Abstract
Nanotechnology is a rapidly growing industry yielding many benefits to society. However, aquatic environments are at risk as increasing amounts of nanoparticles (NPs) are contaminating waterbodies causing adverse effects on aquatic organisms. In this review, the impacts of environmental exposure to NPs, the influence of the physicochemical characteristics of NPs and the surrounding environment on toxicity and mechanisms of toxicity together with NP bioaccumulation and trophic transfer are assessed with a focus on their impacts on bacteria, algae and daphnids. We identify several gaps which need urgent attention in order to make sound decisions to protect the environment. These include uncertainty in both estimated and measured environmental concentrations of NPs for reliable risk assessment and for regulating the NP industry. In addition toxicity tests and risk assessment methodologies specific to NPs are still at the research and development stage. Also conflicting and inconsistent results on physicochemical characteristics and the fate and transport of NPs in the environment suggest the need for further research. Finally, improved understanding of the mechanisms of NP toxicity is crucial in risk assessment of NPs, since conventional toxicity tests may not reflect the risks associated with NPs. Behavioural effects may be more sensitive and would be efficient in certain situations compared with conventional toxicity tests due to low NP concentrations in field conditions. However, the development of such tests is still lacking, and further research is recommended.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute
- Algae
- Aquatic
- Bacteria
- Behavioural
- Bioaccumulation
- Bioavailability
- Chronic
- Crustaceans
- Daphnia
- Ecotoxicity
- Effects
- Exposure
- Fate
- Freshwater
- Invertebrates
- Nanoparticles
- Nanotechnology
- Organisms
- Physicochemical characteristics
- Risk
- Transformation
- Trophic transfer
1 Introduction
Nanoparticles (NPs) have existed for millions of years, formed from natural phenomena such as weathering, volcanic activities and formation of colloids in rivers (Sharma et al. 2015). The use of manufactured NPs started recently (Warheit 2018) and represents a human-made material which is being used increasingly. Currently, nanotechnology is a trillion dollar industry which is increasing exponentially. It can be assumed that naturally produced NPs have been in a form of equilibrium in nature, but engineered NPs are a growing concern among institutions and the public due to their possible negative consequences on living organisms (Moore 2006; Tiede et al. 2009). The knowledge that the scientific world has acquired to date is inadequate to draw conclusions on the actual release and fate of NPs in the natural environment, actual environmental exposure to NPs and the magnitude of harm they incur to living beings (Gottschalk and Nowack 2011; Bäuerlein et al. 2017). Establishing the safety of nanomaterials is important to protect the environment and health of organisms. The effects of NPs depend on many factors including their intrinsic properties, fate and bioavailability in the respective environment and response of the receptor organisms (Lapresta-Fernández et al. 2012). The toxicity of NPs to organisms has been the subject of study for the past decade (Klaine et al. 2008; Moore 2006; Levard et al. 2012); however, coherent, consistent and well-founded data are still lacking (Selck et al. 2016; Giese et al. 2018). Currently available data on exposure to NPs and effects on organisms are currently insufficient to conclude on the risks involved (Ma et al. 2013; Holden et al. 2014; Skjolding et al. 2016; Hjorth et al. 2017a, c), and the detection and quantification of NPs in the environment are challenging (Bundschuh et al. 2016).
The authors appreciate attempts made by several authors in reviewing published literature on freshwater nanoecotoxicology (Handy et al. 2008a, b; Baun et al. 2008; Fabrega et al. 2011; Ma et al. 2013; Vale et al. 2016; Rocha et al. 2017; Lei et al. 2018; Goswami et al. 2017). This review was written in view of giving the reader an exhaustive, holistic and comparable understanding on the effects of NPs on three major groups of freshwater organisms of various classes, in contrast to the majority of reviews which are devoted to specific classes of NPs. By doing this, authors address the issue of information and data heterogeneity. Also, care has been taken in the review to appreciate and stress the importance of the physicochemical characteristics and transformation of NPs in the environment with respect to their toxicity. Furthermore, the number of publications on nanoecotoxicity is increasing every year, and therefore, a concerted effort is needed to cohesively analyse them to better understand potential risks of NPs in a background of ongoing, increasing research and development of nanotechnology applications. This review discusses our current understanding of environmental exposure to NPs, physicochemical characteristics of NPs and the aquatic environment that influence toxicity, their bioavailability, trophic transfer, toxicity, mechanisms of toxicity and behavioural toxicity in view of some key environmentally relevant freshwater organisms. The paper also identifies gaps in research and provides recommendations for future research needs to effectively develop our understanding on the risks of NPs to freshwater aquatic organisms and develop effective strategies to mitigate those risks. This review examined more than 350 articles including review papers with majority of those published in the last decade.
NPs are released into all ecosystems including freshwater, marine water, soil and air. However, the behaviour of NPs in the freshwater environment is likely to differ in each due to their unique environmental characteristics. For example, high salinity in the marine environment causes increased agglomeration, aggregation and precipitation of NPs which affects the bioavailability of NPs (Keller et al. 2010; Gambardella et al. 2015; Buffet et al. 2013). High surface area increases the potentiality of ion release from NPs (Mudunkotuwa and Grassian 2011), while higher aggregation reduces surface area for dissolution and any metal cations released from NPs are likely to be complexed by free chloride (Cl−) ions present in saltwaters (Baker et al. 2014). Moreno-Garrido et al. (2015) report that the EC50 values of NPs for marine algae species are twofold higher than for freshwater species as per the published literature. Also, they claim that OECD documents on safety and toxicity tests for NPs do not have any specific references to marine water. The major source of NPs to soil is through the disposal of wastewater treatment plant (WWTP) sewage sludge, and NPs are unlikely to enter the soil in their original form due to organically rich reactive environments in the WWTP. The attachment of NPs to soil colloids is rapid, and therefore, the mobility of NPs away from the point of source could be limited in soils (El Hadri et al. 2018). Also, the assessment of the form of NPs in soil matrices is hampered by the relative lack of procedures for their characterization compared to aqueous media (Tourinho et al. 2012; Kraas et al. 2017). Due to the variations in the fate and behaviour of NPs and mode of organism exposure in different spheres, the authors have restricted this review to the freshwater environment only. However, this does not undermine the importance for more research related to the nanotoxicity to organisms in other environments which attract comparatively less nanoecotoxicology studies (Minetto et al. 2016).
Metal oxide NPs, metal NPs and carbon nanotubes (CNT) are the most relevant materials in terms of worldwide production volumes and exposure (Bundschuh et al. 2018; Tiede et al. 2016), while the OECD has highlighted silver (Ag), zinc oxide (ZnO), titanium dioxide (TiO2) and cerium dioxide (CeO2) NPs as high interest due to their widespread use, commercial importance and inherent properties (Baker et al. 2014). Rocha et al. (2015) reported that 85% of toxicological studies on marine bivalves are based on inorganic NPs and only 15% are on organic NPs. Also, more than 70% of inorganic NPs examined in saltwater are metal oxides and metals that mainly consist of TiO2, Ag, Au, ZnO and CuO NPs (Minetto et al. 2016; Rocha et al. 2015). A large number of studies (~80%) on the effects of inorganic NPs on the organisms considered in this review also reflect the production, release and exposure risk concerns of inorganic NPs in the freshwater environment (Fig. 2 and Table 2). However, a large proportion of those inorganic NPs are coated with organic capping agents. As an example, citrate and carboxylic acids are the most used reductant and capping agents in the synthesis of AgNPs (Sharma et al. 2014).
Low-throughput tests such as microcosms, mesocosms or field-scale studies are more representative of actual environmental conditions in comparison with high-throughput tests such as in vitro tests which lack environmental complexity. Therefore, using widely accepted key environmental organisms in ecotoxicology with corresponding in vivo tests is still highly regarded in environmental risk assessment of NPs. The European Chemical Agency mentions that in vitro data are relevant information for aquatic toxicity assessments, but also note that there are no EU/OECD guidelines for in vitro tests at the moment (Hjorth et al. 2017b). Therefore the authors have restricted this review to in vivo studies. In vitro assays have a role in hazard identification, but their usefulness in environmental risk assessment is limited (SCENIHR 2009; Mattsson and Simkó 2017). There is a limited correlation between in vitro and in vivo toxicity results (Sharifi et al. 2012). These reasons may have influenced the large number of in vivo studies published compared to in vitro studies as reflected in this review. However, this does not undermine the importance of alternative testing strategies in nanoecotoxicology risk assessment since there is an ongoing discussion and proposals to use those effectively (Hjorth et al. 2017b). Also, certain aspects of NP toxicity studies such as shape-dependent toxicity are based on in vitro experiments (Sharifi et al. 2012; Forest et al. 2017).
2 Nanoparticles (NPs) in the Environment
Several types of NPs are present in the environment and exposure to those particles is a reality. Therefore, it is important to understand the flow of NPs to the environment and exposure to assess the risks (Scown et al. 2010). Release of NPs to the environment may occur from the manufacturer through to the end user who consumes NP-enabled products (Sun et al. 2014). The majority of the products containing NPs belong to cosmetics and personal care products with sunscreen representing the dominant application (Boxall et al. 2007). A significant fraction of the NPs released to soil and air would end up in waterbodies as well while cosmetics, coatings, paints and pigments alone contribute 89–97% of total NP emissions to water (Keller et al. 2013). The data related to NP production and released volumes in the literature have large variations, and Giese et al. (2018) provide a comprehensive summary based on data from literature, their own surveys and modelling. About 250,000 metric tonnes are released to landfills, soil and air every year (Keller and Lazareva 2013), and it is predicted that about 69,000 metric tonnes of NPs are released globally to surface waters directly. This amount is increasing since the predicted NP production in 2019 is close to 600,000 metric tonnes with an annual growth rate of 21.1% of NP production (Vale et al. 2016). It is estimated that around 10–30% of NPs released into the environment would end up in waterbodies in Asia, while it is 3–17% in Europe and 4–19% in North America (Keller and Lazareva 2013).
Usage of NPs are increasingly popular in several consumer and industrial sectors including health and fitness, home and garden, automotive, electronics, contaminant remediation and food and beverage (Vance et al. 2015; Cecchin et al. 2016). The number of inventories with catalogue products which contain NPs is rising globally (Hansen et al. 2016; Vance et al. 2015; Mcgillicuddy et al. 2017). Hansen et al. (2016) summarized the number of products listed yearly until 2015 in the Consumer Product Inventory (CPI 2018) and Nanodatabase (2018) which list products containing NPs or are based on nanotechnology available to the European market. The number of products listed in the Nanodatabase has increased from 2,231 to 3,038 from 2015 to date. There are several classes of engineered nanoparticles (NPs) based on chemical composition and morphology (Kümmerer et al. 2011). Though metal oxide NPs, SiO2 NPs and CNTs are the most produced worldwide (Fig. 1), Silver (Ag) NPs are the most used in consumer products representing 25% of the products containing NPs (Bondarenko et al. 2013; Vance et al. 2015; Bundschuh et al. 2018). Due to their excellent antimicrobial action, Ag NPs are increasingly popular in medicines, cosmetics, personal care and certain clothing products (Boxall et al. 2007; Yameen et al. 2014). Manufacturers are not required to report the use of NPs in products except for a few NPs in some countries (e.g. carbon nanotubes in the USA). Also, manufacturers are not legally bound to label products that contain NPs (Kessler 2011) or may be ignorant with respect to specific information (Giese et al. 2018). A survey conducted by Piccinno et al. (2012) found that the manufacturers are reluctant to provide production amounts with respect to NPs. As per the listed NP-containing products in the Nanodatabase for 2018, the constituent NPs in 64% of the consumer products have not been disclosed (The Nanodatabase 2018), while metallic NPs (19.5%), metal oxide NPs (6.5%) and other types including organic NPs (9.5%) constitute the rest of the products. The majority of organic NPs are constituted of carbon (present in 2% of products), carbon nanotubes (2.1%), bamboo charcoal (1.4%), graphite (0.6%), carbon black (0.5%) and fullerene (0.3%).
Annual production volumes of nanoparticles (adapted from Piccinno et al. 2012)
Emission and environmental concentration levels are mainly estimated by using material flow models following the NP life cycle (Mueller and Nowack 2008; Boxall et al. 2007; Gottschalk et al. 2013; Sun et al. 2014, 2017; Piccinno et al. 2012; Markus et al. 2016, 2017; Jiménez et al. 2016) and analytical methods (Gottschalk et al. 2013; Chang et al. 2017; Aznar et al. 2017; Laborda et al. 2016a, b; Majedi and Lee 2016; Venkatesan et al. 2018; Vidmar et al. 2017; Yang et al. 2016; Gondikas et al. 2014, 2018; Folens et al. 2018; Markus et al. 2018; Hartmann et al. 2013; Chen and Ding 2012; Astefanei et al. 2014). However, there are number of known incorrect assumptions in all the models (Giese et al. 2018). Measured field data are essential to validate predicted environmental concentrations of NPs (Bäuerlein et al. 2017). Most NP analytical studies have so far concentrated on method development, but a rise of efforts to apply these methods to measure actual concentrations in the environment is observed (Aznar et al. 2017; Bäuerlein et al. 2017; Venkatesan et al. 2018; Peters et al. 2018). However, limitations in analytical methods in discriminating engineered NPs from naturally occurring NPs have caused results of models difficult to validate (Giese et al. 2018; Gondikas et al. 2014; Wagner et al. 2014). Also, factors such as transformation of NPs in the environment, aggregation and the copresence of dissolved ions may cause measurement of NP properties and concentrations less accurate (Majedi and Lee 2016). The physicochemical characteristics of the surrounding environment and NP properties such as coating agent and size have a huge impact on their fate and behaviour in the environment which demands careful attention in both modelling and analytical efforts (Ellis et al. 2016; Pu et al. 2016; Luo et al. 2018).Once released in to the environment, NPs undergo transformation and change their characteristics such as size compared to their pristine form (Nowack et al. 2012). For example, NPs may agglomerate in the environment, and the modelling considers agglomerates larger than 100 nm as well and thus targets the complete NP pool. In contrast, analytical methods may consider only the nanofraction, and therefore, the measured concentrations may indeed be smaller than the actual concentrations. However, this was true for certain types of NPs that were measured in a recent study conducted by Bäuerlein et al. (2017) in the Dutch environment, but the measured concentrations of AgNPs were higher than the predicted concentrations in sewage treatment plant effluent.
From various sources, Gottschalk et al. (2013) summarized predicted environmental concentrations of TiO2 (10−3–101 μg L−1), Ag (10−5–100 μg L−1), ZnO (10−4–10−3 μg L−1), CNT (10−6–10−3 μg L−1), fullerenes (10−5–10−4 μg L−1) and CeO2 NPs (10−3–10−1 μg L−1) in freshwater. In the year 2017, the global predicted environmental concentrations of SiO2, CeO2 and Ag NPs in the freshwater were 5,300, 7.0 and 0.3 ng L−1, respectively, and predicted to increase up to 25,300, 46.7 and 2.1 ng L−1 in 2050, respectively. These increased concentrations correlate with the predicted increased release of NPs into the environment (Giese et al. 2018). Based on the per capita contributions from the households, Markus et al. (2018) estimated the concentrations of ZnO, TiO2 and AgNPs in the river Dommel in Netherlands to be 1.4 μg L−1, 1.0 μg L−1 and 13.0 ng L−1, respectively. Relatively few reports are available on actual application of analytical methods to determine the presence, properties and concentrations of NPs in freshwater samples collected from the environment. The concentration of nC60 was found up to 98 ng L−1 by using LC-MS in surface water samples collected from a creek in Hsinchu Science Park, Taiwan (Chen and Ding 2012). Comparatively, C60 and C70 concentrations were reported in using an UPLC-MS method with concentrations between 25 and 330 pg L−1 in freshwater samples collected from several ponds located around Barcelona’s airport (Astefanei et al. 2014). Folens et al. (2018) reported Pt NP concentrations in the range of 0.05–0.9 ng L−1 by measuring with spICP-MS in the road dust leachate of Ghent, Belgium and Gothenburg, Sweden, which might be released into aquatic environment. Peters et al. (2018) reported actual environmental concentrations in the range of 0.3–2.5 ng L−1 for Ag NPs, 1.3–5.2 ng L−1 for CeO2 NPs and 0.2–8.1 μg L−1 for TiO2 NPs measured using spICP-MS in the samples collected from the rivers IJssel and Meuse in the Netherlands. Venkatesan et al. (2018) found TiO2 particle concentration in the range of 260–659 ng L−1 in the Salt River, Arizona, USA, by measuring with spICP-MS. The morphological features of those particles were similar to the NPs present in sunscreens. In the last decade, predicting environmental concentrations of NPs by modelling has received considerable attention, but determining actual concentrations is critical for validating those estimates and reliable risk assessment and for regulating NP industry. However, the development of real-world parameters of NPs and concentrations remains scarce due to several limitations such as lack of appropriate analytical methods and complexity of the real sample matrices (Gondikas et al. 2018).
3 Why Be Concerned About NPs?
Engineered nanoparticles (ENPs) have been around for quite a while, but concerns about the risks associated with them rose a few years ago. Despite huge concerns, due to a lack of sample-related certified standards, analytical procedures and reliable units of measure (Mottier et al. 2017), the presence of NPs in the sources and receiving bodies like waste effluents, surface or groundwaters and sediments was not well documented (Mirzajani et al. 2013; Mitrano et al. 2012). However, the presence of NPs in the environment is a proven phenomenon as per recent studies, as discussed earlier. Bulk materials are usually defined in terms of properties like density, resistivity, magnetism and dielectric constant which are averaged for the whole unit. Compared to their bulk form, NPs possess unusual and different properties which cannot be explained with Newtonian mechanics, but only with quantum mechanics (Throbäck et al. 2007; Bhushan 2010; Poole and Owens 2003). Compared to naturally available NPs, engineered NPs may have different physical and chemical characteristics (Handy et al. 2008b). Unpredictable consequences due to their colloidal nature and the dynamics of NPs in receiving environments represent a huge challenge in assessing their toxicity (Service 2004; Nowack and Bucheli 2007; Blaser et al. 2008; Diegoli et al. 2008; Hassellöv et al. 2008; Tiede et al. 2008). Chemical and physical properties like zeta potential and metal binding capacity are determined by the size of the particles (Madden et al. 2006) which varies significantly in NPs. Due to their small size, the behaviour of NPs in the environment and effects on organisms are different to those of conventional xenobiotics (Scown et al. 2010; Klaine et al. 2012). High surface to volume ratio and abundant reactive sites on the surface are some unique characteristics of NPs, and these along with their mobility could result in unexpected health hazards (Maynard et al. 2006; Wiesner et al. 2006). Also, physicochemical characteristics of both NPs and their surrounding environment and modalities of the suspension decide the attributes of the dispersed nanophase.
It was reported that creation of free radicals and oxidative damage is the main cause of adverse effects in cells (Auffan et al. 2009). Since NPs have a very large surface area in relation to volume, they may cause direct generation of oxyradicals which can attack DNA, proteins and membranes (Brown et al. 2001). Once in the cell, NPs may embed within the cell functional machinery resulting in different toxicological responses compared to conventional toxicants (Moore 2006). In addition to their own toxicity effects, NPs also influence the toxicity of other contaminants which are harmful to aquatic organisms (Tan and Wang 2014; Fan et al. 2016). NPs have the potential to bind toxicants and may carry them to sites in cells where these chemicals would not normally travel (Cheng et al. 2004; Pelkmans and Helenius 2002).
The risk assessment methodologies of NP exposure are still at the research and development level. A number of authors have proposed approaches to NP risk assessment (Dekkers et al. 2016; Domercq et al. 2018; Garner et al. 2017; Hristozov et al. 2016), although a comprehensive risk assessment of NPs, data requirements, models and advancement related to NP production, release, exposure, fate and behaviour, risk characterization, etc. (Scott-Fordsmand et al. 2017) has not yet been developed. The models that are proposed each have their own limitations, and the tools used in characterization of NPs in exposure assessment and toxicology tests are not sufficient for risk assessment (Garner et al. 2017; Mattsson and Simkó 2017). Several parameters influence NP toxicity tests, but there exists a lack of scientific understanding of the importance of each parameter or the interactions between them for the toxicity endpoints in current test guideline (Hjorth et al. 2017c). The available test guidelines are not sufficient to analyse the behaviour of NPs in test media. For example, Wasmuth et al. (2016) concluded that the available OECD guidance document No. 29 which was designed to determine the rate and extent of ion release from metals does not cover analytical methods for NPs. Also, the development of toxicity test guidelines is still at early stages with only a few guidelines available, published recently (OECD 2017a, b, c). Accordingly, the lack of NP toxicity test data which are suited for regulatory decision-making is still a pressing issue (Hjorth et al. 2017c). Also, the currently available remediation or purification technologies may not reduce NPs to environmental permissible levels. Furthermore, increasing NP release may cause further issues in wastewater and sewage sludge treatment plants which may pose a risk to microbes in the digestion systems (Wang and Chen 2016).
4 Physicochemical Characteristics of NPs on Ecotoxicity
NPs exhibit unique physicochemical properties compared to their bulk counterparts. Several researchers have tried to investigate the influence of physicochemical characteristics of NPs such as size, shape, surface properties and charge that could change their toxicity to organisms. Though the results seem to be inconsistent and conflicting, they suggest that the physicochemical properties of NPs could affect the toxicity to organisms. Therefore, these parameters need to be considered in environmental risk assessments and demand further research.
4.1 Size
The uptake and toxicity of NPs depend on the inherent properties of NPs and also the chemistry of the surrounding environment (Park et al. 2015). The behaviour of NPs depends on factors like size, shape, surface chemistry, surface area, functional groups, coatings, charge, aggregation, solubility, photochemistry, crystallinity and the presence of other compounds (Scown et al. 2010; Albanese et al. 2012; Shang et al. 2014; Fröhlich 2012; Clément et al. 2013; Hund-Rinke and Simon 2006; Barbero and Yslas 2016; Garner and Keller 2014). The size of NPs determines the physicochemical properties, adsorption, distribution, metabolism and excretion in the biological systems (Qu et al. 2017). The toxicity of AgNPs is size dependent with smaller particles which are more active (Lok et al. 2007). Choi and Hu (2008) observed that the inhibition of nitrifying organisms correlated with a fraction of AgNPs less than 5 nm in size. This was achieved through examining the correlation between nanoparticle size distribution, photocatalytic reactive oxygen species (ROS) generation, intracellular ROS accumulation and nitrification inhibition. They concluded that NPs of this size could be more toxic to bacteria than any other fractions of NPs.
Choi et al. (2008) saw no indication of internalization of AgNPs (14 nm in size) into the bacteria Escherichia coli since internalization of NPs into bacteria cells depends on the size of the NPs and only smaller NPs (<10 nm in size) could enter (Morones et al. 2005). Jiang et al. (2008) found that binding and activation of membrane receptors and subsequent protein expression in mammalian cells depend on nanoparticle size. Hoecke et al. (2009) reported that the toxicity of CeO2 NPs to Raphidocelis subcapitata increased with decreasing particle size. Lei et al. (2016) observed increased toxicity of zero-valent iron (nZVI) NPs to Chlorella pyrenoidosa with decreasing particle size. Hartmann et al. (2010) studied the ecotoxicity of three TiO2 NPs with different sizes, to the alga Pseudokirchneriella subcapitata. They found that the smallest particle type (<10 nm) resulted in higher inhibition than the other two types (3–300 nm). Kim et al. (2010) investigated the effects of particle size of TiO2 NPs on Daphnia magna. They showed that the particle fraction in between 400 and 800 nm increased antioxidant enzyme activities in comparison with the NPs which were less than 400 nm in size. Cui et al. (2017) reported that the longer Ag nanowires (NWs) (20 μm) were more toxic to Daphnia magna and Daphnia galeata than those that were shorter (10 μm). Similarly, Chae and An (2016) showed that larger Ag nanowires (AgNW) (20 μm) were more toxic to aquatic organisms than smaller ones (10 μm) by exposing Chlamydomonas reinhardtii and Daphnia magna and to Ag NWs.
