Abstract
Worldwide energy consumption has increased drastically over the years due to the increasing population and economic growth. Modern energy services, electricity in particular, are a key enabler of economic and social development of a country. Rapid industrialization has led to the accelerated use of fossil fuels limiting their accessibility and thus causing difficulty to exploit these sources in the future. Thus, to meet the energy demands of the growing population, research has been focussed for the development of clean and green alternative technologies for energy generation. Bioelectrochemical systems (BES) represent such green technologies that utilize biocatalysts for bioenergy generation using wastes and wastewaters as feedstock (Pant et al. 2012a; Rabaey et al. 2009; Sleutels et al. 2012). BESs not only lead to the sustainable renewable energy generation but also help in reducing the costs incurred in waste treatment systems. Over the last couple of years, many possible applications for BESs have been emerged with respect to the oxidation and/or reduction of organic matter at anode and cathode respectively. Among the BESs, most widely studied are the microbial fuel cells (MFCs) that convert chemical energy to electrical energy through microbial oxidation of biodegradable organic matters (Oh et al. 2010; Wang and Ren 2013; Zhang 2012).
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
15.1 Introduction
Worldwide energy consumption has increased drastically over the years due to the increasing population and economic growth. Modern energy services, electricity in particular, are a key enabler of economic and social development of a country. Rapid industrialization has led to the accelerated use of fossil fuels limiting their accessibility and thus causing difficulty to exploit these sources in the future. Thus, to meet the energy demands of the growing population, research has been focussed for the development of clean and green alternative technologies for energy generation. Bioelectrochemical systems (BES) represent such green technologies that utilize biocatalysts for bioenergy generation using wastes and wastewaters as feedstock (Pant et al. 2012a; Rabaey et al. 2009; Sleutels et al. 2012). BESs not only lead to the sustainable renewable energy generation but also help in reducing the costs incurred in waste treatment systems. Over the last couple of years, many possible applications for BESs have been emerged with respect to the oxidation and/or reduction of organic matter at anode and cathode respectively. Among the BESs, most widely studied are the microbial fuel cells (MFCs) that convert chemical energy to electrical energy through microbial oxidation of biodegradable organic matters (Oh et al. 2010; Wang and Ren 2013; Zhang 2012).
Different configurations of MFCs have been employed for simultaneous bioremediation and wastewater treatment along with electricity generation. The unique microbial species used in these systems are known as electroactive bacteria (EAB) that have the ability to donate/accept electrons in the surroundings through their cell surfaces. Several different EABs have been reported for their ability to treat specific pollutant present in the organic wastes. In this chapter, emphasis is laid on the basic principles of MFCs for electricity generation by EABs through degradation of organic matter. Certain specific examples of MFCs for bioremediation and wastewater treatment applications have been described and the current challenges and limitations of MFC technology along with the future directions have been briefly discussed.
15.2 Basic Principles of Power Generation from Organic Wastes in MFC
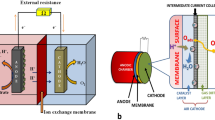
The basic principles of power generation in MFC have been illustrated in detail in Chap. 2. In general, a typical MFC mainly comprises anode and cathode chambers which are separated by an ion exchange membrane (Fig. 15.1). Usually the bacterial degradation of organic matter occurs at anode leading to the generation of protons, electrons and CO2. The protons diffuse through the ion-selective membrane creating a potential difference between the anode and cathode. This in turn causes the electrons to flow and traverse through the external circuit to the cathode where they recombine with the protons and the terminal electron acceptor (usually O2) to form water and electricity. The specific electrode potential depends upon the separate half-cell reactions occurring at the anode and the cathode. These electrode potentials can be deduced via Nernst equation similar to an electrochemical cell (Logan et al. 2006).
In general, the anodic and cathodic reactions of an MFC with acetate as electron donor and O2 as the terminal electron acceptor can be given as:
Thus, a cell voltage of E cell = 1.1 V (E cat – E an) can be obtained from an MFC using acetate as substrate and O2 as electron acceptor.
Apart from O2, certain bacteria can utilize other inorganic compounds as electron acceptors such as nitrate, sulphate, manganese etc. in anaerobic conditions. By utilizing such microbes at cathode, remediation of inorganic pollutant compounds can be achieved. The bioremediation of specific pollutants using MFC technology will be described in details in subsequent sections.
15.3 Electrode Mechanisms
Depending upon the type of pollutant and the electroactive bacteria (EAB), both anode and cathode can be used for bioremediation purpose. Different mechanisms prevail at the electrode surfaces that lead to oxidation/reduction of the organic substrates. As described in Chap. 5, several extracellular electron transfer mechanisms of EABs have been elucidated that directly or indirectly interact with the electrode surfaces to transfer or uptake electrons obtained from the organic pollutant. Depending upon the redox reaction involved, the EABs can either be electrode oxidizing bacteria (at anode) and electrode reducing bacteria (at cathode). Apart from microbial metabolic reactions, partial chemical and electrochemical reactions also prevail in the anode and cathode chambers that influence the pollutant removal and wastewater treatment processes (Venkata Mohan and Srikanth 2011).
15.3.1 Reactions at Anode
Oxidation of substrates at anode can be microbialy catalysed as well as chemically or electrochemically induced. These reactions at anode and the substrate degradation are dependent upon the cathodic reduction processes and the terminal electron acceptor. Oxygen is the most widely used electron acceptor at cathode due to its high electronegativity. At anode, the substrates (pollutant/wastewater) are oxidized by microbial electron transfer reactions which lead to the production of intermediate reductant contaminants or convert to CO2, protons and electrons. In addition, due to the presence of strong oxidizing agents a potential difference is created between anode and cathode which in turn increases the redox potential of the system and induces chemical or electrochemical reactions. These chemical/electrochemical reactions can cause partial oxidation of the substrate leading to the formation of intermediate reductant compounds. The intermediate contaminants/compounds can further be microbialy oxidized or can act as mediators for electron transfer between the bacteria and anode surface (Venkata Mohan and Srikanth 2011). Apart from microbial and induced chemical/electrochemical reactions, electrode sorption also has shown to play an important role for the conversion and removal of trace organic pollutants (Wang et al. 2015a, b). Usually, due to the presence of different types of contaminants present in the wastewater, a combination of different mechanisms as described above can occur simultaneously. A general schematic of possible reactions occurring at the anode and cathode of MFC is shown in Fig. 15.2.
