Abstract
Microorganisms were the first forms of life on Earth and are now part of all living organisms, but the role they played during the evolution of multicellular species is still a mystery. Among other biotic interactions, plants and their herbivorous insects have always occurred under a microbial milieu. During the past 20 years, our understanding of how microorganisms shape the ecology and evolution of plant-insect interactions has increased rapidly. However, the extent to which plant-associated microbes influence insect performance and how insect-associated microbes influence plant defenses remains largely unexplored. Here, we will highlight the potential reciprocal feedbacks between the microbiotas of plants and insects that could affect their interaction. We also bring attention to how network theory can help us understand the potential interactions within and between microbiotas. Finally, we will point out some promising directions for future experimental studies in order to better understand microbe-insect-plant interactions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Despite the historical pairwise perception of the coevolutionary process between plants and herbivorous insects, the environment surrounding plants and their consumers is far from sterile; thus all their interactions take place under a microbial milieu that can significantly alter the ecology and evolution of both plants and insects (Felton and Tumlinson 2008). Metagenomic studies have accelerated our understanding of the fundamental role played by microorganisms in the survival and adaptation of plants (Partida-Martínez and Heil 2011; Pineda et al. 2013) and their herbivorous insects (Chu et al. 2013; Chung et al. 2013; Asplen et al. 2014; Sharpton 2018). Nowadays, we also acknowledge that microorganisms can mediate biochemical communication between plants and insects (Hansen and Moran 2013). Indeed, the field of microbe-insect-plant interactions has been rapidly expanding over the past two decades with excellent reviews about the ecological implications of microorganisms (Biere and Bennet 2013; Biere and Tack 2013; Casteel and Hansen 2014; Sugio et al. 2015; Mason et al. 2019). However, the extent to which plant-associated microbes influence insect performance and how insect-associated microbes influence plant defenses remains largely unknown (but see Schausberger 2018 for induced resistance). Our aim is to provide a conceptual framework to fill this gap through the understanding of possible reciprocal feedbacks between the microbiotas of plants and insects that could affect their evolution. First, we will discuss how the holobiont concept came to be and whether this view actually helps us in understanding the ecology and evolution of hosts and their interactions. Next, we will review evidence about how the phyllosphere affects insect performance and possible feedbacks between the insect microbiota and plant defenses. We will finally point out how network theory can shed light into evaluating coevolutionary processes between the microbiotas of plants and insects.
From Microbes Through Holobionts to Plant-Insect Interactions
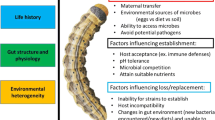
Our understanding of how microorganisms can shape the ecology and evolution of plant-insect interactions has been increasing rapidly since the last 20 years. However, along with all these advances came a general confusion in the terms and concepts frequently used in the context of the host-microbe interactions. The term holobiont was coined by Lynn Margulis (1990) to describe the intimacy between a host and its microbial symbiont. While this term was initially developed to explain the origin of eukaryotic cells, it was latter extended to include other obligatory symbioses (O’Malley 2017). At the beginning of this century, and under the umbrella of the hologenome theory of evolution, the term holobiont was redefined as a host (plant or animal) together with all its associated microorganisms upon which natural selection can operate (Zilber-Rosenberg and Rosenberg 2008; Theis et al. 2016; Rosenberg and Zilber-Rosenberg 2018). Along with this new view of holobionts, other concepts were commonly used although not always with the same meaning. Thus, in 2015 Marchesi and Ravel proposed clear definitions which we will follow throughout this review. The microbiota refers to the assemblage of microorganisms present in a defined host. The microbiome includes the host, all its microorganisms, their genomes, and the surrounding environmental conditions. The hologenome can then be defined as the sum of the genetic information of the host and its microbiota. While there is now plenty of evidence supporting the hologenome hypothesis of evolution, whether the holobiont can function as an evolutionary unit is still under debate (Moran and Sloan 2015; Douglas and Werren 2016; Doolittle and Booth 2017; Doolittle and Inkpen 2018). Holobionts are indeed evolving units, but not evolutionary units upon which natural selection can act because a holobiont is better viewed as an ecological community with interactions that range from parasitic to mutualistic, with horizontal and vertical transmission and multiple levels of fidelity among the partners.
