Abstract
Toxicity of nanoparticles in the aquatic environment is of serious concern as increasing concentration of nanoparticles potentially affects the aquatic plants and animals living in the aquatic ecosystem. Engineered nanoparticles (ENPs) are derived from anthropogenic sources, which are highly stable and uniform in distribution. In the aquatic environment, there is an alarming situation and indefinite safety use for the ENPs. The ENPs interact with aquatic organisms at trophic levels (lower and upper levels) throughout the aquatic food chain. Advancement is rendered in the evaluation of bioaccumulation in recent years, and the transfer in trophic level of ENPs. While findings of numerous studies carried out in different locations of the world have proved the noxious consequences of nanomaterials upon the organism's in the aquatic environment as well as in what manner they impact food chain resulting in bioaccumulation, affecting marine animals’ wellbeing, development, reproduction, and physiology. We are exploring the nanotoxicity in the aquatic food chain and aquatic species, trophic transition, and biomagnification in this chapter. The critical points of the study are that ENPs are able to go up to three trophic stages in the aquatic food chain. Biomagnification of various nanoparticles (quantum dots, nAu, nCeO2 and nTiO2) fit for two trophic levels have a biomagnification ratio greater than one. Not many studies on the third trophic stage nevertheless demonstrated biomagnification. The deposition of ENPs in aquatic plants and animals has also been shown to affect physiological processes of different organisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Aquatic systems
- Bioaccumulation
- Environment
- Nanoparticles
- Phytoplankton
- Reactive oxygen species
- Trophic levels
1 Introduction
Nanotechnology is reportedly expected to hit a market size of $3 trillion by 2020. In the consumer market, more than 1800 nano-enabled items are now available. There has been a tremendous progress in nanoscience and nanotechnology field over the past decade, with nanomaterials being utilized in a wide-ranging field, including business, chemistry, healthcare, medicine, fabric textiles, forestry, wastewater management electronics as well as communications devices (Walters et al. 2016; Bundschuh et al. 2018). Therefore, unintentional liberation of engineered nanoparticles (ENPs) is initiated around the environment, predominantly in waters. On lower and upper trophic stages, ENPs may communicate with food chain species. Advancement has taken place on bioaccumulation evaluation and trophic transition of ENPs in recent years. The released ENPs from nano-enabled products during their life cycle raised environmental health and safety issues.
Nonetheless, ample evidence can be found in recent research articles upon the ecological influences of ENPs (Adiloğlu et al. 2012; Holden et al. 2016; Zhang et al. 2018; Abbas et al. 2020; Attarilaret al. 2020) supporting the forthcoming impacts of ENPs to damage aquatic organisms if existing in abundantly higher concentrations. A previous report by Shi et al. (2013) revealed a massive consumption of ENPs engrained out the toxicological properties in the aquatic ecosystem (Salieri et al. 2015), causing prominent harm to aquatic biota. Studies have shown that the toxicity precisely associated with ZnO-NPs is due to buildup of Zn+2 ions in the water environment (Brun et al. 2014; Zhang et al. 2018). In this chapter, we emphasize on the ecotoxicological consequences of nanoparticles on the aquatic food chain, especially aquatic ecosystems (in plants, marine invertebrates, and fish).
2 Nanotoxicology in the Aquatic Food Chain
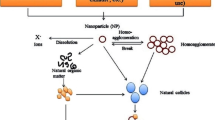
Natural nanoparticles have existed in the atmosphere naturally since centuries while man-made NPs due to their specific surface interactions and properties are related with the design of ENPs providing them different physico-chemical and toxicological characters in contrast to naturally occurring NPs (Handy et al. 2008). Nanotoxicology is a modern and developing research field in toxicology on nanomaterials (Walters et al. 2016; Bundschuh et al. 2018). Evaluating toxicological assets of nanoparticles (NPs) to know if it may pose a threat to the atmosphere or society and its extent is covered in nanotoxicology studies. Nanoparticles toxicity is considered to have a significant impact on plants, animals, and marine organisms (Fig. 1). Indeed, many major chemical manufacturers who produce NPs, discharge effluent into the ocean or rivers. Massive damage to humans and the environment is now happening and is expected to grow significantly. Nanotechnology advancement has not succeeded devoid of questions about its prospective detrimental ecological influences. Much is still unclear, however. Altered interactions with ENPs entail sedimentation, degradation, agglomeration, or else chemical transition, in the same way as absorption plus conversion in the food ecosystem (Vázquez Núñez and De la Rosa-Álvarez 2018).
2.1 Sources of Nanomaterials
Broad classes of substances that consist of particulate elements are called nanoparticles (NPs, which are having one dimension less than 100 nm at the minimum (Laurent et al. 2010; Khan et al. 2019). Nanomaterials are classified into naturally produced nanomaterials that are found in the organism’s body. Further, NPs can be studied under the subgroup of naturally occurring NPs categories based on their origin as they are created by the way as a consequence of engineering activities like vehicle engine exhaust, soldering emissions, ignition activities and forest fires etc. Engineered nanoparticles (ENPs) are man-made having properties for desired applications (Jeevanandam et al. 2018). ENPs include numerous metals based NPs such as fumed silica, titanium dioxide, carbon black, iron oxide, carbon nanotubes (CNTs), etc. (Hristozov and Malsch 2009). Metal nanoparticles (MNPs) possess specific characteristics and have size smaller than twenty to thirty nm. This usually creates additional energy on the exterior of the particles, which makes them extraordinarily reactive and thermodynamic. The scale is also a key factor in reactivity, distribution and toxicity of nanoparticles.
