Abstract
Aims
Litter decomposition affects soil organic carbon storage, and nutrient addition alters exogenous nutrient availability in soil, thereby affecting the endogenous nutrient concentration and extracellular enzyme activity in litter. However, how above- and belowground litter decomposition responds to nutrient addition is not fully understood.
Methods
We conducted a 7-yr field experiment with 5 levels (0, 1, 2.5, 5, and 12.5 g P m−2 yr−1) of phosphorus fertilization (P). Starting in the 6th year, we determine litter decomposition, nutrient elements, and enzyme activity to explore the response mechanisms of above- and belowground litter decomposition to P addition.
Results
All levels of P addition increased the rate of aboveground litter decomposition, but only 2.5 g P m−2 yr−1 increased that of belowground litter. P addition increased the litter initial P and Mn contents, while decreased the C/P and N/P ratios of aboveground litter, which stimulated the activities of β-1,4-glucosidase and β-1,4-N-acetylglucosaminidase, thereby increasing the aboveground litter decomposition rate. The belowground litter decomposition was mainly inhibited by lignin content, and 2.5 g P m−2 yr−1 addition significantly decreased lignin content and increased the phenol oxidase activity, thereby accelerating the decomposition. P addition promoted the accumulation of P and the release of cellulose from aboveground litter and promoted the accumulation of calcium and the release of carbon from belowground litter.
Conclusions
Different mechanisms of above- and belowground litter decomposition under P addition were driven by litter quality and enzyme activity, but moderate P addition increased semiarid grassland litter decomposition, which may contribute to low soil C sequestration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P), is an important regulator of plant growth and development, community dynamics, ecosystem structure and function, and plays an important role in the nutrient cycle and energy flow of grassland ecosystems (Samaddar et al. 2019; Zhu et al. 2021; Sun et al. 2023). Litter decomposition affects the nutrient cycling and soil organic matter formation processes in grassland ecosystems and is highly susceptible to external nutrient changes (Gong et al. 2022). Therefore, studying the effect of exogenous P addition on grassland litter decomposition is helpful to understand the effect of P cycle on C cycle in the grassland ecosystem.
Litter can be divided into aboveground litter and belowground litter, both of which are of great significance to the nutrient cycle of the ecosystem. Above- and belowground litters differ fundamentally in their tissue chemistry heterogeneity and their decomposition microenvironment, so their decomposition may also respond differently to changes in the environment (Xia et al. 2017; DeForest 2019). However, many studies describing organic matter cycling in terrestrial ecosystems are still based on the characterization of aboveground litter and rarely explore both types of substrate decomposition or their response to increased nutrient (especially P) availability, which biases our full understanding of the feedback relationships between plant traits and carbon cycling (Norris et al. 2012; Mariotte et al. 2018). A current study found that P addition simultaneously increased the decomposition rate of above- and belowground litter (Li et al. 2017). Conversely, it has also been found that P addition can increase aboveground litter decomposition but has no effect on belowground litter decomposition (Jiang et al. 2018). Because of these discrepancies and the importance of litter decomposition, it is necessary to study both above- and belowground litter decomposition in a single experiment.
The decomposition rates of above- and belowground litter are affected by many factors. On the ecosystem scale, above- and below-ground litter decomposition are mainly influenced by litter quality, such as chemical elements, lignin, and cellulose, and biological factors, such as microbial enzyme activity (Ochoa-Hueso et al. 2019; Dong et al. 2021). Generally, litter with a high content of water-soluble components and a low content of lignin and cellulose will be more easily decomposed (Wang et al. 2020; Kriiska et al. 2021). In addition, mineral elements such as Mn, Mg, and Ca in above- and belowground litter can participate in the growth and metabolism of microorganisms, thus affecting litter decomposition rate (Berg et al. 2015; Vivanco and Austin 2019). In terms of microorganisms, extracellular enzymes play a critical role in microbial metabolism and litter decomposition, reflecting the relationship between biological nutrient demand and supply (Cui et al. 2019). Microorganisms alter the decomposition process of litter by releasing different extracellular enzymes to decompose organic substances that contain C, N, and P to obtain the nutrients that maintain their metabolism (Jing et al. 2016). P limitation may affect the growth of plants and microorganisms. However, it remains poorly understood how different concentrations of exogenous P inputs regulate litter quality and enzyme activity, and ultimately affect above- and belowground litter decomposition rates.
Two theories are able to explain the changes in litter decomposition processes under P addition: the stoichiometric hypothesis and the microbial mineralization hypothesis. The former suggests that the decomposition rate is higher when the stoichiometry of litter is most similar to that of decomposers (Sterner and Elser 2002). In other words, P addition can accelerate decomposition, especially in nutrient-deficient environments, by increasing nutrient availability and stimulating microbial growth and activity (Moorhead and Sinsabaugh 2006). The latter suggests that the decomposition of litter is driven by microbial nutrient demand (Allison and Vitousek 2005). P addition reduces the limiting effect of nutrients on microorganisms, and the activity of enzymes that obtain this nutrient will decrease, ultimately inhibiting the rate of decomposition of litter. However, it is still unclear how the two mechanisms influence above- and belowground litter decomposition under nutrient deposition.
The temperate grasslands of Inner Mongolia not only provide edible forage for China’s animal husbandry, but also play a crucial role in ecological protection, such as carbon sequestration and water and soil conservation (Bai et al. 2022). However, the consumption of vegetation by livestock grazing leads to a loss of P from grasslands (Chaneton et al. 1996), and N deposition may also cause a P deficiency in grasslands (Tilman et al. 2002). However, little is known about the response of above- and belowground litter decomposition in grassland ecosystems to multiple levels of P addition. To fill this knowledge gap, a 2-yr field litter decomposition experiment (final 2- of a 7-yr experiment) was conducted in this study with 5 P addition levels (0, 1, 2.5, 5, and 12.5 g P m−2 yr−1). The aim was to fully understand the response of above- and belowground litter decomposition to different P addition levels. We hypothesized that: (1) P addition can increase the decomposition rate of above- and belowground litter, but (2) the response mechanisms of above- and belowground litter responded differently to P addition because of litter quality and extracellular enzyme activity. The resulting knowledge helps to fully understand the impact of changes in the P cycle of grassland ecosystems on the C cycle.