In considering the ecology and evolution of holobionts, the fidelity in the transmission of the microbiota along generations is of the most interest. It has been then proposed that the holobiont is constituted by resident microbiota (of vertical transmission), semi-resident microbiota (of vertical and horizontal transmission), and transient microbiota (of horizontal transmission) (Roughgarden et al. 2018). While all three types of microbiota can affect its host fitness (Zakharov 2015; Hurst 2017), the resident microbiota might be more important in evolutionary terms, while the transient microbiota represents an important source of variation affecting the host ecological interactions (Callens et al. 2018; Guégan et al. 2018). In this sense, it is interesting to note that a group of microbes appear to be shared and maintained among most individuals of a single population despite spatial and temporal variation (Roeselers et al. 2011; Lowe et al. 2012; Dougal et al. 2013; Astudillo-García et al. 2017; Kwong et al. 2017). This group of microbes has been termed the core microbiota (Shapira 2016), and while there is still no clear consensus on how to delimit or measure it (Shade and Handelsman 2012; Hurst 2017), considering its function rather than its composition could prove more insightful.
The core microbiota becomes more relevant if functional groups are considered, instead of taxonomic groups, because the latter gives no information about their contribution to the host phenotype (Doolittle and Booth 2017; Foster et al. 2017; Lemanceau et al. 2017). Moreover, considering the functional core microbiota implies that those transient or horizontally transmitted microbes could eventually replace those from the core without altering the host ecology and evolution. Thus, the presence of certain specific lineages of microbes would be sufficient to allow the functional assembly of the holobiont (Roughgarden et al. 2018). This functional contribution could then be relevant even when the microorganisms do not have a common evolutionary history with their host (Catania et al. 2017). Ultimately, the (extended) phenotype expressed by a particular host is the result of not only the presence of different microbes but also their functional contribution. Because this extended phenotype is the one that interacts with the consumers, the functional core microbiota will play a role in plant-insect evolution. Thus, in the context of plant-insect interactions, it is important to understand the effects of the microbiota on its host but also on how this host interacts with other organisms. If the presence/absence of specific lineages in the microbiota affects the fitness of either the plant, the herbivore, or both, a third-party player should be recognized in the battle between plants and herbivorous insects.
The Phyllosphere and Insect Performance
The surface of the leaves is the habitat for large and diverse microbial communities defined as phyllosphere (Ruinen 1956; Lindow and Brandl 2003; Vorholt 2012). All of these microbes are, at some point, inevitably consumed by the insects. While the impact of consuming entomopathogens has been the aim of several studies (Cory and Hoover 2006; Shikano 2017), little is still known about the possible effects of consuming nonpathogenic microbes. Recent evidence suggests that phyllosphere bacteria can indeed colonize the insect midgut (Mason and Raffa 2014; Bansal et al. 2011). Actually, it has been shown that the symbionts in the midgut of the gypsy moth Lymantria dispar are mostly obtained from its host plant (Broderick et al. 2004). However, few studies have evaluated the effect of the phyllosphere on insect performance. To our knowledge only two studies have specifically evaluated the effect of the phyllosphere on insect performance.
Shikano et al. (2015) evaluated the effect of two common bacterial colonizers of the phyllosphere (Pseudomonas fluorescens and P. syringae) on the performance, immunity, and resistance of the cabbage looper Trichoplusia ni. They found that consumption of the phyllosphere bacteria decreased larval growth rate but had no effect on immunity and, while the larval resistance to a baculovirus was not affected, resistance to pathogenic bacteria was concentration-dependent. The phyllosphere, however, can also have positive effects on larval performance. Larvae of the gypsy moth were bigger when consuming diet enriched with bacteria from the phyllosphere of the quaking aspen Populus tremuloides compared than when consuming diet enriched with bacteria previously isolated from their own guts (Mason et al. 2014). That is, bacteria that most benefitted larvae were initially foliar residents, suggesting that toxin-degrading abilities of phyllosphere inhabitants indirectly benefit herbivores upon ingestion (Mason et al. 2014). Interestingly, herbivory can in turn influence the phyllosphere. In the plant Cardamine cordifolia, the abundance of Pseudomonas syringae was higher in herbivore-damaged vs. herbivore-undamaged leaves, while Pedobacter spp. and Pseudomonas fluorescens infections were negatively associated with herbivory (Humphrey et al. 2014). All this evidence suggest that the composition and provenance of the microorganisms involved in the interaction between plants and insects should be identified before further experimental manipulation aimed at demonstrating their functional role.
