Abstract
Colorado potato beetle (CPB) is a leading pest of solanaceous plants. Despite the economic importance of this pest, surprisingly few studies have been carried out to characterize its molecular interaction with the potato plant. In particular, little is known about the effect of CPB elicitors on gene expression associated with the plant’s defense response. In order to discover putative CPB elicitor-responsive genes, the TIGR 11,421 EST Solanaceae microarray was used to identify genes that are differentially expressed in response to the addition of CPB regurgitant to wounded potato leaves. By applying a cutoff corresponding to an adjusted P-value of <0.01 and a fold change of >1.5 or <0.67, we found that 73 of these genes are induced by regurgitant treatment of wounded leaves when compared to wounding alone, whereas 54 genes are repressed by this treatment. This gene set likely includes regurgitant-responsive genes as well as wounding-responsive genes whose expression patterns are further enhanced by the presence of regurgitant. Real-time polymerase chain reaction was used to validate differential expression by regurgitant treatment for five of these genes. In general, genes that encoded proteins involved in secondary metabolism and stress were induced by regurgitant; genes associated with photosynthesis were repressed. One induced gene that encodes aromatic amino acid decarboxylase is responsible for synthesis of the precursor of 2-phenylethanol. This is significant because 2-phenylethanol is recognized by the CPB predator Perillus bioculatis. In addition, three of the 16 type 1 and type 2 proteinase inhibitor clones present on the potato microarray were repressed by application of CPB regurgitant to wounded leaves. Given that proteinase inhibitors are known to interfere with digestion of proteins in the insect midgut, repression of these proteinase inhibitors by CPB may inhibit this component of the plant’s defense arsenal. These data suggest that beyond the wound response, CPB elicitors play a role in mediating the plant/insect interaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interaction of an herbivorous insect with a plant involves a mechanical wound as well as the presence of insect elicitors. In recent years, responses of plants to insect infestation have been profiled in a number of species by using molecular techniques. In particular, identification of plant genes differentially expressed in response to herbivory has been undertaken in Nicotiana, Arabidopsis, Populus, and Picea (Reymond et al. 2000, 2004; Hermsmeier et al. 2001; Schittko et al. 2001; De Vos et al. 2005; Ralph et al. 2006a, b). These studies and others have demonstrated that insect infestation tends to induce expression of genes that are important in direct defenses like proteinase inhibitors, polyphenol oxidases, and lignins, as well as genes involved in indirect defenses such as generation of volatile compounds that are recognized by predatory and parasitic natural enemies of the infesting insects. In contrast, genes responsible for photosynthesis and general metabolism are usually repressed by insect infestation. This compromises plant growth and productivity.
One factor that appears to mediate interactions between an herbivorous insect and its plant host is the composition of the regurgitant or saliva produced by the insect. Specific elicitors isolated from the regurgitant of pest insects applied to mechanically wounded leaves often results in a plant response that more closely mimics the response to insect feeding. A variety of different elicitors have been characterized to date. For example, glucose oxidase from the salivary glands of Helicoverpa zea (Boddie) altered defense responses when applied to wounded leaves of tobacco (Eichenseer et al. 1999; Musser et al. 2002). Recently, a small peptide, inceptin, resulting from the proteolysis of the plant-derived enzyme cATP synthase, was identified as an elicitor from the caterpillar Spodoptera frugiperda (Schmelz et al. 2006). Elicitors can alter the composition of volatile defensive blends that are generated by the plant during the defense response, illustrating that the plant’s response to insect herbivory at the molecular level is different from that to wounding alone. For instance, application of a β-glucosidase isolated from Pieris brassicae (L.) regurgitant to a wounded leaf of cabbage resulted in the generation of a volatile blend that is also produced by herbivory (Mattiacci et al. 1995).
Not all elicitors are peptides. Fatty acid–amino acid conjugates (FACs) are perhaps the best characterized elicitors of volatile production (e.g., Pohnert et al. 1999). Application of volicitin, a FAC component of S. exigua (Hübner) saliva, to a wounded leaf elicited volatiles similar to those produced by insect infestation rather than those resulting from mechanical injury alone (Alborn et al. 1997). Work from the Baldwin group (Halitschke et al. 2001, 2003; Schittko et al. 2001; Roda et al. 2004) has examined the effect of regurgitant from Manduca sexta (L.) on Nicotiana attenuata and has isolated FACs that mimic the effect of the regurgitant. Roda et al. (2004) found that regurgitant treatment led to differential expression of 138 genes, 63 of which were upregulated and 75 that were downregulated. FACs in the regurgitant were responsible for 53 upregulated and 56 downregulated genes. In a comparison of wounded poplar leaves versus application of regurgitant from forest tent caterpillar, Malacosoma disstria Hübner, to negligibly wounded leaves, Major and Constabel (2006) found that 38 genes were induced by both treatments, and these genes tended to be involved in stress and secondary metabolism. In contrast to the study in tobacco, only two genes were unique to regurgitant treatment, suggesting that regurgitant in this case only augmented the wound response. Clearly, a number of insect elicitors may affect the plant response to insect attack. However, it is less clear to what extent these observations of specific plant–insect interactions can be extended to interactions between other species.