Materials and methods
Study area and experimental set-up
The Grassland Ecosystem Research Station (44° 48’ N - 44° 49’ N, 116° 02′ E - 116° 30′ E) was selected as the study area in Inner Mongolia, northern China. The average temperature from June 2018 to June 2020 was 4.3 °C. Annual rainfall was 307.5 mm, mainly concentrated from June to September (the growing season), accounting for about 70% of the total annual rainfall (Fig. S1). The dominant species in the study area are two C3 perennial grasses, the vegetation cover of Leymus chinensis was about 40% and that of Stipa grandis was about 10%. Leymus chinensis contributes approximately 90% of the total aboveground biomass (Wang et al. 2019). The soil is a Calcic-orthic Aridisol in the USDA soil taxonomy that developed from eolian parent material, and the contents of sand, silt, and clay are 60%, 21% and 19%, respectively (Zeng et al. 2010).
The experiment was a randomized block design with three replications. The experiment was established on a flat and uniformly vegetated site in 2014. P treatments were randomly assigned to 5 plots (6 m × 6 m) per block and 15 total plots (5 treatments × 3 blocks). To minimize mutual interference and edge effects, a 1.5-m buffer without fertilization was established between adjacent plots. We designed the P addition based on the fertilization standard for soil in Inner Mongolia, which is 5 g P m−2 yr−1. Five P addition concentrations (0, 1, 2.5, 5, and 12.5 g P m−2 yr−1) were established, and referred to control, P1, P2.5, P5, and P12.5. The P fertilizer used is NaH2PO4 (analytical reagent, test content ≥99.9%), which was applied once per year from 2014 to 2020, and the control was sprayed with only 25 L of water (the amount of dissolved NaH2PO4). Effects of 5-yr of P additions on soil characteristics (in 2018) are shown in Table 1.
Preparation and retrieval of litterbag
At the beginning of June 2018, we randomly established four 1 m × 1 m quadrats in each plot. First, dead material on the ground surface was collected, and soil and other impurities were removed from it. We then used an 8-cm-diameter root drill to collect all dead and living roots to 40 cm of soil, including coarse and fine roots. We used a 2-mm mesh to separate root tissue from the soil and repeated this process until no roots were visible in the soil. Ultimately, we obtained aboveground material and belowground tissue from more species, but mainly those of the dominant species Leymus chinensis and Stipa grandis. These samples were used to represent the above- and belowground litter in the decomposition study. For each plot, the collected litter was homogenized to produce a single homogeneous sample.
The litter decomposition experiments were conducted using the in-situ litterbag method. The litterbag is a 10 cm × 10 cm nylon mesh bag with a 1 mm mesh size. The above- and belowground litterbags were filled with 10 g and 5 g of air-dried litter, respectively. In each plot, 30 bags of aboveground litter and 22 bags of belowground litter were installed. Before placing the aboveground litterbags, we removed any surface litter or stones that would prevent contact with the soil. The aboveground litterbags were then attached to the ground surface using bamboo stakes to ensure they remained in contact with the soil and were distributed evenly throughout the original plots. The belowground litterbags were buried into the soil at a depth of 10 cm and maintained at an angle of 45° to ensure full contact with the soil. Before replacing the original soil above the buried litterbags, we removed enough soil to equal the volume of the litter bag, then provided only enough compression to restore the original soil surface; that is, the density and porosity of the replaced soil did not differ greatly from that of the surrounding soil.
The aboveground litterbags were retrieved at 2, 3, 5, 9, 12, 14, 15, 17, and 24 months and the belowground litterbags were retrieved at 2, 3, 9, 12, 15, and 24 months. On each sampling date, three aboveground and three belowground litter decomposition bags per plot were retrieved. We gently washed away any soil attached to the litter surface in the laboratory with tap water and removed the live roots, which we identified based on their color.
Assay of microbial enzymatic activities
This study determined the activities of five extracellular enzymes in litter, which included one C-acquiring enzyme (β-1,4-glucosidase, BG), one N-acquiring enzyme (β-1,4-N-acetylglucosaminidase, NAG), one P-acquiring enzyme (acid phosphatase, AP), and two oxidases (phenol oxidase, PhOx; peroxidase, PerOx), following a modified method based on the description of Saiya-Cork et al. (2002). Briefly, 1.5 g of litter and 150 ml of Tris buffer (pH = 8) were used for enzyme assays with 4- Methylumbelliferyl-β-D-glucoside (200 μM), 4-Methylumbelliferyl-N-acetyl-β-D- glucosaminide (200 μM) and 4-Methylumvelliferyl Acid phosphate (200 μM) as the BG, NAG and AP substrates, respectively. In addition, 3,4-dihydroxyphenylalanine was used as the PhOx and PerOx substrates. The activity of extracellular enzymes was measured using a microplate reader (Synergy H1, Bio Tek, USA). The detailed methodology is available in the supplementary online material (Appendix 1). Enzyme activity was measured after each collection of above- and belowground litter, and the sample collection time is shown in “Preparation and retrieval of litterbag” section. The average value of multiple determinations was calculated for statistical analysis.
