Abstract
Nano zinc oxide (nZnO) is increasingly used in sunscreen products, with high potential of being released directly into marine environments. This study primarily aimed to characterize the aggregate size and solubility of nZnO and bulk ZnO, and to assess their toxicities towards five selected marine organisms. Chemical characterization showed that nZnO formed larger aggregates in seawater than ZnO, while nZnO had a higher solubility in seawater (3.7 mg L−1) than that of ZnO (1.6 mg L−1). Acute tests were conducted using the marine diatoms Skeletonema costatum and Thalassiosia pseudonana, the crustaceans Tigriopus japonicus and Elasmopus rapax, and the medaka fish Oryzias melastigma. In general, nZnO was more toxic towards algae than ZnO, but relatively less toxic towards crustaceans and fish. The toxicity of nZnO could be mainly attributed to dissolved Zn2+ ions. Furthermore, molecular biomarkers including superoxide dismutase (SOD), metallothionein (MT) and heat shock protein 70 (HSP70) were employed to assess the sublethal toxicities of the test chemicals to O. melastigma. Although SOD and MT expressions were not significantly increased in nZnO-treated medaka compared to the controls, exposure to ZnO caused a significant up-regulation of SOD and MT. HSP70 was increased two to fourfold in all treatments indicating that there were probably other forms of stress in additional to oxidative stress such as cellular injury.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nano zinc oxide (nZnO) particles (with diameter smaller than 100 nm) are used more and more frequently in the manufacture of sunscreens [1]. In fact, nZnO usage may overtake nano-titanium dioxide (nTiO2) in the near future as it can absorb both UV-A and UV-B radiation while nTiO2 can only block UV-B, and thereby offering better protection and improved opaqueness [2]. nZnO is also used in ceramics and rubber processing, wastewater treatment, and as a fungicide [2, 3]. Current production rate of nano-metal oxides (TiO2, ZnO, and Fe2O3) for skincare products is estimated to be at 103 tonnes/a [4]. Italian researchers calculated that at least 25% of the amount of sunscreen applied onto the skin is washed off during bathing and swimming [5], implying that around 250 tonnes of these nanomaterials can be potentially discharged into the marine environment.
Various studies had investigated the aquatic toxicity of nZnO [6–10], but toxicity data on marine species were found to be particularly lacking [11]. It is necessary to look at the effects of nanomaterials on marine organisms since the higher ionic strength and pH of seawater in comparison to freshwater may alter their physicochemical properties [12, 13]. For example, it has been shown that increasing the ionic strength and pH of NaCl suspensions would result in enhanced nTiO2 aggregation rate and size, which in turn may influence their bioavailability in the marine ecosystem [14]. Furthermore, although nanomaterials are theoretically expected to be more toxic than their bulk counterparts due to their greater surface reactivity; in reality, they may form aggregates that approach the size of the bulk materials, and thus are not necessarily more potent in the marine environment [15].
Another crucial factor which may affect the bioavailability of metal oxide nanoparticles is the release of metal ions. It has been shown previously that the toxicity of nZnO was mainly attributed to the dissolved Zn ions [6, 10, 16], yet some evidences of nanoparticle-specific toxicity have also led researchers to caution against such generalization of the toxicity mechanisms of nanomaterials [3, 7].
This study, therefore, aimed to characterize the aggregate size and ion solubility of nZnO and bulk ZnO, and assess their toxicities towards five selected marine organisms. Species with different sensitivity profiles were chosen from three different trophic levels (i.e., primary producer, primary consumer, and predator) for this study, so as to ensure an adequate, comprehensive, evaluation [17]. These included the marine diatoms Skeletonema costatum and Thalassiosia pseudonana, the amphipod Elasmopus rapax, the harpacticoid copepod Tigriopus japonicus, and the marine medaka fish Oryzias melastigma. Some of the species chosen have great ecological importance and the risk assessment associated with them should be highly relevant to practical applications. For instance, S. costatum is the dominant diatom in Hong Kong marine waters, making up to 84% of diatom populations in certain areas [18]. T. japonicus is a harpacticoid copepod that forms a large proportion of the zooplankton communities, which in turn represents 70% of the ocean’s biomass [19]. Harparcticoids are also important as juvenile fish preys in meiobenthic community [19]. The brackish medaka Oryzias latipes has been heavily promoted recently as a model fish complementary to the zebrafish for studying genetic and molecular mechanisms as well as experimental embryology in ecotoxicological studies, since it is more resilient to disease and offers better resolution to questions dealing with sex determination [20]. Its marine counterpart, O. melastigma is also under intense research to boost its worth in toxicological research [21]. In the present study, the toxicities of nZnO and ZnO on these marine organisms were determined based on acute algal growth inhibition tests with the two diatoms, acute lethal tests with the two crustaceans, as well as acute sublethal tests with the medaka fish.
