Abstract
This study aimed to formulate novel microbial consortia isolated from plastic garbage processing areas and thereby devise an eco-friendly approach for enhanced degradation of low-density polyethylene (LDPE). The LDPE degrading bacteria were screened and microbiologically characterized. The best isolates were formulated as bacterial consortia, and degradation efficiency was compared with the consortia formulated using known isolates obtained from the Microbial Culture Collection Centre (MTCC). The degradation products were analyzed by FTIR, GC-FID, tensile strength, and SEM. The bacterial consortia were characterized by 16S ribosomal DNA (rDNA) sequencing. The formulated bacterial consortia demonstrated 81 ± 4 and 38 ± 3 % of weight reduction for LDPE strips and LDPE pellets, respectively, over a period of 120 days. However, the consortia formulated by MTCC strains demonstrated 49 ± 4 and 20 ± 2 % of weight reduction for LDPE strips and pellets, respectively, for the same period. Furthermore, the three isolates in its individual application exhibited 70 ± 4, 68 ± 4, and 64 ± 4 % weight reduction for LDPE strips and 21 ± 2, 28 ± 2, 24 ± 2 % weight reduction for LDPE pellets over a period of 120 days (p < 0.05). The end product analysis showed structural changes and formation of bacterial film on degraded LDPE strips. The 16S rDNA characterization of bacterial consortia revealed that these organisms were novel strains and designated as Enterobacter sp. bengaluru-btdsce01, Enterobacter sp. bengaluru-btdsce02, and Pantoea sp. bengaluru-btdsce03. The current study thus suggests that industrial scale-up of these microbial consortia probably provides better insights for waste management of LDPE and similar types of plastic garbage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the severe threats to global ecosystem is the enormous accumulation and biomagnification of synthetic plastic polymers such as low- and high-density polyethylene due to the lack of efficient and safe approaches for recycling and disposal (North and Halden 2013). The environmental accumulation of plastic debris is a rising concern while the usage exceeds 260 million tonnes of plastic per annum (Barnes et al. 2009; Hopewell et al. 2009). The use of polyethylene increases at a rate of 12 % per year (Usha et al. 2011). Despite the advantages, plastics have many detrimental impacts mainly due to the nonbiodegradable nature of these polymers (Hester and Harrison 2011; Deepika and Jaya 2015; Sen and Rault 2015).
Plastic pollution led to the accumulation of polymers in bionetwork which causes pernicious impact on living systems (Bhardwaj et al. 2012). Approximately, 60 % of total plastic wastes which constitutes 9205 t per day are recycled and about 6137 t are not recycled appropriately. There are many cities in India such as Delhi, Chennai, Mumbai, and Kolkata producing 689, 429, 408, and 425 t of plastic wastes per day, respectively. A survey conducted by the Central Pollution Control Board, Govt. of India (CPCB 2013), found that 15,342 t of plastic wastes are generated every day in 60 major cities which constitute a total of 5,600,000 t per year. Bengaluru is one of the major cities in India that generates more than 4000 t of plastic garbage every day (CPCB 2013).
In recent years, there has been alarming public concern in India over environmental deterioration associated with the improper disposal of plastic wastes. Microbial biotechnology approaches can more efficiently clean up these plastic wastes than conventional methods can and greatly reduce the use of land-based disposal methods. Bioremediation is one of the best approaches in which nutrients are introduced to stimulate the activity of microorganisms already present in the plastic-contaminated soil or by incorporating novel microflora to such polluted areas (bioaugmentation). This approach regarded as the best and safest method possibly produces less toxic end products in comparison with recycling, land filling, and incineration (Kumar et al. 2007). It exploits the utility of microbial metabolism to degrade and transform various hydrocarbon derivatives such as polychlorinated biphenyls (PCBs), polyaromatic hydrocarbons (PAHs), and heterocyclic compounds into less toxic or nontoxic substances (Nakajima-Kambe et al. 1999; Das et al. 2013).
Formulation of novel microbial consortia for efficient and eco-friendly degradation is gaining attention due to considerable advantages over the application of pure isolates in xenobiotic degradation (Sah et al. 2011). This approach is generally accepted as ideal, while microbial consortia enhance the rate of safe detoxification by co-metabolism or gratuitous degradation (Tribedi et al. 2012; Bhardwaj et al. 2012). The screening of microbial consortia from enormously polluted areas offers wide scope and applications. Even though the exploration of microorganisms towards detoxification of xenobiotics are well studied, the screening and utilization of novel microbial consortia with enhanced biodegradation potential still constitute a scope in process development and patent search (Vijaya and Reddy 2008). Hence, the current study aims to characterize and formulate novel microbial consortia from plastic garbage processing areas with high degradation potential in comparison with few known hydrocarbon-degrading isolates.
Materials and methods
Sample collection
Seven plastic garbage processing areas from rural and urban regions of Bengaluru, India, were selected as the sampling spots that included Bommanahalli (Urban Bengaluru, India, 12° 53′ 54″ N and 77° 37′ 4ʺ E), Karnataka Compost Development Corporation (Rural Bengaluru, 12° 53ʹ 46ʺ N and 77° 38′ 58″ E), Bingipura (Urban Bengaluru, 12° 49′ 35″ E and 77° 37′ 58″ E), Mandur (Urban Bengaluru, 134′ 57″ N and 77° 43′ 36″ E), Mavallipura (Urban Bengaluru, 13° 7′ 11″ N and 77° 31′ 23″ E), Kanakapura (Urban Bengaluru, 12° 32′ 46″ N and 77° 25′ 12ʺ E), and Lakshmipura Bande (Urban Bengaluru, 12° 56ʹ 50ʺ N and 77° 33′ 37″ E). The soil samples highly contaminated with plastic debris were collected from selected spots. Three sets of 500 g of soil samples were collected in sterile zip-lock pouches (15 × 15 cm) from each spot, transported with precautions, and stored in cartons with ice packs. The temperature and pH of the soil samples were recorded by thermometer (Bremed Digital Thermometer, BD 1200) and pH strips (Sigma-Aldrich, 7.0–14.0 pH, resolution 0.5 pH unit) at the time of sample collection. The temperature and pH of the soil samples in garbage processing areas were observed to be the ranges of 28–46 °C and 8.0–8.9, respectively. The samples were processed within 12 h of their collection.
