Abstract
Plants and insects live in a microbial world, and the co-existence have shaped their ecology and evolution. These microbial allies play an essential role in the health, well-being, and vigor of their hosts and are often considered as “hidden players” in plant–insect interaction. The present chapter attempts to cover the contribution of microbes as drivers of plant–insect interaction where the microbial companions directly or indirectly influence the plant–insect interaction. The chapter also emphasizes the diversity of microbial communities linked with both plants and insects and their contribution toward plant–insect interaction from an ecological standpoint. It further deals with the recent updates on the use of microorganisms in pest management and the implications of microbes as a toolbox in future IPM strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microbes
- Metagenomics
- Hidden allies
- Toolbox
- Host–microbe interaction
- Microbe-associated molecular patterns (MAMP)
- Symbiosis
- Mycorrhiza-induced resistance (MIR)
- Herbivore-induced plant volatiles (HIPVs)
1 Introduction
The co-existence of plants and insects evolved over 400 million years and shaped the ecosystem. Both plants and insects are engaged in an arms race where plant defenses against insect herbivory, while insects evolve strategies to overwhelm them. Phytophagous insects (generalists and/or specialists) attack diverse plant species for herbivory. In response, plants produce an array of defensive compounds known as plant secondary metabolites to cope with their enemies. Glucosinolates, alkaloids, terpenoids, and phenolics are classic secondary metabolites serving as defensive compounds (Papadopoulou and van Dam 2017), which are either constitutively expressed in plants or induced in response to herbivory (Wu and Baldwin 2010). To counteract these plant defenses, insects secrete elicitors with their salivary secretions that decoy the defense responses (Fürstenberg-Hägg et al. 2013) or detoxify them (Ceja-Navarro et al. 2015).
In the last two decades, biologists have been keen on exploring the role of microbes in shaping the ecology of plants and animals. Studies have revealed that microbial communities associated with plants and insects play an essential role in health, well-being, and vigor of their hosts and are often considered as “hidden players” in plant–insect interaction (Douglas 2018; Sugio et al. 2014); (Biere and Bennett 2013). Various microbial communities (e.g., mycorrhizal fungi, rhizobacteria, root endophytic fungi) promote plant growth and protect them against a wide range of diseases by inducing resistance in systemic tissues (Induced Systemic Resistance – ISR) (Pineda et al. 2010; Van Wees et al. 2008). Furthermore, microbes have profound effects on insect feeding efficiency by helping to digest food or detoxifying entomotoxic compounds, modulating host growth, development, behavior, etc. Microbial allies of insects affect the plant defense mechanisms by either suppressing or counteracting the plant defense response (Sugio et al. 2014; Zhu et al. 2014). Microbes may engage in altering plant metabolisms and/or defense systems that significantly impact the plant–insect interaction either benefiting the plants or insects. Recent advances in high-throughput omics technology have opened up fascinating research area with the possibilities to conduct global analysis on the composition and functional capabilities of microbial symbionts that may contribute to the health and fitness of their host. The fast-moving scientific developments offer excellent potential for in-depth investigation of the fundamental processes and manipulation of the microbiota for effective microbial therapies.
The present chapter attempts to cover the role of microbes as drivers of plant–insect interaction where microbial associates directly or indirectly influence the plant–insect interaction. This chapter centers on the diversity of microbial communities and their contribution to plant–insect interaction from an ecological perspective. It further deals with the recent updates on the use of microorganisms in pest management and the implications of microbes as a toolbox in future IPM strategies.
2 Microbial Contribution in Shaping the Tri-Trophic Interactions in an Ecosystem
A diverse spectrum of microbes are often allied to plants and insects, and the nature of their association may vary from pathogenic to mutualistic interaction depending on underlying ecological factors. Symbiotic microorganisms live in the close interface with the host either permanently or for a considerable part of host’s life cycle and play a key role in their diversification and evolutionary stability (Salem et al. 2015). Most of the intracellular symbionts show maternal inheritance where the symbionts are vertically transmitted from mother to the offspring. The horizontally transmitted symbionts are however transmitted directly from the environment or other conspecific or heterospecific host individuals (Kikuchi et al. 2007). The pathogenic interactions may also shift to the beneficial relationship over the course of time such as Wolbachia infection in Drosophila simulans, leading to an increase in the fecundity over uninfected females (Weeks et al. 2007).
Apart from the two-way interactions between microbes and their hosts (plants or insects), microbes are also engaged in a multi-trophic interaction where microbes interact with plants and insects simultaneously (Biere and Bennett 2013; Biere and Tack 2013). For example, the aphid–barley interaction depends on the interacting aphid species and bacteria present in rhizosphere (Tétard-Jones et al. 2007, 2012). The development of next-generation sequencing (NGS) technologies has enabled assessing the microbial diversity in different ecosystems. Meta-genomic and meta-transcriptomic sequencing combined with bioinformatic tools have assisted in exploring the taxonomic and functional diversity of hitherto hidden microbial association in a given environment (Douglas 2018).
2.1 Microbial Diversity Allied with Plants
Plants harbor diverse microbial communities in different compartments such as the rhizosphere (near the roots), phyllosphere (plant surface like leaves), and endosphere (within the plant and root tissues) (Andreote and e Silva 2017). Microbial communities associated with plants, both belowground and aboveground, benefit their host by aiding in the better uptake of nutrients from the soil for plant growth, increased tolerance to environmental stress (saline stress, drought, and occurrence of heavy metals) (Pineda et al. 2010), and protection against the pathogen (Bulgarelli et al. 2013). Some microbes are also capable of synthesizing plant growth–promoting hormones (Contreras-Cornejo et al. 2009; Van Loon 2007).
2.1.1 Microbes Enhancing Plant Growth and Nutrient Uptake
Plant-associated microbes such as the nitrogen-fixing bacteria (Rhizobium, Bradyrhizobium, Mesorhizobium, Ensifer (Sinorhizobium), and Azorhizobium) are widely studied for its interaction with the host and biogeochemical function (Batterman et al. 2013). The symbiotic association with plants helps in the better nutrient uptake and enables fixing atmospheric nitrogen required for plant growth. These endosymbionts form nitrogen-fixing nodules in the roots of the leguminous plants on expressing the rhizobial nodule–forming (nod) and nitrogen-fixing (nif) genes which are generally located on “symbiosis islands” (Ling et al. 2016). By definition, “symbiosis islands” are the mobile, integrative, conjugative elements that carry genes that enable them to expunge from the chromosome to form closed circular molecule that eventually conjugate and recombine into recipient chromosomes through horizontal gene transfer (HGT) from bacteria present in soil to host leguminous plants (Haskett et al. 2016; Ling et al. 2016). These mobile elements bear various novel traits such as antibiotic resistance, virulence, biofilm formation, degradation of aromatic compounds, and symbiosis (Ling et al. 2016; Okubo et al. 2016). For example, the transfer of the “symbiosis island” of 500 kb from Mesorhizobium loti to Lotus corniculatus and its integration into a phenylalanine-tRNA gene of the host plant chromosome resulted in root nodule formation and nitrogen-fixation in lotus plant (Ramsay and Ronson 2015). A similar example of such symbiotic interaction was documented in S. rostrate-Azorhizobium caulinodans system where the “symbiosis island” of A. caulinodans on integration to glycine-tRNA gene of S. rostrata-induced host nodulation (Ling et al. 2016).
Another interesting symbiotic association, observed between microbes present in plants and soil, is the arbuscular mychorrhizal symbiosis (Hammer et al. 2014; Richardson et al. 2009) where arbuscular mychorrhizal fungi (AMF, obligate biotrophs) belonging to the phylum Glomeromycota colonize on the cortical cells of the plant root. The AMF profit from the host carbon compounds to obtain metabolic energy (Gianinazzi et a. 2010), and in exchange caters better uptake of water and mineral nutrients (such as nitrogen and phosphorous) (Baum et al. 2015; Gutjahr and Parniske 2013) leading to increased host plant biomass, higher tolerance to abiotic stress (salinity, drought, heavy metals) (Singh et al. 2011) and protection against plant diseases (Bernardo et al. 2017). Enhanced plant growth determines increased food supply and improved nutrient quality for the herbivores and in turn influences the plant–insect interaction. Conversely, beneficial microbes accelerate the plant regrowth after herbivory by facilitating the nutrient and water uptake (Herman et al. 2008; Kempel et al. 2009; Kula et al. 2005).
2.1.2 Microbes-Induced Resistance in Plants
Plant defenses against pathogen attack can be either constitutively expressed in plants (passive resistance) or induced after the infection or herbivore attack (induced resistance). Microbes such as plant growth–promoting rhizobacteria (PGPR) and fungi (PGPF) (Segarra et al. 2009; Van Wees et al. 2008) as well as mycorrhizal and endophytic fungi (Stein et al. 2008) often initiate induced systemic resistance (ISR) to mitigate biotic and abiotic stresses in plants (Pozo and Azcón-Aguilar 2007; Shikano et al. 2017; Trillas and Segarra 2009). Induced systemic resistance involves activation of jasmonic acid and ethylene signaling pathways (Van der Ent et al. 2009) either by priming of plant defense genes in response to pathogen or insect attack (Conrath et al. 2001) or on interaction between non-pathogenic microbes with the plant roots. For instance, the establishment of arbuscular mychorrhizal symbiosis activates and boosts plant basal defense mechanisms on pathogen attack through Mycorrhiza-induced Resistance (MIR) (Pozo and Azcón-Aguilar 2007; Song et al. 2015). Based on transcriptomic and proteomic profiling, Fiorilli et al. (2018) demonstrated that the wheat–AMF association was not only benefiting the wheat plant in mineral nutrition but also protecting them against the pathogen (Xanthomonas translucens). Mycorrhizal colonization on plant root induces systemic defense responses via microbe-associated molecular patterns (MAMPs) recognition (Zamioudis and Pieterse 2012). It is interesting to note that the host plant initially perceives this mycorrhizal interaction as a putative pathogen by plant MAMP-recognition receptors and activates MAMP-triggered immunity (MIT) response as the first line of defense to prevent further invasion (Jones and Dangl 2006; Millet et al. 2010). This MIT response induced by mycorrhizal invasion results in transcriptional and hormonal changes that leads to the accumulation of hydrolytic enzymes (chitinase, glucanase), reactive oxygen species in roots, and activation of phenylpropanoid metabolism (García-Garrido and Ocampo 2002; Pozo and Azcón-Aguilar 2007) in the host plant, leading to the establishment of the symbiosis (Schouteden et al. 2015).