Colorado potato beetle (CPB), Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae), is a serious pest of potato, Solanum tuberosum, and other solanaceous crops. This herbivorous insect results in hundreds of millions of dollars of crop losses annually. Despite the economic impact of CPB on solanaceous crops, little is known about the components of CPB regurgitant, or how CPB regurgitant affects the defense response of potato at the molecular level. We have recently begun to characterize CPB regurgitant produced from insects reared on tomato and have concluded that it contains a proteinaceous component that inhibits genes that encode proteinase inhibitors in tomato (Lawrence et al. 2007). Kruzmane et al. (2002) have shown that CPB regurgitant enhances peroxidase and polyphenol oxidase activity in potato; these studies also demonstrated that ethylene is increased in regurgitant-treated wounded leaves. It has been shown that potato plants produce a suite of volatiles—including 2-phenylethanol—upon attack by CPB that attract the CPB predator Perillus bioculatus (F.) (Heteroptera: Pentatomidae; Schütz et al. 1997; Weissbecker et al. 1999). However, a molecular examination of the specific CPB/potato interaction by examining the effect of regurgitant treatment has not been undertaken.
As part of our ongoing studies to characterize CPB–potato interactions at the molecular level, we have used an 11,421 EST Solanaceae microarray to identify additional genes in potato whose expression is altered by putative elicitors present in the regurgitant of CPB raised on potato. Experiments that compared gene expression profiles of mechanically wounded leaves to which CPB regurgitant was applied versus mechanically wounded leaves without regurgitant revealed that the plant’s molecular response to wounding plus CPB regurgitant is not the same as that induced by wounding alone. This set of regurgitant-responsive genes likely comprises both genes that are responsive to regurgitant but not wounding, as well as those genes that are responsive to wounding, but for which this response is augmented by the presence of regurgitant. The addition of CPB regurgitant alters the expression of a suite of defense-associated genes compared to wounding alone, including a gene putatively involved in the synthesis of 2-phenylethanol. Our study provides evidence that CPB modulates the potato’s defense response against this devastating herbivore potentially via elicitors present in its regurgitant.
Methods and Materials
Plant Material
Potato tubers from S. tuberosum var. Kennebec were planted in individual 10-cm pots containing Metromix® (Scotts, Marysville, OH, USA) and Osmocote® (Scotts). Plants were grown for 4 weeks in a greenhouse, and only plants with at least eight leaves were used in the tests. For real-time polymerase chain reaction (PCR) experiments, plants were grown for 4 weeks in a growth chamber with a 16:8 (light to dark, L to D) cycle at 25°C during the light phase and 20°C during the dark phase.
CPB Rearing and Regurgitant Isolation
CPB came from a colony at the USDA—ARS Insect Biocontrol Laboratory that originated from eggs provided by the New Jersey Department of Agriculture in 1996. Field-collected insects from potato fields at the USDA—ARS Beltsville Agricultural Research Center (Beltsville, MD, USA) were introduced annually to maintain genetic diversity. CPB were reared on S. tuberosum var. Kennebec and maintained in a laboratory under a 16:8 (L to D) cycle at approximately 25°C. Regurgitant was collected from the oral cavity of fourth instar CPB with a P200 Pipetteman (Gilson, Oakland, CA, USA). The regurgitant was centrifuged for 5 min at 10,000×g and the supernatant was collected and stored at −80°C.
Wounding, Regurgitant Treatment, and Infestation
Two separate series of experiments were carried out—one for microarray analyses and a second for real-time PCR analyses. Mechanical wounding involved crushing the leaf from the sixth node from the bottom with pliers, while avoiding the major veins, at 1-cm intervals. One hundred microliter of a 1:3 aqueous dilution of the regurgitant or 100 µl of water (control) were added to wounded leaves. Plants were incubated for 4 h after which the wounded leaves were excised and frozen rapidly in liquid nitrogen. For microarray experiments, five plants were pooled for each sample, and the experiments were repeated three times, i.e., there were three biological replicates.
In the second series of experiments for real-time PCR analysis, five treatments were carried out: control (untreated) plants, water-treated wounded plants, regurgitant-treated wounded plants, mildly CPB-infested plants, and acutely CPB-infested plants. Water- and regurgitant-treated wounded plants were treated as described above. For infestation treatments, plants were divided into two groups; the sixth leaf from the bottom was covered with a fine mesh sleeve, and ten third- to fourth-instar CPB larvae were added. The first infestation level was achieved by leaving the ten CPB on the plant for 1 h and then removing them. The plants were harvested 3 h later. The second infestation level was achieved by leaving the ten CPB on the plant for 4 h, removing them, and then harvesting the plant immediately. For real-time PCR (Fig. 1), two plants were pooled for each sample, and experiments were repeated four times.
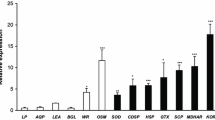
Real-time PCR of five genes from S. tuberosum var. Kennebec that are differentially expressed by treatment of wounded leaves with regurgitant of L. decemlineata. W = wounded + water, W + Reg = wounded + regurgitant 1:3, 1hI3hR = 1 h infestation followed by 3 h recovery, 4hI = 4 h continuous infestation. a STMEV47, ZPT2–13 transcription factor; b STMCK44, carbonic anhydrase; c STMFB59, class IV chitinase; d STMEP88, aromatic amino acid decarboxylase; and e STMDJ96, Zim domain protein 1, JAZ 1, transcription factor. Fold change levels of gene expression were expressed as RQ. As such, an RQ of 1 indicates a sample exhibiting the same relative transcript abundance as the control. The data were log10 transformed prior to graphing; hence, equal relative transcript abundance in control and treatment samples result in a log RQ value of 0. Each bar represents four biological replicates with error bars representing standard error
RNA Isolation for Microarray and Real-Time PCR
For microarray analyses, RNA was isolated from S. tuberosum leaves with Qiagen’s RNeasy kit by using the protocol recommended by the manufacturer (Qiagen, Valencia, CA, USA). The protocol is available at http://www.tigr.org/tdb/potato/microarray_SOPs.shtml. For real-time PCR, RNA was isolated by using Qiagen’s RNeasy Plant Mini kit adding an RNase free DNAse step using the manufacture’s protocol (Qiagen).
