Abstract
The emerging literature on the ecotoxicity of nanoparticles and nanomaterials is summarised, then the fundamental physico-chemistry that governs particle behaviour is explained in an ecotoxicological context. Techniques for measuring nanoparticles in various biological and chemical matrices are also outlined. The emerging ecotoxicological literature shows toxic effects on fish and invertebrates, often at low mg l−1 concentrations of nanoparticles. However, data on bacteria, plants, and terrestrial species are particularly lacking at present. Initial data suggest that at least some manufactured nanoparticles may interact with other contaminants, influencing their ecotoxicity. Particle behaviour is influenced by particle size, shape, surface charge, and the presence of other materials in the environment. Nanoparticles tend to aggregate in hard water and seawater, and are greatly influenced by the specific type of organic matter or other natural particles (colloids) present in freshwater. The state of dispersion will alter ecotoxicity, but many abiotic factors that influence this, such as pH, salinity, and the presence of organic matter remain to be systematically investigated as part of ecotoxicological studies. Concentrations of manufactured nanoparticles have rarely been measured in the environment to date. Various techniques are available to characterise nanoparticles for exposure and dosimetry, although each of these methods has advantages and disadvantages for the ecotoxicologist. We conclude with a consideration of implications for environmental risk assessment of manufactured nanoparticles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanotechnology and the use of nano-scale materials is a relatively new area of science and technology with the global market estimated to be worth $10.5 billion in 2006 (http://www.bccresearch.com/nanotechnology/). Nanotechnology is the intentional and controlled generation, or modification of materials at the nanometer (nm) scale. Although nano-scale materials have been used in the modern context of materials research for at least a decade (e.g., Buckminster fullerenes or C60, Fagan et al. 1991), there is now a wider debate about the risks and benefits of the many manufactured nanomaterials and consumer products now appearing on the market (Royal Society 2004; US EPA 2005; Owen and Depledge 2005; Handy and Shaw 2007; Owen and Handy 2007). The benefits of nanomaterials are potentially enormous, and are still being explored. A diverse range of products and applications are emerging including electronics, optics, textiles, medical devices, cosmetics, food packaging, water treatment technology, fuel cells, catalysts, biosensors and agents for environmental remediation (e.g., Roco 2003; Freitas 2005; Karnik et al. 2005; Aitken et al. 2006; Brody 2006). Although environmental concentrations of manufactured nanoparticles (NPs) have yet to be routinely measured, there are concerns that NPs will be released from these products over their life (e.g., by erosion of the materials with use, or deliberate introduction during remediation of contaminated environmental media), or that product applications could generate wastes containing nanomaterials (e.g., domestic waste-water containing nanomaterials from household products). It is also unclear whether or not sewage treatment works could completely remove NPs from final effluents. Clearly there is a concern that these novel materials could be released into the environment, and that there may be releases from products that are in current use. However, we are only just starting to explore their ecotoxicology and environmental chemistry.
Defining nanomaterials in an ecotoxicological context
There are as yet no internationally agreed formal definitions of nanomaterials (NMs) and NPs, but NMs are usually taken to be material with at least one dimension between about 1 nm and 100 nm (Roco 2003; SCENIHR 2005; Moore 2006). These could be materials such as nanofilms (one dimension), nanowires and nanotubes (two dimensions), or nanoparticles (three dimensions). However, this definition of materials with a <100 nm dimension is arbitrary, and for ecotoxicology we should apply a broader definition that might include materials of a few hundred nm. A precise size threshold in this definition is not intended, since it is the novel toxic effects due to small size that are of interest. However, a pragmatic solution might be to consider materials with a primary dimension of <0.5 μm to differentiate nanoscale from micrometer scale. Whatever pragmatic decision is made about size thresholds to define NPs, some flexibility is needed. For example it could be argued that a single solid particle of 0.5 μm diameter might not be different from the behaviour of a slightly larger particle in the 1–2 μm range. However aggregates with an overall dimension in the μm range, but made of primary particles of <100 nm would be regarded as a NM. Clearly for NPs, the primary particle size should be considered (e.g., the diameter of a single particle). In addition, the sizes of aggregates of NPs, which can be several hundred nanometers or more, and the distribution of particle sizes present in the material also need to be considered. In mammalian toxicology particles sizes (PM, particulate matter) have been defined as coarse particles (diameter between 10 μm and 2.5 μm, PM10–2.5), fine particles (2.5 μm or less, PM2.5), or ultrafine particles (<0.1 μm, PM0.1), so NPs could be regarded as ultrafine particles or smaller.
Why should manufactured nanomaterials be of special concern to ecotoxicologists?
The environment does contain many natural particles at the nm scale such as colloids in freshwater (colloids are materials in the size range 1 μm to 1 nm, Lead and Wilkinson 2006), volcanic dusts in the atmosphere (Ammann et al. 1990), and nm scale particles from soil erosion (Hasegawa et al. 2007). It could be argued that these materials have been in the environment for millions of years and organisms must be adapted to living in the presence of these natural substances. There are also concerns that anthropogenic activity has been incidentally generating nano-scale pollutants such as air-borne particles from car exhausts or nanoparticles generated from the erosion of materials such as car tyres for a long time (see Handy and Shaw 2007 for discussion). Even so, we still have much to learn about the fate and behaviour of natural colloids and their interactions with pollutants (Lead and Wilkinson 2006). We should also consider that manufactured NPs might represent a special case, since they may be designed to have particular surface properties and (surface) chemistries that are less likely to be found in natural particles. They might therefore present enhanced or novel physico-chemical or toxicological properties in comparison to natural NPs.
Manufactured NPs (and natural NPs) often exhibit special physico-chemical properties and reactivities due to their small size and homogeneous composition, structure or surface characteristics, which are not present at the larger scale. So, for example, carbon fullerene NPs (C60 particles), may have a different toxicity compared to fine (μm sized) graphite particles, even though both particles are made of carbon (Barlow et al. 2005). In particular NPs possess a much higher specific surface area (SSA) than their larger counterparts of the same material, and the proportion of atoms on the surface versus the interior of the particle is also much larger for NPs. Together, these factors can give rise to a higher surface reactivity (e.g., adsorption and/or catalytic properties) for the same mass of material. This gives rise to the suggestion that SSA (e.g., m2 g−1 of material) rather than mass concentration (e.g., mg l−1) might sometimes be more important to the toxicity of NPs, and is perhaps a better way to describe the dose–effect of nanomaterials when surface reactivity is a key characteristic (Oberdörster et al. 2007). Of course, the total surface area available will be a function of SSA multiplied by the particle mass concentration, so both are likely to be important in exposure. Also, the importance of shape and particle surface area in the uptake of NPs across the cell membranes of many organisms remains to be established in ecotoxicology. Although, this is an important facet of respiratory toxicity in mammals (Maynard and Aitken 2007).
There are also unusual chemical and physical properties at the lower end of the nanoscale (e.g., 1–10 nm) and at the interface with the atomic scale. In particular, materials in the region from a few nm to several tenths of a nm exhibit properties (electronic states, magnetic and optical properties, catalytic reactivities) that behave differently from both their atomic/molecular level and from their larger particle counterparts. For example, quantum confinement effects have been observed in the electronic states of haematite leading to enhanced oxidation of Mn, even when normalised to SSA (Madden and Hochella 2005). Size also has important control over other physical and chemical properties such as zeta potential and metal binding (Madden et al. 2006). So there is also a concern that fundamental every-day assumptions about the chemical reactivity of molecules and atoms may need to be revisited when considering the ecotoxicity of NPs. Such quantum effects could also impart previously unknown toxic effects.
In addition, the variety of physical structures of NMs, (e.g., different crystal structures of the same material), and the potential for these structures to contain more than one substance (e.g., Ag–Ti composites as antibacterial coatings), or to be manufactured with multiple types of surface ligands, creates a new challenge for ecotoxicity testing.
Aims
The main objective of this review is to describe the known ecotoxicological effects of NMs and NPs and the key aspects of physico-chemistry that are known to affect, or are likely to alter, ecotoxicity. In addition, given the current lack of routine measurement of manufactured NPs in the environment (water, air, or soil/sediments) we suggest on the basis of physico-chemical properties, which environmental compartments might become contaminated with NMs or NPs. We also outline the challenges the novel physico-chemistry of NPs present for environmental risk assessment, which ultimately supports risk management decisions. The implications for human health from exposure via the environment (for a review, see Handy and Shaw 2007), occupational exposure to nanomaterials in the work place (e.g., Aitken et al. 2004), and respiratory toxicity in mammalian models (e.g., rodents, Warheit et al. 2005; Handy and Shaw 2007) are discussed elsewhere.
Ecotoxicity of nanomaterials
There is a rapidly emerging literature on the ecotoxicity of NPs and NMs, with most of the studies to date on aquatic organisms and using only a few types of manufactured NPs that are commercially available. However, particle toxicity has been studied for many years in mammals from the viewpoint of respiratory health and inflammation (Maynard and Aitken 2007). For example, studies with fine and ultrafine TiO2 particles demonstrate some respiratory toxicity and inflammation of the lung in rodents (e.g., Ferin and Oberdörster 1985; Ferin et al. 1991; Oberdörster et al. 1992). In particular, Oberdörster et al. (1992) showed that the level of lung inflammation in rats was associated with particle size, with the smaller ultrafine TiO2 causing more adverse effects. It is therefore worth considering known toxic effects of manufactured NPs on mammals.
Knowledge from mammalian studies
The literature on mammalian models has recently been reviewed (Handy and Shaw 2007). Table 1 gives some examples of the respiratory toxicity of NPs and NMs in small mammals. Carbon nanotubes (CNTs) can cause significant lung damage to mammals when exposed to intratracheal (i.t) doses. For example, mice exposed to a dose of 0.5 mg CNT showed 56% mortality within 7 days of exposure; macrophage granulomas formed beneath the bronchial epithelium, along with necrosis and inflammation of interstitial and peribronchial tissues during the 90-day post-exposure follow-up (Lam et al. 2004). Metal oxides also produce lung injury during respiratory exposure. Rats exposed to cadmium oxide NPs for 6 h showed increased numbers of neutrophils and multifocal alveolar inflammation. In 50% of the rats exposed to 550 μm m−3, an elevated blood cadmium level was also measured, suggesting movement of the particles throughout body systems (Takenaka et al. 2004).
These reports raise a number of concerns from the perspective of ecotoxicology. First, the lung is representative of typical mucous epithelial tissue and it is possible that similar epithelia in aquatic organisms could also show toxic effects. The epithelia of concern would include the gills and gut tissue of fish or invertebrates, as well as specialised epithelial tissue like the mantle of shellfish or the body surface of organisms such as earthworms. We have recently shown epithelial injury to the gill and the intestine in fish exposed to NPs (Federici et al. 2007; Smith et al. 2007). Second, the latent effects of acute respiratory exposure and the inflammation reactions in rat lung raise concerns about the long-term health of organisms after even quite short exposures. However, it could also be argued that the typically milligram doses used in rodent studies are not likely to occur routinely in the environment, except during accidental spills of nanomaterials. Finally, mammalian studies with nanoparticles such as quartz, carbon black and asbestos highlight the fact that not only particle size, but biosolubility and shape may be important factors that influence uptake, toxicity and pathology, at least when exposure is via the airways and lung (Royal Society 2004 and references therein; Maynard and Aitken 2007).
Lethal toxicity of nanoparticles to wildlife
Data on the ecotoxicity of NPs are shown in Tables 2 and 3. There are few published lethal dose values on the ecotoxicity of NPs, although new studies are being published at an increasing rate. Studies on fish and invertebrates (Lovern and Klaper 2006; Zhu et al. 2006) suggest that C60 fullerenes are toxic in the milligram per litre range, but the LC50 values obtained are very dependent on the method of preparation of the material and the addition of dispersants (Table 3). It is possible that dispersed C60 NPs are more toxic than “non-dispersed” material, or that the solvents used have some effects, or somehow change the toxicity of the dispersed NPs themselves. Such studies highlight growing awareness about issues surrounding preparation of NPs (e.g., whether media should be sonicated or solvent-dispersed), how these are administered in ecotoxicological studies, and the associated environmental relevance (e.g., whether a solvent-dispersed solution of NPs is an accurate reflection of how these particles will occur in the environment).