The Gut Microbiota and Plant Defenses
In general, plant tissue consumption results in a reconfiguration of the primary and secondary metabolism. Several studies have reported that after damage, those processes related to the primary metabolism such as growth, photosynthesis, carbon assimilation, respiration, and reallocation of resources decrease (Zangerl et al. 2002; Schwachtje and Baldwin 2008), whereas the secondary metabolism, responsible for the production of chemical defenses, increases (Kessler and Baldwin 2002). These physiological and metabolic changes are closely related to the expression of defensive mechanisms of tolerance and resistance, respectively. It is now recognized that the expression of tolerance is related to changes in primary metabolism that allow tolerant genotypes to reduce the negative effects of herbivory in terms of fitness (Strauss and Agrawal 1999; Stowe et al. 2000; Fornoni 2011). On the other hand, increases in resistance are given by changes in the production and abundance of various secondary metabolites that prevent or limit the loss of foliar tissue (Fritz and Simms 1992; Karban and Baldwin 1997). The triggering of signaling cascades that produce changes in both metabolisms can be either initiated by endogenous biochemical pathways that start when plant cells are damaged (Mithöfer and Boland 2008) or initiated by elicitors of microbial origin present in the regurgitant of herbivorous insects (Felton et al. 2014).
The regurgitant of many insects contains chemical compounds with eliciting properties. Among the substances found in the regurgitant are plant growth promoters such as auxins (Dyer et al. 1995), pectinases (Hori 1975), indoleacetic acid (Miles and Lloyd 1967), epidermal growth factors (Detling and Dyer 1981; Dyer et al. 1995), cytokinins that increase the photosynthetic rate (Giron et al. 2007; Kaiser et al. 2010; Halitschke et al. 2011), and transcription factors involved in the transport of carbon and nitrogen (Steinbrenner et al. 2011) as well as in the reactivation of secondary meristems (Korpita et al. 2014). All these compounds have the potential to alter the tolerance response of plants through different mechanisms. Studies carried out with the tomato plant, Solanum lycopersicum , show that plants treated with Manduca sexta regurgitant recovered more quickly after a defoliation treatment by increasing their growth rate and the reactivation of secondary meristems (Korpita et al. 2014) probably because of metabolites involved in the transport of carbon and nitrogen that come in contact with the plant cells via the regurgitant (Steinbrenner et al. 2011). Some other studies have shown an effect of the regurgitant on plant traits, but the identity and origin of the particular elicitors are still unknown. For example, in the tobacco plant Nicotiana attenuata , it was found that damage by the moth M. sexta decreases the photosynthetic rate; however, when consumed by the hemipteran Tupiocoris notatus , a specific induction of elevated photosynthetic activity was shown (Halitschke et al. 2011). To our knowledge only one study has indeed shown that the gut endosymbionts are responsible for the production of these elicitors. Such is the case of the Lepidoptera Phyllonorycter blancardella , in which its endosymbionts such as Wolbachia spp. produce cytokinins that are deposited on the leaves, via regurgitation, leading to the formation of photosynthetically active “green patches” on damaged leaves (Kaiser et al. 2010). If a general pattern where the gut microbiota participates actively in the production of those compounds present in the regurgitant that triggers some tolerance-related trait, it is still to be confirmed.
On the other hand, there is also evidence that the regurgitant contains elicitors related to resistance mechanisms such as glucose oxidase (Diezel et al. 2009), polyphenol oxidases (Major and Constabel 2006; Ma et al. 2010), and proteinase inhibitors (Korth and Dixon 1997). The presence of such elicitors can sometimes decrease (Bede et al. 2006; Lawrence et al. 2008; Weech et al. 2008; Chung and Felton 2011; Chung et al. 2013) or increase plant resistance (Spiteller et al. 2000; Musser et al. 2002; Ping et al. 2007; Diezel et al. 2009). Many studies have even identified the microbial symbionts known to be responsible for affecting plant resistance. The bacterium Hamiltonella defensa is a facultative endosymbiont of the whitefly Bemisia tabaci , and it is involved in the suppression of JA in tomato plants (Su et al. 2015). Also, in the tomato plant, the larvae of the Colorado potato beetle, Leptinotarsa decemlineata, exploit bacteria in their oral secretions to decrease the production of JA and JA-responsive antiherbivore defenses (Chung et al. 2013). In the same study system, applying bacteria isolated from larval oral secretions to wounded plants confirmed that three microbial symbionts belonging to the genera Stenotrophomonas, Pseudomonas, and Enterobacter were responsible for defense suppression (Chung et al. 2013).
