Abstract
Background
Nitrogen (N) deposition alters litter decomposition and soil carbon (C) sequestration by influencing the microbial community and its enzyme activity. Natural atmospheric N deposition comprises of inorganic N (IN) and organic N (ON) compounds. However, most studies have focused on IN and its effect on soil C cycling, whereas the effect of ON on microbial enzyme activity is poorly understood. Here we studied the effects of different forms of externally supplied N on soil enzyme activities related to decomposition in a temperate steppe. Ammonium nitrate was chosen as IN source, whereas urea and glycine were chosen as ON sources. Different ratios of IN to ON (Control, 10:0, 7:3, 5:5, 3:7, and 0:10) were mixed with equal total amounts of N and then used to fertilize the grassland soils for 6 years.
Results
Our results show that IN deposition inhibited lignin-degrading enzyme activity, such as phenol oxidase (POX) and peroxidase (PER), which may restrain decomposition and thus induce accumulation of recalcitrant organic C in grassland soils. By contrast, deposition of ON and mixed ON and IN enhanced most of the C-degrading enzyme activities, which may promote the organic matter decomposition in grassland soils. In addition, the β-N-acetyl-glucosaminidase (NAG) activity was remarkably stimulated by fertilization with both IN and ON, maybe because of the elevated N availability and the lack of N limitation after long-term N fertilization at the grassland site. Meanwhile, differences in soil pH, soil dissolved organic carbon (DOC), and microbial biomass partially explained the differential effects on soil enzyme activity under different forms of N treatments.
Conclusions
Our results emphasize the importance of organic N deposition in controlling soil processes, which are regulated by microbial enzyme activities, and may consequently change the ecological effect of N deposition. Thus, more ON deposition may promote the decomposition of soil organic matter thus converting C sequestration in grassland soils into a C source.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Extracellular enzymes catalyze the rate-limiting steps of decomposition and nutrient use and mediate the decomposition, transformation and mineralization of soil organic matter (Sinsabaugh 2010). For instance, cellulase can catalyze the degradation of cellulose, and polyphenol oxidase facilitates the biodegradation of lignin and other phenolic compounds in litter and soil (Ljungdahl and Eriksson 1985). In addition, the enzyme activities reflect the metabolic requirements of soil communities in relation to available nutrients (Zeglin et al. 2007). Therefore, the activities of enzymes of soil microorganisms have been used as important indicators to evaluate the effects of nitrogen (N) forms and availability on ecological processes in many simulated N deposition experiments. Most researchers have focused on inorganic N (IN, NH4+, NO3−, or NH4NO3) deposition and its effect on enzyme activity. Cusack (2013) suggested that the relative abundance of NO3− vs. NH4+ may play a role in driving oxidative enzyme activities.
Atmospheric N deposition keeps increasing due to the dramatic increase in emissions of anthropogenic reactive nitrogen compounds over the recent decades, such as the use of nitrogen-based fertilizers, and increased use of fossil fuels in transportation and energy production (Liu et al. 2013; Fenn et al. 2018). Increased N deposition has induced dramatic ecological changes, e.g., in soil carbon (C) sequestration, soil fertility, as well as shifts in vegetation types and soil acidification (Enowashu et al. 2009; Van Groenigen et al. 2017). N deposition alters the soil nutrient cycling through a series of microbe-mediated mechanisms, mainly by affecting the function and composition of the decomposer community, and thus also microbial enzyme activity, which is an important indicator.
