Abstract
Evidence suggests that neurometabolite alterations may be involved in the pathophysiology of autism spectrum disorders (ASDs). We performed a meta-analysis of proton magnetic resonance spectroscopy (1H-MRS) studies to examine the neurometabolite levels in the brains of patients with ASD. A systematic search of PubMed and Web of Science identified 54 studies for the meta-analysis. A random-effects meta-analysis demonstrated that compared with the healthy controls, patients with ASD had lower N-acetyl-aspartate-containing compound (NAA) and choline-containing compound (Cho) levels and NAA/(creatine-containing compound) Cr ratios in the gray matter and lower NAA and glutamate + glutamine (Glx) levels in the white matter. Furthermore, NAA and gamma-aminobutyric acid (GABA) levels, NAA/Cr ratios, and GABA/Cr ratios were significantly decreased in the frontal cortex of patients with ASD, whereas glutamate (Glu) levels were increased in the prefrontal cortex. Additionally, low NAA levels and GABA/Cr ratios in the temporal cortex, low NAA levels and NAA/Cr ratios in the parietal and dorsolateral prefrontal cortices, and low NAA levels in the cerebellum and occipital cortex were observed in patients with ASD. Meta-regression analysis revealed that age was positively associated with effect size in studies analyzing the levels of gray matter NAA and white matter Glx. Taken together, these results provide strong clinical evidence that neurometabolite alterations in specific brain regions are associated with ASD and age is a confounding factor for certain neurometabolite levels in patients with ASD.
Similar content being viewed by others
Introduction
Autism spectrum disorder (ASD) refers to a group of brain-based mental diseases, including autism, Asperger’s syndrome, and pervasive developmental disorders, not otherwise specified, that affect over 1% of the global population [1]. As a representative neurodevelopmental disorder, ASD is behaviorally characterized by deficits in social interactions, impaired verbal communication, and repetitive behavior [2]. Despite considerable efforts in this field, the heterogeneity of the illness features that characterize ASD makes it challenging to elucidate the underlying pathology of the illness and its treatment. Therefore, it is important to identify reliable biomarkers for the early screening and treatment of this disorder.
The direct measurement of neurometabolites is a potential strategy for identifying individuals with psychiatric disorders [3]. Proton magnetic resonance spectroscopy (1H-MRS) is a noninvasive and simultaneous neuroimaging tool that allows for in vivo quantification of brain metabolites in patients [4]. An increasing number of 1H-MRS studies have explored the pathophysiology of ASD. The commonly investigated metabolites include N-acetyl-aspartate-containing compounds (NAA), creatine-containing compounds (Cr), choline-containing compounds (Cho), glutamate + glutamine (Glx), myo-inositol (mI), and gamma-aminobutyric acid (GABA). The NAA signal, which is the most prominent spectral peak, reflects the neuronal density and health [5]. As an important regulator of glutamatergic neurotransmission [6], N-acetyl-aspartyl-glutamate generates a small peak in the brain, which is difficult to distinguish from the N-acetyl-aspartate peak in the 1H-MRS spectrum. Therefore, the NAA signal measured in this study was contributed by both N-acetyl-aspartate and N-acetyl-aspartyl-glutamate. Cr plays an essential role in central nervous system (CNS) energy homeostasis, representing the most stable cerebral metabolite [7]. The benefit of the concentration of creatine or phosphocreatine is relatively similar throughout the brain, and it is often used as an “internal standard” to normalize signals from other metabolites (for example, the NAA/Cr ratio for the measurement of NAA). Typically, creatine and phosphocreatine levels cannot be reliably distinguished using 1H-MRS. In this study, we used the term “Cr” used in the context of 1H-MRS measurements to refer to the combined signal from creatine and phosphocreatine. Cho is often interpreted as a measure of the membrane turnover rate because choline is a constituent of cell membranes [8]. The Cho signal observed by 1H-MRS is composed of phosphatidylcholine metabolites, especially phosphocholine and glycerol-phosphocholine. As an osmolyte in the CNS [9], mI is a key component of the secondary messenger system, is believed to be an essential requirement for cell growth, and has been proposed as a glia-specific marker [10]. Glutamate (Glu) is one of the most abundant mobile metabolites in the brain, while glutamine (Gln) is involved in glutamate recycling and the regulation of brain ammonia metabolism [11]. However, because of the lack of methyl groups in Glu, it produces broad complex peaks that do not generate a prominent single peak in the 1H-MRS spectrum [7]. Unless optimized 1H-MRS methods are used (e.g., a high-field scanner with a short echo time and long acquisition time or a specialized J-editing or J-resolved sequence), the measurements obtained are generally considered to reflect the combined signal from Glu and Gln [12]. Thus, Glx represents overall Glu and Gln levels and their functioning in the brain. In contrast to Glu, GABA is the major inhibitory neurotransmitter in the brain and plays an important role in maintaining the balance of neural circuits [13].
Although 1H-MRS shows promise for profiling ASD by providing chemical compositions of a variety of brain regions, the findings across studies are inconsistent. Taking frontal NAA as a reference, some investigators demonstrated that NAA levels were decreased in patients with ASD compared to those in healthy controls (HCs) [14,15,16], whereas another study documented that NAA levels were increased in patients with ASD [17]. No significant differences in NAA levels were found in any brain region between patients with ASD and HCs [18]. Similarly, inconsistent findings have been reported for other neurometabolites in patients with ASD. These differences may be attributed to differences in the regions of interest (ROIs), MRS methodologies, stages or severity of illness, or medications.