Interestingly, plant pathogens also invade plant tissues through stomata and thus stomatal closure is a part of innate immunity (Melotto et al. 2006). Pseudomonas syringae overwhelms this innate defense by the release of phytotoxin Coronatine (COR) (Zheng et al. 2012) that activates the Jasmonic acid signaling pathway, enabling the reopening of the stomatal pores. Plant-associated microbes have been reported to influence plant metabolic processes to block pathogen invasion (Kumar et al. 2012). A recent study documented that the plant growth–promoting fungi Penicillium simplicissium induces systemic resistance to protect Arabidopsis thaliana against the pathogen Pseudomonas syringae by altering the plant metabolic processes (Desclos-Theveniau et al. 2012; Du et al. 2014). The MYB44 gene product of endophytic fungi acts as stomata-specific enhancer of the plant Abscisic acid (ABA) signaling pathway that promotes stomatal closure thereby by blocking the entry of pathogen through stomata (Hieno et al. 2016; Montillet et al. 2013).
2.1.3 Microbial Toxin Production Against Insects
Microbes colonizing on plants can produce toxic compounds that can be harmful for insects during herbivory (Bizzarri and Bishop 2008; Monnerat et al. 2009). The crystal-like proteins (delta- endotoxins) produced by gram-positive bacteria Bacillus thuringiensis (Bt) on sporulation is known to have insecticidal activity (Palma et al. 2014). These endotoxins constitute Cry (crystal) and Cyt (cytosolic) group of proteins that interact synergistically to have a potential insecticidal effect (Butko 2003). The inactive Cry protoxins are ingested and proteolytically cleaved to yield shorter active toxins of 55-60 kDa in the insect midgut (Bravo et al. 2007). There are several models proposed for the mechanism behind the toxicity of Cry protein (Jurat-Fuentes and Crickmore 2017). It is generally believed that the active toxic molecules bind to specific receptors such as cadherin-like proteins, glycosylphophatidylinositol (GPI)-anchored Alkaline phosphatase (ALP), and GPI -anchored aminopeptidase (APN) at the surface of midgut, forming pores on the membrane, increasing its permeability, and disrupting the transmembrane ionic gradient, resulting in cell lysis and insect death (Pigott and Ellar 2007). Alternatively, the interaction of the Cry toxin with cadherin receptor triggers a signaling cascade involving protein G, adenylate cyclase and protein kinase A, as well as induces the activation of mitogen-activated kinases such as MAPK p38-triggering cell apoptosis (Zhang et al. 2006). More recently, Portugal et al. (Portugal et al. 2017) demonstrated that the binding of Cry toxin to specific receptors activates the phosphorylation of MAPK p38, disrupting the calcium ion influx through pore formation, which leads to cell death. Among different groups of Cry toxins (de Maagd et al. 2003), Cry1A toxin binds to cadherin protein receptors of most lepidopteran species (such as Manduca sexta, Bombyx mori, Heliothis virescens, Helicoverpa armigera, Pectinophora gossypiella, and Ostrinia nubilalis) (Pigott and Ellar 2007). The use of Cry toxins into transgenic crops for targeted and effective pest control has significantly reduced the use of chemical insecticides (Bravo et al. 2011). Another well-characterized rhizospheric bacteria, Pseudomonas protegens, secretes an antimicrobial compound that on ingestion promotes apoptosis in insects (Haas and Keel 2003; Loper and Gross 2007). In addition to the endotoxins, insecticidal proteins are also secreted during bacterial vegetative growth phase known as Vip (vegetative insecticidal proteins) (de Maagd et al. 2003; Estruch et al. 1996) that can be categorized into four groups Vip1, Vip2, Vip3, and Vip4 depending on their amino acid sequences (Chakroun et al. 2016; Zack et al. 2017). Vip1 and Vip2 are binary insecticidal proteins that are toxic to Coleopteran and Hemipteran species (Bi et al. 2015; Chakroun et al. 2016) whereas Vip3 targets against Lepidopteran insects (Song et al. 2016). However, Vip4 protein is not yet reported to have insecticidal activity (Chakroun et al. 2016).
2.2 Microbial Diversity Allied with Insects
Insect-associated microbes colonize mostly in the external cuticle and the gut. However, they can breach the exoskeleton and the gut to gain access to the hemocoel and within the specialized insect cells. Microbial communities present in insect influence several aspects of insect ecology, behavior, and physiology such as responses to the utilization of plant nutrients, immunity, reproduction, detoxification of defensive plant compounds, and protection against natural enemies (Oliver et al. 2010).
2.2.1 Microbes Providing Essential Nutrients
Insects along with other animals are unable to synthesize the essential amino acids and co-factors obligatory for many metabolic enzymes to function. In addition to these essential amino acids, insects cannot synthesize sterols that contribute to membrane architecture (Behmer and Nes 2003). Most insects derive these essential nutrients from their diet while feeding on plant sap. These insects constitute large populations of specific microorganisms localized in specialized cells, called bacteriocytes, within their body. The microbial symbionts present in most of the plant sap–feeding insects (hemipterans) are transmitted vertically from mother to their offspring via ovaries to the cytoplasm of each egg at oviposition that provides essential amino acids and/or vitamin cofactors that are limiting in host diet. Furthermore, the gut symbionts that are deposited externally over the eggs are acquired by the feeding offsprings (Buchner 1965). For instance, the bacterial endosymbiont, Buchnera aphidicola, in aphids is a model for such association where the bacterial symbiont is localized in metabolically active Bucherna cells and uses the insect body as its habitat and in return provides essential nutrients to its host (Wang et al. 2018). Upon the elimination of the symbiotic bacteria by the antibiotic treatment, the capability to synthesize essential amino acids is lost in aphids (Douglas et al. 2001).
Similarly, the symbiotic association of gall midges with fungi is essential for invading the plant stem to access the vascular tissue for nutrients and development of gall (Rohfritsch 2008). The close association of termites with microbes is also one of the best-studied symbiotic relationships in insects. The ability of the termites to harness and feed on nitrogen-deficient wood-based diet is due to the presence of unique consortium of microbes living in the termite gut. The microbial cellulolytic enzymes play a critical role in the digestion by enhancing its digestive efficiency (Peterson and Scharf 2016). The presence of mutualistic gut symbiont Erwinia dacicola in the olive fruit fly Bactrocera oleae benefits the host by providing essential amino acids and protease enzymes to digest the food (Capuzzo et al. 2005). Furthermore, in ants, cockroaches, and termites microbial allies recycle the nitrogenous wastes to essential amino acids (Douglas 2015). The resident microbes often produce glucosyl hydrolase that degrades plant cellulose and hemicellulose to short-chain fatty acids to provide a readily available nutrient source to insect host (Berenbaum 1980; Calderón-Cortés et al. 2012).
2.2.2 Microbes Influencing Insect Immunity
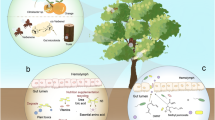
Microbes often contribute to insect innate immunity wherein gut microbes regulate the expression of immune genes (Johnson 2015a). The bacterial symbionts such as Wolbachia pipientis, Spiroplasma species, and Hamiltonella defensa either vertically or horizontally transmitted to the host have shown to influence host immunity (Engel and Moran 2013). For example, the facultative symbiont H. defensa protects aphids against the parasitoid through bacteriophage-encoded gene expression (Oliver et al. 2010). Similarly, Spiroplasma present in Drosophila neotestacea imparts resistance against the parasitic nematode (Jaenike et al. 2010). Furthermore, in mosquitoes, the gut microbiota activates the innate epithelial immunity against Plasmodium infection whereas the elimination of the microbiota renders the mosquitoes susceptible to infection (Dong et al. 2009). Over the years, Wolbachia infection is considered parasitic to insects as they contribute to cytoplasmic incompatibility, leading to reproductive disruption. Nevertheless, studies suggested that Wolbachia infection in Drosophila also deliberates antiviral protection leading to the higher survival of the flies (Johnson 2015b). The presence of Wolbachia in Drosophila induce antiviral resistance against a wide range of RNA viruses (Dicistroviridae, Nodaviridae, Flaviviridae, Togaviridae, and Reoviridae) but not DNA viruses (Teixeira et al. 2008). The reduction of the viral load and the anti-viral protection is reported to be due to the competition between the symbiotic bacteria and the viruses for the cellular resources (Caragata et al. 2013). Alternatively, the symbiotic association of Wolbachia affects the reactive oxygen species (ROS) levels, a key player in the insect immune system. The increase in ROS levels stimulates the Toll pathway, imparting anti-viral protection (Pan et al. 2012). Other studies reported the proliferation of Wolbachia inside the insect body suppresses the viral infection by inducing host immune responses by stimulating the miRNA expression (Hussain et al. 2011), thereby leading to cell death in insect (Brackney 2017; Terradas and McGraw 2017). However, the exact mechanism behind the antiviral protection by Wolbachia is still poorly understood (Yixin et al. 2017) (Fig. 1).