The general lack of LC50 values for fish may also be for technical reasons. Maintaining the high mg l−1 concentrations needed to achieve acute lethal toxicity is difficult. At concentrations above 10 mg l−1 there is significant aggregation of many types of NPs, and even with prolonged sonication plus the addition of dispersants, it remains difficult to achieve reproducible solutions (Handy, personal observations). Interestingly, there are few lethal toxicity values for in vitro assays using non-mammalian cells. Work on acute toxicity to fish and invertebrate cell lines is therefore also required.
Sub-lethal effects of aqueous exposure to nanoparticles in fish
Several authors have exposed teleost fish to NMs (Table 2). These include the effects of C60 fullerenes on large mouth bass (Micropterus salmoides, Oberdörster 2004), fathead minnow (Pimephales promelas, Oberdörster et al. 2006, Zhu et al. 2006), and Japanese medaka (Oryzias latipes, Oberdörster et al. 2006). The effects of CNT’s (Smith et al. 2007) and TiO2 NPs (Federici et al. 2007) on rainbow trout (Oncorhynchus mykiss) have also been studied. These studies have identified potential target organs for NPs. Kashiwada (2006) observed the body distribution of fluorescently labelled NPs in a transparent colour morph of the medaka (O. latipes). This experiment used measurements of fluorescence in the organs of this “see-through” fish to infer the location of the NPs. The fish were exposed to mono-dispersed, non-ionised, fluorescent polystyrene microspheres with a diameter of 39.4 nm at an aqueous concentration of 10 mg l−1 for 7 days. The gills, as expected, showed the greatest increase in fluorescence, shortly followed by the intestine. This suggests that NPs can at least attach to the gill surface, and may even enter the epithelial cells. Whether or not polystyrene microspheres behave like other NPs is unclear. However, similar observations have been made in experiments with trout where CNTs precipitated on the gill mucus during aqueous exposure (Smith et al. 2007). However, in the medaka study the increases in fluorescence in the internal organs were relatively small and, apart from the gall bladder, there were no statistically significant increases in fluorescence in other tissues (brain, liver, kidney or testis; Kashiwada 2006). Of course, this does not mean that the fluorescent NPs were not absorbed into the blood and circulated to the internal organs; it could simply be that exposure time needed to be longer or that excretion rate matched uptake rate (with no net accumulation). However, caution is required when interpreting the results of any experiment with labelled NPs. For example, unequivocal evidence that the fluorescent label remains attached to the NPs inside the tissues is often unavailable.
Both the study on medaka (Kashiwada 2006) and the study on rainbow trout (Smith et al. 2007) demonstrated the presence of NPs in the gut, despite the fact that the delivery route was via aqueous exposure. Freshwater fish do drink a few ml of water kg−1 body mass h−1 (Eddy 1982) and Smith et al. (2007) argue that this could explain the appearance of NPs in the gut. This would be especially important during toxicity, since a stress-induced drinking response could greatly increase the amount of water that is imbibed (Smith et al. 2007). Of course marine teleost fish drink routinely as part of their osmoregulatory strategy (Eddy 1982) and this raises concerns for exposure of the gut in marine species.
Other target organs for nanomaterials in fish are inferred from observations of toxic effects in those organs, rather than demonstrated localisation of NPs within the organ or tissue of concern. This is partly because the techniques for extracting and measuring NPs in tissues are not yet routinely available. However, we should not exclude the possibility that toxic effects occur at doses that are difficult to detect in individual organs (i.e., there is high potency). For CNT’s at least, the liver appears to be an important target organ. Smith et al. (2007) demonstrated pathology in the livers of trout exposed to up to 0.5 mg l−1 CNT for 10 days. Histological changes included altered nuclear morphology with condensed nuclear bodies that had the appearance of apoptotic bodies, and cells with diffuse nuclei in the early stage of cellular necrosis. Biochemical change is also observed in the liver. Smith et al. (2007) found a statistically significant fall in thiobarbituric acid reactive substances (TBARS) in the livers of trout exposed to CNT. In contrast, Oberdörster (2004) found little effect in largemouth bass exposed to C60 for a much shorter time. Oberdörster et al. (2006) also studied the expression of mono-oxygenases (CYP family proteins whose role includes metabolism of foreign compounds) in the liver of fathead minnows exposed to 0.5 mg l−1 C60 fullerenes for 96 h. They found no effects on either hepatic CYP mRNA levels or the proteins themselves. However, they did report a statistically significant reduction in PMP70 protein, an isoenzyme involved in hepatic lipid metabolism (Oberdörster et al. 2006). Interestingly, the reduction in PMP70 may be species or exposure-time dependent, since measurements of PMP70 in medaka exposed to the same material for a shorter time period (48 h) showed no effect on protein levels (Oberdörster et al. 2006).
The brain is also a potential target organ, at least for carbon-based NMs (Oberdörster 2004; Smith et al. 2007). Oberdörster (2004) first suggested the possibility of brain injury in fish when a significant elevation in lipid peroxidation was observed in the brains of juvenile largemouth bass exposed to 0.5 mg l−1 C60 fullerenes, although there was no apparent dose–response relationship. There is some logic to this concern about the brain given that several biomedical studies have attempted to exploit the permeability of the blood–brain barrier (BBB) to natural lipid micelles and particulates (by endocytosis) to develop nano-drug delivery techniques for the brain (Cui et al. 2005; Dobson 2001; Kreuter 2005). Smith et al. (2007) have similarly demonstrated brain pathology in rainbow trout exposed to 0.5 mg l−1 CNT for 10 days. These pathologies included injury to the cerebral vasculature (suspected aneurisms) and more minor cellular injuries such as individual necrotic bodies and small foci of vacuolisation in parts of the brain (Smith et al. 2007). However, brain injury was not a feature of TiO2 exposure in rainbow trout (Federici et al. 2007), and it seems likely that this brain injury will depend on the nature of the particles, with other types of NPs targeting different organs.
The Smith et al. (2007) study used a body systems approach to investigate organ effects, and was able to show that CNTs are a respiratory toxicant but did not cause major haematological disturbances. However, there are many organ systems for which data are not available, including skeletal muscle and possible locomotion effects, and effects on spleen, kidney or bone functions. More ADME (absorption, distribution, metabolism and excretion) data, and a good understanding of the distribution and localisation of NMs in body systems are needed before a definitive list of all the target organs for different NMs can be produced. Nonetheless, available sub-lethal exposure studies do at least identify some common themes in toxicity mechanisms that appear to be similar to those found in mammalian studies. These mechanisms include oxidative stress, disturbances to trace element metabolism, and vascular injury, as well as expected injury to the gill during aqueous exposure (see Smith et al. 2007). For example, several studies report lipid peroxidation or changes in TBARS in tissues (Oberdörster, 2004; Zhu et al. 2006; Smith et al. 2007). Changes in anti-oxidant defences in tissues are also implicated. Smith et al. (2007) observed a compensatory increase in total glutathione in the gill and intestine during CNT exposure over 10 days, while much shorter exposures to C60 fullerenes might deplete tissue glutathione (Oberdörster 2004). Recently, Zhu et al. (2007) also implicated oxidative stress in the developmental toxicity of C60 particles to zebrafish.
Observations of oxidative stress, and the well known sensitivity of DNA to oxidising agents, suggest that genotoxicity may also be a potential mechanism of toxicity worthy of investigation (e.g., via the COMET assay or micronucleus induction). Recent studies (e.g., Linse et al. 2007) further suggest that interaction of NPs with proteins may also be important in sublethal responses. Linse et al. (2007) used in vitro experiments with buffers containing soluble proteins and exposure to hydrophobic NPs (CeO, MWCNTs, polymer-coated quantum dots) to show an increased rate of protein fibril nucleation (albeit at pH 2, which is far lower than physiological pH). The mechanism of nucleation was unclear but the authors speculated that it might result from particles increasing the probability of homogeneous nucleation events (by providing locally higher concentrations of monomers), or that interaction with particle surfaces could promote protein conformational changes.
Sub-lethal aqueous toxicity of nanoparticles to invertebrates
There are a few reports on the effects of NPs to invertebrates (Table 2). The use of the waterflea, Daphnia magna, as a standard ecotoxicology test species is well known, and it is perhaps no surprise that early studies have focused on this organism. Several studies have used Daphnia to estimate LC50 values (Table 3). Oberdörster et al. (2006) have also investigated chronic effects in D. magna and other invertebrates. D. magna were exposed to up to 5 mg l−1 C60 fullerenes over 21 days. At the highest C60 concentration (5.0 mg l−1), mortality of 40% was observed, along with fewer offspring and a delay in moulting. In the same study, exposure of the freshwater invertebrate, Hyallela azteca, to 7 mg l−1 C60 fullerenes for 96 h was reported to have no effect on mobility, moulting or feeding behaviour (Oberdörster et al. 2006). More recently, several studies have noted behavioural effects of NPs in D. magna (Lovern et al. 2007; Roberts et al. 2007). Lovern et al. (2007) noted changes in locomotor behaviour (hopping frequency and appendage movement) and Roberts et al. (2007) found that D. magna use the organic lysophosphatidylcholine coating on SWCNTs as a food source. Published reports on the ecotoxicity of manufactured NPs to soil invertebrates and other terrestrial invertebrates appear to be lacking, although we are aware of preliminary studies with earthworms (e.g., Scott-Fordsmand et al. 2007).
Bacteria, algae and aquatic plants
Similar to the situation for aquatic invertebrates, there are very few published ecotoxicology studies on environmentally relevant bacteria, algae and plants. Hund-Rinke and Simon (2006) exposed algae (Desmodesmus subspicatus) in the growth inhibition test to titanium NPs, and depending on the method of preparation of the material, found EC50 values ranging from 44 mg l−1 to no effects at the highest concentration used (50 mg l−1). However, it has been noted that the surface structure or matrix of plant cell walls can act as a surface to grow NPs. Scarano and Morelli (2003) noted that stable nano-crystals form on marine phytoplankton when exposed to Cd. This raises the possibility that metal NP exposure in marine algae could simply arise from the presence of appropriate conditions for crystal formation at the surface of the organism during aqueous metal exposures. Plant viruses are also used as scaffolds for NP construction (Barnhill et al. 2007). These observations raise the concern that organisms do not necessarily need to be exposed to NMs added directly to the water, and the possibility that viruses could act as vectors for the movement or growth of NPs. Plant cells have also been used as tools during the development of imaging technology for NMs, and this has generated some incidental information on uptake. Wu et al. (2007) found that zinc oxide NPs aggregate on the surface of plant cells.
Published studies on the ecotoxicity of NMs to bacterial species are limited, even though the bacteriocidal properties of NMs have been reported in the biomedical literature. For example, it is well known that TiO2 NPs and silver NPs kill bacteria (e.g., Fu et al. 2005; Duran et al. 2007), and have been used for sterilization of medical devices (Sekiguchi et al. 2007). One might therefore expect some of these materials to be toxic to microbes in the environment. The bacterium Shewanella algae, has been demonstrated to deposit platinum NPs, and this is suggested as a biotechnology application to recover platinum (Konishi et al. 2007). Perhaps bacteria may be useful in the bioremediation of NMs. Tong et al. (2007) recently investigated the effects of C60 NPs on the bacterial diversity in soil. Using DNA and fatty acid profiling of the soil, they found little impact on the microbial community after a 30-day exposure to 1 mg C60/g of soil. However, C60 in suspension does have effects on bacterial cultures in the laboratory. Lyon et al. (2005) report minimum inhibitory concentrations of C60 of 0.5–1.0 and 1.5–3.0 mg l−1 respectively for Escherichia coli and Bacillus subtilis growth. Interestingly, the C60 tended to associate more with the Gram-negative E. coli, suggesting that the surface properties of the bacterial cell membrane may be a significant factor in toxicity. However for C60 at least, small aggregates are more toxic to B. subtilis, but the change in toxicity with particle size is only partially explained by surface area effects, and interactions with the cell membrane are implicated (Lyon et al. 2006). ZnO NPs appear to disrupt the Gram-negative cell membrane structure in E. coli (Brayner et al. 2006), and it is proposed that NPs with a positive charge such as cerium oxide could bind the Gram negative cell membrane by electrostatic attraction (Thill et al. 2006). Clearly, the intimate relationship between the physico-chemistry of the medium and membrane biology of the microbe is emerging as a key factor in NP toxicity to microorganisms.
