Abstract
Microbial ecology is the key to understanding the function of soil biota for organic matter cycling after a single amendment of organic waste in semiarid soils. Therefore, in this paper, the long-term effect (17 years) of adding different doses of a solid municipal waste to an arid soil on humus–enzyme complexes, a very stable and long-lasting fraction of soil enzymes, as well as on microbial and plant abundance, was studied. Humic substances were extracted by 0.1 M pH 7 sodium pyrophosphate from soil samples collected in experimental plots amended with different doses of a solid municipal waste (0, 65, 130, 195, and 260 t/ha) 17 years before. The activity of different hydrolases related with the C (β-glucosidase), N (urease), and P (alkaline phosphatase) cycles and with the formation of humic substances (o-diphenol oxidase) were determined in this extract. The density and diversity of plant cover in the plots, as well as the fungal and bacterial biomass (by analyzing phopholipid fatty acids) were also determined. In general, the amended plots showed greater humic substance-related enzymatic activity than the unamended plots. This activity increased with the dose but only up to a certain level, above which it leveled off or even diminished. Plant diversity and cover density followed the same trend. Fungal and bacterial biomass also benefited in a dose-dependent manner. Different signature molecules representing gram+ and gram− bacteria, and those corresponding to monounsaturated and saturated fatty acids showed a similar behavior. The results demonstrate that organic amendment had a noticeable long-term effect on the vegetal development, humic substances-related enzyme activity and on the development of bacteria and fungi in semiarid conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Measurements of enzymatic activity provide information about soil microbial activity [30] and reflect the effect of numerous factors, including climate, type of amendment, management techniques, and type of crops. Enzymatic activities also integrate information about edaphic properties [31] and have been considered as indicators of soil quality due to their sensitivity to any changes that take place therein [13].
In SE Spain, semiarid climatic conditions (where average annual temperature and precipitation are 18°C and 300 mm, respectively) mean that a stable plant cover that would contribute organic matter to the soil is difficult to establish [26]. In this scenario, the addition of organic amendments to the soil is considered suitable because not only does it increase the organic matter content of the soil but it also improves its microbiological and biochemical quality [20]. The application of organic amendments to the soil such as of compost, sewage sludge, or municipal solid waste (MSW) reactivates the soil’s biogeochemical cycles [34], which involves greater enzymatic activity intervening in these cycles. However, free enzymes normally have a short-lived activity because they can be rapidly denatured, degraded, or irreversibly inhibited. Extracellular enzymes are usually associated with and protected by soil colloids, such as clays or humic substances [11], and act as a stable nucleus of soil activity. These humus–enzymes exhibit a great resistance against thermal denaturation, dehydration, and proteolysis [30].
The addition of organic amendment is not only reflected at the biochemical level but it also involves greater microbial proliferation and greater vegetal development [39] and even a change in the structure of the microbial populations. The phospholipid fatty acid profile gives quantitative information about community structure [14], is used as a measure of soil microbial biomass, and is sensitive to soil type and management [9], heavy metal contamination [18], vegetal composition [7, 21], and organic amendments [38]. For these reasons, the phopholipid fatty acid (PLFA) profile could help to understand soil ecology’s response to different actions or management activities.
The aim of the present paper was to study the long-term response of humus-associated enzymes and microbial communities to a single application of municipal waste to a semiarid soil. We have chosen activity measurements of humus–enzyme complexes because these fractions represent the most important fractions of extracellular enzymes that are stabilized and protected by organic matter [12, 30]. Activities of humus-associated enzymes involved in C-, N-, and P-cycling should be linked to the presence or absence of specific signature PLFAs providing information about possible changes of microbial community composition of this semiarid environment.