Interestingly, the effects of the bacterial symbionts on plant resistance seem to be host-dependent. For example, bacterial isolates from oral secretions of the false potato beetle Leptinotarsa juncta belonging to the genera Pantoea, Acinetobacter, Enterobacter, and Serratia were found to suppress polyphenol oxidase activity in the non-preferred host tomato, while only Pantoea sp. was observed to suppress the same activity in the preferred host horsenettle (Wang et al. 2016). There is even evidence of potential trade-offs among resistance traits mediated by bacterial symbionts. The bacterium Pantoea ananatis, isolated from the oral secretions of the armyworm Spodoptera frugiperda , downregulates the activity of the proteins polyphenol oxidase and trypsin proteinase inhibitors, but upregulates the peroxidase activity in the tomato plant (Acevedo et al. 2017). In turn, plant chemical defense can also affect the composition and structure of the insect microbial community. In the trembling aspen, Populus tremuloides , phenolic glycosides and condensed tannins affected the relative abundances of Ralstonia and Acinetobacter in the midgut of the gypsy moth Lymantria dispar (Mason et al. 2015). Taken together, these examples show the potential feedbacks between insect microbial communities and plant resistance. However, future studies should be designed to specifically test reciprocal feedbacks between the gut microbiota and plant resistance. Overall, all the evidence points at microorganisms modulating the expression of tolerance and resistance mechanisms against herbivory. We visualized two approaches that can complement each other to disentangle the evolutionary role of plant and insect microbiotas: network theory and experimental studies.
Network Theory and Interactions Among Microbiotas
Over the last 20 years, it has been recognized the value and importance of networks in a myriad of applications in biology. The concepts and tools developed from graph theory have provided new insights into evolutionary ecology as well as a valuable conceptual framework to address new challenges. Fundamental concepts from ecological systems – such as communities – to networks of biological interactions among their components provide a way to summarize large amounts of information within single objects. Perhaps, one of the most successful examples of the application of network theory in biology is plant science where key regulators, functional modules, and novel phenotypes have been identified through gene regulatory networks (Álvarez-Buylla et al. 2007). In the field of microbial evolution, network theory has been used to identify new targets for probiotic treatments (Lemon et al. 2012), find the most influential biotic and abiotic factors that structurally change the structure of the microbial community (Fisher and Mehta 2014), and distinguish the topological properties of microbial networks between health and disease states (Sánchez et al. 2017; Sommer et al. 2017). Microbial networks thus constitute a heuristic tool that could help us model and understand the complexity of the interactions within the microbiota and among microbiotas of interacting hosts.
In general, ecological networks can be divided in static or dynamic networks. Static networks focus on the study of its topological properties: measures of centrality (the relative importance of the nodes, each node representing a particular microorganism), the distribution of links to other nodes (degree distribution), identifying modules or clusters, and finding overrepresented or recurrent subgraphs (motifs). These kinds of networks are useful because they take into account any binary dependence between the elements, that is, the presence-absence of interactions among species. Weighted networks are a generalization of static networks, adding to the edges a measure of the relevance or certainty of its links. This value can be represented by the frequency or the strength of the different interactions. On the other hand, dynamical or evolving networks represent not only the species (i.e., microorganisms) and the topology/strength of the interactions but also the dynamic nature of the whole system (i.e., the holobiont). However, these kinds of networks are more difficult to infer from empirical data because they need a detailed and specific information almost never available. Due to the fact that static networks are much easier to obtain from data, the ample majority of ecological networks belong to this kind.
In microbiome studies, particular approaches have been adapted and combined to infer co-ocurrence networks of OTUs, interaction networks, and a new multi-network approach involving networks of microorganisms with its host. In a seminal paper, Gould and collaborators (2018) mapped the interactions between individual species of bacteria against several fitness traits of its hosts, the fruit fly. They showed that the same bacterial interactions that shape microbial abundances in the microbiota also determine the fly fecundity. In a recent study, Huitzil et al. (2018) proposed an evolutionary computational model in which a network representing the host can adapt in order to perform a predefined function related to its host. In this model, the host network interacts with its microbial network, and these complex interactions can explain the presence of dysbiosis, specialization, and microbial diversity.
One of the most important goals in microbial networks is to identify the so-called keystone taxa in microbial communities and to determine the factors that influence its function in a given environment. The dominant taxa could be the most abundant or the most important, structurally speaking, in terms of the topology of the network. In an extraordinary example, Flores et al. (2013) observed that complex networks between host and parasites or between bacteria and phages are at the same time, but at different scales, modular and nested. This observation suggests that different evolutionary regimes operate at different scales. The next natural step would be to integrate various layers of information via multiple single networks. This approach is called multilayer networks or multi-networks and could be defined as an amalgamation of networks that interact and evolve with each other (Bianconi 2018). Multilayer network applied to whole holobionts and to the interactions among holobionts could be an extremely valuable tool in understanding microbe-insect-plant interactions. Each one of these networks (plant microbiota network and insect microbiota network) will then form a meta-network, which will undoubtedly be a more realistic approach to the study of ecological interactions among holobionts. Thus, multilayer networks have the potential to take into account multitrophic interactions (Mirzaei and Maurice 2017).