Litter quality
Litter samples were ground using an electric mill (1093 Cyclotec, FossTecator, Högnäs, Sweden) to pass through a 40-mesh stainless-steel sieve. The total C and N contents were determined by an automatic elemental analyzer (Vario EL, Elementar, Langenselbold, Germany) (Shi et al. 2021). The total P, calcium (Ca), magnesium (Mg), and Mn contents were determined by axial-view inductively coupled plasma spectrometry (SPECTRO Analytical Instruments, Kleve, Germany) (Li et al. 2022). The cellulose and lignin contents were determined using the Van Soest method (Van Soest 1967). The detailed methodology is available in the supplementary online material (Appendix 2). The measurement time of the above indicators is after each sample is recovered, and the sample collection time is shown in “Preparation and retrieval of litterbag” section.
Soil sampling and analyses
When retrieving the litterbags in August 2018, soil samples were collected by soil drills with an inner diameter of 8 cm from the 0–10 cm layer under the litterbags. Soil samples were brought back to the laboratory in crisper boxes with ice packs, and impurities were removed using a 2-mm sieve. The contents of soil total C (STC), N (STN) and P (STP) were determined by the same method as litter. We determined the soil NH4+-N and NO3−-N contents using a continuous flow analyzer (AA3, Bran+Luebbe, Norderstedt, Germany). Soil pH was measured using a calibrated pH meter. The soil available P (Pavail) was determined using molybdenum blue spectrophotometry with a UV spectrometer (756 PC, Jinghua, China), while soil organic P (Porg) was determined using molybdenum blue spectrophotometry with the same UV spectrometer after combustion of the sample.
Data analysis
Litter decomposition rate and mass remaining
We determined the constant for the decomposition rate (k) after 24 months of litter decomposition using a single-negative exponential model (Olson 1963). We then calculated the mass remaining (MR) as the ratio of residual litter mass to the initial litter mass after a given duration of decomposition (Gao et al. 2015):
where X0 (g) is the dry weight of litter at the beginning of the experiment, Xt (g) is the residual mass of litter at decomposition time, t, e is the base of the natural logarithm, and k is the litter decomposition rate.
Nutrient accumulation index
The release or accumulation of each element in litter can be characterized by the nutrient accumulation index (R). When R < 100%, it indicated that the litter nutrient had a net release; when R > 100%, it indicated that the net accumulation (McGroddy et al. 2004).
where C0 (mg·g−1) and M0 (g) represent the initial concentrations of litter elements and dry weight, Ct (mg·g−1) and Mt (g) represent the concentration and dry weight after t time, respectively.
Statistical analysis
Although we collected triplicate decomposition bags per plot per time period, we mixed them into a composite sample for the determination of the above indicators. All statistical analyses and graphing were performed using the R software (version 4.1.0). One-way analysis of variance (ANOVA) and the Tukey test were used the “multcomp” package to detect the effect of different P addition levels on soil properties, decomposition rate, litter mass remaining, initial litter quality and extracellular enzyme activities. Normality and homogeneity of variance tests were performed using the Shapiro-Wilk and Bartlett tests, respectively, to ensure the validity and reliability of the research results. A linear mixed effects model was used to determine the effects of P addition, sampling times and their interaction on litter element release, P addition and sampling time were fixed effects, block and block - sampling time interactions as random effect. Models were run using the “lmer” function from the “lmerTest” package for R. We used Pearson correlation to analyze the relationship between the litter decomposition rate and the litter quality and enzyme activity. To compare and analyze the effects of litter chemical properties and biological factors on the decomposition rate of above- and belowground litter under P addition, we constructed a priori model of partial least squares path modeling (Fig. S2). In the model, the chemical properties of litter were divided into three categories: nutrient element (N, P, Ca, Mg, Mn), carbon quality (C, Cel, Lig), ecological stoichiometry (C/N, C/P, N/P, Lig/N). We also considered the biological factors, including enzyme activity (BG, NAG, AP, PhOx, PerOx), and microbial ecological stoichiometry (ln BG/ln NAG, ln BG/ln AP, ln NAG/ln AP) that respond to microbial nutrient requirements. We used the “plspm” package in R to calculate the path coefficients of the model. To improve the model fit, we eliminated factors with loading less than 0.7 and used goodness of fit (GOF) values to evaluate the model fit.
Results
Initial litter quality
Compared to the control, P5 significantly increased the aboveground litter initial C, P, Mg, and Mn contents, while it significantly decreased the C/P and N/P ratios (p < 0.05; Table 2). For belowground litter, P2.5 significantly increased the initial C and P contents, and significantly decreased the Ca and lignin contents and the C/P, N/P, and lignin/N ratios (p < 0.05; Table 2). However, the differences between the initial litter quality for the two litter types and the control values were often only significant at high or intermediate P addition levels.
Litter mass remaining and decomposition rate
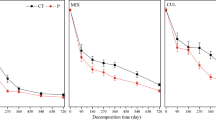
The litter mass remaining showed an alternating trend of rapid decline and then slow decline during the whole decomposition process. After 24 months, the litter mass remaining was significantly different between the different P additions (p < 0.05; Fig. 1a, b). P addition significantly increased aboveground litter decomposition, with the highest value in the P5, which was 2.08 times higher than the control (p < 0.05; Table 3). The belowground litter decomposition rate increased significantly only at P2.5, which was 1.17 times higher than the control (p < 0.05; Table 3).