The tri-trophic interactions between plant-microbe-insect. (1) Plant endophytic microbes producing toxin against insect herbivore. (2) Microbial volatiles mixed with host plant volatiles attract parasitoids. (3) Insect gut microbiome enables detoxifying defensive plant compounds. (4) Indirect interaction between the plant endophytes and insect gut microbiome wherein the insect ultimately confiscates the plant defenses. (5) Soil microbes produce different metabolites that are detrimental to belowground herbivores. (6) Plant growth–promoting rhizobia as well as mycorrhizal fungi interact with plant roots, influencing the JA/ ET signaling pathways inducing resistance against aboveground herbivores
2.2.3 Microbes Influencing Detoxification of Plant-Defensive Compounds
Microbes are considered as the drivers promoting plant specialization in herbivorous insects (Janson et al. 2008). The acquisition of symbiotic microbes enabled the different sap-feeding insects to colonize on several plants. Plant-defensive secondary metabolites (terpenoids, phenolics, alkaloids, glucosinolates, and alliinins) are an essential determinant in plant–insect interaction. The ability of the insects to detoxify these toxic plant allelochemicals is often attributed to microorganisms associated with insects (Boone et al. 2013; Douglas 2013; García-Fraile 2018; Howe et al. 2018). Some herbivores often neutralize toxic phenolic compounds by increasing their gastrointestinal mucus production, by recruiting the gut microorganisms for degradation, and/or by secreting phenol-binding proteins in the saliva (Dearing et al. 2005). Insect symbionts can inhibit or counteract the host plant defenses through the direct or indirect production of enzymes targeting plant-defensive compounds (Broderick et al. 2004; Dowd and Shen 2011). The symbiotic fungus, Leucocoprinus gongylophorus, is present in the nest of the leaf-cutting ant, Acromyrmex echinatior, and aids to overwhelm plant-defensive phenolic compounds. Precisely, leaf-cutting ants preferentially feed on the fungal hyphae called gongylidia that expresses the laccase coding genes. On ingestion, the laccase molecules pass through the gut of these ants, released on defecation onto the ingested plant materials, and degrade plant defense compounds, for example, flavonoids and tannins (De Fine Licht et al. 2013).
Similarly, Ceja-Navarro et al. (2015) demonstrated the role of gut microbiome of the coffee berry borer (Hypothenemus hampei) in the detoxification of toxic plant alkaloid (Caffeine). H. hampei is a devastating insect pest of coffee that resulted in 80% crop loss on infestation. Caffeine, produced by the coffee plants, acts as a defense mechanism in response to herbivory. Interestingly, the coffee borer H. hampei possesses a core gut microbiota that is responsible for detoxification of caffeine in the insect gut and supports in the survival of the insect in a hostile environment. The gut bacteria such as Pseudomonas possess caffeine demethylase genes that aid in caffeine detoxification. Upon treatment with antibiotic that confiscates the insect gut microflora, eliminates the caffeine degradation ability of the beetle. However, the re-inoculation of Pseudomonas strain re-establishes the caffeine detoxification ability, thus certifying the pivotal role of microbial associates in caffeine degradation. Similarly, gut microbiota of the velvet bean caterpillar Anticarsia gemmatalis is involved in the production of serine and cysteine proteases and contributes to the insect’s tolerance to dietary protease inhibitors in soy plant (Pilon et al. 2013). Another interesting study showed that in the mountain pine beetle, Dendroctonus ponderosae, females initiate mass colonization through the production of aggregation pheromone trans-verbenol (Vité and Pitman 1968) by confiscating the host plant defense mechanisms. The trans-verbenol is a product of oxidative degradation of plant secondary defense compound monoterpene a-pinene (Renwick et al. 1976).
Recent studies have reported symbiont-mediated terpene degradation and verbenone production in beetles (Fig. 2) (Berasategui et al. 2017; Cao et al. 2018). Shotgun DNA sequencing on the gut microbiome of the mountain pine beetle revealed the presence of terpene-degrading bacteria belonging to genera Pseudomonas, Rahnella, Serratia, and Burkholderia (Adams et al. 2013).
2.2.4 Impact on Insect Pheromone Production and Reproduction
Pheromones are chemical compounds that serve as cues/signals for communication between individuals of the same species. These chemical compounds are involved in courtship, mating, defense, trail marking, aggregation, kin recognition, etc. (Howard and Blomquist 2005; Regnier and Law 1968). Though pheromones are generally encoded by insect gene, studies have shown that host-associated microbes also play a significant role in modulating their host chemical profiles, mating preference, and social behavior (Engl and Kaltenpoth 2018). Such modulation of the chemical signals occur either directly by influencing the biosynthetic pathway of pheromone production (Marshall et al. 2016) or by manipulating the host metabolic pool and allocating resources into pheromone production (Engl et al. 2018). The microbial symbiont in saw-toothed grain beetle Oryzaephilus surinamensis modulates the cuticle synthesis, resulting in the thinner cuticle and thereby rendering the beetles more susceptible to desiccation (Engl et al. 2018). Several insects exhibit reduced attractiveness and fecundity on the disruption of resident microbes with antibiotic treatment. This suggests a microbial role in mate choice and sexual communication (Ben-Yosef et al. 2008). For instance, the disruption of gut microbiota by the administration of antibiotics in Tephritid fruit fly, Ceratitis capitata, showed increased oviposition rates of females under nutritional stress as well as prolonged mating latency in males on a standard diet (Ben-Yosef et al. 2008). The reduced attractiveness of the female oriental fruit flies, Bactrocera dorsalis, to the males on antibiotic treatment could be reversed by the re-establishment of the gut microbiota into the female flies through feeding (Engl and Kaltenpoth 2018). Another fascinating example showed that Drosophila flies reared on different diets exhibit a strong mating preference where flies fed on the same diet preferred to mate with each other but not with files reared on a different diet (Sharon et al. 2010). Such preference in mate choice could be mediated by diet itself or by diet-associated gut microbial shifts. Sharon et al. (2010) showed evidence of resident gut bacteria influencing the mating preference in D. melanogaster, which could be abolished by antibiotic treatment. Interestingly, the lost preference for mate choice could be re-established by infecting the axenic D. melanogaster flies with the microbiota of the healthy flies on a diet. However, this study was controversial as several researchers tried to replicate the experiment that had conflicting results where the assortative mating pattern was only observed in inbreed fly lines before the transfer to different diets (Najarro et al. 2015). Others showed no stability of the results within the replicates (Arbuthnott et al. 2016). Thus, extensive research is needed to elucidate the factors influencing the mating preference and success in D. melanogaster.
Microbes-associated with insects often manipulate host reproduction by feminizing genetic males, inducing parthenogenesis or male killing, and by inducing cytoplasmic incompatibility (i.e., reproductive sterility when infected males mate with uninfected or infected females with a different symbiont strain) (Hughes et al. 2012; Miller and Schneider 2012; Werren et al. 2008). Wolbachia, Arsenophonus, Cardinium, Rickettsia, and Spiroplasma are among the universal reproductive manipulators that influence host reproduction (Engelstädter and Hurst 2009). Spiroplasma in pea aphid induces male killing to prevent competition with the infected females and avoidance of inbreeding depression (Simon et al. 2011). Increased female bias in infected female whiteflies was observed due to the invasion of Rickettsia (Himler et al. 2011) that swayed the population dynamics of whiteflies. The virus LbFv decreases the competitive ability of the parasitoid Leptopilina boulardi to infect Leptopilina heterotoma by manipulating the reproductive behavior of the parasitoid (Patot et al. 2012). Reproductive manipulators may serve as novel targets to be exploited in the development of alternative control strategies. These reproductive manipulators indirectly impact the plant–insect interactions by regulating the population dynamics and in so doing minimize the genetic diversity and/or recombination rates in infected species (Engelstädter and Hurst 2009) which in turn influence their co-evolutionary dynamics and functioning of ecological networks (Ferrari and Vavre 2011).
2.3 Crosstalk in Signaling Pathways – Decoy of Plant Defenses
Plants are armed with a plethora of defense mechanisms to combat against insect and pathogen attack. These defensive mechanisms are either constitutively present or activated upon insect or pathogen invasion (Pieterse and Dicke 2007). On perceiving the pathogen or insect attack, plants initially retort through its primary immune response and also activate effective systemic broad-spectrum resistance known as induced resistance against attackers (Walters et al. 2007). The phytohormones – salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) – are documented as key players in the regulation of plant defense signaling pathways (Koornneef and Pieterse 2008). In response to pathogen or insect attack, plants emit alarm signals with the production of SA, JA, and ET that contributes to plant defense response. SA-mediated defense responses are generally induced by microbial pathogens whereas insect invasion is usually dissuaded by JA/ET-mediated defenses (Kessler and Baldwin 2002; Thomma et al. 2001). However, in nature plants often encounter raid by different aggressors (pathogens and or herbivores) either simultaneously or by consequent invasion (Stout et al. 2006). Therefore, the crosstalk between the defense signaling pathways delivers a powerful defensive mechanism. These signaling pathways can be either mutually antagonistic or synergistic that allows the plant to combat against its invaders (Bostock 2005). Intriguingly, insect herbivores and pathogens have evolved to decoy the plant defenses for their own benefit by overwhelming the defense mechanisms modulating the plant’s signaling network (Pieterse and Dicke 2007). Herbivores often exploit its symbionts to overwhelm the anti-herbivore defenses by dodging the plant perception (Giron and Glevarec 2014; Sugio et al. 2014). For instance, the bacteria present in oral secretion of the Colorado potato beetle, Leptinotarsa decemlineata, activate the plant defense response through the stimulation of the SA signaling pathway as a response to microbial pathogen attack which in turn downregulates the JA anti-herbivore response, ensuring improved larval growth (Chung et al. 2013). A similar example, herbivorous silverleaf whitefly nymphs (Bemisia tabaci), activates SA signaling pathway as a decoy strategy to overcome JA-mediated defense to enhance larval performance (Zarate et al. 2007). Microbial pathogens often have the ability to produce phytohormones or their functional mimics and thereby manipulate plant signaling network (Robert-Seilaniantz et al. 2007). For instance, P. syringae bacteria produce a potent mimic of JA-Ile called coronatine that activates JA-Ile responses and suppresses SA-dependent defenses, resulting in enhanced pathogen growth (Nomura et al. 2005). The induction of SA signaling pathways results in the activation of pathogenesis-related protein encoding genes having antimicrobial activity (van Loon et al. 2006). Some of the prominent molecular players in the crosstalk between SA/JA signaling pathways are the regulatory protein NONEXPRESSOR OF PR GENES1 (NPR1), WRKY transcription factors, glutaredoxin GRX480, and Mitogen-activated protein (MAP) kinases. These regulatory components are essential for the activation of the SA signaling pathway which in turn suppresses the JA-induced response, resulting in overcoming plant defense against herbivore attack (Koornneef and Pieterse 2008).
2.4 Soil Microbial Diversity Influencing the Plant–Insect Interaction
Apart from the plant- and insect-associated microbes, the soil microbial community also plays a crucial role not only in enhancing plant growth and increased tolerance to abiotic stress but also in influencing aboveground insect herbivores through biochemical changes in plant-mediated mechanisms (Pineda et al. 2017). For example, the foliar-feeding Aphis jacobaea population depends on the soil microbial communities of its host plant ragwort (Senecio jacobaea). A different consortium of free-living soil-borne microbes influences the concentration of amino acids in the plant phloem sap, thereby affecting the aphid population (Kos et al. 2015). Additionally, inoculation of the distinct microbiome in soil manipulates the leaf metabolome of Arabidopsis, making it resistant against caterpillar Trichoplusia ni (Badri et al. 2013).
