Abstract
Transcranial Doppler ultrasound (TCD) has been used in pediatric traumatic brain injury for more than 20 years. Although there is solid evidence of its utility, many centers don’t use it in neurocritical care settings. In this chapter we will revise the role of TCD in pediatric traumatic brain injury. Namely, the role of TCD in estimating intracranial pressure and cerebral perfusion pressure; evaluating cerebrovascular autoregulation and continuous monitoring; detecting regional variations on cerebral hemodynamics and in the diagnosis of brain death. A critical literature review and the author’s own experience will hopefully help the reader in a better understanding of this powerful instrument of non-invasive neuromonitoring.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Traumatic brain injury
- Transcranial Doppler
- Intracranial pressure
- Cerebral perfusion pressure
- Cerebral autoregulation
Traumatic brain injury (TBI) is the leading cause of trauma-related death and permanent disability in children. Worldwide, it affects more than three million children annually [1] and in the United States alone, TBI contributes to the death of more than 1000 children every year [2].
When a child is admitted to the hospital after a moderate or severe TBI, management is targeted at avoiding secondary damage to the injured brain. In order to achieve this goal, maintaining an adequate cerebral blood flow (CBF) is crucial. Guidelines have traditionally used intracranial pressure (ICP) monitoring and treatment of increased ICP as the main objective to improve outcome following TBI. In children, adequate randomized controlled studies to evaluate the role of ICP monitoring and treatment have not been performed and the strength of recommendation of the latest guidelines on ICP monitoring and ICP treatment thresholds is weak (Level III) [3].
Cerebral perfusion pressure (CPP) is defined as the difference between mean arterial blood pressure (ABP) and mean ICP, and it is the pressure gradient driving cerebral blood flow. In normal conditions, CBF is autoregulated to maintain an adequate oxygen and glucose delivery to the brain across physiological range of CPP. After TBI, cerebral autoregulation might be impaired and decreases in CPP could lead to cerebral ischemia. Thresholds for adequate CPP in children with TBI have recently been published suggesting that CPP targets should be age-specific: above 40 mmHg in children under 6 years-old and above 50 mmHg in children from 6 to 17 years-old [4]. If CPP is the driving pressure of CBF, it is logical that treatment protocols should focus on CPP, rather then on ICP. CPP by definition can be manipulated by changing ICP or ABP.
Traditionally, an ICP bolt and an arterial line are used to monitor ICP and CPP invasively. In children, invasive ICP-CPP monitoring is reserved for patients in whom the severity of the clinical conditions demand ICP-CPP guided treatment. Otherwise, the risks associated with invasive neuromonitoring, such as bleeding and infection, may not represent a beneficial intervention. In these cases, non-invasive methods, like transcranial Doppler ultrasonography (TCD), for assessment of these parameters could offer an alternative for treatment or a screening tool to determine the need for invasive monitoring.
Role of transcranial Doppler ultrasonography on Pediatric TBI:
Non-invasive Estimate of ICP
One of the most studied roles of TCD in TBI is the ability to estimate or predict ICP non-invasively.
There are two indices commonly used to estimate ICP with TCD:
-
Resistance index (Pourcelot) [5]:
-
(peak systolic velocity – end diastolic velocity)/peak systolic velocity
-
-
Pulsatility index (Gosling) [6]:
-
(peak systolic velocity – end diastolic velocity)/mean velocity
-
Although the accuracy of TCD to estimate ICP in adult patients with TBI has been studied over the years with generally good results [7], there is less evidence in children and results are conflicting. Some authors state that transcranial Doppler pulsatility index is not a reliable indicator of intracranial pressure in children with severe traumatic brain injury, based in data from 34 children and 275 examinations [8]. A threshold PI of 1 was used to detect ICP 20 mm Hg or higher and the sensitivity and specificity was 25% and 88%, respectively. But if the PI threshold was increased to 1.2 the specificity would be 100%. This is in line with our experience that a high PI, in face of a normal arterial pressure and normal pCO2, implies a high ICP. There are also studies with good results in children [9,10,11]. The largest study, included 117 children with severe TBI and PI >1.31 had a sensitivity of 94% and a specificity of 41% to identify patients with ICP >20 mmHg. The authors conclude that TCD examination is a safe, reproducible, and reliable method to identified children at increased risk of ICH and decreased CPP after severe TBI, and its use should be encouraged in PICU [11]. In our own experience we evaluated 18 children with severe TBI with TCD and invasive ICP. Sixteen patients had ICP values above 20 mmHg, with a mean highest value of 35.7 ± 11.2 mmHg. The first measurement of PI had a mean of 1.12 ± 0.33. There was a significant correlation between the first PI determination and the corresponding ICP value (Pearson correlation coefficient r = 0.755, p < 0.0001) [10].
