Abstract
The Polish Arctowski Station is situated in the maritime Antarctic on the western shore of Admiralty Bay and encompasses terrestrial habitats which are not permanently covered by ice, in contrast to more than 90% of the island’s surface area. Over the past several decades, studies exploring the soils of those habitats have revealed a considerable diversity of bacteria, filamentous fungi, and, to a lesser extent, yeasts; however, characterization of this complex microbiome, especially at the molecular level, is still far from satisfactory. The isolates were assigned to their respective genera and species based on genetic analysis of the D1/D2 and ITS1-5.8S-ITS2 regions of rDNA. In the studied soil samples, the most abundant microorganisms belonged to the genera Cryptococcus, Rhodotorula, and Debaryomyces. Physiological and biochemical analysis of Cryptococcus gilvescens (pro tempore Goffeauzyma gilvescens) and Rhodotorula mucilaginosa showed only a limited level of intraspecies diversity. Cellular DNA content and karyotypes were determined using flow cytometry and pulsed-field gel electrophoresis for several selected strains. For the first time, genome size and electrophoretic karyotypes were investigated in C. gilvescens (pro tem G. gilvescens), Cryptococcus saitoi (pro tem Naganishia globosa), Cryptococcus gastricus (pro tem Goffeauzyma gastrica), and Cryptococcus albidus (pro tem Naganishia albida). In addition, plate tests showed Antarctic yeasts to be a potential source of biotechnologically important enzymes. This study in biodiversity, presenting physiological and molecular characterization of psychrotolerant yeast strains isolated from the soils of western Admiralty Bay, contributes to a better understanding of the microbial ecology of this unique ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temperature is one of the main factors influencing microbial development by affecting the growth rate and metabolic activity of microorganisms as well as the physical and chemical properties of the environment. According to the current knowledge of the ecology and biodiversity of the Earth’s cryosphere, metabolically active microbial populations can be found even at sites with extremely low temperatures (Margesin and Miteva 2011). Microorganisms which colonize such ecosystems and are capable of growth at 0 °C or less are known as psychrophilic. Among them, obligate psychrophiles grow best at less than 15 °C with a maximum of 20 °C, while facultative ones grow optimally at 20–25 °C (Morita 1975). Cold-adapted organisms include a vast array of archaea, bacteria, yeasts, filamentous fungi, cyanobacteria, and algae. The least is known about psychrotolerant yeasts despite the fact that they constitute a versatile group of eukaryotic microorganisms characterized by diverse nutritional preferences and a surprising survival ability in extreme environments of widely varying physical and geochemical parameters. It has even been suggested that yeasts are better adapted to cold habitats than bacteria (Buzzini and Margesin 2014).
Psychrophilic and psychrotolerant yeasts have consistently been found in numerous cold habitats, including permafrost, snow, cold deserts, glacial ice, meltwater, sediments, deep sea, as well as frozen and refrigerated foods (Buzzini and Margesin 2014). To date, the greatest number of psychrophilic yeasts (13 ascomycetes and 57 basidiomycetes, a total of 70) has been isolated from soil, water, ice, and snow samples in Antarctica. Most of them have been assigned to the genera Candida, Dioszegia, Rhodotorula, Mrakia, Mrakiella, Sporobolomyces, Glaciozyma, Malassezia, Saccharomyces, Clavispora, and Cryptococcus based on phenotypic and molecular data (Thomas-Hall and Watson 2002; Thomas-Hall et al. 2002, 2010; Guffogg et al. 2004; Buzzini et al. 2012; Connell et al. 2014). Phenotypic methods of yeast identification mostly focus on analysis of morphological traits (shape, size, and biomass pigmentation), physiological traits (sporulation and cellular inclusions), and biochemical profiles (enzymatic activity) (Carrasco et al. 2012; Kurtzmann et al. 2011; Kishida et al. 2009; Turchetti et al. 2008; Moreira et al. 2001). In turn, molecular methods have greatly improved the ability to rapidly detect, identify, and classify microorganisms, and also to establish taxonomic relationships among closely related genera and species. These include techniques such as nucleic acid hybridization (Southern blot analysis or Solution-phase hybridization) and amplification-based or polymerase chain reaction (PCR) technologies, which compare sequences of conserved genomic regions (such as 18S and 26S rRNA or the internal transcribed spacer—ITS), restriction fragment length polymorphisms (RFLP), amplified fragment length polymorphisms (AFLP), or G + C % content in genomic DNA with corresponding data for known organisms (Turchetti et al. 2008; Jeyaram et al. 2008; Diaz and Fell 2005).
Currently, in research of cold-adapted yeasts, the focus has shifted from taxonomy, growth conditions, and the relationship with the abiotic environment to their biotechnological, and especially enzymatic, potential. Due to their ability to grow and conduct metabolism at low temperatures, they are thought to produce unique biocatalysts with potentially valuable industrial applications. Examples include lipases A and B secreted by Candida antarctica (now Pseudozyma antarctica), which are used in a range of biotransformations in the food, pharmaceutical, and cosmetic industries (Shivaji and Prasad 2009; Szczęsna-Antczak et al. 2014).
The objective of this study was to isolate yeast strains from soil samples collected from King George Island, the largest of the South Shetland Islands, Antarctica. The isolates were characterized physiologically and biochemically and identified at the molecular level using the D1/D2 and ITS1-5.8S-ITS2 regions of rDNA. Cellular DNA content and karyotypes were established for selected yeast strains using flow cytometry (FCM) and pulsed-field gel electrophoresis (PFGE). In addition, a series of extracellular enzyme activity tests were performed to determine the biotechnological potential of the isolated strains.
Materials and methods
Sampling
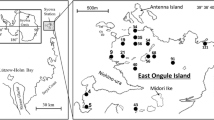
Soil samples were collected from ten different sites in the vicinity of the Henryk Arctowski Polish Antarctic Station on King George Island (62°09′41″S, 58°28′10″W) at the beginning of March 2010 (Table 1). The samples were stored in sterile plastic tubes at 4 °C until analysis.
Yeast isolation and diazonium blue B staining
All chemicals used in the study were of analytical grade. They were purchased from Chempur (Poland) and POCh (Poland) unless otherwise stated.
The yeast isolation procedure was as follows. 2.5 g of each soil sample was suspended in 25 mL 0.5% glucose and incubated for 48 h at 10 °C on an orbital shaker (Infors, Switzerland) at 150 rpm for sample rehydration and cell regeneration. Subsequently, 1 mL of yeast suspension was transferred to YPG medium [yeast extract (BD, USA) 10 g/L, bactopeptone (BD, USA) 20 g/L, glucose 20 g/L], and incubated for another 48 h at 20 °C and 150 rpm for non-selective enrichment of microorganisms. After 24 and 48 h, the cultured yeast suspensions, both undiluted and diluted tenfold, were plated in the amount of 200 µL on a solid YPG medium supplemented with antibiotics inhibiting bacterial growth [chloramphenicol (Sigma–Aldrich, USA) 0.25 mg/mL and ampicillin (Sigma–Aldrich, USA) 0.1 mg/mL] for yeast isolation. After 72 h of incubation at 20 °C, the yeast cultures were streaked onto YPG agar medium and pure yeast cultures were transferred onto YPG agar slants. Yeast isolates were classified as basidiomycetes or ascomycetes in accordance with the procedure described by Kurtzmann et al. (2011), based on staining with diazonium blue B (Sigma–Aldrich, USA).
