Abstract
Introduction
Cerebral perfusion pressure (CPP) is one of the most important parameters in preventing ischemic brain insults. Guidelines have used CPP values to guide treatment of traumatic brain injury (TBI) for many years. We tested the feasibility of a novel non-invasive method for CPP estimation (nCPP) in children with severe TBI.
Methods
Retrospective analysis of prospectively monitored pediatric TBI patients with invasive intracranial pressure (ICP) monitoring, arterial blood pressure, and Transcranial Doppler (TCD) studies was performed daily. A novel estimator of CPP (nCPP) was calculated using TCD-spectral accounting method. We analyzed the correlation coefficient and correlation in time domain between CPP and nCPP, prediction ability of nCPP to detect low CPP, and the confidence intervals for CPP prediction (95% CI).
Results
We retrospectively analyzed 69 TCD recordings from 19 children (median age 15 years, range 3–16 years). There was a good correlation between CPP and nCPP (Spearman correlation coefficient, R = 0.67 (p < 0.0001), and a good mean correlation in time domain (R = 0.55 ± 0.42). The ability of nCPP to predict values of CPP below 70 mmHg was excellent as demonstrated by an area under the curve of 0.908 (95% CI = 0.83–0.98) using a receiver operating curve analysis. Bland-Altman analysis revealed that nCPP overestimated CPP by 19.61 mmHg with a wide 95% CI of ± 40.4 mmHg.
Conclusions
nCPP monitoring with TCD appears to be a feasible method for CPP assessment in pediatric TBI. The novel spectral CPP tested in this study has a decent correlation with invasive CPP and can predict low CPP with excellent accuracy at the 70-mmHg threshold.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maintaining an adequate cerebral blood flow (CBF) after a traumatic brain injury (TBI) is one of the most important goals to avoid secondary damage to the injured brain. Guidelines have traditionally used intracranial pressure (ICP) monitoring and treatment of increased ICP as the main objective to improve outcome following TBI [1]. Although this is a standard of care in developed countries, a recent randomized study failed to show a difference in outcome when patients with TBI were randomly assigned to invasive ICP monitoring vs clinical and radiological assessment [2]. In children, evidence is even less robust and adequate randomized controlled studies to evaluate the role of ICP monitoring and treatment have not been performed. There is evidence that sustained ICP > 20 mmHg is associated with poor outcome, but there is no data to support an absolute ICP target in children with TBI [3].
Cerebral perfusion pressure (CPP) is defined as the difference between mean arterial pressure (ABP) and mean ICP, and it is the pressure gradient driving cerebral blood to flow. In normal conditions, CBF is autoregulated to maintain an adequate oxygen and glucose delivery to the brain across physiological range of CPP. After TBI, cerebral autoregulation might be impaired and decreases in CPP could lead to cerebral ischemia. Thresholds for adequate CPP in children with TBI have recently been published suggesting that CPP targets should be age-specific [4]. This report, from Allen and colleagues (2014), describes a new method to establish a goal of CPP above specified thresholds (above 40 mmHg in children under 6 years old and above 50 mmHg in children from 6 to 17 years old).
If CPP is the driving pressure of CBF, it is logical that treatment protocols should focus on CPP, first of all and then on ICP. CPP can be manipulated by changing ICP or ABP. Both strategies can be used depending on the clinical situation, but ultimately, the goal should be to maintain an adequate CBF by maintaining adequate CPP and avoiding intracranial hypertension.
In children, invasive ICP-CPP monitoring is reserved for patients in whom the severity of the clinical conditions demands ICP-CPP-guided treatment. Otherwise, the risks associated with invasive neuromonitoring, such as bleeding and infection [3], may not represent a beneficial intervention.
In these cases, non-invasive methods for assessment of these parameters could offer an alternative for treatment or a screening tool to determine the need for invasive monitoring. Among the several non-invasive methods reported for CPP assessments (nCPP) [5,6,7], ultrasound-based alternatives are of special interest since these techniques are low-cost and widely available in the neurocritical care settings. Transcranial Doppler ultrasonography (TCD) has been one of the most used methods for nCPP in TBI [6]. Several studies have tested the feasibility of TCD for these purposes in children [8, 9]. There are contrasting results on the application of the TCD-derived pulsatility index for predicting cerebral hypoperfusion. Nevertheless, other studies on mathematical models for continuous non-invasive ICP prediction using simultaneous measurements of systemic arterial blood pressure and transcranial Doppler flow velocity waveforms have shown better ability of TCD to estimate and track ICP changes [10,11,12].