Other organisms and routes of exposure
Most of the emerging literature on wildlife has inevitably tended to be on organisms that are used in aquatic ecotoxicity testing. There are significant gaps in the literature for other organisms. For example, there appear to be no reports on terrestrial plants, amphibians, reptiles, or birds; although Ek et al. (2004) suggest that long-range transport of nanoparticulate metals in the air could be a factor in the bioaccumulation of platinum group metals by raptors. Furthermore, despite some detailed information on small mammals in the biomedical literature, there is still a need to examine relevant small mammals in the wild, such as voles and shrews. It would also be important to examine risks to farm animals and the human food chain (Handy and Shaw 2007).
Most of the ecotoxicology studies to date (Table 2) have used aqueous exposures. Other routes of exposure need to be investigated including laboratory-based dietary exposure to clarify concerns about toxicity via food, as well as simple food web or mesocosm approaches to allow ecological interpretation of possible effects. Dietary toxicity was identified in trout, where incidental ingestion of test water (stress-induced drinking) caused pathology in the gut, and included biochemical evidence of oxidative stress in the gut epithelium (CNTs, Smith et al. 2007; TiO2 NPs, Federici et al. 2007). There was also evidence of direct NP precipitation on the gut mucosa in Smith et al. (2007), and this is perhaps no surprise given that particles tend to aggregate even in modest saline conditions (Stolpe and Hassellöv 2007), such as would be found in the gut lumen. For similar reasons, the physico-chemical behaviour of NPs (see below) suggests that NPs would aggregate and adsorp on to many types of surfaces. This could be adsorption to the surfaces of aquatic sediments, algal mats, biofilms, soils, and even the exterior surfaces of organisms (e.g., CNT precipitation on fish gills, Smith et al. 2007).
Physico-chemical properties of manufactured nanoparticles and ecotoxicity
Manufactured NMs and NPs are constructed to impart material-specific physico-chemical properties that are relevant to the type of product application (e.g., oxidising properties associated with antibacterial nano-products). Given the diverse range of products and applications, it is no surprise that generalisations about the chemistry of NMs and NPs should be made with caution. The diversity of manufactured NPs is reflected in the ecotoxicological reports to date (Table 2) which have used several different types of NPs of varying chemical composition, sizes, shapes, and ability to disperse in solution. Nonetheless, it is worth considering some of the fundamentals of particle and colloid chemistry (Stumm 1993; Elimelech et al. 1995; Birdi 1997), their behaviours in environmental systems (Buffle and van Leuween 1992; Grasso et al. 2002; Buffle and van Leuween 1993; Lead and Wilkinson 2006), and how this might apply to ecotoxicity. Many issues arise including the agglomeration or aggregation of NPs, dispersion of NPs by interactions with natural organic matter (NOM) in the water (or by dispersing agents that could be added in a toxicity test), and the ability of particles to adsorb onto surfaces. These behaviours will partly be a function of the surface chemistry of the NPs, the composition of NPs, the presence of any coatings (“capping agents”), dissolution of material from the particle surface into solution (dissolution), and the presence of any readily soluble substances in the preparation (e.g., metal salts in CNT preparations, Smith et al. 2007). Nonetheless, we attempt to summarise the key physico-chemical behaviours of NPs for the ecotoxicologist below, and describe what abiotic factors (salinity/ionic strength, pH, hardness, etc.) have been investigated in ecotoxicity studies so far.
Chemical terminology: dissolved versus colloids
In order to study the ecotoxicology of manufactured NPs to aquatic organisms the first step is to introduce the particles to an aquatic medium, i.e., to disperse them. A stable dispersion of NPs in a liquid is called a colloidal system or colloidal dispersion. A colloidal dispersion or a sol is one phase (the solid) homogeneously distributed in another phase (the water). There is no such thing as a colloidal solution, and colloids are dispersed rather than dissolved in a medium. Dispersion has to be strictly separated from the process of dissolution. The term “colloid” applies to particle sizes or other suspended material in the 1 nm–1 μm size range (Lead and Wilkinson 2006). In colloid chemistry, a “stabilized” dispersion (kinetically stable when dispersed i.e., over long time scales, but still thermodynamically unstable) describes a liquid where the particles may collide by Brownian motion or shear flow, but do not stick together after the collision. This will tend to keep particles dispersed, and we need to be clear when using the word “stable” in nanoecotoxicology. For example, an ecotoxicologist might use the phrase “stable solution” to imply consistent properties of a stock solution used for a toxicity test, whereas the particle chemist uses “stable” to describe the dispersion of the suspension and its thermodynamic state; in terms of solution behaviour the chemist’s interpretation it is the exact opposite of the ecotoxicologist’s interpretation! (see below). In this review we will therefore only use “stable” to describe the thermodynamic state of the solution.
Colloidal stability and aggregation
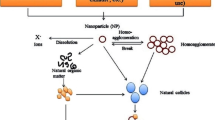
A colloidal dispersion is thermodynamically unstable and will always tend to aggregate and separate; however, the process may be slow (hours-days), so that the dispersion appears to be virtually stable. The processes important for the separation of a colloidal dispersion are mainly particle collisions, and attachment resulting in aggregation with accompanied settling. The particle–particle collisions originate from three fundamental processes: Brownian motion of particles leads to perikinetic aggregation, particles travelling at different velocities in a shear flow experience orthokinetic (shear) aggregation, and particles of different size or density undergo differential settling (Fig. 1). Aggregation may occur as homoaggregation (particles of the same type aggregating together), or heteroaggregation (particles attaching to other particle types present). In Fig. 1, the collision rate for perikinetic aggregation and differential settling is lowest for particles of the same size, hence a monodisperse dispersion will be more stable than polydisperse dispersions. Also, for small particles perikinetic aggregation is the dominant mechanism. After the initial interactions there may be more particle–particle, particle-cluster and cluster–cluster aggregation processes to consider. Aggregation phenomena have practical implications, such as the attachment of particles to the walls of experimental equipment (glassware, fish tanks, or the inner workings of scientific instruments), and the tendency of NPs to aggregate in natural waters where other colloid material or micro-organisms may be present, or on the test organisms themselves (Smith et al. 2007).
The three collision mechanisms and associated rate coefficients for the aggregation of 1 μm particles with particles of diameter dp; Temperature is 12°C, particle density 2.6 g ml−1 and shear rate 35 s−1. The cartoons represent the processes of perikinetic, orthokinetic, or differential settling respectively. Dotted arrow indicates the graph relating to each cartoon (process). In this example, perikinetic processes (e.g., Brownian motion) dominate aggregation rates at very small particle size, but when particle sizes exceed 1 μm orthokinetic (shear processes) and differential settling become more important
In a fluid at rest and for particles below 100 nm settling can usually be neglected over very short time scales (minutes or hours) since the settling velocity is equal to, or less than, the Brownian displacement. Hence aggregation processes such as differential settling or shear flow may be slow, or irrelevant, as long as no larger particles are present. The NPs in the dispersion diffuse by Brownian motion, and temperature and the particle number concentration (e.g., number of particles l−1) determine the particle–particle collision frequency. If the dispersion is fully destabilized then each collision is successful, hence the collision efficiency is unity. For spherical particles and collisions between particles and aggregates that are not too different in size the resulting perikinetic aggregation can be described by the Smoluchowski equation:
With N T the total number concentration (primary particles and aggregates), k a the rate constant, k B the Boltzmann constant, T the temperature, η the dynamic viscosity and t the time.
However, this simplification does not take into account effects which stabilize colliding particles, resulting in a collision efficiency <1. The stabilization originates from forces between the particles. The particles affect each other by attractive and repulsive forces that act on different length scales (fractions of nanometers to several nanometers). The forces usually accounted for are Borne repulsion, diffuse double layer potential, and van der Waals attraction. These forces are treated in the extended DLVO theory. DLVO theory was developed by Derjaguin and Landau (1941), and Verwey and Overbeck (1948) (hence the name DLVO); and balances the attractive and repulsive forces acting on two closely adjacent particles (Fig. 2). However, DLVO theory does not include the effects of particle shape, charge heterogeneity, and surface roughness, which may also influence the collision efficiency (Elimelech et al. 1995; Bhattacharjee et al. 1998; Grasso et al. 2002). It must be emphasized that DLVO theory is only applicable if there is no interference with such diffusive or attractive forces. If the electrostatic diffuse double layer (EDL, see Fig. 2) surrounding adjacent particles overlap, then other factors are involved. This may be the case for particles of <20 nm at ionic strength of <1 mM (Kallay and Zalac 2002).
A schematic diagram showing (left panel) the electrical double layer (EDL) on the surface of a particle, with the different potentials to be considered and the Debye length 1/κ which is the length where the potential has fallen to a value of 1/e of the Stern potential. An increased ionic strength (addition of salt ions) will cause additional charge screening of the surface and effectively compress the EDL. The Debye length can range between fractions of a nm (seawater) and nearly 1 μm (ultrapure water). The right hand panel shows a simplified graph summarising the DLVO interaction energies and the resulting sum function. The top graph shows a situation where the repulsive forces (e.g., electrostatic charge repulsion) are working against the attractive forces (van der Waals) and an activation energy is required to achieve particle–particle attachment in either the secondary or primary minimum. The bottom graph shows three possible situations: fully stabilised system, a system having secondary and primary minimum and a fully destabilised system where the energy barrier for attachment in the primary minimum has vanished. Attachment in the primary or secondary minimum has certain consequences for the reversibility of attachment: escape from the secondary minimum can be achieved by slight energy input (e.g., ultrasonic power) or reduction in ionic strength, escape from primary minimum is often impossible or can be achieved by charge reversal if attachment was due to opposite charge of particles. Abbreviations: zeta potential (ζ), electrostatic potential (ψ), electrostatic potential at the stern layer (ψS), Euler’s number (e), Boltzmann constant (k). X is a distance from the surface, Xs is the distance where ions and molecules are mobile and can be sheared off (shear plane), and potential here is measured as the zeta potential. The diffusion layer is an unstirred layer of water adjacent to the surface, and the bulk solution is the free moving water (e.g., seawater, freshwater)
While van der Waals forces between surfaces separated by a medium can be regarded as material constants and are always attractive, the EDL consists of the layer of charge at the surface of a particle and the electric field generated by the charged surface. This can have a net negative or positive charge, depending on the surface ligands of the particle. These forces are generally repulsive (i.e., like charges of two identical particles will repel each other). If the repulsive forces are strong enough the colloidal dispersion can be virtually stable. The repulsive forces are electrostatic (equal net charge), and act on fairly large length scales involving the outer layer of the particle, hence the term electrostatic double layer (EDL). However, now consider the effect of adding salt ions (e.g., NaCl) to the medium. Clearly, opposite charges will attract, and some of these salt ions will accumulate in the EDL and screen some of the surface charge of the NPs. This will reduce the EDL thickness and the length scale that repulsive (stabilizing) forces act on. Of course the salt does not have to be NaCl. Di- and trivalent ions are especially effective at charge shielding, and can act even more effective as soon as they are specifically sorbed to the particle’s surface. The net surface charge of the particles is difficult to measure directly, and zeta potential (ζ) is often measured. However, zeta potentials are only the potential at the shear plane which divides the ions and molecules fixed to the surface from those that can freely move with the liquid relative to the bulk aqueous phase (Fig. 2; van Leeuwen and Galceran 2004). So, strictly speaking, zeta potentials are not measurements of the surface charge of the particle itself, but a voltage reflecting the effects of surface charge and flow dynamics near the surface.
The addition of salts to the media (thus increasing ionic strength) will compress the EDL. Two particles can now approach each other more closely and start to be affected by attractive forces that are acting on shorter length scales (e.g.,van der Waals forces, hydrophobic interaction forces or electrostatic attraction due to surface charge heterogeneity). Such attractive forces can mean that a collision leads to an attachment (first step of aggregation) of the two particles. In addition to the importance of ionic strength for influencing aggregation behaviour, in natural waters it is well known that highly negatively charged natural acids (e.g., humic and fulvic acids) will bind to natural mineral NPs (positively charged). This may lead to a reduction in surface charge of the particles (positive charged particles, low concentration of humics), a net neutral surface charge (intermediate concentration of humics), or even provide them with a net negative surface charge by charge reversal that stabilizes the colloidal dispersions (elevated concentration of humics). However, the outcome of interactions with humics and particles cannot be easily predicted, but may be analyzed experimentally, e.g., by acid-base titrations and measurement of zeta potential. This chemistry clearly has implications for ecotoxicity experiments. For example, NP dispersion will depend on the type and amount of natural organic matter in the water (Hyung et al. 2007; Giasuddin et al. 2007), and the salt content. It is likely that manufactured NPs will quickly aggregate in natural seawater, even at low salinity.