Nanomaterials are known to be common stressors and can cause damage in organisms, for example via oxidative stress-induced- [22, 23], or genotoxicity caused by mechanical injury to the cell [24]. Hence, the present study also aimed to reveal the potential stress proteomic responses in the medaka O. melastigma after exposure to the nZnO and ZnO using western blot analysis. The selected stress markers included superoxide dismutase (SOD), an enzymatic antioxidant; metallothioneins (MT), a group of heat-stable and cysteine-rich metal-binding proteins involved in a range of protective stress responses (in particular, zinc regulation and distribution in cells) [25]; and a heat shock protein, HSP70, often up-regulated to relieve stress-induced apoptosis [26]. The results of the biomarker responses were essential to determine the sublethal toxicities of the test chemicals to O. melastigma and provide insights into toxic mechanisms of nZnO.
Materials and methods
Chemicals preparation and characterization
nZnO powder (average particle size (APS), 20 nm) was purchased from Nanostructured & Amorphous Materials Inc. (New Mexico, USA); ZnO powder (i.e., bulk ZnO) was purchased from BDH Chemical Ltd. (Poole, England) while zinc sulfate heptahydrate (ZnSO4·7H2O) was bought from Sigma Chemical Co. (St. Louis, Missouri, USA).
To determine the APS distribution, nZnO and ZnO powders were examined under transmission electron microscope (TEM; Tecnai G2 20 S-TWIN at 200 kV, Philips, Eindhoven, The Netherlands), while for aggregate size distribution, 100 mg L−1 of nZnO and ZnO suspensions were dispersed in filtered artificial seawater (salinity, 30 ± 0.5‰; pH, 8.0 ± 0.1; sea salt: Tropic Marine, Germany; filtered through 0.45 μm membrane filter) and inspected by laser diffractometry (LD; LS 13 320 Series, Beckman Coulter Inc., Fullerton, USA).
To quantify the dissolved metal ion concentrations for nZnO and ZnO, test solutions of 80 mg L−1 (in filtered artificial seawater) were shaken on a rotary shaker (Lab-line Orbit-Environ Shaker, Lab-Line Instruments Inc., Melrose Park, IL) operating at ∼200 rpm under room temperature (25 ± 2 °C). At each sampling time point, aliquots were withdrawn and filtered through a 0.1-µm sterile syringe filter. The Zn2+ concentration in the filtrate was then measured by inductively coupled plasma with optical emission spectrometry (ICP-OES; Optima 2100 DV, Perkin-Elmer, Norwalk, USA). The method of standard additions was utilized to account for matrix interference [27].
Acute algal growth inhibition tests
Stock solutions (100 mg L−1) of nZnO, ZnO, and ZnSO4·7H2O powders were made by dispersing 0.025 g of each chemical in 250 mL filtered artificial seawater. The nZnO and ZnO suspensions were stirred continuously with a magnetic stirrer (∼200 rpm) at room temperature (25 ± 2 °C) for at least 3 days before experimentation. The stirring method was employed instead of using surfactants or sonication to disperse the chemicals, in order to simulate an environmentally relevant scenario.
The test procedures were modified from ASTM and OECD guidelines [28, 29]. Initial algal concentrations of 4 × 104 cells mL−1 for S. costatum (CCMP 1335; CCMP, USA) and 105 cells mL−1 for T. pseudonana (CCAP 1177/3; CCAP, UK) were exposed to different concentrations of the test chemicals dispersed in f2-Si medium in 24-well multidishes (Nunc; Naterville, USA; n = 4). In order to assess possible shading effects imposed by the insoluble solids of the metal oxides, an experimental design adapted from Hund-Rinke and Simon [30] was utilized. A transparent multidish was stacked on top of another, and the algae were only incubated in the lower chamber. To test the toxicity effects of chemicals, the test compounds and algae were mixed together in the lower plate wells with only f2-Si medium in the upper wells, while to assess the shading effects, the test materials and algae were separated in the upper and lower wells, respectively. The walls and undersides of the multidishes were then covered with black paper such that light could only pass through the upper wells to reach the lower dish. To ensure more uniform dispersal of the metal oxides, which tend to aggregate and settle down over time, the dishes were shaken atop a titer plate shaker for 15 min every 2 h at a mediocre speed of ∼100 rpm. The whole set-up was incubated in an environmental chamber with 16 h light:8 h dark cycle and 25 ± 2 °C for 96 h. Initial and final cell counts were determined using a hemocytometer to calculate the average growth rate during the test period.
