Abstract
Insect herbivores have ubiquitous associations with microorganisms that have major effects on how host insects may interact in their environment. Recently, increased attention has been given to how insect gut microbiomes mediate interactions with plants. In this paper, I discuss the ecology and physiology of gut bacteria associated with insect herbivores and how they may shape interactions between insects and their various host plants. I first establish how microbial associations vary between insects with different feeding styles, and how the insect host physiology and ecology can shape stable or transient relationships with gut bacteria. Then, I describe how these relationships factor in with plant nutrition and plant defenses. Within this framework, I suggest that many of the interactions between plants, insects, and the gut microbiome are context-dependent and shaped by the type of defense and the isolates present in the environment. Relationships between insects and plants are not pairwise, but instead highly multipartite, and the interweaving of complex microbial interactions is needed to fully explore the context-dependent aspects of the gut microbiome in many of these systems. I conclude the review by suggesting studies that would help reduce the unsureness of microbial interactions with less-defined herbivore systems and identify how each could provide a path to more robust roles and traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insects comprise the largest portion of animal biomass (Bar-on et al. 2018), and insect herbivores can cause significant impacts on managed and unmanaged ecosystems. Understanding how plant nutritional and defensive traits mediate insect population dynamics is imperative for predicting herbivore outbreaks and manipulating plant traits for management. However, plant-herbivore interactions seldom occur in isolation. A seminal paper by Mcfall-Ngai et al. (2013) outlined the ubiquity of microbial interactions (symbiosis) in the ecology and evolution of animals, and suggested that multiple fields of animal biology would benefit from a greater understanding of how microbiome perturbations alter species interactions. Over the past two decades, there has been greater incorporation, understanding, and acceptance of microorganisms as key players in interactions among plants and arthropod herbivores (Douglas 2015; Engel and Moran 2013; Hansen and Moran 2014; Moran et al. 2019). However, we still only have a nascent understanding of how plants alter the temporal dynamics, efficacy, and evolution of insect gut microbes. Here, I review recent progress in this area using some of the most well-studied systems and provide a roadmap for future research directions.
Plants have evolved suites of defenses that limit feeding, performance, reproduction, and survival of insect herbivores. These diverse defenses include chemical and physical components that are present constitutively or as inducible phenotypes (Agrawal 2019; Karban & Baldwin, 2007; Mithöfer & Boland, 2012; Schuman and Baldwin 2016). The usefulness of plant defenses as a means of preserving some measure of plant fitness is contingent upon balancing the magnitude and efficacy of defenses with metabolic costs, temporal aspects of herbivore attack, and feeding patterns and traits of specific herbivores (Agrawal 2019). While there is clear diversity in plant defense types, expression and synthesis locations, effects typically converge on herbivore digestion as a target for disrupting attacks. Impeding herbivore digestion can occur through multiple pathways acting singly or in combination, including reducing consumption, causing herbivores to incur metabolic costs for detoxification or tissue repair, outright toxicity, disruption of nutrient digestion and/or absorption, and increasing susceptibility to pathogens (see reviews by: Chen, 2008; Erb & Reymond, 2019; Shikano 2017).
Like many other animals, insect herbivore guts are often populated with communities of microorganisms (the gut microbiome) that can contain bacteria, fungi, and protists. However, we are only beginning to elucidate how herbivore gut microbiomes may respond to, mitigate, or exacerbate plant defenses targeted at the host insect. In this review, I provide a synthesis of our current understanding of insect-gut bacteria interactions, how they integrated with plant defenses, and how life history may contribute to variation in microbiomes across insect taxa. I will primarily focus on plant defense relationships with foliage-feeding Coleoptera, Lepidoptera, and the suborder Heteroptera within the order Hemiptera, as these groups are the most well-studied with regard to gut bacteria and plant defense. Additionally, while it is important to acknowledge the nutritional and defensive importance of endosymbiotic bacteria inhabiting specialized organs (bacteriocytes), the specific focus of this review will cover interactions within the gut tissues. An improved understanding of the reciprocal interactions among plants and insect herbivore gut microbes will provide insight into new mechanisms by which insects may circumvent plant defenses, and the broader mechanisms by which insect microbiomes mediate ecological interactions.
Microbial Ecology and Functioning of gut Microbiomes
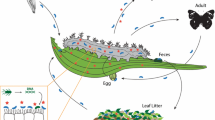
The functions, evolutionary ecology, and metabolic potential of various members of the gut microbiome have been reviewed previously and provide foundational understanding of the diversity of interactions occurring in arthropods (Douglas 2015; Engel and Moran 2013; Hansen and Moran 2014; Moran et al. 2019). Here, I provide an overview of the microbial ecology and how herbivore life history, physiology, and interactions in the environment can shape gut microbiomes in larval lepidopterans and coleopterans and how they may contrast that of hemipterans (Fig. 1). Access to reasonably-priced next-generation sequencing has produced a large amount of information on the gut bacterial communities within the last decade. These studies have revealed that there is enormous variation in community membership, fidelity of associations, and potential functions in these systems. In this section, I focus on the most pertinent information related to how gut microbes may interact with plant defenses.
Potential impacts of host physiological ecology influencing the acquisition, establishment, and persistence and proliferation of microbial communities in insect herbivore guts. There are overarching ecological influences that can govern the finer-scale interactions. Herbivore life history, how they exploit their diets, and the heterogeneity of their environment likely have effects on what set or sets of microbes ultimately populates their gut tissues. These larger forces can shape how open or closed the system is to new members. Accompanying these larger ecological forces are the smaller scale physiological ecology and adaptions the insects and microbes have. The sources of acquisition can be diverse and change through time. The members that establish and populate the gut tissues require the ability to tolerate their new environment and obtain adequate nutritional components to complete proliferation. If these factors fail, or the particular microbes are incapable of competing with new colonizers, they will ultimately be lost from the system
Insect Life History and Feeding Strategies Shaping Gut Microbiome Ecology
Insect herbivores can have dramatically different feeding styles, which produce different levels of exposure to plant defenses. In the case of large, chewing folivores, insects typically ingest substantially more physical and chemical defenses compared to sap feeders. Additionally, insects occupy different degrees of specialization within feeding guilds, and encounter substrates with different nutrient and chemical compositions. The different evolutionary trajectories of herbivore feeding groups also likely shapes host microbial interactions. For example, core microbial taxa may populate and be supportive where they supplement nutritionally poor substrates or confer some sort of additive benefit. Variable taxa may arise in systems encountering increased taxonomic and genetic diversity populating their gut tissues, with microbial turnover occurring rapidly. Fundamentally, many of the ecological and evolutionary forces that shape plant-herbivore interactions may also have impacts in insect-microbe relationships, whether that is in the form of ancient horizontal gene transfers or adaption of specific bacterial partners to the insect environment. Why some insect lineages exhibit poor fidelity with set(s) of bacteria are largely unknown, and additional comparative and manipulative studies will help elucidate these gaps.
Foliage-feeding lepidopteran larvae have relatively simple gut morphologies. The lepidopteran gut tissues are adapted for rapid transit of food through a simple tube. The midgut comprises most of the gut tissue, with small fore- and hind-gut segments. The epithelial layer of the midgut of these systems is protected by a peritrophic matrix (PM), a mucosal-analogous barrier. Bacteria present in lepidopteran larvae are generally not restricted to different regions of the gut, colonizing the cuticle in the fore- and hindgut while being present along the peritrophic matrix that separates the lumen and food bolus from the epithelial layer in the midgut (Teh et al. 2016). The gut microbiomes in lepidopterans studied thus far are simple, consisting of <5–7 dominant bacterial taxa, mostly belonging to the genera Enterococcus, Klebsiella, Enterobacter, and Pseudomonas, as well as a few documented fungi (Chen et al. 2020; Mason et al. 2019a; Paniagua Voirol et al. 2018). These bacterial genera have frequently emerged as the dominant taxa occupying the gut tissues of different lepidopteran species (Broderick et al. 2004; Priya et al. 2012; Hammer et al. 2014; Staudacher et al. 2016; Wang et al. 2017; Chen et al. 2018; Phalnikar & Kunte, 2018; Jones et al. 2019). For lepidopteran folivores, no study thus far has established an essential nutritional function of the gut symbionts that is analogous to the nutritional functions provided by bacterial symbionts in other insect orders , such as Hemiptera (Paniagua Voirol et al. 2018). However, lepidopteran gut microbe communities are metabolically active, producing RNA transcripts in the gut (Chen et al. 2018), and undergoing changes in population sizes with the growth and life cycle progression of their larval host (Mason et al. 2020). As larvae mature into adults, their gut systems are dramatically altered and reduced as they prepare for a new dietary lifestyle. Multiple studies have found similar gut microbial community members in adults that are commonly found in larvae, but the makeup generally differs from juveniles (Hammer et al. 2014; Ravenscraft et al. 2019a, b). Global mechanisms of gut microbiome transfer between mother and offspring are also not apparent for these systems, and the ecological and evolutionary consequences of why this may be the case are still being considered (Moran et al. 2019). This could be due to the availability of microbes in the environment coupled with the general lack of essential functions, but we currently do not have a good basis in what may drive these relationships. Further development and emergence of model will help answer these questions.