Methods
Study Area, Experimental Plot Design and Sampling
The experimental plots were located in Murcia (southeast Spain, 38°11′53.84″ N, 1°02′33.34″ W) in an area greatly affected by soil degradation processes such as erosion, agricultural abandonment, scarce organic matter, etc. The climate is Mediterranean semiarid. The mean annual rainfall was 272.2 mm and mean annual temperature was 16.7°C. Potential evapotranspiration reaches 1,000 mm year−1. Soil is a Xeric Torriorthent [40]. In October 1988, 15 3 × 5-m plots were established in the experimental area after a randomized block design. This area was agricultural abandoned and the soil was totally bare. The organic fraction of a fresh MSW without composting or grinding was used as soil amendment, after 15–20 days of natural stabilization. The inert and gross components (plastics, glass) of the MSW were removed by sieving before the remaining organic fraction was incorporated into the soil. The main characteristics of this material are given in Table 1.
Four rates of MSW (65, 130, 195 and 260 t ha−1) were added in triplicate to the top 20-cm soil layer using a rotovator. The control plots (Cs) (without MSW) were also tilled by rotovator. The MSW amendment levels were designed to raise the soil organic matter content by 0.5% (low amendment, L), 1.0% (medium amendment, M), 1.5% (high amendment, H), and 2.0% (very high amendment, VH) with regard to the C. The MSW was added to the soil only once (in 1988), at the beginning of the experiment (17 years before the present). The plots were sampled in the spring of 2005. For each sampling, eight subsamples per plot were randomly collected with hand-driven probes to a depth of 15 cm and then mixed to constitute a single sample per plot. The samples were sieved to <2 mm and stored at 4°C until analysis. The main characteristics of amended soil are shown in Table 2.
Chemical Analyses
Humic substances were extracted with a 0.1-M, pH 7.1 sodium pyrophosphate solution (w/v ratio = 1:10) by mechanical shaking for 24 h. The centrifuged and filtered (0.2-μm Millipore membrane, Billerica, MA, USA) extracts were dialyzed against distilled water with a membrane of 12,000–14,000 molecular weigh cut off and 25-Å pore diameter (Visking® dialysis tube, Serva GmbH, Heidelberg, Germany) to obtain a purified humic extract. Humic substance C was determined in this extract by a C analyzer for liquid samples (Shimazdu 5050A), and water-soluble proteins were determined by the Folin–Lowry method [27].
Soil electrical conductivity and pH were measured in a 1/5 (w/v) aqueous solution in a Crison mod.2001 conductivimeter and pH meter. Total organic C (TOC) was determined by oxidation with K2CrO7 in an acid medium and evaluating the excess of dichromate with (NH4)2Fe(SO4)2 [43]. Total carbonates were measured in a Bernard calcimeter according to the method described by Guitian and Carballas [22]. Total N was determined by the Kjeldahl method modified by Bremmer and Mulvaney [10]. Available P was determined as to the Olsen method [32] and available K was analyzed by ammonium displacement of the change cations [33]. Heavy metals were determined by atomic absorption in the suitable dilutions of a nitric-perchloric 1:1 extract (concentration of nitric and perchloric acid was 70%).
Enzymatic Assays
Enzymatic activities were determined using 1 ml pyrophosphate extract obtained from the soil using the following methods. Urease activity was determined by the buffered method of Kandeler and Gerber [23], using urea as substrate. Alkaline phosphatase and β-glucosidase activities were determined following the methods reported by Tabatabai and Bremner [42] and Eivazi and Tabatabai [15], respectively. Substrates of these activities were 0.025 M p-nitrophenyl phosphate and 0.025 M p-nitrophenyl β-d-glucopiranoside, respectively. o-Diphenol oxidase activity was determined as reported by Perucci et al. [37] using 0.2 M cathecol as substrate.