Schematic illustration of the possible bio-electrochemical reactions occurring at anode and biocathode during MFC operation: (a) Microbial oxidation of substrate; (b) Formation of intermediate redox compounds by chemical/electrochemical/microbial induced reactions; (c) Anaerobic oxidation/reduction of intermediate redox compounds by microbes; (d) Electrochemical reduction; (e) Microbial reduction using aerobic or anaerobic biocathode; (f) Formation of intermediate redox compounds by microbial/chemical/electrochemical induced reactions; (g) Microbial oxidation/reduction of intermediate redox compounds by aerobic biocathode; (h) Microbial oxidation/reduction of intermediate redox compounds by anaerobic biocathode; and (i) Formation of intermediate redox compounds by microbial metabolism and further reduction using chemical/electrochemical reactions
15.3.2 Reactions at Cathode
The electrons obtained by the substrate oxidation at anode flow via the external circuit to cathode where the reduction reaction takes place in the presence of terminal electron acceptors (TEA). Depending upon the type of MFC system and its application, TEA can be varied at the cathode. As described previously, oxygen is the most commonly used TEA as it is highly electronegative, abundant in nature and sustainable due to its reduction product being water (Dopson et al. 2016). It is observed that in the absence of O2 environmental contaminants such as nitrate, hydrocarbons, azo dyes etc. can be utilized as potential electron acceptors in MFC systems thereby resulting in effective remediation of such pollutant containing waste streams at cathode (Pant et al. 2012).
Similar to anode, depending upon the microenvironment, different reactions are possible at cathode (Fig. 15.2). The microenvironment at cathode can be chosen based on the type and nature of the pollutant to be treated. Generally, in the absence of oxygen, the reduction reaction is catalysed by microbial metabolic reactions which can be either aerobic or anaerobic in nature. Like in anode, the prevailing chemical/electrochemical reactions can also lead to partial reduction of the pollutant resulting in an intermediate oxidant formation. This intermediate oxidant again can further be reduced microbially or can induce certain chemical/electrochemical reaction to generate product of interest. The energy output and treatment efficiency for this process, however, varies with the type of reaction occurring at cathode.
15.4 MFC Configurations
Several designs and configurations of MFCs have been developed for simultaneous wastewater treatment, pollutant remediation and bioelectricity generation. In general, they can be broadly classified into double chambered, single chambered, U-tube, upflow and stack MFCs (Fig. 15.3). The type of MFC used depends upon the purpose of its application. The most primitive MFC design is a double chambered MFC in which the anode and cathode chambers are separated by a cation or proton exchange membrane. These systems have high internal resistance and complex design and, therefore, scale up of double chambered MFCs is challenging.
To minimize the complexity and cost of the process, single chambered air-cathode MFCs were developed that could obtain high volumetric power densities (Logan et al. 2006). In this configuration, a single chamber (anode) is attached to the membrane cathode assembly (MCA) such that one side of the cathode is bonded to the membrane while the other side is air-facing. This design negates the use of external air supply to the cathode and thus proves to be cost effective.
For bulk-scale wastewater treatment and simultaneous electricity generation, upflow and U-tube MFCs are considered to be a promising configuration (Deng et al. 2010). This configuration is a hybrid of upflow anaerobic sludge blanket (UASB) and MFC that combines the advantages of both the systems. In this design, the substrate is fed continuously to the reactor from the bottom of the anodic chamber such that an up-flow hydraulic pattern is created. Due to this, continuous mixing of the anolyte is ensured and thus the use of a mechanical agitator can be avoided. Upflow MFCs have shown high power outputs as compared to the single chambered.
A scalable configuration of MFCs which has been used for practical application is the Stack MFCs. In this configuration, several MFCs are interconnected either in series or in parallel. Since current is an extensive property with respect to the surface area of electrodes, stack MFCs are considered to be the most appropriate configuration for obtaining high voltage and current outputs along with higher wastewater treatment efficiencies (Aelterman et al. 2006; Pasupuleti et al. 2015).
15.5 Microbial Remediation Using MFC-Based Technologies
Bioremediation is the process of consumption or degradation of environmental pollutants by the use of naturally occurring or deliberately introduced microorganisms to clean a polluted site. Though bioremediation process is considered to be an efficient, cost effective and environmentally friendly technology, the major challenge for this process is the lack of contact between the pollutant and the microbes and the slow kinetics (Wang et al. 2015b). MFC-based technologies emerge as alternative bioremediation processes by utilizing EABs to oxidize/reduce pollutant at a specific site. Sometimes the pollutants themselves can act as mediators in electron transfer and can be treated in the process. Over the past few decades, several studies related to the removal of specific environmental pollutants such as azo dyes, polycyclic hydrocarbons and its derivatives, heavy metals, radioactive compounds etc. using MFC-based technologies have been explored (Table 15.1). These studies reflect the practical feasibility of MFC-based technologies for real-time removal of environmental pollutants at contaminated sites in a sustainable and economical manner.