Therefore, we conducted a systematic review and meta-analysis to analyze the neurometabolite levels in different regions of the brain of patients with ASD. We also explored the influence of age, sex, full-scale intelligence quotient (FSIQ), and magnetic field strength on the neurometabolite levels in the brains of patients with ASD.
Method
We conducted a systematic review and meta-analysis of ASD in accordance with the guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement [19].
Data sources and study selection
We performed a systematic search of peer-reviewed publications in the English language using the PubMed and Web of Science databases through October 14, 2022, without year limitations. We used the search terms “Autism” AND “MRS” OR “magnetic resonance spectroscopy”. In addition, the references of the relevant articles were manually searched to identify additional studies. Studies utilizing 1H-MRS to examine neurometabolite levels in patients with ASD and HCs were included. Studies were excluded in the following conditions: (1) review articles, (2) MRS was measured in animal models, (3) lack of HCs, (4) samples overlapped with other studies, (5) in vitro data, (6) lack of necessary data, (7) participants suffering from serious complications, (8) postmortem samples, (9) non-English publications, and (10) articles with individual metabolites studied in < three articles.
Data extraction
Two investigators independently extracted data from the included articles. Primary outcomes of sample size, mean neurometabolite levels, standard deviation (SD), and P-values were collected to generate the effective size (ES) from eligible studies. Data on the last name and country of the first author, publication year, mean age, sex distribution (male %), FSIQ, and criteria and diagnosis of the participants were extracted for potential moderator analyses. The ROIs were as follows: (1) cerebellar cortex, (2) prefrontal cortex, (3) dorsolateral prefrontal cortex (DLPFC), (4) frontal cortex, (5) occipital cortex, (6) parietal cortex, (7) temporal cortex, including the hippocampus, (8) thalamus, and (9) BG. When data from the bilateral lobes were reported separately, the left lobe was used because it has been examined in most studies. The demographic and clinical characteristics of the included studies are summarized in eTable.
Statistical analysis
The Comprehensive Meta-Analysis Version 3 software (Biostat, Englewood, NJ, USA) was used for all statistical analyses. In most cases, ES was obtained using sample size, mean neurometabolite levels, and SDs. If neurometabolite levels and SDs were not available, sample sizes and P-values were used to obtain the ES. ES was calculated as the standardized mean difference in brain metabolite levels between patients with ASD and HCs and then converted to Hedges’ g, and a random-effects model was employed. Hedges’g statistic provides a correction factor to address potential biases resulting from the sample size. If between-study heterogeneity exists, the random-effects model can allocate the study weight based on the inverse of the total variance, producing a wider 95% confidence interval than the fixed-effects model [20].
Between-study heterogeneity was assessed using the Cochrane Q test and I2 statistics. For the Cochrane Q test, P < 0.10 was considered to be statistically significant. The I2 index determined the inconsistencies across the original studies and subgroups and evaluated the impact of heterogeneity. I2 values of 0%, 25%, 50%, 75%, and 100% indicated no, small, moderate, and high heterogeneity, respectively. We then performed subgroup analyses and unrestricted maximum-likelihood random-effects meta-regression of ES to address the between-study heterogeneity.
We first performed a visual inspection of funnel plots to investigate publication bias. Funnel plots were plotted by the effect sizes against precision (inverse of SE). We also used the Egger’s test to evaluate statistical significance.
The current meta-analyses included studies with considerable heterogeneity and confounders, including categorical variables (assessed brain region, magnetic field strength, and medication status) and continuous variables (sample size, age, sex, publication year, course of the disease, and disease severity). Therefore, we performed meta-regression analyses to investigate the influence of these potential sources in studies showing significant differences in metabolites between patients with ASD and HCs.
We considered P < 0.05 to be statistically significant unless otherwise noted; a P-value below 0.1 was reported as a trend.
Results
Characteristics of the included studies
We performed a systematic search, which produced 244 records from PubMed, 253 records from Web of Science, and 5 additional records from the reference lists of the retrieved articles. After scanning the titles and abstracts, 78 articles relevant to the present meta-analysis were identified for a full-text review. A total of 54 studies (including 1,501 patients and 1,294 HCs) that met the criteria were included in this meta-analysis (Fig. 1) [14,15,16,17,18, 21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69].
Most studies were conducted on children (N = 34, 63%). The sample sizes ranged from 8 to 114 for patients with ASD and 8 to 70 for HCs. The average of each study ranged from 1.9 to 40.54 for patients with ASD and from 2.0 to 41.08 for HCs. Twenty-six (48%) studies were performed at a magnetic field strength of 3 T, while 27 (50%) studies were performed at 1.5 T. 1H-MRS protocols and methodological information, including measurement techniques and parameters, are described in Table 1.
Meta-analysis for neurometabolite level measurements
We compared seven neurometabolite levels across the gray and white matter of the brain between patients with ASD and HCs. Our research revealed a statistically significant decrease in NAA concentrations in both the gray matter and white matter of patients with ASD compared with those in controls (gray matter: nine studies, Hedges’ g = −3.03, 95% CI = −0.603 to −0.003, P = 0.047; white matter: six studies, Hedges’ g = -0.690, 95% CI = −1.168 to -0.213, P = 0.005; Table 1). A significant decrease in the NAA/Cr ratio was observed in the gray matter of patients with ASD (five studies, Hedges’ g = -0.302, 95% CI = -0.569 to -0.034, P = 0.027; Table 1; Fig. 2A). For Glx, there was a significant decrease in the white matter in patients with ASD, while a trend for a significant difference in the gray matter was observed between cases and controls (white matter: five studies, Hedges’ g = −0.743, 95% CI = −1.288 to -0.197, P = 0.008; gray matter: eight studies, Hedges’ g = −0.263, 95% CI = −0.567 to 0.041, P = 0.090; Table 1; Fig. 2B). Additionally, although we found a significant decrease in Cho (six studies, Hedges’ g = −0.701, 95% CI = −1.297 to -0.106, P = 0.021, Fig. 2C) and a trend for a significant difference in Cr (six studies, Hedges’ g = −0.425, 95% CI = −0.859 to −0.009, P = 0.055) in the gray matter of children with ASD, subgroup analysis showed that there was no difference between any specific brain regions (Table 2).