2.2 Physicochemical Properties of Engineered Nanoparticles Influencing Their Toxicity
Engineered nanoparticles (ENPs) may have the probability of toxicity risk. Still, it is also dependent on (a) amount and extent of exposure, (b) integral and inherent nanoparticles toxicity, (c) persistence in body of the nanoparticles, and (d) susceptibility of the organism (Dhasmana et al. 2017). Nanoparticle toxicity is primarily based on properties like (Fig. 2): (i) outer layer of the surface can change the physico-chemical properties of nanoparticles and consequently influence noxiousness, (ii) total area of the surface is amplified with the rise in the chemical activity of nanoparticles and is also a significant factor accountable for toxicity, (iii) composition of nanoparticle (chemically) and toxicity is dependent upon the phase of nanoparticles, i.e. the chemistry and crystalline, (iv) size: smaller size from the same material will be more toxic than bulk, and larger particles, (v) interface along with toxins accessible in the water, and (vi) ENPs functional behaviour (Walters et al. 2016; Dhasmana et al. 2017; Mahaye et al. 2017). The active behaviour of ENPs are the dissolution of ENPs and generation of reactive oxygen species (ROS) into metal ions in the water (Mahaye et al. 2017). Ionic structure of metals endures being less lethal than nanomaterials (Bielmyer et al. 2006; Batley et al. 2013). Higher levels of ENPs of nearly one mg L−1 was stated as the precise cause for death than small levels of about 5–50 μg L−1 which caused physical alterations, chromosomal modifications and oxidative strain (Bystrzejewska-Piotrowska et al. 2009; Barbara Rasco 2013).
Properties of nanomaterials influencing their toxicity (based on Turan 2019)
2.3 Transformation Processes
The reliant on the inbuilt assets and on water properties dispersed by nanoparticles are subjected to several conversion pursuits. The key routes are physical, biological or else, chemical alterations that later outline the performance of nanoparticles in the aqueous system (Stone et al. 2010; Lowry et al. 2012). Hetero and homo aggregation, deposition, agglomeration, as well as sedimentation, are some of the physical processes, whereas suspension and redox effects (oxidation, sulfidation) and photochemical reaction are chemical processes. The chief instances of the biological processes are microbial mediated biodegradation and bio-modification activities (Lead et al. 2018). The kind of nanoparticles and factors such as the chemistry of water, pH, the strength of ion and natural organic matter (NOM) make a difference in transformations. Outcome and behaviour in water will be affected by the collaboration of distributed nanoparticles with NOM according to the properties of surface establishing a diverse natural coating (Biswas and Sarkar 2019).
The reduction and oxidation processes are outlined by electron transfer among the chemical moieties in the environment. Reduction and oxidation processes are commenced by silver and iron (Shah et al. 2015). There is ample oxygen in the oxidizing natural environment, e.g. aerated soils as well as natural waters, whereas the reductive ecosystem is drained of oxygen (Lowry et al. 2012). Sunlight-catalyzed redox reactions like photooxidation and photoreduction alter oxidation status of nanoparticles, persistence, ROS, and coating. For example, it was observed that TiO2 and carbon nanotubes (CNTs) are instinctively photoactive and capable of generating ROS (Chen and Jafvert 2011). Dissolution and sulfidation processes have significant impacts on the surface properties, persistence and toxicity of the nanomaterials (Levard et al. 2011). Adsorption of inorganic and organic ligands and macromolecules on NPs alter the behaviour and exterior interface of NPs substantially.
A physical change like aggregation is an unalterable process that reduces the surface area, the surge in NPs size altering, in turn, their reactivity, transport, sedimentation and toxicity. Consequently, reduction in surface area of the NPs leads to the decrease in toxicity which in turn alters ROS generation or dissolution (Nichols et al. 2002; Oberdörster et al. 2006; Sellers et al. 2008; Aitken et al. 2010; Lowry et al. 2012; Rist and Hartmann 2018). The photocatalytic reactions in the presence of sunlight resulted in lowering of the pH of the medium which further resulted in high ionic strength and presence of divalent ions (Hartmann et al. 2014; Yin et al. 2015). Porous aggregates can be available as sediment rather than compact ones that remain suspended in water due to erosion and disaggregation processes that create smaller pieces which consumes natural organic matter (NOM) around them (Chekli et al. 2015). The redox reactions change coating, nanoparticles’ reactivity, toxicity, surface charging and aggregation state properties which change these transformations (Lowry et al. 2012). Biotransformation on modified NPs of the bioavailable poly(ethylene glycol) (PEG) coatings initiates their aggregation (Kirschling et al. 2011). Due to exclusive change in seawater and freshwater at high dilutions, toxicity of ENPs in all aquatic habitats is not consistent (Renzi and Guerranti 2015; Ju-Nam and Lead 2008).
