainable production of energy from biodegradable compounds. The complexities of the underlying biochemical processes were the reason for an inevitable setback for MFCs, when compared to the advancement of their alternative competitive technologies, such as solar photovoltaic systems. MFCs came under the limelight when the National Aeronautics and Space Administration (NASA) became interested in exploiting opportunities to recycle organic wastes into electricity during long space flights [17]. Three unique features, which are less sludge production, energy and cost-saving approach, and less energy consumption, make MFCs stand out from other existing technologies [13].
3 Structure and mechanism of a typical MFC
A typical MFC (as shown in Fig. 1) comprises of three major components: an anaerobic anodic chamber, an aerobic cathodic chamber, and a separator connecting the two chambers, which is responsible for exchanging protons [18]. In the anodic chamber, electroactive microorganisms oxidize the added substrates and generate electrons and free H+ ions in the process. The electrons produced are transported to the anode and flow to the cathode through an external circuit operated under a load, i.e., resistor [11]. Although the separator plays an increasingly important role in MFCs relative to catalytic and electrode materials, it increases the internal strength and reduces the efficiency of the MFCs, thus significantly restricting the practical applications of MFCs. Therefore, a conventional MFC is usually half biological since only the anode side contains electrochemically active microorganisms while the cathode is abiotic [13].
Oxygen, ferricyanide, and hydrogen peroxide are used mainly as terminal electron acceptors in the cathode. Among them, especially for the air cathode MFCs (which consists of a sheet of catalysts, an electrode, and a separator), oxygen is considered the most suitable terminal electron acceptor mostly due to its sustainability and amount. Compared to the cathode, a high concentration of H+ ions near the anode results in an electrochemical gradient that pushes protons to the cathode through the semi-permeable membrane. With the electrons and the dissolved oxygen at the cathode, the migrated protons unite to form pure water [13]. Electrode reactions are shown below using acetate as a model substrate:
-
Anodic reaction:
-
Cathodic reaction:
The ultimate reaction thus describes the bio-oxidative breakdown of acetate to CO2 with the simultaneous generation of electricity. Centered on the movement of the emitted electrons from the media to the anode by active microorganisms, MFCs can be classified into two categories: mediator MFCs and mediator-less MFCs [3]. A component-wise analysis of MFCs is given below.
3.1 Anode and the anodic chamber
Electrons from anodic exo-electrogenic microbes are used by cathodic electron acceptors followed by charge neutralization by protons crossing the proton exchange membrane (PEM) [19, 20]. A substrate, a mediator (although, optional), a microorganism microbial culture, and the anode as the electron acceptor are packed in the compartment to provide an efficient oxidative atmosphere [16]. One of the factors responsible for anode performance is the electrode material. Anodic materials must tick all the five boxes—good electrical conductivity and low resistance, strong biocompatibility, large surface area, chemical stability and anti-corrosion nature, and sufficient mechanical strength and toughness [21]. Metal anodes made up of non-corrosive stainless steel mesh can also be used [22]. Graphite rods are the most common materials used for anode electrodes because they are simple to treat, have a considerable surface area, and are affordable. The surface area is a major factor to account for while scaling up the MFC because of an increase in current with an overall increase in total surface area. Carbon, containing graphite fiber brush, is the most widely used material for an MFC anode [23]. Table 1 describes the advantages and disadvantages of the commonly used materials for anodes and their effects on MFC performance.
The microbial electron transfer rate can be improved by adding artificial electron carriers (or mediators) and changing the composition of the cell. By anaerobic oxidation of organic matter, bacteria produce electrons and transfer them to respiratory enzymes that are generally attached to the inner cell membrane [32]. These artificial electron carriers may be used to move electrons from inside the cell to an external electrode. Mn (IV), Fe (III), covalently bonded neutral red, anthraquinone-2,6-disulfonate (AQDS), or high humic acid concentrations [33], for example, can be used as mediators. They are known to mediate the transition of electrons between the microbial film and the anode [34]. Researchers have also shown that directing water flow through the electrode can effectively increase the power output [35]. Another efficient way to enhance the MFC power output is to use modified carbon and metal-based anodes with conductive polymers [36].
3.2 Cathode and the cathodic chamber
Via the PEM, H+ ions from the anodic chamber migrated to the cathodic chamber to complete the circuit. The produced electrons are taken to the anode and flow through an external circuit controlled by a resistor to the cathode. The migrated protons combine with the electrons and dissolved oxygen at the cathode to form pure water [13] that spreads along with the aid of catalysts through the ion-permeable membrane on the cathode as the protons which migrated through the PEM combine with the electrons [11] (from the external circuit along the resistor) and dissolved oxygen to form pure water, as shown below [37]:
The electron acceptor’s concentration, chemical identity, proton availability, catalytic performance, catalytic ability, and electrode structure affect the cathode’s reaction yield. Ferricyanide ion, [Fe(CN)6]−3, is a very common electron acceptor used in MFC because of its excellent efficiency [38]. The most significant advantage of ferricyanide is the low overpotential using a plain carbon cathode, which results in a cathode working potential close to its open circuit potential. However, a major drawback is the absence of oxygen re-oxidation, which allows the catholyte to be replaced periodically [39]. In addition, the diffusion of ferricyanide through the cation exchange membrane (CEM) and into the anode chamber will impair the efficiency of the long-term device [17].
The choice of cathode material has a paramount influence on MFC performance. Materials with high proton absorptivity and high redox potential, which include graphite, carbon fabric, and carbon paper, can be used as cathodic materials [40, 41].
When platinum was used with graphite felt as a cathode, a maximum power density of 150 mW/m2 was derived. The power density observed was three times greater than that of a pure graphite cathode [42]. Granulated and porous cathodes are known to greatly impact MFC performances; however, power output remains more or less unaffected [43, 44]. A close microbe-cathode interaction has been established and demonstrated which relates the oxidation of soluble electron donors with the reduction of cathode oxygen diffusion [45]. A study [46] reveals that the ratio of oxidized to reduced mediator species in a microorganism affects the ability for electrons to be transported in or out of the cell by microorganisms. Table 2 shows some of the commonly used cathodes in MFCs with their respective maximum generated power.
Platinum is a widely used abiotic catalyst, as it lowers the energy of activation and increases the rate of reaction [51, 52], but due to its poisoning nature against the components in the substrate and its high cost, it is avoided [39]. Oxygen is the most effective electron acceptor for an MFC because of its elevated oxidizing performance, accessibility, low cost, longevity, and zero chemical waste production, as H2O is produced as the only end product.
3.3 Proton exchange membrane
A PEM primarily separates the anode and the cathode to prevent back diffusion of oxygen to the anode by maintaining the concentration of substrate in the anode and to complete the electrical circuit. It also helps in increasing coulombic efficiency, and also to ensure sustainable operation over time. pH splitting (increasing pH in the cathode chamber and reducing pH in anode chamber) [53], increasing overall internal resistance, and the overall cost of MFCs are some of the challenges of utilizing a separator [54]. Nafion is a more frequently used membrane and is the best choice despite being expensive. Experiments to substitute Nafion separators with other membranes such as sulfonated polyether ether ketone membranes [55, 56], anion and cation exchange membranes (AEMs and CEMs) [57], ultrafiltration and microfiltration membranes, bipolar membranes, and forward osmosis membranes have recently been demonstrated by a group of researchers. It should be remembered when using PEM that it can be permeable to chemicals such as ferricyanide, oxygen, and a variety of other ions, or to the organic matter used as the substrate. Ion exchange membranes have grabbed the researchers’ attention, and it is in popular demand recently. More systematic studies are necessary to evaluate the membrane’s effect on performance and long-term stability [41].
4 Effects of different parameters on the performance of MFCs
The laboratory-scale version of an MFC has been still lower than what is shown in an ideal case. The efficiency of an MFC is affected by several parameters which are mentioned below, including the range of microbes, variety and concentration of fuel biomass, and ionic strength and configuration of the reactor [58].
4.1 Effect of pH buffer
Since the anodic chamber generates protons that flow to the cathodic chamber to produce water, continuous operation of MFCs can make the anodic chamber acidic due to the accumulation of protons. Likewise, owing to the constant absorption of protons by the oxygen reduction reaction, the cathodic side suffers alkalinization due to the insufficient supply of protons from the anodic chamber. This pH gap raises the driving force of the diffusion of protons from the anode to the cathode chamber, and a complex equilibrium is formed that essentially places an electrochemical or thermodynamic limit on the power production of the MFC [59]. According to the Nernst equation, the declining capacity of oxygen should be increased with a decline in the pH value. Increasing pH values will thus reduce the current performance values drastically [60]. For their optimal development, bacteria typically need a pH close to 6.9–7 (neutral) and respond to changes in internal and external pH by controlling their activity [61]. A study [62] shows that, at a neutral pH, anodic bacterial behavior is most desirable, and the rate of cathodic reaction improved at a higher pH. At acidophilic conditions, the highest power production was located around the pH of 6.3 [63]. One of the key reasons for sinking voltage efficiencies is the pH gradient between anode and cathode; to tackle which, phosphate/bicarbonate buffer is added [12]. A study [64] studied the effects of different widely used buffers (phosphate, MES, HEPES, and PIPES) on single-chamber MFC power generation and compared it to non-buffered control, and their internal resistance and set neutral pH analysis revealed that phosphate and PIPES are the most effective buffers since pH was held close to the buffer pKa, thus optimizing the energy of the buffer and buffering potential [65, 66]. Growing phosphate concentration across particular ranges has been shown to increase power production [67].
Phosphate is, however, inefficient for wastewater treatment because of its expense and the eutrophication caused by it [60]. Instead, if one takes care of its concentration levels, bicarbonate buffer is inexpensive and can be a great alternative to phosphate [68].
In spite of such advantages, buffers are known to create varying conductivities (which affect ohmic resistances and output power) [66]. To reduce that and to guarantee a continuous feed of a cheaper buffer, researchers have performed experiments to add an acidic buffer carbon dioxide to the cathode compartment which mixes bicarbonate and carbonate with hydroxide ion in the cathode to create carbon dioxide/carbonate or bicarbonate buffered catholyte structures [69]. The final results showed that, by feeding air with carbon dioxide in the cathodic chamber, power density and cell voltage increased with a decreased pH imbalance.
4.2 Effect of temperature
Temperature fluctuations influence the kinetics of devices, mass transfer (activation energy, mass transfer coefficient, and conductivity of solution) [70], thermodynamics (free Gibbs energy and electrode potential), and the presence and distribution of microbial communities [70, 71]. Temperature has proven to be an important parameter for the removal of COD and electricity generation, showing a change in the removal of COD and an increase in power density with an increase in temperature [3, 72]. The reasons given for the rise in power production with an increase in temperature are mainly due to increased microbial metabolic rates [3], increased permeability of the membrane [70], and decrease in ohmic resistances due to the higher conductivity of the liquid solution [73]. Temperature linearly increases with decreasing internal resistance due to increasing conductivity [70, 73]. Temperature exponentially increases power generation of MFCs due to the anodic biofilm formation which affects biocatalytic activity and hence greater efficiency [74,75,76]. Biofilms are observed to exhibit their most excellent electrocatalytic activity at 30–45 °C [3]. Since multiple exo-electrogenic bacteria have different acceptable temperature ranges and have evolved at different temperatures, the temperature has importance during the initial growth process as it determines the concentration and distribution of the other microbial species within the biofilm matrix [27, 77]. Following this period, the MFCs can operate over a broad range of temperatures without a significant decrease in performance [78]. These observations are essential for the wastewater treatment application of MFCs. As it is obvious that the initial steps of the process will describe the MFC’s final performance, we initially use higher temperatures and then run the MFC at lower temperatures, minimizing heating costs without any apparent reduction in performance [79].
