Abstract
According to the recent studies, approximately 380 million tons of plastic is being generated across the world per year and 90% of it is recycled, so that it is converted into a pollutant. The majority of plastic waste has been sent to landfills; therefore, the soil acts as a major sink for plastic wastes. During the process of plastic breakdown in the soil, the plastic debris will be changed into micro-nanoplastics (MNPs), which will have a negative impact on the flora and fauna in the ecosystem, including the human health. Hence, appropriate degradation methods are needed to overcome this issue. Microbial biodegradation is the best method and is considered to be a more profitable and more effective and a highly accepted method. The microorganisms which are responsible for the biodegradation are differing from one another and have their own optimal growth conditions in the soil. Many kinds of microorganisms are involved in the biodegradation of MNPs. Among these biodegrade microorganisms, the bacteria are easier to grow and degrade MNPs compared to others. The objectives of this chapter are (1) to summarize the bacterial degradation of MNPs in soil and (2) to list out various kinds of bacteria and enzymes, which are involved in the degradation of MNPs in the soil system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
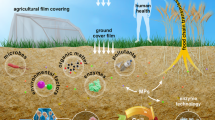
Plastics are organic polymers containing molecules composed of long carbon chains like back-bones formed during the polymerization (Koushal et al., 2014). They are made of carbon and hydrogen, with nitrogen, sulfur, and other various organic and inorganic materials derived from fossil fuels (Kumari & Murthy, 2013). Many of the same units (or mers) are connected together to form a long chain or polymer or macromolecules. Plastics are polymers that, when heated, become mobile and can be molded into required shapes. Plastic-derived materials can be pushed into any required shape because they are non-metallic compounds. Plastics are predominantly used in the packaging business, which includes industries such as food, pharmaceuticals, and cosmetics. Polyethylene (LDPE, MDPE, HDPE, LLDPE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC), polybutyrene tetraphthalate (PBT), and nylon are the most regularly used polymers in the industries. Since the last six decades, when the commercial production of plastics began, we are depending on plastic as an affordable, versatile, and durable material. The production is accelerating so rapidly that it has created 8.3 billion tons of plastic, and unfortunately over 90% of it is not being recycled. As of 2018, approximately 380 million tons of plastic is produced worldwide each year and to combat the problem of plastic waste, the strategies of reuse, reduction, and recycling are now widely adopted. However, this method is less effective, especially for plastics waste that has been mixed with other types of waste (Drzyzga & Prieto, 2018). So that the majority of plastic materials has been sent to landfills and yet we are still producing and consuming more plastic. The decomposition process of plastic polymers takes thousands of years, and the landfill plastics waste processing requires large space, and incineration plastics waste processing can produce toxic gases into the environment (Kumar et al., 2017). As a result, people commonly burn plastic debris to combat the accumulation of plastic waste in the environment; however, this activity pollutes the air. It emits hazardous substances like CO2 and dioxins into the atmosphere, which are causes of lung diseases and cancer (Kale et al., 2015). Plastic waste is a contaminant that pollutes the land, air, and water ecosystems, harming the biosphere including human beings (Soud, 2019; Sowmya et al., 2014). Micro and nanoplastics (MNPs) are pieces of any plastic material having a size less than 5 mm in length that form as a result of bigger plastic goods degrading in the environment due to natural processes such as weathering. In recent years, the MNPs are abundantly found in the sea, freshwater, terrestrial environment, and organisms. MNPs contamination is becoming a major issue, and it is considered to be the second-most important scientific topic in the study of environment and ecology. Microplastics are seen as a serious threat to terrestrial ecosystems, including the soil, which potentially holds more plastic than the seas (Hurley & Nizzetto, 2018). Microplastics were abundantly found in floodplain soils (Scheurer & Bigalke, 2018), coastal beach soils (Zhou et al., 2018), and farming soils (Liu et al., 2018). Microplastics entered in soil will get stored, translocated, cause erosion, degradation and leach the groundwater, and thus threaten organisms and further effect human health (Hurley & Nizzetto, 2018). Microplastics accumulation can be influenced by soil biota. Microplastics can be consumed by soil fauna and transformed into smaller MNPs in their gizzard. Digging mammals, such as gophers and moles, can incidentally contribute to the further abrasion into nanoplastics and translocation of microplastics (Rillig et al., 2017). Microplastics pollution can have negative impact on organisms in soils. Plastics are manufactured with multiple types of chemical ingredients to enhance their quality, including plasticizers, stabilizers, flame retardants, and monomers (de Souza Machado et al., 2018). The chemical ingredients can be leached out during the life cycle of the product, especially in the soil environment. On the other hand, plastics can also absorb other toxicants such as metals, polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), and organochlorine pesticides (e.g. DDT, HCH) due to their hydrophobic surface. The annual plastic release into soil is approximately 4 to 23 times higher than that of the sea (Horton et al., 2017). The study of plastic pollution in the oceans preceded that of soil contamination (da Costa et al., 2016). The terrestrial environment can’t cope with this amount of plastic polluting MNPs; thus, a proper method of processing plastic waste is necessary. The plastic degradation mainly comprises of the following three types: photodegradation, oxy-photodegradation, and biodegradation (Shah et al., 2008). Among the best methods until date is biodegradation, as it uses microbes to degrade plastic, which is advantageous and efficient as well as widely accepted. In biodegradation, several types of plastic are degraded by various microbes and decomposers, such as actinomycetes, algae, bacteria, fungi, and others (Agrawal & Singh, 2016). They have the ability to create enzymes (both intracellular and extracellular) that aid in the decomposition of polymeric polymers. Bacteria are easier than fungi to grow and break down polymeric materials because fungi require more stable conditions to develop and degrade (Amobonye et al., 2021). The aim of this chapter is to discuss the state of soil pollution and highlight the major knowledge points on the microplastics and nanoplastics (MNPs) degradation by using of bacteria in the soil environment. This will help to improve our knowledge on the exposure, effect, and risks of MNPs degradation in contaminated soil by bacteria.
2 Types of Most Commonly Used Plastics
There are different types of plastics, classified on the basis of their origin, chemical structure, and physical properties (Fig. 14.1).
2.1 Classification Based on the Origin of the Plastics
Based on the origin, the plastics are divided into three types.
-
(a)
Natural plastics:
Natural plastics are material, which can be moulded in its natural form (Tar, shellac, tortoiseshell, animal horn, cellulose, amber, and latex from tree sap).
-
(b)
Semi-synthetic plastics:
Semi-synthetic plastics are chemically altered natural materials. Celluloid and vulcanized rubber were the first polymers, which are chemically modified from natural polymers, such as cellulose and latex.
-
(c)
Synthetic plastics:
Synthetic polymers are synthesized entirely in the lab, usually by polymerizing monomers sourced from oil or gas, and plastics are made from them by adding different chemical ingredients. Bakelite is the first complete synthetic polymer. The use of synthetic plastics is widespread in the packaging of products, such as pharmaceuticals, food, cosmetics, chemicals, and detergents.
3 Classification Based on the Structure of the Atoms
Plastic polymers are classified into two groups based on their atomic structure (Kumar et al., 2013).
-
(a)
C–C backbone polymers: C–C backbone polymers, including PE, PVC, PS, and PP, represent 77% of the total market share. (A) Polypropylene or PP (e.g., bottle caps, drinking straws, medicine bottles, car batteries, disposable syringes). (B) Polyvinyl chloride or PVC (e.g., bottles of juice, cling films, raincoats, visors, shoe soles, garden hoses, and electrical wiring pipes). (C) Polystyrene or PS (e.g., disposable cups, plates, trays, and cutlery, as well as packing materials and laboratory ware). (D) Polyethylene (PE): Polyethylene ((C2H4)n) is the most common plastic used for packaging. PE is usually a mixture of similar polymers of ethylene, with various values of n. The commonly used PEs are as follows: (1) High-density polyethylene or HDPE (e.g., water bottles, trash, and retail bags). (2) Low-density polyethylene or LDPE (e.g., frozen food bags, squeezable bottles, flexible container lids). (3) Medium-density polyethylene or MDPE (e.g., gas pipes and fittings, sacks, shrink film, packaging film, carrier bags). (4) Linear low-density or LLDPE (e.g., cable coverings, toys, lids, buckets, containers, and pipe).
-
(b)
C–O backbone polymers or hetero atomic polymers: C–C backbone polymers, including PET and PU, represent ~18% of the market share. (A) Polyethylene terephthalate or PET (e.g., soft drink, water and dressing bottles, peanut butter, and jam bars). (B) Polyurethane or PU (e.g., bedding, truck seating).
4 Classification on the Basis of Thermal Properties
Plastics can be classified into three kinds based on their thermal properties.
-
(a)
Thermosetting plastics: Thermosets are hard and have a very tight-meshed, branched molecular structure. These plastics can withstand high temperatures and once hardened these cannot be reformed or recycled even with the application of heat. Thermosets are used, for example, to make light switches (e.g., bakelite, polyurethane, epoxy resin, vinyl ester resin, and vulcanized rubber).
-
(b)
Elastomers: Elastomers also have a cross-linked structure and a looser mesh than thermosets, allowing for elasticity. Elastomers also cannot be reshaped with heat once they have been shaped (e.g., automobile tires).
-
(c)
Thermoplastics: Thermoplastics usually have low melting points, which allow them to be remolded or recycled easily. They have a linear or branched molecular structure that determines their strength and thermal behavior; they are flexible at ordinary temperatures. At approx. 120–180 °C, thermoplastics become a pasty/liquid mass. The service temperature range for thermoplastics is lower than that of thermosets. Most plastics are thermoplastics, which are commonly used in packaging (e.g., polyvinyl chloride, polyethylene Polystyrene, Teflon, Acrylic, and Nylon are some of the thermoplastic materials).
5 Classification on the Basis of Degradability
5.1 Non-biodegradable Plastics
Non-biodegradable plastics also known as synthetic plastics are derived from petrochemicals and are very high molecular weight polymers. They do not degrade naturally and hence accumulate in environment.
5.2 Biodegradable Plastics
Biodegradable plastics are derived from natural substances such as components of algae, plants, and animals, which provide cellulose, starch, and protein needed for their production. They can easily be destroyed by UV radiation, water, enzymes, pH changes, and other factors. They are further divided into four groups
-
(i)
Bio-based bioplastics: Plastics whose entire carbon content is produced from agricultural and forestry resources such as corn starch, soybean protein, and so on.
