Abstract
In the past years, neuroinflammation has been widely investigated in Alzheimer’s disease (AD). Evidence from animal, in vivo and post-mortem studies has shown that inflammatory changes are a common feature of the disease, apparently happening in response to amyloid-beta and tau accumulation. Progress in imaging and fluid biomarkers now allows for identifying surrogate markers of neuroinflammation in living individuals, which may offer unprecedented opportunities to better understand AD pathogenesis and progression. In this context, inflammatory mediators and glial proteins (mainly derived from microglial cells and astrocytes) seem to be the most promising biomarkers. Here, we discuss the biological basis of neuroinflammation in AD, revise the proposed neuroinflammation biomarkers, describe what we have learned from anti-inflammatory drug trials, and critically discuss the potential addition of these biomarkers in the AT(N) framework.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since its first description in 1906, Alzheimer’s disease (AD) has been consistently associated with amyloid-beta (Aβ) plaques and tau neurofibrillary tangles. The pathological potential of glial cells has also been described, but less mentioned, by Alois Alzheimer’s original reports (1). Neuroinflammation was later associated with the disease’s pathological process, likely playing a fundamental role in potentiating Aβ and tau pathologies in the brain (2).
In the last years, AD diagnosis shifted from a clinical construct to a biological definition, allowing for identifying pre-symptomatic AD stages — the so-called preclinical AD. The National Institute on Aging — Alzheimer’s Associacion (NIA-AA) 2018 proposed a research framework based on biomarkers, independently from clinical presentations — the AT(N) system (3). It essentially defines AD according to the biomarker positivity for Aβ (A), Tau (T), and neurodegeneration (N). Moreover, this dynamic system may incorporate novel candidate biomarkers to provide information about additional pathophysiological mechanisms represented by other letters. Hampel and colleagues propose using the letter “X” to represent an additional group of promising pathological markers, with neuroinflammation being of high interest (4).
Neuroinflammation in AD is typically associated with glial changes in the brain. This complex process includes microglial cells and astrocytes associated with a cascade of inflammatory mediators and modulators (2). A growing body of evidence suggests that the inflammatory process may occur in the early stages of AD, potentiating the accumulation of insoluble Aβ and tau (5). It is thought that inflammatory changes are a response to Aβ and tau pathologies, but one cannot rule out that they are triggering the deposition of Aβ and tau in the brain (6–8). Also, early inflammation might protect against protein accumulation, which could explain why some studies have shown conflicting results (9, 10). Thus, biomarkers of neuroinflammation may become useful for early diagnosis, prognosis, and potential drug-target engagement in the secondary prevention of AD.
In this review, we explore the biological basis of neuroinflammation in AD, especially the roles of microglial cells and astrocytes, and provide detailed information on current biomarkers of neuroinflammatory changes. We also describe advances and contributions of anti-inflammatory drug trials and discuss the potential of adding biomarkers of neuroinflammation in the AT(N) framework.
The biology of neuroinflammation
To understand the biology of neuroinflammation, we first need to discuss the basic concepts of inflammation. According to the Nature Portfolio, inflammation is “a biological response to harmful stimuli, such as pathogens, damaged cells or irradiation. It is a protective attempt by the organism to remove injurious stimuli and to initiate the healing process. It is characterized by pain, redness, heat, swelling and disturbance of function” (11). These cardinal signs of inflammation were described in the first century by the Roman encyclopedist Aulus Celsus. The absence of signs of inflammation in diseases of the central nervous system (CNS) was historically behind the dogma of the brain as an “immune-privileged” organ (12, 13). This idea changed in the last decades when histopathological studies identified neuroinflammatory changes in the brain. Currently, neuroinflammation refers mainly to the dynamic responses of microglia and astrocytes and has been associated with neurodegenerative disorders such as AD (14).
As for the brain, it is important to differentiate the adaptive immune response, a core feature of multiple sclerosis and autoimmune encephalitis, from the innate immune response, mostly as seen in neurodegenerative diseases (15). The first involves adaptive immune cells such as T and B lymphocytes and NK cells, along with myeloid cells like monocytes, invading the CNS through the blood-brain barrier (BBB) and directly provoking local modifications. On the other hand, the innate response involves mostly microglia and astrocytes becoming active/reactive in response to various stimuli. In an attempt to halt hazardous threats, this response happens by promoting local alterations in tissue homeostasis, phagocytosis and degradation of small aggregates, as well as the release of signaling and toxic molecules (16). Clinical evidence suggests that innate and adaptive immnune responses occur in AD and seem dependent on the disease stage (17).
Microglial cells, the brain-resident immune cells, are continuously active and change their phenotypes in response to inflammatory stimuli. Indeed, microglia play a complex role in the trajectories of AD, acquiring multiple phenotypes through the activation of different pathways (18). For a long time, microglial responses were considered exclusively detrimental to AD progression. However, more recently, protective subtypes of microglial responses were identified in AD (19). While there is still room for discussion about the protective/detrimental role of microglia in AD, it seems clear that its activation is stage-dependent and that multiple phenotypes may co-exist in particular disease stages. Activated microglia have been extensively studied in immune surveillance, debris phagocytosis, regulation of neuronal apoptosis, synaptic plasticity, and pruning. Depending on the original insult, microglia can recognize abnormal molecules, become activated into heterogeneous morphologies, and promote a context-specific response. This response usually involves internalizing and degrading molecules using different endocytic pathways and releasing pro-inflammatory factors, such as cytokines, chemokines, and reactive oxygen species (ROS). It is proposed that repeated exposure to hazardous stimuli might cause microglia to become aberrantly active, sustaining noxious conditions to the CNS and, eventually, leading to, or accelerating, neurodegeneration (20, 21).
Astrocytes, an abundant glial cell type in the human brain, carry many important physiological roles in the CNS, such as regulation of synaptic function, maintenance of the BBB, calcium signaling, energy supply to neurons, homeostasis of neurotransmitters and ions, and release of gliotransmitters (22). Along with these homeostatic roles, astrocytes can also respond to inflammatory stimuli by becoming reactive. Reactive astrogliosis refers to molecular, functional, and morphological changes that may impact the brain environment during pathological stimuli, such as hypoxia, cytokines, misfolded proteins, low glucose supply, neurotransmitter imbalance, and ROS (23–26). Reactive astrocytes have been reported in numerous diseases and are typically observed in post-mortem AD brains, colocalizing with Aβ and tau pathologies (27). While reactive astrocytes were initially considered a homogeneous population, nowadays, there is a consensus that astrocytes assume multiple phenotypes in response to different pathological stimuli (26, 28). The interplay between microglial cells and astrocytes deserves attention since microglia can trigger astrocyte reactivity, increasing the release of cytokines and chemokines and generating more ROS (29). In addition to this orchestrated response, in vitro studies demonstrated that astrocytes can independently react to inflammatory insults by activating classical inflammation-related pathways (30, 31). However, unlike microglia, astrocytes are not primarily inflammatory cells, and their repertoire of functions is broader. Thus, it is important to highlight that astrocyte responses are not always associated with inflammatory changes.
Interestingly, activated microglia and reactive astrocytes colocalize with Aβ plaques and tau tangles in post-mortem studies, suggesting that inflammation is associated with AD core pathological features. Additionally, it was shown that misfolded proteins act as danger-associated molecular patterns, triggering microglial activation and astrocyte reactivity via surface and toll-like receptors (32). In addition, the recent genetic risk factors described for AD are mainly from inflammatory pathways, strengthening the theory of an amyloid-inflammatory cascade in the disease pathogenesis (33). Another important hypothesis in the genesis of neurodegenerative diseases is inflammaging (34). It proposes that an impairment in any of the highly interconnected seven pillars of aging — stem cell regeneration, metabolism, inflammation, proteostasis, macromolecular damage, stress, and epigenetics — affects all other pillars. The insidious disruption in this aging network builds up to a persistent state of low-grade inflammation, chronically activating the immune system and accelerating senescence. It is proposed that inflammaging byproducts in the periphery can enter the CNS and trigger proinflammatory states in glial cells, eventually leading to neurodegeneration (35, 36). Thus, the inflammatory component of AD cannot be neglected and deserves further investigation. To date, a few indirect fluid and imaging biomarkers of neuroinflammation have been investigated and provided the initial basis for understanding the role of neuroinflammation in AD.
Biomarkers of neuroinflammation in AD
Fluid biomarkers
Microglia
In the brain, the triggering receptor expressed on myeloid cells 2 (TREM2) is almost uniquely expressed by microglia and is mostly upregulated on by these cells around amyloid plaques in AD. Secretase shedding of the receptor ectodomain gives rise to soluble TREM2 (sTREM2), which can be detected in the blood and cerebrospinal fluid (CSF). An increase in sTREM2 proteolytic shedding seems directly related to decreased TREM2 activity (37). The activation of TREM2 in animal models has produced controversial results, either ameliorating pathological phenotypes (38) or exacerbating AD pathology’s spreading (39).
The sTREM2 has been increasingly explored as a biomarker of AD, however, its performance in discriminating between clinical diagnoses is still under investigation. While some studies observed increased significant differences between cognitively unimpaired (CU) and AD dementia individuals’ (40, 41) other reports found no alterations (42–48). However, CSF sTREM2 levels seem to fluctuate according to changes in the hallmarks of AD pathology. Specifically, CSF sTREM2 levels decrease in response to CSF Aβ1–42 (49, 50) but increase in response to CSF tau elevation (total tau or phosphorylated tau) (50), suggesting it is a stage-specific biomarker. Additionally, high levels of sTREM2 are associated with high levels of neurofilament light chain (NfL), and with an increased CSF/plasma albumin ratio, which indicates lower BBB integrity (42). Cross-sectional studies pointed to a protective effect of sTREM2 in mild cognitive impairment (MCI), as individuals with higher sTREM2 present a higher gray matter volume in the bilateral inferior and medial temporal cortices and precuneus as in the left supramarginal gyrus (48).
Longitudinal studies were key to better understanding the dynamic changes in microglial states. In line with this, it was demonstrated that higher baseline levels of sTREM2 are associated with slower gray matter volumetric loss in parahippocampal gyrus, left fusiform cortex, left middle temporal gyrus, and left lateral occipital cortex (51). Baseline CSF sTREM2 levels predicted longitudinal memory decline but not longitudinal worse executive functioning (42, 43, 52). Importantly, individuals with presymptomatic AD with a steeper longitudinal increase in sTREM2 presented a slower rate of cognitive decline (53). Similarly, higher sTREM2 levels are associated with slower clinical decline in the dementia stage of AD (54, 55). A higher annual increase in sTREM2 is associated with a diminished annual rate of Aβ accumulation in presymptomatic carriers of pathogenic variants (53) and individuals across the AD continuum (56). The temporal changes of microglial activation fluid biomarkers in AD can be seen in Figure 1.
Temporal progression of neuroinflammation fluid biomarkers in Alzheimer’s disease
Hypothetical curves of fluid neuroinflammatory biomarkers across the AD continuum. Plasma biomarker findings from previous studies are represented by rectangles, while CSF biomarker findings are represented by ellipses. The dotted lines show hypothetical relative biomarker abnormalities and are not based on previous studies. Curves are color-coded, with orange indicating astrocyte biomarkers and blue indicating microglia biomarkers. It’s important to note that the magnitudes of other biomarkers presented in the graph are relative to their own normal range and should not be compared across different biomarkers. Abbreviations: AD — Alzheimer’s disease; MCI — mild cognitive impairment; CSF — cerebrospinal fluid; sTREM2 — soluble triggering receptor expressed in myeloid cells 2; YKL-40 — chitinase-3-like protein 1; GFAP — glial fibrillary acidic protein; S100B — calcium-binding protein B
Astrocytes
The glial fibrillary acidic protein (GFAP) is an intermediate filament protein of astrocytes. Because it has been considered a canonical marker of reactive astrocytes for a long time, it was the first astrocyte protein explored as a biomarker in AD. Initial studies on CSF GFAP in AD date from the 90s and have already pointed to a significant increase in GFAP levels in patients with AD dementia compared to CU controls (57, 58). GFAP in the CSF seems to have diagnostic value and correlated with disease severity (59). However, CSF GFAP does not seem sensitive enough to detect the early phases of dementia, as no differences were found between CU and MCI Aβ-positive (60, 61). However, an association was observed between CSF GFAP and Aβ pathology in CU individuals, as assessed by PET and CSF biomarkers (62, 63). It is important to consider that an age-related increase in CSF GFAP levels was observed in CU individuals, contributing to the idea that GFAP is an unspecific marker (57, 64). Additionally, CSF GFAP levels are increased in other neurodegenerative conditions, such as frontotemporal lobe dementia (65, 66), Creutzfeldt-Jakob disease (67), Parkinson’s disease (65), and vascular dementia (58).