However, natural atmospheric N deposition is a complex of compounds including not only IN sources (NH4+, NO3−) but also sources of organic N (ON, urea and amino acids) (Neff et al. 2002; Zhang et al. 2012). Hence, investigation of the responses of soil microbial activities to different ratios of IN to ON fertilization is highly recommended. The average contribution of ON to total N deposition is 30% globally and 28% in China and even up to 40% in the grassland in Inner Mongolia of China (Cornell 2011; Zhang et al. 2012). Different forms of N deposition can influence the activity and expression of microbial enzymes that mediate decomposition of leaf litter (Dong et al. 2019, 2020). Most studies, however, just have focused on deposition of inorganic N and its effects on decomposition of litter as well as its microbial communities. For example, addition of IN usually has reduced soil microbial biomass, changed the microbial composition and respiration, and showed negative effects on soil microbial activity on a global level (Zhang et al. 2018). Some studies have found that IN fertilization stimulates the synthesis of hydrolytic enzymes, including C-degrading enzymes, and those for acquisition of both N and P in litter decomposition. For example, IN addition improved the activities of β-1,4-glucosidase and cellobiohydrolase, which are involved in degradation of labile C compounds (mainly polysaccharides and cellulosic organic matter). Furthermore, IN additions also promoted the activities of β-1,4-N-acetyl-glucosaminidase and acid phosphatase, which related to degradation of chitin (N-compounds) and hydrolysis of ester-bound phosphate (P-compounds), respectively (Keeler et al. 2009; Hobbie et al. 2012). Fertilization with IN has also been reported to impede the activity of oxidative enzymes, such as phenol oxidase and peroxidase important for the degradation of recalcitrant C compounds, including polyphenols and lignin in soil and litter (Gallo et al. 2004; Sun et al. 2016). It is not clear, however, if the response of microbial decomposers to ON deposition in grassland soils follows the IN pattern.
In this work, we chose ammonium nitrate as IN source and urea and glycine as source for organic N. Mixed N with different IN-to-ON ratios (Control, 10:0, 7:3, 5:5, 3:7, and 0:10) was used to fertilize temperate grassland soils in Inner Mongolia (China) for 6 years. The overall objective of this work is to compare the response of soil microbial enzyme activities to different forms of N deposition in temperate grassland soils. Our working hypothesis was that added IN would increase the activity of polysaccharide-degrading enzymes (such as β-1,4-glucosidase and cellobiohydrolase) as well as N- and P-acquiring enzymes (β-1,4-N-acetyl-glucosaminidase and acid phosphatase), and repress the activity of phenol oxidase and peroxidase. Furthermore, we hypothesize that this increase would be strengthened and the repression would be weakened with the increasing in ON-to-IN ratios because of the lower N assimilation costs.
Materials and methods
Site description
We collected samples at a long-term experimental site (fenced since 2013) in a temperate steppe, which is managed by Erguna Forest-Steppe Ecotone Research Station of Institute of Applied Ecology, Chinese Academy of Sciences (CAS). The study site is located in Erguna River Basin, in the northeastern part of Inner Mongolia, China (50° 10′ 46.1ʺ N; 119° 22′ 56.4ʺ E), with a relatively flat land and most typical chernozem soil. Mean annual precipitation in this area is 360 mm, and mean annual temperature is − 2.4 °C (Dong et al. 2020). Mean N deposition is 8.1 kg N ha−1 year−1 during the entire growing seasons (Li et al. 2015). Leymus chinensis and Stipa baicalensis could account for almost 85% of the total aboveground biomass in the grassland site (Luo et al. 2017).
N fertilization experiment
NH4NO3 was chosen as the IN source, while urea and glycine were mixed in equal proportions and chosen as the ON source, because urea and free amino acids were expected to be higher with possibility of direct injection into the atmosphere (Neff et al. 2002). Urea could contribute 40% and dissolved free amino acids could contribute 20–50% of dissolved organic N (Cornell 2011). Thirty-six plots (each plot 6 m × 6 m) received one of the following six fertilization treatments with different IN-to-ON ratios (N0, Control; N1, 10:0; N2, 7:3; N3, 5:5; N4, 3:7; and N5, 0:10) (n = 6). Plots were separated by 1 m wide buffer zones, and each one received a total amount of 10 g N m−2 year−1 in solution or just water (control) once a year, starting in May 2014.
Soil collection and chemical analysis
Topsoil samples (0–10 cm) were collected from each plot in July, August and September in 2019 after 6 years of N fertilization. Ten soil cores were collected from each plot using an auger with diameter of 30 mm, and mixed into a homogeneous sample. Soil samples were sieved through a 2 mm mesh sieve to remove fine roots and small stones. All fresh soil samples were stored in a refrigerator at 4 °C until later analysis and enzyme activity measurements.