Acute lethal tests for crustaceans
The copepod T. japonicus was sampled from a single high-shore rock pool at the Cape d’Aguilar Marine Reserve, Hong Kong (22°13′N, 114°12′E) in December 2008, while the amphipod E. rapax was obtained from the outdoor aquarium tanks at the Swire Institute of Marine Science (SWIMS), which were also supplied with running natural seawater from Cape d’Aguilar. The selected amphipods were measured linearly for the first to fourth thoracic segments because of their body curvature [31] and averaged 0.39 ± 0.05 mm in length (mean ± s.d.; n = 20). The animals were acclimated in laboratory conditions for at least 2 days prior to the start of experiments, during which the T. japonicus culture was fed with a diet mixture of S. costatum and the green alga Ulva lactua, while the amphipods remained unfed during the acclimation and experimental periods. For T. japonicus, gravid females were selected from the culture and isolated in 24-well multidishes where they were fed with S. costatum. Nauplii born within 24 h were then used for the tests.
There were three replicates for each treatment, each consisting of a 250-mL glass bowl containing ten E. rapax individuals, or a 24-well multidish containing 8 T. japonicus nauplii placed individually in each well with 105 cells mL−1 of S. costatum provided as food. Two strips of nylon mesh (30 × 10 mm) were added to each glass bowl to provide a substrate for the amphipods [32]. Both tests were conducted under experimental conditions described before. The duration of each test was 96 h, with test solutions being renewed once midway. Mortality was monitored daily and dead animals were removed.
Stress responses in fish after acute exposure
Based on the results of ion solubility of nZnO and ZnO, exposure concentrations of 4 and 40 mg L−1 for nZnO and ZnO, and 14 mg L−1 for ZnSO4·7H2O were selected, since at such concentrations all treatments should have comparable Zn2+ ion concentrations. The exposure conditions and procedures were similar to those for E. rapax, and each treatment consisted of four replicates, each with ten O. melastigma larvae (<24 h) placed in a 250-mL glass bowl. At the end of 96 h, the fish were harvested and homogenized on ice in lysis buffer (50 mM Tris–HCl (pH 8.0), 1% (v/v) Triton X-100, 150 mM NaCl, 10 mM DTT) mixed with 1× protease inhibitor cocktail (Roche Molecular Biochemicals, USA). Afterwards, they were centrifuged for 15 min at 14,000×g, and the supernatant extracted for protein quantification (2D-Quant Kit; Amersham Biosciences, USA) followed by Western blot analysis.
For Western blot analysis, equal amounts of protein extract (10 µg) were separated by electrophoresis on SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked with 5% (w/v) nonfat dry milk in TBS containing 0.1% Tween-20 (TBS-T) and incubated with primary antibodies using various selected stress proteins, i.e., the rabbit anti-cod polyclonal MT (Cat. No. M04406201-500; Biosense Laboratories AS, Norway), rabbit anti-Cu/Zn polyclonal SOD (Cat. No. SPC-116C/D; StressMarq Biosciences Inc., Canada) and mouse anti-HSP70 monoclonal IgG1 (Cat. No. MA3-007; Affinity BioReagents, USA). After incubation with the secondary antibodies, peroxidase-goat anti-rabbit IgG (H + L; Zymed Laboratories, USA) for the former two and peroxidase-rabbit anti-mouse IgG (H + L; Zymed Laboratories) for the latter, the membranes were washed again using TBS-T. Membranes were treated with ECL (for SOD and HSP70) or Advance ECL (for MT) kits (Amersham Biosciences, UK), and exposed to X-ray film. The developed films of the blots were scanned using densitometer GS-800 (Biorad Laboratories, USA), and the protein levels (i.e., the band volumes) were then quantified using Quantity One software (BioRad Laboratories).
Statistical analysis
Data analysis was performed using GraphPad Prism version 5.00 (GraphPad Software, San Diego), SPSS version 15 (SPSS Inc., Chicago) and PRIMER version 6 (Primer-E Ltd, Plymouth). A Monte Carlo method was employed to compare the APS and aggregate size distribution of nZnO and ZnO. Two thousand random values were generated based on the mean and standard deviation provided by laser diffractometry, and student’s t test was used to test for differences between the two treatment groups.