4.3 Effect of organic loading rate
Organic loading rate (OLR) and sludge loading rate (SLR) are two other parameters responsible for determining the reactor’s capacity to convert organic substrates per unit volume and per unit mass of microorganisms [3]. Thus, suitable values for SLR or OLR are important for a good coulombic effectiveness and power density [80, 81].
For the application of wastewater treatment, a study [60] demonstrates that COD removal efficiencies increased with an increase in SLR. When MFCs were operated at an SLR of 0.25 kg COD kg VSS−1day−1, the maximum efficiency of COD removal was 47.8% when the feed pH was 6.0, and 40.4% at pH 8.0. When SLR was kept at 0.75 kg COD kg VSS−1day−1, these efficiencies further improved to 61.9% and 58.8%, respectively. A further increase in SLR resulted, however in a decline in the elimination of organic matter. Complex reactions including hydrolysis, acidogenesis, and electrogenesis tend to occur in the environment during the handling of wastewater containing fermentable organic matter. Subsequent reactions have greatly impacted the aggregation of high concentrations of intermediate products. The suspended sludge content in the anode chamber decreased with an increase in SLR from 0.75 to 1.25 kg COD kg VSS−1day−1, and the high concentration of volatile fatty acids produced a toxic impact on the bacteria, which further degraded the efficiency of MFCs in terms of organic matter elimination [60].
High OLR, equivalent to highly saturated anode surface area conditions, can aid in competition between exo-electrogenic microorganisms and other types of bacteria, leading to greater removal of organic matter, which is not associated with current generation. This reduces coulombic efficiency, but improves substratum degradation. The coulombic efficiency is better at low OLR since a reduced volume of substrate is required for the processing of methane [82, 83]. Thus, enhanced electron recovery does not guarantee a better MFC performance.
4.4 Effects of feed rate and shear stress
Flow intensity and the resultant hydraulic retention time (HRT) and shear stresses are essential factors that really need to be solved before MFCs can be efficiently used in wastewater treatment plants because the continuous operation of MFCs entails hydrodynamic issues that can drastically affect the performance of MFCs [84, 85]. Optimally increased flow rates are known to produce good power densities; however, even higher flow rates have a negative impact [86]. Therefore, optimum efficiency is said to be achieved only after the microbial population propagates and nutrient capture and the amount of substrate hydrolysis are most favorable [87]. Coulombic efficiency and COD removal are inversely proportional to the flow rate [82]. The hydraulic retention time decreases as the flow rate rises, thus reducing the time taken by microbes to degrade the organic matter. The average efficiency of elimination of COD thus decreases.
Higher shear rates (although, lower than tensile strength) lead to thicker and denser biofilms that can pass electrons more quickly, thereby increasing the power density. A decrease in the current output and thickness of the biofilm is observed if the shear rate is very strong (above the tensile strength), indicating cell detachment [88, 89]. Increased shear concentrations have also been found to limit microbial diversity causing biofilm homogeneity [90, 91].
5 Applications of MFCs
MFCs have emerged as the best choice for maximum energy recovery and in situ energy conversion. Some of the recent breakthrough applications of MFC are described below.
5.1 Bioelectricity production in MFCs
As previously discussed, an MFC produces electricity with the help of the applied potential difference combined with the electrons released by anaerobic microbial oxidation of carbohydrate substrates which come from rural and urban wastes [92].
Even though these systems have deficient energy levels, research from the last two decades has proven that MFC can still be a terrific technology since fuel is transformed directly to electricity without heat generation [93]; as a consequence of which, the Carnot loop, which restricts thermal energy conversion, is eliminated, allowing higher conversion efficiency (> 70%) to be obtained [94]. Studies show that using different compounds as substrates in MFC yields different power densities. Table 3 shows the power densities, as obtained by some of the compounds when used as substrates in MFCs.
5.2 Biohydrogen production in MFCs
Hydrogen is recognized as an impermanent renewable energy carrier. Much focus is paid to the worldwide use of H2 as a source of energy fuel. Its advantages are numerous: it is clean, efficient, and renewable, and generates no toxic by-product. Electrolysis has been used as an efficient way for high-purity hydrogen generation [98]. MFCs can be easily adjusted to the harvest of biohydrogen, instead of producing electricity, by combining electrolysis with MFC. This setup is known as microbial electrolysis cell or MEC (discussed with details later) [3].
Deriving protons and electrons from water hydrolysis generally requires a high external voltage. Therefore, by using a form of hybridized MFC system that does not require oxygen, we can directly produce hydrogen at low voltages by deriving protons and electrons from organic matter instead of water. However, there is a thermodynamic infeasibility associated with hydrogen production from electron and proton combination. This, however, can be overcome by application of an external electrical potential to augment the MFC’s cathode potential [3]. Cathodic oxygen has the potential to permeate through the PEM and inhibit anaerobic respiration. The anodic potential must be raised to an additional voltage of around 0.23 V or more [16] in order to produce hydrogen gas in a typical MEC. In contrast to the standard 4 mol H2 mol−1 glucose obtained in conventional fermentation, MFCs can contain 8–9 mol H2 mol−1 glucose [3].
5.3 Wastewater treatment
Wastewater has immense potential as an energy source. Energy conservation, sludge volume control, and bioenergy generation are the key benefits of MFC for wastewater treatment [99]. Various forms of wastewater, such as wastewater from food production, swine wastewater, and sanitary waste, contain various biodegradable organic substances that can be used to power MFCs [16]. MFCs were first used to treat wastewater in 1991 [100]. Reasons for their usage are enlisted as follows:
-
It enables direct recovery of electric energy and value-added products [101]
-
Strong effluent efficiency and low environmental impact can be accomplished by successfully integrating biological and electrochemical processes [102]
-
Real-time monitoring and control of MFC is possible, which benefits good operating stability [102]
-
In the wastewater treatment phase, the amount of power produced by MFCs can theoretically halve the energy used in a traditional treatment course that consumes a lot of electricity during activated sludge aeration [60]
-
Generates 50–90% less solid wastes [3]
-
Organic molecules, such as acetate and butyrate, can be broken down into CO2 and H2O [103]
In recent years, scientists have demonstrated that biological treatment has proven to be a safe and an extremely cost-effective process for the removal of nitrogenous and organic matter from the leachate [104]. Some microbes used in MFC have a special capacity to eliminate sulfides, which is an essential step in wastewater treatment [58]. The development of electrochemically active microbes during the wastewater treatment process can be increased by MFCs [105]. A study [32] reported up to 80% of COD removal and a coulombic efficiency as high as 80%.
MFC could be an efficient method of electricity generation and odor removal, and a study [16] demonstrated that the rate of odor elimination is accelerated by MFC-based technologies when electricity production exceeds a limit of 228 mW/m2. The scaling up of MFCs for wastewater treatment (containing suspended solids) is limited due to the biofouling of membrane and high initial costs. Thus, if we eliminate the use of a membrane, scaling up of MFC will become easier. A study [106] demonstrated a mediator-less and membrane-less MFC (MLMFC) to treat synthetic wastewater in which 88% of COD and 87% of BOD (biological oxygen demand) and 45–50% of Kjeldahl nitrogen removal efficiencies were reported. In bioelectricity generation from chemical wastewater treatment [92], a dual-chambered microbial fuel cell (MFC) was demonstrated without the use of an anode chamber mediator. When a cathode made of potassium ferricyanide was used, current densities in the range of 747.96 to 862.85 mA/m2 were observed.
5.4 Application of MFCs in biosensors
MFCs can also act as quantitative sensors for microbial respiration [107]. The current produced in an MFC, which is a result of anaerobic respiration by electron transfer to the anode, can be used to calculate metabolic rates of the microbes [108] and substrate concentrations over time [10]. In the past few decades, many different types of MFC-based biosensors have been proposed and tested, like immune-sensors [109], enzymatic biosensors [110], DNA biosensors [111], and cell-based biosensors [112]. One type of enzymatic biosensors measures the amount of lack of oxygen and the amount of produced hydrogen peroxide in a cell [113]. A study [114] demonstrates the development and activity of a microliter-sized biosensor based on MFC for the monitoring of radioactive substances in water. It uses formaldehyde as a harmful ingredient and records the detection of swift and sensitive current responses in the biosensor with 0.2V added to the anode as a way to maintain the baseline signal over a concentration range of 0.001–0.1%.
6 MFC hybrid systems
MFCs can also be integrated with other advantageous concepts/devices, in order to produce hydrogen fuel and several other valuable products, which provides an exciting solution to improve MFC feasibility and broaden the domains of MFC applications. The limitations that typical MFCs are endowed with can be overcome by using such hybrid technologies, which are still very recent advancements in fuel and bioenergy generation [58]. Much research has been conducted on the effects of the addition of algae in the cathode, the effects of modification of electrode and membrane materials, the impact of variable bacterial electrogenicity, the addition of electro-catalysts and photocatalysts, etc. This research has directly given importance to the study of multitudes of MFC hybrid systems and technologies associated with/derived from MFCs, like MFC-Fenton hybrid systems [115], MFC-PEC (photoelectrochemical) hybrid systems [116], MDCs (microbial desalination cells) [117], and MFC-MEC (microbial electrolysis cell) hybrid system [118]. The techniques mentioned above are descriptively reviewed in the following part of the article.
6.1 MFC-Fenton hybrid systems
The power generated in the MFCs drives the electrochemical reactions that take place in this hybrid system. In this system, oxygen from the cathodic chamber accepts electrons to either produce H2O2 or drive a Fenton process [119, 120]. In the Fenton process, the following reaction takes place, leading to the production of hydroxyl radicals along with hydroxyl anions and ferric ions [121]:
The electrons from the cathode are absorbed by Fe3+ ions to give back Fe2+ ions, which react back with H2O2 to give more hydroxyl radicals and anions. These hydroxyl radicals are excellent oxidants and are capable of potentially degrading many organic molecules [122]. If an air cathode is used, oxygen can be reduced to form H2O2 as
The high efficiency of the H2O2 used in Fenton reagent makes this process highly advantageous to use with MFCs [3]. MFC-Fenton systems are known to degrade several bio-refractory pollutants, including phenol and p-nitrophenol [123]. Based on the location where the Fenton reactions take place in the hybrid system, we can have in situ and ex situ processes.
6.1.1 In situ Fenton process integrated with MFCs
Here, bio-oxidation of the organic source takes place in the anodic chamber, which is separated from the cathodic chamber by a PEM [124]. The bio-oxidation process releases electrons at the anode, which follow the external circuit path to reach the cathode to help in the Fenton reactions. In the cathodic chamber, H2O2 is produced using O2 and protons, which migrated through the PEM [58, 122].
If the concentration of dissolved oxygen is too low for H2O2 production, cathodes with larger surface areas or grooves or layers can be used. External energy can also be supplied to the system for boosting H2O2 production [125]. Adding Fe2+ to the system is both advantageous (due to the number of different reactive species formed) and disadvantageous (due to its loss as a result of sludge production) [58]. To avoid sludge production, one may use heterogeneous catalysts like α-Fe2O3 and Fe3O4 [126]. Power densities as great as 0.823 W cm−2 were obtained when γ-FeOOH was used as the iron source in the cathodic chamber, along with electrodes coated with conductive films, whereas using FeVO4/carbon felt (CF), the power density rose to 850 mW cm−3, with an output voltage of 327 mV [105]. H2O2 production can also be increased to 136 μmol L−1 using Fe/Fe2O3/graphite felt with triphenyltin chloride as the degradation material. When Fenton process was combined with photocatalytic fuel cells (PFCs), there was a 39–62% increase in the effluent removal process and a 1.21–2.04 times greater electricity production as compared to single PFCs [127].
H2O2 is known to have a corrosive behavior on the CEM, as a result of which MFC-Fenton systems need improvements. Also, the salts required to generate ferrous ions for the reaction in the cathodic chamber lead to high running costs [223].
