Abstract
The work is devoted to improving the technology of obtaining organo-mineral fertilizers by modifying the composition of the organic shell, which allows the formation of its developed nanoporous structure. Perspective directions of the “green” transition of agriculture in the concept of sustainable innovative outpacing are substantiated. The relevance of using granular products with a porous structure in chemical production and the agricultural industry is given. A review of methods for obtaining granules with a nanoporous structure is also carried out. The necessity of creating a new type of fertilizers, combining nutrients included in the composition of mineral fertilizers and organic elements, has been substantiated. The technological bases of encapsulation of mineral fertilizers with an organic shell are presented. A new composition of the shell for encapsulation has been proposed, in which biochar is used as an additional pore-forming agent. The results of studying the structure of granules of organo-mineral fertilizers with biochar in the shell are presented.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Nanoporous structure
- Organic-mineral fertilizers
- Granulation
- Concept of green innovative outpacing/proactivity
1 Introduction
Tackling climate risks, resulting from anthropogenic pressures on ecosystems, involves global reductions in greenhouse gas emissions in all sectors of the economy and a “green” transition of national economies to climate neutrality and sustainable development [1,2,3] in accordance with the documents “The European Green Deal” [4] and “Fit for 55”: delivering the EU's 2030 Climate Target on the way to climate neutrality [5], adopted by the European Commission in response to climate and environmental-related challenges [6].
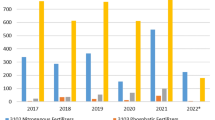
According to the European Environment Agency [7], the largest polluter in terms of anthropogenic N2O greenhouse gas emissions in the EU is the agricultural sector, which accounted for 74.59% of nitrogen oxide emissions (or 174,965.9 kt CO2 eq.) in 2019. The overall dynamics of greenhouse gas formation in the EU countries is shown in Fig. 1. In Ukraine, according to the annual inventory submissions 2021 [8, 9], the volume of nitrogen oxide emissions in the agricultural sector was 111.3 kt in 2019, 78% of which are direct and indirect emissions from agricultural land (as a result of the use of organic and mineral fertilizers).
Dynamics of greenhouse gas emissions in the EU, thousand kt (developed by authors based on data [7])
The dynamics of fertilizer use in the agricultural sector of Ukraine for the period 2000–2020 indicates an increase in consumption of mineral fertilizers by almost 886% (from 0.28 million tons in 2000 to 2.78 million tons in 2020) while reduction of organic fertilizers by 60.6% compared to 2000 (from 29 million tons to 11.4 million tons). Meanwhile, the share of agricultural lands treated with mineral fertilizers increased only by 252.7% and in 2020 amounted to 39.5%, and the share of agricultural lands treated with organic fertilizers increased by 41.2% and in 2020 amounted to 2.4% [10]. These data indicate that while maintaining current trends, the volume of greenhouse gases in agriculture will increase, and hence, the “green” transition to organic production will take place at an extremely slow pace, even if it is focused on achievement of the climate neutrality goals by 2060 or even by 2070, as defined in [11, 12].
The need to accelerate the “green” transition of Ukraine to climate neutrality through the introduction of environmental innovations is identified in the National Economic Strategy for the period up to 2030 [11]. Moreover, the trigger for a “green” transition in Europe is the ever-growing hybrid threats from the Russian Federation, which intensify migration processes, including due to climate change, and affect the food, energy, environmental, and national security of European countries. In this regard, according to the concept of sustainable innovative outpacing, one of means of accelerating the “green” transition in agriculture is to use new generation fertilizers that are environmentally safe at all stages of their ecological and economic cycle, including the stages of life cycle and customization one [13,14,15], and based on an innovative approaches to the technologies of the fertilizer production and the use of only clean energy sources in the production process [16,17,18,19].
Obtaining granules with a nanoporous structure is a prerequisite for the implementation of some processes in chemical technology and ensuring the necessary characteristics of a granulated product at the stage of its economic use. For example, for blasting operations in the mining industry using ANFO, a prerequisite is the use of ammonium nitrate with a developed network of nanopores (porous ammonium nitrate, PAN) [20,21,22] simultaneously with the development of the technological foundations of the process [23, 24] and the determination of the laws of hydrodynamics [25] and heat and mass transfer processes [26,27,28]. The porosity of adsorbents and catalysts is also a prerequisite for successfully implementing processes in chemical technology [29, 30].