Microarrays
Two-color spotted cDNA microarrays were used to carry out gene expression profiling experiments. The TIGR 10K EST Solanaceae microarray contains 11,412 annotated cDNA clones spotted as randomized duplicates on the array. Because our primary objective was to identify regurgitant-responsive genes, hybridizations were carried out in which wounding plus regurgitant leaf samples were cohybridized (paired) with wounding plus water leaf samples. This direct comparison provides greater ability to identify genes differentially expressed in response to regurgitant. The variance is smaller in the direct comparison technique than in indirect comparisons where arrays pairing wounding plus water vs. control leaf samples are compared to arrays pairing wounding plus regurgitant vs. control leaf samples (Dobbin and Simon 2002). For each treatment–control comparison, three biological replicates were analyzed; for each biological replicate, a dye swap of technical replicates was performed. In total, six arrays for wound and wound plus regurgitant were carried out. The TIGR Solanaceae Expression Profiling Service performed all the microarray procedures including cDNA labeling, hybridization, data quantification, and data normalization by using LOWESS (protocols available at http://www.tigr.org/tdb/potato/microarray_SOPs.shtml). The data from the microarray experiments are available from the TIGR Solanaceae Gene Expression Database (http://www.tigr.org/tigr-scripts/tdb/potato/study/potato_study_hybs.pl? study=86&user=&pass=&sort=id&order=asc).
Exported data were analyzed in R (Ihaka and Gentleman 1996) with the BioConductor suite of packages (Gentleman et al. 2004). Quality assessment of the raw and background-corrected data was carried out by inspection of ratio–intensity plots (also known as minus–add (MA) plots)—pairwise correlations of ratio (M) values between slides; and distribution and density of intensity (A) values. Data were analyzed with the linear models for microarray data package (Smyth 2005) and exploratory analysis for two-color spotted microarray data (marray) package (Yang and Paquet 2005) by using methods described in Dudoit and Yang (2002), Smyth and Speed (2003), Smyth (2004), and Smyth et al. (2005). Within-array data were normalized by two-dimensional spatial loess and print-tip loess detrending procedure. Data were then scaled to have the same median-absolute-deviation across arrays. Nonspecific filtering was applied to reduce false discovery rate by removing invalid and low intensity genes. Intensity filtering was done with the genefilter package to remove genes whose A values were smaller than 7 in at least 75% of the samples. Linear models were fitted to the normalized data by using duplicate correlations, and empirical Bayes analysis was used to compute moderated t-statistics, which were then used to obtain P-values. For multiple testing, the P-value adjustment method of Benjamini and Hochberg (1995) was applied to control the false discovery rate (i.e., expected proportion of truly nondifferentially expressed genes among the rejected hypotheses). An adjusted P-value cutoff of 0.01 was used to generate differentially expressed gene lists. Differentially expressed genes were chosen if the fold change was >1.5 (induced) or <0.67 (repressed).
Real-time PCR
TaqMan Reverse Transcription reagents (Applied Biosystems, Foster City, CA, USA) were used to synthesize cDNA. Reaction conditions were 1× TaqMan RT buffer, 5.5 mM MgCl2, 500 μM dNTPs, 2.5 μM random hexamers, 0.4 U/μl RNase Inhibitor, and 1.25 U/μl multiscribe reverse transcriptase and incubated at 25°C 10 min, 48°C 30 min, and finally 95°C 5 min.
Real-time PCR was performed with a 7500 Real-Time PCR System (Applied Biosystems) by using the following parameters: 50°C 2 min, 95°C 10 min, followed by 40 cycles of 95°C 15 s, 60°C 1 min. Power SYBR Green PCR Master Mix (Applied Biosystems) was used in a final reaction volume of 25 μl. Target gene primers were used at a final concentration of 900 nM and 18S ribosomal endogenous control primers at 100 nM.
To utilize the comparative CT method of relative quantitation of gene expression, validation experiments were performed on all target gene primers (primer pairs listed in Table 1). The primers used for 18S rRNA were as described in Nicot et al. (2005). All target gene primers had amplification efficiency similar to the 18S amplicon (absolute value of the slope of ΔCT (target gene-18S) vs. log input RNA were all <0.1). Dissociation curves were performed for all primer pairs to check specificity of primers for the target gene. Fold change levels of gene expression were expressed as relative quantitation (RQ) values by using a “calibrator” sample as a reference with Sequence Detection Software version 1.4 (Applied Biosystems). As such, an RQ of 1 indicates a sample exhibiting the same relative transcript abundance as the control. The data were log10 transformed prior to graphing; hence, equal relative transcript abundance in control and treatment samples resulted in a log RQ value of 0.
