Abstract
Deschampsia antarctica is the only hair grass that has been able to successfully colonize the Antarctic continent. However, there is little research on the role of microorganisms associated with the rhizosphere that may participate in its growth and development. The objective of this research was to characterize a psychrotolerant bacterial strain isolated from the rhizosphere of D. antarctica. Biochemical and molecular studies were performed to characterize this bacterium. It was determined that this strain secretes a neutral polysaccharide that presents different compositions at different temperatures (4 and 20 °C). Based on biochemical and phylogenetic analyses, the Antarctic rhizobacterium could be a new species of Pseudomonas. To determine their ability to solubilize different sources of inorganic phosphate, qualitative and quantitative analyses were conducted to determine P released at 4 °C. The Antarctic strain of Pseudomonas sp. was able to solubilize all sources of phosphates, and 34.2 mg P/L was released from rock phosphate. Growth physiological parameters were evaluated for seedlings of D. antarctica inoculated with the rhizobacteria. It was found that the bacterial inoculation promoted plant root development. SEM analysis of the roots showed that the bacterium is mainly located in the root hairs of D. antarctica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Antarctic continent is a place where climate and soil present extreme characteristics; nevertheless, it is full of life, and its unique features still allow species of plants and animals to adapt to harsh environments. D. antarctica Desv is one of the most studied plant species in Antarctica because it is one of the two vascular plants able to withstand the extreme conditions (Alberdi et al. 2002).
Most of the research done on D. antarctica has been devoted to studying the adaptation of this plant to the cold environment and to understand its physiological and molecular mechanisms of tolerance to UV radiation and high salinity (Ruhland et al. 2005; Parnikoza et al. 2011; Chew et al. 2012). However, there are few reports on external factors that may facilitate the adaptation and dissemination of this plant in the Antarctic (Gielwanowska et al. 2011; Chew et al. 2012).
The ability of plants to adapt to adverse environmental conditions defines their long-term survival and geographical and environmental distribution. Sometimes, such adaptations are the result of interactions between roots and soil microorganisms. Furthermore, it has been determined that rhizosphere microorganisms play an important role in the biogeochemical cycle of macronutrients important for plant development. In particular, P is an essential element for the growth and development of plants and microorganisms. Although P is abundant in soils, in both organic and inorganic forms, plants are only able to absorb it from the soil solution as phosphate anions, and often, available P is a limiting factor for plant growth. This is due mainly to the fact that phosphate anions are extremely reactive and can be immobilized by precipitations with cations such as Ca2+, Mg2+, Fe3+, and Al3 +, reducing its availability to plants (Gyaneshwar et al. 2002). This can be a serious problem in soil fertility, especially those derived from volcanic ash, such as the case in Antarctic soils.
Some species of microorganisms, especially those associated with the plant roots, have the ability to solubilize P from the soil, making it readily available for plant uptake (Cakmakci et al. 2006). The insoluble inorganic phosphate may be converted into P available through metabolic activities, for example, through organic acid secretion which in turn dissolves phosphate rock, and forming highly stable calcium chelates, originating from the phosphate salts, thereby releasing the soluble P into the soil solution (Chung et al. 2005). The production of microbial metabolites results in a decrease in soil pH, which probably plays an important role in P solubilization (Stevenson 2005). Most studies on phosphate solubilizing bacteria are limited to mesophilic temperatures (Chung et al. 2005; Chen et al. 2006). A few species of bacteria such as Pseudomonas corrugata (Trivedi and Sa 2008) and Pseudomonas fluorescens (Katiyar and Goel 2003), both mutant strains, and native psychrotolerant bacteria such as Pantoea dispersa (Selvakumar et al. 2008), Pseudomonas fragi (Selvakumar et al. 2009), and Pseudomonas lurida M2RH3 (Selvakumar et al. 2011) are known to solubilize phosphates at low temperature. However, the inorganic phosphate solubilization activity at low temperature has not been previously reported for bacterial strains isolated from Antarctic soil or from the rhizosphere of D. antarctica. In addition, there is no information in previous work that had been published about the interaction at the physiological level of phosphate solubilizing bacteria with D. antarctica. In an earlier study (Barrientos et al. 2008), several bacterial strains from the rhizospheric soil D. antarctica were isolated and characterized. A wide range of psychrotolerant bacteria were found in Antarctic soil samples, and they were found to be resistant to antibiotics and tolerant to heavy metals. Based on strain identification using the 16S rRNA gene, most of the bacterial isolates appeared to be Pseudomonas species, and some also belonged to the genera Flavobacterium and Arthrobacter.
The aim of the present research was to conduct a molecular and biochemical characterization of one of the bacterial strains isolated from the rhizosphere of D. Antarctica and to determine their ability to solubilize inorganic phosphate from different sources, at low temperature. Specifically, we tested whether the strain might be classified as Plant Growth-Promoting Rhizobacteria (PGPR). In addition, we will be using D. antarctica and the bacterial strain on a plant–microbe interaction study at physiological level. In this regard, we want to understand the role of Antarctic soil microorganisms associated with of D. antarctica rhizosphere and if they are able to help this plant in its adaptation and survival in extreme environmental conditions of Antarctica.
Materials and methods
Biological material. The organism used in this study corresponds to the Antarctic strain Pseudomonas sp. Da-bac TI-8 PTA 8990. Its 16S rRNA gene sequence has been deposited in GenBank under accession number JQ598792. 1. This strain was isolated from rhizosphere soil samples of D. antarctica, in the work carried out by Barrientos et al. (2008). Prior evaluation showed that it was a psychrotolerant bacterium, having an optimum growth at temperature around 20 °C. Because of this, subsequent analyses were performed with two different incubation temperatures (4 and 20 °C).
Growth at different temperatures. Strain Da-bac TI-8 was grown in triplicate in liquid medium at 4 and 20 °C. The LB (Luria–Bertani) culture medium was used, consisting of 10 g tryptone, 10 g NaCl, and 5 g of yeast extract in 1,000 mL of distilled water. The medium pH was adjusted to 7 before being sterilized by autoclaving. The experiment was conducted in shake flasks with a capacity of 1,000 mL with constant stirring, containing 250 mL of LB medium and an initial inoculum of 1 mL of the bacterial strain removed from a culture during logarithmic phase of growth at 20 °C. To quantitate the bacteria, aliquots of 1 mL were periodically taken in the case of incubation at 20 °C, sampling times were every 3 h and for incubation at 4 °C, the sampling intervals were every 24 h. Measuring the absorbance was performed spectrophotometrically MBA 2000 (Perkin Elmer) at a wavelength of 520 nm, in triplicate, and the results were expressed as mean values. The technique used to estimate biomass was dry weight. This technique was supplemented by indirect measurement of medium turbidity by measuring absorbance. In addition, we analyzed the following bacterial growth parameters such as specific growth rate (μ), generation time (g) according to the methodology described by Nedwell and Rutfer (1994).