The recent development of ultrasensitive techniques allowed the detection of brain-derived proteins in the blood in low concentrations. Surprisingly, plasma GFAP seems to better predict AD pathology than CSF GFAP (60, 61, 65), detecting amyloid load before symptoms onset. Part of the explanation of plasma GFAP’s better performance compared to CSF relies on sample stability. Specifically, GFAP in the blood is less vulnerable to freeze-thaw cycles, making it a better matrix than CSF (68). Specifically, studies in CU older individuals at risk for AD (i.e., Aβ-positive) showed that plasma GFAP associates with Aβ (69, 70). Additionally, individuals with autosomal dominantly inherited familial AD (mutation carriers) have higher levels of plasma GFAP, even in the presymptomatic phase, compared to non-mutation carriers (71). Plasma GFAP is associated with Aβ but not tau pathology (61, 72), which might explain why plasma GFAP is a better marker of early AD pathology. It was demonstrated that plasma GFAP increases the ability of other plasma biomarkers to distinguish between Aβ-negative and Aβ-positive individuals (69). Increased plasma GFAP levels are also associated with other biological findings in AD, such as decreased cortical thickness (72, 73), decreased hippocampal volume (73), and white matter hyperintensity (72, 74).
Longitudinal studies also point the prognostic utility of plasma GFAP. Initial findings showed that plasma GFAP measures in MCI can predict conversion to AD within a five-year window (75). Plasma GFAP can also be combined with other AD plasma biomarkers to add predictive value in detecting clinical progression (76). A recent study demonstrated that plasma GFAP levels were associated with a greater risk of clinical AD incidence more than a decade before diagnosis (77). Higher baseline GFAP levels were associated with a steeper rate of decline in cognitive domains (76, 78). Like CSF GFAP, plasma GFAP seems to increase as a function of aging (69).
YKL-40 is a secreted glycoprotein expressed in several tissues and involved in immune system activation. In the brain, it is mainly produced by reactive astrocytes during neuroinflammatory conditions (79). Through numerous studies, CSF YKL-40 levels are increased in MCI and AD (80–82), but its role in AD pathophysiology is still poorly understood. Notably, only ∼10% of astrocytes express YKL-40 in the human cortex and hippocampus (79). Thus, YKL-40 measures likely reflect a more specific astrocyte population than GFAP. Additionally, temporal analysis of AD biomarkers shows that CSF YKL-40 levels increase later than other biomarkers, which might indicate that this biomarker changes in response to AD pathology (i.e., Aβ and tau) (83). However, it was estimated that in individuals with familial AD, CSF YKL-40 levels start to increase at least 15 years prior to symptoms onset (84). These apparently contradictory findings might highlight important pathological differences between sporadic and familial AD cases.
In addition, CSF YKL-40 levels increase with age, with a steeper elevation observed in at-risk APOE ε4 carriers (85–87). Although neuropathological studies have identified YKL-40—positive astrocytes in clusters near Aβ plaques, its levels in the CSF are more related to tau (either fluid or PET biomarkers) than Aβ pathology in CU and cognitively impaired (CI) individuals (79, 88–90). Interestingly, CSF YKL-40 seems to positively associate with hippocampal atrophy and cognitive impairment in individuals in the AD continuum (88); however, in CU individuals, CSF YKL-40 was associated with higher grey matter volumes and [18F]Fluorodeoxyglucose ([18F] FDG) metabolism (91), which might represent a transient astrocytic response to AD pathology in the early stages of the disease. Longitudinal studies corroborate cross-sectional findings demonstrating the YKL-40 association with brain atrophy and cortical thickness (92). Baseline levels of both YKL-40 in MCI predicted progression to AD with a hazard ratio of 3 (82).
CSF YKL-40 might be altered in other neurodegenerative conditions, as already demonstrated in frontotemporal dementia (93), and Creutzfeldt-Jakob disease (79). Additionally, in individuals with behavioral variant FTD, the presence of positive AD biomarkers is associated with higher levels of YKL-40 compared to those with negative AD biomarkers (87). Similarly, it was observed that YKL-40 is only increased in Lewy body dementia presenting AD as a co-pathology (47), suggesting that the increase is driven by AD-related neurodegeneration.
YKL-40 is significantly produced outside of the brain (e.g., macrophages, chondrocytes, vascular smooth muscle cells, and some types of cancer cells), which might make it difficult to use plasma YKL-40 as a proxy of brain neuroinflammation. Indeed, studies observed only a modest correlation between CSF and plasma YKL-40 levels (94, 95). Differently from CSF, studies diverge about the utility of plasma YKL-40 for distinguishing between CU and AD individuals. Additionally, no correlation between plasma YKL-40 and Aβ42, tau, p-tau181, or cortical amyloid load was observed (95). Finally, because plasma YKL-40 is increased in several non-neurodegenerative diseases, adding numerous confounding factors, its utility as a plasma AD biomarker might be limited.
In the brain, S100B is mostly expressed in astrocytes with a minor expression in other glial cell types, such as oligodendrocytes (96). Despite the fact that S100B biological functions are still not precisely described, it is known that under non-pathological conditions, small amounts of this protein are released and present neurotrophic effects. However, its increased release by reactive astrocytes seems to enhance neuroinflammation (97). The S100B expression is not confined to the CNS, and because many non-neural cell types produce and release S100B, the use of blood measures of this biomarker as a proxy of brain pathology is limited. In this context, only a moderate correlation between plasma and CSF S100B was observed (98). Thus, the CSF measures of S100B may represent a more reliable measure of reactive astrogliosis.
However, findings regarding CSF S100B in AD are contradictory (90, 98–101). A few studies demonstrated a moderate increase in CSF S100B in AD, especially in patients with mild to moderate AD (CDR = 1–2) (99, 100). However, no differences between CU and AD were observed in later studies (101, 102). In fact, our recent meta-analysis synthesized the literature findings about changes in CSF S100B levels in AD and found no significant differences compared to healthy controls (80). Additionally, no associations between S100B and CSF Aβ1–42 levels were observed in AD patients (103), and no difference was found in S100B levels between CU Aβ-negative and CU Aβ-positive individuals (104). Finally, as observed for other astrocyte biomarkers, levels of CSF S100B were increased in other neurodegenerative diseases, such as Lewy body dementia and Parkinson’s disease (98). The temporal changes in fluid biomarkers of reactive astrocytes across the AD continuum is depicted in Figure 1.
Other fluid biomarkers of inflammation
Other inflammatory fluid biomarkers measured in AD are non-cell and non-disease specific such as cytokines, chemokines, and growth factors. Cytokines are a heterogeneous group of proteins that can be synthesized and secreted by most cells in the human body. Cytokines can be classified as pro- or anti-inflammatory according to their response to a foreign threat. The coordinated and time-limited action of these inflammatory mediators is key to eliminating the invading pathogens and re-establishing the body’s homeostasis. Although several pro-inflammatory proteins [e.g., interleukin (IL) 6, IL-1β and transforming growth factor beta (TGF- β)] were already found around Aβ plaques in the AD brain (105), their changes represent unspecific alterations of the immune system. Because numerous peripheral factors might affect CSF cytokine levels, studies measuring these proteins in body fluids are heterogeneous. Their use as a biomarker in AD seems limited due to several confounding factors (106). Finally, the lack of longitudinal studies evaluating cytokine expression/release undermines the interpretation of these cytokines as prognostic markers in AD.
PET Biomarkers
Microglia
Due to its response to pathological alterations in the AD brain, especially in the early stages, microglial changes are promising imaging biomarkers of neuroinflammation. PET imaging targeting microglia has been a challenge because of the complexity of microglial responses and the inability of the exams to capture different microglial phenotypes (107, 108). Currently, a few molecular targets have been proposed to assess microglial alterations using PET: 18kDa translocator protein (TSPO), colony-stimulating factor-1 receptor (CSF1R), cannabinoid receptor type 2 (CB2R), P2Y12 receptor (P2Y12R), P2X7 receptor (P2X7R), and TREM1 and TREM2. From the ones described above, the most widely investigated PET microglial target is the TSPO.
In the brain, TSPO is present in the outer mitochondrial membrane of microglial cells, astrocytes, and endothelial cells. Under pathological conditions, TSPO is upregulated in microglial cells. Thus, TSPO-PET has been proposed as a marker of microglial activation (109). Indeed, the TSPO-PET signal is increased in vulnerable regions of AD. The [11C](R)PK11195-PET, the first TSPO tracer, presents high binding in AD-related brain regions such as frontal, temporal, parietal, and occipital cortices and hippocampus in dementia stages (110). Fan et al. (2015) (111) observed a persistent increase in the TSPO density at different stages of the disease in a 17-month longitudinal study.
The second-generation of TSPO tracers also identified increased binding in AD patients. AD participants that underwent [11C]DPA713-PET imaging presented a widespread increase in binding potential (BPND) values compared to young and older healthy controls, whereas [11C](R)PK11195 binding increase was restricted to only a few regions in the same patients (112). In another study, higher [11C]DAA1106 binding was observed in MCI individuals that developed dementia within 5 years. However, it was unable to predict the clinical outcome in terms of the type of dementia due to similar patterns of [11C]DAA1106 uptake in AD and Lewy body dementia converters (113). Conversely, [11C]PBR-28-PET had conflicting results. Kreisl et al (2016) (114) observed higher binding in cortical regions of AD patients. However, in a similar study (115), AD patients displayed displayed a trend for increased uptake in some brain regions, but they were not significant. It should be noted that a common single-nucleotide polymorphism (rs6971) in exon 4 of the TSPO gene is responsible for a significant variation of binding affinity of the second generation TSPO radiopharmaceuticals, which may undermine its use without genetic testing. A new generation of TSPO radiotracers has been recently developed aiming to avoid the need of genetic testing (116). The [18F]GE-180, a third-generation TSPO tracer, showed increased PET signal in cortical areas compared to healthy controls, supporting the hypothesis of increased microglial activation in AD (40, 117). The [11C]ER176 presents favorable kinetics (118), high signal-to-noise ratio, and volume of distribution stability (119), however, no data with AD patients is available yet. In summary, TSPO tracers have been useful to identify TSPO overexpression in the human brain, but the lack of specificity for microglial cells is a limiting factor that should be carefully considered and debated (120, 121).
The selectivity of microglial PET tracers has been a matter of debate, with multiple targets being explored. In this context, the CSF1R is only expressed on the cell surface of microglia and macrophages (122). At the moment, only a few CSF1R tracers have been developed, such as [11C]CPPC and [11C]AZ683. The [11C] CPPC-PET consistently identified higher binding in the cortex, hippocampus, and cerebellum of a mouse model of amyloidosis (16-month-old APP mice), suggesting its capability of detecting microglial changes (123). No human PET studies with these tracers have been conducted so far, but histological analysis in post-mortem tissue confirmed increased CSF1R density in the AD brain (124, 125).
The CB2R is a key player in the endocannabinoid system but is very little expressed in in the homeostatic brain. The upregulation of C2BR occurs in microglial cells as a response to immune activation. However, the [11C] NE40, a novel PET tracer for CB2R, identified overall lower CB2R availability in AD mouse model brains, which remains to be better explored. The authors suggest that CB2R plays an important role in microglial functions related to initiation, maintenance, and removal of Aβ plaques (126).
Purinergic receptors are also explored as potential targets for imaging microglial cells with PET. P2Y12R is a cell-surface protein expressed by microglia in the brain, its expression in other brain cells remains unclear (127). Under neuroinflammatory conditions, activated microglia overexpresses PRY12R. To date, three PET tracers have been synthesized, but their BBB permeability needs to be improved. Similarly, P2X7R is expressed in microglial cells, oligodendrocytes and Schwann cells (128). In brain autoradiography experiments, a P2X7 analogue, [11C]SMW139, showed no difference between AD and healthy individuals (129). Finally, although no clinical study has used PET imaging with TREM tracers, a few advances have been made using novel TREM1 and TREM2 tracers in animal models of neurodegenerative disorders (124, 130). The temporal changes in imaging biomarkers targeting microglial activation in AD can be seen in Figure 2.
Temporal progression of neuroinflammation imaging biomarkers in Alzheimer’s disease
Hypothetical curves of imaging neuroinflammatory biomarkers across the AD continuum. Neuroimaging biomarkers from previous studies are represented by polygons. The dotted lines show hypothetical relative biomarker abnormalities and are not based on previous studies. Curves are color-coded, with orang indicating astrocyte biomarkers and blue indicating microglia biomarkers. It’s important to note that the magnitudes of other biomarkers presented in the graph are relative to their own normal range and should not be compared across different biomarkers. Abbreviations: AD — Alzheimer’s disease; MCI — mild cognitive impairment; TSPO — 18 kDa translocator protein; I2-BS — imidazoline2 binding sites; MAO-B — monoamine oxidase B.