Litter mass remaining of above (a) and belowground litter (b) under the P addition. Significance: The * mark indicates that litter mass remaining has a significant difference among different P addition treatments at the same sampling time (ANOVA followed by LSD, p < 0.05). Error bars show standard errors of means for n = 3
Litter N, P, and mineral element dynamics
During the litter decomposition process, aboveground litter N accumulated in the first 5 months and was then released (Fig. 2a), while P showed accumulation, with significant differences observed under different P addition treatments (p < 0.01; Fig. 2c). Belowground litter N and P showed continuous release, and P addition had no significant effect (p = 0.53, 0.15; Fig. 2b, d). Above- and belowground litter Ca accumulated, with significant differences observed under different P addition treatments (p < 0.01; Fig. 3a, b). Mg and Mn showed accumulation in aboveground litter and release in belowground litter, while P addition did not significantly affect the Mg and Mn remaining in above- and belowground litter (Fig. 3c to f).
Litter C, cellulose, and lignin dynamics
During the litter decomposition process, above- and belowground litter C showed release, and the average C remaining after two years were 33.0% and 53.9%, respectively (Fig. 4a, b). The cellulose release trend of aboveground litter shows significant differences under different P additions (p < 0.05, Fig. 4c). Compared with the control, P1 to P12.5 reduced the cellulose remaining in aboveground litter. The cellulose of belowground litter accumulated first and was then released, and the remaining was not significantly different between different P additions (p = 0.42; Fig. 4d). Above- and belowground litter lignin showed accumulation, and P addition had no significant effect on the lignin remaining (p = 0.12, 0.85; Fig. 4e, f).
Litter extracellular enzyme activities
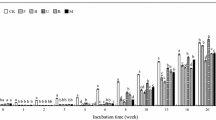
P addition significantly increased the activities of C- and N-acquiring enzymes in aboveground litter, and the activities of BG and NAG reached the highest values in P12.5 and P5, respectively (p < 0.05; Fig. 5a, b). In the belowground litter, the activity of P-acquiring enzymes (AP) decreased significantly in the P2.5 and P5 treatments, with the lowest value at the P2.5, and PhOx activity reached the maximum at the P2.5 treatment (Fig. 5c, d). The enzyme activity in the belowground litter was lower than that in the aboveground litter, and the mean (for all P levels) BG, NAG, and AP activity decreased by 78%, 81%, and 56%, respectively, compared with the aboveground litter, whereas PhOx and PerOx activity decreased by 1% and 14%, respectively (Fig. 5e).
Enzyme activity in the above- and belowground litter under the P addition. Abbreviations: BG is β-1,4-glucosidase, NAG is β-1,4-N-acetylglucosaminidase, AP is acid phosphatase, PhOx is phenol oxidase, and PerOx is peroxidase. Bars for a given enzyme labeled with different letters differ significantly between P addition levels, p < 0.05. Error bars show standard errors of means for n = 3
Main factors affecting the above- and belowground litter decomposition
The correlation matrix showed the aboveground litter decomposition rate was significantly positively correlated with the NAG and BG activities (p < 0.05), BG/AP (p < 0.05) and NAG/AP ratios (p < 0.01; Fig. 6a); it was significantly negatively correlated with the initial C/P, N/P, and BG/NAG ratios (p < 0.05). The belowground litter decomposition rate was significantly positively correlated with the PhOx activity (p < 0.01) and negatively correlated with the initial lignin content (P < 0.01; Fig. 6b). Across the experimental P fertilization gradient, we observed divergent drivers of decomposition of above- and belowground litter.
The correlation between aboveground (a) and belowground (b) litter decomposition rate and nutrient element, carbon quality, ecological stoichiometry, and enzyme activity. Cel is cellulose, Lig is lignin, NAG is β-1,4-N-acetylglucosaminidase, AP is acid phosphatase, BG is β-1,4-glucosidase, PhOx is phenol oxidase, and PerOx is peroxidase, BG/NAG is the ln BG/ln NAG ratio, BG/AP is the ln BG/ln AP ratio, NAG/AP is the ln NAG/ln AP ratio, and Lignin/N is lignin/N ratio. Significance: * p < 0.05, ** p < 0.01
Using partial least squares-path modeling, we investigated the mechanism of P addition on the decomposition rate of above- and belowground litter (Fig. 7). P addition had a significant positive effect on aboveground litter nutrient elements and a significant negative effect on carbon quality and ecological stoichiometry, with standard direct path effects of 0.87, −0.85, and − 0.37, respectively (Fig. 7a). Aboveground litter decomposition rate was controlled by enzyme activity with a standard direct path effect of 0.96 (Fig. 7a, Fig. S3). P addition had a significant negative effect on belowground litter nutrient elements and ecological stoichiometry, with standard direct path effects of −0.54 and − 0.85, respectively (Fig. 7b). The belowground litter decomposition rate was controlled by carbon quality, ecological stoichiometry, and enzyme activity with standard direct path effects of −0.60, 0.51, and 0.52, respectively (Fig. 7b, Fig. S3). In general, P addition had a positive effect on aboveground litter decomposition with a standard total path effect of 0.34 and a negative effect on belowground litter decomposition with a standard total effect of −0.38.
