Abstract
Antarctica is covered with ice and therefore the coldest, driest and least populated continent of the Earth. Apart from the challenges of sustaining the life in such environment, the microbial diversity of archaea, bacteria and fungi can thrive here by maintaining the membrane fluidity, producing the antifreeze proteins, cold-shock proteins, cryoprotectants, osmolytes, antioxidants, cold active enzymes, alteration in DNA, etc. and thereby adapted for the cold environments. In Antarctica, these microbes are the main basis for the biogeocycling of nutrients in extreme environments. The enzymes produced by these psychrophilic organisms are cold active and therefore gained the spotlight owing to their significance in environment and research. The behaviour of these cold-active enzymes has a wide and potentially biotechnological applications in fields as wide as the detergent, textile and food industries, medical and pharmaceutical preparations, bioremediation, etc. Therefore, the current manuscript illustrates the native microbial diversity and their significance and application aspect in environment and industry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Antarctica is located close to the South Pole. The entire continent covers an area of 14 million km2 of which only 0.3% is not covered in ice. A massive ice sheet covering almost the whole continent has more than 26,106 km3 of ice, an amount which if calculated in terms of sea-level rise will be equivalent to about 58 m [1]. Average thickness of the ice sheet of the continent is about 2.1 km. The four main geographical regions of Antarctica are West and East Antarctica, the Antarctic Peninsula and the Sub-Antarctic region. Antarctica and its residents on daily basis are subjected to extremely harsh climatic conditions such as low humidity, frequent freeze-thaw and wet-dry cycles, low temperatures, variable UV radiation and strong desiccating winds [2]. Extreme environmental conditions result in formation of oligotrophic environments in which only exceedingly particular organisms can thrive. The primary basis of ecosystem processes in Antarctica is formed by the microbial autotrophs, which are crucial to the processes of primary colonization and stabilization, thereby paving the way to secondary colonization and succession by other microbiota, plants and metazoans. Advancement in molecular techniques and their increased use for the study of microbes dwelling in Antarctic region have revealed the presence of a very rich microbial diversity, including viral diversity [3]. Numerous microbes have successfully colonized the harsh environment of Antarctica, and the richness of these microorganisms is now well known. The dynamics of microbial communities and their function in the Antarctic at present and in the upcoming future have been revealed by recent metagenomic data. Sadaiappan et al. [4] conducted Qiime analysis and revealed that the reads were signified by the presence of archaea and bacteria. The reads altogether represented microbes belong to 412 genera, 86 classes and 38 phyla. With 96.8% of the total readings, bacteria were found to have a larger diversity than archaea, which had only 1.7%. A total of 33 phyla of bacteria with the dominance of proteobacteria (87%) were reported, which was found to be followed by phylum Bacteroidetes (4.2%) and Firmicutes (1.70%). Major types of the proteobacteria reported were Gammaproteobacteria (58%), Alphaproteobacteria (29%), and Deltaproteobacteria (0.4%). Acidobacteria, Actinobacteria, Bacteroidetes, Chloroflexi, Cyanobacteria, Deinococcus-Thermus, Firmicutes, Nitrospirae, Planctomycetes, Proteobacteria, and Verrucomycetes made up the maximum variety of the bacteria and 1.5% of the total reads remained unclassified. Archaeal diversity was composed of five major phyla, namely, Crenarchaeota, Diapherotrites, Euryarchaeota, Hadesarchaeaota and Thaumarchaeota. These are typically psychrophilic or psychrotolerant bacteria, which have been evolved a variety of adaptations to withstand the harsh effects of such cold surroundings [5]. These bacteria use a variety of techniques to thrive in such extreme environments, including maintenance of membrane fluidity, producing antifreeze proteins, cryoprotectants, cold-shock proteins, cold active enzymes, etc. Enzymes produced by these microorganisms are being increasingly reported as essential components responsible for their adaptation. The enzymes produced by these psychrophilic organisms are constantly in the spotlight owing to their significance in both basic and applied research. The properties of cold-adapted enzymes offer a wide range of potentially beneficial biotechnological applications in fields as wide as the detergent, textile and food industries, medical and pharmaceutical preparations, bioremediation, etc. The search for cold-adapted enzymes with potential utility in biotechnological sectors is consequently receiving more attention. The current manuscript discusses the diversity of bacteria found in the Antarctic, their coping mechanisms and potential ecological and industrial applications.
15.2 Challenges for Life in Antarctica
The environmental and geographical conditions of various locations impart various direct and indirect stresses and thereby play an important role in evolution of species as well as their distribution. The Earth harbours almost around 10 million species which are the result of long-term evolution in various ecosystems from high mountains to deep oceans and from tropics to the arctic tundra. Antarctica is covered by ice sheet that can be up to 3 km thick and surrounded by an annual ice pack, and surprisingly, these ice sheets represent 97% of the Earth’s ice and 70% of its freshwater. Life in the largest freezer of Earth, i.e. Antarctica, is subjected to dangerous environmental stresses, which is more than any other desert or terrestrial ecosystem. Various chemical and physical gradients along with harsh climatic conditions such as very low temperature, humidity, low availability of water, high salinity, low carbon and nutrient concentration, inexhaustible freeze-thaw cycle, low annual precipitation, strong winds causing abrasions, high sublimation, seasonal day length variations, evaporation, limited nutrition and high exposure to ultraviolet radiations are few to be named [6]. The combination and interactions of these environmental stresses act as limiting factors for the survival of living organisms thus determining the taxonomical diversity of the continent. The soil of this continent is usually considered biologically depauperate or very simple in this term. The diversity of photosynthetic autotrophs is only covered by lower plants such as algae, mosses and lichens. Due to low diversity of flora, the terrestrial faunal diversity is also low due to lack of sufficient food and mostly consists of simple communities of invertebrates, nematodes, springtails, mites, tardigrades and rotifers [7].
The food web of the Antarctic continent is thought to be simplest on the planet, yet we lack fundamental understanding between the relationship of abiotic factors and biological diversity. Various studies have revealed that the chemistry of the soil is one of the most primary drivers for the establishment of soil biota than any other environmental factor. Magalhaes et al. [7] showed that the soil with lower conductivity and high C/N ratio were found to have higher diversity of microbial biota. The study also indicated that as the pH of the soil increases, the abundance of the cyanobacteria also increases, and this correlation is also relevant in alpine environment. The microbiota of soil also plays an important role in recycling of organic and inorganic nutrients and decomposition. Low level of microbiota insures flow of nutrients in low diversity of food web and vice versa. Similar correlation of soil chemistry is observed with diversity of invertebrates, lichens and algae, i.e. low salt concentration, higher pH and high C/N ratio [8]. The studies have shown that soil moisture ranging from 0.7 to 11.3% acted as an important determinant for the presence of species in the Antarctic continent [7]. The other studies have indicated that composition of organic matter, soil salinity and availability of liquid water also acts as strong limiting factors for biological colonization. Magalhaes et al. [7] also found that both the bacteria and cyanobacteria respond to physical and chemical parameters in almost similar ways, but the abundance and diversity of bacteria were found to be greater than cyanobacteria which could imply that bacteria possess high tolerance levels to the environmental factors than the cyanobacteria. It is often observed that primary colonizer of ice-free areas are bacteria, algae and cyanobacteria which bind the soil before the development of higher plants such as mosses and lichens on the substratum. Convey et al. [9] hypothesized that the primary colonizers including mosses and lichens are subjected to environmental stress to much greater extent than higher colonizer as they are unprotected from greater water retention and buffering of temperature and soil pH. The studies have also revealed that the presence of invertebrates, lichens and algae were found to be more abundant in the areas where the diversity of bacteria and cyanobacteria was more, indicating the importance of well-developed microbial biota for the development of complex multi-tropical community.
Terrestrial ecosystem of Antarctica covers a little proportion (0.32%) of the whole continent’s area. Except high cliffs and exposed ridges of mountains, most of the habitats also suffer extended seasonal snowfall or ice cover which helps in protection of biotic components from extremely low temperatures and windy abrasions. But the same ice cover unavoidably limits biological activity for a long period of time. The little possibility of biological activity under ice is also limited to seasonal duration [9]. Some researchers have found that even in the ice-free areas, the diversity and abundance of living organisms were irregular. This makes it unclear to intemperate that these irregularities are due to edaphic factors, topology and vegetation or due to microclimatic conditions. Studies conducted in the ice-free areas showed seasonal variation in the flora and fauna of the region with respect to abiotic factors. For example, in summers, the moisture content of the ice-free area increases due to the increase in the temperature, the abundance and diversity of the microbes, and photosynthetic autotrophs, and microfaunal species increases drastically. Similarly, the coastal areas also experience higher temperature and thus higher water availability which results in more suitable habitats and hence higher production of biomass.
The presence of human beings in Antarctica is very low due to harsh conditions, but still this continent has not escaped the negative effects of anthropogenic activities worldwide. The global climatic changes have also shown deleterious effects on the physical, chemical and biological components of this continent. The most obvious and known effect of anthropogenic activities on Antarctica is the creation of ozone hole which had serious consequences to biological systems including humans. However in case of marine biota, the negative effects of the global warming are more prominent. The marine life of Antarctica usually thrives at temperature range of −1.9 °C to −0.5 °C. Various studies have been conducted on various marine animals of Antarctica in order to understand the effect of elevated temperature on them. The most obvious result of these studies was that most of the Antarctic marine species are very poor in surviving elevated temperatures. As the temperature increases, the levels of available oxygen decrease, and concentration of oxygen increases in water which creates a problem for numerous species due to increased cell damage from reactive oxygen species. The other negative impact of increased temperature is reduced oxygen availability and increased metabolic rates which would in turn affect the energy production. Apart from global activities affecting various aspects of the continent, local activities such as fishing, shipping traffic, tourism, pollutants released by research stations of various countries like the USA, Argentina, China, Russia, etc. are also the cause of great concern [10]. The contaminants released by different anthropogenic activities include leachate from historic disposal sites, petrochemicals from fuel spillage, organic enrichments, disposed chemicals through sewage systems and other harmful gases like SO2, NO2, CO, CO2, etc. During the early periods of fishing, almost all the types of finfishes and their predators were fished causing a sharp decline in their number over a period of time. Shipping traffic is another major problem which is used for many purposes. These ships not only contribute to air and water pollution by secreting poisonous gases, sewage waste and polluted liquids but also create noise pollution which creates disturbance for the marine life.