Forest plot for random-effects meta-analysis on differences in various brain neurometabolite (A, NAA/Cr ratio in gray matter; B, Glx in white matter; C, Cho in gray matter) concentrations between autism spectrum disorder (ASD) patients and healthy controls. The sizes of the squares are proportional to the study weight. The diamond marker indicates the pooled effect size. CI Confidence interval.
Next, we performed a region-specific meta-analysis and found that patients with ASD had significant reductions in NAA in most parts of the brain (DLPFC, frontal cortex, occipital cortex, parietal cortex, and temporal cortex) (DLPFC, 5 studies: Hedges’ g = −0.349, 95% CI = −0.694 to −0.004, P = 0.048; frontal cortex, 24 studies: Hedges’ g = −0.234, 95% CI = −0.419 to −0.048, P = 0.014; occipital cortex, 4 studies, Hedges’ g = −0.667, 95% CI = −1.026 to −0.309, P < 0.001; parietal cortex, 8 studies: Hedges’ g = −0.318, 95% CI = −0.535 to −0.102, P = 0.004; temporal cortex, 11 studies: Hedges’ g = −0.354, 95% CI = −0.672 to −0.037, P = 0.029; Table 2; Fig. 3A, B). Notably, NAA level changes in patients with ASD were age-dependent, as we found that NAA level reductions in the cerebellum, frontal cortex, and temporal cortex were specific to children with ASD (cerebellum, 5 studies: Hedges’ g = −0.305, 95% CI = −0.558 to −0.053, P = 0.018; frontal cortex, 13 studies: Hedges’ g = −0.288, 95% CI = −0.534 to −0.043, P = 0.022; temporal cortex, 6 studies: Hedges’ g = −0.514, 95% CI = −0.917 to −0.111, P = 0.012; Table 2). In line with the age-dependent NAA alterations in specific brain regions, analyses of the NAA/Cr ratio in the frontal and parietal cortices revealed a reduced NAA/Cr ratio (frontal cortex, 12 studies: Hedges’ g = −0.383, 95% CI = −0.625 to −0.141, P = 0.002; parietal cortex, 3 studies: Hedges’ g = −0.341, 95% CI = −0.666 to −0.016, P = 0.040) in patients with ASD when compared with that in controls, and the NAA/Cr ratio (nine studies: Hedges’ g = −0.406, 95% CI = −0.687 to −0.124, P = 0.005) remained low in the frontal cortex of children but not in that of adults with ASD (Table 2).
Forest plot for random-effects meta-analysis on differences in frontal cortex (A) and parietal cortex (B) NAA concentrations between autism spectrum disorder patients and healthy controls. The sizes of the squares are proportional to the study weight. The diamond marker indicates the pooled effect size. CI Confidence interval.
Moreover, the comparison of metabolic differences in Glx across various brain regions revealed no statistically significant differences between the cases and controls. Although the number of related studies was limited, we found that Glu levels increased significantly in the prefrontal cortex of patients with ASD (five studies: Hedges’ g = 0.461, 95% CI = 0.043 to 0.880, P = 0.031; Table 2; Fig. 4A), which was not observed in the BG (three studies: Hedges’ g = −0.065, 95% CI = −0.644 to 0.515, P = 0.827; Table 2). The results further showed that the concentration of GABA (six studies: Hedges’ g = -0.418, 95% CI = −0.773 to 0.064, P = 0.021) as well as the GABA/Cr ratio (four studies: Hedges’ g = -0.702, 95% CI = −1.064 to 0.340, P < 0.001) decreased significantly in the frontal cortex of patients with ASD; a decreased GABA/Cr ratio (three studies: Hedges’ g = −0.862, 95% CI = −3.086 to −0.638, P = 0.003) was also found in the temporal cortex of patients with ASD (Table 2, Fig. 4B-D).
Forest plot for random-effects meta-analysis on differences in various brain neurometabolite (A, Glu in prefrontal cortex; B, GABA in frontal cortex; C, GABA/Cr ratio in temporal cortex; GABA/Cr ratio in frontal cortex) concentrations between autism spectrum disorder patients and healthy controls. The sizes of the squares are proportional to the study weight. The diamond marker indicates the pooled effect size. CI Confidence interval.
Investigation of heterogeneity
Most of the between-study heterogeneities were at low-to-moderate levels in the meta-analysis; next, we performed meta-regression analyses, as shown in eTable 2.
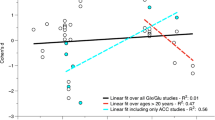
The current meta-regression for NAA in the gray matter revealed that the mean age of the participants was positively associated with ES (coefficient [95% CI], 0.0337 [0.0113–560]; P = 0.0032), but not with sex, FSIQ, or publication year (eFig. 1A). For Glx in the white matter, the results showed that mean age was significantly associated with ES (coefficient [95% CI], 0.1914 [0.0504–3324]; P = 0.0078), whereas sex and publication year had no moderating effect on the outcome of the meta-analysis (eFig. 1B). Furthermore, publication year had a moderating effect on the outcome of the meta-analysis (coefficient [95% CI], 0.1799 [0.0446–3152]; P = 0.0092) for studies measuring frontal cortex GABA levels, but not mean age, as shown in eFig. 1C. There were no associations between any covariates and NAA or NAA/Cr ratios in the specific brain regions (eTable 2).