PLFA and Ergosterol Content
Phospholipids (PLFA) were extracted from 4 g of soil using a chloroform–methanol extraction, fractionated, and quantified using the procedure described by Frostegard et al. [19] and Bardgett et al. [4]. The fatty acid nomenclature is that used by Frostegard et al. [19]. The fatty acids i15:0, a15:0, 15:0, i16:0, 16:1ω7c, 17:0, i17:0, cy17:0, 18:1ω9c, and cy19:0 were chosen to represent bacterial biomass (bacPLFA) [4, 19], and 18:2ω6 (fungPLFA) was taken to indicate fungal biomass [17]. The ratio of bacPLFA-to-fungPLFA (bacPLFA/fungPLFA) represents the ratio between bacterial and fungal biomass. The Gram-positive (G+ PLFA) specific fatty acids i15:0, a15:0, i16:0, and i17:0 and the Gram-negative (G− PLFA) specific fatty acids cy17:0, 18:1ω9c, and cy19:0 were taken as a measure of the ratio between Gram-positive and Gram-negative bacterial biomass (G+:G− ratio). The fatty acids 15:1, 16;1ω7, 16:1ωn5, 17:1, 18:1ω9c, and 18:1ω7 represent monounsaturated fatty acid, whereas the fatty acids 14:0, i15:0, a15:0, 15:0, i16:0, 16:0, i17:0, cy17:0, 17:0, 18:0, 20:0, 22:0 and 24:0 represent saturated fatty acids. The monounsaturated PLFA to saturated PLFA ratio was expressed as mono:sat. The diversity of the fatty acids was calculated with the Shannon-index H (H PLFA):
where pi is the relative abundance of each fatty acid in the total sum and n is the number of detected fatty acids. Ergosterol was extracted with ethanol and measured by high-performance liquid chromatography [24].
Plant Cover and Biodiversity
The percentage of plant cover was estimated by a grid-line intersect method. The diversity of vegetation species was calculated with the Shannon-index (H veg) using the equation:
where pi is the relative abundance of each vegetal species in the total sum and n is the number of detected vegetal species.
Statistical Analyses
Data were submitted to one-way ANOVA. To determine significant differences between the means of the treatments a multiple range test at the 95% confidence level was performed. The post-hoc test applied was Fisher’s least significant difference method. Previously, normality of data was tested by Kolmogorov–Smirnov test. The correlation matrix at different significance levels was established to study the possible correlation between the different parameters studied. A factor analysis was carried out for relative quantity of specific PLFA to explore possible differences between treatments. Software used was SPSS 13.0.
Results
Chemical Parameters and Enzymatic Activities
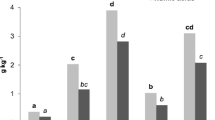
Organic amendment significantly influenced on several chemical parameters (Tables 1 and 3). TOC increased significantly with dose regarding the control in plots with L, M, and H organic amendments. On the contrary, VH treatment could not further increase TOC, which remained at levels similar to H (Table 2). The total Zn and Cu contents of M, H, and VH treatments were significantly higher than those recorded for the control and L (Table 2), but the amounts of heavy metal of all plots were below the limits established by EU legislation for soil contamination (European directive 86/278/EEC). The humic substances C, protein, and polyphenol content in the extract of the humic substances increased with amendment dose up to the H treatment, which showed the highest values (Fig. 1).
Humic substances C, proteins, polyphenols, and enzymatic activities (urease, β-glucosidase, alkaline phosphatase, and o-diphenol oxidase) in extracts of 0.1 M sodium pyrophosphate pH = 7 from the control and the amended plots. Bars represent standard deviation. For each parameter, bars with the same letter are not significantly different according to the least significant difference test (P ≤ 0.05)
Organic amendment significantly influenced extracted proteins, extracted polyphenols, and enzyme activities (Table 3). The enzymatic activities in the humic substances extract (urease, β-glucosidase, alkaline phosphatase, and o-diphenoloxidase) increased significantly in all the amended plots in comparison to the control. H rates of municipal waste favored humus-associated enzyme activities to a greater extent than either low and medium or very high application rates (Fig. 1). There was only one exception of this trend: Low municipal waste amendment did not change the activity of humus-associated o-diphenol oxidase (Fig. 1).
PLFA Profile and Ergosterol Content
In general, a single application of different amounts of municipal waste increased total contents of PLFAs, as well as bacterial and fungal indicators (Table 4). M, H, and VH treatments showed higher bacterial and fungal PLFA contents than the two other treatments (C and L) (Table 4). The bacterial-to-fungal ratio of PLFAs revealed no significant differences between treatments (Table 4). G− PLFA biomarkers (16:1ω7, cyc17:0. 18:1ω9c, and 18:1ω7) and G+ PLFA biomarkers (i15:0, a15:0, i16:0, and i17:0) increased with increasing amounts of organic waste (Table 4). However, the G+/G− ratio remained constant in all the treatments (Table 4). Monounsaturated and saturated fatty acids increased with municipal waste dose, H and VH treatments showing significantly higher values than the others (Table 4). There are no significant differences in the monounsaturated/saturated PLFA ratio (Table 4). Fatty acid 17:1 only appeared in the H treatment. The diversity Shannon Index (H PLFA) was higher in H and VH treatments than in control, L, and M treatments (Table 4). Ergosterol content increased significantly in all amended plots in comparison to the control (Table 4).