15.5.1 MFC-Assisted Biodegradation of Azo Dyes
Azo dyes are aromatic compounds comprising one or more azo groups (−N=N–) and are the most widely used synthetic dyes used in commercial applications (Solanki et al. 2013). These compounds when degraded result in production of mutagenic or carcinogenic degraded products and if released into the environment can pose serious threat to human health and the natural environment (Chen 2015). These dyes are water soluble and highly stable in nature and at present harsh physicochemical methods (coagulation, flocculation, adsorption, membrane filtration etc.) are used for their removal from industrial effluents (Pandey et al. 2007). These physicochemical methods are energy and cost intensive and they often lead to the production of secondary waste streams that need further treatment and/or disposal. Biological processes for azo dye degradation have also been studied extensively using enzymes or whole cells (aerobic/anaerobic) and prove to be effective alternatives to the physicochemical methods of decolourization of wastewater. Nevertheless, the huge cost of enzymes, product inhibition, incomplete degradation and slow kinetics are the major challenges for the application of these processes at commercial scale. Enzymatic decolourization is now widely used for the decolourization of dye wastewater. However, this method is also facing several problems such as cost of enzymes, enzyme stability and product inhibition.
Recently, MFCs have been employed for the application of azo dye treatment by utilizing both the anode and cathode chambers (Chen 2015; Solanki et al. 2013). In anode, anaerobic degradation of azo dye occurs via co-metabolism i.e. in the presence of another organic compound (carbon source) which acts as a primary or co-substrate. The EABs utilize primary substrate as electron donor and a portion of electron released are used to generate electricity while the other portion is utilized for azo dye reduction thereby competing with anode for electrons. Apart from co-metabolism, direct anaerobic degradation of azo dyes has also been reported in MFCs in the absence of other organic compounds (Solanki et al. 2013). In such systems dye decolourization occurs via breakdown of the azo bond at the anode while complete degradation of intermediates occurs at cathode. Anaerobic degradation of various dyes such as congo red (Cao et al. 2010), active brilliant red (Sun et al. 2009), acid orange (Mu et al. 2009) etc. have been reported at anode with 75–90% removal efficiencies (Solanki et al. 2013).
Azo dyes can also be degraded in the cathode chamber by receiving electrons from the cathode electrode. Such reactions are already well established for electrochemical cells in which the chromophoric linkage of azo dyes is reduced to degradable colourless amines. Similar mechanisms can be employed in MFCs systems in which the pollutants with high redox potentials such as nitro-aromatic compounds, metal ions like manganese (VII), chromium (VI) or uranium (VI) etc. can be used and treated. The feasibility of utilizing different toxic azo dyes as electron acceptors at cathode was demonstrated by Liu et al. Several dyes such as methyl orange, orange I, orange II etc. were studied for the degradation in cathode (Liu et al. 2009). The mechanisms of dye degradation in cathode are similar to the anaerobic anodic degradation. However, additional protons and electrons are transferred to the cathode via the membrane and the external circuit respectively which can also be utilized for the degradation of dye.
The performance of an MFC for azo dye degradation depends on several factors such as type and structure of dye used, its concentration, operating pH, wastewater quality, external resistance used etc. (Solanki et al. 2013). These factors not only influence the degradation process but also affect power generation capacity of MFC system as a whole.
15.5.2 Bioremediation of Hydrocarbons and Their Derivatives
Organic compounds comprising hydrogen and carbon are known as hydrocarbons while their derivatives have a functional group in place of hydrogen atom. Major hydrocarbon pollutants include BTEX (benzene, toluene, ethylbenzene and xylenes), PAHs (polycyclic aromatic hydrocarbons) and TPHs (total petroleum hydrocarbons) which impose serious health and environmental concerns and thus require to be eliminated. BTEXs are usually found in petroleum derivatives such as petrol (gasoline) and have harmful effects on the central nervous system of humans. They also lead to the contamination of soil and groundwater that are near to the petroleum and/or natural gas production sites. The amount of BTEX at a site is used to assess the relative risk or seriousness of contamination at that particular site. PAHs consist of two or more fused benzene rings and/or pentacyclic molecules arranged in various structural configurations. Due to their low water solubility they can persist in the environment for longer duration. Though they are found in ubiquitous environments, they are most prevalent contaminants in soils (Sherafatmand and Ng 2015). TPHs are mixtures of hydrocarbons that are found in crude oil and can contaminate the environment. They mainly comprise hexane, benzene, toluene, xylenes, naphthalene, fluorine, gasoline constituents, mineral oils etc. Like BTEXs, some TPH compounds can affect the central nervous system or can cause serious effects on the blood, immune system, lungs, skin and eyes. Some TPH compounds have also been shown to affect reproduction and the developing foetus in animals. At present, all the hydrocarbon pollutants (BTEXs, PAHs and TPHs) and their derivatives are degraded using different bioremediation techniques such as biosparging, biostimulation, bioaugmentation etc. However, these techniques have several disadvantages such as low kinetics, low contact between the microbe and the pollutant, competition for survival between the new and the already present microbes etc. (Wang et al. 2015a).
Several studies have suggested MFCs to be an alternate remediation technology that could help mitigate the problems associated with the existing bioremediation techniques (Morris and Jin 2008; Sherafatmand and Ng 2015; Wang et al. 2012). MFCs can couple the hydrocarbon degradation to energy production (in the form of electricity) by employing electrogenic bacteria that could utilize hydrocarbons as electron donors at anode. For this purpose, major studies have reported the use of sediment MFC (SMFC) that utilizes indigenous microbes present in the soil/sediments to remove organic compounds. SMFCs typically consist of an anode buried inside the soil at the site of interest and a cathode in the top of the soil exposed to air (De Schamphelaire et al. 2008). Sherafatmand and Ng reported bioremediation of PAHs in water originated from soil with consistent power generations of 6.02 ± 0.34 and 3.63 ± 0.37 mW/m2 by the aerobic and anaerobic SMFC respectively. The bioremediation capabilities of 41.7%, 31.4% and 36.2% removal of naphthalene, acenaphthene and phenanthrene, respectively in the aerobic environment and 76.9%, 52.5% and 36.8%, respectively in the anaerobic environment were achieved (Sherafatmand and Ng 2015). Wang et al. reported the use of U-tube MFCs for enhanced degradation of TPHs. They reported the degradation rates to be enhanced by 120% with simultaneous 125 ± 7 C of charge output (0.85 ± 0.05 mW/m2 in the tested period (25 days). These studies suggest that utilizing MFCs for hydrocarbon remediation is the most successful technology nearing commercialization with several pilot and field studies as compared to other applications (Wang et al. 2015a).