2.4 Pathway of Exposure in the Aquatic Environment
Fundamental mechanisms of toxicity for numerous nanoparticles are studied in vitro at the cellular level to oxidative stress. Oxidative stress creates reactive oxygen in species (Oberdorster et al. 2005; Nel et al. 2006). Physical injury to cell membranes may cause toxicity (Stoimenov et al. 2002). Route of uptake is by adhesion of nanoparticles to the cell coat and disconnection of soluble toxic species (Klaine et al. 2008). The type of organisms, uni- or multicellular level and its trophic level determines the absorption of nanoparticles and its toxicity in aquatic biota. For example, the mechanism of crossing the cell membrane (viz. direct or via endocytosis) in unicellular organisms remains a significant issue. However, endocytosis has been observed as the preferred pathway for internalization of nanoparticles in eukaryotic organisms (Moore 2006; Nowack and Bucheli 2007). In the case of higher organisms, the nanoparticles might be absorbed by the gill or the external surface epithelia. In contrast, interaction with the aquatic plants may include root surface adsorption, cell wall integration, or intercellular space diffusion (Nowack and Bucheli 2007). Another contaminant uptake pathway is through the food chain, mostly via direct ingestion. The water fleas (Daphnia magna) ingested and metabolized the lipid-coated nanotubes present in the aquatic system as its normal feeding behaviour (Roberts et al. 2007). Similarly, Bouldin et al. (2008) reported the absorption of quantum dots in the water fleas (Ceriodaphnia dubia) through dietary mechanism from an algal food.
The toxicity of ENPs in aquatic animals is particulate dependent and depends on how they penetrate the cells of the organism (Singh et al. 2011). The technique of the process of entry into the cell starts with their adhesion to the pores of the cell membrane followed by their final entry into the cell by endocytosis or by ion transfer systems (Fig. 3). Interference with the electron transport mechanism or the development of reactive oxygen species (ROS) caused during the entry of ENPs has substantial adverse effects; beginning with cell membrane damage (Ross et al. 2007). The nanoparticles ability to enhance cell damage (by reactive oxygen generation) governs the toxic effects of ENPs in the aquatic system. For example, Smith et al. (2007) demonstrated that the single-walled carbon nanotubes (SWCNTs) increase in oxidative stress and iono-regulatory disturbance in the gut lumen of fish when exposed to sub-lethal concentration for 10-days.
Pathway of exposure in the aquatic environment (based on Walters et al. 2016)
3 Nanotoxicity to Individual Species in Aquatic Food Chain
After the release of nanomaterials in the environment, the aquatic system is the main sink of ENPs. ENPs can influence not only the growth of aquatic species but also the whole ecological equilibrium in the aquatic system. Some studies on nanomaterials and its effect on the aquatic ecosystem have been discussed in the following sub-sections.
3.1 Microbial Toxicity
The consequences of ENPs are of considerable significance in the ecological process. In reaction to high nAg levels, the composition of a bacterial population shifted, while its metabolic processes remained usual (Das et al. 2012). There is significant proof that nanoparticles are moved trophically within the food chain. These hazards were observed in nTiO2 toxicity, where biofilm-accumulated TiO2 was relocated to biofilm-exhausting snails which caused trophic harm (Yeo and Nam 2013; Banerjee and Choudhury 2019). Pakrashi et al. (2014) detected related deteriorating consequences on nAu-exposed algae, carboxyl quantity. Biomagnification of these inter-trophic transitions has also not been observed (Laws et al. 2016). Banerjee and Choudhury (2019) emphasize another hypothesis stating that the potential for transferring ENPs across ecosystem boundaries also lies. ENPs can be transported via floods or evolving insects from the aquatic to the terrestrial ecosystem. This perspective requires confirmation by additional studies.
Engineered nanoparticles (ENPs) also seem to be non-toxic to specific populations of microorganisms, because they are trapped within biofilm's extra polymeric material. Lone organisms, such as leaf dwelling bacteria and fungi, are generally immune to nCuO and nAg. These findings indicate the effects of ENPs across microorganisms on the community and evolution (Bundschuh et al. 2016; Banerjee and Choudhury 2019). The absorption of metal ENPs like ZnO and CuO in water depends on the original scale of the nanoparticles (Hanna et al. 2013). In the case of nAg, the uptake rate was observed to increase with a change in the size of the ENP (Pan et al. 2012; Banerjee and Choudhury 2019). Zhao and Wang (2012) have noted a contrary reverse trend, however. Related data were well accessible (Handy et al. 2008; Klaine et al. 2008). Various nanomaterials, particularly silver, indicate bactericidal properties (Sondi and Salopek-Sondi 2004; Morones et al. 2005; Banerjee and Choudhury 2019). The titanium dioxide also shows strong antimicrobial activity (Wolfrum et al. 2002).
3.2 Toxicity to Aquatic Plants
Less research has been done on the impact of the ENPs on aquatic plants. Synchrotron-based micro X-ray fluorescence mapping and extended X-ray absorption structure spectroscopy revealed deposits of the fraction of Ag2S and silver thiol species in the roots of duckweed after exposure to 24 h of ENP (Stegemeier et al. 2017; Banerjee and Choudhury 2019). The development of Ag derivatives in the plant roots was possibly due to the plant molecular defence system to retort the intake of Ag-ENPs (Stegemeier et al. 2017). Kim et al. (2011) reported hindrances to the development of Lemna paucicostata plants exposed to Ag-ENP (even at a low concentration of 1 ppm) and TiO2-ENP (at a higher concentration of 250 ppm).