-
(ii)
Biodegradable bioplastics: A biodegradable bioplastic is commonly made of renewable raw materials, microorganisms, petrochemicals, or a combination of all three. These plastics degrade completely by microorganisms into biogases and biomass (primarily carbon dioxide and water) without releasing harmful compounds. The use of biodegradable plastics is common in disposable items such as packaging, crockery, cutlery, medical devices, personal hygiene products, and foodservice containers. Several biodegradable bioplastics have been developed over the past few years, including polyhydroxyalkanoates (PHAs), polylactides, polycaprolactone, and polysaccharides. The polyhydroxyalkanoates (PHAs) were first observed in bacteria in 1888 by Martinus Beijerinck. There are two main types of biodegradable plastics.
-
(a)
Oxo-biodegradable bioplastics (OBP): OBP is produced by mixing ordinary plastics with a little portion of fatty acid compounds obtained from transition metals.
-
(b)
Hydro-biodegradable bioplastics (HBP): HBP is made from bio-based sources like corn, wheat, sugar cane, petroleum-based sources, or a combination of both. Both types of degradation begin with a chemical breakdown (oxidation and hydrolysis, respectively), followed by a biodegradation process. In both cases, degrading plastic emits CO2, but hydro-biodegradable plastics can emit methane as well. Examples for biodegradable plastics are polyglycolic acid (PGA), polyhydroxy butyrate (PHB), polylactic acid (PLA), polycaprolactone (PCL), poly hydroxyl alkanoates (PHA), polyhydroxyl valerate (PHV), polyvinyl alcohol (PVOH), and polyvinyl acetate (PVAc)
-
(a)
-
(iii)
Compostable bioplastics: When composted, these bioplastics decompose at a similar rate as other compostable materials without leaving behind any toxic residues.
-
(iv)
Photodegradable bioplastics: A photodegradable bioplastic is composed of light-sensitive groups attached to its backbone; therefore, prolonged exposure to UV light disintegrates their polymeric structure, making them prone to further degradation by microbes.
6 Classification Based on the Fragment Size
Plastic fragments are categorized as micro nanoplastics (MNPs) and micro, macro, and megaplastics based on their size in the environment. Micro and nano-sized plastic particles are produced by the physico-chemical breakdown of plastic waste and are referred to as micro-nanoplastics (MNPs). MNPs are split into two groups based on their source of origin: primary MNPs and secondary MNPs.
-
(i)
Primary MNPs:
Primary MNPs are derived from household items, cosmetics, and polymeric raw materials from the plastics industry, such as polyethylene (PE), polystyrene (PS), and polypropylene (PE).The primary microplastics contain micro-beads in personal care products, tiny beads used for exfoliation, the abrasives in toothpastes, the plastic pellets used for grinding and polishing in industrial production, or the tiny debris originally produced in the manufacturing process (Wang et al., 2020).
-
(ii)
Secondary MNPs:
Secondary MNPs are formed due to fragmentation of extensive plastic waste from exposure to abiotic factors such as temperature, UV radiation, and microbial degradation. Secondary MPs are mainly from the industrial and daily plastic goods discarded in the environment (e.g., bottles, packaging bags, boxes, clothing, various instruments, and production wastes) (Ammala et al., 2011).
Toxicological Effect of Micro-Nanoplastics (MNPs)
There are two types of pollutants transported together with MNPs that damage the ecosystem: The first are chemicals applied to plastics to increase their performance; the second is pollutants (chemical substances or pathogens) acquired and carried by MNPs from their surroundings in the continual transfer process in the environment (Yuan et al., 2020). When plastic ages in the ecosystem, it promotes the absorption of contaminants (such as heavy metal ions, POPs, and microbes) (Mao et al., 2020). Moreover, when soil MPs are becoming more abundant, the interaction between MPs and microorganisms becomes more frequent (Sangeetha Devi et al., 2015). MPs can be consumed or attached to organisms at various trophic levels in the soil, and then transported to organisms at higher trophic levels in the food chain, resulting in MP flow in the food web (Kumar Sen & Raut, 2015). The movements of MPs in the food web can cause physical and chemical damages to organisms. Pollutants or pathogens spread by plastic particles will enter the food web and travel up in the food chain. Chlorinated plastic can release toxic soil, affecting the environment and groundwater. MNPs have been detected for the first time in human blood (Leslie et al., 2022), warning that the ubiquitous particles may be making their way into organs. According to a Dutch study, half of the blood samples showed traces of PET plastic, which is widely used to make drink bottles, and more than third contained polystyrene, which is widely used in disposable food containers and other products. According to the study, MNPs might have entered the body by a variety of means, including air, water, and food, as well as toothpaste, lip glosses, and tattoo ink. Methane gas, a major greenhouse gas generated during the decomposition process, affects significantly to global warming (Hester & Harrison, 2011).
7 Degradation of Plastics
There are two ways to degrade plastic waste. (1) Abiotic methods, (2) Biotic methods
7.1 Abiotic Methods
The degradation process of plastics is influenced by abiotic factors, which involve mechanical and chemical forces. The process will convert plastics into brittle materials, which leads to the formation of MNPs. The MW of the polymer is decreased during chemical fragmentation, but not during mechanical fragmentation. It is controlled by a number of factors, including polymer chain length, intramolecular forces, mechanical stability, polymer crystallinity, and plastic weight. Polymer degradation has been classified as follows based on the nature of the causing agents.
-
(i)
Photo-oxidative degradation:
A photo-oxidation process, also known as ultraviolet degradation, degrades polymeric materials by exposing them to terrestrial light energy in combination with a chemical oxidizer, such as air. Certain plastics are naturally susceptible to photo-oxidation due to their structure and functional groups. As a result of adequate light energy input, these functional groups (chromospheres) cleave and produce free radicals, which is very similar to thermal oxidation. In essence, light energy accelerates the generation of free radicals, which initiate the degradation reaction. The photo-oxidation reaction reduces the molecular weight of a polymer by incorporating oxygen into its backbone structure as carbonyl groups. Its rate of initiation is very slow; once the plastic started to degrade, it propagates very fast. It is an environmentally friendly method, but it is quite expensive.
-
(ii)
Thermo-oxidative degradation:
Thermal oxidation is the process by which polymeric materials are degraded by contacting a chemical oxidizer. Most polymers are susceptible to thermal oxidation, and it is by far the most common degradation process for plastics.
In oxidation, oxygen is added into the molecular structure of a polymer, creating a type of carbon–oxygen bond known as carbonyl functionality. The process of oxidation produces a permanent change in a plastic by shortening its chains by reducing its molecular weight. The oxidation is driven by the formation of free radicals within the plastic. In plastic formulations, free radicals can be unintentional byproducts of polymerization, as additives to formulations, or as contaminants. These free radicals are reactive and attack the covalent bonds in the polymer backbone. Polymer chains are cleaved through thermal oxidation, and the resulting shortened chains are terminated by oxygenated functional groups, such as carboxylic acids, esters, ketones, and aldehydes. In this method, oxygen is needed as well as heat (75–200 °C, a temperature higher than ambient). Various harmful gases are emitted into the environment at high temperatures. This approach is very quick, but that’s not widely accepted.
-
(iii)
Hydrolytic degradation:
The destruction of a polymeric material by contact with water, specifically hydrogen cations (H+) or hydroxyl anions, is known as hydrolysis (OH−). The degradation of plastic materials can be caused by immersion in water, condensation cycles, or exposure to steam. It can also be caused by interaction with acids (high H+ concentration) or bases (high OH− concentration), both of which can speed up the process significantly.
7.2 Biotic Methods
The microbial aspect of the synthetic plastic degradation is mostly due to the action of diverse microbial populations that have been identified as potential xenobiotics degraders based on their adaptability to and use these compounds as growth and energy substrates. These organisms use their diverse enzyme systems to break down polymers into intermediates that can then be absorbed and metabolized to meet their energy requirements. In this regard, the ability to biodegrade certain plastic polymers has recently been explored. Microbial degradation is a practical, clean, and affordable way to remediate MNPs contaminants. In this process, the plastic gets modified chemically, physically, and mechanically through surface degradation caused by diverse microbes and decomposer organisms such as actinomycetes, algae, bacteria, as well as fungi. As a result of microbial activity, various metabolic reaction pathways are involved in the conversion of organic molecules (MNPs) into biogas and residual biomass. The biodegradation of plastic waste is an efficient, profitable, and economically viable method. These bacteria can produce a variety of enzymes, both intracellular and extracellular, which can catalyze the degradation of plastic polymers into small and safe fragments (Agrawal & Singh, 2016). The use of microbial cells to break down plastic C–C linkages is considered to be more successful (Wei & Zimmermann, 2017). Microbial degradation is a specific enzymatic reaction. Certain enzymes are responsible for the breakdown of specific substrates (Adamcová & Vaverková, 2014).
8 Mechanism of Plastic Biodegradation
Abiotic degradation occurs before biodegradation and is triggered by thermal, hydrolytic, or UV light in the environment. Smaller polymer fragments are generated by abiotic breakdown can penetrate through the cell membrane and be biodegraded by enzymatic action inside microbial cells; nevertheless, some microorganisms secrete extracellular enzymes that can act on specific plastic polymers. The entire process of microbial degradation can be summarized in four essential stages (Fig. 14.2):
-
(i)
Biodeterioration or colonization (Adherence of microbes to the surface of polymer superficially): The first step of biodegradation is the biodeterioration that includes the combined action of microbial communities. Physico-chemical reactions lead to the incorporation of aquaphilic groups, which make the polymer more hydrophilic and reduce surface energy. It may allow the polymer’s carbon to be used for microbial growth and development. Deterioration is a type of surface degradation that affects a material’s mechanical, physical, and chemical properties. This process will be accelerated by biofilms formed by microorganisms on the plastic surface. Biofilm is a colony of living organisms. Microbes attach to one another in a polymer matrix and colonize the surface of the material to form biofilms with the help of polysaccharides and proteins extracellular polymeric substances (EPS) are produced by themselves to break down the plastic surface. The EPS contains polysaccharides, proteins, and nucleic acids. The EPS penetrates into the surface pores of the plastic, causing them to expand. Microbes and bacteria have been enhanced in their ability to degrade plastic polymers, produce holes, and promote the physical deterioration of plastic polymers. Furthermore, the growth of biofilms on plastic surfaces supports the formation of different acid compounds (nitrous acid, nitric acid, sulfuric acid, citric, fumaric, gluconic, glutaric, glyoxylic, oxalic, and oxaloacetic acid) affecting the pH of plastic polymers and causing changes in the microstructures of the polymer, called chemical plastic deterioration.