Fatty acid analysis. The analysis of methyl esters fatty acid was performed by GLC according to the instructions of the Microbial Identification System (MIDI). Fatty acids were analyzed and determined with a GC—Clarus 500 equipment (Perkin Elmer) equipped with a FID (Flame Ionization Detector) and a specific column for fatty acids SP™ Fused Silica Capillary Column 2380 (60 m × 0.25 mm × 0.2 μm film thickness, SUPELCO) using a temperature program of 150 °C for 1 min, then increased at 1 °C/min to 168 °C held there for 11 min, increase at 6 °C/min to 230 °C and held for 8 min. Fatty acids were identified and quantified by comparison of the methyl esters fatty acids (FAME Mix C4–C24 and octadecadienoic acid, methyl conjugates).
Phenotypic characterization. Test using carbon sources was determined by the method API 50 CH (BioMerieux, France). Samples were incubated 48 h at 20 °C and 72 h at 4 °C. Furthermore, the enzymatic activities of this strain were determined by using the semiquantitative method API ZYM (BioMerieux SA, France), incubating the samples at 20° and 4 °C for 16 h. Subsequently, the readings were performed according to manufacturer’s instructions.
16S rDNA sequence analysis of. The 16S rRNA gene fragment from the Antarctic strain Da-bac TI-8 was amplified by PCR with primers F8 (forward) 5′-AGAGTTTGATCCTGGCTCAG-3′ and R1492 (reverse) 5′-GGTTACCTTGTTACGACTT-3′, which amplified a product of 1,500 bp (Weisburg et al. 1991; Baker et al. 2003). The PCR was performed according to the method described by Barrientos et al. (2008). Subsequently, these samples were sequenced with an ABI 3730XL (Applied Biosystems) sequencer by the company Macrogen (Korea). The sequence was analyzed and identified through the database “Living Tree Project” (LTP http://www.arb-silva.de/projects/living-tree) with the collaboration of Dr. Ramon Rosselló-Mora “from the Mediterranean Institute for Advanced Studies”(IMEA).
Estimate of the production of exopolysaccharide (EPS)
The EPS extraction process was performed according to the methodology described by Quezada et al. (1993). We started with a 5 ml preinoculum of Da-bac TI-8 strain in LB medium, obtained after 24 h of growth at 20 °C, from this culture, 1 mL was removed for inoculation of 500 mL Erlenmeyer flasks containing 250 mL of LB medium. From these flasks, some were incubated at 20 °C and others at 4 °C. Every 24 h, 50 mL of bacterial culture was removed and centrifuged at 10,000 rpm for 60 min (Beckman Centrifuge). Three volumes of cold ethanol 96 % (v/v) were added to the supernatant and then stored at 4 °C during 12 h. Then, the white precipitate of extracellular material (EPS) was collected by centrifugation at 7,000 rpm for 10 min. The precipitate was washed two times with absolute ethanol and then dried at room temperature. The remaining solid was dissolved in distilled water and then dialyzed using a Midicell dialysis membrane pore size 12–14,000 Dalton, for 72 h in distilled water, and finally, it was lyophilized and weighed to determine yields.
Characterization EPS
Colorimetric analysis. Protein content in the EPS was determined by the Bradford method (1976), with bovine albumin serum as a standard, and the total neutral sugar content was determined by the method described by Dubois et al. (1956), using glucose as a standard.
Monosaccharide analysis. All samples were analyzed by high performance anion exchange chromatography with pulsed amperometric detection (HPAEC–PAD) (Taboada et al. 2010). Total monosaccharide compositions were determined after one step hydrolysis using 72 % (v/v) H2SO4 at 120 °C for 60 min. The hydrolyzed samples were filtered, and 100 μL samples were made to 5 mL with water UP (ultra pure). Samples of 4 mL were used for injection in the HPAEC-PAD system. Chromatography of the samples was performed using a Dionex LC system coupled to an AS 50 autosampler. The HPAEC system was equipped with a CarboPac PA10 column (4 × 250 mm) in combination with a CarboPac guard column and run at 30 °C. Separation was performed with a flow rate of 0.7 mL/min in the column and a total flow rate of 1 mL/min in the post-column using a combined gradient of two eluents prepared using degassed distilled HPLC grade water (Fisher Chemical): eluent A, 0.17 M sodium acetate in 0.2 M NaOH solution; eluent B, distilled water. Gradients of A and B were used in sequence to elute monosaccharides. This resulted in the following gradient: A 0–5 min, 100 %; B 6–31.5 min. Samples (5 μL) were injected at 15.5 min. NaOH (0.3 M) was used post-column at a flow rate of 0.3 mL/min. The effluent was monitored using a pulsed-electrochemical detector in the pulse-amperometric mode with a gold working electrode and a Ag/AgCl reference electrode to which potentials of E1 = 0.1 V, E2 = −2.0 V, E3 = 0.6 V, and E4 = −0.1 V were applied for duration times t1 = 0.4 s, t2 = 0.02 s, t3 = 0.01 s, and t4 = 0.07 s, respectively. Quantification of the samples was performed using the response factors calculated from the peak areas of the mixed standard solutions for six sugars (glucose, mannose, rhamnose, galactose, arabinose, and xylose). Fucose was used as internal standard.
Analysis of mineral phosphate solubilization
The strain solubilizing phosphate activity was determined according to the protocol of Pikovskaya (1948). In summary, the bacterial strain was grown in PVK agar medium. The utilized phosphate sources were tricalcium phosphate [Ca3(PO4)2], monobasic calcium phosphate [Ca(HPO4) 2H2O], and rock phosphate (Gafsa). During the evaluation of phosphate rock solubilization, a pH indicator (bromophenol blue) was added to the culture medium (to improve visibility of the halo produced). The amount of P applied was equivalent to 400 mg P/L for each of the sources used. The inoculation was accomplished with an aliquot (5 μL) of the bacterial suspension of a known concentration (1 × 106 CFU/mL). Subsequently, the plates were incubated at 15 °C for 120 h and analyzed visually. Using as a criterion for phosphate solubilization, a halo formation (solubilization) around the colony (Das 1989; Singal et al. 1991), the ratio between the diameter of the halo and the diameter of the colony was calculated.
Quantitative analysis of phosphate solubilization
Quantitative estimation of solubilized phosphorus was carried out by growing the bacteria in 25 mL of PVK liquid medium, supplemented with phosphate rock (400 mg P/L) in 125 mL Erlenmeyer flasks on a rotary shaker (125 rpm) at 4 °C 120 h. Control flasks were not inoculated with bacteria. The strain was tested in quintuplicate. Subsequently, samples were centrifuged at 10,000 rpm for 10 min. at 4 °C, and the content of soluble phosphorus was determined in the supernatant using the Phosphomolibdic—ascorbic acid method (Murphy and Riley 1962). Five replicates per treatment were used, and the results were statically analyzed by ANOVA and Tukey Means Comparison test (P ≤ 0.05), using the statistical software SPSS 11.0.