Coleopterans have unparalleled diversity among herbivores. As such, beetle have extensive variation in host plant specialization, the tissues that they consume, and, most likely, how they interact with their gut microbes. Unlike lepidopterans, the immature gut tissues can be highly segmented and contain different modifications and enlargements to their tissues that is typically tied to their diet. However, like the lepidopterans, coleopteran midguts are protected with a PM. These modifications are especially apparent in wood feeding larvae and are linked with distinct microbial communities (Ceja-Navarro et al. 2013), but are generally far less explored in beetles with other feeding styles. Some foliage-feeding coleopterans can have marked variability in their gut communities, and others have stable interactions with few members. This is evident in members of the Chyrsomelidae family. For example, the bacterial communities associated with the guts of the Colorado potato beetle (Chung et al. 2017; Wang et al., 2020), western corn rootworm (Chu et al. 2013; Dematheis et al. 2012), and Cephaloleia spp. (Blankenchip et al. 2018) can shift in response to different diet stimuli. While the role of maternal transfer is unclear for the majority of these systems, the Cephaloleia spp. show shared bacterial membership between adult guts and egg surfaces (Blankenchip et al. 2018). The leaf beetle systems have been found to have abundant taxa such as Pseudomonas, Enterobacter, and Rickettsia, but they are understudied compared to the lepidopteran systems, so the breadth of these patterns is unclear. It is also unclear if the bacteria in the aforementioned leaf beetle systems produce essential or facultative functions. In contrast, the tortoise beetle Cassida rubiginosa has a microbiome limited to a reduced bacterial membership (specifically Stammera capulata) in specific regions of their guts (Salem et al. 2017) that are stably transferred via specialized egg structures, and perform essential functions by secreting digestive pectinases in the foregut. Greater emphasis on comparing life history strategies and host plant exploitation of closely related species needed to fully parse where stable microbial functions persist in coleopterans vs. instances where the community membership and potential roles are more varied.
As an important contrast with the foliage-feeding holometabolous insects, hemipterans have substantial modifications to their gut tissues and how they integrate their microbiome. Phloem and xylem feeding hemipterans (Auchenorrhyncha and Sternorrhyncha) consume extreme diets and have substantial modifications to their digestive tissues to process the osmotic and nutritional challenges with their diet. For sap feeders, their guts lack a PM and are organized by multiple layering to prevent water loss. The bacteria associated with sap-feeding insects is primarily concentrated on the nutritional symbionts present in specialized bacteriocyte cells (Douglas 2015). Depending upon the insect’s species and lineage, there may be one or multiple symbionts present in one or more bacteriocytes (Baumann 2005). These symbionts function as critical mediators of nutrient provisioning, wherein their substantially reduced genome works dynamically with the host (Smith and Moran 2020). Sap feeding insects also possess a variety of heritable secondary symbionts that provide a variety of facultative functions to the host insect but are outside of the scope of this paper. Exhaustive syntheses of the impact of these bacteria on insect herbivores have been performed elsewhere (Oliver et al. 2010; Guo et al. 2017).
The gut morphology and associated microbiome differs in the Hemiptera suborder Heteroptera. Heteropterans feed on the cell contents of a variety of tissue types, ranging from fruits to leaves. Many investigations on heteropteran gut microbiomes have been completed, and there is a large diversity in functional associations. Heteropteran midguts are commonly multisegmented and have specialized crypts which are critical to facilitation of microbiome establishment (Kikuchi et al. 2011a; Sudakaran et al. 2017). Microbiome compositions are often host-insect specific; some insect lineages have strong relationships with various bacterial species across the phyla Alpha-Proteobacteria (for ex. Acetobacter), Beta-Proteobacteria (Burkholderia), Gamma-Proteobacteria (for ex. Pantoea, Klebsiella), and Actinobacteria (for ex. Coriobacterium) (Kikuchi et al. 2011b; Sudakaran et al. 2012; Taylor et al. 2014). Disruption of the heteropteran microbiome often leads to severe effects to insect development, survival, and reproduction (Hosokawa et al. 2012; Salem et al. 2012). Heteroperans have multiple mechanisms of transmission, with some maternal members depositing secretions to facilitate transfer while others acquire associates every generation from the environment (Salem et al. 2015). Patterns of transmission are evident in the decay of microbial genomes, where those with intimate associations with insect hosts exhibit a more narrow metabolic potential (Salem et al. 2015).
Collectively, the microbiome variation (or lack thereof) is influenced first and foremost by how the insect can consume food and whether there are essential functions needed to supplement nutritional needs. In some instances, this is achieved by coevolved strategies of transmission between conspecifics, while in others environmental acquisition is common. Maternal transfer provides greater insurance that the metabolic process is fulfilled. The environmental acquisition and establishment may facilitate short-term adaption to novel host plants and enable leaps in herbivore phenotypes. For this latter process to occur, the ability to colonize the gut is needed (Fig. 1), and there are a series of hurdles the bacteria may need to overcome. Divergent acquisition strategies are not necessarily at odds, but obviously depend upon the ecological context and the ability for novel microbes to establish in the gut interface with existing microbiome members.
The Insect Gut as an Environment for Bacteria
The insect gut can represent either a harsh or welcoming environment for different microbes. Depending upon the herbivore, the gut will have a range of pH, oxygen tensions, physical substrates and/or crypts, and antimicrobial responses. Nonetheless, bacteria can establish and thrive in many of these seemingly challenging environments. The insect gut is essentially a sieve; filtering microbes that cannot function in their environment while retaining those which can. This is especially important for insects that frequently encounter novel microbiota entering their tissues.
The range of physicochemical conditions in insect gut tissues varies widely across different herbivorous insect species, and can also fluctuate within species, especially across life stages. Coleopteran herbivores generally range from neutral to slightly alkaline midguts (Felton et al. 1992; Johnson and Rabosky 2000). Lepidopteran larvae represent a more extreme environment, with several species having a pH exceeding 9 (Appel and Maines 1995; Johnson and Felton 1996). For both Coleopteran and lepidopteran herbivores, oxygen tensions are low in the center of the lumen, but along the edge of the lumen oxygen levels are higher (Johnson and Barbehenn 2000). Cross section imaging of lepidopteran midguts have shown a higher portion of bacteria along the edge of the lumen (Shao et al. 2014). Some heteropterans have been reported to have a gut pH that is slightly acidic or neutral (Bandani et al. 2010; Bell et al. 2005), but broader conclusions should be tempered as studies are limited.
While pH can alter microbial compositions in the environment, we currently do not have strong evidence that it has an overriding effect on the microbial community structure in the insect gut. Presumably, the bacteria that are establishing and propagating in the gut tissues have some capacity to tolerate a dramatically different environment. Some support for lepidopteran gut bacteria exhibiting plastic expression of genes involved in pH tolerance has recently been documented (Mazumdar et al., 2020), but how pH tolerance varies across a range of potential colonizers is unknown. One would expect that there is pH tolerance for bacteria capable of colonizing these tissues versus those that fail. This is a significant omission in understanding since pH is often referred to as a key element in structuring microbiomes in surveys of gut bacteria.
The physical structure of the gut also plays a role in selecting for certain microbiome communities and facilitating, or hindering, microbial proliferation. There are multiple sites within even simple gut systems that are suitable for bacterial colonization. The fore- and hindgut are comprised of cuticle and are shed with each instar molt. Cuticle can provide structure for bacterial colonization in both organs, but the bacteria that make use of these surfaces would need to be non-specialized enough to persist outside the gut after molting (e.g., to colonize other habitats), or possess adaptations to facilitate recolonization after molting. The site of midgut colonization could be along the PM for holometabolous insects (Shao et al. 2014) or in specialized gut compartments known as crypts (Kikuchi et al. 2011a). Each of these may select for microbes possessing specific traits. The production of specialized crypts in heteropterans certainly limits compatible members, some of which is mediated by other antimicrobial activities and the bacterial synthesis of polysaccharides. Microbiome colonization in coleopteran and lepidopteran gut tissues and what governs these processes are unknown.
After colonization of gut tissues and tolerance of physicochemical conditions, the gut microbiome then needs to contend with the expression of antimicrobial peptides in the gut. Insect immune responses in the gut are usually in the form of various antimicrobial peptides (Wu et al. 2016), but understanding of how they shape the composition and population densities of resident gut microbiomes is not well-documented for lepidopteran and coleopterans. Immune responses are a dynamic process that gut microbiomes can induce, and presumably some members are more susceptible to these dynamics. The recognition of particular gut members likely results from a combination of Toll-like or immune deficiency (IMD) pathways (Buchon et al. 2013, 2014; Feldhaar and Gross 2008; Carvalho et al. 2012; Nishide et al. 2019; Sansone et al. 2015), but underlying mechanisms and downstream upregulation would vary between species and microbial stimuli.
Initial establishment of the gut microbiome and additional consumption of bacteria can induce immune-related responses which can influence microbial populations. For example, establishment of Burkholderia in the midguts of the heteropteran Riptortus pedestris triggers expression of different immune-related genes in the hemolymph and fat body (Kim et al. 2016; Kim et al. 2015). The antimicrobial peptides produced by R. pedestris were antagonistic against symbiotic Burkholderia, yet the symbionts were able to suppress the immune defenses to facilitate colonization (Kate et al. 2016). Similar upregulation of immunity pathways has been observed for the heteropteran Dysdercus fasciatus for associations with gut bacteria in the Coriobacterium and Gordonibacter genera (Bauer et al. 2014). Unlike work with R. pedstris, the various members of the gut microbiome were insensitive to the immune responses mounted by D. faciatus (Onchuru and Kaltenpoth 2019). Lepidopteran larvae also exhibit systemic responses when non-pathogenic bacteria colonize their gut tissues. When the caterpillars Trichoplusia ni and Galleria mellonella were fed non-pathogenic bacteria and compared to those which were not, there was a substantial difference in the immune response of the caterpillar, where there was modulation of antimicrobial activity in response to the challenge (Freitak et al. 2007, 2014). Orally administered nonpathogenic Escherichia coli and Micrococcus stimulated immune responses in larvae (Freitak et al. 2007) and across generations (Freitak et al. 2014). Similarly, corn earworm (Helicoverpa zea) that ingested Enterobacter had increased antimicrobial lysozyme and glucose oxidase in the midgut and salivary enzymes compared to those fed diet not containing the bacteria (Wang et al. 2018). Increased immune-related responses were observed with fall armyworm (Spodoptera frugiperda), where larvae having guts populated with Enterobacter had elevated hemocytes and phenoloxidase expression in their hemolymph compared to larvae lacking microbes (Mason et al. 2019b). We currently do not know how strongly bacterial populations are regulated by antimicrobial properties of the gut in Lepidoptera, and whether differences in microbiome composition are observed. For coleopterans, expression of antimicrobial peptides has been observed by gut bacteria in the red palm weevil Rhynchophorus ferrugineus, where the induction of the IMD pathway limits the proliferation of gut microbes (Xiao et al. 2019). For R. ferrugineus, the inhibition of the IMD pathway did not result in community compositional shifts (Xiao et al. 2019).