To determine the IC50 (i.e., median inhibition concentration, 50% inhibition of algal growth) and LC50 (i.e., median lethal concentration, lethal to 50% of the test animals) values, data were fit to a sigmoidal log(dose)–response curve (variable slope) with a four-parameter logistic equation.
The toxicities of nZnO and ZnO were compared through comparisons of their IC50 or LC50 values for each test organism. The IC50 or LC50 values were log-transformed to normalize the distributions, and then a ratio test with bootstrapping (based on [33] with modification) was applied. The IC50 or LC50 ratio was defined as
where ζ refers to the ratio of IC50 or LC50 values, and a and b are two independent populations. If the 95% confidence interval (CI) of the IC50 or LC50 ratio contained 0, it would be concluded that there is no difference between the two IC50 or LC50 values.
The mean expression levels of SOD, MT, and HSP70 among the different treatments were compared using Student’s t test (two-tailed, unpaired) and one-way ANOVA with Tukey’s post-hoc comparisons. Multivariate analysis (non-metric multidimensional scaling (MDS), analysis of similarity (ANOSIM), and similarity percentage analysis (SIMPER)) were conducted to assess overall dissimilarities between the treatments based on these three response variables. In ANOSIM, the null hypothesis (Ho) that there are no differences in medaka stress responses between treatments was tested by computing a test statistic R. R is approximately zero if Ho is true, and equals to one if the treatments are completely dissimilar [34].
Results and discussion
Determination of aggregate size distributions
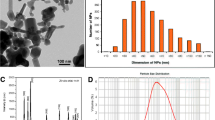
TEM images showed that the APS of nZnO was 26.2 ± 5.1 nm (mean ± s.d., n = 102), which corroborated closely to the manufacturer’s information, while the APS of ZnO was significantly larger (student’s t test, t = 25.907, p < 0.001) at 216.2 ± 73.2 nm (n = 100; Fig. 1). nZnO particles were more uniform in shape, being spherical to slightly elliptical, while ZnO particles were more angular and irregularly shaped.
The hydrodynamic diameters (D H) of the metal oxides, on the other hand, portrayed a radically dissimilar picture (Fig. 2). nZnO tended to form aggregates in seawater in the micrometer range (2.3 ± 1.6 µm), which were even bigger than those formed by its bulk equivalent (1.7 ± 1.2 µm; t = 4.183, p < 0.01). Previous studies also reported similar observations in other nano-oxide particles and suggested that the smaller the primary particle size, the larger the aggregate would be formed [35, 36].
Determination of nanoparticle dissolution
Based on the results of aggregate size distributions, it was deemed that a 0.1-µm filter was sufficient to separate the metal oxide particles from the released Zn2+ ions. This decision was also supported by results of a freshwater study that the measurement of dissolution rates and solubility equilibrium of nZnO and ZnO were unaffected by passing the solution through either a dialysis membrane (molecular weight cutoff, 1,000) or a 0.1-µm filter [6].
For both nZnO and ZnO, the equilibrium was achieved within 72 h after initial dispersion of both metal oxides in seawater (Fig. 3). The dissolved Zn concentration for nZnO and ZnO was 3.7 ± 0.6 and 1.6 ± 0.5 mg Zn L−1, respectively, at equilibrium (n = 2; Fig. 3). To our knowledge, this was the first study to investigate the solubility of nZnO in a saltwater environment. The solubility of ZnO at pH 8.0 was close to values (1.1–2.5 mg Zn L−1) postulated in the literature [37–39]. In contrast to previous research where the solubilities of nZnO and ZnO were found to be the same in distilled water [6, 34], the current study showed that nZnO displays a higher solubility than its bulk equivalent in seawater, possibly because of its smaller size, larger surface area and curvature [40].
Acute toxicities of nZnO, ZnO and ZnSO4 on various marine organisms
nZnO and ZnO did not induce the same toxicity in the test algae and crustaceans, as none of the 95% CI of log(ζ) constructed in the LC50 ratio test enclosed 0 (Table 1). In general, nZnO appeared to be more toxic than ZnO towards the algae while ZnO was shown to have more adverse impact on the crustaceans than nZnO, and shading did not appear to play an important role in affecting the toxicity of both chemicals in the algal growth inhibition tests (data not shown). While many studies had shown that the toxicity of nZnO and ZnO were not different [6–10], there were also exceptions [7, 15, 35]. Based on our results, different taxonomic groups likely exhibit different responses and sensitivities towards nZnO and ZnO. Species or taxon-specific responses to nZnO merit further studies with a view to making robust generalization.