Particles can also be sterically stabilized by surface orientation, or binding of surfactant molecules, or polymers, which physically hinder two particles from approaching each other. Steric stabilization can stabilize colloidal dispersions even at higher levels of ionic strength when DLVO theory would predict strong aggregation. This means that we might use chemical agents to disperse NPs, as long as the appropriate “solvent controls” are included in any ecotoxicology experiment (e.g., Smith et al. 2007 used sodium dodecyl sulphate, SDS, detergent to disperse CNT). It is also probable that the presence of solvents and dispersants in waste-water going to sewage treatment works are likely to influence the behaviour of NPs during treatment. For example, dispersants in the influent water could lead to a slower rate of NP aggregation than predicted by particle size, dissolved organic matter, or salinity. This influence would presumably also change during sewage treatment as dispersants are degraded (e.g., by microbes in the clinker beds).
It is also possible to achieve the opposite effect (aggregation) by adding multi-charged polymer reagents to the media. The opposite destabilizing process will be polymer bridging by a multi-charged polymer that binds to several particles while the polymer forms a bridge between them. This process is utilized in sewage and water treatment facilities, and also occurs in nature where charged polysaccharides (such as exudates from algae) promote aggregation. It is well known that organic substances from surface waters may promote aggregation while soil-derived organic matter tends to stabilize natural NPs (Wilkinson et al. 1997). Multiple charged cations and anions (Ca2+, SO4 2−) may show the same effect of promoted aggregation.
Variable charge and surface charge
Another important consideration for the stability of colloidal dispersions is the variability of charge with the pH of the surrounding medium. Some NPs show a net negative charge over wide pH ranges (e.g., clay minerals where charge is often only slightly pH dependent). Other particles, for example, many oxides and carbonates show a positive charge at low to circumneutral pH, or, as with iron oxides, have their point of zero charge (PZC) in the pH region of natural waters (pH 6 to 8). The PZC will also be affected by other factors such as surface sorbed organic matter, but not by ionic strength (Hendershot and Lavkulich 1979). As long as the latter particle types (neutral charged) do not interact with natural organic matter or show specific adsorption of charge (e.g., carbonate ions), they will have low stability and will quickly aggregate. Charge heterogeneity is also an important consideration; clay minerals may show aggregation even at net negative charge due to charge heterogeneity, in which the edges of the particles are positively charged while the faces are negative (edge-face or card house aggregates). Clearly, surface charge screening on particles can be altered by H+ concentration in a predictable manner. In practice, measuring the zeta potential as a function of pH can give reasonably good predictions of colloidal stability. If this approach is combined with titration of other naturally occurring substances in the medium (humic acids, carbonate, mono- and divalent cations), then the stability may be estimated for relatively complex media (e.g., natural waters).
Dispersion of nanoparticles
The production of colloidal dispersions is an area of intense activity in the field of colloid chemistry and clay science (Seta and Karathanasis 1996; Carrado et al. 2006; Lagaly 2006). The dispersion of a solid is often achieved by the removal of substances which promote aggregation or by the addition of surface active agents. This may not be desirable from an ecotoxicological perspective, because modifying a natural water to remove NOM or carbonates so that manufactured NPs will disperse could be regarded as removing ecological realism from the experiment. Alternatively, adding materials such as detergents or solvents to the test medium might result in surface modification of the NPs (e.g., surfactant adsorption to the particles); with implications for ecological realism and interference with the very toxicological properties ecotoxicologists are trying to investigate.
Most of the manufactured NPs used in ecotoxicological studies so far have been particle systems not designed to be in pure aqueous solutions (e.g., fullerenes, carbon nanotubes, carbon black) and it has been very obvious that dispersing these particles is virtually impossible in pure water by physical means alone (e.g., Smith et al. 2007). Some of the NPs (e.g., metal oxides or sulfides) would be slightly more dispersible due to hydroxylation, or at least hydration, but most of the systems also have strong attractive forces. This suggests a stark choice for the ecotoxicological experimenter: disperse the NPs and risk the criticisms outlined above, or accept that they may aggregate during the experiment. The chemistry suggests that NPs will aggregate in many types of natural waters (e.g., hard freshwater and seawater), and it may sometimes be argued that it is more ecologically relevant to use the natural aggregated NPs for experiments. Clearly, careful consideration of the solution chemistry of the water/environmental sample, and the possible methods of dispersion for the specific NP being investigated is needed (see Crane and Handy 2007 for a detailed practical discussion).
Ecotoxicologists have essentially used three basic approaches to achieve dispersion of NPs (Table 2); solvents or surfactants, sonication, or prolonged stirring. It is worth considering these approaches in the context of the fundamental physico-chemistry discussions above. First, consider solvents. Solvents such as tetrahydrofuran (THF) which has been used to disperse C60 fullerenes (e.g., Oberdörster 2004), act as a co-solvent rather than as a surfactant. THF provides a “solubilization” of the fullerene molecules in the water-THF mixture by making the solvent-water mixture less polar. This inevitably changes the behaviour of the NPs in the water. Furthermore as Smith et al. (2007) suggest, the choice of dispersant is problematic since some of the best dispersants from the viewpoint of chemistry (e.g., amides and furans (Ham et al. 2005)) are also likely to be toxic to organisms. For example, although THF has been used in ecotoxicity studies (e.g., Oberdörster 2004), there are concerns about its toxic effects, or effects of contaminants in the THF solvent (Henry et al. 2007). Zhu et al. (2006) exposed two populations of D. magna to C60 fullerenes, one dispersed in THF, the other stirred into water for a minimum of 2 months. The 48h LC50 values were 0.8 mg l−1 and >35 mg l−1 for THF and water-stirred particles respectively. These findings suggest that the toxicity of C60 particles was increased by the THF. Fortner et al. (2005) argues that this could be due to residual THF being trapped in the centre of C60 aggregates, or some other unpredicted effect of THF on particle shape or size. A compromise is possible in which a less powerful dispersant is used that will still give reasonably good dispersion, but with less risk of solvent toxicity to the test organism. This approach was used by Smith et al. (2007) who used the detergent SDS to disperse carbon nanotubes. In any case, it is likely to be essential to measure the relevant physical-chemical properties at the start and end of any ecotoxicity experiment to ensure minimal during the experiment. This is time consuming, but gives further confidence in experimental results and will help with data interpretation.
There are also some fundamental conceptual problems with the use of prolonged stirring or sonication to disperse NPs. Stirring or sonication simply provides energy that may break up aggregates, and will enable initial dispersion, but if the solution chemistry does not provide colloidal stabilization the NPs will eventually aggregate again. If prolonged stirring increases the dispersability it means that there must have been a spontaneous surface modification (e.g., hydroxylation of surface groups), and the stirring itself would not be the driving force for the dispersion. One might argue that prolonged stirring could modify the NPs so that any ecotoxicological test using NPs dispersed by this method may not be representative of the original material. These arguments must of course, be balanced against the practical issue of getting the NPs into a liquid phase to enable handling of the material during a toxicity test.
Evidence that physico-chemistry alters the ecotoxicity of nanoparticles
As a research community, we are able to describe some of the fundamental physico-chemical behaviour of colloids and other particles, and we recognise that generally the ecotoxicology of chemicals (and particles) is altered by abiotic factors such as pH, salinity, water hardness, temperature, and the presence of dissolved organic matter in the water etc. However, this is an area where research is particularly lacking for manufactured NPs. To date, there have been few systematic ecotoxicological studies to investigate how changes in abiotic factors such as pH, hardness, ionic strength, or the presence of organic ligands in the water influence ecotoxicity. Many basic questions remained to be answered. For example, for metals, do the same rules about the protective effects of increased water hardness or altered pH on some organisms apply to metals in nanoparticulate form? We might argue that they should if the functional material on the surface of the metal particle is a metal ion. Alternatively, elevation of hardness would be expected to increase aggregation due to specific sorption and/or compression of the EDL (Fig. 2), but we do not know if these effects decrease or increase toxicity yet. The ecotoxicity of NPs in seawater compared to freshwater is also unclear. Experimental evidence from colloid chemistry in saline conditions suggests that even small increases in salinity above that of freshwater (e.g., 2.5 parts per thousand, ppt) can dramatically decrease colloid concentrations by aggregation and precipitation processes (Stolpe and Hassellöv 2007). For many estuarine organisms such a small salinity change alone would have little biological effect, but this chemistry study predicts the rapid loss of colloid from the freshwater as soon as it enters the estuarine zone. This implies that toxicity tests in freshwater are not likely to be informative of toxicity in sea water, and further implies that sediment dwelling and particle filtering organisms in estuarine and marine ecosystems are likely to be important receptors for hazard assessment. Furthermore, the mechanisms of toxicity could be fundamentally different because organisms in freshwater and seawater will experience different physico-chemical forms of the same NP because of salinity effects on aggregation. At least one study has included some salinity experiments. Kashiwada (2006) exposed medaka eggs to fluorescent NPs (30 mg l−1) at a variety of different salinities. An increase in toxicity was seen with increasing salinity, along with a greater tendency for the particles to form aggregates. At a salinity of approximately 18.5 ppt the greatest fluorescence in the tissue (accumulation) was reached and 100% of the eggs were dead within 24 h. At higher salinities approaching that of normal seawater, accumulation decreased but the egg mortality rate remained high.
Does particle size and shape alter ecotoxicity?
Another possibility is that it is not just the colloid chemistry of the particles, but their size or shape that influences toxic effects (an idea well established in mammalian respiratory toxicity, Maynard and Aitken 2007). Clearly, a central question is whether or not small size alone is the cause of toxic effects? There are almost no ecotoxicological data that have systematically investigated particle-size effects, although as discussed above size will affect various physico-chemical properties. Such studies are important as they inform the need for additional hazard assessments for materials in nano form as compared to bulk form. Kashiwada (2006) showed a particle-size effect on the accumulation of fluorescent NPs in the Japanese medaka, with the smaller particles accumulating more quickly. Alternatively, studies on mammalian immune cells show no difference in toxicity of ultrafine carbon dust compared to much smaller carbon NPs (Barlow et al. 2005). There are also reports of differences in the acute toxicity of dispersed NPs compared to larger aggregates of the same material in invertebrates (Lovern and Klaper 2006; Table 3). There are not enough data to reach a consensus on this issue in ecotoxicology, but this does suggest that we ought to measure particle size, shape, and surface area during experiments (see below).
Does particle surface area alter ecotoxicity?
The fundamental concerns are that NPs are very small, and therefore present a very large surface area relative to the particle volume, or that the surface of manufactured NPs may be reactive. There is at least some speculation that the ability of NPs to generate reactive oxygen species (ROS) at the surface of the NPs, when adjacent to cell membranes, might initiate inflammation reactions or immune responses (Barlow et al. 2005). Oxidative stress has been reported in the tissues of aquatic organisms during NP exposure (CNT, Smith et al. 2007; TiO2 Federici et al. 2007). For TiO2 NPs at least, the catalytic properties of the particle surface (Watanabe et al. 1999; Hirano et al. 2005) might reflect toxic effects (see discussion in Federic et al. 2007).
A particular concern for metal-based NPs in relation to small size and large surface area is the dissolution of soluble metal ions from the surface of the particle, and perhaps the eventual complete dissolution of the particle so that only metals in solution remain. Even with solubility of a few percent, a 1 mg l−1 solution of a metal oxide NP might generate μg l−1 concentrations of metal ions in solution, and so there are concerns that some NPs will act as delivery vehicles for free metal ions. Clearly, free metal ion concentrations can be monitored during experiments, but it would be useful to know the solubility of the NP in advance. Solubility can be measured with reasonable accuracy by a combination of a size fractionation method (dialysis, ultracentrifugation, ultrafiltration, or field flow fractionation) followed by total metal analysis by ICP-MS or GF-AAS (e.g., Lyvén et al. 2003). However, these combined approaches are not routine, and become technically difficult below 10 nm (separation is less exact). In addition, contamination or sorptive loss of the metals can become important at low nanomolar concentrations.