Yeast strains and culture medium
Strains were stored in the form of glycerol stocks (1:1) at −80 °C. The cultures were grown using YPG agar medium (15 g/L), and a liquid YPG medium was used for batch culture. Medium pH was in the range of 5.5–6.0.
Determination of yeast growth temperature, carbon assimilation ability, and enzymatic potential
YPG agar plates were spot inoculated with four droplets of a pure yeast culture and incubated for 48 h at 20 °C; subsequently, replicates were made using fresh media and incubated at 4, 10, 15, 20, 30, and 37 °C. Strain growth was monitored over 7 days. The ability to utilize different carbon sources and enzymatic activity were evaluated at 20 °C using API 20 C AUX and API ZYM (API System; bioMerieux, France) tests, respectively. Analysis was conducted following the manufacturer’s instructions.
rDNA sequencing and molecular phylogenetic analysis
DNA extraction
Following 48 h culture, the yeast biomass was centrifuged (5000×g, 10 min, 4 °C) and a 100 mg sample was ground to homogeneous powder under liquid nitrogen. DNA was extracted over 30 min at room temperature in an extraction buffer [300 mM Tris–HCl (Sigma–Aldrich, USA), 375 mM NaCl, 37.5 mM EDTA (Sigma–Aldrich, USA), 3% SDS (Sigma–Aldrich, USA), pH 8.5] with the addition of phenol:chloroform:isoamyl alcohol mixture (Sigma–Aldrich, USA) (25:24:1). Then, 5 µL RNase A (Sigma–Aldrich, USA) (10 mg/mL) was added to the aqueous phase, which was incubated for 1 h at 37 °C and extracted with an equal volume of phenol:chloroform:isoamyl alcohol mixture. DNA was precipitated from the aqueous phase by adding 0.1 volume of 3 M sodium acetate at pH 5.2 and 0.6 volume of chilled isopropanol. Following overnight incubation at −20 °C, the extract was centrifuged (16,000×g, 15 min, 4 °C), and the resulting DNA pellets were washed twice with 70% ethanol, dried, and dissolved in sterile water. The concentration of the isolated genomic DNA was measured using the µDrop system (Thermo Scientific, USA), with the quality determined electrophoretically. Depending on the concentration, 1–5 µL DNA was used for PCR.
Amplification
The D1/D2 domains of the large subunit (LSU) rRNA gene region and the internal transcribed spacers (ITS1 and ITS2 regions) including the 5.8S rRNA gene were amplified by cycle sequencing using the following forward and reverse primers, respectively: ITS5 (5′-GGA AGT AAA AGT CGT AAC AAG G-3′) and LR6 (5′-CGC CAG TTC TGC TTA CC-3′) for LSU; V9 (5′-TGC GTT GAT TAC GTC CCT GC-3′) and RLR3R (5′-GGT CCG TGT TTC AAG AC-3′) for the ITS regions. The iProof™ High-Fidelity DNA Polymerase Kit (Bio-Rad, USA) was used for PCR. Following the reaction, the product was cleaned by means of the commercially available Wizard® SV Gel and PCR Clean-Up System (Promega, USA). The amplified fragments were sequenced by Genomed S.A. (Warsaw, Poland) using the following forward and reverse primers, respectively: NL1FWD (5′-GCA TAT CAA TAA GCG GAG GAA AAG-3′) and NL4REV (5′-GGT CCG TGTTTC AAG ACG G-3′) for LSU and ITS1 (5′-TCC GTA GGT GAA CCT GCG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) for the ITS regions. The sequences were aligned, analyzed, and corrected using Nucleic Acid Sequence Massager software, which is available online at http://www.cmbn.no/tonjum/seqMassager-saf.htm, and compared with sequences from the international GenBank database (http://www.ncbi.nlm.nig.gov/) using the BLASTn search tool.
Intracellular protein profiles
Isolation of intracellular proteins
Following 48 h culture in YPG medium, the yeast biomass was ground under liquid nitrogen and extracted in 20 mM Tris–HCl buffer at pH 7.4 with the addition of 0.1% Triton X-100, 100 mM KCl, 8 mM MgCl2, 150 mM NaCl, and 1 mM PMSF (Sigma–Aldrich, USA). Protein concentration was determined in the supernatant obtained after extract centrifugation using the Quick Start Bradford Protein Assay (Bio-Rad, USA). Intracellular protein profiles were analyzed by means of large-scale electrophoresis using a Protean II XL System (Bio-Rad, USA) according to the method described by Laemmli (1970). Proteins were visualized by Bio-Safe Coomassie staining according to the manufacturer’s protocol (Bio-Rad, USA).
Analysis of intracellular proteins
To compare the profiles of intracellular proteins, electrophoregrams were digitized by means of a GS-800™ Calibrated Densitometer, and then analyzed using the Quantity One® software (Bio-Rad, USA) with Complete Linkage clustering.
Extracellular enzyme activities of isolated yeast strains
Solid agar media (YP and YPG) supplemented with a substrate (enzymatic activity inducer: 1–8) were spot inoculated with 2 µL of standardized suspension of yeast cells.
-
1.
Amylolytic activity: After 11 days of incubation, agar plates (YP) supplemented with 2% soluble starch were flooded with iodine solution. Estimation of amylolytic activity was based on coloring of the medium to dark-violet and observation of clear zones around colonies.
-
2.
Cellulolytic activity: After 11 days of incubation, agar plates (YP) supplemented with 2% carboxymethylcellulose (Sigma–Aldrich, USA) were flooded with 1.4 mM Congo-Red (Sigma–Aldrich, USA) solution and incubated for 15 min at room temperature. Then, 10 mL of 1 M NaCl was added to remove excess stain. Cellulolytic activity was indicated by the formation of an opaque clear zone around colonies.
-
3.
Pectinolytic activity: After 11 days of incubation, agar plates (YP) containing 2% apple pectin (Sigma–Aldrich, USA) were flooded with 1% hexadecyltrimethylammonium bromide (Sigma–Aldrich, USA) solution. Pectinolytic activity was indicated by a clear zone around colonies.
-
4.
Lipolytic activity: Agar plates (YP) were supplemented with 1% tributyrin (Fluka, USA) and lipolytic activity was indicated by a clear halo around colonies.
-
5.
Proteolytic activity: Agar plates (YPG) were supplemented with 2% skim milk and proteolytic activity was indicated by a clear halo around colonies.
-
6.