Tourism in Antarctica has extended for its exclusive “expedition cruises”, and it is expected to increase even more in the near future. This huge number could be a potential threat to the wildlife as the increasing pollution, risks of invasive species and disruption of scientific research activities. This ever-growing tourism with the landing of the tourists greatly disturbs the fragile polar system and has great negative potential towards both terrestrial and marine habitats. Major source of marine pollution is sewage outfalls, abandoned dump sites accidental oil spills and exhaust emissions. These contaminants are persistent as they continue to leach for a very long durations into the marine environment majorly effecting benthic communities. Apart from these contaminants, Padeiro et al. [11] reported high levels of heavy metals such as Zn, Pb, Cd, Cr and Ni in the soil samples of Antarctica due to human activities. Ogaki et al. [12] also reported the effect of heavy metal on the fungal diversity of different lakes of Antarctica. They observed that lakes which were in close proximity to human had high sedimentation levels of heavy metals and reduced diversity and richness of fungi. In contrast, low levels of sedimented heavy metals and high diversity and richness of the same were reported in the lakes which were far away from human impact.
15.3 Diversity of Microorganisms Found in Antarctic
As mentioned in the above section, abiotic factors are the major determining factor of biotic diversity in an ecosystem. The Antarctic region has vast variation in the abiotic factors; hence, a huge diversified microbial community has been developed, which has been reported by various scientists. Some of them are discussed below:
-
(a)
Fungal Diversity
In Antarctica, microorganism-dominated food chains are mainly present in different pristine ecosystems. Concerning to fungal diversity, two basic forms, i.e. yeast and filamentous fungal forms of fungi, are mainly found in Antarctica, which represent colonies with different colours and morphologies [13]. These colonies display a very high level of genetic plasticity, which in turn allows them to survive under very unfavourable conditions of low temperatures, freeze-thaw cycles, variable pH levels, high UV irradiation, strong winds, dehydration, minimal nutrient concentration and osmotic stress conditions. The Antarctic fungal community includes taxa belonging to the major fungal groups like Ascomycota, Zygomycota, Basidiomycota, Glomeromycota and Zygomycota. In addition to these taxa, some assemblages also include Mycetozoa (slime moulds) and stramenopiles (oomycota). But, according to some recent taxonomic studies considering phylogenetic analysis as well as characterization of uncultivable taxa, this traditional taxonomic hierarchy is changing [14]. Tedersoo et al. [14] reported colonization of fungi belonging to 18 different phyla, i.e. Ascomycota, Basidiobolomycota, Aphelidiomycota, Basidiomycota, Chytridiomycota, Calcarisporiellomycota, Entorrhizomycota, Entomophthoromycota, Glomeromycota, Muccoromycota, Kickxellomycota, Mortierellomycota, Monoblepharomycota, Neocallimastigomycota, Zoopagomycotina, Olpidiomycota and Rozaellomycota. Bridge and Spooner [15] documented about the 1000 fungal species (without lichen forms) of Antarctic region and also concluded that true diversity of Antarctic fungi is far more than the recorded estimate.
Several recent studies have concluded that there is a vast difference between the fungal diversity of Antarctic peninsula and continental Antarctica environments because the harsh condition of Antarctic peninsula is milder than that of continental regions as well as the Antarctic peninsula has more life in the form of macroalgae, plants, vertebrates, invertebrates etc., which provides nutrients and organic matter to survive the fungi and thus creating various ecological niche and microenvironments for the fungal food web. In contrast, in Continental Antarctica, nutrients and organic matter are very limited due to the absence of life forms. Fungi in the region of continental Antarctica are majorly present in lichen symbiosis (symbiotically associated with lichens), while the presence of free living fungi in soils is poorly understood [13]. On the other hand, cosmopolitan psychrotolerant fungi are ectotypes with mesophilic psychrotolerant nature. Species like Penicillium antarcticum, Penicillium chrysogenum, Antarctomyces psychrotrophicus, Antarctomyces pellizariae, Thelebolus spp., Metschnikowia australis, Mortierella antarctica, etc. are considered as endemic psychrophilic species, and different species of Aspergillus, Penicillium, Cladosporium, Colletotrichum and Rhodotorula are known as cosmopolitan cold-tolerant taxa [16].
-
(b)
Bacterial, Cyanobacterial and Archaeal Diversity
Antarctic, which is also known as driest, coldest continent, is separated from other continents via the Antarctic circumpolar current and the southern ocean. Massive ice sheets present here reflect 40–90% of incident solar radiation and cause a mass of cold dense air to accumulate on the polar plateau. In these very harsh conditions, only cold-adapted microorganisms like microorganisms, tardigrades, mites, tundra vegetation, penguins, seals and several types of algae can survive. In spite of these extreme conditions, prokaryotes and archaea dominate in the most Antarctic ecosystem and thus play major role in biogeochemical cycles, food webs and mineralization of pollutants. A major phylum of gram-negative bacteria, namely, Proteobacteria, is frequently found in Antarctic soil. Several other studies which are mainly focused on microbial diversity in Antarctic soil suggested Actinobacteria and Proteobacteria as the major phyla; on the other hand, the phyla Cyanobacteria and Firmicutes are common but less frequent [17].
Various new approaches like multi-omics approach reveal the several communities of prokaryotes present with in soil at Edmondson point (ice-free region on eastern slope at the base of Mount Melbourne, Victoria Land, Antarctica). The above region consists of Actinobacteria, Proteobacteria, Acidobacteria, Planctomycetes, Verrucomicrobia, Bacteroidetes and Chloroflexi. Bacteroidetes, Proteobacteria, Actinobacteria and Firmicutes are majorly reported through cultivation-based methods [18]. Some advanced analysis as well as 16S rRNA sequencing also reveals that the anaerobic, spore-forming Firmicutes are the most abundant group present in the rhizosphere of the Antarctic vascular plants [19]. During the summer season, snow melts and Antarctic soil becomes free and receives nutrients from the ocean as well as animal life (i.e. penguin guano), which enrich the soil with phosphorus and nitrogen, hence create an environment that supports abundant microbial growth. O’Brien et al. [20] reported that the ornithogenic soil samples of Antarctica support high bacterial yield counts (6–9 log CFU g-1) with respect to other samples. Some cold-adapted Burkholderia species were also discovered in the coastal region of Ross sea in Antarctica.
With the help of applied metatranscriptomics, metaproteomics and metagenomics to permafrost, thermokarst soil, it can be concluded that Actinobacterial lineages are the most active as well as numerically dominated members of the prokaryotic community in the seasonally thawed soil [21]. Actinobacteria also showed the flexible nature towards both short- and long-term changes towards environmental conditions. On the other hand, Acidobacteria has been found very common to a vast range of Antarctic and Arctic soil biotopes. Despite the fact that Actinobacteria, Acidobacteria and Proteobacteria are numerically most abundant, cyanobacteria also plays an impressive role in terms of significant colonist in the cold soil. Cyanobacteria like Nostoc commune are majorly present in both Antarctic and Arctic soil and hence drive several important functional processes related to the nitrogen and carbon cycling. Increasing soil temperature due to global warming may extend microbial growth period. New meta-analysis reveals that the warming of cold soil directly increases the abundance of microbes, which indirectly impact the stored carbon. Thus, there is an urgent need of more extensive datasets on the effect of different climatic conditions on microbial community’s functional processes and composition.
15.4 Survival Strategy of Antarctica Bacteria
Microbes residing in the Antarctica region face numerous challenges. Among them, cold temperature conditions are the most and consistent stress. In order to grow in such environmental conditions, microbes have developed some survival strategies. Out of which some are discussed below:
15.4.1 Production of Cryoprotectant and Osmolytes
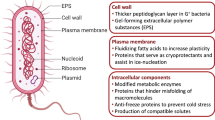
The microbes in Antarctica are exposed to cold environment, which accelerates the osmotic damage and dehydration resulting in disruption of the functioning of cells and their survival by deactivating the enzymes and causing several negative effects. But to avert this cold aggregation of protein, there are many psychrophiles that are reported to secrete various ice-nucleating proteins, extracellular polymeric substances (EPS), compatible solutes and bio-surfactants. All these exopolymeric substances or osmolytes help to maintain optimum membrane fluidity and prevent cell shrinkage by simply accumulating within the cell and suppressing the solutes to freeze inside the cell membrane that protects the cell from cryo-injuries [22]. During exposure of the cells to cryoprotectants, ice disrupts the cell membranes mechanically, which is protected by secreting extracellular polymeric substances (EPS) within the cell; similarly, a sea ice bacterium, namely, Colwellia psychrerythraea, secretes EPS that protect the cell membrane. Moreover, the severe damage caused by ice crystals is protected through biofilms that are formed by psychrophilic microbial cells. Not only this, biofilms are also necessary to acquire the nutrients within the channels. On growing Mesorhizobium sp. strain N33 at 4 °C, it was reported that threonine, valine and sarcosine were accumulated in a higher amount which is considered as a cryoprotectant for microbes [23]. Certain type of growth-promoting substance such as glycine, and betaine, also known as compatible solutes has the ability to protect bacteria at low temperature. These compatible solutes avoid the cold-induced cellular protein aggregation. Moreover, these osmolytes also influence the fluidity of the bacterial membrane adapted to the cold stress.