Publication bias
Publication bias was assessed quantitatively and qualitatively using linear regression analysis and visual inspection of the funnel plots for each group and brain region. No significant publication bias was detected for most region-specific metabolites, except for Glx in the white matter (P = 0.04191, Table 1), NAA in the DLPFC (P = 0.00125, Table 2), and GABA in the frontal cortex (P = 0.01774, Table 2).
Discussion
We conducted a comprehensive systematic review and meta-analysis to compare commonly investigated neurometabolites, such as NAA, Cho, Cr, Glu, Glx, mI, and GABA, in specific brain regions between patients with ASD and controls, while also considering factors that could affect metabolite levels, such as age and intelligence quotient. Our study provided the results of the quantitative synthesis of data from 54 1H-MRS studies, of which 1501 were patients with ASD and 1294 were HCs. Our main findings were as follows.
(1) The comparison of metabolic differences between patients with ASD and HCs across the gray matter of the brain revealed statistically significant differences in NAA levels and NAA/Cr ratios, accompanied by a trend for significant differences between patients with ASD and HCs for Cr and Glx analyses.
(2) In the white matter, there were significant reductions in NAA levels and Glx concentrations between patients with ASD and HCs, and no statistically significant differences were found for Cr, Cho, and mI.
(3) A region-stratified anatomical focus on NAA showed that there were significant reductions in NAA in many brain regions (including the DLPFC and frontal, occipital, parietal, and temporal regions), but not in the prefrontal cortex and thalamus.
(4) Patients with ASD showed a significant decrease in the NAA/Cr ratio in the DLPFC, frontal cortex, and parietal cortex, whereas no differences were observed in the cerebellum, prefrontal cortex, and temporal regions.
(5) Significant differences were found for GABA and the GABA/Cr ratio between patients with ASD and HCs in the frontal cortex, while the GABA/Cr ratio decreased in the temporal cortex of patients with ASD.
(6) Glu increased significantly in the prefrontal cortex of patients with ASD.
(7) Age was a confounding factor in this meta-analysis.
In addition to ASD, it has been suggested that abnormalities in the neurometabolites in the brain are implicated in the pathophysiology of other neurological diseases such as MDD, Alzheimer’s disease, and schizophrenia. Previous meta-analyses have demonstrated that Glx levels are significantly decreased in patients with major depressive disorder [70], which was not observed in our meta-analysis assessing Glx levels in patients with ASD. Another meta-analysis showed that there was a decrease in NAA levels in patients with schizophrenia [71]. Together with our findings of decreased NAA levels in the brains of patients with ASD, this supports the hypothesis that there is a strong association between ASD and schizophrenia [72]. Interestingly, the present meta-analysis revealed alterations in Cho, Cr, Glu, and GABA levels in different brain regions between patients with ASD and HCs. It is very likely that patients with ASD have a unique neurometabolite factor profile compared to those with other neuropsychiatric diseases, which may partially explain the pathogenesis of ASD.
NAA is a reliable marker of mitochondrial activity and is often used as a chemical marker of neuronal integrity [73]. Autopsy reports suggest that a decrease in NAA may activate and increase astroglia with an increased inflammatory response [74, 75] thus negatively affecting tissue damage and nerve repair in the brain [26], which is consistent with our findings of NAA reductions in the brains of children with ASD using 1H-MRS. Additionally, metabolite differences associated with NAA and the NAA/Cr ratio were found to be age-dependent. A significant decrease in both NAA levels and the NAA/Cr ratio was observed only in the frontal cortex of children with ASD. Children with ASD have an enlarged frontal cortex in their infancy [76] with the presence of inflammation or hypoperfusion, as suggested by signs, including decreased serum levels of adhesion molecules and a correlation with their head circumferences [74, 77]. As a key region of the human brain for communication and cognitive function, the frontal cortex contributes to early developmental defects in children with ASD, including glial activation, migration defects, and abnormal apoptosis [78]. Therefore, it is reasonable to conclude that NAA abnormalities in ASD are age-dependent. This result is consistent with previous studies suggesting premature aging and neurodegenerative processes in autistic brain, as Ivashko-Pachima et al. discovered a genomic autism activity-dependent neuroprotective protein mutation in postmortem Alzheimer’s disease brains correlating with increasing tauopathy [79], and data from Grigg further suggested that tauopathy may explain brain imaging results of atrophy in ASD children [80]. Nevertheless, future research should examine NAA longitudinally over the life course, both in humans and rodent models of ASD, to determine the complete profile of the NAA system in ASD.
Another neurometabolite that was significantly decreased in the frontal cortex was GABA, which is consistent with the decrease in GABAergic interneuron subtype cells found in the prefrontal cortex of postmortem autistic brains [81]. Notwithstanding a previous meta-analysis suggesting that GABA levels are lower in patients with ASD than those in controls [82], our findings further revealed the specific areas of the brain with dysregulated GABA levels in patients with ASD and analyzed the absolute and relative GABA levels. Consistently, various animal models have revealed a correlation between low GABA levels and ASD (for a review, refer [82]). Furthermore, an examination of ASD in post-mortem studies indicated the downregulation of enzymes involved in GABA activation [83, 84]. Clinical studies have demonstrated that the density, number, and protein subunits of GABA receptors are decreased in patients with ASD [85,86,87]. The low frontal cortex GABA system in ASD could be a result of the loss of GABAergic interneurons [88], indicating that impaired GABA processing could become a novel therapeutic target for autism [89]. Consistent with this hypothesis, we found a significant decrease in the GABA/Cr ratio in the temporal cortex of children with ASD. However, due to the limitations of the included literature, the relationship between GABA and other brain regions requires further study.