Does particle surface charge alter ecotoxicity?
The idea that surfaces will exchange or adsorb pollutants is not new and this has been investigated in the context of colloids (Guo et al. 2002; Wilkinson and Buffle 2004; Lamelas et al. 2005). For the ecotoxicology community, adsorption phenomena have been applied to metal binding on fish gills (for a review see Handy and Eddy 2004) which is controlled by physical factors such as the net charge of the surface (e.g., polyanions on cell membranes), the ionic mobility of counter ions in the external medium (partly defined by charge density and the hydrated ionic radius of the ion), and competition with other ions in the medium such as H+ (Handy and Eddy 1991). Such experiments have led to potentially predictive equilibrium toxicological models such as the biotic ligand model (Paquin et al. 2002), and bioavailability models based on dynamic concepts (van Leeuwen et al. 2005). Some of these ideas can also be applied to NPs.
Some NMs have a net negative surface charge (e.g., fullerenes, CNTs) and will therefore bind cationic pollutants such as metals. Charge also largely determines the rate and extent of aggregation, as predicted by DLVO theory (see above). There is evidence that NPs do interact with trace metals, and surface charge may be fundamentally involved in these effects. Li et al. (2003) have shown that multi-walled CNTs (MWCNTs) have mg g−1 levels of Pb, Cd and Cu. Although this level of either metals or CNTs is unlikely in the environment, it shows a great capacity for metal interactions with NMs. In addition iron oxide particles strongly bind trace metals such as Cu and greater binding is observed at smaller particle sizes (Madden et al. 2006). Thus the presence of NPs can enhance the toxicity or bioaccumulation of other contaminants. For example, carp exposed to cadmium (Cd) in the presence of TiO2 NPs showed much greater Cd accumulation than in the presence of Cd alone (Zhang et al. 2007). Fish exposed to Cd alone had a Cd concentration of 9.07 μg g−1 dry weight compared to 23.3 μg g−1 in the Cd + TiO2 treatment. Some interactions may not relate to surface charge, but the absence of charge or the hydrophobicity of the surface. Moore et al. (1997) exposed mussels (Mytilus edulis) to nano-size particles of sucrose polyester alongside the polyaromatic hydrocarbon (PAH), anthracene. The uptake of anthracene was increased by 160%, and cellular toxicity by 122%, in the presence of NPs. One explanation is that the surface of the NP is involved in the delivery of the PAH. Clearly, the interactions of the surface of manufactured NPs with existing chemicals is worthy of further investigation, and may be as important as the intrinsic toxicity of the NPs themselves.
Concentrations of manufactured nanoparticles and nanomaterials in the environment
Handy and Shaw (2007) recently reviewed environmental exposure to manufactured NPs in the context of human health. They identified several anthropogenic sources including air pollution, the use of NMs in agriculture and farming products with consequent issues for the contamination of the food chain, as well as applications in water treatment or water-related technology that imply some potential exposure for aquatic systems. Environmental remediation is one specific application where there will be deliberate introduction of significant levels of manufactured NMs to soils and groundwaters (Tratnyek and Johnson 2006). In addition, there is a risk of diffuse pollution during the product life cycle of NMs, and concerns about disposal by landfill and incineration (Handy and Shaw 2007). However, even though we can logically predict potential sources of NP pollution, there appear to be no measurements of concentrations of manufactured NPs in field-collected samples. There are certainly no routine environmental monitoring programs for manufactured NPs. Nonetheless, simple models based on estimated product usage and NP concentration in the products could be used as a starting point to predict worse case scenarios (Boxall et al. 2007).
One counter argument to worries about manufactured NPs is that natural NPs have been in the environment for millions of years from weathering processes and natural geochemical cycles, but as we point out in the introduction, it is the novel chemistry of manufactured NPs that are also a concern. Natural NPs are not necessarily harmless (e.g., respiratory toxicity from volcanic dusts, Forbes et al. 2003). One challenge is therefore to measure both naturally occurring and manufactured NPs in the environment, and to differentiate between the toxicity of these.
The question also arises of whether effluents and waste-waters containing NMs can be cleaned up. The above physico-chemistry suggests that most NPs are likely to aggregate, given the high concentrations and types of organic matter and solids at sewage treatment works. So, existing sewage treatment methods might be effective. Alternatively, some manufactured NPs do have bacteriocidal properties (e.g., nano silver) and we do not know what the possible effects will be on the micro-organisms involved in secondary waste treatment. However, these concerns do need to be balanced by the potential benefits of nanotechnology. In the case of sewage treatment, the addition of TiO2 NPs in the presence of UV light (photocatalysis) can remove 70% of total organic carbon from waste-water (Le-Clech et al. 2006).
One final source of exposure is accidental releases and spillages of manufactured NPs directly into the environment. This is a risk with any manufactured material, not just NMs. There is little information on the persistence of manufactured NMs in environmentally relevant matrices, and there are some concerns that the standard octanol-water partition coefficients (K ow) tests may not work with NPs (Crane and Handy 2007) and therefore may not be useful for predicting bioaccumulation potential or chronic effects. As discussed above, NMs mixed with river water containing NOM remain in suspension over long time periods (Giasuddin et al. 2007; Hyung et al. 2007), although chemical interactions between the two may change the behaviour of both natural and manufactured materials. In addition, changes in pH, ionic strength and Ca concentration may promote aggregation. From the viewpoint of a spill into a river, we might therefore expect the material to stay initially in the aqueous phase, but to aggregate and settle out as it moves down stream. In either case, the material is still present in the environment and will persist. Presumably the only way these NMs will be removed from the environment is through solubilisation or diagenetic processes. Thus both these processes are of considerable importance in our understanding of environmental concentrations. However, for persistent NMs we might expect sediments, sludges and soils to be sinks for these materials, and so soil and benthic organisms should be a particular focus of ecotoxicology research.
Challenges for measuring manufactured NPs in the environment
The detection of NMs in the environment is complicated by three issues:- (i) low concentrations of NMs seem likely in the environment (ng l−1 or low μg l−1, Boxall et al. 2007), (ii) relatively high concentration of natural NPs (e.g., colloids) or organic carbon (mg l−1) may make the detection of manufactured carbon-based NPs very difficult, (iii) similar arguments apply to metal-based NPs and background levels of trace metals in the environment, in the dissolved or colloidal forms. This latter point rules out the bulk measurement of total metal NPs (e.g., by ICP-MS alone) directly in environmental samples as a means of quantifying NPs; fractionation prior to ICP-MS is needed (e.g., Lyvén et al. 2003). However, there are many approaches to measuring NPs in the laboratory (see below) and it is a question of applying these techniques to extract NPs from environmental matrices, and then to separate the NPs in the extract using existing size-separation techniques. Therefore a priority research issue is the extraction of NMs from soil and sediments, or other complex matrices. However, these challenges are no more difficult than extracting other complex contaminants (e.g., organic pollutants from soil matrices). We believe it is possible to develop physical and chemical measurement methods for field collected samples, to validate them, and then to apply them to monitoring programs. Most likely these methodologies will involve size fractionation, total element or isotope analysis, along with suitable labelling (fluorescent or isotopic) and ancillary measurements. This viewpoint is consistent with the view of Maynard et al. (2007), who primarily focused on atmospheric particle pollution and human health.
Practical issues of measuring and characterising NPs for the ecotoxicologist
Clearly, for many ecotoxicologists, particle chemistry and the practical methods needed to measure NPs may be new techniques that have not been applied in ecotoxicological studies. We therefore attempt to summarise some of the methods for measuring and characterising NPs here, and focus on some of the common methods where the equipment might be reasonably found in a typical materials science or physical chemistry laboratory in academia or industry. These methods are summarised in Tables 4 and 5. Some of these techniques require considerable training and experience, so it is inevitable that many ecotoxicologists will need to collaborate with materials scientists and chemists to collect characterisation data.
Measuring particle concentration and chemical composition
Concentrations can be based on mass, SSA or number. Ecotoxicologists are used to working with mass concentration (e.g., mg l−1) and there are several approaches for determining particle concentration. Most of the simple techniques listed in Table 4 such as gravimetric methods, turbidity, or spectrophotometry of the samples can detect mg l−1 concentrations of material and the equipment needed is familiar and available in most laboratories. These approaches might be useful for a lethal dose test where high concentrations are used. However, such approaches generally cannot detect μg l−1 concentrations or less, and might be of limited use for chronic studies, or for some field-collected samples where concentrations of NPs may be low. In addition, choice of standards to relate instrument signal to concentration is problematic in these complex systems. More sensitive methods, such as laser induced breakdown detection (LIBD) might be employed for the latter, but this is a more specialist technique and the equipment is not routinely available in most laboratories. LIBD, like all laser techniques is more difficult to perform with non-spherical particles or porous organic material that might alter the behaviour of the laser light on the surface of the material, but it would work well with, for example, hard metal oxide spheres. For metal-based manufactured NPs, existing methods of trace element detection can be employed such as ICP-MS, ICP-OES, and GFAAS (or flame AAS at high concentrations) that have been used for many years in metal toxicity studies (e.g., Handy and Depledge 1999). However, some caution is also needed. Routine protocols for trace metals should also be validated with the metal NP of interest. For example, there is some evidence that ICP-MS for TiO2 NPs behaves differently from Ti metal solutions (Federici et al. 2007) and there are concerns that NPs may not fully atomise in the furnace of the instruments. Of course, ICP-MS and similar traditional metal analysis techniques can be used to overview the elemental composition of a liquid sample. However, for natural waters or field-collected samples this will also include the background metal levels, and it is not always possible to differentiate the metal composition of the manufactured NP from this background.
An alternative approach is to look at the elemental composition of individual NPs using electron microscopy techniques. These can be coupled to methods for analysing element concentrations and speciation within a single particle such as X-ray diffraction and reflection methods (e.g., energy dispersive X-ray transmission electron microscopy, TEM-EDX methods). These have been used by geochemists and biologists to look at naturally occurring metal granules and NPs (e.g., Wu et al. 2002). These techniques offer spatial resolution at the nm level, and for metals, it is possible to detect the main components in a particle (e.g., heavy metals and major anions like phosphorus). The main limitation for the ecotoxicologist is that some skill in electron microscopy is needed, but these facilities are available at many universities.
Particle number (i.e., number of particles/unit volume), perhaps reflecting the cumulative toxic effect associated with shape or surface area of each particle, may be as critical as the measured mass concentration in particle toxicity (Oberdörster et al. 2007). Particle number can be determined by visual counting methods such as light or electron microscopy (EM). Light microscopy has a μm resolution, and will detect large aggregates, or polymerised strands of NMs (e.g., Lam et al. 2004), but cannot detect individual NPs. EM techniques offer nm scale resolution, making it possible to count individual particles and detect shape. Whether scanning, transmission, or atomic force microscopy (AFM) is used, all these techniques suffer some draw backs. Firstly, the NPs need to be transferred from their dispersed state to a dried or vacuum state on a support grid in order to be placed in the microscope. The drying inevitably will alter the colloid behaviour of the material, and there is no way of being certain that aggregates present on an EM grid are truly representative of the sample, or whether they are just an artefact of preparation. Secondly, EM techniques use very small volumes, and many grids from repeat sub-samples of the dispersion would need to be counted in order to give a statistically robust measure of particle number per litre of dispersion. Other techniques that count particle number in the whole suspension may therefore be more practicable. Nonetheless microscopy techniques are very valuable to visualize and determine the shape of particles, but the inherent limitations above should be kept in mind and characterization should preferably not solely be based on microscopy.
Measuring particle shape, size, and surface area
Measurement of particle shape, size and surface area (in addition to particle numbers) could be used merely to confirm the structure of the material being used for experiments, but equally there are suggestions that particle surface area may be a better metric than concentration to describe the dose–response relationship (Oberdörster et al. 2007). In addition, there are well known examples in the respiratory toxicology literature where particle size and shape have been critical to uptake and toxicity (e.g., the uptake of asbestos fibres into lung epithelial cells, Bonner 2007).