15.4.2 Maintain Membrane Fluidity
To overcome the adverse effects of harsh environments, modulation of the fluidity of the membrane and providing interface between the external and internal environment are a strategy adopted by microbes in the freezing environment. Activation of the membrane-associated sensor, upregulating membrane fluidity and increase in membrane rigidity are the major aspects of maintaining the membrane fluidity. At low temperature, the function and physical properties of membranes are affected, leading to decrease in the membrane fluidity, the beginning of gel-phase transitions and the loss of cell function. In accordance to the function of bacterial membrane, some significant adaptations are present in bacterial membrane including the degree of unsaturation, branched fatty acids chain and chain length. Cell membrane associated with the modification of lipid fatty acyl chains helps to maintain optimum membrane fluidity. Increase in the methyl branched fatty acid helps cold microbial cells to survive harsh environmental conditions. The presence of desaturase enzyme in cold-adapted bacteria converts saturated acyl fatty acids to unsaturated acyl chains by removing two hydrogen atoms and helps in cold-adapted bacterial survival. Apart from this, steric constraints reduce number of interaction in membrane as well as changing packing order which playing important role in membrane fluidity of psychrophiles. Increase in the degree of unsaturated fatty acid in Leucosporidium, Candida and Torulopsis has been reported by Nagy and Kerekes [24]. Additionally, the reported study shows that the level of wax ester synthase increases when Psychrobacter arcticus exposed to cold temperatures [25].
15.4.3 Antifreezing Protein
For cold environment, psychrophiles have antifreezing proteins (AFPs) as a survival strategy. By synthesizing such unique proteins, they prevent the growth and recrystallization of ice. Bacteria reported to have AFP activity are Psychrobacter sp., Rhodococcus sp., Stenotrophomonas maltophilia, P. fluorescens, Marinomonas protea and Enterobacter agglomerans [26]. Thermal hysteresis (TH) is the difference in freezing and melting point which results in the adsorption of AFP on crystal surface of the ice, which results in the ice growth on convex surface between the adjoining AFPs thereby decreases in freezing point. Isolated antifreeze protein (AFN) helps maintaining the frozen food cell structure, showing its high value for frozen food industry isolated from the Antarctic bacterial culture GU3.1.1 [27].
15.4.4 Alterations in DNA Replication
Temperature-induced conformational or physicochemical changes in proteins, RNA and DNA could form basis for sensing temperature. Compaction might be due to one or more reasons like (1) temperature-induced conformational change in the DNA, (2) alteration in the activity and/or amounts of the DNA proteins like DNA gyrase, histone-like proteins including H-NS and topoisomerase I and/or impaired synthesis of the proteins that influence condensation of nucleoid [28]. DNA conformation is essential for cold adaptation, and the expression of many genes is dependent on the DNA conformation that in turn depends on the temperature-dependent changes in DNA supercoiling. Demonstration of link between expression of DNA supercoiling and cold-inducible genes inhibits the negative supercoiling leading to inhibition of the cold-inducible gene expression. The nucleoid-associated proteins like H-NS and topoisomerase II and I regulate supercoiling in bacteria at low temperature and thereby control cold-inducible gene [29]. Under cold conditions, expression of such genes induces and the product takes part in the adaptation.
15.4.5 Antioxidant Production
Bacteria in Antarctica have to resist not only to cold temperatures but also other stress conditions like high UV radiation. These bacteria have developed different antioxidant systems to survive in this extreme environment by avoiding the oxidative stress caused by reactive oxygen species (ROS). Hydrogen peroxide (H2O2), anion superoxide (O2−) and hydroxyl radicals (OH) are the most common species of ROS. Oxidative damage occurs in the microbial cell despite adverse conditions, but some psychrophilic bacteria have the ability to counter the situation and reduce oxidative stress through certain defence mechanisms [30]. Glutathione, vitamins and some pigments like carotenoids are the examples of nonenzymatic antioxidant defence, which are reported to present in high amounts in psychrophiles. Carotenoids are pigment antioxidants that neutralize free radicals through various reactions in the microbial cell. For example, the interaction of carotenoids with singlet oxygen (1O2) occurs via transferring excitation energy to the carotenoid or by chemical quenching of 1O2. Carotenoids pigment can interact with oxygen radicals in three main ways: electron transfer, hydrogen abstraction and the addition of radical species [31]. Apart from this, various psychrophiles are reported to produce various antioxidant enzymes, which can neutralize the free oxygen radicals and thereby protect microbes from oxidative damage.
15.5 Ecological Significance of Microbes
The microbes are the basis of nutrient cycling and community development at any naive regions. The microbes that reside in the Antarctica region are also reported to play crucial roles in ecosystem.
15.5.1 Biogeochemical Cycling
Microorganisms are the major drivers of biogeochemical cycles on Earth. They have a significant impact on carbon (C) as well as breakdown of organic matter, nitrogen (N) efflux and phosphorus (P) mobilization in the environment [32, 33]. These processes may result in CO2 elevation, greenhouse gas release, nutrient loading and water consumption [34, 35]. The Earth’s biosphere is dominated by cold environments, and the cold biosphere is dominated by microbes. Microorganisms in cold Southern Ocean waters are recognized for having key roles in global biogeochemical cycles.
-
(i)
Carbon Cycle
The carbon cycle involves several microbial processes such as CO2 fixation, organic compound decomposition, mineralization to CO2, oxidation of methane (methanotrophy) and methane production (methanogenesis). The latter is solely anaerobic process, while the other activities may occur under both aerobic and anaerobic conditions. In the cryosphere, microorganisms are believed to carry out biogeochemical cycles within soil snow, lakes, hills and both in supraglacial and subglacial environments [36, 37]. Supraglacial microbial communities (within the snowpack or within 0.1–3 mm dark granular aggregates, cryoconite debris) are known to cycle carbon through photosynthesis and respiration pathways. Antarctic cryoconite holes (CHs) are water-filled depressions upon the glacier surface which contain coating of cryoconite debris that constitute important features on glaciers and ice sheets. Once hydrologically connected, these microbially dominated mini-ecosystems supply nutrients and biota for downstream environments. Within cryoconite holes, and especially in cryoconite debris, diverse communities of eubacteria, archaebacteria and eukaryotes exist. These microbial communities play a crucial role in carbon cycling such as primary producers like cyanobacteria and non-cyanobacterial group which fix inorganic carbon via photosynthesis and provide nutrients for the heterotrophic fraction of the community. Previous morphological studies of the Antarctic region suggested the presence of several different species of cyanobacteria, including Hormathonema spp., Gloeocapsa spp., Anabaena spp., Aphanocapsa spp., Lyngbya spp., etc. [38]. Heterotrophic community was dominated by relatively few taxa of Gammaproteobacteria, Sphingobacteria–Flavobacteria and Alphaproteobacteria reported by Straza et al. [39] who demonstrated substrate utilization by these taxa. Several mechanisms of carbon dioxide fixation are known of which Calvin-Benson-Bassham (CBB) cycle is the most important autotrophic pathway. The first rate-limiting step is catalysed by the ribulose1,5-biphosphate carboxylase/oxygenase (RubisCO) enzyme. Four different natural types of RuBisCO are known: type I which is encoded by cbbL genes which have been found within green-like autotrophic bacterial groups, green algae, cyanobacteria and representatives of some Alpha-, Beta- and Gammaproteobacteria and red-like autotrophic bacterial groups, including non-green algae and representatives of some Alpha- and Betaproteobacteria and autotrophic eukaryotes. Type II (cbbM gene) is found in purple non-sulphur bacteria, aerobic and facultative anaerobic chemoautotrophic bacteria and dinoflagellates. RuBisCO type III has only been found in Archaea. Finally, type IV RuBisCO is designated as RuBisCO-like and is considered not to be involved in the Calvin cycle [40].
However, complexity of genes and processes involved in the carbon cycling makes it difficult to understand how these complex processes could be affected by global change. Another carbon cycle is methane cycle which is probably one of the most studied carbon-related processes and has been shown to occur widely in Antarctic soils. Production of methane from organic matter is a multistep process, in which methanogenesis, carried out by microorganisms from the Archaea domain, is the final step. Oxidation of methane is a bacterial process, while carbon dioxide fixation can be carried out by chemolithotrophic bacteria and by oxygenic and anoxygenic photosynthetic organisms. The carbon source/sink ratio is, in part, determined by the equilibrium between methylotrophy and methanogenesis. According to in situ measurements, polar soils are a net source of CO2 emissions but a sink for methane [41]. However, there is evidence that increased soil temperatures (thaw) result in increased methanogen diversity of both active layer and permafrost soils as well as considerable increase in methane production [41]. According to Tveit et al. [42], methane productivity changes were linked to a shift from formate- and H2-using Methanobacteriales to Methanomicrobiales and from the acetotrophic Methanosarcinaceae to Methanosaetaceae. Very little is known concerning the distribution and abundance of methanogens in Antarctic soils. However, it is reasonable to predict that a warming climate may lead to more anaerobic soil conditions, which could ultimately result in these soils becoming net methane producers.