15.5.3 Removal of Heavy Metals
Heavy metals are group of metals and metalloids (such as Cd, Cr, Cu, Ni, As, Pb, Zn etc.) that have atomic density of greater than 4000 kg/m3. They are used extensively in industrial, medical and household applications. However, when exposed in the environment they can pose various health and environmental concerns since they are not biodegradable and can accumulate in living tissues causing serious diseases and disorders (Wang et al. 2015a, b). Due to the high market value of these metals, studies have been focused on recovery of these metals rather than their degradation. Numerous approaches have been studied for the development of cheaper and more effective technologies for heavy metal recovery from contaminated wastewater such as adsorption, membrane separation (including ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO), electrotreatments (such as electrodialysis), photocatalytic processes etc. (Barakat 2011). These conventional methods are energy intensive and become ineffective if metals concentrations are below 1–100 mg/L (Barakat 2011).
MFCs appear to be effective in recovering heavy metals from wastewater (Mathuriya and Yakhmi 2014). Metal ions can be reduced and deposited by bacteria by utilizing both anode and cathode chambers of MFC. In principle, metal pollutants can be recovered at anode by the oxidative action of microbes while they can serve as alternative electron acceptors at cathode in place of oxygen. Nitrate, trichloroethene, perchlorate etc. have been demonstrated as effective electron acceptors in the MFC cathode chamber (Mathuriya and Yakhmi 2014). Different mechanisms have been elucidated for simultaneous wastewater treatment and heavy metal recovery in MFCs (Wang et al. 2015a, b) which include:
-
1.
Metal with a redox potential higher than the MFC anode potential can be spontaneously reduced e.g. Au(III), Ag(I), Cu(II) etc.
-
2.
Metals with lower redox potentials can be recovered by applying an external power supply (microbial electrolysis cell) to force the electrons to travel from the anode to the abiotic cathode e.g. Ni(II), Cd(II), Zn(II) etc.
-
3.
Microbial reduction of metal oxides on the cathode with or without using an external potential.
The recovery of metallic species in MFCs has several benefits as compared to other conventional methods such as eliminating the need of external energy input for the treatment process, recovery of metals present in lower concentrations etc. Nevertheless, metals with low redox potentials still require stringent operating conditions and external input to drive the reaction in MFCs and thus extensive research is required to optimize and enhance the process efficiency.
15.5.4 Other Pollutants
Apart from the applications described above, MFCs have also been employed to treat several other pollutants such as chlorinated organic compounds, perchlorate reduction, sulfide removal, trace organic compounds etc. (Pant et al. 2012; Wang et al. 2015a, b). These pollutants can be effectively removed in MFCs since they provide a unique environment where both oxidation and reduction reactions can occur simultaneously along with the different microbial reactions as described in Fig. 15.1. Though most of these studies were conducted in lab scale, successful demonstration of MFCs for environmental remediation in pilot scale have also been reported (Wang et al. 2015a, b). Apart from treatment of one specific pollutant at a time, different contaminants that co-exist in soil, sediment or groundwater can also be removed using MFC technology though more studies are required to understand the mechanisms of treatment under such conditions.
15.6 Organic Wastes and Wastewater as Potential Feedstocks for MFCs
Over the past few decades, numerous studies have reported the application of MFCs to treat wastes and wastewaters with simultaneous electricity generation (Pant et al. 2012c). In these systems, the organic content is degraded with the help of electrogenic bacteria which convert the chemical energy of the organic waste/wastewater directly into electricity. Removal efficiency as high as 95% have been reported so far. MFCs prove to be attractive technologies for the waste/wastewater treatment as they reduce the input energy requirement as compared to the conventional wastewater treatment technologies. They also produce less sludge during the treatment making it environmentally friendly process. Several solid wastes and wastewaters like food wastes, cattle manure, domestic, industrial, and agricultural wastewaters have been studied in MFCs for bioelectricity generation which are elucidated in subsequent sections.
15.6.1 Solid Residual Wastes
Solid residual wastes usually cannot be reused, recycled or composted and need stringent disposal technologies such as landfill and incineration. A strategy to remove these materials and products from the waste stream is the use of MFC-based technologies. The primary goal is to obtain high organic removal rather than achieving higher power outputs. However, to make the MFC system self-sufficient, the exploitation of MFC for simultaneous power generation and waste treatment is necessary. The solid waste residues are majorly composed of complex molecules such as cellulose and hemicellulose which can be actively utilized for bioelectricity generation. Due to the different operational conditions, reactor configurations, types of electrodes, membranes and microorganisms involved, it is difficult to compare the performances of MFCs. However, a rough approximation can be made to evaluate the performance in terms of volumetric power densities and removal efficiencies. Under different operational and experimental conditions of MFCs, the power densities achieved with different solid residual wastes such as corn stover, cattle manure, food and vegetable waste etc. range 2–100 W/m3 with COD removal efficiencies ranging from 40 to 90% (Table 15.2). These studies suggest that the energy-generating capacities of MFCs vary significantly, depending on the composition, strength and solution chemistry of wastes.
Complex substrates present in wastes require higher energy to break down as compared to simple substrates and thus in turn yield lower energy outputs as compared to pure substrates. Certain complex substrates like lignocellulosic biomass can be detrimental to electrogenic bacteria and may require the use of pretreatment strategies prior to substrate utilization by MFCs. Different pretreatment methods such as mechanical, thermal, chemical, biological and the combination of these have been reported in literature for the hydrolysis of complex substrates into simple sugars or low-molecular weight compounds (Ariunbaatar et al. 2014). These hydrolysis products are ideal substrates to support bioelectricity generation in MFC systems.