6.1.2 Ex situ Fenton process integrated with MFCs
When the abovementioned two parts of the MFC-Fenton system are located separately in two different chambers, an ex situ MFC-Fenton system is formed, as shown in Fig. 2. Here, the anodic chamber of the MFC releases electrons which are sent to the anodic Fenton treatment (AFT) chamber and then back to the cathodic chamber of the MFC. The H2O2 produced in the AFT chamber is reacted with the ferrous ions to give hydroxyl radicals [128]. High dissolved oxygen levels increase H2O2 production. Iron zeolites showed better results than Fe2+ in energy production, achieving dye decolorization of up to 95%, with an operational duration of 6 h [129]. Dyes like methylene blue showed 97% decolorization during an operational duration of 8 h, alongside the production of H2O2 using an MFC-MEC-Fenton hybrid system [130, 131].
A large electrode surface area increases H2O2 production. Increasing Fe2+ and Fe3+ also dramatically affects the effluent degradation rates. For medicinal effluent components like paracetamol, graphite-felt electrodes were required in an MFC-Fenton process, and a pH of 2 was obtained as the optimal pH for the subsequent reactions [132], whereas with FeVO4 as a catalyst, a pH of 3 was found out to be optimum [133]. The ex situ design, where a single-chambered MFC powers the AFT system, reduces our need for an external electrical supply and hence reduces costs. Table 4 reviews data collected from various studies regarding H2O2 production, power generation, and pollutants targeted by the respective MFC-Fenton systems. An anode of a dual-chamber MFC, modified with polypyrrole/AQDS, has been studied to give an exceptionally high power density of 1.303 W m−2, a value which is almost thirteen times more than that generated by a typical MFC [134]. Natural organic pollutants like amaranth have been degraded in MFCs using an anode made of graphite and a pure spectrographic graphitic cathode, to generate 28.3 W m−3 power and 73–80 mg L−1 H2O2 [129, 234]. Using a graphite electrode and an anthraquinone-based catalyst, pollutants like congo red can be degraded to generate 0.808 W m−3 of power, using MFC-Fenton systems with iron phthalocyanine as a source of iron [137]. A large electrode surface area increases H2O2 production. Increasing Fe2+ and Fe3+ also dramatically affects the effluent degradation rates.
6.2 MFC-PEC hybrid systems
Hydrogen and methane have long been known as the drivers for non-polluting vehicles and are generally produced from fossil fuels and biomass. They can be used as substitutes for fossil fuels in ICEs [224]. Turning wastewater into hydrogen gas calls for a photoelectrochemical cell (PEC) [138]. MFCs are known to have exhibited problems such as their non-dynamic electron transfer process, costly CEMs, and subsequent biofouling around it, causing a reduction in their performance due to the hindrance in charge permeability and the decrease in the potential of the cathode, the long activation/stimulation, and operational durations, etc. [102]. Compared to MFCs, PECs involve a more dynamic and unhindered electron transfer process, lesser heat intolerance, and the deletion of the problems associated with the use of biotic materials [139, 140]. Based on converting light energy into electricity, a PEC consists of two electrodes, out of which at least one is made up of a semiconducting material and is exposed to light. Hence, we can either have the anode as an n- or a p-type semiconductor paired with a metallic cathode and vice versa or have a p-type anode and an n-type cathode and vice versa.
Electronic charge carriers (electrons and holes) are formed as a result of light-induced intrinsic ionization of the semiconducting material (due to transfer of electrons from the VB to the CB). At the photo-anode, water gets oxidized by these holes to form protons, which move to the cathode to get reduced to hydrogen gas [141].
Upon combining the above system with an MFC, we get an MPEC (microbial photoelectrochemical cell) as shown in Fig. 3, where the bacteria present in the anodic chamber digest the organic compounds from effluents to produce electrons [142].
When the PEC is illuminated with light, these electrons are accelerated towards the PEC along an external circuit where they form hydrogen gas by reducing protons. The total system requires only light as an energy input. The generated current further helps in preventing recombination of electrons with holes, thus boosting hydrogen production [58]. There are two ways in which the pollutants can get degraded—directly (by the already oxidative holes) and indirectly (by the help of the products of the reaction between holes or electrons and the oxygenated molecules which are highly oxidative species) [140]. Hence, an MFC can generate electricity to power the PEC process without the help of an external power supply and also get an increase in its cathode potential.
Recombination of holes and electrons still remains a most significant problem in employing semiconductor devices with MFCs. They can be efficiently reduced by using a fuel cell composed of a bioanode coupled with a photocathode. In this cell, the cathodic photo-generated holes combine with the anodic electrons, and not with the cathodic photo-generated electrons. This action not only prevents recombination but also provides us with plenty of photo-generated electrons to help in reduction reactions at the cathode [143]. Photocatalysts like TiO2 [225], ZnO [226], Cu2O [227], and CdS are the prominently used semiconducting materials in previously conducted studies involving MFC-PEC systems [116]. However, TiO2 is known to absorb only UV light due to its broad bandgap [142].
To decrease this bandgap, a hybrid nano-structure of TiO2 and Ag [144], or addition of another semiconductor like graphitic carbon nitride (g-C3N4) [145] or tungsten trioxide (WO3) [146], could be used. Instead of using commercial rutile, the use of narrow bandgap TiO2 can also enhance its action and energy recovery, owing to the reduced electron transfer resistance [147, 140]. Using a TiO2 nanowire arrayed photoanode, coupled with a platinum-based cathode and 0.1 W cm−2 of photo-illumination, a current per unit area of 0.012 A cm−2 and 0.7 V voltage has been generated [148]. One study reported the use of a Nb2O5-xNPs anode, a platinum-based cathode, and 0.1 W cm−2 of light illumination to generate a current density of 0.90 mA cm−2 [149].
6.3 MFC-MEC hybrid systems
While MFCs generate electricity from the organic decomposition of effluents, MECs (shown in Fig. 4) generate hydrogen (by a process called electro-hydrogenesis [233]) or methane or H2O2 from the decomposition of organic compounds on the application of electrical energy depending upon the substrates/reactions involved. Exo-electrogenic bacteria in the anodic biofilm generate an electrical potential of 0.3 V by consuming energy and releasing positive and negative charges. Then, upon addition of an external supplementary potential gradient to the cell, protons are reduced to produce hydrogen gas. This supplied voltage requirement for MECs is generally greater than 0.2 V, which is much lower than the 1.8 V required for electrolysis of water [150].
The electrons in organic effluents released during bio-oxidation are delivered to the cathode externally along the circuit, where they either release hydrogen gas by proton reduction or produce H2O2 by O2 reduction. The absence of need for any expensive anodic catalyst or any substrates [118] makes MECs a highly capable and efficient option for hydrogen production [151].
As a substrate, acetic acid is used to yield H2 gas with great efficiencies at an applied voltage of 0.2–0.8 V. On subjecting 0.6 V to the system, the efficiency reaches 288% (i.e., when we neglect the additional energy provided to the system from the enthalpy of combustion of acetic acid, and 82% otherwise), with a H2 gas emission rate of 1.1 m3 per m3 of reactor fed per day [152]. Increase in the concentration and conductivity of the acetate is known to support H2 gas production [118]. Increase in the buffer concentration of phosphate improved the conductivity of the feed solution, resulting in a significant increase in hydrogen gas production rate from 2.9 to 7.9 mL per liter feed per day [153].
Excellent yields of hydrogen gas were obtained from several other acids like acetic acid at a range of overall energy efficiencies, ranging between 64 and 82% enlisted in Table 5 [154]. A good hydrogen production from cellulose, with an overall efficiency of 63%, at 0.11 m3 per cubic meter of feed per day has been observed. The slow production rate is justified by the slow rate of hydrolysis of cellulose particles [155]. Four to five times greater recovery rates were achieved using glucose instead of cellulose for hydrogen production [156]. The production rate was observed to be 1.23 m3 per cubic meter of feed per day. Although the overall recovery rate is lower than that of acetate at 71%, the production rates are approximately comparable. Hence, we have a process that provides a useful method for hydrogen production from environment-friendly biomass resources. If need be, one might have to reduce the excess overpotential that must be overcome to initiate hydrogen production. To do so, platinum may be used as a catalyst, but due to its high cost, a much cheaper stainless steel plate is used as the preferred electrode material [157].
Since the additional voltage (of at least 2 V) is essential in hydrogen production in an MEC, we can combine an MEC with an MFC (which generates power) and hence escape the requirement of external power input [25]. Using a two-chamber MFC-MEC system, 1.5 mL of hydrogen per liter feed per day is observed, with a yield of 1.6 moles of hydrogen per mole of acetate, in the presence of 0.1 M buffer [158]. Using a low external resistance, a greater hydrogen gas production rate was obtained in the MEC [159].
Alternatively, one can use multiple MFCs to charge parallel capacitors to construct the MFC-MEC hybrid, such that the capacitors can supply continuous power to the MECs when they discharge. The hydrogen production observed without such capacitors was 0.31 m3 per cubic meter feed per day which increased to 0.72 m3 per cubic meter feed per day in the presence of capacitors [159].
MFC-MEC hybrid systems have also been investigated in the areas of heavy metal removal where metal ions like cobalt [160], lead [161], and Cu+2 and Ni+2 [162] were removed from wastewater alongside contaminant degradation [163].
Carbon dioxide reduction in MFC-MEC systems has also been observed [164]. When powered by an MFC with a power density of 18.5 W m−2, it was shown that the MFC-MEC hybrid system reduced carbon dioxide to carbon monoxide at a rate of 0.06 mmol m−2 h−1 [162].
Removal of nutrients like nitrogen and phosphorous from wastewater using MFC-MEC hybrid systems has also been studied extensively [165]. Phosphorous, in the form of magnesium and ammonium containing struvite, is removed from wastewater. In a study conducted [166], struvite formation alongside hydrogen gas production was observed in the MEC unit. The struvite coagulated at the rate of 0.3–0.9 g/m2/h in a single-chamber unit, leading to up to 40% phosphate removal at an efficiency of around 73% and a hydrogen gas production rate of 0.7 to 2.3 m3 per cubic meter feed per day. The experimental data obtained from this study suggests that the energy required of struvite production in an MEC can be considerably controlled and brought down in the form of energy recovered as hydrogen, thus significantly lowering the operational costs involved in struvite recovery [167].
The combination of MFC-MEC systems also promotes ANAMMOX (anaerobic ammonium oxidation) in the absence of any external organic source [58, 168].
6.4 Microbial desalination cells
The function of a microbial desalination cells (MDC) is primarily ion removal from water to reduce its salinity. There are other widely accepted methods as well, which can give fresh drinking water by desalination of seawater, like reverse osmosis (RO). The problem with RO is that it requires high pressure, or, in other words, high energy input (about 3–4 kWh/m3 of seawater). Other distillation techniques require even more energy input—multi-stage flash distillation (MSF), multi-effect distillation (MED), membrane distillation [228], etc. [169]. To do so, the MDC has to generate power by effluent treatment.
A typical MDC (represented in Fig. 5) consists of a desalination chamber placed in between two ion exchange membranes, one between the desalination chamber and the anodic chamber and one between the desalination chamber and the cathodic chamber. The two membranes are termed as AEM and CEMs, respectively. In the desalination process, the exo-electrogenic anodic microbial biofilm conducts bio-oxidation of organic compounds present in the substrate of the anodic chamber and releases the electrons which migrate across bacterial cell walls to the anode and subsequently to the cathode by following the external circuit path [229]. This creates a potential difference which causes anions and cations present in the desalination chamber to move towards the anode and the cathode, respectively, by crossing their respective ion exchange membranes.