3.3 Toxicity to Phytoplankton
Phytoplanktons are an essential means of the marine food web system and are the most significant consumers in aquatic habitats. Where ENPs have significant toxic effects on phytoplankton, the whole ecosystem is affected due to phytoplankton toxicity as they hold crucial importance in the aquatic food chain. The toxicity to phytoplankton and reduction in their growth will automatically allow the entire food system to fail or collapse. Therefore, ecotoxicological studies on phytoplankton are of particular importance for aquatic systems. As expected, the algae were the most sensitive group of aquatic organisms to ENP. It was found that ZnO exhibited maximum toxicity in freshwater plankton Pseudokirchneriella subcapitata among other metal and metal oxide ENPs, with substantial growth reduction (EC50) at 42 mg l−1 (Aruoja et al. 2009; Banerjee and Choudhury 2019). In the marine algae, Thalassiosa pseudonanathe EC50 for ZnO was found to be 4.6 mg L−1 (Wong et al. 2010). Particles of Nano-C60 impaired the growth of P. subcapitata at a concentration of 90 mg L−1 nearly 30%. The C60 ENPs’ contact with the algal cells has facilitated the entrance into the cells of other contaminants. This stimulated more significant damage to algal cells and improved cellular apoptosis (Sigg et al. 2014; Banerjee and Choudhury 2019). Toxic effects of NiO ENPs on the alga Chlorella vulgaris have been tested. The tests showed 32.28 mg L−1 EC50 values with 72 h sensitivity to NiO. NiO toxicity of thylakoid systems in Chlorella vulgaris has caused plasmolysis, cell membrane damage, and disorder. The most alarming discovery was that the NiO effects could be transmitted to herbivores at a higher trophic level, devouring the NiO effect (Gong et al. 2011; Banerjee and Choudhury 2019).
The exposure of nanomaterials to phytoplankton and the deposition in phytoplankton will directly or indirectly impact the whole marine environment because they are the primary consumers of the nutrient in aquatic environments. Phytoplanktons are the primary producers, so the nanocrystals lying on the exteriors of this biota enter up in the food chain. The iron nanoparticles hamper the growth of marine phytoplankton. There is also inhibition of development of marine phytoplankton species Isochrysis galban due to presence of iron nanoparticles (Keller 2012). In photosynthesis of phytoplankton, chlorophyll is of a, b, and c types (Chen et al. 2012). As the Fe3O4 nanoparticle intensity enhanced, the chlorophyll a matter tends to decline in C. vulgaris (Chen et al. 2012). There has been a significant toxic effect of Fe3O4 nanoparticles on CO2 absorption and the net photosynthetic rate. In lipid peroxidation and cellular oxidative, the malondialdehyde (MDA) is an important marker in C. vulgaris, which steadily rises as the Fe3O4 nanoparticle concentration rises (Chen et al. 2012). This has demonstrated that the MDA content in C. vulgaris has been increased due to stress-induced by Fe3O4 nanoparticles (Chen et al. 2012).
3.4 Fish Nanotoxicity
Fish is a common aquatic vertebrate, serving an essential ecological role in aquatic systems. It is also an important food source for humans—a study on the toxicity and behaviour of ENPs in fish directly related to human safety. To forecast the toxicity of a specific material, different stages in the fish life cycle are studied. Harmful ENPs can be highly toxic to many invertebrate organisms, including fish species which are also part of the aquatic food chain. Marine invertebrates such as Hediste diversicolor and Scrobicularia sp. had been chosen for the study of their behavioural and biochemical reactions to Cu NPs. Impaired coping habits were found at Scrobicularia sp. for Cu-ENPs or Cu soluble as well; however, H. diversicolor reflected harmful effects only on soluble Cu. All species showed no variations in their cholinesterasic behaviour, demonstrating that either the Cu-ENPs or the soluble Cu did not induce neurotoxicity (Buffet et al. 2011; Banerjee and Choudhury 2019). When nanoparticles enter their digestive glands and gills, ENPs injure suspension-fed invertebrates and detritivores. ENPs typically reach cells along endocytotic pathways, causing damage to large tissues, particularly in tissues that contain highly phagocytic cells (Moore 2006; Banerjee and Choudhury 2019). The bivalve mollusk is another important invertebrate that can be used for research into the effects of ENPs in both fresh and coastal waters.
The oxidative stress in fish causes toxic possessions in the liver and gills (Aschberger et al. 2011). Cu-ENP in zebrafish triggered damage to gills and may cause severe, dangerous effects (Griffitt et al. 2007). Gills and liver were having Ag-ENPs as well as Cu-ENPs of main targets for accumulation as investigated by histological tests, interpreting these nanoparticles tremendously poisonous to zebrafish as the concentration of LC50 was 1.5 mg L−1 for 48 h (Bilberg et al. 2010; Sigg et al. 2014). ZnO-ENPs and ZnO microparticles showed a dose-dependent effect in the degree of injury, though Al2O3 and TiO2-ENPs did not cause any substantial harm (Sigg et al. 2014). Nano-C60 and nano-C70 particles in zebrafish embryos also showed the same impacts (Usenko et al. 2008; Vieira et al. 2009; Sigg et al. 2014). Nanoparticle ecotoxicity on fish is significant since fish are the primary species in the aquatic environment as well as potent bioindicators of environmental waste and toxicology studies. Daphnia magna can filter and feed on synthesized particles ranging from 0.4 to 40 μm (e.g. algal cells, bacteria, and other organic or inorganic particles) (Xu et al. 2019). Thus, it is inevitable that NPs may enter into the body of D. magna as food. Indeed, uptake of NPs has been found in many reports. Another comparative study reported was done on nanotoxicity of metals on zebrafish. 48 h exposure of Cu on zebrafish eggs revealed deformity and late hatching, although no teratogenic effects for a similar time under Au–NPs was observed. The indicator for toxicant contact is done on model water fleas of genus Daphnia members. In supplement, to the entire accessibility of the comprehensive genome sequence, Daphnia has a significant fraction of genes familiar with humans (Sá-Pereira et al. 2018). With the surge in TiO2-NP concentration, there has been a growth in mortality rate when TiO2-NP was exposed to D. magna.