15.6.2 Organic Wastewater
Wastewater treatment at present utilize aerobic and anaerobic biologic treatment technologies which include activated sludge, trickling filters, sequencing batch reactors (SBR), upflow anaerobic sludge blankets (UASB), anaerobic filters, constructed wetlands, or a combination of these. These technologies provide sufficient effluent quality. They are usually energy and cost intensive. MFCs on the other hand could be used for generating energy along with wastewater treatment and thus can offset the operational costs of wastewater treatment plants (Pant et al. 2012c). Apart from reducing the overall energy consumption in the treatment process, MFCs produce much less secondary sludge making the process environmental friendly. In fact while performing energy balance analysis for MFC systems, it was observed that theoretically the energy generated by MFC process is much higher as compared to the energy consumption (Kelly and He 2014). However, practically the energy recoveries from the MFCs have been much lower due to the prevailing internal resistance of the systems. The presence of alternate electron acceptors such as nitrate, nitrite, sulphate etc. can impair energy recovery from wastewater.
Several wastewater streams originating from different sources such as distillery waste, brewery waste, food processing waste, palm oil mill effluent etc. which are readily available and are rich in organic content have been used in MFCs for power generation (Table 15.3). Most of these studies have been conducted using mixed cultures so as to avoid stringent aseptic conditions and to their ability to utilize wide variety of substrates. Usually (Pant et al. 2012c) MFCs have shown to be effective in a COD range of 3–5 g/L. The absence of microbial growth inhibiting agents in these wastewaters adds up to an additional advantage. Since the COD concentration of the wastewater originating from industries or agriculture is much higher, integrated treatment systems coupling MFCs with other wastewater treatment technologies such as dark fermentation or anaerobic digestion processes etc. have also been employed to treat wastewaters and enhance the overall energy recovery (Pandit et al. 2014; Varanasi et al. 2015). In the integration process, the complex wastewater is first converted to volatile fatty acids (VFA) via the acidogenic pathway and these VFAs in turn are utilized in MFCs by the EAB. The integrated MFC technologies have proved to be better treatment systems achieving removal efficiencies of 70–90% with the overall energy recoveries 30–40% (Chookaew et al. 2014; Pasupuleti et al. 2015; Varanasi et al. 2015; Wang et al. 2011).
15.7 Challenges
Several attempts have been made in the development of various MFC technologies for enhanced energy recovery and simultaneous waste/wastewater treatment. Their practical real-field applications have been limited due to the associated operational and economic challenges. Although many pilot scale studies have been performed using real-time wastewater, the outputs obtained is far behind from those obtained with the bench studies under similar operational conditions (Du et al. 2007). Several operational factors such as limited membrane transport, ohmic losses, activation losses, unstable voltage for long duration of time, columbic losses etc. limit the performance of MFCs during large scale operation (Logan 2010). Though stacks cells provide appreciable outputs and stability, they are limited by the voltage reversal arising at high current densities. Growth of excessive unwanted biomass and biofouling of membranes can also severely affect the long-term performance of pilot-scale MFCs. To utilize MFCs in real-world applications such as environmental remediation and wastewater treatment, more flexible reactor configurations will be required that can adapt to the physico-chemical environment to which they are constructed. Use of expensive electrodes and membranes materials, their pretreatment methods, installation and operational costs, use of extra current collectors and precious metal catalysts etc. contribute to the economic constraints for the large scale production of these systems (Pant et al. 2012c). These constraints can be overcome by utilizing cheaper electrodes and treatment strategies. Use of aeration in cathode chamber also leads to the increasing costs of MFCs for wastewater treatment and utilizing biocathodes or single chambered air-cathode MFCs could be a possible solution for such systems. Implementing biocathodes not only reduces the costs but can also lead to the production of value added compounds (Huang et al. 2011b). Developing membrane-less MFCs is another strategy that could be used to further reduce the overall costs and improve the performance and treatment efficiencies of MFCs. However, in such a scenario, the distance between the electrodes might increase and thus in-turn the internal resistance of the systems may increase. Further research should be made to compare the performances of membrane and membraneless systems and if needed cheaper membrane materials or membrane cathode assemblies should be used which can further improve the performance of the system. Though at present the outputs of MFCs are far behind than the theoretical values, the ongoing research to tackle the above mentioned challenges can lead to successful commercialization of these technologies.
15.8 Conclusions and Future Prospects
Direct waste to energy conversion by employing MFC-based technologies appears to be the most promising solution to tackle the global energy and wastewater management related issues. The present chapter discusses in brief the recent advances made with respect to environmental remediation as well as waste/wastewater treatment by employing MFCs. Several studies suggest that MFCs achieve as high as 90% removal efficiencies though the energy recoveries are poor. To make this process energy efficient, considerable attention must be given to the complex reactor configurations, type of electrodes, membranes and the external circuit components which collectively affect the internal resistance of the system. To avoid strict aseptic conditions, enrichment of electrogen-rich consortium is desirable. For achieving high energy recoveries from MFCs, pre-treatment of wastes and/or integration with existing wastewater treatment technologies like fermentation, anaerobic digestion, activated sludge process etc. appear to be more realistic, cost-efficient and feasible. With the recent developments of novel cost effective materials and cell components, superior performance is expected from MFCs that could expand their applicability for real-field applications. Wide applications of MFCs have emerged using biocathodes which include bioproduct development and its recovery along with power generation. It is anticipated that with these upcoming improvements and the few pilot-scale studies, the commercialization of MFCs is the next step for a sustainable and economical bioenergy production.
References
Aelterman, P., Rabaey, K., & Verstraete, W. (2006). Continuous electricity generation at high voltages and currents using stacked MFCs. Environmental Science & Technology, 40, 3388–3394.
An, B.-M., Song, Y.-H., Shin, J.-W., & Park, J.-Y. (2015). Two-chamber MFC to simultaneously remove ethanolamine and nitrate. Desalination and Water Treatment, 57(17), 7866–7873.