Isolation and screening of plastic-degrading bacteria
The soil samples were serially diluted by standard methods (Geldreich et al. 1972). Different forms of plastic used in the study were low-density polyethylene (LDPE) strips (5 × 1 cm) and LDPE pellets (1.0 g l−1). Isolation and screening of plastic-degrading bacteria were performed using minimal media plates (Hemashenpagam et al. 2013) containing LDPE powder (1.0 g l−1) as nutritional source. The LDPE powder was obtained from Sarvodaya polymers, one of the main plastic manufacturers in Bengaluru, India. The minimal media contained ammonium sulfate (1.0 g l−1), di-potassium phosphate (7.0 g l−1), potassium phosphate (2.0 g l−1), magnesium sulfate (0.1 g l−1), bacteriological agar (2.0 g l−1), and LDPE powder (1.0 g l−1).
Study of the rate of LDPE degradation
The isolates were cultivated on minimal media and incubated at 37 °C for 96 h, and the plates were observed for bacterial colonies. The number of bacteria from all the plates were enumerated (CFU/g), and physiological characteristics of the bacterial colonies were studied. The LDPE-degrading bacteria were identified by zone clearance method (Dey et al. 2012) using coomassie blue (Howard and Hilliard 1999). The plates without LDPE were used as the control. Further, the bacterial isolates were inoculated in minimal media broth containing LDPE strips (5 × 1 cm) and LDPE pellets (1.0 g l−1). The broth cultures were incubated at 37 °C for 120 days. The degradation was periodically monitored by measuring the weight of LDPE pellets and strips. The plastics in minimal media with bacterial isolates were used as test samples, and the tubes with plastics exposed to media without any bacteria were used as control. The percentage LDPE degradation was estimated by the following formula.
Microbial characterization of isolated bacteria
The morphological features of five main isolates from selected samples were studied by Gram staining (Beveridge 2001). The cultural characteristics of these isolates were studied by plating them on selective and differential media such as MacConkey’s agar (Mossel et al. 1962) and Blood agar (Pelczar et al. 1977) (Himedia India). The isolates were further characterized by standard biochemical tests (Murray et al. 2003; Lee et al. 2003; Stager et al. 1983; Taylor and Achanzar 1972; Titters and Sancholzer 1936; Klein et al. 1991; Jurtshuk and McQuitty 1976).
Study of growth parameters
The three isolates which demonstrated maximum LDPE degradation was selected, and plastic degradation studies were performed under varying conditions of pH and temperature. This is performed to study ideal environmental conditions for LDPE degradation. The varying conditions of pH used in the study were 5.54, 6.13, 7.27, 8.55, and 9.02. The varying temperature conditions were 4, 25, 37, and 45 °C. The control and test samples were prepared, and degradation study was carried out for a period of 120 days.
Formulation of microbial consortia and determination of rate of LDPE degradation
The pure cultures of Enterobacter spp. (IS2 and IS3) and Pantoea spp. (IS5) that showed maximum percentage of degradation for LDPE strips and pellets were selected to formulate the consortia. These three isolates were effectively grown in minimal media and showed best degradation for LDPE strips and pellets. Approximately 5 g of LDPE strips (5 × 1 cm) were incorporated in 1000 ml of minimal media containing 3 ml of microbial consortia. Similarly, 5 g of LDPE pellets were incorporated in 1000 ml of minimal media containing 3 ml of microbial consortia. The microbial consortia were prepared by inoculating 1 ml each freshly prepared log phase cultures of IS2, IS3, and IS5 into a conical flask containing 1000 ml minimal media broth. The optical density of individual cultures was maintained to be 0.7–0.9 at 600 nm. The numbers of cells present in each inoculum were enumerated by a hemocytometer and which were found to be approximately 1.8 × 109 cells ml−1. The test and control samples were incubated at 45 °C for 120 days in a bacteriological incubator. The percentage of degradation for LDPE strips and LDPE pellets by microbial consortia was periodically estimated by weight loss method by the following formula:
The numbers of viable bacterial count in the degradation phase (after 120 days) of LDPE strips and pellets in minimal media at 45 °C were enumerated by serial dilution techniques (CFU ml−1) to ensure the growth and survival of bacteria at this temperature and high alkaline pH.