3.5 Toxicity to Human Health
Severe threat to human health may arise due to the direct or indirect contact of ENPs. Due to the contact with water comprising the residue of ENPs leads to direct contact, which usually occurs by the use of industrial effluents released into aquatic systems. Breathing of water aerosols, skin, inhalation or ingestion or intake of polluted and contaminated drinking water is some of the immediate interaction practices (Daughton 2004). Predicted environmental concentrations (PECs) of nanoparticles regularly used in aquatic systems have been outlined in the following Table 1.
4 Conclusion
The existence of nanoparticles influences aquatic life. ENPs toxicity can be initiated or mitigated by the occurrence of chemical stressors and DOM (dissolved organic matter). This chapter assesses ENPs properties on the aquatic environment with ecotoxic effects due to its event. To improve nanoparticle risk assessment, there is a need for more research. Toxicity evaluation needs to be begun on formulations of nanoparticles evaluated involving at least five species from different trophic levels for extracting the predicted no-effect concentrations (PNECs) for identifying the species sensitivities to other species. Moreover, evaluation of the toxicity to different natural and artificial aquatic and soil systems should also be performed to demonstrate the toxicity of nanomaterials at a holistic scale.
References
Abbas Q, Yousaf B, Amina AMU, Munir MAM, El-Naggar A et al (2020) Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: a review. Environ Int 138:105646. https://doi.org/10.1016/j.envint.2020.105646
Adiloğlu SI, Yu C, Chen R, Li JJ, Li JJ et al (2012) We are IntechOpen, the world’s leading publisher of Open Access books Built by scientists, for scientists TOP 1%. Intech, i(tourism):13. https://doi.org/10.1016/j.colsurfa.2011.12.014
Aitken RJ, Peters SAK, Jones AD, Stone V (2010) Regulation of carbon nanotubes and other high aspect ratio nanoparticles: approaching this challenge from the perspective of asbestos. In: International handbook on regulating nanotechnologies. https://doi.org/10.4337/9781849808125.00020
Aruoja V, Dubourguier HC, Kasemets K, Kahru A (2009) Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ 407:1461–1468
Aschberger K, Micheletti C, Sokull-Klüttgen B, Christensen FM (2011) Analysis of currently available data for characterizing the risk of engineered nanomaterials to the environment and human health-lessons learned from four case studies. Environ Int 37:1143–1156. https://doi.org/10.1016/j.envint.2011.02.005
Attarilar S, Yang J, Ebrahimi M, Wang Q, Liu J (2020) The toxicity phenomenon and the related occurrence in metal and metal oxide nanoparticles: a brief review from the biomedical perspective, vol 8. https://doi.org/10.3389/fbioe.2020.00822
Banerjee A, Choudhury AR (2019) Nanomaterials in plants, algae and microorganisms concepts and controversies, vol 22019. Academic Press, pp 129–141. https://doi.org/10.1016/B978-0-12-811488-9.00007-X
Barbara Rasco MO (2013) Impact of engineered nanoparticles on aquatic organisms. J Fish Livestock Prod. https://doi.org/10.4172/2332-2608.1000e106
Batley GE, Kirby JK, McLaughlin MJ (2013) Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc Chem Res. https://doi.org/10.1021/ar2003368
Bielmyer GK, Grosell M, Brix KV (2006) Toxicity of silver, zinc, copper, and nickel to the copepod Acartia tonsa exposed via a phytoplankton diet. Environ Sci Technol. https://doi.org/10.1021/es051589a
Bilberg K, Malte H, Wang T, Baatrup E (2010) Silver nanoparticles and silver nitrate cause respiratory stressin Eurasian perch (Perca fluviatilis). Aquat Toxicol 96:159–165
Biswas JK, Sarkar D (2019) Nanopollution in the aquatic environment and ecotoxicity: no nano issue! Current Pollut Rep. https://doi.org/10.1007/s40726-019-0104-5
Bouldin JL, Ingle TM, Sengupta A, Alexander R, Hannigan RE, Buchanan RA (2008) Aqueous toxicity and food chain transfer of quantum dots (TM) in freshwater algae and Ceriodaphnia dubia Environ. Toxicol Chem 27(9):1958–1963
Brun NR, Lenz M, Wehrli B, Fent K (2014) Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleuthero-embryos: importance of zinc ions. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2014.01.053
Buffet PE, Tankoua OF, Pan JF, Berhanu D, Herrenknecht C, Poirier L, Amiard-Triquet C, Amiard JC, Bérard JB, Risso C, Guibbolini M, Roméo M, Reip P, Valsami-Jones E, Mouneyrac C (2011) Behavioural and biochemical responses of two marine invertebrates Scrobicularia plana and Hediste diversicolor to copper oxide nanoparticles. Chemosphere 84:166–174. https://doi.org/10.1016/j.chemosphere.2011.02.003
Bundschuh M, Seitz F, Rosenfeldt RR, Schulz R (2016) Effects of nanoparticles in fresh waters: risks, mechanisms and interactions. Freshw Biol 61:2185–2196
Bundschuh M, Filser J, Lüderwald S, McKee MS, Metreveli G, Schaumann GE et al (2018) Nanoparticles in the environment: where do we come from, where do we go to? Environ Sci Eur. https://doi.org/10.1186/s12302-018-0132-6