β-Galactosidase activity: Agar plates (YPG) were supplemented with 0.3 g/L S-Gal (Sigma–Aldrich, USA) and β-galactosidase activity was indicated by the formation of a black precipitate.
-
7.
Phytase activity: Three different minimal solid media were used for the assay according to Nuobarience et al. (2010): a phytate medium containing phytic acid dipotassium salt (Sigma–Aldrich, USA) as a phosphate source, a positive control medium containing phosphate, and a phosphate-free negative control medium.
-
8.
Salinity tolerance: Agar plates (YPG) were supplemented with 10, 15, and 20% NaCl.
All yeast cultures were conducted at 20 °C and their enzymatic potential was evaluated after 7 and 11 days of incubation as a ratio between the diameter of the clear zone and that of the colony (d z/d c).
Flow cytometry
Cell preparation and DNA staining
All isolated Antarctic yeasts were grown in a YPG batch culture as described above up to a certain growth phase, defined on the basis of the growth curves previously prepared for the studied microorganisms. The analyzed cells were collected from the early and late logarithmic phases, as well as from the stationary phase. The cells were prepared for cytometric analysis pursuant to a modified procedure described by Sabatinos and Forsburg (2009). For this purpose, 107 cells were transferred to a sterile Eppendorf tube and centrifuged (10,000×g; 5 min), after which the supernatant was removed. Subsequently, 1 mL of cold 70% ethanol was added slowly, while stirring the suspension. The samples were incubated overnight at 4 °C to fix the cells. Then, 0.3 mL of the suspension (approx. 2–3 × 106 cells) was taken and centrifuged again (10,000×g; 5 min). The pellet was resuspended in 1 mL 50 mM sodium citrate. Following another centrifugation at 10,000×g for 5 min, the cells were suspended in 0.5 mL 50 mM sodium citrate containing RNase A (Sigma–Aldrich, USA) at a concentration of 1 mg/mL. The suspension was incubated at 37 °C for 16 h, and then 20 µL of Proteinase K (Sigma–Aldrich, USA) was added at a concentration of 20 mg/mL. After incubation at 50 °C for 1 h, samples were centrifuged (10,000×g; 5 min) and the pellet was resuspended in 0.5 mL 50 mM sodium citrate and left overnight at 4 °C. Then, 0.5 mL 50 mM sodium citrate supplemented with 2 µM SYTOX Green (Life Technologies, USA) was added, and the samples were sonicated for 45 s in an ultrasonic water bath. The cells of Saccharomyces cerevisiae reference strains (BY4742 and BY4743, which are haploid and diploid, respectively; purchased from EUROSCARF, Germany) were prepared pursuant to the same procedure.
Cell samples were subjected to DNA content analysis by flow cytometry (FCM). DNA content in yeast cells was calculated based on a calibration curve for the relationship between fluorescence intensity and genome size, prepared on the basis of analysis of S. cerevisiae BY4742 and BY4743 reference strains.
Flow cytometry analysis
FCM was conducted in a FACS Canto II apparatus (BD Biosciences, USA) at the Department of Haemostasis and Haemostatic Disorders, Medical University in Lodz, Poland. Laser parameters were set using the Cytometer Setup and Tracking Beads system (BD Biosciences, USA) prior to each measurement. Green fluorescence detection was conducted using channel FL1 at a wavelength of 530 nm and a bandwidth of 30 nm. Photomultiplier voltages for the FSC, SSC, and FL1 detectors were 27, 231, and 450 V (or 430 V), respectively. The limit of detection for the FSC detector was 200. In each independent experiment, 30,000 cells were analyzed. Fluorescence intensities were expressed as mean and median values. The results were interpreted using the Facs/Diva ver. 6.0 software (BD Biosciences, USA). DNA content in yeast cells was computed based on a calibration curve reflecting the relationship between fluorescence and genome size prepared on the basis of analysis of S. cerevisiae BY4742 and BY4743 reference strains according to the formula:
\({\text{DNA content}} = 0.3747 \times {\text{MFI}}\;({\text{for}}\, 430\;{\text{V}})\)
or
\({\text{DNA content}} = 0.2463 \times {\text{MFI}}\;({\text{for}}\, 450\;{\text{V}}),\)
where MFI—mean fluorescence intensity.
All experiments and measurements were conducted in triplicate. Results are presented as mean ± SD.
Pulsed-field gel electrophoresis of genomic DNA
Immobilization of yeast DNA in agarose plugs
The yeasts were cultured at 20 °C for 24 h until they reached an optical density of >1 at 660 nm. Then, 5 × 108 cells were transferred onto a fresh YPG medium and incubated overnight at 20 °C. DNA plugs were prepared according to the protocol described by Saracli et al. (2006). The time of incubation with a lysing enzyme from Trichoderma harzianum (Sigma-Aldrich, USA) at a concentration of 75 mg/200 µL cell suspension was varied depending on the strain.
Pulsed-field gel electrophoresis
Electrophoretic separations were conducted using a CHEF-DR II pulsed-field gel electrophoresis system from Bio-Rad Laboratories (USA), equipped with a buffer cooling module, also from Bio-Rad (USA). Electrophoresis was carried out in MegaBase agarose gel (Bio-Rad, USA) with a size of 13 × 14 cm and a thickness of 8–10 mm and in 1 × TAE buffer (40 mM Tris–acetate, pH 7.5; 2 mM EDTA, pH 8.0) circulating at a flow rate of approx. 1 L/min. Buffer temperature was maintained at a constant level of 14 °C using a cooler (Bio-Rad, USA). The basic separation conditions were as follows: 1% MegaBase agarose, switch ramp 100–200 s at 3.5 V for 16 h followed by 200–300 s at 4.0 V for 29 h. Depending on DNA separation quality, conditions were modified for different yeast strains. Hansenula wingei chromosomal DNA (Bio-Rad, USA) and S. cerevisiae strains BY4742 and BY4743 were used as size markers. Following electrophoresis, the gel was stained with ethidium bromide to visualize the results. The gel was incubated in an ethidium bromide solution at a concentration of 0.5 µg/mL for 30 min. The gel was scanned using Gel Doc (Bio-Rad, USA), and the image was processed using the Image Lab software (Bio-Rad, USA).
Results
Isolation and preliminary characterization of Antarctic yeasts
A total of 57 yeast strains were isolated from soil samples collected from various sampling sites in Antarctica (Table 1). The greatest number of isolates was obtained from the soil taken from an old skua nest located on the moraine of the Ecology Glacier and from soil over grown with mosses (22 and 15 isolates, respectively). The number of strains isolated from sites over grown with lichens and mosses located in the old tundra on Puchalski Hill and on Henryk Arctowski Station Hill was much lower. The fewest yeasts were found in soil samples collected from penguin rookeries (1, 2, 6, 7).