-
(ii)
Biofragmentation or depolymerization (Exploitation of polymer as a food/carbon source to the microbes): Cleavage of the primary carbon chain takes place by catalytic agents (depolymerase enzymes), which are secreted by microorganisms and result in the formation of low molecular weight fragments such as oligomers, dimers, and monomers. Microbe-secreted extracellular and intracellular depolymerase enzymes play a significant role in the breakdown of plastic waste degradation. The released enzymes will break down complex polymers into smaller and simpler chains during the breakdown process. These decomposed small molecules will be easily dissolved in water and then absorbed by microorganisms through their semi-permeable cell membranes and utilized as carbon and energy sources.
-
(iii)
Assimilation: Assimilation is the process of integrating molecules transported in the cytoplasm into the microbial metabolism to generate energy, biomass, vesicles, and numerous primary and secondary metabolites. Bacteria thus secrete some enzymes which played a significant part in the degradation process. The main end products of biodegradation of plastic in an aerobic environment are CO2, H2O, and biomass, whereas in anaerobic conditions, the main products are CO2, H2O, biomass, and CH4, while the main products of biodegradation of plastic in a sulfidogenic environment are H2S, CO2, and water.
-
(iv)
Mineralization: Mineralization refers to the excretion of simple and different salts, and also complex metabolites that reach the extracellular surroundings. In this process, hazardous compounds are transformed into more environmentally friendly compounds. In mineralization, biodegradable materials or biomass are converted into gases, water, salt, minerals, and other residues. These gases include carbon dioxide, methane, and nitrogen. Mineralization will be completed when all biodegradable compounds have been consumed by microorganisms and all carbon has been converted to carbon dioxide.
Biodegradation is influenced by various factors, including polymer characteristics, organism type, and pretreatment method. The polymer characteristics such as its mobility, tactility, crystallinity, molecular weight, the type of functional groups and substituents present in its structure, and plasticizers or additives added to the polymer all play an important role in its degradation. Environmental conditions mediate the interaction between microbes and the degradative pathway during degradation. At commercial level, additives, antioxidants, and stabilizers get attached to the surface of polymers which may be proven harmful and susceptible to organisms in the environment and may also lead to slowing down of the speed of biodegradation process. The majority of plastics deteriorate at first on the surface, which is exposed and vulnerable to chemical or enzymatic attack. Therefore, degradation of microplastics proceeds faster than meso- and microplastics, as microplastics has a higher surface-to-volume ratio.
9 Plastics Biodegradation Bacteria
First Report of Plastic Degradation by Bacteria: For the first time, comparative degradation assay of lignin and paraffin’s was studied due to action of bacteria (Fuhs, 1961) by growing bacteria on different kinds of alkenes as the only source of carbon. They further reported that bacteria can deteriorate only polymers with molecular weight up to 4800. Later, reports on plastic degradation by microbes started increasing significantly in the literature from various regions. Similarly, bacterial strains can degrade plastic polymeric substances in contaminated water or soil. Several studies have reported that plastics biodegradation by specialized bacteria can be a promising bioremediation strategy for contaminated ecosystems (Yoshida et al., 2016). Bacterial strains such as Pseudomonas spp., Bacillus spp., and Streptomyces spp. have exhibited high degradation efficiency against various plastic polymers (Li et al., 2020; Matjašič et al., 2021). In many cases, the plastics degradation rates by fungi exceed those achieved by bacterial strains (Muhonja et al., 2018). According to Amobonye et al. (2021), bacteria are easier to grow and degrade polymeric materials than fungi that need more stable conditions.
9.1 Plastic-Degrading Bacteria
Bacteria are considered to be the engine of the earth’s nutrients, as they are responsible for the conversion and cycling of nutrients in the environment. Bacteria use the contaminants for their growth, nutrition, and reproduction. This is the main reason behind bacterial transformation of different contaminants which are organic in nature. Microorganisms get carbon (C) from Organic Carbon (OC). Carbon (C) is essential for bacteria and other microorganisms as it acts as a building block for new cell. Carbon (C) is also a source of energy utilized by the organisms (Mondal & Palit, 2019). Most of the identified bacteria belong to the phyla proteobacteria (48%), firmicutes (37.4%) and actinobacteria (9.8%). Research has reported a wide range of plastic-degrading bacteria, summarized below.
-
(a)
C–C backbone plastic polymer degradation bacteria in soil:
PE, PP, PVC, and PS are the four main types of synthetic plastics in the C–C backbone group. The polymer’s structure renders it resistant to biodegradation. Furthermore, their short tenure in natural ecosystems (a few decades) is inadequate for nature to evolve new enzymatic systems that can degrade these synthetic polymers (Mueller, 2006).
-
(i)
PE, HDPE, and LDPE Biodegradation Bacteria:
Polyethylene is a thermoplastic polymer made from ethylene gas and serves as a basis for multiple plastic products. Polyethylene is the most produced plastic in the world, which contains high hydrophobic level and high-molecular weight. The most commonly used PEs is LDPE and HDPE. Low-Density Polyethylene (LDPE) is the most extensively used packaging material, due to its outstanding mechanical qualities, water barrier capabilities, low cost, lightweight, and high energy effectiveness. HDPE is a denser version of polyethylene which is used to make water and drain pipes because of its rigidity and crystalline structure. In its natural form, it cannot be degraded easily by microorganisms. As early as the 1970s, Albertsson carried out experiments on microbial degradation of 14C-labeled PE by using three different soil microbiotas as inocula (Albertsson, 1978). After that, Kawai et al. claimed that the upper limit of molecular weight for PE degradation by microorganisms was about 2000 Da based on the results of a numerical simulation (Kawai et al., 2004). Actinobacter sp. can partially break down lower molecular weight PE oligomers (MW = 600–800), whereas high molecular weight PE cannot be degraded. (Ghosh et al., 2013). In order to make it biodegradable, the crystallinity molecular weight and mechanical properties of the PE have to be modified. PE is activated by UV light at the beginning of the degradation process, which acts as an activator. As part of a similar study, PE was exposed to UV light as well as treated with nitric acid (Hasan et al., 2007). The pretreated polymer was applied to a microbial treatment. More than 20 bacterial genera have been shown to degrade different types of PE, those include various Gram-negative and Gram-positives species belonging to the genera Pseudomonas, Ralstonia, tenotrophomonas, Klebsiella, and Acinetobactor and Rhodococcus, Staphylococcus, Streptococcus, Streptomyces, and Bacillus (Danso et al., 2019). Majority of these bacterial strains can degrade the surface of PE and/or form a biofilm over it. In the process of biodegradation, the PE or paraffin molecules containing carbonyl group first get converted into an alcohol (containing −OH group) (Fig. 14.3) by a mono-oxygenase enzyme. The alcohol is then oxidized to an aldehydes (containing -CHO group) by alcohol dehydrogenase enzyme. An aldehydes dehydrogenase converts aldehydes to a fatty acid (containing -COOH group). This fatty acid then undergoes β-oxidation pathway inside cells (Hasan et al., 2007). Pseudomonas species has the unique ability to degrade and metabolize polymers with extracellular oxidative and/or hydrolytic enzymes, which facilitate uptake and degradation of polymer fragments and control the interaction between biofilms and polymer surfaces (Wilkes & Aristilde, 2017). Brevibacillus borstelensis, a thermophilic soil bacterium, utilizes BLDPE as the sole carbon and energy source, thus causing a reduction of 30% in the molecular weight of PE film after 30 days of incubation (Hadad et al., 2005). After thermal treatment, Klebsiella pneumoniae degraded the HDPE. This strain was able to adhere strongly to HDPE surfaces, leading to an increase in biofilm thickness while simultaneously decreasing the weight and tensile strength of the HDPE film by 18.4% and 60%, respectively, after 60 days (Awasthi et al., 2017). In the soil mixed with municipal waste, the decreasing order of degradation susceptibility of polymers was PE>>>LDPE>HDPE as determined by analyzing the weight loss of samples, changes in tensile strength, changes in FTIR, and bacterial activity in the soil (Orhan et al., 2004). Table 14.1 shows that the soil-isolated microbial strains can degrade PE, HDPE, and LDPE.