Ariunbaatar, J., Panico, A., Esposito, G., Pirozzi, F., & Lens, P. N. L. (2014). Pretreatment methods to enhance anaerobic digestion of organic solid waste. Applied Energy, 123, 143–156.
Barakat, M. A. (2011). New trends in removing heavy metals from industrial wastewater. Arabian Journal of Chemistry, 4, 361–377.
Baranitharan, E., Khan, M. R., Prasad, D. M. R., Teo, W. F. A., Tan, G. Y. A., & Jose, R. (2015). Effect of biofilm formation on the performance of MFC for the treatment of palm oil mill effluent. Bioprocess and Biosystems Engineering, 38, 15–24.
Behera, M., Jana, P. S., More, T. T., & Ghangrekar, M. M. (2010). Bioelectrochemistry Rice mill wastewater treatment in MFCs fabricated using proton exchange membrane and earthen pot at different pH. Bioelectrochemistry, 79, 228–233.
Bonmatí, A., Sotres, A., Mu, Y., Rozendal, R., & Rabaey, K. (2013). Oxalate degradation in a bioelectrochemical system: Reactor performance and microbial community characterization. Bioresource Technology, 143, 147–153.
Cao, Y., Hu, Y., Sun, J., & Hou, B. (2010). Explore various co-substrates for simultaneous electricity generation and Congo red degradation in air-cathode single-chamber MFC. Bioelectrochemistry, 79, 71–76.
Catal, T., Fan, Y., Li, K., Bermek, H., & Liu, H. (2011). Utilization of mixed monosaccharides for power generation in MFCs. Journal of Chemical Technology and Biotechnology, 86, 570–574.
Chen, B. (2015). Exploring MFC-assisted bioremediation of textile dyes. Energy Conversion Energy Reviews, 1(1), 11–18.
Cheng, H.-Y., Liang, B., Mu, Y., Cui, M.-H., Li, K., Wu, W.-M., & Wang, A.-J. (2015). Stimulation of oxygen to bioanode for energy recovery from recalcitrant organic matter aniline in MFCs (MFCs). Water Research, 81, 72–83.
Chookaew, T., Prasertsan, P., & Ren, Z. J. (2014). Two-stage conversion of crude glycerol to energy using dark fermentation linked with MFC or microbial electrolysis cell. New Biotechnology, 31, 179–184.
De Schamphelaire, L., Rabaey, K., Boeckx, P., Boon, N., & Verstraete, W. (2008). Outlook for benefits of sediment MFCs with two bio-electrodes. Microbial Biotechnology, 1, 446–462.
Deng, Q., Li, X., Zuo, J., Ling, A., & Logan, B. E. (2010). Power generation using an activated carbon fiber felt cathode in an upflow MFC. Journal of Power Sources, 195, 1130–1135.
Dopson, M., Ni, G., & Sleutels, T. H. (2016). Possibilities for extremophilic microorganisms in microbial electrochemical systems. FEMS Microbiology Reviews, 40, 164–181.
Du, H., & Li, F. (2015). Size effects of potato waste on its treatment by MFC. Environmental Technology, 37(10), 1305–1313.
Du, Z., Li, H., & Gu, T. (2007). A state-of-the-art review on MFCs: A promising technology for wastewater treatment and bioenergy. Biotechnology Advances, 25, 464–482.
Fang, C., Min, B., & Angelidaki, I. (2011). Nitrate as an oxidant in the cathode chamber of a MFC for both power generation and nutrient removal purposes. Applied Biochemistry and Biotechnology, 164(4), 464–474.
Frattini, D., Falcucci, G., Minutillo, M., Ferone, C., Cioffi, R., & Jannelli, E. (2016). On the effect of different configurations in air-cathode MFCs fed by composite food waste for energy harvesting. Chemical Engineering Transactions, 49, 85–90 ISBN 978-88-95608-40-2.
Guo, W., Feng, J., Song, H., & Sun, J. (2014). Simultaneous bioelectricity generation and decolorization of methyl orange in a two-chambered MFC and bacterial diversity. Environmental Science and Pollution Research, 21, 11531–11540.
Hiegemann, H., Herzer, D., Nettmann, E., Lübken, M., Schulte, P., Schmelz, K.-G., Gredigk-Hoffmann, S., & Wichern, M. (2016). An integrated 45L pilot MFC system at a full-scale wastewater treatment plant. Bioresource Technology, 218, 115–122.
Hosseinpour, M., Asadi, M., Eliato, T. R., Vossoughi, M., & Alemzadeh, I. (2016). Ethylene glycol biodegradation in MFC. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 38(8), 1096–1102.
Huang, J., Yang, P., Guo, Y., & Zhang, K. (2011a). Electricity generation during wastewater treatment: An approach using an AFB-MFC for alcohol distillery wastewater. Desalination, 276, 373–378.
Huang, L., Regan, J. M., & Quan, X. (2011b). Electron transfer mechanisms, new applications, and performance of biocathode MFCs. Bioresource Technology, 102, 316–323.
Jayashree, C., Tamilarasan, K., Rajkumar, M., Arulazhagan, P., Yogalakshmi, K. N., Srikanth, M., & Banu, J. R. (2016). Treatment of seafood processing wastewater using upflow MFC for power generation and identification of bacterial community in anodic biofilm. Journal of Environmental Management, 180, 351–358.
Jong, B. C., Liew, P. W. Y., Juri, M. L., Kim, B. H., Mohd Dzomir, A. Z., Leo, K. W., & Awang, M. R. (2011). Performance and microbial diversity of palm oil mill effluent MFC. Letters in Applied Microbiology, 53(6), 660–667.
Karluvalı, A., Köroğlu, E. O., Manav, N., Çetinkaya, A. Y., & Özkaya, B. (2015). Electricity generation from organic fraction of municipal solid wastes in tubular MFC. Separation and Purification Technology, 156, 502–511.