Intriguingly, belowground microbes have been shown to influence plant–insect interaction by modulating herbivore-induced plant volatile (HIPV) emission (Pineda et al. 2015). Plants in response to herbivore attack emit varieties of volatile organic compounds (HIPVs) in order to attract the potential predator. For example, the volatiles emitted by Nerium oleander plants in response to Aphis nerii attack signal the predator Chrysoperla carnea which could be altered by the presence or absence of soil microbial communities (Benítez et al. 2017). It was interesting to note C. carnea females preferred the HIPV blend emitted from plants grown on soil inoculated with microbes to those emitted from plants grown on control sterile soil (Benítez et al. 2017).
Furthermore, certain beneficial soil microbes can synthesize the phytohormones that enhance the plant growth and can mitigate abiotic stress (salinity, drought, heavy metals) (Egamberdieva et al. 2011; Egamberdieva et al. 2017; Liu et al. 2013). For example, root-colonizing soil bacterium B. licheniformis can synthesize indole-acetic-acid (IAA), which promotes wheat plant under saline stress (Singh and Jha 2016). Recent studies have demonstrated a linkage between the leaf microbiome and soil microbial communities (Pineda et al. 2017), wherein belowground microbial entities impact the aboveground insect herbivory as well as the composition of symbiotic “phytobiome” (i.e., plant microbiome). For instance, entomopathogenic fungi (Beauveria bassiana and Metarhizium anisopliae) that are typically present in soil colonize in different parts of broad bean (Vicia faba) plant and enhance plant growth as well as exhibit resistance against insects by translocating nitrogen to the plant from the insect cadavers via their fungal mycelia (Behie et al. 2012; Jaber and Enkerli 2016). Another example of such interaction is observed by a fungus Trichoderma, thought to be restricted to the soil, have now been known to colonize on the leaves and can suppress insect pests such as thrips (Muvea et al. 2014).
In the quest for crop protection, there is extensive use of insecticides that pose a threat of insecticide resistance (Whalon et al. 2008). One of the common organophosphorus insecticides used worldwide is fenitrothion that targets acetylcholine esterases and exhibits insect-specific toxicities (Stenersen 2004). Extensive application of such insecticides have led to an increased population of fenitrothion-degrading microbes in the soil that convert the toxic fenitrothion to non-toxic 3-methyl-4-nitrophenol and utilize it for their growth (Itoh et al. 2018). Riptortus pedestris (bean bug), a severe pest of leguminous crops, harbors Burkholderia in its midgut in sac-like tissues called “crypts” during its larval second instar stage that enables the bean bug to circumvent the toxic compounds, conferring insecticide resistance (Kikuchi et al. 2005). Notably, such symbiotic association ensures not only host survival but also an increase in body size, growth, and higher fecundity of the host (Kikuchi et al. 2005) .
2.5 Role of Microbial Volatiles in Plant–Insect Interaction
Similar to plants and animals, microbes also emit a plethora of volatile organic compounds (VOCs) in the course of their metabolic processes (Bitas et al. 2013). These compounds are usually lipophilic in nature that belong to the class of alcohols, aldehydes, esters, terpenoids, thiols, and fatty acid derivatives and have low molecular weight (<300 g mol−1), low boiling point, and high vapor pressure (0.01 kPa at 20 °C) (Kanchiswamy et al. 2015a, b). The volatile compounds are perceived from a distance as chemical signals to communicate with each other and contribute significantly in multitrophic interaction (Schulz-Bohm et al. 2017). Over the years, the role of microbial volatile compounds (mVOCs) in plant physiology has gained attention. mVOCs affect hormonal balance, metabolism, sugar concentration, and the acquisition of essential nutrients in plants, thereby inducing growth and regulating stress response. For instance, volatiles released from Bacillus subtilis have been shown to stimulate growth and salt tolerance in Arabidopsis thaliana (Ryu et al. 2003; Zhang et al. 2008a). The underpinning mechanism behind the contribution of VOC in A thaliana was demonstrated using proteome analysis in combination with other biochemical experiments (Kwon et al. 2010). The VOCs released by B. subtilis upregulates the iron-regulated transporter 1 (IRT1) gene expression, facilitating the iron uptake from soil. Iron is an essential micronutrient in photosynthesis. Its increased uptake enhances the photosynthesis efficiency and the chlorophyll content thus, inducing plant growth (Fincheira and Quiroz 2018). Salt tolerance in A. thaliana in response to mVOCs resulted in the regulation of HKT1 gene that encodes high-affinity Na+ transporter (Zhang et al. 2008b). Similarly, Pseudomonas chlororaphis releases 2, 3- butanediol that induces shoot growth and confers resistance in the tobacco leaves against the soft-rot pathogen Erwinia carotovora (Han et al. 2006). The VOC-mediated resistance requires JA/SA/ET signaling pathways (Farag et al. 2013). Apart from the synergistic effect, the microbes also influence antagonistically to plants. Some bacterial species belonging to genera Burkholderia, Pseudomonas, Serratia, Chromobacterium release a wide array of volatiles that exhibit phytotoxicity and inhibit plant growth (Bailly and Weisskopf 2012; Kai et al. 2009).
Microbial volatiles are equally crucial to insects and their natural enemies. Insects rely on olfactory cues to locate their host as food resource or as oviposition site and exhibit defense against pathogens (Davis et al. 2013). For instance, the gut bacteria in locust produce antimicrobial phenolic compounds to protect against other microbial pathogens as well as aggregation pheromone “guaiacol” that promotes mating in locust (Dillon et al. 2000, 2002). In Rhagoletis pomonella (apple maggot fly) the oviposition behavior is influenced by the release of volatiles emitted by Enterobacter agglomerans present on the fruit (Lauzon et al. 1998). Insects are often attracted to fermented fruit that is inhabited by microbes. The mVOCs emitted enable insects to locate their food source (DeVries 1987). Yeast volatiles have also been reported to modulate sexual behavior and mating in Drosophila melanogaster (Gorter et al. 2016). Not only this, microbial volatiles contribute significantly to tri-trophic interaction (Hulcr et al. 2005). The volatiles released by plants or microbes associated with plants provide cues for the natural enemies to locate attacked plants (Hulcr et al. 2005). Interestingly, the yeast volatiles deployed by Ogataea pini inhibit the growth of entomopathogenic fungus (B. bassiana) on bark beetle. Understanding mVOCs and its role in plant–insect interaction provides a great platform to develop novel, eco-friendly, cost-effective, sustainable pest management strategies (Bitas et al. 2013).
3 Microbes as a Toolbox: Integration of Microbes in Pest Management
The world population is predicted to upsurge from a present population of 6 billion to 9 billion in 30 years, and the need for increased food production to meet the demands of the ever-increasing population is a major challenge (Lacey et al. 2015). Approximately 42% of the total crop loss is caused by pest infestation and is anticipated to rise to 83% without any crop protection (Oerke and Dehne 2004). In the quest of increasing crop yield, farmers have embraced a wide range of conventional pesticides such as organochlorines, organophosphates, carbamates, and pyrethroids. The use of chemical pesticides to control devastating pest has been undoubtedly a great success but suffers from many limitations. Extensive pesticide usage and the constant evolutionary dynamics of insects have led to the selection for pesticide resistance in target species as well as killed a number of non-target beneficial insect species, including pollinators and natural enemies. These chemicals often pollute the surface water and are harmful to birds, humans, and domestic and aquatic animals (Usta 2013). It is high time to reduce the use of chemical pesticides, so as not to gamble with the ecosystem, and to choose an eco-friendly alternative to pest control. As discussed earlier, microbes play a crucial role in host physiology and traits and contribute significantly to plant–insect interaction. Harnessing the potential of the microbes as a toolbox in controlling pests is indeed a smarter alternative approach toward sustainable IPM strategy for crop protection. The development of biocontrol against insect pests by exploiting the microbial potential has progressed tremendously over the last 20 years. However, the European legislation is making continuous efforts to promote the use of biopesticides through policies to restrict the broad-spectrum chemical pesticide practice and ban certain pesticides, but still, it holds no more than 3% of the total global pesticide market (Lacey et al. 2015). The use of microbial entomopathogens in agriculture is an excellent substitute for chemical fertilizer. Several entomopathogenic microbes are available in the global market as microbial control agents (MCAs) (Lacey et al. 2015). These entomopathogenic microbes easily invade the insect body while feeding where they multiply and confiscate the host, ultimately causing insect death.
Most of the commercially available biopesticides target one specific pest, and although it is advantageous for the safety of the environment and the non-target species, such low range of effectivity has restricted biopesticides to a niche market (Lacey et al. 2015). For example, entomopathogenic viral formulations are commercially available to control insect pests such as codling moth, Cydia pomonella, that are highly selective for the target pest and often sensitive to environmental conditions such as solar radiation (Lacey et al. 2008). To achieve commercially successful biopesticides, improvements are needed on the insecticidal activity spectra, persistence to environmental variations, and delivery to target-specific sites of pest occurrence and should be cost-effective (Glare et al. 2012).
Interestingly, by transferring microbial symbionts from one insect species to another species that do not harbor such microbes naturally can have a drastic effect on the insect physiology and behavior. For instance, Wolbachia isolated from Drosophila and introduced to mosquitoes by injecting into the A. aegypti embryos has remarkably reduced the virus load and viral transmission by mosquitoes (Fraser et al. 2017). Moreover, in addition to suppression of viral transmission, Wolbachia infection also causes cytoplasmic incompatibility, leading to reproductive disruption and population reduction (Ferguson et al. 2015; Joshi et al. 2017). This target-specific control strategy could be an effective alternative to control disease outbreaks. Another strategy could either be the mass release of sterile male insect or release of Wolbachia infected incompatible females into the environment, resulting in reproductive disruption, thereby controlling the pest population (Nikolouli et al. 2018). However, these strategies suffer certain drawbacks. The mass production of sterile insects is often challenging and not cost-effective. Moreover, environmental factors such as temperature change might have an effect on anti-viral protection and cytoplasmic incompatibility imparted by Wolbachia (Ross et al. 2017). Additionally, anti-viral protection depends on the bacterial load that considerably affects the physiology and fitness of the insect host (Martinez et al. 2015).