Plant Cover and Diversity
The density of the plant cover was significantly lower in the control, L, and M treatments than in the H and VH treatments (Table 5). Plots with high doses of organic amendment showed the highest density of plant cover. The Shannon diversity index was significantly lower in the control than in the other plots (Table 5). The abundance of Stipa capensis Thunb. decreased with increasing rates of municipal waste (Table 5) and Suaeda vera Forssk. increased in H and VH treatments in comparison with the other treatments. Chenopodium murale L. was only observed in H and VH treatments (Table 5), whereas Plantago albicans L. and Artemisia barrelieri Besser only appeared in the control.
Correlation Analysis
The correlation matrix between the different parameters is shown in Table 6. TOC and humic substance C were positively and significantly correlated with all the enzymatic activities analyzed (urease, β-glucosidase, alkaline phosphatase and o-diphenyloxidase), with bacPLFA, fungPLFA, G+ PLFA, G− PLFA, and the ergosterol content. FungPLFA was also positively and significantly correlated with ergosterol. The Shannon biodiversity index for vegetation (H veg) was positively and significantly correlated with the Shannon biodiversity index for PLFAs. Positive correlation coefficients were observed for plant cover density and all the parameters analyzed. Total nitrogen was positively correlated with urease, β-glucosidase, and o-diphenyloxidase activities.
Factor Analysis
The factor analysis made with the 26 PLFAs analyzed provided three factors (Fig. 2), factor 1 explaining 27.52% of the variance, factor 2 20.61%, and factor 3 15.92%. Attending to factor 1, the ANOVA of coordinates established that H and VH treatments are significantly different in comparison to the control. Load factor of each fatty acid is presented in Fig. 2. Weight of fungal PLFA biomarker was lower than bacterial PLFA indicators.
Discussion
Hydrolase activities are related with the element cycles N (urease), P (phosphatase), and C (β-glucosidase), and catalyze the hydrolysis of complex molecules to simpler molecules, permitting their assimilation by roots and microorganisms [1, 25]. Other enzymes, such as o-diphenol-oxidase, are directly involved in humus formation [41]. In this work, we have found positive correlations between total N and different humus–enzyme activities (urease, alkaline phosphatase, and o-diphenol-oxidase) (Table 6). The short-term increase in the enzymatic activity of an amended soil, widely reported, may be due to the biodegradation of the organic matter present, which generates compounds that may act as substrates for the enzymatic activity [6], whereas some authors [36] even suggested that the enzymatic load of the organic amendments is responsible for these increases in the soil’s enzymatic activity. In a larger time scale, the plant growth resulting from amendment provides root exudates that may act as substrates for enzymatic activities [35]. In addition, vegetal rests could contribute, in long-term scale, into the pool of humic substances that could be linked to enzymes. Moreover, in our long-term field experiment, the increased level of TOC and humic substance C 17 years after amendment may have been the result of the consequent vegetation growth [34]. Indeed, significant positive correlations were observed between both organic C content and the plant cover (Table 6).
Bastida et al. (2006) [5] has observed a relationship between total extracellular enzymatic activity and the state of the vegetation cover, a decrease in the one being reflected in a degradation of the other. In this work, the different enzymatic activities studied in the pyrophosphate extract also increased in parallel with the plant cover, with a positive and significant correlation between the two (Table 6), each showing a similar tendency. Also, there was a positive and significant correlation between o-diphenoloxidase activity and the polyphenol content, suggesting that this type of compound functions as substrate for this enzyme.