Microbial biodegradation pathways of synthetic plastic material. (Adapted from Ru et al. (2020))
-
(ii)
Polypropylene (PP) biodegradation bacteria:
PP is a thermoplastic polymer resin with a semi-crystalline structure, which is the second most commonly used plastic in the world. Most commercial PP is isotactic and has an intermediate level of crystallinity between that of LDPE and HDPE due to its durability and outstanding characteristics, it is used in a variety of applications to include packaging for consumer products, plastic moldings, plastic tubs, stationary folders, packaging materials non-absorbable sutures, diapers, automotive industry, and textiles. It can be degraded when exposed to ultraviolet UV light from the sun, and it can also be oxidized at high temperatures. Even though PP is a polyolefin, it has the same oxidative degradation susceptibility as PE. However, its substitution of methyl for hydrogen in the ß position allows it to be more resistant to microbial degradation. Microbial degradation of PP was firstly assessed by Cacciari et al. (1993) by cultures enriched from sandy soils containing PE wastes. These isolated bacterial communities from soil samples mixed with starch have been shown to be capable of degrading PP. Biodegradation of isotactic PP without any treatment is reported with one of the community designated as 3S among the four microbial communities (designated as 1S, 2S, 3S, and 6S) adapted to grow on starch containing PE obtained from enrichment culture. Pseudomonas chlororaphis, Pseudomonasstuzeri, and Vibrio species were identified in the community 3S. It is reported that UV-treated PP sample is more susceptible to degradation (Sameh et al., 2006). Pseudomonas and Bacillus bacterial species were isolated from the soil of a plastic-dumping site, could utilize PP as their carbon source for growth and degrade 0.05–5% of PP after incubation for 12 months (Arkatkar et al., 2010). A mixed consortium of four bacterial isolates from waste management landfills and sewage treatment plants could degrade the PP strips and pellets, lost 44.2–56.3% of their weight after 140 days (Skariyachan et al., 2018). Bacillus Rhodococcus, Bacillus gottheilii were isolated from mangrove sediments and also able to grow in aqueous synthetic media containing PP microplastics and resulting in a weight loss of 4.0–6.4% after 40 days (Auta et al., 2018). Helen AS et al. reported in 2017 that B. cereus had a PP degradation capacity of 0.003 grams per day and S.globispora had a PP degradation capacity of 0.002 g per day. Table 14.1 shows that the soil-isolated microbial strains can degrade the Polypropylene (PP)
-
(iii)
PVC biodegradation bacteria:
PVC is a strong plastic that resists abrasion and chemicals. It also has low moisture absorption properties. There are a lot of studies about the thermal and photodegradation of PVC, but only a few studies on the biodegradation of this material. PVC is the primary synthetic plastic type with the highest percentage of plasticizers (up to 50%). Plasticized PVC is susceptible to microbial attack because plasticizers can be utilized as a carbon source by bacteria. Microorganisms degrading plasticized PVC just break down components of the plasticizer [e.g., bis (2-Ethylhexyl) phthalate, DEHP] rather than the backbone of PVC. The degradation of both PVC and plasticizers by microorganisms has not been observed so far. Therefore, we do not know what enzymes are responsible for the microbial degradation of PVC. Nevertheless, a number of bacterial varieties have been reported to be able to degrade the plasticized PVC, including those isolated from garden soil (Nakamiya et al., 2005; Giacomucci et al., 2019), landfill leachate, waste disposal sites (Latorre et al., 2012; Anwar et al., 2016), and marine environments (Kumari et al., 2019). Table 14.1 shows a list of soil-isolated bacteria used in the degradation of Polyvinyl chloride (PVC).
-
(iv)
PS biodegradation bacteria:
The PS polymer (C8H8)n is an aromatic polymer with a high molecular weight, which is made of monomer styrene. PS can be solid or foamed, while styrene monomer is liquid. The general purpose polystyrene (GPPS) is clear, rigid, and brittle. In many aspects of human life and industry, polystyrene is widely used due to its properties such as low cost, lightweight, ease of manufacture and versatility, thermal efficiency, durability, and water resistance. PS is used in the manufacture of disposable cups, packaging materials, and laboratory ware, as well as in certain electronic products. It is used for its lightweight, stiffness, and excellent thermal insulation. Polystyrene is extremely stable and difficult to degrade in the environment due to its hydrophobic nature, making it resistant to hydrolysis (Albertsson & Karlsson, 1993). Styrene, benzene, toluene, and acrolein are released when it is decomposed by thermal or chemical processes. There are limited publications on PS biodegradation, however, a few researchers have reported on the microbial decomposition of its monomer, styrene. There are several ways of styrene catabolism; however, a predominant pathway involves the oxidation of styrene to phenyl acetate, which is then converted via the TCA cycle. This pathway is shown in Fig. 14.2. According to Kaplan et al., PS breakdown is less than 1% after 90 days in farmed soils with a wide range of fungi, bacteria, and invertebrates, with no notable rise in degradation rate after this one time (Kaplan et al., 1979). Otake et al., on the other hand, observed that a PS sheet buried in soil for 32 years showed no signs of degradation (Otake et al., 1995). The Rhodococcus ruber has been demonstrated to create biofilms on PS and partially break ot down (Mor & Sivan, 2008). A biofilter made up of Brevibacillus sp. has been found to remove 3 kg of styrene in a day (Baggi et al., 1983). Oikawa et al. was isolated and identified Pseudomonas sp. and Bacillus sp. for styrene degradation, and also Xanthomonas sp. and Sphingobacterium sp. for PS decomposition by 16 S ribosomal DNA analyses from soil (Oikawa et al., 2003). Four microbial strains have been isolated from garden soil after 8 months of buried samples of PS and EPS solution (2%) in chloroform. They were identified as Microbacterium sp. NA23, Paenibacillus urinalis NA26, Bacillus sp. NB6, and Pseudomonas aeruginosa NB26. They were able to extract some carbon from the complex molecules of PS, but the process was very slow and did not cause any significant chemical changes on the surface (Atiq et al., 2010). The biodegradation of PS involved Gram-positive coccobacillus, Gram-negative cocci, Gram-negative rod-shaped bacillus, Gram-positive cocci (in clusters) in Garden soil, and Gram-negative cocci (in singles) in garbage soil with weight loss up to 30% (Asmita et al, 2015). Krueger et al. (2015) found a reduction in molecular mass of polystyrene sulfonate (PSS) by 50% within 20 days as a result of the activity of Gloeophyllum trabeum DSM 1398. Citrobacter sedlakii, Enterobacter sp., Alcaligenes sp., and Brevundimonas diminuta were isolated and identified by Sekhar et al. (2016), and the highest PS degradation rate was estimated to be 12.4% within 30 days of Enterobacter. Pseudomonas spp. and Bacillus spp. were reported to be able to breakdown high-impact PS (HIPS) film by Mohan et al. (2016). Bacillus spp., in particular, succeeded a reduction of plastic weight loss by 23% after 30 days. Table 14.1 shows list of soil-isolated bacteria used in the degradation of Poly styrene (PS)
-
(b)
C–O backbone plastic polymer degradation bacteria in soil:
Two synthetic plastic polymers lie under the C–O backbone category, namely PU and PET. However, this type of plastic material can be hydrolyzed due to the presence of ester bonds
-
(i)
PU biodegradation bacteria:
Polyurethanes (PUs) are an important branch of synthetic plastics belonging to the thermosetting group, which can be re-used for production. PUs can be broadly categorized as follows: flexible, semi-rigid, rigid, microcellular, viscoelastic, or thermoplastic urethanes. The polyurethanes industries were laid in the late 1930s with the discovery by German scientist Otto Bayer (Szycher, 1999). Since that time, scientists have been finding its use in an ever-increasing number of applications, and polyurethanes are now all around us, playing a vital role in many industries—from furniture to footwear, construction to cars, i.e., furniture coatings, adhesives, construction materials, flame retardants, fibers, paints, elastomeric parts, and synthetic skins are just a few examples. Polyurethanes have also been employed in a variety of biomedical applications, including vascular prostheses, prosthetic skin, pericardial patches, soft-tissue adhesive, drug delivery devices, and tissue engineering scaffolds (Young & Lovell, 1994). Now Polyurethanes (PU) represent almost 8% of produced plastics which place them as the sixth most used polymer in the world (Kemona & Piotrowska, 2020). PU is the condensation product of polyisocyanate and polyol having intramolecular urethane bonds (carbonate ester bond –NHCOO–) (Sauders & Frisch, 1964). There are two types of PU when it comes to biodegradation: polyester polyurethane (PSPU) and polyether polyurethane (PEPU) which have several applications in the industrial field. Microbial degradation (fungal) of PU was firstly reported by Darby and Kaplan in 1968 (Darby & Kaplan, 1968). In comparison to PEPU, PSPU was easier to biodegrade. Following that, numerous microorganisms were shown to be capable of degrading polyester PU. The potential of Staphylococcus epidermidis KH11 to break down polyether PU was examined by Jansen et al. (1991). As well as three esterases purified from Pseudomonas chlororaphis (Ruiz et al., 1999), the protease purified from Pseudomonas fluorescens (Vega et al., 1999), and a lipase purified from B. subtilis (Rowe & Howard, 2002) have the ability to degrade PSPU. In addition, they also cloned a gene named pulA from Pseudomonas fluorescens (Ruiz & Howard, 1999) and two genes, pue A and pue B, from Pseudomonas chlororaphis (Stern & Howard, 2000; Howard et al., 2001b). These genes encoded three different esterases involved in the microbial degradation of emulsified polyester PU by Pseudomonas fluorescens and Pseudomonas chlororaphis. The list of bacterial strains were degrading different kinds of polyurethane (Kemona & Piotrowska, 2020). Table 14.1 shows the list of soil-isolated bacteria used in the degradation of various polyurethanes (PUs).
-
(ii)
Polyethylene terephthalate (PET) biodegradation bacteria:
PET, a synthetic polymer generated from crude oil, is today one of the most widely used plastics (Liu et al., 2019), contributing to more than 10% of the plastic market share (Carr et al., 2020). This polymer is made up of terephthalic acid (TPA) and ethylene glycol repeating units (EG) (Fig. 14.1). PET is convenient both in terms of manufacture and utility, as it is utilized in containers, films, and fibers, in addition to bottles, due to its lightweight, durability, and mold ability. It is resistant to biodegradation due to the polymer’s backbone’s high stability, as well as its crystallinity and surface hydrophobicity, which are some of the underlying elements that limit the natural breakdown of this plastic. B.subtilis, S.aureus, and S.pyogenes are considered as important PET and PS degrading bacteria (Asmita et al., 2015). Ideonella sakaiensis was also reported to degrade PET polymer (Yoshida et al., 2016; Oberbeckmann & Labrenz, 2020).Table 14.1 shows the list of soil-isolated bacteria used in the degradation of Polyethylene terephthalate (PET).
9.2 Plastic-Degrading Actinomycetes
Actinomycetes are a phylum of gram positive bacteria. They are prokaryotic organisms with a primitive unicellular organization. Actinomycetes are anaerobic microorganisms. On solid substrates, they have filamentous and branching growth patterns that resemble fungal mycelia. Their colonies, like myceliums, are large. Many genera of actinomycetes have aerial hyphae. Some actinomycetes have flagella and are motile. Actinomycetes can be found in both soil and water. Actinomycetes include the Streptomyces groups, Rhodococcus ruber, Actinomadura spp., and the thermophilic Thermo actinomycetes species have been isolated from different ecological zones and demonstrated to possess significant plastic biodegradative potentials (Auta et al., 2018; Jabloune et al., 2020). Their ability to produce a wide range of hydrolytic enzymes as well as other bioactive metabolites has previously been emphasized. These hydrolytic enzymes are one of the most important components in their ability to grow on a variety of plastic polymers and degrade the large molecular weight molecules into simpler ones. They can produce extracellular polymers such as dextrin, glycogen, levan, and N-acetyl glucosamine-rich slime polysaccharides which probably facilitate their attachment to plastic surfaces for subsequent microbial action. Biofilm formation has been found to be an important factor in the actinomycetal colonization of plastics, similar to the bacteria PET and other polymers including p-nitro phenyl esters, cutin, and suberin were found to be degraded by Streptomyces scabies, isolated from potatoes (Jabloune et al., 2020). Nocardiopsis sp., an endophytic actinomycetes isolated from the hibiscus, was similarly found to break down PE and fuel. The effectiveness of actinomycetal plastic degradation has also been highlighted in a microbial consortium with a substantial fraction of actinomycetal species degrading polyurethane and different chemical additives.