However, Matzke et al. (2014) observed no clear-cut relationship between NP toxicity and size of NPs when the bacterium Pseudomonas putida was exposed to AgNPs. Li et al. (2010a) studied the effects of three different sized (36, 52 and 66 nm) Ag NPs but concluded that the toxicity was not a function of size possibly due to the large degree of aggregation of NPs in synthetic freshwater. Also, Lopes et al. (2014) studied the effects of ZnO NPs with two different particle sizes (30 and 80–100 nm) and ionic Zn. They found that the acute toxicity of ZnO NPs did not depend on particle size. Iswarya et al. (2017) exposed the alga to PVP-coated Au NPs in different sizes but observed no size-dependent toxicity. However, the toxicity of citrate-coated Au NPs depended on the size with the smaller particles being less toxic. The smaller-sized NPs reacted rapidly with the substances in the solution causing aggregation which may have caused less toxicity. Wiench et al. (2009) reported that the acute toxicity to Daphnia magna was independent of particle size, type of coating, aggregation of particles or the type of medium for TiO2 and ZnO NPs.
4.2 Shape
Peng et al. (2011) observed that rod-shaped zinc oxide NPs (ZnO NPs) were more toxic to the alga Phaeodactylum tricornutum than sphere-shaped NPs. Bacchetta et al. (2018) observed higher internalization of spherical- and tube-shaped CNTs into Daphnia magna compared to the cubic NPs. They also reported that NP shape influenced the severity of pathogenesis with cubic NPs being more effective in terms of physical damage and cellular degeneration. Liu et al. (2018) observed that star-shaped Au NPs were more toxic to the fungus Aspergillus niger, Mucor hiemalis and Penicillium chrysogenum compared to the toxicity of spherical-shaped ones. Also, the toxicity of star-shaped NPs increased with smaller sizes. Nasser et al. (2016) suggested that shape and charge played an important role in the toxicity and uptake of Au NPs to Daphnia magna. Abramenko et al. (2018) observed higher toxicity of spherical-shaped Ag NPs to Danio rerio embryos compared with Ag nano-plates. In contrast Dai et al. (2015) saw no effect of NP form or shape on the toxicity of CuO NPs to Capitella teleta. Also, Silva et al. (2014) claimed that particle shape did not contribute to the toxicity of organo-coated Ag NPs to Escherichia coli and Daphnia magna. Chauhan et al. (2011) claimed that the rod-shaped CdSe/CdS NPs penetrated tumour cells more rapidly than spherical NPs. Truong et al. (2015) suggested that nonspherically shaped, such as filamentous, discs or wormlike NPs were better as drug delivery carriers. However, Chithrani et al. (2006) observed higher uptake of spherical-shaped Au NPs into mammalian cells than the rod-shaped Au NPs.
4.3 Surface Properties
Though NP size still remains central in determining toxicity, studies suggest that other inherent factors like coating agents should be considered in toxicity studies (Silva et al. 2014). The role of the surface properties of NPs is poorly understood and cannot be generalized to determine the risks (Baumann et al. 2014; Saei et al. 2017). Surface properties of NPs are key factors in determining behaviour of NPs; multiple types of surface ligands pose new challenges in understanding the toxicity of NPs (Yu et al. 2013). NPs are highly reactive because of their large surface area. The surface chemistry and reactivity of NPs determine their interactions with the surface lining layers of biological tissues (Hoet et al. 2004) and transfer of NPs to higher levels through the food web (Geitner et al. 2016). Many NPs which are in development are complex and carry different coatings which can alter their surface properties (Nune et al. 2009; Daima et al. 2014). Currently many different types of compounds are being used as capping agents in commercial NP production (Table 1). The physicochemical characteristics of these different coatings lead NPs to behave differently in the environment. Different ligands impart different chemical properties and affect charge, particle size, surface area and aggregation of NPs (Elsaesser and Howard 2012; Lapresta-Fernández et al. 2012; Rana and Kalaichelvan 2013; Cupi et al. 2016b). NPs are stabilized against aggregation and other chemical reactions like oxidation and sulfidation through adsorption or covalent attachment of organic compounds which provide electrostatic, steric or electrosteric repulsive forces between particles (Phenrat et al. 2008; Hotze et al. 2010). However, the impacts of different coatings on toxicity have been scarcely explored (Dominguez et al. 2015). It was shown that fullerene can cause oxidative damage in mammalian cells, and their toxicity is related to lipophilicity; reduction of lipophilicity with modification of the surface of fullerene by introducing aliphatic and hydroxyl groups resulted in reduced toxicity (Colvin 2003; Sayes et al. 2004). It has been reported that uncoated colloidal fullerenes may damage the brain of largemouth bass (Oberdörster 2004). Iron oxide NPs coated with ascorbate and dextran have been shown to be more toxic to the freshwater cladoceran Daphnia magna in comparison with the same NPs coated with citrate and polyvinylpyrrolidone (Baumann et al. 2014). Bozich et al. (2014) found that both the type of ligand and the charge of the NP surface affected the toxicity of Au NPs to Daphnia magna at acute and chronic level. Bone et al. (2012) found that the silver speciation from silver NPs (Ag NPs) varied significantly by coating type (gum Arabic and polyvinylpyrollidone) and the presence of plants (Potamogeton diversifolius and Egeria densa) in the medium, which reduced the toxicity of NPs to Daphnia magna. Interestingly, the fate and behaviour of NPs are changed by organisms as well. Adeleye and Keller (2016) observed charge reversal and change of surface properties of TiO2 NPs by the extracellular polymeric substances (EPS) produced by Chlamydomonas reinhardtii. The presence of EPS may affect the bioavailability of NPs, their interactions with organisms and overall effects. Therefore, the authors suggested that the fate and effects of NPs cannot be simply predicted by the physicochemical characteristics of NPs.
4.4 Charge
The surface charge of NPs, measured as zeta potential, contributes to the adhesion of NPs on cell surfaces and hence is important in the toxicity of NPs. The NPs with the highest positive charge are the most toxic to the algae cells. Algal cells, having a negative charge on their surface, attract positive NPs to neutralize the charge, and this causes surface alterations resulting in cell death (Karunakaran et al. 2015). El Badawy et al. (2010) observed a surface charge-dependent toxicity of Ag NPs to bacteria (Bacillus sp.) when they were exposed to four different Ag NPs with different surface charges. Dominguez et al. (2015) showed that different types of coatings and the charge of NPs had an impact on ROS formation and gene expression in Daphnia magna. Nasser et al. (2016) suggested charge played an important role in toxicity and uptake of Au NPs to Daphnia magna.
5 Effects of the Surrounding Environment on NP Toxicity
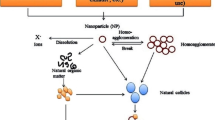
OECD (2014) highlights the importance in identifying transformation, degradation and dissolution in the characterization of NPs in toxicity tests (Cupi et al. 2016a). Transformation of Ag NPs affects their surface properties, transportation, reactivity and toxicity in the environment (Xiu et al. 2011; Liu et al. 2010; Levard et al. 2011a, b). It is important to further assess the effects of the transformed NPs as well as fresh NPs to clearly understand how the transformation of NPs in the aquatic environment affects organisms (Levard et al. 2012). Biological systems have not evolved in the presence of ENPs which are produced today, and hence, the lack of knowledge about transport and fluxes of such particles present problems (Hoet et al. 2004; Dowling 2004). Generally, abiotic factors like pH, salinity, hardness of water and chemical oxygen demand (COD) influence the aquatic toxicity of chemicals (Li et al. 2013; Fabrega et al. 2011). The fate and toxicity of NPs in the aquatic environment are governed by physicochemical pathways which include aggregation and subsequent sedimentation, dissolution, adsorption to particulate and other solid surfaces, stabilization via surfactants and binding to natural organic matter (NOM) (Wang et al. 2016c; Boncel et al. 2015; Apul and Karanfil 2015; Köser et al. 2017; Ellis et al. 2018). Biological degradation, abiotic degradation, oxidation and reduction could also be of concern in some aquatic environments (Batley and Mclaughlin 2007). It was reported that the surface coatings change or are replaced with new coatings during their transit in water (Jarvie and King 2010). Less is known about the comparative toxicity of metallic NPs and their ionic forms (Xiu et al. 2012; Wang et al. 2016a). It has been found that NPs release ions into water over time and the rate and the degree of dissolution depend on their surface functionalization. Therefore, the biological toxicity of aged and freshly prepared NPs differ (Kittler et al. 2010). Strigul et al. (2009) studied the toxicity of boron NPs (B NPs) to Daphnia magna. Depending on the age of the test solution, the calculated 48 h LC50 values for B NPs ranged from 56 to 66 mg/L, and the difference in toxicity was attributed to dissolution of NPs releasing free ions. Once released into the environment, the toxicity also depends on the oxidation state of the NPs (Conway et al. 2015). Lei et al. (2016) found that the toxicity of nZVI to the alga Chlorella pyrenoidosa decreased after NPs was aged for 3 months in the medium, in comparison with the toxicity of fresh NPs. They attributed this to the surface oxidation of the NPs.
5.1 Media and Exposure System
Abiotic factors like pH, salinity, water hardness, temperature, different organic ligands and the components in the media affect the ecotoxicity of NPs (Handy et al. 2008b; Jin et al. 2010; Djurišić et al. 2015). The fate and transport of NPs in aquatic systems largely depend on the chemical characteristics of water (Garner and Keller 2014). Physicochemical factors in freshwater are different from brackish or seawater. Therefore the behaviour and effects of NPs identified in one medium cannot readily be applied to other media.
Li et al. (2011) assessed the toxicity of ZnO NPs to Escherichia coli in five different media (ultrapure water, 0.85% NaCl, phosphate-buffered saline (PBS), minimal Davis (MD) and Luria-Bertani (LB)). They observed different toxicity levels in a range of media and recommended that attention be paid to the physicochemical characteristics of NPs and media in bacterial toxicity tests. Li et al. (2013) found that the toxicity of ZnO NPs to Escherichia coli depended on the dissolution of NPs. Interestingly, toxicity was reduced by the presence of Ca2+ and Mg2+ in the medium which could compete with toxic Zn+ ions for binding sites on the organisms. Lopes et al. (2012) observed higher bacterial toxicity in Milli-Q water than in ASTM hard water which may be due to the interference of ions in ASTM hard water causing higher aggregation. von Moos et al. (2015) exposed Chlamydomonas reinhardtii to CuO NPs (10 mg/L) in five different exposure media. They observed that the media was decisive in determining toxicity regardless of the effects from NPs or ions. Similarly, Aravantinou et al. (2015) observed that the different sensitivity of the algae Chlorococcum sp. and Scenedesmus rubescens to ZnO NPs strongly depended on the algae medium. Zhang et al. (2016a) observed that media chemistry had profound effects on aggregation, dissolution and toxicity of TiO2, ZnO and Ag NPs and CNTs to Chlorella pyrenoidosa. Seo et al. (2014) observed different toxicity levels in different media (ISO and moderately hard water (MHW)) when Daphnia magna was exposed to Ag, Cu and Zn NPs. Though the dissolution rate of NPs was higher in ISO medium, the toxicity was highest in MHW. Muna et al. (2017) found increased total Cu body burden from Cu NPs after exposing Daphnia magna in natural freshwater compared with OECD202 artificial medium. The Cu body burden in daphnids in natural freshwater bodies may be higher than laboratory predictions carried out using artificial media. Also, the total Cu body burden was higher in daphnids exposed to Cu NPs than Cu salt. Hu et al. (2017) reported higher toxicity of AgNPs and AgNO3 to Daphnia magna in M4 medium in comparison with the surface water. For both forms of Ag, daphnids took up less and depurated more in the surface water. The authors suggested a reduced toxicity for the observation. However, Salieri et al. (2015) did not observe any significant influence of test media on toxicity of TiO2 NPs to Daphnia magna. They believe this may be due to fast and strong agglomeration of NPs in all media, creating secondary particle size in the micrometre range. They did however report that the exposure volume of the medium had a significant influence on toxicity.
Nicolas et al. (2016) conducted standard algal growth inhibition tests (OECD 2011) with Raphidocelis subcapitata to test how the exposure system (24-well microplate, cylindrical vials and Erlenmeyer flasks) influenced the toxicity of TiO2 NPs. They found that the exposure system significantly affected the results and recommended attention be paid during the algal growth inhibition test. Sørensen and Baun (2015) exposed the alga Raphidocelis subcapitata to AgNPs and AgNO3 for 2 and 48 h in standard algal toxicity tests. Similar toxicity levels were observed for Ag+ in the two tests, whereas the toxicity of AgNPs was less toxic in 2 h test compared to 48 h test. Interestingly, ageing AgNPs in the medium for 48 h before performing the 2 h test increased the toxicity, while ageing beyond 48 h prior to testing reduced the toxicity. Xiao et al. (2018) observed higher toxicity in a dynamic exposure system with a vibration speed of 140 rpm in comparison with static exposure when Daphnia magna was exposed to CuO NPs. The aggregation of NPs in the dynamic system was less, and therefore, they hypothesized that the reduced toxicity may be due to the lower hydrodynamic diameter (HDD) of NPs. Sørensen et al. (2016b) claimed that the acute toxicity of Ag NPs and CuO NPs to Daphnia magna after pulse exposure (1–2 h) was comparable to the effects levels of 24 h continuous exposure. They attributed this to rapid toxicokinetic and toxicodynamic features of NPs causing the same level of toxicity following a few hours of exposure, concluding that the dissolved fractions of NPs are responsible for the toxicity. With this, they suggested that the use of pulse exposure was more environmentally relevant for NP toxicity assessments than standard continuous exposure tests.
Media critically influence the toxicity of NPs; this is due to several reasons. The physicochemical characteristics of the medium affect the fate and behaviour of NPs. The constituents of the medium may react with dissolved ions from NPs causing complexation and aggregation or compete with them for binding sites on the organisms. In addition, an organism’s sensitivity and response to NP exposure also depend on the medium. Due to these reasons, the toxicity of NPs in one medium cannot be readily applied to other media. Other than the media, the outcome of toxicity testing of NPs is highly influenced by the test duration, the time from the moment NPs are added to the test medium, dynamic vs static exposure and pulse vs continuous exposure.
5.2 Natural Organic Matter
Studies suggest that the presence of some organic and inorganic substances in the medium could change the properties of NPs which contribute to determining the fate and toxicity of NPs (Metreveli et al. 2016; Gunsolus et al. 2015; Wang et al. 2018; Luoma et al. 2016). NPs may be more stable in natural waters than in synthetic waters where no NOM is present (Batley and Mclaughlin 2007). NOM present in media could form a layer on NPs and increase the stability of NPs (Baalousha and Lead 2013; Omar et al. 2014). Once released to the aquatic environment, NOM coated on NPs changes the reactivity and bioavailability of NPs to organisms (Aiken et al. 2011). Also, DOM may promote the mobility of the NPs in the aquatic environment (Ren et al. 2017). Liberation of ions from NPs is influenced by the presence of NOM in water (Wang et al. 2015). Jiang et al. (2015) observed that NOM affected the dissolution kinetics of ZnO NPs and found that the dissolution rate constants and dissolved Zn concentrations increased with increased NOM concentration. In addition, they found that the aromatic carbon content of NOM played a key role in promoting dissolution. Li et al. (2016b) studied the effects of DOM in the medium on the generation of ROS and the acute toxicity of metal oxide NPs to Escherichia coli. They observed that different photo-reactivity of humic and fulvic acids resulted in different effects on ROS generation and acute toxicity of NPs. Seitz et al. (2015) found that the pH and dissolved organic matter (DOM) in water considerably influenced the acute and chronic toxicity of Ag NPs to Daphnia magna. Xiao et al. (2018) observed that the toxicity of CuO NPs to Daphnia magna was mitigated in the presence of DOM. There are different views about the effects of NOM on the toxicity of NPs. However, NOM have been shown to influence the stability, dissolution, reactivity, bioavailability and mobility of NPs which directly or indirectly affected the toxicity.
5.3 Sulfidation
In natural waters, Ag NPs will preferentially transform to Ag2S or AgCl as per the thermodynamic constraints. Also, the transformation will depend on pH and redox potential (Eh) and the composition of natural waters; by knowing those values, it is possible to predict the speciation of silver in simple systems. Under aerobic conditions, formation of silver chloride species is predicted, but under anaerobic conditions, sulfidation is predicted (Levard et al. 2012). Bioavailability and toxicity of ions change with sulfidation, and it was found that the toxicity of Ag+ to Daphnia magna decreased by about fivefold in the presence of environmentally relevant levels of sulphide (Bianchini and Wood 2008; Bianchini et al. 2002). Guo et al. (2017) observed that the toxicity depended on sulfidation rate when the bacteria Escherichia coli was exposed to Ag NPs. Reinsch et al. (2012) observed decreasing toxicity of Ag NPs to Escherichia coli with increasing sulfidation (Ag2S:Ag0 ratio).
5.4 Other Factors
The interactions between NPs and bacteria can be affected by several other factors such as the pH and ionic strength of the medium and the photocatalytic activity of NPs under different irradiation conditions (Djurišić et al. 2015). Pagnout et al. (2012) observed toxicity changed with different electrolytes (NaCl, CaCl2, Na2SO4) in the medium when Escherichia coli was exposed to TiO2 NPs. Also, they observed that the toxicity changed with pH which may cause changes in the surface charge of NPs resulting in different interactions between bacteria and NPs. Bhuvaneshwari et al. (2015) observed increased toxicity of ZnO NPs to Scenedesmus obliquus under UV-C irradiation compared with that under visible light. They ascribed this to increased ROS production in UV-C irradiated algal cells compared with cells under other irradiation conditions. Sendra et al. (2017) reported a significant increase in the toxicity of TiO2 NPs and bulk TiO2 under UVA irradiation in comparison with that observed invisible light. Ratti et al. (2016) observed light-enhanced antimicrobial activity of NPs when Escherichia coli and Bacillus subtilis were exposed to AgNPs. Lee and An (2013) exposed Raphidocelis subcapitata to ZnO and TiO2 NPs under visible, UVA and UVB irradiation conditions. Though the growth of algae was inhibited under all conditions, there was no significant toxicity difference among the light conditions.
Physicochemical characteristics of NPs alter upon environmental release with time under the influence of the surrounding environment, thereby affecting their impact on organisms. Several environmental factors such as media composition, exposure scenario, sulfidation, irradiation, pH and ionic strength of media influence the toxicity of NPs. Most ecotoxicological studies (Table 2) to date have focused on the effects of as-prepared NPs on organisms; few studies have evaluated the effects of the transformation of NPs on toxicity. More studies focusing on this aspect which are biologically and environmentally relevant are warranted.
5.5 NP Stability and Aggregation
Aggregation, sulfidation and oxidation are examples of changes that could happen to varying degrees (Fortner et al. 2005; Brant et al. 2005; Teeguarden et al. 2007; Garner and Keller 2014; Conway et al. 2015). Size and aggregation are the crucial factors in determining the ecotoxicity of carbon NPs, while solubility and speciation determine the toxicity of metal oxide NPs (Blinova et al. 2010). The degree, kinetics and size range of aggregates depend on the characteristics of the particles, the characteristics of the environment and the concentrations of the particles (Phenrat et al. 2007; Hyung et al. 2007). Negatively charged NPs are electrostatically stabilized when the negative charge is strong enough to repel NPs from each other to overcome attractive forces. However, the presence of counterions in the solution will reduce the repulsive forces resulting in decreased stability. Several researchers provided supportive evidence for this phenomenon claiming that the different ionic strengths of the environment affect the aggregation and stability of NPs (El Badawy et al. 2010; Li et al. 2010c; Liu et al. 2011; Delay et al. 2011). Even a slight increase in salinity decreases colloids by particle aggregation and precipitation (Stolpe and Hassellöv 2010). The ionic strength of freshwater systems ranges from 1 to 10 mM and that of seawater is about 700 mM (Levard et al. 2012). However, there is a tendency for less aggregation when NPs are stabilized sterically other than solely by surface charge. Attachment of certain polymers causes steric stabilization, and several researchers demonstrated that adsorption of compounds in natural waters induced steric forces that resist aggregation (Fabrega et al. 2009; Delay et al. 2011; Chinnapongse et al. 2011; Cumberland and Lead 2009). Polyelectrolytes exhibit additional electrosteric forces in addition to steric stabilization which makes them excellent in stabilizing NPs (Badawy et al. 2010).
Aggregation is a crucial factor in determining NP toxicity. In general, the majority of studies support the idea that the aggregation of NPs reduces the toxicity to organisms though some researchers have claimed otherwise. Several researchers reported a correlation between aggregation of NPs and their toxicity to isolated strains of bacteria (Kvitek et al. 2008; Lok et al. 2007; Bradford et al. 2009). They demonstrated that aggregation mitigates the potential toxicity of NPs. It is generally accepted that aggregation reduces toxicity to aquatic organisms. Low environmental concentrations lead to less aggregation, and hence, unlike traditional toxicants, it is possible that low concentrations are more toxic than higher concentrations with time (Tiede et al. 2009). Lok et al. (2007) observed higher aggregation in high salt media resulting in loss of antibacterial activities of AgNPs to Escherichia coli. Fernandes et al. (2006) suggested that NPs would disaggregate in the presence of household or industrial detergents. Limbach et al. (2008) found that protein breakdown products and surfactants in wastewater change the zeta potential of NPs causing stabilization. Oleszczuk et al. (2015) found that certain surfactants decrease the toxicity of TiO2 NPs to Daphnia magna, and they hypothesized that the surfactants increase the aggregation of NP, reducing the bioavailability to daphnids. In contrast, there are instances where higher toxicity was observed with the aggregation of NPs. In one such study, Kashiwada (2006) found increased salinity caused higher toxicity by NPs to medaka eggs though NPs showed higher aggregation in saline media.
5.6 Influence of NPs on Other Contaminant Effects
There is also evidence that NPs influence the toxicity of other contaminants (Deng et al. 2017) and that influence is mitigated by the characteristics of the aquatic environment, such as the presence of organic matter. Fan et al. (2016) observed a reduction in Cu accumulation in Daphnia magna in the presence of TiO2 NPs, but humic acids decreased that reducing effect. In a similar study, Rosenfeldt et al. (2015) observed a twofold decrease in Cu toxicity to Daphnia magna in the presence of TiO2 NPs in the medium. They attributed this to the adsorption of Cu to NPs leading to a reduction in the bioavailability of Cu as the cause of toxicity reduction. In another study, Liu et al. (2015) found that TiO2 NPs increased Cu accumulation in Daphnia magna, while TiO2 nanosheets decreased Cu accumulation. Interestingly, the presence of Cu2+ in the medium caused agglomeration and sedimentation of TiO2 NPs causing decreased NP bioaccumulation. Hartmann et al. (2010) investigated the toxicity of cadmium (Cd2+) ions in the presence of TiO2 NPs. Toxicity from Cd was reduced in the presence of TiO2 NPs compared to Cd alone due to the decreased bioavailability of Cd resulting from the sorption of Cd to NPs. Kim et al. (2016) observed decreased bioaccumulation of Cu, while both acute toxicity and bioaccumulation of Cd increased in the presence of citrate-coated Ag NPs after 24 h exposure of Daphnia magna. Simon et al. (2015) observed a reduction in the toxicity of triclocarban to Daphnia magna in the presence of multiwalled carbon nanotubes (MWCNT). In contrast, Wang et al. (2014) observed increased toxicity of Nickel (Ni) to Daphnia magna in the presence of hydroxylated MWCNTs. They found that this was due to the uptake of Ni-adsorbed NPs. NPs could also influence the multixenobiotic resistance (MXR) of aquatic organisms. Georgantzopoulou et al. (2016) reported similar findings when they exposed Daphnia magna to Ag NPs. Zhang et al. (2017) evaluated the joint toxicities of TiO2 NPs with four different organochlorine contaminants (OC) towards the alga Chlorella pyrenoidosa. The results indicated that there were synergistic, antagonistic and additive effects between TiO2 NPs and OCs on the alga. Similarly, Yu et al. (2018) reported synergistic and antagonistic effects of TiO2, SiO2 and Ag NPs and CdTe/CdS QDs on Cd2+ toxicity towards alga Chlamydomonas reinhardtii. Li et al. (2017a) also observed increased toxicity of Cd2+ ions to Daphnia magna in the presence of TiO2 NPs. These studies show that the NPs influence the effects of existing environmental contaminants on organisms and therefore highlight the importance of systematic studies of toxicological effects of NPs due to their own effects plus their influence on other contaminant effects in environmental risk assessment.