Soil chemistry was analyzed on soil samples (0–10 cm) collected in September 2019. Soil C and N contents were determined using a Vario MACRO Cuber Analyzer (Elementar Analysensysteme GmbH, Germany). Available phosphorus was determined according to Olsen and Sommers (1982). Soil pH was determined on a 1:2.5 w/v suspension in distilled water. Soil microbial biomass was determined using chloroform fumigation–extraction as described by Brookes et al. (1985). Briefly, we extracted soluble C and N from 10 g soil samples before and after fumigation with a 0.5 M K2SO4 solution. Dissolved organic C and N (DOC and DON, respectively) were measured on a Multi N/C 3100 analyzer (Analytik Jena, Germany). Conversion factors of 0.45 for C and 0.54 for N were used to calculate microbial biomass C and N (MBC and MBN, respectively) (Brookes et al. 1985; Beck et al. 1997).
Enzyme activity assays
We measured the activities of six extracellular enzymes involved in C decomposition, N acquisition, and P acquisition in grassland soils. Two hydrolytic enzymes, namely, β-1,4-glucosidase (BG) and cellobiohydrolase (CBH) related to degradation of labile-C compounds, contribute to the degradation of cellulose and other beta-1,4 glucans (Ljungdahl and Eriksson 1985; Hobbie et al. 2012). Two oxidative enzymes, namely, phenol oxidase (POX, e.g., laccase) and peroxidase (PER, e.g., lignin peroxidase), involved in degradation of recalcitrant C compounds, such as the complex and recalcitrant lignin, tannin and their degradation products (Kirk and Farrell 1987; Wang et al. 2018). The β-1,4-N-acetyl-glucosaminidase (NAG) is involved in chitin degradation and other β-1,4-linked glucosamine polymers that are analogous to the role of BG in cellulose degradation, thus equivalent to the acquisition of organic N for soil microbes (Keeler et al. 2009). The acid phosphatase (AP) hydrolyzes ester-bound phosphate, releasing phosphate, and represents an index of microbial investment in P acquisition.
The enzyme activities were determined by spectrophotometry using commercial chemical assay kits (Comin Biotechnology Co., Ltd. Suzhou, China) according to methods described by Saiya-Cork et al. (2002) and Sinsabaugh (2010). In brief, we homogenized the air-dried soil samples quantitatively, extracted them in specific buffer solutions (from the kits provided by the above commercial company) for each enzyme assay. Then the soil suspensions were incubated at 30 °C for 2–5 h on a shaker. After that, the soil extractions, substrate suspensions, and chromogenic reagents were successively dispensed into 96-well microplates using the substrates of p-nitrophenyl-β-d-glucopyranoside for BG, p-nitrophenyl-β-d-cellobioside for CBH, L-3,4-dihydroxyphenylalanine (L-DOPA) for POX, L-DOPA + hydrogen peroxide for PER, p-nitrophenyl-N-acetyl-β-d-glucosaminide for NAG, and disodium phenyl phosphate hydrate for AP. All chromogenic reactions of the mixtures were allowed to react in the dark. The absorbance of all soil samples was determined on a microplate spectrophotometer (Biotek Epoch, Vermont, USA) at 400 nm for BG, 540 nm for CBH, 430 nm for POX and PER, 450 nm for NAG, and 660 nm for AP. Enzyme activity was estimated as the average of three samplings, and expressed as micromol substrate converted per gram of soil sample per hour (μmol h−1 g−1).
Statistical analysis
We used one-way analysis of variance (ANOVA) to determine the effect of different forms of N fertilization on soil characteristics and enzyme activities. Tukey’s HSD and LSD test in post hoc multiple comparisons were conducted whenever the results of ANOVA indicated a significant difference (P < 0.05). For soil enzymes assayed on multiple dates, we used repeated-measures ANOVA to determine the effect of sampling date (July, August and September), and treatment (control and different N additions) on enzyme activity. We found no significant date by treatment interactions using repeated-measures ANOVA so we averaged across three dates to calculate a mean value for enzyme activity for each replicate of each treatment for further analyses. Linear regression was used to determine if the response of enzyme activity to different forms of N addition was related to the effect of those N addition on soil characteristics across 36 plots, including soil chemistry (pH), SOM characteristics (soil C, C:N, DOC, and DON), soil P availability, soil microbial biomass (MBC and MBN). We also examined relationships among enzyme activities and root decomposition using simple linear regression. Root decomposition rates were the average decay constants of five dominant species taken from an earlier paper (Dong et al. 2020). Significant differences were accepted at P < 0.05. All statistical analyses were performed using SPSS 19.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Soil properties and microbial biomass
Added IN (N1) and mixed N (N2, N3 and N4) did not change total soil C concentrations, while ON addition (N5) marginally promoted total soil C as compared to control (Table 1). All forms of N addition increased total soil N concentrations and decreased soil C:N after 6 years of N fertilization (Table 1). DOC increased significantly with all forms of N addition, especially after ON fertilization (N5) to 5.7 times that of control, and to 4.7 times higher than IN addition. Dissolved organic N increased significantly by 86% (N1) to 123% (N5) from control but showed no significant difference among different forms of N fertilization (Table 1). Added IN (N1) decreased soil pH significantly (P < 0.01), while the mixed N treatments alleviated the decline of soil pH, and ON addition showed no significant effect on pH compared with control treatments (Table 1).