The plastics of poly (β-hydroxybutyrate) (PHB)-and poly (ε-caprolactone) (PCL) were degraded by aerobic microorganisms that persist in the natural environment. Plastic depolymerizing microbes can be found over many kinds of material, including landfill leachate, compost, sewage sludge, forest soil, farm soil, paddy soil, weed field soil, roadside sand, and pond sediment (Nisida & Tokiwa, 1993). Actinomycetes strains Streptomyces genus and Micromonospora genus were isolated and screened for the capability to degrade poly (ethylene succinate) (PES), poly (ε-caprolactone) (PCL), and poly (β-hydroxybutyrate) (PHB) from the upstream and downstream regions of the Touchien River in Taiwan (Hoang et al., 2007). Streptoverticillium kashmirense AF1 can degrade a natural polymer; poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) was isolated from municipal sewage sludge by soil burial technique. Extracellular enzymes PHBV depolymerase secreted by Streptoverticillium kashmirense AF1 was purified and degrade PHBV film (Shah et al., 2007) Actinomadura, Microbispora, Streptomyces, Thermo actinomyces, and Saccharomonospora were thermophilic actinomycetes strains able to degrade poly (ethylene succinate) (PES), poly (ε-caprolactone) (PCL), and poly (β-hydroxybutyrate) (PHB). Thermophilic actinomycetes Microbispora rosea, Excellospora japonica, and E. viridilutea were able to degrade aliphatic polyester, poly (tetramethylene succinate) (100 mg PTMS film) (Jarerat & Tokiwa, 2001) Rhodococcusruber (C208) Rhodococcus sp. strain RHA1, strong polychlorinated biphenyl (PCB) degrader has diverse biphenyl/PCB degradative genes and harbors huge linear plasmids, including pRHL1 (1100 kb), pRHL2 (450 kb), and pRHL3 (330 kb). Linear plasmids of Rhodococcus sp. strain RHA1 having degradative genes such as bphB2, etbD2, etbC, bphDEF, bphC2, and bphC4 (Shimizu et al., 2001) Amycolatopsis strains, poly(L-lactide) degrader stain has the ability to absorb breakdown products such as poly lactic acids (Pranamuda et al., 1999). Polylactide (PLA)-degrading microorganisms are sparsely distributed in soil environments. Totally 34 different kinds of marine Actinomycetes isolates were discovered in marine soil. Five of the most common Actinomycetes cultures were tested for plastic degradation such as Streptomyces sp., Pseudonocardia sp., Actinoplanes sp., Sporichthya sp. Among them, Streptomyces sp. has shown significant reduction (20%) when compared to other tested organisms (Sathya et al., 2012). Table 14.2 summarizes Actinomycetes strains associated with various plastics biodegradation in the soil.
10 Plastic-Degrading Bacterial Enzymes
Plastic-degrading bacteria and other microorganisms are involved in plastic biodegradation by producing a variety of essential enzymes. This polymer biodegradation process involves two reactions: Hydrolysis, and Oxidation. Hydrolysis is the breakdown of polymers catalyzed by hydrolases enzymes, which are one of the most important aspects in their ability to grow on various polymers and degrade high molecular weight to simpler ones. Hydrolase enzymes catalyze the hydrolysis of esters, carbonates, amides, and glycosidic linkages to create monomers from various hydrolyzed polymers. Oxidation is a biodegradation process that is conducted by oxidoreductase enzymes. Meanwhile, oxidoreductase enzymes catalyze ethylene, carbonate, amide, urethane, and other oxidizing and reducing processes (Ganesh et al., 2017). Some polymer compounds cannot be degraded by certain enzymes, the other appropriate enzymes will work together to break down those compounds. This phenomenon is known as oxidation. Plastic biodegradation enzymes are classified into two broad categories, viz., extracellular and intracellular enzymes (Gu, 2003).
Extracellular Enzymes
These types of enzymes are involved in heterogonous reactions, as these act on the macromolecules at the surface of the solid plastic while it is in a liquid state (Chinaglia et al., 2018). Additionally, other groups of enzymes are involved in the surface functionalization of hydrophobic plastic surfaces, the degradation of the plastic metabolic intermediates into monomeric units, and the mineralization of the final monomeric intermediates.
Intracellular Enzymes
These enzymes convert intermediates into compounds that can be assimilated by microbes via aerobic and anaerobic processes (Pathak, 2017).
Enzyme technology has recently been investigated for the production, isolation, purification, and providing the enzymes for the degradation of plastics. These enzymes are non-toxic and biodegradable. In the last decade, a few polymer plastics chains (PE, PP, PS, and PVC) are subjected to degrade by a distinct group of enzymes as shown in Table 14.3. Many enzymes like esterases, protease, cutinase, and laccase have shown significant results in the breakdown of MNPs. A bacterium named Ideonella sakaiensisis can utilize PET as its primary carbon and energy source (Yoshida et al., 2016). By the presence of two active enzymes (PETase and MHET ase), this bacterium converts PET into its monomers terephthalic acid and ethylene glycol (Palm et al., 2019). Recent research on the enzymatic degradation of plastics has generated a lot of interest in protein/enzyme engineering to improve enzyme activity.
An engineered PETase mutant from Ideonella sakaiensis exhibits an increase in the three mutants (R61A, L88F, and I179F) by 1.4-fold, 2.1-fold, and 2.5-fold, in comparison to the wild type strain. It has been demonstrated that enzyme activity can be significantly improved by rational protein engineering and by modifying key hydrophobic grooves of substrate binding sites (Ma et al., 2018). Surprisingly, a recent study found that protein-engineered enzymes were effective in degrading MNPs (Islam et al., 2019). According to the study, the degradation of the MNPs of PU has increased by about 6.7 times. These remarkable results indicate that protein/enzyme modification could be one of the approaches for more effectively removing MNPs. Immobilized enzyme techniques have recently been used to degrade MNPs in the environment (Shakerian et al., 2020). Bis phenol A (BPA), a monomer of polycarbonate plastics, is one of the most produced chemicals on the planet (Hacıosmanoğlu et al., 2019). Laccase enzyme was reported to be the most commonly used enzyme in immobilized systems to break down the BPA (Piao et al., 2019). When compared to free enzymes, immobilization of oxidative enzymes (laccase and horseradish peroxide) has shown high stability, durability, reusability, and cost-effectiveness (Shakerian et al., 2020). Hence, the combination of membranes and enzymes/microbial technology is expected to have a promising future in the degradation of MNPs from the soil and other eco systems.
11 Conclusions
The biodegradation of MNPs by using plastic-degrading bacteria is a viable and cost-effective plastic waste degradation technique that can be easily implemented in real-time to maintain the environmental quality of the soil caused by MNPs. This process has minimal or no side effects on the environment. The degradation of MNPs involves some intra and extracellular enzymes (Hydrolase and Oxidase), which are produced by bacteria. This enzymatic process breaks down the recalcitrant plastic polymers into microbial biomass and other environmentally safe compounds through several steps, including biodeterioration, depolymerization, assimilation, and mineralization. Optimizing the right environmental factors is the main factor to enhance the ability of bacteria to degrade plastics waste. Many advanced techniques like enzyme/protein engineering and enzymatic immobilization techniques have been developed to facilitate the biodegradation of MNPs.
References
Abraham, J., Ghosh, E., Mukherjee, P., & Gajendiran, A. (2017). Microbial degradation of low density polyethylene. Environmental Progress & Sustainable Energy, 36, 147–154.
Adamcová, D., & Vaverková, M. (2014). Degradation of biodegradable/degradable plastics in municipal solid-waste landfill. Polish Journal of Environmental Studies, 23(4), 1071–1078.
Agrawal, P., & Singh, R. K. (2016). Breaking down of polyethylene by Pseudomonas species. International Journal of Scientific & Engineering Research, 7(3), 124–127.
Akutsu, Y., Nakajima-Kambe, T., Nomura, N., & Nakahara, T. (1998). Purification and properties of a polyester polyurethane-degrading enzyme from Comamonasacidovorans TB-35. Applied and Environmental Microbiology, 64, 62–67.
Albertsson, A. C. (1978). Biodegradation of synthetic polymers. II. A limited microbial conversion of 14C in polyethylene to 14CO2 by some soil fungi. Journal of Applied Polymer Science, 22, 3419–3433.
Albertsson, A. C., & Karlsson, S. (1993). Aspects of biodeterioration of inert and degradable polymers. International Biodeterioration & Biodegradation, 31, 161–170.
Allen, A. B., Hilliard, N. P., & Howard, G. T. (1999). Purification and characterization of a soluble polyurethane degrading enzyme from Comamonasacidovorans. International Biodeterioration and Biodegradation, 43, 37–41.
Ammala, A., Bateman, S., Dean, K., Petinakis, E., Sangwan, P., Wong, S., Yuan, Q., Yu, L., Patrick, C., & Leong, K. H. (2011). An overview of degradable and biodegradable Polyolefins. Progress in Polymer Science, 36(8), 1015–1049.
Amobonye, A., Bhagwat, P., Singh, S., & Pillai, S. (2021). Plastic biodegradation: Frontline microbes and their enzymes. Science of the Total Environment, 759, 143536.
Anwar, M. S., Kapri, A., Chaudhry, V., Mishra, A., Ansari, M. W., Souche, Y., et al. (2016). Response of indigenously developed bacterial consortia in progressive degradation of polyvinyl chloride. Protoplasma, 253, 1023–1032.
Arkatkar, A., Juwarkar, A. A., Bhaduri, S., Uppara, P. V., & Doble, M. (2010). Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. International Biodeterioration & Biodegradation, 64, 530–536.