It is necessary to highlight the direction of obtaining granules with a porous structure for further use as fertilizers. This is especially important when it is required to ensure a time-controlled dissolution of granules in the soil. Moreover, in the case of using multilayer fertilizers, the structure of each layer should ensure the release of components (nutrients) according to a given law.
Obtaining a porous structure of granules is possible both with special processing and simultaneously with the formation of granules:
-
1.
Introduction of pore-forming and modifying additives in the process of granule formation.
-
2.
Introduction of pore-forming and modifying additives in the process of additional processing of already formed granules.
-
3.
Moistening and heat treatment of the formed granules with pore formation due to moisture removal, incl. with components to the humidifier (except for pore-forming and modifying), which can be further used as nutrients.
Research into the needs of the fertilizer market has shown that the most promising direction for increasing the efficiency of nitrogen granules is their encapsulation with phosphate-containing shells. Considering the highest nitrogen losses from urea granules in the soil, it is adopted as the core of the encapsulated granule. The well-known property of phosphates to dissolve more slowly in comparison with nitrogen fertilizers predetermined to take them as the basis of the shell. At the same time, changes in climatic conditions for growing agricultural plants and a decrease in the humus content in the soil require different solutions to increase it. One of the alternatives to compensate for the lack of organic matter is the use of sodium and potassium humates. Considering the removal of significant amounts of nutrients with the harvest, including calcium, we have developed a technical solution for the production and use of calcium humate, which was tested in the conditions of vegetation and small-scale experiments and confirmed its effectiveness.
The basic scheme for obtaining organo-mineral fertilizers can be presented as shown in Fig. 2.
Model installation for encapsulating fertilizers [13]: 1—phosphorite bunker; 2—microelements bunker; 3—mixer; 4—dispenser; 5—potassium-magnesium solution tank; 6—humate solution tank; 7—potassium-magnesium and humate mixing tank; 8—urea granule feed unit; 9—nozzle; 10—disc granulator; 11—tray for granulated product
Obtaining a nanoporous structure of the granule shell is one of the ways to ensure a given rate of dissolution of fertilizer with soil moisture. The amount of moisture that can penetrate into the membrane and stay there to ensure the transfer of nutrients to the kidney depends on the pores’ structure, size, and volume. Thus, in this work, two problems are simultaneously solved: the proposal of a new shell composition for encapsulation and the study of the properties of the nanoporous structure of granules of organic-mineral fertilizers.
2 Technological, Constructive, and Optimization Calculation
The developed technical solution for obtaining encapsulated organo-mineral fertilizers consists of forming a phosphate-containing shell on a granule (prill) of carbamide in a pelletizing granulator. The process is carried out by the method of agglomeration of fine powders by wetting them with a plasticizer, which is used as one of the types of mineral or organic fertilizers. Common shells for encapsulation of nitrogen fertilizers are sulfur, polymer, and suspension coatings. The main substance of the shell is sulfur and polymeric materials applied to the granules in the form of solutions or suspensions. The introduction of polymer components into the shell composition reduces the overall nutritional value of the fertilizer. It requires special conditions to apply a uniform thickness of a thin solid coating, which significantly increases the cost of fertilizers.
To increase the nutritional value of the encapsulated fertilizer, shells based on suspensions of ammophos or powdered ammoniated superphosphate and phosphorite concentrates have recently been used. Aqueous solutions of other fertilizers are used as a plasticizer to form the shell based on powdered phosphates. However, the use of solutions of inorganic fertilizers in the composition of such a shell does not allow to create its stable porous structure, which when dissolving the shell increases its pore size and increases the rate of dissolution of the nitrogen core of the granule.
The shells of encapsulated fertilizers formed on the core of nitrogen, phosphorus, or potassium fertilizers are known. They include an inorganic substance in the form of a natural phosphorus-glauconite concentrate as the base of the shell, and a binder in the form of aqueous solutions of potassium or nitrogen fertilizers, or combinations thereof. The disadvantage of such granule structure forming is the use of aqueous solutions of nitrogen and potassium fertilizers as a plasticizer, which leads to the rapid dissolution of these fertilizers in the soil and does not allow the creation of a porous shell structure for the entire period of dissolution of the granule core.
The prototype is the shells of nitrogen fertilizers, which include powdered ammoniated superphosphate moistened with calcium humate as a plasticizer, which is formed by agglomeration, followed by rolling and drying of the encapsulated product. Shell formation is carried out at a ratio of inorganic coating substance and calcium humate 1 (0.0012 ÷ 0.01).