Different forms of N addition significantly influenced total soil microbial biomass. For example, all forms of externally supplied N increased MBC and MBN in soil (P < 0.05), but this enhancement increased with the increasing ratio of organic N in N fertilization (Table 1). N5 treatments (IN:ON, 0:10) had the strongest effect on MBC and MBN concentration among all the N-fertilized treatments (P < 0.001, Table 1).
Microbial enzyme activity
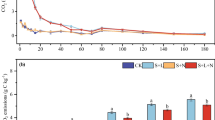
Nitrogen fertilization had positive, none, and negative effects on soil enzyme activity, depending on the form of added N and on the enzyme assayed (Fig. 1). Activities of all enzymes differed significantly among the different forms of N additions (P < 0.05 in most cases, Fig. 1). As regards cellulose degrading enzymes, added IN slightly enhanced the activities of BG and CBH, and this increase was pronounced to a range of 8–24% for BG, and 8–33% for CBH with increasing ratio of ON in the fertilizer mixture (P < 0.05, Fig. 1). For the oxidative enzymes involved in lignin degradation, added IN decreased the activity by 16% for POX and by 5% for PER as compared to control and thus showed a negative effect on POX (P < 0.05) and a marginally negative effect on PER (P < 0.1). The negative effects of added IN on POX and PER shifted to be positive as the ratios of ON increased (P < 0.05, Fig. 1). In ON treatments, the activities of POX and PER increased at its most by 19% and 43%, respectively (P < 0.01). For N-acquiring enzymes, there were significant, positive effects on NAG by all forms of N addition (Fig. 1). The activity of NAG was increased by 25% (N5) to 39% (N1) relative to control after addition of different forms of N (P < 0.01), but showed no significant difference between IN, ON and mixed N addition although there was a slowly decreasing tendency with the increasing ratios of ON-to-IN (Fig. 1). For P-acquiring enzymes, added N showed no significant effect on AP activity, with the exception of a marginally positive effect caused by IN treatment (N1, P = 0.078, Fig. 1).
Percentage difference in soil extracellular enzyme activities following different forms of N treatment (Ntrt) compared to control treatment (CK) calculated as [(Ntrt − CK)/CK × 100%]. Asterisks (*) indicate significant differences for Tukey’s HSD test in multiple comparisons of different forms of N treatment from control (*P < 0.05; **P < 0.01). IN (N1, IN:ON = 10:0); Mixed N (N2, N3, N4, IN:ON = 7:3, 5:5, 3:7); ON (N5, IN:ON = 0:10). BG, β-1,4-glucosidase; CBH, cellobiohydrolase; POX, phenol oxidase; PER, peroxidase; NAG, β-1,4-N-acetyl-glucosaminidase; AP, acid phosphatase
Correlation between soil properties and enzyme activity
Soil pH was negatively related to the activity of NAG and AP across all experimental plots (Table 2). Soil BG and CBH enzymes and peroxidase were positively related to DOC in the soil and soil microbial biomass C (R2 = 0.41–0.63, P < 0.05, Table 2). Soil P availability was unrelated to AP activity. C:N ratios for soil and for soil microbial biomass were unrelated to soil enzyme activity (Table 2).
Discussion
After 6 years of N fertilization in a temperate grassland, soil total N, soil DOC, and microbial biomass were promoted irrespective of whether inorganic or organic N was added. Moreover, this increase was enhanced with an increasing ON-to-IN ratio in the mixed-N fertilization. This result is in part consistent with the findings that organic fertilizers can promote accumulation of organic matter and increase both soil nutrients and microbial biomass (Bastida et al. 2007; Elfstrand et al. 2007). Meanwhile, our results are contrast to those of other studies in forest and farmland soils, which have reported that added inorganic N decreased or did not change total soil organic C, and N, or microbial biomass (Keeler et al. 2009; Ai et al. 2012; Zhang et al. 2018). These contradictory results suggest that in the N-limited grassland system, addition of organic N and mixed N may play more important roles in stimulating microbial activity, soil fertility and productivity than just inorganic N.