Beyond direct effects of host insect physiology and gut structure, communities of microbes in insect guts are also structured by competition for resources and colonization sites. Host-specific gut conditions will select for a reduced subset of bacteria that can adapt. However, since there are common species that populate guts across different insect lineages, there may be common mechanisms to contend with gut physicochemistry while obtaining suitable nutritents across the different bacterial groups. There are probably commonalities in how these associates contend with the high pH environment (especially in the case of Lepidoptera), acquire nutrients to proliferate, and evade host immune responses to persist and populate in these environments. Further exploration of establishment processes is needed, especially in cases where there is weak evidence of vertical transfer of the gut microbiome. Understanding how bacteria establishes across space, time, and diets would facilitate a better knowledge of why some systems exhibit high variation.
Environmental Heterogeneity and Resulting Impacts on Insect Gut Microbiomes
Mobile insects commonly experience changes in their environment through space and time. There are several ways that heterogenous environments can alter microbial interactions within a host. First, insect populations may experience different abiotic conditions. Second, they may encounter new microbes in the environment that can facilitate new processes or present as opportunistic pathogens. Finally, herbivores may encounter plants with different compositions of nutrients and chemical defenses, which can alter microbe community, proliferation, and gene expression. All these processes feed into how the gut microbiome varies temporally and spatially, how it interacts directly with the host insect, and how it might indirectly influence phenotypic aspects of plants being fed upon by insect hosts.
Among the main taxa discussed within this review, lepidopterans tend to have the most variation in gut microbe composition. Not surprisingly, lepidopteran gut microbiomes also undergo the most drastic changes in response to ingestion of environmental bacteria. However, recent work indicates that these fluctuations may be family- or species-specific. In using 16S-rRNA sequencing, some studies have identified diets and diet-associated microbes as contributors to lepidopteran gut microbiomes, (Chen et al. 2018; Chaturvedi et al. 2017; Hammer et al. 2017; Jones et al. 2019; Su’ad et al. 2019) while other studies have observed no such effects (Minard et al. 2019; Whitaker et al. 2016). Notably, many of these studies used different insects occupying distinct families. Additionally, a recent study has shown that the gut community of the cabbage moth is influenced by the host insects contacting soil (Hannula et al. 2019). Some studies have indicated some vertical transmission of associates (Chen et al. 2016), but others have shown that these early acquisitions are subsequently dampened by acquiring additional members from the environment (Chen et al. 2018; Mason and Raffa 2014).
For lepidopteran systems, several studies have noted that there is a simplification of these communities in laboratory settings. Namely, laboratory colonies or larvae feeding on artificial diets have relatively few members and are dominated by Enterococcus compared to those feeding on plant-based diets and in field settings (Broderick et al. 2004; Mason et al. 2019a, 2019b; Staudacher et al. 2016). When lab-based larvae historically maintained on wheat germ artificial diets are provided plant-based food sources, there is an increase in microbial diversity associated with their guts. For Lepidoptera, there are two considerations that need to be made when inferring interactions with their gut microbiome. First, laboratory-based colonies maintained on artificial ingredients are unlikely to reflect those fed on plants and/or collected from the field. Fundamentally, the interpretations produced by laboratory-maintained colonies on plant-insect-gut microbiome may certainly differ compared to those in the field or feeding on plants. Second, the ingestion of novel microbes may produce a level of environmental noise, but also potential phenotypic plasticity. While some work has inferred that there is a very transient component of microbes associated with the lepidopteran gut (Hammer et al. 2017), at least some species are capable of maintaining members in their gut environment without repeated introduction (Mason et al. 2020). Overall, better parsing of environmental noise from core establishment is needed for these lepidopteran systems, especially as it pertains to interactions with host plants.
Coleopterans can also exhibit differences in gut microbiomes depending upon population and plant species. Diet-driven effects on shifting gut microbiomes have been documented for several species (Blankenchip et al. 2018; Chu et al. 2013; Chung et al. 2017). Currently the role of host plant-associated bacteria in establishing and outcompeting resident bacteria in these systems is unclear. For many leaf-feeding beetles, there are limited useful artificial diets for laboratory-maintained colonies; usually they are reared entirely on plants. This may allow the larvae to maintain certain groups and therefore have less observable plasticity in their microbiome composition. However, explicit comparisons are generally lacking. In wood-boring beetles feeding on a synthetic artificial diet, similar patterns emerge as those in lepidopterans (Geib et al. 2009). Recruitment and replacement may be occurring in these systems, but the mechanisms remain unexplored and evidence of this occurring is scant.
While environmental heterogeneity can influence heteropteran success as it relates to exploiting dietary resources, the way it may integrate with gut microbiomes is more nuanced. In instances where members are acquired every generation (Itoh et al. 2018a), different environments may allow for the host to exhibit greater phenotypic diversity from the unique genetic potential obtained from the symbionts. Alternatively, for stably transmitted microbes, environmental factors may contribute less to the overall structure and functioning of microbial communities (Sudakaran et al. 2012). This is not to say that replacement or shifts in the gut microbiome is not possible in these systems. For example, the Welwitschia bug, Probergrothius angolensis, possesses dramatically different community compositions between different host plants despite a stably transmitted microbiome through time (Martinez et al. 2019). How the environment interfaces with any of these systems varies depending the host insect, the microbes encountered, and the host plant being exploited.
When encountering novel microbes in the environment, there is a risk an insect may acquire opportunistic pathogens as a component of their gut microbiome. While the most noted example commonly discussed is Serratia, there are other members that could be antagonistic. Proteases and hemolytic activities that have been ascribed to Serratia virulence can also be expressed among other taxa in the gut (Givskov et al. 1997, Mason unpublished data). Various strains and/or species of bacteria residing in the gut can also be pathogens, such as Klebsiella, Enterobacter, and Enterococcus (Bulla Jr et al. 1975; Mason et al. 2011; Insua et al. 2013). Antagonistic gut bacteria need to breach host preventative mechanisms and avoid immune defenses (Vallet-Gely et al. 2008), and plant defenses may contribute to entomopathogens by aiding either of these processes.
Several studies have noted that some herbivorous insects harbor distinct bacterial gut communities when they feed on different host plants (Broderick et al. 2004; Chung et al. 2017; Jones et al. 2019).There are two intersecting functions for how this may occur. First, there may be delivery of bacteria that are resident on the foliage to the insect (Hammer et al. 2017). Second, the ingestion of various phytochemicals may stimulate growth responses of some members of the community, or have more negative effects on others (Mason et al. 2015). Phytochemicals are diverse (Richards et al., 2016), and have distinct properties that can be both insecticidal and antimicrobial. The effects of plant chemistry on microbiomes lie within questions about life history of the insect and associated microbes. For example: Is the insect a specialist or a generalist on a set of host plants? Does it have a stable or variable community? How toxic are the chemicals to the host and microbes? How adapted are specific microbes to the phytochemicals? The ability a microbiome to contend with different antimicrobial plant defenses may relate to the frequency they interact with that set of compounds, and there may be feedbacks in establishment of bacteria and the ability of the host to use a substrate.
Insect gut microbiome Interactions with Plant Defenses
Plant defenses can target and disrupt herbivore digestion by altering digestive efficacy, protective barriers in the gut, and by damaging cells in the gut tissues (Chen, 2008; Konno and Mitsuhashi 2019; Mithöfer & Boland, 2012). Gut microbiome influences on the efficacy of plant defenses potentially span a range of effects, from synergistic (enhancing defense efficacy) to antagonistic (decreasing efficacy) (Fig. 2). Current research is focused on identifying patterns in gut microbiome effects, specifically whether microbial impacts on plant defense depend on the type of defense and its mode of action. As outlined previously, there is a diversity of microbe taxa and genotypes in insect guts which vary temporally and spatially depending on the insect taxa being colonized. The relative permanence or transience of gut flora will therefore further influence the outcomes for plant defense efficacy across modes of action. Here, I focus on reviewing evidence of gut microbiome impacts on defense efficacy with these modifiers in mind.