Results and Discussion
Genes are Both Induced and Repressed by CPB Regurgitant
As an important step towards our long-term goal of better understanding the contribution of CPB to the CPB–potato interaction, we set out to use microarray gene expression profiling to identify genes whose expression is modulated by the presence of CPB regurgitant. Regurgitant-responsive genes can serve as vital reporter genes for investigations of CPB–potato interactions and particularly for identification of components of CPB regurgitant that act as elicitors to induce or repress the expression of these genes. Accordingly, leaves were wounded and treated with either CPB regurgitant at a 1:3 dilution or with water. Two-colored microarrays were performed to compare these two conditions. This experimental design provided the most direct means to identify genes whose expression is modulated by CPB regurgitant, since our primary objective was to identify regurgitant-responsive genes rather than investigate the effect of regurgitant on the expression of wounding-responsive genes. Four hours following wounding was chosen as the most appropriate time point for these analyses, based on the rationale that most genes that are induced early in the defense response would still be upregulated at 4 h; genes that are induced only later in the defense response would be commencing upregulation at 4 h, and genes that are differentially expressed only as an indirect consequence of the wounding plus or minus regurgitant treatment would not yet have been upregulated at 4 h, i.e., allowing us to minimize secondary effects of the plant/insect interaction. Korth and Dixon (1997) have demonstrated that while the timing of maximum expression of genes in response to wounding or wounding plus regurgitant can differ over the course of the defense response, both treatments result in a local induction of gene expression over baseline levels within a few hours of treatment, and that this induction persists for many hours. This and other studies (e.g., Christopher et al. 2004; Delessert et al. 2004) suggest that while the timing of sample harvest is critical to detect peak levels of gene expression during the defense response, a relatively broad window of opportunity exists to detect statistically different levels of gene expression for the majority of genes differentially expressed during the defense response. Consequently, we decided to focus on a single time point following wounding of the leaves for our microarray analysis. A limitation to this approach is that the time course of the regurgitant-associated induction or repression of gene expression is not known, which affects to some extent our ability to make biological inferences from the data. It is also possible that the analysis failed to identify those regurgitant-responsive genes that are significantly induced or repressed very early or very late in response to the treatment but not at 4 h following wounding. Similarly, this analysis would not reveal regurgitant-responsive genes that are not present on the TIGR Solanaceae microarray.
Analyses of the microarray data revealed 127 genes whose transcript abundance was significantly different in plants treated with mechanical wounding plus regurgitant compared to plants that were treated with mechanical wounding alone. The genes selected as significantly differentially expressed had an adjusted P-value of <0.01 and a fold changed of >1.5 (induced) or <0.67 (repressed). The regurgitant-responsive gene set can include genes that are regurgitant but not wounding responsive, as well as genes that are wounding responsive but whose expression patterns are intensified by regurgitant. Annotations for these differentially expressed genes are based on similarities to annotated sequences at the National Center for Biotechnology Information queried using Basic Local Alignment Search Tool (BLAST)X (Altschul et al. 1997).
There were 73 genes found to be induced (adjusted P-value < 0.01, fold change > 1.5) by adding CPB regurgitant to wounded leaves compared to the addition of water to wounded leaves (Table 2). They include proteins involved in secondary metabolism, general metabolism, protein expression, transcriptional regulation, stress, and pathogen responses. Not unexpectedly, a number of genes that encode proteins of unknown function were also induced. This can be in part ascribed to the lack of complete coding sequence information for a number of cDNAs represented on the array, which reduces the probability of retrieving a significant match from a sequence similarity search.
Interestingly, a total of 54 genes were repressed (adjusted P-value < 0.01, fold change < 0.67) in wounded leaves in the presence of regurgitant (Table 3). Genes associated with photosynthesis and stress were the most prevalent among downregulated genes. Genes were considered repressed if, in addition to a P-value of ≤0.01, the fold change was <0.67. Genes of secondary metabolism were more abundant when compared to genes repressed by regurgitant treatment (Tables 2 and 3).
Expression patterns of five genes found to be differentially expressed by CPB regurgitant with the microarray were confirmed with real-time PCR with independent experimental material (Fig. 1). The genes were chosen to represent different functional categories. These experiments further compared transcript abundance in water-treated and regurgitant-treated wounded leaves to that of control (unwounded) leaves as well as to two infestation treatments. The latter one provided an additional control to determine whether wounding plus regurgitant was a reasonable substitute for infestation in generating a defense response. All five genes showed expression patterns in regurgitant-treated wounded leaves and water-treated wounded leaves that were in agreement with the microarray data. For four genes—STMEV47 (similar to ZPT2–13 zinc finger protein), STMFB59 (similar to class IV chitinase), STMDJ96 (similar to ZIM domain protein), and STMEP88 (aromatic amino acid decarboxylase)—real-time PCR data demonstrated that transcript abundance in regurgitant-treated wounded leaves was significantly higher than in water-treated wounded leaves. With the exception of STMEP88, each of these genes was also upregulated by wounding alone relative to control untreated plants; for these three genes, the presence of regurgitant served to amplify the induction of gene expression over wounding alone. The fifth gene that was tested, STMCK44 (similar to carbonic anhydrase), was downregulated by wounding and further downregulated by wounding plus regurgitant, consistent with the microarray data. Each of these five genes was also differentially expressed in response to CPB infestation of leaves in the same pattern produced by mechanical wounding plus regurgitant (Fig. 1), lending validation to the wounding/regurgitant method. Two different infestation treatments were carried out to contrast the effect with the addition of regurgitant to wounded leaves. Infestation consisted of either 4 h of continuous infestation (4hI) or the leaves were subjected to 1 h of infestation followed by 3 h of recovery (1hI3hR). Neither of these infestation treatments was specifically designed to mimic the wounding treatment in intensity or timing and, as such, comparison of the amplitudes of gene responses to mechanical wounding plus or minus regurgitant to those evoked by the infestation treatments should be interpreted with care. Each of the genes examined by real-time PCR showed a greater response to 4hI than to 1hI3hR; additionally, transcript abundance for most genes exhibited greater variance in the 1hI3hR treatment. Taken together, the quantitative real-time PCR experimental data demonstrated that four genes are differentially expressed by wounding, the expression is enhanced by regurgitant and infestation also results in differential expression of these genes.