Effects on C-degrading enzyme activity
Consistent with our hypothesis, all forms of N fertilization stimulated the production of BG and CBH enzymes, which contributed to the degradation of cellulose after 6 years of N fertilization in a temperate grassland. The overall positive effect of N addition on the activities of these enzymes has been reported for many forest and grassland sites (Saiya-Cork et al. 2002; Keeler et al. 2009; Wang et al. 2011; Dong et al. 2019). Furthermore, we found that this positive effect was enhanced with increasing ON-to-IN ratios in the mixed-N fertilizer, and increased most for just organic N. Furthermore, our results demonstrate that the effects of N addition on phenol oxidase activity shifted from negative as induced by IN to positive by ON. This is probably due to that inorganic N stimulates nitrification, which in turn acidifies soil (Krusche et al. 2003), and that organic N addition alleviated the decline of soil pH (Kirk et al. 2010; Jiang et al. 2018). Simultaneously, the amino acids included in ON can provide both readily bioavailable N and an available C source for priming microbial activity (Peierls and Paerl 1997; Hobbie 2015). On the other hand, this improvement of two classes of oxidase activity by ON addition may be related to the enhanced C:N in microbial biomass caused by addition of ON (Table 1) in a direction consistent with a more fungus-dominated community (with higher C:N) in soil (Paul and Clark 1996; Kellner et al. 2008). For example, a fungal isolate identified as Rhizoctonia solani, from a grassland soil produced laccase, lignin peroxidase, and Mn peroxidase and thus induced higher oxidase activity (McErlean et al. 2006).
Some different results were observed in our previous study, reporting leaf litter decomposition and the litter’s enzyme activity (Dong et al. 2019). Specifically, the activities of leaf litter enzymes, including BG, CBH, POX, and PER, were greatly promoted by addition of mixed N in our previous research, while in the present work, the activities of soil enzymes were most enhanced by addition of just ON. These inconsistent results suggest that the C-degrading enzyme activities of both soil- and litter-dwelling microbial communities were enhanced after ON addition, but showed different response to the form of N and to the ON-to-IN ratio. This finding is in line with some studies reporting that the forms of N in the fertilizer and ON-to-IN ratio strongly influences the microbial composition, microbial activities and microbial biomass as well as the C cycling (Knorr et al. 2005; Guo et al. 2011; Du et al. 2014). Furthermore, there are different responses of the enzyme activities to different forms of N in soil and litter. Earlier, these responses have been related to microbial biomass and its C:N ratio, because the microorganisms were influenced indirectly by factors, such as nutrient availability and environmental conditions (Keeler et al. 2009). For example, following the ON addition and increased soil organic C and N (Table 1), microbial biomass and enzyme activity in the soil increased strongly due to favourable temperature and humidity, as well as more available nutrients in soil, which consequently led to higher enzyme activity than in litter layers with low moisture and relatively low substrate quality (such as lignin) (Ai et al. 2012; Dong et al. 2019). On the other hand, the enzyme activity is also dependent on soil microbial carbon and nutrient constraints, which in turn are driven by soil physicochemical properties in different decomposition environments (Jing et al. 2020). Moreover, it has been reported that real priming induced by organic compounds was not much affected by N availability, and microbial synthesis of degrading enzymes was modulated more by added C than by added N (Mason-Jonesa et al. 2018). These results suggest that C availability may be one of the most important limiting factors in priming microbial activity.
Effects on N- and P-acquiring enzymes’ activity
In contrast to our hypothesis, we found that all forms of N fertilization strongly enhanced N-acquiring enzymes, but without any significant difference among different forms of N (Fig. 1). This result suggests that in the N-limited systems, the addition of N, regardless of its form, promoted the activity of N-acquiring enzymes. On the other hand, the NAG activity was most strongly stimulated by addition of IN and more so than by addition of ON. This may be due to that IN fertilizer (viz. NH4+, and NO3−, as available nitrogen sources) can be efficiently taken up and utilized by microorganisms and thus stimulates microbial activity and metabolic efficiency, leading to an increase in N acquiring enzymes (Cusack 2013). In addition, just ON fertilizer (urea and amino acids) provided C sources as well as N for the microorganisms, and enhanced the activity of C-degrading enzymes, which produced glucose and other monosaccharides as energy source and also nutrient requirement.