Naturally, the kinetics and other aspects of this process much depend on the current generated by the bacteria. Hence, any method of increasing the current density works well for the desalination process. One such way is to decrease solution resistance by using high salt concentration [170]. When the concentration of the salt in the desalination chamber is kept at 20 g L−1, the resistance of the solution is 39 Ω. When the concentration is decreased to 6 g L−1, the resistance increases to 256 Ω [171].
In the presence of a harsh anode potential, power and current densities can sometimes shoot to lower values which can significantly affect current generation in MDCs, despite low resistance. This is due to a phenomenon known as type D power overshoot [172] which can be overcome if we set the reactors to optimally low values of resistances [173]. Anolytes like acetate and xylose which are more readily biodegradable than other organic compounds show COD removal values greater than 70% which are higher than when domestic wastewater is used as an anolyte, whose COD removal stands at about 54% [174]. Instead of the typical reactions responsible for oxygen reduction at the cathode, one could employ ferricyanide reduction reaction in cathodes of MDCs, which can generate power up to 65 Wm−3; however, due to the non-sustainability and uneconomical nature of this reaction, it is not preferred to use in MDCs [175].
Upon application of an external electrical voltage to a desalination cell, we get what is called a microbial electrolysis desalination cell (MEDC) which happens to be a hybrid between MDCs and MECs [176]. Hence, the H2 gas production is observed in the cathodic chamber of MEDCs. The inter-membrane distance is 1–2.4 cm, which causes excessive impedance in current and power generation due to high internal resistance [174]. The approaches used to decrease the inter-membrane distance optimally are in current-day research. Even pH imbalances can significantly affect MDC performances [230]. Since the anion exchange membrane (AEM) only permits anion migration across the anodic chamber, it leads to anion accumulation in that chamber. Protons released from bio-oxidation of organic compounds also get accumulated in the anodic chamber. This increase in the concentration of anions and protons is harmful to the exo-electrogenic bacteria present in the anodic biofilm [177]. One obvious solution is the addition of buffers to control the pH. Still, it is not a practical solution since it can, in turn, add ions across the AEM into the desalination chamber and hamper the process to even further extents. A plausible solution would be to reprocess/recapitulate the anodic and the cathodic solutions uninterruptedly. Recirculation helps to stabilize the pH by maintaining it at 6, despite lower buffer concentrations [178]. However, due to the aerobes present in the cathodic chamber, the percentage of the substrate which is acted upon by the anodic microbes for current production drastically decreases with recirculation since the aerobes consume oxygen present in the cathodic chamber and degrade the local substrate inefficiently, making it a fundamentally defective fuel cell. Hence, there is the need for optimal recirculation so that unnecessary substrate degradation and wastage are prohibited [179]. Another problem faced in applying MDCs and MEDCs in practical life is membrane fouling due to ion accumulation. Even scale formation on the CEM surfaces has been observed due to cations found in saltwater [174]. These problems hamper the prospects of extensive scale application of such useful technology.
7 Challenges faced by MFCs in scaling up to an efficient level
Over years, a large majority of technology has been developed to focus on the particularly important task of evaluation of biomass. This reduces the waste material by utilizing it to generate harness able forms of energy. It has been theoretically proven that MFCs have huge potential and have many advantages over conventional methods and devices of energy production and waste management.
The capacity of a MFC to generate power has been significantly improved since its inception by the use of better architecture, cost-effective materials, and materials with better conductivity [180].
The power generation capacity of MFCs has been significantly improved since its inception by the use of better architecture, cost-effective materials, and materials with better conductivity [10]. Several varying reactor configurations have also been suggested to improve the power efficiency of MFCs to large extents [181]. Even with the huge potential that it has shown theoretically, MFCs still have a long way to go before they actually step into the market [44]. They can not only generate electrical energy for later use but also evaluate waste and offer energy recovery from wastewater [182].
Even with all the advantages and theoretically proven potential, MFCs still have a long way to go, and this is because of the complex nature of the system [44]. There are a lot of issues; to begin with, most of which have hindered the process of scaling up of the MFCs to a stage where it can be widely used with complete reliability for both energy generation and wastewater treatment [183]. The challenges faced by MFCs can be broadly categorized into the following sub-headings:
-
Expenses - of anodes/cathodes, electrolytes, ion exchange membranes, exo-electrogenic microbial community, other essential components, labor- and expertise-related costs.
-
Low efficiency - ohmic losses (due to resistances of materials), activation energy losses (energy consumed to cross energy barrier), losses associated with mass transfer, low harnessable power output, inconsistent and unstable power output, etc.
-
The differences in laboratory results and actual results due to idealistic simulations in the laboratory.
-
Competing waste management and energy generation technologies
7.1 Costs
One of the reasons MFCs are difficult to use for commercial purposes is the cost associated with setting them up and maintaining them. Since MFCs need not be recharged like several other batteries [184], it provides continuous energy supply, and it is much easier if the materials used in the cell last longer in order to reduce the maintenance costs for the cell which is why the material used has to be of desirable quality and also should be inexpensive and readily available [185]. Costs related to microbial fuel cells are generally divided into three categories—material-related costs, component-related costs, and labor costs [231].
7.1.1 Materials
Several studies like [186] have in the past estimated the costs incurred due to different categories of materials used in the cell. These not only take into account the current costs incurred but also project the future costs keeping in mind the technological advancements. As a result, it was concluded that 61.1% of the total costs due to materials are incurred due to conducting materials including the cathode (47%), the anode (9.4%), and other conducting materials used in the cell (4.7%). It was also noticed that 38% of total costs corresponded to the separation membrane used in the cell
Electrodes and conducting materials
Owing to the fact that majority of the costs incurred in an MFC are corresponding to the electrode material, there have been many recent studies with the aim of coming up with a better alternative to the conventionally used electrode materials by understanding the importance of the electrodes and how results change with changing materials and conditions in the cell to a deeper level [187].
Older and recent studies like refs. [60, 188], and [189] explored the use of conventionally used electrode materials including carbon; materials that are derived from carbon such as plain carbon paper, carbon rod, carbon fiber, carbon cloth, carbon sheet, graphite rod, graphite sheet, graphite fibers, graphite cloth, and graphite brushes; metal/metal oxides (gold (Au), copper (Cu), aluminum (Al), silver (Ag)); and several conducting polymers. However, all of these materials were either not biocompatible or could not deliver the desired results in terms of conductivity [190]. While discussing what electrodes can be used for, the price comes into the picture. The following properties are suitable for a good electrode material for an MFC:
In many studies, it has been observed that larger internal resistances have generally led up to lower power output. This is why the conventionally used materials, which are also not very cost-effective, do not provide desirable results. However, several materials that can be used as electrodes have been suggested that reduce costs and at the same time improve the power output. With that said, this does not affect the power density [191].
The surface area of the electrode not only affects the resistance of the electrode but also provides more active sites for bacteria growth and reactions taking place on the anode [192].
Since the anode is constantly in contact with the bacterial culture and their respiratory process, the material used for the electrode should not be corrosive as that will hinder the growth of the bacteria. This is why gold, copper, etc. are not considered to be very successful electrode materials for MFCs. The cost incurred and the general availability of the material to be used matter a great deal. Gold, platinum, etc. are therefore not very suitable for use as electrodes in MFCs [193].
The electrode stays in the electrolyte solution in contact with the highly active microorganism culture. If the electrode is not stable, it swells and reduces the active sites for bacteria. The swelling is caused by three significant reasons—corrosion, thermal instability, and low mechanical strength.
As mentioned, most of the conventionally used electrode materials are not favorable to produce an adequate amount of electric energy alongside evaluating wastewater efficiently since they lack in one or more of the abovementioned “suitable” qualities for an electrode. For this reason, several recent studies like refs. [38, 194, 195], and [196] have explored on new materials and better methods of modification of the existing electrodes to improve the quality of the results achieved from microbial fuel cells. Several other studies like refs. [197,198,199], and [200] have also explored new and innovative methods to improve performances by experimenting with different materials and observing their respective effects.
The reaction kinetics under normal conditions are not as favorable. For this reason, a catalyst is needed to be used on the cathode electrode. Platinum, which is used as a catalyst at the cathode to help cross the cathodic barrier, is also an expensive material, to begin with, and hence adds significantly to the total cost incurred. Recent studies have also focused on developing the use of better catalyst materials which give desirable results and are comparatively cheaper and widely available. He et al. [201] explore the use of alternative catalysts using Fe-N-C derived from 5H-dibenz [b,f] azepine-5-carboxamide (carbamazepine) which turns out to be much cheaper. The maximum power density produced by cathode-limiting MFCs with Fe-CBZ-Cats air cathodes was 431 ± 23 mW cm2 which was higher than MFCs with Pt/C air cathodes. Mixed transition metal-manganese oxides have also been tested as catalysts and have achieved fair results. NiMn2O4, which is a transition metal-manganese oxide, achieves 80% (439 m W/m3) out of the power density obtained with platinum (549 m W/m3) [232]
Separation media
In the earliest models of MFCs, ion exchange membranes were used [202]. These membranes not only did not support enough power densities but also are expensive in their fabrication and are not as widely available as needed to scale up microbial fuel cells commercially [203].
Many membrane-less structures of microbial fuel cells have also been explored, but they fail to provide desirable results because electron recovery in these cases is reduced significantly.
However, there still is scope for improvement; several studies have come up with cheap and commonly available materials that can be used to make separator membranes in microbial fuel cells. Khalili et al. [204] explored the use of unglazed wall ceramic (UGWC) and unglazed floor ceramic (UGFC) as materials for separating media [205, 206]. It was observed that UGFC-based microbial fuel cells produced a maximum power density of 106.89mW m−2 and UGWC-based microbial fuel cells produced a maximum power density of 321mW m−2. Both of these were lower than the conventionally used Nafion-17, which produces a power density of 602mW m−2. So even though the material used was cheaper, power density was sacrificed. Use of novel zeolite-loaded sulfonated polyethersulfone/polyvinylidene fluoride membrane was also documented by a recent study [207]. This method enabled higher proton conductivity and lower oxygen diffusion, thereby increasing the power generated.
Components
MFC units not only use materials but also have larger components that act as a one-time investment in many cases, provided they are tough and robust enough to be used for a long time, which makes this a very important investment. These usually include reactor materials and compartments. Conventionally, the use of two-compartment cells has been prevalent for the better part of a century. With that being said, recent studies such as refs. [196, 194], and [38] have shown the use of much more efficient and inexpensive alternatives to these electrodes and electrolytes that have been conventionally used for a long time.
7.1.2 Labor costs
As in any other industry, skilled labor is required to produce the final product, which can be (however, dependent on location) highly expensive. This cost can be reduced by mass-producing the product. Use of readily available and cheaply transported materials can reduce the fabrication costs effectively [208].
7.2 Energy losses
One of the major threats for microbial fuel cells is the high amounts of energy losses during production. Even though MFCs have higher energy retention of 44% than conventional biogas energy plants which have retention of about 30%, the losses of energy in an MFC are a highly contributing factor in reducing its total power generation to a value lower than the theoretical value [3, 209]. The following contributing factors to these losses are shown in Fig. 6.
7.2.1 Ohmic losses
Ohmic losses include the losses in energy due to the internal resistances of the materials used. There are majorly two sources of losses that exist due to internal resistances of the materials used in the cell. There are losses in energy due to the resistance in the flow of electrons through the electrodes and the external circuit. This is the reason why the materials used as conductors have to be good enough and inexpensive at the same time (especially the cathode). These losses of the first type can be avoided without compensating much on the quality of the material or the cost, by reducing the separation between the two electrodes [186].
There are also losses due to the resistance offered by the separation media and the electrolyte used in the cell. Various studies (as stated above) have been made in the field to reduce the costs of these materials while increasing the power generated, and the power density; more of these include refs. [210] and [211] which focus on the effect of the membrane material and fabrication techniques on the performance of a microbial fuel cell.