Diazonium blue B (DBB) staining showed 84% of the isolated yeasts to be basidiomycetes, with the remaining 16% being ascomycetes. The determination of culture traits (macroscopic yeast growth in solid and liquid YPG media), morphological characteristics (cell size and shape), physiological characteristics (ability to grow in the temperature range of 4–37 °C on solid and liquid YPG media), as well as biochemical characteristics (assimilation of different carbon sources tested with API 20 C AUX and enzymatic activity tested with API ZYM) for 57 yeast isolates revealed some strains with identical growth and metabolic patterns (data not shown). On that basis, the 57 isolates were preliminarily classified into 18 physiological groups.
Taxonomic identification of yeast isolates
To determine the genetic identity of 57 isolates of Antarctic yeasts, genomic DNA was isolated (see “Materials and methods”) and amplification was carried out for DNA fragments encoding the D1/D2 region of the 26S rDNA large ribosomal subunit as well as the ITS1 and ITS2 region located between the genes encoding the 18S and 26S rDNA subunits. Following amplification, DNA fragments were sequenced and aligned with homologous sequences deposited in the GenBank database (Table 2). The obtained amplicon sequences were deposited in GenBank (accession numbers are given in Table 2). The taxonomic identification of yeasts was consistent with the results presented above. This means that the strains which were preliminarily classified to one physiological group based on their morphological, physiological, and biochemical characteristics, did, indeed, belong to the same genus and species (Table 2).
Among the studied isolates, the largest taxonomic group was Cryptococcus, accounting for approx. 60% of all strains. The most abundant species of the genus was Cryptococcus gilvescens (pro tem Goffeauzyma gilvescens) with 38 strains isolated from an old skua nest located on the oldest moraine of the Ecology Glacier. Other isolates belonging to the same genus included Cryptococcus gastricus (pro tem Goffeauzyma gastrica), Cryptococcus saitoi (pro tem Naganishia globosa), Cryptococcus albidus (pro tem Naganishia albida), and Cryptococcus adeliensis (pro tem Naganishia adeliensis), with one strain per species. The remaining six isolates belonging to the phylum Basidiomycota were conclusively classified as Rhodotorula mucilaginosa. This species seems to be very widespread on King George Island as its four isolated strains come from four different soil sampling sites.
The ascomycetes were classified as Debaryomyces hansenii (five isolates), Debaryomyces macquariensis, and Leuconeurospora sp.
Figure 1 presents the phylogenetic relationship between the yeast cultures isolated in this study and the D1/D2 GenBank sequences of published species. However, it should be noted that this analysis does not reflect the differences observed in the preliminary study on the physiology and metabolic activity of the strains which were assigned to the same genus and species based on genetic identification. For instance, strains which were genetically assigned to the species C. gilvescens (pro tem G. gilvescens) differ in some of the aforementioned characteristics. As a result, in the course of further study, a more detailed biochemical, biophysical, and genetic characterization was established for 18 selected strains.
Biochemical and biophysical characterization of Antarctic yeasts
Based on the biochemical test API 20 C AUX, most of the 18 yeast strains were found to grow on glucose, calcium 2-ketogluconate, arabinose, galactose, cellobiose, and maltose; and many of them assimilated xylose, inositol, and lactose (Table 3). In terms of carbon sources, the most versatile strains were D. hansenii IBT-D1 and C. gastricus IBT-D7 (pro tem G. gastrica), which utilized 16 out of 19 studied compounds; growth was inhibited only on substrates containing inositol, xylose, and raffinose (Table 3).
The catalytic capacity of Antarctic yeasts studied with the API ZYM test showed that they mostly produced acidic and alkaline phosphatase, β-galactosidase, leucine arylamidase, esterolytic enzymes, and naphthol-AS-BI-phosphohydrolase. However, no strain exhibited lipase (C14), trypsin-like or chymotrypsin-like proteases, α-galactosidase, β-glucuronidase, N-acetyl-β-glucosaminidase, α-mannosidase, or α-fucosidase activity (Fig. 2).
API 20 C AUX tests showed that the strains genetically classified to the same species differed in their catalytic activity and ability to assimilate selected carbon sources (Table 3). The tests confirmed that 7 strains belonging to the species C. gilvescens (pro tem G. gilvescens; IBT-D13, IBT-D15, IBT-D16, IBT-D19, IBT-D20, IBT-D23, and IBT-D60) differed from one another in terms of both physiological and biochemical characteristics (growth temperature range as well as API 20 C AUX and API ZYM test results; see Table 3; Fig. 2). This proves that these strains should be assigned to different physiological groups. This is also the case for the four strains of the species Rh. mucilaginosa. Some small differences were also found in the intracellular protein profiles of these two species (Fig. 3).
Electrophoretic intracellular protein profiles of Antarctic strains and their digitalized diagrams obtained by the Quantity One® Software (Bio-Rad, USA); a species G. gilvescens: lane 1 IBT-D13, lane 2 IBT-D15, lane 3 IBT-D16, lane 4 IBT-D19, lane 5 IBT-D20, lane 6 IBT-D23, lane 7 IBT-D60; b species Rh. mucilaginosa: lane 1 IBT-D12, lane 2 IBT-D34, lane 3 IBT-D38, lane 4 IBT-D42
Extracellular enzyme activities of isolated yeast strains
The enzymatic potential of the 18 selected Antarctic yeast strains is presented in Table 4. The most widespread extracellular enzymatic activity, exhibited by all the studied yeast strains, was lipolytic activity. Cryptococcus saitoi IBT-D37 (pro tem N. globosa) produced the largest clear zone (d z/d c—3.2 after 11 days). These results are inconsistent with API ZYM tests, in which none of the studied strains revealed lipase C14 activity. However, it should be noted that the substrate in the selective medium was tributyrin, an ester composed of butyric acid and glycerol. The positive results in plate screening tests may indicate that the studied strains cleave only triacylglycerols comprising short-chain fatty acids. Almost all strains showed the ability to utilize phytate in the form of phytic acid dipotassium salt from the medium. Rhodotorula mucilaginosa IBT-D12 and C. gilvescens IBT-D19 (pro tem. G. gilvescens) were observed to grow most vigorously on a medium containing phytic acid dipotassium salt as a sole phosphorus source. The third most widespread activity was that of β-galactosidase, which was found in 11 out of the 18 studied strains. Proteolytic activity was less frequent. Only 4 strains were able to utilize skimmed milk, with the largest clear zone (d z/d c—2.9) noted in C. saitoi IBT-D37 (pro tem N. globosa) and C. albidus IBT-D60 (pro tem N. albida). Cryptococcus adeliensis IBT-D58 (pro tem N. adeliensis) was the only strain with pectinolytic activity. Moreover, the tests showed that it had the greatest biotechnological potential as it exhibited as many as six out of the seven studied enzymatic activities. Amylolytic activity was revealed by ten strains, while cellulase activity was found in none.