We found that N fertilization had no effect on the activity of P-acquiring enzymes. This result differs from many previous investigations, which have found positive effects of added N on AP activity (Stursova et al. 2006; Zeglin et al. 2007; Keeler et al. 2009; Wang et al. 2011; Yang et al. 2017). According to the economy-based resource-allocation theories, microorganisms may use resources that are available in excess to capture those that are limited (Vitousek and Farrington 1997; Treseder and Vitousek 2001). We may conclude that P was not the limiting nutrient in microbial resource acquisition as there was no significant difference in soil available P related to different forms of N addition (Table 1), but possibly due to its nutrient return from litter input.
Enzyme activity and decomposition
Among different forms of N fertilization, none of the investigated soil enzyme activities was related to root decomposition rate (Dong et al. 2020). This lack of correlation between soil enzymes’ activities and root decomposition rates may have one or more explanations:
-
1.
The composition of microbial communities is different in soil and in root decomposing environments;
-
2.
There is a high temporal heterogeneity of enzyme activity, namely, a higher frequency of pulse events in enzyme activity than can be observed in three samplings during a growing season. Thus, this study may not reflect the integrated process of root decomposition that occurs on a 2-year time scale.
-
3.
Decomposition of litter relies on a complex suite of enzymes, while only a small number of them were measured in this study. Consequently, the variation in extracellular enzyme activities following different forms of N addition did not explain the effect of N-form on decomposition, but a shift from negative to positive effect on activity for oxidative enzymes after ON addition may be one of the mechanisms that could explain the positive effect of mixed N on root decomposition.
Conclusions
Different forms of N deposition show variable effects on the activity of decomposing enzymes in a temperate grassland soil. Inorganic N deposition had a slightly positive effect on the activity of cellulose-degrading enzymes (BG and CBH), but had negative or no effect on the activity of the ligninolytic enzymes (POX and PER). In contrast, the deposition containing organic N strongly promoted the activities of both cellulose- and lignin-degrading enzymes. On the other hand, N fertilization, regardless of its form, significantly stimulated the activity of N-acquiring enzymes in soil but showed little effect on those for P-acquisition. Our results emphasize that future studies should consider the effects of organic N deposition on soil carbon cycle processes, such as soil respiration, and litter decomposition, as well as effects on microbial community and enzymatic activities, which may change the effects of N deposition.
Availability of data and materials
All data are available in the main text or in the additional materials and raw data are available upon request to the corresponding author.
Abbreviations
- C:
-
Carbon
- N:
-
Nitrogen
- P:
-
Phosphorus
- IN:
-
Inorganic nitrogen
- ON:
-
Organic nitrogen
- DOC:
-
Dissolved organic carbon
- DON:
-
Dissolved organic nitrogen
- MBC:
-
Microbial biomass carbon
- MBN:
-
Microbial biomass nitrogen
- BG:
-
β-1,4-Glucosidase
- CBH:
-
Cellobiohydrolase
- POX:
-
Phenol oxidase
- PER:
-
Peroxidase
- NAG:
-
β-1,4-N-Acetyl-glucosaminidase
- AP:
-
Acid phosphatase
References
Ai C, Liang G, Sun J, Wang X, Zhou W (2012) Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 173–174:330–338
Bastida F, Kandeler E, Hernández T, García C (2007) Long-term effect of municipal solid waste amendment on microbial abundance and humus-associated enzyme activities under semiarid conditions. Microb Ecol 55:651–661
Beck T, Joergensen RG, Kandeler E, Makeschin F, Nuss E, Oberholzer HR, Scheu S (1997) An inter-laboratory comparison of ten different ways of measuring soil microbial biomassC. Soil Biol Biochem 29:1023–1032