The aim of our study is to test the feasibility and accuracy of a novel TCD-based method for nCPP assessment in children with TBI to estimate CPP absolute values and changes of CPP over time.
Methods
Patients
We retrospectively studied digital recordings of MCA flow velocity, ICP, and arterial blood pressure from pediatric TBI patients admitted to Addenbrooke’s Hospital, Cambridge, UK, and recruited prospectively between 1992 and 2009 to a project aimed at daily assessment of cerebral autoregulation in children with severe TBI. All patients presenting with severe (admission Glasgow Coma Score (GCS) < 8) or moderate (admission GCS < 12) traumatic brain injury (TBI), with secondary neurological deterioration requiring intubation and mechanical ventilation, were eligible for inclusion in this study. All patients were sedated and paralyzed as per relevant time-matching local guidelines for management of TBI in children.
TBI data collection was approved by the Institutional Review Board (REC 97/290, 1997). For patients recruited before 1997, The Neurosciences Users’ Committee allowed TCD examinations for the assessment of TBI patients. Further use of the anonymized data was allowed as a part of clinical audits.
Monitoring and data analysis
ABP was measured directly from the radial artery calibrated at the level of the heart (Baxter Health Care Corp., CardioVascular Group). ICP was monitored continuously using a microtransducer placed in the brain parenchyma (MicroSensors ICP Transducer; Codman and Shurtleff, Inc.). CPP was calculated as the difference between the mean ABP and ICP.
Arterial cerebral blood flow velocity (CBFVa) was obtained bilaterally from the left and right middle cerebral arteries (MCA) by using a TCD ultrasonography system (DWL Multidop X4, DWL Elektronische Systeme GmbH), with the probe held in place during the entire recording using a head frame provided by the TCD device manufacturer. Mean CBFVa was calculated as the average between left and right CBFVa.
Raw signals were digitized using an analog–digital converter (DT 2814, Marlborough, CA, USA) sampled at a frequency of 100 Hz and recorded with ICM+® (Cambridge Enterprise, https://icmplus.neurosurg.cam.ac.uk/). The recorded signals were subjected to manual artifacts removal and analyzed with ICM+®. All parameters were calculated and averaged. All calculations were performed over a 10-s long-sliding window.
Non-invasive CPP calculation
Formula was created using arithmetics described previously [13]:
where a1 represents the pulse amplitude of the first harmonic of the ABP waveform, sPI denotes the spectral pulsatility index; CVR, cerebrovascular resistance; Ca, compliance of arteries and arterioles; HR, heart rate (expressed in Hz). A detailed description of the spectral model development and formula is provided in the appendix.
Statistical analysis
The analysis of the data was conducted with R Studio software (R version 3.4.1). Multiple recordings were considered as independent events. Data were tested for normal distribution using the Shapiro-Wilk test and are presented as median and interquartile range. All parameters assessed were non-parametric in nature.
To assess the performance of the proposed method, the correlation between CPP and the nCPP were verified using the Spearman correlation coefficient (R, with the level of significance set at 0.05), as well as the correlation coefficient in the time domain during monitoring period.
The Bland-Altman method was used to determine the agreement between absolute values of invasive CPP and nCPP, with the respective bias and 95% confidence intervals (CI) for CPP prediction.
The area under the curve (AUC) of the receiver operating characteristic (ROC) curve was performed to determine the ability of nCPP to detect low CPP. Different low CPP thresholds of 50, 60, and 70 mmHg were tested, according to Allen et al.’s established age-specific CPP thresholds for TBI patients [4]. The prediction ability is considered reasonable when the AUC is higher than 0.7, strong when the AUC exceeds 0.8 and excellent when the AUC exceeds 0.9 [14].
Results
Nineteen patients were included in the study. Their median age was 15 years (interquartile range (IQR), 13–16; range 3–16 years; 63% males). We analyzed 69 TCD recordings from these patients and all other variables collected simultaneously during that period.
In Table 1, we present the median (IQR) values of the neurophysiologic variables analyzed. The percentage of measurements presenting low CPP at the threshold ≤ 50 mmHg was 10% (N = 7), at ≤ 60 mmHg was 28% (N = 19), and at ≤ 70 mmHg was 52% (N = 36). The median initial GCS score was 6.5 (IQR, 5–7; range 3–9).