6 The Toxicity of NPs to Freshwater Organisms
Human and industrial wastes enter waterways, and hence, it is inevitable that NPs also end up in waterbodies due to the mass use of products containing NPs (Daughton 2004; Moore 2002). Ingestion and inhalation are considered as the major routes of NP uptake by terrestrial organisms (Dowling 2004; Warheit 2004; Moore and Allen 2002). In addition, there might be other routes of exposure in aquatic organisms, such as uptake through gills and surface epithelia (Moore 2006; Oberdörster 2004). Once internalized into invertebrates, the gut epithelium, the cellular immune system and the hepatopancreas are likely targets for reactive mechanisms, while the liver is a probable target in fish (Moore 1990; Moore et al. 2004). Eukaryotes have developed advanced mechanisms, endocytosis (100 nm or less) and phagocytosis (100–10,000 nm) for cellular internalization of particles (Na et al. 2003; Pelkmans and Helenius 2002; Moore 2006). Contamination of waterways is not only harmful for aquatic biota but also to terrestrial organisms including humans by direct or indirect exposure to NPs via direct ingestion, inhalation of water aerosols, skin contact or food (Daughton 2004). The ability of water treatment plants to treat NPs is still doubtful, and in particular, uncharged or anionic NPs could pass through into the sewage effluent. Also, some studies showed that there is a potential for NPs to harm important bacteria in sewage treatment plants (Choi et al. 2008; Kwak et al. 2001; Ghafari et al. 2008; Nyberg et al. 2008) which may put freshwater aqueous ecosystems under threat from other contaminants.
Metallic NPs have the potential to dissolve and release ions into the aquatic media. Some researchers claim that these liberated ions are the only cause of toxicity to aquatic organisms, while other studies indicate that NPs are the major cause of toxicity (Li et al. 2017b; Abramenko et al. 2018). This debate is still prevalent though the effects of metallic NPs have been intensively studied in the past decades (Wang et al. 2016a). In general, the toxicity of NPs is compared to the toxicity of the counterpart bulk material, usually metal salts, to test this hypothesis (Djurišić et al. 2015). Evaluation of acute and chronic toxicity and mechanism of toxicity is crucial in environmental risk assessment of NPs in protecting the organisms and setting up guidelines. A considerable number of studies have been undertaken to date in assessing the toxicity of different NPs and the sensitivity of organisms to NPs and on the mechanisms of toxicity.
6.1 The Toxicity of NPs to Bacteria
Toxicity to bacteria is an area of concern due to the possibility that important biogeochemical processes and other organisms in the aquatic environment may be affected detrimentally by the release of NPs to aquatic systems (Neal 2008). Several studies have evaluated the effects on non-pathogenic or environmentally relevant bacteria (Bondarenko et al. 2013; Hwang et al. 2008; Strigul et al. 2009; Jiang et al. 2009; Bradford et al. 2009; Baek and An 2011; Choi et al. 2008; Lopes et al. 2012; Heinlaan et al. 2008; von Moos and Slaveykova 2014). Also, due to the antimicrobial effects of certain NPs, the effects on pathogenic bacteria have been heavily studied due to the potential applications of NPs in medical and healthcare products. Studies on silver dominate these as a result of the excellent antimicrobial properties of Ag NPs (Marambio-Jones and Hoek 2010; Rai et al. 2009; Atiyeh et al. 2007; Durán et al. 2010; Fabrega et al. 2009).
6.1.1 Acute and Chronic Toxicity
More than 60% of the studies have looked into the acute toxicity of NPs to bacteria, while approximately 40% of studies have assessed other cellular effects such as membrane damage, morphological changes, oxidative stress, uptake, internalizations, enzyme activity, protein damage and DNA damage (Table 2). Currently, there are no standard bacterial species recommended for toxicity assessments. However, Escherichia coli has been the most preferred organism since 45% of studies have used it to assess the effects of NPs on bacteria (Fig. 2a). More than 40% of studies have assessed the effects of Ag NPs on bacteria (Fig. 2d) possibly due to the increased antimicrobial properties of Ag compared to other metals which is a huge concern in environmental toxicity and also due to the importance of Ag NPs in the medical field. Additionally, ZnO and TiO2 NPs which have antimicrobial properties have been used in 15% and 11% of studies, respectively. Most studies have focused on single species, while there are a few studies at the community level (Miao et al. 2018; Colman et al. 2012; Kumar et al. 2012; Pradhan et al. 2011; Ge et al. 2013; Frenk et al. 2013; Li et al. 2016a; Asadishad et al. 2018; Ma et al. 2015) which report on changes in microbial biomass, community activity, community composition, microbial diversity, community richness, genome and structural diversity. Risk assessment of the effects of NPs on bacterial communities is particularly important due to the detrimental effects on treatment processes.
6.1.2 Toxicity from NPs or Ions
The toxicity of NPs is widely attributed to the dissolved ions rather than NPs themselves (Jiang et al. 2009). Fabrega et al. (2009) investigated the impact of Ag NPs on Pseudomonas fluorescens in the presence of river humic acids (RHA) at different pH values. They found that the bacterial growth was entirely inhibited at 2 mg/L under all conditions and adversely affected at 0.2 mg/L concentration under some conditions. A similar toxicity was observed in the absence of RHA at pH 9 only. With these results, the authors concluded that there was a specific nanoparticle effect which could not be explained by just dissolved Ag+. Jin et al. (2010) studied the influence of inorganic aquatic chemistry on Ag NP stability and bacterial viability. They found that the antibacterial activity of Ag NPs was much lower than Ag+, when compared on the basis of mass added. Choi et al. (2010) found Ag+ was more toxic to Escherichia coli biofilms than Ag NPs with minimum bactericidal concentrations of 1.2 mg/L and 10 mg/L, respectively. Ag NPs were highly aggregated in the presence of biofilms causing increased size of NPs by a factor of 40 causing reduced toxicity. The aggregation may be due to a change of ionic strength in the medium caused by biofilms and interactions with various complexing agents produced by biofilms. Xiu et al. (2012) suggested that the antimicrobial effects of AgNPs are primarily from Ag+ released from NPs rather than the morphological properties of the particles after exposing Escherichia coli to AgNPs with different coatings and sizes. Dorobantu et al. (2015) observed no damage to membranes of the bacteria Pseudomonas aeruginosa and Staphylococcus aureus when exposed to Ag NPs. However, though AgNPs were toxic to bacteria, only AgNO3 caused membrane damage, and therefore, they decided that only Ag+ ions were responsible for biological action against microorganisms. However, Lok et al. (2006) claimed that Ag NPs were more toxic to Escherichia coli than Ag+ though they found the mode of action of NPs was similar to Ag+. The effective concentrations for toxicity of AgNPs and Ag+ were at nanomolar and micromolar level, respectively. Similarly, Baek and An (2011) attributed the toxicities to particle-specific toxicity rather than ionic effects when Escherichia coli, Bacillus subtilis and Streptococcus aureus were exposed to metal oxide NPs. Most studies on metallic NP toxicity support the hypothesis that the toxicity of NPs to bacteria is primarily caused by the ions released from NPs and the toxicity of NPs is less than their bulk form. However, as above, there are instances where authors conclude that the NPs are more toxic than the ions.
6.1.3 Mechanisms of Toxicity
Sondi and Salopek-Sondi (2004) found that Ag NP-treated Escherichia coli cells were damaged, while Ag NPs were found accumulated in the bacterial membrane. They concluded that such changes in morphology would significantly increase the permeability of the membrane resulting in death of the cell. Jiang et al. (2009) suggested that the toxicity to Bacillus subtilis, Escherichia coli and Pseudomonas fluorescens was affected by the attachment of NPs to the surface of bacteria upon exposure to Al2O3, SiO2 and ZnO NPs. Thill et al. (2006) found that CeO2 NPs come into contact with Escherichia coli cells to cause toxicity. They observed a strong absorption of NPs to the surface of bacterial cells and reduction of NPs which was correlated with the cytotoxicity. Su et al. (2009) found that the nanohybrids made up of Ag and silica were adhered to the surface of the bacteria cells. Subsequent toxicity studies revealed that the toxicity was related to the cell death caused by loss of membrane integrity due to the formation of ROS. Lok et al. (2006) observed that AgNPs destabilized the outer membrane, collapsed the plasma membrane potential and depleted the levels of intracellular ATP when Escherichia coli was exposed to AgNPs and Ag+. Li et al. (2010b) observed that NPs destroyed the permeability of the membrane of Escherichia coli and some membrane enzymes were depressed upon exposure to AgNPs. Brayner et al. (2006) observed the membrane disorganization of Escherichia coli cells as a result of exposure to ZnO NPs which led to NP accumulation in the bacterial membrane and internalization as verified by TEM images. Feng et al. (2015) studied the toxicity of cationic and anionic Au NPs to the Gram-negative bacteria Shewanella oneidensis and the Gram-positive bacteria Bacillus subtilis. Au NPs coated with cationic polyelectrolyte PAH were associated most with the bacterial surfaces and caused greatest membrane damage causing highest toxicity. Hossain and Mukherjee (2012) observed morphological changes of Escherichia coli cells to the filamentous form followed by filamentation-associated clumping with increased CdO NP concentrations. Also, the cell surface was severely damaged, and cell division proteins were affected upon exposure to NPs. The researchers attributed intracellular oxidative stress as a cause of these changes. Kumar et al. (2011) assessed the toxicity of TiO2 and ZnO NPs to Escherichia coli and observed that the exposure caused oxidative stress leading to genotoxicity and cytotoxicity. Both NPs caused induction of reactive oxygen species (ROS) and DNA damage. Genotoxic effects were also reported by Lopes et al. (2012) when bacterium Salmonella typhimurium was exposed to sodium dodecyl sulphate and didodecyl dimethylammonium bromide NPs. The mechanisms of toxicity of NPs to bacteria are complex although membrane damage by the production of ROS and physical damage by NPs have attracted most attention (Hwang et al. 2008). Membrane damage causes severe effects including the inability to properly regulate transportation through the plasma membrane. Attachment of NPs onto the surface of the bacteria is emphasized, while accumulation of NPs in the membrane has also been observed. In addition, some researchers have reported additional adverse effects such as DNA and protein damage and enzyme inactivation (Kumar et al. 2011).
6.2 The Toxicity of NPs to Freshwater Algae
Freshwater microalgae are primary producers in the environment and hence carry out a pivotal role in the food chain. Therefore, any abnormal structural or population changes of the organism will affect higher organisms which directly or indirectly feed on them (Nyholm and Peterson 1997). This highlights the importance of assessing any causes for such changes and the effects due to such causes. Their main habitats, freshwater bodies, are always under threat of chemicals released by households and industries. Also, toxicity tests with algae are recommended internationally by organizations as a source of basic information to understand environmental hazards (OECD 2011; ASTM 2012).
6.2.1 Acute and Chronic Toxicity
Most acute toxicity tests have been performed over 72 h (>40%), a time recommended for algae by the OECD; 18 and 15% of studies report 96 and 48 h toxicity tests, respectively. Several methodologies have been used to measure the acute toxicity of NPs to algae, although growth inhibition has been predominantly used. Growth inhibition can be evaluated using several techniques and methodologies including cell counting, ATP measurement, optical density measurement and chlorophyll content. Other endpoints assessed include membrane damage, oxidative stress, uptake, accumulation, cell morphology, mitochondrial dysfunction, cellular growth and metabolic profiling (Table 2). Raphidocelis subcapitata (31%), Chlamydomonas reinhardtii (24%), Chlorella pyrenoidosa (12%), Chlorella vulgaris (8%) and Euglena gracilis (8%) have been the most preferred species in NP studies (Fig. 2b). Most studies on algae have assessed the effects of Ag NPs (24%) followed by TiO2 (23%), ZnO (11%) and CeO2 (8%) (Fig. 2e).
6.2.2 Toxicity from NPs or Ions
Lee and An (2013) exposed the alga Raphidocelis subcapitata to ZnO NPs and concluded that the observed toxicity was almost entirely caused by the dissolved free Zn2+ ions. Li et al. (2015b) exposed the alga Euglena gracilis to AgNPs in the presence and absence of cysteine which is a strong silver ligand. The effects of NPs on photosynthesis decreased in the presence of cysteine suggesting that the effects of AgNPs were mediated by the dissolved Ag+. Müller et al. (2016) exposed the alga Chlamydomonas reinhardtii to Cu NPs and corresponding dissolved fraction of Cu2+ ions and observed that the toxicity was similar. Also, when the same experiments were performed in the presence of EDTA which is a strong metal ion chelator, the toxicity of both NPs and Cu2+ decreased. These results indicated that the toxicity of Cu NPs arises mostly from the dissolved fraction of Cu2+ ions. Despite Zn2+ being toxic, Iswarya et al. (2017) saw a reduction in toxicity of Au NPs to the alga Scenedesmus obliquus with the addition of Zn2+ ions to the medium. Navarro et al. (2008) examined the short-term toxicity of Ag+ and Ag NPs to photosynthesis in Chlamydomonas reinhardtii. They found that the toxicity of Ag+ in terms of EC50 was about 18 times higher compared to Ag NPs. However, the observed toxicity by Ag NPs could not be fully explained relative to the Ag+ measured in the Ag NP suspension, and the toxicity of Ag NPs appeared to be much higher when compared as a function of Ag+ concentration. When the alga Raphidocelis subcapitata was exposed to Ag NPs, the toxicity from 2 to 48 h did not increase at the corresponding ionic release rate. Also, the addition of cysteine in equimolar concentrations to silver did not eliminate toxicity. Therefore, Sørensen and Baun (2015) suggested that the dissolution cannot be the only process which contributes to the algal toxicity.
6.2.3 Mechanisms of Toxicity
Angel et al. (2015) found that the presence of dissolved organic carbon (DOC) reduced the toxicity of NPs to Raphidocelis subcapitata. The presence of DOC substantially reduced the sorption of NPs to the algal cells, and therefore, they concluded that sorption was the cause of the toxic mechanism. However, though they stopped ROS generation by using UV filters, the toxicity observed was still similar to the levels when ROS was present. They concluded, in contrast to many other findings, that the toxicity was not caused by localized exposure to ROS. Rogers et al. (2010) assessed the effects of CeO2 NPs and CeO2 macro-particles (<5 μm) to Raphidocelis subcapitata. They concluded that the effects were due to membrane damage of cells by lipid peroxidation caused by the production of hydroxyl radicals. Sørensen et al. (2016a) observed growth inhibition in Raphidocelis subcapitata and Chlamydomonas reinhardtii following exposure to Pt NPs and attributed toxicity to oxidative stress caused by ROS production. Higher body burden of NPs was found in Raphidocelis subcapitata, possibly due to favoured binding of NPs to the polysaccharide-rich cell wall. Interestingly, the accumulation of intracellular ROS levels was comparatively less in Raphidocelis subcapitata though it was the most sensitive species. Membrane damage was not observed in both algae species. Bhuvaneshwari et al. (2015) noted significant toxicity correlated with intracellular ROS generation in the alga Scenedesmus obliquus when exposed to ZnO NPs. Substantial membrane damage and a significantly enhanced lactate dehydrogenase (LDH) enzyme release into the medium were also observed. Nogueira et al. (2015) exposed the alga Raphidocelis subcapitata to grapheme oxide NPs and observed increased ROS production and membrane damage in algal cells which was suggested as the cause of observed growth inhibition. Oukarroum et al. (2017) suggested that several cellular alterations, such as the inhibition in cellular division processes, the deterioration of photosynthetic apparatus and the generation of ROS, caused the cell viability in alga Chlorella vulgaris to decrease when exposed to NiO NPs. Qian et al. (2016) saw increased ROS production and lipid peroxidation in the cyanobacterium Microcystis aeruginosa when exposed to AgNPs. They also showed that ROS inhibited SOD and POD transcription and expression. In contrast, ROS production was mediated by the induction of SOD and POD activity and the expression of the antioxidant enzyme glutamine synthetase in Chlorella vulgaris at same exposure scenario. Dauda et al. (2017) reported a significant increase in GST and peroxidase (POD) enzymes in Chlorella vulgaris upon exposure to TiO2 NPs.
Miao et al. (2010) studied the behaviour and toxicity of Ag NPs to the freshwater alga Ochromonas danica to determine whether there were any other mechanisms in algal toxicity other than due to the Ag+ liberated outside the cells. They demonstrated that the Ag NPs were taken inside the cells where they exerted their toxic effects. However, they did not discuss how the NPs exerted toxic effects inside the cells. Dorobantu et al. (2015) observed that Ag NPs caused membrane damage in the alga Euglena gracilis, but not in Chlorella protothecoides. In addition Ag NPs caused morphological changes in Euglena gracilis altering the shapes from spindle to round with the cells showing increased diameter. Ag+ ions from AgNO3 caused membrane damage in both algae causing intracellular material leaking out of the cells resulting in a depressed volume of cells. Hartmann et al. (2010) evaluated the toxicity of TiO2 NPs to the alga Pseudokirchneriella subcapitata and suggested that the observed decreased growth rate could be caused by the adhesion of NPs onto the algal cell surfaces. Ozkaleli and Erdem (2018) observed lipid peroxidation of the alga Raphidocelis subcapitata cell membrane upon exposure to TiO2 NPs, resulting in the deformation of the membrane structure. Li et al. (2015b) reported a doubling of cell volume when the alga Euglena gracilis was exposed to AgNPs. They suggested that the enlargement was a result of unspecific interactions of Ag+ ions released from AgNPs with the thiol groups of glycoproteins in the pellicle. However, they did not observe any internalization of NPs into the algal cells. Ji et al. (2011) excluded the effects of ions or shading for the observed toxicity of ZnO NPs to the alga Chlorella sp. but concluded that the toxicity was caused by entrapping and wrapping by the NPs.
Zhou et al. (2016) observed increased toxicity and cell internalization of Ag NPs in the absence of EPS compared to the presence of EPS. EPS could bind both NPs and Ag+ reducing the internalization and toxicity. Stevenson et al. (2013) investigated the toxicity of Ag NPs to the populations of the alga Chlamydomonas reinhardtii at different phases of batch culture and found that the toxicity was highest for the cultures at early phases in growth. Dynamic process modelling, incorporating algal growth rate, dissolution, bioaccumulation and extracellular DOC production, revealed that the DOC was a strong factor mitigating the toxicity of NPs. Kadukova (2016) exposed the alga Parachlorella kessleri to AgNO3 and noted that the Ag+ was removed from the medium by biosorption by algae. Interestingly, the majority of Ag was released back into the medium in the next 14 days, while the algal cells had formed Ag NPs inside, within that period. Those NPs were comparatively less toxic against algal cells than Ag+ ions at the same Ag concentrations. The surface charge of the algae cells and the NPs is a major determinant in causing toxicity. There is a high tendency for negatively charged NPs to bind on the positively charged algal surfaces. Also, once bound to the cells, the charge density of the NPs decreases which favours the adsorption of more NPs resulting in large clusters. It is widely accepted that the sorption of NPs on the algal surfaces facilitates the localized exposure to ROS resulting in oxidative damage to the cell membranes. However, sorption of NPs might cause toxicity even without the production of ROS. Certain other effects were also reported including adverse effects to morphology, cell division, gene expression and even physical effects. Also, algae have their own mechanisms to mitigate the NP toxicity into certain extent.
6.3 The Toxicity of NPs to Daphnia
Invertebrates are the most widely distributed living macroorganisms on earth. Their presence in almost all ecological niches, fast and high rate of reproduction, short life span and relatively high sensitivity to pollutants make them excellent candidates for ecotoxicological studies. Among invertebrates, Daphnia sp. is the first choice for standard toxicity tests among control agencies (Jonczyk and Gilron 2005). Except in extreme environments, this organism is present in all aquatic habitats and possesses all the above-mentioned positive characteristics for standard tests (Cattaneo et al. 2009). Daphnids exert strong grazing effects and support the aquatic food web. They feed on several sources like bacteria, algae, other invertebrates and plants and enter the trophic chain at intermediary level by being a preferential prey for larger organisms like fish, birds and humans. This also makes them a possible important linkage for passing contaminants through the food chain which should be studied for any such contaminants which are suspected of being capable of bioconcentration and bioaccumulation (Zhu et al. 2010b).
6.3.1 Acute and Chronic Toxicity
The Daphnia spp. 48 h acute test is one of the most widely used aquatic standardized tests, and this is reflected in NP toxicity studies. However, there are some suggestions to improve the sensitivity by prolonged exposure up to 72 h or the 48 h test duration followed by a 24 h recovery period (Novak et al. 2018). LC50 and EC50 are the most common endpoints used, while other effects such as uptake, accumulation, feeding rate, reproduction, enzyme activity, oxidative stress and morphological changes are reported (Table 2). More than 87% of studies have used Daphnia magna as the test species (Fig. 2c) possibly as a result of its inclusion in regulatory chemical testing, guidelines and international standards (Baun et al. 2008). The majority of studies have tested against Ag NPs (27%) followed by TiO2 (23%), Au NPs (11%) ZnO NPs (10%) and CuO NPs (10%) (Fig. 2f).
6.3.2 Toxicity from NPs or Ions
There are different views on whether NPs or liberated ions from NPs cause the toxicity to Daphnia sp. Some evidence suggests that the ions are the cause and the NPs merely represent a source of ions, while several other studies suggest cumulative effects or more adverse effects from NPs. Li et al. (2015a) observed significant changes in the metabolomic profile of Daphnia magna after exposure to Ag NPs and Ag+ for 48 h. The changes in metabolites of daphnids exposed to Ag NPs were identical to those exposed to Ag+, and therefore, they concluded that Ag+ is the dominant cause of toxicity. Sakamoto et al. (2015) observed higher toxicity for Daphnia magna, Daphnia galeata and Bosmina longirostris after exposure to Ag NPs compared to Ag+. However, the 48 h EC50 values of Ag NPs based on Ag+ concentrations were comparable with those of Ag+, and therefore, they concluded that the effects of NPs were due to liberated Ag+ from AgNPs. Zhao and Wang (2011) observed no toxicity from AgNPs to Daphnia magna when the liberated Ag+ ions were complexed by cysteine, suggesting that the toxicity was primarily caused by Ag+. Shen et al. (2015) exposed Daphnia magna to seven types of Ag NPs with different sizes and coatings in NaNO3 medium for 8 h to identify Ag species responsible for acute toxicity. The LC50 values of the seven Ag NPs as free Ag+ agreed well with that of AgNO3, and therefore, they concluded that the Ag+ is exclusively responsible for acute toxicity. Bacchetta et al. (2016) noted the toxicity of ZnO NPs to Daphnia magna was similar to the toxicity from Zn2+ and therefore concluded that the toxicity was caused by released ions from NPs. Adam et al. (2014) found chronic effects of ZnO NPs (EC50, 0.112 mg/L) and ionic Zn (EC50: 0.082 mg/L) in Daphnia magna following exposure for 21 days. They studied the influence of free, dissolved and aggregated Zn fractions in the medium and concluded that the dissolved fraction was largely responsible for the chronic toxicity. Adam et al. (2015b) concluded that the ions from the dissolution of Cu NPs caused toxicity to Daphnia magna by exposing them to CuO and ZnO NPs and Cu and Zn salts for 21 days.