Brookes PC, Landrnan A, Pruden G et al (1985) Chloroformfumigation and the release of soil nitrogen: a rapiddirect extraction method to measure microbial biomassnitrogen in soil. Soil Biol Biochem 17:837–842
Cornell SE (2011) Atmospheric nitrogen deposition: revisiting the question of the importance of the organic component. Environ Pollut 159:2214–2222
Cusack DF (2013) Soil nitrogen levels are linked to decomposition enzyme activities along an urban-remote tropical forest gradient. Soil Biol Biochem 57:192–203
Dong L, Sun T, Berg B, Zhang L, Zhang Q, Wang Z (2019) Effects of different forms of N deposition on leaf litter decomposition and extracellular enzyme activities in a temperate grassland. Soil Biol Biochem 134:78–80
Dong L, Berg B, Sun T, Wang Z, Han X (2020) Response of fine root decomposition to different forms of N deposition in a temperate grassland. Soil Biol Biochem 147:107845. https://doi.org/10.1016/j.soilbio.2020.107845
Du YH, Guo P, Liu JQ, Wang CY, Yang N, Jiao ZX (2014) Different types of nitrogen deposition show variable effects on the soil carbon cycle process of temperate forests. Glob Change Biol 20(10):3222–3228
Elfstrand S, Hedlund K, Martensson A (2007) Soil enzyme activities, microbial community composition and function after 47 years of continuous green manuring. Appl Soil Ecol 35:610–621
Enowashu E, Poll C, Lamersdorf N, Kandeler E (2009) Microbial biomass and enzyme activities under reduced nitrogen deposition in a spruce forest soil. Appl Soil Ecol 43:11–21
Fenn ME, Bytnerowicz A, Schilling SL, Vallano DM, Zavaleta ES, Weiss SB, Morozumi C, Geiser LH, Hanks K (2018) On-road emissions of ammonia: an underappreciated source of atmospheric nitrogen deposition. Sci Total Environ 625:909–919
Gallo M, Amonette R, Lauber C, Sinsabaugh RL, Zak DR (2004) Microbial community structure and oxidative enzyme activity in nitrogen-amended north temperate forest soils. Microb Ecol 48:218–229
Guo P, Wang CY, Jia Y, Wang Q, Han GM, Tian XJ (2011) Responses of soil microbial biomass and enzymatic activities to fertilizations of mixed inorganic and organic nitrogen at a subtropical forest in East China. Plant Soil 338:355–366
Hobbie SE (2015) Plant species effects on nutrient cycling: revisiting litter feedbacks. Trends Ecol Evol 30(6):357–363
Hobbie SE, Eddy WC, Buyarski CR, Adair EC, Ogdahl ML, Weisenhorn P (2012) Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr 82:389–405
Jiang J, Wang YP, Yu MX (2018) Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem Geol 501:86–94
Jing X, Chen X, Fang J, Ji C, Shen H, Zheng C, Zhu B (2020) Soil microbial carbon and nutrient constraints are driven more by climate and soil physicochemical properties than by nutrient addition in forest ecosystems. Soil Biol Biochem 141:107657. https://doi.org/10.1016/j.soilbio.2019.107657
Keeler BL, Hobbie SE, Kellogg LE (2009) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15
Kellner H, Luis P, Zimdars B, Kiesel B, Buscot F (2008) Diversity of bacterial laccase-like multicopper oxidase genes in forest and grassland Cambisol soil samples. Soil Biol Biochem 40(3):638–648
Kirk TK, Farrell RL (1987) Enzymatic “combustion”: the microbial degradation of lignin. Annu Rev Microbiol 41:465–505
Kirk GJD, Bellamy PH, Lark R (2010) Changes in soil pH across England and Wales in response to decreased acid deposition. Glob Change Biol 16:3111–3119
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Krusche AV, De Camargo PB, Cerri CE, Ballester MV, Lara LBLS, Victoria RL, Martinelli LA (2003) Acid rain and nitrogen deposition in a subtropical watershed (Piracicaba): ecosystem consequences. Environ Pollut 121:389–399
Li X, Shi H, Xu W, Liu W, Wang X, Hou L et al (2015) Seasonal and spatial variations of bulk nitrogen deposition and the impacts on the carbon cycle in the arid/semiarid grassland of Inner Mongolia, China. PLoS ONE 10(12):e0144689
Liu XJ, Zhang Y, Han WX, Tang A, Shen JL, Cui ZL, Vitousek P, Erisman JW, Goulding K, Christie P, Fangmeier A, Zhang FS (2013) Enhanced nitrogen deposition over China. Nature 494:459–462
Ljungdahl LG, Eriksson KE (1985) Ecology of microbial cellulose degradation. Adv Microb Ecol 8:237–299