Megacopta punctatissima, a soybean crop pest, utilizes its gut bacterial symbiont Ishikawaella to thrive on soybean. However, a closely related species M. cribraria shows high mortality on soybean. Administrating Ishikawaella from M. punctatissima into the newly hatched nymphs of M. cribraria enabled successfully thriving on soybean whereas M. punctatissima lost the ability to survive on soybean (Hosokawa et al. 2007). This suggests that the potential of microbial symbionts could be used as an approach for the manipulation of insect host range.
The use of genetically modified (GM) crop variety is expressing microbial endotoxins or inducing RNA interference (RNAi) to target-specific insect species also holds excellent potential against pest control (Zhang et al. 2017). However, it is not feasible to engineer all vulnerable crop varieties as polyphagous pests have a broad host range. Insects do not only attack for feeding but also vectors plant pathogens. An alternative approach to this could be genetically modifying the microbes to deliver RNA interference to the insect by knocking down the genes essential for insect metabolic processes (Whitten et al. 2016). The delivery of dsRNA for RNA interference can be easily achieved through genetically modifying the microbes that are invariably ingested by the insects where it can proliferate in the gut and spread through feces. For example, administration of the genetically modified bacterial strain, expressing dsRNA against insect α-tubulin gene, to the western flower thrips (F. occidentalis) significantly increases the insect mortality (Whitten et al. 2016). However, RNAi technique holds high potential to control insect pest population, though a fundamental problem exists, i.e., dissemination of genetically modified microbes to non-target hosts through horizontal transfer. However, the development of highly specific dsDNA for RNAi to target genes of a particular pest species can mitigate the limitation (Arora and Douglas 2017).
Microbial symbionts are an integral part of the insect life cycle that often influence different aspects: host physiology, behavior, immunity, and reproduction. The elimination of these obligate microbial partners could be a promising strategy to control insect pests. The use of antimicrobial peptides such as melittin, cecropin, or toxin proteins to target obligate symbionts would compromise the insect pest. However, the delivery of such antimicrobial agents to a specific site to target gut symbionts is a challenge. Nevertheless, Husseneder et al. (2016) used genetically engineered Kluyveromyces lactics (as a microbial delivery vehicle) that expresses melittin against the termite Coptotermes formosanus, which resulted in the elimination of termite gut symbiont thereby losing its cellulose degrading capability.
Furthermore, manipulating the genetic pool of the microorganisms for specific expression in different habitat could provide a much safer strategy to target pest insects. In particular, various entomopathogenic microbes (Metarhzium and Photorhabdus) have been identified to possess promoters that express toxin gene only in insect habitat (Fang et al. 2011; Münch et al. 2008). Several bacterial suicidal genes are available that degrade in a non-permissive habitat (Li and Wu 2009). The encapsulation of the microbes enables microbial release on insect feeding and in insect gut under particular environmental conditions (such as a change in pH, hydrostatic pressure, or high protease activity) (Arora et al. 2015).
4 Conclusion and Future Perspective
To satisfy the ever-augmenting demands of the growing population, the need for increased food production and crop protection is a major challenge. An army of researchers has been engaged over the years in the development of robust IPM strategies, but most cropping systems to date are hugely dependent on chemical pesticides (Stenberg 2017). There is a clear need for a holistic approach for sustainable pest management as well as to minimize the associated risks. The recent development in technologies has opened up new dimensions in crop protection. The advent of genomics and next-generation sequencing has made it practicable to explore the full spectrum of microbial diversity as there are no longer “hidden players” in plant–insect interaction. The recent advancement in omics technologies is anticipated to have a considerable impact on the development of biocontrol strategies by harvesting the knowledge in the interaction between insects and their microbial allies. The characterization of microbial diversity together with metabolic fingerprinting plays a crucial role in an in-depth understanding of host–microbe interaction (Douglas 2018). Exploiting microbial partners can serve as a potential candidate for future pest management. Furthermore, recent advancements in RNAi and CRISPR-Cas9 technology have led to breakthroughs in agriculture by manipulating host-associated microorganisms as control strategies against pest insects (Arora and Douglas 2017; Gao 2018). The recent genetic engineering of gut microbiota in honeybee through state-of-the-art CRISPR-CAS9 technology has proven to be an excellent toolkit to characterize and manipulate the gut microbiome in insect host physiology (Leonard et al. 2018). The reproductive alteration mediated by bacterium Wolbachia by inducing cytoplasmic incompatibility in the host insect also serves as a potent strategy for pest control (Arora and Douglas 2017). However, it is essential to consider the risk associated with the release of genetically modified microbes to the environment. The application of antagonistic microbes is an alternative eco-friendly approach toward crop protection where the antagonistic microbe competes and/or inhibits the growth of plant pathogens (Feichtmayer et al. 2017). Not only microbes but also microbial-volatile compounds (mVOCs) are potential candidates in biocontrol (Bailly and Weisskopf 2017). It is important to consider that each of these strategies has its limitations that need to be considered in advance. Insects and microbes have a relatively short generation time and are in a constant evolutionary race to overwhelm our control endeavors. The continuous improvement of existing strategies and development of new avenues are pivotal to get rid of crop losses due to insect infestation in future.
References
Adams AS, Aylward FO, Adams SM, Erbilgin N, Aukema BH, Currie CR et al (2013) Mountain pine beetles colonizing historical and naive host trees are associated with a bacterial community highly enriched in genes contributing to terpene metabolism. Appl Environ Microbiol, AEM:00068–00013
Andreote FD, e Silva M d CP (2017) Microbial communities associated with plants: learning from nature to apply it in agriculture. Curr Opin Microbiol 37:29–34
Arbuthnott D, Levin TC, Promislow DE (2016) The impacts of Wolbachia and the microbiome on mate choice in Drosophila melanogaster. J Evol Biol 29(2):461–468
Arora, A. K., & Douglas, A. E. (2017). Hype or opportunity? Using microbial symbionts in novel strategies for insect pest control. J Insect Physiol, 103, 10-17. doi:https://doi.org/10.1016/j.jinsphys.2017.09.011
Arora AK, Forshaw A, Miller TA, Durvasula R (2015) A delivery system for field application of paratransgenic control. BMC Biotechnol 15(1):59
Badri DV, Zolla G, Bakker MG, Manter DK, Vivanco JM (2013) Potential impact of soil microbiomes on the leaf metabolome and on herbivore feeding behavior. New Phytol 198(1):264–273
Bailly A, Weisskopf L (2012) The modulating effect of bacterial volatiles on plant growth: current knowledge and future challenges. Plant Signal Behav 7(1):79–85
Bailly A, Weisskopf L (2017) Mining the Volatilomes of plant-associated microbiota for new biocontrol solutions. Front Microbiol 8:1638. https://doi.org/10.3389/fmicb.2017.01638
Batterman SA, Hedin LO, Van Breugel M, Ransijn J, Craven DJ, Hall JS (2013) Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature 502(7470):224
Baum C, El-Tohamy W, Gruda N (2015) Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: a review. Sci Hortic 187:131–141
Behie S, Zelisko P, Bidochka M (2012) Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 336(6088):1576–1577
Behmer ST, Nes WD (2003) Insect sterol nutrition and physiology: a global overview. Adv Insect Physiol 31(1)
Benítez E, Paredes D, Rodríguez E, Aldana D, González M, Nogales R et al (2017) Bottom-up effects on herbivore-induced plant defences: a case study based on compositional patterns of rhizosphere microbial communities. Sci Rep 7(1):6251
Ben-Yosef M, Jurkevitch E, Yuval B (2008) Effect of bacteria on nutritional status and reproductive success of the Mediterranean fruit fly Ceratitis capitata. Physiol Entomol 33(2):145–154
Berasategui A, Salem H, Paetz C, Santoro M, Gershenzon J, Kaltenpoth M, Schmidt A (2017) Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Mol Ecol 26(15):4099–4110
Berenbaum M (1980) Adaptive significance of midgut pH in larval Lepidoptera. Am Nat 115(1):138–146
Bernardo L, Morcia C, Carletti P, Ghizzoni R, Badeck FW, Rizza F, Terzi V (2017) Proteomic insight into the mitigation of wheat root drought stress by arbuscular mycorrhizae. J Proteome 169:21–32
Bi Y, Zhang Y, Shu C, Crickmore N, Wang Q, Du L et al (2015) Genomic sequencing identifies novel Bacillus thuringiensis Vip1/Vip2 binary and Cry8 toxins that have high toxicity to Scarabaeoidea larvae. Appl Microbiol Biotechnol 99(2):753–760
Biere A, Bennett AE (2013) Three-way interactions between plants, microbes and insects. Funct Ecol 27(3):567–573
Biere A, Tack AJ (2013) Evolutionary adaptation in three-way interactions between plants, microbes and arthropods. Funct Ecol 27(3):646–660