Photosynthesis and Carbohydrate Metabolism
Twenty percent of the genes repressed by regurgitant encoded photosynthetic proteins (Table 3). Examples include genes involved in photosynthetic electron transport such as a photosystem II reaction center protein and a thylakoid lumenal 16.5 kDa protein. This observed downregulation of photosynthetic genes is consistent with the findings of Hermsmeier et al. (2001). A number of genes associated with the utilization of carbon resources were repressed by regurgitant, including carbonic anhydrase, glyceraldehyde-3-phosphate dehydrogenase, and Rubisco. However, this was not an overriding pattern, as other genes involved in carbohydrate metabolism were induced when regurgitant was added to wounded leaves. For example, genes that encode pyruvate decarboxylase, glyceralydehyde-3-phosphate dehydrogenase, and uridine diphosphate (UDP)-glycosyltransferase were induced by regurgitant. Glyceraldehyde-3-phosphate dehydrogenase poses an interesting case in our study, as different genes that encode this enzyme showed upregulation or downregulation at 4 h after wounding plus regurgitant. Enzymes encoded by different members of this gene family are targeted to different cellular compartments and participate in different biochemical pathways: Chloroplastic glyceraldehyde-3-phosphate dehydrogenase catalyzes a step of the reduction phase of the Calvin cycle (photosynthetic carbon reduction cycle), while cytosolic glyceraldehyde-3-phosphate dehydrogenase is an enzyme of glycolysis. Furthermore, different forms of cytosolic glyceraldehyde-3-phosphate dehydrogenase exist that allow carbon flux through glycolysis even under nonfavorable energy status conditions, such as when low concentrations of ATP are encountered (Dennis and Blakeley 2000). Clearly, CPB exerts an influence through its regurgitant not only on the plant’s carbon acquisition via photosynthesis but also on the plant’s utilization of its carbon resources. That some genes associated with carbon metabolism are upregulated by regurgitant while others are downregulated indicate that this modulation is not a wholesale induction or repression of resource utilization; rather, it would appear that the presence of CPB regurgitant signals changes to the plant that result in more subtle redirection of carbon flux through metabolic networks in the attacked leaves. It will be of interest to determine how CPB regurgitant influences carbon flux through the biochemical pathways associated with the genes shown to be regurgitant responsive in this study.
Protein Synthesis and Nitrogen Metabolism
We detected a suite of differentially expressed genes associated with nitrogen-based biochemical pathways. Notably, genes that encode allantoinase were induced by CPB regurgitant in potato. Allantoinase is an enzyme of ureide metabolism that catalyzes the conversion of allantoin to allantoate. The ureides are nitrogen-rich organic compounds that are important for nitrogen transport and remobilization. Ureides are best characterized in nitrogen-fixing legumes, but recently enzymes implicated in ureide metabolism have been described in nonureide-type legume (Yang and Han 2004) and nonlegume species (Yang and Han 2004; Todd and Polacco 2006). The potato putative allantoinase genes, along with a putative allantoinase gene from tomato, show high sequence similarity to a functionally characterized Arabidopsis allantoinase; these genes were distinct from genes that encode a phylogenetically related enzyme, dihydroorotase (Yang and Han 2004). Complementation studies with the Arabidopsis allantoinase gene confirmed the biochemical activity of the enzyme encoded by this gene, and mutagenesis studies were used to show that allantoinase allows the plant to utilize allantoin as a sole nitrogen source. In black locust, there is an upregulation of allantoinase in the fall and spring and, hence, is proposed to play a role in seasonal nitrogen cycling (Yang and Han 2004). Allantoinases have been linked previously to plant defense in potato. A putative allantoinase was induced in potato tuber by treatment with a crude elicitor made from hyphal wall components of the fungus Phytophthora infestans (Nakane et al. 2003).
Other genes associated with nitrogen utilization that were upregulated in the presence of regurgitant include glutamine synthetase (likely associated with nitrogen reassimilation and recycling) and a gene that encodes a protein similar to an amino acid transporter. Together, these results suggest that plants alter their nitrogen utilization strategies in response to elicitation by regurgitant over and beyond changes brought about in response to wounding. Our data suggest that these changes invoke enhanced recycling and remobilization of nitrogen resources in attacked leaves. While considerable attention has been paid to the effect of soil nitrogen availability on allocation of plant resources to defense and the effect of leaf nitrogen content on the performance of herbivorous insects (e.g., Stamp 2003 and references therein), much less is known about repartitioning and reallocation of nitrogen resources in plants upon infestation and how this may affect not only the insect’s performance but also the plant’s ability to resist attack to promote damage repair and to resume growth and development following attack. Clearly, nitrogen resource utilization in plants under attack by herbivorous insects requires further investigation.
Secondary Metabolism
Genes involved in secondary metabolism were prominent in the list of upregulated genes of plants that had been wounded and treated with regurgitant. Eight different genes that encode cytochrome P450s were induced. Cytochrome P450s are a complex superfamily. In fact, 272 genes have been identified in the Arabidopsis genome (Schuler and Werck-Reichhart 2003). They are involved in biosynthetic reactions that produce such compounds as phenylpropanoids, alkaloids, and terpenoids. Terpenoids make up a portion of the volatiles created by infestation-induced plants, which could play a part in attraction of predator insects and parasitoids to the infested potato. Two genes encoding proteins involved in terpene synthesis are induced in these plants as well. In addition, several genes that may encode proteins involved in phenylpropanoid biosynthesis are induced: tyramine hydroxycinnamoyl transferase, copper amine oxidase, and peroxidase.