Astrocytes
Although considered the brain’s homeostatic cells, astrocytes can also play important roles in neuroinflammation. Astrocyte reactivity has been evaluated using different molecular targets such as monoamine oxidase B (MAO-B), imidazoline2 binding sites (I2-BS), acetate metabolism, and the organic anion transporter 1C1 (OATP1C1).
The first astrocyte-enriched target to have a PET tracer developed was MAO-B. This enzyme is located in the outer mitochondrial membrane and is mainly responsible for catalyzing the oxidative deamination of amines. In the brain, MAO-B is primarily found in astrocytes and radial glia, but also, in a smaller amount, in monoaminergic neurons (131, 132). MAO-B seems to be upregulated in reactive astrocytes (133), but it is important to note that this does not seem to be the case for all reactive astrocytes, but rather for a subpopulation of these cells (134, 135). Using tritiated MAO-B inhibitors, Saura et al., found MAO-B increased activity — up to three-fold — in cortical and hippocampal plaque-associated astrocytes (136).
The first radiotracer for MAO-B used in AD research was [11C]Deuterium-L-Deprenyl ([11C]DED). [11C] DED was first developed as [11C]-L-Deprenyl and later improved with the addition of deuterium, which reduces the tracer rate of trapping and improves sensitivity to changes in MAO-B. L-Deprenyl is an irreversible inhibitor oxidized by MAO-B to produce a reactive intermediate that covalently binds to the enzyme (137). Initial autoradiographic and PET studies using L-Deprenyl or [11C]DED seemed to point to a significant and widespread increase in MAO-B in the brain of AD patients compared to healthy controls. However, subsequent, more consistent evidence has suggested that this increase occurs earlier in the disease course, in prodromal AD stages. For example, an autoradiographic study in AD patients using [3H]-L-Deprenyl identified a significant increase in MAO-B activity in gray matter regions, such as the frontal and temporal cortices, basal ganglia, thalamus, and white matter in comparison to controls (138). On the other hand, a much more recent autoradiographic study using [11C]-L-Deprenyl only identified an increase in its binding in AD versus age-matched controls in the temporal cortex and white matter (139).
A similar outcome was also observed in PET studies. In the first PET work (140), a higher [11C]DED binding was observed in the parietal, temporal, and medial temporal lobe in AD patients compared to healthy controls. It is important to note that this study used [11C]DED slopes/intercept rate values as a measurement of radiotracer binding (considered a suboptimal measure) and that the group of AD patients analyzed included moderate to severe dementia (MMSE Score = 14.4 ± 2.07), different from subsequent studies, which used mild dementia cases (MMSE Score = 24.4 ± 5.7). Cross-sectional studies from Prof. Nordberg’s group using an optimized PET analysis did not find differences between AD and controls. But, more interestingly, they identified an increased [11C] DED binding in the prodromal stages of AD. A peak in [11C]DED binding was observed in MCI Aβ-positive patients (141) and presymptomatic autosomal dominant Alzheimer’s disease (ADAD) mutation carriers (142). Moreover, a longitudinal work (over a mean period of 2.8 ± 0.6 years) observed an initial higher [11C]DED binding in presymptomatic stages of ADAD, which declined as amyloid load increased. The same decline in MAO-B levels was not seen in sporadic AD patients, which increased in MCI Aβ-positive patients and remained unchanged during the study (143). These findings suggest that (i) the astrocyte response in ADAD and sporadic AD may be distinct; or (ii) may result from a short study follow-up period since it has been already observed that ADAD might have an accelerated rate of evolution in comparison to sporadic AD (144).
Despite being, by far, the most used PET radiotracer to investigate changes in astrocytes in AD, [11C]DED findings still have to be reproduced in larger and more diverse AD populations. One advancement that would potentially help is the development of an equivalent radiotracer using the [18F] isotope. Several radiotracers have been developed with this purpose, such as DL-4-[18F]fluorodeprenyl (145), [18F]fluororasagilineD2 (146) and [18F]fluorodeprenyl-D2 (147). However, most of the DED fluorinated analogous radiotracers presented undesired characteristics such as brain permeable radiometabolites, poor brain uptake or complex radiosynthesis.
Other molecules with affinity to MAO-B, but not analogous to DED, have also been developed. The [11C] SL25.1188 (148), a reversible MAO-B radiotracer, has already gone through its first-in-human clinical studies, and efforts to produce a fluorinated analogous have already begun (149, 150). Another interesting reversible MAO-B radiotracer being developed is the [18F]SMBT-1 (151), which was originally designed to detect Tau tangles, but was found to bind MAO-B with high affinity (152). [18F]SMBT-1 was developed via lead optimization from [18F]THK-5351 and already showed promising results on its first human trials, which included a few MCI and AD patients (153). In a more recent work, [18F] SMBT-1-PET (154) was investigated in a higher number of patients across the AD continuum, showing an increase in cortical brain regions in the Aβ-positive groups, including Aβ-positive cognitively unimpaired (CU) individuals. Further studies, especially with a higher number of AD and MCI patients, are necessary to corroborate these encouraging findings.
Another PET target for reactive astrocytes is the I2-BS, mostly found in astrocytes’ outer mitochondria membrane (155). Coincidently, it has been reported that I2-BS binding site is located in MAO-B (156). An autoradiography study with the radiotracer [11C]BU99008, which binds to I2-BS, showed that [11C]BU99008 and [11C] DED have similar regional distribution, but [11C]BU99008 has a higher specific binding in AD brains compared to CU individuals, (157). [11C]BU99008-PET studies in MCI and AD patients have identified a similar outcome to DED with an elevation on [11C]BU99008 uptake in Aβ-positive individuals; particularly, this elevation was superior in MCI than AD patients (158, 159).
A different approach for imaging astrocytes is to use a radiolabeled molecule that, in the brain, is mainly transported or metabolized by these cells, which seems to be the case of acetate (160). Therefore, the radiotracer [11C]acetate has been suggested as a marker of astrocyte metabolism (161). In Aβ-positive MCI patients, it was observed that [11C]acetate uptake was significantly elevated in the medial temporal lobe (162). An additional work, showed that [11C]acetate uptake was elevated in AD-vulnerable brain regions, such as the entorhinal cortex and the hippocampus, if compared to healthy controls (163).
Moreover, an alternative tracer developed for imaging astrocytes is Sulforhodamine 101 N-(3-[18F]Fluoropropyl) sulfonamide ([18F]2BSRF101) (164). [18F]2BSRF101 was developed based on the fluorescent dye Sulforhodamine 101 (SR101), which has been extensively used as an astrocyte marker. For a long time, the specific binding point or transporter of SR101 in astrocytes was unknown until the thyroid hormone transporter OATP1C1 (also known as SLCO1C1) was identified as the SR101-uptake transporter (165). It is important to note that SR101 is not specific to astrocytes, also labeling, to a minor degree, oligodendrocytes, although it is still dependent on SR101 uptake by astrocytes OATP1C1 transporters (166). [18F]2BSRF101 has not been tested in humans yet, but its binding was elevated in the cortex and hippocampus of the 3xTg, a mouse model that presents amyloid and tau pathologies. By contrast, no changes were found using [11C]DED in the same model, suggesting that [18F]2BSRF101-PET might bring additional information about the astrocyte heterogeneity in AD (167). The temporal changes in imaging biomarkers targeting astrocytes across AD continuum can be seen in Figure 2.
Other imaging biomarkers of neuroinflammation
Apart from the radiotracers primarily targeting glial cells, other available radiotracers targeting brain alterations may also be implicated in brain inflammatory changes such as glucose brain metabolism, cyclooxygenase (COX) isoenzymes, inducible nitric oxide synthase (iNOS) and ROS production.
The glucose brain metabolism has been widely investigated using the [18F]FDG tracer, a glucose analog molecule. In AD, a specific [18F]FDG-PET hypometabolism is seen in the later stages of the disease (for review, see Chételat, 2020 (168)). Interestingly, recent evidence identified a transient [18F]FDG-PET brain hypermetabolism in MCI patients (169). In parallel, correlations between glucose metabolism measured by [18F]FDG-PET and the astrocyte biomarkers, measured trough [11C]DED or plasma GFAP, have been found in ADAD patients (170) and sporadic AD patients (89). Indeed, studies have been consistently demonstrating that glial cells and inflammatory changes impact the brain [18F]FDG-PET signal. For instance, [18F]FDG-PET is sensitive to activation/deactivation of astrocytes and microglial cells (171–175). Thus, it is thought that [18F]FDG-PET may capture neuroinflammatory changes in AD.
Another target being used for radiotracers development are the COX enzymes. COX-1 and COX-2 are enzymes involved in forming prostaglandins and thromboxane (176). COX-1 is considered a housekeeping enzyme not induced by inflammation; however, a study observed an expression of COX-1 in microglia in association with Aβ plaques of AD patients. A proradiotracer of ketoprofen, [11C]ketoprofen-methylester ([11C]KTP-Me), a non-selective inhibitor of COX, however, did not find statistical differences in MCI and AD individuals compared to healthy controls. Although predominantly neuronal, COX-2 is increased during neuroinflammation and is associated with the immunomodulation of the brain tissue (177). Indeed, [11C]MC1 binding, a COX-2 tracer, was higher in the brain lesion site of rhesus macaques injected with lipopolysaccharide in the right putamen (178). However, [11C]MC1 has not been used in AD patients or models.
The iNOS enzyme has also been a target for radiotracers development. In the brain, iNOS is expressed by microglial cells, astrocytes, neurons, and endothelial cells (179, 180). The expression of iNOS is low in healthy or non-inflammatory states but it is stimulated by inflammatory stimuli (181). PET radiotracers targeting iNOS, such as [18F]NOS (182) and [18F]FBAT (183), are promising to track neuroinflammation, but have not yet been tested in AD patients.
Additionally, ROS has also been a focus of PET radiotracers. ROS are a byproduct of physiological cellular functioning and are also signaling molecules. Abnormal ROS production or clearing and resulting oxidative stress are linked to aging and degenerative disease in general (184). Even though this is not a specific neuroinflammatory process, it can cause DNA damage and mitochondrial dysfunction (i) in neurons, promoting a proinflammatory environment through the production of damage-associated molecular patterns; and (ii) in glial cells, resulting in glial changes and cell death (185). The brain is especially prompt to oxidative stress-related damage because it is the most energetically active organ in the human body, meaning intense cellular metabolism and, consequently, intense ROS production. In the specific context of AD, along with mitochondrial production of ROS, there is evidence that Aβ plaques may also be a source of ROS production (186, 187). Some effort has been made to develop PET tracers that target ROS, such as [11C]Ascorbic acid, [11C]dehydroascorbic acid and [18F]dihydromethidine, but these tracers were not yet investigated in the context of AD (188, 189). On the other hand, [18F]ROStrace was associated with amyloid burden in a mouse model, and [64Cu]ATSM was shown to be related to Aβ accumulation in a preliminary study comparing individuals with early biomarker-evidenced of AD (190, 191). A list of proposed PET radiotracers targeting neuroinflammation is presented in Table 1.
Neuroinflammatory biomarkers as prognostic markers
Converging evidence suggests that the pathogenesis of neurodegenerative disorders begins years before symptom onset. Theoretically, a perfect marker of disease progression should track different stages since the earliest pathological alterations. In AD, prognostic biomarkers have promising clinical applications because drug therapies are supposed to start before symptoms (192). Indeed, AD biomarkers positivity has been consistently demonstrated in preclinical phases, which is thought to be the most suitable timing for disease-modifying treatments and secondary prevention strategies. Together with target engagement biomarkers, prognostic markers may also be a method to enrich participants’ inclusion in clinical trials with early AD.
Prognostic markers diverge from diagnostic markers in many aspects. Biomarkers of disease progression should be sensitive to cognitive deterioration and the pathological burden of the disease (193). Currently available evidence has pointed out a myriad of markers of disease progression, including neuroimaging, cognitive testing, and fluid markers. Peripheral biomarkers of neuroinflammation have been increasingly mentioned as promising prognostic markers due to their early identification in peripheral tissue in different conditions (194). However, current evidence of the validity of these markers is still under debate. In the following session we in-depth analyze the prognostic value and test accuracy of neuroinflammation biomarkers of AD.
Therapeutic targets of neuroinflammation
Recent advances in the biomarker field have allowed for classifying individuals in the AD spectrum before the onset of symptoms. The importance of using biomarkersto develop treatments that prevent the development of dementia was brought to light (195). Therapies targeting amyloid plaques, such as aducanumab (FDA-approved) and lecanemab (FDA-approved), can remove amyloid aggregates (196). Still, it is unclear whether a single target will halt the progression of a complex disease like AD. Thus, there is a need to explore other treatment possibilities, including drugs targeting inflammatory changes. A list of ongoing clinical trials targeting neuroinflammatory pathways is presented in Table 2.