-
(ii)
Nitrogen Cycle
Nitrogen is generally used for proteins and nucleic acid synthesis. The primary sources of bio-available nitrogen (nitrate, nitrite, ammonia and organic nitrogen) are internal remineralization; external sources like snowmelt, aerial deposition and complex organic material decomposition; as well as microbial-mediated atmospheric nitrogen fixation (by cyanobacteria or some microbial groups associated with plant roots) [43]. Additionally, certain types of sedimentary and metasedimentary bedrocks may have ecologically important amount of nitrogen, which if liberated could impact biological nitrogen cycling in soils. Most Antarctic soils are severely oligotrophic and are particularly low in organic nitrogen. Ice-free continental Antarctica is completely deprived from higher plants; thus, much of the soil nitrogen cycling is thought to be driven by microbial communities such as cyanobacteria including Leptolyngbya, Phormidium, Oscillatoria, Nostoc, Calothrix, Dichothrix, Nodularia and Hydrocoryne [44]. These are widely considered to be the central regulators of nitrogen cycling in soils and play crucial role as “ecosystem engineers” [44]. Martínez-Pérez et al. [45] demonstrated the role of small unicellular diazotrophic symbiont; UCYN-A with algal partner potentially contributes significantly to N2 fixation from Artic to Antarctic circle. UCYN-A lacks key genes for CO2 fixation and therefore lives in association with alga from which it receives fixed carbon. In return, UCYN-A transfers up to 95% of its recently fixed N to the algal partner [45]. Similarly, many fungi including yeasts are commonly found in Antarctic habitats such as Rhodotorula muscorum, Rhodotorula mucilaginosa, Cryptococcus aerius and Cryptococcus albidus; by producing enzymes such as urease and protease, they play an essential role in nitrogen mineralization or ammonification. In addition, nitrate and nitrite can be also used by many fungi, including yeasts, in dissimilatory pathways such as denitrification. In a study, a bacterial isolate, i.e. Candida sp. and a mesophilic isolate of Trichosporon cutaneum, is found in Antarctic soils governing the process of denitrification [46]. The genes of nitrogen cycling have been extensively studied in Antarctic soils, primarily by targeting the nitrogenase (nifH) gene. These soils have a high prevalence of diazotrophy, which is mostly but not entirely associated with cyanobacterial lineages. Genes implicated in nitrite oxidation and ammonia oxidation, the nxrA and amoA genes, respectively, have also been reported in Antarctic soil metagenomes [47]. Denitrification, a process that generates the “greenhouse” gas N2O and for which the narG gene is the genetic marker, is mostly linked to Actinobacteria and Proteobacteria in Antarctic soils. However, PCR-dependent and metagenomic gene surveys have suggested that the nitrogen cycle is severely truncated in these soils, with key enzymes implicated in some crucial steps (such as dissimilatory nitrate reductase and nitrous oxide reductases) either present at very low abundance or undetectable.
-
(iii)
Phosphorus Cycle
Phosphorus (P) is a key limiting nutrient for organisms, and its biogeochemical cycling is believed to regulate primary productivity and ecosystem structure. Phosphorus is derived primarily from the weathering of apatite in soils following exposure of the parent substrate [48,49,50]. Unlike soil carbon (C) and nitrogen (N), which reside primarily in organic pools, soil P is typically dominated by inorganic pools consisting of unweathered and largely biologically unavailable material, and also its biogeochemical cycle differs from nitrogen and carbon cycle in that it does not include a gas phase. The release of bioavailable P from the lithosphere into the soil ecosystem is governed by a variety of physical, chemical and biological weathering processes. Rain and weathering cause rocks to release phosphate ions and other minerals, which initiate the phosphorous cycle [51, 52]. Inorganic phosphate is then distributed in soils and water where it is taken up by living organisms to form biomass. The organic phosphate returns to the soil when organisms decompose and die. Within the soil, organic forms of phosphate can be hydrolysed releasing phosphate to the environment in a process known as mineralization [53]. Microbial phosphatases play an important role in this process. In soils, organic phosphorus is mainly found as phytates (inositol hexa- and penta-phosphates) from which phosphate is released by the action of specific phosphatases called phytases, which remains in the surrounding in the available form [54, 55]. Several phytase-producing bacterial and yeast strain have been reported from Antarctic region including Papiliotrema laurentii, Rhodotorula mucilaginosa, Cryptococcus laurentii and Pseudomonas sp. [56].
15.5.2 Role as PGPR
Antarctic plants have developed a set of survival mechanisms including adjustment in cellular and physiological molecular responses such as modification in the membrane lipid composition and the production of antioxidants, osmoprotectants and cold-shock proteins, among others to grow and survive in this hostile environment. Thus, the ability of plants to adapt to adverse environmental conditions defines their long-term survival and geographical and environmental distribution. In addition to these biochemical and physiological changes, external factors also aid in the adaptation and dissemination of plants in Antarctica. Sometimes, such adaptations are the result of interactions between roots and soil microorganisms. For example, microorganisms produce molecules that actively cooperate in the establishment and development of plants known as plant growth-promoting microbes (PGPM) [57, 58]. PGPMs have also adapted to live and perform all their functions in extreme conditions, and they also contribute to enhance the Antarctic plant biomass [59]. Most of the microbial species found in the rhizosphere are organotrophic. Among positive effects performed by PGPM, a few microorganisms produce chemicals involved in the acquisition of nutrients (e.g. the acquisition of iron by bacterial siderophores or phosphorus by the secretion of organic acids from mycorrhizal fungi) and/or the development of roots (e.g. by the production of phytohormones) [60, 61]. Fardella et al. [62] demonstrated that fungal endophytes isolated from Antarctic plants improved the survival and water use efficiency of several tree and shrub species. Similarly, Berrıos et al. [63] reported that an Antarctic strain of Pseudomonas sp. imparts a beneficial effect on plant Deschampsia antarctica growth, probably because of their contribution of hormones and nutrients (P) supplementation. Moreover, various studies have reported beneficial effect of PGPM including Pseudomonas tolaasii (resistance to antibiotics and heavy metals), P. trivialis (tolerance to temperature) and Arthrobacter sp. (antimicrobial properties) associated with Antarctic vascular plants Deschampsia antarctica [64, 65]. Thus, certain strains of rhizobacteria from Antarctica may be useful tools in promoting abiotic stress tolerance and productivity in important crop species by altering root systems.
15.5.3 Role in Bioremediation
Bioremediation is defined as a method of using microbes, plants or microbial or plant enzymes to detoxify contaminants within soil and other environments. The concept also includes biodegradation, which is defined as a partial, and occasionally complete transformation by microbes and plants [66, 67]. For at least 40 years, bioremediation has been considered as a way to promote recovery of contaminated habitats at both higher and lower temperatures. In comparison to other methods, microbial biodegradation is the most effective and economically viable method that also poses the least risk to the environment. Rhodococcus is one of the bacterial strains that has been isolated from the Antarctic continent and has received the most attention for its substantial role in soil ecosystems and outstanding metabolic capability. This bacterial group was able to break down aromatic molecules as well as alkanes with chain lengths ranging from C6 to C20, which are referred to as persistent fractions in Antarctic soils [68]. Another significant hydrocarbon degrader in Antarctic soil has been identified as the Acinetobacter genus. In a microcosm experiment, the strain Acinetobacter B-2-2 was combined with a Rhodococcus ADH strain and was able to degrade 81.1% of the oil in pristine soil that had been contaminated for the experiment, as opposed to the 75% degradation that the strain Acinetobacter B-2-2 achieved when used alone in a prior study [69]. One of the main types of bacteria that degrade hydrocarbons is known as Pseudomonas. After 6 days in the presence of 3.5% diesel oil (v/v), the Antarctic Peninsula isolate of Pseudomonas sp. J3 displayed impressive cellular development [70]. Sphingomonas is another bacterial species isolated from Antarctic environments that has been demonstrated to be able to use hydrocarbons as a special source of carbon [71]. At low temperatures ranging from 1 to 35 °C, the Scott Base-Antarctic strain Ant 17 was able to degrade the aromatic component of numerous different crude oils, although the optimal circumstance was pH 6.4 at 22 °C. Additionally, Sphingomonas Ant 17 demonstrated resistance to freeze-thaw cycles and UV radiation, which is highly helpful in Antarctic regions where both conditions are commonly present. Additionally, fungi and yeast from the Aspergillus, Candida, Penicillium and Rhodotorula genera have been widely reported as potential candidates in hydrocarbon degradations [72], (Table 15.1).
The Maritime Antarctic is the best area in Antarctica for successful bioremediation. In this region, temperature above 0 °C is typical in summers, as seen at King George Island. Though the Antarctica has a pH range of 6 on the island and 9 on the coast and soils with pH values exceeding 8.8 have been found to have more effective hydrocarbon biodegradation [68], bioremediation degradation is more efficient under aerobic conditions rather than anaerobic conditions, as the major aerobic hydrocarbon breakdown pathways are faster and generate more energy. Approximately 0.3 g of oxygen are required for every gram of oil oxidized [83]. Once the general biodegradation of substances takes place inside the cell, the microbial community—which promote biodegradation via aerobiosis—is also be able to internalize the substrate. The ability of microbial cells to contact and internalize a substrate is known as bioavailability and is critical for the biodegradation process. The cytoplasmic matrix can freeze, and the channels across the cell membrane may seal in conditions below the freezing point, inhibiting cell function.
Low temperatures further increase oil viscosity, and water solubility decreases evaporation thus slowing down biodegradation process. Therefore, the susceptibility of biodegradation of petroleum hydrocarbons decreases from paraffins > branched alkanes > olefins > monocyclic aromatic hydrocarbons (MAHs) > naphthenes > polycyclic aromatic hydrocarbons (PAHs) [84]. Because of the above-mentioned factors, bioremediation treatments are mostly recommended during summers when the soils are not frozen, temperatures are higher and water is readily available. Moreover, by adding nutrients, carbon sources or electron donors to the native bacteria or fungi (biostimulation, biorestoration), or by adding an enriched culture of microorganisms that have particular properties that allow them to degrade the desired contaminant more quickly (bioaugmentation), the process of bioremediation increases the rate of the natural microbial degradation of contaminants (Fig. 15.1). Low temperatures, however, can be advantageous in the case of spills since snow can contain as a containment boom and slows the spilt oil penetration by acting as an absorbent.