Salt tolerance of Antarctic yeast isolates
The initial screening for tolerance of high NaCl concentrations in the medium was done by cultivation on solid media containing 10, 15, and 20% NaCl and examination of growth after 4, 7, and 11 days. Among the 18 studied strains, two (C. gilvescens IBT-D16 and IBT-D60; pro tem G. gilvescens) did not tolerate such salt concentrations at all (Table 4). Most yeasts tolerated a 15% concentration and two (C. gilvescens IBT-D15 and IBT-D23; pro tem G. gilvescens) tolerated a 20% concentration.
Genome size and electrophoretic karyotypes
Cellular DNA content was determined in the studied Antarctic yeasts using flow cytometry (FCM) and pulsed-field gel electrophoresis (PFGE) (see “Materials and methods” and Table 5). A comparison of FCM genome size estimation with the chromosome length established by PFGE karyotyping (Fig. 5) enabled determination of the ploidy of the studied yeasts, and chromosomal polymorphisms were checked for strains classified in the same species (Table 5).
According to FCM analysis, D. hansenii IBT-D1 is a haploid strain as DNA content in its cells is similar to that observed for the haploid reference strain of S. cerevisiae BY4742. The genome size estimated by cytometric analysis for D. hansenii IBT-D1, that is, 12.7 Mb, is similar to that reported by other authors (Petersen and Jespersen 2004; Corredor et al. 2003). The other Debaryomyces species (D. macquariensis IBT-D11) was initially thought to be diploid due to a cellular DNA content of 22–24 Mb, but it was eventually found to be haploid as electrophoretic separation of its genomic DNA revealed the presence of 9 chromosomes with a total size corresponding to FCM results.
Cellular DNA content in C. gastricus IBT-D7 (pro tem G. gastrica) and all the studied C. gilvescens strains (pro tem G. gilvescens) (approx. 12–13 Mb) indicated a haploid chromosome number (Fig. 4), which was corroborated by PFGE results (Fig. 5). The genomes of C. gastricus IBT-D7 (pro tem G. gastrica) and C. gilvescens (pro tem G. gilvescens) strains consisted of six chromosomes of a total size of 14.1 and 11.5–11.9 Mb, respectively (Fig. 5). All C. gilvescens (pro tem G. gilvescens) strains gave identical electrophoretic images of chromosomes. No chromosomal polymorphisms were observed. The determination of ploidy in C. adeliensis IBT-D58 (pro tem N. adeliensis) and C. albidus IBT-D62 (pro tem N. albida) strains required PFGE karyotype analysis, which showed IBT-D62 to be a haploid strain with a relatively large genome of approx. 15.8 Mb, consisting of 16 chromosomes with a length ranging from 0.5 to 2.4 Mb. In turn, C. adeliensis IBT-D58 (pro tem N. adeliensis) was found to be an aneuploid strain, as the ratio of FCM genome size to the size of an individual copy determined using PFGE was 1.59. A value of 1 would indicate haploid cells, while 2—diploid cells. Thus, 1.59 suggests that some chromosomes may occur in one copy, while others in two copies. For the yeast Rh. mucilaginosa, the observed fluorescence intensity and the genome size estimated on that basis indicated the presence of two copies of all chromosomes. Genomic DNA analysis revealed ten chromosomes with lengths varying from 0.8 to 3.1 Mb, with a total size of 15.1 Mb (Fig. 5). A comparison of genome size obtained by FCM (31.1–33.8 Mb) with PFGE analysis results showed that all the studied Rh. mucilaginosa strains were diploid. Furthermore, no differences in chromosome number or size were observed between the four strains of the species. The DNA content of C. saitoi IBT-D37 (pro tem N. globosa) estimated with FCM is 41.3 Mb, which indicated that this was also probably a diploid strain. The haploid genome of the IBT-D37 strain was the largest among the studied yeasts, amounting to approx. 20 Mb. Unfortunately, it was impossible to examine the karyotype of that strain in greater detail as PFGE analysis did not lead to clear chromosome separation, probably due to the fact that the cell wall of C. saitoi IBT-D37 (pro tem N. globosa) was very resistant to the lytic enzymes used for analysis.
Karyotypes and chromosome size estimation of Antarctic yeast strains. a Diagram of electrophoretic karyotypes of Antarctic yeast strains obtained by pulsed-field gel electrophoresis (PFGE). b Pulsed-field gel electrophoresis image; lane 1 H. wingei (standard); lane 2 and 6 S. cerevisiae BY4743 (standard); lane 3 G. gilvescens IBT-D13; lane 4 Leuconeurospora sp. IBT-D59; lane 5 G. gastrica IBT-D7
Discussion
Antarctica is characterized by the most extreme climatic conditions and is considered one of the driest places in the world. Sub-Antarctic regions, such as the South Shetland archipelago, exhibit higher temperatures and their soils are richer in organic material, also from marine animals, due to the absence of a year-round snow and ice cover. Nevertheless, frequent cycles of soil freezing and thawing are also stressful and limiting to life (Carrasco et al. 2012). The Antarctic zone has been a rich source of psychrophilic and psychrotrophic microorganisms including bacteria, archaea, yeasts, and filamentous fungi. Interest in these microbes has greatly increased over the past 20 years as a result of their considerable biotechnological potential, which may be difficult to estimate, but which could contribute to many areas of economy, medicine, and environmental protection (Kumar et al. 2011; Martinez-Rosales and Castro-Sowinski 2011; Vaz et al. 2011; Carrasco et al. 2012; Vasileva-Tonkova et al. 2014).
In the course of the presented study, 57 strains of psychrotolerant yeasts were isolated from soil samples collected on King George Island in maritime Antarctica. Among these isolates, as many as 48 (84%) were classified as basidiomycetes, and only 9 (16%) as ascomycetes. This stark difference in the number of representatives of the two phyla is not surprising as, according to the literature data (Butinar et al. 2007; Branda et al. 2010; Carrasco et al. 2012), Basidiomycota species are prevalent in cold environments. For instance, in the study of Carrasco et al. (2012), the ratio of basidiomycetes to ascomycetes was almost identical to the one reported in this work (80:20). Similar results were reported by Butinar et al. (2007), with a corresponding ratio of 85:15, while Branda et al. (2010) identified only one Ascomycota species among a total of 28 yeast isolates.
Amplification of the D1/D2 and ITS1&ITS2 regions showed the genus Cryptococcus to be the most abundant yeast taxon in the studied samples of Antarctic soil (11 out of 18 isolates, or approx. 60%, belonged to that genus). Currently, according to the new classification proposed by Liu et al. (2016), some Cryptococcus species have been reassigned to other genera, e.g., Cystofilobasidium, Holtermaniella, Dioszegia, Dimennazyma, Papiliotrema, Bandonia, Goffeauzyma, and Naganishia. A great abundance of Cryptococcus has also been reported by several other research teams investigating the microbiome of extreme environments. In the studies of Vaz et al. (2011) and Arenz et al. (2006), who examined samples from King George Island and Dry Valley, respectively, Cryptococcus strains also constituted the largest group of isolates. Similar findings were reported for microbiome analysis of an Arctic region (Butinar et al. 2007) and an Alpine glacier (Turchetti et al. 2008), with Cryptococcus accounting for 50–60% of isolates. The predominance of Cryptococcus species in soil has been attributed to the polysaccharide capsules it produces, which are particularly important when competing with bacteria in arid soils. Moreover, in contrast to ascomycetes, Cryptococcus can use the nutrients available in oligotrophic systems (Vishniac 2006).