Comparative analysis of LDPE degradation using known isolates
Three bacteria which are known to degrade polyethylene (Obradors and Aguilar 1991), included Pseudomonas putida MTCC 2445 (designated as MTCC1), Pseudomonas stutzeri MTCC 2643 (designated as MTCC2), and Bacillus subtilis MTCC 9447(designated as MTCC3) which were obtained from the Microbial Type Culture Collection Centre and GenBank (MTCC), Chandigarh, India. These bacterial strains were studied for LDPE degradation in minimal media (pH 8.5) at 45°. The optical density of individual strains was maintained to be 0.7–0.9 at 600 nm. These growth parameters were selected based on our previous findings (Skariyachan et al. 2015). These strains were inoculated to minimal media containing LDPE strips and pellets. Similarly, separate sets were prepared by inoculating the isolates described in the current study which showed significant degradation towards LDPE strips and pellets. The LDPE strips and pellets incorporated in the minimal media with bacterial consortia were used as test samples, and the tubes with these plastics exposed to media without any bacterial consortia were used as control. The test and control tubes were incubated at 45 °C for 120 days in a bacteriological incubator. The biodegradation was periodically monitored by weight loss method, and the percentage of weight loss for LDPE strips and pellets were estimated using the formula mentioned in the previous section.
The degradation efficiency of microbial consortia formulated in the current study was further compared with microbial consortia formulated using the strains of MTCC1, MTCC2, and MTCC3. The MTCC consortia were formulated by inoculating 1 ml of each of the three cultures (OD 0.7–0.9 at 600 nm) into a conical flask containing 1000 ml minimal media with the same environmental parameters as mentioned previously. Approximately 5 g of LDPE strips (5 × 1 cm) and LDPE pellets were separately incorporated in 1000 ml of minimal media each containing 3 ml of MTCC consortia. The test and control samples were incubated at 45 °C for the period of 120 days in a bacteriological incubator. The percentage of degradation for LDPE strips and LDPE pellets by MTCC consortia was periodically estimated by weight loss method.
Molecular characterization of the best isolates by 16S rDNA sequencing
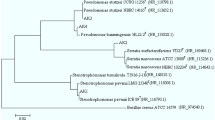
The bacterial isolates IS2, IS3 and IS5 which exhibited maximum biodegradation properties towards LDPE strips and pellets were subjected to 16S ribosomal DNA (rDNA) gene sequencing. This procedure was carried out at BioAxis DNA Research Center Private Limited, Hyderabad, India. The sequence obtained was subjected to BLAST (www.ncbi.nlm.nih.gov/BLAST) search for retrieving the best homologous sequences. These sequences were analyzed for evolutionary relationship by constructing phylograms using distance-based (neighbor joining) method (Saitou and Nei 1987), and the tree was visualized by TreeView software (Page 2002). The 16S rDNA sequences of the isolates were deposited to GenBank (www.ncbi.nlm.nih.gov/GenBank).
Fourier transform infrared analysis
The LDPE strips (exposed to the microbial consortia in culture flasks) recovered after an incubation period of 120 days were carefully rinsed with distilled water and air dried. The air-dried LDPE strips were mixed with potassium bromide (KBr) at a temperature of 27 °C for 5 days. KBr pellets of the strips were prepared by freezing under liquid nitrogen a 1.50-mg plastic sample, in a stainless steel vial containing two stainless steel balls. The vial was shaken for 25 s, and this step was repeated to convert the film into fine powder. The finely powdered film sample was mixed with 450 mg powdered KBr for 10 s in the same vial and the 350-mg portion of the mixture was pressed to form the KBr disk and the transmission measurement of the test and control (the untreated plastic strips) were carried out (Shimadzu IR spectrophotometer, 8400S, Japan). Similar protocols were followed for the FTIR analysis of LDPE pellets.
Gas chromatography flame ionization detector analysis
Gas chromatography flame ionization detector (GC-FID) analysis was carried out by a DB 5 column (Agilent 7890A, 6890N, Thermo Trace GC Ultra) to analyze the degradation end products. The degraded LDPE films were pre-treated with di-ethyl ether, and 1 μl of sample was injected into the column with helium as carrier gas. The flow rate was adjusted to 2.0 ml m−1, and the monomers released as a result of degradation were analyzed. The dimensions of the GC column were 30 ml × 0.30 mm ID × 0.25 μm film thickness. Temperature ramp used in this study is as follows: initial temperature 40 °C; holding time 2 m. The ramp rate is 10 °C m−1, the temperature is 310 °C, and holding time is 10 min.
Tensile strength analysis
The LDPE strips incubated in minimal media over a period of 120 days with bacterial consortia were washed by sterile distilled water and dried at ambient temperature. The LDPE strips were cut into 2.5 × 1-cm pieces and placed on universal testing machine (Instron) under axial loading. The strain rate used in the study was 5 mm min−1. The reduction in tensile strength of LDPE treated with bacterial culture was compared with the control using the instrument (Mecmesin instrument ILC Load Cell 500N). The results were analyzed based on the peak loads. Similar studies were also conducted for degraded LDPE pellets.
Scanning electron microscopy
The physical changes and bacterial colonization on the surface of degraded LDPE strips were analyzed by high-resolution scanning electron microscopy (Model Geminr; specifications: w-filament, low vacuum and humidity capability, secondary E-T and solid-state back-scattered electron detector, ultrathin window EDS system (EDAX), and resolution at 20 kV:3 nm in high vacuum). The degraded LDPE strips were used as test samples and LDPE strips collected from the plastic processing areas (without microbial consortia or incubation in minimal media), and fresh LDPE strips were used as the controls. The LDPE strips incubated in minimal media broth over a period of 120 days with bacterial consortia were washed with 2 % (v/v) aqueous sodium dodecyl sulfate (SDS) and distilled water frequently through mild shaking for 5 min and flushed with 70 % alcohol. These steps were repeated for control samples. The samples were further gold coated (13 nm width) for 20 min and placed on the holder and scanned under different magnifications.
Statistical analysis
The experimental protocols mentioned in the study were replicated as three independent trails, and one-way analysis of variance (ANOVA) was performed to estimate the statistical significance at p < 0.05 (Kruskala and Wallisa 1952).