Some attention has been paid to this in the past. The ADAPT trial (197) was released in 2008, with negative results regarding preventing dementia onset using non-steroidal anti-inflammatories celecoxib and naproxen. Not only no evidence for efficacy was achieved in delaying cognitive decline or preventing dementia onset, but the trial also held the possibility of naproxen use being associated with increased cognitive decline. The study was terminated because the intervention group showed increased cardiovascular risk.
Another attempt to halt disease progression in cognitively unimpaired individuals using NSAID naproxen was the INTREPAD trial. For this trial, a composite primary endpoint comprising cognitive, neurologic, and biomarker data was developed and used. As occurred in the ADAPT study, naproxen did not show good outcomes and was associated with important side effects (198). It is important to note that, even with limited statistical power, these studies were marked by a tendency for adverse results.
Other attempts to treat AD targeting inflammatory processes were made. For instance, minocycline was found to have an anti-neuroinflammatory effect, inhibiting microglia in animal studies, but clinical trials did not show clinical impact (199). Rosiglitazone, a PPAR inhibitor developed as an anti-diabetic, was also considered a good candidate to prevent AD due to its modulating neuroinflammatory response (200, 201). But, once again, results in clinical trials were not able to achieve its primary clinical outcome.
A possible explanation for previous failures is that anti-inflammatory drugs impact both protective and harmful inflammatory states of glial cells (20). As we develop knowledge toward a more specific understanding of the inflammatory states of glial cells, more selective therapies may be developed to inhibit processes related to harmful phenotypes selectively. An example is the Etanercept, a TNF-α blocker that has been considered a plausible approach for reducing detrimental inflammation in AD 202).
Another approach that has been recently explored is the use of drugs with pleiotropic effects that may be repurposed for AD prevention by acting on neuroinflammation. Pioglitazone, another PPAR inhibitor, is being studied despite the initial unsatisfactory results of similar drugs (203). Candesartan, an ACE inhibitor, is a potential candidate, with preclinical studies indicating immune modulation towards a more protective state in animal models of AD (204). Also, the anti-inflammatory effects of statins, a lipid-reducing class of drugs, has been gaining attention in the context of AD (205). Semaglutide, a GLP-1 receptor agonist, is entering phase 3 evaluation for treating early AD (206). It is proposed that pleiotropic anti-inflammatory effects are behind its anti-dementia potential (207). Multiple phytotherapeutic and antioxidant drugs, such as resveratrol, omega-3 fatty acids, and folic acid, have been suggested as supplements to treating AD due to their anti-inflammatory potential (208–210). Even though these drugs tend to have little, unspecific effects, they are well-tolerated and safe interventions that may be further explored as adjuvant therapies.
In conclusion, no therapy targeting inflammation has effectively prevented or halted the cognitive decline in AD. Still, with an improved understanding of biological processes and biomarkers, a more specific approach may warrant a better response. With the recent advances in Aβ-targeted therapies for AD, neuroinflammation may be a secondary target aiming for effective disease-modifying treatments.
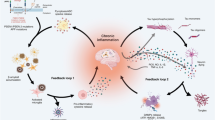
Neuroinflammation in the AT(N) framework
The AT(N) system is proposed to be dynamic and open for including novel biomarkers indicating additional pathophysiological processes in AD. The question is straightforward, are we ready to include neuroinflammation as an “Im” (microglial positivity), “Ia” (astrocytic positivity), “G” (glial positivity) or simply “I” (neuroinflammation)? First, they add little information as diagnostic tools compared to AD core biomarkers — Aβ and tau. Second, the biological interpretation of neuroinflammation imaging biomarkers is far from conclusive, relying on the overexpression of proteins in immune-associated cells. Also, these proteins are rarely specific to the cell type of interest. Third, as happens to fluid biomarkers, proteins that leak or are secreted by immune-associated brain cells are used as surrogate markers of neuroinflammation. Forth, similarly to systemic inflammatory biomarkers such as the C-reactive protein, biomarkers of neuroinflammation present low specificity for AD. In clinical research, however, neuroinflammation biomarkers have been useful in elucidating additional pathophysiological mechanisms and proposing therapeutic targets in AD. A summary of neuroinflammatory-related imaging targets and fluid proteins measured in AD can be seen in Figure 3.
Imaging and fluid biomarker targets for tracking neuroinflammation in Alzheimer’s disease
Schematic representation of cell-derived origins of imaging and fluid biomarkers of neuroinflammation in AD. Abbreviations: AD — Alzheimer’s disease; TSPO — 18 kDa translocator protein; I2-BS — imidazoline2 binding sites; MAO-B — monoamine oxidase B; sTREM2 — soluble triggering receptor expressed in myeloid cells 2; YKL-40 — chitinase-3-like protein 1; GFAP — glial fibrillary acidic protein; S100B — calcium-binding protein B; iNOS — inducible nitric oxidase; P2X7R — P2X7 receptor; ROS — reactive oxygen species; COX-1 — cyclooxygenase 1; COX-2 — cyclooxygenase 2; OATP1C1 — organic anion transporter 1C1; P2Y12R — P2Y12 receptor; CB2R — cannabinoid receptor type 2.
Concluding remarks
In summary, neuroinflammatory markers of AD have potential clinical relevance. However, their role in clinical practice remains elusive. Large longitudinal, multicentric studies in diverse populations with AD core biomarkers associated with neuroinflammation biomarkers in the AD continuum are needed. In addition, it is necessary to advance these biomarkers’ biological interpretation. Thus, although promising, more evidence is needed to propose a new biomarker group representing neuroinflammation in the AT(N) framework.
References
Rodríguez JJ, Olabarria M, Chvatal A, Verkhratsky A. Astroglia in dementia and Alzheimer’s disease. Cell Death Differ. Mar 2009;16(3):378–85. doi: https://doi.org/10.1038/cdd.2008.172
Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. Apr 2015;14(4):388–405. doi: https://doi.org/10.1016/s1474-4422(15)70016-5
Jack CR, Jr., Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018/4 2018;14(4):535–562. doi: https://doi.org/10.1016/j.jalz.2018.02.018
Hampel H, Cummings J, Blennow K, Gao P, Jack CR, Vergallo A. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat Rev Neurol. Sep 2021;17(9):580–589. doi: https://doi.org/10.1038/s41582-021-00520-w
Pascoal TA, Benedet AL, Ashton NJ, et al. Microglial activation and tau propagate jointly across Braak stages. Nat Med. Sep 2021;27(9):1592–1599. doi: https://doi.org/10.1038/s41591-021-01456-w
Lee JW, Lee YK, Yuk DY, et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. Aug 29 2008;5:37. doi: https://doi.org/10.1186/1742-2094-5-37
Minter MR, Taylor JM, Crack PJ. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J Neurochem. Feb 2016;136(3):457–74. doi: https://doi.org/10.1111/jnc.13411
Maphis N, Xu G, Kokiko-Cochran ON, et al. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain. Jun 2015;138 (Pt 6):1738–55. doi: https://doi.org/10.1093/brain/awv081
Meyer PF, Savard M, Poirier J, et al. Bi-directional Association of Cerebrospinal Fluid Immune Markers with Stage of Alzheimer’s Disease Pathogenesis. J Alzheimers Dis. 2018;63(2):577–590. doi: https://doi.org/10.3233/JAD-170887
Pereira JB, Janelidze S, Strandberg O, et al. Microglial activation protects against accumulation of tau aggregates in nondemented individuals with underlying Alzheimer’s disease pathology. Nature Aging. 2022/12/01 2022;2(12):1138–1144. doi: https://doi.org/10.1038/s43587-022-00310-z
Group NP. Nature Portfolio. Accessed September, 29th, 2022
Rustenhoven J, Kipnis J. Brain borders at the central stage of neuroimmunology. Nature. 2022/12/01 2022;612(7940):417–429. doi: https://doi.org/10.1038/s41586-022-05474-7
Louveau A, Harris TH, Kipnis J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. Oct 2015;36(10):569–577. doi: https://doi.org/10.1016/j.it.2015.08.006
Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. Nov 26 2020;9(1):42. doi: https://doi.org/10.1186/s40035-020-00221-2
Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. Jun 2015;16(6):358–72. doi: https://doi.org/10.1038/nrn3880
Sarlus H, Heneka MT. Microglia in Alzheimer’s disease. J Clin Invest. Sep 01 2017;127(9):3240–3249. doi: https://doi.org/10.1172/JCI90606
Bettcher BM, Tansey MG, Dorothee G, Heneka MT. Peripheral and central immune system crosstalk in Alzheimer disease — a research prospectus. Nat Rev Neurol. Nov 2021;17(11):689–701. doi: https://doi.org/10.1038/s41582-021-00549-x
Paolicelli RC, Sierra A, Stevens B, Tremblay ME, Aguzzi A, Ajami B, Amit I, Audinat E, Bechmann I, Bennett M, Bennett F, Bessis A, Biber K, Bilbo S, Blurton-Jones M, Boddeke E, Brites D, Brône B, Brown GC, Butovsky O, Carson MJ, Castellano B, Colonna M, Cowley SA, Cunningham C, Davalos D, De Jager PL, de Strooper B, Denes A, Eggen BJL, Eyo U, Galea E, Garel S, Ginhoux F, Glass CK, Gokce O, Gomez-Nicola D, González B, Gordon S, Graeber MB, Greenhalgh AD, Gressens P, Greter M, Gutmann DH, Haass C, Heneka MT, Heppner FL, Hong S, Hume DA, Jung S, Kettenmann H, Kipnis J, Koyama R, Lemke G, Lynch M, Majewska A, Malcangio M, Malm T, Mancuso R, Masuda T, Matteoli M, McColl BW, Miron VE, Molofsky AV, Monje M, Mracsko E, Nadjar A, Neher JJ, Neniskyte U, Neumann H, Noda M, Peng B, Peri F, Perry VH, Popovich PG, Pridans C, Priller J, Prinz M, Ragozzino D, Ransohoff RM, Salter MW, Schaefer A, Schafer DP, Schwartz M, Simons M, Smith CJ, Streit WJ, Tay TL, Tsai LH, Verkhratsky A, von Bernhardi R, Wake H, Wittamer V, Wolf SA, Wu LJ, Wyss-Coray T. Microglia states and nomenclature: A field at its crossroads. Neuron. 2022 Nov 2;110(21):3458–3483. doi: https://doi.org/10.1016/j.neuron.2022.10.020. PMID: 36327895; PMCID: PMC9999291.