Within the genus Cryptococcus (pro tem Goffeauzyma and Naganishia), the predominant species was C. gilvescens (pro tem G. gilvescens), which accounted for approx. 40% of all the studied isolates. Reports on C. gilvescens collection sites show that the species has a preference for regions with all-year low temperatures, such as mountain glaciers (Turchetti et al. 2008; Butinar et al. 2007) and Antarctica (Carrasco et al. 2012). Among the seven isolated C. gilvescens strains, three (IBT-D16, IBT-D19, and IBT-D20) exhibited amylolytic activity and four (IBT-D16, IBT-D19, IBT-D20, and IBT-D23) β-galactosidase activity. Marked phytase activity was found in three strains (IBT-D16, IBT-D19, and IBT-D20) with two others (IBT-D13 and IBT-D23) showing weaker activity. All C. gilvescens isolates exhibited lipolytic activity towards tributyrin. This is in line with the findings of Turchetti et al. (2008) and Carrasco et al. (2012), who studied C. gilvescens strains isolated from Italian Alpine glaciers and King George Island soil, respectively. Similarly to our results, no strain revealed cellulolytic activity, with the dominant activities being lipolytic and amylolytic.
Another identified Cryptococcus (pro tem Goffeauzyma) taxon was C. gastricus IBT-D7 (pro tem G. gastrica). Our tests showed that C. gastricus degraded starch and tributyrin; however, in contrast to the strain isolated by Carrasco et al. (2012), isolate IBT-D7 did not exhibit cellulolytic activity and was halotolerant (grew in the presence of 15% NaCl). A strain belonging to the same species, described by Pathan et al. (2010), tolerated only 4% salt.
One Cryptococcus strain was assigned to C. saitoi IBT-D37 (pro tem N. globosa), capable of growth in the temperature range of 4–30 °C. Plate screening tests showed that isolate IBT-D37 had a rich enzymatic potential, synthesizing hydrolases degrading starch, lactose, casein, and tributyrin at 20 °C, with the activity against the last of these substrates being the highest. These enzymatic activities suggest that isolate IBT-D37 may be a source of new enzymes with a wide range of applications, e.g., in the production of detergents for cold washing, chemicals, pharmaceuticals, and food. In addition to its attractive enzymatic potential, C. saitoi IBT-D37 (pro tem N. globosa) is halotolerant (it grows in the presence of 10% NaCl) in contrast to the strain isolated by Butinar et al. (2007) from an Arctic glacier. Among the studied yeasts, similar tolerance to salinity was exhibited by the strain C. albidus IBT-D62 (pro tem N. albida) isolated from a soil sample collected from the Jersak Hills. Due to its halotolerance, this strain may be considered a potential source of other useful molecules, such as extremolytes, which increase intracellular osmotic potential and protect cellular structures. IBT-D62 revealed amylolytic, lipolytic, proteolytic, and β-galactosidase activity. Another identified yeast strain was C. adeliensis IBT-D58 (pro tem N. adeliensis). Its assimilation profile and growth temperature range were identical to those described by Scorzetti et al. (2000) for the C. adeliensis type strain. IBT-D58 manifested rich enzymatic activity (Table 4), and was the only pectinolytic psychrotolerant yeast strain in this study.
The other basidiomycete strains isolated in this work were conclusively identified as Rh. mucilaginosa. Isolates IBT-D12, IBT-D34, IBT-D38, and IBT-D42 were assigned to different physiological groups based on their biochemical properties and protein profiles. The strains also differed in terms of plate test results. All exhibited lipolytic activity, in line with the study of Tansel Yalcin et al. (2014). The strain IBT-D12 had unique phytase activity, while IBT-D42 could tolerate 15% NaCl. Nonetheless, PFGE and FCM analyses showed that the four studied Rh. mucilaginosa strains had an identical karyotype consisting of ten chromosomes with a total size of approx. 15 Mb. A prior karyotyping study of clinical Rh. mucilaginosa isolates by Saracli et al. (2003) revealed nine chromosomes.
Ascomycetes were less numerous, with the identified strains being D. hansenii, D. macquariensis, and Leuconeurospora sp. Most of the strains belonging to the first species (including isolate IBT-D1) reveal an interesting lipolytic potential. For instance, the strain D. hansenii YLL29 obtained from dry-salted olives cv. Thassos produces lipase with an activity of 7.44 U/mL (Papagora et al. 2013). Debaryomyces hansenii strains are halo- and osmotolerant (Aggarwal and Mondal 2009; Breuer and Harms 2006), and can grow even in the presence of 4 M NaCl, although for isolate IBT-D1, the upper limit is slightly lower (2.6 M NaCl). The genome of D. hansenii IBT-D1 consists of six chromosomes, varying from 1.5 to 2.9 Mb, with the total genome size estimated at 12.5 Mb. These results are consistent with the reports of other authors. Debaryomyces hansenii has many varieties, which were studied by Petersen and Jespersen (2004). The strains belonging D. hansenii var. hansenii have genomes with a size of approx. 12.6 Mb consisting of six chromosomes with a length of 1.36–3.14 Mb, while D. hansenii var. fabryi has seven chromosomes (from 0.31 to 2.68 Mb) with a total genome size of 11.9 Mb. Some other D. hansenii strains have been found to contain from seven to ten chromosomes with a length of 0.31–3.14 Mb. In conclusion, the results of the present study show that the chromosomal arrangement of D. hansenii is very heterogenic with distinct chromosome polymorphism. The average number of chromosomes in D. hansenii is most likely to be six, for which the total genome size varies between 9.4 and 12.6 Mb. However, additional differences can be seen in both chromosome number and genome size. Furthermore, PFGE has been proved to have a very high discriminative power for strain typing in D. hansenii (Petersen and Jespersen 2004). In addition to D. hansenii, Antarctic soil collected from grassy penguin rookeries contained a strain which was identified as D. macquariensis (IBT-D11) based on D1/D2 and ITS1 and ITS2 sequence alignments. The genome of the IBT-D11 isolate was found to have nine chromosomes with a total size of 20.9 Mb, in contrast to D. hansenii IBT-D1 with six chromosomes with a total size of 12.5 Mb. The presence of this species in an extreme environment has been reported by Duarte et al. (2013), who isolated a D. macquariensis strain from ornithogenic soil inhabited by penguins (just as was the case in the present work). Both strains are characterized by marked lipolytic activity.