The maximum immobilized enzyme activity observed in the H treatment accompanied by the maximum plant cover was one of the most noteworthy findings and suggests that the long-term biochemical and biological response of the soil to the addition of organic wastes is linear up to a given threshold value, conditioned, possibly, by the vegetation cover. An explanation about the lower plant cover observed in the VH treatment compared with the H treatment remains unclear. High amounts of some heavy metals (not measured) or recalcitrant phytotoxic compounds could be responsible of this phenomenon. The threshold for enzymatic activities in the pyrophosphate extract and plant cover above the H dose cannot be attributed to measured heavy metals nor to increased salt and electrical conductivity concentrations because there were no significant differences for these parameters between H and VH treatments (Table 2). There is a clear match between humic substance C and the enzymes of the extract (note the positive and significant correlation between both parameters) (Table 6).
Soil fungi and bacteria are the major organisms responsible for nutrient cycling and for controlling the amounts of available nutrients to plants. To carry out these functions, they possess a potent enzymatic system. Plants, in turn, add energy to the soil subsystem in the form of litter and root exudates. The higher development of microbial population could support the higher production of enzymes. Some of these extracellular enzymes could be associated to humic compounds after their production. Indeed, there are positive correlations coefficients between fungal and bacterial PLFA and these enzymes activities. The highly positive and significant correlation between fungal PLFA and ergosterol suggests that, although some plants also contribute fatty acid 18:2ω6, this acid is a good measure of fungal biomass [2, 3].
The results point to a degree of microbial development parallel to the amendment dose added up to the high dose, whereas no differences existed between high and very high additions. This behavior was similar in fungi and bacteria, and even within the different bacterial groups (gram+ and gram−). Marschner et al. [29] showed that bacterial development was parallel to organic C content in amended soils. This behavior could be reflected in the positive correlation between bacterial PLFA and TOC.
As in the case of enzymatic activities, several authors have observed that not only the plant cover but also the diversity of the cover affects the soil microbial communities [21, 28] and their activity. From the greater plant diversity (Shannon index) observed in high and very high doses, it seems that the addition of organic amendment to a soil could be related to a generation of niches for plants not observed in the control treatment. In this sense, we have observed a decrease in S. capensis Thumb. cover in plots with high and very high organic amendment addition (H and VH), whereas an increase in C. murale L. was observed in the same plots comparing to control. The positive correlation coefficients between plant cover or diversity and the activity of some immobilized enzymes indicate that vegetation is a key aspect in the soil biochemical cycles. Moreover, the higher PLFA diversity index observed in the treatments with high rates of organic amendment (H and VH) with respect to the other treatments suggests a change in the microbial diversity associated with the change in the above-ground diversity in this long-term experiment. The presence of a different grouping in PLFA factor analysis could indicate a change in the structure of soil microbial community even 17 years after amendment. This fact means that the stabilization of soil microbial community structure could take a longer amount of time under semiarid climatic conditions. However, molecular methods could increase the knowledge about these changes.
Several authors have investigated fungi/bacteria ratio of disturbed ecosystems and fertilized ecosystems. Elfstrand et al. (2007) [16] observed, also in long-term scale, that there were no significant differences in bacterial/fungal and G+/G− ratio of Eutric Cambisol when green manure is applied to soil, comparing with unfertilized soils. However, they found differences between green manure and other types of amendments. Marschner et al. (2003) [29] found significant differences between different types of fertilization in a calcareous regosol in a long-term scale, manure sewage sludge and straw showing the highest values on these ratios, rather than mineral fertilization. Bossio and Scow (1998) [8] observed that the monounsaturated/saturated PLFA ratio increased with high straw inputs, indicating that this ratio could act as an indicator of C availability in soil. However, our results indicate that there are no significant differences for this ratio between different treatments. In this sense, we should keep in mind that this is a long-term experiment and, maybe, this ratio would be more suitable in a short-term one. In our work, the absence of any difference in the fungi/bacteria or G+/G− ratios between treatments and even the control (despite the substantial increase in microbial biomass) suggests that, in the semiarid climatic conditions of the study area, there was a parallel increase in bacterial (G+ and G−) and fungal biomass related with the increase in vegetal cover, humus formation, and creation of enzyme–humus complexes.