Other studies on mathematical models for continuous non-invasive ICP prediction using simultaneous measurements of systemic arterial blood pressure and transcranial Doppler flow velocity waveforms have shown better ability of TCD to estimate and track ICP changes [12,13,14].
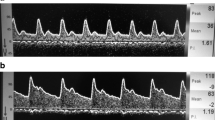
In summary, TCD can accurately predict a raised ICP in pediatric TBI, especially if a higher cut-off value for PI is used. In our clinical practice we use a threshold of 1.4 using the Gosling PI. We have to take into consideration arterial blood pressure and pCO2 as these parameters can change PI and give false negatives, in case of arterial hypertension, and false positives, in case of hypotension or hyperventilation. This non-invasive technique can be extremely useful at admission to help determine the level of care and prioritize actions to take in children who suffered a TBI [15]. This is best exemplified by the case of a patient where TCD at admission revealed a very high PI and prompted an emergent surgery instead of invasive ICP monitoring in the PICU (Fig. 1).
Non-invasive Estimate of CPP
Among the several non-invasive methods reported for CPP assessment (nCPP) [7, 16, 17], ultrasound-based alternatives are of special interest since these techniques are low-cost and widely available in the neurocritical care settings. TCD has been one of the most used methods for determination of nCPP in TBI [7]. Several studies have tested the feasibility of TCD for these purposes in children [8, 11, 18]. Although Figaji et al. concluded that PI was not a reliable indicator of ICP, they found that the correlation of PI with CPP was much better and significantly related (p = 0.001) [8]. These data were corroborated in more recent studies that found a sensitivity of 80% of PI to detect a CPP of less than 50 mmHg [11] and in another study where a novel estimator of CPP was calculated using TCD-spectral accounting method that showed a good correlation of nCPP and CPP (Spearman correlation coefficient, R = 0.67 (p < 0.0001) and the ability of nCPP to predict values of CPP below 70 mmHg was excellent as demonstrated by an area under the curve of 0.91 (95% CI = 0.83–0.98) using a receiver operating curve analysis [18].
It is not a surprise that PI correlates better with CPP than with ICP. It has been elegantly demonstrated by de Riva et al. that PI is not dependent solely on cerebrovascular resistance but it is a product of the interplay between CPP, pulse amplitude of arterial pressure, cerebrovascular resistance and compliance of the cerebral arterial bed as well as the heart rate. Therefore, PI is not an accurate estimator of ICP and it describes CPP in a more accurate manner [19]. This is consistent with our practice where we have found cases of pediatric TBI with high PI and normal ICP in patients with low arterial blood pressure (Fig. 2) [15].
Autoregulation and Continuous Monitoring of TCD Signals
Cerebrovascular autoregulation is a hemodynamic mechanism that allows cerebral blood flow to remain constant with changes of CPP. This is fundamental to protect the brain against inappropriate CBF. If cerebrovascular autoregulation is impaired, CBF becomes dependent on CPP and any changes in arterial blood pressure will reflect directly on CBF. It has been shown that after a TBI, impaired autoregulation is independently associated with a worse outcome and mortality [20,21,22].
The requirements to measure and monitor dynamic autoregulation over time are:
-
Continuous arterial blood pressure monitoring (invasive or non-invasive)
-
A surrogate for CBF:
-
Non-invasive (TCD, Near-infrared spectroscopy – NIRS)
-
Invasive (PbtO2, ICP, Laser Doppler Flow)
-
-
A mathematical model to quantificate the relationship between ABP and CBF
-
Time domain analysis (PRx, COx, Mx, Lx, ORx)
-
Frequency domain analysis (coherence, gain of transfer, phase shift)
-
In the case of TCD, autoregulation monitoring uses the signals of ABP, ICP and cerebral blood flow velocities to calculate indices of autoregulation [23]:
-
Mx index is the correlation coefficient between mean flow velocity and CPP
-
Sx index is the correlation coefficient between systolic flow velocity and CPP
If Mx and Sx are positive it means autoregulation is impaired and this is associated with a bad outcome after TBI. In the example below, we can see a patient with adequate autoregulation and a negative Mx (Fig. 3).