Organic acid identification
Identification of organic acids released by the bacteria into the PVK liquid culture media was performed from analysis of the culture supernatant using HPLC–UV equipment (Hwangbo et al. 2003). A Kromasil column C18 RP (4.6 × 250 mm, 5 μm) was used as a mobile phase 0.1 % H3PO4, with a flow of 0.5 mL/min. The corresponding peaks for each organic acid were detected at 220 nm and identified according to retention time of commercial standards: oxalic acid, citric acid, gluconic acid, and malic acid.
Indole acetic acid (IAA) production
To determine the IAA produced, we adopted the methodology described by Bric et al. (1991). The measurements were made by analyzing three replicates of the strain T1-8 grown in 3 mL of LB medium supplemented with l-tryptophan (1, 2 and 5 mg/mL). Control treatments did not receive l-tryptophan supplementation. Cultures were maintained on a gyratory shaker (120 rpm) and were incubated at two temperatures (4° and 20 °C) for 48 and 216 h, respectively, as described by Ahmed et al. (2005). Subsequently, the bacterial cultures were centrifuged at 4,800 rpm for 15 min at 4 °C. For IAA analysis, and 1 mL of the supernatant was withdrawn and mixed with 2 mL of Salkowski reagent (20 mL of FeCl3, 57.45 mL H2O, and 22.55 mL H2SO4), under stirring for 15 s with a vortex mixer. The mixture was allowed to stand for 30 min, and then, the absorbance at 530 nm was read in a Nanodrop device. A pink color development indicates the production of IAA. IAA concentration produced in the culture medium was estimated using a standard curve of IAA. The results were analyzed by ANOVA and Dunnet’s mean comparison (P ≤ 0.05), using the statistical software SPSS 11.0.
Analysis of plant growth promotion
Deschampsia antarctica plants grown in vitro were inoculated with a bacterial concentration of 103 CFU/mL in order to study the effect of different temperatures (13 and 22 °C) on plant–microbe interaction. In all cases, D. antarctica seedlings were inoculated with 0.1 mL of bacteria. The seedlings were placed in square Petri dishes (12 × 12 cm) containing 50 % Murashige–Skoog medium. For each treatment, 30 seedlings were inoculated, 10 plates, three replicates per treatment. Once the seedlings were inoculated with the respective treatments and placed in culture dishes, they were incubated for 2 months. At the end of the test, the following biometric parameters were measured: root number, root length, leaf dry mass, and root dry mass. All biometric results were subjected to a variance analysis (ANOVA) for one factor, and means were compared by the Dunnet test for P ≤ 0.05, using the statistical program SPSS 11.0.
Scanning electron microscopy (SEM)
Bacterial cell presence on the root surface of D. antarctica were visualized by SEM on 5 mm root samples from inoculated and non-inoculated plants. Root pieces were fixed in 2.5 % glutaraldehyde (100 mM buffer phosphate, pH 7) for 4 h at room temperature. Samples were dehydrated using a series of ethanol washes followed by a series of acetone washes and then dried at critical point using CO2 (Sorvall Critical Point Drying System, Connecticut, USA). Samples were covered with 99.99 % gold–palladium (60:40 Au:Pd ratio) (Pelco, Watford, UK), in a Pelco Sputter 9100 (Polaron Equip-ment Ltd., Watford, UK). Root samples were examined using a scanning electron microscope, LEO 1420 VP (LEO Electron Microscopy Ltd., Cambridge, UK) (Fig. 1).
Results
Molecular and biochemical characterization of strain Da-bac TI-8
Growth of Pseudomonas sp. Da-bac TI-8. In order to understand the growth of Pseudomonas sp. Da-bac TI-8 strain at different temperatures, the bacterial growth curves were determined, and the results are shown in Fig. 1. The bacterial strain Pseudomonas sp., isolated from the Antarctic soil, showed a classical bacterial growth curve in LB medium. Bacteria cultured at 20 °C showed a phase delay of 3 h, followed by an exponential phase lasting 33 h achieving a growth rate of 0.045 g/h. After this time, there was a steady growth, which was continued for 72 h. On the other hand, when the bacteria were incubated at 4 °C, there was a greater duration of each growth phase, the adaptation phase occurred during the first 15 h, the exponential growth started at 216 h, and the stationary phase was maintained until 500 h of incubation. The rate of bacterial growth at this temperature was 0.027 g/h. The bacterial strain in optimum temperature conditions (20 °C) doubled every 1.31 h, while at 4 °C, the doubling time was every 4.31 h. Growth assays at 37 °C failed because growth was totally inhibited.
Fatty acid profile. Results of the fatty acid analysis at different growth temperatures are shown in Table 1. Fatty acid analysis revealed that the methyl ester derivatives of fatty acids of this strain grown at 4 °C, consisted of C15:1, C16:1, C17:1, and C15:1 as unsaturated fatty acids. Among the constituents, C15:1 was the major component comprising 42.71 % of the total fatty acids. Saturated fatty acids consisted of C13:0, C16:0, and C17:0. Among the polyunsaturated fatty acids, C18:1nt and C24:1n9 were observed. When the bacteria were grown at 20 °C, they showed a fatty acid profile where new short chain fatty acids such as C8:0 were present. Fatty acid C13:0 was most abundant at this temperature (24.99 %).
Phenotypic characterization. Physiological and biochemical characteristics (enzymatic activity and carbon source) of this strain are listed in Tables 2 and 3.
16S rRNA gene sequence analysis. To identify the bacterial strain Da-bac TI-8 isolated from the D. antarctica rhizosphere, a phylogenetic analysis was performed. As shown in the phylogenetic tree (Fig. 2), the similarity of the 16S rRNA gene sequence of strain Da-bac TI-8 with the Pseudomonas species is greater than 99 %. Strain Da-bac TI-8 showed a high similarity with Pseudomonas brenneri AF268969 (99.5 %), Pseudomonas migulae AF074383 (99.3 %), Pseudomonas proteolytica AJ537603 (99.2 %), and Pseudomonas panacis AY787208 (99.1 %). However, there is an obvious gap between the Antarctic strain and other strains in the phylogenetic tree. This prompted speculation that this organism may constitute a new species of Pseudomonas. In order to obtain a better characterization of this strain, several biochemical studies were performed as described below.
Production and Characterization of Exopolysaccharides. Figure 3 shows the kinetics of exopolysaccharide (EPS) production at two different temperatures (4° and 20 °C). In both cases, the production of exopolysaccharides increased progressively during the logarithmic growth phase and reaches a maximum in the stationary phase. This suggests that the production of EPS by Pseudomonas sp. strain Da-bac TI-8 is a process that could be associated bacterial growth.