7.2.2 Activation barrier
This corresponds to the energy lost to cross the cathodic barrier and the activation energy of the redox reactions. The losses occur in the transportation of electrons during the reaction at the surface of the electrode. One way to reduce these losses is to increase the surface area of the electrode, thereby providing more surface area and more active sites for the reaction to take place. Another one is to pre-process the waste to some extent.
7.2.3 Mass transfer hindrance
Mass transfer losses are the losses that occur due to the hindrance in the flow of electrons through the conducting materials such as the electrode (i.e., current flowing through the material) due to the mass transfer to or/and from the electrodes [212]. Usually, this occurs in the presence of high current densities due to incomplete and limited mass transfer of chemical species by diffusion towards the electrode. As a result of insufficient mass transfer, the reactant depletes or causes the product to accumulate. To reduce these mass transfer losses, maintaining high concentrations and even distribution of oxidants like O2 across the cathode compartment might be useful. In addition, optimization of MFC operating conditions, electrode material, and cathode compartment design can reduce losses due to mass transfer.
7.3 Miscellaneous challenges
There are many other reasons that are not so significant and not very simple to state but have a negative impact on the cell output due to their presence. One of them is the substrate crossover in the cathode compartment. As explained in [56], the substrate crossover in the cathode compartment reduces the total power generated by the cell because it contributes to the restriction in flow, which increases the lost power from the cell. This is also a difference in the theoretical and practical observations of the cell. Theoretically, the membrane should not allow the reactant molecules to pass through it and should only let the ions to pass through. But as seen in various studies [152, 210, 211, 213], membrane transport crossover of the reactant molecules does take place in the real world. Increasing the supplied power can initially help in reducing the crossover. Other ways can be to dilute the reactant solutions, and by improving the membrane materials that have better efficiency at being selective to the molecules they let through can help too, but these methods are not very inexpensive.
Another way to reduce losses due to the ohmic voltages can be to reduce the separation between the electrodes, which in turn reduce the resistance and as a result decreases the ohmic polarization in the cell. The selection of highly conductive electrodes for electron transport is crucial, so if the electrodes are electrolyte supported, both the electrodes and electrolyte should exhibit high electronic and ionic conductivity, respectively [190].
7.4 Differences in the theoretical and actual performances of MFCs
This point has been studied over many times and is probably the only challenge that has a definitive face. Many studies and applications have been discussed over and over again, where the uses of MFCs have been emphasized to evaluate wastewater or waste material in general. But the main issue with these studies is that most of them are lab scale and are done in controlled environments [213]. This reduces the losses made due to various industry-related reasons. Since the studies are lab scale, the cost factor which makes the entire commercial space is not given a high weightage.
Solutions to make a move for the commercialization of MFCs is to take up all the challenges discussed above on a small scale, preferably a lab-scale study, and try to eliminate them with as high efficiency as possible. As discussed, stacking is difficult for MFCs but is probably one of the most practical ways to take the product to market [214]. As seen, a single type of cell has not been able to produce larger power results that the cells have potential for; this indicates that a possible solution would be to stack these single MFCs to create better results. But there are a few important points to keep in mind while stacking is considered. Theoretically stacking MFCs leads to increase in power, but in practical applications, it is lower because of all the losses that have been extensively seen in this text [215]. However, there is not much progress in the field of stacking of MFCs and would require extensive research in the future considering mainly the losses.
7.5 Competing wastewater treatment technologies
As mentioned at the beginning of this section, MFCs are more or less related to biochemical processes involving technologies that are majorly focused on evaluating waste material. These include technologies from worm pits to huge biogas plants. On the one hand, these technologies have helped in getting ideas in the development of MFCs because of their similarity, but on the other hand, these technologies have also proven to be the biggest competitors for MFCs [216,217,218]. Especially in developing countries, conventional modes of production of energy and treatment of waste might not be as efficient but are cheaper and widely accepted. This poses a challenge to commercially accepting MFCs as an alternative. A major reason is the non-availability of the materials locally and the high costs associated with importing them and preparing an MFC unit.
8 Conclusion
Microbial fuel cells and their modified derivative systems are breakthrough methods to treat biodegradable effluents efficiently with simultaneous generation of electricity, thus catering to the rapidly growing demands of energy production and wastewater treatment. They have, on multiple occasions, proven to be of great use in small/laboratory scales as a source of hydrogen, as a method of desalination, etc. However, the use of MFCs and its hybrid systems has been restricted on industrial scales because of multiple reasons such as low output power densities, high cost of reactor construction and operation, long residence time, and contamination or clogging of the ion exchange membranes. Although hybrid systems are known to give greater outputs than standalone MFCs, they still need improvement in multiple aspects like up-scaling, system integration and maintenance, and electrode characterization. The expensive and rare platinum/gold-based electrodes and photocatalysts and membranes need replacement with more easily available and sustainable electrodes. During up-scaling, with an increase in the volume of an MFC, power output decreases due to an increase in internal resistance. This needs to be handled with optimization by negotiating both the influencing aspects. The wastewater which is used to conduct experiments in the laboratories is made highly conductive and is quite unlike the real world form of wastewater. Hence, our focus needs to be shifted to actual wastewaters so as to help the industries which need this technology. Employing stacked MFCs in series and parallel can generate higher power densities if we take care of the overpotential, which inhibits current density from increasing in the series connection.
Abbreviations
- AEM:
-
anion exchange membrane
- AFT:
-
anodic Fenton treatment
- ANAMMOX:
-
anaerobic ammonium oxidation
- AQDS:
-
anthraquinone-2,6-disulphonicsalt
- BSA:
-
bovine serum albumin
- BOD:
-
biological oxygen demand
- CEM:
-
cation exchange membrane
- CF:
-
carbon felt
- COD:
-
chemical oxygen demand
- HEPES:
-
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid
- MD:
-
membrane distillation
- MDC:
-
microbial desalination cell
- MEC:
-
microbial electrolysis cell
- MED:
-
multi-effect distillation
- MEDC:
-
microbial electrolysis desalination cell
- MES:
-
2-Morpholinoethanesulfonic acid
- MFC:
-
microbial fuel cell
- MLMFC:
-
membrane-less microbial fuel cell
- MPEC:
-
microbial photoelectrochemical cell
- MSF:
-
multi-stage flash distillation
- OLR:
-
organic loading rate
- PEC:
-
photoelectrochemical cell
- PEM:
-
proton exchange membrane
- PFC:
-
photocatalytic fuel cells
- pH:
-
power of hydrogen
- PIPES:
-
piperazinediethanesulfonic acid
- PPy:
-
polypyrrole
- RO:
-
reverse osmosis
- SLR:
-
sludge loading rate
- UV:
-
ultraviolet
- VSS:
-
volatile suspended solid
References
James C, Hema Meenal S, Elakkiya S, Logarshani S (2020) Sustainable environment through treatment of domestic sewage using MFC. Mater Today Proc. https://doi.org/10.1016/j.matpr.2020.07.110
Sugawara E, Nikaido H (2014) Properties of AdeABC and AdeIJK efflux systems of Acinetobacter baumannii compared with those of the AcrAB-TolC system of Escherichia coli 58:7250–7257
Santoro C, Arbizzani C, Erable B, Ieropoulos I (2017) Microbial fuel cells: From fundamentals to applications. A review. J Power Sources 356:225–244
Pandey P, Shinde VN, Deopurkar RL et al (2016) Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl Energy 168:706–723
Agarwal A, Upadhyay U, Sreedhar I et al (2020) A review on valorization of biomass in heavy metal removal from wastewater. J Water Process Eng
Logan BE, Elimelech M (2012) Membrane-based processes for sustainable power generation using water. Nature 488:313–319
Mansor M, Timmiati SN, Lim KL et al (2019) Recent progress of anode catalysts and their support materials for methanol electrooxidation reaction. Int J Hydrog Energy 44:14744–14769
Logan BE, Rabaey K (2012) Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 337(80):686–690
Palanisamy G, Jung HY, Sadhasivam T et al (2019) A comprehensive review on microbial fuel cell technologies: processes, utilization, and advanced developments in electrodes and membranes. J Clean Prod 221:598–621
Slate AJ, Whitehead KA, Brownson DAC, Banks CE (2019) Microbial fuel cells: an overview of current technology. Renew Sust Energ Rev 101:60–81
Do MH, Ngo HH, Guo WS et al (2018) Challenges in the application of microbial fuel cells to wastewater treatment and energy production: a mini review. Sci Total Environ 639:910–920
Aghababaie M, Farhadian M, Jeihanipour A, Biria D (2015) Effective factors on the performance of microbial fuel cells in wastewater treatment–a review. Environ Technol Rev 4:71–89
Sreedhar I, Upadhyay U, Roy P et al (2020) Carbon capture and utilization by graphenes-path covered and ahead. J Clean Prod
Flimban SGA, Ismail IMI, Kim T, Oh SE (2019) Overview of recent advancements in the microbial fuel cell from fundamentals to applications: design, major elements, and scalability. Energies 12:12173390
Virdis B, Freguia S, Rozendal RA, et al (2011) Microbial fuel cells. In: Treatise on water science. 641–665
Rahimnejad M, Adhami A, Darvari S et al (2015) Microbial fuel cell as new technology for bioelectricity generation: a review. Alexandria Eng J 54:745–756
Nitisoravut R, Regmi R (2017) Plant microbial fuel cells: a promising biosystems engineering. Renew Sust Energ Rev 76:81–89
Nemeth T, Schröer P, Kuipers M, Sauer DU (2020) Lithium titanate oxide battery cells for high-power automotive applications – electro-thermal properties, aging behavior and cost considerations. J Energy Storage 31:101656
Tatinclaux M, Gregoire K, Leininger A et al (2018) Electricity generation from wastewater using a floating air cathode microbial fuel cell. Water-Energy Nexus. https://doi.org/10.1016/j.wen.2018.09.001
Salar-Garcia MJ, Obata O, Kurt H et al (2020) Impact of inoculum type on the microbial community and power performance of urine-fed microbial fuel cells. Microorganisms 8:1–16
Yaqoob AA, Ibrahim MNM, Rafatullah M et al (2020) Recent advances in anodes for microbial fuel cells: an overview. Materials (Basel) 13:2078