It has been proposed that nZnO is antibacterial by direct interaction with the bacterial external membrane surface [35, 41], and causes damage either mechanically or via generation of reactive oxygen species (ROS). Surface charges were found to be important in initiating contact between the nanomaterials and cells [42]. The same toxicity mechanism may be applied for the microalgae as such adsorption is important during metal uptake. The surfaces of S. costatum and Thalassiosira sp. were found to be dominantly negatively charged [43], and therefore might have attracted Zn2+ ions and nZnO particles, which have positive zeta potential at pH 8.0 [44]. As nZnO has higher solubility relative to ZnO (Fig. 3), it might have released more Zn2+ ions to attach onto the algae surface, thus causing greater toxicity.
nZnO could also cause injury to crustaceans by adhering to their exoskeleton or the gut wall if it gets ingested by the organisms [45]. Carbon nanotubes can accumulate in Daphnia’s gut without being absorbed by cell tissues [46], but its physiochemical properties may also be altered with the pH changes in such an environment [47]. Both Tigriopus sp. and Elasmopus sp. are described as generalist feeders [19, 48]. Copepod nauplii might have found it easier to feed on the smaller aggregates of ZnO (Fig. 2), and compensatory feeding of metal oxide particles with low or no nutritional value [48] could have exacerbated their toxicity.
Except for T. japonicus, the toxicity of nZnO to the other three species could be attributed mainly to dissolved Zn ions (Table 2). This is in agreement with other studies [6, 10, 49]. However, exclusions to this “rule” exist: nano-specific toxicity was observed in ryegrass exposed to nZnO which were able to enter root cells via mechanical breakdown and subsequently impeded root growth [50]. At the high nZnO concentration (1,000 mg/L) employed in the ryegrass experiment [50], it is certain that insoluble Zn should exist in the bulk nutrient solution. Hence, the physical injury to the ryegrass is most probably incurred by the insoluble aggregates. Similarly, in this study, the T. japonicus nauplii were observed to be swarmed by the nZnO colloids, with their movement being hampered by the nanomaterials attached onto their tests, providing further evidence that aggregation may also play a part in conferring toxicity through mechanical injuries besides solubility. It is commonly acknowledged that aggregation of nanomaterials in aqueous suspensions have prevented their entry across cell membranes and barriers, thus limiting their potential to cause greater toxicity. Still, as efforts are devoted into reducing aggregation sizes of nanomaterials so as to enhance their applicability, along with the few demonstrations that nZnO can actually create holes in the cell barriers of ryegrass [50], it would be prudent to primarily keep researching on the effects of released metal ions from metal oxides.
Two distinct isoforms of MT with a molecular weight of about 7 kDa were detected by Western blot (Fig. 4a), which were labeled as MT-I and MT-II. They appeared to be “housekeeping” enzymes in O. melastigma since the basal expressions in controls were quite high. Rainbow trout, Gibel, and Crucian carps were also shown to have the potential to produce two types of MT [51]. nZnO did not induce increased MT or SOD expression compared to the control, at the concentrations used in this experiment (Fig. 5). In another experiment, the eggs and adults of freshwater medaka O. latipes exposed to 5 mg L−1 of nano-iron displayed a decrease in SOD activities for the first few days, but recovery soon took place and the final SOD activities were not different from the control [52]. As O. melastigma was harvested only at one time point (96 h) in this study, it was possible for such adjustment to have taken place, thus accounting for the lack of change observed. nZnO of 5 mg L−1 had significantly depleted GSH and SOD activities in mouse embryo fibroblast cells [24], but it is noted that the powder was dispersed in fetal bovine serum followed by sonication, which might have reduced the aggregate size (not reported in the paper) and induced greater responses.