The highest EPS production was obtained at 20 °C (0.43 mg/mL), which corresponds to 0.15 mg EPS/mg dry biomass at 72 h. When the Antarctic bacteria was incubated at 4 °C, it was observed that at 25 h of incubation, the EPS production (0.1 mg/mL) was lower than reached at the same time at 20 °C. However, the ratio EPS/biomass was higher at 4 °C. This suggests that at 4 °C, the bacteria are producing and secreting this polysaccharide to the culture medium perhaps to act as a viscosifying agent. At this temperature, the maximum production was 2.11 mg EPS/mL of culture medium, corresponding to 1.7 mg EPS/mg of dry biomass at 200 h. It is important to note that the weight ratio of EPS/biomass at 4 °C is 11 times higher than at 20 °C. This suggests that the production and excretion of this metabolite could somehow be associated with the bacterial adaptation mechanisms to low temperature.
In order to know more about the nature of bacterial EPS, produced and secreted by Pseudomonas sp. strain Da-bac TI-8 at different temperatures, its chemical composition was determined. The EPS were dialyzed to remove excess salt and low molecular weight sugars that may be present as impurities. Once the EPS was dried, the protein content was determined, and it was very low for both temperatures obtaining values of 1.24 % and 2.48 % (w/w) when grown at 20° and 4 °C, respectively. The analysis of the EPS sugar composition after total hydrolysis revealed the presence of three neutral sugars at 20 °C: mannitol, galactose, and glucose.
In contrast, the EPS obtained at 4 °C yielded four neutral sugars residues. In addition, from above sugars, rhamnose was also found, but only in trace amounts. The sugars were found in a molar ratio mannitol:galactose:glucose (1.0:0.7:0.7). In both cases, mannitol was the most abundant sugar residue of EPS produced by Pseudomonas sp. strain Da-bac TI-8.
Solubilization of P. In order to understand the functionality of this psychrotolerant strain of Pseudomonas sp. Da-bac TI-8 that was isolated from the rhizosphere of D. antarctica, we assessed the P solubilization ability from different inorganic substrates. First, a qualitative analysis was performed by measuring the halo that was formed around the inoculation site of the bacteria in the medium on PVK-solid agar enriched with various phosphate substrates. As an insoluble substrate, we used calcium phosphate dehydrate, calcium hydrogen phosphate and phosphate rock (Gafsa). The results obtained are shown below in Table 4.
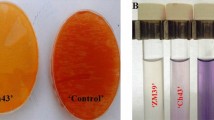
The solubilizing capacity of inorganic P, of the strain Pseudomonas sp. Da-bac TI-8, was visible in the agar medium-PVK, where a clear halo was produced around the bacteria (Fig. 4). The diameter of the halo formed by the bacteria varied between 3.3 and 5.3 mm, with the larger halo being when Ca (HPO4)2 H2O was P source. Differences in size of the solubilization halo may be due to the variation in the growth rate by the strain for each of the substrates studied.
The solubilizing capacity of inorganic P at 4 °C and the final pH of the liquid culture medium are presented in Table 4. It was observed that this strain solubilized 34.3 mg/L of P from a natural mineral like phosphate rock. On the other hand, the control showed a small concentration of solubilized phosphorus (2.5 mg/L to P). According to some authors (Goldstein 2000; Goenadi et al. 2000), this should be the natural phosphate solubilization mechanism, characterized by the slow release of P to the environment, and especially in the case of phosphate rock. Moreover, the final pH of the medium decreased from 6.5 to 4.2, indicating that compounds with acidic properties (protons) were released into the medium.
In a previous study, conducted at room temperature (21 °C), it was demonstrated that some bacteria were capable of solubilizing phosphate from phosphate rock in an amount ranging from 70 to 140 mg/L of P.
IAA production. The bacterial production of IAA, at different temperatures, after adding different concentrations of tryptophan to the nutrient broth is presented in Fig. 5. It could be observed that the amount of IAA produced increases along with the amount of tryptophan added to culture broth, being higher the production of this phytohormone at 20° than at 4 °C, respectively. This difference could be related to the different growth rate of this specie at such temperatures, considering that IAA maybe a secondary metabolite that would require a specific growth phase of bacterial strain for its production.
Effect of inoculating D. antarctica with PGPR bacteria
In order to determine whether the strain of psychrotolerant Pseudomonas sp. isolated from the rhizosphere of D. antarctica has any promoting effect on plant growth, a direct inoculation test was performed. The results of the physiological changes that occur in the plant at different temperatures are shown in Fig. 6 and Table 5. The measurements were performed at 13 °C because it is the optimum growth temperature of D. antarctica. The lower inoculum concentrations (101 and 102 CFU/mL) tested caused no significant effect on growth of Deschampsia. In this study, concentrations greater than 103 CFU/mL of bacteria caused plant growth inhibition.
In vitro inoculation assay revealed that inoculated seedling D. antarctica, at a concentration of 103 CFU/mL of bacteria, had no effect on plant root length at both temperatures. At 22 °C, there was no effect on plant shoot dry weigh or root dry weight, but there was a significant effect on the shoot dry weight/root dry weight ratio regarding control treatment.
By other side, at 13 °C, the inoculation with psychrotolerant bacteria do not have any difference with control treatment on root length, but it have significant differences on root dry weight, shoot dry weight as well as in the shoot dry weight/root dry weight ratio. It is interesting to note that the shoot/root ratio was higher than control at 22 °C but lower than control at 13 °C.
SEM analysis
In order to have a confirmation that antarctic Pseudomona sp., isolated from the rhizosphere of D. antarctica, could be attached to its root surface on their natural environment, and a SEM analysis of plant roots previously inoculated with the bacterium was performed. The SEM micrographs are shown in Fig. 7. In Fig. 7a, the topography of Deschampsia root hairs is presented, and they showed a morphology similar to other species from the same family (Gramineae). Figure 7b shows that bacterial colonization occurred preferentially in the absorbent hairs of D. antarctica where a large number of individual cells were in this area. On the contrary, bacterial colonization showed a low number of single cells on the surface of the primary roots (Fig. 7c). Finally, there were no detectable bacteria on the surface of the root of control plants (Fig. 7d). These results suggest that in the Antarctic environment, the Pseudomonas sp. could habitat near the surface of Deschampsia roots.