Experimental Studies
To date most studies about microbe-insect-plant interactions have concentrated on the description of the microbiotas within multicellular species as well as in the sources of possible environmental regulation. One of the major assumptions behind these studies is that microorganisms directly affect the survival, performance, and fitness of their hosts as well as the interactions between hosts and other community members. Correlative evidence support this premise but have not yet identified the functional role of most microbial lineages. If microorganisms have the potential to regulate the ecological and evolutionary dynamics between plants and insects, then our technological efforts to reduce or control herbivory levels should not neglect the effects of microorganisms. Pests are usually controlled with the use of insecticides and/or genetically modified crops, whereas the manipulation of microorganisms within plants or insects is still underdeveloped. We highlight two experimental approximations that can shed light into the functional role of microorganisms within the context of a plant-insect interaction. First, performing cross-infection experiments (i.e., reciprocal transplants) of microorganisms from different host populations or environmental conditions can provide relevant information on their role for plant and insect coadaptation to each other. These kinds of experiments are also fundamental to differentiate the relative importance of natural selection and genetic drift in the conformation and function of the microbial communities. Second, experiments where the microbiota composition is manipulated, either the presence/absence or the relative abundance of certain microorganisms, can identify their functional role on the plant-insect interaction. These experimental approximations, among others, will demonstrate whether the understanding and manipulation of plants and insects require the recognition that the evolution of the interaction depends not only upon the plant and insect genetics within a specific environmental context but also on the microbiome as well.
References
Acevedo FE, Peiffer M, Tan CW, Stanley BA, Stanley A, Wang J, Jones AG, Hoover K, Rosa C, Luthe D, Felton G (2017) Fall armyworm-associated gut bacteria modulate plant defense responses. Mol Plant-Microbe Interact 30:127–137
Alvarez-Buylla ER, Benitez M, Dávila EB, Chaos A, Espinosa-Soto C, Padilla-Longoria P (2007) Gene regulatory network models for plant development. Curr Opin Plant Biol 10:83–91
Asplen MK, Bano N, Brady CM, Desneux N, Hopper KR, Malouines C, Oliver KM, White JA, Heimpel GE (2014) Specialisation of bacterial endosymbionts that protect aphids from parasitoids. Ecol Entomol 39:736–739
Astudillo-García C, Bell JJ, Webster NS, Glasl B, Jompa J, Montoya JM, Taylor MW (2017) Evaluating the core microbiota in complex communities: a systematic investigation. Environ Microbiol 19:1450–1462
Bansal R, Hulbert S, Schemerhorn B, Reese JC, Whitworth RJ, Stuart JJ, Chen MS (2011) Hessian fly-associated bacteria: transmission, essentiality, and composition. PLoS One 6:e23170
Bede JC, Musser RO, Felton GW, Korth KL (2006) Caterpillar herbivory and salivary enzymes decrease transcript levels of Medicago truncatula genes encoding early enzymes in terpenoid biosynthesis. Plant Mol Biol 60:519–531
Bianconi G (2018) Multilayer networks: structure and function. Oxford University Press, Oxford
Biere A, Bennet AE (2013) Three-way interactions between plants, microbes and insects. Funct Ecol 27:567–573
Biere A, Tack AJM (2013) Evolutionary adaptation in three-way interactions between plants, microbes and arthropods. Funct Ecol 27:646–660
Broderick NA, Raffa KF, Goodman RM, Handelsman J (2004) Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl Environ Microbiol 70:293–300
Callens M, Watanabe H, Kato Y, Miura J, Decaestecker E (2018) Microbiota inoculum composition affects holobiont assembly and host growth in Daphnia. Microbiome 6:56
Casteel CL, Hansen AK (2014) Evaluating insect-microbiomes at the plant-insect interface. J Chem Ecol 40:836–847
Catania F, Krohs U, Chittò M, Ferro D, Ferro K, Lepennetier G, Görtz HD, Schreiber RS, Kurtz J, Gadau J (2017) The hologenome concept: we need to incorporate function. Theory Biosci 136:89–98