In contrast, there are reports by some researchers regarding the toxicity of NPs which cannot be explained by ionic effects (Navarro et al. 2008; Fabrega et al. 2009; Yin et al. 2011). Allen et al. (2010) observed that coffee-coated AgNPs were more toxic to Daphnia magna than Ag+. Pakrashi et al. (2017) observed that AgNPs significantly affected the reproduction process of the first two broods in comparison with AgNO3 which affected only the first brood. Based on this, they suggested that AgNPs may have longer adverse effects than Ag+ ions. Bhuvaneshwari et al. (2016) claimed that the relative contribution of dissolved ions from NPs towards acute toxicity to Ceriodaphnia dubia was less than that of ZnO NPs. When Daphnia magna was exposed to CuO NPs and CuSO4, Kim et al. (2017) observed that the dissolved Cu2+ ion concentration from CuO NPs after 72 h was much less than the 72 h median effective concentration of CuSO4. These authors therefore suggested that the observed median toxicity of CuO NPs at 72 h was caused by the particles rather than by the dissolved ions. Xiao et al. (2015) reported that the relative percentage contributions of dissolved ions from CuO and ZnO NPs were 26% and 31%, respectively, when Daphnia magna was exposed to NPs. Therefore, they concluded that the particles rather than the dissolved ions were the main source of toxicity.
6.3.3 Ingestion into Daphnia
Ingestion via active and passive diffusion is the most common way of NP uptake by daphnids. Many NPs are lipophilic, and the ingested NPs are highly likely to be found in storage cells which contain lipids such as triacylglycerol and glycogen (Goulden and Hornig 1980; Moore 2006). The size of the particles daphnids can uptake depends on their body size. Daphnia magna can ingest particles up to about 70 μm, and the minimum size depends on the distances between setulae on thoracic limbs, which do not depend on the age or size since the gap is constant throughout (Burns 1968; Geller and Müller 1981). Zhao and Wang (2011) observed a linear and positive correlation between Ag concentration in the daphnids and the concentration in the medium after exposing Daphnia magna to Ag NPs. Also, at same Ag exposure concentration levels, the Ag body burden from Ag NPs was two to three orders of magnitude higher than that from AgNO3, showing the potential of daphnids to accumulate Ag NPs due to ingestion of NPs into their gut environment. Zhao and Wang (2012b) demonstrated that the Ag NP influx rate of Daphnia magna decreased with increased NP size. Also, they found 60% of Ag distributed in the gut of daphnids and concluded that ingestion was the dominant uptake pathway. Similarly, Skjolding et al. (2014a) observed a higher uptake of smaller mercaptoundecanoic acid-coated Au NPs than bigger particles. However, no such correlation was observed for citrate-coated Au NPs. In contrast, Rosenkranz et al. (2009) reported a lower uptake of smaller carboxylated polystyrene NPs (20 nm) in terms of mass compared to larger particles (1,000 nm). Tan et al. (2016b) observed that the uptake of polyacrylate-coated TiO2 NPs by Daphnia magna depended on the calcium concentration in the medium. At low Ca concentrations, NPs were ingested via endocytosis and passive drinking and distributed throughout the body, with the highest NP concentration at the abdominal zone and gut. In contrast, NPs were actively ingested and concentrated only in the gut at high Ca concentration levels in the medium. Conine and Frost (2017) found that the presence of food reduced the toxicity of AgNPs in terms of the growth and survival of Daphnia magna. They also found that toxicity was greater for animals fed with P-rich algae compared to P-poor algae. The algal-bound AgNPs were not toxic at any tested concentrations, and they suggested that the reduced toxicity in daphnids fed with P-rich algae was due to higher removal efficiency of Ag NPs by P-rich algae from the medium leaving less for uptake by daphnids. They also suggested that the algae may convert NPs to non-toxic form to daphnids, while the nutrition and overall health of daphnids also play a role in responding to NPs. Skjolding et al. (2014b) studied the influence of surface functionalization of ZnO NPs and observed fast uptake of ZnO NPs and ZnO-octyl NPs compared to ZnO-OH NPs. Daphnids ingest NPs via active and passive diffusion, while the body size of the daphnids and the concentration of NPs in the medium positively correlate with ingestion. The body burden of NPs may be higher than their bulk counterparts due to the higher NP accumulation in the guts. The size of the NPs influences the ingestion though there are conflicting views on the correlation of size and ingestion rate. Also, several other factors such as media composition, the presence of food and the surface functionalization of NPs influence the ingestion.
6.3.4 Mechanisms of Toxicity
The widely accepted key toxic mechanism for acute toxicity from metals and metal NPs to invertebrates such as daphnids is the inhibition of Na+/K+-ATPase activity and the prevention of the absorption of Na+ ions which could induce ionoregulatory failure and finally cause the death of the organism (Bianchini and Wood 2003; Kennedy et al. 2012; Rüdel et al. 2015). In addition to this, several other effects are reported at acute and chronic level. Bacchetta et al. (2016) exposed Daphnia magna to ZnO NPs and noted morphological changes in the digestive epithelium. They attributed these effects to the dissolved Zn2+ from NPs. Zn2+ ions enter into the gut enterocyte cytoplasm and resulted in altered mitochondria membrane permeability causing ROS production, which stimulates the extensive autophagy process eventually causing cell and animal death. Chae and An (2016) reported structural damage to the digestive organs of Daphnia magna along with the production of lipid droplets and concluded that AgNPs adversely affected nutrient uptake leading to immobility and death. Das et al. (2013) suggested that the observed decreased reproduction, growth inhibition and erratic behaviour of Daphnia magna from chronic exposure to TiO2 and Ag NPs could be due to the uptake of NPs in their gut plus decreased enzyme activity. Zhu et al. (2010a) observed growth retardation and reproductive defects in Daphnia magna upon exposure to TiO2 NPs. A significant amount of NPs accumulated in the body interfered with food intake which could conceivably be the cause. Blinova et al. (2017) saw long-term effects on reproductive potential with decreased number of neonates hatched from ephippia when Daphnia magna was exposed to Fe3O4 NPs. Lv et al. (2017) observed reduced digestive enzyme activities in Daphnia magna upon exposure to C60 and Si NPs. They also reported a concentration-dependent increase in SOD and LPO levels. However, the SOD activity decreased at a higher dose of C60 exposure after 72 h along with increased levels of MDA. They suggested this may be due to the breakdown of the antioxidant system at high concentrations over lengthy exposures. Ulm et al. (2015) found increased GSH, CAT and AChE activity levels in Daphnia magna upon exposure to TiO2 NPs. When Dabrunz et al. (2011) exposed Daphnia magna to TiO2 NPs for 96 h, they observed that the second moulting was disrupted due to the biological surface coating of NPs on the daphnids. Disruption to moulting directly results in reduced reproduction rates. Vijayakumar et al. (2016) noted ingestion of ZnO NPs in Ceriodaphnia cornuta and Moina micrura which caused blackening of the intestine, rupture of intestinal wall, shrinkage of the abdomen and loss of carapace and antennae leading to structural deformities. Rainville et al. (2014) reported increased protein carbonylation indicating ROS, changed vitellogenin levels and higher haemoglobin levels indicating cellular respiration from Ag NP exposure in Daphnia magna. NPs would have adverse effects on ionoregulatory processes, digestive system, growth, reproduction, behaviour, oxidative stress and moulting. Daphnia acute and chronic tests are widely used by regulatory regimes. However, the toxicity of NPs might not be reflected within the scope of the tests, and careful consideration of the mechanisms of toxicity is important to analyse effects. It is also reported that daphnids release certain proteins creating eco-corona around NPs (Nasser et al. 2016) resulting in heightened uptake and toxicity which warrants careful investigation of NP risks under environmentally relevant scenarios.
6.4 Bioaccumulation and Trophic Transfer of NPs
NPs in current use are expected to persist in the aquatic environment in different forms. Bioaccumulation of NPs is significant and calls for more research even though the emission of NPs to the aquatic environment is low, because of their limited degradability combined with the probability that they will be fed on by many invertebrates (Baun et al. 2008). To understand the trophic transfer of NPs through the food web, it is important to understand the mode of action at cellular and higher levels within individual organisms (Aschberger et al. 2011). Cells use different routes to internalize NPs, and a particular preferred route is chosen based on NP properties like size, shape and surface characteristics (Yameen et al. 2014). Any foreign materials which the cell finds harmful are transported to the lysosomes where they are digested. Therefore, in medical nanotechnology, many NPs are designed to enter the target cell through the caveolae to avoid degradation (Na et al. 2003; Panyam and Labhasetwar 2003). Once they are released into the environment after use, their non-degradative nature might negatively affect aquatic organisms. Bioaccumulation and biomagnification of NPs through the food webs are yet to be properly understood, with more research required on the influential physicochemical characteristics of NPs and trophic transfers (Zhu et al. 2009). The potential of accumulation and biomagnification of NPs may be higher in comparison with conventional contaminants, but the current testing paradigms do not emphasize the importance of evaluating the ecological impacts in this context (Wu et al. 2017a).
6.4.1 Bioaccumulation of NPs
Cellular uptake and accumulation of NPs may determine the toxicity (Taylor et al. 2016a). However, studies show contradictory results regarding the internalization mechanisms and where NPs accumulate inside the algae cells. Some reports claim that NPs enter into the cells, while others claim that they are just absorbed onto the cellular surface of algal cells or restricted to the outer region including the cell wall or periplasmic space. Taylor et al. (2016a) noted NPs in the periplasmic space of Chlamydomonas reinhardtii algal cells when they were exposed to Ag NPs. However, there were no Ag NPs accumulated inside the vesicle or the endosome around the cell, excluding the possibility of endocytosis or passive diffusion which is proposed to be the most feasible route (von Moos et al. 2014; Behra et al. 2013) for cellular internalization. In contrast, they observed Ag2S particles in the cytoplasm which they suggested were present as a result of sulfidation of Ag+ ions from Ag NPs. Sulfidation is widely accepted as a mechanism for the complexation and sequestration of heavy metals in plants to mitigate the toxicity (Chen et al. 2013). Lee et al. (2015) found that the bioaccumulation of Au NPs in Euglena gracilis was higher than in Chlamydomonas reinhardtii and noted that the reason might be the difference in the physical structure of organisms and the surface area available for interaction with NPs. They also observed the transfer of NPs to Daphnia magna after feeding them with Au NP-treated algae. Zhao et al. (2016) observed internalization of CuO NPs into Chlorella pyrenoidosa cells by endocytosis followed by storage in the vacuole. Yue et al. (2017) observed cell-associated Ag in the alga Euglena gracilis when exposed to Ag NPs. However, Ag NPs did not enter into the algal cells, only absorbed onto the algal surface.
Several studies have looked into the bioaccumulation of NPs in daphnids. Waterborne exposure and diet are the major routes for uptake of NPs in daphnids. NPs may enter the body or be retained by attaching to the body surface including the carapace. The concentration of NPs, media composition and physicochemical characteristics of NPs such as size and charge influence the uptake and retention of NPs in daphnids. Ribeiro et al. (2017) concluded that waterborne exposure to Ag NPs causes more accumulation of Ag than dietary exposure in Daphnia magna. However, more Ag from AgNO3 was accumulated through the diet. Similarly, Wu et al. (2017a) observed a higher uptake, retention of NPs and attachment to the carapace surface of Daphnia magna upon waterborne dermal exposure to CuO NPs when compared to feeding exposure. Oral exposure was predominant in feeding exposure through NP-treated algae, and the ingested Cu was regulated within the body and transferred to other biological compartments such as neonates and carapaces which may have caused less toxicity. Botha et al. (2016) observed that the uptake of Au NPs into Daphnia magna was related to NP concentration in the medium and the charge of NPs. NPs were seen adsorbed to the surface of daphnids and in the gut, but there were no evidence of NP internalization into the body cavity. No effects on reproduction or moulting patterns were observed. Wray and Klaine (2015) observed that the uptake and elimination of Au NPs by Daphnia magna were influenced by the size and surface charge of NPs, whereas shape of NPs was non-influential. However, they also found no evidence for NP internalization into the body with NPs restricted to the gut lumen and the carapace. Adam et al. (2014) found increased concentrations of Zn in Daphnia magna with increased ZnO NPs and Zn2+ concentrations in the media after exposing them for 21 days. In a similar study, Adam et al. (2015a) observed localization of CuO NPs in the gut of Daphnia magna when they were exposed to CuO NPs for 10 days. However, CuO were not internalized in the cells and were easily eliminated. Khan et al. (2014) observed accumulation of Au NPs in the gut lumen of Daphnia magna, but there was no internalization into the gut epithelial cells. Zhu et al. (2010a) found that significant amount of TiO2 NPs accumulated in the body and Daphnia magna and had difficulty in eliminating these NPs. Tan et al. (2016a) reported that Ca concentration in the medium influenced NP uptake into Daphnia magna. They observed TiO2 NPs distributed throughout the daphnid while NPs were concentrated in the gut at high Ca concentrations. Vijayakumar et al. (2016) observed the bioaccumulation of ZnO NPs in the gut region of Ceriodaphnia cornuta and Moina micrura. Pakrashi et al. (2017) exposed Daphnia magna to AgNPs and saw the NPs accumulated in the gut and non-gut tissues. Interestingly, a higher degree of positive correlation between the concentration of Ag in the non-gut tissue was found. Xiao et al. (2015) reported that the bioaccumulation of NPs or dissolved ions from NPs were concentration dependent. At low concentrations, Daphnia magna accumulated more dissolved ions from Cu and ZnO NPs (0.05 and 0.5 mg/L, respectively), while the particles were accumulated more at high concentrations (0.1 and 1 mg/L). Scanlan et al. (2013) observed similar or higher concentrations of Ag levels in the haemolymph of Daphnia magna in comparison with the initial concentration of Ag NWs in the medium indicating effective bioaccumulation during filter feeding. Lovern et al. (2008) used electron microscopy to observe accumulation and to investigate the presence and distribution of Au NPs in gut tissues of Daphnia magna exposed for 24 h. They observed movement of NPs to the posterior region of the gut, and there were no large blockages, and minimal deaths were observed. Therefore, they concluded that the particles are cleared with time in waste pellets.
Correlation between accumulation of NPs in daphnids and their eggs is also reported. Sá-Pereira et al. (2018) found NPs in the digestive tract, mainly in the gut and in the eggs of the brood pouch of Daphnia magna when exposed to TiO2 NPs. Also, the penetration of Ti into epithelial region was higher at higher concentration levels. When Daphnia magna was exposed to polystyrene NPs, Brun et al. (2017) noted accumulation of NPs in or on the lipophilic cells in the early stages of embryonic development, while the embryo is still surrounded by a chorion. However, they did not observe any NPs accumulated neither in the gut epithelium nor in lipid droplets in the adults. Sakka et al. (2016) observed higher mortality and reproductive effects in Daphnia magna correlated with the uptake of Ag NPs.
6.4.2 Trophic Transfer of NPs
Studies show that NPs are taken up and accumulated inside organisms which are transferred to higher trophic levels. Transfer of nanoparticles up through the food chain is a primary concern since it affects the balance of the ecosystem putting ecosystem health at risk (Bhuvaneshwari et al. 2018a; Wu et al. 2017b). Since organisms may feed on NP-contaminated food, it is important to understand the role of the trophic route (Bour et al. 2015). Dietary intake of NPs may cause significant effects on growth, survival and reproduction (Bhuvaneshwari et al. 2018a). Certain metals and NPs are accumulated more through dietary intake than waterborne exposure (Ribeiro et al. 2017), while certain other NPs are accumulated more through the waterborne exposure (Bhuvaneshwari et al. 2018b). The effects from NPs ingested via dietary intake may have different mechanism of toxicity compared to direct exposure (Bour et al. 2015). Werlin et al. (2011) showed that the CdSe quantum dots can be transferred from the bacteria Pseudomonas aeruginosa to the protozoa Tetrahymena thermophile with the Cd concentration in the protozoa five times higher than that found in the bacteria. Chae and An (2016) observed that the Ag NWs were transferred from the alga Chlamydomonas reinhardtii to Danio rerio through Daphnia magna. Renault et al. (2008) showed that Au NPs were transferred from the freshwater alga Scenedesmus subspicatus to Corbicula fluminea. Bouldin et al. (2008) observed the transfer of carboxyl quantum dots from Raphidocelis subcapitata to Ceriodaphnia dubia through dietary intake. Chen et al. (2016) found that the trophic transfer of fullerene NPs from Scenedesmus obliquus to Daphnia magna was dependent on subcellular distribution of NPs in alga cell. They observed that the highest NP transfer occurs via the cell wall followed by cell organelle and cell membrane. McTeer et al. (2014) reported the transfer of Ag to Daphnia magna from AgNP- and AgNO3-treated alga, Chlamydomonas reinhardtii. Bhuvaneshwari et al. (2018a) observed the transfer of ZnO NPs from the alga Scenedesmus obliquus to Ceriodaphnia dubia with the BMF found to be nearly one causing ultrastructural damage and degradation of internal organs in Daphnia. Larguinho et al. (2014) reported that Au NPs transferred from the alga Dunaliella salina to the bivalve Mytilus galloprovincialis. However, they did not observe any significant morphological alterations in mussel digestive glands or activation of any antioxidant enzymes tested. Zhu et al. (2010b) observed trophic transfer of TiO2 NPs from Daphnia magna to Danio rerio by dietary exposure. Although they observed lower biomagnification from dietary intake than from aqueous exposure, the higher body burden in the dietary exposure group led them to conclude that trophic transfer is a major route of potential NP exposure. Skjolding et al. (2014b) observed trophic transfer of ZnO NPs and ZnO-octyle NPs from Daphnia magna to Danio rerio. However, daphnids did not uptake ZnO-OH NPs, and therefore, these NPs were not available for trophic transfer. This demonstrates that surface functionalization influences the trophic transfer of NPs. Cano et al. (2018) observed the trophic transfer of MWCNTs from Daphnia magna to Pimephales promelas which was found to be dependent on the size of the particles. However, Bhuvaneshwari et al. (2017) did not observe any transfer of nZVIs from the treated alga Scenedesmus sp. to Ceriodaphnia dubia though the algae had taken up NPs. Similarly, Bhuvaneshwari et al. (2018b) did not observe any trophic transfer of TiO2 NPs from the treated alga Dunaliella salina to Artemia salina. However, Hu et al. (2017) observed the transfer of Ag from AgNP-treated Chlorella pyrenoidosa to Daphnia magna. In this case the biomagnification factor (BMF) was 0.5, and therefore, they concluded that there was no biological magnification of NPs from algae to daphnids.
6.5 Effects of NPs on Behaviour of Aquatic Organisms
In addition to the direct effects of the contaminants to organisms, behavioural effects are also critically important. Behaviour is a sensitive measure of an organism’s response to stress, and noticeable changes can be observed at concentrations of contaminants which are orders of magnitude less than that which cause mortality (Weis and Candelmo 2012). Behavioural ecotoxicity tests are becoming increasingly popular because of their high sensitivity at low concentrations and early response (Yeardley et al. 1996). Though the importance of behavioural tests is appreciated in ecotoxicology tests, far less attention has been received (Melvin and Wilson 2013). Most currently available standard tests mention the obligation to document abnormal behaviour, but this is not quantitatively sufficient for any risk assessments (Postma and Keijzers 2014). Most of the behavioural activities which are used in experiments are related to feeding (feeding rate, filtration rate, predator response) or movement (swimming, avoidance, burrowing).
There are a few studies on the effects of the NPs on the behaviour of aquatic species with most relating to daphnids. The adhesion of aggregates of NPs to the exoskeleton of Daphnia sp. may lead to different probability of survival, loss of mobility and physical damage (Baun et al. 2008). Stanley et al. (2016) exposed Daphnia magna to multiwalled carbon nanotubes (MWCNTs) for 48 h and found LC50 as 29.3 mg/L and EC50 (swimming velocity) as 6.7 mg/L. They concluded that behavioural tests are more sensitive than traditional acute toxicity tests for materials which are toxic physically rather than chemically. Also, they suggested that use of only survival endpoints to set environmental guidelines could underestimate potential hazards and risks of NPs to the environment. Lovern and Klaper (2006) observed Daphnia magna showing abnormal behaviour such as sporadic swimming in small circles, persistent ramming to vessel walls and inability to swim down from the surface when exposed to fullerene NPs. Artells et al. (2013) studied the effects of CeO2 NPs on the survival and swimming behaviour of Daphnia similis and Daphnia pulex. Swimming velocities decreased in the range of 30–40% in both species when treated with 1 mg/L NPs. At higher concentrations (10 and 100 mg/L), the swimming velocity of Daphnia similis was more impacted than Daphnia pulex. Noss et al. (2013) studied the swimming behaviour of Daphnia magna after treating with TiO2 NPs. They observed a treatment-dependent swarming in the centre of the test vessels during the initial period. The swimming velocities increased with increased body length but significantly reduced after 96 h of exposure. Vijayakumar et al. (2016) observed abnormal behaviour in Ceriodaphnia cornuta and Moina micrura upon exposure to ZnO NPs. The restricted and reduced movements were attributed to the adhesion and agglomeration of NPs on the carapace and the filter apparatus. When Strigul et al. (2009) exposed Daphnia magna to 2.5 mg L−1 B NPs, they were actively swimming compared with the control group. However, when the concentration increased to 8 mg L−1, they were less active, while they were very slow at 25 mg L−1.
O’Keefe et al. (1998) suggested that the predation risk of daphnids depends on their swimming behaviour. Pokhrel and Dubey (2012) investigated the potential impacts of citrate-coated Ag NPs on the behaviour of Daphnia magna in the presence of the predatory dragonfly Anax junius. In the absence of Ag NPs, daphnids avoided predators with both horizontal and vertical movements which are different to the control. However, they did not show any difference in vertical movement when treated with Ag NPs suggesting that Ag NPs may have potential implications on daphnid populations with increased vulnerability to predation. Lovern et al. (2007a) quantified the behavioural responses of Daphnia magna at sublethal concentrations of TiO2 and fullerene NPs. Both treatments caused significant increase in hopping frequency and appendage movement suggesting increased risk of predation and reproductive decline. Lu et al. (2017) observed a decrease in the ingestion and filtration rate of algae by Daphnia magna upon exposure to increased concentrations of TiO2 NPs, and the researchers attributed this to the observed chronic toxicity. Similarly, Lv et al. (2017) saw a reduction in ingestion and filtration rate of Daphnia magna upon exposure to C60 and Si NPs. Heinlaan et al. (2017) suggested that the reduced algal feeding rate of Co3O4 and Mn2O3 NPs-exposed Daphnia magna was not particle specific since similar results were obtained for daphnids exposed to relevant metals. Gaiser et al. (2011) observed reduced feeding in Daphnia magna when they were exposed to CeO2 NPs which was ascribed to potential replacement or coating of algae by NPs and filling the intestine with particles. Zhu et al. (2010a) observed drastic reductions in food intake when Daphnia magna was exposed to TiO2 NPs. The chronic toxicity of NPs was ascribed to poor food intake and malnutrition. McTeer et al. (2014) observed a significant reduction of feeding when Daphnia magna were fed with Ag NP- and AgNO3-contaminated algae compared to the control. They concluded that this reduction was due to the presence of Ag in algae.
In general, behavioural tests are fast and more sensitive than conventional acute and chronic ecotoxicity tests. These characteristics are particularly useful in assessing NP toxicity. NPs tend to transform and aggregate in the medium exerting huge challenges in assessing toxicity by conventional tests. Also, due to numerous types of NPs entering into the market, it is a huge challenge to assess the toxicity due to time consuming nature and the cost of conventional tests. These issues can be overcome by choosing comparatively faster and cheaper behavioural tests. There is a growing interest to develop lab-on-a-chip behavioural tests (Wang et al. 2017; Cartlidge et al. 2017) which are fast and sensitive with added advantages.
7 Conclusions and Recommendations
-
1.