Results and discussion
Isolation and screening of polyethylene-degrading bacteria
The initial screening of polyethylene-degrading bacteria was performed by incorporating LDPE powder in minimal media. After an incubation period of 96 h, small, circular, cream-colored, convex, and translucent colonies were observed in all the minimal media plates. A total of 821 CFU/g were isolated in the minimal media from all the sampling spots. When these colonies were stained with coommasie blue, clear halos were evident around the bacterial colonies. This was the initial indication of the abilities of bacterial isolates for LDPE degradation in minimal media (Supplementary File, Fig 1). Based on further microbiological characterization, it was observed that 821 CFU/g belonged to five main isolates which were designated as IS1, IS2, IS3, IS4, and IS5. From this study, it was clear that the bacteria isolated from plastic garbage processing areas are capable of utilizing polyethylene as nutritional sources. There are studies which depicted that the microorganisms screened from hydrocarbon-contaminated soil demonstrated degradation capabilities (Yoon et al. 2012; Duddu et al. 2015). Studies also revealed that Gram-negative bacteria could easily adapt to the environment rich in polyethylene and similar polymers (Dey et al. 2012).
Determination of rate of LDPE degradation
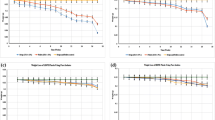
The rate of LDPE degradation was determined by weight loss method wherein LDPE films and pellets were incorporated in minimal media as nutritional source by the isolates. This study demonstrated that IS1, IS2, IS3, IS4, and IS5 showed 59 ± 3, 70 ± 4, 68 ± 4, 52 ± 2, and 64 ± 4 % degradation for LDPE films over a period of 120 days, respectively. Similarly, IS1, IS2, IS3, IS4, and IS5 showed 16 ± 2, 21 ± 2, 28 ± 2, 19 ± 2, and 24 ± 2 % degradation for LDPE pellets over a period of 120 days, respectively (Fig.1). However, there were no reduction in the weight of LDPE strips and pellets used as controls. Similarly, the known isolates obtained from MTCC indicated that MTCC1, MTCC2, and MTCC3 showed 38 ± 3, 45 ± 4, and 31 ± 3 % degradation for LDPE strips and 15 ± 2, 11 ± 2, and 13 ± 2 % degradation for LDPE pellets for 120 days, respectively (Fig. 1). The data was found to be statistically significant (p < 0.05). From this data, it is clear that the isolates described in the current study demonstrated greater degradation of LDPE than did known isolates obtained from MTCC. Furthermore, this study noticed that the growth rate of MTCC isolates was slower than that of our isolates. Previous studies reported 20 % of weight reduction for LDPE films by few American Type Culture Collection (ATCC) strains over a period of 120 days (Kyaw et al. 2012). However, the current study suggested 52–70 % of degradation for LDPE films by the bacterial isolates.
Biodegradation potential of the bacterial isolates screened from various plastic garbage processing areas towards low-density polyethylene (LDPE) in comparison with known LDPE-degrading strains obtained from MTCC studied by weight loss method. IS1, IS2, IS3, IS4, and IS5 indicate the major types of screened isolates, and MTCC1, MTCC, and MTCC3 indicate the known LDPE-degrading strains. Figure shows the degradation potential of isolates (IS1–1S5) towards LDPE strips and pellets over a period of 120 days (p < 0.05)
The LDPE films and pellets are the two important forms of plastic used in the current study. This was due to the regional importance and availability of LDPE films and pellets for the plastic manufacturers. The survey was conducted prior to the sampling; this study noticed that the LDPE pellets were routinely used for the making of most of the hard plastic materials in the city, whereas the LDPE films were commonly used for the manufacturing of soft polyethylene carry bags, sheets, decorative, wrappers, etc. These types of plastic materials were very common in the sampling site during the time of sample collection. Hence, LDPE strips and pellets were used in this study.
Microbial characterization of the isolated bacteria
Most of the bacterial isolates were identified as Gram-negative bacilli after Gram staining (Beveridge 2001). When the isolates (n = 5) were grown over MacConkey’s agar (Mossel et al. 1962), 60 % of the isolates (IS2, IS3, and IS5) showed lactose-fermenting, circular, large, convex, shiny colonies. Forty percent (IS1 and IS4) exhibited nonlactose-fermenting, circular, medium-sized translucent colonies. Likewise, when plated to blood agar (Pelczar et al. 1977), 100 % the isolates were demonstrated as nonhemolytic bacteria. Hence, the bacterial isolates considered as generally recognized as safe (GRAS) and scaling up of such isolates as potential polyethylene-degrading bacteria probably offer scope and applications.
The biochemical characteristics of the five main isolates are shown in Table 1. Based on biochemical characterization, the main LDPE-degrading isolates, IS1, IS2, IS3, IS4, and IS5, were identified to be Proteus spp., Enterobacter spp., Enterobacter spp., Pseudomonas spp., and Pantoea spp., respectively. The best LDPE-degrading isolates IS2, IS3, and IS5 were grown luxuriously in minimal media by utilizing LDPE as nutritional source, and they were identified to be Enterobacter spp. and Pantoea spp. Previous studies suggested that most of the hydrocarbon-degrading organisms were Gram-negative bacteria (Kyaw et al. 2012; Nanda et al. 2010; John et al. 2012; Usha et al. 2011) in which Pseudomonas spp. constitute major populations (Kyaw et al. 2012; John et al. 2012; Tribedi et al. 2012). This study showed that Enterobacter spp. and Pantoea spp. demonstrated better degradation potential than di Pseudomonas spp. (Fig. 1). Furthermore, in the presence of LDPE, IS2, IS3, and IS5 showed enhanced growth in minimal media and demonstrated better percentage degradation for polyethylene in comparison with similar previous reports (Duddu et al. 2015; Dey et al. 2012; Usha et al. 2011).