Bitas V, Kim H-S, Bennett JW, Kang S (2013) Sniffing on microbes: diverse roles of microbial volatile organic compounds in plant health. Mol Plant-Microbe Interact 26(8):835–843
Bizzarri M, Bishop A (2008) The ecology of Bacillus thuringiensis on the phylloplane: colonization from soil, plasmid transfer, and interaction with larvae of Pieris brassicae. Microb Ecol 56(1):133–139
Boone CK, Keefover-Ring K, Mapes AC, Adams AS, Bohlmann J, Raffa KF (2013) Bacteria associated with a tree-killing insect reduce concentrations of plant defense compounds. J Chem Ecol 39(7):1003–1006
Bostock RM (2005) Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu Rev Phytopathol 43:545–580
Brackney DE (2017) Implications of autophagy on arbovirus infection of mosquitoes. Curr Opin Insect Sci 22:1–6
Bravo A, Gill SS, Soberón M (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49(4):423–435. https://doi.org/10.1016/j.toxicon.2006.11.022
Bravo A, Likitvivatanavong S, Gill SS, Soberón M (2011) Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem Mol Biol 41(7):423–431. https://doi.org/10.1016/j.ibmb.2011.02.006
Broderick NA, Raffa KF, Goodman RM, Handelsman J (2004) Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl Environ Microbiol 70(1):293–300
Buchner P (1965) Endosymbiosis of animals with plant microorganims
Bulgarelli D, Schlaeppi K, Spaepen S, van Themaat EVL, Schulze-Lefert P (2013) Structure and functions of the bacterial microbiota of plants. Annu Rev Plant Biol 64:807–838
Butko P (2003) Cytolytic toxin Cyt1A and its mechanism of membrane damage: data and hypotheses. Appl Environ Microbiol 69(5):2415–2422. https://doi.org/10.1128/AEM.69.5.2415-2422
Calderón-Cortés N, Quesada M, Watanabe H, Cano-Camacho H, Oyama K (2012) Endogenous plant cell wall digestion: a key mechanism in insect evolution. Annu Rev Ecol Evol Syst 43:45–71
Cao Q, Wickham JD, Chen L, Ahmad F, Lu M, Sun J (2018) Effect of oxygen on Verbenone conversion from cis-Verbenol by gut facultative anaerobes of Dendroctonus valens. Front Microbiol 9:464
Capuzzo C, Firrao G, Mazzon L, Squartini A, Girolami V (2005) ‘Candidatus Erwinia dacicola’, a coevolved symbiotic bacterium of the olive fly Bactrocera oleae (Gmelin). Int J Syst Evol Microbiol 55(4):1641–1647
Caragata EP, Rancès E, Hedges LM, Gofton AW, Johnson KN, O’Neill SL, McGraw EA (2013) Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog 9(6):e1003459
Ceja-Navarro JA, Vega FE, Karaoz U, Hao Z, Jenkins S, Lim HC et al (2015) Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat Commun 6:7618
Chakroun M, Banyuls N, Bel Y, Escriche B, Ferré J (2016) Bacterial vegetative insecticidal proteins (Vip) from entomopathogenic bacteria. Microbiol Mol Biol Rev 80(2):329–350
Chung SH, Rosa C, Scully ED, Peiffer M, Tooker JF, Hoover K et al (2013) Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc Nat Acad Sci:201308867
Conrath U, Thulke O, Katz V, Schwindling S, Kohler A (2001) Priming as a mechanism in induced systemic resistance of plants. Eur J Plant Pathol 107(1):113–119
Contreras-Cornejo HA, Macías-Rodríguez L, Cortés-Penagos C, López-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol 149(3):1579–1592
Davis TS, Crippen TL, Hofstetter RW, Tomberlin JK (2013) Microbial volatile emissions as insect semiochemicals. J Chem Ecol 39(7):840–859
De Fine Licht HH, Schiøtt M, Rogowska-Wrzesinska A, Nygaard S, Roepstorff P, Boomsma JJ (2013) Laccase detoxification mediates the nutritional alliance between leaf-cutting ants and fungus-garden symbionts. Proc Natl Acad Sci U S A 110(2):583–587. https://doi.org/10.1073/pnas.1212709110
de Maagd RA, Bravo A, Berry C, Crickmore N, Schnepf HE (2003) Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu Rev Genet 37(1):409–433
Dearing MD, Foley WJ, McLean S (2005) The influence of plant secondary metabolites on the nutritional ecology of herbivorous terrestrial vertebrates. Annu Rev Ecol Evol Syst 36:169–189
Desclos-Theveniau M, Arnaud D, Huang T-Y, Lin GJ-C, Chen W-Y, Lin Y-C, Zimmerli L (2012) The Arabidopsis lectin receptor kinase LecRK-V. 5 represses stomatal immunity induced by Pseudomonas syringae pv. tomato DC3000. PLoS Pathogens 8(2):e1002513
DeVries PJ (1987) The butterflies of Costa Rica and their natural history
Dillon RJ, Vennard CT, Charnley AK (2000) Pheromones: exploitation of gut bacteria in the locust. Nature 403(6772):851
Dillon R, Vennard C, Charnley A (2002) A note: gut bacteria produce components of a locust cohesion pheromone. J Appl Microbiol 92(4):759–763
Dong Y, Manfredini F, Dimopoulos G (2009) Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog 5(5):e1000423. https://doi.org/10.1371/journal.ppat.1000423
Douglas AE (2013) Microbial brokers of insect-plant interactions revisited. J Chem Ecol 39(7):952–961
Douglas AE (2015) Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol 60:17–34
Douglas AE (2018) Omics and the metabolic function of insect-microbial symbioses. Curr Opin Insect Sci
Douglas AE, Minto LB, Wilkinson TL (2001) Quantifying nutrient production by the microbial symbionts in an aphid. J Exp Biol 204(2):349
Dowd PF, Shen SK (2011) The contribution of symbiotic yeast to toxin resistance of the cigarette beetle (Lasioderma serricorne). Entomol Exp Appl 56(3):241–248
Du M, Zhai Q, Deng L, Li S, Li H, Yan L et al (2014) Closely related NAC transcription factors of tomato differentially regulate stomatal closure and reopening during pathogen attack. The Plant Cell 114:128272
Egamberdieva D, Kucharova Z, Davranov K, Berg G, Makarova N, Azarova T et al (2011) Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol Fertil Soils 47(2):197–205
Egamberdieva D, Wirth S, Jabborova D, Räsänen LA, Liao H (2017) Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J Plant Interact 12(1):100–107
Engel P, Moran NA (2013) The gut microbiota of insects–diversity in structure and function. FEMS Microbiol Rev 37(5):699–735
Engelstädter J, Hurst GD (2009) The ecology and evolution of microbes that manipulate host reproduction. Annu Rev Ecol Evol Syst 40:127–149
Engl T, Kaltenpoth M (2018) Influence of microbial symbionts on insect pheromones. Nat Prod Rep 35(5):386–397
Engl T, Eberl N, Gorse C, Krüger T, Schmidt TH, Plarre R et al (2018) Ancient symbiosis confers desiccation resistance to stored grain pest beetles. Mol Ecol 27(8):2095–2108
Estruch JJ, Warren GW, Mullins MA, Nye GJ, Craig JA, Koziel MG (1996) Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc Natl Acad Sci 93(11):5389–5394
Fang W, Vega-Rodríguez J, Ghosh AK, Jacobs-Lorena M, Kang A, Leger RJS (2011) Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science 331(6020):1074–1077
Farag MA, Zhang H, Ryu C-M (2013) Dynamic chemical communication between plants and Bacteria through airborne signals: induced resistance by bacterial volatiles. J Chem Ecol 39(7):1007–1018. https://doi.org/10.1007/s10886-013-0317-9
Feichtmayer J, Deng L, Griebler C (2017) Antagonistic microbial interactions: contributions and potential applications for controlling pathogens in the aquatic systems. Front Microbiol 8:2192. https://doi.org/10.3389/fmicb.2017.02192
Ferguson NM, Kien DTH, Clapham H, Aguas R, Trung VT, Chau TNB, McGraw EA (2015) Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Trans Med 7(279):279ra237–279ra237
Ferrari J, Vavre F (2011) Bacterial symbionts in insects or the story of communities affecting communities. Philos Trans R Soc London B: Biol Sci 366(1569):1389–1400
Fincheira P, Quiroz A (2018) Microbial volatiles as plant growth inducers. Microbiol Res
Fiorilli V, Vannini C, Ortolani F, Garcia-Seco D, Chiapello M, Novero M, Bagnaresi P (2018) Omics approaches revealed how arbuscular mycorrhizal symbiosis enhances yield and resistance to leaf pathogen in wheat. Sci Rep 8(1):9625
Fraser JE, De Bruyne JT, Iturbe-Ormaetxe I, Stepnell J, Burns RL, Flores HA, O’Neill SL (2017) Novel Wolbachia-transinfected Aedes aegypti mosquitoes possess diverse fitness and vector competence phenotypes. PLoS Pathog 13(12):e1006751
Fürstenberg-Hägg J, Zagrobelny M, Bak S (2013) Plant defense against insect herbivores. Int J Mol Sci 14(5):10242–10297
Gao C (2018) The future of CRISPR technologies in agriculture. Nat Rev Mol Cell Biol 19:275. https://doi.org/10.1038/nrm.2018.2
García-Fraile P (2018) Roles of bacteria in the bark beetle holobiont–how do they shape this forest pest? Ann Appl Biol 172(2):111–125
García-Garrido JM, Ocampo JA (2002) Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. J Exp Bot 53(373):1377–1386
Gianinazzi S, Gollotte A, Binet M-N, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20(8):519–530
Giron D, Glevarec G (2014) Cytokinin-induced phenotypes in plant-insect interactions: learning from the bacterial world. J Chem Ecol 40(7):826–835
Glare T, Caradus J, Gelernter W, Jackson T, Keyhani N, Köhl J et al (2012) Have biopesticides come of age? Trends Biotechnol 30(5):250–258. https://doi.org/10.1016/j.tibtech.2012.01.003
Gorter JA, Jagadeesh S, Gahr C, Boonekamp JJ, Levine JD, Billeter J-C (2016) The nutritional and hedonic value of food modulate sexual receptivity in Drosophila melanogaster females. Sci Rep 6:19441. https://doi.org/10.1038/srep19441
Gutjahr C, Parniske M (2013) Cell and developmental biology of arbuscular mycorrhiza symbiosis. Annu Rev Cell Dev Biol 29:593–617
Haas D, Keel C (2003) Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu Rev Phytopathol 41(1):117–153
Hammer EC, Balogh-Brunstad Z, Jakobsen I, Olsson PA, Stipp SL, Rillig MC (2014) A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol Biochem 77:252–260
Han SH, Lee SJ, Moon JH, Park KH, Yang KY, Cho BH et al (2006) GacS-dependent production of 2R, 3R-butanediol by Pseudomonas chlororaphis O6 is a major determinant for eliciting systemic resistance against Erwinia carotovora but not against Pseudomonas syringae pv. tabaci in tobacco. Mol Plant-Microbe Interact 19(8):924–930