Nanotechnology is a booming industry and more applications are continuously being found. Therefore, release of NPs to the aquatic environment during their manufacture or use is unavoidable. The exact concentrations of NPs in waterbodies are yet to be assessed, and only limited predicted data are available with huge assumptions since there is also a lack of published data on NP-containing products. There has been increasing interest in research on the fate and effects of NPs in the environment, but the scientific community has not been able to come to a general consensus to accurately design regulatory requirements or guidelines.
-
2.
Efforts have been taken to assess the flow of NPs into the environment and the exposure levels. Recent developments in material flow modelling are noteworthy. Also, recent efforts to accurately measure the environmental concentrations of NPs by analytical methods are a positive sign which also support in verifying the values predicted by models. However, factors such as the complexity of real sample matrices, transformation and aggregation of NPs once released into the environment and limitations in the analytical methods are causing a huge challenge in accurately measuring the environmental concentrations, while there is considerable uncertainty in models resulting in lack of reliable data. Therefore, estimates of more refined levels are needed, and further research is needed for determination of actual environmental concentrations of NPs for reliable risk assessment and for regulating NP industry.
-
3.
NPs possess special physicochemical characteristics due to their smaller size which may have different effects on organisms compared to their bulk counterparts. However, the presence of NPs in the environment is still not well documented due to a lack of sample-related certified standards, analytical procedures and reliable units of measurement. Also, toxicity tests and risk assessment methodologies specific to NPs are still at the research and development stage. Further, the available technologies are not sufficient to remediate them to environmental permissible levels.
-
4.
The effect of the particle properties of NPs on toxic responses has been heavily studied in the last decade, and it has been found that certain physicochemical properties such as size, shape and surface functionality of NPs influence toxicity. However, conflicting and inconsistent results demand further research to make sound conclusions to protect the organisms from adverse effects of NPs. It is recommended to focus on the NP properties that are already known to influence toxicity. In the meantime, it is required to put more attention into systematic approaches to design NP structures with minimal adverse effects to the environment.
-
5.
The surrounding environment largely influences the transformation of NPs once they are released into the aquatic environment. The presence of NOM, media constituents and kinetics of transformations make it significantly difficult to predict transformations in complex natural environments. Further research is required to develop methodologies and generate data on the fate and transport of NPs in the environment and how these affect organisms. Site-specific studies are recommended since mechanisms of transformation depend on the characteristics of any particular environment. Also, knowledge gaps exist, and further studies are required regarding effects of ageing of NPs since kinetics of particle degradation and kinetics of biological impact are extremely important to tease out mechanisms of interaction and mode of action. NPs interact with other chemicals in the environment. These interactions influence their very own toxicity and also the effects of those chemicals to aquatic organisms. Therefore, it is required to take multiple chemical interactions into account in environmental risk assessment of NPs.
-
6.
Opinions still differ in what causes the effects of metallic NPs on living organisms. Contradictory views on whether NPs, ions released from NPs or a combination of both cause toxicity is still an issue to understand the nature of the metallic NPs’ toxicity as well as their toxicity mechanisms. In general, the effects seem to depend on several factors such as the type of NPs, the surrounding environment and the organism. Therefore, more focused research is required to address this to better understand the toxic potential of the metallic NPs and make accurate risk assessments on them.
-
7.
The toxicity data generated even with standard toxicity tests are not consistent. Compared with the traditional contaminants, there are several variables in assessing the effects of NPs to organisms. The influence of particle properties on the toxicity of NPs is a major challenge to compare and make conclusive decisions based on obtained acute and chronic toxicity values. Therefore, it is necessary to develop robust procedures to generate data with a high degree of credibility. With such variable results, it is difficult to extrapolate the sensitivity of different species, and therefore, it is recommended that toxicity tests be conducted with a broad range of taxa to protect different organisms in aquatic environments.
-
8.
Mechanisms of NP toxicity are also still not well understood. The underlying mechanisms for the toxic interactions of NPs are complex, possibly involving various processes mediated through reactive oxygen species (ROS) generation and oxidative stress. These mechanisms are currently regarded as the best-developed paradigm for NP toxicity. Other toxicity mechanisms include membrane damage, protein denaturation, DNA damage, behavioural effects, physical damage, etc. Improved understanding on the mechanisms of NP toxicity is crucial in risk assessment of NPs since conventional toxicity tests may not reflect the risks associated with NPs.
-
9.
NPs can be ingested and accumulated inside the organism or absorbed onto the surface which may lead to trophic transfer of NPs through the food web. Studies on the bioaccumulation and trophic transfer of NPs are very limited, and therefore, more research is recommended to understand the effects on organisms at higher levels.
-
10.
Due to the low NP concentrations in field conditions, the toxicity or any other physiological effects in organisms are unlikely to be prominent enough for detection. Behavioural effects may be more sensitive and would be efficient in certain situations to evaluate effects. Also, behavioural toxicity tests are fast and cheaper which could be helpful in assessing the toxicity of ever-increasing varieties of NPs. Further, behavioural tests may be more relevant in addressing challenges posed by NPs such as transformation and aggregation. However, attention to such tests is still lacking, and further research is recommended.
8 Summary
Both the use and the number of applications of nanoparticles have expanded rapidly in recent years. This has led to increasing concern regarding the impact of nanoparticles on ecosystem health. Toxicological research in this area is therefore of utmost importance in order to determine the risks of nanoparticles to organisms in the environment. The goal of this review is to analyse recent literature in this interdisciplinary research field, with special focus on the freshwater environment. The paper begins with summarizing knowledge of current production and use of nanoparticle production and exposure concerns in the environment. The major physicochemical characteristics of NPs are examined and their subsequent fate, behaviour and toxicity to aquatic organisms. We review literature regarding the toxicity of nanoparticles to freshwater organisms at different trophic levels involving studies on bacteria, algae and Daphnia. Finally this review examines the less understood behavioural effects of nanoparticles on freshwater organisms. This aspect necessarily focuses on inorganic nanoparticles due to their industrial use and production although the effects of organic nanoparticle should not be overlooked. It is a huge challenge to accurately predict the environmental concentrations of nanoparticles, their fate and behaviour in the environment and to assess the risks posed to aquatic organisms. However, the work carried out by the nanotoxicology community over recent years is commendable. Through analysis, this review contributes to improved understanding on the effects of NPs while also identifying current research gaps and suggesting future research areas in nanotoxicology.
References
Abramenko NB, Demidova TB, Abkhalimov ЕV, Ershov BG, Krysanov EY, Kustov LM (2018) Ecotoxicity of different-shaped silver nanoparticles: case of zebrafish embryos. J Hazard Mater 347:89–94
Adam N, Schmitt C, Galceran J, Companys E, Vakurov A, Wallace R, Knapen D, Blust R (2014) The chronic toxicity of ZnO nanoparticles and ZnCl2 to Daphnia magna and the use of different methods to assess nanoparticle aggregation and dissolution. Nanotoxicology 8:709–717
Adam N, Leroux F, Knapen D, Bals S, Blust R (2015a) The uptake and elimination of ZnO and CuO nanoparticles in Daphnia magna under chronic exposure scenarios. Water Res 68:249–261
Adam N, Vakurov A, Knapen D, Blust R (2015b) The chronic toxicity of CuO nanoparticles and copper salt to Daphnia magna. J Hazard Mater 283:416–422
Adeleye AS, Keller AA (2016) Interactions between algal extracellular polymeric substances and commercial TiO2 nanoparticles in aqueous media. Environ Sci Technol 50:12258–12265
Aiken GR, Hsu-Kim H, Ryan JN (2011) Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ Sci Technol 45:3196–3201
Akhil K, Sudheer Khan S (2017) Effect of humic acid on the toxicity of bare and capped ZnO nanoparticles on bacteria, algal and crustacean systems. J Photochem Photobiol B Biol 167:136–149
Albanese A, Tang PS, Chan WC (2012) The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng 14:1–16
Allen HJ, Impellitteri CA, Macke DA, Heckman JL, Poynton HC, Lazorchak JM, Govindaswamy S, Roose DL, Nadagouda MN (2010) Effects from filtration, capping agents, and presence/absence of food on the toxicity of silver nanoparticles to Daphnia magna. Environ Toxicol Chem 29:2742–2750
Angel BM, Vallotton P, Apte SC (2015) On the mechanism of nanoparticulate CeO 2 toxicity to freshwater algae. Aquat Toxicol 168:90–97
Apul OG, Karanfil T (2015) Adsorption of synthetic organic contaminants by carbon nanotubes: a critical review. Water Res 68:34–55
Aravantinou AF, Tsarpali V, Dailianis S, Manariotis ID (2015) Effect of cultivation media on the toxicity of ZnO nanoparticles to freshwater and marine microalgae. Ecotoxicol Environ Saf 114:109–116
Artells E, Issartel J, Auffan M, Borschneck D, Thill A, Tella M, Brousset L, Rose J, Bottero J-Y, Thiéry A (2013) Exposure to cerium dioxide nanoparticles differently affect swimming performance and survival in two daphnid species. PLoS One 8:e71260
Aruoja V, Dubourguier H-C, Kasemets K, Kahru A (2009) Toxicity of nanoparticles of CuO, ZnO and TiO 2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ 407:1461–1468
Aruoja V, Pokhrel S, Sihtmäe M, Mortimer M, Mädler L, Kahru A (2015) Toxicity of 12 metal-based nanoparticles to algae, bacteria and protozoa. Environ Sci Nano 2:630–644
Asadishad B, Chahal S, Akbari A, Cianciarelli V, Azodi M, Ghoshal S, Tufenkji N (2018) Amendment of agricultural soil with metal nanoparticles: effects on soil enzyme activity and microbial community composition. Environ Sci Technol 52:1908–1918
Aschberger K, Micheletti C, Sokull-Klüttgen B, Christensen FM (2011) Analysis of currently available data for characterising the risk of engineered nanomaterials to the environment and human health – lessons learned from four case studies. Environ Int 37:1143–1156
Astefanei A, Núñez O, Galceran MT (2014) Analysis of C60-fullerene derivatives and pristine fullerenes in environmental samples by ultrahigh performance liquid chromatography–atmospheric pressure photoionization-mass spectrometry. J Chromatogr A 1365:61–71
ASTM (2012) Standard guide for conducting static toxicity tests with microalgae. ASTM International, West Conshohocken
Atiyeh BS, Costagliola M, Hayek SN, Dibo SA (2007) Effect of silver on burn wound infection control and healing: review of the literature. Burns 33:139–148
Auffan M, Rose J, Wiesner MR, Bottero J-Y (2009) Chemical stability of metallic nanoparticles: a parameter controlling their potential cellular toxicity in vitro. Environ Pollut 157:1127–1133
Aznar R, Barahona F, Geiss O, Ponti J, José Luis T, Barrero-Moreno J (2017) Quantification and size characterisation of silver nanoparticles in environmental aqueous samples and consumer products by single particle-ICPMS. Talanta 175:200–208
Baalousha M, Lead J (2013) Characterization of natural and manufactured nanoparticles by atomic force microscopy: effect of analysis mode, environment and sample preparation. Colloids Surf A Physicochem Eng Asp 419:238–247
Bacchetta R, Maran B, Marelli M, Santo N, Tremolada P (2016) Role of soluble zinc in ZnO nanoparticle cytotoxicity in Daphnia magna: a morphological approach. Environ Res 148:376–385
Bacchetta R, Santo N, Valenti I, Maggioni D, Longhi M, Tremolada P (2018) Comparative toxicity of three differently shaped carbon nanomaterials on Daphnia magna: does a shape effect exist? Nanotoxicology 12:201–223
Badawy AME, Luxton TP, Silva RG, Scheckel KG, Suidan MT, Tolaymat TM (2010) Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ Sci Technol 44:1260–1266
Baek Y-W, An Y-J (2011) Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci Total Environ 409:1603–1608
Baker TJ, Tyler CR, Galloway TS (2014) Impacts of metal and metal oxide nanoparticles on marine organisms. Environ Pollut 186:257–271
Barbero CA, Yslas EI (2016) Ecotoxicity effects of nanomaterials on aquatic organisms: nanotoxicology of materials. Appl Nanotechnol Environ Sustain:330
Batley G, Mclaughlin MJ (2007) Fate of manufactured nanomaterials in the Australian environment. CSIRO Land and Water, Acton
Bäuerlein PS, Emke E, Tromp P, Hofman JAMH, Carboni A, Schooneman F, de Voogt P, van Wezel AP (2017) Is there evidence for man-made nanoparticles in the Dutch environment? Sci Total Environ 576:273–283
Baumann J, Köser J, Arndt D, Filser J (2014) The coating makes the difference: acute effects of iron oxide nanoparticles on Daphnia magna. Sci Total Environ 484:176–184
Baun A, Hartmann NB, Grieger K, Kusk KO (2008) Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicology 17:387–395
Becaro AA, Jonsson CM, Puti FC, Siqueira MC, Mattoso LH, Correa DS, Ferreira MD (2015) Toxicity of PVA-stabilized silver nanoparticles to algae and microcrustaceans. Environ Nanotechnol Monit Manag 3:22–29
Behra R, Sigg L, Clift MJ, Herzog F, Minghetti M, Johnston B, Petri-Fink A, Rothen-Rutishauser B (2013) Bioavailability of silver nanoparticles and ions: from a chemical and biochemical perspective. J R Soc Interface 10:20130396
Bhushan B (2010) Springer handbook of nanotechnology. Springer, Berlin
Bhuvaneshwari M, Iswarya V, Archanaa S, Madhu GM, Kumar GKS, Nagarajan R, Chandrasekaran N, Mukherjee A (2015) Cytotoxicity of ZnO NPs towards fresh water algae Scenedesmus obliquus at low exposure concentrations in UV-C, visible and dark conditions. Aquat Toxicol 162:29–38
Bhuvaneshwari M, Iswarya V, Nagarajan R, Chandrasekaran N, Mukherjee A (2016) Acute toxicity and accumulation of ZnO NPs in Ceriodaphnia dubia: relative contributions of dissolved ions and particles. Aquat Toxicol 177:494–502
Bhuvaneshwari M, Kumar D, Roy R, Chakraborty S, Parashar A, Mukherjee A, Chandrasekaran N, Mukherjee A (2017) Toxicity, accumulation, and trophic transfer of chemically and biologically synthesized nano zero valent iron in a two species freshwater food chain. Aquat Toxicol 183:63–75
Bhuvaneshwari M, Iswarya V, Vishnu S, Chandrasekaran N, Mukherjee A (2018a) Dietary transfer of zinc oxide particles from algae (Scenedesmus obliquus) to Daphnia (Ceriodaphnia dubia). Environ Res 164:395–404
Bhuvaneshwari M, Thiagarajan V, Nemade P, Chandrasekaran N, Mukherjee A (2018b) Toxicity and trophic transfer of P25 TiO2 NPs from Dunaliella salina to Artemia salina: effect of dietary and waterborne exposure. Environ Res 160:39–46
Bianchini A, Wood CM (2003) Mechanism of acute silver toxicity in Daphnia magna. Environ Toxicol Chem 22:1361–1367
Bianchini A, Wood CM (2008) Does sulfide or water hardness protect against chronic silver toxicity in Daphnia magna? A critical assessment of the acute-to-chronic toxicity ratio for silver. Ecotoxicol Environ Saf 71:32–40
Bianchini A, Bowles KC, Brauner CJ, Gorsuch JW, Kramer JR, Wood CM (2002) Evaluation of the effect of reactive sulfide on the acute toxicity of silver (I) to Daphnia magna. Part 2: toxicity results. Environ Toxicol Chem 21:1294–1300
Binaeian E, Rashidi A, Attar H (2012) Toxicity study of two different synthesized silver nanoparticles on bacteria Vibrio fischeri. WASET 67:1219–1225
Blaser SA, Scheringer M, Macleod M, Hungerbühler K (2008) Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles. Sci Total Environ 390:396–409
Blinova I, Ivask A, Heinlaan M, Mortimer M, Kahru A (2010) Ecotoxicity of nanoparticles of CuO and ZnO in natural water. Environ Pollut 158:41–47
Blinova I, Niskanen J, Kajankari P, Kanarbik L, Käkinen A, Tenhu H, Penttinen O-P, Kahru A (2013) Toxicity of two types of silver nanoparticles to aquatic crustaceans Daphnia magna and Thamnocephalus platyurus. Environ Sci Pollut Res 20:3456–3463
Blinova I, Kanarbik L, Irha N, Kahru A (2017) Ecotoxicity of nanosized magnetite to crustacean Daphnia magna and duckweed Lemna minor. Hydrobiologia 798:141–149
Boncel S, Kyzioł-Komosińska J, Krzyżewska I, Czupioł J (2015) Interactions of carbon nanotubes with aqueous/aquatic media containing organic/inorganic contaminants and selected organisms of aquatic ecosystems – a review. Chemosphere 136:211–221
Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A (2013) Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol 87:1181–1200
Bone AJ, Colman BP, Gondikas AP, Newton KM, Harrold KH, Cory RM, Unrine JM, Klaine SJ, Matson CW, Di Giulio RT (2012) Biotic and abiotic interactions in aquatic microcosms determine fate and toxicity of Ag nanoparticles: part 2–toxicity and Ag speciation. Environ Sci Technol 46:6925–6933
Botha TL, Boodhia K, Wepener V (2016) Adsorption, uptake and distribution of gold nanoparticles in Daphnia magna following long term exposure. Aquat Toxicol 170:104–111
Bouldin JL, Ingle TM, Sengupta A, Alexander R, Hannigan RE, Buchanan RA (2008) Aqueous toxicity and food chain transfer of quantum dots™ in freshwater algae and Ceriodaphnia dubia. Environ Toxicol Chem 27:1958–1963
Bour A, Mouchet F, Silvestre J, Gauthier L, Pinelli E (2015) Environmentally relevant approaches to assess nanoparticles ecotoxicity: a review. J Hazard Mater 283:764–777
Boxall AB, Chaudhry Q, Sinclair C, Jones A, Aitken R, Jefferson B, Watts C (2007) Current and future predicted environmental exposure to engineered nanoparticles. Central Science Laboratory, Department of the Environment and Rural Affairs, London, p 89
Bozich JS, Lohse SE, Torelli MD, Murphy CJ, Hamers RJ, Klaper RD (2014) Surface chemistry, charge and ligand type impact the toxicity of gold nanoparticles to Daphnia magna. Environ Sci Nano 1:260–270
Bradford A, Handy RD, Readman JW, Atfield A, Mühling M (2009) Impact of silver nanoparticle contamination on the genetic diversity of natural bacterial assemblages in estuarine sediments. Environ Sci Technol 43:4530–4536
Brant J, Lecoanet H, Wiesner MR (2005) Aggregation and deposition characteristics of fullerene nanoparticles in aqueous systems. J Nanopart Res 7:545–553
Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fiévet F (2006) Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett 6:866–870
Brown DM, Wilson MR, Macnee W, Stone V, Donaldson K (2001) Size-dependent proinflammatory effects of ultrafine polystyrene particles: a role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicol Appl Pharmacol 175:191–199
Brun NR, Beenakker MMT, Hunting ER, Ebert D, Vijver MG (2017) Brood pouch-mediated polystyrene nanoparticle uptake during Daphnia magna embryogenesis. Nanotoxicology 11:1059–1069
Buffet P-E, Pan J-F, Poirier L, Amiard-Triquet C, Amiard J-C, Gaudin P, Risso-De Faverney C, Guibbolini M, Gilliland D, Valsami-Jones E (2013) Biochemical and behavioural responses of the endobenthic bivalve Scrobicularia plana to silver nanoparticles in seawater and microalgal food. Ecotoxicol Environ Saf 89:117–124
Bundschuh M, Seitz F, Rosenfeldt RR, Schulz R (2016) Effects of nanoparticles in fresh waters: risks, mechanisms and interactions. Freshw Biol 61:2185–2196
Bundschuh M, Filser J, Lüderwald S, Mckee MS, Metreveli G, Schaumann GE, Schulz R, Wagner S (2018) Nanoparticles in the environment: where do we come from, where do we go to? Environ Sci Eur 30:6
Burns CW (1968) The relationship between body size of filter-feeding cladocera and the maximum size of particle ingested. Limnol Oceanogr 13:675–678
Cano AM, Maul JD, Saed M, Irin F, Shah SA, Green MJ, French AD, Klein DM, Crago J, Cañas-Carrell JE (2018) Trophic transfer and accumulation of multiwalled carbon nanotubes in the presence of copper ions in Daphnia magna and Fathead minnow (Pimephales promelas). Environ Sci Technol 52:794–800
Cartlidge R, Nugegoda D, Wlodkowic D (2017) Millifluidic Lab-on-a-Chip technology for automated toxicity tests using the marine amphipod Allorchestes compressa. Sensors Actuators B Chem 239:660–670
Cattaneo AG, Gornati R, Chiriva-Internati M, Bernardini G (2009) Ecotoxicology of nanomaterials: the role of invertebrate testing. Invertebr Surviv J 6:78–97
Cecchin I, Reddy KR, Thomé A, Tessaro EF, Schnaid F (2016) Nanobioremediation: integration of nanoparticles and bioremediation for sustainable remediation of chlorinated organic contaminants in soils. Int Biodeter Biodegr 119:419–428
Chae Y, An Y-J (2016) Toxicity and transfer of polyvinylpyrrolidone-coated silver nanowires in an aquatic food chain consisting of algae, water fleas, and zebrafish. Aquat Toxicol 173:94–104
Chang Y-J, Shih Y-H, Su C-H, Ho H-C (2017) Comparison of three analytical methods to measure the size of silver nanoparticles in real environmental water and wastewater samples. J Hazard Mater 322:95–104