15.5.4 Bio-prospecting of Antarctica Microbes
Although there are numerous definitions of “bioprospecting”, there isn’t a single one that is universally acknowledged. Rogan [85] defines bioprospecting as “a range of activities related with the search for a novel biodiversity, whose constituent parts may be employed in a product or process and developed for commercialization”. Antarctica is an important location for bioprospecting. Its microbial diversity is still poorly studied and may contain microbes with very relevant abilities for white (industrial), green (agricultural) and red (pharmaceutical) biotechnologies. Microbes have developed a variety of adaptation strategies to enable growth in the harsh Antarctic environment. These adaptations are frequently accompanied by changes to metabolic and gene regulatory systems. These adaptations include the production of antifreeze proteins and cold-active enzymes, as well as the storage of cryoprotectants such as sugars and polyols in the cell to maintain turgor pressure, high levels of unsaturated membrane phospholipids to stabilise membranes and fungal melanin for protection against freezing and UV radiation. Thus, it turns out that microbial extremophiles are a chemical reservoir of extremolytes and extremozymes that have the potential to be excellent resources for the growth of a bio-based economy [86].
Actinobacteria represent a considerable portion of the microbial community in the majority of soils including the Antarctic region. Additionally, Actinobacteria found in rhizosphere soil have been linked to the development of beneficial compounds and antimicrobials [87]. The genus Streptomyces has demonstrated potential as a biocontrol agent for fungal diseases in commercial crops. Additionally, grape-derived Streptomyces spp. displayed antifungal action towards pathogenic yeast and fungus from the same habitat [88]. While the genus Arthrobacter is well known for producing secondary bioactive metabolites and for bioconversions, it has been regularly found in Antarctic and Arctic regions. Rojas et al. [89] searched for novel metabolites produced by Antarctic bacteria found novel molecules linked to cyclic thiazolyl peptides that were active against gram-positive pathogens and produced by Arthrobacter agilis from Lake Hoare and Lake Fryxell in the McMurdo Dry Valley region of Antarctica (Fig. 15.2).
15.5.5 Industrial Significance of Antarctic Dwelling Microbes
Microbes that live in Antarctica produce a number of industrially useful enzymes (Table 15.2). For example, the fungus Cladosporium sp. isolated from a marine sponge and the Penicillium species isolated from various Antarctic marine creatures (such as sea stars, mollusks and macroalgae) are both capable of producing xylanase. For instance, some yeasts from the genera Cryptococcus, Leucosporidium, Metschnikowia, Candida, Yarrowia and Saccharomyces isolated from Antarctic marine samples have been reported to produce microbial lipases, which are significant enzymes used in a variety of applications in the dairy, bakery, oil, meat and fish processing, and beverage industries, for improving the food quality, as well as for the detergent and cosmetic industry.
These psychrophilic enzymes have been reported to have tremendous applications in industries. Some of the industrial applications of Antarctic living microorganisms have been described below:
Food Industry
The use of colour to make the food appear rich and tasty is an important factor in the food industry. So far, most of the colours added to the food are synthetic and therefore unhealthy. Recently, the focus has shifted towards natural colouring agents to avoid the adverse health effects of synthetic colours. A number of microbial pigments such as Arpink red™ from Penicillium oxalicum, astaxanthin produced by Xanthophyllomyces dendrorhous, lycopene from Fusarium sporotrichioides and Erwinia uredovora, riboflavin from Ashbya gossypii and β-carotene produced by Blakeslea trispora enhance the colour of different foods when supplemented [103]. Spray-dried prodigiosin from S. marcescens has been reported to be an effective colouring agent in milk, yogurt and carbonated drinks [104]. In addition, cold-active enzymes are a very potent tool for the food business. Foods are increasingly being processed in industrial settings at low temperatures in an effort to preserve energy while avoiding negative impacts on flavour, texture and nutritional value. A potentially significant enzyme in the dairy business is cold-active-galactosidase, which hydrolyses lactose to glucose and galactose at refrigeration temperature. A cold-active-galactosidase from the Antarctic psychrophile Pseudoalteromonas haloplanktis was patented by Hoyoux et al. in [105] for its ability to hydrolyse lactose during milk storage at low temperatures. Lipase is used to degum the vegetable oil and to alter the value of oil and fats to obtain more valuable products that are rich in poly unsaturated fatty acids. Lipases are also used for producing coco butter substitutes and human milk fat substitutes through the processes of esterification. Psychrophilic lipases reported from Antarctic region include many Moraxella sp., Halomonas, Pseudoalteromonas haloplanktis and Psychrobacter sp. [106].
Textile Industries
1.3 million tonnes of synthetic dyes and chemicals are used in the textile industry, 15% of which leak as effluents after use. Unfortunately, a significant part of these colours bypass traditional wastewater treatment methods and exist as a potentially harmful environmental contaminant with negative effects on human health and the environment. As a result, there is a huge concern about using ecologically friendly colours in place of synthetic dyes in the textile business. Microorganisms can produce environmentally friendly pigments that are deemed suitable for use in the textile industry. The textile industry uses microbial pigments extensively, which could boost their market value. As a result, it is vital to investigate novel microbial sources for pigment synthesis. The cryosphere harbours immense microbial diversity that has the ability to produce pigments useful for the textile industry. The use of cold-adapted cellulases in denim finishing and fabric production could improve the smoothness and softness of tissues, decolorize textile effluents and enable the development of ecologically friendly fibre processing techniques [107].
Medical and Pharmaceutical Applications
Studying psychrophilic bacteria as potential new tools for pharmacological and cosmetic applications is gaining popularity. Tomova et al. [64] observed that numerous promising psychrophilic strains were found to be a valuable source of novel active antibacterial chemicals at low temperatures. In recent years, substances produced by the halophilic actinomycete Nocardioides sp. Strain A-1 showed potential for use in agriculture for plant protection since it had antimicrobial activity against the bacteria Xanthomonas oryzae, which causes bacterial blight disease in rice. Antarticine-NF3, an antifreeze glycoprotein produced by the Antarctic bacterium Pseudoalteromonas, has been found to have effective results in scar treatment and is therefore being included in cosmetic regeneration creams [108]. Additionally, cold-adapted enzymes are highly effective in the field of pharmaceuticals. For example, cold-adapted dehalogenases have significant effects on the synthesis of optically pure pharmacological intermediates like halo-alkanoic acids. Cold-adapted lipases that are obtained from Candida antarctica can be used for several applications, such as to enhance the quality of beauty products, modifying sugars and their related compounds, synthesizing optically active drug intermediates, production of various cosmetic products, fragrance esters and pharmaceuticals [109].
Detergent and Cleaning Industry
Currently, the detergent industry accounts for 30–40% of all enzyme production worldwide and needs enzymes that can function at low temperatures in order to conserve energy. Cold-adapted enzymes such as lipases, proteases and α-amylases are vigorously used in manufacturing detergent, which can improve the efficacy of detergents and also decrease the amount of chemicals used in detergents, thereby protecting the texture and colours of fabrics and reduce wear and tear during washing. Esterases and lipases are vital enzymes since they can catalyse the cleavage of ester bonds and also help in reversing the reactions in organic solvents. The inclusion of lipases in detergents or cleaning solutions can also improve the detergents’ stain-removing abilities. Currently, cold-active subtilisins, isolated from Antarctic Bacillus species, are being used in the manufacture of cold-active detergents that can help in alkaline stability and cold activity needed for optimal washing results. Proteases, amylases and lipases are a few examples of cold-active enzymes that have shown to be quite effective in cleaning procedures at low temperatures. Wipes or other formulations containing psychrozymes can quickly clean solid objects that cannot be heated for washing. Due to their considerable market share in the enzyme industry, proteases are also shown to be important enzymes in detergents and washing powders. As detergent additions for cold washing, proteases isolated from Acinetobacter sp., Bacillus sp., Planococcus sp., Pseudomonas aeruginosa and Serratia marcescens can be employed [110].
Biodiesel Production
The production of biodiesel at industrial scale is done mostly by using vegetable oils through a process called transesterification. However, the use of vegetable oils for this purpose is an expensive affair and also leads to competition with the food sector due to which potential alternative raw materials for the process are under investigation. A practical alternative to traditional methods of producing biodiesel is microorganisms. The most crucial enzymes employed in the manufacture of biodiesel are lipases. Long-chain triacylglycerols are hydrolysed by lipases, producing free fatty acids, glycerol and mono- and diglycerols. Lipases catalyse the two-step transesterification of vegetable, animal and algal oils in the manufacture of biodiesel. Lipases obtained from Antarctic microbes have peculiar stability characteristics. Lipase obtained from soil bacterium Janibacter sp. R02 presents a halophilic, alkaliphilic and thermophilic profile [111]. A cold-tolerant lipase isolated from Pseudomonas sp. AMS8 exhibited excellent stability when organic solvents were present. It displayed 92%, 109% and 88% activity in the presence of 25% (v/v) xylene, octane and methanol, respectively [112]. For these applications, isolated Rhodotorula species from Antarctica also make great candidates. They have the capacity to accumulate significant amounts (50–70%, w/w) of “single cell oils” (SCOs) with a fatty acid composition characterized by a predominance of palmitic (16:0), oleic (18:1) and linoleic (18:2) acids, which is suitable for the biodiesel industry [113].