Bystrzejewska-Piotrowska G, Golimowski J, Urban PL (2009) Nanoparticles: their potential toxicity, waste and environmental management. Waste Manag. https://doi.org/10.1016/j.wasman.2009.04.001
Chekli L, Zhao Y, Tijing L, Phuntsho S, Donner E, Lombi E, Gao B, Shon H (2015) Aggregation behaviour of engineered nanoparticles in natural waters: characterizing aggregate structure using on-line laser light scattering. J Hazard Mater 284:190–200
Chen CY, Jafvert CT (2011) The role of surface functionalization in the solar light-inducedproduction of reactive oxygen species by single-walled carbon nanotubes in water. Carbon 49(15):5099–5106. https://doi.org/10.1016/j.carbon.2011.07.029
Chen G, Liu X, Su C (2012) Distinct effects of humic acid on transport and retention of TiO2 rutile nanoparticles in saturated sand columns. Environ Sci Technol 46:7142–7150. https://doi.org/10.1021/es204010g
Daughton CG (2004) Non-regulated water contaminants: emerging research. Environ Impact Assess Rev 24:711–732
Das P, Xenopoulos MA, Williams CJ, Hoque ME, Metcalfe CD (2012) Effects of silver nanoparticles on bacterial activity in natural waters. Environ Toxic Chem 31:122–130
Dhasmana A, Firdaus S, Singh KP, Raza S, Jamal QMS, Kesari KK et al (2017) Nanoparticles: applications, toxicology and safety aspects. Environ Sci Eng (Subseries: Environ Sci). https://doi.org/10.1007/978-3-319-46248-6_3
Dumont E, Johnson AC, Keller VDJ, Williams RJ (2015) Nano silver and nano zinc‐oxide in surface waters—Exposure estimation for Europe at high spatial and temporal resolution. Environ Pollut 196:341–349
Giese B, Klaessig F, Park B, Kaegi R, Steinfeldt M, Wigger H, et al. (2018) Risks, release and concentrations of engineered nanomaterial in the environment. Sci Rep 8(1):S. 1565. https://doi.org/10.1038/s41598-018-19275-4
Gong N, Shao KS, Feng W, Lin ZZ, Liang CH, Sun YQ (2011) Biotoxicity of nickel oxide nanoparticlesand bio-remediation by microalgae Chlorella vulgaris. Chemosphere 83:510–516
Gottschalk F, Lassen C, Kjoelholt J, Christensen F, Nowack B (2015) Modeling flows and concentrations of nine engineered nanomaterials in the Danish environment. Int J Environ Res Pub Health. https://doi.org/10.3390/ijerph120505581
Griffitt RJ, Weil R, Hyndman KA, Denslow ND, Powers K et al (2007) Exposure to copper nanoparticlescauses gill injury and acute lethality in zebrafish (Danio rerio). Environ Sci Technol 41:8178–8186
Handy RD, Von Der Kammer F, Lead JR, Hassellöv M, Owen R, Crane M (2008) The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology. https://doi.org/10.1007/s10646-008-0199-8
Hanna SK, Miller RJ, Zhou DX, Keller AA, Lenihan HS (2013) Accumulation and toxicity of metaloxide nanoparticles in a soft-sediment estuarine amphipod. Aquat Toxicol 142:441–446
Hartmann NIB, Skjolding LM, Hansen SF, Baun A, Kjølholt J, Gottschalk F (2014) Environmental fate and behaviour of nanomaterials: new knowledge on important transfomation processes
Holden PA, Gardea-Torresdey JL, Klaessig F, Turco RF, Mortimer M, Hund-Rinke K et al (2016) Considerations of environmentally relevant test conditions for improved evaluation of ecological hazards of engineered nanomaterials. Environ Sci Technol. https://doi.org/10.1021/acs.est.6b00608
Hristozov D, Malsch I (2009) Hazards and Risks of engineered nanoparticles for the environment and human health. Sustainability. https://doi.org/10.3390/su1041161
Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK (2018) Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. https://doi.org/10.3762/bjnano.9.98
Jing H, Zhou Y, Wang C, Li S, Wang X (2017) Toxic effects and molecular mechanism of different types of silver nanoparticles to the aquatic crustacean Daphnia magna. Environ Sci Technol 51(21):12868–12878. https://doi.org/10.1021/acs.est.7b03918
Ju-Nam Y, Lead JR (2008) Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci Total Environ 400:396–414
Keller AA, Garner K, Miller RJ, Lenihan HS (2012) Toxicity of nano-zero valent iron to freshwater and marine organisms. PLoS One 7(8):e43983
Khan R, Inam MA, Khan S, Park DR, Yeom IT (2019) Interaction between persistent organic pollutants and ZnO NPs in synthetic and natural waters. Nanomaterials 9. https://doi.org/10.3390/nano9030472
Kim E, Kim SH, Kim HC, Lee SG, Lee SJ, Jeong SW (2011) Growth inhibition of aquatic plant caused by silver and titanium oxide nanoparticles. Toxicol Environ Health Sci 3:1–6
Kirschling TL, Golas PL, Unrine JM, Matyjaszewski K, Gregory KB, Lowry GV, Tilton RD (2011) Microbial bioavailability of covalently bound polymer coatings on model engineered nanomaterials. Environ Sci Technol 45(12):5253–5259. https://doi.org/10.1021/es200770z
Klaine SJ, Alvarez PJJ, Batley GE, Fernes TF, Hy RD, Lyon DY, Mahendra S, Mclaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27:1825–1851
Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN (2010) Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev 2574–2574