Chauhan VP, Popović Z, Chen O, Cui J, Fukumura D, Bawendi MG, Jain RK (2011) Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew Chem Int Ed 50:11417–11420
Chen H-C, Ding W-H (2012) Determination of aqueous fullerene aggregates in water by ultrasound-assisted dispersive liquid–liquid microextraction with liquid chromatography–atmospheric pressure photoionization-tandem mass spectrometry. J Chromatogr A 1223:15–23
Chen S, Goode AE, Sweeney S, Theodorou IG, Thorley AJ, Ruenraroengsak P, Chang Y, Gow A, Schwander S, Skepper J (2013) Sulfidation of silver nanowires inside human alveolar epithelial cells: a potential detoxification mechanism. Nanoscale 5:9839–9847
Chen Q, Hu X, Yin D, Wang R (2016) Effect of subcellular distribution on nC 60 uptake and transfer efficiency from Scenedesmus obliquus to Daphnia magna. Ecotoxicol Environ Saf 128:213–221
Cheng X, Kan AT, Tomson MB (2004) Naphthalene adsorption and desorption from aqueous C60 fullerene. J Chem Eng Data 49:675–683
Chinnapongse SL, Maccuspie RI, Hackley VA (2011) Persistence of singly dispersed silver nanoparticles in natural freshwaters, synthetic seawater, and simulated estuarine waters. Sci Total Environ 409:2443–2450
Chithrani BD, Ghazani AA, Chan WC (2006) Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 6:662–668
Choi O, Hu Z (2008) Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 42:4583–4588
Choi O, Deng KK, Kim N-J, Ross L, Surampalli RY, Hu Z (2008) The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res 42:3066–3074
Choi O, Yu C-P, Fernández GE, Hu Z (2010) Interactions of nanosilver with Escherichia coli cells in planktonic and biofilm cultures. Water Res 44:6095–6103
Clément L, Hurel C, Marmier N (2013) Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants – effects of size and crystalline structure. Chemosphere 90:1083–1090
Colman BP, Wang S-Y, Auffan M, Wiesner MR, Bernhardt ES (2012) Antimicrobial effects of commercial silver nanoparticles are attenuated in natural streamwater and sediment. Ecotoxicology 21:1867–1877
Colvin VL (2003) The potential environmental impact of engineered nanomaterials. Nat Biotechnol 21:1166–1170
Conine AL, Frost PC (2017) Variable toxicity of silver nanoparticles to Daphnia magna: effects of algal particles and animal nutrition. Ecotoxicology 26:118–126
Conway JR, Adeleye AS, Gardea-Torresdey J, Keller AA (2015) Aggregation, dissolution, and transformation of copper nanoparticles in natural waters. Environ Sci Technol 49:2749–2756
CPI (2018) Consumer products inventory [Online]. http://www.nanotechproject.org/cpi/. Accessed 11 Mar 2018
Cui R, Chae Y, An Y-J (2017) Dimension-dependent toxicity of silver nanomaterials on the cladocerans Daphnia magna and Daphnia galeata. Chemosphere 185:205–212
Cumberland SA, Lead JR (2009) Particle size distributions of silver nanoparticles at environmentally relevant conditions. J Chromatogr A 1216:9099–9105
Cupi D, Hartmann NB, Baun A (2016a) Influence of pH and media composition on suspension stability of silver, zinc oxide, and titanium dioxide nanoparticles and immobilization of Daphnia magna under guideline testing conditions. Ecotoxicol Environ Saf 127:144–152
Cupi D, Sørensen SN, Skjolding LM, Baun A (2016b) Toxicity of engineered nanoparticles to aquatic invertebrates. Engl Nanopart Environ Biophys Processes Toxicity 4
Dabrunz A, Duester L, Prasse C, Seitz F, Rosenfeldt R, Schilde C, Schaumann GE, Schulz R (2011) Biological surface coating and molting inhibition as mechanisms of TiO2 nanoparticle toxicity in Daphnia magna. PLoS One 6:e20112
Dai L, Banta GT, Selck H, Forbes VE (2015) Influence of copper oxide nanoparticle form and shape on toxicity and bioaccumulation in the deposit feeder, Capitella teleta. Mar Environ Res 111:99–106
Daima HK, Selvakannan P, Kandjani AE, Shukla R, Bhargava SK, Bansal V (2014) Synergistic influence of polyoxometalate surface corona towards enhancing the antibacterial performance of tyrosine-capped Ag nanoparticles. Nanoscale 6:758–765
Das P, Xenopoulos MA, Metcalfe CD (2013) Toxicity of silver and titanium dioxide nanoparticle suspensions to the aquatic invertebrate, Daphnia magna. Bull Environ Contam Toxicol 91:76–82
Dauda S, Chia MA, Bako SP (2017) Toxicity of titanium dioxide nanoparticles to Chlorella vulgaris Beyerinck (Beijerinck) 1890 (Trebouxiophyceae, Chlorophyta) under changing nitrogen conditions. Aquat Toxicol 187:108–114
Daughton CG (2004) Non-regulated water contaminants: emerging research. Environ Impact Assess Rev 24:711–732
Dekkers S, Oomen AG, Bleeker EAJ, Vandebriel RJ, Micheletti C, Cabellos J, Janer G, Fuentes N, Vázquez-Campos S, Borges T, Silva MJ, Prina-Mello A, Movia D, Nesslany F, Ribeiro AR, Leite PE, Groenewold M, Cassee FR, Sips AJAM, Dijkzeul A, van Teunenbroek T, Wijnhoven SWP (2016) Towards a nanospecific approach for risk assessment. Regul Toxicol Pharmacol 80:46–59
Delay M, Dolt T, Woellhaf A, Sembritzki R, Frimmel FH (2011) Interactions and stability of silver nanoparticles in the aqueous phase: influence of natural organic matter (NOM) and ionic strength. J Chromatogr A 1218:4206–4212
Deng R, Lin D, Zhu L, Majumdar S, White JC, Gardea-Torresdey JL, Xing B (2017) Nanoparticle interactions with co-existing contaminants: joint toxicity, bioaccumulation and risk. Nanotoxicology 11:591–612
Diegoli S, Manciulea AL, Begum S, Jones IP, Lead JR, Preece JA (2008) Interaction between manufactured gold nanoparticles and naturally occurring organic macromolecules. Sci Total Environ 402:51–61
Djurišić AB, Leung YH, Ng A, Xu XY, Lee PK, Degger N (2015) Toxicity of metal oxide nanoparticles: mechanisms, characterization, and avoiding experimental artefacts. Small 11:26–44
Domercq P, Praetorius A, Boxall AB (2018) Emission and fate modelling framework for engineered nanoparticles in urban aquatic systems at high spatial and temporal resolution. Environ Sci Nano 5:533–543
Dominguez GA, Lohse SE, Torelli MD, Murphy CJ, Hamers RJ, Orr G, Klaper RD (2015) Effects of charge and surface ligand properties of nanoparticles on oxidative stress and gene expression within the gut of Daphnia magna. Aquat Toxicol 162:1–9
Dorobantu LS, Fallone C, Noble AJ, Veinot J, Ma G, Goss GG, Burrell RE (2015) Toxicity of silver nanoparticles against bacteria, yeast, and algae. J Nanopart Res 17:172
Dowling AP (2004) Development of nanotechnologies. Mater Today 7:30–35
Durán N, Marcato PD, Conti RD, Alves OL, Costa F, Brocchi M (2010) Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J Braz Chem Soc 21:949–959
El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM (2010) Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol 45:283–287
El Hadri H, Louie SM, Hackley VA (2018) Assessing the interactions of metal nanoparticles in soil and sediment matrices – a quantitative analytical multi-technique approach. Environ Sci Nano 5:203–214
Ellis L-JA, Valsami-Jones E, Lead JR, Baalousha M (2016) Impact of surface coating and environmental conditions on the fate and transport of silver nanoparticles in the aquatic environment. Sci Total Environ 568:95–106
Ellis L-JA, Baalousha M, Valsami-Jones E, Lead JR (2018) Seasonal variability of natural water chemistry affects the fate and behaviour of silver nanoparticles. Chemosphere 191:616–625
Elsaesser A, Howard CV (2012) Toxicology of nanoparticles. Adv Drug Deliv Rev 64:129–137
Fabrega J, Fawcett SR, Renshaw JC, Lead JR (2009) Silver nanoparticle impact on bacterial growth: effect of pH, concentration, and organic matter. Environ Sci Technol 43:7285–7290
Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR (2011) Silver nanoparticles: behaviour and effects in the aquatic environment. Environ Int 37:517–531
Fan W, Peng R, Li X, Ren J, Liu T, Wang X (2016) Effect of titanium dioxide nanoparticles on copper toxicity to Daphnia magna in water: role of organic matter. Water Res 105:129–137
Feng ZV, Gunsolus IL, Qiu TA, Hurley KR, Nyberg LH, Frew H, Johnson KP, Vartanian AM, Jacob LM, Lohse SE (2015) Impacts of gold nanoparticle charge and ligand type on surface binding and toxicity to Gram-negative and Gram-positive bacteria. Chem Sci 6:5186–5196
Fernandes M, Rosenkranz P, Ford A, Christofi N, Stone V (2006) Ecotoxicology of nanoparticles (NPs). SETAC Globe:43–45
Folens K, van Acker T, Bolea-Fernandez E, Cornelis G, Vanhaecke F, Du Laing G, Rauch S (2018) Identification of platinum nanoparticles in road dust leachate by single particle inductively coupled plasma-mass spectrometry. Sci Total Environ 615:849–856
Forest V, Leclerc L, Hochepied J-F, Trouvé A, Sarry G, Pourchez J (2017) Impact of cerium oxide nanoparticles shape on their in vitro cellular toxicity. Toxicol In Vitro 38:136–141
Fortner J, Lyon D, Sayes C, Boyd A, Falkner J, Hotze E, Alemany L, Tao Y, Guo W, Ausman K (2005) C60 in water: nanocrystal formation and microbial response. Environ Sci Technol 39:4307–4316
Frenk S, Ben-Moshe T, Dror I, Berkowitz B, Minz D (2013) Effect of metal oxide nanoparticles on microbial community structure and function in two different soil types. PLoS One 8:e84441
Fröhlich E (2012) The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine 7:5577–5591
Gaiser BK, Biswas A, Rosenkranz P, Jepson MA, Lead JR, Stone V, Tyler CR, Fernandes TF (2011) Effects of silver and cerium dioxide micro-and nano-sized particles on Daphnia magna. J Environ Monit 13:1227–1235
Gambardella C, Costa E, Piazza V, Fabbrocini A, Magi E, Faimali M, Garaventa F (2015) Effect of silver nanoparticles on marine organisms belonging to different trophic levels. Mar Environ Res 111:41–49
Garner KL, Keller AA (2014) Emerging patterns for engineered nanomaterials in the environment: a review of fate and toxicity studies. J Nanopart Res 16:2503
Garner KL, Suh S, Keller AA (2017) Assessing the risk of engineered nanomaterials in the environment: development and application of the nanoFate model. Environ Sci Technol 51:5541–5551
Ge Y, Priester JH, van de Werfhorst LC, Schimel JP, Holden PA (2013) Potential mechanisms and environmental controls of TiO2 nanoparticle effects on soil bacterial communities. Environ Sci Technol 47:14411–14417
Geitner NK, Marinakos SM, Guo C, O’brien N, Wiesner MR (2016) Nanoparticle surface affinity as a predictor of trophic transfer. Environ Sci Technol 50:6663–6669
Geller W, Müller H (1981) The filtration apparatus of Cladocera: filter mesh-sizes and their implications on food selectivity. Oecologia 49:316–321
Georgantzopoulou A, Cambier S, Serchi T, Kruszewski M, Balachandran YL, Grysan P, Audinot J-N, Ziebel J, Guignard C, Gutleb AC (2016) Inhibition of multixenobiotic resistance transporters (MXR) by silver nanoparticles and ions in vitro and in Daphnia magna. Sci Total Environ 569:681–689
Ghafari P, St-Denis CH, Power ME, Jin X, Tsou V, Mandal HS, Bols NC, Tang XS (2008) Impact of carbon nanotubes on the ingestion and digestion of bacteria by ciliated protozoa. Nat Nanotechnol 3:347–351
Giese B, Klaessig F, Park B, Kaegi R, Steinfeldt M, Wigger H, Gleich A, Gottschalk F (2018) Risks, release and concentrations of engineered nanomaterial in the environment. Sci Rep 8:1565
Gökçe D, Köytepe S, Özcan İ (2018) Effects of nanoparticles on Daphnia magna population dynamics. Chem Ecol 34:301–323
Gonçalves RA, de Oliveira Franco Rossetto AL, Nogueira DJ, Vicentini DS, Matias WG (2018) Comparative assessment of toxicity of ZnO and amine-functionalized ZnO nanorods toward Daphnia magna in acute and chronic multigenerational tests. Aquat Toxicol 197:32–40
Gondikas AP, Kammer F, Reed RB, Wagner S, Ranville JF, Hofmann T (2014) Release of TiO2 nanoparticles from sunscreens into surface waters: a one-year survey at the old Danube recreational lake. Environ Sci Technol 48:5415–5422
Gondikas A, von der Kammer F, Kaegi R, Borovinskaya O, Neubauer E, Navratilova J, Praetorius A, Cornelis G, Hofmann T (2018) Where is the nano? Analytical approaches for the detection and quantification of TiO2 engineered nanoparticles in surface waters. Environ Sci Nano 5:313–326
Goswami L, Kim K-H, Deep A, Das P, Bhattacharya SS, Kumar S, Adelodun AA (2017) Engineered nano particles: nature, behavior, and effect on the environment. J Environ Manag 196:297–315
Gottschalk F, Nowack B (2011) The release of engineered nanomaterials to the environment. J Environ Monit 13:1145–1155
Gottschalk F, Sun T, Nowack B (2013) Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ Pollut 181:287–300
Goulden CE, Hornig LL (1980) Population oscillations and energy reserves in planktonic cladocera and their consequences to competition. Proc Natl Acad Sci 77:1716–1720
Greulich C, Braun D, Peetsch A, Diendorf J, Siebers B, Epple M, Köller M (2012) The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv 2:6981–6987
Gunsolus IL, Mousavi MP, Hussein K, Bühlmann P, Haynes CL (2015) Effects of humic and fulvic acids on silver nanoparticle stability, dissolution, and toxicity. Environ Sci Technol 49:8078–8086
Guo Z, Chen G, Zeng G, Yan M, Huang Z, Jiang L, Peng C, Wang J, Xiao Z (2017) Are silver nanoparticles always toxic in the presence of environmental anions? Chemosphere 171:318–323
Handy RD, Henry TB, Scown TM, Johnston BD, Tyler CR (2008a) Manufactured nanoparticles: their uptake and effects on fish – a mechanistic analysis. Ecotoxicology 17:396–409
Handy RD, von der Kammer F, Lead JR, Hassellöv M, Owen R, Crane M (2008b) The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 17:287–314
Hansen SF, Heggelund LR, Besora PR, Mackevica A, Boldrin A, Baun A (2016) Nanoproducts – what is actually available to European consumers? Environ Sci Nano 3:169–180
Hartmann NB, von der Kammer F, Hofmann T, Baalousha M, Ottofuelling S, Baun A (2010) Algal testing of titanium dioxide nanoparticles – testing considerations, inhibitory effects and modification of cadmium bioavailability. Toxicology 269:190–197
Hartmann G, Hutterer C, Schuster M (2013) Ultra-trace determination of silver nanoparticles in water samples using cloud point extraction and ETAAS. J Anal At Spectrom 28:567–572
Hassellöv M, Readman JW, Ranville JF, Tiede K (2008) Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Ecotoxicology 17:344–361
Heinlaan M, Ivask A, Blinova I, Dubourguier H-C, Kahru A (2008) Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 71:1308–1316
Heinlaan M, Muna M, Juganson K, Oriekhova O, Stoll S, Kahru A, Slaveykova VI (2017) Exposure to sublethal concentrations of Co3O4 and Mn2O3 nanoparticles induced elevated metal body burden in Daphnia magna. Aquat Toxicol 189:123–133
Hjorth R, Coutris C, Nguyen NHA, Sevcu A, Gallego-Urrea JA, Baun A, Joner EJ (2017a) Ecotoxicity testing and environmental risk assessment of iron nanomaterials for sub-surface remediation – recommendations from the FP7 project NanoRem. Chemosphere 182:525–531
Hjorth R, Holden PA, Hansen SF, Colman BP, Grieger K, Hendren CO (2017b) The role of alternative testing strategies in environmental risk assessment of engineered nanomaterials. Environ Sci Nano 4:292–301
Hjorth R, Skjolding LM, Sørensen SN, Baun A (2017c) Regulatory adequacy of aquatic ecotoxicity testing of nanomaterials. NanoImpact 8:28–37
Hoecke KV, Quik JT, Mankiewicz-Boczek J, Schamphelaere KAD, Elsaesser A, Meeren PVD, Barnes C, Mckerr G, Howard CV, Meent DVD (2009) Fate and effects of CeO2 nanoparticles in aquatic ecotoxicity tests. Environ Sci Technol 43:4537–4546
Hoet PH, Brüske-Hohlfeld I, Salata OV (2004) Nanoparticles – known and unknown health risks. J Nanobiotechnol 2:12
Holden PA, Klaessig F, Turco RF, Priester JH, Rico CM, Avila-Arias H, Mortimer M, Pacpaco K, Gardea-Torresdey JL (2014) Evaluation of exposure concentrations used in assessing manufactured nanomaterial environmental hazards: are they relevant? Environ Sci Technol 48:10541–10551
Hossain ST, Mukherjee SK (2012) CdO nanoparticle toxicity on growth, morphology, and cell division in Escherichia coli. Langmuir 28:16614–16622
Hotze EM, Phenrat T, Lowry GV (2010) Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J Environ Qual 39:1909–1924
Hristozov D, Gottardo S, Semenzin E, Oomen A, Bos P, Peijnenburg W, van Tongeren M, Nowack B, Hunt N, Brunelli A (2016) Frameworks and tools for risk assessment of manufactured nanomaterials. Environ Int 95:36–53
Hu Y, Chen X, Yang K, Lin D (2017) Distinct toxicity of silver nanoparticles and silver nitrate to Daphnia magna in M4 medium and surface water. Sci Total Environ 618:838–846
Hund-Rinke K, Simon M (2006) Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids (8 pp). Environ Sci Pollut Res 13:225–232
Hwang ET, Lee JH, Chae YJ, Kim YS, Kim BC, Sang BI, Gu MB (2008) Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small 4:746–750
Hyung H, Fortner JD, Hughes JB, Kim J-H (2007) Natural organic matter stabilizes carbon nanotubes in the aqueous phase. Environ Sci Technol 41:179–184
Iswarya V, Bhuvaneshwari M, Chandrasekaran N, Mukherjee A (2016) Individual and binary toxicity of anatase and rutile nanoparticles towards Ceriodaphnia dubia. Aquat Toxicol 178:209–221
Iswarya V, Johnson J, Parashar A, Pulimi M, Chandrasekaran N, Mukherjee A (2017) Modulatory effects of Zn2+ ions on the toxicity of citrate-and PVP-capped gold nanoparticles towards freshwater algae, Scenedesmus obliquus. Environ Sci Pollut Res 24:3790–3801
Jaiswal S, Mishra P (2018) Antimicrobial and antibiofilm activity of curcumin-silver nanoparticles with improved stability and selective toxicity to bacteria over mammalian cells. Med Microbiol Immunol 207:39–53
Jarvie HP, King SM (2010) Just scratching the surface? New techniques show how surface functionality of nanoparticles influences their environmental fate. Nano Today 5:248–250
Jensen LHS, Skjolding LM, Thit A, Sørensen SN, Købler C, Mølhave K, Baun A (2017) Not all that glitters is gold – electron microscopy study on uptake of gold nanoparticles in Daphnia magna and related artifacts. Environ Toxicol Chem 36:1503–1509
Ji J, Long Z, Lin D (2011) Toxicity of oxide nanoparticles to the green algae Chlorella sp. Chem Eng J 170:525–530
Jiang W, Kim BY, Rutka JT, Chan WC (2008) Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol 3:145–150
Jiang W, Mashayekhi H, Xing B (2009) Bacterial toxicity comparison between nano-and micro-scaled oxide particles. Environ Pollut 157:1619–1625
Jiang C, Aiken GR, Hsu-Kim H (2015) Effects of natural organic matter properties on the dissolution kinetics of zinc oxide nanoparticles. Environ Sci Technol 49:11476–11484
Jiménez AS, Sun T, van Tongeren M, Wohlleben W (2016) The flows of engineered nanomaterials from production, use, and disposal to the environment. Indoor Outdoor Nanopart 48:209
Jin X, Li M, Wang J, Marambio-Jones C, Peng F, Huang X, Damoiseaux R, Hoek EM (2010) High-throughput screening of silver nanoparticle stability and bacterial inactivation in aquatic media: influence of specific ions. Environ Sci Technol 44:7321–7328
Jonczyk E, Gilron G (2005) Acute and chronic toxicity testing with Daphnia Sp. In: Blaise C, Férard JF (eds) Small-scale freshwater toxicity investigations. Springer, Dordrecht
Kadukova J (2016) Surface sorption and nanoparticle production as a silver detoxification mechanism of the freshwater alga Parachlorella kessleri. Bioresour Technol 216:406–413
Karunakaran G, Suriyaprabha R, Rajendran V, Kannan N (2015) Toxicity evaluation based on particle size, contact angle and zeta potential of SiO2 and Al2O3 on the growth of green algae. Adv Nano Res 3:243–255
Kashiwada S (2006) Distribution of nanoparticles in the see-through medaka (Oryzias latipes). Environ Health Perspect 114:1697–1702
Keller AA, Lazareva A (2013) Predicted releases of engineered nanomaterials: from global to regional to local. Environ Sci Technol Lett 1:65–70
Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R, Ji Z (2010) Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ Sci Technol 44:1962–1967
Keller AA, Mcferran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15:1–17
Kennedy AJ, Chappell MA, Bednar AJ, Ryan AC, Laird JG, Stanley JK, Steevens JA (2012) Impact of organic carbon on the stability and toxicity of fresh and stored silver nanoparticles. Environ Sci Technol 46:10772–10780
Kessler R (2011) Engineered nanoparticles in consumer products: understanding a new ingredient. Environ Health Perspect 119:A120–A125
Khan FR, Kennaway GM, Croteau M-N, Dybowska A, Smith BD, Nogueira AJA, Rainbow PS, Luoma SN, Valsami-Jones E (2014) In vivo retention of ingested Au NPs by Daphnia magna: no evidence for trans-epithelial alimentary uptake. Chemosphere 100:97–104
Kim KT, Klaine SJ, Cho J, Kim S-H, Kim SD (2010) Oxidative stress responses of Daphnia magna exposed to TiO2 nanoparticles according to size fraction. Sci Total Environ 408:2268–2272
Kim I, Lee B-T, Kim H-A, Kim K-W, Kim SD, Hwang Y-S (2016) Citrate coated silver nanoparticles change heavy metal toxicities and bioaccumulation of Daphnia magna. Chemosphere 143:99–105
Kim S, Samanta P, Yoo J, Kim W-K, Jung J (2017) Time-dependent toxicity responses in Daphnia magna exposed to CuO and ZnO nanoparticles. Bull Environ Contam Toxicol 98:502–507
Kittler S, Greulich C, Diendorf J, Koller M, Epple M (2010) Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater 22:4548–4554
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, Mclaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27:1825–1851
Klaine SJ, Koelmans AA, Horne N, Carley S, Handy RD, Kapustka L, Nowack B, von der Kammer F (2012) Paradigms to assess the environmental impact of manufactured nanomaterials. Environ Toxicol Chem 31:3–14
Köser J, Engelke M, Hoppe M, Nogowski A, Filser J, Thöming J (2017) Predictability of silver nanoparticle speciation and toxicity in ecotoxicological media. Environ Sci Nano 4:1470–1483
Kraas M, Schlich K, Knopf B, Wege F, Kägi R, Terytze K, Hund-Rinke K (2017) Long-term effects of sulfidized silver nanoparticles in sewage sludge on soil microflora. Environ Toxicol Chem 36:3305–3313
Kumar A, Pandey AK, Singh SS, Shanker R, Dhawan A (2011) Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic Biol Med 51:1872–1881
Kumar V, Kumari A, Guleria P, Yadav SK (2012) Evaluating the toxicity of selected types of nanochemicals. Rev Environ Contam Toxicol 215:39–121