Excitatory neurotransmission in the brain is primarily mediated by glutamatergic neurons. The correlation between dysfunction of the glutamatergic system and ASD is widely recognized. Glutamatergic receptor expression, along with its function, is altered in patients with ASD, which is in line with a growing body of evidence from various animal models of ASD (for a review, refer [90]). Glu is a major excitatory neurotransmitter widely distributed throughout the CNS. A meta-analysis suggested that blood Glu levels are higher in patients with ASD than those in controls [91], which is consistent with our results of increased Glu levels in the prefrontal cortex of patients with ASD, suggesting that Glu dysfunction may be regionally specific in ASD. Because GABA levels are decreased in the frontal cortex of patients with ASD, the increase in Glu in these patients may be due to the reduced levels of glutamic acid decarboxylase in the brain that converts Glu into GABA [84, 92]. Therefore, our findings support Fatemi’s hyperglutamatergic hypothesis of autism [93]. Genetic studies have suggested that mutations in the GRIN gene encoding the N-methyl-d-aspartate receptor are associated with ASD [94, 95]. Thus, further research is needed to explore whether the Glu level can be used as a potential biomarker for diagnosis in the future. However, Glu and Gln are difficult to separate; therefore, Glx has been used instead in most studies. Our findings revealed low Glx levels in the white matter of children with ASD, and the decrease in Glx may not simply be reflected as a decrease in Glu or Gln because the ratio of Glu to Gln in these patients is unknown.
The strength of this work is that it is the most comprehensive study of neurometabolites in patients with ASD to date. Furthermore, the between-study heterogeneity was not high, suggesting the robustness of our meta-analysis. The potential moderators that we analyzed included the brain region source, magnetic field strength, sample size, publication year, age, and sex. Some unexplained heterogeneity may be due to other variables that were not analyzed, such as medication status, disease severity, and lifestyle. Importantly, our analyses showed a specific decrease in the NAA levels in the frontal and temporal cortices of individuals with ASD, which was not reported in a previous meta-analysis of NAA levels in patients with ASD published in 2012 due to the limited sample size [96].
This meta-analysis has some limitations. First, although we categorized ROIs into different brain areas, the classification might have been oversimplified considering the functional variability within each subarea. Voxel sizes also varied among the studies, and these differences might have skewed the results of the ROI analyses. Second, the field strength was ≤ 3 T, which limited the accurate differentiation of homologous neurometabolites. For example, Glx is composed of both Glu and Gln, but Glu accounts for approximately 80% of Glx levels at 1.5 T or 3 T [97]. Moreover, it is difficult to differentiate N-acetyl-aspartyl-glutamate from N-acetyl-aspartate and Glu using in vivo 1H-MRS because of the similarity in their structures and spectra [98]. Moreover, 1H-MRS does not provide information regarding cell types. Future studies should increase the field strength to improve the separation of individual signal spectra. Third, most studies included in the meta-analysis did not consider food or smoking information as potential confounders. Food intake can influence neurometabolic levels in the brain, and a study showed decreased Glx, Cho, NAA, and Cr levels in the human brain after food intake and that the frontal cortex is the most affected by neurometabolite levels [99]. Furthermore, smoking has been reported to directly or indirectly affect Glu and Cho levels in the frontal cortex [100]. Finally, due to the limited number of studies and small sample sizes assessing neurometabolite levels in the DLPFC and occipital and parietal cortices, we did not perform subgroup analyses on these brain regions.
In conclusion, the results of our meta-analysis suggest that compared with HCs, patients with ASD have lower NAA and Cho levels and NAA/Cr ratios in the gray matter, and ASD is associated with decreased levels of NAA in the DLPFC and frontal, occipital, parietal, and temporal cortices. Low levels of Glx were observed in the white matter of children with ASD, whereas Glu levels were found to be increased in the prefrontal cortex. Our study further revealed decreased frontal cortex GABA/Cr and NAA/Cr ratios as manifestations of ASD. These results strengthen the clinical evidence of an abnormal neurometabolite profile in the brains of patients with ASD.
References
Baxter AJ, Brugha TS, Erskine HE, Scheurer RW, Vos T, Scott JG. The epidemiology and global burden of autism spectrum disorders. Psychol Med. 2015;45:601–13.
Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health. 2017;38:81–102.
Shen L, Zhao Y, Zhang H, Feng C, Gao Y, Zhao D, et al. Advances in biomarker studies in autism spectrum disorders. Adv Exp Med Biol. 2019;1118:207–33.
Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. 2014;39:1–36.
Paslakis G, Traber F, Roberz J, Block W, Jessen F. N-acetyl-aspartate (NAA) as a correlate of pharmacological treatment in psychiatric disorders: A systematic review. Eur Neuropsychopharmacol.2014;24:1659–75.
Benarroch EE. N-acetylaspartate and N-acetylaspartylglutamate: neurobiology and clinical significance. Neurology. 2008;70:1353–7.
Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Curr Top Behav Neurosci. 2012;11:199–251.
Boulanger Y, Labelle M, Khiat A. Role of phospholipase A(2) on the variations of the choline signal intensity observed by 1H magnetic resonance spectroscopy in brain diseases. Brain Res Brain Res Rev. 2000;33:380–9.
Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: Homeostasis, metabolism and functional significance. J Neurochem. 2002;82:736–54.
Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15:289–98.
Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–94.