Summary of effects of plant defenses on herbivore midgut epithelium (ME), peritrophic matrix (PM), and lumen (Lu) and how gut bacteria may interact with those processes. While the effect produced by the plant defenses vary between different specialized phytochemicals and plant species, understanding the damage they cause can provide a basis for the effects of different gut bacteria. In some instances, the interactive effects of plant defenses and gut bacteria may be negative on the insect host, while others may be benign or positive. However, ultimate phenotypes that arise from these interactions are likely heavily influenced by the species/isolates of microbes present in the system. How these processes may vary between insects with different plant life histories are unknown
Plant Defenses Altering the Peritrophic Matrix Integrity and Microbial Associations
For Coleoptera and Lepidoptera, the epithelium of the gut lumen is protected by the peritrophic matrix (PM): a thin layer of cuticle that protects cells from the food bolus. The PM is comprised of a mixture of glycoproteins (peritrophins and mucins) and chitin. Exhaustive reviews on the functions, metabolism, physical formation, and evolutionary ecology of the PM have been previously published (Hegedus et al. 2009; Konno and Mitsuhashi 2019). The ability of the PM to enhance digestive efficiency (Bolognesi et al. 2008), and protect against potential infections (Kuraishi et al. 2011), abrasions (Sudha and Muthu 1988), or oxidative stress (Barbehenn 2001; Bi and Felton 1995;) makes it critical for keeping host-microbe interactions in check. While the PM is a protective barrier, some plant defenses can weaken and/or create perforations that can destabilize its important functions.
Plant defenses that impact PM and epithelial layer are plentiful (Konno and Mitsuhashi 2019). The PM can be thickened by some proteins, such as MLX56 found in mulberry, which impairs digestive efficiency (Konno et al. 2018; Wasano et al. 2009;). Alternatively, several defensive proteins and chemicals can weaken the PM. Proteases (Mohan et al. 2008; Pechan et al. 2002), lectins (Harper et al. 1998; Hopkins and Harper 2001; Walski et al. 2014), and chitinases (Freitas et al. 2016; Lawrence and Novak 2006; Mason et al. 2019a) can destabilize and alter PM integrity. Physical aspects of plant defenses can also be disruptive to the PM by penetrating the barrier and necessitating repair by the insect. Non-glandular trichomes in particular have been shown to breach the PM and induce performance costs to the insect, as has been shown for horsenettle (Solanum carolinense) and Manduca sexta (Kariyat et al. 2017). Similar results have been observed for fall armyworm and maize, where plants with different sized trichomes altered the integrity of the PM (Mason et al. 2019a). Effectiveness of chemicals and trichomes in disrupting the PM likely varies between various plant species and genotypes.
Plant defenses thinning or perforating the PM can create an increased risk of microbial infection. Among known viral, bacterial, and fungal pathogens of insects, alteration of PM integrity is a ubiquitous strategy for tissue invasion that is achieved via diverse mechanisms (Berini et al. 2015; Hoover et al., 2010; Peng et al., 1999; Rees et al., 2009; Valaitis and Podgwaite 2013). While the weakening of the PM barrier by plant defenses can synergize with manipulation of the PM by entomopathogens, it can also create avenues for resident gut microbes to become pathogenic, especially when those microbes populate the PM as a site of replication. This was recently shown with fall armyworm and the maize system, where the breakdown of the PM by proteases and chitinases encouraged invasion of an Enterobacter isolate into the body cavity of the host (Mason et al. 2019a, 2019b). The negative effects of bacteria on the caterpillar host differed between individual isolates of Enterobacter, however none of those that were tested were alone strongly pathogenic. The ability for plants to break down the insect PM creates a stressful scenario for insects, which must repair the immediate damage and protect against opportunistic microbiota. Combined assaults on the gut directly and indirectly through the microbiome can overwhelm the herbivore. The ability for the insect gut microbiome to exploit damage incurred by the PM is likely influenced in part by the members present and the degree of damage to the structure. Currently, we do not know the ecological and evolutionary consequences of the negative functions of these bacteria, but this may provide an avenue for novel means for selecting antagonists.
Interactions between Plant Chemical Defenses and Gut Microbes
Plant chemical defenses are abundant and diverse and reviewed in detail elsewhere. In general, chemical defenses can have direct toxicity, induce oxidative stress, damage epithelial cells, and disrupt digestion. Mechanisms and efficacy of chemical defenses vary between specific chemicals and herbivore systems. Currently, our understanding of these interactions and how they confer effects into herbivore systems are still limited, but present potential avenues for future research, particularly in how they are linked to the greater system. So far, in cases where bacteria are mediating defense interactions, the microbes appear to augment the herbivores’ intrinsic capacities to contend with the defenses.
Plant phytochemicals have antimicrobial properties and so the ability to metabolize various groups of compounds by bacteria are likely common (Janssen et al. 2005). It seems likely that there may be some direct interactions in the herbivore gut that results in metabolism of toxic phytochemicals. Bacteria associated with insect herbivores possess the capability of metabolizing plant chemicals (reviewed in: Itoh et al. 2018b; Mason et al. 2019a; van den Bosch and Welte 2017), but the alleviation of impacts on host insects has only been observed in a subset of systems. Whether that metabolism extends to the host depends upon metabolic potential of the isolate, the ability to establish within a host, and whether the level of defenses encountered do not overwhelm the system.
Some examples of bacterial-mediated detoxification have been documented. Metabolism of chemicals by the insect gut microbiome may be present in systems that frequently encounter plant defenses and are stable across generations, such as those observed in conifer-attacking insects (Berasategui et al. 2017; Boone et al. 2013; Xu et al. 2015;) where bacteria were able to metabolize terpenoids to benefit the insects. Alternatively, environmental sources of bacteria may provide novel means to supplement the host insects’ intrinsic detoxification activities. In gypsy moth (Lymantria dispar), alleviation of salicinoid phenolic glycosides was observed with specific communities of bacteria obtained from the host plant (Mason et al. 2014). The responses observed in that study were dependent upon the community of bacteria present. Comparable studies done in bean bugs (Riptortus pedestris) indicated the metabolism of insecticides was observed with specific strains of Burkholdaria (Kikuchi et al. 2012). In both instances, the bacteria that mediated the responses were environmentally derived from plants or soil. Recently, bacteria associated with fall armyworm were shown to metabolize insecticides (Gomes et al. 2020), but that activity differed between laboratory and field sources of the insect. This is important because it shows that the environmental context may be important in understanding how gut microbiomes can have facultative functions in these insects. In all the cases that have been shown, the processes are augmentative to the herbivores’ intrinsic metabolic capabilities.
Through primary or secondary activity, some plant chemicals (phenolics) can induce oxidative stress in insect gut tissues. Oxidative damage can vary between specific phytochemicals (Barbehenn et al. 2006, 2008; Summers and Felton 1994). Reactive oxygen species formation can be cytotoxic and damage the epithelial layer. Gut bacteria can potentially impact reactive oxygen species through metabolism of the initial phenolic or by quenching the activity of free radicals. For example, some strains of Enterococcus mundtii isolated from the beet armyworm (Spodoptera littoralis) midgut possess catalase and superoxide dismutase enzymes which have the potential to decrease oxidative stress (Mazumdar et al. 2018). Diet manipulations coupled with establishment of distinct microbial communities in the gut can facilitate better evaluation of microbial effects on oxidative stress.
Plant proteinase inhibitors (PIs) are forms of plant defenses that interfere with herbivore digestion (Napoleão et al. 2019) and may interact with herbivore gut microbiota in a variety of ways. In one instance, bacteria may be able to supplement herbivore proteases that are affected by plant PIs. One example of this was observed in western corn rootworm (Diabrotica virgifera virgifera), where certain bacterial communities allowed greater survival on hosts with PIs and removal with antibiotics decreased survival (Chu et al. 2013). Comparable studies have been completed with lepidopteran caterpillars. One study found that bacteria isolated from the gut of the velvetbean caterpillar, Anticarsia gemmatalis, have the ability to counteract trypsin inhibitors in culture and demonstrated that multiple bacteria strains were able to perform this function (Pilon et al. 2017). A separate study with the velvetbean caterpillar showed that when the resident community was disrupted with antibiotics, the microbiome exhibited minimal effects on larval survival, but led to faster development time and increased weight gain (Visôtto et al. 2009). Microbiome supplementation of PIs may be common in Lepidoptera and Coleoptera and may enable adaption to challenging diets. Similar to testing effects of oxidative stress, evaluating the integration of gut microbiomes with these defenses could be accomplished with diet and microbiome manipulations. However, the ability of bacteria to supplement proteases would require the excreted protease to function within the physiochemical (pH) conditions of the herbivore’s gut.
Effects of Gut Microbes on Modulation of Plant Defense Induction
Gut bacteria can also modulate the expression of plant defenses by enabling the insect to avoid heightened levels of induced defenses. Generally, this occurs through exploitation of the antagonistic relationships between the plant jasmonic (JA) and salicylic acid (SA) signaling pathways (Thaler et al. 2012). Typically, JA is responsive to effectors associated with chewing insects while SA is responsive to piercing-sucking insects and bacterial pathogens (Erb et al. 2012; Howe 2004; Walling 2008). This JA/SA antagonism has been shown to be exploited by the gut symbionts of chewing herbivores in both direct and indirect mechanisms. The Colorado potato beetle (Leptinotarsa decemlineata) and false potato beetle (Leptinotarsa juncta) possess bacteria in the oral secretions that reduces the induction of JA-dependent defenses (Chung et al. 2013; Wang et al. 2016). Effectors associated with gut Pseudomonas and Enterobacter isolates downregulate the expression of the tomato defenses targeting the herbivore, allowing the insect to better exploit the host plant (Chung et al. 2013; Sorokan et al. 2019). In both cases, the response is achieved by the bacteria being physically deposited onto the plant to achieve the antagonism. Similarly, fall armyworm can alter plant responses by directly regurgitating bacteria on plants (Acevedo et al. 2017). However, directionality of induction by tomato and maize to fall armyworm bacteria differed, where tomato defenses were suppressed, but maize defenses elevated (Acevedo et al. 2017). There were differences between the effectiveness of individual isolates and how much induction or suppression they produced. Similarly, gut bacteria can indirectly modify plant defenses by altering the expression of salivary effectors in different caterpillars. In H. zea, gut bacteria increased salivary glucose oxidase, which increased defense expression in tomato (Wang et al. 2017) and maize (Wang et al. 2018).