Kümmerer K, Menz J, Schubert T, Thielemans W (2011) Biodegradability of organic nanoparticles in the aqueous environment. Chemosphere 82:1387–1392
Kvitek L, Panáček A, Soukupova J, Kolar M, Vecerova R, Prucek R, Holecová M, Zboril R (2008) Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J Phys Chem C 112:5825–5834
Kwak S-Y, Kim SH, Kim SS (2001) Hybrid organic/inorganic reverse osmosis (RO) membrane for bactericidal anti-fouling. 1. Preparation and characterization of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane. Environ Sci Technol 35:2388–2394
Laborda F, Bolea E, Cepriá G, Gómez MT, Jiménez MS, Pérez-Arantegui J, Castillo JR (2016a) Detection, characterization and quantification of inorganic engineered nanomaterials: a review of techniques and methodological approaches for the analysis of complex samples. Anal Chim Acta 904:10–32
Laborda F, Bolea E, Jiménez-Lamana J (2016b) Single particle inductively coupled plasma mass spectrometry for the analysis of inorganic engineered nanoparticles in environmental samples. Trends Environ Anal Chem 9:15–23
Lapresta-Fernández A, Fernández A, Blasco J (2012) Nanoecotoxicity effects of engineered silver and gold nanoparticles in aquatic organisms. TrAC Trends Anal Chem 32:40–59
Larguinho M, Correia D, Diniz MS, Baptista PV (2014) Evidence of one-way flow bioaccumulation of gold nanoparticles across two trophic levels. J Nanopart Res 16:2549
Lee W-M, An Y-J (2013) Effects of zinc oxide and titanium dioxide nanoparticles on green algae under visible, UVA, and UVB irradiations: no evidence of enhanced algal toxicity under UV pre-irradiation. Chemosphere 91:536–544
Lee W-M, Yoon S-J, Shin Y-J, An Y-J (2015) Trophic transfer of gold nanoparticles from Euglena gracilis or Chlamydomonas reinhardtii to Daphnia magna. Environ Pollut 201:10–16
Lei C, Zhang L, Yang K, Zhu L, Lin D (2016) Toxicity of iron-based nanoparticles to green algae: effects of particle size, crystal phase, oxidation state and environmental aging. Environ Pollut 218:505–512
Lei C, Sun Y, Tsang DCW, Lin D (2018) Environmental transformations and ecological effects of iron-based nanoparticles. Environ Pollut 232:10–30
Levard C, Michel FM, Wang Y, Choi Y, Eng P, Brown GE (2011a) Probing Ag nanoparticle surface oxidation in contact with (in) organics: an X-ray scattering and fluorescence yield approach. J Synchrotron Radiat 18:871–878
Levard C, Reinsch BC, Michel FM, Oumahi C, Lowry GV, Brown GE Jr (2011b) Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: impact on dissolution rate. Environ Sci Technol 45:5260–5266
Levard C, Hotze EM, Lowry GV, Brown GE Jr (2012) Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ Sci Technol 46:6900–6914
Li T, Albee B, Alemayehu M, Diaz R, Ingham L, Kamal S, Rodriguez M, Bishnoi SW (2010a) Comparative toxicity study of Ag, Au, and Ag–Au bimetallic nanoparticles on Daphnia magna. Anal Bioanal Chem 398:689–700
Li W-R, Xie X-B, Shi Q-S, Zeng H-Y, Ou-Yang Y-S, Chen Y-B (2010b) Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol 85:1115–1122
Li X, Lenhart JJ, Walker HW (2010c) Dissolution-accompanied aggregation kinetics of silver nanoparticles. Langmuir 26:16690–16698
Li M, Zhu L, Lin D (2011) Toxicity of ZnO nanoparticles to Escherichia coli: mechanism and the influence of medium components. Environ Sci Technol 45:1977–1983
Li M, Lin D, Zhu L (2013) Effects of water chemistry on the dissolution of ZnO nanoparticles and their toxicity to Escherichia coli. Environ Pollut 173:97–102
Li L, Wu H, Ji C, van Gestel CA, Allen HE, Peijnenburg WJ (2015a) A metabolomic study on the responses of Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Ecotoxicol Environ Saf 119:66–73
Li X, Schirmer K, Bernard L, Sigg L, Pillai S, Behra R (2015b) Silver nanoparticle toxicity and association with the alga Euglena gracilis. Environ Sci Nano 2:594–602
Li D, Li B, Wang Q, Hou N, Li C, Cheng X (2016a) Toxicity of TiO2 nanoparticle to denitrifying strain CFY1 and the impact on microbial community structures in activated sludge. Chemosphere 144:1334–1341
Li Y, Niu J, Shang E, Crittenden JC (2016b) Influence of dissolved organic matter on photogenerated reactive oxygen species and metal-oxide nanoparticle toxicity. Water Res 98:9–18
Li L, Sillanpää M, Schultz E (2017a) Influence of titanium dioxide nanoparticles on cadmium and lead bioaccumulations and toxicities to Daphnia magna. J Nanopart Res 19:223
Li Y, Qin T, Ingle T, Yan J, He W, Yin J-J, Chen T (2017b) Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch Toxicol 91:509–519
Limbach LK, Bereiter R, Müller E, Krebs R, Gälli R, Stark WJ (2008) Removal of oxide nanoparticles in a model wastewater treatment plant: influence of agglomeration and surfactants on clearing efficiency. Environ Sci Technol 42:5828–5833
Liu J, Sonshine DA, Shervani S, Hurt RH (2010) Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 4:6903–6913
Liu W, Zhou Q, Liu J, Fu J, Liu S, Jiang G (2011) Environmental and biological influences on the stability of silver nanoparticles. Chin Sci Bull 56:2009–2015
Liu L, Fan W, Lu H, Xiao W (2015) Effects of the interaction between TiO2 with different percentages of exposed {001} facets and Cu2+ on biotoxicity in Daphnia magna. Sci Rep 5:11121
Liu Y, Fan W, Xu Z, Peng W, Luo S (2017) Transgenerational effects of reduced graphene oxide modified by Au, Ag, Pd, Fe3O4, Co3O4 and SnO2 on two generations of Daphnia magna. Carbon 122:669–679
Liu K, He Z, Byrne HJ, Curtin J, Tian F (2018) Investigating the role of shape and size of gold 2 nanoparticles on their toxicities to fungi 3. Int J Environ Res Public Health 15(5):E998. https://doi.org/10.3390/ijerph15050998
Lok C-N, Ho C-M, Chen R, He Q-Y, Yu W-Y, Sun H, Tam PK-H, Chiu J-F, Che C-M (2006) Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res 5:916–924
Lok C-N, Ho C-M, Chen R, He Q-Y, Yu W-Y, Sun H, Tam PK-H, Chiu J-F, Che C-M (2007) Silver nanoparticles: partial oxidation and antibacterial activities. J Biol Inorg Chem 12:527–534
Lopes I, Ribeiro R, Antunes F, Rocha-Santos T, Rasteiro M, Soares A, Gonçalves F, Pereira R (2012) Toxicity and genotoxicity of organic and inorganic nanoparticles to the bacteria Vibrio fischeri and Salmonella typhimurium. Ecotoxicology 21:637–648
Lopes S, Ribeiro F, Wojnarowicz J, Łojkowski W, Jurkschat K, Crossley A, Soares AM, Loureiro S (2014) Zinc oxide nanoparticles toxicity to Daphnia magna: size-dependent effects and dissolution. Environ Toxicol Chem 33:190–198
Lovern SB, Klaper R (2006) Daphnia magna mortality when exposed to titanium dioxide and fullerene (C60) nanoparticles. Environ Toxicol Chem 25:1132–1137
Lovern SB, Strickler JR, Klaper R (2007a) Behavioral and physiological changes in Daphnia magna when exposed to nanoparticle suspensions (titanium dioxide, nano-C60, and C60HxC70Hx). Environ Sci Technol 41:4465–4470
Lovern SB, Strickler JR, Klaper R (2007b) Behavioral and physiological changes in Daphnia magna when exposed to nanoparticle suspensions (titanium dioxide, nano-C(60), and C(60)HxC(70)Hx). Environ Sci Technol 41:4465–4470
Lovern SB, Owen HA, Klaper R (2008) Electron microscopy of gold nanoparticle intake in the gut of Daphnia magna. Nanotoxicology 2:43–48
Lu G, Yang H, Xia J, Zong Y, Liu J (2017) Toxicity of Cu and Cr nanoparticles to Daphnia magna. Water Air Soil Pollut 228:18
Luo P, Roca A, Tiede K, Privett K, Jiang J, Pinkstone J, Ma G, Veinot J, Boxall A (2018) Application of nanoparticle tracking analysis for characterising the fate of engineered nanoparticles in sediment-water systems. J Environ Sci 64:62–71
Luoma SN, Stoiber T, Croteau M-N, Römer I, Merrifeld R, Lead JR (2016) Effect of cysteine and humic acids on bioavailability of Ag from Ag nanoparticles to a freshwater snail. NanoImpact 2:61–69
Lv X, Huang B, Zhu X, Jiang Y, Chen B, Tao Y, Zhou J, Cai Z (2017) Mechanisms underlying the acute toxicity of fullerene to Daphnia magna: energy acquisition restriction and oxidative stress. Water Res 123:696–703
Ma H, Williams PL, Diamond SA (2013) Ecotoxicity of manufactured ZnO nanoparticles – a review. Environ Pollut 172:76–85
Ma Y, Metch JW, Vejerano EP, Miller IJ, Leon EC, Marr LC, Vikesland PJ, Pruden A (2015) Microbial community response of nitrifying sequencing batch reactors to silver, zero-valent iron, titanium dioxide and cerium dioxide nanomaterials. Water Res 68:87–97
Madden AS, Hochella MF, Luxton TP (2006) Insights for size-dependent reactivity of hematite nanomineral surfaces through Cu2+ sorption. Geochim Cosmochim Acta 70:4095–4104
Majedi SM, Lee HK (2016) Recent advances in the separation and quantification of metallic nanoparticles and ions in the environment. TrAC Trends Anal Chem 75:183–196
Marambio-Jones C, Hoek EM (2010) A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res 12:1531–1551
Markus A, Parsons J, Roex E, de Voogt P, Laane R (2016) Modelling the transport of engineered metallic nanoparticles in the river Rhine. Water Res 91:214–224
Markus AA, Parsons JR, Roex EW, de Voogt P, Laane RW (2017) Modelling the release, transport and fate of engineered nanoparticles in the aquatic environment – a review. Rev Environ Contam Toxicol 243:53–87
Markus A, Krystek P, Tromp P, Parsons J, Roex E, de Voogt P, Laane R (2018) Determination of metal-based nanoparticles in the river Dommel in the Netherlands via ultrafiltration, HR-ICP-MS and SEM. Sci Total Environ 631:485–495
Mattsson M-O, Simkó M (2017) The changing face of nanomaterials: risk assessment challenges along the value chain. Regul Toxicol Pharmacol 84:105–115
Matzke M, Jurkschat K, Backhaus T (2014) Toxicity of differently sized and coated silver nanoparticles to the bacterium Pseudomonas putida: risks for the aquatic environment? Ecotoxicology 23:818–829
Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdörster G, Philbert MA, Ryan J, Seaton A, Stone V (2006) Safe handling of nanotechnology. Nature 444:267–269
Mcgillicuddy E, Murray I, Kavanagh S, Morrison L, Fogarty A, Cormican M, Dockery P, Prendergast M, Rowan N, Morris D (2017) Silver nanoparticles in the environment: sources, detection and ecotoxicology. Sci Total Environ 575:231–246
Mcteer J, Dean AP, White KN, Pittman JK (2014) Bioaccumulation of silver nanoparticles into Daphnia magna from a freshwater algal diet and the impact of phosphate availability. Nanotoxicology 8:305–316
Melvin SD, Wilson SP (2013) The utility of behavioral studies for aquatic toxicology testing: a meta-analysis. Chemosphere 93:2217–2223
Metreveli G, Frombold B, Seitz F, Grün A, Philippe A, Rosenfeldt RR, Bundschuh M, Schulz R, Manz W, Schaumann GE (2016) Impact of chemical composition of ecotoxicological test media on the stability and aggregation status of silver nanoparticles. Environ Sci Nano 3:418–433
Metzler D, Erdem A, Huang C (2018) Influence of Algae Age and population on the response to TiO2 nanoparticles. Int J Environ Res Public Health 15:585
Miao A-J, Luo Z, Chen C-S, Chin W-C, Santschi PH, Quigg A (2010) Intracellular uptake: a possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica. PLoS One 5:e15196
Miao L, Wang P, Wang C, Hou J, Yao Y, Liu J, Lv B, Yang Y, You G, Xu Y, Liu Z, Liu S (2018) Effect of TiO2 and CeO2 nanoparticles on the metabolic activity of surficial sediment microbial communities based on oxygen microelectrodes and high-throughput sequencing. Water Res 129:287–296
Minetto D, Volpi Ghirardini A, Libralato G (2016) Saltwater ecotoxicology of Ag, Au, CuO, TiO2, ZnO and C60 engineered nanoparticles: an overview. Environ Int 92-93:189–201
Mirzajani F, Askari H, Hamzelou S, Farzaneh M, Ghassempour A (2013) Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol Environ Saf 88:48–54
Mitrano DM, Lesher EK, Bednar A, Monserud J, Higgins CP, Ranville JF (2012) Detecting nanoparticulate silver using single-particle inductively coupled plasma–mass spectrometry. Environ Toxicol Chem 31:115–121
Moore MN (1990) Lysosomal cytochemistry in marine environmental monitoring. Histochem J 22:187–191
Moore MN (2002) Biocomplexity: the post-genome challenge in ecotoxicology. Aquat Toxicol 59:1–15
Moore M (2006) Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int 32:967–976
Moore MN, Allen JI (2002) A computational model of the digestive gland epithelial cell of marine mussels and its simulated responses to oil-derived aromatic hydrocarbons. Mar Environ Res 54:579–584
Moore MN, Depledge MH, Readman JW, Leonard DP (2004) An integrated biomarker-based strategy for ecotoxicological evaluation of risk in environmental management. Mutat Res Fundam Mol Mech Mutagen 552:247–268
Moreno-Garrido I, Pérez S, Blasco J (2015) Toxicity of silver and gold nanoparticles on marine microalgae. Mar Environ Res 111:60–73
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346
Mottier A, Mouchet F, Pinelli É, Gauthier L, Flahaut E (2017) Environmental impact of engineered carbon nanoparticles: from releases to effects on the aquatic biota. Curr Opin Biotechnol 46:1–6
Mudunkotuwa IA, Grassian VH (2011) The devil is in the details (or the surface): impact of surface structure and surface energetics on understanding the behavior of nanomaterials in the environment. J Environ Monit 13:1135–1144
Mueller NC, Nowack B (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42:4447–4453
Müller E, Behra R, Sigg L (2016) Toxicity of engineered copper (Cu0) nanoparticles to the green alga Chlamydomonas reinhardtii. Environ Chem 13:457–463
Muna M, Heinlaan M, Blinova I, Vija H, Kahru A (2017) Evaluation of the effect of test medium on total Cu body burden of nano CuO-exposed Daphnia magna: a TXRF spectroscopy study. Environ Pollut 231:1488–1496
Na K, Bum Lee T, Park K-H, Shin E-K, Lee Y-B, Choi H-K (2003) Self-assembled nanoparticles of hydrophobically-modified polysaccharide bearing vitamin H as a targeted anti-cancer drug delivery system. Eur J Pharm Sci 18:165–173
The Nanodatabase (2018) http://nanodb.dk/
Nasser F, Davis A, Valsami-Jones E, Lynch I (2016) Shape and charge of gold nanomaterials influence survivorship, oxidative stress and moulting of Daphnia magna. Nano 6:222
Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R (2008) Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol 42:8959–8964
Neal AL (2008) What can be inferred from bacterium–nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicology 17:362
Nicolas M, Séverine LM, Anne B-N, Pascal P (2016) Effect of two TiO2 nanoparticles on the growth of unicellular green algae using the OECD 201 test guideline: influence of the exposure system. Toxicol Environ Chem 98:860–876
Nogueira PFM, Nakabayashi D, Zucolotto V (2015) The effects of graphene oxide on green algae Raphidocelis subcapitata. Aquat Toxicol 166:29–35
Noss C, Dabrunz A, Rosenfeldt RR, Lorke A, Schulz R (2013) Three-dimensional analysis of the swimming behavior of Daphnia magna exposed to nanosized titanium dioxide. PLoS One 8:e80960
Novak S, Jemec Kokalj A, Hočevar M, Godec M, Drobne D (2018) The significance of nanomaterial post-exposure responses in Daphnia magna standard acute immobilisation assay: example with testing TiO2 nanoparticles. Ecotoxicol Environ Saf 152:61–66
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150:5–22
Nowack B, Ranville JF, Diamond S, Gallego-Urrea JA, Metcalfe C, Rose J, Horne N, Koelmans AA, Klaine SJ (2012) Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ Toxicol Chem 31:50–59
Nune SK, Chanda N, Shukla R, Katti K, Kulkarni RR, Thilakavathy S, Mekapothula S, Kannan R, Katti KV (2009) Green nanotechnology from tea: phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J Mater Chem 19:2912–2920
Nyberg L, Turco RF, Nies L (2008) Assessing the impact of nanomaterials on anaerobic microbial communities. Environ Sci Technol 42:1938–1943
Nyholm N, Peterson HG (1997) Laboratory bioassays with microalgae. CRC Press, Boca Raton
O’Keefe TC, Brewer MC, Dodson SI (1998) Swimming behavior of Daphnia: its role in determining predation risk. J Plankton Res 20:973–984
Oberdörster E (2004) Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ Health Perspect 112:1058
OECD (2011) Test No. 201: Freshwater alga and cyanobacteria, growth inhibition test [Online]. OECD Publishing. Available: https://www.oecd-ilibrary.org/environment/test-no-201-alga-growthinhibition-test_9789264069923-en. Accessed 13 March 2018
OECD (2014) Ecotoxicology and environmental fate of manufactured nanomaterials: test guidelines [Online]. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO%282014%291&doclanguage=en. Accessed 04 Apr 2018
OECD (2017a) 28-day (Subacute) inhalation toxicity study [Online]. http://www.oecd-ilibrary.org/docserver/download/9741201e.pdf?expires=1520925030&id=id&accname=guest&checksum=936D2F4A91857FF5F257958C9BD2664A. Accessed 13 Mar 2018
OECD (2017b) 90-day (Subchronic) inhalation toxicity study [Online]. http://www.oecd-ilibrary.org/docserver/download/9741301e.pdf?expires=1520925206&id=id&accname=guest&checksum=A86B5A2CC113CC021C4108A044C45AC4. Accessed 13 Mar 2018
OECD (2017c) Dispersion stability of nanomaterials in simulated environmental media [Online]. http://www.oecd-ilibrary.org/docserver/download/9717701e.pdf?expires=1520923737&id=id&accname=guest&checksum=A807794DAB0F036965D880F8711F459C. Accessed 13 Mar 2018
Oleszczuk P, Jośko I, Skwarek E (2015) Surfactants decrease the toxicity of ZnO, TiO2 and Ni nanoparticles to Daphnia magna. Ecotoxicology 24:1923–1932
Omar FM, Aziz HA, Stoll S (2014) Aggregation and disaggregation of ZnO nanoparticles: influence of pH and adsorption of Suwannee River humic acid. Sci Total Environ 468:195–201
Oukarroum A, Bras S, Perreault F, Popovic R (2012) Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotoxicol Environ Saf 78:80–85
Oukarroum A, Zaidi W, Samadani M, Dewez D (2017) Toxicity of nickel oxide nanoparticles on a freshwater green algal strain of Chlorella vulgaris. Biomed Res Int 2017:8. https://doi.org/10.1155/2017/9528180
Ozkaleli M, Erdem A (2018) Biotoxicity of TiO2 nanoparticles on Raphidocelis subcapitata microalgae exemplified by membrane deformation. Int J Environ Res Public Health 15:416
Pacheco A, Martins A, Guilhermino L (2018) Toxicological interactions induced by chronic exposure to gold nanoparticles and microplastics mixtures in Daphnia magna. Sci Total Environ 628–629:474–483
Pagnout C, Jomini S, Dadhwal M, Caillet C, Thomas F, Bauda P (2012) Role of electrostatic interactions in the toxicity of titanium dioxide nanoparticles toward Escherichia coli. Colloids Surf B: Biointerfaces 92:315–321
Pakrashi S, Tan C, Wang WX (2017) Bioaccumulation-based silver nanoparticle toxicity in Daphnia magna and maternal impacts. Environ Toxicol Chem 36:3359–3366
Panyam J, Labhasetwar V (2003) Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev 55:329–347
Park Y, Hong Y, Weyers A, Kim Y, Linhardt R (2011) Polysaccharides and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol 5:69–78
Park S, Woodhall J, Ma G, Veinot JG, Boxall A (2015) Do particle size and surface functionality affect uptake and depuration of gold nanoparticles by aquatic invertebrates? Environ Toxicol Chem 34:850–859
Pelkmans L, Helenius A (2002) Endocytosis via caveolae. Traffic 3:311–320
Peng X, Palma S, Fisher NS, Wong SS (2011) Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquat Toxicol 102:186–196
Peters RJB, van Bemmel G, Milani NBL, Den Hertog GCT, Undas AK, van der Lee M, Bouwmeester H (2018) Detection of nanoparticles in Dutch surface waters. Sci Total Environ 621:210–218
Phenrat T, Saleh N, Sirk K, Tilton RD, Lowry GV (2007) Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ Sci Technol 41:284–290
Phenrat T, Saleh N, Sirk K, Kim H-J, Tilton RD, Lowry GV (2008) Stabilization of aqueous nanoscale zerovalent iron dispersions by anionic polyelectrolytes: adsorbed anionic polyelectrolyte layer properties and their effect on aggregation and sedimentation. J Nanopart Res 10:795–814
Piccinno F, Gottschalk F, Seeger S, Nowack B (2012) Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res 14:1–11
Pokhrel LR, Dubey B (2012) Potential impact of low-concentration silver nanoparticles on predator–prey interactions between predatory dragonfly nymphs and Daphnia magna as a prey. Environ Sci Technol 46:7755–7762
Poole CP Jr, Owens FJ (2003) Introduction to nanotechnology. Wiley, Hoboken
Postma J, Keijzers C (2014) Behavior as response parameter. A literature review on the relevance for population sustainability. Weesp, The Netherlands: Ecofide. Report No. 74
Pradhan A, Seena S, Pascoal C, Cássio F (2011) Can metal nanoparticles be a threat to microbial decomposers of plant litter in streams? Microb Ecol 62:58–68
Pu Y, Tang F, Adam P-M, Laratte B, Ionescu RE (2016) Fate and characterization factors of nanoparticles in seventeen subcontinental freshwaters: a case study on copper nanoparticles. Environ Sci Technol 50:9370–9379
Qian H, Zhu K, Lu H, Lavoie M, Chen S, Zhou Z, Deng Z, Chen J, Fu Z (2016) Contrasting silver nanoparticle toxicity and detoxification strategies in Microcystis aeruginosa and Chlorella vulgaris: new insights from proteomic and physiological analyses. Sci Total Environ 572:1213–1221
Qin G, Xiong Y, Tang S, Zhao P, Doering JA, Beitel SC, Hecker M, Wang M, Liu H, Lu H (2015) Impact of predator cues on responses to silver nanoparticles in Daphnia carinata. Arch Environ Contam Toxicol 69:494–505
Qu H, Linder SW, Mudalige TK (2017) Surface coating and matrix effect on the electrophoretic mobility of gold nanoparticles: a capillary electrophoresis-inductively coupled plasma mass spectrometry study. Anal Bioanal Chem 409:979–988
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Rainville L-C, Carolan D, Varela AC, Doyle H, Sheehan D (2014) Proteomic evaluation of citrate-coated silver nanoparticles toxicity in Daphnia magna. Analyst 139:1678–1686
Rana S, Kalaichelvan P (2013) Ecotoxicity of nanoparticles. ISRN Toxicol 2013:11
Ratti M, Naddeo J, Tan Y, Griepenburg JC, Tomko J, Trout C, O’malley SM, Bubb DM, Klein EA (2016) Irradiation with visible light enhances the antibacterial toxicity of silver nanoparticles produced by laser ablation. Appl Phys A 122:346
Reinsch B, Levard C, Li Z, Ma R, Wise A, Gregory K, Brown G Jr, Lowry G (2012) Sulfidation of silver nanoparticles decreases Escherichia coli growth inhibition. Environ Sci Technol 46:6992–7000