Growth parameters for efficient degradation
When the species of Enterobacter and Pantoea were grown at varying pH and temperature conditions, these bacteria exhibited maximum growth at 45 °C under pH 8.5 and showed effective LDPE degradation. The viable bacterial count estimated from the minimal media (with degraded LDPE film after 120 days of incubation) showed that the number of bacterial isolates for IS2, IS3, and IS5 were found to be 1.63 × 105, 1.44 × 104, and 2.16 × 104 CFU ml−1 of inoculums, respectively. The viable counts of microbial consortia at the same conditions were found to be 2.31 × 105 CFU ml−1 by serial dilution techniques. Similarly, the viable bacterial count estimated from the minimal media composed of LDPE pellets demonstrated that the number of bacterial isolates for IS2, IS3, IS5, and microbial consortia was found to be 1.02 × 102, 1.43 × 103, 1.12 × 103, and 1.12 ×103 CFU ml−1 of inoculums, respectively (Supplementary Materials, Table 1). This is an indication of the growth and survival of these organisms in the minimal media (pH 8.5) at 45 °C by utilizing LDPE as their nutritional source. Furthermore, the number of bacteria enumerated (CFU ml−1) from the minimal media plates maintained at 37 °C was found to be less than that of those plates incubated at 45 °C. The percentage weight reductions for LDPE strips and pellets under these conditions were found to be 52–70 ± 4 and 16–28 ± 2 %, respectively, over a period of 120 days. Most of the Gram-negative mesophiles prefer an optimum pH of 7.2–7.5 for their growth and survival. The current study showed that the isolates were effectively grown at pH 8.5 suggesting that the massive discharges of plastic garbage raise the soil pH and the bacteria became adaptable to such environments. The underlying processes as a selection pressure involving possible toxicity of plastics to microbes and use of plastics as sole nutritional source probably contribute the microbial adaptability to the plastic-contaminated soil environment. Further, the exact mechanism of LDPE degradation at an unusual temperature of 45 °C needs to be analyzed by more sophisticated approaches or molecular level studies. A preceding study on biodegradation of polyhydroxyl butyrate by microbial isolates suggested that one of the ideal parameters for effective biodegradation of various polymeric substances is 45 °C and pH 7.0 (Lodhi et al. 2011).
Biodegradation potential of microbial consortia over MTCC consortia
The microbial consortia formed using IS2, IS3, and IS5 showed maximum biodegradation properties in comparison with individual application of IS2, IS3, and IS5 and MTCC consortia. This study demonstrated that formulated microbial consortia comprising IS2, IS3, and IS5 showed 81 ± 4 and 38 ± 3 % degradation for LDPE strips and LDPE pellets, respectively, over a period of 120 days. In contrast, consortia formulated by MTCC1, MTCC2, and MTCC3 demonstrated weight reduction of 49 ± 4 and 20 ± 2 % for LDPE strips and LDPE pellets, respectively, over a period of 120 days. Furthermore, the isolates in their individual application exhibited 70 ± 4, 68 ± 4, and 64 ± 4 % weight reduction for LDPE strips and 21 ± 2, 28 ± 2, 24 ± 2 % weight reduction for LDPE pellets over a period of 120 days which were also less in comparison with the percentage of weight reduction showed by microbial consortia (Fig. 2). When the study replicated as independent trails, all the data were found to be statistically significant (p < 0.05). From this study, it is clear that the formulated bacterial consortia showed greater percentage of LDPE degradation than the known MTCC isolates did. Furthermore, the formulation of various bacterial strains in the form of consortia enhances the percentage of degradation in comparison with the application of isolates in their pure form, and this approach is one of the efficient and eco-friendly approaches towards the degradation of various plastic polymers as suggested in our previous study (Skariyachan et al. 2015). Various studies conducted on bioremediation using microbial consortia over pure isolates suggested that consortia harbor high biodegradation potential in comparison with pure isolates (Patel et al. 2012; Sah et al. 2011).
An important point noticed in this study is the unequal proportions of various forms of LDPE degradation by the formulated bacterial consortia. This study suggested that two different forms of LDPE are not degraded at the same proportion by the screened isolates. The degradation of LDPE strips by the isolates was greater in comparison with LDPE pellets. This is probably due to the high surface area and lower thickness of LDPE film where the bacterial colonization might have aroused faster in comparison with LDPE pellets. However, the exact fact behind this concept and scope of various forms of plastics in degradation studies needed to be explored. Few reports suggested that surface area features are one of the main parameters for bacterial colonization which demonstrated direct correlations to the degradation potential of various hydrocarbon-degrading microorganisms (Tribedi and Sil 2013). The current study formulated the bacterial consortia by mixing IS2, IS3 (Enterobacter spp.), and IS5 (Pantoea spp.) which showed the best biodegradation activities. The study can be further extended by combining all the five isolates described in the study. The mutualistic relationship and survival abilities of all these strains in minimal media needed to be further studied. Several combinations of clonal bacterial strains can be selected in appropriate approaches, and the biodegradation potential of the best combinations can be further screened.