Nanoparticles Production
Through bio-mineralization processes, some bacteria are exceptional makers of inorganic nanoparticles from organic compounds. The Antarctic microbial population has been identified as possible biomineralizing candidates, although they are still unexplored. For instance, peroxide-resistant psychrophilic strains of Pseudomonas spp. are able to synthesize cadmium sulphide (CdS) when grown at 15 °C in a medium with added H2O2 and CdCl2 [114]. Magnetotactic cocci belonging to Alphaproteobacteria class and isolated from Antarctica marine sediments have the ability to produce elongated magnetite magnetosomes that can be used to produce iron nanoparticles. Purified magnetosomes have been used in a variety of applications, including contrast agents for magnetic resonance imaging, alternating magnetic field-induced hyperthermia agents for cancer treatments and support for enzyme immobilization. It has been discovered that bacteria from the genera Pseudomonas, Psychrobacter and Shewanella were able to synthesise CdS and CdTe QDs. Biosynthesized QDs post purification exhibit broad spectra of absorption and emission characteristics similar to biogenic Cd nanoparticles [114]. Brevundimonas, Bacillus and Rhodococcus, three recently isolated bacterial strains from a consortium linked to the psychrophilic Antarctic marine ciliate Euplotes focardii, were employed by John et al. [115] to synthesise silver nanoparticles (AgNPs).
15.6 Conclusion and Future Prospective
Due to the cold temperature of Antarctica region, their microbial diversity has developed various adaptations for surviving under such extreme cold environment. Nutrient mobilizing property and enzymes producing potential of these microorganisms made glory for them as they can efficiently work under low temperature and therefore have a wide application in agriculture, industry, medical, etc. Besides, they are responsible for the biogeocycling of the nutrients in the environment. So studies could be conducted to determine the role of specific microbial diversity in biogeocycling. Due to their lower richness, Antarctic lakes are excellent model systems to study how microbes affect geochemistry since it is easy to take in a significant amount of the variety and link certain taxa to specific processes. New knowledge on how microbes have adapted to the Antarctic environment under a wide range of chemical and physical circumstances is being gained through the use of molecular approaches. Managers of human activity, policy-makers, scientists and the general public can learn more about the causes, impacts and consequences of human activity, including anthropogenic climate change, by being given critical sentinel indicators.
References
Fretwell P, Pritchard HD, Vaughan DG, Bamber JL et al (2013) Bedmap 2: improved ice bed, surface and thickness datasets for Antarctica. Cryosphere 7:375–393
Cowan DA, Tow LA (2004) Endangered Antarctic environments. Annu Rev Microbiol 58:649–690. https://doi.org/10.1146/annurev.micro.57.030502.090811
Chong CW, Pearce DA, Convey P (2015) Emerging spatial patterns in Antarctic prokaryotes. Front Microbiol 6:1058. https://doi.org/10.3389/fmicb.2015.01058
Sadaiappan B, Kannan S, Palaniappan S, Manikkam R, Ramasamy B, Anilkumar N, Subramanian M (2020) Metagenomic 16S rDNA amplicon data of microbial diversity and its predicted metabolic functions in the Southern Ocean (Antarctic). Data Br 28:104876
Cavicchioli R, Charlton T, Ertan H, Mohd Omar S, Siddiqui KS, Williams TJ (2011) Biotechnological uses of enzymes from psychrophiles. J Microbial Biotech 4:449
Bottos EM, Laughlin DC, Herbold CW, Lee CK, McDonald IR, Cary SC (2020) Abiotic factors influence patterns of bacterial diversity and community composition in the Dry Valleys of Antarctica. FEMS Microbiol Ecol 96(5):fiaa042
Magalhaes C, Stevens MI, Cary SC, Ball BA, Storey BC, Wall DH, Ruprecht U (2012) At limits of life: multidisciplinary insights reveal environmental constraints on biotic diversity in continental Antarctica. PLos One 7(9):e44578. https://doi.org/10.1371/journal.pone.0044578
Velasco-Castrillon A, Schultz MB, Colombo F, Gibson JA, Davies KA, Austin AD, Stevens MI (2014) Distribution and diversity of soil microfauna from East Antarctica: assessing the link between biotic and abiotic factors. PLoS One 9(1):e87529
Convey P (1997) How are the life history strategies of Antarctic terrestrial invertebrates influenced by extreme environmental conditions? J Therm Biol 22(6):429–440
Aronson RB, Thatje S, McClintock JB, Hughes KA (2011) Anthropogenic impacts on marine ecosystems in Antarctica. Ann N Y Acad Sci 1223(1):82–107
Padeiro A, Amaro E, Dos Santos MM, Araújo MF, Gomes SS, Leppe M et al (2016) Trace element contamination and availability in the Fildes Peninsula, King George Island, Antarctica. Environ Sci Process Impacts 18(6):648–657
Ogaki MB, Coelho LC, Vieira R, Neto AA, Zani CL, Alves TM et al (2020) Cultivable fungi present in deep-sea sediments of Antarctica: taxonomy, diversity, and bioprospecting of bioactive compounds. Extremophiles 24:227–238
Rosa Luiz H et al (2019) Fungi in Antarctica: diversity, ecology, effects of climate change, and bioprospection for bioactive compounds. In: Fungi of Antarctica. Springer, Cham, pp 1–17
Tedersoo L, Sánchez-Ramírez S, Köljalg U, Bahram M, Döring M, Schigel D, May T, Ryberg M, Abarenkov K (2018) High-level classification of the fungi and a tool for evolutionary ecological analyses. Fungal Div 90:135–159
Bridge PD, Spooner BM (2012) Non-lichenized Antarctic fungi: transient visitors or members of a cryptic ecosystem? Fungal Ecol 5:381–394
Ruisi S, Barreca D, Selbmann L, Zucconi L, Onofri S (2007) Fungi in Antarctica. Rev Environ Sci Biotechnol 6:127–141
Cary SC, McDonald IR, Barrett JE, Cowan DA (2010) On the rocks: the microbiology of Antarctic Dry Valley soils. Nat Rev Microbiol 8:129–138
Núñez-Montero K, Barrientos L (2018) Advances in Antarctic research for antimicrobial discovery: A comprehensive narrative review of bacteria from Antarctic environments as potential sources of novel antibiotic compounds against human pathogens and microorganisms of industrial importance. Antibiotics 7:90. https://doi.org/10.3390/antibiotics7040090
Teixeira LCRS, Peixoto RS, Cury JC, Sul WJ, Pellizari VH, Tiedje J, Rosado AS (2010) Bacterial diversity in rhizosphere soil from Antarctic vascular plants of Admiralty Bay, maritime Antarctica. ISME J 4:989
O’Brien A, Sharp R, Russell NJ, Roller S (2004) Antarctic bacteria inhibit growth of food-borne microorganisms at low temperatures. FEMS Microbiol Ecol 48:157–167
Hultman J, Waldrop M, Mackelprang R et al (2015) Multi-omics of permafrost, active layer and thermokarst bog soil microbiomes. Nature 521:208–212. https://doi.org/10.1038/nature14238
Dhaulaniya AS, Balan B, Agrawal PK, Singh DK (2019) Cold survival strategies for bacteria, recent advancement and potential industrial applications. Arch Microbiol 201(1):1–16
Ghobakhlou AF, Johnston A, Harris L, Antoun H, Laberge S (2015) Microarray transcriptional profiling of Arctic Mesorhizobium strain N33 at low temperature provides insights into cold adaption strategies. BMC Genomics 16(1):1–14
Nagy G, Kerekes R (1981) Influence of cultivation temperature on the TTC-reducing capacity of psychrophilic, psychotropic, and mesophilic pseudomonas strains. ZentralblattfürBakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. ZweiteNaturwissenschaftlicheAbteilung: Mikrobiologie der Landwirtschaft, der Technologie und des Umweltschutzes 136(3):185–188
Ayala-del-Río HL, Chain PS, Grzymski JJ, Ponder MA, Ivanova N, Bergholz PW et al (2010) The genome sequence of Psychrobacter arcticus 273-4, a psychoactive Siberian permafrost bacterium, reveals mechanisms for adaptation to low-temperature growth. Appl Environ Microbiol 76(7):2304–2312
Celik Y, Graham LA, Mok YF, Bar M, Davies PL, Braslavsky I (2010) Superheating of ice crystals in antifreeze protein solutions. Proc Natl Acad Sci 107(12):5423–5428
Muñoz PA, Márquez SL, González-Nilo FD, Márquez-Miranda V, Blamey JM (2017) Structure and application of antifreeze proteins from Antarctic bacteria. Microb Cell Factories 16(1):1–13
Woldringh CL, Jensen PR, Westerhoff HV (1995) Structure and partitioning of bacterial DNA: determined by a balance of compaction and expansion forces? FEMS Microbiol Lett 131(3):235–242
Shivaji S, Prakash JS (2010) How do bacteria sense and respond to low temperature? Arch Microbiol 192(2):85–95
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141(2):312–322
Correa-Llantén DN, Amenábar MJ, Blamey JM (2012) Antioxidant capacity of novel pigments from an Antarctic bacterium. J Microbiol 50(3):374–379
Khan A, Singh AV, Pareek N, Arya P, Upadhayay VK, Kumar Jugran A, Kumar Mishra P, Goel R (2023a) Credibility assessment of cold adaptive Pseudomonas jesenni MP1 and P. palleroniana N26 on growth, rhizosphere dynamics, nutrient status, and yield of the kidney bean cultivated in Indian Central Himalaya. Front Plant Sci 14:1042053. https://doi.org/10.3389/fpls.2023.1042053