The relative expression levels of MT-I, MT-II, SOD and HSP70 in the medaka O. melastigma after exposure to 4 and 40 mg L−1 of nZnO and ZnO. ZnO was shown to have significantly different MT-I (t = 3.634, *p < 0.05) and SOD (t = 5.058, **p < 0.01) expressions from nZnO at 4 mg L−1. Error bars ±2 s.e.m. (n = 3)
ZnO appeared to invoke greater responses in all biomarkers than nZnO (Figs. 4 and 5), although significant difference was only detected for MT-I (t = 3.634, p < 0.05) and SOD (t = 5.058, p < 0.01) at 4 mg L−1. This might suggest that ZnO was slightly more bioavailable for the medaka larvae than nZnO. Once again, the smaller ZnO aggregates might have found an easier entry into the fish via gills or ingestion. Adult medaka exposed to latex nanoparticles showed that most accumulation occurred in the gills and intestine [12]. Change in expression relative to the control was the greatest for HSP70, for which the protein level increased two to fourfold (Figs. 5 and 6). Clearly, MT and SOD expressions were not augmented by nZnO, signifying no or low oxidative stress. The rise in HSP70 levels, therefore, implied that there were other additional (non-oxidative) stresses imposed by dissolved Zn2+ ions, given that the prime function of HSP70 is to provide cytoprotection [53]. It has been speculated that smaller particles, once gained entry into cells, could cause mechanical injury that led to increased genotoxicity independent of oxidative stress [24]. The damage incurred in the medaka (including oxidative stress) was generally more potent in ZnO treatments.
The relative expression levels of MT-I, MT-II, SOD, and HSP70 in the medaka O. melastigma after exposure to 4 and 40 mg L−1 of nZnO and 14 mg L−1 of ZnSO4·7H2O, referred to as Treatment A, B, and C, respectively. ZnSO4·7H2O was shown to have significant effect on the expression levels of MT-I (one-way ANOVA: F 2,6 = 13.074, p < 0.01), MT-II (F 2,6 = 16.223, p < 0.01) and HSP70 (F 2,6 = 9.15, p < 0.05) as compared to the other two treatments. Bars with the same letter are statistically indifferent. Error bars ±2 s.e.m. (n = 3)
In this study, ZnSO4·7H2O of 14 mg L−1 induced significantly higher response levels of MT-I, MT-II, and HSP70 as compared to nZnO of 4 and 40 mg L−1. This again indicated that the toxicity of nZnO was primarily due to dissolved Zn2+ ions. The response levels were not the same for nZnO and ZnSO4·7H2O, probably because there was uncertainty involved in the exact determination of the dissolved Zn2+ concentration from nZnO, leading to slightly higher Zn2+ concentrations being employed for the ZnSO4·7H2O treatments.
The MDS plot illustrated that the biomarker responses of medaka in the control and nZnO treatments were highly similar (Fig. 7), with R statistic <0.3 between the three groups (Table 3). Regardless, the global R statistic was 0.535 (p < 0.001), and thus the null hypothesis that there was no overall difference between treatments was rejected. Both ZnO treatments were separated from the control and 4 mg nZnO L−1 treatment (R statistic >0.6). A high variability of the biomarker responses was observed in the 40 mg nZnO L−1 treatment, but in general they were more closely resembled to the responses to ZnO treatments (Fig. 7). ZnSO4·7H2O was highly differentiated from the control and other treatments (except for 40 mg ZnO L−1), with R statistic being close to or equals to 1. The results indicated that the biomarker responses in the fish were highly dependent on exposure concentrations and chemical forms.
Conclusions
In contrary to prior studies conducted in freshwater, nZnO did not display the same toxicity as ZnO towards marine organisms. Plausible explanations included the change in D H as they formed aggregations, and the alteration to surface charge as pH of the environment increased from neutral to 8.0. Surface charge in turn affected the particle’s attachment onto organisms’ cells, and thus modified its toxicity. The toxicity of nZnO was shown to be influenced significantly (but not solely) by the release of Zn2+ ions into the solution, which is also supported by other research studies. In freshwater, the amount of dissolved Zn for the same concentration of nZnO and ZnO was similar, whereas in seawater, their solubility were altered by the change in pH, and this might have additionally contributed towards their differential toxicities. Aside from investigating the toxicity of nanomaterials such as nZnO, it would be essential to further elucidate if these nanomaterials could considerably interact with other common chemical pollutants (e.g., polycyclic aromatic hydrocarbons) in seawater by changing the form, structure, and adsorption efficient of the chemical pollutants and eventually lead to increased (i.e., additive or synergistic) toxicity to marine organisms.