SEM micrograph of roots D. antarctica grown at 13 °C. a Deschampsia root hairs morphology. b Root hairs surface of plant colonized by Pseudomonas sp. Da-bac TI-8 strain, 2 months after inoculation. c Root surface of plant colonized by Pseudomonas sp. Da-bac TI-8 strain, 2 months after inoculation. d Root surface of non-inoculated plant free of bacteria (control)
Discussion
Molecular and biochemical characterization of strain Da-bac TI-8
Growth of Pseudomonas sp. Da-bac TI-8
Results of growth at different temperatures indicate that Pseudomonas sp. Da-bac TI-8 is a microorganism psychrotolerant because it can grow at 4° and 20 °C, but not at 37 °C. It has been shown that the temperature variation in the psychrotolerant bacterial cultures influences the bacterial growth phases duration. Pseudomonas sp. Dabac T1-8 cultivated 4 °C showed slow growth with long lasting on each growth stages. Panicker et al. (2002) obtained similar results when assessing the growth of a Pseudomonas sp. isolated from Antarctica. They observed in a bacterial media grown at 30° and 35 °C, respectively, a decrease in the number of cells after been cultured during 50 h. On the contrast, the cultures grown at 0 or 4 °C exhibited a relatively slow growth during the initial 24 h of incubation, concluding that this could be due to the fact that at lower temperature there is a reduction in the rate of substrates uptake, taken up by active transport, mainly due to the reduction of the fluidity of the cell membrane, as described Nedwell (1999).
Nevot et al. (2008) showed that a reduction in the incubation temperature causes an increase in the lag phase. Results obtained by Nevot et al. (2008), determined that the lag phase in the psychrotolerant bacterium Pseudoalteromonas antarctica lasted 24 h at 5 °C, 3–4 h at 25 °C and above 25 °C the duration decreased until it reached a value of zero at 30 °C. This has been commonly observed in other cold-adapted microorganisms.
As the results show, the fatty acid composition of the bacterial strain Da-bac TI-8 varies with the growth temperature. In the present study, it was observed that the content of unsaturated fatty acid increased when cells were grown at 4 °C compared with when cells were grown at 20 °C. In order to maintain the membrane fluidity, psychrophilic microorganisms have a mechanism of adaptation to low temperatures consisting in the increase in monounsaturated fatty acids content and the elongation of fatty acid chains, involving an even number of carbons (Annous et al. 1997; Chintalapati et al. 2004; Pucci and Pucci 2006). In the lipid analysis of this bacterium, it was found fatty acids that are characteristic of Pseudomonas. The main fatty acids present in all species of the genus Pseudomonas are C16:0 (saturated), C16:1, and C18:1 (unsaturated), as well as the presence of fatty acids hydroxycyclopropanes and branched chain fatty acids (Palleroni 2005). Based on the results of fatty acid profile, this strain belongs to psychrotolerant Pseudomonas group.
Phylogenetic analysis based on 16S rRNA gene sequence showed that strain Da-bac TI-8 belongs of the genus Pseudomonas with more than 99 % sequence similarity with the 16S rRNA gene of species such as P. brenneri AF268969, P. migulae AF074383, P. proteolytica AJ537603, and P. panacis AY787208. This assay suggests that the bacterial strain Pseudomonas sp. Da-bac TI-8 could be a new species of psychrotolerant bacteria; however, more work have to be done in order to clarify this issue. Furthermore, the novel Pseudomonas strain can be differentiated phenotypically from most closely related species, as described in Tables 2 and 3.
In relation to the enzymatic assay, it is important to mention that the same enzymatic profile is obtained when the strain was growth at 4 and 20 °C, respectively (Table 2). The enzymatic activities of Da-bac TI-8 compare favorably with those previously reported in the literature for other strains phylogenetically close to Da-bac TI-8 or that are also psychrotolerant. The P. breneri showed similar enzymatic activities than the strain of Pseudomonas sp. Da-bac TI-8, except for the arylamidase activities (Baida et al. 2001). Furthermore, P. brenneri has no phosphatase activity and, in contrast, P. panacis showed enzymes to produce cystine arylamidase, instead of leucine and valine arylamidase (Park et al. 2005). Unlike other strains, Pseudomonas proteolytic only showed lipase enzymatic activity (Reddy et al. 2004). All the enzymatic results suggest that these strains are biochemically different.
Once the bacterial growth curve was determined, it was decided to study several carbon sources that Pseudomonas sp. Da-bac TI-8 could assimilate and use for its growth using API CH test. Results shown in Table 3 were compared with that obtained by other phylogenetically close strains. Under optimal growth conditions (20 °C), the Pseudomonas sp. Da-bac TI-8 strain has the capacity to utilize most of carbon sources assayed for its growth. Previous reported psychrophilic bacterium agrees with present work bacteria only in the fact that all of them were able to assimilate simplest sugars like glycerol and erythritol. They were also able to use D-trehalose, which is also a known cryoprotectant that is very useful at low temperatures. With regard to the rest of carbon sources, there are several differences among all the species. These show that Pseudomonas sp. Da-bac TI-8 has a unique behavior that makes it different from phylogenetically closest strains.
Production and characterization of exopolysaccharides
In general, the growth phase where the production of exopolysaccharides occurs differs between one microorganism to another. EPS synthesis may be associated with growth, as in the case of the polysaccharide pullulan, produced by Aureobasidium pullulans, the alginate polysaccharide produced by Azotobacter vinelandi or heteropolysaccharides produced by many acid lactic bacteria (Weldman and Maddox 2003; Corsaro et al. 2004). It is less common that exopolysaccharides production occurs only when growth has stopped completely, as happens with curdlan polysaccharide obtained from Alcaligenes faecalis (Sutherland 1990) or the exopolysaccharides produced by Aeromonas salmonicida (Bonet et al. 1993). In case of xanthan, the synthesis occurs in all phases of growth, reaching a maximum EPS production, once the bacterial growth stopped (Sutherland 1990, 1993).
In previous research (Nichols et al. 2005), the effect of temperature on the production of exopolysaccharides by a marine bacterium isolated from Antarctic was studied. The EPS yields at −2 and 10 °C, respectively, were 10 times greater than at 20 °C, which is the optimum temperature for many psychrotolerant bacteria. These results are consistent with results obtained in present work.
Some authors have suggested that the protein content of microbial EPS could be between 1 and 18 % (w/w), depending on the EPS kind (Beech et al. 1999), which is in agreement with results obtained in this work. According to Mancuso et al. (2005), EPS produced by Antarctic bacterial strains could have different chemical composition. These authors suggested that glucose, galactose, mannose, rhamnose, and fucose sugars were the ones most often found in bacterial EPS. Corsaro et al. (2004), analyzed exopolysaccharides produced by an isolation of Antarctic Pseudoalteromonas haloplanktis TAC 125. These authors found that these EPS were mainly composed of residues of mannose and some traces of glucose. These reports are consistent with the results obtained in this work. It is important to note that other functions attributed to bacterial EPS are related to cell adhesion (Kumar et al. 2007).
Solubilization of P and organic acids production
The decrease of pH in the medium during the solubilization of rock phosphate has been attributed to the secretion of organic acids by bacterial strains (Hwangbo et al. 2003; Chen et al. 2006).