In conclusion, humic substance-related enzymatic activities, abundance of soil microorganisms, and abundance and diversity of plant cover have been changed even 17 years after a single addition of a MSW. The addition of such a waste leads to the long-term increase in the organic matter content and water-soluble carbon fractions, and of the enzymatic activities of urease, β-glucosidase, alkaline phosphatase, and o-diphenyloxidase associated with humic substances, improving the biochemical quality of the soil. Also, an increase in microbial abundance of both fungi and bacteria has occurred 17 years after organic amendment, accompanied by a change in soil microbial community structure under different treatments. However, such materials should be used rationally because the response of the enzymatic activity associated with the pyrophosphate extract or of the plant cover was not parallel with the dose of waste used; in the semiarid conditions in which the experiment was conducted, a threshold value existed, above which any increase in dose was not matched by an improvement in the chemical and biochemical characteristics of the soil.
References
Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic, San Diego
Bååth E, Anderson TH (2003) Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA based techniques. Soil Biol Biochem 35:955–963
Bardgett RD, Hobbs PJ, Frostegard A (1996) Changes in soil fungal:bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol Fertil Soils 22:261–264
Bardgett RD, McAlister E (1999) The measurement of soil fungal:bacterial biomass ratios as an indicator of ecosystem self-regulation in temperate meadow grasslands. Biol Fertil Soils 29:282–290
Bastida F, Moreno JL, Hernández T, García C (2006) Microbiological degradation index of soils in a semiarid climate. Soil Biol Biochem 38:3463–3473
Benítez E, Sainz H, Nogales R (2005) Hydrolytic enzyme activities of extracted humic substances during the vermicomposting of a lignocellulosic olive waste. Bioresour Technol 96:785–790
Bezemer TM, Lawson CS, Hedlund K, Edwards AR, Brook AJ, Igual JM, Mortimer SR, Van der Putten WH (2006) Plant species and functional groups effects on abiotic and microbial soil properties and plant–soil feedback responses in two grasslands. J Ecol 94:893–904
Bossio DA, Scow KM (1998) Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb Ecol 35:265–278
Bossio DA, Scow KM, Gunapala N, Graham KJ (1998) Determinants of soil microbial communities: effects of agricultural management, season, and soil type on phospholipids fatty acid profiles. Microb Ecol 36:1–12
Bremmer JM, Mulvaney RL (1978) Urease activity in soils. In: Burns RG (ed) Soil enzymes. Academic, New York, pp 595–624
Burns RG (1982) Enzyme activity in soil: location and a possible role in microbial ecology. Soil Biol Biochem 14:423–427
Ceccanti B, Nannipieri P, Cervelli S, Sequi P (1978) Fractionation of humus–urease complexes. Soil Biol Biochem 10:39–45
Dick WA, Tabatabai MA (1993) Significance and potential uses of soil enzymes. In: Metting FB (ed) Soil microbial ecology: application in agricultural and environmental management. Marcel Dekker, New York, pp 95–127
Ebersberger D, Werrnbter N, Niklaus P, Kandeler E (2004) Effects of long term CO2 enrichment on microbial community structure in calcareous grassland. Plant Soil 264:313–323
Eivazi F, Tabatabai MA (1987) Glucosidases and galactosidases in soils. Soil Biol Biochem 20:601–606
Elfstrand S, Hedlund K, Mårtensson A (2007) Soil enzyme activities, microbial community composition and function after 47 years of continuous green manuring. Appl Soil Ecol 35:610–621
Federle TW, Dobbins DC, Thornton-Manning JR, Jones DD (1986) Microbial biomass, activity, and community structure in subsurface soils. Ground Water 24:365–374
Frostegard A, Tunlid A, Baath E (1993) Phospholipid fatty-acid composition, biomass, and activity of microbial communities from 2 soils types experimentally exposed to different heavy-metals. Appl Environ Microbiol 59:3606–3617
Frostegard A, Tunlid A, Baath E (1993) Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol Biochem 26:723–730