Hancu I. Optimized glutamate detection at 3T. J Magn Reson Imaging. 2009;30:1155–62.
Di J, Li J, O’Hara B, Alberts I, Xiong L, Li J, et al. The role of GABAergic neural circuits in the pathogenesis of autism spectrum disorder. Int J Dev Neurosci. 2020;80:73–85.
Carvalho Pereira A, Violante IR, Mouga S, Oliveira G, Castelo-Branco M. Medial frontal lobe neurochemistry in autism spectrum disorder is marked by reduced N-Acetylaspartate and unchanged Gamma-Aminobutyric Acid and Glutamate + Glutamine Levels. J Autism Dev Disord. 2018;48:1467–82.
Mori K, Toda Y, Ito H, Mori T, Goji A, Fujii E, et al. A proton magnetic resonance spectroscopic study in autism spectrum disorders: amygdala and orbito-frontal cortex. Brain Dev. 2013;35:139–45.
Tebartz van Elst L, Maier S, Fangmeier T, Endres D, Mueller GT, Nickel K, et al. Disturbed cingulate glutamate metabolism in adults with high-functioning autism spectrum disorder: evidence in support of the excitatory/inhibitory imbalance hypothesis. Mol Psychiatry. 2014;19:1314–25.
Murphy DG, Critchley HD, Schmitz N, McAlonan G, Van Amelsvoort T, Robertson D, et al. Asperger syndrome: A proton magnetic resonance spectroscopy study of brain. Arch Gen Psychiatry. 2002;59:885–91.
Horder J, Petrinovic MM, Mendez MA, Bruns A, Takumi T, Spooren W, et al. Glutamate and GABA in autism spectrum disorder-a translational magnetic resonance spectroscopy study in man and rodent models. Transl Psychiatry. 2018;8:106.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34.
Qin XY, Feng JC, Cao C, Wu HT, Loh YP, Cheng Y. Association of Peripheral blood levels of brain-derived neurotrophic factor with autism spectrum disorder in children: A systematic review and meta-analysis. JAMA Pediatr. 2016;170:1079–86.
Goji A, Ito H, Mori K, Harada M, Hisaoka S, Toda Y, et al. Assessment of Anterior Cingulate Cortex (ACC) and Left Cerebellar Metabolism in Asperger’s Syndrome with Proton Magnetic Resonance Spectroscopy (MRS). PLoS One. 2017;12:e0169288.
Ito H, Mori K, Harada M, Hisaoka S, Toda Y, Mori T, et al. A Proton Magnetic Resonance Spectroscopic Study in Autism Spectrum Disorder Using a 3-Tesla Clinical Magnetic Resonance Imaging (MRI) System: The Anterior Cingulate Cortex and the Left Cerebellum. J Child Neurol. 2017;32:731–9.
Robertson CE, Ratai EM, Kanwisher N. Reduced GABAergic action in the Autistic brain. Curr Biol. 2016;26:80–85.
Rojas DC, Singel D, Steinmetz S, Hepburn S, Brown MS. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage. 2014;86:28–34.
Brix MK, Ersland L, Hugdahl K, Gruner R, Posserud MB, Hammar A, et al. “Brain MR spectroscopy in autism spectrum disorder-the GABA excitatory/inhibitory imbalance theory revisited”. Front Hum Neurosci. 2015;9:365.
Libero LE, Reid MA, White DM, Salibi N, Lahti AC, Kana RK. Biochemistry of the cingulate cortex in autism: An MR spectroscopy study. Autism Res. 2016;9:643–57.
Cochran DM, Sikoglu EM, Hodge SM, Edden RA, Foley A, Kennedy DN, et al. Relationship among Glutamine, gamma-Aminobutyric Acid, and Social Cognition in Autism Spectrum Disorders. J Child Adolesc Psychopharmacol. 2015;25:314–22.
Brown MS, Singel D, Hepburn S, Rojas DC. Increased glutamate concentration in the auditory cortex of persons with autism and first-degree relatives: a (1)H-MRS study. Autism Res. 2013;6:1–10.
Port RG, Gaetz W, Bloy L, Wang DJ, Blaskey L, Kuschner ES, et al. Exploring the relationship between cortical GABA concentrations, auditory gamma-band responses and development in ASD: Evidence for an altered maturational trajectory in ASD. Autism Res. 2017;10:593–607.
Endres D, Tebartz van Elst L, Meyer SA, Feige B, Nickel K, Bubl A, et al. Glutathione metabolism in the prefrontal brain of adults with high-functioning autism spectrum disorder: an MRS study. Mol Autism. 2017;8:10.
Drenthen GS, Barendse EM, Aldenkamp AP, van Veenendaal TM, Puts NA, Edden RA, et al. Altered neurotransmitter metabolism in adolescents with high-functioning autism. Psychiatry Res Neuroimaging. 2016;256:44–49.
Puts NAJ, Wodka EL, Harris AD, Crocetti D, Tommerdahl M, Mostofsky SH, et al. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Res. 2017;10:608–19.
Horder J, Lavender T, Mendez MA, O’Gorman R, Daly E, Craig MC, et al. Reduced subcortical glutamate/glutamine in adults with autism spectrum disorders: a [(1)H]MRS study. Transl Psychiatry. 2013;3:e279.
Joshi G, Biederman J, Wozniak J, Goldin RL, Crowley D, Furtak S, et al. Magnetic resonance spectroscopy study of the glutamatergic system in adolescent males with high-functioning autistic disorder: a pilot study at 4T. Eur Arch Psychiatry Clin Neurosci. 2013;263:379–84.