Asmita, K., Shubhamsingh, T., & Tejashree, S. (2015). Isolation of plastic degrading microorganisms from soil samples collected at various locations in Mumbai, India. International Research of Environetmental Sciences, 4(3), 77–85.
Atiq, N., Safia, A., & Ali, M. I. (2010). Isolation and identification of polystyrene biodegrading bacteria from soil. African Journal of Microbiology Research, 4, 1537–1541.
Auta, H. S., Emenike, C. U., Jayanthi, B., & Fauziah, S. H. (2018). Growth kinetics and biodeterioration of polypropylene microplastics by Bacillus sp. and Rhodococcus sp. isolated from mangrove sediment. Marine Pollution Bulletin, 127, 15–21.
Awasthi, S., Srivastava, P., Singh, P., Tiwary, D., & Mishra, P. K. (2017). Biodegradation of thermally treated high-density polyethylene (HDPE) by Klebsiella pneumoniae CH001. 3 Biotech, 7, 332.
Baggi, G., Boga, M. M., & Catelani, D. (1983). (1983). Styrene catabolism by a strain of Pseudomonas fluorescens. Systematic and Applied Microbiology, 4, 141–147.
Begum, M. A., Varalakshmi, B., & Umamagheswari, K. (2015). Biodegradation of polythene bag using bacteria isolated from soil. International Journal of Current Microbiology and Applied Sciences, 4(11), 674–680.
Bhardwaj, H., Gupta, R., & Tiwari, A. (2012). Microbial population associated with plastic degradation. Scientific Reports, 5, 272–274.
Cacciari, I., Quatrini, P., Zirletta, G., Mincione, E., Vinciguerra, V., Lupattelli, P., et al. (1993). Isotactic polypropylene biodegradation by a microbial community: Physicochemical characterization of metabolites produced. Applied and Environmental Microbiology, 59, 3695–3700.
Carr, C. M., Clarke, D. J., & Dobson, A. D. W. (2020). Microbial polyethylene terephthalate hydrolases: Current and future perspectives. Frontiers in Microbiology, 11, 571265.
Chinaglia, S., Tosin, M., & Degli-Innocenti, F. (2018). Biodegradation rate of biodegradable plastics at molecular level. Polymer Degradation and Stability, 147, 237–244.
da Costa, J. P., Santos, P. S. M., Duarte, A. C., & Rocha-Santos, T. (2016). (Nano) plastics in the environment—Sources, fatesandeects. Science of the Total Environment, 566–567, 15–26.
Danso, D., Chow, J., & Streit, W. R. (2019). Plastics: Environmental and biotechnological perspectives on microbial degradation. Applied and Environmental Microbiology, 85, 1–14.
Darby, R. T., & Kaplan, A. M. (1968). Fungal susceptibility of polyurethanes. Applied Microbiology, 16, 900–905. https://doi.org/10.1128/aem.16.6.900-905.1968
de Souza Machado, A. A., Kloas, W., Zarfl, C., Hempel, S., & Rillig, M. C. (2018). Microplastics as an emerging threat to terrestrial ecosystems. Global Change Biology, 24(2018), 1405–1416.
Deepika, S., & Jaya, M. R. (2015). Biodegradation of low density polyethylene by microorganisms from garbage soil. Journal of Experimental Biology and Agricultural Sciences, 3, 15–21.
Delacuvellerie, A., Cyriaque, V., Gobert, S., Benali, S., & Wattiez, R. (2019). The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low density polyethylene degradation. Journal of Hazardous Materials, 380, 1–11.
Drzyzga, O., & Prieto, A. (2018). Plastic waste management, a matter for the community. Microbial Biotechnology, 12(1), 66–68.
Duddu, M. K., Tripura, K. L., Gantuku, G., & Divya, D. S. (2015). Biodegradation of low density polyethylene (LDPE) by a new biosurfactant-producing thermophilic Streptomyces coelicoflavus NBRC 15399T. African Journal of Biotechnology, 14(4), 327–340.
El-Sayed, A. H. M. M., Mahmoud, W. M., Davis, E. M., & Coughlin, R. W. (1996). Biodegradation of polyurethane coatings by hydrocarbon-degrading bacteria. International Biodeterioration and Biodegradation, 37, 69–79.
El-Shafei, H. A., El-Nasser, N. H. A., Kansoh, A. L., & Ali, A. M. (1998). Biodegradation of disposable polyethylene by fungi and Streptomyces species. Polymer Degradation and Stability, 62, 361–365.
Farzi, A., Dehnad, A., Shirzad, N., & Norouzifard, F. (2017). Biodegradation of high density polyethylene using Streptomyces species. Journal of Coastal Life Medicine, 5, 474–479.
Farzi, A., Dehnad, A., & Fotouhi, A. F. (2019). Biodegradation of polyethylene terephthalate waste using Streptomyces species and kinetic modeling of the process. Biocatalysis and Agricultural Biotechnology, 17, 25–31.
Fernandes, I. P., Barbosa, M., Amaral, J. S., Pinto, V., Rodrigues, J. L., Ferreira, M. J., & Barreiro, M. F. (2016). Biobased additives as biodegradability enhancers with application in TPU-based footwear components. Journal of Renewable Materials, 4, 47–56.
Fuhs, G. W. (1961). Der mikrobielleAbbau von Kohlenwasserstoffen. Archiv fur Mikrobiologie, 39, 374–422.
Ganesh, P., Dineshraj, D., & Yoganathan, K. (2017). Production and screening of depolymerasing enzymes by potential bacteria and fungi isolated from plastic waste dump yard sites. International Journal of Applied Research, 3(3), 693–695.
Gautam, R., Bassi, A. S., Yanful, E. K., & Cullen, E. (2007). Biodegradation of automotive waste polyester polyurethane foam using Pseudomonas chlororaphis ATCC55729. International Biodeterioration and Biodegradation, 60, 245–249.
Ghosh, S. K., Pal, S., & Ray, S. (2013). Study of microbes having potentiality for biodegradation of plastics. Environmental Science and Pollution Research, 20, 4339–4355.
Giacomucci, L., Raddadi, N., Soccio, M., Lotti, N., & Fava, F. (2019). Polyvinyl chloride biodegradation by Pseudomonas citronellolis and Bacillus flexus. New Biotechnology, 52, 35–41.
Gu, J. D. (2003). Microbiological deterioration and degradation of synthetic polymeric materials: Recent research advances. International Biodeterioration and Biodegradation, 52, 69–91.
Hacıosmanoğlu, G. G., Doğruel, T., Genç, S., Oner, E. T., & Can, Z. S. (2019). Adsorptive removal of 525 bisphenol a from aqueous solutions using phosphonatedlevan. Journal of Hazardous Materials, 374(526), 43–49.
Hadad, D., Geresh, S., & Sivan, A. (2005). Biodegradation of polyethylene by the thermophilic bacterium Brevibacillusborstelensis. Journal of Applied Microbiology, 98, 1093–1100.
Hasan, F., Shah, A. A., Hameed, A., & Ahmed, S. (2007). Synergistic effect of photo and chemical treatment on the rate of biodegradation of low density polyethylene by fusarium sp. AF4. Journal of Applied Polymer Science, 105, 1466–1470.
Helen, A. S., Uche, E. C., & Hamid, F. S. (2017). Screening of polyptopylene degradation potential of bacteria isolated from mangrove ecosystems in Peninsular Malaysia. International Journal of Bioscience, Biochemistry and Bioinformatics, 7(4), 245–251.
Hester, R. E., & Harrison, R. M. (2011). Marine pollution and human health. Issues in environmental science and technology (Vol. 33, pp. 84–85). Royal Society of Chemistry.
Hoang, K. C., Lee, C. Y., Tseng, M., & Chu, W. S. (2007). Polyester degrading actinomycetes isolated from the Touchien River of Taiwan. World Journal of Microbiology and Biotechnology, 23(2), 201–205.
Horton, A. A., Walton, A., Spurgeon, D. J., Lahive, E., & Svendsen, C. (2017). Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Science of the total environment, 586(2017), 127–141.
Howard, G. T., Crother, B., & Vicknair, J. (2001a). Cloning, nucleotide sequencing and characterization of a polyurethanase gene (pueB) from pseudomonas chlororaphis. International Biodeterioration and Biodegradation, 47, 141–149.
Howard, G. T., Vicknair, J., & Mackie, R. I. (2001b). Sensitive plate assay for screening and detection of bacterial polyurethanase activity. Letters in Applied Microbiology, 32, 211–214. [CrossRef].
Howard, G. T., Norton, W. N., & Burks, T. (2012). Growth of Acinetobacter gerneri P7 on polyurethane and the purification and characterization of a polyurethane enzyme. Biodegradation, 23, 561–573.
Hurley, R. R., & Nizzetto, L. (2018). Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Current Opinion in Environmental Science & Health, 1, 6–11.
Hussein, A. A., Al-Mayaly, I. K., & Khudeir, S. H. (2015). Isolation, screening and identification of low density polyethylene (LDPE) degrading bacteria from contaminated soil with plastic wastes. Mesopotamia Environmental Journal, 1(4), 1–14.
Islam, S., Apitius, L., Jakob, F., & Schwaneberg, U. (2019). Targeting microplastic particles in the 541 void of diluted suspensions. Environment International, 123, 428–435.
Jamil, S. U. U., Zada, S., Khan, I., Sajjad, W., Rafiq, M., Shah, A. A., & Hasan, F. (2017). Biodegradation of polyethylene by bacterial strains isolated from Kashmir cave, Buner, Pakistan. Journal of Cave and Karst Studies, 79(1), 73–80.
Jansen, B., Schumacher-Perdreau, F., Peters, G., & Pulverer, G. (1991). Evidence for degradation of synthetic polyurethanes by Staphylococcus epidermidis. Zentralblatt für Bakteriologie, 276, 36–45.
Jarerat, A., & Tokiwa, Y. (2001). Degradation of poly (tetramethylene succinate) by thermophilic actinomycetes. Biotechnology Letters, 23(8), 647–651.
Jeon, H. J., & Kim, M. N. (2015). Functional analysis of alkane hydroxylase system derived from Pseudomonas aeruginosa E7 for low molecular weight polyethylene biodegradation. International Biodeterioration and Biodegradation, 103, 141–146.