García C, Hernández T, Costa F (1994) Microbial activity in soils under Mediterranean environmental conditions. Soil Biol Biochem 26:1185–1191
Grayston SJ, Wang SQ, Campbell CD, Edwards AC (1998) Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem 30:369–378
Guitian F, Carballas T (1976) Técnicas de análisis de suelos. Pico Sacro. Santiago de Compostela
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils 6:68–72
Kandeler E, Kampichler C, Joergensen RG, Molter K (1999) Effects of mesofauna in a spruce forest on soil microbial communities and N cycling in field mesocosms. Soil Biol Biochem 31:1783–1792
Kandeler E (2007) Physiological and biochemical methods for studying soil biota and their function. In: Paul EA (ed) Soil microbiology, biochemistry and soil ecology. Elsevier, San Diego, pp 53–80
Lopez Bermúdez F, Albaladejo J (1990) Factores ambientales de la degradación del suelo en el Área mediterránea. In: Albaladejo J, Stocking MA, Díaz E (eds) Soil degradation and rehabilitation in mediterranean environmental conditions. CEBAS-CSIC, Madrid, pp 15–42
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurements with the folin phenol reagent. J Biol Chem 193:265–275
Marschner P, Yang CH, Lieberei R, Crowley DE (2001) Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol Biochem 33:1437–1445
Marschner P, Kandeler E, Marschner B (2003) Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol Biochem 35:453–461
Nannipieri P, Grego S, Ceccanti B (1990) Ecological significance of the biological activity in soils. In: Bollag JM, Stotzky G (eds) Soil biochemistry. Marcel Dekker, New York, pp 293–355
Naseby DC, Lynch JM (1997) Rhizosphere soil enzymes as indicators of perturbations caused by enzyme substrate addition and inoculations of a genetically modified strain of Pseudomonas fluorescens on wheat seed. Soil Biol Biochem 29:1253–1362
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties. Second edition. SSSA, Madison, pp 403–430
Page AL, Miller RH, Keeney DR (1982) Methods of soil analysis. Part 2. Chemical and microbiological properties. Second edition. SSSA, Madison
Pascual JA, García C, Hernández T, Ayuso M (1997) Changes in the microbial activity of an arid soil amended with urban organic wastes. Biol Fertil Soils 24:429–434
Pascual JA, García C, Hernandez T (1999) Lasting microbiological and biochemical effects of the addition of municipal solid waste to an arid soil. Biol Fertil Soils 30:1–6
Perucci P (1992) Enzyme activity and microbial biomass in a field soil amended with municipal refuse. Biol Fertil Soils 14:54–60
Perucci P, Casucci C, Dumontet S (2000) An improved method to evaluate o-diphenol oxidase activity of soil. Soil Biol Biochem 32:1927–1933
Petersen SO, Henriksen K, Mortensen GK, Krogh,PH, Brandt KK, Sorensen J, Madsen T, Petersen J, Gron C (2003) Recycling of sewage sludge and household compost to arable land: fate and effects of organic contaminants, and impact on soil fertility. Soil Tillage Res 72:139–152
Ros M, Hernandez MT, García C (2003) Soil microbial activity after restoration of a semiarid soil by organic amendments. Soil Biol Biochem 35:463–469
Soil Survey Staff (1998) Keys of soil taxonomy. USDA-NCRS, Washington, DC
Stevenson FJ (1982) Humus chemistry. Genesis, composition, reactions. Wiley, New York
Tabatabai MA, Bremmer JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Yeomans J, Bremmer JM (1989) A rapid and precise method for routine determination of organic carbon in soil. Commun Soil Sci Plan Anal 19:1467–1476
Acknowledgment
Felipe Bastida thanks the Spanish Ministry of Science and Technology for the financial support of his Spanish FPU Fellowship and his stage in the University of Hohenheim. In addition, the authors want to thank the members of Prof. Kandeler’s team at the University of Hohenheim for providing support during PLFA analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bastida, F., Kandeler, E., Hernández, T. et al. Long-term Effect of Municipal Solid Waste Amendment on Microbial Abundance and Humus-associated Enzyme Activities Under Semiarid Conditions. Microb Ecol 55, 651–661 (2008). https://doi.org/10.1007/s00248-007-9308-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9308-0