Microbes shaping plant responses undoubtedly depend upon the ability of the plant to perceive and respond to the various stimuli. All insects possess some receptors that plants can exploit to upregulate defense responses upon attack (Acevedo et al. 2015). Microbial-induced variation in plant responses hinges upon the types of microbial and herbivore effectors and the plasticity of their expression, the way the plants perceive the responses, and how they up- or down-regulate defenses. The fact that gut bacteria modulate the expression levels of salivary glucose oxidase (Wang et al. 2018) and glucose oxidase is expressed in many lepidopteran salivary glands (Eichenseer et al. 2010) suggests broader roles of gut bacteria in modifying plant responses to herbivore.
Parsing Complex Interactions between Insect Hosts, gut Microbes, and Plant Defenses
Variation in insect life histories and the difficulty of performing manipulative experiments on gut microbe communities creates some inherent challenges in dissecting interplay among folivorous insects, their microbes, and host plants. Despite these constraints, we have been able to discern some similarities in the bacterial communities that populate gut tissues, which suggests that the microbes in this region share some are phylogenetic and trait-based features. Whether the microbes that inhabit these tissues can have positive, negative, or neutral effects depend upon the context of the interaction. In this section, I discuss the different research avenues needed to identify broader patterns of insect gut microbiome effects on hosts, the importance of plant defenses in mediating these relationships, and the consequences for the evolution of the organisms involved.
Intersections between Plant Defenses, Microbes, and Immune Signaling
As discussed in the previous sections, insect-associated microbes can stimulate immune responses in their insect hosts. Plant defenses and insecticides are also capable of interacting with and augmenting host immune systems (James and Xu 2012; Smilanich et al. 2009, 2011). Therefore, it is logical to surmise that gut microbiome and plant-mediated effects on insect immunity will intersect, with the potential for counteractive, additive, or even synergistic effects. For example, one recent study focused on caterpillar microbiomes showed that different host plants altered systemic immune responses and gut bacterial composition (Su’ad et al. 2019). In addition, ingestion of bacteria on plants by caterpillars can increase immune responses (Olsen et al. 2017). It is well recognized that plant nutrition and defense expression can alter herbivore responses to pathogen stressors (Shikano 2017), and this could easily extend to the resident microbiome. Recently it was postulated that herbivore self-medication may act through changes in the gut microbiome rather than the defenses themselves (Harris et al. 2019). There is currently little empirical support for this in insect systems, but it is certainly worth exploring given that self-medication has been documented in many animal systems, and many plants produce compounds that may affect differnt microbes. More studies are needed across a greater diversity of systems to discern how the insect immune system mediates indirect interactions between plants and gut microbes, and to parse the contributions of plant defenses as direct modifiers of microbial communities. Contextualization and incorporating environmental variables (in the case of laboratory reared animals) will be essential components of these studies. This is especially the case for Lepidoptera which undergo dramatic simplification of their gut communities compared to conspecifics collected from the field (Paniagua Voirol et al. 2018).
Impacts of Plant Microbes on Microbe Establishment and Succession
Plants can serve as a reservoir for microbes that also colonize insect guts, but very little is known about how microbes on or within plants alter existing insect gut community assembly or the resulting insect immune responses to newly ingested bacteria. In some cases, plants may facilitate the colonization of potentially pathogenic bacteria if the appearance of these microbes in the gut alongside plant defenses results in the opportunity to invade and exploit insect tissues (Mason et al. 2019a). Through similar mechanisms, plant defenses may also create opportunities for new non-pathogenic colonizers by destabilizing existing community members through direct (inhibitory) or indirect (immunity-mediated) mechanisms. Conversely, an existing microbiome may function to limit such invasions (Dillon et al. 2005), either through competitive inhibition or differential activation and tolerance of host antimicrobial defenses. For most of the taxa discussed within this review, the establishment, persistence, and maintenance of gut bacteria are not well-defined, so it has been difficult to determine the impact they may have on colonization by new environmentally acquired taxa. Using defined bacterial strains and/or communities and evaluating impacts of new strains would facilitate evaluating these relationships.
There is also a significant need to understand how insects acquire microbes, variation in communities in the field, and how these two components interact to alter host survival and fitness. When microbial surveys are conducted on insects found in nature, there is an inherent survivor bias, leading researchers to wonder whether these are the insects that have acquired the “correct” suite of microbes. If this is the case, it is reasonable to hypothesize that an important component of early instar survival involves acquiring certain microbial assemblages. Empirically testing this is not easy and will require researchers to perform experiments in the field that manipulate several variables simultaneously, such as host plant identity, insect developmental stage, insect genotype, and presence of potentially pathogenic bacteria. These experiments can integrate with techniques for microbiome profiling, many of which have come a long way towards accounting for biases introduced by technical aspects. These experiments would help us answer many questions about how insect life stage and life history impact gut microbiomes, and perhaps why certain bacterial taxa frequently emerge as components of the gut microbiome in these systems.
Determining What Makes a Gut a Suitable Environment and Bacteria Good Tenants
Beyond just the identities of gut microbes, it is important to begin thinking about how gut microbes may differ in their metabolic capabilities or their ability to inhabit certain hosts. Namely, are the array of microbes that colonize insect guts stochastic or do they have similar core metabolic processes and needs regardless of the host they inhabit? Even though individual gut microbiomes may be relatively simple or transient (e.g., for Lepidoptera), across insect taxa, marked microbial diversity (e.g., different Phyla) have been documented to be able to colonize insect gut tissues. Thus, we would expect that there is a concurrently diverse metabolic potential among bacterial taxa known to inhabit insect guts, but we do not know how this contributes to the communities that we observe, or how host plants might play a role in determining the suite of metabolic activities that are maintained in gut microbe genomes. If these can be defined for a set or subset of insects, this may enable predictions of functions (or lack thereof) in other insect species. However, to fully understand the roles that these bacteria may or may not play is a better connection of pattern to process, through a combination of observation and manipulative experiments in comparative contexts. For example, comparative and functional metabolomics and (meta)genomics for insects harboring different microbial assemblages would facilitate some of these comparisons. These types of experiments would reduce speculation of function and integrate the necessary ecological complexity and nuance to the different systems.
Conclusions
The synthesis of literature reviewed here demonstrates that gut microbes can influence interactions between insects and their host plants through multiple mechanisms, but that we have only a nascent understanding of the evolutionary context and ecological significance of these effects. Work in a greater diversity of insect herbivore systems is needed to establish a framework for evaluating drivers of microbial diversity, host-microbe interactions, and extended phenotypes. So far, we know that the roles that microbes play as mediators of interactions between insects and plants defenses vary in somewhat predictable ways across the taxa explored here (Coleoptera, Lepidoptera, and Hemiptera), but this conclusion is based on very limited studies, many of which involve laboratory-reared organisms with highly simple gut microbiomes. Future works should focus on expanding the use of comparative approaches between closely related insect herbivore groups that differ in life history or diet breadth, manipulative experiments to parse gut microbe establishment and community assembly, and how gut microbes interact with the immune systems of their hosts. Once these aspects are more well explored, we will be able to design more robust studies to understand how host plant defenses shape insect-gut microbe interactions and fitness outcomes.