Kelly, P. T., & He, Z. (2014). Understanding the application niche of MFCs in a cheese wastewater treatment process. Bioresource Technology, 157, 154–160.
Kiran Kumar, A., Venkateswar Reddy, M., Chandrasekhar, K., Srikanth, S., & Venkata Mohan, S. (2012). Endocrine disruptive estrogens role in electron transfer: Bio-electrochemical remediation with microbial mediated electrogenesis. Bioresource Technology, 104, 547–556.
Lee, D.-J., Liu, X., & Weng, H.-L. (2014). Sulfate and organic carbon removal by MFC with sulfate-reducing bacteria and sulfide-oxidising bacteria anodic biofilm. Bioresource Technology, 156, 14–19.
Li, X. M., Cheng, K. Y., & Wong, J. W. C. (2013). Bioelectricity production from food waste leachate using MFCs: Effect of NaCl and pH. Bioresource Technology, 149, 452–458.
Liu, L., Li, F., Feng, C., & Li, X. (2009). MFC with an azo-dye-feeding cathode. Applied Microbiology and Biotechnology, 85, 175–183.
Logan, B. E. (2010). Scaling up MFCs and other bioelectrochemical systems. Applied Microbiology and Biotechnology, 85, 1665–1671.
Logan, B. E., Hamelers, B., Rozendal, R., Schröder, U., Keller, J., Freguia, S., Aelterman, P., Verstraete, W., & Rabaey, K. (2006). MFCs: Methodology and technology. Environmental Science & Technology, 40, 5181–5192.
Luo, H., Liu, G., Zhang, R., & Jin, S. (2009). Phenol degradation in MFCs. Chemical Engineering Journal, 147, 259–264.
Luo, Y., Liu, G., Zhang, R., & Zhang, C. (2010). Power generation from furfural using the MFC. Journal of Power Sources, 195(1), 190–194.
Mathuriya, A. S., & Yakhmi, J. V. (2014). MFCs to recover heavy metals. Environmental Chemistry Letters, 12, 483–494.
Md Khudzari, J., Tartakovsky, B., & Raghavan, G. S. V. (2016). Effect of C/N ratio and salinity on power generation in compost MFCs. Waste Management, 48, 135–142.
Mohammadi Khalfbadam, H., Cheng, K. Y., Sarukkalige, R., Kaksonen, A. H., Kayaalp, A. S., & Ginige, M. P. (2016). A bio-anodic filter facilitated entrapment, decomposition and in situ oxidation of algal biomass in wastewater effluent. Bioresource Technology, 216, 529–536.
Mohan, S. V., & Chandrasekhar, K. (2011). Self-induced bio-potential and graphite electron accepting conditions enhances petroleum sludge degradation in bio-electrochemical system with simultaneous power generation. Bioresource Technology, 102, 9532–9541.
Mohanakrishna, G., Venkata Mohan, S., & Sarma, P. N. (2010). Bio-electrochemical treatment of distillery wastewater in MFC facilitating decolorization and desalination along with power generation. Journal of Hazardous Materials, 177, 487–494.
Morris, J. M., & Jin, S. (2008). Feasibility of using MFC technology for bioremediation of hydrocarbons in groundwater. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering, 43, 18–23.
Mu, Y., Rabaey, K., Rozendal, R. A., Yuan, Z., & Keller, J. (2009). Decolorization of Azo dyes in bioelectrochemical systems. Environmental Science & Technology, 43, 5137–5143.
Nimje, V. R., Chen, C.-Y., Chen, H.-R., Chen, C.-C., Huang, Y. M., Tseng, M.-J., Cheng, K.-C., & Chang, Y.-F. (2012). Comparative bioelectricity production from various wastewaters in MFCs using mixed cultures and a pure strain of Shewanella oneidensis. Bioresource Technology, 104, 315–323.
Oh, S. T., Kim, J. R., Premier, G. C., Lee, T. H., Kim, C., & Sloan, W. T. (2010). Sustainable wastewater treatment: How might MFCs contribute. Biotechnology Advances, 28, 871–881.
Pandey, A., Singh, P., & Iyengar, L. (2007). Bacterial decolorization and degradation of azo dyes. International Biodeterior and Biodegradation, 59, 73–84.
Pandit, S., Balachandar, G., & Das, D. (2014). Improved energy recovery from dark fermented cane molasses using MFCs. Frontiers of Chemical Science and Engineering, 8, 43–54.
Pant, D., Singh, A., Van Bogaert, G., Irving Olsen, S., Singh Nigam, P., Diels, L., & Vanbroekhoven, K. (2012). Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Advances, 2, 1248–1263.
Pant, D., Arslan, D., Van Bogaert, G., Gallego, Y. A., De Wever, H., Diels, L., & Vanbroekhoven, K. (2013). Integrated conversion of food waste diluted with sewage into volatile fatty acids through fermentation and electricity through a fuel cell. Environmental Technology, 34, 1935–1945.
Pasupuleti, S. B., Srikanth, S., Venkata Mohan, S., & Pant, D. (2015). Continuous mode operation of MFC stack with dual gas diffusion cathode design for the treatment of dark fermentation effluent. International Journal of Hydrogen Energy, 40, 12424–12435.
Rabaey, K., Angenent, L., & Schroder, U. (2009). Bioelectrochemical systems: From extracellular electron transfer to biotechnological application. London: IWA Publishing.
Rakoczy, J., Feisthauer, S., Wasmund, K., Bombach, P., Neu, T. R., Vogt, C., & Richnow, H. H. (2013). Benzene and sulfide removal from groundwater treated in a MFC. Biotechnology and Bioengineering, 110, 3104–3113.
Sevda, S., Dominguez-Benetton, X., Vanbroekhoven, K., De Wever, H., Sreekrishnan, T. R., & Pant, D. (2013). High strength wastewater treatment accompanied by power generation using air cathode MFC. Applied Energy, 105, 194–206.