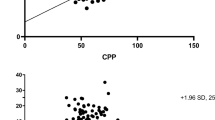
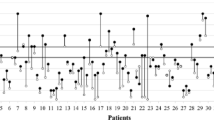
There was a good correlation between nCPP and invasive CPP (R = 0.67 [p < 0.0001]) (Fig. 1). The averaged correlation in the time domain between CPP and nCPP was R = 0.55 ± 0.42 (95% CI, 0.45–0.65). There were three cases where the correlation in time domain was almost perfect (R > 0.97) (Fig. 2). The accuracy of the method obtained with Bland-Altman analysis revealed not only a wide 95% CIs for prediction of ± 40.4 mmHg but also a negative bias of − 19.61 (Fig. 3), means that nCPP overestimated true CPP by about 20 mmHg.
The ability of nCPP to predict CPP ≤ 70 mmHg was excellent, presenting an AUC = 0.91 (95% CI, 0.83–0.99). The optimal cut-off values for prediction of a CPP < 70 mmHg was a nCPP of 73.95 mmHg. The sensitivity and specificity and positive and negative predictive powers for this threshold were 0.46 and 0.97 and 0.94 and 0.63 respectively (Fig. 4). This means that a nCPP < 74 mmHg very likely predicts a CPP < 70 mmHg.
The ability of nCPP to predict at the thresholds of 60 and 50 mmHg was also good (AUC = 0.80 and AUC = 0.86 respectively), but there were only five episodes with nCPP < 60 mmHg (Table 2).
Discussion
This is, to our knowledge, the first study to evaluate the accuracy of TCD in estimating CPP in a cohort of children with severe TBI. We have demonstrated that it can predict CPP values below 70 mmHg and that it also has a good correlation in time domain. This means that TCD-based nCPP allows for monitoring of the patients’ CPP over time and detects improvement or deterioration. It can also aid in assessing response to treatment or other interventions.
The threshold of 70 mmHg for CPP is much higher than the current guidelines recommendations for children. Nonetheless, some of our patients were treated before current guidelines were issued and a higher CPP was targeted. We tested three different thresholds of nCPP: 50, 60, and 70 mmHg. They all had a good agreement with CPP, but the performance of our new model was best at the higher threshold. This does not necessarily have a clinical implication and that was not the aim of this study. There are recent studies about cerebral autoregulation and optimal CPP in children with TBI suggesting that higher CPP targets could be linked to better outcomes [15], but more data are needed to define the best strategy and CPP goals in the treatment of pediatric TBI.
Unfortunately, our results also demonstrate that currently the accuracy of this method to estimate absolute CPP values is not good enough to be applied in the clinical practice and to substitute invasive measurement of CPP, since nCPP values overestimated CPP values by about 20 mmHg. We believe that more data are needed to test this technique and to make the model more accurate. With larger sample sizes it could be possible to improve the model taking into account this difference and adding a correction factor to the formula.
Nonetheless, for clinical decisions, measuring the absolute value of nCPP is not the only thing that matters; its trend and how it changes in response to treatment or insults seems equally important. With our study of correlations in time domain, we prove that this can be done and in some cases with excellent results (Fig. 2).
Another objective of our study was to test the prediction ability of nCPP to detect low CPP. For a threshold of 70 mmHg, results were excellent with an AUC of 0.91. This test was very specific (97%) and with very high positive predictive value (94%). Although the values of the AUC for thresholds of 60 and 50 mmHg were also good, and are clinically more relevant to the situation of children below the age of 13 years, we cannot generalize the results because of the low number of episodes with nCPP < 60 mmHg. As this is a retrospective data from a historical cohort, the target CPP limits used were higher than used in current guidelines to manage TBI in children; it is difficult to extrapolate these results to present day CPP targets. We do however prove feasibility of the equation to predict nCPP in this small cohort of children with TBI. Further work is required to see if nCPP predictions would be still applicable to lower CPP in the range of 40–60 mmHg to match the current thresholds used clinically.
Impaired autoregulation is associated with a poor prognosis, and observational data suggests that optimal neurologic outcome and survival are associated with optimal perfusion pressure both in pediatric and adult population [16, 17].
Thresholds of ICP and CPP are not clear and might be dependent on the autoregulatory state of the patients [18]. However, no randomized, controlled, interventional data are available to assess autoregulation monitoring after pediatric traumatic brain injury.