Partial least squares path modeling of the effect of P addition on the above- and belowground litter decomposition rate. NAG is β-1,4-N-acetylglucosaminidase, BG is β-1,4-glucosidase, PhOx is phenol oxidase, Cel is cellulose, Lig is Lignin and Lignin/N is lignin/N ratio. GOF is goodness of fit. Significance: * p < 0.05, ** p < 0.01
Discussion
The effects of P addition on nutrient dynamics in above- and belowground litter
Litter decomposition is accompanied by dynamic changes of nutrients, and the release or accumulation of nutrients mainly depends on the balance between the chemical composition of the litter and the nutrient requirements of microorganisms (Martínez-García et al. 2021). In our study, both above- and belowground litter C showed a continuous release process, which was consistent with litter mass loss, and P addition reduced C remaining (Fig. 4). C is the main constituent element of litter quality, and the precipitation during the growing season had a strong physical leaching effect on non-structural carbohydrates in the litter, resulting in a gradual decrease in C content (Dong et al. 2021). The addition of P reduced the litter C/P ratio, which stimulated microbial demand for C, and microorganisms accelerated the decomposition of C by increasing the secretion of BG (Gong et al. 2022). N showed a trend of accumulation followed by release in aboveground litter and continuous release in belowground litter (Fig. 2). This is mainly because the C/N ratio of aboveground litter was relatively high, which cannot meet the demand of decomposers for N (Chen et al. 2016). High concentrations of P addition (P5 and P12.5) promoted the accumulation of P in the aboveground litter and inhibited the release of P in the belowground litter, which was similar to other studies (Jiang et al. 2018). We know that microorganisms decompose organic matter to obtain P requires a high cost, and P addition results in a surplus of readily available P in the soil, which inhibits microbial metabolism (Zheng et al. 2017). The initial N/P of aboveground litter was higher than that of belowground litter. This indicated that the P content of aboveground litter may not be sufficient for its own nutrient requirements and that it needs to absorb P from the outside, so P showed accumulation (Cleveland et al. 2006).
The metal element Ca, as a structural component of litter, plays an important role in litter decomposition. The underlying mechanism of litter Ca accumulation may be related to the microbial demand for Ca (Berg 2014; García-Palacios et al. 2016). In our study, the accumulation of Ca was highest at low concentration of P (P1), suggesting that moderate P addition can enhance microbial activity and promote calcium demand. However, the high P concentration would increase the soil pH, thereby promoting the mineral binding of P and Ca, resulting in a decrease in Ca accumulation (Hobbie et al. 2006; Reich et al. 2005). Our study revealed an interesting phenomenon, Mg and Mn showed an accumulation trend in aboveground litter, but a release trend in belowground litter. Mg and Mn act as regulators of cell growth and division, as well as cofactors of enzyme synthesis, which have significant effects on microbial growth and reproduction (Xu et al. 2006; Vivanco and Austin 2019). Aboveground litter had lower initial Mg and Mn compared to belowground litter, which could not meet the demands of microbial growth. Consequently, the acquisition of exogenous nutrients by microorganisms led to the accumulation of Mg and Mn in aboveground litter (Zheng et al. 2017).
The effects of P addition on enzyme activities in above- and belowground litter
Microorganisms are the ultimate decomposers of litter, and their functional activities can be characterized by differences in extracellular enzyme activities. P addition can affect microbial species and thereby affect the activities of extracellular enzymes by altering nutrient availability to these microbes (Dong et al. 2019). We found that the BG and NAG activities increased in the aboveground litter with P addition (Fig. 5). This may be because P addition stimulated microbial activity and increased the demand for C and N. BG could hydrolyze the litter cellulose into glucose that the microbes could utilize (Chen et al. 2018), and NAG can degrade chitin and peptidoglycan in the litter, and help microorganisms obtain nitrogen sources for growth and reproduction (Shi et al. 2021). These results support the microbial resource allocation theory of litter decomposition, which suggests that when the P availability increases, the original balance among microbial C, N, and P is disrupted, microorganisms redistribute resources to increase the production of enzymes that acquire limiting elements to maintain their elemental balance (Sinsabaugh et al. 2002).
The microbial mining hypothesis of litter decomposition proposes that increasing nutrient availability, will decrease the limiting effect of nutrients on microorganisms, and the resource allocation by microorganisms to obtain such nutrients will decrease (Allison and Vitousek 2005). In other words, an increase in P concentration may reduce AP activity, thereby inhibiting litter decomposition (Xiao et al. 2018). However, our study found that the AP activity of aboveground litter was only inhibited at P12.5, and the AP activity of belowground litter was inhibited at P2.5 and P5. Interestingly, the litter decomposition rate was not the lowest under these gradients, which reflects that the response of AP to P addition is not the key to causing the change of litter decomposition rate. P addition had no significant effect on the activities of two hydrolases (BG and NAG) in the belowground litter but increased the activities of PhOx. This may represent a trade-off between the enzymes responsible for obtaining easily available C (such as cellulose) and the enzymes responsible for depolymerization of more difficult to degrade C compounds (such as lignin) to obtain nutrients (DeForest 2019).
The contrasting mechanisms for decomposition of above- and belowground litter under P addition
Previous studies on the decomposition of grassland litter mainly focused on the growing season, neglecting the slow decomposition process of litter in the non-growing season. This may have led to a significant overestimation of the turnover time of litter in grassland ecosystems (Zhao et al. 2016). In this study, the decomposition process showed a three-step pattern with time, with a significantly faster decomposition rate during the growing season than in the non-growing season. On the one hand, precipitation during the growing season can cause the litter to be washed and broken down, resulting in the loss of organic matter through physical leaching (Dong et al. 2021). In addition, the soil microbial environment improves during the growing season, and microbial activity and metabolism are more vigorous, leading to an accelerated litter decomposition rate (Trevathan-Tackett et al. 2020). The solar radiation during the growing season is stronger than in the non-growing season, which promotes photochemical reactions in the recalcitrant compounds of litter and also accelerates litter decomposition (Gallo et al. 2006).