Among the studied strains, only one (Leuconeurospora sp. IBT-D59) was not assigned to a species due to the low correspondence between its DNA sequences and the homologous sequences of reference strains or due to the absence of a reference sequence in GenBank (in the case of ITS1 and ITS2). The closest type species determined on the basis of D1/D2 sequencing was Leuconeurospora pulcherrima, showing 98% correspondence with IBT-59 (13 nucleotide mismatch and 1 gap in 534 bp sequence). Similar results were reported by Carrasco et al. (2012), who identified one of the yeast strains isolated from King George Island as Leuconeurospora sp. with only 98% similarity to L. pulcherrima (14 nucleotide mismatch and 1 gap in 583 bp sequence).
In summary, the isolation and genetic identification of cultivable yeasts from extreme environments not only contributes to knowledge about the biodiversity and ecology of cold ecosystems, but also enriches microorganism collections with new yeast species. The enzymatic potential of yeasts isolated from cold environments is considerable for a number of industries. These enzymes may be used in many processes requiring high activity at mild temperatures or a rapid heat-inactivation rate. Such biocatalysts have a wide range of potential applications, including the food and dairy industry, the brewing and wine industry, the textile industry, the laundry industry, etc. It should be noted that few data concerning the karyotypes of cold-adapted yeasts have been reported in the literature so far. To our knowledge, this is the first FCM and PFGE study analyzing the genome size and electrophoretic karyotypes of C. gilvescens (pro tem G. gilvescens), C. saitoi (pro tem N. globosa), C. gastricus (pro tem G. gastrica), and C. albidus (pro tem N. albida).
References
Aggarwal M, Mondal AK (2009) Debaryomyces hansenii: an osmotolerant and halotolerant yeast. In: Satyanarayana T, Kunze G (eds) Yeast biotechnology: diversity and application, Springer, Berlin, pp 65–84. doi:10.1007/978-1-4020-8292-4_4
Arenz BE, Held BW, Jurgens JA, Farrell RL, Blanchette RA (2006) Fungal diversity in soils and historic wood from the Ross Sea region of Antarctica. Soil Biol Biochem 38:3057–3064. doi:10.1016/j.soilbio.2006.01.016
Branda E, Turchetti B, Diolaiuti G, Pecci M, Smiraglia C, Buzzini P (2010) Yeast and yeast-like diversity in the southernmost glacier of Europe (Calderone Glacier, Apennines, Italy). FEMS Microbiol Ecol 72:354–369. doi:10.1111/j.1574-6941.2010.00864.x
Breuer U, Harms H (2006) Debaryomyces hansenii—an extremophilic yeast with biotechnological potential. Yeast 23:415–437. doi:10.1002/yea.1374
Butinar L, Spencer-Martins I, Gunde-Cimerman N (2007) Yeasts in high Arctic glaciers: the discovery of a new habitat for eukaryotic microorganisms. Antonie van Leeuwenhoek 91:277–289. doi:10.1007/s10482-006-9117-3
Buzzini P, Margesin R (2014) Cold-adapted yeasts: a lesson from the cold and a challenge for the XXI century. In: Buzzini P, Magresin R (eds) Cold-adapted yeasts. Biodiversity, adaptation strategies and biotechnological significance. Springer, Berlin, pp 3–32. doi:10.1007/978-3-642-39681-6_1
Buzzini P, Branda E, Goretti M, Turchetti B (2012) Psychrophilic yeasts from worldwide glacial habitats: diversity, adaptation strategies and biotechnological potential. FEMS Microbiol Ecol 82:217–241. doi:10.1111/j.1574-6941.2012.01348.x
Carrasco M, Rozas JM, Barahona S, Alcaino J, Cifuentes V, Baeza M (2012) Diversity and extracellular enzymatic activities of yeasts isolated from King George Island, the sub-Antarctic region. BMC Microbiol 12:251. doi:10.1186/1471-2180-12-251
Connell LB, Rodriguez RR, Redman RS, Dalluge JJ (2014) Cold-adapted yeasts in Antarctic deserts. In: Buzzini P, Magresin R (eds) Cold-adapted yeasts. Biodiversity, adaptation strategies and biotechnological significance. Springer, Berlin, pp 75–98. doi:10.1007/978-3-642-39681-6_4
Corredor M, Davila AM, Casaregola S, Gaillardin C (2003) Chromosomal polymorphism in the yeast species Debaryomyces hansenii. Antonie von Leeuwenhoek 84:81–88. doi:10.1023/A:1025432721866
Diaz MR, Fell JW (2005) Use of a suspension array for rapid identification of the varieties and genotypes of the Cryptococcus neoformans species complex. J Clin Microbiol 43:3662–3672. doi:10.1128/JCM.43.8.3662-3672.2005
Duarte AWF, Dayo-Owoyemi I, Nobre FS, Pagnocca FC, Chaud LCS, Pessoa A, Felippe MGA, Sette LD (2013) Taxonomic assessment and enzymes production by yeasts isolated from marine and terrestrial Antarctic samples. Extremophiles 17:1023–1035. doi:10.1007/s00792-013-0584-y
Guffogg S, Thomas-Hall S, Holloway P, Watson K (2004) A novel psychrotolerant member of the hymenomycetous yeasts from Antarctica: Cryptococcus watticus sp. nov. Int J Syst Evol Micr 54:275–277. doi:10.1099/ijs.0.02877-0
Jeyaram K, Singh WM, Capece A, Romano P (2008) Molecular identification of yeast species associated with ‘Hamei’—a traditional starter used for rice wine production in Manipur, India. Int J Food Microbiol 124:115–125. doi:10.1016/j.ijfoodmicro.2008.02.029 (Epub 2008 Mar 6)
Kishida M, Seike Y, Kawasaki H (2009) Identification and characterization of a psychrophilic yeast strain newly isolated from the fermentative starter (Loog-pang) of a traditional drink in Thailand. Biocontrol Sci 14:119–122. doi:10.4265/bio.14.119
Kumar L, Awasthi G, Singh B (2011) Extremophiles: a novel source of industrially important enzymes. Biotechnology 10:121–135. doi:10.3923/biotech.2011.121.135
Kurtzmann CP, Fell JW, Boekhout T, Robert V (2011) Methods for isolation, phenotypic characterization and maintenance of yeasts. In: Kurtzman CP, Fell JW, Boekhout T (eds) The yeast: a taxonomic study. Elsevier, Amsterdam pp 87–110. doi:10.1016/B978-0-444-52149-1.00007-0
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0
Liu XZ, Wang QM, Göker M, Groenewald M, Kachalkin AV, Lumbsch HT, Millanes AM, Wedin M, Yurkov AM, Boekhout T, Bai FY (2016) Towards an integrated phylogenetic classification of the Tremellomycetes. Stud Mycol 81:85–147. doi:10.1016/j.simyco.2015.12.001 (Epub 2016 Jan 8)