These acids play an important role in the medium acidification that facilitates the phosphorus solubilization. The results obtained by these authors indicate that the pH change depends on the type of phosphate tested. The strong drop in pH of the medium was associated with the dissolution of phosphate rock; however, this was not the case with other phosphates used in the study. The inverse relationship between the decrease in pH and the amount of solubilized phosphate has also been reported by other authors (Illmer 1995; Hwangbo et al. 2003; Chung et al. 2005).
Most studies of phosphate solubilizing bacteria have been restricted to mesophilic temperatures (Chung et al. 2005; Chen et al. 2006). Some species of bacteria such as P. corrugata (Trivedi and Sa 2008), P. dispersa (Selvakumar et al. 2008), Pseudomonas fluoresecens (Katiyar and Goel 2003). All of the above strains are psychrotolerant mutants, with the exception of the strain of P. fragi (Selvakumar et al. 2009), and are known to be phosphate solubilizing at low temperatures. It should be noted that the psychrotolerant strain of Pseudomonas sp. T1-8 showed high capacity of phosphate solubilization at low temperature. This fact suggests that one of their roles in the rhizosphere of D. antarctica could be the solubilization of the highly immobilized P from the Antarctic soil, which is volcanic soil.
That is why, it became interesting to know what would be the solubilization mechanism that this bacterium is employing. There are several reports in the literature, which describe that some bacteria have solubilization mechanism from mineral phosphate associated with the release of organic acids of low molecular weight (Deubel and Gransee 2000). These acids have chemical structures (hydroxyl and carboxyl groups) that are able to chelate metal cations to form a very stable complex (Stevenson 2005). Thus, the phosphates are released, becoming in soluble forms available for subsequent absorption by plants (Reyes et al. 2007).
The obtained chromatographic results show that the strain Da-bac TI-8 produces and excretes gluconic acid in the culture medium that contains calcium phosphate. Therefore, this could be the way in which solubilization of mineral phosphate occurs. It has been reported that the main organic acid produced by phosphate solubilizing bacteria such as Pseudomonas sp. Erwinia herbicola and Burkholderia cepacia is gluconic acid (Rodriguez and Fraga 1999; Lin et al. 2006; Song et al. 2008). Another organic acid which is found in strains with the ability to solubilize phosphate is the 2-ketogluconic acid, present in Bacillus and Rhizobium species, being also reported in Pseudomonas (Richardson 2001, Gulati et al. 2007). Some strains of Bacillus amyloliquefaciens and Bacillus licheniformis produce mixtures of lactic, isovaleric, isobutyric, and acetic acids. Other organic acids such as glycolic, oxalic, malic, and succinic acids have also been identified between solubilizing bacteria (Ryan et al. 2001).
IAA production
The Antarctic strain Da-bac TI-8 produced a lower level of IAA at a lower temperature. However, the concentrations obtained from this bacterium can be sufficient to have a direct influence on the plant growth, given that phytohormones operate at very low concentrations (Ahmad et al. 2005). Some studies have found that microorganisms produce auxins in the presence of a suitable precursor such as l-tryptophan. The addition of tryptophan to the culture medium favors the production of IAA, based on the fact that tryptophan is an intermediate in the biosynthetic pathway of the IAA (Idris et al. 2007).
When taken together, the combined results suggest that the strain of Pseudomonas sp. Da-bac TI-8 could be considered as a Plant Growth-Promoting Rhizobacteria (PGPR). From the ecological point of view, one might speculate that this bacterium could influence the growth and adaptation of D. antarctica to the Antarctic environment.
Effect of inoculating D. antarctica with PGPR bacteria
In this work, it was obtained that 103 CFU/mL was at least the lower amount of inoculum required to promote a plant growth effect after two month of Deschampsia inoculation. Even when this amount could be considered low, regarding any commercial biofertilizer formulation, these are concentrations that could be found in soil bacterial communities that habitat in plant rhizosphere. Furthermore, despite to the fragile root structure of Deschampsia plants, the Pseudomonas was able to attach to their surface as demonstrated by SEM. Such fact suggests that the bacterial inoculum could be able to growth on the hairy root area and to promote plant growth through several mechanisms early explained through this work.
At 22 °C, it was obtained an increase in the shoot/root ratio regarding control, which could be a positive outcome from the plant perspective because it put less resource in roots and more into leaves and photosynthetic tissues. Such result could be due to the higher amount of IAA secreted by the bacteria at this temperature, as previously presented (see Fig. 5), in addition to the fact that bacterial growth rate was high at 22 °C. The higher phytohormone concentration could produce a slight inhibition of root development, which could lead to the lower root dry weight observed in inoculated plants regarding control treatment. Dobbelaere et al. (2002) observed an inhibitory effect of the high inoculum concentrations on root development; which was attributed to the large number of bacterial cells that produce supraoptimal amounts of hormones causing inhibition of root growth.
On the other side, the lower shoot/root ratio observed at 13 °C on Deschampsia plants regarding control treatment could be due to the effect of IAA released by the bacterial inoculum at the hairy root surface. It is known that lower bacterial concentrations produce intermediate levels of IAA, promoting increases in root development. This indicates that only small doses of the plant phytohormone may achieve positive effects. The growth promotion effect was better at 13 °C, probably because D. antarctica presents an optimal photosynthetic activity at this temperature (Alberdi et al. 2002).
The positive effect caused by the bacteria in the plant development incubated at 13 °C could be related to the ability of this strain to synthesize IAA and to solubilize inorganic phosphates at low temperatures. There is a significant number of bacterial species, many of them associated with plants rhizosphere, which are capable of exerting a beneficial effect on plant growth. These groups of bacteria are called PGPR, and they are bacteria species of Pseudomonas, Azospirillum, Burkholderia, Bacillus, Enterobacter, Rhizobium, Erwinia, Serratia, Alcaligenes, Arthrobacter, Acinetobacter, and Flavobacterium (Rodriguez and Fraga 1999; Rosas et al. 2006). This paper demonstrates that in the Antarctic soil could habitat some PGPR bacterial species that could be playing important roles in the distribution and survival of one of two vascular plants that inhabit the Antarctic continent.
SEM analysis
The hair root zone the root tip was found to be preferential site of bacterial colonization, and this pattern has been explained by an elevated metabolic activity associated with exudation from the root (Hansen et al. 1997; Parsello-Cartieaux et al. 2001). Thus, in the study, it is shown that this bacterium strain is capable of binding to the roots of D. antarctica, preferably to the absorbent hairs. This would allow the plant to benefit directly from the advantages of the presence of the bacterium, such as phytohormones (IAA) excretion and solubilization of insoluble P present in the rhizosphere.