Schneider K, Thorne RJ, Cameron PJ (2016) An investigation of anode and cathode materials in photomicrobial fuel cells. Philos Trans R Soc A Math Phys Eng Sci 374:20150080
Paquin F, Rivnay J, Salleo A et al (2015) Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J Mater Chem C 3:10715–10722
Kim JR, Jung SH, Regan JM, Logan BE (2007) Electricity generation and microbial community analysis of alcohol powered microbial fuel cells. Bioresour Technol 98:2568–2577
Liu H, Cheng S, Logan BE (2005) Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ Sci Technol 39:658–662
Rahimnejad M, Ghoreyshi AA, Najafpour G, Jafary T (2011) Power generation from organic substrate in batch and continuous flow microbial fuel cell operations. Appl Energy 88:3999–4004
Ahn Y, Logan BE (2010) Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour Technol 101:469–475
Ishii S, Watanabe K, Yabuki S et al (2008) Comparison of electrode reduction activities of Geobacter sulfurreducens and an enriched consortium in an air-cathode microbial fuel cell. Appl Environ Microbiol 74:7348–7355
Zou Y, Xiang C, Yang L et al (2008) A mediatorless microbial fuel cell using polypyrrole coated carbon nanotubes composite as anode material. Int J Hydrog Energy 33:4856–4862
Jung RK, Dec J, Bruns MA, Logan BE (2008) Removal of odors from swine wastewater by using microbial fuel cells. Appl Environ Microbiol 74:2540–2543
Logan BE, Murano C, Scott K et al (2005) Electricity generation from cysteine in a microbial fuel cell. Water Res 39:942–952
Chakraborty I, Das S, Dubey BK, Ghangrekar MM (2020) Novel low cost proton exchange membrane made from sulphonated biochar for application in microbial fuel cells. Mater Chem Phys 239:122025
Lin CW, Wu CH, Chiu YH, Tsai SL (2014) Effects of different mediators on electricity generation and microbial structure of a toluene powered microbial fuel cell. Fuel 125:30–35
Yu F, Wang C, Ma J (2016) Applications of graphene-modified electrodes in microbial fuel cells. Materials (Basel):9
Firdous S, Jin W, Shahid N et al (2018) The performance of microbial fuel cells treating vegetable oil industrial wastewater. Environ Technol Innov 10:143–151
Ghadge AN, Jadhav DA, Pradhan H, Ghangrekar MM (2015) Enhancing waste activated sludge digestion and power production using hypochlorite as catholyte in clayware microbial fuel cell. Bioresour Technol 182:225–231
Chouler J, Padgett GA, Cameron PJ et al (2016) Towards effective small scale microbial fuel cells for energy generation from urine. Electrochim Acta 192:89–98
Fu Y, Xu Q, Zai X et al (2014) Low electrical potential anode modified with Fe/ferric oxide and its application in marine benthic microbial fuel cell with higher voltage and power output. Appl Surf Sci 289:472–477
Liu YF, Zhang XL, Li CJ (2020) Advances in carbon-based anode materials for microbial fuel cells. Gongcheng Kexue Xuebao/Chinese J Eng 42:270–277
Sonawane JM, Pant D, Ghosh PC, Adeloju SB (2019) Fabrication of a carbon paper/polyaniline-copper hybrid and its utilization as an air cathode for microbial fuel cells. ACS Appl Energy Mater 2:1891–1902
Abourached C, English MJ, Liu H (2016) Wastewater treatment by microbial fuel cell (MFC) prior irrigation water reuse. J Clean Prod 137:144–149
Liu YF, Zhang XL, Li CJ (2020) Advances in carbon-based anode materials for microbial fuel cells. Gongcheng Kexue Xuebao/Chinese. J Eng Des
Pattanayak P, Pramanik N, Papiya F et al (2020) Metal-free keratin modified poly(pyrrole-co-aniline)-reduced graphene oxide based nanocomposite materials: a promising cathode catalyst in microbial fuel cell application. J Environ Chem Eng 8:103813
Ucar D, Zhang Y, Angelidaki I (2017) An overview of electron acceptors in microbial fuel cells. Front Microbiol 8:00643
Huang L, Regan JM, Quan X (2011) Electron transfer mechanisms, new applications, and performance of biocathode microbial fuel cells. Bioresour Technol 102:316–323
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7:375–381
Deng Q, Li X, Zuo J et al (2010) Power generation using an activated carbon fiber felt cathode in an upflow microbial fuel cell. J Power Sources 195:1130–1135
Ter Heijne A, Hamelers HVM, De Wilde V et al (2006) A bipolar membrane combined with ferric iron reduction as an efficient cathode system in microbial fuel cells. Environ Sci Technol 40:5200–5205
You S, Zhao Q, Zhang J et al (2008) Increased sustainable electricity generation in up-flow air-cathode microbial fuel cells. Biosens Bioelectron 23:1157–1160
Chen GW, Choi SJ, Lee TH et al (2008) Application of biocathode in microbial fuel cells: cell performance and microbial community. Appl Microbiol Biotechnol 79:379–388
Papiya F, Pattanayak P, Kumar V et al (2020) Sulfonated graphene oxide and titanium dioxide coated with nanostructured polyaniline nanocomposites as an efficient cathode catalyst in microbial fuel cells. Mater Sci Eng C 108:110498
Tajdid Khajeh R, Aber S, Nofouzi K (2020) Efficient improvement of microbial fuel cell performance by the modification of graphite cathode via electrophoretic deposition of CuO/ZnO. Mater Chem Phys 240:122208
Merino-Jimenez I, Celorrio V, Fermin DJ et al (2017) Enhanced MFC power production and struvite recovery by the addition of sea salts to urine. Water Res 109:46–53
Harnisch F, Schröder U (2009) Selectivity versus mobility: separation of anode and cathode in microbial bioelectrochemical systems. ChemSusChem 2:921–926
Chen Y, Zhao Z, Weng Z, et al (2019) Performance study of polypyrrole-nanowires based microbial fuel cells. In: 2019 3rd International Conference on Circuits, System and Simulation, ICCSS 2019. 235–238
Ghasemi M, Wan Daud WR, Alam J et al (2016) Sulfonated poly ether ether ketone with different degree of sulphonation in microbial fuel cell: application study and economical analysis. Int J Hydrog Energy 41:4862–4871
Asensio Y, Fernandez-Marchante CM, Lobato J et al (2018) Influence of the ion-exchange membrane on the performance of double-compartment microbial fuel cells. J Electroanal Chem 808:427–432
Zhang Y, Liu M, Zhou M et al (2019) Microbial fuel cell hybrid systems for wastewater treatment and bioenergy production: synergistic effects, mechanisms and challenges. Renew Sust Energ Rev 103:13–29
Choudhury P, Prasad Uday US, Bandyopadhyay TK et al (2017) Performance improvement of microbial fuel cell (MFC) using suitable electrode and Bioengineered organisms: a review. Bioengineered 8:471–487
Goswami R, Mishra VK (2018) A review of design, operational conditions and applications of microbial fuel cells. Biofuels 9:203–220
Li M, Zhou M, Tian X et al (2018) Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol Adv 36:1316–1327
Ramachandran R, Chen SM, peter G k G (2015) Enhancement of different fabricated electrode materials for microbial fuel cell applications: an overview. Int J Electrochem Sci 10:7111–7137
Naina Mohamed S, Thota Karunakaran R, Manickam M (2018) Enhancement of bioelectricity generation from treatment of distillery wastewater using microbial fuel cell. Environ Prog Sustain Energy. https://doi.org/10.1002/ep.12734
Nam JY, Kim HW, Lim KH et al (2010) Variation of power generation at different buffer types and conductivities in single chamber microbial fuel cells. Biosens Bioelectron 25:1155–1159
Srivastava P, Yadav AK, Mishra BK (2015) The effects of microbial fuel cell integration into constructed wetland on the performance of constructed wetland. Bioresour Technol 195:223–230
Kim C, Lee CR, Song YE et al (2017) Hexavalent chromium as a cathodic electron acceptor in a bipolar membrane microbial fuel cell with the simultaneous treatment of electroplating wastewater. Chem Eng J 328:703–707
Wu S, Liang P, Zhang C et al (2015) Enhanced performance of microbial fuel cell at low substrate concentrations by adsorptive anode. Electrochim Acta 161:245–251
Saba B, Christy AD, Yu Z, Co AC (2017) Sustainable power generation from bacterio-algal microbial fuel cells (MFCs): an overview. Renew Sust Energ Rev 73:75–84
Torres CI, Lee HS, Rittmann BE (2008) Carbonate species as OH- carriers for decreasing the pH gradient between cathode and anode in biological fuel cells. Environ Sci Technol 42:8773–8777
Ren H, Jiang C, Chae J (2017) Effect of temperature on a miniaturized microbial fuel cell (MFC). Micro Nano Syst Lett 5:13
Wang XL, Wu C, Zhang JQ, et al (2011) Acclimation stage on the performance of microbial fuel cells subjected to variation in COD, temperature, and electron acceptor. In: Advanced Materials Research 2346–2350
Gadkari S, Fontmorin JM, Yu E, Sadhukhan J (2020) Influence of temperature and other system parameters on microbial fuel cell performance: numerical and experimental investigation. Chem Eng J 388:124176
Uria N, Ferrera I, Mas J (2017) Electrochemical performance and microbial community profiles in microbial fuel cells in relation to electron transfer mechanisms. BMC Microbiol 17:208
Gonzalez del Campo A, Lobato J, Cañizares P et al (2013) Short-term effects of temperature and COD in a microbial fuel cell. Appl Energy 101:213–217
Commault AS, Barrière F, Lapinsonnière L et al (2015) Influence of inoculum and anode surface properties on the selection of Geobacter-dominated biofilms. Bioresour Technol 195:265–272
Patil SA, Harnisch F, Kapadnis B, Schröder U (2010) Electroactive mixed culture biofilms in microbial bioelectrochemical systems: the role of temperature for biofilm formation and performance. Biosens Bioelectron 26:803–808
Michie IS, Kim JR, Dinsdale RM et al (2011) The influence of psychrophilic and mesophilic start-up temperature on microbial fuel cell system performance. Energy Environ Sci 4:1011–1019
Cheng S, Xing D, Logan BE (2011) Electricity generation of single-chamber microbial fuel cells at low temperatures. Biosens Bioelectron 26:1913–1917
Oliveira VB, Simões M, Melo LF, Pinto AMFR (2013) Overview on the developments of microbial fuel cells. Biochem Eng J 73:53–64
Behera M, Ghangrekar MM (2009) Performance of microbial fuel cell in response to change in sludge loading rate at different anodic feed pH. Bioresour Technol 100:5114–5121
Martin E, Savadogo O, Guiot SR, Tartakovsky B (2010) The influence of operational conditions on the performance of a microbial fuel cell seeded with mesophilic anaerobic sludge. Biochem Eng J 51:132–139
Di Lorenzo M, Scott K, Curtis TP, Head IM (2010) Effect of increasing anode surface area on the performance of a single chamber microbial fuel cell. Chem Eng J 156:40–48
Velvizhi G, Venkata Mohan S (2012) Electrogenic activity and electron losses under increasing organic load of recalcitrant pharmaceutical wastewater. Int J Hydrog Energy 37:5969–5978
Moon H, In SC, Jae KJ, Kim BH (2005) Residence time distribution in microbial fuel cell and its influence on COD removal with electricity generation. Biochem Eng J 27:59–65
Aelterman P, Versichele M, Marzorati M et al (2008) Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresour Technol 99:8895–8902
Ieropoulos I, Winfield J, Greenman J (2010) Effects of flow-rate, inoculum and time on the internal resistance of microbial fuel cells. Bioresour Technol 101:3520–3525
Juang DF, Yang PC, Chou HY, Chiu LJ (2011) Effects of microbial species, organic loading and substrate degradation rate on the power generation capability of microbial fuel cells. Biotechnol Lett 33:2147–2160
Herbert-Guillou D, Tribollet B, Festy D (2001) Influence of the hydrodynamics on the biofilm formation by mass transport analysis. Bioelectrochemistry 53:119–125
Pham HT, Boon N, Aelterman P et al (2008) High shear enrichment improves the performance of the anodophilic microbial consortium in a microbial fuel cell. Microb Biotechnol 1:487–496