Chu CC, Spencer JL, Curzi MJ, Zavala JA, Suefferheld MJ (2013) Gut bacteria facilitate adaptation to crop rotation in the western corn rootworm. Proc Natl Acad Sci 110:11917–11,922
Chung SH, Felton GW (2011) Specificity of induced resistance in tomato against specialist lepidopteran and coleopteran species. J Chem Ecol 37:378–386
Chung SH, Rosa C, Scully ED, Peiffer M, Tooker JF, Hoover K, Luthe DS, Felton GW (2013) Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc Natl Acad Sci 110:15728–15733
Cory JS, Hoover K (2006) Plant-mediated effects in insect-pathogen interactions. Trends Ecol Evol 21:278–286
Detling JK, Dyer MI (1981) Evidence for potential plant growth regulators in grasshoppers. Ecology 62:485–488
Diezel C, von Dahl CC, Gaquerel E, Baldwin IT (2009) Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol 150:1576–1586
Doolittle WF, Booth A (2017) It’s the song, not the singer: an exploration of holobiosis and evolutionary theory. Biol Philos 32:5–24
Doolittle WF, Inkpen SA (2018) Processes and patterns of interaction as unit of selection: and introduction to ITSNTS thinking. Proc Natl Acad Sci 115:4006–4014
Dougal K, de la Fuente G, Harris PA, Girdwood SE, Pinloche E, Newbold CJ (2013) Identification of a core bacterial community within the large intestine of the horse. PLoS One 8:1–12
Douglas AE, Werren JH (2016) Holes in the hologenome: why host-microbe symbioses are not holobionts. Am Soc Microbiol 7:e02099–e02015
Dyer MI, Moon AM, Brown MR, Crossley DA (1995) Grasshopper crop and midgut extract effects on plants: an example of reward feedback. Proc Natl Acad Sci 92:5475–5478
Felton GW, Tumlinson JH (2008) Plant-insect dialogs: complex interactions at the plant-insect interface. Curr Opin Plant Biol 11:457–463
Felton GW, Chung SH, Estrada-Hernández MG, Louis J, Peiffer M, Tian D (2014) Herbivore oral secretions are the first line of protection against plant-induced defences. Annu Plant Rev 47:37–76
Fisher CK, Mehta P (2014) Identifying keystone species in the human gut microbiome from metagenomic time-series using sparse linear regression. PLoS One 9:e102451
Flores CO, Valverde S, Weitz JS (2013) Multi-scale structure and geographic drivers of cross-infection within marine bacteria and phages. ISME J 7:520–532
Fornoni J (2011) Ecological and evolutionary implications of plant tolerance to herbivory. Funct Ecol 25:399–407
Foster KR, Schluter J, Coyte KZ, Rakoff-Nahoum S (2017) The evolution of the host microbiome as an ecosystem on a leash. Nature 548:43–51
Fritz RS, Simms EL (1992) Plant resistance to herbivores and pathogens: ecology, evolution and genetics. Chicago University Press, Chicago
Giron D, Kaiser W, Imbault N, Casas J (2007) Cytokinin-mediated leaf manipulation by a leafminer caterpillar. Biol Lett 3:340–343
Gould AL, Zhang V, Lamberti L, Jones EW, Obadia B, Korasidis N, Gavryushkin A, Carlson JM, Beerenwinkel N, Ludington WB (2018) Microbiome interactions shape host fitness. Proc Natl Acad Sci 115:E11951–E11960
Guégan M, Zouache K, Démichel C, Minard G, Van VT, Potier P, Mavingui P, Moro CV (2018) The mosquito holobiont: fresh insight into mosquito-microbiota interactions. Microbiome 6:49
Halitschke R, Hamilton JG, Kessler A (2011) Herbivore-specific elicitation of photosynthesis by mirid bug salivary secretions in the wild tobacco Nicotiana attenuata. New Phytol 191:528–535
Hansen AK, Moran NA (2013) The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol Ecol 23:1473–1496
Hori K (1975) Pe`ctinase and plant growth-promoting factors in the salivary glands of the larva of the bug, Lygus disponsi. J Insect Physiol 21:1271–1274
Huitzil S, Sandoval-Motta S, Frank A, Aldana M (2018) Modeling the role of the microbiome in evolution. Front Physiol 9:1836
Humphrey PT, Nguyen TT, Villalobos MM, Whiteman NK (2014) Diversity and abundance of phyllosphere bacteria are linked to insect herbivory. Mol Ecol 23:1497–1515
Hurst GDD (2017) Extended genomes: symbiosis and evolution. Interface Focus 7:20170001
Kaiser W, Huguet E, Casas J, Commin C, Giron D (2010) Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. Proc R Soc B 277:2311–2319
Karban R, Baldwin IT (1997) Induced responses to herbivory. Chicago University Press, Chicago