Conclusion
Phylogenetic and biochemical assays suggest that the bacterial strain Pseudomonas sp. Da-bac TI-8 showed differences with other Pseudomonas species; however, further studies would be needed on this matter in order to be sure its new specie. This strain is capable of solubilizing P from different sources through the excretion of gluconic acid and producing phytohormones; therefore, it behaves as a PGPR psychrotolerant. Inoculation tests in D. antarctica indicated that it produces a beneficial effect on plant growth, probably because of their contribution of hormones and nutrients (P). SEM analyses indicate that this bacterium mainly binds to the plant root hairs. Therefore, its effect is probably exerted by means of its proximity to the roots of this plant. The overall results suggest that some microorganisms from the rhizosphere of D. antarctica may play an important role in the survival and adaptation to the Antarctic environment from this plant.
References
Ahmad F, Ahmad I, Saghir M (2005) Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turk J Biol 29:29–34
Alberdi M, Bravo L, Gutierrez A, Gidekel M, Corcuera L (2002) Ecophysiology of Antarctic vascular plants. Physiol Plant 115:479–486
Annous BA, Becker LA, Bayles DO, Labeda DP, Wilkinson BJ (1997) Critical Role of Anteiso-C15: 0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl Environ Microbiol 63:3887–3894
Baida N, Yazourhb A, Singer E, Izard D (2001) Pseudomonas sp brenneri. nov., a new species isolated from natural mineral waters. Res Microbiol 152:493–502
Baker GC, Smith JJ, Cowan DA (2003) Review and re-analysis of domain-specific 16S primers. J Microbiol Meth 55:541–555
Barrientos L, Gidekel M, Gutiérrez A (2008) Characterization of rhizospheric bacteria isolated from Deschampsia antarctica Desv. World J Microbiol Biotech 24:2289–2296
Beech I, Hanjagsit L, Kalaji M, Neal A, Zinkevich V (1999) Chemical and structural characterization of exopolymers produced by Pseudomonas sp. NCIMB 2021 in continuous culture. Microbiol 145:1491–1497
Bonet R, Simon-Pujol MD, Assembled F (1993) Effects of nutrients on exopolysaccharide production and surface propierties of Aeromonas salmonicida. Appl Environ Microbiol 59:2437–2441
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bric J, Bostock R, Silverstone S (1991) Rapid in situ assay for indoleacetic acid production by bacteria inmobilized on a nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Cakmakci R, Donme F, Aydin Sahin AF (2006) Growth promotion of plants by plant growth promoting rhizobacteria under greenhouse and two field different soil conditions. Soil Biol Biochem 38:1482–1487
Chen YP, Rekha PD, Arun AB, Shen FT, Lai WA, Young SC (2006) Phosphate solubilizing bacteria from subtropical soil tricalcium phosphate solubilizing and their abilities. Soil Ecol 34:33–41
Chew O, Lelean S, John UP, Spangenberg GC (2012) Cold acclimation you induce rapid and dynamic changes file in freeze tolerance in the cryophile mechanisms Deschampsia antarctica E. Desv. Plant Cell Environ 35:829–837
Chintalapati S, Kiran MD, Shivaji S (2004) Role of membrane lipid fatty acids in cold adaptation. Cell Mol Biol (Noisy-le-grand) 50:631–642
Chung H, Park M, Madhaiyan M, Seshadri S, Song J, Cho H, Sa T (2005) Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol Biochem 37:1970–1974
Corsaro MM, Lanzetta R, Parrilli E, Parrilli M, Tutino ML, Ummarino S (2004) Influence of growth temperature on lipid and phosphate contents of surface polysaccharides from the Antarctic bacterium Pseudoalteromonas haloplanktis TAC 125. J Bacteriol 186:29–34
Das AC (1989) Utilization of insoluble phosphates by soil fungi. Indian J Soc Soil Sci 58:1208–1211
Deubel A, Gransee Merbach W (2000) Transformation of organic rhizodeposits byn rhizoplane bacteria and its influence on the availability of tertiary calcium phosphate. J Plant Nutr Soil Sci 163:387–392
Dobbelaere S, Croonenborghs A, Thys A, Ptacek D, Okon Y, Vanderleyden J (2002) Effect of inoculation with wild type Azospirrillum brasilense and A. Irakense strains on development and nitrogen uptake of spring wheat and grain maize. Biol Fertil Soils 36:283–297
Dubois M, Giles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Giełwanowska I, Pastorczyk M, Kellmann-Sopyła W (2011) Influence of environmental changes on polar physiology and development of vascular plants. Papers on Global Change IGBP 18:53–62
Goenadi DH, Siswanto, Sugiarto Y (2000) Bioactivation of poorly soluble phosphate rocks with a phosphorus-solubilizing fungus. Soil Sci Soc Am J 64:927–932
Goldstein AH (2000) Bioprocessing of rock phosphate ore: essential technical considerations for the development of a successful commercial technology. In: Proceedings of the 4th International Fertilizer Association Technical conference, New York
Gulati A, Rahi P, Vyas P (2007) Characterization of phosphate-solubilizing fluorescent pseudomonads from the rhizosphere of seabuckthorn growing in the cold deserts of Himalayas. Curr Microbiol 56:73–79
Gyaneshwar P, Kumar GN, Parekh LJ, Poole PS (2002) Role of soil microorganisms in Improving P nutrition of plants. Plant Soil 245:83–93
Hansen M, Kragelund L, Ybroe O, Sorensen J (1997) Early colonization of barley roots by Pseudomonas fluorescens studied by immunofluorescence technique and confocal laser scanning microscopy. FEMS Microbiol Ecol 23:353–360
Hwangbo H, Park RD, Kim YW, Rim YS, Park KH, Kim TH, Suh JS, Kim KY (2003) 2-Ketogluconic production and phosphate solubilization by Enterobacter intermedium. Curr Microbiol 47:87–92
Idris EE, Iglesias DJ, Talon M, Borris R (2007) Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol Plant Microbe Interact 20:619–626
Illmer P (1995) Solubilization of hardly-soluble AIPO4 with P solubilizing microorganism. Soil Biol Biochem 27:265–270
Katiyar V, Goel A (2003) Solubilization of inorganic phosphate and plant growth promotion by cold tolerant mutants of Pseudomonas fluorescens. Microbiol Res 158:163–168
Kumar AS, Mody K, Jha B (2007) Review Article. Bacterial exopolysaccharides—a perception. J Basic Microbiol 47:103–117
Lin TF, Huang HI, Shen FT, Young CC (2006) The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC Al74. Bioresour Technol 97:957–960
Mancuso C, Garon S, Bowman J, Nichols P, Gibson J, Guézennec J (2005) Chemical Characterization of exopolysaccharides from Antarctic marine bacteria. Microbial Ecol 49:578–589