Rickard AH, McBain AJ, Stead AT, Gilbert P (2004) Shear rate moderates community diversity in freshwater biofilms. Appl Environ Microbiol 70:7426–7435
Rochex A, Godon JJ, Bernet N, Escudié R (2008) Role of shear stress on composition, diversity and dynamics of biofilm bacterial communities. Water Res 42:4915–4922
Gajda I, Greenman J, Melhuish C, Ieropoulos I (2015) Self-sustainable electricity production from algae grown in a microbial fuel cell system. Biomass Bioenergy 82:87–93
Li J, Yang W, Zhang B, et al (2018) Electricity from microbial fuel cells. Green Energy and Technology 54:745–756
Tharali AD, Sain N, Osborne WJ (2016) Microbial fuel cells in bioelectricity production. Front Life Sci 9:252–266
Logan BE (2004) Extracting hydrogen and electricity from renewable resources. Environ Sci Technol 38:160–167
Min B, Kim JR, Oh SE et al (2005) Electricity generation from swine wastewater using microbial fuel cells. Water Res 39:4961–4968
Heilmann J, Logan BE (2006) Production of electricity from proteins using a microbial fuel cell. Water Environ Res 78:531–537
Jafary T, Wan Daud WR, Ghasemi M et al (2019) Clean hydrogen production in a full biological microbial electrolysis cell. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2018.01.010
Hu J, Zhang Q, Lee DJ, Ngo HH (2018) Feasible use of microbial fuel cells for pollution treatment. Renew Energy. https://doi.org/10.1016/j.renene.2017.02.001
Habermann W, Pommer EH (1991) Biological fuel cells with sulphide storage capacity. Appl Microbiol Biotechnol 35:128–133
Naina Mohamed S, Ajit Hiraman P, Muthukumar K, Jayabalan T (2020) Bioelectricity production from kitchen wastewater using microbial fuel cell with photosynthetic algal cathode. Bioresour Technol. https://doi.org/10.1016/j.biortech.2019.122226
Li WW, Yu HQ, He Z (2014) Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ Sci 7:911–924
Yu D, Wang G, Xu F et al (2013) Electricity generation from artificial wastewater using an upflow microbial fuel cell electricity generation from artificial wastewater using an upflow microbial fuel cell. Bioresour Technol 39:5262–5267
Kumar SS, Kumar V, Malyan SK et al (2019) Microbial fuel cells (MFCs) for bioelectrochemical treatment of different wastewater streams. Fuel. https://doi.org/10.1016/j.fuel.2019.05.109
Xu P, Xu H, Shi Z (2018) A novel bio-electro-Fenton process with FeVO4/CF cathode on advanced treatment of coal gasification wastewater. Sep Purif Technol 194:457–461
Miran W, Nawaz M, Jang J, Lee DS (2016) Conversion of orange peel waste biomass to bioelectricity using a mediator-less microbial fuel cell. Sci Total Environ 547:197–205
Kumar SS, Kumar V, Kumar R et al (2019) Microbial fuel cells as a sustainable platform technology for bioenergy, biosensing, environmental monitoring, and other low power device applications. Fuel. https://doi.org/10.1016/j.fuel.2019.115682
Lee H, Yang W, Wei X, et al (2015) A microsized microbial fuel cell based biosensor for fast and sensitive detection of toxic substances in water. In: Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS). 573–576
Felix FS, Angnes L (2018) Electrochemical immunosensors – a powerful tool for analytical applications. Biosens Bioelectron 102:470–478
Rocchitta G, Spanu A, Babudieri S et al (2016) Enzyme biosensors for biomedical applications: strategies for safeguarding analytical performances in biological fluids. Sensors (Switzerland) 16:780
Mehrotra P (2016) Biosensors and their applications - a review. J Oral Biol Craniofacial Res 6:153–159
Justino CIL, Gomes AR, Freitas AC et al (2017) Graphene based sensors and biosensors. Trends Anal Chem 91:53–66
Gerritsen M, Jansen JA, Lutterman JA (1999) Performance of subcutaneously implanted glucose sensors for continuous monitoring. Neth J Med 54:167–179
Yang W, Wei X, Fraiwan A et al (2016) Fast and sensitive water quality assessment: a μl-scale microbial fuel cell-based biosensor integrated with an air-bubble trap and electrochemical sensing functionality. Sensors Actuators B Chem 226:191–195
Zhuang L, Zhou S, Li Y et al (2010) In situ Fenton-enhanced cathodic reaction for sustainable increased electricity generation in microbial fuel cells. J Power Sources 195:1379–1382
Lu A, Li Y, Jin S et al (2012) Growth of non-phototrophic microorganisms using solar energy through mineral photocatalysis. Nat Commun 3:105497
Morel A, Zuo K, Xia X et al (2012) Microbialdesalination cells packed with ion-exchange resin to enhance water desalination rate. Bioresour Technol 118:243–248
Sun M, Sheng GP, Mu ZX et al (2009) Manipulating the hydrogen production from acetate in a microbial electrolysis cell-microbial fuel cell-coupled system. J Power Sources 191:338–343
Ardakani MN, Badalians Gholikandi G (2020) Microbial fuel cells (MFCs) in integration with anaerobic treatment processes (AnTPs) and membrane bioreactors (MBRs) for simultaneous efficient wastewater/sludge treatment and energy recovery -a state-of-the-art review. Biomass Bioenergy 141:105726
Nordin N, Ho LN, Ong SA et al (2017) Hybrid system of photocatalytic fuel cell and Fenton process for electricity generation and degradation of Reactive Black 5. Sep Purif Technol 177:135–141
Feng CH, Li FB, Mai HJ, Li XZ (2010) Bio-electro-Fenton process driven by microbial fuel cell for wastewater treatment. Environ Sci Technol 44:1875–1880
Rozendal RA, Leone E, Keller J, Rabaey K (2009) Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system. Electrochem Commun 11:1752–1755
Zhu X, Ni J (2009) Simultaneous processes of electricity generation and p-nitrophenol degradation in a microbial fuel cell. Electrochem Commun 11:274–277
Singh HM, Pathak AK, Chopra K et al (2019) Microbial fuel cells: a sustainable solution for bioelectricity generation and wastewater treatment. Biofuels. https://doi.org/10.1080/17597269.2017.1413860
Chen JY, Zhao L, Li N, Liu H (2015) A microbial fuel cell with the three-dimensional electrode applied an external voltage for synthesis of hydrogen peroxide from organic matter. J Power Sources 287:291–296
Ramirez JH, Costa CA, Madeira LM et al (2007) Fenton-like oxidation of Orange II solutions using heterogeneous catalysts based on saponite clay. Appl Catal B Environ 71:44–56
Zhao K, Zeng Q, Bai J et al (2017) Enhanced organic pollutants degradation and electricity production simultaneously via strengthening the radicals reaction in a novel Fenton-photocatalytic fuel cell system. Water Res 108:293–300
Zhu X, Logan BE (2013) Using single-chamber microbial fuel cells as renewable power sources of electro-Fenton reactors for organic pollutant treatment. J Hazard Mater 252–253:198–203
de DMAF, Iglesias O, Bocos E et al (2014) Application of benthonic microbial fuel cells and electro-Fenton process to dye decolourisation. J Ind Eng Chem 20:3754–3760
Xu H, Quan X, Xiao Z, Chen L (2018) Effect of anodes decoration with metal and metal oxides nanoparticles on pharmaceutically active compounds removal and power generation in microbial fuel cells. Chem Eng J 335:539–547
Zhang Y, Wang Y, Angelidaki I (2015) Alternate switching between microbial fuel cell and microbial electrolysis cell operation as a new method to control H2O2 level in Bioelectro-Fenton system. J Power Sources 291:108–116
Zhang L, Yin X, Li SFY (2015) Bio-electrochemical degradation of paracetamol in a microbial fuel cell-Fenton system. Chem Eng J 276:185–192
Luo Y, Zhang R, Liu G et al (2011) Simultaneous degradation of refractory contaminants in both the anode and cathode chambers of the microbial fuel cell. Bioresour Technol 102:3827–3832
Feng C, Ma L, Li F et al (2010) A polypyrrole/anthraquinone-2,6-disulphonic disodium salt (PPy/AQDS)-modified anode to improve performance of microbial fuel cells. Biosens Bioelectron 25:1516–1520
Feng C, Li F, Liu H et al (2010) A dual-chamber microbial fuel cell with conductive film-modified anode and cathode and its application for the neutral electro-Fenton process. Electrochim Acta 55:2048–2054
Yong XY, Gu DY, Wu YD et al (2017) Bio-Electron-Fenton (BEF) process driven by microbial fuel cells for triphenyltin chloride (TPTC) degradation. J Hazard Mater 324:178–183
Yuan GE, Li Y, Lv J et al (2017) Integration of microbial fuel cell and catalytic oxidation reactor with iron phthalocyanine catalyst for Congo red degradation. Biochem Eng J 120:118–124
Kment SK, Naldoni A et al (2020) FeO-based nanostructures and nanohybrids for photoelectrochemical water splitting. Prog Mater Sci
Lianos P (2017) Review of recent trends in photoelectrocatalytic conversion of solar energy to electricity and hydrogen. Appl Catal B Environ 210:235–254
Antolini E (2019) Photoelectrocatalytic fuel cells and photoelectrode microbial fuel cells for wastewater treatment and power generation. J Environ Chem Eng 7:103241
Bak T, Nowotny J, Rekas M, Sorrell CC (2002) Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects. Int J Hydrog Energy 27:991–1022
Wang Y, Zu M, Zhou X et al (2020) Designing efficient TiO2-based photoelectrocatalysis systems for chemical engineering and sensing. Chem Eng J
Fischer F (2018) Photoelectrode, photovoltaic and photosynthetic microbial fuel cells. Renew Sust Energ Rev 90:16–27
Fu S, Deng B, Ma D et al (2018) Visible-light-driven photocatalytic fuel cell with an Ag-TiO2 carbon foam anode for simultaneous 4-chlorophenol degradation and energy recovery. ChemEngineering 2:1–10
Yu T, Liu L, Li L, Yang F (2016) A self-biased fuel cell with TiO2/g-C3N4 anode catalyzed alkaline pollutant degradation with light and without light - what is the degradation mechanism? Electrochim Acta 210:122–129
Zeng Q, Bai J, Li J et al (2018) Highly-stable and efficient photocatalytic fuel cell based on an epitaxial TiO2/WO3/W nanothorn photoanode and enhanced radical reactions for simultaneous electricity production and wastewater treatment. Appl Energy 220:127–137
Khan ME, Khan MM, Min BK, Cho MH (2018) Microbial fuel cell assisted band gap narrowed TiO2 for visible light induced photocatalytic activities and power generation. Sci Rep 8
Wang H, Qian F, Wang G et al (2013) Self-biased solar-microbial device for sustainable hydrogen generation. ACS Nano 7:8728–8735
Li M, He X, Zeng Y et al (2015) Solar-microbial hybrid device based on oxygen-deficient niobium pentoxide anodes for sustainable hydrogen production. Chem Sci 6:6799–6805
Logan BE, Call D, Cheng S et al (2008) Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ Sci Technol 42:8630–8640
Chorbadzhiyska E, Apostolova D, Bardarov I et al (2020) Hybrid MFC-MEC systems: principles and applications. Bulg Chem Commun. https://doi.org/10.34049/bcc.52.A.178
Jung RK, Cheng S, Oh SE, Logan BE (2007) Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ Sci Technol 41:1004–1009
Wang A, Sun D, Cao G et al (2011) Integrated hydrogen production process from cellulose by combining dark fermentation, microbial fuel cells, and a microbial electrolysis cell. Bioresour Technol 102:4137–4143
Cheng S, Logan BE (2007) Sustainable and efficient biohydrogen production via electrohydrogenesis. Proc Natl Acad Sci U S A 104:18871–18873
Rezaei F, Richard TL, Brennan RA, Logan BE (2007) Substrate-enhanced microbial fuel cells for improved remote power generation from sediment-based systems. Environ Sci Technol 41:4053–4058
Ren Z, Ward TE, Logan BE, Regan JM (2007) Characterization of the cellulolytic and hydrogen-producing activities of six mesophilic Clostridium species. J Appl Microbiol 103:2258–2266
Azwar MY, Hussain MA, Abdul-Wahab AK (2014) Development of biohydrogen production by photobiological, fermentation and electrochemical processes: a review. Renew Sust Energ Rev 31:158–173