The addition of exogenous nutrients altered the litter decomposition rate. P addition significantly increased the aboveground litter decomposition rate, but for belowground litter, the decomposition rate was lower than that of aboveground litter, and only the P2.5 significantly promoted litter decomposition. One important reason is that P addition changed the above- and belowground litter quality to P addition, but their responses to P addition were not the same. P addition significantly decreased the C/P and N/P ratios of the aboveground litter, which may cause C and N to become the elements that limit the growth of microorganisms. The acquisition of C and N by microorganisms may accelerate the decomposition of aboveground litter. We also found that mineral elements, particularly Mn, played an important role in litter decomposition, and P addition increased the content of Mn in aboveground litter. It can form Mn peroxidase (Berg et al. 2015) and regulate the activity of fungi that produce lignin degrading enzymes, and accelerate litter decomposition (Vivanco and Austin 2019). In addition, the study found that the decomposition rate of belowground litter in the control plot was higher than that of aboveground litter, but P addition reversed this pattern. We speculate that this may be due to the lower Mn content in the aboveground litter of the control, which is not conducive to its decomposition. The carbon quality of litter also affects its decomposition. Compared with aboveground litter, belowground litter contains a higher initial lignin content. These insoluble substances can combine with soil inorganic nitrogen ions to form new compounds that are more difficult to decompose, resulting in a further slowing of the decomposition rate (Xia et al. 2017).
Microbial extracellular enzymes represent trade-offs based on the availability of nutrient resources, and the litter extracellular enzyme stoichiometry can reflect changes in nutrient availability and in the demand for energy and nutrients to support microbial growth, ultimately affecting litter decomposition rate (Xiao et al. 2020; Xu et al. 2022). In this study, the microbes of leaf litter invested more in C- and N-acquisition enzymes under P addition, thereby accelerating litter decomposition (Shi et al. 2021). This is also an important reason why the decomposition rate of aboveground litter was higher than that of belowground litter under P addition. In addition, there was a negative correlation between aboveground litter decomposition rate and the ln BG/ln NAG ratio, which indicates that microorganisms have a greater demand for N. It also showed that P addition can accelerate N cycling in the grassland ecosystem, thereby accelerating litter decomposition. PhOx activity in the belowground litter significantly affected its decomposition. PhOx is mainly produced by white rot fungi, which can oxidize the benzene rings in phenolic compounds and promote the decomposition of refractory substances such as lignin (Luo et al. 2019). Belowground has a particularly high decomposition rate at P2.5, and PhOx plays a key role. The enzymatic activities and stoichiometry of above- and belowground litter under P addition produced different effects on decomposition, which may be related to the different microenvironments (Deng et al. 2019). In general, the factors affecting the decomposition rates of above- and belowground litter were different, but appropriate P addition can promote litter decomposition, thereby improving the recycling of grassland nutrients. It could also lead to a reduction in soil carbon, potentially resulting in soils emitting more carbon than they store.
Conclusions
The response of litter decomposition to P addition varied with litter types and P addition gradients. P addition significantly increased the decomposition rate of aboveground litter. The application of P fertilizer increased the aboveground litter initial P and Mn contents and decreased the C/P and N/P ratios, improving the chemical properties of the litter, and reducing the difficulty of decomposition. This also caused the redistribution of resources by microorganisms, increased the investment in C- and N-acquiring enzymes, and further promoted the litter decomposition rate. Different from aboveground litter, only 2.5 g P m−2 yr−1 addition significantly increased the decomposition rate of belowground litter. This concentration of P addition reduced the initial lignin content of belowground litter, increased the activity of polyphenol oxidase, accelerated the decomposition of refractory substances, and thus increased the decomposition. P addition also promoted carbon return by promoting the release of cellulose and carbon from above- and belowground litter. Overall, our results suggest that the different mechanisms of above- and belowground litter decomposition under P addition were driven by litter quality and enzyme activity. The resulting knowledge is enhancing our comprehension of the effects of nutrient addition on litter decomposition, as well as the role of P to the carbon cycle.
References
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37:937–944. https://doi.org/10.1016/j.soilbio.2004.09.014
Bai X, Zhao W, Wang J, Ferreira CSS (2022) Reducing plant community variability and improving resilience for sustainable restoration of temperate grassland. Environ Res 207:112149. https://doi.org/10.1016/j.envres.2021.112149
Berg B (2014) Decomposition patterns for foliar litter – a theory for influencing factors. Soil Biol Biochem 78:222–232. https://doi.org/10.1016/j.soilbio.2014.08.005
Berg B, Kjønaas OJ, Johansson MB et al (2015) Late stage pine litter decomposition: relationship to litter N, Mn, and acid unhydrolyzable residue (AUR) concentrations and climatic factors. For Ecol Manag 358:41–47. https://doi.org/10.1016/j.foreco.2015.08.032
Chaneton EJ, Lemcoff JH, Lavado RS (1996) Nitrogen and phosphorus cycling in grazed and ungrazed plots in a temperate subhumid grassland in Argentina. J Appl Ecol 33:291–302. https://doi.org/10.2307/2404751