Keren-Shaul H, Spinrad A, Weiner A, et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell. Jun 15 2017;169(7):1276–1290.e17. doi: https://doi.org/10.1016/j.cell.2017.05.018
Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. Mar 2021;17(3):157–172. doi: https://doi.org/10.1038/s41582-020-00435-y
Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. Jun 2016;12(6):719–32. doi: https://doi.org/10.1016/j.jalz.2016.02.010
Lee HG, Wheeler MA, Quintana FJ. Function and therapeutic value of astrocytes in neurological diseases. Nat Rev Drug Discov. May 2022;21(5):339–358. doi: https://doi.org/10.1038/s41573-022-00390-x
Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. Jan 2010;119(1):7–35. doi: https://doi.org/10.1007/s00401-009-0619-8
Hashioka S, Wu Z, Klegeris A. Glia-Driven Neuroinflammation and Systemic Inflammation in Alzheimer’s Disease. Curr Neuropharmacol. 2021;19(7):908–924. doi: https://doi.org/10.2174/1570159X18666201111104509
Azevedo FA, Carvalho LR, Grinberg LT, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. Apr 10 2009;513(5):532–41. doi: https://doi.org/10.1002/cne.21974
Escartin C, Galea E, Lakatos A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. Mar 2021;24(3):312–325. doi: https://doi.org/10.1038/s41593-020-00783-4
Serrano-Pozo A, Mielke ML, Gomez-Isla T, et al. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am J Pathol. Sep 2011;179(3):1373–84. doi: https://doi.org/10.1016/j.ajpath.2011.05.047
Galea E, Weinstock LD, Larramona-Arcas R, et al. Multi-transcriptomic analysis points to early organelle dysfunction in human astrocytes in Alzheimer’s disease. Neurobiol Dis. May 2022;166:105655. doi: https://doi.org/10.1016/j.nbd.2022.105655
Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. Jan 26 2017;541(7638):481–487. doi: https://doi.org/10.1038/nature21029
Bellaver B, Rocha AS, Souza DG, et al. Activated peripheral blood mononuclear cell mediators trigger astrocyte reactivity. Brain Behav Immun. Aug 2019;80:879–888. doi: https://doi.org/10.1016/j.bbi.2019.05.041
Acaz-Fonseca E, Ortiz-Rodriguez A, Azcoitia I, Garcia-Segura LM, Arevalo M-A. Notch signaling in astrocytes mediates their morphological response to an inflammatory challenge. Cell Death Discovery. 2019/04/03 2019;5(1):85. doi: https://doi.org/10.1038/s41420-019-0166-6
Heneka MT. Inflammasome activation and innate immunity in Alzheimer’s disease. Brain Pathol. Mar 2017;27(2):220–222. doi: https://doi.org/10.1111/bpa.12483
Uddin MS, Kabir MT, Jalouli M, et al. Neuroinflammatory Signaling in the Pathogenesis of Alzheimer’s Disease. Curr Neuropharmacol. 2022;20(1):126–146. doi: https://doi.org/10.2174/1570159X19666210826130210
Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. Oct 2018;14(10):576–590. doi: https://doi.org/10.1038/s41574-018-0059-4
Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. Jun 2000;908:244–54. doi: https://doi.org/10.1111/j.1749-6632.2000.tb06651.x
Kosyreva AM, Sentyabreva AV, Tsvetkov IS, Makarova OV. Alzheimer’s Disease and Inflammaging. Brain Sci. Sep 13 2022;12(9)doi: https://doi.org/10.3390/brainsci12091237
Schlepckow K, Monroe KM, Kleinberger G, et al. Enhancing protective microglial activities with a dual function TREM2 antibody to the stalk region. EMBO Mol Med. Apr 7 2020;12(4):e11227. doi: https://doi.org/10.15252/emmm.201911227
Zhong L, Xu Y, Zhuo R, et al. Soluble TREM2 ameliorates pathological phenotypes by modulating microglial functions in an Alzheimer’s disease model. Nat Commun. Mar 25 2019;10(1):1365. doi: https://doi.org/10.1038/s41467-019-09118-9
Jain N, Lewis CA, Ulrich JD, Holtzman DM. Chronic TREM2 activation exacerbates Aβ-associated tau seeding and spreading. J Exp Med. Jan 2 2023;220(1)doi: https://doi.org/10.1084/jem.20220654
Rauchmann BS, Brendel M, Franzmeier N, et al. Microglial Activation and Connectivity in Alzheimer Disease and Aging. Ann Neurol. Nov 2022;92(5):768–781. doi: https://doi.org/10.1002/ana.26465
Nordengen K, Kirsebom BE, Henjum K, et al. Glial activation and inflammation along the Alzheimer’s disease continuum. J Neuroinflammation. Feb 21 2019;16(1):46. doi: https://doi.org/10.1186/s12974-019-1399-2
Winfree RL, Dumitrescu L, Blennow K, et al. Biological correlates of elevated soluble TREM2 in cerebrospinal fluid. Neurobiol Aging. Oct 2022;118:88–98. doi: https://doi.org/10.1016/j.neurobiolaging.2022.06.013
Chen YH, Lin RR, Huang HF, Xue YY, Tao QQ. Microglial Activation, Tau Pathology, and Neurodegeneration Biomarkers Predict Longitudinal Cognitive Decline in Alzheimer’s Disease Continuum. Front Aging Neurosci. 2022;14:848180. doi: https://doi.org/10.3389/fnagi.2022.848180
Li TR, Lyu DY, Liu FQ. Cerebrospinal Fluid sTREM2 in Alzheimer’s Disease Is Associated with Both Amyloid and Tau Pathologies but not with Cognitive Status. J Alzheimers Dis. Oct 6 2022;doi: https://doi.org/10.3233/jad-220598
Diaz-Lucena D, Kruse N, Thüne K, et al. TREM2 expression in the brain and biological fluids in prion diseases. Acta Neuropathol. Jun 2021;141(6):841–859. doi: https://doi.org/10.1007/s00401-021-02296-1
Knapskog AB, Henjum K, Idland AV, et al. Cerebrospinal fluid sTREM2 in Alzheimer’s disease: comparisons between clinical presentation and AT classification. Sci Rep. Sep 28 2020;10(1):15886. doi: https://doi.org/10.1038/s41598-020-72878-8
Morenas-Rodríguez E, Alcolea D, Suárez-Calvet M, et al. Different pattern of CSF glial markers between dementia with Lewy bodies and Alzheimer’s disease. Sci Rep. May 24 2019;9(1):7803. doi: https://doi.org/10.1038/s41598-019-44173-8
Gispert JD, Suárez-Calvet M, Monté GC, et al. Cerebrospinal fluid sTREM2 levels are associated with gray matter volume increases and reduced diffusivity in early Alzheimer’s disease. Alzheimers Dement. Dec 2016;12(12):1259–1272. doi: https://doi.org/10.1016/j.jalz.2016.06.005
Ma LZ, Tan L, Bi YL, et al. Dynamic changes of CSF sTREM2 in preclinical Alzheimer’s disease: the CABLE study. Mol Neurodegener. Apr 10 2020;15(1):25. doi: https://doi.org/10.1186/s13024-020-00374-8
Suárez-Calvet M, Morenas-Rodríguez E, Kleinberger G, et al. Early increase of CSF sTREM2 in Alzheimer’s disease is associated with tau related-neurodegeneration but not with amyloid-β pathology. Mol Neurodegener. Jan 10 2019;14(1):1. doi: https://doi.org/10.1186/s13024-018-0301-5
Leng F, Zhan Z, Sun Y, et al. Cerebrospinal Fluid sTREM2 Has Paradoxical Association with Brain Structural Damage Rate in Early- and Late-Stage Alzheimer’s Disease. J Alzheimers Dis. 2022;88(1):117–126. doi: https://doi.org/10.3233/jad-220102
Edwin TH, Henjum K, Nilsson LNG, et al. A high cerebrospinal fluid soluble TREM2 level is associated with slow clinical progression of Alzheimer’s disease. Alzheimers Dement (Amst). 2020;12(1):e12128. doi: https://doi.org/10.1002/dad2.12128
Morenas-Rodríguez E, Li Y, Nuscher B, et al. Soluble TREM2 in CSF and its association with other biomarkers and cognition in autosomal-dominant Alzheimer’s disease: a longitudinal observational study. Lancet Neurol. Apr 2022;21(4):329–341. doi: https://doi.org/10.1016/s1474-4422(22)00027-8
Pillai JA, Khrestian M, Bena J, Leverenz JB, Bekris LM. Temporal Ordering of Inflammatory Analytes sTNFR2 and sTREM2 in Relation to Alzheimer’s Disease Biomarkers and Clinical Outcomes. Front Aging Neurosci. 2021;13:676744. doi: https://doi.org/10.3389/fnagi.2021.676744
Hu WT, Ozturk T, Kollhoff A, et al. Higher CSF sTNFR1-related proteins associate with better prognosis in very early Alzheimer’s disease. Nature Communications. 2021/06/28 2021;12(1):4001. doi: https://doi.org/10.1038/s41467-021-24220-7
Ewers M, Biechele G, Suárez-Calvet M, et al. Higher CSF sTREM2 and microglia activation are associated with slower rates of beta-amyloid accumulation. EMBO Mol Med. Sep 7 2020;12(9):e12308. doi: https://doi.org/10.15252/emmm.202012308
Rosengren LE, Wikkelsø C, Hagberg L. A sensitive ELISA for glial fibrillary acidic protein: application in CSF of adults. J Neurosci Methods. Mar 1994;51(2):197–204. doi: https://doi.org/10.1016/0165-0270(94)90011-6
Wallin A, Blennow K, Rosengren LE. Glial fibrillary acidic protein in the cerebrospinal fluid of patients with dementia. Dementia. Sep–Oct 1996;7(5):267–72. doi: https://doi.org/10.1159/000106891
Fukuyama R, Izumoto T, Fushiki S. The cerebrospinal fluid level of glial fibrillary acidic protein is increased in cerebrospinal fluid from Alzheimer’s disease patients and correlates with severity of dementia. Eur Neurol. 2001;46(1):35–8. doi: https://doi.org/10.1159/000050753
Benedet AL, Milà-Alomà M, Vrillon A, et al. Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA Neurol. Dec 1 2021;78(12):1471–1483. doi: https://doi.org/10.1001/jamaneurol.2021.3671
Pereira JB, Janelidze S, Smith R, et al. Plasma GFAP is an early marker of amyloid-β but not tau pathology in Alzheimer’s disease. Brain. Dec 16 2021;144(11):3505–3516. doi: https://doi.org/10.1093/brain/awab223
Salvadó G, Milà-Alomà M, Shekari M, et al. Cerebral amyloid-β load is associated with neurodegeneration and gliosis: Mediation by p-tau and interactions with risk factors early in the Alzheimer’s continuum. Alzheimers Dement. May 2021;17(5):788–800. doi: https://doi.org/10.1002/alz.12245
Milà-Alomà M, Salvadó G, Gispert JD, et al. Amyloid beta, tau, synaptic, neurodegeneration, and glial biomarkers in the preclinical stage of the Alzheimer’s continuum. Alzheimers Dement. Oct 2020;16(10):1358–1371. doi: https://doi.org/10.1002/alz.12131
Lauridsen C, Sando SB, Møller I, et al. Cerebrospinal Fluid Aβ43 Is Reduced in Early-Onset Compared to Late-Onset Alzheimer’s Disease, But Has Similar Diagnostic Accuracy to Aβ42. Front Aging Neurosci. 2017;9:210. doi: https://doi.org/10.3389/fnagi.2017.00210
Oeckl P, Halbgebauer S, Anderl-Straub S, et al. Glial Fibrillary Acidic Protein in Serum is Increased in Alzheimer’s Disease and Correlates with Cognitive Impairment. J Alzheimers Dis. 2019;67(2):481–488. doi: https://doi.org/10.3233/jad-180325
Ishiki A, Kamada M, Kawamura Y, et al. Glial fibrillar acidic protein in the cerebrospinal fluid of Alzheimer’s disease, dementia with Lewy bodies, and frontotemporal lobar degeneration. J Neurochem. Jan 2016;136(2):258–61. doi: https://doi.org/10.1111/jnc.13399
van Eijk JJ, van Everbroeck B, Abdo WF, Kremer BP, Verbeek MM. CSF neurofilament proteins levels are elevated in sporadic Creutzfeldt-Jakob disease. J Alzheimers Dis. 2010;21(2):569–76. doi: https://doi.org/10.3233/jad-2010-090649
Simrén J, Weninger H, Brum WS, et al. Differences between blood and cerebrospinal fluid glial fibrillary Acidic protein levels: The effect of sample stability. Alzheimers Dement. Oct 2022;18(10):1988–1992. doi: https://doi.org/10.1002/alz.12806
Chatterjee P, Pedrini S, Stoops E, et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer’s disease. Transl Psychiatry. Jan 11 2021;11(1):27. doi: https://doi.org/10.1038/s41398-020-01137-1
Prins S, de Kam ML, Teunissen CE, Groeneveld GJ. Inflammatory plasma biomarkers in subjects with preclinical Alzheimer’s disease. Alzheimers Res Ther. Aug 3 2022;14(1):106. doi: https://doi.org/10.1186/s13195-022-01051-2
O’Connor A, Abel E, Benedet AL, et al. Plasma GFAP in presymptomatic and symptomatic familial Alzheimer’s disease: a longitudinal cohort study. J Neurol Neurosurg Psychiatry. Aug 10 2022;doi: https://doi.org/10.1136/jnnp-2022-329663
Shir D, Graff-Radford J, Hofrenning EI, et al. Association of plasma glial fibrillary acidic protein (GFAP) with neuroimaging of Alzheimer’s disease and vascular pathology. Alzheimers Dement (Amst). 2022;14(1):e12291. doi: https://doi.org/10.1002/dad2.12291
Rajan KB, Aggarwal NT, McAninch EA, et al. Remote Blood Biomarkers of Longitudinal Cognitive Outcomes in a Population Study. Ann Neurol. Dec 2020;88(6):1065–1076. doi: https://doi.org/10.1002/ana.25874
Asken BM, VandeVrede L, Rojas JC, et al. Lower White Matter Volume and Worse Executive Functioning Reflected in Higher Levels of Plasma GFAP among Older Adults with and Without Cognitive Impairment. J Int Neuropsychol Soc. Jul 2022;28(6):588–599. doi: https://doi.org/10.1017/s1355617721000813
Cicognola C, Janelidze S, Hertze J, et al. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res Ther. Mar 27 2021;13(1):68. doi: https://doi.org/10.1186/s13195-021-00804-9
Ebenau JL, Pelkmans W, Verberk IMW, et al. Association of CSF, Plasma, and Imaging Markers of Neurodegeneration With Clinical Progression in People With Subjective Cognitive Decline. Neurology. Mar 29 2022;98(13):e1315–e1326. doi: https://doi.org/10.1212/WNL.0000000000200035