There are several optical methods that may be used to determine information about particle shape, size distribution, and sometimes, additional information on the number of particles in the dispersion can be obtained (Table 5). Given that the dispersion of NPs is sensitive to the aggregation chemistry outlined above, techniques that give minimal perturbation and without any additional sample preparation are preferred. This is partly why probing methods using electromagnetic radiation (e.g., light, X-rays or neutrons) are preferred, and the scattering patterns can be related to physical or even structural properties of the particles. Of these approaches, the only method that is readily available in many laboratories is dynamic light scattering (DLS) where the fluctuations in the scattered light from single NPs can be mathematically related to the diffusion coefficient (and hydrodynamic diameter) of the particles. DLS is relatively simple to perform, but has two important limitations. Firstly, it is not suitable to determine very broad size distributions (e.g., combinations of nano and fine particles), but it would work well for typical samples of manufactured NPs. For example, where the average particle size is expected to be 50 nm, there may be a range of sizes ± 30 nm about the mean. Secondly, DLS is an intensity-based technique and the determined size is biased towards larger components of the samples (due to the non-linear relationship of light scattering intensity as a function of size, Holthoff et al. 1996). Therefore even a very small fraction of dust or other micrometer sized particles will distort or even cover the signal from the NPs. It should be emphasised that at constant number concentration the intensity of the scattered light scales with d6 when the particles are much smaller than the laser wavelength and still with d2 if particles are similar or larger than the wavelength. Hence with some 1 μm particles in the sample even a high number of 10 nm particles will not even show up in the measurement. Additionally it has to be pointed out that the intensity weighted distribution functions from DLS cannot be transformed into volume, surface or number distributions without exact knowledge of particle shape, structure and refractive index; and multi-angle measurements should always be performed for such conversions (Filella et al. 1997). Nonetheless, DLS when applied carefully can be used to clarify particle sizes in test materials, and is useful for monitoring changes in aggregation behaviour during experiments. DLS could also be used to characterize fractions collected from other size separation techniques.
Another minimum perturbation method for determining the size distribution of NPs is Field-Flow Fractionation (FFF) that is a chromatography-like size-fractionating method without a stationary phase. FFF separates the NPs according to their particle size (diffusion coefficient) only by means of hydrodynamic forces present as the particles flow gently down an open channel (e.g., Taylor and Simkiss 2004). Some kind of detector is required to monitor the size distributions emerging from the channel. Some of these techniques are also used for concentrations measurements (e.g., as in Table 4), including UV absorbance, ICP-MS etc. Therefore, the detection method needs considering in addition to the FFF itself.
BET analysis (originally reported by Brunauer et al. 1938) is perhaps the most readily available method for determining particle surface area, and in addition, net surface charge (related to zeta potentials) may be useful for describing the possible chemical or biological reactivity of the particle surface. Surface area, or rather specific surface area (SSA, e.g., m2/g of material), can be measured by calculation using transmission EM measurements of the particle dimensions. However, EM is labour intensive, and cannot easily take into account the complex surface shape or porosity that may influence surface area measurements. BET analysis may therefore have some advantages over EM techniques (Table 5), but the SSA measured by BET in a dry sample may not coincide with the apparent surface area in aqueous dispersion, especially for aggregating particles (Waychunas et al. 2005). It is therefore better to use a combination of BET, EM, and other techniques for both accuracy and confidence in the data collected. There will also need to be an ecotoxicological interpretation of any surface area measurements in relation to the aggregation chemistry of the NPs. We may need to consider “apparent” surface area (surface area lost due to particle–particle contact in an aggregate, which may be dominant or negligible depending on the aggregate type) and whether the measurement method would reflect this. Also we may need to consider the issue of the “bioreactive” surface area, similar to the issues in soil matrix ecotoxicology (Reid et al. 2000), and how much of the surface area is actually accessible to the organism. Alternatively, from a chemistry perspective, the “active” surface area may be defined as that fraction of the total SSA which contains potentially biologically active material such as metal ions. Measurement of the latter parameters is not well defined, but nonetheless these are important concepts for the ecotoxicologist to consider when interpreting surface area data. A word of caution must also be inserted here. Both TEM and BET analyses are performed under ultra high vacuum conditions (UHV) which means that the sample is completely dehydrated prior to measurement. We should be mindful that in ecotoxicological experiments we will be looking at surface area effects in the solution phase and the behaviour of the particle surface (unstirred layers, charge shielding etc) will be different to the behaviour of the dry nanopowder. Importantly, because of the physico-chemistry outlined above, the bioreactive surface area is likely to be less than the measured total SSA. In addition, because of these complexities the SSA measured in the “solution phase” may not be the same as the dry powder, even when using an identical measurement technique.
Considerations for hazard and risk assessment
Our review highlights some of the challenges facing ecotoxicologists, (academic, industrial and regulatory) and risk assessors as they attempt to assess the hazards and environmental risks of manufactured NMs. A critical issue is to ensure that the paradigm shift from (e.g., dissolved) chemical ecotoxicology to manufactured NP ecotoxicology is an informed one, building on information already known about naturally occurring and anthropogenic particle properties, behaviour and toxicity. There is clearly an overarching need to bring the physical, chemical and biological communities together in this regard. This is of particular importance both at the early stage of design of ecotoxicological experiments and in their execution. It also highlights the need to take this interdisciplinary approach when undertaking problem formulation (e.g., endpoint selection) within environmental risk assessment (Owen and Handy 2007). Nanoecotoxicology will inevitably develop into a rigorous discipline as these science communities come together and exchange knowledge, helping to develop better source—pathway—receptor linkages as part of problem formulation within environmental risk assessment.
This review highlights the fact that a primary consideration for hazard and risk assessment is the need to understand both the properties of the starting nanomaterial and the physico-chemical nature of the test and potential receptor systems before commencing an ecotoxicological study. Although the temptation to launch into an ecotoxicological test is great, the above review shows that an understanding of intrinsic characteristics of the starting material (e.g., particle size and ligand chemistry) and an understanding of how the material will behave in the study medium (e.g., by measuring zeta potential as a function of media pH to assess likely colloid stability; titration of humic substances in the media, etc.) are fundamental to understanding the likely fate, behaviour, uptake and ecotoxicity of the material under environmental conditions. This knowledge must be used to inform the design of the study. An evaluation of intrinsic properties is in fact already recognised as an important component of environmental risk assessment for chemicals. Development of a consensus is important on a base set of properties to be assessed for the starting NM characterisation, and measurements of NM and relevant abiotic parameters to be made during organism exposure: some of these issues are currently being explored by the Organisation for Economic Cooperation and Development (OECD), which has established a Working Party on Manufactured NMs (WPMN) to assess, among other things, the fitness for purpose of test guideline methods (including ecohazard) (http://www.oecd.org/env/nanosafety).
At the moment it is too early to say with any certainty whether the endpoints currently employed within regulatory chemicals ecohazard tests (e.g., survival, growth, and reproduction) need to be amended for NMs (Crane and Handy 2007). These authors reviewed the use of regulatory ecotoxicity tests with NPs, concluding that there appears to be a majority view that the general approach for undertaking a risk assessment and the general framework for applying existing regulatory ecotoxicity tests should be acceptable for NPs (Crane and Handy 2007). However, they point out that some modifications of sample preparation and handling will be needed to accommodate the physico-chemistry of NPs. They also suggest that careful consideration should be given to the use of dispersed versus non-dispersed NPs, and whether or not the use of any reference materials should be part the experimental design for regulatory tests. If reference materials are used, then we must consider if these are intended as positive or negative controls, and how similar the reference material is in size, shape and chemical reactivity to the test material. Many of the reference materials used as positive controls in mammalian toxicology (e.g., carbon black, silica particles) may not be toxic to aquatic species and remain to be experimentally proven as useful controls for ecotoxicology (Crane and Handy 2007; Federici et al. 2007). These studies and our review both show that the exposure aspects of ecohazard assessment (e.g., dosimetry-measurement and characterisation in test media and exposed organisms; associated abiotic factors that influence behaviour) and how such exposure data are then reported in studies (e.g., surface area, particle number or mass concentration) are critical areas where collaboration involving the materials science, particle chemistry and ecotoxicological communities could be highly profitable in terms of developing consensus and good practice. In the same way that standard ecotoxicological tests for dissolved chemicals advocate the reporting of actual rather than nominal concentration data, nanoecotoxicology will need to rise to a similar challenge for manufactured NPs in terms of measuring and reporting exposure data. Hand in hand with this is the need for routine techniques to be available to the ecotoxicologist for measurement and characterisation of NMs, some of which have been reviewed above. A further significant issue to be addressed is the need for standard reference materials for bench marking mammalian toxicological tests (Aitken et al. 2007), and how these bench marks should be applied in ecotoxicology. Federici et al. (2007) argues that some of the reference materials used for mammalian respiratory toxicity are not appropriate for aquatic species, and we may need to develop our own reference materials for ecotoxicology.
Is the complexity and diversity of NMs overwhelming for the regulator?
The chemical classification of NMs can fall into broad categories such as, (i) carbon based structures including carbon nanotubes and carbon fullerenes; (ii) metal-containing NPs such as metal oxides or metal particles; or (iii) quantum dots. Alternatively, manufactured NMs could be classified by material type (coatings, composite materials etc; see Handy and Shaw 2007 for discussion). Regardless of how these materials are classified for regulatory purposes, even within a single chemical there will be immense diversity in crystal structures, sizes, morphologies, SSAs, and surface charges. Consequently, there is likely to be diversity in chemical reactivity and ecotoxicity. In addition, even with batches of the same material there can be differences in particle size distributions or the level of metal contaminants in the material.
This complexity presents a real challenge to the rational development of toxicity testing strategies and the risk assessment of these new materials (Owen and Handy 2007). Nevertheless, it is only quantitatively, and not qualitatively, different from the situation for other pollutants. For instance, in the area of metal bioavailability and toxicity, much progress has been made in combining metal speciation and bioavailability/toxicity into a single coherent framework, initially based on chemical thermodynamics (Slaveykova and Wilkinson 2005; Paquin et al. 2002) and more recently based on biological, physical and chemical dynamic concepts (van Leeuwen et al. 2005; Croteau and Luoma 2007). Consideration of individual metals and toxicity to particular biological species can be performed within this framework. The point is that a mechanistic approach involving understanding of the chemistry and mechanisms of biological effect has enabled this complexity to be unravelled. We need to do the same with NMs. Although we have outlined the fundamental physico-chemical features of particles here, and report recent observations on toxic effects of NMs, a mechanistic understanding of the toxic effects remains to be established. If ecotoxicologists study the mechanisms involved and can relate these in a predictable way to physico-chemistry and abiotic factors measured in the environment; it will be easier for risk assessors to relate environmental quality with toxic effects within a regulatory framework.
The complexity of behaviour of NPs in environmental systems described above may drive ecotoxicologists to adopt a mixtures approach to ecohazard assessment from the outset: it appears almost impossible to undertake an assessment of NP ecohazard (and indeed exposure) without some understanding of mixture effects and the abiotic (and biotic) controls on NP behaviour in natural systems. A better understanding of mixture ecotoxicity may be one of the enduring benefits of research into manufactured NPs. Another may be that while in the longer term it may transpire that environmental exposure to manufactured NPs, is with some exceptions, rather small, the ongoing research could provide huge insights into the fate, behaviour and toxicity of incidentally-formed NPs in the environment (e.g., plastic particles) where exposure may be far greater, and a better understanding of how these interact with chemicals to influence their bioavailability and toxicity.
Conclusions, recommendations, and knowledge gaps
Overall, the ecotoxicology literature on NPs and NMs is only just beginning, but toxic effects have been identified in a range of fish and invertebrates that raise sufficient concern that NPs in the environment could have adverse effects on wildlife, if present at high enough levels. There are several significant knowledge gaps in the ecotoxicology. For example, information is generally lacking on bacteria, plants, and higher vertebrate species. We therefore recommend research on these taxa if credible source-pathway-receptor linkages can be demonstrated which would lead to exposure concentrations of NPs likely to cause harm. Furthermore, the studies reported so far are also largely observational, and often use high mg l−1 doses to ensure biological effect (proof of principle experiments). This is perhaps no surprise, and before we can evolve credible hypotheses on mechanisms of ecotoxicity some period of observation and data collection is needed. Clearly we are still in this data collection phase regarding biological effects, and information on effects at much lower concentrations and over longer timescales will be needed as information on environmental exposure emerges.