A gene that encodes aromatic amino acid decarboxylase was induced, and confirmation of this result by real time RT-PCR is presented in Fig. 1d. This enzyme is involved in the production of phenylalanine-derived volatile compounds important for insect attraction (Tieman et al. 2006). Phenylpropanoids are often anti-insecticidal compounds. Aromatic amino acid decarboxylase is the enzyme responsible for the precursor of 2-phenylethanol (Tieman et al. 2006), which is a volatile specific to CPB-damaged plants (Schütz et al. 1997) and is found to be particularly attractive to the CPB predator P. bioculatis (Weissbecker et al. 1999). This suggests that 2-phenylethanol is a volatile produced in response to regurgitant treatment of wounded potato plants. This plant response is part of a tritrophic interaction, which may indirectly result in plant defense.
Genes Implicated in Pathogen Defense and/or General Stress Response
Many of the genes identified as differentially expressed in response to regurgitant application to wounded leaves have been associated either with defense against pathogens or as part of general stress response. Generally, pathogen-induced genes are induced by the phytohormone salicylic acid, while wounding-associated genes are induced by jasmonic acid. However, overlap in these pathways is well-documented, and it will be of interest to test the regulation of these genes by these compounds.
Chitinases were among those pathogen-associated genes affected by regurgitant application. Two chitinase genes were identified as regurgitant-induced (a class IV and class II). The class IV chitinase identified by BLAST search and the class II chitinase were found to be involved with the response to pathogen attack (Büchter et al. 1997; Shinya et al. 2007). The induction of the class IV chitinase is confirmed by real-time PCR data in Fig. 1c. Three genes with homology to pathogenesis protein 10 were also induced by regurgitant. The homolog of this gene is induced by methyl jasmonate and salicylic acid in S. surattense (Liu et al. 2006). This suggests that the protein may have a broad defense function.
A noteworthy category of repressed genes are those genes associated with stress. In tomato, we have found that proteinase inhibitors PinI and PinII are repressed by CPB regurgitant (Lawrence et al. 2007). There are 16 type 1 and type 2 proteinase inhibitor clones on the potato microarray. Three of these inhibitors are repressed by CPB regurgitant. Since proteinase inhibitors act by inhibiting digestion of proteins in the insect midgut, this repression may represent a mechanism by which the insect subverts the plant’s defense mechanism.
Other potentially interesting genes that are repressed by regurgitant are the germins. Of the eight germin genes found on the potato microarray, four are repressed by CPB regurgitant. Germins are a large multigene family, which may contain superoxide dismutase activity, and some subfamilies may be induced by pathogens and herbivores (Lou and Baldwin 2006; Zimmermann et al. 2006). For example, the silencing of a germin from N. attenuata results in increased mass of M. sexta feeding on the plant and increases Tupiocoris notatus preference for the transgenic plant (Lou and Baldwin 2006). In Arabidopsis, 32 germins have been identified (Zimmermann et al. 2006). An extensive study of germins from barley examined members of each of the six identified subfamilies (Zimmermann et al. 2006). The barley sequence of HvGER2a showed the greatest similarity to the potato CPB repressed germin genes when examined by using BLASTX. The gene in barley is wound and cold-induced (Zimmermann et al. 2006) but the protein does not seem to have superoxide dismutase activity. Therefore, it is unclear how this protein or the potato germins might function. Perhaps knowing how CPB regurgitant inhibits germins in potato may offer insight into transgenic approaches that may circumvent this repression.
In summary, we have presented the first examination of transcriptional profiling of genes affected by CPB regurgitant. This is a valuable first step given the agronomic importance of this plant/insect interaction. It also affords the opportunity to characterize promoters from a number of regurgitant-induced genes. We found both induced and repressed genes due to regurgitant addition to wounded leaves. Characterization of the regurgitant may further elucidate the components involved in induction of potato genes by CPB regurgitant. CPB regurgitant affects the expression of a diverse set of genes: While many of these genes can be associated with classical stress and defense-associated mechanisms, including defensive chemistry biosynthesis, a number of these genes are related to the utilization of carbon and nitrogen resources. Interestingly, at least one of these regurgitant-responsive genes may be implicated in tritrophic interactions. As such, this study demonstrates that CPB regurgitant affects the expression of genes beyond those typically associated with the host’s defensive arsenal and illustrates the complexity of the interactions that occur between herbivorous insects and host plants and potentially with other organisms.
References
Alborn, H. T., Turlings, T. C. J., Jones, T. H., Stenhagen, G., Loughrin, J. H., and Tumlinson, J. H. 1997. An elicitor of plant volatiles from beet armyworm oral secretions. Science 276:945–949.
Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D. J. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402.
Benjamini, Y., and Hochberg, G. Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. (Britain) 57:289–300.
Büchter, R., Strömberg, A., Schmelzer, E., and Kombrink, E. 1997. Primary structure and expression of acidic (class II) chitinase in potato. Plant Mol. Biol. 35:749–761.
Christopher, M. E., Miranda, M., Major, I. T., and Constabel, C. P. 2004. Gene expression profiling of systemically wound-induced defenses in hybrid poplar. Planta 219:936–947.
Delessert, C., Wilson, I. W., Van Der,D., Straeten, Dennis, E. S., and Dolferus, R. 2004. Spatial and temporal analysis of the local response to wounding in Arabidopsis leaves. Plant Mol. Biol. 55:165–181.