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328
Korpita T, Gómez S, Orians CM (2014) Cues from a specialist herbivore increase tolerance to defoliation in tomato. Funct Ecol 28:395–401
Korth KL, Dixon RA (1997) Evidence for chewing insect-specific molecular events distinct from a general wound response in leaves. Plant Physiol 115:1299–1305
Kwong WK, Medina LA, Koch H, Sing KW, Soh EJY, Ascher JS, Jaffé R, Moran NA (2017) Dynamic microbiome evolution in social bees. Sci Adv 3:e1600513
Lawrence SD, Novak NG, Ju CJT, Cooke JEK (2008) Potato, Solanum tuberosum, defense against Colorado potato beetle, Leptinotarsa decemlineata (Say): microarray gene expression profiling of potato by Colorado potato beetle regurgitant treatment of wounded leaves. J Chem Ecol 34:1013–1025
Lemanceau P, Blouin M, Muller D, Moënne-Loccoz Y (2017) Let the core microbiota be functional. Trends Plant Sci 22:583–595
Lemon KP, Armitage GC, Relman DA, Fischbach MA (2012) Microbiota-targeted therapies: an ecological perspective. Sci Transl Med 4:137rv5
Lindow SE, Brandl MT (2003) Microbiology of the phyllosphere. Appl Environ Microbiol 69:1875–1883
Lowe B, Marsh T, Isaacs-Cosgrove N, Kirkwood R, Kiupel M, Mulks M (2012) Defining the “core microbiome” of the microbial communities in the tonsils of healthy pigs. BMC Microbiol 12:20
Ma R, Chen JL, Cheng DF, Sun JR (2010) Activation of defense mechanism in wheat by polyphenol oxidase from aphid saliva. J Agric Food Chem 58:2410–2418
Major IT, Constabel CP (2006) Molecular analysis of poplar defense against herbivory: comparison of wound- and insect elicitor-induced gene expression. New Phytol 172:617–635
Marchesi JR, Ravel J (2015) The vocabulary of microbiome research: a proposal. Microbiome 3:31
Margulis L (1990) Words as battle cries: symbiogenesis and the new field of endocytobiology. Bioscience 40:673–677
Mason CJ, Raffa KF (2014) Acquisition and structuring of midgut bacterial communities in gypsy moth (Lepidoptera: Erebidae) larvae. Environ Entomol 43:595–604
Mason CJ, Couture JJ, Raffa KF (2014) Plant-associated bacteria degrade defense chemicals and reduce their adverse effects on an insect defoliator. Oecologia 175:901–910
Mason CJ, Rubert-Nason KF, Lindroth RL, Raffa KF (2015) Aspen defense chemicals influence midgut bacterial community composition of gypsy moth. J Chem Ecol 41:75–84
Mason CJ, Jones AG, Felton GW (2019) Co-option of microbial associates by insects and their impact on plant-folivore interactions. Plant Cell Environ 42:1078–1086
Miles PW, Lloyd J (1967) Synthesis of a plant hormone by the salivary apparatus of plant-sucking Hemiptera. Nature 213:801–802
Mirzaei MK, Maurice CF (2017) Ménage à trois in the human gut: interactions between host, bacteria and phages. Nat Rev Microbiol 15:397–408
Mithöfer A, Boland W (2008) Recognition of herbivory-associated molecular patterns. Plant Physiol 146:825–831
Moran NA, Sloan DB (2015) The hologenome concept: helpful or hollow? PLoS Biol 13:1–10
Musser RO, Hum-Musser SM, Eichenseer H, Peiffer M, Ervin G, Murphy JB, Felton GW (2002) Caterpillar saliva beats plant defences. Nature 416:599–600
O’Malley MA (2017) From endosymbiosis to holobionts: evaluating a conceptual legacy. J Theor Biol 434:34–41
Partida-Martínez LP, Heil M (2011) The microbe-free plant: fact or artifact? Front Plant Sci 2:100
Pineda A, Dicke M, Pieterse CJ, Pozo MJ (2013) Beneficial microbes in changing environment: are they always helping plants to deal with insects? Funct Ecol 27:574–586
Ping L, Büchler R, Mithöfer A, Svatoš A, Spiteller D, Dettner K, Gmeiner S, Piel J, Schlott B, Boland W (2007) A novel Dps-type protein from insect gut bacteria catalyses hydrolysis and synthesis of N-acyl amino acids. Environ Microbiol 9:1572–1583
Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, Rawls JF (2011) Evidence for a core gut microbiota in the zebrafish. ISME J 5:1595–1608
Rosenberg E, Zilber-Rosenberg I (2018) The hologenome concept of evolution after 10 years. Microbiome 6:78
Roughgarden J, Gilbert SF, Rosenberg E, Zilber-Rosenberg I, Lloyd EA (2018) Holobionts as units of selection and a model of their population dynamics and evolution. Biol Theory 13:44–65