Non-invasive assessment of CPP and therefore of ICP has been more widely studied in the adult population using TCD [19]. In a series of 25 consecutive patients with head injury, non-invasive CPP was calculated as “ABP × FVd/FVm + 14” (FVd, FVm diastolic, and mean CBFVa, respectively) and compared with invasive CPP. The absolute difference between direct CPP and nCPP was less than 10 mmHg in 89% of measurements and less than 13 mmHg in 92% of measurements. The 95% confidence range for predictors was no wider than ± 12 mmHg [20].
In order to use nCPP in clinical practice, the values must be calculated in real-time analysis. This can be done with ICM+ software. As shown in the time domain analysis examples (Fig. 4), one can detect changes in CPP by following the trends of nCPP. This is particularly valuable in patients without invasive ICP monitoring. There are two potential groups of patients who could benefit from non-invasive CPP monitoring; patients with a contraindication to invasive ICP monitoring (like coagulopathy or scalp infection) and patients without a clear indication for invasive monitoring who could potentially benefit from it (moderate TBI with potential to deteriorate, patients with TBI, and confounders that prevent adequate neurological assessment like alcohol or drugs intake or previous neurological lesions). Another reason to use nCPP would be centers without neurosurgery specialists or expertise in invasive ICP monitoring.
Our study has some limitations. There were a low number of episodes with CPP below 60 mmHg in this cohort. Treatment protocols used in these patients focused on maintaining an appropriate CPP and episodes of low CPP were aggressively treated. The number of TCD recordings was adequate, but the total number of patients is small so that no speculations regarding lower CPP thresholds used currently in children can be made. According to data from the Centers for Disease Control and Prevention (https://www.cdc.gov/traumaticbraininjury/data/rates_hosp_byage.html), TBI in children is steadily decreasing over the years. A multicenter project would allow collection of more robust data and generalization of the model proposed in the current study.
Conclusions
Non-invasive CPP monitoring with TCD appears to be a feasible method for CPP assessment in pediatric patients with TBI. The novel spectral CPP tested in this study has a decent correlation with invasive CPP and can predict low CPP with excellent accuracy at the 70-mmHg threshold.
Further work is required to test the non-invasive CPP predictions using this equation for CPP thresholds below 70 mmHg.
References
Carney N, Totten AM, OʼReilly C, et al (2017) Guidelines for the management of severe traumatic brain injury, Fourth Edition. Neurosurgery 80:6–15. https://doi.org/10.1227/NEU.0000000000001432
Kochanek PM, Adelson PD, Ashwal S, Bell MJ, Bratton S, Carson S, Chesnut RM, Ghahar J, Goldstein B, Grant GA, Kissoon N, Peterson K, Selden NR, Tong KA, Tasker RC, Vavilala MS, Wainwright MS, Warden CR (2012) Chapter 11. Barbiturates. Pediatr Crit Care Med 13:S49–S52. https://doi.org/10.1097/PCC.0b013e31823f672e
Kochanek PM, Carney N, Adelson PD et al (2012) Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med 13(Suppl 1):S1–S82. https://doi.org/10.1097/PCC.0b013e31823f435c
Allen BB, Chiu Y, Gerber LM, Ghajar J, Greenfield JP (2014) Age-specific cerebral perfusion pressure thresholds and survival in children and adolescents with severe traumatic brain injury. Pediatr Crit Care Med 15:62–70. https://doi.org/10.1097/PCC.0b013e3182a556ea
Robba C, Bacigaluppi S, Cardim D, Donnelly J, Bertuccio A, Czosnyka M (2015) Non-invasive assessment of intracranial pressure. Acta Neurol Scand 134:4–21. https://doi.org/10.1111/ane.12527
Cardim D, Robba C, Bohdanowicz M, Donnelly J, Cabella B, Liu X, Cabeleira M, Smielewski P, Schmidt B, Czosnyka M (2016) Non-invasive monitoring of intracranial pressure using transcranial Doppler ultrasonography: is it possible? Neurocrit Care 25:473–491. https://doi.org/10.1007/s12028-016-0258-6