The ecotoxicity of NMs and NPs requires a multi-disciplinary approach, and ecotoxicologists need to understand the main issues in particle chemistry in order to interpret ecotoxicological data correctly. These include the effects of particle size, shape and surface area, and the interactions of the particles with other material in the water or environmental matrix. Our knowledge of the effects of abiotic factors on the ecotoxicity of NPs is poor, and work on salinity, pH, water hardness, the presence of NOM or other colloid materials need to be investigated. All the above parameters need to be monitored in ecotoxicology experiments with NPs. There are also some less intuitive concerns. NPs and NMs may be able to adsorb other chemicals, and there is a risk that NMs will be delivery vehicles for other toxic substances, or that there may be synergistic toxic effects when NPs are present in mixtures of other chemicals.
Finally, the situation regarding environmental monitoring is problematic. Physico-chemical methods to detect manufactured NPs in the environment need to be developed in order to facilitate chemical monitoring. Also, there are no biomarkers for NPs that could be used as part of a biological monitoring program. Although existing regulatory toxicity tests may work (with modifications) for NPs, the risk analysis would remain incomplete without measurement of real concentrations of NPs in the environment.
References
Aitken RJ, Chaudhry MQ, Boxall ABA, Hull M (2006) Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med 56:300–306
Aitken RJ, Creely KS, Tran CL (2004) Nanoparticles: an occupational hygiene review. Research Report 274, HSE books, Norwich
Aitken RJ, Tran CL, Donaldson K, Stone V, Cumpson P, Johnstone J, Chaudhry Q, Cash S (2007) Reference materials for engineered nanoparticle toxicology and metrology. Preliminary Note May 2007. Report by the Institute of Occupational Medicine (IOM) for the Department of the Environment and Rural Affairs (DEFRA), Report No. ACHS/07/09A 5 June 2007. Available at: http://www.defra.gov.uk/environment/chemicals/achs/070605/ACHS0709A.pdf
Ammann M, Burtscher H, Siegmann HC (1990) Monitoring volcanic activity by characterization of ultrafine aerosol emissions. J Aerosol Sci 21:S275–S278
Barlow PG, Donaldson K, MacCallum J, Clouter A, Stone V (2005) Serum exposed to nanoparticle carbon black displays increased potential to induce macrophage migration. Toxicol Lett 155:397–401
Barnhill HN, Reuther R, Ferguson PL, Dreher T, Wang Q (2007) Turnip yellow mosaic virus as a chemoaddressable bionanoparticle. Bioconjugate Chem 18:852–859
Bermudez E, Mangum JB, Wong BA, Asgharian B, Hext PM, Warheit DB, Everitt JI (2004) Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci 77:347–357
Bhattacharjee S, Chen JY, Elimelech M (1998) DLVO interaction energy between spheroidal particles and a flat surface. Colloid Surface A 165:143–156
Birdi KS (ed) (1997) Handbook of surface and colloid chemistry. CRC Press, Boca Raton, 763 pp
Bonner JC (2007) Lung fibrotic responses to particle exposure. Toxicol Pathol 35:148–153
Boxall ABA, Chaudhry Q, Sinclair C, Jones A, Aitken R, Jefferson B, Watts C (2007) Current and future predicted environmental exposure to engineered nanoparticles. Report by the Central Science Laboratory (CSL) York for the Department of the Environment and Rural Affairs (DEFRA), UK. Available at: http://www.defra.gov.uk/science/Project_Data/DocumentLibrary/CB01098/CB01098_6270_FRP.pdf
Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fievet F (2006) Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett 6:866–870
Brody AL (2006) Nano and food packaging technologies converge. Food Technol 60:92–94
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Buffle J, Leeuwen HP (eds) (1992) Environmental particles vol 1. IUPAC environmental analytical and physical chemistry series. Lewis Publishers, Boca Raton, 554 pp
Buffle J, Leeuwen HP (eds) (1993) Environmental particles vol 2. IUPAC environmental analytical and physical chemistry series. Lewis Publishers, Boca Raton, 42 pp
Carrado KA, Decarreau A, Petit S, Bergaya F, Lagaly G (2006) Synthetic clay minerals and purification of natural clays. In: Bergaya F, Theng BKG, Lagaly G (eds) Handbook of clay science. Elsevier, Amsterdam pp 115–140
Crane M, Handy RD (2007) An assessment of regulatory testing strategies and methods for characterizing the ecotoxicological hazards of nanomaterials, Report for Defra, London, UK. Available at: http://randd.defra.gov.uk/Document.aspx?DocumentID=2270
Croteau M-N, Luoma SN (2007) Characterizing dissolved Cu and Cd uptake in terms of the biotic ligand and biodynamics using enriched stable isotopes. Environ Sci Technol 41:3140–3145
Cui DX, Tian FR, Ozkan CS, Wang M, Gao HJ (2005) Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett 155:73–85
Derjaguin BV, Landau LD (1941) Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Phys Chim 14:733–762
Dobson J (2001) Nanoscale biogenic iron oxides and neurodegenerative disease. FEBA Lett 496:1–5
Duran N, Marcato PD, De Souza GIH, Alves OL, Esposito E (2007) Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J Biomed Nanotech 3:203–208
Eddy FB (1982) Osmotic and ionic regulation in captive fish with particular reference to salmonids. Comp Biochem Physiol B 73:125–141
Ek KH, Rauch S, Morrison GM, Lindberg P (2004) Platinum group elements in raptor eggs, faeces, blood, liver and kidney. Sci Total Environ 334–335:149–159
Elimelech M, Gregory J, Jia X, Williams RI (1995) Particle deposition and aggregation: measurement, modelling and simulation. Butterworth-Heinemann, Woburn, 441 pp
Fagan PJ, Calabrese JC, Malone B (1991) The chemical nature of Buckminster fullerene (C60) and the characterization of a platinum derivative. Science 252:1160–1161
Ferin J, Oberdörster G (1985) Biological effects and toxicity assessment of titanium dioxides—anatase and rutile. Am Ind Hyg Assoc J 46:69–72
Ferin J, Oberdörster G, Soderholm SC, Gelein R (1991) Pulmonary tissue access of ultrafine particles. J Aerosol Med 4:57–68
Filella M, Zhang J, Newman M, Buffle J (1997) Analytical applications of photon correlation spectroscopy for size distribution measurements of natural colloidal suspensions: capabilities and limitations. Colloid Surface A 120:27–46
Forbes L, Jarvis D, Potts J, Baxter PJ (2003) Volcanic ash and respiratory symptoms in children on the island of Montserrat, British West Indies. Occup Environ Med 60:207–211
Fortner JL, Lyon D, Sayer CM, Boyd A, Frehner JG, Hotze EM, Alemann LB, Hughes J. (2005) C60 in water: nanocrystal formation and microbial response. Environ Sci Technol 39:4307–4316
Federici G, Shaw BJ, Handy RD (2007) Toxicity of titanium dioxide nanoparticles to rainbow trout, (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat Toxicol 84:415–430
Freitas RA (2005) What is nanomedicine? Nanomedicine 1:2–9
Fu GF, Vary PS, Lin CT (2005) Anatase TiO2 nanocomposites for antimicrobial coatings. J Phys Chem 109:8889–8898
Giasuddin ABM, Kanel SR, Choi H (2007) Adsorption of humic acid onto nanoscale zerovalent iron and its effect on arsenic removal. Environ Sci Technol 41:2022–2027
Grasso D, Subramaniam K, Butkus M, Strevett K, Bergendahl J (2002) A review of non-DLVO interactions in environmental colloidal systems. Rev Environ Sci Biotechnol 1:17–38
Grubek-Jaworska H, Nejman P, Czuminska K, Przybylowski T, Huczko A, Lange H, Bystrzejewski M, Baranowski P, Chazan R (2006) Preliminary results on the pathogenic effects of intratracheal exposure to one-dimensional nanocarbons. Carbon 44:1057–1063
Guo LD, Santschi PH, Ray SM (2002) Metal partitioning between colloidal and dissolved phases and its relation with bioavailability to American oysters. Marine Environ Res 54:49–64
Ham HT, Choi YS, Chung IJ (2005) An explanation of dispersion states of single-walled carbon nanotubes in solvents and aqueous surfactant solutions using solubility parameters. J Colloid Interface Sci 286:216–223
Handy RD, Depledge MH (1999) Physiological responses: their measurement and use as environmental biomarkers in ecotoxicology. Ecotoxicology 8:329–349
Handy RD, Eddy FB (1991) Effects of inorganic cations on Na+ adsorption to the gill and body surface of rainbow trout, Oncorhynchus mykiss, in dilute solutions. Can J Fish Aquat Sci 48:1829–1837
Handy RD, Eddy FB (2004) Transport of solutes across biological membranes in eukaryotes: an environmental perspective. In: van Leeuwen HP, Köster W (eds) Physicochemical kinetics and transport at chemical-biological interphases, IUPAC series. John Wiley, Chichester, pp 337–356
Handy RD, Shaw BJ (2007) Toxic effects of nanoparticles and nanomaterials: implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc 9:125–144
Hasegawa S, Wakamatsu S, Ohara T, Itano Y, Saitoh K, Hayasaki M, Kobayashi S (2007) Vertical profiles of ultrafine to supermicron particles measured by aircraft over Osaka metropolitan area in Japan. Atmos Environ 41:717–729
Hendershot WH, Lavkulich LM (1979) Effect of sodium chloride saturation and organic matter removal on the value of zero-point of charge. Soil Sci 128:136–141
Henry TB, Menn F-M, Fleming JT, Wilgus J, Compton RN, Sayler GS (2007) Attributing effects of aqueous C60 nano-aggregates to tetrahydrofuran decomposition products in larval zebrafish by assessment of gene expression. Environ Health Perspect 115:1059–1065
Hirano K, Nitta H, Sawada K (2005) Effect of sonication on the photo-catalytic mineralization of some chlorinated organic compounds. Ultrasonics Sonochem 12:271–276
Holthoff H, Egelhaaf SU, Borkovec M, Schurtenberger P, Sticher H (1996) Coagulation rate measurements of colloidal particles by simultaneous static and dynamic light scattering. Langmuir 12:5541–5549
Hund-Rinke K, Simon M (2006) Ecotoxic effect of photocatalytic active nanoparticles TiO2 on algae and daphnids. Environ Sci Pollut Res 13:225–232
Hyung H, Fortner JD, Hughes JB, Kim J-H (2007) Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environ Sci Technol 41:179–184
Kallay N, Zalac S (2002) Stability of nanodispersions: a model for kinetics of aggregation of nanoparticles. J Colloid Interface Sci 253:70–76
Kammer FVD (2005) Characterization of environmental colloids applying field-flow fractionation—multi detection analysis with emphasis on light scattering techniques. PhD thesis, Hamburg University of Technology, pp 13–15
Karnik BS, Davies SH, Baumann MJ, Masten SJ (2005) Fabrication of catalytic membranes for the treatment of drinking water using combined ozonation and ultrafiltration. Environ Sci Technol 39:7656–7661
Kashiwada S (2006) Distribution of nanoparticles in the see-through Medaka (Oryzias latipes). Environ Health Perspect 114:1697–1702
Konishi Y, Ohno K, Saitoh N, Nomura T, Nagamine S, Hishida H, Takahashi Y, Uruga T (2007) Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J Biotechnol 128:648–653
Koyama S, Endo M, Kim YA, Hayashi T, Yanagisawa T, Osaka K, Koyama H, Haniu H, Kuroiwa N (2006) Role of systemic T-cells and histopathological aspects after subcutaneous implantation of various carbon nanotubes in mice. Carbon 44:1079–1092
Kreuter J (2005) Application of nanoparticles for the delivery of drugs to the brain. In: de Boer AG (ed) Drug transport(ers) and the diseased brain, international congress series, 1277. Elsevier, Amsterdam, pp 85–94
Lagaly G (2006) Colloid clay science. In: Bergaya F, Theng BKG, Lagaly G (eds) Handbook of clay science. Elsevier, Amsterdam pp 141–246