Dennis, D. T., and Blakeley, S. D. 2000. Carbohydrate metabolism, pp. 630–675, in B. Buchanan, W. Gruissem, and R. Jones (eds.). Biochemistry and Molecular Biology of Plants American Society of Plant Physiologists, Rockville, MD.
De, Vos, M., Van, Osten, V. R., Van, Poecke, R. M., Van, Pelt, J. A., Pozo, M. J., Mueller, M. J., Buchala, A. J., Métraux, J. P., Van, Loon, L. C., Dicke, M., and Pieterse, C. M. 2005. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant-Microb. Interact. 18:923–937.
Dobbin, K., and Simon, R. 2002. Comparison of microarray designs for class comparison and class discovery. Bioinformatics 18:1438–1445.
Dudoit, S., and Yang, J. Y. H. 2002. Bioconductor R packages for exploratory analysis and normalization of cDNA microarray data, pp. 73–101, in G. Parmigiani, E. S. Garrett, R. A. Irizarry, and S. L. Zeger (eds.). The Analysis of Gene Expression Data: Methods and SoftwareSpringer, New York.
Eichenseer, H., Mathews, M. C., Bi, J. L., Murphy, J. B., and Felton, G. W. 1999. Salivary glucose oxidase: multifunctional roles for Helicoverpa zea. Arch. Insect Biochem. Physiol. 42:99–109.
Gentleman, R. C., Carey, V. J., Bates, D. M., Bolstad, B., Dettling, M., Dudroit, S., Ellis, B., Gautier, L., Ge, Y., Gentry, J., Hornik, K., Hothorn, T., Huber, W., Iacus, S., Irizarry, R., Leisch, F., Li, C., Maechler, M., Rossini, A. J., Sawitzki, G., Smith, C., Smyth, G., Tierney, L., Yang, J. Y. H., and Zhang, J. 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5:R80.
Halitschke, R., Schittko, U., Pohnert, G., Boland, W., and Baldwin, I. T. 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata III. Fatty acid–amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 125:711–717.
Halitschke, R., Gase, K., Hui, D., Schmidt, D. D., and Baldwin, I. T. 2003. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata VI. Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid–amino acid conjugates. Plant Physiol. 131:1894–1902.
Hermsmeier, D., Schittko, U., and Baldwin, I. T. 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. I. Large-scale changes in the accumulation of growth- and defense-related plant mRNAs. Plant Physiol. 125:683–700.
Ihaka, R., and Gentleman, R. 1996. R: A language for data analysis and graphics. J. Comp. Graph. Stat. 5:299–314.
Korth, K. L., and Dixon, R. A. 1997. Evidence for chewing insect-specific molecular events distinct from a general wound response in leaves. Plant Physiol. 115:1299–1305.
Kruzmane, D., Jankevica, L., and Ievinsh, G. 2002. Effect of regurgitant from Leptinotarsa decemlineata on wound responses in Solanum tuberosum and Phaseolus vulgaris. Physiol. Plant. 115:577–584.
Lawrence, S. D., Novak, N. G., and Blackburn, M. B. 2007. Inhibition of proteinase inhibitor transcripts by Leptinotarsa decemlineata regurgitant in Solanum lycopersicum. J. Chem. Ecol. 33:1041–1048.
Liu, X., Huang, B., Lin, J., Fei, J., Chen, Z., Pang, Y., Sun, X., and Tang, K. 2006. A novel pathogenesis-related protein (SsPR10) from Solanum surattense with ribonucleolytic and antimicrobial activity is stress- and pathogen-inducible. J. Plant Physiol. 163:546–556.
Lou, Y., and Baldwin, I. T. 2006. Silencing of a germin-like gene in Nicotiana attenuata improves performance of native herbivores. Plant Physiol. 140:1126–1136.
Major, I. T., and Constabel, P. C. 2006. Molecular analysis of poplar defense against herbivory: comparison of wound- and insect elicitor-induced gene expression. New Phytol. 172:617–635.
Mattiacci, L., Dicke, M., and Posthumus, M. A. 1995. Beta-glucosidase: an elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc. Nat. Acad. Sci. U.S.A. 92:2036–2040.
Musser, R. O., Hum-Musser, M. C., Bi, J. L., Murphy, J. B., and Felton, G. W. 2002. Caterpillar saliva beats plant defences. Nature 416:599–600.
Nakane, E., Kawakita, K., Doke, N., and Yoshioka, H. 2003. Elicitation of primary and secondary metabolism during defense in the potato. J. Gen. Plant Pathol. 69:378–384.
Nicot, N., Hausman, J. F., Hoffmann, L., and Evers, D. 2005. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 56:2907–2914.
Pohnert, G., Jung, V., Haukioja, E., Lempa, K., and Boland, W. 1999. New fatty acid amides from regurgitant of lepidopteran (Noctuidae, Geometridae) caterpillars. Tetrahedron 55:11275–11280.
Ralph, S., Oddy, C., Cooper, D., Yueh, H., Jancsik, S., Kolosova, N., Philippe, R. N., Aeschliman, D., White, R., Huber, D., Ritland, C. E., Benoit, F., Rigby, T., Nantel, S., Butterfield, Y. S. N., Kirkpatrick, R., Chun, E., Liu, J., Palmquist, D., Wynhoven, B., Stott, J., Yang, G., Barber, S., Holt, R. A., Siddiqui, A., Jones, S. J. M., Marra, M. A., Ellis, B. E., Douglas, C. J., Ritland, K., and Bohlmann, J. 2006a. Genomics of hybrid poplar (Populus trichocarpa x deltoides) interacting with forest tent caterpillars (Malacosoma disstria): normalized and full-length cDNA libraries, expressed sequence tags, and a cDNA microarray for the study of insect-induced defences in poplar. Mol. Ecol. 15:1275–1297.