The disadvantage of these shells of nitrogen fertilizer granules is the use of powdered ammoniated superphosphate, which requires pre-acid decomposition of phosphorite and low pH of the fertilizer as a whole, which affects the degree of crop yield.
The new model is based on the task of creating a microporous coating of nitrogen fertilizer granules, which effectively acts to increase crop yields on all types of soils.
The problem is solved by using phosphate-glauconite concentrate with the addition of biochar in the amount of 5–10% by weight of phosphate-glauconite concentrate and an aqueous solution of calcium humate. The ratio of a mixture of phosphate-glauconite concentrate with biochar and calcium humate is 1: (0.09–0.11).
The physical model of encapsulation can be represented as follows. A fraction (2–4) mm of carbamide granules, powdered phosphate-glauconite concentrate with a particle size of fewer than 200 microns, and an aqueous solution of calcium humate containing up to 85% water are preliminarily prepared by sieving. Urea granules are encapsulated in a disc granulator (Fig. 3) on the pellet surface while simultaneously dosing urea granules, powdered phosphate-glauconite concentrate into the granulator, and wetting the surface of the granules, and powder with a plasticizer in the form of an aqueous calcium humate solution.
When the granulator plate rotates, the moistened carbamide granules move (roll) along the trajectory of an irregular spiral, and as they increase in size, approach the plate side, and are discharged from it. The process of granule growth occurs due to the adhesion of moist small particles of phosphate-glauconite concentrate. In the process of pellet rolling, these particles are compacted on the surface of the granule, then after the next cycle of irrigation and rolling of the granule, additional adhesion occurs, followed by compaction of other particles, and so on until the required thickness of the granule shell is reached. At a certain, given diameter, it rolls over the side of the plate. Further, the encapsulated granule is sent for drying, followed by the separation of the commercial fraction.
Implementing the encapsulation process on a pan granulator has its advantages. After reaching the required size, the granules are removed from the apparatus, and visual control of the granule growth process allows you to quickly track changes in the technological parameters of encapsulation.
To obtain encapsulated organo-mineral fertilizers when conducting laboratory studies, carbamide granules of fraction 2–4 mm were used, with 46.1% nitrogen, phosphate-glauconite concentrate with a total content of P2O5 14%, and a particle size of no more than 200 microns, potassium and calcium humates with a mass moisture content of 85 and 87%, and the mass fraction of potassium and calcium humates (in terms of dry matter) 18 and 16%, respectively. At the same time, the humate solution has a pH = 10.8, which allows us to consider it as a blocker of heavy elements in the soil. Biochar powder was prepared by sieving to a fraction of fewer than 200 microns, with a carbon content of 93%. The production of potassium and calcium humates was carried out on a model homogenizer.
The general technique of the experiment is described in [15].
The choice of a plasticizer in the form of potassium and calcium humates is due to the positive results of agrochemical tests, which showed an additional increase in yield compared to a plasticizer based on potassium (an aqueous solution of potassium mag). In the first series of experiments, the dependence of the strength of the applied coating on the amount of plasticizer-calcium humate was determined (Table 1).
As follows from the experimental data obtained, the dependence of the strength of the granules on the moisture content of the granulated phosphate-containing mixture is extreme depends on the rheological properties of the phosphate-containing additive, and the optimal amount of moisture is 12.5%, which correlates with the data of other researchers—8–15%. When the moisture content of the phosphate-containing charge is less than 10%, not all of the charge is uniformly moistened and cannot be involved in the agglomeration process. In this case, the rolling process is disrupted, and the strength of the phosphate-containing shell is low, with low adhesion of the shell to the carbamide granule. When the moisture content of the charge is more than 15%, the phosphate-containing charge is granulated to a moisture content of 12.5%. Then, the over moistening of the charge follows with the formation of stuck together granules and ultimately, the disruption of the agglomeration process. Additional studies on introducing a carbon-containing nanoporous additive—biochar into the shell composition—showed an insignificant change in the moisture content of the charge while maintaining the trend of optimal moisture content. It was interesting to determine the maximum amount of biochar that can be introduced into the composition of the phosphate-containing shell. In experiment 1, biochar was not introduced into the composition of the shell (comparison experiment). In experiments 2–5, it was introduced in various quantities with its preliminary mixing with phosphate-glauconite concentrate. The results of experimental studies are presented in Table 2.