Lam CW, James JT, McCluskey R, Hunter RL (2004) Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci 77:126–134
Lamelas C, Wilkinson KJ, Slaveykova VI (2005) Influence of the composition of natural organic matter on Pb bioavailability to microalgae. Environ Sci Technol 39:6109–6116
Lead JR, Wilkinson KJ (2006) Aquatic colloids and nanoparticles: current knowledge and future trends. Environ Chem 3:159–171
Le-Clech P, Lee EK, Chen V (2006) Hybrid photocatalysis/membrane treatment for surface waters containing low concentrations of natural organic matters. Water Res 40:323–330
Li JF, Yu WH, Chen CS, Wei WCJ (2003) Modelling nanosized colloidal particle interactions with Brownian dynamics using a discrete element method. Nanotech 2:566–569
Linse S, Cabaleiro-Lago C, Xue W-F, Lynch I, Lindman S, Thulin E, Radford SE, Dawson KA (2007) Nucleation of protein fibrillation by nanoparticles. PNAS USA 104:8691–8696
Lovern SB, Klaper RD (2006) Daphnia magna mortality when exposed to titanium nanoparticles and fullerene (C60) nanoparticles. Environ Toxicol Chem 25:1132–1137
Lovern SB, Strickler JR, Klaper R (2007) Behavioral and physiological changes in Daphnia magna when exposed to nanoparticle suspensions (titanium dioxide, nano-C-60, and C(60)HxC(70)Hx). Environ Sci Technol 41:4465–4470
Lyon DY, Fortner JD, Sayes CM, Colvin VL, Hughes JB (2005) Bacterial cell association and antimicrobial activity of a C-60 water suspension. Environ Toxicol Chem 24:2757–2762
Lyon DY, Adams LK, Falkner JC, Alvarez PJJ (2006) Antibacterial activity of fullerene water suspensions: effects of preparation method and particle size. Environ Sci Technol 40:4360–4366
Lyvén B, Hassellöv M, Turner DR, Haraldsson C, Andersson K (2003) Competition between iron- and carbon-based colloidal carriers for trace metals in a freshwater assessed using flow field-flow fractionation coupled to ICPMS. Geochim Cosmochim Acta 67:3791–3802
Madden AS, Hochella MF (2005) A test of geochemical reactivity as a function of mineral size: manganese oxidation promoted by hematite nanoparticles. Geochim Cosmochim Acta 69:389–398
Madden AS, Hochella MF, Luxton TP (2006) Insights for size-dependent reactivity of hematite nanomineral surfaces through Cu2+ sorption. Geochim Cosmochim Acta 70:4095–4104
Maynard AD, Aitken RJ (2007) Assessing exposure to airborne nanomaterials: current abilities and future requirements. Nanotoxicology 1:26–41
Maynard AD, Ku BK, Emery M, Stolzenburg M, McMurry PH (2007) Measuring particle size-dependent physicochemical structure in airborne single walled carbon nanotube agglomerates. J Nanoparticle Res 1:85–92
Moore MN (2006) Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int 32:967–976
Moore MN, Lowe DM, Soverchia C, Haigh SD (1997) Uptake of a non-calorific, edible sucrose polyester oil and olive oil by marine mussels and their influence on uptake and effects of anthracene. Aquat Toxicol 39:307–320
Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, Arras M, Fonseca A, Nagy JB, Lison D (2005) Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Applied Pharmacol 207:221–231
Oberdörster E (2004) Manufactured nanomaterials (Fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ Health Perspect 112:1058–1062
Oberdörster G, Ferin J, Gelein R, Soderholm SC, Finkelstein J (1992) Role of the alveolar macrophage in lung injury—studies with ultrafine particles. Environ Health Perspect 97:193–199
Oberdörster E, Zhu SQ, Blickley TM, Clellan-Green P, Haasch ML (2006) Ecotoxicology of carbon-based engineered nanoparticles: effects of fullerene (C-60) on aquatic organisms. Carbon 44:1112–1120
Oberdörster G, Oberdörster E, Oberdörster J (2007) Concepts of nanoparticle dose metric and response metric. Environ Health Perspect 115:A290–A291
Owen R, Depledge MH (2005) Nanotechnology and the environment: risks and rewards. Marine Pollut Bull 50:609–612
Owen R, Handy RD (2007) Formulating the problems for environmental risk assessment of nanomaterials. Environ Sci Technol 41:5582–5588
Paquin PR, Gorsuch JW, Apte S, Batley GE, Bowles KC, Campbell PGC, Delos CG, Di Toro DM, Dwyer RL, Galvez F, Gensemer RW, Goss GG, Hogstrand C, Janssen CR, Mcgeer JC, Naddy RB, Playle RC, Santore RC, Schneider U, Stubblefield WA, Wood CM, Wu KB (2002) The biotic ligand model: a historical overview. Comp Biochem Physiol C 133:3–35
Reid BJ, Jones KC, Semple KT (2000) Bioavailability of persistent organic pollutants in soils and sediments—a perspective on mechanisms, consequences and assessment. Environ Pollut 108:103–112
Roberts AP, Mount AS, Seda B, Souther J, Qiao R, Lin SJ, Ke PC, Rao AM, Klaine SJ (2007) In vivo biomodification of lipid-coated carbon nanotubes by Daphnia magna. Environ Sci Technol 41:3025–3029
Roco MC (2003) Nanotechnology: convergence with modern biology and medicine. Curr Opin Biotechnol 14:337–346
Royal Society (2004) Nanoscience and nanotechnologies: opportunities and uncertainties. Report by the Royal Society and The Royal Academy of Engineering. [http://www.nanotec.org.uk/finalReport.htm]
Scarano G, Morelli E (2003) Properties of phytochelatin-coated CdS nanocrystallites formed in a marine phytoplanktonic alga (Phaeodactylum tricornutum, Bohlin) in response to Cd. Plant Sci 165:803–810
SCENIHR (2005) Opinion on the appropriateness of existing methodologies to assess the potential risks associated with engineered and adventitious products of nanotechnologies. Scientific Committee on Emerging and Newly Identified Health Risks, European Commission SCENIHR/002/05
Scott-Fordsmand J, Krogh PH, Johansen A, Schaefer M (2007) Toxicity of nanoparticles to earthworms. Poster presented at SETAC Europe 17th Annual Meeting, Multiple stressors for the environment and human health present and future challenges and perspectives, Porto, Portugal, 20–24 May 2007
Sekiguchi Y, Yao Y, Ohko Y, Tanaka K, Ishido T, Fujishima A, Kubota Y (2007) Self-sterilizing catheters with titanium dioxide photocatalyst thin films for clean intermittent catheterization: basis and study of clinical use. Int J Urol 14:426–430
Serita F, Kyono H, Seki Y (1999) Pulmonary clearance and lesions in rats after a single inhalation of ultrafine metallic nickel at dose levels comparable to the threshold limit value. Ind Health 37:353–363
Seta AK, Karathanasis AD (1996) Water dispersible colloids and factors influencing their dispersibility from soil aggregates. Geoderma 74:255–266
Slaveykova VI, Wilkinson KJ (2005) Predicting the bioavailability of metals and metal complexes: critical review of the biotic ligand model. Environ Chem 2:9–24
Smith CJ, Shaw BJ, Handy RD (2007) Toxicity of single walled carbon nanotubes on rainbow trout, (Oncorhynchus mykiss): respiratory toxicity, organ pathologies, and other physiological effects. Aquat Toxicol 82:94–109
Stolpe B, Hassellöv M (2007) Changes in size distribution of fresh water nanoscale colloidal matter and associated elements on mixing with seawater. Geochim Cosmochim Acta 71:3292–3301
Stumm W (1993) Aquatic colloids as chemical reactants: surface structure and reactivity. Colloids Surface A 73:1–18
Takenaka S, Karg E, Kreyling WG, Lentner B, Schulz H, Ziesenis A, Schramel P, Heyder J (2004) Fate and toxic effects of inhaled ultrafine cadmium oxide particles in the rat lung. Inhal Toxicol 16:83–92
Taylor MG, Simkiss K (2004) Transport of colloids and particles across cell membranes. In: van Leeuwen HP, Köster W (eds) Physicochemical kinetics and transport at chemical-biological interphases, IUPAC series. John Wiley, Chichester, pp 271–336
Thill A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, Flank AM (2006) Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ Sci Technol 40:6151–6156
Tong Z, Bischoff M, Nies L, Applegate B, Turco RF (2007) Impact of fullerene on a soil microbial community. Environ Sci Technol 41:2985–2991
Tratnyek PG, Johnson RL (2006) Nanotechnologies for environmental cleanup. NanoToday 1:44–48
US EPA (2005) Nanotechnology White, External Review Draft 2nd December 2005. Science Policy Council, U.S. Environmental Protection Agency, Washington DC
Van Leeuwen HP, Galceran J (2004) Biointerfaces and mass transfer. In: van Leeuwen HP, Köster W (eds) Physicochemical kinetics and transport at chemical-biological interphases, IUPAC series. John Wiley, Chichester, pp 113–146
Van Leeuwen HP., Town RM, Buffle J, Cleven RFML, Davison W, Puy J, van Riemsdijk WH, Sigg L (2005) Dynamic speciation analysis and bioavailability of metals in aquatic systems. Environ Sci Technol 39:8545–8556
Verwey EJW, Overbeek JThG, (1948) Theory of the stability of lyophobic colloids: the interaction of sol particles having an electric double layer. Elsevier, New York, 205 pp
Warheit DB (2006) What is currently known about the health risks related to carbon nanotube exposures? Carbon 44:1064–1069
Warheit DB, Brock WJ, Lee KP, Webb TR, Reed KL (2005) Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: impact of surface treatments on particle toxicity. Toxicol Sci 88:514–524
Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GAM, Webb TR (2004) Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci 77:117–125
Watanabe T, Nakajima A, Wang R, Minabe M, Koizumi S, Fujishima A, Hashimoto K (1999) Photocatalytic activity and photoinduced hydrophilicity of titanium dioxide coated glass. Thin Solid Films 351:260–263
Waychunas GA, Kim CS, Banfield JF (2005) Nanoparticulate iron oxide minerals in soils and sediments: unique properties and contaminant scavenging mechanisms. J Nanopart Res 7:409–433
Wilkinson KJ, Buffle J (2004) Critical evaluation of the physicochemical parameters and processes for modelling the biological uptake of trace metals in environmental (aquatic) systems. In: van Leeuwen HP, Köster W (eds) Physicochemical kinetics and transport at chemical-biological interphases, IUPAC series. John Wiley, Chichester, pp 445–533
Wilkinson KJ, Joz-Roland A, Buffle J (1997) Different roles of pedogenic fulvic acids and aquagenic hiopolymers on colloid aggregation and stability in freshwaters. Limnol Oceanogr 42:1714–1724
Wu R, Wu J, Xie C, Zhang J, Wang A (2002) Morphological characteristic of Zn/ZnO nanopowders and the optical properties. Mat Sci Eng A 328:196–200
Wu YL, Lim CS, Fu S, Tok AIY, Lau HM, Boey FYC, Zeng XT (2007) Surface modifications of ZnO quantum dots for bio-imaging. Nanotechnology 18: Article No. 215604, 30th May 2007
Zhang X, Sun H, Zhang Z, Niu Q, Chen Y, Crittenden JC (2007) Enhanced bioaccumulation of cadmium in carp in the presence of titanium dioxide nanoparticles. Chemosphere 67:160–166
Zhu S, Oberdörster E, Haasch ML (2006) Toxicity of an engineered nanoparticle (fullerene, C60) in two aquatic species, Daphnia and fathead minnow. Mar Environ Res 62:S5–S9
Zhu XS, Zhu L, Li Y, Duan ZH, Chen W, Alvarez PJJ (2007) Developmental toxicity in zebrafish (Danio rerio) embryos after exposure to manufactured nanomaterials: Buckminster fullerene aggregates (nC(60)) and fullerol. Environ Toxicol Chem 26:976–979
Acknowledgements
Dr Richard Handy was funded by a NERC grant (NE/E014348/1) whilst writing this review. Dr Jamie Lead was also funded by NERC (Nanonet, NE/E002889/1). Dr Martin Hassellöv was funded by the Swedish Research Council FORMAS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Handy, R.D., von der Kammer, F., Lead, J.R. et al. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 17, 287–314 (2008). https://doi.org/10.1007/s10646-008-0199-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0199-8