Jabloune, R., Khalil, M., Ben Moussa, I. E., Simao-Beaunoir, A. M., Lerat, S., Brzezinski, R., & Beaulieu, C. (2020). Enzymatic degradation of p-Nitrophenyl Esters, Polyethylene Terephthalate, Cutin, and Suberin by Sub1, a Suberinase encoded by the plant pathogen streptomyces scabies. Microbes Environments, 35(1):ME19086. https://doi.org/10.1264/jsme2.ME19086. PMID: 32101840; PMCID: PMC7104285.
Kale, S. K., Deshmukh, A. G., Dudhare, M. S., & Patil, V. B. (2015). Microbial degradation of plastic: A review. Journal of Biochemical Technology, 6(2), 952–961.
Kaplan, D. L., Roy, H., & Jim, S. (1979). Biodegradation of polystyrene, poly(metnyl methacrylate), and phenol formaldehyde. Applied and Environmental Microbiology, 38, 551–553.
Kawai, F., Watanabe, M., Shibata, M., Yokoyama, S., Sudate, Y., & Hayashi, S. (2004). Comparative study on biodegradability of polyethylene wax by bacteria and fungi. Polymer Degradation and Stability, 86, 105–114.
Kay, M. J., Morton, L. H. G., & Prince, E. L. (1991). Bacterial degradation of polyester polyurethane. International Biodeterioration, 27, 205–222.
Kemona, A., & Piotrowska, M. (2020). Polyurethane recycling and disposal: Methods and prospects. Polymers, 12, 1752.
Koushal, V., Sharma, R., Sharma, M., Sharma, R., & Sharma, V. (2014). Plastics: Issues challenges and remediation. International Journal of Waste Resources, 4(1), 1–6.
Krueger, M. C., Hofmann, U., Moeder, M., & Schlosser, D. (2015). Potential of wood-rotting fungi to attack polystyrene sulfonate and its depolymerisation by Gloeophyllumtrabeum via hydroquinone-driven Fenton chemistry. PLoS One, 10, e0131773.
Kumar Sen, S., & Raut, S. (2015). Microbial degradation of low density polyethylene (LDPE): A review. Journal of Environmental Chemical Engineering, 3(1), 462–473.
Kumar, S., Das, M. L., Rebecca, J., & Sharmila, S. (2013). Isolation and identification of LDPE degrading fungi from municipal solid waste. Journal of Chemical and Pharmaceutical Research, 5(3), 78–81.
Kumar, V. R., Kanna, G. R., & Elumalai, S. (2017). Biodegradation of polyethylene by green photosynthetic microalgae. Journal of Bioremediation & Biodegradation, 8(1), 1–8. OMICS Publishing Group.
Kumari, P., & Murthy, N. S. (2013). A novel mathematical approach for optimization of plastic degradation. International Journal of Engineering Trends and Technology, 4(8), 3539–3542.
Kumari, A., Chaudhary, D. R., & Jha, B. (2019). Destabilization of polyethylene and polyvinylchloride structure by marine bacterial strain. Environmental Science and Pollution Research International, 26, 1507–1516.
Latorre, I., Hwang, S., & Montalvo-Rodriguez, R. (2012). Isolation and molecular identification of landfill bacteria capable of growing on di-(2-ethylhexyl) phthalate and deteriorating PVC materials. Journal of Environmental Science and Health, Part A, 47, 2254–2262.
Leslie, H. A., van Velzen, M. J. M., Brandsma, S. H., Vethaak, D., Garcia-Vallejo, J. J., & Lamoree, M. H. (2022). Discovery and quantification of plastic particle pollution in human blood. Environment International, 163, 107199. ISSN 0160-4120.
Li, J., Kim, H. R., Lee, H. M., Yu, H. C., Jeon, E., Lee, S., & Kim, D.-H. (2020). Rapid biodegradation of polyphenylene sulfide plastic beads by pseudomonas sp. Science of the Total Environment, 720, 137616.
Liu, M., Lu, S., Song, Y., Lei, L., Hu, J., Lv, W., Zhou, W., Cao, C., Shi, H., Yang, X., & He, D. (2018). Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environmental Pollution, 242(2018), 855–862.
Liu, C., Shi, C., Zhu, S., Wei, R., & Yin, C.-C. (2019). Structural and functional characterization of polyethylene terephthalate hydrolase from Ideonellasakaiensis. Biochemical and Biophysical Research Communications, 508, 289–294.
Ma, Y., Yao, M., Li, B., Ding, M., He, B., Chen, S., Zhou, X., & Yuan, Y. (2018). Enhanced poly(ethylene terephthalate) hydrolase activity by protein engineering. Engineering, 4, 888–893.
Mao, R., Lang, M., Yu, X., Wu, R., Yang, X., & Guo, X. (2020). Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. Journal of Hazardous Materials, 393, 122515.
Matjašič, T., Simčič, T., Medvešček, N., Bajt, O., Dreo, T., & Mori, N. (2021). Critical evaluation of biodegradation studies on synthetic plastics through a systematic literature review. Science of the Total Environment, 752, 141959.
Mohan, A. J., Sekhar, V. C., Bhaskar, T., & Nampoothiri, K. M. (2016). Microbial assisted high impact polystyrene (HIPS) degradation. Bioresource Technology, 213, 204–207.
Mondal, S., & Palit, D. (2019). Effective role of microorganism in waste management and environmental sustainability. In M. Jhariya, A. Banerjee, R. Meena, & D. Yadav (Eds.), Sustainable agriculture, forest and environmental management. Springer. https://doi.org/10.1007/978-981-13-6830-1_14
Mor, R., & Sivan, A. (2008). Biofilm formation and partial biodegradation of polystyrene by the actinomycetes Rhodococcusruber: Biodegradation of polystyrene. Biodegradation, 19, 851–858.
Mueller, R. J. (2006). Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process Biochemistry, 41, 2124–2128.
Muhonja, C. N., Makonde, H., Magoma, G., & Imbuga, M. (2018). Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS One, 13(7), e0198446.
Mukherjee, S., & Chatterjee, S. (2014). A Comparative study of commercially available plastic carry bag biodegradation by microorganisms isolated from hydrocarbon effluent enriched soil. International Journal of Current Microbiology and Applied Sciences, 3(5), 318–325.
Nair, S., & Kumar, P. (2007). Molecular characterization of a lipase-producing Bacillus pumilus strain (NMSN-1d) utilizing colloidal water-dispersible polyurethane. World Journal of Microbiology and Biotechnology, 23, 1441–1449.
Nakajima-Kambe, T., Onuma, F., Kimpara, N., & Nakahara, T. (1995). Isolation and characterization of a bacterium which utilizes polyester polyurethane as a sole carbon and nitrogen source. FEMS Microbiology Letters, 129, 39–42.
Nakajima-Kambe, T., Onuma, F., Akutsu, Y., & Nakahara, T. (1997). Determination of the polyester polyurethane breakdown products and distribution of the polyurethane degrading enzyme of Comamonasacidovorans strain TB-35. Journal of Fermentation and Bioengineering, 83, 456–460.
Nakamiya, K., Hashimoto, S., Ito, H., Edmonds, J. S., Yasuhara, A., & Morita, M. (2005). Microbial treatment of bis (2-ethylhexyl) phthalate in polyvinyl chloride with isolated bacteria. Journal of Bioscience and Bioengineering, 99, 115–119.
Nakkabi, A., Sadiki, M., Fahim, M., Ittobane, N., IbnsoudaKoraichi, S., Barkai, H., & El abed, S. (2015). Biodegradation of poly(ester urethane)s by Bacillus subtilis. International Journal of Environmental Research, 9, 157–162.
Nisida, H., & Tokiwa, Y. (1993). Distribution of poly (β-hydroxybutyrate) and poly (ε-caprolacton) aerobic degrading microorganisms in different environments. Journal of Environmental Polymer Degradation, 1, 227–233.
Oberbeckmann, S., & Labrenz, M. (2020). Marine microbial assemblages on microplastics: Diversity, adaptation, and role in degradation. Annual review of marine. Science, 12(1), 209–232.
Oceguera-Cervantes, A., Carrillo-García, A., López, N., Bolaños-Nuñez, S., Cruz-Gómez, M. J., Wacher, C., & Loza-Tavera, H. (2007). Characterization of the polyurethanolytic activity of two Alicycliphilus sp. strains able to degrade polyurethane and N-methylpyrrolidone. Applied and Environmental Microbiology, 73, 6214–6223.
Oikawa, E., Linn, K. T., & Endo, T. (2003). Isolation and characterization of polystyrene degrading microorganisms for zero emission treatment of expanded polystyrene. Environmental Engineering Research, 40, 373–379.
Orhan, Y., Hrenovic, J., & Buyukgungor, H. (2004). Biodegradation of plastic compost bags under controlled soil conditions. Acta Chimica Slovenica, 51, 579–588.
Otake, Y., Kobayashi, T., & Asabe, H. (1995). Biodegradation of low-density polyethylene, polystyrene, polyvinyl chloride, and urea formaldehyde resin buried under soil for over 32 years. Journal of Applied Polymer Science, 56, 1789–1796.
Palm, G. J., Reisky, L., Böttcher, D., Müller, H., Michels, E. A., Walczak, M. C., Berndt, L., Weiss, M. S., Bornscheuer, U. T., & Weber, G. (2019). Structure of the plastic-degrading Ideonellasakaiensis MHETase bound to a substrate. Nature Communications, 10, 1–10.
Pathak, V. M. (2017). Review on the current status of polymer degradation: A microbial approach. Bioresources and Bioprocessing, 4, 15.
Patil, R. C. (2018). Screening and characterization of plastic degrading bacteria from garbage soil. British Journal of Environmental Sciences, 6(4), 33–40.
Peng, Y. H., Shih, Y. H., Lai, Y. C., Liu, Y. Z., Liu, Y. T., & Lin, N. C. (2014). Degradation of polyurethane by bacterium isolated from soil and assessment of polyurethanolytic activity of a pseudomonas putida strain. Environmental Science and Pollution Research, 21, 9529–9537.
Pérez-Lara, L. F., Vargas-Suárez, M., Lõpez-Castillo, N. N., Cruz-Gõmez, M. J., & Loza-Tavera, H. (2016). Preliminary study on the biodegradation of adipate/phthalate polyester polyurethanes of commercial-type by Alicycliphilus sp. BQ8. Journal of Applied Polymer Science, 133, 1–9.