As follows from the presented experimental results, for a given phosphate-containing mixture, the maximum content of biochar based on the strength characteristics of the shell is not more than 10%. At the same time, according to other researchers’ data, its effect on increasing fertilizer efficiency begins to show itself even when it is present in 1%. Thus, an increase in the content of biochar with nanosized pores will make it possible to more efficiently use the nutrient elements of the granule and accordingly, increase the yield of agricultural crops. This statement was confirmed during test vegetation experiments and showed an increase in yield compared to the sample without nanoporous additives by 23%.
3 Phase Composition and Crystal Structure of Granules of Organic-Mineral Fertilizers
The main characteristics of samples of organic-mineral fertilizers are presented in Table 3.
Samples 5 and 6 contained biochar in various compositions. According to the results of the experiments, a composition with 10% biochar addition into a phosphate shell with calcium humate as a plastiziser was more successful for obtaining the nanoporous shell. The cross-section analysis of obtained samples showed that with the addition of biochar into phosphate coating of the granule, the attachment between granule of carbamide and phosphate shell increased, and the interface between layers became more uniform structure (Fig. 4). The addition of biochar slightly decreases the density of the granules but makes such fertilizers more appropriate for agricultural application.
SEM images of granules cross section containing carbamide in phosphate shell with various plastizisers 1—calcium humate, 2—potassium humate, 3—potassium-magnesium, 4—calcium humate with complex Avatar, 5—potassium humate with biochar addition to the shell, 6—calcium humate with addition of 10% biochar into phosphate shell
The nanoporous structure of biochar is presented in the Fig. 5a. A micrograph of biochar microstructure is shown in the Fig. 5b, c.
4 Conclusions
The research results have shown that the improvement of the composition of the organic shell in the process of encapsulating the mineral fertilizer makes it possible to create a developed nanoporous structure of this shell. The introduction of biochar into the shell leads to the formation of an ordered structure of organic-mineral fertilizer and the creation of conditions for a controlled process of its solubility in soil.
The introduction of a new type of fertilizer will increase the security of producers and consumers, improve national security, global competitiveness, inclusive, and “green” economic growth, and accelerate the achievement of the Sustainable Development Goals and climate neutrality.
The research results obtained allow in future to assess the competitiveness of a new type of fertilizer in the context of the concept of sustainable innovative outpacing.
References
Wang C (2015) Explaining connotation of regional green transition. In: Proceedings of the 2015 International conference on industrial technology and management science (ITMS 2015), 34, 1203–1205
Sutherland LA, Darnhofer I, Wilson GA, Zagata L (2015) Transition pathways towards sustainability in agriculture: case studies from Europe. CABI Publishing
Shen J, Zhu Q, Jiao X, Ying H, Wang H, Wen X, Xu W, Li T, Cong W, Liu X, Hou Y, Cui Z, Oenema O, Davies WJ, Zhang F (2020) Agriculture green development: a model for China and the world. Front Agri Sci Eng 7(1):5–13
European Commission (2021) The European Green Deal
European Commission (2021) Communication from the commission to the European Parliament, the Council, the European economic and social committee and the committee of the regions ‘Fit for 55’: delivering the EU's 2030 Climate Target on the way to climate neutrality. COM/2021/550 final
World Economic Forum (2021) The Global Risks Report 2021
European Environment Agency (2021) EEA greenhouse gases—data viewer. Data viewer on greenhouse gas emissions and removals, sent by countries to UNFCCC and the EU Greenhouse Gas Monitoring Mechanism (EU Member States)
UNFCCC (2021). National Inventory Submissions 2021
UNFCCC (2021) Ukraine. 2021 Common Reporting Format (CRF)
State Statistics Service of Ukraine (2021) Application of mineral and organic fertilizers (1919–2020)
Resolution of the Cabinet of Ministers of Ukraine (2021) On approval of the National Economic Strategy for the period up to 2030” from 03.03.2021 No. 179
Ministry of Environmental Protection and Natural Resources of Ukraine (2021) Draft Concept of “green” energy transition of Ukraine until 2050