Stocker H, Beyer L, Perna L, et al. Association of plasma biomarkers, p-tau181, glial fibrillary acidic protein, and neurofilament light, with intermediate and long-term clinical Alzheimer’s disease risk: Results from a prospective cohort followed over 17 years. Alzheimers Dement. Mar 2 2022;doi: https://doi.org/10.1002/alz.12614
Verberk IMW, Laarhuis MB, van den Bosch KA, et al. Serum markers glial fibrillary acidic protein and neurofilament light for prognosis and monitoring in cognitively normal older people: a prospective memory clinic-based cohort study. Lancet Healthy Longev. Feb 2021;2(2):e87–e95. doi: https://doi.org/10.1016/s2666-7568(20)30061-1
Llorens F, Thüne K, Tahir W, et al. YKL-40 in the brain and cerebrospinal fluid of neurodegenerative dementias. Mol Neurodegener. Nov 10 2017;12(1):83. doi: https://doi.org/10.1186/s13024-017-0226-4
Bellaver B, Ferrari-Souza JP, Uglione da Ros L, et al. Astrocyte Biomarkers in Alzheimer Disease: A Systematic Review and Meta-analysis. Neurology. May 5 2021;doi: https://doi.org/10.1212/wnl.0000000000012109
De Kort AM, Kuiperij HB, Alcolea D, et al. Cerebrospinal fluid levels of the neurotrophic factor neuroleukin are increased in early Alzheimer’s disease, but not in cerebral amyloid angiopathy. Alzheimers Res Ther. Sep 24 2021;13(1):160. doi: https://doi.org/10.1186/s13195-021-00899-0
Kester MI, Teunissen CE, Sutphen C, et al. Cerebrospinal fluid VILIP-1 and YKL-40, candidate biomarkers to diagnose, predict and monitor Alzheimer’s disease in a memory clinic cohort. Alzheimers Res Ther. Sep 17 2015;7(1):59. doi: https://doi.org/10.1186/s13195-015-0142-1
Palmqvist S, Insel PS, Stomrud E, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer’s disease. EMBO Mol Med. Dec 2019;11(12):e11170. doi: https://doi.org/10.15252/emmm.201911170
Schindler SE, Li Y, Todd KW, et al. Emerging cerebrospinal fluid biomarkers in autosomal dominant Alzheimer’s disease. Alzheimers Dement. May 2019;15(5):655–665. doi: https://doi.org/10.1016/j.jalz.2018.12.019
Sutphen CL, Jasielec MS, Shah AR, et al. Longitudinal Cerebrospinal Fluid Biomarker Changes in Preclinical Alzheimer Disease During Middle Age. JAMA Neurology. 2015;72(9):1029–1042. doi: https://doi.org/10.1001/jamaneurol.2015.1285
Henson RL, Doran E, Christian BT, et al. Cerebrospinal fluid biomarkers of Alzheimer’s disease in a cohort of adults with Down syndrome. Alzheimers Dement (Amst). 2020;12(1):e12057. doi: https://doi.org/10.1002/dad2.12057
Woollacott IOC, Nicholas JM, Heller C, et al. Cerebrospinal Fluid YKL-40 and Chitotriosidase Levels in Frontotemporal Dementia Vary by Clinical, Genetic and Pathological Subtype. Dement Geriatr Cogn Disord. 2020;49(1):56–76. doi: https://doi.org/10.1159/000506282
Ferrari-Souza JP, Ferreira PCL, Bellaver B, et al. Astrocyte biomarker signatures of amyloid-β and tau pathologies in Alzheimer’s disease. Mol Psychiatry. Aug 10 2022;doi: https://doi.org/10.1038/s41380-022-01716-2
Salvadó G, Milà-Alomà M, Shekari M, et al. Reactive astrogliosis is associated with higher cerebral glucose consumption in the early Alzheimer’s continuum. European Journal of Nuclear Medicine and Molecular Imaging. 2022/11/01 2022;49(13):4567–4579. doi: https://doi.org/10.1007/s00259-022-05897-4
Van Hulle C, Jonaitis EM, Betthauser TJ, et al. An examination of a novel multipanel of CSF biomarkers in the Alzheimer’s disease clinical and pathological continuum. Alzheimers Dement. Mar 2021;17(3):431–445. doi: https://doi.org/10.1002/alz.12204
Salvadó G, Shekari M, Falcon C, et al. Brain alterations in the early Alzheimer’s continuum with amyloid-β, tau, glial and neurodegeneration CSF markers. Brain Commun. 2022;4(3):fcac134. doi: https://doi.org/10.1093/braincomms/fcac134
Morar U, Izquierdo W, Martin H, et al. A study of the longitudinal changes in multiple cerebrospinal fluid and volumetric magnetic resonance imaging biomarkers on converter and non-converter Alzheimer’s disease subjects with consideration for their amyloid beta status. Alzheimers Dement (Amst). 2022;14(1):e12258. doi: https://doi.org/10.1002/dad2.12258
Alcolea D, Martinez-Lage P, Sanchez-Juan P, et al. Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology. Aug 18 2015;85(7):626–33. doi: https://doi.org/10.1212/WNL.0000000000001859
Blanco-Palmero VA, Rubio-Fernández M, Antequera D, et al. Increased YKL-40 but Not C-Reactive Protein Levels in Patients with Alzheimer’s Disease. Biomedicines. Aug 27 2021;9(9)doi: https://doi.org/10.3390/biomedicines9091094
Craig-Schapiro R, Perrin RJ, Roe CM, et al. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer’s disease. Biol Psychiatry. Nov 15 2010;68(10):903–12. doi: https://doi.org/10.1016/j.biopsych.2010.08.025
Michetti F, D’Ambrosi N, Toesca A, et al. The S100B story: from biomarker to active factor in neural injury. J Neurochem. Jan 2019;148(2):168–187. doi: https://doi.org/10.1111/jnc.14574
Piazza O, Leggiero E, De Benedictis G, et al. S100B induces the release of pro-inflammatory cytokines in alveolar type I-like cells. Int J Immunopathol Pharmacol. Apr-Jun 2013;26(2):383–91. doi: https://doi.org/10.1177/039463201302600211
Schulz I, Kruse N, Gera RG, et al. Systematic Assessment of 10 Biomarker Candidates Focusing on α-Synuclein-Related Disorders. Mov Disord. Dec 2021;36(12):2874–2887. doi: https://doi.org/10.1002/mds.28738
Green AJ, Harvey RJ, Thompson EJ, Rossor MN. Increased S100beta in the cerebrospinal fluid of patients with frontotemporal dementia. Neurosci Lett. Oct 10 1997;235(1–2):5–8. doi: https://doi.org/10.1016/s0304-3940(97)00701-5
Peskind ER, Griffin WS, Akama KT, Raskind MA, Van Eldik LJ. Cerebrospinal fluid S100B is elevated in the earlier stages of Alzheimer’s disease. Neurochem Int. Nov-Dec 2001;39(5–6):409–13. doi: https://doi.org/10.1016/s0197-0186(01)00048-1
Maetzler W, Berg D, Synofzik M, et al. Autoantibodies against amyloid and glial-derived antigens are increased in serum and cerebrospinal fluid of Lewy body-associated dementias. J Alzheimers Dis. 2011;26(1):171–9. doi: https://doi.org/10.3233/jad-2011-110221
Andreasen N, Gottfries J, Vanmechelen E, et al. Evaluation of CSF biomarkers for axonal and neuronal degeneration, gliosis, and beta-amyloid metabolism in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. Oct 2001;71(4):557–8. doi: https://doi.org/10.1136/jnnp.71.4.557
Teitsdottir UD, Jonsdottir MK, Lund SH, Darreh-Shori T, Snaedal J, Petersen PH. Association of glial and neuronal degeneration markers with Alzheimer’s disease cerebrospinal fluid profile and cognitive functions. Alzheimers Res Ther. Aug 4 2020;12(1):92. doi: https://doi.org/10.1186/s13195-020-00657-8
Milà-Alomà M, Shekari M, Salvadó G, et al. Cognitively unimpaired individuals with a low burden of Aβ pathology have a distinct CSF biomarker profile. Alzheimers Res Ther. Jul 27 2021;13(1):134. doi: https://doi.org/10.1186/s13195-021-00863-y
Zheng C, Zhou X-W, Wang J-Z. The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Translational Neurodegeneration. 2016/04/05 2016;5(1):7. doi: https://doi.org/10.1186/s40035-016-0054-4
Brosseron F, Krauthausen M, Kummer M, Heneka MT. Body fluid cytokine levels in mild cognitive impairment and Alzheimer’s disease: a comparative overview. Mol Neurobiol. Oct 2014;50(2):534–44. doi: https://doi.org/10.1007/s12035-014-8657-1
Beaino W, Janssen B, Vugts DJ, de Vries HE, Windhorst AD. Towards PET imaging of the dynamic phenotypes of microglia. Clin Exp Immunol. Dec 2021;206(3):282–300. doi: https://doi.org/10.1111/cei.13649
Kreisl WC, Kim MJ, Coughlin JM, Henter ID, Owen DR, Innis RB. PET imaging of neuroinflammation in neurological disorders. Lancet Neurol. Nov 2020;19(11):940–950. doi: https://doi.org/10.1016/S1474-4422(20)30346-X
Zhang L, Hu K, Shao T, et al. Recent developments on PET radiotracers for TSPO and their applications in neuroimaging. Acta Pharm Sin B. Feb 2021;11(2):373–393. doi: https://doi.org/10.1016/j.apsb.2020.08.006
Femminella GD, Ninan S, Atkinson R, Fan Z, Brooks DJ, Edison P. Does Microglial Activation Influence Hippocampal Volume and Neuronal Function in Alzheimer’s Disease and Parkinson’s Disease Dementia? J Alzheimers Dis. 2016;51(4):1275–89. doi: https://doi.org/10.3233/JAD-150827
Fan Z, Okello AA, Brooks DJ, Edison P. Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer’s disease. Brain. Dec 2015;138 (Pt 12):3685–98. doi: https://doi.org/10.1093/brain/awv288
Yokokura M, Terada T, Bunai T, et al. Depiction of microglial activation in aging and dementia: Positron emission tomography with [J Cereb Blood Flow Metab. Mar 2017;37(3):877–889. doi: https://doi.org/10.1177/0271678X16646788
Yasuno F, Kosaka J, Ota M, et al. Increased binding of peripheral benzodiazepine receptor in mild cognitive impairment-dementia converters measured by positron emission tomography with [11C]DAA1106. Psychiatry Res. Jul 30 2012;203(1):67–74. doi: https://doi.org/10.1016/j.pscychresns.2011.08.013
Kreisl WC, Lyoo CH, Liow JS, et al. (11)C-PBR28 binding to translocator protein increases with progression of Alzheimer’s disease. Neurobiol Aging. Aug 2016;44:53–61. doi: https://doi.org/10.1016/j.neurobiolaging.2016.04.011
Dani M, Wood M, Mizoguchi R, et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain. Sep 01 2018;141(9):2740–2754. doi: https://doi.org/10.1093/brain/awy188
Mizrahi R, Rusjan PM, Kennedy J, et al. Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J Cereb Blood Flow Metab. Jun 2012;32(6):968–72. doi: https://doi.org/10.1038/jcbfm.2012.46
Xiang X, Wind K, Wiedemann T, et al. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci Transl Med. Oct 13 2021;13(615):eabe5640. doi: https://doi.org/10.1126/scitranslmed.abe5640
Ikawa M, Lohith TG, Shrestha S, et al. 11C-ER176, a Radioligand for 18-kDa Translocator Protein, Has Adequate Sensitivity to Robustly Image All Three Affinity Genotypes in Human Brain. J Nucl Med. Feb 2017;58(2):320–325. doi: https://doi.org/10.2967/jnumed.116.178996
Fujita M, Kobayashi M, Ikawa M, et al. Comparison of four. EJNMMI Res. Oct 16 2017;7(1):84. doi: https://doi.org/10.1186/s13550-017-0334-8
Delage C, Vignal N, Guerin C, et al. From positron emission tomography to cell analysis of the 18-kDa Translocator Protein in mild traumatic brain injury. Sci Rep. Dec 14 2021;11(1):24009. doi: https://doi.org/10.1038/s41598-021-03416-3
Nutma E, Fancy N, Weinert M, et al. Translocator protein is a marker of activated microglia in rodent models but not human neurodegenerative diseases. bioRxiv. 2022:2022.05.11.491453. doi: https://doi.org/10.1101/2022.05.11.491453
Hagan N, Kane JL, Grover D, et al. CSF1R signaling is a regulator of pathogenesis in progressive MS. Cell Death Dis. Oct 23 2020;11(10):904. doi: https://doi.org/10.1038/s41419-020-03084-7
Horti AG, Naik R, Foss CA, et al. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proc Natl Acad Sci U S A. Jan 29 2019;116(5):1686–1691. doi: https://doi.org/10.1073/pnas.1812155116
Akiyama H, Nishimura T, Kondo H, Ikeda K, Hayashi Y, McGeer PL. Expression of the receptor for macrophage colony stimulating factor by brain microglia and its upregulation in brains of patients with Alzheimer’s disease and amyotrophic lateral sclerosis. Brain Res. Mar 07 1994;639(1):171–4. doi: https://doi.org/10.1016/0006-8993(94)91779-5
Walker DG, Tang TM, Lue LF. Studies on Colony Stimulating Factor Receptor-1 and Ligands Colony Stimulating Factor-1 and Interleukin-34 in Alzheimer’s Disease Brains and Human Microglia. Front Aging Neurosci. 2017;9:244. doi: https://doi.org/10.3389/fnagi.2017.00244
Ruiz de Martín Esteban S, Benito-Cuesta I, Terradillos I, et al. Cannabinoid CB2 Receptors Modulate Microglia Function and Amyloid Dynamics in a Mouse Model of Alzheimer’s Disease. Front Pharmacol. 2022;13:841766. doi: https://doi.org/10.3389/fphar.2022.841766
Gómez Morillas A, Besson VC, Lerouet D. Microglia and Neuroinflammation: What Place for P2RY12? Int J Mol Sci. Feb 06 2021;22(4)doi: https://doi.org/10.3390/ijms22041636
Andrejew R, Oliveira-Giacomelli Á, Ribeiro DE, et al. The P2X7 Receptor: Central Hub of Brain Diseases. Front Mol Neurosci. 2020;13:124. doi: https://doi.org/10.3389/fnmol.2020.00124
Janssen B, Vugts DJ, Wilkinson SM, et al. Identification of the allosteric P2X. Sci Rep. Apr 26 2018;8(1):6580. doi: https://doi.org/10.1038/s41598-018-24814-0
Lucot KL, Stevens MY, Bonham TA, et al. Tracking Innate Immune Activation in a Mouse Model of Parkinson’s Disease Using TREM1 and TSPO PET Tracers. J Nucl Med. Oct 2022;63(10):1570–1578. doi: https://doi.org/10.2967/jnumed.121.263039
Levitt P, Pintar JE, Breakefield XO. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci U S A. Oct 1982;79(20):6385–9.