One of the major challenges in using TCD signals to evaluate autoregulation is the necessity to be able to record flow velocities for a long period of time. This can be accomplished with probe holders, but the signal can be lost with positioning of the patient or spontaneous movement. Children represent an additional challenge because of different head sizes and some holders are difficult to use in small children. More recently, new devices using robotic probes allow for continuous monitoring over extended time periods with good results for at least 4 hours of monitoring [24].
In summary, dynamic cerebrovascular autoregulation monitoring can be done non-invasively with TCD but it is difficult to accomplish due to the necessity of long-term acquisition of the TCD signals. New technological advances in this area will make it more usable in clinical practice.
Detect Regional Variations on Cerebral Hemodynamics
One of the challenges in studying the injured brain is that many devices only allow for measurements in one particular area of the brain. This is the case with ICP bolts or PbtO2 probes. TCD has the major advantage of allowing insonation of different territories. This is particularly important in pathologies like TBI that can have focal lesions. Although a raised ICP, especially if severe, will ultimately be transmitted to the whole brain, we have found cases with important asymmetries at an initial phase (Fig. 4).
Diagnosis of Brain Death
Use of TCD as a tool to aid in the diagnosis of brain death is beyond the scope of this chapter. Nonetheless, TBI is one of the major indications for organ donation and TCD can identify cerebral circulatory arrest and can be extremely useful in determined circumstances. Although TCD is not accepted in all countries for the diagnosis of brain death, it is commonly used in others. The indications for using an ancillary test of no cerebral blood flow are:
-
Impossibility to complete components of the examination or the apnea test
-
Uncertainties about the results of the neurological examination
-
If a medication effect may be present
-
To allow a shorter period of time between the two examinations (in children an interval of 12 h is necessary if no ancillary test is used)
In our practice we use TCD in every patient that is considered for organ donation. We find it reassuring for both family members and staff.
Conclusions
An experienced operator only needs a few minutes to understand if CBF is normal or compromised when performing a TCD. PI is calculated instantaneously and, as previously described, it will be high in cases with decreased CPP. This can be extremely useful for point of care decisions at the bedside in cases of pediatric TBI.
Although TCD can and has been used for cerebrovascular autoregulation monitoring this is more difficult to accomplish in clinical practice and is often performed in investigation settings. New technological advances will make this tool easier to use and help guide patient management.
References
Dewan MC, Mummareddy N, Wellons JC, Bonfield CM. Epidemiology of global pediatric traumatic brain injury: qualitative review. World Neurosurg. 2016;91:497–509.e1. https://doi.org/10.1016/j.wneu.2016.03.045.
Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury–related emergency department visits, hospitalizations, and deaths — United States, 2007 and 2013. MMWR Surveill Summ. 2017;66:1–16. https://doi.org/10.15585/mmwr.ss6609a1.
Kochanek PM, Tasker RC, Carney N, et al. Guidelines for the management of pediatric severe traumatic brain injury, third edition. Pediatr Crit Care Med. 2019;20:S1–S82. https://doi.org/10.1097/PCC.0000000000001735.
Allen BB, Chiu Y, Gerber LM, et al. Age-specific cerebral perfusion pressure thresholds and survival in children and adolescents with severe traumatic brain injury. Pediatr Crit Care Med. 2014;15:62–70. https://doi.org/10.1097/PCC.0b013e3182a556ea.
Planiol T, Pourcelot L, Pottier JM, Degiovanni E. [Study of carotid circulation by means of ultrasonic methods and thermography]. Rev Neurol (Paris). 1972;126:127–41.
Gosling R, King D. Arterial assessment by Doppler-shift ultrasound. Proc Roy Soc Med. 1974;67:447–9.
Cardim D, Robba C, Bohdanowicz M, et al. Non-invasive monitoring of intracranial pressure using transcranial Doppler ultrasonography: is it possible? Neurocrit Care. 2016;25:473–91. https://doi.org/10.1007/s12028-016-0258-6.