References
Seshadri R (2004) Oxide nanoparticles. In: Rao CNR, Müller A, Cheetham AK (eds) The chemistry of nanoparticles: synthesis, properties and applications. Wiley, Weinheim, pp 94–112
Theodore L (2006) Nanotechnology: basic calculations for engineers and scientists. Wiley, Hoboken
Wang X, Lu J, Xu M, Xing B (2008) Sorption of pyrene by regular and nanoscaled metal oxide particles: influence of adsorbed organic matter. Environ Sci Technol 42:7267–7272
Pikethly MJ (2004) Nanomaterials—the driving force. Mat Today 7:20–29
Danovaro R, Bongiorni L, Corinaldesi C, Giovannelli D, Damiani E, Astolfi P, Greci L, Pusceddu A (2008) Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect 116:441–447
Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS (2007) Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol 41:8484–8490
Heinlaan M, Ivask A, Blinova I, Dubourguier H, Kahru A (2008) Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 71:1308–1316
Wang H, Wick RL, Xing B (2009) Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ Pollut 157:1171–1177
Zhu X, Zhu L, Duan Z, Qi R, Li Y, Lang Y (2008) Comparative toxicity of several metal oxide nanoparticles aqueous suspensions to zebrafish (Danio rerio) early developmental stage. J Environ Sci Heal A 43:278–284
Aruoja V, Dubourguier H, Kasemets K, Kahru A (2009) Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcaptitata. Sci Total Environ 407:1461–1468
Handy RD, Owen R, Valsami-Jones E (2008) The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicology 17:315–325
Kashiwada S (2006) Distribution of nanoparticles in the see-through medaka (Oryzias latipes). Environ Health Perspect 114:1697–1702
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability, and effects. Environ Toxicol Chem 27:1825–1851
French RA, Jacobson AR, Kim B, Isley SL, Lee Penn R, Baveye PC (2009) Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ Sci Technol 43:1354–1359
Adams LK, Lyon DY, Alvarez PJJ (2006) Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res 40:3527–3532
Brunner TJ, Wick P, Manser P, Spohn P, Grass RN, Limbach LK, Bruinink A, Stark WJ (2006) In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environ Sci Technol 40:4374–4381
Kahru A, Dubourguier HC, Blinova I, Ivask A, Kasemets K (2008) Biotests and biosensors for ecotoxicology of metal oxide nanoparticles: a minireview. Sensors 8:5153–5170
Environmental Protection Department (EPD) (2006) Marine water quality in Hong Kong. Environment Protection Department, Hong Kong
Raisuddin S, Kwok KWH, Leung KMY, Schlenk D, Lee JS (2007) The copepod Tigriopus: a promising marine model organism for ecotoxicology and environmental genomics. Aquat Toxicol 83:161–173
Wittbrodt J, Shima A, Schart M (2002) Medaka—a model organism from the far east. Nat Rev Genet 3:53–64
Kong RYC, Giesy JP, Wu RSS, Chen EXH, Chiang MWL, Lim PL, Yuen BBH, Yip BWP, Mok HOL, Au DWT (2008) Development of a marine fish model for studying in vivo molecular responses in ecotoxicology. Aquat Toxicol 86:131–141
Oberdörster E (2004) Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ Health Perspect 112:1058–1062
Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI, Wiesner MR, Nel AE (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 6:1794–1807
Yang H, Liu C, Yang D, Zhang H, Xi Z (2009) Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. J Appl Toxicol 29:69–78
Andrews GK (2000) Regulation of metallothionein gene expression by oxidative stress and metal ions. Biochem Pharmacol 59:95–104
Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B (1997) Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol 17:5317–5327
Elmer P (1982) Analytical methods for atomic absorption spectrophotometry. Perkin Elmer, Norwalk, USA
ASTM (1993) Standard guide for conducting static toxicity tests with microalgae. American Society for Testing and Material (ASTM) E1218-04. Philadelphia, Pennsylvania
OECD (1984) Guideline for testing of chemicals—freshwater alga and cyanobacteria, growth inhibition test. Organization for Economic Co-operation and Development (OECD) Guidelines for Testing of Chemicals 201. Paris, France
Hund-Rinke K, Simon M (2006) Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environ Sci Pollut R 13:225–232
Hacker SD, Steneck RS (1990) Habitat architecture and the abundance and body-size-dependent habitat selection of a phytal amphipod. Ecology 71:2269–2285