Khan A, Panthari D, Sharma RS, Punetha A, Singh AV, Upadhayay VK (2023b) Biofertilizers: a microbial-assisted strategy to improve plant growth and soil health. In: Advanced microbial techniques in agriculture, environment, and health management. Academic Press, pp 97–118
Bardgett RD, Van Der Putten WH (2014) Belowground biodiversity and ecosystem functioning. Nature 515(7528):505–511
Khan A, Singh AV (2021) Multifarious effect of ACC deaminase and EPS producing Pseudomonas sp. and Serratia marcescens to augment drought stress tolerance and nutrient status of wheat. World J Microbiol Biotechnol 37(12):1–17
Upadhayay VK, Singh AV, Khan A (2022a) Cross talk between zinc-solubilizing bacteria and plants: A short tale of bacterial-assisted zinc biofortification. Frontiers in Soil Science 1(788170):10–3389
Khan A, Upadhayay VK, Panwar M, Singh AV (2020a) Soil microbiota: A key bioagent for revitalization of soil health in hilly regions. In: Microbiological advancements for higher Altitude Agro-Ecosystems & Sustainability. Springer, Singapore, pp 183–200
de la Torre JR, Goebel BM, Friedmann EI, Pace NR (2003) Microbial diversity of cryptoendolithic communities from the McMurdo Dry Valleys, Antarctica. Appl Environ Microbiol 69(7):3858–3867
Straza TR, Cottrell MT, Ducklow HW, Kirchman DL (2009) Geographic and phylogenetic variation in bacterial biovolume as revealed by protein and nucleic acid staining. Appl Environ Microbiol 75(12):4028–4034
Badger MR, Bek EJ (2008) Multiple Rubisco forms in proteobacteria: their functional significance in relation to CO2 acquisition by the CBB cycle. J Exp Bot 59(7):1525–1541
Allan J, Ronholm J, Mykytczuk NCS, Greer CW, Onstott TC, Whyte LG (2014) Methanogen community composition and rates of methane consumption in Canadian high Arctic permafrost soils. Environ Microbiol Rep 6(2):136–144
Tveit AT, Urich T, Frenzel P, Svenning MM (2015) Metabolic and trophic interactions modulate methane production by Arctic peat microbiota in response to warming. Proc Natl Acad Sci 112(19):E2507–E2516
Khan A, Joshi M, Singh AV (2020b) Rhizospheric microbial community: ecology, methods, and functions. In: Sharma SK, Singh UB, Sahu PK, Singh HV, Sharma PK (eds) Rhizosphere microbiology. Springer, Singapore, pp 127–148
Ortiz M, Bosch J, Coclet C, Johnson J, Lebre P, Salawu-Rotimi A et al (2020) Microbial nitrogen cycling in Antarctic soils. Microorganisms 8(9):1442
Martínez-Pérez C, Mohr W, Löscher CR, Dekaezemacker J, Littmann S, Yilmaz P et al (2016) The small unicellular diazotrophic symbiont, UCYN-A, is a key player in the marine nitrogen cycle. Nat Microbiol 1(11):1–7
Vishniac HS (2006) Yeast biodiversity in the Antarctic. In: Biodiversity and ecophysiology of yeasts. Springer, Berlin, Heidelberg, pp 419–440
Chan Y, Van Nostrand JD, Zhou J, Pointing SB, Farrell RL (2013) Functional ecology of an Antarctic dry valley. Proc Natl Acad Sci 110(22):8990–8995
Singh J, Singh AV, Upadhayay VK, Khan A, Chandra R (2022) Prolific contribution of Pseudomonas protegens in Zn biofortification of wheat by modulating multifaceted physiological response under saline and non-saline conditions. World J Microbiol Biotechnol 38(12):1–20
Khan A, Singh AV, Upadhayay VK, Ballabh A, Prasad B (2022a) Influence of PGPR on growth and yield of oat (Avena sativa L.) under field conditions. Indian. J Ecol 49(4):1351–1356
Upadhayay VK, Singh AV, Khan A, Singh J, Pareek N, Raghav A (2022b) FE-SEM/EDX based zinc mobilization analysis of Burkholderia cepacia and Pantoea rodasii and their functional annotation in crop productivity, soil quality, and zinc biofortification of Paddy. Front Microbiol 13. https://doi.org/10.3389/fmicb.2022.852192
Khan A, Singh AV, Kumar R, Kukreti B, Bundela V, Upadhayay VK (2022b) Relative impact of PGPR inoculation on biofortification and yield of wheat under field conditions and their performance assessment through statistical tools. J Pharm Innov 11(8):490–495
Kaviya N, Upadhayay VK, Singh J, Khan A, Panwar M, Singh AV (2019) Role of microorganisms in soil genesis and functions. In: Mycorrhizosphere and Pedogenesis. Springer, Singapore, pp 25–52
Khan A, Singh J, Upadhayay VK, Singh AV, Shah S (2019) Microbial biofortification: A green technology through plant growth promoting microorganisms. In: Sustainable green technologies for environmental management. Springer, Singapore, pp 255–269
Punetha A, Punetha S, Khan A (2022) Soil community composition and ecosystem processes. In: Rukhsana, Alam A (eds) Agriculture, environment and sustainable development. Springer, Cham, pp 217–236
Upadhayay VK, Singh J, Khan A, Lohani S, Singh AV (2019) Mycorrhizal mediated micronutrients transportation in food based plants: A biofortification strategy. In: Mycorrhizosphere and Pedogenesis. Springer, Singapore, pp 1–24
Duarte AWF, Dos Santos JA, Vianna MV, Vieira JMF, Mallagutti VH, Inforsato FJ et al (2018) Cold-adapted enzymes produced by fungi from terrestrial and marine Antarctic environments. Crit Rev Biotechnol 38(4):600–619
Upadhayay VK, Khan A, Singh J, Singh AV (2021a) Bacterial assisted improved Zn consignment in root and shoot of rice plant by zinc solubilizing Serratia marcescens bearing plant probiotic traits. Adv Biores 13(1):1–8
Upadhayay VK, Singh AV, Khan A, Pareek N (2021b) Influence of zinc solubilizing bacterial co-inoculation with zinc oxide supplement on rice plant growth and Zn uptake. J Pharm Innov 10:113–116
Jorquera MA, Shaharoona B, Nadeem SM, de la Luz Mora M, Crowley DE (2012) Plant growth-promoting rhizobacteria associated with ancient clones of creosote bush (Larrea tridentata). Microb Ecol 64(4):1008–1017
Roshani KA, Singh AV, Upadhayay VK, Prasad B (2020) Development of potential microbial consortia and their assessment on wheat (Triticum aestivum) seed germination. Environ Ecol 38(1):6–16
Parveen H, Singh AV, Khan A, Prasad B, Pareek N (2018) Influence of plant growth promoting rhizobacteria on seed germination and seedling vigor of green gram. Int J Chem Stud 6(4):611–618
Fardella C, Oses R, Torres-Díaz C, Molina-Montenegro MA (2014) Antarctic fungal endophytes as tool for the reintroduction of native plant species in arid zones. Bosque 35:235–239. https://doi.org/10.4067/S0717-92002014000200011
Berríos G, Cabrera G, Gidekel M, Gutiérrez-Moraga A (2013) Characterization of a novel antarctic plant growth-promoting bacterial strain and its interaction with antarctic hair grass (Deschampsia Antarctica Desv). Polar Biol 36(3):349–362
Tomova I, Stoilova-Disheva M, Lazarkevich I, Vasileva-Tonkova E (2015) Antimicrobial activity and resistance to heavy metals and antibiotics of heterotrophic bacteria isolated from sediment and soil samples collected from two Antarctic islands. Front Life Sci 8(4):348–357
Presta L, Inzucchi I, Bosi E, Fondi M, Perrin E, Miceli E et al (2016) Draft genome sequence of Flavobacterium sp. strain TAB 87, able to inhibit the growth of cystic fibrosis bacterial pathogens belonging to the Burkholderia cepacia complex. Genome Announc 4(3):e00410–e00416
Khan A, Kukreti B, Makarana G, Suyal DC, Singh AV, Kumar S (2023c) Role of microorganisms in alleviation of arsenic toxicity in plants. In: Unravelling plant-microbe synergy. Academic Press, pp 263–281
Khan A, Sharma RS, Panthari D, Kukreti B, Singh AV, Upadhayay VK (2023d) Bioremediation of heavy metals by soil dwelling microbes: an environment survival approach. In: Pandey SC, Pande V, Sati D, Samant M (eds) Advanced microbial techniques in agriculture, environment and health management. Elsevier, London, pp 167–190
Ismail W, Gescher J (2012) Epoxy coenzyme A thioester pathways for degradation of aromatic compounds. Appl Environ Microbiol 78(15):5043–5051
Dias RL, Ruberto L, Hernández E, Vázquez SC, Balbo AL, Del Panno MT, Mac Cormack WP (2012) Bioremediation of an aged diesel oil-contaminated Antarctic soil: evaluation of the “on site” biostimulation strategy using different nutrient sources. Int Biodeterior Biodegradation 75:96–103
Pérez-de-Mora A, Engel M, Schloter M (2011) Abundance and diversity of n-alkane-degrading bacteria in a forest soil co-contaminated with hydrocarbons and metals: a molecular study on alkB homologous genes. Microb Ecol 62:959–972
Powell SM, Ferguson SH, Bowman JP, Snape I (2006) Using real-time PCR to assess changes in the hydrocarbon-degrading microbial community in Antarctic soil during bioremediation. Microb Ecol 52:523–532
Reyes-César A, Absalon AE, Fernández FJ, González JM, Cortés-Espinosa DV (2014) Biodegradation of a mixture of PAHs by non-ligninolytic fungal strains isolated from crude oil-contaminated soil. World J Microbiol Biotechnol 30:999–1009
Pini F, Grossi C, Nereo S, Michaud L, Giudice AL, Bruni V et al (2007) Molecular and physiological characterisation of psychotropic hydrocarbon-degrading bacteria isolated from Terra Nova Bay (Antarctica). Eur J Soil Biol 43(5–6):368–379