Figaji AA, Zwane E, Fieggen AG, et al. Transcranial Doppler pulsatility index is not a reliable indicator of intracranial pressure in children with severe traumatic brain injury. Surg Neurol. 2009;72:389–94. https://doi.org/10.1016/j.surneu.2009.02.012.
O’Brien NF, Maa T, Reuter-Rice K. Noninvasive screening for intracranial hypertension in children with acute, severe traumatic brain injury. J Neurosurg Pediatr. 2015;16:420–5. https://doi.org/10.3171/2015.3.PEDS14521.
Vieira F, Cardoso K, Abecasis F, et al. Doppler transcraniano na monitorização do traumatismo craniencefálico grave em pediatria. Acta Pediátrica Port. 2012;43:239–45.
Melo JRT, Di Rocco F, Blanot S, et al. Transcranial Doppler can predict intracranial hypertension in children with severe traumatic brain injuries. Childs Nerv Syst. 2011;27:979–84. https://doi.org/10.1007/s00381-010-1367-8.
Schmidt B, Czosnyka M, Raabe A, et al. Adaptive noninvasive assessment of intracranial pressure and cerebral autoregulation. Stroke. 2003;34:84–9. https://doi.org/10.1161/01.STR.0000047849.01376.AE.
Kashif FM, Verghese GC, Novak V, et al. Model-based noninvasive estimation of intracranial pressure from cerebral blood flow velocity and arterial pressure. Sci Transl Med. 2012;4:129ra44-129ra44. https://doi.org/10.1126/scitranslmed.3003249.
Cardim D, Schmidt B, Robba C, et al. Transcranial Doppler monitoring of intracranial pressure plateau waves. Neurocrit Care. 2016;1–9 https://doi.org/10.1007/s12028-016-0356-5.
Abecasis F, Oliveira V, Robba C, Czosnyka M. Transcranial Doppler in pediatric emergency and intensive care unit: a case series and literature review. Childs Nerv Syst. 2018;34:1465–70. https://doi.org/10.1007/s00381-018-3877-8.
Robba C, Bacigaluppi S, Cardim D, et al. Non-invasive assessment of intracranial pressure. Acta Neurol Scand. 2015; https://doi.org/10.1111/ane.12527.
Zhang X, Medow JE, Iskandar BJ, et al. Invasive and noninvasive means of measuring intracranial pressure: a review. Physiol Meas. 2017;38:R143–82.
Abecasis F, Cardim D, Czosnyka M, et al. Transcranial Doppler as a non-invasive method to estimate cerebral perfusion pressure in children with severe traumatic brain injury. Childs Nerv Syst. 2020;36:125–31. https://doi.org/10.1007/s00381-019-04273-2.
de Riva N, Budohoski KP, Smielewski P, et al. Transcranial Doppler pulsatility index: what it is and what it isn’t. Neurocrit Care. 2012;17:58–66. https://doi.org/10.1007/s12028-012-9672-6.
Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med. 2006;34:1783–8. https://doi.org/10.1097/01.CCM.0000218413.51546.9E.
Radolovich DK, Aries MJH, Castellani G, et al. Pulsatile intracranial pressure and cerebral autoregulation after traumatic brain injury. Neurocrit Care. 2011;15:379–86. https://doi.org/10.1007/s12028-011-9553-4.
Chaiwat O, Sharma D, Udomphorn Y, et al. Cerebral hemodynamic predictors of poor 6-month Glasgow outcome score in severe pediatric traumatic brain injury. J Neurotrauma. 2009;26:657–63. https://doi.org/10.1089/neu.2008.0770.
Czosnyka M, Smielewski P, Kirkpatrick P, et al. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–34. https://doi.org/10.1161/01.STR.27.10.1829.
Zeiler FA, Smielewski P. Application of robotic transcranial Doppler for extended duration recording in moderate/severe traumatic brain injury: first experiences. Crit Ultrasound J. 2018;10:16. https://doi.org/10.1186/s13089-018-0097-0.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Abecasis, F. (2022). Traumatic Brain Injury – Pediatric. In: Ziai, W.C., Cornwell, C.L. (eds) Neurovascular Sonography . Springer, Cham. https://doi.org/10.1007/978-3-030-96893-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-96893-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-96892-2
Online ISBN: 978-3-030-96893-9
eBook Packages: MedicineMedicine (R0)