Sherafatmand, M., & Ng, H. Y. (2015). Using sediment MFCs (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresource Technology, 195, 122–130.
Shin, J.-W., Seo, S.-J., Maitlo, H. A., & Park, J.-Y. (2015). The enhancement of ammonium removal from ethanolamine wastewater using air-cathode MFCs coupled to ferric reduction. Bioresource Technology, 190, 466–473.
Sleutels, T. H. J. a., Ter Heijne, A., Buisman, C. J. N., & Hamelers, H. V. M. (2012). Bioelectrochemical systems: An outlook for practical applications. ChemSusChem, 5, 1012–1019.
Solanki, K., Subramanian, S., & Basu, S. (2013). MFCs for azo dye treatment with electricity generation: A review. Bioresource Technology, 131, 564–571.
Sulonen, M. L. K., Kokko, M. E., Lakaniemi, A.-M., & Puhakka, J. A. (2015). Electricity generation from tetrathionate in MFCs by acidophiles. Journal of Hazardous Materials, 284, 182–189.
Sun, J., Hu, Y., Bi, Z., & Cao, Y. (2009). Simultaneous decolorization of azo dye and bioelectricity generation using a microfiltration membrane air-cathode single-chamber MFC. Bioresource Technology, 100, 3185–3192.
Varanasi, J. L., Roy, S., Pandit, S., & Das, D. (2015). Improvement of energy recovery from cellobiose by thermophillic dark fermentative hydrogen production followed by MFC. International Journal of Hydrogen Energy, 40, 8311–8321.
Velvizhi, G., & Mohan, S. V. (2012). Electrogenic activity and electron losses under increasing organic load of recalcitrant pharmaceutical wastewater. International Journal of Hydrogen Energy, 37, 5969–5978.
Venkata Mohan, S., & Srikanth, S. (2011). Enhanced wastewater treatment efficiency through microbially catalyzed oxidation and reduction: Synergistic effect of biocathode microenvironment. Bioresource Technology, 102, 10210–10220.
Venkata Mohan, S., Mohanakrishna, G., & Sarma, P. N. (2010). Composite vegetable waste as renewable resource for bioelectricity generation through non-catalyzed open-air cathode MFC. Bioresource Technology, 101, 970–976.
Wang, H., & Ren, Z. J. (2013). A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnology Advances, 31, 1796–1807.
Wang, X., Feng, Y., Wang, H., Qu, Y., Yu, Y., Ren, N., Li, N., Wang, E., Lee, H., & Logan, B. E. (2009). Bioaugmentation for electricity generation from corn Stover biomass using MFCs. Environmental Science & Technology, 43(15), 6088–6093.
Wang, A., Sun, D., Cao, G., Wang, H., Ren, N., Wu, W.-M., & Logan, B. E. (2011). Integrated hydrogen production process from cellulose by combining dark fermentation, MFCs, and a microbial electrolysis cell. Bioresource Technology, 102, 4137–4143.
Wang, X., Cai, Z., Zhou, Q., Zhang, Z., & Chen, C. (2012). Bioelectrochemical stimulation of petroleum hydrocarbon degradation in saline soil using U-tube MFCs. Biotechnology and Bioengineering, 109, 426–433.
Wang, Z., Lee, T., Lim, B., Choi, C., & Park, J. (2014). Microbial community structures differentiated in a single-chamber air-cathode MFC fueled with rice straw hydrolysate. Biotechnology for Biofuels, 7, 9.
Wang, H., Luo, H., Fallgren, P. H., Jin, S., & Ren, Z. J. (2015a). Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnology Advances, 33, 317–334.
Wang, L., Wu, Y., Zheng, Y., Liu, L., Zhao, F., et al. (2015b). Efficient degradation of sulfamethoxazole and the response of microbial communities in MFCs. RSC Advances, 5, 56430–56437.
Wen, Q., Wu, Y., Cao, D., Zhao, L., & Sun, Q. (2009). Electricity generation and modeling of MFC from continuous beer brewery wastewater. Bioresource Technology, 100, 4171–4175.
Wu, C.-H., I, Y.-P., Chiu, Y.-H., & Lin, C.-W. (2014). Enhancement of power generation by toluene biodegradation in a MFC in the presence of pyocyanin. Journal of the Taiwan Institute of Chemical Engineers, 45, 2319–2324.
Yuan, S.-J., Sheng, G.-P., Li, W.-W., Lin, Z.-Q., Zeng, R. J., Tong, Z.-H., & Yu, H.-Q. (2010). Degradation of organic pollutants in a Photoelectrocatalytic system enhanced by a MFC. Environmental Science & Technology, 44(14), 5575–5580.
Zhang, Y. (2012). Energy recovery from waste streams with MFC-based technologies. Doctoral dissertation.
Zhang, C., Li, M., Liu, G., Luo, H., & Zhang, R. (2009a). Pyridine degradation in the MFCs. Journal of Hazardous Materials, 172, 465–471.
Zhang, Y., Min, B., Huang, L., & Angelidaki, I. (2009b). Generation of electricity and analysis of microbial communities in wheat straw biomass-powered MFCs. Applied and Environmental Microbiology, 75, 3389–3395.
Zhao, G., Ma, F., Wei, L., Chua, H., Chang, C.-C., & Zhang, X.-J. (2012). Electricity generation from cattle dung using MFC technology during anaerobic acidogenesis and the development of microbial populations. Waste Management, 32, 1651–1658.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Capital Publishing Company, New Delhi, India
About this chapter
Cite this chapter
Varanasi, J.L., Das, D. (2018). Bioremediation and Power Generation from Organic Wastes Using Microbial Fuel Cell. In: Das, D. (eds) Microbial Fuel Cell. Springer, Cham. https://doi.org/10.1007/978-3-319-66793-5_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-66793-5_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-66792-8
Online ISBN: 978-3-319-66793-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)