Aislabie JM, Balks MR, Foght JM, Waterhouse EJ (2004) Hydrocarbon spills on Antarctic soils: effects and management. Environ Sci Technol 38(5):1265–1274
Ruberto LA, Vazquez S, Lobalbo A, Mac Cormack WP (2005) Psychrotolerant hydrocarbon-degrading Rhodococcus strains isolated from polluted Antarctic soils. Antarct Sci 17(1):47–56
Stallwood B, Shears J, Williams PA, Hughes KA (2005) Low temperature bioremediation of oil-contaminated soil using biostimulation and bioaugmentation with a Pseudomonas sp. from maritime Antarctica. J Appl Microbiol 99(4):794–802
Saul DJ, Aislabie JM, Brown CE, Harris L, Foght JM (2005) Hydrocarbon contamination changes the bacterial diversity of soil from around Scott Base, Antarctica. FEMS Microbiol Ecol 53(1):141–155
Ma Y, Wang L, Shao Z (2006) Pseudomonas, the dominant polycyclic aromatic hydrocarbon-degrading bacteria isolated from Antarctic soils and the role of large plasmids in horizontal gene transfer. Environ Microbiol 8(3):455–465
Ferrari BC, Zhang C, Van Dorst J (2011) Recovering greater fungal diversity from pristine and diesel fuel contaminated sub-Antarctic soil through cultivation using both a high and a low nutrient media approach. Front Microbiol 2:217
Hua Z, Chen J, Lun S, Wang X (2003) Influence of biosurfactants produced by Candida Antarctica on surface properties of microorganism and biodegradation of n-alkanes. Water Res 37(17):4143–4150
Martorell MM, Ruberto LAM, Fernández PM, Castellanos de Figueroa LI, Mac Cormack WP (2017) Bioprospection of cold-adapted yeasts with biotechnological potential from Antarctica. J Basic Microbiol 57(6):504–516
Hughes KA, Bridge P, Clark MS (2007) Tolerance of Antarctic soil fungi to hydrocarbons. Sci Total Environ 372(2–3):539–548
Confalonieri F, Sommer S (2011) Bacterial and archaeal resistance to ionizing radiation. J Phys Conf Ser 261(1):012005. IOP Publishing
Das N, Chandran P (2011) Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int 2011:941810. https://doi.org/10.4061/2011/941810
Rogan-Finnemore M (2005) The legal implications of bioprospecting in the Antarctic region. UC Research Repository
Zucconi L, Canini F, Temporiti ME, Tosi S (2020) Extracellular enzymes and bioactive compounds from Antarctic terrestrial fungi for bioprospecting. Int J Environ Res Public Health 17(18):6459
Zainal N, Ser HL, Yin WF, Tee KK, Lee LH, Chan KG (2016) Streptomyces humi sp. nov., an actinobacterium isolated from soil of a mangrove forest. Antonie Van Leeuwenhoek 109:467–474
Vercesi A, Nasini G, Locci R (1992) Biological and chemical characterization of the antibiotic activity of Streptomyces species isolated from grapevine carposphere. Actinomycetes 3(1)
Rojas JL, Martín J, Tormo JR, Vicente F, Brunati M, Ciciliato I et al (2009) Bacterial diversity from benthic mats of Antarctic lakes as a source of new bioactive metabolites. Mar Genomics 2(1):33–41
Teoh CP, Koh SP, Ling CMWV (2020) Characterisation of an Antarctic yeast, Glaciozyma Antarctica PI12. Borneo Int J Biotechnol 1:89
Fenice M (2016) The Psychrotolerant Antarctic fungus Lecanicillium muscarium CCFEE 5003: A powerful producer of cold-tolerant Chitinolytic enzymes. Molecules 21:447
Martorell MM, Ruberto LAM, de Figueroa LIC, Mac Cormack WP (2019) Antarctic Yeasts as a Source of Enzymes for Biotechnological Applications. In: Rosa LH (ed) Fungi of Antarctica: diversity, ecology and biotechnological applications. Springer International Publishing, Cham, pp 285–304
Baeza M, Alcaíno J, Cifuentes V, Turchetti B, Buzzini P (2017) Cold-active enzymes from cold-adapted yeasts. In: Sibirny AA (ed) Biotechnology of yeasts and filamentous fungi. Springer International Publishing, Cham, pp 297–324
He L, Mao Y, Zhang L, Wang H, Alias SA, Gao B (2017) Functional expression of a novel α-amylase from Antarctic psychrotolerant fungus for the baking industry and its magnetic immobilization. BMC Biotechnol 17:22
Mao Y, Yin Y, Zhang L, Alias SS, Gao B, Wei D (2015) Development of a novel Aspergillus uracil deficient expression system and its application in expressing a cold-adapted α-amylase gene from Antarctic fungi Geomyces pannorum. Process Biochem 50:1581–1590
Yu P, Wang XT, Liu JW (2015) Purification and characterization of a novel cold-adapted phytase from strain JMUY14 isolated from the Antarctic: characterization of a novel cold-adapted phytase. J Basic Microbiol 55:1029–1039
Białkowska AM, Szulczewska KM, Krysiak J, Florczak T, Gromek E, Kassassir H, Kur J, Turkiewicz M (2017) Genetic and biochemical characterization of yeasts isolated from Antarctic soil samples. Polar Biol 40:1787–1803
Salwoom L, Raja Abd Rahman RNZ, Salleh AB, Mohd Shariff F, Convey P, Pearce D, Mohamad Ali MS (2019) Isolation, characterisation, and lipase production of a cold-adapted bacterial strain Pseudomonas sp. LSK25 isolated from Signy Island, Antarctica. Molecules 24(4):715
Furbino LE, Pellizzari FM, Neto PC, Rosa CA, Rosa LH (2018) Isolation of fungi associated with macroalgae from maritime Antarctica and their production of agarolytic and carrageenolytic activities. Polar Biol 41:527–535
Marizcurrena JJ, Martı’nez-Lo’pez W, Ma H, Lamparter T, Castro-Sowinski S (2018) A highly efficient and cost-effective recombinant production of a bacterial photolyase from the Antarctic isolate Hymenobacter sp. UV11. Extremophiles 23:49
Gil-Dura’n C, Ravanal MC, Ubilla P, Vaca I, Cha’vez R (2018) Heterologous expression, purification and characterization of a highly thermolabile endoxylanase from the Antarctic fungus Cladosporium sp. Fungal Biol 122:87582
See-Too WS, Convey P, Pearce DA, Chan KG (2018) Characterization of a novel N-acyl homoserine lactonase, AidP, from Antarctic Planococcus sp. Microb Cell Factories 17:179
Dharmaraj S, Ashokkumar B, Dhevendaran K (2009) Fermentative production of carotenoids from marine actinomycetes. Iran J Microbiol 1(4):36–41
Namazkar S, Ahmad WA (2013) Spray-dried prodigiosin from Serratia marcescens as a colorant. Biosci Biotechnol Res Asia 10(1):69
Hoyoux A, Jennes I, Dubois P, Genicot S, Dubail F, François JM et al (2001) Cold-adapted β-galactosidase from the Antarctic psychrophile Pseudoalteromonas haloplanktis. Appl Environ Microbiol 67(4):1529–1535
Kavitha M (2016) Cold active lipases—an update. Front Life Sci 9:226–238
Araujo R, Casal M, Cavaco-Paulo A (2008) Application of enzymes for textile fibres processing. Biocatal Biotransformation 26:332–349. https://doi.org/10.1080/10242420802390457
Bisht S (2011) Cold active proteins in food and pharmaceutical industry [article on the Internet]. Biotech Articles. [cited 2017 Dec. 15]. Available from http://www.biotecharticles.com/Biotechnology-products-Article/ColdActive-Proteins-in-Food-and-Pharmaceutical-Industry-719
Hamid B, Bashir Z, Yatoo AM, Mohiddin F, Majeed N, Bansal M, Poczai P, Almalki WH, Sayyed RZ, Shati AA et al (2022) Cold-active enzymes and their potential industrial applications—a review. Molecules 27:5885. https://doi.org/10.3390/molecules27185885
Furhan J, Awasthi P, Sharma S (2019) Biochemical characterization and homology modeling of cold-active alkophilic protease from Northwestern Himalayas and its application in detergent industry. Biocatal Agric Biotechnol 17:726–735
Castilla A, Giordano SR, Irazoqui G (2022) Extremophilic lipases and esterases: characteristics and industrial applications. In: Kuddus M (ed) Microbial Extremozymes. Academic Press, pp 207–222
Ganasen M, Yaacob N, Rahman RN, Leow AT, Basri M, Salleh AB et al (2016) Cold-adapted organic solvent tolerant alkalophilic family I.3 lipase from an Antarctic pseudomonas. Int J Biol Macromol 92:1266–1276
Viñarta SC, Maza DD, Fernández PM, Aybar MJ, de Figueroa LIC (2022) Integrated production of biodiesel and industrial wastewater treatment by culturing oleaginous microorganisms. In: Kumar V, Kumar M (eds) Integrated environmental technologies for wastewater treatment and sustainable development. Elsevier, Amsterdam, pp 81–101
Plaza DO, Gallardo C, Straub YD, Bravo D, Pérez-Donoso JM (2016) Biological synthesis of fluorescent nanoparticles by cadmium and tellurite resistant Antarctic bacteria: exploring novel natural nanofactories. Microb Cell Factories 15(1):1–11
John MS, Nagoth JA, Ramasamy KP, Ballarini P, Mozzicafreddo M, Mancini A et al (2020) Horizontal gene transfer and silver nanoparticles production in a new Marinomonas strain isolated from the Antarctic psychrophilic ciliate Euplotes focardii. Sci Rep 10(1):1–14
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Khan, A. et al. (2023). Microbial Community Dynamics of Antarctica: Their Ecological Potential and Industrial Importance. In: Soni, R., Suyal, D.C., Morales-Oyervides, L. (eds) Microbial Bioactive Compounds. Springer, Cham. https://doi.org/10.1007/978-3-031-40082-7_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-40082-7_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40081-0
Online ISBN: 978-3-031-40082-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)