16S rDNA characterization
The sizes of 16S rDNA sequences of IS2, IS3, and IS5 were identified to be 1496, 1409, and 1405 bp, respectively. From the BLAST analysis, it is evident that the 16S rDNA sequence of IS2 showed 99 % sequence identity to Enterobacter cloacae, while IS3 and IS5 showed 98 % sequence identities to Enterobacter ludwigii and Pantoea agglomerans, respectively. The evolutionary relationship established by the neighbor-joining approach revealed that there is a high evolutionary homologous relationship and sequence conservation among 16S rDNA sequences of the isolates and the best homologous sequences. The GenBank accession numbers for 16S rDNA sequences of IS2, IS3, and IS5 are KT334807, KT334808, and KT334809, respectively, which were designated as Enterobacter sp. bengaluru-btdsce01, Enterobacter sp. bengaluru-btdsce02, and Pantoea sp. bengaluru-btdsce03, respectively. To the best of our knowledge, this study is probably the first of its kind which report enhanced LDPE degradation capabilities of Enterobacter spp. and Pantoea spp. by growing in minimal media at high salinity. These isolates were deposited to MTCC, Chandigarh, India.
FTIR and GC-FID analysis
The structural variations observed in the test LDPE strips, pellets, and controls are shown in Fig. 3. The FTIR spectra of one of the degraded LDPE strip demonstrated several peaks in the range of 500–1700 cm−1 in comparison with the control (Fig 3a, b). Similarly, the FTIR spectra of one of the degraded LDPE pellets also illustrated little structural variation (peaks between 721–1630 and 2852–3435 cm−1) in comparison with the control (Fig 3c, d). This demonstrated that various forms of LDPE treated with bacterial consortia underwent major structural changes which are a direct indication of biodegradation (Corti et al. 2010; Esmaeili et al. 2013) by the bacterial consortia. Furthermore, GC-FID results of LDPE strips revealed that there are major structural changes in the degraded LDPE strips in comparison with the control at RT 6.1 and 8.5 min. Many peaks at low responses were also observed in the test sample when compared to the control (Fig. 4). The current study thus illuminated structural changes responsible for weight loss in degraded LDPE strips and pellets by the selected microbial consortia. However, further experimental studies such as nuclear magnetic resonance or similar techniques such as gel permeation chromatography or viscometric analysis are required to appreciate the structural variations and other changes which resulted due to the microbial degradation of plastic polymers.
The FTIR spectra of the degraded polyethylene (LDPE strips and pellets) by bacterial consortia after 120 days: a FTIR spectrum of LDPE strip (control); b FTIR spectrum of degraded LDPE strips (test); c FTIR spectrum of LDPE pellet (control); d FTIR spectrum of degraded LDPE strips (test). The marked regions show major structural variation
Tensile strength analysis
There were considerable differences in the percentage of elongation of degraded LDPE films after 120 days by microbial isolates in comparison with the control. The tensile strength (TS) and extension at break (EAB) for the control LDPE strips were found to be 19 ± 3 % MPa and 18 ± 3 % mm, respectively. However, the TS and EAB of one of the degraded LDPE strips by microbial consortia were found to be 16 ± 3 % and 13 ± 3 % mm after 120 days, respectively (Fig. 5a). The decrease in TS and EAB indicates the change in tensile strength by bacterial degradation of LDPE strips. Similarly, the reduction in compression of LDPE pellets analyzed by the tensile strength analysis showed that the force required to deform one of the tested LDPE pellets to be 40 ± 3 % N in comparison with the control where the deformation force was estimated to be 50 ± 3 % N (Fig. 5b). The decrease in deformation force for tested in comparison with the control samples indicates structural changes of LDPE pellets by the bacterial consortia. All the findings related to tensile strength analysis of the degraded polymers are in accordance with the previous reports by Lee et al. (1991); Orhan and Büyükgüngör (2000); Jakubowicz et al. (2011), and Nowak et al. (2011).
Tensile strength analysis of degraded polyethylene end products by bacterial consortia. a Reduction in elongation of the LDPE strips (control and test) analyzed for tensile strength. The dark peak indicates the tensile strength of control. The light peak indicates the tensile strength of degraded LDPE strips by bacterial consortia after 120 days. b The reduction in compression of the LDPE pellets analyzed for tensile strength. The dark peak indicates the tensile strength of control. The light peak indicates the tensile strength of degraded LDPE strips by bacterial consortia after 120 days
SEM analysis
After 120 days of incubation in minimal media, erosion, formation of pits and cavities, and attachment and colonization of rod-shaped bacteria were apparent on the surface of LDPE films (Fig. 6). However, the control samples showed no defects or bacterial biofilm (Fig. 6a, c). The adhesion of bacterial film to the polymer surface is one of the major reasons for biodegradation (Das and Kumar 2015). The presence of pits and cavities is probably due to the lack of a uniform distribution of short branches or photodegradable products in the polymer matrix suggesting that the bacterial consortia might have better interactions with the plastic surface during the degradation (Manzur et al. 2004). The surface erosion mechanism involved in the degradation of plastic products is probably due to enzymatic reaction by bacterial consortia (Bhatia et al. 2014). However, further studies are required to identify the exact mechanism for degradation by microbial consortia. The current study certainly provides insights for all the future perspectives.