Ralph, G. S., Yueh, H., Friedmann, M., Aeschliman, D., Zeznik, J. A., Nelson, C. C., Butterfield, Y. S. N., Kirkpatrick, R., Liu, J., Jones, S. J. M., Marra, M. A., Douglas, C. J., Ritland, K., and Bohlmann, J. 2006b. Conifer defence against insects: microarray gene expression profiling of Sitka spruce (Picea sitchensis) induced by mechanical wounding or feeding by spruce budworms (Choristoneura occidentalis) or white pine weevils (Pissodes strobi) reveals large-scale changes of the host transcriptome. Plant Cell Environ. 29:1545–1570.
Reymond, P., Weber, H., Damond, M., and Farmer, E. E. 2000. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12:707–719.
Reymond, P., Bodenhausen, N., Van, Poecke, M. P., Krishnamurthy, V., Dicke, M., and Farmer, E. E. 2004. A conserved transcript pattern in response to a specialist and a generalist herbivore. Plant Cell 16:3132–3147.
Roda, A., Halitschke, R., Steppuhn, A., and Baldwin, I. T. 2004. Individual variability in herbivore-specific elicitors from the plant’s perspective. Mol. Ecol. 13:2421–2433.
Schittko, U., Hermsmeier, D., and Baldwin, I. T. 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. II Accumulation of plant mRNAs in response to insect-derived cues. Plant Physiol. 125:701–710.
Schmelz, E. A., Carroll, M. J., Leclere, S., Phipps, S. M., Meredith, J., Chourey, P. S., Alborn, H. T., and Teal, P. E. A. 2006. Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. U.S.A. 103:8894–8899.
Schuler, M. A., and Werck-Reichhart, D. 2003. Functional genomics of P450s. Annu. Rev. Plant Biol. 54:629–667.
Schütz, S., Weissbecker, B., Klein, A., and Hummel, H. E. 1997. Host plant selection of the Colorado potato beetle as influenced by damage induced volatiles of the potato plant. Naturwissenschaften 84:212–217.
Shinya, T., Hanai, K., Gális, I., Suvuki, K., Matsuoka, K., Matsuoka, H., and Saito, M. 2007. Characterization of NtChitIV, a class IV chitinase induced by ß-1,3-,1,6-glucan elicitor from Alternaria alternata 102: antagonistic effect of salicylic acid and methyl jasmonate on the induction of NtChitIV. Biochem. Biophys. Res. Commun. 353:311–317.
Smyth, G. K. 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3:1–26.
Smyth, G. K. 2005. Limma: linear models for microarray data, pp. 397–420, in R. Gentleman, V. Carey, S. Dudoit, R. Irizarry, and W. Huber (eds.). Bioinformatics and Computational Biology Solutions using R and BioconductorSpringer, New York.
Smyth, G. K., and Speed, T. 2003. Normalization of cDNA microarray data. Methods 31:265–273.
Smyth, G. K., Michaud, J., and Scott, H. S. 2005. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics 21:2067–2075.
Stamp, N. 2003. Out of the quagmire of plant defense hypotheses. Q. Rev. Biol. 78:23–55.
Tieman, D., Taylor, M., Schauer, N., Fernie, A. R., Hanson, A. D., and Klee, H. J. 2006. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc. Nat. Acad. Sci. U.S.A. 103:8287–8292.
Todd, C. D., and Polacco, J. C. 2006. AtAAH encodes a protein with allantoate amidohydrolase activity from Arabidopsis thaliana. Planta 223:1108–1113.
Weissbecker, B., Van, Loon, J. J. A., and Dicke, M. 1999. Electroantennogram responses of a predator Perillus bioculatus and its prey Leptinotarsa decemlineata, to plant volatiles. J. Chem. Ecol. 25:2313–2325.
Yang, J., and Han, K.-H. 2004. Functional characterization of Allantoinase genes from Arabidopsis and a nonureide-type legume black locust. Plant Physiol. 134:1039–1049.
Yang, Y. H., and Paquet, A. C. 2005. Preprocessing two-color spotted arrays, pp. 49–69, in R. Gentleman, V. Carey, S. Dudoit, R. Irizarry, and W. Huber (eds.). Bioinformatics and Computational Biology Solutions using R and BioconductorSpringer, New York.
Zimmermann, G., Bäumlein, H., Mock, H. -P., Himmelbach, A., and Schweizer, P. 2006. The multigene family encoding germin-like proteins of barley. Regulation and function in basal host resistance. Plant Physiol. 142:181–192.
Acknowledgments
We are indebted to the TIGR Expression Profiling Service for providing and performing the microarrays and the data acquisition, to Y. Lui for assistance with the statistical analyses of the microarray data, and to R. Bennett for providing the potato plants. We thank E. Clark and L. Liska for providing CPB. Finally, we thank S. Seybold and three anonymous reviewers for constructive feedback on earlier versions of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lawrence, S.D., Novak, N.G., Ju, C.JT. et al. Potato, Solanum Tuberosum, Defense Against Colorado Potato Beetle, Leptinotarsa Decemlineata (Say): Microarray Gene Expression Profiling of Potato by Colorado Potato Beetle Regurgitant Treatment of Wounded Leaves. J Chem Ecol 34, 1013–1025 (2008). https://doi.org/10.1007/s10886-008-9507-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9507-2