Margesin R, Miteva V (2011) Diversity and ecology of psychrophilic microorganisms. Res Microbiol 162:346–361. doi:10.1016/j.resmic.2010.12.004
Martinez-Rosales C, Castro-Sowinski S (2011) Antarctic bacterial isolates that produce cold-active extracellular proteases at low temperature but are active and stable at high temperature. Polar Res 30:7123. doi:10.3402/polar.v30i0.7123
Moreira SR, Schwan RF, Pinheiro de Carvalho E, Wheals AE (2001) Isolation and identification of yeasts and filamentous fungi from yoghurts in Brazil. Braz J Microbiol 32:117–122. doi:10.1590/S1517-83822001000200009
Morita RY (1975) Psychrophilic bacteria. Bacteriol Rev 39:144–167
Nuobarience L, Hansen A, Jespersen L, Arneborg N (2010) Phytase-active yeasts from grain-based food and beer. J Appl Microbiol 110:1370–1380. doi:10.1111/j.1365-2672.2011.04988.x
Papagora C, Roukas T, Kotzekidou P (2013) Optimization of extracellular lipase production by Debaryomyces hansenii isolates from dry-salted olives using response surface methodology. Food Bioprod Process 91:413–420. doi:10.1016/j.fbp.2013.02.008
Pathan AAK, Bhadra B, Begum Z, Shivaji S (2010) Diversity of yeasts from puddles in the vicinity of Midre Lovenbreen glacier, Arctic and bioprospecting for enzymes and fatty acids. Curr Microbiol 60:307–314. doi:10.1007/s00284-009-9543-3
Petersen KM, Jespersen L (2004) Genetic diversity of the species Debaryomyces hansenii and the use of chromosome polymorphism for typing of strains isolated from surface-ripened cheeses. J Appl Microbiol 97:205–213. doi:10.1111/j.1365-2672.2004.02293.x
Sabatinos SA, Forsburg SL (2009) Measuring DNA content by flow cytometry in fission yeast. Methods Mol Biol 521:449–461. doi:10.1007/978-1-4939-2596-4_5
Saracli MA, Sener K, Gonlum A, Yildiran ST, Wickes BL (2003) Genotyping of clinical Rhodotorula mucilaginosa isolates by pulsed field gel electrophoresis. Mycoses 46:487–491. doi:10.1046/j.0933-7407.2003.00925.x
Saracli MA, Yildiran ST, Sener K, Gonlum A, Doganci L, Keller SM, Wickes BL (2006) Genotyping of Turkish environmental Cryptococcus neorformans var. neoformans isolates by pulsed field gel electophoresis and mating type. Mycoses 49:124–129. doi:10.1111/j.1439-0507.2006.01203.x
Scorzetti G, Petrescu I, Yarrow D, Fell JW (2000) Cryptococcus adeliensis sp. nov., a xylanase producing basidiomycetous yeast from Antarctica. Antonie van Leeuwenhoek 77:153–157. doi:10.1023/A:1002124504936
Shivaji S, Prasad GS (2009) Antarctic yeast: biodiversity and potential application. In: Satyanarayana T, Kunze G (eds) Yeast biotechnology: diversity and applications. Springer, Berlin, pp 3–16. doi:10.1007/978-1-4020-8292-4_1
Szczęsna-Antczak M, Kamińska J, Florczak T, Turkiewicz M (2014) Cold-active yeast lipases: recent issues and future prospects. In: Buzzini P, Margesin R (eds) Cold-adapted yeasts: biodiversity, adaptation strategies and biotechnological significance. Springer, Berlin, pp 353–375. doi:10.1007/978-3-642-39681-6_16
Tansel Yalcin H, Corbaci C, Ucar FB (2014) Molecular characterization and lipase profiling of the yeasts isolated from environments contaminated with petroleum. J Basic Microbiol 54:S85–S92. doi:10.1002/jobm.201300029
Thomas-Hall S, Watson K (2002) Cryptococcus nyarrowii sp. nov., a basidiomycetous yeast from Antarctica. Int J Syst Evol Microbiol 52:1033–1038. doi:10.1099/ijs.0.01940-0
Thomas-Hall S, Watson K, Scorzetti G (2002) Cryptococcus statzelliae sp. nov. and three novel strains of Cryptococcus victoriae, yeasts isolated from Antarctic soils. Int J Syst Evol Micr 52:2303–2308. doi:10.1099/ijs.0.02293-0
Thomas-Hall S, Turchetti B, Buzzini P, Branda E, Boekhout T, Theelen B, Watson K (2010) Cold-adapted yeasts from Antarctica and the Italian Alps—description of three novel species: Mrakia robertii sp. nov., Mrakia blollopis sp. nov. and Mrakiella niccombsii sp. nov. Extremophiles 14:47–59. doi:10.1007/s00792-009-0286-7
Turchetti B, Buzzini P, Goretti M, Branda E, Diolaiuti G, D’Agata C, Smiraglia C, Vaughan-Martini A (2008) Psychrophilic yeasts in glacial environments of Alpine glaciers. FEMS Microbiol Ecol 63:73–83. doi:10.1111/j.1574-6941.2007.00409.x
Vasileva-Tonkova E, Romanovskaya V, Gladka G, Goulianova D, Tomova I, Stoilova-Disheva M, Tashyrev O (2014) Ecophysiological properties of cultivable heterotrophic bacteria and yeasts dominating in phytocenoses of Galindez Island, maritime Antarctica. World J Microbiol Biotechnol 30:1387–1398. doi:10.1007/s11274-013-1555-2
Vaz ABM, Rosa LH, Vieira MLA, de Garcia V, Brandao LR, Teixeira LCRS, Moline M, Libkind D, van Broock M, Rosa CA (2011) The diversity, extracellular enzymatic activities and photoprotective compounds of yeasts isolated in Antarctica. Braz J Microbiol 42:937–947. doi:10.1590/S1517-83822011000300012
Vishniac H (2006) A multivariate analysis of soil yeasts isolated from a latitudinal gradient. Microb Ecol 52:90–103. doi:10.1007/s00248-006-9066-4
Acknowledgements
The authors are grateful to the hydromicrobiologist Dorota Górniak, Ph.D. (Department of Microbiology, University of Warmia and Mazury, Olsztyn, Poland) for fruitful discussion concerning sampling sites and for taking the samples. The authors would like to extend their gratitude to the microbiologist Alina Kunicka-Styczyńska, Ph.D., D.Sc. (Institute of Fermentation Technology and Microbiology, Lodz University of Technology, Poland) for consultation and assistance in the isolation and characterization of Antarctic yeasts. The presented results were obtained as part of the PLATPROT project (PBS1/A9/7/2012). Financial support was provided by the Polish National Center for Research and Development (NCBiR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Kur J.: Deceased.
Rights and permissions
About this article
Cite this article
Białkowska, A.M., Szulczewska, K.M., Krysiak, J. et al. Genetic and biochemical characterization of yeasts isolated from Antarctic soil samples. Polar Biol 40, 1787–1803 (2017). https://doi.org/10.1007/s00300-017-2102-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-017-2102-7