Surface analysis of degraded LDPE by bacterial consortia analyzed using SEM: a LDPE strip control; b degraded LDPE strips after 120 days; c one of the plastic residues collected from the plastic garbage processing area (control); d degradation of the plastic residue by bacterial consortia after 120 days (d, e) Attachment of rod-shaped bacterial consortia and formation of biofilms over the degraded LDPE strips after 120 days
Conclusions
This study aimed to devise an eco-friendly approach by formulating novel bacterial consortia of Enterobacter spp. and Pantoea spp. screened from plastic garbage processing areas towards the degradation of LDPE films and pellets. The bacteria were able to utilize LDPE as the nutritional source and grown at a temperature of 45 °C and pH 8.5. The formulated microbial consortia demonstrated greater percentage of LDPE degradation in comparison with the application of pure cultures and bacterial consortia formulated using three known isolates obtained from MTCC. The degradation products analyzed for FTIR, GC-FID, tensile strength, and SEM revealed major structural variations in the degradation of end products and formation of bacterial biofilims on the surface of the LDPE film over a period of 120 days. Thus, the current study suggests that utility of microbial consortia with enhanced biodegradation potential is an eco-friendly approach for plastic waste management. The current study is probably the first of its kind which demonstrates the LDPE degradation potential of bacterial consortia formulated by Enterobacter spp. and Pantoea spp. This study paves a profound scope for industrial scale-up and utilization of this microbial formulation as secure remedies for low-density polyethylene and similar types of polymer eradication from the plastic-polluted environments.
References
Barnes DK, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos Trans R Soc Lond Ser B Biol Sci 364:1985–1998
Beveridge TJ (2001) Use of gram stain in microbiology. Biotech Histochem 76:111–118
Bhardwaj H, Gupta R, Tiwari A (2012) Microbial population associated with plastic degradation. Open Access Sci Rep 1:272. doi:10.4172/scientificreports.272
Bhatia M, Girdhar A, Tiwari A, Nayarisseri A (2014) Implications of a novel Pseudomonas species on low density polyethylene biodegradation: an in vitro to in silico approach. Springerplus 3:497. doi:10.1186/2193-1801-3-497
Central Pollution Control Board (2013) Annual Report 2011–12. PR Division, Ministry of Environment & Forests, Govt. of India. (http://cpcb.nic.in/upload/AnnualReports/AnnualReport_43_AR_2011-12)
Corti A, Muniyasami S, Vitali M, Imam SH, Chiellini E (2010) Oxidation and biodegradation of polyethylene films containing pro-oxidant additives: synergistic effects of sunlight exposure, thermal aging and fungal biodegradation. Polym Degrad Stab 95:1106–1114
Das MP, Kumar S (2015) An approach to low-density polyethylene biodegradation by Bacillus amyloliquefaciens. 3Biotech 5:81–86
Das MP, Kumar S, Rebecca JL, Sharmila S (2013) Isolation and identification of LDPE degrading fungi from municipal solid waste. J Chem Pharm Res 5:78–81
Deepika S, Jaya MR (2015) Biodegradation of low density polyethylene by microorganisms from garbage soil. JEBAS 3:15–21
Dey U, Mondal NK, Das K, Dutta S (2012) An approach to polymer degradation through microbes. IOSR J Pharm 2:385–388
Duddu MK, Tripura LK, Guntuku G, Divya DS (2015) Biodegradation of LDPE by a new bio-surfactant producing thermophillic Streptomyces coelicoflavus NBRC 15399. Afr J Biotechnol 14:327–340
Esmaeili A, Pourbabaee AA, Alikhani HA, Shabani F, Esmaeili E (2013) Biodegradation of low-density polyethylene (LDPE) by mixed culture of Lysinibacillus xylanilyticus and Aspergillus niger in soil. PLoS One. doi:10.1371/journal.pone.0071720
Geldreich EE, Nash HD, Reasoner DJ, Taylor RH (1972) The necessity of controlling bacterial populations in potable waters: community water supply. J Am Water Works Assoc 64:596–602
Hemashenpagam N, Growther L, Murgalatha N, Raj VS, Vimal SS (2013) Isolation and characterization of a bacterium that degrades PBSA. Int J Pharm Bio Sci 4:335–342
Hester RE, Harrison RM (2011) Marine pollution and human health. RSC Publishing, London
Hopewell J, Dvorak R, Kosior E (2009) Plastics recycling: challenges and opportunities. Philos Trans R Soc Lond Ser B Biol Sci 364:2115–2126
Howard GT, Hilliard NP (1999) Use of coomassie blue-polyurethane interaction in detection of polyurethanease proteins and polyurethanolytic bacteria. Int Biodeterior Biodegrad 43:23–30
Jakubowicz I, Yarahmadi N, Arthurson V (2011) Kinetics of abiotic and biotic degradability of low-density polyethylene containing pro-degradant additives and its effect on the growth of microbial communities. Polym Degrad Stab 96:919–928
John RC, Essien JP, Akpan SB, Okpokwasili GC (2012) Polycyclic aromatic hydrocarbon-degrading bacteria from aviation fuel spill site at Ibeno, Nigeria. Bull Environ Contam Toxicol 88:1014–1019
Jurtshuk P Jr, McQuitty DNM (1976) Use of quantitative oxidase test for characterizing oxidative metabolism in bacteria. Appl Environ Microbiol 31:668–679
Klein PD, Graham DY, Gaillour A (1991) Water source as risk factor for Helicobacter pylori infection in Peruvian children. Lancet 337:1503–1506
Kruskala WH, Wallisa WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47:583–621
Kumar S, Hatha AAM, Christi KS (2007) Diversity and effectiveness of tropical mangrove soil micro flora on the degradation of polythene carry bags. Rev Biol Trop 55:777–786
Kyaw BM, Champakalakshmi R, Sakharkar MK, Lim CS, Sakharkar KR (2012) Biodegradation of low density polythene (LDPE) by Pseudomonas spp. Indian J Microbiol 52:411–419
Lee B, Pometto AL, Fratzke A, Bailey TB (1991) Biodegradation of dagradable plastic polyethylene by Phanerochate and Streptomyces species. Appl Environ Microbiol 57:678–685
Lee YJ, Kim KS, Kwon YK, Tak RB (2003) Biochemical characteristics and antimicrobials susceptibility of Salmonella gallinarum isolated in Korea. J Vet Med Sci 4:161–166
Lodhi AF, Hasan F, Shah Z, Hameed A, Faisal S, Shah AA (2011) Optimization of culture conditions for the production of poly (3-Hydroxybutyrate) depolymerase from newly isolated Aspergillus fumigates from soil. Pak J Bot 43:1361–1372
Manzur A, Limon GM, Favela TE (2004) Biodegradation of physiochemically treated LDPE by a consortium of filamentous fungi. J Appl Polym Sci 92:265–271
Mossel DAA, Mengerink WHJ, Scholts HH (1962) Use of modified MacConkey agar medium for the selective growth and enumeration of Enterobacteriaceae. Appl Microbiol 84:235–240
Murray PR, Baron JH, Pfaller MA, Jorgensen JH, Yolken RH (2003) Manual of clinical microbiology, 8th edn. American Society for Microbiology, Washington D.C.