Sun M, Sheng GP, Zhang L et al (2008) An MEC-MFC-coupled system for biohydrogen production from acetate. Environ Sci Technol 42:8095–8100
Rago L, Monpart N, Cortés P et al (2016) Performance of microbial electrolysis cells with bioanodes grown at different external resistances. Water Sci Technol 73:1129–1135
Huang L, Yao B, Wu D, Quan X (2014) Complete cobalt recovery from lithium cobalt oxide in self-driven microbial fuel cell - microbial electrolysis cell systems. J Power Sources 259:54–64
Li Y, Wu Y, Liu B et al (2015) Self-sustained reduction of multiple metals in a microbial fuel cell-microbial electrolysis cell hybrid system. Bioresour Technol 192:238–246
Li Z, Wang Q, Liu D et al (2013) Ionic liquid-mediated electrochemical CO2 reduction in a microbial electrolysis cell. Electrochem Commun 35:91
Liu H, Leng F, Guan Y et al (2017) Simultaneous pollutant removal and electricity generation in a combined ABR-MFC-MEC system treating fecal wastewater. Water Air Soil Pollut 228:179
Zhao H, Zhang Y, Zhao B et al (2012) Electrochemical reduction of carbon dioxide in an MFC-MEC system with a layer-by-layer self-assembly carbon nanotube/cobalt phthalocyanine modified electrode. Environ Sci Technol 46:5198–5204
Wang X, Tian Y, Liu H et al (2019) Optimizing the performance of organics and nutrient removal in constructed wetland–microbial fuel cell systems. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2018.11.005
Cusick RD, Logan BE (2012) Phosphate recovery as struvite within a single chamber microbial electrolysis cell. Bioresour Technol 107:110–115
Gude VG (2016) Wastewater treatment in microbial fuel cells - an overview. J Clean Prod 122:287–307
Elabed A, El Abed S, Ibnsouda S, Erable B (2019) Sustainable approach for tannery wastewater treatment: bioelectricity generation in bioelectrochemical systems. Arab J Sci Eng 44:10057–10066
Sevda S, Yuan H, He Z, Abu-Reesh IM (2015) Microbial desalination cells as a versatile technology: functions, optimization and prospective. Desalination 371:9–17
Yuan L, Yang X, Liang P et al (2012) Capacitive deionization coupled with microbial fuel cells to desalinate low-concentration salt water. Bioresour Technol 110:735–738
Chen X, Xia X, Liang P et al (2011) Stacked microbial desalination cells to enhance water desalination efficiency. Environ Sci Technol 45:2465–2470
Watson VJ, Logan BE (2011) Analysis of polarization methods for elimination of power overshoot in microbial fuel cells. Electrochem Commun 13:54–56
Pandit S, Sarode S, Das D (2017) Fundamentals of microbial desalination cell. In: Microbial fuel cell: a bioelectrochemical system that converts waste to watts. 18:353–371
Luo H, Xu P, Jenkins PE, Ren Z (2012) Ionic composition and transport mechanisms in microbial desalination cells. J Membr Sci 409–410:16–23
Harnisch F, Schröder U (2010) From MFC to MXC: chemical and biological cathodes and their potential for microbial bioelectrochemical systems. Chem Soc Rev 39:4433–4448
Borjas Z, Esteve-Núñez A, Ortiz JM (2017) Strategies for merging microbial fuel cell technologies in water desalination processes: start-up protocol and desalination efficiency assessment. J Power Sources. https://doi.org/10.1016/j.jpowsour.2017.02.052
Luo H, Jenkins PE, Ren Z (2011) Concurrent desalination and hydrogen generation using microbial electrolysis and desalination cells. Environ Sci Technol 45:340–344
Zhang L, Zhu X, Kashima H et al (2015) Anolyte recirculation effects in buffered and unbuffered single-chamber air-cathode microbial fuel cells. Bioresour Technol 179:26–34
Kim Y, Logan BE (2013) Microbial desalination cells for energy production and desalination. Desalination 308:122–130
Oguz Koroglu E, Civelek Yoruklu H, Demir A, Ozkaya B (2019) Scale-up and commercialization issues of the MFCs. In: Microbial electrochemical technology
Prestigiacomo C, Fernandez-Marchante CM, Fernández-Morales FJ et al (2016) New prototypes for the isolation of the anodic chambers in microbial fuel cells. Fuel 181:704–710
Kuntke P, Sleutels THJA, Saakes M, Buisman CJN (2014) Hydrogen production and ammonium recovery from urine by a microbial electrolysis cell. Int J Hydrog Energy 39:4771–4778
Papillon J, Ondel O, Maire É (2021) Scale up of single-chamber microbial fuel cells with stainless steel 3D anode: Effect of electrode surface areas and electrode spacing. Bioresour Technol Reports 13:100632
Pasternak G, Greenman J, Ieropoulos I (2017) Self-powered, autonomous biological oxygen demand biosensor for online water quality monitoring. Sensors Actuators B Chem 244:815–822
Koroglu EO, Yoruklu HC, Demir A, Ozkaya B (2018) Scale-up and commercialization issues of the MFCs: challenges and implications. In: Biomass, biofuels, biochemicals: microbial electrochemical technology: sustainable platform for fuels, chemicals and remediation. Elsevier, 565–583
Kim B, Chang IS, Dinsdale RM, Guwy AJ (2021) Accurate measurement of internal resistance in microbial fuel cells by improved scanning electrochemical impedance spectroscopy. Electrochim Acta 366:137388
Ghasemi B, Yaghmaei S, Ghaderi S et al (2020) Effects of chemical, electrochemical, and electrospun deposition of polyaniline coatings on surface of anode electrodes for evaluation of MFCs’ performance. J Environ Chem Eng 8:104039
Wei J, Liang P, Huang X (2011) Recent progress in electrodes for microbial fuel cells. Bioresour Technol 102:9335–9344
Nwaokocha CN, Giwa SO, Layeni AT et al (2020) Microbial fuel cell: bio-energy production from Nigerian corn starch wastewater using iron electrodes. Mater Today Proc. https://doi.org/10.1016/j.matpr.2020.09.345
Peera SG, Maiyalagan T, Liu C et al (2020) A review on carbon and non-precious metal based cathode catalysts in microbial fuel cells. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2020.07.252
Stoll ZA, Ma Z, Trivedi CB et al (2016) Sacrificing power for more cost-effective treatment: a techno-economic approach for engineering microbial fuel cells. Chemosphere 161:10–18
Serra PMD, ES A (2021) Sourcing power with microbial fuel cells: a timeline. J Power Sources 482:228921
Yaqoob AA, Ibrahim MNM, Rodríguez-Couto S (2020) Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): an overview. Biochem Eng J 164:107779
Babauta JT, Kerber M, Hsu L et al (2018) Scaling up benthic microbial fuel cells using flyback converters. J Power Sources 395:98–105
López-Hincapié JD, Picos-Benítez AR, Cercado B et al (2020) Improving the configuration and architecture of a small-scale air-cathode single chamber microbial fuel cell (MFC) for biosensing organic matter in wastewater samples. J Water Process Eng 38:101671
Neethu B, Ghangrekar MM (2017) Electricity generation through a photo sediment microbial fuel cell using algae at the cathode. Water Sci Technol 76:3269–3277
Narayanasamy S, Jayaprakash J (2018) Improved performance of Pseudomonas aeruginosa catalyzed MFCs with graphite/polyester composite electrodes doped with metal ions for azo dye degradation. Chem Eng J 343:258–269
Nguyen DT, Taguchi K (2019) Enhancing the performance of E. coli-powered MFCs by using porous 3D anodes based on coconut activated carbon. Biochem Eng J 151:107357
Tajdid Khajeh R, Aber S, Zarei M (2020) Comparison of NiCo2O4, CoNiAl-LDH, and CoNiAl-LDH@NiCo2O4 performances as ORR catalysts in MFC cathode. Renew Energy 154:1263–1271
Theodosiou P, Greenman J, Ieropoulos I (2019) Towards monolithically printed Mfcs: development of a 3d-printable membrane electrode assembly (mea). Int J Hydrog Energy 44:4450–4462
He W, Wei J, Huang T, Zhang E (2020) Enhanced electricity production in single-chamber MFCs with air cathodes decorated by Fe–N–C catalysts derived from 5H-dibenz [b,f] azepine-5-carboxamide (Carbamazepine). Int J Hydrog Energy 45:17525–17532
Juang DF, Lee CH, Hsueh SC (2012) Comparison of electrogenic capabilities of microbial fuel cell with different light power on algae grown cathode. Bioresour Technol 123:23–29
Bakonyi P, Koók L, Rózsenberszki T et al (2020) Development and application of supported ionic liquid membranes in microbial fuel cell technology: a concise overview. Membranes (Basel) 10:10011016
Khalili HB, Mohebbi-Kalhori D, Afarani MS (2017) Microbial fuel cell (MFC) using commercially available unglazed ceramic wares: low-cost ceramic separators suitable for scale-up. Int J Hydrog Energy 42:8233–8241
Das I, Das S, Dixit R, Ghangrekar MM (2020) Goethite supplemented natural clay ceramic as an alternative proton exchange membrane and its application in microbial fuel cell. Ionics (Kiel). https://doi.org/10.1007/s11581-020-03472-1
You J, Fan H, Winfield J, Ieropoulo IA (2020) Complete microbial fuel cell fabrication using additive layer manufacturing. Molecules. https://doi.org/10.3390/molecules25133051
Nagar H, Aniya V (2020) Microporous material induced composite membrane with reduced oxygen leakage for MFC application. J Environ Chem Eng 8:104117
Koroglu EO, Yoruklu HC, Demir A, Ozkaya B (2018) Scale-up and commercialization issues of the MFCs: challenges and implications. In: Biomass, biofuels, biochemicals: microbial electrochemical technology: sustainable platform for fuels, chemicals and remediation. Elsevier, 565–583
Taşkan E, Bulak S, Taşkan B et al (2019) Nitinol as a suitable anode material for electricity generation in microbial fuel cells. Bioelectrochemistry 128:118–125
Ardakani MN, Badalians Gholikandi G (2020) Microbial fuel cells (MFCs) in integration with anaerobic treatment processes (AnTPs) and membrane bioreactors (MBRs) for simultaneous efficient wastewater/sludge treatment and energy recovery - a state-of-the-art review. Biomass Bioenergy 141
Li C, Song Y, Wang X, Zhang Q (2020) Synthesis, characterization and application of S-TiO2/PVDF-g-PSSA composite membrane for improved performance in MFCs. Fuel 264:116847
Yang W, Li J, Fu Q et al (2021) Minimizing mass transfer losses in microbial fuel cells: theories, progresses and prospectives. Renew Sustain Energy Rev 136:110460
Kardi SN, Ibrahim N, Rashid NAA, Darzi GN (2019) Investigating effect of proton-exchange membrane on new air-cathode single-chamber microbial fuel cell configuration for bioenergy recovery from Azorubine dye degradation. Environ Sci Pollut Res 26:21201–21215
Fischer F, Sugnaux M, Savy C, Hugenin G (2018) Microbial fuel cell stack power to lithium battery stack: pilot concept for scale up. Appl Energy 230:1633–1644
Tremouli A, Greenman J, Ieropoulos I (2018) Investigation of ceramic MFC stacks for urine energy extraction. Bioelectrochemistry 123:19–25
Huang S, Zhang J, Pi J et al (2021) Long-term electricity generation and denitrification performance of MFCs with different exchange membranes and electrode materials. Bioelectrochemistry 140:107748
Chakraborty I, Sathe SM, Dubey BK, Ghangrekar MM (2020) Waste-derived biochar: applications and future perspective in microbial fuel cells. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.123587
He L, Du P, Chen Y et al (2017) Advances in microbial fuel cells for wastewater treatment. Renew Sust Energ Rev 71:388–403
Acknowledgements
The authors of this article would like to express their gratitude to the Association of Chemical Engineers, BITS Pilani, Hyderabad Campus, for facilitating and giving them the opportunity to conduct the studies and research involved in the making of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siddiqui, S., Bhatnagar, P., Dhingra, S. et al. Wastewater treatment and energy production by microbial fuel cells. Biomass Conv. Bioref. 13, 3569–3592 (2023). https://doi.org/10.1007/s13399-021-01411-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01411-2