Westlund KN, Denney RM, Rose RM, Abell CW. Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience. May 1988;25(2):439–56. doi: https://doi.org/10.1016/0306-4522(88)90250-3
Ekblom J, Jossan SS, Bergstrom M, Oreland L, Walum E, Aquilonius SM. Monoamine oxidase-B in astrocytes. Glia. Jun 1993;8(2):122–32. doi: https://doi.org/10.1002/glia.440080208
Ekblom J, Jossan SS, Oreland L, Walum E, Aquilonius SM. Reactive gliosis and monoamine oxidase B. J Neural Transm Suppl. 1994;41:253–8. doi: https://doi.org/10.1007/978-3-7091-9324-2_33
Olsen M, Aguilar X, Sehlin D, et al. Astroglial Responses to Amyloid-Beta Progression in a Mouse Model of Alzheimer’s Disease. Mol Imaging Biol. Aug 2018;20(4):605–614. doi: https://doi.org/10.1007/s11307-017-1153-z
Saura J, Luque JM, Cesura AM, et al. Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience. Sep 1994;62(1):15–30. doi: https://doi.org/10.1016/0306-4522(94)90311-5
Fowler JS, Logan J, Shumay E, Alia-Klein N, Wang GJ, Volkow ND. Monoamine oxidase: radiotracer chemistry and human studies. J Labelled Comp Radiopharm. Mar 2015;58(3):51–64. doi: https://doi.org/10.1002/jlcr.3247
Jossan SS, Gillberg PG, Gottfries CG, Karlsson I, Oreland L. Monoamine oxidase B in brains from patients with Alzheimer’s disease: a biochemical and autoradiographical study. Neuroscience. 1991;45(1):1–12. doi: https://doi.org/10.1016/0306-4522(91)90098-9
Gulyas B, Pavlova E, Kasa P, et al. Activated MAO-B in the brain of Alzheimer patients, demonstrated by [11C]-L-deprenyl using whole hemisphere autoradiography. Neurochem Int. Jan 2011;58(1):60–8. doi: https://doi.org/10.1016/j.neuint.2010.10.013
Santillo AF, Gambini JP, Lannfelt L, et al. In vivo imaging of astrocytosis in Alzheimer’s disease: an (1)(1)C-L-deuteriodeprenyl and PIB PET study. Eur J Nucl Med Mol Imaging. Dec 2011;38(12):2202–8. doi: https://doi.org/10.1007/s00259-011-1895-9
Carter SF, Scholl M, Almkvist O, et al. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J Nucl Med. Jan 2012;53(1):37–46. doi: https://doi.org/10.2967/jnumed.110.087031
Scholl M, Carter SF, Westman E, et al. Early astrocytosis in autosomal dominant Alzheimer’s disease measured in vivo by multi-tracer positron emission tomography. Sci Rep. Nov 10 2015;5:16404. doi: https://doi.org/10.1038/srep16404
Rodriguez-Vieitez E, Saint-Aubert L, Carter SF, et al. Diverging longitudinal changes in astrocytosis and amyloid PET in autosomal dominant Alzheimer’s disease. Brain. Mar 2016;139 (Pt 3):922–36. doi: https://doi.org/10.1093/brain/awv404
Stanley K, Walker Z. Do patients with young onset Alzheimer’s disease deteriorate faster than those with late onset Alzheimer’s disease? A review of the literature. Int Psychogeriatr. Dec 2014;26(12):1945–53. doi: https://doi.org/10.1017/S1041610214001173
Plenevaux A, Fowler JS, Dewey SL, Wolf AP, Guillaume M. The synthesis of no-carrier-added DL-4-[18F]fluorodeprenyl via the nucleophilic aromatic substitution reaction. Int J Rad Appl Instrum A. 1991;42(2):121–7. doi: https://doi.org/10.1016/0883-2889(91)90060-e
Nag S, Lehmann L, Kettschau G, et al. Development of a novel fluorine-18 labeled deuterated fluororasagiline ([(18)F]fluororasagiline-D2) radioligand for PET studies of monoamino oxidase B (MAO-B). Bioorg Med Chem. Nov 1 2013;21(21):6634–41. doi: https://doi.org/10.1016/j.bmc.2013.08.019
Nag S, Fazio P, Lehmann L, et al. In Vivo and In Vitro Characterization of a Novel MAO-B Inhibitor Radioligand, 18F-Labeled Deuterated Fluorodeprenyl. J Nucl Med. Feb 2016;57(2):315–20. doi: https://doi.org/10.2967/jnumed.115.161083
Bramoullé Y, Puech F, Saba W, et al. Radiosynthesis of (S)-5-methoxymethyl-3-[6-(4,4,4-trifluorobutoxy)benzo[d]isoxazol-3-yl] oxazolidin-2-[11C]one ([11C] SL25.1188), a novel radioligand for imaging monoamine oxidase-B with PET. Journal of Labelled Compounds and Radiopharmaceuticals. 2008;51(3):153–158. doi: https://doi.org/10.1002/jlcr.1492
Hicks JW, Sadovski O, Parkes J, et al. Radiosynthesis and ex vivo evaluation of [(18)F]-(S)-3-(6-(3-fluoropropoxy)benzo[d]isoxazol-3-yl)-5-(methoxymethyl) oxazolidin-2-one for imaging MAO-B with PET. Bioorg Med Chem Lett. Jan 15 2015;25(2):288–91. doi: https://doi.org/10.1016/j.bmcl.2014.11.048
Dahl K, Bernard-Gauthier V, Nag S, et al. Synthesis and preclinical evaluation of [(18)F]FSL25.1188, a reversible PET radioligand for monoamine oxidase-B. Bioorg Med Chem Lett. Jul 1 2019;29(13):1624–1627. doi: https://doi.org/10.1016/j.bmcl.2019.04.040
Harada R, Hayakawa Y, Ezura M, et al. (18)F-SMBT-1: A Selective and Reversible PET Tracer for Monoamine Oxidase-B Imaging. J Nucl Med. Feb 2021;62(2):253–258. doi: https://doi.org/10.2967/jnumed.120.244400
Ng KP, Pascoal TA, Mathotaarachchi S, et al. Monoamine oxidase B inhibitor, selegiline, reduces (18)F-THK5351 uptake in the human brain. Alzheimers Res Ther. Mar 31 2017;9(1):25. doi: https://doi.org/10.1186/s13195-017-0253-y
Villemagne VL, Harada R, Dore V, et al. First-in-Humans Evaluation of (18)F-SMBT-1, a Novel (18)F-Labeled Monoamine Oxidase-B PET Tracer for Imaging Reactive Astrogliosis. J Nucl Med. Oct 2022;63(10):1551–1559. doi: https://doi.org/10.2967/jnumed.121.263254
Villemagne VL, Harada R, Doré V, et al. Assessing Reactive Astrogliosis with. J Nucl Med. Oct 2022;63(10):1560–1569. doi: https://doi.org/10.2967/jnumed.121.263255
Regunathan S, Feinstein DL, Reis DJ. Expression of non-adrenergic imidazoline sites in rat cerebral cortical astrocytes. J Neurosci Res. Apr 15 1993;34(6):681–8. doi: https://doi.org/10.1002/jnr.490340611
Kimura A, Tyacke RJ, Minchin MC, Nutt DJ, Hudson AL. Identification of an I(2) binding protein from rabbit brain. Ann N Y Acad Sci. Dec 2003;1009:364–6. doi: https://doi.org/10.1196/annals.1304.048
Kumar A, Koistinen NA, Malarte ML, et al. Astroglial tracer BU99008 detects multiple binding sites in Alzheimer’s disease brain. Mol Psychiatry. Oct 2021;26(10):5833–5847. doi: https://doi.org/10.1038/s41380-021-01101-5
Calsolaro V, Matthews PM, Donat CK, et al. Astrocyte reactivity with late-onset cognitive impairment assessed in vivo using (11)C-BU99008 PET and its relationship with amyloid load. Mol Psychiatry. Oct 2021;26(10):5848–5855. doi: https://doi.org/10.1038/s41380-021-01193-z
Livingston NR, Calsolaro V, Hinz R, et al. Relationship between astrocyte reactivity, using novel (11)C-BU99008 PET, and glucose metabolism, grey matter volume and amyloid load in cognitively impaired individuals. Mol Psychiatry. Apr 2022;27(4):2019–2029. doi: https://doi.org/10.1038/s41380-021-01429-y
Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. Jul 15 1998;18(14):5225–33. doi: https://doi.org/10.1523/JNEUROSCI.18-14-05225.1998
Kato H, Okuno T. Functional imaging of astrocyte activity. Neural Regen Res. Jun 2021;16(6):1206–1207. doi: https://doi.org/10.4103/1673-5374.300432
Duong MT, Chen YJ, Doot RK, et al. Astrocyte activation imaging with 11C-acetate and amyloid PET in mild cognitive impairment due to Alzheimer pathology. Nucl Med Commun. Nov 1 2021;42(11):1261–1269. doi: https://doi.org/10.1097/MNM.0000000000001460
Nam M-H, Ko HY, Lee S, et al. Visualization of reactive astrocytes in living brain of Alzheimer’s disease patient. bioRxiv. 2021:2021.04.13.439744. doi: https://doi.org/10.1101/2021.04.13.439744
Kreimerman I, Porcal W, Olivera S, Oliver P, Savio E, Engler H. Synthesis of [18F]2B-SRF101: A Sulfonamide Derivative of the Fluorescent Dye Sulforhodamine 101. Curr Radiopharm. Nov 10 2017;10(3):212–220. doi: https://doi.org/10.2174/1874471010666170928112853
Schnell C, Shahmoradi A, Wichert SP, et al. The multispecific thyroid hormone transporter OATP1C1 mediates cell-specific sulforhodamine 101-labeling of hippocampal astrocytes. Brain Struct Funct. Jan 2015;220(1):193–203. doi: https://doi.org/10.1007/s00429-013-0645-0
Hagos L, Hulsmann S. Unspecific labelling of oligodendrocytes by sulforhodamine 101 depends on astrocytic uptake via the thyroid hormone transporter OATP1C1 (SLCO1C1). Neurosci Lett. Sep 19 2016;631:13–18. doi: https://doi.org/10.1016/j.neulet.2016.08.010
Kreimerman I, Reyes AL, Paolino A, et al. Biological Assessment of a (18) F-Labeled Sulforhodamine 101 in a Mouse Model of Alzheimer’s Disease as a Potential Astrocytosis Marker. Front Neurosci. 2019;13:734. doi: https://doi.org/10.3389/fnins.2019.00734
Chételat G, Arbizu J, Barthel H, et al. Amyloid-PET and. Lancet Neurol. Nov 2020;19(11):951–962. doi: https://doi.org/10.1016/S1474-4422(20)30314-8
Ashraf A, Fan Z, Brooks DJ, Edison P. Cortical hypermetabolism in MCI subjects: a compensatory mechanism? Eur J Nucl Med Mol Imaging. Mar 2015;42(3):447–58. doi: https://doi.org/10.1007/s00259-014-2919-z
Carter SF, Chiotis K, Nordberg A, Rodriguez-Vieitez E. Longitudinal association between astrocyte function and glucose metabolism in autosomal dominant Alzheimer’s disease. Eur J Nucl Med Mol Imaging. Feb 2019;46(2):348–356. doi: https://doi.org/10.1007/s00259-018-4217-7
Zimmer ER, Parent MJ, Souza DG, et al. [(18)F]FDG PET signal is driven by astroglial glutamate transport. Nat Neurosci. Mar 2017;20(3):393–395. doi: https://doi.org/10.1038/nn.4492
Rocha A, Bellaver B, Souza DG, et al. Clozapine induces astrocyte-dependent FDG-PET hypometabolism. Eur J Nucl Med Mol Imaging. Jun 2022;49(7):2251–2264. doi: https://doi.org/10.1007/s00259-022-05682-3
Xiang X, Wind K, Wiedemann T, et al. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci Transl Med. Oct 13 2021;13(615):eabe5640. doi: https://doi.org/10.1126/scitranslmed.abe5640
Zimmer ER, Pascoal TA, Rosa-Neto P, Nordberg A, Pellerin L. Comment on “Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases”. Sci Transl Med. Aug 24 2022;14(659):eabm8302. doi: https://doi.org/10.1126/scitranslmed.abm8302