References
Acevedo FE, Peiffer M, Tan C et al (2017) Fall armyworm-asscoaciated gut bacteria modualte plant defense responses. Mol Plant-Microbe Interact 30:127–137
Acevedo FE, Rivera-Vega LJ, Chung SH et al (2015) Cues from chewing insects — the intersection of DAMPs, HAMPs, MAMPs and effectors. Curr Opin Plant Biol 26:80–86. https://doi.org/10.1016/j.pbi.2015.05.029
Agrawal AA (2019) A scale-dependent framework for trade-offs, syndromes, and specialization in organismal biology †. Ecology. https://doi.org/10.1002/ecy.2924,101
Appel HM, Maines LW (1995) The influence of host plant on gut conditions of gypsy moth (Lymantria dispar) caterpillars. J Insect Physiol 41:241–246. https://doi.org/10.1016/0022-1910(94)00106-Q
Bandani AR, Kazazi M, Alah Y (2010) Gut pH, and isolation and characterization of digestive α-D-Glucosidase of Sunn Pes. J Agric Sci Technol 12:265–274
Bar-on YM, Phillips R, Milo R (2018) The biomass distribution on earth. Proc Nat Acad Sci 115:6506–6511. https://doi.org/10.1073/pnas.1711842115
Barbehenn RV (2001) Roles of peritrophic membranes in protecting herbivorous insects from ingested plant allelochemicals. Arch Insect Biochem Physiol 47:86–99. https://doi.org/10.1002/arch.1039
Barbehenn RV, Jones CP, Hagerman AE et al (2006) Ellagitannins have greater oxidative activities than condensed tannins and galloyl glucoses at high pH: potential impact on caterpillars. J Chem Ecol 32:2253–2267. https://doi.org/10.1007/s10886-006-9143-7
Barbehenn RV, Maben RE, Knoester JJ (2008) Linking phenolic oxidation in the midgut lumen with oxidative stress in the midgut tissues of a tree-feeding caterpillar Malacosoma disstria (Lepidoptera: Lasiocampidae). Environ Entomol 37:1113–1118. https://doi.org/10.1603/0046-225X(2008)37[1113:LPOITM]2.0.CO;2
Bauer E, Salem H, Marz M et al (2014) Transcriptomic immune response of the cotton stainer dysdercus fasciatus to experimental elimination of vitamin-supplementing intestinal Symbionts. PLoS One 9:12. https://doi.org/10.1371/journal.pone.0114865
Baumann P (2005) Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol 59:155–189. https://doi.org/10.1146/annurev.micro.59.030804.121041
Bell H, Down R, Edwards JP, Gatehouse J (2005) Digestive proteolytic activity in the gut and salivary glands of the predatory bug Podisus maculiventris Heteroptera: Pentatomidae); effect of proteinase inhibitors Euro J Entomol 102:139–145. https://doi.org/10.14411/eje.2005.022
Berasategui A, Salem H, Paetz C et al (2017) Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Mol Ecol 26:4099–4110
Berini F, Caccia S, Franzetti E et al (2015) Effects of Trichoderma viride chitinases on the peritrophic matrix of Lepidoptera. Pest Manag Sci 72:980–989. https://doi.org/10.1002/ps.4078
Bi J, Felton G (1995) Foliar oxidative stress and insect herbivory: primary compounds , secondary metabolites, and reactive oxygen species as components of induced resistance. J Chem Ecol 2:1511–1530
Blankenchip CL, Michels DE, Braker HE, Goffredi SK (2018) Diet breadth and exploitation of exotic plants shift the core microbiome of Cephaloleia, a group of tropical herbivorous beetles. Peer J. https://doi.org/10.7717/peerj.4793,6,e4793
Bolognesi R, Terra WR, Ferreira C (2008) Peritrophic membrane role in enhancing digestive efficiency: theoretical and experimental models. J Insect Physiol 54:1413–1422
Boone CK, Keefover-Ring K, Mapes AC et al (2013) Bacteria associated with a tree-killing insect reduce concentrations of plant defense compounds. J Chem Ecol 39:1003–1006. https://doi.org/10.1007/s10886-013-0313-0
Broderick NA, Raffa KF, Goodman RM, Handelsman J (2004) Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl Environ Microbiol 70:293–300. https://doi.org/10.1128/AEM.70.1.293
Buchon N, Broderick NA, Lemaitre B (2013) Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol 11:615–626. https://doi.org/10.1038/nrmicro3074
Buchon N, Silverman N, Cherry S (2014) Immunity in Drosophila melanogaster-from microbial recognition to whole-organism physiology. Nat Rev Immunol 14:796–810. https://doi.org/10.1038/nri3763
Bulla LA Jr, Rhodes RA, St. Julian G (1975) Bacteria as insect pathogens. Annu Rev Microbiol 29:163–190
Carvalho FA, Aitken JD, Vijay-Kumar M, Gewirtz AT (2012) Toll-like receptor–gut microbiota interactions: perturb at your own risk! Annu Rev Physiol 74:177–198
Ceja-Navarro JA, Nguyen NH, Karaoz U et al (2013) Compartmentalized microbial composition , oxygen gradients and nitrogen fixation in the gut of Odontotaenius disjunctus. ISME J 8:6–18. https://doi.org/10.1038/ismej.2013.134
Chaturvedi S, Rego A, Lucas LK, Gompert Z (2017) Sources of variation in the gut microbial community of Lycaeides melissa caterpillars. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-11781-1
Chen M-S (2008) Inducible direct plant defense against insect herbivores: A review. Insect Sci 15:101–114. https://doi.org/10.1111/j.1744-7917.2008.00190.x
Chen B, Du K, Sun C et al (2018) Gut bacterial and fungal communities of the domesticated silkworm (Bombyx mori) and wild mulberry-feeding relatives. ISME J 12:2252–2262. https://doi.org/10.1038/s41396-018-0174-1
Chen B, Teh B, Sun C et al (2016) Biodiversity and activity of the gut microbiota across the life history of the insect herbivore Spodoptera littoralis. Sci Rep 6:1–14. https://doi.org/10.1038/srep29505
Chen X, Peiffer M, Tan CW, Felton GW (2020) Fungi from the black cutworm Agrotis ipsilon oral secretions mediate plant–insect interactions. Arthropod-Plant Interact:1–10
Chu C-C, Spencer JL, Curzi MJ et al (2013) Gut bacteria facilitate adaptation to crop rotation in the western corn rootworm. Proc Natl Acad Sci 110:11917–11922. https://doi.org/10.1073/pnas.1301886110
Chung SH, Rosa C, Scully ED et al (2013) Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc Natl Acad Sci U S A 110:15728–15733. https://doi.org/10.1073/pnas.1308867110
Chung SH, Scully ED, Peiffer M et al (2017) Host plant species determines symbiotic bacterial community mediating suppression of plant defenses. Sci Rep 7:1–13. https://doi.org/10.1038/srep39690
Dematheis F, Kurtz B, Vidal S, Smalla K (2012) Microbial communities associated with the larval gut and eggs of the Western corn rootworm. PLoS One 7:e44685. https://doi.org/10.1371/journal.pone.0044685
Dillon RJ, Vennard CT, Buckling A, Charnley AK (2005) Diversity of locust gut bacteria protects against pathogen invasion. Ecol Lett 8:1291–1298. https://doi.org/10.1111/j.1461-0248.2005.00828.x
Douglas AE (2015) Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol 60:17–34. https://doi.org/10.1146/annurev-ento-010814-020822
Eichenseer H, Mathews MC, Powell JS, Felton GW (2010) Survey of a salivary effector in caterpillars: glucose oxidase variation and correlation with host range. J Chem Ecol 36:885–897. https://doi.org/10.1007/s10886-010-9830-2
Engel P, Moran NA (2013) The gut microbiota of insects - diversity in structure and function. FEMS Microbiol Rev 37:699–735. https://doi.org/10.1111/1574-6976.12025
Erb M, Meldau S, Howe GA (2012) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17:250–259. https://doi.org/10.1016/j.tplants.2012.01.003
Erb M, Reymond P (2019) Molecular interactions between plants and insect herbivores. Annu Rev Plant Biol 70:527–557. https://doi.org/10.1146/annurev-arplant-050718-095910
Feldhaar H, Gross R (2008) Immune reactions of insects on bacterial pathogens and mutualists. Microbes Infect 10:1082–1088. https://doi.org/10.1016/j.micinf.2008.07.010
Felton GW, Workman J, Duffey SS (1992) Avoidance of antinutritive plant defense: role of midgut pH in Colorado potato beetle. J Chem Ecol 18:571–583
Freitak D, Schmidtberg H, Dickel F et al (2014) The maternal transfer of bacteria can mediate trans-generational immune priming in insects. Virulence 5:547–554. https://doi.org/10.4161/viru.28367
Freitak D, Wheat CW, Heckel DG, Vogel H (2007) Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biol 13:1–13. https://doi.org/10.1186/1741-7007-5-56
Freitas CDT, Viana CA, Vasconcelos IM et al (2016) Plant physiology and biochemistry first insights into the diversity and functional properties of chitinases of the latex of Calotropis procera. Plant Phys Biochem 108:361–371. https://doi.org/10.1016/j.plaphy.2016.07.028
Geib SM, Jimenez-gasco MDM, Carlson JE, Hoover K (2009) Effect of host tree species on cellulase activity and bacterial community composition in the gut of larval Asian longhorned beetle. Environ Entomol 38:686–699
Givskov M, Eberl L, Molin S (1997) Control of exoenzyme production, motility and cell differentiation in Serratia liquefaciens. FEMS Microbiol Lett 148:115–122
Gomes AFF, Omoto C, Cônsoli FL (2020) Gut bacteria of field-collected larvae of Spodoptera frugiperda undergo selection and are more diverse and active in metabolizing multiple insecticides than laboratory-selected resistant strains. J Pest Sci (2004) 93:833–851
Guo J, Hatt S, Wang Z et al (2017) Nine facultative endosymbionts in aphids. A review J Asia Pac Entomol 20:794–801. https://doi.org/10.1016/j.aspen.2017.03.025
Hammer TJ, Janzen DH, Hallwachs W et al (2017) Caterpillars lack a resident gut microbiome. Proc Natl Acad Sci 201707186
Hammer TJ, McMillan WO, Fierer N (2014) Metamorphosis of a butterfly-associated bacterial community. PLoS One 9:e86995. https://doi.org/10.1371/journal.pone.0086995