Libero LE, DeRamus TP, Lahti AC, Deshpande G, Kana RK. Multimodal neuroimaging based classification of autism spectrum disorder using anatomical, neurochemical, and white matter correlates. Cortex. 2015;66:46–59.
Corrigan NM, Shaw DW, Estes AM, Richards TL, Munson J, Friedman SD, et al. Atypical developmental patterns of brain chemistry in children with autism spectrum disorder. JAMA Psychiatry. 2013;70:964–74.
Gaetz W, Bloy L, Wang DJ, Port RG, Blaskey L, Levy SE, et al. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 2014;86:1–9.
Aoki Y, Abe O, Yahata N, Kuwabara H, Natsubori T, Iwashiro N, et al. Absence of age-related prefrontal NAA change in adults with autism spectrum disorders. Transl Psychiatry. 2012;2:e178.
Hardan AY, Fung LK, Frazier T, Berquist SW, Minshew NJ, Keshavan MS, et al. A proton spectroscopy study of white matter in children with autism. Prog Neuropsychopharmacol Biol Psychiatry. 2016;66:48–53.
Bejjani A, O’Neill J, Kim JA, Frew AJ, Yee VW, Ly R, et al. Elevated glutamatergic compounds in pregenual anterior cingulate in pediatric autism spectrum disorder demonstrated by 1H MRS and 1H MRSI. PLoS One. 2012;7:e38786.
Kubas B, Kulak W, Sobaniec W, Tarasow E, Lebkowska U, Walecki J. Metabolite alterations in autistic children: A 1H MR spectroscopy study. Adv Med Sci. 2012;57:152–6.
Say GN, Sahin B, Aslan K, Akbas S, Lncesu L, Ceyhan M. Increased Laterality of the Thalamus in Children and Adolescents with Asperger’s Disorder: An MRI and Proton Spectroscopy Study. Psychiatry Investig. 2014;11:237–42.
Hegarty JP 2nd, Gu M, Spielman DM, Cleveland SC, Hallmayer JF, Lazzeroni LC, et al. A proton MR spectroscopy study of the thalamus in twins with autism spectrum disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:153–60.
Bernardi S, Anagnostou E, Shen J, Kolevzon A, Buxbaum JD, Hollander E, et al. In vivo 1H-magnetic resonance spectroscopy study of the attentional networks in autism. Brain Res. 2011;1380:198–205.
Suzuki K, Nishimura K, Sugihara G, Nakamura K, Tsuchiya KJ, Matsumoto K, et al. Metabolite alterations in the hippocampus of high-functioning adult subjects with autism. Int J Neuropsychopharmacol. 2010;13:529–34.
Fujii E, Mori K, Miyazaki M, Hashimoto T, Harada M, Kagami S. Function of the frontal lobe in autistic individuals: a proton magnetic resonance spectroscopic study. J Med Invest. 2010;57:35–44.
Harada M, Taki MM, Nose A, Kubo H, Mori K, Nishitani H, et al. Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 tesla instrument. J Autism Dev Disord. 2011;41:447–54.
Kleinhans NM, Richards T, Weaver KE, Liang O, Dawson G, Aylward E. Brief report: biochemical correlates of clinical impairment in high functioning autism and Asperger’s disorder. J Autism Dev Disord. 2009;39:1079–86.
Hardan AY, Minshew NJ, Melhem NM, Srihari S, Jo B, Bansal R, et al. An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Res. 2008;163:97–105.
DeVito TJ, Drost DJ, Neufeld RW, Rajakumar N, Pavlosky W, Williamson P, et al. Evidence for cortical dysfunction in autism: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 2007;61:465–73.
Vasconcelos MM, Brito AR, Domingues RC, da Cruz LC,Jr., Gasparetto EL, Werner J,Jr. et al. Proton magnetic resonance spectroscopy in school-aged autistic children. J Neuroimaging. 2008;18:288–95.
Kleinhans NM, Schweinsburg BC, Cohen DN, Muller RA, Courchesne E. N-acetyl aspartate in autism spectrum disorders: regional effects and relationship to fMRI activation. Brain Res. 2007;1162:85–97.
Endo T, Shioiri T, Kitamura H, Kimura T, Endo S, Masuzawa N, et al. Altered chemical metabolites in the amygdala-hippocampus region contribute to autistic symptoms of autism spectrum disorders. Biol Psychiatry. 2007;62:1030–7.
Page LA, Daly E, Schmitz N, Simmons A, Toal F, Deeley Q, et al. In vivo 1H-magnetic resonance spectroscopy study of amygdala-hippocampal and parietal regions in autism. Am J Psychiatry. 2006;163:2189–92.
Friedman SD, Shaw DW, Artru AA, Dawson G, Petropoulos H, Dager SR. Gray and white matter brain chemistry in young children with autism. Arch Gen Psychiatry. 2006;63:786–94.
Fayed N, Modrego PJ. Comparative study of cerebral white matter in autism and attention-deficit/hyperactivity disorder by means of magnetic resonance spectroscopy. Acad Radio. 2005;12:566–9.
Hisaoka S, Harada M, Nishitani H, Mori K. Regional magnetic resonance spectroscopy of the brain in autistic individuals. Neuroradiology. 2001;43:496–8.
Otsuka H, Harada M, Mori K, Hisaoka S, Nishitani H. Brain metabolites in the hippocampus-amygdala region and cerebellum in autism: an 1H-MR spectroscopy study. Neuroradiology. 1999;41:517–9.
Hashimoto T, Tayama M, Miyazaki M, Yoneda Y, Yoshimoto T, Harada M, et al. Differences in brain metabolites between patients with autism and mental retardation as detected by in vivo localized proton magnetic resonance spectroscopy. J Child Neurol. 1997;12:91–96.