Laws J, Heppell CM, Sheahan D, Liu CF, Grey J (2016) No such thing as a free meal: organotin transferacross the freshwater-terrestrial interface. Freshwater Biol 61:2051–2062
Lead JR, Batley GE, Alvarez PJJ, Croteau MN, Handy RD, McLaughlin MJ et al (2018) Nanomaterials in the environment: behavior, fate, bioavailability, and effects—an updated review. Environ Toxic Chem. https://doi.org/10.1002/etc.4147
Levard C, Reinsch BC, Michel FM, Oumahi C, Lowry GV, Brown GE (2011) Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: impact on dissolution rate. Environ Sci Technol. https://doi.org/10.1021/es2007758
Liu HH, Cohen Y (2014) Multimedia environmental distribution of engineered nanomaterials. Environ Sci Technol. https://doi.org/10.1021/es405132z
Lowry GV, Gregory KB, Apte SC, Lead JR (2012) Transformations of nanomaterials in the environment. Environ Sci Technol. https://doi.org/10.1021/es300839e
Mahaye N, Thwala M, Cowan DA, Musee N (2017) Genotoxicity of metal based engineered nanoparticles in aquatic organisms: a review. Mutation Res Rev Mutation Res. https://doi.org/10.1016/j.mrrev.2017.05.004
Meesters JAJ, Quik JTK, Koelmans AA, Hendriks AJ, Van De Meent D (2016) Multimedia environmental fate and speciation of engineered nanoparticles: a probabilistic modeling approach. Environ Sci Nano. https://doi.org/10.1039/c6en00081a
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
Moore MN (2006) Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int 32:967–976
Musee N (2011) Simulated environmental risk estimation of engineered nanomaterials: a case of cosmetics in Johannesburg City. Human Exp Toxicol. https://doi.org/10.1177/0960327110391387
Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311:622–627
Nichols G, Byard S, Bloxham MJ, Botterill J, Dawson NJ, Dennis A, Diart V, North NC, Sherwood JD (2002) A review of the terms agglomerate and aggregate with a recommendation for nomenclature used in powder and particle characterization. J Pharm Sci 91:2103–2109
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150(1):5–22. https://doi.org/10.1016/j.envpol.2007.06.006
O’Brien N, Cummins E (2010) Nano-scale pollutants: fate in irish surface and drinking water regulatory systems. Human Ecol Risk Assess. https://doi.org/10.1080/10807039.2010.501270
Oberdörster G, Oberdörster E, Oberdörster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839
Oberdörster E, Zhu S, Blickley TM, McClellan-Green PML (2006) Haasch Ecotoxicology of carbon-based engineered nanoparticles: effects of fullerene (C60) on aquatic organisms. Carbon 44:1112–1120
Pakrashi S, Dalai S, Chandrasekaran N and Mukherjee A (2014) Trophic transfer potential of aluminium oxide nanoparticles using representative primary producer (Chlorella ellipsoides) and a primary consumer (Ceriodaphnia dubia). Aquat Toxicol 152:74–81
Pan JF, Buffet PE, Poirier L, Amiard-Triquet C, Gilliland D, Joubert Y et al (2012) Size dependent bioaccumulation and ecotoxicity of gold nanoparticles in an endobenthic invertebrate: the tellinid clam Scrobicularia plana. Environ Pollut 168:37–43
Praetorius A, Scheringer M, Hungerbühler K (2012) Development of environmental fate models for engineered nanoparticles—a case study of TiO2 nanoparticles in the rhine river. Environ Sci Technol. https://doi.org/10.1021/es204530n
Renzi M, Guerranti C (2015) Ecotoxicity of nanoparticles in aquatic environments: a review based on multivariate statistics of meta-data. J Environ Anal Chem 2:149
Rist S, Hartmann NB (2018) Aquatic ecotoxicity of microplastics and nanoplastics: lessons learned from engineered nanomaterials. In: Handbook of environmental chemistry. https://doi.org/10.1007/978-3-319-61615-5_2
Roberts AP, Mount AS, Seda B, Souther J, Qiao R, Lin S, Ke PC, Rao AM, Klaine SJ (2007) In vivo biomodification of lipid-coated carbon nanotubes by Daphnia magna. Environ Sci Technol 41(8):3025–3029
Ross JRM, Flegal AR, Brown CL, Squire S, Scelfo GM, Hibdon S (2007) Spatial and temporal variations in silver contamination and toxicity in San Francisco Bay. Environ Res 105:34–52
Salieri B, Pasteris A, Baumann J, Righi S, Köser J, D’Amato R et al (2015) Does the exposure mode to ENPs influence their toxicity to aquatic species? A case study with TiO2 nanoparticles and Daphnia magna. Environ Sci Poll Res. https://doi.org/10.1007/s11356-014-4005-2
Sani-Kast N, Scheringer M, Slomberg D, Labille J, Praetorius A, Ollivier P, Hungerbühler K (2015) Addressing the complexity of water chemistry in environmental fate modeling for engineered nanoparticles. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2014.12.025
Sá-Pereira P, Diniz MS, Moita L, Pinheiro T, Mendonça E, Paixão SM, Picado A (2018) Protein profiling as early detection biomarkers for TiO2 nanoparticle toxicity in Daphnia magna. Ecotoxicology 27:430–439
Sellers K, Mackay C, Bergeson LL, Clough SR, Hoyt M, Chen J, Henry K, Hamblen J (2008) Nanotechnology and the environment. CRC Press