Piao, M., Zou, D., Ren, X., Gao, S., Qin, C., & Piao, Y. (2019). High efficiency biotransformation of bisphenol A in a fluidized bed reactor using stabilized laccase in porous silica. Enzyme and Microbial Technology, 126, 1–8.
Pranamuda, H., Chollakup, R., & Tokiwa, Y. (1999). Degradation of polycarbonate by a polyester-degrading strain, Amycolatopsis sp. strain HT-6. Applied and Environmental Microbiology, 65(9), 4220–4222.
Ren, X., Tang, J., Liu, X., & Liu, Q. (2020). Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environmental Pollution. (Barking, Essex: 1987), 256, 113347.
Riandi, M. I., Kawuri, R., & Sudirga, S. K. (2017). Potential of pseudomonas sp. and Ochrobacterum sp. isolated from various soil sample as degrading bacteria of high density polyethylene (HDPE) and low density polyethylene (LDPE) plastic. Symbiosis Journal of Biological Science, 5(2), 58–63.
Rillig, M. C., Ziersch, L., & Hempel, S. (2017). Microplastic transport in soil by earthworms. Scientific Reports, 7(2017), 1362.
Ronkvist, A. M., Xie, W., Lu, W., & Gross, R. A. (2009). Cutinase-catalyzed hydrolysis of poly (ethylene terephthalate). Macromolecules, 42, 5128–5138.
Rowe, L., & Howard, G. T. (2002). Growth of Bacillus subtilis on polyurethane and the purification and characterization of a polyurethanaselipase enzyme. International Biodeterioration & Biodegradation, 50, 33–40.
Ru, J., Huo, Y., & Yang, Y. (2020). Microbial degradation and valorization of plastic wastes. Frontiers in Microbiology, 11, 442.
Ruiz, C., & Howard, G. T. (1999). Nucleotide sequencing of a polyurethanase gene (pulA) from Pseudomonas fluorescens. International Biodeterioration & Biodegradation, 44, 127–131.
Ruiz, C., Main, T., Hilliard, N. P., & Howard, G. T. (1999). Purification and characterization of two polyurethanase enzymes from Pseudomonas chlororaphis. International Biodeterioration & Biodegradation, 43, 43–47.
Russell, J. R., Huang, J., Anand, P., Kucera, K., Sandoval, A. G., Dantzler, K. W., Hickman, D., Jee, J., Kimovec, F. M., & Koppstein, D. (2011). Biodegradation of polyester polyurethaneby endophytic fungi. Applied and Environmental Microbiology, 77, 6076–6084.
Sameh, A. S., Alariqi Kumar, A. P., Rao, B. S. M., & Singh, R. P. (2006). Biodegradation of γ-sterilized biomedical polyolefins under composting and fungal culture environments. Journal of Polmer Degradation and Stability, 91, 1105–1116.
Saminathan, P., Sripriya, A., Nalini, K., Sivakumar, T., & Thangapandian, V. (2014). Biodegradation of plastics by Pseudomonas putida isolated from garden soil samples. Journal of Advanced Botany and Zoology, 1(3), 1–4.
Sangeetha Devi, R., Rajesh Kannan, V., Nivas, D., Kannan, K., Chandru, S., & Robert Antony, A. (2015). Biodegradation of HDPE by aspergillus Spp. from marine ecosystem of gulf of Mannar, India. Marine Pollution Bulletin, 96(1–2), 32–40.
Sathya, R., Ushadevi, T., & Panneerselvam, A. (2012). Plastic degrading actinomycetes isolated from mangrove sediments. International Journal of Current Research, 4(10), 001–003.
Sauders, J. H., & Frisch, K. C. (1964). Polyurethanes: Chemistry and technology, part II technology. Inter-Science Publishers.
Scheurer, M., & Bigalke, M. (2018). Microplastics in Swiss floodplain soils. Environmental Science & Technology, 52(2018), 3591–3598.
Sekhar, V. C., Nampoothiri, K. M., Mohan, A. J., Nair, N. R., Bhaskar, T., & Pandey, A. (2016). Microbial degradation of high impact polystyrene (HIPS), an e-plastic with decabromodiphenyl oxide and antimony trioxide. Journal of Hazardous Materials, 318, 347–354.
Shah, A. A., Hasan, F., Hameed, A., & Ahmed, S. (2007). Isolation and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) degrading actinomycetes and purification of PHBV depolymerase from newly isolated Streptoverticilliumkashmirense AF1. Annals of Microbiology, 57(4), 583–588.
Shah, A. A., Hasan, F., Akhter, J. I., Hameed, A., & Ahmed, S. (2008). Degradation of polyurethane by novel bacterial consortium isolated from soil. Annals of Microbiology, 58, 381–386. [CrossRef].
Shah, Z., Hasan, F., Krumholz, L., Atkas, D., & Shah, A. A. (2013). Degradation of polyester polyurethane by newly isolated Pseudomonas aeruginosa strain MZA-85 and analysis of degradation products by GC-MS. International Biodeterioration and Biodegradation, 77, 114–122.
Shah, Z., Gulzar, M., Hasan, F., & Shah, A. A. (2016). Degradation of polyester polyurethane by an indigenously developed consortium of Pseudomonas and Bacillus species isolated from soil. Polymer Degradation and Stability, 134, 349–356.
Shahnawaz, M., Sangale, M. K., & Ade, A. B. (2016). Rhizosphere of Avicennia marina (Forsk.) Vierh. As a landmark for polythene degrading bacteria. Environmental Science and Pollution Research, 23, 14621–14635.
Shakerian, F., Zhao, J., & Li, S.-P. (2020). Recent development in the application of immobilized oxidative enzymes for bioremediation of hazardous micro pollutants–a review. Chemosphere, 239, 124716.
Shimizu, S., Kobayashi, H., Masai, E., & Fukuda, M. (2001). Characterization of the 450-kb linear plasmid in a polychlorinated biphenyl degrader, Rhodococcus sp. strain RHA1. Applied and Environmental Microbiology, 67(5), 2021–2028.
Shresta, J. K., Joshi, D. R., Regmi, P., & Badahit, G. (2019). Isolation and identification of low density polyethylene (LDPE) degrading bacillus spp. from a soil of landfill site. Acta Scientific Microbiology, 2(4), 30–34.
Skariyachan, S., Megha, M., Kini, M. N., Mukund, K. M., Rizvi, A., & Vasist, K. (2015). Selection and screening of microbial consortia for efficient and ecofriendly degradation of plastic garbage collected from urban and rural areas of Bangalore, India. Environmental Monitoring and Assessment, 187(1), 4174.
Skariyachan, S., Manjunatha, V., Sultana, S., Jois, C., Bai, V., & Vasist, K. S. (2016). Novel bacterial consortia isolated from plastic garbage processing areas demonstrated enhanced degradation for low density polyethylene. Environmental Science and Pollution Research International, 23(18), 18307–18319.
Skariyachan, S., Patil, A. A., Shankar, A., Manjunath, M., Bachappanavar, N., & Kiran, S. (2018). Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillussps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polymer Degradation and Stability, 149, 52–68.
Soud, S. A. (2019). Biodegradation of polyethylene LDPE plastic waste using locally isolated Streptomyces sp. Journal of Pharmaceutical Sciences and Research, 11(4), 1333–1339.
Sowmya, H., Ramalingappa, V., Krishnappa, M., & Thippeswamy, B. (2014). Low density polyethylene degrading fungi isolated from local dumpsite of Shivamogga district. International Journal of Biological Research, 2(2), 39–43.
Stern, R. V., & Howard, G. T. (2000). The polyester polyurethanase gene (pueA) from Pseudomonas chlororaphis encodes a lipase. FEMS Microbiology Letters, 185, 163–168.
Szycher, M. (1999). Szycher’s handbook of polyurethanes. CRC Press.
Tribedi, P., & Sil, A. K. (2013). Low-density polyethylene degradation by Pseudomonas sp. AKS2 biofilm. Environmental Science and Pollution Research, 20, 4146–4153.
Usha, R., Sangeetha, T., & Palaniswamy, M. (2011). Screening of Polyethylene degrading microorganisms from garbage soil. Libyan Agriculture Research Center Journal International, 2, 200–204.
Vega, R. E., Main, T., & Howard, G. T. (1999). Cloning and expression in Escherichia coli of a polyurethane-degrading enzyme from Pseudomonas fluorescens. International Biodeterioration & Biodegradation, 43, 49–55.
Wang, W., Ge, J., Yu, X., & Li, H. (2020). Environmental fate and impacts of microplastics in soil ecosystems: Progress and perspective. Science of the Total Environment, 708, 134841.
Wei, R., & Zimmermann, W. (2017). Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: How far are we? Microbial Biotechnology, 10(6), 1308–1322.
Wilkes, R. A., & Aristilde, L. (2017). Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp. capabilities and challenges. Journal of Applied Microbiology, 123, 582–593.
Yoon, M. G., Jeon, H. J., & Kim, M. N. (2012). Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. Journal of Bioremediation & Biodegradation, 3, 1–8.
Yoshida, S., Hiraga, K., Takehana, T., Taniguchi, I., Yamaji, H., Maeda, J., Toyohara, K., Miyamoto, K., Kimura, Y., & Oda, K. (2016). A bacterium that degrades and assimilates poly(ethylene terephthalate). Science, 351(6278), 1196–1199.
Young, R. J., & Lovell, P. A. (Eds.). (1994). Introduction to polymers (2nd ed.). Chapman & Hall.
Yuan, J., Ma, J., Sun, Y., Zhou, T., Zhao, Y., & Yu, F. (2020). Microbial degradation and other environmental aspects of microplastics/plastics. Science of the Total Environment, 715, 136968.
Zhou, Q., Zhang, H., Fu, C., Zhou, Y., Dai, Z., Li, Y., Tu, C., & Luo, Y. (2018). The distribution andmorphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea. Geoderma, 322(2018), 201–208.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jukuri, S., Lavudi, S. (2023). Bacterial Remediation of Micro-Nanoplastics (MNPs): Contaminated Soil. In: Maddela, N.R., Reddy, K.V., Ranjit, P. (eds) Micro and Nanoplastics in Soil. Springer, Cham. https://doi.org/10.1007/978-3-031-21195-9_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-21195-9_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-21194-2
Online ISBN: 978-3-031-21195-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)