Hannula S, Zhu F, Heinen R, Bezemer T (2019) Foliar-feeding insects acquire microbiomes from the soil rather than the host plant. Nat Commun 10:1254. https://doi.org/10.1038/s41467-019-09284-w
Hansen AK, Moran NA (2014) The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol Ecol 23:1473–1496
Harper MS, Hopkins TL, Czapla TH (1998) Effect of wheat germ agglutinin on formation and structure of the peritrophic membrane in European corn borer (Ostrinia nubilalis) larvae. Tissue Cell 30:166–176
Harris EV, De Roode JC, Id NMG (2019) Diet – microbiome – disease : investigating diets influence on infectious disease resistance through alteration of the gut microbiome. PLoS Pathog 15:e1007891. https://doi.org/10.1371/journal.ppat.1007891
Hegedus D, Erlandson M, Gillott C, Toprak U (2009) New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol 54:285–302. https://doi.org/10.1146/annurev.ento.54.110807.090559
Hopkins TL, Harper MS (2001) Lepidopteran peritrophic membranes and effects of dietary wheat germ agglutinin on their formation and structure. Arch Insect Biochem Physiol 47:100–109
Hosokawa T, Hironaka M, Mukai H et al (2012) Mothers never miss the moment: a fine-tuned mechanism for vertical symbiont transmission in a subsocial insect. Anim Behav 83:293–300. https://doi.org/10.1016/j.anbehav.2011.11.006
Hoover K, Humphries MA, Gendron AR, Slavicek JM (2010) Impact of viral enhancin genes on potency of Lymantria dispar multiple nucleopolyhedrovirus in L. dispar following disruption of the peritrophic matrix. J Invertebr Pathol 104:150–152
Howe GA (2004) Jasmonates as signals in the wound response. J Plant Growth Regul 23:223–237. https://doi.org/10.1007/s00344-004-0030-6
Insua JL, Llobet E, Moranta D, et al (2013) Modelling Klebsiella pneumoniae pathogenesis by infecting the wax moth Galleria mellonella. Infect Immun IAI-00391
Itoh H, Hori T, Sato Y et al (2018a) Infection dynamics of insecticide-degrading symbionts from soil to insects in response to insecticide spraying. ISME J 12:909–920
Itoh H, Tago K, Hayatsu M, Kikuchi Y (2018b) Detoxifying symbiosis: microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat Prod Rep 35:434–454
James RR, Xu J (2012) Mechanisms by which pesticides affect insect immunity. J Invertebr Pathol 109:175–182
Janssen DB, Dinkla IJT, Poelarends GJ, Terpstra P (2005) Bacterial degradation of xenobiotic compounds: evolution and distribution of novel enzyme activities. Env Microbiol 7:1868–1882. https://doi.org/10.1111/j.1462-2920.2005.00966.x
Johnson KS, Barbehenn RV (2000) Oxygen levels in the gut lumens of herbivorous insects. J Insect Physiol 46:897–903
Johnson KS, Felton GW (1996) Potential influence of midgut pH and redox potential on protein utilization in insect herbivores. Arch Insect Biochem Physiol 105:85–105
Johnson KS, Rabosky D (2000) Phylogenetic distribution of cysteine proteinases in beetles : evidence for an evolutionary shift to an alkaline digestive strategy in Cerambycidae. Comp Biochem Phys Part B: Biochem Molec Biol 126:609–619
Jones A, Mason C, Felton G, Hoover K (2019) Host plant and population source drive diversity of microbial gut communities in two polyphagous insects. Sci Rep 9:1–11
Karban R, Baldwin IT (2007) Induced responses to herbivory. University of Chicago Press
Kariyat RR, Smith JD, Stephenson AG et al (2017) Non-glandular trichomes of Solanum carolinense deter feeding by Manduca sexta caterpillars and cause damage to the gut peritrophic matrix. Proc Roy Soc B: Biol Sci 284:20162323
Kim J, Beom J, Rang Y et al (2015) Burkholderia gut symbionts enhance the innate immunity of host Riptortus pedestris. Dev Comp Immunol 53:265–269. https://doi.org/10.1016/j.dci.2015.07.006
Kim J, Beom J, Am H et al (2016) Understanding regulation of the host-mediated gut symbiont population and the symbiont-mediated host immunity in the Riptortus-Burkholderia symbiosis system. Dev Comp Immunol 64:75–81. https://doi.org/10.1016/j.dci.2016.01.005
Kikuchi Y, Hayatsu M, Hosokawa T et al (2012) Symbiont-mediated insecticide resistance. Proc Natl Acad Sci 109:8619–8622. https://doi.org/10.1073/pnas.1200231109/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1200231109
Kikuchi Y, Hosokawa T, Fukatsu T (2011a) An ancient but promiscuous host-symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J 5:446–460. https://doi.org/10.1038/ismej.2010.150
Kikuchi Y, Hosokawa T, Fukatsu T (2011b) Specific developmental window for establishment of an insect-microbe gut symbiosis. Appl Environ Microbiol 77:4075–4081. https://doi.org/10.1128/AEM.00358-11
Konno K, Mitsuhashi W (2019) The peritrophic membrane as a target of proteins that play important roles in plant defense and microbial attack. J Insect Physiol 117:103912. https://doi.org/10.1016/j.jinsphys.2019.103912
Konno K, Shimura S, Ueno C, Arakawa T (2018) Phytochemistry abnormal swelling of the peritrophic membrane in Eri silkworm gut caused by MLX56 family defense proteins with chitin-binding and extensin domains. Phytochemistry 147:211–219. https://doi.org/10.1016/j.phytochem.2018.01.005
Kuraishi T, Binggeli O, Opota O et al (2011) Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proc Natl Acad Sci 108:15966–15971. https://doi.org/10.1073/pnas.1105994108
Lawrence SD, Novak NG (2006) Expression of poplar chitinase in tomato leads to inhibition of development in Colorado potato beetle. Biotechnol Lett 28:593–599
Martinez AJ, Onchuru TO, Ingham CS et al (2019) Angiosperm to gymnosperm host-plant switch entails shifts in microbiota of the Welwitschia bug, Probergrothius angolensis (distant, 1902). Mol Ecol 28:5172–5187
Mason CJ, Clair AS, Peiffer M et al (2020) Diet influences proliferation and stability of gut bacterial populations in herbivorous lepidopteran larvae. PLoS One 15:e0229848. https://doi.org/10.1371/journal.pone.0229848
Mason CJ, Couture JJ, Raffa KF (2014) Plant-associated bacteria degrade defense chemicals and reduce their adverse effects on an insect defoliator. Oecologia 175:901–910. https://doi.org/10.1007/s00442-014-2950-6
Mason CJ, Jones AG, Felton GW (2019a) Co-option of microbial associates by insects and their impact on plant–folivore interactions. Plant Cell Environ 42:1078–1086. https://doi.org/10.1111/pce.13430
Mason CJ, Raffa KF (2014) Acquisition and structuring of midgut bacterial communities in gypsy moth (Lepidoptera: Erebidae) larvae. Environ Entomol 43:595–604. https://doi.org/10.1603/EN14031
Mason CJ, Ray S, Shikano I, et al (2019b) Plant defenses interact with insect enteric bacteria by initiating a leaky gut syndrome. 6–11. https://doi.org/10.5061/dryad.7254t7d
Mason CJ, Rubert-Nason KF, Lindroth RL, Raffa KF (2015) Aspen defense chemicals influence midgut bacterial community composition of gypsy moth. J Chem Ecol 41:75–84s. https://doi.org/10.1007/s10886-014-0530-1
Mason KL, Stepien TA, Blum JE et al (2011) From commensal to pathogen: translocation of enterococcus faecalis from the midgut to the hemocoel of Manduca sexta. MBio 2:1–7. https://doi.org/10.1128/mBio.00065-11.Editor
Mazumdar T, Teh B-S, Boland W (2018) The microbiome of Spodoptera littoralis: development, control and adaptation to the insect host. Metagenomics Gut Microbes 77
Mazumdar T, Teh BS, Murali A et al (2020) Survival strategies of Enterococcus mundtii in the gut of Spodoptera littoralis: a live report. bioRxiv
Mcfall-Ngai M, Had MG, Bosch TCG et al (2013) Animals in a bacterial world, a new imperative for the life sciences. Proc Nat Acad Sci 110:3229–3236. https://doi.org/10.1073/pnas.1218525110
Minard G, Tikhonov G, Ovaskainen O, Saastamoinen M (2019) The microbiome of the Melitaea cinxia butterfly shows marked variation but is only little explained by the traits of the butterfly or its host plant. Environ Microbiol 21:4253–4269
Mohan S, Ma PWK, Williams WP, Luthe DS (2008) A naturally occurring plant cysteine protease possesses remarkable toxicity against insect pests and synergizes Bacillus thuringiensis toxin. PLoS One 3:1–7. https://doi.org/10.1371/journal.pone.0001786
Moran NA, Ochman H, Hammer TJ (2019) Evolutionary and ecological consequences of gut microbial communities. Annu Rev Ecol Syst 50:451–475
Napoleão TH, Albuquerque LP, Santos NDL et al (2019) Insect midgut structures and molecules as targets of plant-derived protease inhibitors and lectins. Pest Manag Sci 75:1212–1222
Nishide Y, Kageyama D, Yokoi K et al (2019) Functional crosstalk across IMD and toll pathways: insight into the evolution of incomplete immune cascades. Proc Roy Soc B 286:20182207
Oliver KM, Degnan PH, Burke GR, Moran NA (2010) Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55:247–266
Olsen GL, Myers JH, Hemerik L, Cory JS (2017) Phylloplane bacteria increase the negative impact of food limitation on insect fitness. Ecol Entomol 42:411–421
Onchuru T, Kaltenpoth M (2019) Established cotton stainer gut bacterial mutualists evade regulation of host antimicrobial peptides. Appl Environ Microbiol 85:1–14
Paniagua Voirol LR, Frago E, Kaltenpoth M et al (2018) Bacterial symbionts in Lepidoptera: their diversity, transmission, and impact on the host. Front Microbiol 9:556
Pechan T, Cohen A, Williams WP, Luthe DS (2002) Insect feeding mobilizes a unique plant defense protease that disrupts the peritrophic matrix of caterpillars. Proc Natl Acad Sci 99:13319–13323