Luo Q, Gong J, Yang L et al (2017) Impacts of nitrogen addition on the carbon balance in a temperate semiarid grassland ecosystem. Biol Fertil Soils 53:911–927
Mason-Jonesa K, Schmückera N, Yakov K (2018) Contrasting effects of organic and mineral nitrogen challenge the N-Mining Hypothesis for soil organic matter priming. Soil Biol Biochem 124:38–46
McErlean C, Marchant R, Banat IM (2006) An evaluation of soil colonisation potential of selected fungi and their production of ligninolytic enzymes for use in soil bioremediation applications. Antonie Van Leeuwenhoek 90:147–158
Neff JC, Holland EA, Dentener FJ, McDowell WH, Russell KM (2002) The origin, composition and rates of organic nitrogen deposition: a missing piece of the nitrogen cycle? Biogeochemistry 57:99–136
Olsen S, Sommers L (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd edn. SSSA, Madison
Paul EA, Clark FE (1996) Soil microbiology and biochemistry, 2nd edn. Academic Press, San Diego, p 340
Peierls BL, Paerl HW (1997) Bioavailability of atmospheric organic nitrogen deposition to coastal phytoplankton. Limnol Oceanogr 42:1819–1823
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Sinsabaugh RL (2010) Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42:391–404
Stursova M, Crenshaw CL, Sinsabaugh RL (2006) Microbial responses to long-term N deposition in a semiarid grassland. Microb Ecol 51:90–98
Sun T, Dong L, Wang Z, Lü X, Mao Z (2016) Effects of long-term nitrogen deposition on fine root decomposition and its extracellular enzyme activities in temperate forests. Soil Biol Biochem 93:50–59
Treseder KK, Vitousek PM (2001) Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82:946–954
Van Groenigen JW et al (2017) Sequestering soil organic carbon: a nitrogen dilemma. Environ Sci Technol 51:11503–11504
Vitousek PM, Farrington H (1997) Nutrient limitation and soil development: experimental test of a biogeochemical theory. Biogeochemistry 37:63–75
Wang C, Han G, Jia Y, Feng X, Guo P, Tian X (2011) Response of litter decomposition and related soil enzyme activities to different forms of nitrogen fertilization in a subtropical forest. Ecol Res 26:505–513
Wang X, Yao B, Su X (2018) Linking enzymatic oxidative degradation of lignin to organics detoxification. Int J Mol Sci 19:3373
Yang S, Xu ZW, Wang RZ et al (2017) Variations in soil microbial community composition and enzymatic activities in response to increased N deposition and precipitation in Inner Mongolian grassland. Appl Soil Ecol 119:275–285
Zeglin LH, Stursova M, Sinsabaugh RL, Collins SL (2007) Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia 154:349–359
Zhang Y, Song L, Liu XJ et al (2012) Atmospheric organic nitrogen deposition in China. Atmos Environ 46:195–204
Zhang TA, Chen HYH, Ruan HH (2018) Global negative effects of nitrogen deposition on soil microbes. ISME J 12:1817–1825
Acknowledgements
We want to thank the reviewers for their insightful comments towards improving the manuscript. We acknowledge the valuable help from all the staff of the Erguna Forest-Steppe Ecotone Ecosystem Research Station of IAE, CAS.
Funding
The funding for this research was supported by the National Natural Science Foundation of China (32022054 and 31901137), and China Postdoctoral Science Foundation (2018M640263), Instrument Developing Project of CAS (YJKYYQ20190079), Strategic Priority Research Program of CAS (Grant No. XDA28120100) and Youth Innovation Promotion Association of CAS (2019198).
Author information
Authors and Affiliations
Contributions
LD conceived the idea, wrote the first draft of manuscript and carried out the analyses. TS and BB contributed to improve the paper and the English. Other authors contributed to the data collection and paper writing and improving. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors agreed and approved the manuscript for publication in Ecological Processes.
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dong, L., Berg, B., Gu, W. et al. Effects of different forms of nitrogen addition on microbial extracellular enzyme activity in temperate grassland soil. Ecol Process 11, 36 (2022). https://doi.org/10.1186/s13717-022-00380-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13717-022-00380-2