Chen Y, Sayer EJ, Li Z et al (2016) Nutrient limitation of woody debris decomposition in a tropical forest: contrasting effects of N and P addition. Funct Ecol 30:295–304. https://doi.org/10.1111/1365-2435.12471
Chen X, Ding Z, Tang M, Zhu B (2018) Greater variations of rhizosphere effects within mycorrhizal group than between mycorrhizal group in a temperate forest. Soil Biol Biochem 126:237–246. https://doi.org/10.1016/j.soilbio.2018.08.026
Cleveland CC, Reed SC, Townsend AR (2006) Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 87:492–503. https://doi.org/10.1890/05-0525
Cui Y, Fang L, Deng L et al (2019) Patterns of soil microbial nutrient limitations and their roles in the variation of soil organic carbon across a precipitation gradient in an arid and semi-arid region. Sci Total Environ 658:1440–1451. https://doi.org/10.1016/j.scitotenv.2018.12.289
DeForest JL (2019) Chronic phosphorus enrichment and elevated pH suppresses Quercus spp. leaf litter decomposition in a temperate forest. Soil Biol Biochem 135:206–212. https://doi.org/10.1016/j.soilbio.2019.05.005
Deng J, Chong Y, Zhang D et al (2019) Temporal variations in soil enzyme activities and responses to land-use change in the Loess Plateau, China. Appl Sci 9:3129. https://doi.org/10.3390/app9153129
Dong L, Sun T, Berg B et al (2019) Effects of different forms of N deposition on leaf litter decomposition and extracellular enzyme activities in a temperate grassland. Soil Biol Biochem 134:78–80. https://doi.org/10.1016/j.soilbio.2019.03.016
Dong X, Gao P, Zhou R et al (2021) Changing characteristics and influencing factors of the soil microbial community during litter decomposition in a mixed Quercus acutissima Carruth. And Robinia pseudoacacia L. forest in northern China. CATENA 196:104811. https://doi.org/10.1016/j.catena.2020.104811
Gallo ME, Sinsabaugh RL, Cabaniss SE (2006) The role of ultraviolet radiation in litter decomposition in arid ecosystems. Appl Soil Ecol 34:82–91. https://doi.org/10.1016/j.apsoil.2005.12.006
Gao J, Kang F, Li T et al (2015) Assessing the effect of leaf litter diversity on the decomposition and associated diversity of fungal assemblages. Forests 6:2371–2386. https://doi.org/10.3390/f6072371
García-Palacios P, McKie BG, Handa IT et al (2016) The importance of litter traits and decomposers for litter decomposition: a comparison of aquatic and terrestrial ecosystems within and across biomes. Funct Ecol 30:819–829. https://doi.org/10.1111/1365-2435.12589
Gong J, Zhang Z, Zhu C et al (2022) The response of litter decomposition to phosphorus addition in typical temperate grassland in Inner Mongolia. J Arid Environ 197:104677. https://doi.org/10.1016/j.jaridenv.2021.104677
Hobbie SE, Reich PB, Oleksyn J et al (2006) Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 87:2288–2297. https://doi.org/10.1890/0012-9658(2006)87[2288:tseoda]2.0.co;2
Jiang L, Kou L, Li S (2018) Alterations of early-stage decomposition of leaves and absorptive roots by deposition of nitrogen and phosphorus have contrasting mechanisms. Soil Biol Biochem 127:213–222. https://doi.org/10.1016/j.soilbio.2018.09.037
Jing X, Yang X, Ren F et al (2016) Neutral effect of nitrogen addition and negative effect of phosphorus addition on topsoil extracellular enzymatic activities in an alpine grassland ecosystem. Appl Soil Ecol 107:205–213. https://doi.org/10.1016/j.apsoil.2016.06.004
Kriiska K, Lõhmus K, Frey J et al (2021) The dynamics of mass loss and nutrient release of decomposing fine roots, needle litter and standard substrates in hemiboreal coniferous forests. Front For Glob Change 4:686468. https://doi.org/10.3389/ffgc.2021.686468
Li W, Qiu X, Bai L, Yang D (2017) Effects of nitrogen and phosphorus addition on litter decomposition on the Stipa baicalensis steppe. Acta Prataculturae Sin 26:43–53
Li Y, Gong J, Zhang Z et al (2022) Grazing directly or indirectly affect shoot and root litter decomposition in different decomposition stage by changing soil properties. CATENA 209:105803. https://doi.org/10.1016/j.catena.2021.105803
Luo R, Fan J, Wang W et al (2019) Nitrogen and phosphorus enrichment accelerates soil organic carbon loss in alpine grassland on the Qinghai-Tibetan plateau. Sci Total Environ 650:303–312. https://doi.org/10.1016/j.scitotenv.2018.09.038
Mariotte P, Mehrabi Z, Bezemer TM et al (2018) Plant–soil feedback: bridging natural and agricultural sciences. Trends Ecol Evol 33:129–142. https://doi.org/10.1016/j.tree.2017.11.005
Martínez-García LB, Korthals GW, Brussaard L et al (2021) Litter quality drives nitrogen release, and agricultural management (organic vs. conventional) drives carbon loss during litter decomposition in agro-ecosystems. Soil Biol Biochem 153:108115. https://doi.org/10.1016/j.soilbio.2020.108115
McGroddy ME, Silver WL, Oliveira RC de (2004) The effect of phosphorus availability on decomposition dynamics in a seasonal lowland Amazonian forest. Ecosystems 7:172–179. https://doi.org/10.1007/s10021-003-0208-y
Moorhead D, Sinsabaugh R (2006) A theoretical model of litter decay and microbial interaction. Ecol Monogr 76:151–174. https://doi.org/10.1890/0012-9615(2006)076[0151:ATMOLD]2.0.CO;2
Norris M, Avis P, Reich P, Hobbie S (2012) Positive feedbacks between decomposition and soil nitrogen availability along fertility gradients. Plant Soil 367:347–361. https://doi.org/10.1007/s11104-012-1449-3
Ochoa-Hueso R, Delgado-Baquerizo M, An King PT et al (2019) Ecosystem type and resource quality are more important than global change drivers in regulating early stages of litter decomposition. Soil Biol Biochem 129:144–152. https://doi.org/10.1016/j.soilbio.2018.11.009
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331. https://doi.org/10.2307/1932179
Reich PB, Oleksyn J, Modrzynski J et al (2005) Linking litter calcium, earthworms and soil properties: a common garden test with 14 tree species. Ecol Lett 8:811–818. https://doi.org/10.1111/j.1461-0248.2005.00779.x
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315. https://doi.org/10.1016/S0038-0717(02)00074-3