Xiang X, Tahirovic S, Ziegler S, Haass C, Brendel M. Response to Comment on “Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases”. Sci Transl Med. Aug 24 2022;14(659):eabn5104. doi: https://doi.org/10.1126/scitranslmed.abn5104
Rouzer CA, Marnett LJ. Cyclooxygenases: structural and functional insights. J Lipid Res. Apr 2009;50 Suppl(Suppl):S29–34. doi: https://doi.org/10.1194/jlr.R800042-JLR200
Ohnishi A, Senda M, Yamane T, et al. Exploratory human PET study of the effectiveness of (11)C-ketoprofen methyl ester, a potential biomarker of neuroinflammatory processes in Alzheimer’s disease. Nucl Med Biol. Jul 2016;43(7):438–44. doi: https://doi.org/10.1016/j.nucmedbio.2016.04.005
Shrestha S, Kim MJ, Eldridge M, et al. PET measurement of cyclooxygenase-2 using a novel radioligand: upregulation in primate neuroinflammation and first-in-human study. J Neuroinflammation. May 02 2020;17(1):140. doi: https://doi.org/10.1186/s12974-020-01804-6
Murphy S, Simmons ML, Agullo L, et al. Synthesis of nitric oxide in CNS glial cells. Trends Neurosci. Aug 1993;16(8):323–8. doi: https://doi.org/10.1016/0166-2236(93)90109-y
Heneka MT, Feinstein DL. Expression and function of inducible nitric oxide synthase in neurons. J Neuroimmunol. Mar 1 2001;114(1–2):8–18. doi: https://doi.org/10.1016/s0165-5728(01)00246-6
Licinio J, Prolo P, McCann SM, Wong ML. Brain iNOS: current understanding and clinical implications. Mol Med Today. May 1999;5(5):225–32. doi: https://doi.org/10.1016/S1357-4310(99)01453-7
Zhou D, Lee H, Rothfuss JM, et al. Design and synthesis of 2-amino-4-methylpyridine analogues as inhibitors for inducible nitric oxide synthase and in vivo evaluation of [18F]6-(2-fluoropropyl)-4-methyl-pyridin-2-amine as a potential PET tracer for inducible nitric oxide synthase. J Med Chem. Apr 23 2009;52(8):2443–53. doi: https://doi.org/10.1021/jm801556h
Yeh SH, Huang WS, Chiu CH, et al. Automated Synthesis and Initial Evaluation of (4′-Amino-5′,8′-difluoro-1′H-spiro[piperidine-4,2′-quinazolin]-1-yl)(4-[(18)F]fluorophenyl)methanone for PET/MR Imaging of Inducible Nitric Oxide Synthase. Mol Imaging. 2021;2021:9996125. doi: https://doi.org/10.1155/2021/9996125
Ionescu-Tucker A, Cotman CW. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol Aging. Nov 2021;107:86–95. doi: https://doi.org/10.1016/j.neurobiolaging.2021.07.014
Simpson DSA, Oliver PL. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants (Basel). Aug 13 2020;9(8)doi: https://doi.org/10.3390/antiox9080743
Butterfield DA. Brain lipid peroxidation and alzheimer disease: Synergy between the Butterfield and Mattson laboratories. Ageing Res Rev. Dec 2020;64:101049. doi: https://doi.org/10.1016/j.arr.2020.101049
Teleanu DM, Niculescu AG, Lungu, II, et al. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int J Mol Sci. May 25 2022;23(11)doi: https://doi.org/10.3390/ijms23115938
Carroll VN, Truillet C, Shen B, et al. [(11)C]Ascorbic and [(11)C] dehydroascorbic acid, an endogenous redox pair for sensing reactive oxygen species using positron emission tomography. Chem Commun (Camb). Apr 7 2016;52(27):4888–90. doi: https://doi.org/10.1039/c6cc00895j
Egami H, Nakagawa S, Katsura Y, et al. (18)F-Labeled dihydromethidine: positron emission tomography radiotracer for imaging of reactive oxygen species in intact brain. Org Biomol Chem. Apr 1 2020;18(13):2387–2391. doi: https://doi.org/10.1039/d0ob00126k
Hsieh CJ, Hou C, Zhu Y, et al. [(18)F]ROStrace detects oxidative stress in vivo and predicts progression of Alzheimer’s disease pathology in APP/PS1 mice. EJNMMI Res. Jul 27 2022;12(1):43. doi: https://doi.org/10.1186/s13550-022-00914-x
Okazawa H, Ikawa M, Tsujikawa T, et al. Cerebral Oxidative Stress in Early Alzheimer’s Disease Evaluated by (64)Cu-ATSM PET/MRI: A Preliminary Study. Antioxidants (Basel). May 22 2022;11(5)doi: https://doi.org/10.3390/antiox11051022
Huang LK, Chao SP, Hu CJ. Clinical trials of new drugs for Alzheimer disease. J Biomed Sci. Jan 06 2020;27(1):18. doi: https://doi.org/10.1186/s12929-019-0609-7
Yagi T, Kanekiyo M, Ito J, et al. Identification of prognostic factors to predict cognitive decline of patients with early Alzheimer’s disease in the Japanese Alzheimer’s Disease Neuroimaging Initiative study. Alzheimers Dement (N Y). 2019;5:364–373. doi: https://doi.org/10.1016/j.trci.2019.06.004
Peluso MJ, Sans HM, Forman CA, et al. Plasma Markers of Neurologic Injury and Inflammation in People With Self-Reported Neurologic Postacute Sequelae of SARS-CoV-2 Infection. Neurol Neuroimmunol Neuroinflamm. Sep 2022;9(5)doi: https://doi.org/10.1212/NXI.0000000000200003
Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. May 2011;7(3):280–92. doi: https://doi.org/10.1016/j.jalz.2011.03.003
van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. Nov 29 2022;doi: https://doi.org/10.1056/NEJMoa2212948
Martin BK, Szekely C, Brandt J, et al. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Arch Neurol. Jul 2008;65(7):896–905. doi: https://doi.org/10.1001/archneur.2008.65.7.nct70006
Meyer PF, Tremblay-Mercier J, Leoutsakos J, et al. INTREPAD: A randomized trial of naproxen to slow progress of presymptomatic Alzheimer disease. Neurology. Apr 30 2019;92(18):e2070–e2080. doi: https://doi.org/10.1212/WNL.0000000000007232
Kobayashi K, Imagama S, Ohgomori T, et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. Mar 07 2013;4(3):e525. doi: https://doi.org/10.1038/cddis.2013.54
Escribano L, Simón AM, Gimeno E, et al. Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology. Jun 2010;35(7):1593–604. doi: https://doi.org/10.1038/npp.2010.32
Gold M, Alderton C, Zvartau-Hind M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, doubleblind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30(2):131–46. doi: https://doi.org/10.1159/000318845
Butchart J, Brook L, Hopkins V, et al. Etanercept in Alzheimer disease: A randomized, placebo-controlled, double-blind, phase 2 trial. Neurology. May 26 2015;84(21):2161–8. doi: https://doi.org/10.1212/WNL.0000000000001617
Burns DK, Alexander RC, Welsh-Bohmer KA, et al. Safety and efficacy of pioglitazone for the delay of cognitive impairment in people at risk of Alzheimer’s disease (TOMMORROW): a prognostic biomarker study and a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol. Jul 2021;20(7):537–547. doi: https://doi.org/10.1016/S1474-4422(21)00043-0
Elkahloun AG, Saavedra JM. Candesartan Neuroprotection in Rat Primary Neurons Negatively Correlates with Aging and Senescence: a Transcriptomic Analysis. Mol Neurobiol. Mar 2020;57(3):1656–1673. doi: https://doi.org/10.1007/s12035-019-01800-9
Huang W, Li Z, Zhao L, Zhao W. Simvastatin ameliorate memory deficits and inflammation in clinical and mouse model of Alzheimer’s disease via modulating the expression of miR-106b. Biomed Pharmacother. Aug 2017;92:46–57. doi: https://doi.org/10.1016/j.biopha.2017.05.060
Chen SD, Chuang YC, Lin TK, Yang JL. Alternative role of glucagon-like Peptide-1 receptor agonists in neurodegenerative diseases. Eur J Pharmacol. Jan 05 2023;938:175439. doi: https://doi.org/10.1016/j.ejphar.2022.175439
Nowell J, Blunt E, Edison P. Incretin and insulin signaling as novel therapeutic targets for Alzheimer’s and Parkinson’s disease. Mol Psychiatry. Jan 2023;28(1):217–229. doi: https://doi.org/10.1038/s41380-022-01792-4
Moussa C, Hebron M, Huang X, et al. Resveratrol regulates neuroinflammation and induces adaptive immunity in Alzheimer’s disease. J Neuroinflammation. Jan 03 2017;14(1):1. doi: https://doi.org/10.1186/s12974-016-0779-0
Canhada S, Castro K, Perry IS, Luft VC. Omega-3 fatty acids’ supplementation in Alzheimer’s disease: A systematic review. Nutr Neurosci. Oct 2018;21(8):529–538. doi: https://doi.org/10.1080/1028415X.2017.1321813
Chen H, Liu S, Ji L, et al. Folic Acid Supplementation Mitigates Alzheimer’s Disease by Reducing Inflammation: A Randomized Controlled Trial. Mediators Inflamm. 2016;2016:5912146. doi: https://doi.org/10.1155/2016/5912146
Acknowledgement and Funding
ERZ serves in the scientific advisory board of Next Innovative Therapeutics (Nintx). The other authors declare that they have no competing interests. We thank Marco De Bastiani from Universiade Federal do Rio Grande do Sul for the fruitful discussion and for revising the final draft. P.R-N and SG are funded by Colin J. Adair Charitable Foundation, THE Weston Brain Institute, Canadian Institutes of Health Research (CIHR) [MOP-11-51-31; RFN 152985, 159815, 162303], Canadian Consortium of Neurodegeneration and Aging (CCNA; MOP-11-51-31 -team 1), the Alzheimer’s Association [NIRG-12-92090, NIRP-12-259245], Brain Canada Foundation (CFI Project 34874; 33397), the Fonds de Recherche du Québec — Santé (FRQS; Chercheur Boursier, 2020-VICO-279314). ERZ is funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [#312410/2018- 2; #435642/2018-9; #312306/2021-0; 409066/2022-2]; Fundação de Amparo a pesquisa do Rio Grande do Sul (FAPERGS) [21/2551-0000673-0]; Alzheimer’s Association [AARGD-21-850670]; Alzheimer’s Association and National Academy of Neuropsycology [ALZ-NAN-22-928381].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest: ERZ serves in the scientific advisory board of Next Innovative Therapeutics (Nintx). The other authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Bieger, A., Rocha, A., Bellaver, B. et al. Neuroinflammation Biomarkers in the AT(N) Framework Across the Alzheimer’s Disease Continuum. J Prev Alzheimers Dis 10, 401–417 (2023). https://doi.org/10.14283/jpad.2023.54
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2023.54