Peng J, Zhong J, Granados RR (1999) A baculovirus enhancin alters the permeability of a mucosal midgut peritrophic matrix from lepidopteran larvae. J Insect Physiol 45:159–166
Phalnikar K, Kunte K, Agashe D (2018) Dietary and developmental shifts in butterfly-associated bacterial communities. Royal Society Open Science 5(5):171559
Phalnikar K, Kunte K, Agashe D (2019) Disrupting butterfly caterpillar microbiomes does not impact their survival and development. Proc R Soc B 286:20192438
Pilon FM, da Silva CR, Visôtto LE et al (2017) Purification and characterization of trypsin produced by gut bacteria from Anticarsia gemmatalis. Arch Insect Biochem Physiol 96:e21407. https://doi.org/10.1002/arch.21407
Priya NG, Ojha A, Kajla MK et al (2012) Host plant induced variation in gut bacteria of Helicoverpa armigera. PLoS One 7:e30768
Ravenscraft A, Berry M, Hammer T et al (2019a) Structure and function of the bacterial and fungal gut microbiota of Neotropical butterflies. Ecol Monogr 89:e01346. https://doi.org/10.1002/ecm.1346
Ravenscraft A, Kish N, Peay K, Boggs C (2019b) No evidence that gut microbiota impose a net cost on their butterfly host. Mol Ecol 28:2100–2117
Rees JS, Jarrett P, Ellar DJ (2009) Peritrophic membrane contribution to Bt Cry δ-endotoxin susceptibility in Lepidoptera and the effect of Calcofluor. J Invertebr Pathol 100:139–146
Richards LA, Glassmire AE, Ochsenrider KM et al (2016) Phytochemical diversity and synergistic effects on herbivores. Phytochem Rev 15:1153–1166. https://doi.org/10.1007/s11101-016-9479-8
Salem H, Bauer E, Kirsch R et al (2017) Drastic genome reduction in an herbivore’s pectinolytic symbiont. Cell 171:1520–1531.e13. https://doi.org/10.1016/j.cell.2017.10.029
Salem H, Florez L, Gerardo N, Kaltenpoth M (2015) An out-of-body experience: the extracellular dimension for the transmission of mutualistic bacteria in insects. Proc R Soc London B Biol Sci 282:20142957
Salem H, Kreutzer E, Sudakaran S, Kaltenpoth M (2012) Actinobacteria as essential symbionts in firebugs and cotton stainers (Hemiptera, Pyrrhocoridae). Environ Microbiol 15:1956–1968. https://doi.org/10.1111/1462-2920.12001
Sansone CL, Cohen J, Yasunaga A et al (2015) Microbiota-dependent priming of antiviral intestinal immunity in Drosophila. Cell Host Microbe 18:571–581
Schuman MC, Baldwin IT (2016) The layers of plant responses to insect herbivores. Annu Rev Entomol 61:373–394. https://doi.org/10.1146/annurev-ento-010715-023851
Shao Y, Arias-Cordero E, Guo H et al (2014) In vivo pyro-SIP assessing active gut microbiota of the cotton Leafworm, Spodoptera littoralis. PLoS One 9:e85948. https://doi.org/10.1371/journal.pone.0085948
Shikano I (2017) Evolutionary ecology of multitrophic interactions between plants, insect herbivores and entomopathogens. J Chem Ecol 43:586–598. https://doi.org/10.1007/s10886-017-0850-z
Smilanich AM, Dyer LA, Chambers JQ, Bowers MD (2009) Immunological cost of chemical defence and the evolution of herbivore diet breadth. Ecol Lett 12:612–621. https://doi.org/10.1111/j.1461-0248.2009.01309.x
Smilanich AM, Vargas J, Dyer LA, Bowers MD (2011) Effects of ingested secondary metabolites on the immune response of a polyphagous caterpillar Grammia incorrupta. J Chem Ecol 37:239–245
Smith TE, Moran NA (2020) Coordination of host and symbiont gene expression reveals a metabolic tug-of-war between aphids and Buchnera. Proc Nat Acad Sci 117:2113–2121. https://doi.org/10.1073/pnas.1916748117
Sorokan AV, Burkhanova GF, Benkovskaya GV, Maksimov IV (2019) Colorado potato beetle microsymbiont Enterobacter BC - 8 inhibits defense mechanisms of potato plants using crosstalk between jasmonate - and salicylate - mediated signaling pathways. Arthropod Plant Interact 14:161–168. https://doi.org/10.1007/s11829-019-09732-w
Staudacher H, Kaltenpoth M, Breeuwer JAJ et al (2016) Variability of bacterial communities in the moth heliothis virescens indicates transient association with the host. PLoS One 11:e0154514. https://doi.org/10.5061/dryad.dv35j.Funding
Su’ad AY, Harrison JG, Philbin CS et al (2019) Host plant-dependent effects of microbes and phytochemistry on the insect immune response. Oecologia 191:141–152
Sudakaran S, Kost C, Kaltenpoth M (2017) Symbiont acquisition and replacement as a source of ecological innovation. Trends Microbiol 25:375–390
Sudakaran S, Salem H, Kost C, Kaltenpoth M (2012) Geographical and ecological stability of the symbiotic mid-gut microbiota in European firebugs, Pyrrhocoris apterus (Hemiptera, Pyrrhocoridae). Mol Ecol 21:6134–6151. https://doi.org/10.1111/mec.12027
Sudha PM, Muthu SP (1988) Damage to the midgut epithelium caused by food in the absence of peritrophic membrane. Curr Sci 57:624–625
Summers CB, Felton GW (1994) Prooxidant effects of phenolic acids on the generalist herbivore. J Chem Ecol 24:943–953
Taylor CM, Coffey PL, DeLay BD, Dively GP (2014) The importance of gut symbionts in the development of the brown marmorated stink bug, Halyomorpha halys (Stål). PLoS One 9:e90312. https://doi.org/10.1371/journal.pone.0090312
Teh B-S, Apel J, Shao Y, Boland W (2016) Colonization of the intestinal tract of the polyphagous pest Spodoptera littoralis with the GFP-tagged indigenous gut bacterium Enterococcus mundtii. Front Microbiol 7:928
Thaler JS, Humphrey PT, Whiteman NK (2012) Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci 17:260–270
Valaitis AP, Podgwaite JD (2013) Bacillus thuringiensis Cry1A toxin-binding glycoconjugates present on the brush border membrane and in the peritrophic membrane of the Douglas-fir tussock moth are peritrophins. J Invertebr Pathol 112:1–8
Vallet-Gely I, Lemaitre B, Boccard F (2008) Bacterial strategies to overcome insect defences. Nat Rev Microbiol 6:302–313. https://doi.org/10.1038/nrmicro1870
van den Bosch TJM, Welte CU (2017) Detoxifying symbionts in agriculturally important pest insects. Microb Biotechnol 10:531–540
Visôtto LE, Oliveira MG, Guedes RNC et al (2009) Contribution of gut bacteria to digestion and development of the velvetbean caterpillar, Anticarsia gemmatalis. J Insect Physiol 55:185–191. https://doi.org/10.1016/j.jinsphys.2008.10.017
Walling LL (2008) Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol 146:859–866
Walski T, Van Damme EJM, Smagghe G (2014) Penetration through the peritrophic matrix is a key to lectin toxicity against Tribolium castaneum. J Insect Physiol 70:94–101. https://doi.org/10.1016/j.jinsphys.2014.09.004
Wang J, Chung SH, Peiffer M et al (2016) Herbivore oral secreted bacteria trigger distinct defense responses in preferred and non-preferred host plants. J Chem Ecol 42:463–474. https://doi.org/10.1007/s10886-016-0712-0
Wang J, Gao Z, Yang M et al (2020) geographically isolated Colorado potato beetle mediating distinct defense responses in potato is associated with the alteration of gut microbiota. J Pest Sci 93:379–390. https://doi.org/10.1007/s10340-019-01173-x
Wang J, Hoover K, Felton GW et al (2017) Helicoverpa zea gut-associated bacteria indirectly induce defenses in tomato by triggering a salivary elicitor (s). New Phytol 214:1294–1306. https://doi.org/10.1111/nph.14429
Wang J, Yang M, Song Y et al (2018) Gut-associated bacteria of Helicoverpa zea indirectly trigger plant defenses in maize. J Chem Ecol 44:609–699
Wang J, Gao Z, Yang M, Xue R, Yan H, Kaiyun F, Zhang Z, Guo W, Felton GW, Zeng R (2020) Geographically isolated Colorado potato beetle mediating distinct defense responses in potato is associated with the alteration of gut microbiota. J Pest Sci 93(1):379–390
Wasano N, Konno K, Nakamura M, et al (2009) Phytochemistry A unique latex protein , MLX56, defends mulberry trees from insects. Phytochemistry 70:880–888. https://doi.org/10.1016/j.phytochem.2009.04.014
Whitaker MRL, Salzman S, Sanders J et al (2016) Microbial communities of lycaenid butterflies do not correlate with larval diet. Front Microbiol 7:1920
Wu K, Yang B, Huang W et al (2016) Gut immunity in lepidopteran insects. Dev Comp Immunol 64:65–74. https://doi.org/10.1016/j.dci.2016.02.010
Xiao R, Wang X, Xie E et al (2019) An IMD-like pathway mediates the intestinal immunity to modulate the homeostasis of gut microbiota in Rhynchophorus ferrugineus Olivier (Coleoptera : Dryophthoridae). Dev Comp Immunol 97:20–27. https://doi.org/10.1016/j.dci.2019.03.013
Xu L, Lou Q, Cheng C et al (2015) Gut-associated bacteria of dendroctonus valens and their involvement in verbenone production. Microb Ecol 70:1012–1023. https://doi.org/10.1007/s00248-015-0625-4
Acknowledgements
I appreciate the discussions with Alissa Hanshew, Swayamjit Ray, Ikkei Shikano, Asher Jones, Kelli Hoover, and Gary Felton that helped form the core ideas in this paper. I thank Nick Sloff for the illustration depicted in Fig. 1. I would also like to thank the anonymous reviewers and Kerry Mauck for guidance and suggestions in earlier draft iterations. Funding was provided by a United States Department of Agriculture NIFA Postdoctoral Fellowship (2018-67012-27979).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mason, C.J. Complex Relationships at the Intersection of Insect Gut Microbiomes and Plant Defenses. J Chem Ecol 46, 793–807 (2020). https://doi.org/10.1007/s10886-020-01187-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-020-01187-1