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nedwell DB (1999) Effect of low temperature on microbial growth: lowered affinity for substrates limits growth at low temperature. FEMS Microbiol Ecol 30:101–111
Nedwell D, Rutfer M (1994) Influence of temperature on growth rate and competition between two psychrotolerant Antarctic bacteria: diminishes affinity for low temperature substrate uptake. Appl Environ Microbiol 60:1984–1992
Nevot MV, Deroncele Ma, Forestry J, Mercade E (2008) Effect of incubation temperature on growth parameters of Pseudoalteromonas antarctica NF3 production of extracellular and its polymeric substances. J Appl Microbiol 105:255–263
Nichols CM, Bowman JP, Guezennec J (2005) Effects of incubation temperature on growth and production of exopolysaccharides by an Antarctic sea ice bacterium grown in batch culture. Appl Environ Microbiol 71:3519–3523
Palleroni NJ (2005) Genus I. Pseudomonas Migula 1894, 237AL. In: Brenner DJ, Krieg NR, Staley JT (eds) Bergey’s manual of systematic bacteriology volume 2: the Proteobacteria Part B the Gammaproteobacteria. Springer, New York, pp 322–379
Panicker G, Aislabie J, Saul D, Bej AK (2002) Cold tolerance of Pseudomonas sp. 30–3 isolated from oil-contaminated soil, Antarctica. Polar Biol 25:5–11
Park Y, Lee Y, Yi H, Kim Y, Bae K, Choi J, Sung H, Jung HS, Chun J (2005) Pseudomonas sp panacis. nov., isolated from the surface of rusty roots of Korean ginseng. Int J Syst Evol Micr 55:1721–1724
Parnikoza I, Kozeretska I, Kunakh V (2011) Vascular plants of the maritime Antarctic: origin and adaptation. Am J Plant Sci 2:381–395
Parsello-Cartieaux F, Pascale D, Sarrobert C, Thibaud MC, Achouak W, Robaglia C, Nussaume L (2001) Utilization of mutants to analyze the interaction between Arabidopsis thaliana and its naturally root-associated Pseudomonas. Planta 212:190–198
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Microbiologiya 17:362–370
Pucci G, Pucci O (2006) Changes in membrane fatty acids ester aromaticum Microbacterium GNP-5 at different temperatures and osmolarities. Argent J Microbiol 11:61–73
Quezada E, Bejar V, Calvo C (1993) Exopolysaccharide production by Volcaniella euryhaline. Experientia 49:1037–1041
Reddy G, Matsumoto G, Schumann P, Stackebrandt E, Shivaji S (2004) Psychrophilic Pseudomonas from Antarctica: pseudomonas antarctica sp. nov., Pseudomonas sp meridian. nov. and Pseudomonas sp proteolytica. nov. Int J Syst Evol Micr 54:713–719
Reyes I, Valery A, Valduz Z (2007) Phosphate solubilizing microorganisms isolated from rhizospheric and bulk soils of plants at an abandoned colonizer phosphate rock mine. In: Velázquez E, Rodriguez-Barrueco C (eds) First international meeting on microbial phosphate solubilization, pp 69–75
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol 28:897–906
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Rosas SB, Andrew JA, Rovera M, Correa N (2006) Phosphate-solubilizing Pseudomonas putida can rhizobia-legume Influence the symbiosis. Soil Biol Biochem 38:3502–3505
Ruhland CT, Xiong FS, Clark D, Day T (2005) The Influence of Ultraviolet-B radiation on growth, hydroxycinnamic acids and flavonoids of Deschampsia antarctica during springtime ozone depletion in Antarctica. Photobio Photoch J 81:1086–1093
Ryan PR, Delhaize E, Jones DL (2001) Function and mechanism of organic anion exudation from plant roots. Annl Rev Plant Physiol Plant Mol Biol 52:527–560
Selvakumar G, Kundu S, Joshi P, Nazim S, Gupta AD, Mishra P, Gupta KHS (2008) Characterization of a cold-tolerant plant growth-promoting bacterium isolated from Pantoea 1A dispersed to sub-alpine soil in the North Western Indian Himalayas. World J Microb Bio 24:955–960
Selvakumar G, Joshi P, Nazim S, Mishra P, Bisht J, Gupta H (2009) Phosphate Solubilization and growth promotion by Pseudomonas fragi CS11RH1 (MTCC 8984), a psychrotolerant bacterium isolated from a high altitude Himalayan rhizosphere. Biology 64:239–245
Selvakumar G, Joshi P, Suyal P, Mishra PK, Joshi GK, Bisht JK, Bhatt C, Gupta HS (2011) Pseudomonas lurida M2RH3 (MTCC 9245), a psychrotolerant bacterium from the Uttarakhand Himalayas, solubilizes phosphate and promotes wheat seedling growth. World J Microb Biot 27:1129–1135
Singal R, Gupta R, Kuhad RC, Saxena RK (1991) Solubilization of inorganic phosphates by a fungus Basidiomycetous cuathus. Indian J Microbiol 31:397–401
Song OR, Lee SJ, Lee YS, Lee SC, Kim KK, Choi YL (2008) Solubilization of insoluble inorganic phosphate by Burkholderia cepacia DA23 isolated from cultivated soil. Braz J Microbiol 39:151–156
Stevenson FJ (2005) Cycles of soil: carbon, nitrogen, phosphorus, sulfur, micronutrients. Wiley, New York
Sutherland IW (1990) Biotechnology of microbial exopolysaccharide. In: Baddiley J, Carey NH, Higgins IJ, Pitter WG (eds) Cambridge studies in biotechnology, vol 9. Cambridge University Press, Cambridge
Sutherland IW (1993) Xanthan. In: Swing JG, Civerolo EL (eds) Xanthomonas. Chapman and Hall, London, pp 363–388
Taboada E, Fisher P, Jara R, Zúñiga E, Gutierrez A, Cabrera JC, Gidekel M, Villalonga R, Cabrera G (2010) Isolation and characterization of pectic substances from murta (Ugni molinae) fruits. Food Sci 123:669–678
Trivedi P, Sa T (2008) Pseudomonas corrugata (NRRL B-30409) Mutants increased phosphate solubilization, organic acid production, and plant growth at lower temperatures. Curr Microbiol 56:140–144
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification forphylogenetic study. J Bacteriol 173:697–703
Weldman AD, Maddox IS (2003) Exopolysaccharides from lactic acid bacteria: perspectives and challenges. Trends Microbiol 21:269–274
Acknowledgments
The authors thank Ramon Rossello Mora for his assistance with phylogenetic analyses. The authors thank Charles Guy for his help in the translation and proofreading of the manuscript. Monica Ramirez and Yamile Bernardo are thanked for their assistance with HPLC Analyses. The authors thank INACH 0301 Grants; UXMAL S.A.: Doctorate Fellowship CONICYT Graciela Berríos.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berríos, G., Cabrera, G., Gidekel, M. et al. Characterization of a novel antarctic plant growth-promoting bacterial strain and its interaction with antarctic hair grass (Deschampsia antarctica Desv). Polar Biol 36, 349–362 (2013). https://doi.org/10.1007/s00300-012-1264-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-012-1264-6