Zhang X, Medow JE, Iskandar BJ, Wang F, Shokoueinejad M, Koueik J, Webster JG (2017) Invasive and noninvasive means of measuring intracranial pressure: a review. Physiol Meas 38:R143–R182
Figaji AA, Zwane E, Fieggen AG, Siesjo P, Peter JC (2009) Transcranial Doppler pulsatility index is not a reliable indicator of intracranial pressure in children with severe traumatic brain injury. Surg Neurol 72:389–394. https://doi.org/10.1016/j.surneu.2009.02.012
Melo JRT, Di Rocco F, Blanot S et al (2011) Transcranial Doppler can predict intracranial hypertension in children with severe traumatic brain injuries. Childs Nerv Syst 27:979–984. https://doi.org/10.1007/s00381-010-1367-8
Schmidt B, Czosnyka M, Raabe A, Yahya H, Schwarze JJ̈, Sackerer D, Sander D, Klingelhöfer J̈ (2003) Adaptive noninvasive assessment of intracranial pressure and cerebral autoregulation. Stroke 34:84–89. https://doi.org/10.1161/01.STR.0000047849.01376.AE
Kashif FM, Verghese GC, Novak V, Czosnyka M, Heldt T (2012) Model-based noninvasive estimation of intracranial pressure from cerebral blood flow velocity and arterial pressure. Sci Transl Med 4:129ra44–129ra44. https://doi.org/10.1126/scitranslmed.3003249
Cardim D, Schmidt B, Robba C, Donnelly J, Puppo C, Czosnyka M, Smielewski P (2016) Transcranial Doppler monitoring of intracranial pressure plateau waves. Neurocrit Care 26:1–9. https://doi.org/10.1007/s12028-016-0356-5
de Riva N, Budohoski KP, Smielewski P, Kasprowicz M, Zweifel C, Steiner LA, Reinhard M, Fábregas N, Pickard JD, Czosnyka M (2012) Transcranial Doppler pulsatility index: what it is and what it isn’t. Neurocrit Care 17:58–66. https://doi.org/10.1007/s12028-012-9672-6
Hosmer D, Lameshow S (1989) Applied logistic regression. John Wiley & Sons, New York
Hockel K, Diedler J, Neunhoeffer F, Heimberg E, Nagel C, Schuhmann MU (2017) Time spent with impaired autoregulation is linked with outcome in severe infant/paediatric traumatic brain injury. Acta Neurochir 159:2053–2061. https://doi.org/10.1007/s00701-017-3308-8
Donnelly JE, Young AMH, Brady K (2017) Autoregulation in paediatric TBI—current evidence and implications for treatment. Childs Nerv Syst 33:1735–1744. https://doi.org/10.1007/s00381-017-3523-x
Aries MJH, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, Hutchinson PJ, Brady KM, Menon DK, Pickard JD, Smielewski P (2012) Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury*. Crit Care Med 40:2456–2463. https://doi.org/10.1097/CCM.0b013e3182514eb6
Flechet M, Meyfroidt G, Piper I, et al (2018) Visualizing cerebrovascular autoregulation insults and their association with outcome in adult and paediatric traumatic brain injury. In: Heldt T. (eds) Intracranial Pressure & Neuromonitoring XVI. Acta Neurochirurgica Supplement, vol 126. Springer, Cham. https://doi.org/10.1007/978-3-319-65798-1_57
Rasulo FA, Bertuetti R, Robba C, Lusenti F, Cantoni A, Bernini M, Girardini A, Calza S, Piva S, Fagoni N, Latronico N (2017) The accuracy of transcranial Doppler in excluding intracranial hypertension following acute brain injury: a multicenter prospective pilot study. Crit Care 21:44. https://doi.org/10.1186/s13054-017-1632-2
Schmidt EA, Czosnyka M, Gooskens I et al (2001) Preliminary experience of the estimation of cerebral perfusion pressure using transcranial Doppler ultrasonography. J Neurol Neurosurg Psychiatry 70:198–204
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MC has financial interest in a part of licensing fee for ICM+ software (Cambridge Enterprise LTd, UK).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Francisco Abecasis and Danilo Cardim share first authorship.
Electronic supplementary material
ESM 1
(DOCX 2642 kb)
Rights and permissions
About this article
Cite this article
Abecasis, F., Cardim, D., Czosnyka, M. et al. Transcranial Doppler as a non-invasive method to estimate cerebral perfusion pressure in children with severe traumatic brain injury. Childs Nerv Syst 36, 125–131 (2020). https://doi.org/10.1007/s00381-019-04273-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04273-2