Samaddar S, Chatterjee P, Truu J et al (2019) Long-term phosphorus limitation changes the bacterial community structure and functioning in paddy soils. Appl Soil Ecol 134:111–115. https://doi.org/10.1016/j.apsoil.2018.10.016
Shi J, Gong J, Baoyin T et al (2021) Short-term phosphorus addition increases soil respiration by promoting gross ecosystem production and litter decomposition in a typical temperate grassland in northern China. CATENA 197:104952. https://doi.org/10.1016/j.catena.2020.104952
Sinsabaugh RL, Carreiro MM, Repert DA (2002) Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24. https://doi.org/10.1023/A:1016541114786
Sterner RW, Elser JJ (2002) Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press. https://www.jstor.org/stable/j.ctt1jktrp3
Sun Q, Jia R, Qin J et al (2023) Grassland management regimes regulate soil phosphorus fractions and conversion between phosphorus pools in semiarid steppe ecosystems. Biogeochemistry 163:33–48. https://doi.org/10.1007/s10533-023-01019-w
Tilman D, Cassman KG, Matson PA et al (2002) Agricultural sustainability and intensive production practices. Nature 418:671–677. https://doi.org/10.1038/nature01014
Trevathan-Tackett SM, Jeffries TC, Macreadie PI et al (2020) Long-term decomposition captures key steps in microbial breakdown of seagrass litter. Sci Total Environ 705:135806. https://doi.org/10.1016/j.scitotenv.2019.135806
Van Soest PJ (1967) Development of a comprehensive system of feed analyses and its application to forages. J Anim Sci 26:119–128. https://doi.org/10.2527/jas1967.261119x
Vivanco L, Austin AT (2019) The importance of macro- and micro-nutrients over climate for leaf litter decomposition and nutrient release in Patagonian temperate forests. For Ecol Manag 441:144–154. https://doi.org/10.1016/j.foreco.2019.03.019
Wang B, Gong J, Zhang Z et al (2019) Nitrogen addition alters photosynthetic carbon fixation, allocation of photoassimilates, and carbon partitioning of Leymus chinensis in a temperate grassland of Inner Mongolia. Agric For Meteorol 279:107743. https://doi.org/10.1016/j.agrformet.2019.107743
Wang C, Wang W, Sardans J et al (2020) Higher fluxes of C, N and P in plant/soil cycles associated with plant invasion in a subtropical estuarine wetland in China. Sci Total Environ 730:139124. https://doi.org/10.1016/j.scitotenv.2020.139124
Xia M, Talhelm AF, Pregitzer KS (2017) Chronic nitrogen deposition influences the chemical dynamics of leaf litter and fine roots during decomposition. Soil Biol Biochem 112:24–34. https://doi.org/10.1016/j.soilbio.2017.04.011
Xiao W, Chen X, Jing X, Zhu B (2018) A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol Biochem 123:21–32. https://doi.org/10.1016/j.soilbio.2018.05.001
Xiao L, Liu G, Li P et al (2020) Ecoenzymatic stoichiometry and microbial nutrient limitation during secondary succession of natural grassland on the Loess Plateau. China Soil Tillage Res 200:104605. https://doi.org/10.1016/j.still.2020.104605
Xu X, Shibata H, Enoki T (2006) Decomposition patterns of leaf litter of seven common canopy species in a subtropical forest: dynamics of mineral nutrients. J For Res 17:1–6. https://doi.org/10.1007/s11676-006-0001-9
Xu M, Li W, Wang J et al (2022) Soil ecoenzymatic stoichiometry reveals microbial phosphorus limitation after vegetation restoration on the Loess Plateau. China Sci Total Environ 815:152918. https://doi.org/10.1016/j.scitotenv.2022.152918
Zeng D, Li L, Fahey TJ et al (2010) Effects of nitrogen addition on vegetation and ecosystem carbon in a semi-arid grassland. Biogeochemistry 98:185–193. https://doi.org/10.1007/s10533-009-9385-x
Zhao Y, Wu F, Yang W et al (2016) Variations in bacterial communities during foliar litter decomposition in the winter and growing seasons in an alpine forest of the eastern Tibetan plateau. Can J Microbiol 62:35–48. https://doi.org/10.1139/cjm-2015-0448
Zheng Z, Mamuti M, Liu H et al (2017) Effects of nutrient additions on litter decomposition regulated by phosphorus-induced changes in litter chemistry in a subtropical forest, China. For Ecol Manag 400:123–128. https://doi.org/10.1016/j.foreco.2017.06.002
Zhu X, Fang X, Wang L et al (2021) Regulation of soil phosphorus availability and composition during forest succession in subtropics. For Ecol Manag 502:119706. https://doi.org/10.1016/j.foreco.2021.119706
Acknowledgments
This study was funded by the National Natural Science Foundation of China (Grant No. 32230065), and the National Key Research and Development Program of China (Grant No. 2022YFF1303404). We thank the staff of the Grassland Ecosystem Research Station of Inner Mongolia University for their help during data collection, and the staff of Laboratory Analysis and Testing Center of the State Key Laboratory of Surface Earth Processes and Resource Ecology for their guidance during the laboratory analysis and data analysis. We appreciate the valuable input and suggestions provided by Editor Luca Bragazza and the anonymous reviewers, which have contributed to improving the quality and clarity of this manuscript.
Author information
Authors and Affiliations
Contributions
Jirui Gong: Funding acquisition, Supervision, Project administration. Xuede Dong: Conceptualization, Data curation, Writing–original draft, Methodology, Formal analysis, Writing–review & editing. Xiaobing Li: Conceptualization, Project administration. Kexin Yue: Investigation, Data curation. Jiayu Shi: Investigation. Liangyuan Song: Investigation. Zihe Zhang: Investigation. Weiyuan Zhang: Investigation. Ying Li: Investigation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Luca Bragazza.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 229 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gong, J., Dong, X., Li, X. et al. Phosphorus fertilization affects litter quality and enzyme activity in a semiarid grassland. Plant Soil 492, 91–108 (2023). https://doi.org/10.1007/s11104-023-06153-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06153-w