Haskett TL, Terpolilli JJ, Bekuma A, O’Hara GW, Sullivan JT, Wang P et al (2016) Assembly and transfer of tripartite integrative and conjugative genetic elements. Proc Natl Acad Sci 113(43):12268–12273
Herman M, Nault B, Smart C (2008) Effects of plant growth-promoting rhizobacteria on bell pepper production and green peach aphid infestations in New York. Crop Prot 27(6):996–1002
Hieno A, Naznin HA, Hyakumachi M, Higuchi-Takeuchi M, Matsui M, Yamamoto YY (2016) Possible involvement of MYB44-mediated stomatal regulation in systemic resistance induced by Penicillium simplicissimum GP17-2 in Arabidopsis. Microbes Environ 31(2):154–159
Himler AG, Adachi-Hagimori T, Bergen JE, Kozuch A, Kelly SE, Tabashnik BE et al (2011) Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science 332(6026):254–256
Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T (2007) Obligate symbiont involved in pest status of host insect. Proc R Soc Lond B Biol Sci 274(1621):1979–1984
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50(1):371–393. https://doi.org/10.1146/annurev.ento.50.071803.130359
Howe M, Keefover-Ring K, Raffa KF (2018) Pine engravers carry bacterial communities whose members reduce concentrations of host monoterpenes with variable degrees of redundancy, specificity, and capability. Environ Entomol 47(3):638–645
Hughes DP, Brodeur J, Thomas F (2012) Host manipulation by parasites. Oxford University Press, Oxford
Hulcr J, Pollet M, Ubik K, Vrkoc J (2005) Exploitation of kairomones and synomones by Medetera spp.(Diptera: Dolichopodidae), predators of spruce bark beetles. Eur J Entomol 102(4):655
Hussain M, Frentiu FD, Moreira LA, O’Neill SL, Asgari S (2011) Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc Natl Acad Sci 108(22):9250–9255
Husseneder C, Donaldson JR, Foil LD (2016) Genetically engineered yeast expressing a lytic peptide from Bee Venom (Melittin) kills symbiotic protozoa in the gut of Formosan subterranean termites. PLoS One 11(3):e0151675
Itoh H, Tago K, Hayatsu M, Kikuchi Y (2018) Detoxifying symbiosis: microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat Prod Rep 35(5):434–454
Jaber LR, Enkerli J (2016) Effect of seed treatment duration on growth and colonization of Vicia faba by endophytic Beauveria bassiana and Metarhizium brunneum. Biol Control 103:187–195
Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ (2010) Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329(5988):212–215
Janson EM, Stireman JO III, Singer MS, Abbot P (2008) Phytophagous insect–microbe mutualisms and adaptive evolutionary diversification. Evol Int J Org Evol 62(5):997–1012
Johnson K (2015a) The impact of Wolbachia on virus infection in mosquitoes. Viruses 7(11):5705–5717
Johnson KN (2015b) Bacteria and antiviral immunity in insects. Curr Opin Insect Sci 8:97–103
Jones JD, Dangl JL (2006) The plant immune system. Nature 444(7117):323
Joshi D, Pan X, McFadden MJ, Bevins D, Liang X, Lu P et al (2017) The maternally inheritable Wolbachia wAlbB induces refractoriness to Plasmodium berghei in Anopheles stephensi. Front Microbiol 8:366
Jurat-Fuentes JL, Crickmore N (2017) Specificity determinants for cry insecticidal proteins: insights from their mode of action. J Invertebr Pathol 142:5–10
Kai M, Haustein M, Molina F, Petri A, Scholz B, Piechulla B (2009) Bacterial volatiles and their action potential. Appl Microbiol Biotechnol 81(6):1001–1012
Kanchiswamy CN, Malnoy M, Maffei ME (2015a) Bioprospecting bacterial and fungal volatiles for sustainable agriculture. Trends Plant Sci 20(4):206–211
Kanchiswamy CN, Malnoy M, Maffei ME (2015b) Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front Plant Sci 6:151
Kempel A, Brandl R, Schädler M (2009) Symbiotic soil microorganisms as players in aboveground plant–herbivore interactions–the role of rhizobia. Oikos 118(4):634–640
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53(1):299–328
Kikuchi Y, Meng X-Y, Fukatsu T (2005) Gut symbiotic bacteria of the genus Burkholderia in the broad-headed bugs Riptortus clavatus and Leptocorisa chinensis (Heteroptera: Alydidae). Appl Environ Microbiol 71(7):4035–4043
Kikuchi Y, Hosokawa T, Fukatsu T (2007) Insect-microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl Environ Microbiol 73(13):4308–4316
Koornneef A, Pieterse CM (2008) Cross talk in defense signaling. Plant Physiol 146(3):839–844
Kos M, Tuijl MA, de Roo J, Mulder PP, Bezemer TM (2015) Species-specific plant–soil feedback effects on above-ground plant–insect interactions. J Ecol 103(4):904–914
Kula AA, Hartnett DC, Wilson GW (2005) Effects of mycorrhizal symbiosis on tallgrass prairie plant–herbivore interactions. Ecol Lett 8(1):61–69
Kumar AS, Lakshmanan V, Caplan JL, Powell D, Czymmek KJ, Levia DF, Bais HP (2012) Rhizobacteria Bacillus subtilis restricts foliar pathogen entry through stomata. Plant J 72(4):694–706
Kwon YS, Ryu C-M, Lee S, Park HB, Han KS, Lee JH et al (2010) Proteome analysis of Arabidopsis seedlings exposed to bacterial volatiles. Planta 232(6):1355–1370
Lacey LA, Thomson D, Vincent C, Arthurs SP (2008) Codling moth granulovirus: a comprehensive review. Biocontrol Sci Tech 18(7):639–663. https://doi.org/10.1080/09583150802267046
Lacey L, Grzywacz D, Shapiro-Ilan D, Frutos R, Brownbridge M, Goettel M (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41
Lauzon C, Sjogren R, Wright S, Prokopy R (1998) Attraction of Rhagoletis pomonella (Diptera: Tephritidae) flies to odor of bacteria: apparent confinement to specialized members of Enterobacteriaceae. Environ Entomol 27(4):853–857
Leonard SP, Perutka J, Powell JE, Geng P, Richhart DD, Byrom M, Moran NA (2018) Genetic engineering of bee gut microbiome bacteria with a toolkit for modular assembly of broad-host-range plasmids. ACS Synth Biol 7(5):1279–1290
Li Q, Wu Y-J (2009) A fluorescent, genetically engineered microorganism that degrades organophosphates and commits suicide when required. Appl Microbiol Biotechnol 82(4):749–756
Ling J, Wang H, Wu P, Li T, Tang Y, Naseer N et al (2016) Plant nodulation inducers enhance horizontal gene transfer of Azorhizobium caulinodans symbiosis island. Proc Natl Acad Sci 113(48):13875–13880
Liu F, Xing S, Ma H, Du Z, Ma B (2013) Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl Microbiol Biotechnol 97(20):9155–9164
Loper JE, Gross H (2007) Genomic analysis of antifungal metabolite production by Pseudomonas fluorescens Pf-5. In: New perspectives and approaches in plant growth-promoting rhizobacteria research. Springer, Dordrecht, pp 265–278
Marshall D, Jackson T, Unelius CR, Wee S, Young S, Townsend R, Suckling D (2016) Morganella morganii bacteria produces phenol as the sex pheromone of the New Zealand grass grub from tyrosine in the colleterial gland. Sci Nat 103(7-8):59
Martinez J, Ok S, Smith S, Snoeck K, Day JP, Jiggins FM (2015) Should symbionts be nice or selfish? Antiviral effects of Wolbachia are costly but reproductive parasitism is not. PLoS Pathog 11(7):e1005021
Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126(5):969–980
Miller WJ, Schneider D (2012) Endosymbiotic microbes as adaptive manipulators of arthropod behavior and natural driving sources of host speciation. Host Manipulation by Parasites, 119–137
Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, Werck-Reichhart D, Ausubel FM (2010) Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 22(3):973–990
Monnerat RG, Soares CM, Capdeville G, Jones G, Martins ÉS, Praça L et al (2009) Translocation and insecticidal activity of Bacillus thuringiensis living inside of plants. Microb Biotechnol 2(4):512–520
Montillet J-L, Leonhardt N, Mondy S, Tranchimand S, Rumeau D, Boudsocq M et al (2013) An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol 11(3):e1001513
Münch A, Stingl L, Jung K, Heermann R (2008) Photorhabdus luminescens genes induced upon insect infection. BMC Genomics 9(1):229
Muvea AM, Meyhöfer R, Subramanian S, Poehling H-M, Ekesi S, Maniania NK (2014) Colonization of onions by endophytic fungi and their impacts on the biology of Thrips tabaci. PLoS One 9(9):e108242
Najarro MA, Sumethasorn M, Lamoureux A, Turner TL (2015) Choosing mates based on the diet of your ancestors: replication of non-genetic assortative mating in Drosophila melanogaster. Peer J 3:e1173
Nikolouli K, Colinet H, Renault D, Enriquez T, Mouton L, Gibert P et al (2018) Sterile insect technique and Wolbachia symbiosis as potential tools for the control of the invasive species Drosophila suzukii. J Pest Sci:1–15
Nomura K, Melotto M, He S-Y (2005) Suppression of host defense in compatible plant–Pseudomonas syringae interactions. Curr Opin Plant Biol 8(4):361–368
Oerke E-C, Dehne H-W (2004) Safeguarding production—losses in major crops and the role of crop protection. Crop Prot 23(4):275–285
Okubo T, Piromyou P, Tittabutr P, Teaumroong N, Minamisawa K (2016) Origin and evolution of nitrogen fixation genes on symbiosis islands and plasmid in Bradyrhizobium. Microbes Environ 31(3):260–267
Oliver KM, Degnan PH, Burke GR, Moran NA (2010) Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55:247–266
Palma L, Muñoz D, Berry C, Murillo J, Caballero P (2014) Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins 6(12):3296–3325
Pan X, Zhou G, Wu J, Bian G, Lu P, Raikhel AS, Xi Z (2012) Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci 109(1):E23–E31
Papadopoulou GV, van Dam NM (2017) Mechanisms and ecological implications of plant-mediated interactions between belowground and aboveground insect herbivores. Ecol Res 32(1):13–26
Patot S, Allemand R, Fleury F, Varaldi J (2012) An inherited virus influences the coexistence of parasitoid species through behaviour manipulation. Ecol Lett 15(6):603–610
Peterson BF, Scharf ME (2016) Lower termite associations with microbes: synergy, protection, and interplay. Front Microbiol 7:422