Shah M, Guo QX, Fu Y (2015) The colloidal synthesis of unsupported nickel-tin bimetallic nanoparticles with tunable composition that have high activity for the reduction of nitroarenes. Catal Commun 65:85–90
Shi H, Magaye R, Castranova V, Zhao J (2013) Titanium dioxide nanoparticles: a review of current toxicological data. Particle Fibre Toxicol. https://doi.org/10.1186/1743-8977-10-15
Sigg L, Behr R, Groh K, Isaacson C, Odzak N et al (2014) Chemical aspects of nanoparticle ecotoxicology. Chimia 68:806–811
Singh S, Dosani T, Karakoti AS, Kumar A, Seal S, Self WT (2011) A phosphate-dependent shift in redox state of cerium oxide nanoparticles and its effects on catalytic properties. Biomaterials 32:6745-6753. https://doi.org/10.1016/j.biomaterials.2011.05.073
Smith CJ, Shaw BJ, Handy RD (2007) Toxicity of single walled carbon nanotubes on rainbow trout, (Oncorhynchus mykiss): respiratory toxicity, organ pathologies, and other physiological effects. Aquat Toxicol 82:93–109
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study 620 on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275:177–182
Stegemeier JP, Colman BP, Schwab F, Wiesner MR, Lowry GV (2017) Uptake and distribution of silver in the aquatic plant Landoltia punctata (Duckweed) exposed to silver and silver sulphide nanoparticles. Environ Sci Technol 51:4936–4943
Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ (2002) Metal oxide nanoparticles as bactericidal agents. Langmuir 18:6679–6686. https://doi.org/10.1021/la0202374
Stone V, Nowack B, Baun A, van den Brink N, von der Kammer F, Dusinska M et al (2010) Nanomaterials for environmental studies: classification, reference material issues, and strategies for physico-chemical characterization. Sci Total Environ 408(7):1745–1754. https://doi.org/10.1016/j.scitotenv.2009.10.035
Sun TY, Bornhöft NA, Hungerbühler K, Nowack B (2016) Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ Sci Technol. https://doi.org/10.1021/acs.est.5b05828
Sun TY, Gottschalk F, Hungerbühler K, Nowack B (2014) Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ Poll. https://doi.org/10.1016/j.envpol.2013.10.004
Turan NB, Erkan HS, Engin GO, Bilgili MS (2019) Nanoparticles in the aquatic environment: usage, properties, transformation and toxicity—a review. Process Saf Environ Prot 130:238–249. https://doi.org/10.1016/j.psep.2019.08.014
Usenko CY, Harper SL, Tanguay RL (2008) Fullerene C60 exposure elicits an oxidative stress response in embryonic zebrafish. Toxicol Appl Pharmacol 229:44–55
Vieira LR, Gravato C, Soares AMVM, Morgado F, Guilhermino L (2009) Acute effects of copper and mercury on the estuarine fish Pomatoschistus microps: linking biomarkers to behaviour. Chemosphere 76:1416–1427
Vázquez Núñez E, de la Rosa-Álvarez G (2018) Environmental behavior of engineered nanomaterials in terrestrial ecosystems: uptake, transformation and trophic transfer. Curr Opi Environ Sci Health. https://doi.org/10.1016/j.coesh.2018.07.011
Walters C, Pool E, Somerset V (2016) Nanotoxicology: a review. Toxicol New Aspects Sci Conundrum. https://doi.org/10.5772/64754
Wolfrum EJ, Huang J, Blake DM, Maness PC, Huang Z, Fiest J (2002) Photocatalytic oxidation of bacteria, bacterial and fungal spores, and model biofilm components to carbon dioxide on titanium dioxide–coated surfaces. Environ Sci Technol 36:3412–3419
Wong SWY, Leung PTY, Djurisi AB, Leung KMY (2010) Toxicities of nano zinc oxide to five marine organisms: influences of aggregate size and ion solubility. Anal Bioanal Chem 396:609–618
Xu Z, Liu Y, Wang Y (2019) Application of Daphnia magna for nanoecotoxicity study methods in molecular biology book. Nanotoxicity. https://doi.org/10.1007/978-1-4939-8916-4_21
Yin Y, Yang X, Zhou X, Wang W, Yu S, Liu J, Jiang G (2015) Water chemistry controlled aggregation and photo-transformation of silver nanoparticles in environmental waters. J Environ Sci 34:116–125
Yeo MK, Nam DH (2013) Influence of different types of nanomaterials on their bioaccumulation in a paddy microcosm: a comparison of TiO2 nanoparticles and nanotubes. Environ Poll 178:166–172
Zhang W, Xiao B, Fang T (2018) Chemical transformation of silver nanoparticles in aquatic environments: mechanism, morphology and toxicity. Chemosphere 191(7):324–334. https://doi.org/10.1016/j.chemosphere.2017.10.016
Zhao CM, Wang WX (2012) Size-dependent uptake of silver nanoparticles in Daphnia magna. Environ Sci Technol 46:11345–11351
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Krishna, D., Sachan, H.K. (2021). Nano-toxicity and Aquatic Food Chain. In: Singh, P., Singh, R., Verma, P., Bhadouria, R., Kumar, A., Kaushik, M. (eds) Plant-Microbes-Engineered Nano-particles (PM-ENPs) Nexus in Agro-Ecosystems. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-030-66956-0_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-66956-0_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66955-3
Online ISBN: 978-3-030-66956-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)