Artyukhov A, Vakal S, Shkola V, Vakal V, Yanovska A (2021) Obtaining of the novel organo-mineral fertilizers in pan granulators: technological fundamentals. In: Ivanov V, Pavlenko I, Liaposhchenko O, Machado J, Edl M (eds) Advances in design, simulation and manufacturing IV. DSMIE 2021. Lecture Notes in Mechanical Engineering, 207–217
Shkola V, Prokopenko O, Stoyka A, Nersesov V, Sapiński A (2021) Green project assessment within the advanced innovative development concept. Estudios de Economia Aplicada 39(5)
Vakal S, Yanovska A, Vakal V, Artyukhov A, Shkola V, Yarova T, Dmitrikov V, Krmela J, Malovanyy M (2021) Minimization of soil pollution as a result of the use of encapsulated mineral fertilizers. J Ecol Eng 22(1):221–230
Kotenko O, Domashenko M, Shkola V (2019) Production costs decreasing by introduction of energy-efficient technologies within the enterprise’s counter-crisis management strategy. Inter J Ecol Econ Statis 40(3):88–97
Kurbatova T, Skibina T (2019) Renewable energy policy in Ukraine's household sector: measures, outcomes, and challenges. In: IEEE International Conference on Modern Electrical and Energy Systems, 234–237
Kurbatova T, Sotnyk I, Kubatko O, Baranchenko Y, Arakpogun E, Roubik H (2020) State support policy for renewable energy development in emerging economies: the case of Ukraine. Inter J Global Environ Issues 19(1–3):26–52
Trypolska G, Kruvda O, Kurbatova T, Andrushchenko O, Suleymanov C, Brydun Y (2021) Impact of new renewable energy capacities on employment in Ukraine in 2021–2030. Inter J Energy Econ Policy 11(6):98–105
Artyukhov A, Artyukhova N, Krmela J, Krmelová V (2020) Granulation machines with highly turbulized flows: creation of software complex for technological design. In: IOP Conference Series: Materials Science and Engineering 776(1):Article no. 012018
Artyukhov A, Artyukhova N, Krmela J, Krmelová V (2020) Complex designing of granulation units with application of computer and software modeling: case “Vortex granulator”. Mat Sci Eng 776(1):Article no. 012016
Artyukhov A, Artyukhova N (2018) Utilization of dust and ammonia from exhaust gases: new solutions for dryers with different types of fluidized bed. J Environ Health Sci Eng 16(2):193–204
Artyukhov AE, Artyukhova NO (2019) Technology and the main technological equipment of the process to obtain N4HNO3 with Nanoporous Structure. Springer Proc Phys 221:585–594
Artyukhov AE, Ivaniia AV (2017) Obtaining porous ammonium nitrate in multistage and multifunctional vortex granulators. Naukovyi Visnyk Natsionalnoho Hirnychoho Universytetu 6:68–75
Ivaniia AV, Artyukhov AY, Olkhovyk AI (2019) Hydrodynamic and thermodynamic conditions for obtaining a nanoporous structure of ammonium nitrate granules in vortex granulators. Springer Proc Phys 221:257–268
Artyukhov AE, Krmela J, Gavrylenko OM (2019) Evaluation of the impact made by the hydrodynamic regime of the granulation equipment operation on the nanoporous structure of N4HNO3 granules. J Nano Elect Phys 11(3):03033
Yukhymenko M, Ostroha R, Artyukhov A (2016) Hydrodynamic and kinetic processes of the mineral fertilizer granules encapsulating in the multistage device with suspended layer. Eastern-European J Enter Technol 6(6–84):22–28
Ostroha R, Yukhymenko M, Yakushko S, Artyukhov A (2017) Investigation of the kinetic laws affecting the organic suspension granulation in the fluidized bed. Eastern-European J Enterprise Technol 4(1):4–10
Chuah CY, Li W, Yanqin Yang Y, Bae T-H (2020) Evaluation of porous adsorbents for CO2 capture under humid conditions: the importance of recyclability. Chem Eng J Adv 3:100021
Broom D (2021) Characterizing adsorbents for gas separations Measurement needs and laboratory techniques (white paper). Hiden Isochema
Acknowledgements
This research work had been supported by the Ministry of Science and Education of Ukraine under the project No 0120U102003 “process of formation of the novel ecologically safe fertilizers with prolonged action based on the phosphorite deposits raw materials”.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Vakal, S.V., Vakal, V.S., Artyukhov, A.E., Shkola, V.Y., Yanovska, A.O. (2023). Selection of Optimal Technological Parameters for Obtaining Encapsulated Organic-Mineral Fertilizers with Nanoporous Structure. In: Fesenko, O., Yatsenko, L. (eds) Nanomaterials and Nanocomposites, Nanostructure Surfaces, and Their Applications . Springer Proceedings in Physics, vol 279. Springer, Cham. https://doi.org/10.1007/978-3-031-18096-5_23
Download citation
DOI: https://doi.org/10.1007/978-3-031-18096-5_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-18095-8
Online ISBN: 978-3-031-18096-5
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)