King CK, Gale SA, Hyne RV, Stauber JL, Simpson SL, Hickey CW (2005) Sensitivities of Australian and New Zealand amphipods to copper and zinc in waters and metal-spiked sediments. Chemosphere 63:1466–1476
Wheeler MW, Park RM, Bailer AJ (2006) Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ Toxicol Chem 25:1441–1444
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-E, Plymouth
Jiang W, Mashayekhi H, Xing B (2009) Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ Pollut 157:1619–1625
Grassian VH (2008) When size really matters: size-dependent properties and surface chemistry of metal oxide nanoparticles in gas and liquid phase environments. J Phys Chem C 112:180303–18313
Bénézeth P, Palmer DA, Wesolowski DJ, Xiao C (2002) New measurements of the solubility of zinc oxide from 150 to 350 °C. J Solut Chem 31:947–973
Dutra AJB, Paiva PRP, Tavares LM (2005) Alkaline leaching of zinc from electric arc furnace steel dust. Miner Eng 19:478–485
Yebra DM, Kiil S, Weinell CE, Dam-Johansen K (2006) Dissolution rate measurements of sea water soluble pigments for antifouling paints: ZnO. Prog Org Coat 56:327–337
Cao G (2004) Nanostructures & nanomaterials: synthesis, properties & applications. World Scientific, Singapore
Zhang L, Jiang Y, Ding Y, Povey M, York D (2007) Investigation into the antibacterial behavior of suspensions of ZnO nanoparticles (ZnO nanofluids). J Nano Res 9:479–489
Neal AL (2008) What can be inferred from bacterium-nanoparticle interactions about the potential consequences of environmental exposure to nanoparticles? Ecotoxicol 17:362–371
Gélabert A, Pokrovsky OS, Viers J, Schott J, Boudou A, Feurtet-Mazel A (2006) Interaction between zinc and freshwater and marine diatom species: surface complexation and Zn isotope fractionation. Geochim Cosmochim Ac 70:839–857
Liufu S, Xiao H, Li Y (2004) Investigation of PEG adsorption on the surface of zinc oxide nanoparticles. Powder Technol 145:20–24
Baun A, Hartmann NB, Grieger K, Kusk KO (2008) Ecotoxicity of engineered nanoparticles to aquatic invertebrates: a brief review and recommendations for future toxicity testing. Ecotoxicol 17:387–395
Petersen EJ, Akkanen J, Kukkonen JVK, Weber WJ (2009) Biological uptake and depuration of carbon nanotubes by Daphnia magna. Environ Sci Technol 43:2969–2975
Roberts AP, Mount AS, Seda B, Souther J, Qiao R, Lin S, Ke PC, Rao AM, Klaine S (2007) In vivo biomodification of lipid-coated carbon nanotubes by Daphnia magna. Environ Sci Technol 41:3025–3029
Cruz-Rivera E, Hay ME (2000) Can quantity replace quality? Food choice, compensatory feeding, and fitness of marine mesograzers. Ecology 81:201–219
Mortimer M, Kasemets K, Heinlaan M, Kurvet I, Kahru A (2008) High throughput kinetic Vibrio fischeri bioluminescence inhibition assay for study of toxic effects of nanoparticles. Toxicol In Vitro 22:1412–1417
Lin D, Xing B (2008) Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol 42:5580–5585
Muto N, Ren H, Hwang G, Tominaga S, Itoh N, Tanaka K (1999) Induction of two major isoforms of metallothionein in crucian carp (Carassius cuvieri) by air-pumping stress, dexamethasone, and metals. Comp Biochem Phys C 122:75–82
Li H, Zhou Q, Wu Y, Fu J, Wang T, Jiang G (2009) Effects of waterborne nano-iron on medaka (Oryzias latipes): antioxidant enzymatic activitiy, lipid peroxidation and histopathology. Ecotoxicol Environ Safe 72:684–692
Kiang JG, Tsokos GC (1998) Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Therapeut 80:183–201
Acknowledgments
This research is partially funded by the Area of Excellence Scheme under the University Grants Committee of the Hong Kong Special Administration Region, China (Project No. AoE/P-04/2004) and supported by the Strategic Research Theme of Sustainable Environment and the Faculty of Science of the University of Hong Kong (HKU). Stella Wong thanks HKU to partially support her PhD studentship. We are grateful to Dr. X.Y. Li, Civil Engineering Department, HKU for allowing us to use the laser diffractometry instrument. We also thank the anonymous reviewer for his/her valuable comments on this manuscript, and thank Helen Leung and Cecily Law for their technical support throughout the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, S.W.Y., Leung, P.T.Y., Djurišić, A.B. et al. Toxicities of nano zinc oxide to five marine organisms: influences of aggregate size and ion solubility. Anal Bioanal Chem 396, 609–618 (2010). https://doi.org/10.1007/s00216-009-3249-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-3249-z