Ruinen J (1956) Occurrence of Beijerinckia species in the ‘phyllosphere’. Nature 177:220–221
Sánchez B, Delgado S, Blanco-Míguez A, Lourenço A, Gueimonde M, Margolles A (2017) Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res 61:1600240
Schausberger P (2018) Herbivore-associated bacteria as potential mediators and modifiers of induced plant defense against spider mites and thrips. Front Plant Sci 9:1107
Schwachtje J, Baldwin IT (2008) Why does herbivore attack reconfigure primary metabolism? Plant Physiol 146:845–851
Shade A, Handelsman J (2012) Beyond the Venn diagram: the hunt for a core microbiome. Environ Microbiol 14:4–12
Shapira M (2016) Gut microbiotas and host evolution: scaling up symbiosis. Trends Ecol Evol 31:539–549
Sharpton TJ (2018) Role of the gut microbiome in vertebrate evolution. mSystems 3:e00174–e00117
Shikano I (2017) Evolutionary ecology of multitrophic interactions between plants, insect herbivores and entomopathogens. J Chem Ecol 43:586–598
Shikano I, Olson GL, Cory JS (2015) Impact of non-pathogenic bacteria on insect disease resistance: importance of ecological context. Ecol Entomol 40:620–628
Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P (2017) The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol 15:630–638
Spiteller D, Dettner K, Boland W (2000) Gut bacteria may be involved in interactions between plants, herbivores and their predators: microbial biosynthesis of N-acylglutamine surfactants as elicitors of plant volatiles. Biol Chem 381:755–762
Steinbrenner AD, Gómez S, Osorio S, Fernie AR, Orians CM (2011) Herbivore-induced changes in tomato (Solanum lycopersicum) primary metabolism: a whole plant perspective. J Chem Ecol 37:1294–1303
Stowe KA, Marquis RJ, Hochwender CG, Simms EL (2000) The evolutionary ecology of tolerance to consumer damage. Annu Rev Ecol Syst 31:565–595
Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14:179–185
Su Q, Oliver KM, Xie W, Wu Q, Wang S, Zhang Y (2015) The whitefly-associated facultative symbiont Hamiltonella defensa suppresses induced plant defences in tomato. Funct Ecol 29:1007–1018
Sugio A, Dubreuil G, Giron D, Simon JC (2015) Plant-insect interactions under bacterial influence: ecological implications and underlying mechanisms. J Exp Bot 66:467–478
Theis KR, Dheilly NM, Klassen JL, Brucker RM, Baines JF, Bosch TCG, Cryan JF, Gilbert SF, Goodnight CJ, Lloyd EA, Sapp J, Vandenkoornhuyse P, Zilber-Rosenberg I, Rosenberg E, Bordenstein SR (2016) Getting the hologenome concept right: an eco-evolutionary framework for hosts and their microbiomes. mSystems 1:e00028–e00016
Vorholt JA (2012) Microbial life in the phyllosphere. Nat Rev Microbiol 10:828–840
Wang J, Chung SH, Peiffer M, Rosa C, Hoover K, Zeng R, Felton GW (2016) Herbivore oral secreted bacteria trigger distinct defense responses in preferred and non-preferred host plants. J Chem Ecol 42:463–474
Weech MH, Chapleau M, Pan L, Ide C, Bede JC (2008) Caterpillar saliva interferes with induced Arabidopsis thaliana defence responses via the systemic acquired resistance pathway. J Exp Bot 59:2437–2448
Zakharov IA (2015) Intracellular symbionts as a factor of insect evolution. Biol Bull Rev 5:99–108
Zangerl AR, Hamilton JG, Miller TJ, Crofts AR, Oxborough K, Berenbaum MR, de Lucia EH (2002) Impact of folivory on photosynthesis is greater than the sum of its holes. Proc Natl Acad Sci 99:1088–1091
Zilber-Rosenberg I, Rosenberg E (2008) Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiol Rev 32:723–735
Acknowledgments
We want to thank the GMEE group for fruitful discussions about biotic interactions. This work was supported by grants from FOFI-UAQ 2018 (Programa: 205014190826, Fondo: 1299) to RAM, PAPIIT IN 210617 to JF, and FNB-2019-05 to EG.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mayoral-Peña, Z., Álvarez-Martínez, R., Fornoni, J., Garrido, E. (2020). The Extended Microbiota: How Microbes Shape Plant-Insect Interactions. In: Núñez-Farfán, J., Valverde, P. (eds) Evolutionary Ecology of Plant-Herbivore Interaction. Springer, Cham. https://doi.org/10.1007/978-3-030-46012-9_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-46012-9_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46011-2
Online ISBN: 978-3-030-46012-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)