Ren M, Horn H, Frimmel FH (2017) Aggregation behavior of TiO2 nanoparticles in municipal effluent: Influence of ionic strengthen and organic compounds. Water Res 123:678–686
Renault S, Baudrimont M, Mesmer-Dudons N, Gonzalez P, Mornet S, Brisson A (2008) Impacts of gold nanoparticle exposure on two freshwater species: a phytoplanktonic alga (Scenedesmus subspicatus) and a benthic bivalve (Corbicula fluminea). Gold Bull 41:116–126
Ribeiro F, Gallego-Urrea JA, Jurkschat K, Crossley A, Hassellöv M, Taylor C, Soares AM, Loureiro S (2014) Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci Total Environ 466:232–241
Ribeiro F, van Gestel CAM, Pavlaki MD, Azevedo S, Soares AMVM, Loureiro S (2017) Bioaccumulation of silver in Daphnia magna: waterborne and dietary exposure to nanoparticles and dissolved silver. Sci Total Environ 574:1633–1639
Rocha TL, Gomes T, Sousa VS, Mestre NC, Bebianno MJ (2015) Ecotoxicological impact of engineered nanomaterials in bivalve molluscs: an overview. Mar Environ Res 111:74–88
Rocha TL, Mestre NC, Sabóia-Morais SMT, Bebianno MJ (2017) Environmental behaviour and ecotoxicity of quantum dots at various trophic levels: a review. Environ Int 98:1–17
Rodea-Palomares I, Boltes K, Fernández-Piñas F, Leganés F, García-Calvo E, Santiago J, Rosal R (2011) Physicochemical characterization and ecotoxicological assessment of CeO2 nanoparticles using two aquatic microorganisms. Toxicol Sci 119:135–145
Rogers NJ, Franklin NM, Apte SC, Batley GE, Angel BM, Lead JR, Baalousha M (2010) Physico-chemical behaviour and algal toxicity of nanoparticulate CeO2 in freshwater. Environ Chem 7:50–60
Rosenfeldt RR, Seitz F, Senn L, Schilde C, Schulz R, Bundschuh M (2015) Nanosized titanium dioxide reduces copper toxicity: the role of organic material and the crystalline phase. Environ Sci Technol 49:1815–1822
Rosenkranz P, Chaudhry Q, Stone V, Fernandes TF (2009) A comparison of nanoparticle and fine particle uptake by Daphnia magna. Environ Toxicol Chem 28:2142–2149
Rüdel H, Muñiz CD, Garelick H, Kandile NG, Miller BW, Munoz LP, Peijnenburg WJ, Purchase D, Shevah Y, van Sprang P (2015) Consideration of the bioavailability of metal/metalloid species in freshwaters: experiences regarding the implementation of biotic ligand model-based approaches in risk assessment frameworks. Environ Sci Pollut Res 22:7405–7421
Saei AA, Yazdani M, Lohse SE, Bakhtiary Z, Serpooshan V, Ghavami M, Asadian M, Mashaghi S, Dreaden EC, Mashaghi A, Mahmoudi M (2017) Nanoparticle surface functionality dictates cellular and systemic toxicity. Chem Mater 29:6578–6595
Sakamoto M, Ha J-Y, Yoneshima S, Kataoka C, Tatsuta H, Kashiwada S (2015) Free silver ion as the main cause of acute and chronic toxicity of silver nanoparticles to cladocerans. Arch Environ Contam Toxicol 68:500–509
Sakka Y, Skjolding LM, Mackevica A, Filser J, Baun A (2016) Behavior and chronic toxicity of two differently stabilized silver nanoparticles to Daphnia magna. Aquat Toxicol 177:526–535
Salieri B, Pasteris A, Baumann J, Righi S, Köser J, D’amato R, Mazzesi B, Filser J (2015) Does the exposure mode to ENPs influence their toxicity to aquatic species? A case study with TiO2 nanoparticles and Daphnia magna. Environ Sci Pollut Res 22:5050–5058
Sá-Pereira P, Diniz MS, Moita L, Pinheiro T, Mendonça E, Paixão SM, Picado A (2018) Protein profiling as early detection biomarkers for TiO2 nanoparticle toxicity in Daphnia magna. Ecotoxicology 27:430–439
Sapsford KE, Algar WR, Berti L, Gemmill KB, Casey BJ, Oh E, Stewart MH, Medintz IL (2013) Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev 113:1904–2074
Sayes CM, Fortner JD, Guo W, Lyon D, Boyd AM, Ausman KD, Tao YJ, Sitharaman B, Wilson LJ, Hughes JB (2004) The differential cytotoxicity of water-soluble fullerenes. Nano Lett 4:1881–1887
Scanlan LD, Reed RB, Loguinov AV, Antczak P, Tagmount A, Aloni S, Nowinski DT, Luong P, Tran C, Karunaratne N (2013) Silver nanowire exposure results in internalization and toxicity to Daphnia magna. ACS Nano 7:10681–10694
SCENIHR (2009) Health effects of exposure to EMF [Online]. http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_022.pdf. Accessed 16 Mar 2018
Scott-Fordsmand JJ, Peijnenburg WJ, Semenzin E, Nowack B, Hunt N, Hristozov D, Marcomini A, Irfan MA, Jiménez AS, Landsiedel R (2017) Environmental risk assessment strategy for nanomaterials. Int J Environ Res Public Health 14:1251
Scown T, van Aerle R, Tyler C (2010) Review: do engineered nanoparticles pose a significant threat to the aquatic environment? Crit Rev Toxicol 40:653–670
Seitz F, Rosenfeldt RR, Storm K, Metreveli G, Schaumann GE, Schulz R, Bundschuh M (2015) Effects of silver nanoparticle properties, media pH and dissolved organic matter on toxicity to Daphnia magna. Ecotoxicol Environ Saf 111:263–270
Selck H, Handy RD, Fernandes TF, Klaine SJ, Petersen EJ (2016) Nanomaterials in the aquatic environment: an EU-USA perspective on the status of ecotoxicity testing, research priorities and challenges ahead. Environ Toxicol Chem SETAC 35:1055
Sendra M, Moreno-Garrido I, Yeste MP, Gatica JM, Blasco J (2017) Toxicity of TiO2, in nanoparticle or bulk form to freshwater and marine microalgae under visible light and UV-A radiation. Environ Pollut 227:39–48
Seo J, Kim S, Choi S, Kwon D, Yoon T-H, Kim W-K, Park J-W, Jung J (2014) Effects of physiochemical properties of test media on nanoparticle toxicity to Daphnia magna straus. Bull Environ Contam Toxicol 93:257–262
Service RF (2004) Nanotoxicology. Nanotechnology grows up. Science (New York, NY) 304:1732
Shang L, Nienhaus K, Nienhaus GU (2014) Engineered nanoparticles interacting with cells: size matters. J Nanobiotechnol 12:1
Sharifi S, Behzadi S, Laurent S, Forrest ML, Stroeve P, Mahmoudi M (2012) Toxicity of nanomaterials. Chem Soc Rev 41:2323–2343
Sharma VK, Siskova KM, Zboril R, Gardea-Torresdey JL (2014) Organic-coated silver nanoparticles in biological and environmental conditions: fate, stability and toxicity. Adv Colloid Interf Sci 204:15–34
Sharma VK, Filip J, Zboril R, Varma RS (2015) Natural inorganic nanoparticles – formation, fate, and toxicity in the environment. Chem Soc Rev 44:8410–8423
Shen M-H, Zhou X-X, Yang X-Y, Chao J-B, Liu R, Liu J-F (2015) Exposure medium: key in identifying free Ag+ as the exclusive species of silver nanoparticles with acute toxicity to Daphnia magna. Sci Rep 5:9674
Shukla R, Nune SK, Chanda N, Katti K, Mekapothula S, Kulkarni RR, Welshons WV, Kannan R, Katti KV (2008) Soybeans as a phytochemical reservoir for the production and stabilization of biocompatible gold nanoparticles. Small 4:1425–1436
Silva T, Pokhrel LR, Dubey B, Tolaymat TM, Maier KJ, Liu X (2014) Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: comparison between general linear model-predicted and observed toxicity. Sci Total Environ 468–469:968–976
Simon A, Preuss TG, Schäffer A, Hollert H, Maes HM (2015) Population level effects of multiwalled carbon nanotubes in Daphnia magna exposed to pulses of triclocarban. Ecotoxicology 24:1199–1212
Singla M, Kumar M (2009) Optical characterization of ZnO nanoparticles capped with various surfactants. J Lumin 129:434–438
Skjolding LM, Kern K, Hjorth R, Hartmann N, Overgaard S, Ma G, Veinot J, Baun A (2014a) Uptake and depuration of gold nanoparticles in Daphnia magna. Ecotoxicology 23:1172–1183
Skjolding LM, Winther-Nielsen M, Baun A (2014b) Trophic transfer of differently functionalized zinc oxide nanoparticles from crustaceans (Daphnia magna) to zebrafish (Danio rerio). Aquat Toxicol 157:101–108
Skjolding LM, Sørensen SN, Hartmann NB, Hjorth R, Hansen SF, Baun A (2016) Aquatic ecotoxicity testing of nanoparticles – the quest to disclose nanoparticle effects. Angew Chem Int Ed 55:15224–15239
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275:177–182
Song JE, Phenrat T, Marinakos S, Xiao Y, Liu J, Wiesner MR, Tilton RD, Lowry GV (2011) Hydrophobic interactions increase attachment of gum arabic-and PVP-coated Ag nanoparticles to hydrophobic surfaces. Environ Sci Technol 45:5988–5995
Sørensen SN, Baun A (2015) Controlling silver nanoparticle exposure in algal toxicity testing – a matter of timing. Nanotoxicology 9:201–209
Sørensen SN, Engelbrekt C, Lützhøft H-CH, Jiménez-Lamana J, Noori JS, Alatraktchi FA, Delgado CG, Slaveykova VI, Baun A (2016a) A multimethod approach for investigating algal toxicity of platinum nanoparticles. Environ Sci Technol 50:10635–10643
Sørensen SN, Holten Lützhøft H-C, Rasmussen R, Baun A (2016b) Acute and chronic effects from pulse exposure of D. magna to silver and copper oxide nanoparticles. Aquat Toxicol 180:209–217
Stanley JK, Laird JG, Kennedy AJ, Steevens JA (2016) Sublethal effects of multiwalled carbon nanotube exposure in the invertebrate Daphnia magna. Environ Toxicol Chem 35:200–204
Stevenson LM, Dickson H, Klanjscek T, Keller AA, Mccauley E, Nisbet RM (2013) Environmental feedbacks and engineered nanoparticles: mitigation of silver nanoparticle toxicity to Chlamydomonas reinhardtii by algal-produced organic compounds. PLoS One 8:e74456
Stolpe B, Hassellöv M (2010) Nanofibrils and other colloidal biopolymers binding trace elements in coastal seawater: significance for variations in element size distributions. Limnol Oceanogr 55:187–202
Strigul N, Vaccari L, Galdun C, Wazne M, Liu X, Christodoulatos C, Jasinkiewicz K (2009) Acute toxicity of boron, titanium dioxide, and aluminum nanoparticles to Daphnia magna and Vibrio fischeri. Desalination 248:771–782
Su H-L, Chou C-C, Hung D-J, Lin S-H, Pao IC, Lin J-H, Huang F-L, Dong R-X, Lin J-J (2009) The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials 30:5979–5987
Sun TY, Gottschalk F, Hungerbühler K, Nowack B (2014) Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ Pollut 185:69–76
Sun TY, Mitrano DM, Bornhöft NA, Scheringer M, Hungerbühler K, Nowack B (2017) Envisioning nano release dynamics in a changing world: using dynamic probabilistic modeling to assess future environmental emissions of engineered nanomaterials. Environ Sci Technol 51:2854–2863
Tan C, Wang W-X (2014) Modification of metal bioaccumulation and toxicity in Daphnia magna by titanium dioxide nanoparticles. Environ Pollut 186:36–42
Tan C, Wang W-X (2017) Influences of TiO2 nanoparticles on dietary metal uptake in Daphnia magna. Environ Pollut 231:311–318
Tan L-Y, Huang B, Xu S, Wei Z-B, Yang L-Y, Miao A-J (2016a) TiO2 nanoparticle uptake by the water flea Daphnia magna via different routes is calcium-dependent. Environ Sci Technol 50:7799–7807
Tan L-Y, Huang B, Xu S, Wei Z, Yang L, Miao A-J (2016b) Aggregation reverses the carrier effects of TiO2 nanoparticles on cadmium accumulation in the waterflea Daphnia magna. Environ Sci Technol 51:932–939
Taylor C, Matzke M, Kroll A, Read DS, Svendsen C, Crossley A (2016a) Toxic interactions of different silver forms with freshwater green algae and cyanobacteria and their effects on mechanistic endpoints and the production of extracellular polymeric substances. Environ Sci Nano 3:396–408
Taylor NS, Merrifield R, Williams TD, Chipman JK, Lead JR, Viant MR (2016b) Molecular toxicity of cerium oxide nanoparticles to the freshwater alga Chlamydomonas reinhardtii is associated with supra-environmental exposure concentrations. Nanotoxicology 10:32–41
Teeguarden JG, Hinderliter PM, Orr G, Thrall BD, Pounds JG (2007) Particokinetics in vitro: dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol Sci 95:300–312
Thill A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, Flank AM (2006) Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ Sci Technol 40:6151–6156
Throbäck IN, Johansson M, Rosenquist M, Pell M, Hansson M, Hallin S (2007) Silver (Ag+) reduces denitrification and induces enrichment of novel nirK genotypes in soil. FEMS Microbiol Lett 270:189–194
Tiede K, Boxall AB, Tear SP, Lewis J, David H, Hassellöv M (2008) Detection and characterization of engineered nanoparticles in food and the environment. Food Addit Contam 25:795–821
Tiede K, Hassellöv M, Breitbarth E, Chaudhry Q, Boxall AB (2009) Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. J Chromatogr A 1216:503–509
Tiede K, Hanssen SF, Westerhoff P, Fern GJ, Hankin SM, Aitken RJ, Chaudhry Q, Boxall ABA (2016) How important is drinking water exposure for the risks of engineered nanoparticles to consumers? Nanotoxicology 10:102–110
Tourinho PS, van Gestel CA, Lofts S, Svendsen C, Soares AM, Loureiro S (2012) Metal-based nanoparticles in soil: fate, behavior, and effects on soil invertebrates. Environ Toxicol Chem 31:1679–1692
Truong NP, Whittaker MR, Mak CW, Davis TP (2015) The importance of nanoparticle shape in cancer drug delivery. Expert Opin Drug Deliv 12:129–142
Ulm L, Krivohlavek A, Jurašin D, Ljubojević M, Šinko G, Crnković T, Žuntar I, Šikić S, Vinković Vrček I (2015) Response of biochemical biomarkers in the aquatic crustacean Daphnia magna exposed to silver nanoparticles. Environ Sci Pollut Res 22:19990–19999
Vale G, Mehennaoui K, Cambier S, Libralato G, Jomini S, Domingos RF (2016) Manufactured nanoparticles in the aquatic environment-biochemical responses on freshwater organisms: a critical overview. Aquat Toxicol 170:162–174
Vance ME, Kuiken T, Vejerano EP, Mcginnis SP, Hochella MF Jr, Rejeski D, Hull MS (2015) Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol 6:1769–1780
Venkatesan AK, Reed RB, Lee S, Bi X, Hanigan D, Yang Y, Ranville JF, Herckes P, Westerhoff P (2018) Detection and sizing of Ti-containing particles in recreational waters using single particle ICP-MS. Bull Environ Contam Toxicol 100:120–126
Vidmar J, Milačič R, Ščančar J (2017) Sizing and simultaneous quantification of nanoscale titanium dioxide and a dissolved titanium form by single particle inductively coupled plasma mass spectrometry. Microchem J 132:391–400
Vijayakumar S, Malaikozhundan B, Gobi N, Vaseeharan B, Murthy C (2016) Protective effects of chitosan against the hazardous effects of zinc oxide nanoparticle in freshwater crustaceans Ceriodaphnia cornuta and Moina micrura. Limnol Ecol Manag Inland Waters 61:44–51
von Moos N, Slaveykova VI (2014) Oxidative stress induced by inorganic nanoparticles in bacteria and aquatic microalgae – state of the art and knowledge gaps. Nanotoxicology 8:605–630
von Moos N, Bowen P, Slaveykova VI (2014) Bioavailability of inorganic nanoparticles to planktonic bacteria and aquatic microalgae in freshwater. Environ Sci Nano 1:214–232
von Moos N, Maillard L, Slaveykova VI (2015) Dynamics of sub-lethal effects of nano-CuO on the microalga Chlamydomonas reinhardtii during short-term exposure. Aquat Toxicol 161:267–275
Wagner S, Gondikas A, Neubauer E, Hofmann T, Kammer F (2014) Spot the difference: engineered and natural nanoparticles in the environment-release, behavior, and fate. Angew Chem Int Ed 53:12398–12419
Wang D, Chen Y (2016) Critical review of the influences of nanoparticles on biological wastewater treatment and sludge digestion. Crit Rev Biotechnol 36:816–828
Wang C, Wei Z, Feng M, Wang L, Wang Z (2014) The effects of hydroxylated multiwalled carbon nanotubes on the toxicity of nickel to Daphnia magna under different pH levels. Environ Toxicol Chem 33:2522–2528
Wang L-F, Habibul N, He D-Q, Li W-W, Zhang X, Jiang H, Yu H-Q (2015) Copper release from copper nanoparticles in the presence of natural organic matter. Water Res 68:12–23
Wang D, Lin Z, Wang T, Yao Z, Qin M, Zheng S, Lu W (2016a) Where does the toxicity of metal oxide nanoparticles come from: the nanoparticles, the ions, or a combination of both? J Hazard Mater 308:328–334
Wang S, Lv J, Ma J, Zhang S (2016b) Cellular internalization and intracellular biotransformation of silver nanoparticles in Chlamydomonas reinhardtii. Nanotoxicology 10:1129–1135
Wang Z, Zhang L, Zhao J, Xing B (2016c) Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter. Environ Sci Nano 3:240–255
Wang X, Cheng E, Burnett IS, Huang Y, Wlodkowic D (2017) Automatic multiple zebrafish larvae tracking in unconstrained microscopic video conditions. Sci Rep 7:17596
Wang X, Fan W, Dong Z, Liang D, Zhou T (2018) Interactions of natural organic matter on the surface of PVP-capped silver nanoparticle under different aqueous environment. Water Res 138:224–233
Warheit DB (2004) Nanoparticles: health impacts? Mater Today 7:32–35
Warheit D (2018) Hazard and risk assessment strategies for nanoparticle exposures: how far have we come in the past 10 years? F1000Res 7:376. https://doi.org/10.12688/f1000research.12691.1. [version 1; referees: 2 approved]
Wasmuth C, Rüdel H, Düring R-A, Klawonn T (2016) Assessing the suitability of the OECD 29 guidance document to investigate the transformation and dissolution of silver nanoparticles in aqueous media. Chemosphere 144:2018–2023
Weis JS, Candelmo A (2012) Pollutants and fish predator/prey behavior: a review of laboratory and field approaches. Curr Zool 58:9–20
Werlin R, Priester JH, Mielke RE, Krämer S, Jackson S, Stoimenov PK, Stucky GD, Cherr GN, Orias E, Holden PA (2011) Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Nat Nanotechnol 6:65–71
Wiench K, Wohlleben W, Hisgen V, Radke K, Salinas E, Zok S, Landsiedel R (2009) Acute and chronic effects of nano-and non-nano-scale TiO2 and ZnO particles on mobility and reproduction of the freshwater invertebrate Daphnia magna. Chemosphere 76:1356–1365
Wiesner MR, Lowry GV, Alvarez P, Dionysiou D, Biswas P (2006) Assessing the risks of manufactured nanomaterials. Environ Sci Technol 40:4336–4345
Wray AT, Klaine SJ (2015) Modeling the influence of physicochemical properties on gold nanoparticle uptake and elimination by Daphnia magna. Environ Toxicol Chem 34:860–872
Wu F, Bortvedt A, Harper BJ, Crandon LE, Harper SL (2017a) Uptake and toxicity of CuO nanoparticles to Daphnia magna varies between indirect dietary and direct waterborne exposures. Aquat Toxicol 190:78–86
Wu Y-S, Huang S-L, Chung H-C, Nan F-H (2017b) Bioaccumulation of lead and non-specific immune responses in white shrimp (Litopenaeus vannamei) to Pb exposure. Fish Shellfish Immunol 62:116–123
Xiao Y, Vijver MG, Chen G, Peijnenburg WJ (2015) Toxicity and accumulation of Cu and ZnO nanoparticles in Daphnia magna. Environ Sci Technol 49:4657–4664
Xiao Y, Peijnenburg WJ, Chen G, Vijver MG (2018) Impact of water chemistry on the particle-specific toxicity of copper nanoparticles to Daphnia magna. Sci Total Environ 610:1329–1335
Xiu Z-M, Ma J, Alvarez PJ (2011) Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ Sci Technol 45:9003–9008
Xiu Z-M, Zhang Q-B, Puppala HL, Colvin VL, Alvarez PJ (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12:4271–4275
Yameen B, Choi WI, Vilos C, Swami A, Shi J, Farokhzad OC (2014) Insight into nanoparticle cellular uptake and intracellular targeting. J Control Release 190:485–499
Yang Y, Long C-L, Li H-P, Wang Q, Yang Z-G (2016) Analysis of silver and gold nanoparticles in environmental water using single particle-inductively coupled plasma-mass spectrometry. Sci Total Environ 563–564:996–1007
Yeardley RB, Gast LC, Lazorchak JM (1996) The potential of an earthworm avoidance test for evaluation of hazardous waste sites. Environ Toxicol Chem 15:1532–1537
Yin L, Cheng Y, Espinasse B, Colman BP, Auffan M, Wiesner M, Rose J, Liu J, Bernhardt ES (2011) More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol 45:2360–2367
Yu S-J, Yin Y-G, Liu J-F (2013) Silver nanoparticles in the environment. Environ Sci: Processes Impacts 15:78–92
Yu Z, Hao R, Zhang L, Zhu Y (2018) Effects of TiO2, SiO2, Ag and CdTe/CdS quantum dots nanoparticles on toxicity of cadmium towards Chlamydomonas reinhardtii. Ecotoxicol Environ Saf 156:75–86
Yue Y, Li X, Sigg L, Suter MJ, Pillai S, Behra R, Schirmer K (2017) Interaction of silver nanoparticles with algae and fish cells: a side by side comparison. J Nanobiotechnol 15:16
Zhang L, Li J, Yang K, Liu J, Lin D (2016a) Physicochemical transformation and algal toxicity of engineered nanoparticles in surface water samples. Environ Pollut 211:132–140
Zhang Y-Q, Dringen R, Petters C, Rastedt W, Köser J, Filser J, Stolte S (2016b) Toxicity of dimercaptosuccinate-coated and un-functionalized magnetic iron oxide nanoparticles towards aquatic organisms. Environ Sci Nano 3:754–767
Zhang S, Deng R, Lin D, Wu F (2017) Distinct toxic interactions of TiO2 nanoparticles with four coexisting organochlorine contaminants on algae. Nanotoxicology 11:1115–1126
Zhao CM, Wang WX (2011) Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ Toxicol Chem 30:885–892
Zhao C-M, Wang W-X (2012a) Importance of surface coatings and soluble silver in silver nanoparticles toxicity to Daphnia magna. Nanotoxicology 6:361–370
Zhao C-M, Wang W-X (2012b) Size-dependent uptake of silver nanoparticles in Daphnia magna. Environ Sci Technol 46:11345–11351
Zhao J, Cao X, Liu X, Wang Z, Zhang C, White JC, Xing B (2016) Interactions of CuO nanoparticles with the algae Chlorella pyrenoidosa: adhesion, uptake, and toxicity. Nanotoxicology 10:1297–1305
Zhou K, Hu Y, Zhang L, Yang K, Lin D (2016) The role of exopolymeric substances in the bioaccumulation and toxicity of Ag nanoparticles to algae. Sci Rep 6:32998
Zhu X, Zhu L, Chen Y, Tian S (2009) Acute toxicities of six manufactured nanomaterial suspensions to Daphnia magna. J Nanopart Res 11:67–75
Zhu X, Chang Y, Chen Y (2010a) Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere 78:209–215
Zhu X, Wang J, Zhang X, Chang Y, Chen Y (2010b) Trophic transfer of TiO2 nanoparticles from Daphnia to zebrafish in a simplified freshwater food chain. Chemosphere 79:928–933
Acknowledgements
This material is based upon work which was supported by the Australian Government to Sam Lekamge through an “Australian Government Research Training Program Scholarship”. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the Australian Government.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lekamge, S., Ball, A.S., Shukla, R., Nugegoda, D. (2018). The Toxicity of Nanoparticles to Organisms in Freshwater. In: de Voogt, P. (eds) Reviews of Environmental Contamination and Toxicology Volume 248. Reviews of Environmental Contamination and Toxicology, vol 248. Springer, Cham. https://doi.org/10.1007/398_2018_18
Download citation
DOI: https://doi.org/10.1007/398_2018_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-14705-1
Online ISBN: 978-3-030-14706-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)