Pieterse CM, Dicke M (2007) Plant interactions with microbes and insects: from molecular mechanisms to ecology. Trends Plant Sci 12(12):564–569
Pigott CR, Ellar DJ (2007) Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol Mol Biol Rev 71(2):255–281
Pilon F, Visôtto L, Guedes R, Oliveira M (2013) Proteolytic activity of gut bacteria isolated from the velvet bean caterpillar Anticarsia gemmatalis. J Comp Physiol B 183(6):735–747
Pineda A, Zheng S-J, Van Loon JJ, Pieterse CM, Dicke M (2010) Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends Plant Sci 15(9):507–514
Pineda A, Soler R, Pozo MJ, Rasmann S, Turlings TC (2015) Above-belowground interactions involving plants, microbes and insects. Front Plant Sci 6:318
Pineda A, Kaplan I, Bezemer TM (2017) Steering soil microbiomes to suppress aboveground insect pests. Trends Plant Sci 22(9):770–778
Portugal L, Muñóz-Garay C, de Castro DLM, Soberón M, Bravo A (2017) Toxicity of Cry1A toxins from Bacillus thuringiensis to CF1 cells does not involve activation of adenylate cyclase/PKA signaling pathway. Insect Biochem Mol Biol 80:21–31
Pozo MJ, Azcón-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10(4):393–398
Ramsay J, Ronson C (2015) Genetic regulation of symbiosis island transfer in Mesorhizobium loti. In Biological Nitrogen Fixation
Regnier FE, Law JH (1968) Insect pheromones. J Lipid Res 9(5):541–551
Renwick J, Hughes P, Krull I (1976) Selective production of cis-and trans-verbenol from (−)-and (+)-alpha by a bark beetle. Science 191(4223):199–201
Richardson AE, Barea J-M, McNeill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321(1-2):305–339
Robert-Seilaniantz A, Navarro L, Bari R, Jones JD (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10(4):372–379
Rohfritsch O (2008) Plants, gall midges, and fungi: a three-component system. Entomol Exp Appl 128(1):208–216
Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, Hoffmann AA (2017) Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog 13(1):e1006006
Ryu C-M, Farag MA, Hu C-H, Reddy MS, Wei H-X, Paré PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci 100(8):4927–4932
Salem H, Florez L, Gerardo N, Kaltenpoth M (2015) An out-of-body experience: the extracellular dimension for the transmission of mutualistic bacteria in insects. Proc R Soc B 282(1804):20142957
Schouteden N, De Waele D, Panis B, Vos CM (2015) Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: a review of the mechanisms involved. Front Microbiol 6:1280
Schulz-Bohm K, Martín-Sánchez L, Garbeva P (2017) Microbial volatiles: small molecules with an important role in intra- and inter-kingdom interactions. Front Microbiol 8(2484). https://doi.org/10.3389/fmicb.2017.02484
Segarra G, Van der Ent S, Trillas I, Pieterse C (2009) MYB72, a node of convergence in induced systemic resistance triggered by a fungal and a bacterial beneficial microbe. Plant Biol 11(1):90–96
Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E (2010) Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci U S A 107(46):20051–20056. https://doi.org/10.1073/pnas.1009906107
Shikano I, Rosa C, Tan C-W, Felton GW (2017) Tritrophic interactions: microbe-mediated plant effects on insect herbivores. Annu Rev Phytopathol 55:313–331
Simon J-C, Boutin S, Tsuchida T, Koga R, Le Gallic J-F, Frantz A et al (2011) Facultative symbiont infections affect aphid reproduction. PLoS One 6(7):e21831
Singh RP, Jha PN (2016) A halotolerant bacterium Bacillus licheniformis HSW-16 augments induced systemic tolerance to salt stress in wheat plant (Triticum aestivum). Front Plant Sci 7:1890
Singh LP, Gill SS, Tuteja N (2011) Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal Behav 6(2):175–191
Song Y, Chen D, Lu K, Sun Z, Zeng R (2015) Enhanced tomato disease resistance primed by arbuscular mycorrhizal fungus. Front Plant Sci 6:786
Song F, Lin Y, Chen C, Shao E, Guan X, Huang Z (2016) Insecticidal activity and histopathological effects of Vip3Aa protein from Bacillus thuringiensis on Spodoptera litura. J Microbiol Biotechnol
Stein E, Molitor A, Kogel K-H, Waller F (2008) Systemic resistance in Arabidopsis conferred by the mycorrhizal fungus Piriformospora indica requires jasmonic acid signaling and the cytoplasmic function of NPR1. Plant Cell Physiol 49(11):1747–1751
Stenberg JA (2017) A conceptual framework for integrated pest management. Trends Plant Sci 22(9):759–769
Stenersen J (2004) Chemical pesticides mode of action and toxicology. CRC Press, Boca Raton
Stout MJ, Thaler JS, Thomma BP (2006) Plant-mediated interactions between pathogenic microorganisms and herbivorous arthropods. Annu Rev Entomol 51:663–689
Sugio A, Dubreuil G, Giron D, Simon J-C (2014) Plant–insect interactions under bacterial influence: ecological implications and underlying mechanisms. J Exp Bot 66(2):467–478
Teixeira L, Ferreira Á, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6(12):e1000002
Terradas G, McGraw EA (2017) Wolbachia-mediated virus blocking in the mosquito vector Aedes aegypti. Curr Opin Insect Sci 22:37–44
Tétard-Jones C, Kertesz MA, Gallois P, Preziosi RF (2007) Genotype-by-genotype interactions modified by a third species in a plant-insect system. Am Nat 170(3):492–499
Tétard-Jones C, Kertesz MA, Preziosi RF (2012) Identification of plant quantitative trait loci modulating a rhizobacteria-aphid indirect effect. PLoS One 7(7):e41524
Thomma BP, Penninckx IA, Cammue BP, Broekaert WF (2001) The complexity of disease signaling in Arabidopsis. Curr Opin Immunol 13(1):63–68
Trillas M, Segarra G (2009) Interactions between nonpathogenic fungi and plants. Adv Bot Res 51:321–359
Usta C (2013) Microorganisms in biological pest control—a review (bacterial toxin application and effect of environmental factors). In Current progress in biological research: Intech
Van der Ent S, Van Wees SC, Pieterse CM (2009) Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 70(13–14):1581–1588
Van Loon L (2007) Plant responses to plant growth-promoting rhizobacteria. In: New perspectives and approaches in plant growth-promoting rhizobacteria research. Springer, Dordrecht, pp 243–254
van Loon LC, Rep M, Pieterse CM (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44:135–162
Van Wees SC, Van der Ent S, Pieterse CM (2008) Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol 11(4):443–448
Vité J, Pitman G (1968) Bark beetle aggregation: effects of feeding on the release of pheromones in Dendroctonus and Ips. Nature 218(5137):169
Walters D, Newton A, Lyon G (2007) Induced resistance for plant defence. Wiley Online Library
Wang D, Huang Z, He H, Wei C (2018) Comparative analysis of microbial communities associated with bacteriomes, reproductive organs and eggs of the cicada Subpsaltria yangi. Arch Microbiol 200(2):227–235
Weeks AR, Turelli M, Harcombe WR, Reynolds KT, Hoffmann AA (2007) From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol 5(5):e114
Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6(10):741
Whalon ME, Mota-Sanchez D, Hollingworth RM (2008) Global pesticide resistance in arthropods. Cabi
Whitten MM, Facey PD, Del Sol R, Fernández-Martínez LT, Evans MC, Mitchell JJ et al (2016) Symbiont-mediated RNA interference in insects. Proc R Soc B 283(1825):20160042
Wu J, Baldwin IT (2010) New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 44:1–24
Yixin HY, Seleznev A, Flores HA, Woolfit M, McGraw EA (2017) Gut microbiota in Drosophila melanogaster interacts with Wolbachia but does not contribute to Wolbachia-mediated antiviral protection. J Invertebr Pathol 143:18–25
Zack MD, Sopko MS, Frey ML, Wang X, Tan SY, Arruda JM et al (2017) Functional characterization of Vip3Ab1 and Vip3Bc1: two novel insecticidal proteins with differential activity against lepidopteran pests. Sci Rep 7(1):11112
Zamioudis C, Pieterse CM (2012) Modulation of host immunity by beneficial microbes. Mol Plant-Microbe Interact 25(2):139–150
Zarate SI, Kempema LA, Walling LL (2007) Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol 143(2):866–875
Zhang X, Candas M, Griko NB, Taussig R, Bulla LA (2006) A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc Natl Acad Sci 103(26):9897–9902
Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S, Paré PW (2008a) Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J 56(2):264–273
Zhang H, Kim M-S, Sun Y, Dowd SE, Shi H, Paré PW (2008b) Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol Plant-Microbe Interact 21(6):737–744
Zhang J, Khan SA, Heckel DG, Bock R (2017) Next-generation insect-resistant plants: RNAi-mediated crop protection. Trends Biotechnol 35(9):871–882. https://doi.org/10.1016/j.tibtech.2017.04.009
Zheng X-Y, Spivey NW, Zeng W, Liu P-P, Fu ZQ, Klessig DF et al (2012) Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe 11(6):587–596
Zhu F, Poelman EH, Dicke M (2014) Insect herbivore-associated organisms affect plant responses to herbivory. New Phytol 204(2):315–321
Acknowledgments
AC would like to acknowledge “EVA4.0”, No. CZ.02.1.01 /0.0 /0.0 /16_ 019/0000803 financed by OP RDE” and IGA, Faculty of Forestry and Wood Sciences, Czech University of Life Sciences, Prague for the financial support during the preparation of the book chapter. AR is supported by the EU project “EXTEMIT - K,” No. CZ.02.1.01/0.0/0.0/15_003/0000433 financed by OP RDE and IGA, Faculty of Forestry and Wood Sciences, Czech University of Life Sciences, Prague.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Chakraborty, A., Roy, A. (2021). Microbial Influence on Plant–Insect Interaction. In: Singh, I.K., Singh, A. (eds) Plant-Pest Interactions: From Molecular Mechanisms to Chemical Ecology. Springer, Singapore. https://doi.org/10.1007/978-981-15-2467-7_14
Download citation
DOI: https://doi.org/10.1007/978-981-15-2467-7_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-2466-0
Online ISBN: 978-981-15-2467-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)