Nakajima-Kambe T, Shigeno-Akutsu Y, Nomura N, Onuma F, Nakahara T (1999) Microbial degradation of polyurethane, polyester polyurethanes and polyether polyurethanes. Appl Microbiol Biotechnol 51:134–140
Nanda S, Sahu SS, Abraham J (2010) Studies on the biodegradation of natural and synthetic polyethylene by Pseudomonas spp. J Appl Sci Environ Manag 14:57–60
North EJ, Halden RU (2013) Plastics and environmental health: the road ahead. Rev Environ Health 28:1–8
Nowak B, Pajak J, Drozd BM, Rymarz G (2011) Microorganisms participating the biodegradation of modified polyethylene films in different soils under laboratory conditions. Int Biodeterior Biodegrad 65:757–767
Obradors N, Aguilar J (1991) Efficient biodegradation of high-molecular-weight polyethylene glycols by pure cultures of Pseudomonas stutzeri. Appl Environ Microbiol 57:2383–2388
Orhan Y, Büyükgüngör H (2000) Enhancement of biodegradability of disposable polyethylene in controlled biological soil. Int Biodeterior Biodegrad 45:49–55
Page RD (2002) Visualizing phylogenetic trees using TreeView. Curr Protoc Bioinformatics. Chapter 6: Unit 6.2. doi:10.1002/0471250953.bi0602s01
Patel V, Jain S, Madamwar D (2012) Naphthalene degradation by bacterial consortium (DV-AL) developed from Alang-Sosiya ship breaking yard, Gujarat, India. Bioresour Technol 107:122–130
Pelczar MJ Jr, Reid RD, Chan ECS (1977) Microbiology, 4th edn. Tata McGraw-Hill Publishing Company Ltd, New Delhi
Sah A, Negi H, Kapri A, Anwar S, Goel R (2011) Comparative shelf life and efficacy of LDPE and PVC degrading bacterial consortia under bioformulation. Ekologija 57:55–61
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sen SK, Rault S (2015) Microbial degradation of low density polyethylene (LDPE): a review. JECE 3:462–473
Skariyachan S, Megha M, Kini MN, Mukund KM, Rizvi A, Vasist K (2015) Selection and screening of microbial consortia for efficient and ecofriendly degradation of plastic garbage collected from urban and rural areas of Bangalore. India Environ Monit Assess 187:4174. doi:10.1007/s10661-014-4174-y
Stager CE, Erikson E, Davis JR (1983) Rapid method for detection, identification and susceptibility testing of enteric pathogens. J Clin Microbiol 17:79–84
Taylor WI, Achanzar D (1972) Catalase test as an aid to the identification of Enterobacteriaceae. Appl Microbiol 29:58–61
Titters RR, Sancholzer LA (1936) The use of semi-solid agar for the detection of bacterial motility. J Bacteriol 31:575–580
Tribedi P, Sil AK (2013) Cell surface hydrophobicity: a key component in the degradation of polyethylene succinate by Pseudomonas sp. AKS2. J Appl Microbiol 116:295–303
Tribedi P, Sarkar S, Mukherjee K, Sil AK (2012) Isolation of a novel Pseudomonas sp. from soil that can efficiently degrade polyethylene succinate. Environ Sci Pollut Res 19:2115–2124
Usha R, Sangeetha T, Palaniswamy M (2011) Screening of polyethylene degrading microorganisms from garbage soil. Libyan Agric Res Center J Int 2:200–204
Vijaya CH, Reddy RM (2008) Impact of soil composting using municipal solid waste on biodegradation of plastics. Indian J Bacteriol 8:235–259
Yoon MG, Jeon HJ, Kim MN (2012) Biodegradation of polyethylene by a soil bacterium and alkB cloned recombinant cell. J Bioremedediat Biodegrad 3:145
Acknowledgments
The authors sincerely acknowledge Karnataka State Council for Science and Technology (KSCST), Indian Institute of Science, Bangalore, for the financial support (Proj. Ref. No. 38S0142).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gerald Thouand
Electronic supplementary material
ESM 1
(DOC 783 kb)
Rights and permissions
About this article
Cite this article
Skariyachan, S., Manjunatha, V., Sultana, S. et al. Novel bacterial consortia isolated from plastic garbage processing areas demonstrated enhanced degradation for low density polyethylene. Environ Sci Pollut Res 23, 18307–18319 (2016). https://doi.org/10.1007/s11356-016-7000-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7000-y