Naaijen J, Zwiers MP, Amiri H, Williams SC, Durston S, Oranje B, et al. Fronto-striatal glutamate in autism spectrum disorder and obsessive compulsive disorder. Neuropsychopharmacology. 2017;42:2466–7.
Hashimoto T, Kawano N, Fukuda K, Endo S, Mori K, Yoneda Y, et al. Proton magnetic resonance spectroscopy of the brain in three cases of Rett syndrome: Comparison with autism and normal controls. Acta Neurol Scand. 1998;98:8–14.
Levitt JG, O’Neill J, Blanton RE, Smalley S, Fadale D, McCracken JT, et al. Proton magnetic resonance spectroscopic imaging of the brain in childhood autism. Biol Psychiatry. 2003;54:1355–66.
O’Brien FM, Page L, O’Gorman RL, Bolton P, Sharma A, Baird G, et al. Maturation of limbic regions in Asperger syndrome: A preliminary study using proton magnetic resonance spectroscopy and structural magnetic resonance imaging. Psychiatry Res. 2010;184:77–85.
Oner O, Devrimci-Ozguven H, Oktem F, Yagmurlu B, Baskak B, Munir KM. Proton MR spectroscopy: Higher right anterior cingulate N-acetylaspartate/choline ratio in Asperger syndrome compared with healthy controls. AJNR Am J Neuroradiol. 2007;28:1494–8.
Gabis L, Wei H, Azizian A, DeVincent C, Tudorica A, Kesner-Baruch Y, et al. 1H-magnetic resonance spectroscopy markers of cognitive and language ability in clinical subtypes of autism spectrum disorders. J Child Neurol. 2008;23:766–74.
Margari L, De Giacomo A, Craig F, Palumbi R, Peschechera A, Margari M, et al. Frontal lobe metabolic alterations in autism spectrum disorder: a (1)H-magnetic resonance spectroscopy study. Neuropsychiatr Dis Treat. 2018;14:1871–6.
Kolodny T, Schallmo MP, Gerdts J, Edden RAE, Bernier RA, Murray SO. Concentrations of Cortical GABA and Glutamate in young adults with autism spectrum disorder. Autism Res. 2020;13:1111–29.
Jimenez-Espinoza C, Marcano Serrano F, Gonzalez-Mora JL. N-Acetylaspartyl-Glutamate metabolism in the cingulated cortices as a biomarker of the etiology in ASD: A (1)H-MRS Model. Molecules. 2021;26:675.
Wood ET, Cummings KK, Jung J, Patterson G, Okada N, Guo J, et al. Sensory over-responsivity is related to GABAergic inhibition in thalamocortical circuits. Transl Psychiatry. 2021;11:39.
Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, et al. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry. 2019;24:952–64.
Iwata Y, Nakajima S, Plitman E, Mihashi Y, Caravaggio F, Chung JK, et al. Neurometabolite levels in antipsychotic-naive/free patients with schizophrenia: A systematic review and meta-analysis of (1)H-MRS studies. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:340–52.
Zheng Z, Zheng P, Zou X. Association between schizophrenia and autism spectrum disorder: A systematic review and meta-analysis. Autism Res. 2018;11:1110–9.
Psomiades M, Mondino M, Fonteneau C, Bation R, Haesebaert F, Suaud-Chagny MF, et al. N-Acetyl-Aspartate in the dorsolateral prefrontal cortex in men with schizophrenia and auditory verbal hallucinations: A 1.5 T Magnetic Resonance Spectroscopy Study. Sci Rep. 2018;8:4133.
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81.
Menassa DA, Sloan C, Chance SA. Primary olfactory cortex in autism and epilepsy: Increased glial cells in autism. Brain Pathol. 2017;27:437–48.
Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–51.
Tsuchiya KJ, Hashimoto K, Iwata Y, Tsujii M, Sekine Y, Sugihara G, et al. Decreased serum levels of platelet-endothelial adhesion molecule (PECAM-1) in subjects with high-functioning autism: a negative correlation with head circumference at birth. Biol Psychiatry. 2007;62:1056–8.
Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–30.
Ivashko-Pachima Y, Hadar A, Grigg I, Korenkova V, Kapitansky O, Karmon G, et al. Discovery of autism/intellectual disability somatic mutations in Alzheimer’s brains: mutated ADNP cytoskeletal impairments and repair as a case study. Mol Psychiatry. 2021;26:1619–33.
Grigg I, Ivashko-Pachima Y, Hait TA, Korenkova V, Touloumi O, Lagoudaki R, et al. Tauopathy in the young autistic brain: novel biomarker and therapeutic target. Transl Psychiatry. 2020;10:228.
Hong T, Falcone C, Dufour B, Amina S, Castro RP, Regalado J, et al. GABAARalpha2 is decreased in the Axon initial segment of pyramidal cells in specific areas of the prefrontal cortex in autism. Neuroscience. 2020;437:76–86.
Schur RR, Draisma LW, Wijnen JP, Boks MP, Koevoets MG, Joels M, et al. Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) H-MRS studies. Hum Brain Mapp. 2016;37:3337–52.
Collins AL, Ma D, Whitehead PL, Martin ER, Wright HH, Abramson RK, et al. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics. 2006;7:167–74.
Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002;52:805–10.
Fatemi SH, Reutiman TJ, Folsom TD, Rustan OG, Rooney RJ, Thuras PD. Downregulation of GABAA receptor protein subunits alpha6, beta2, delta, epsilon, gamma2, theta, and rho2 in superior frontal cortex of subjects with autism. J Autism Dev Disord. 2014;44:1833–45.
Oblak AL, Gibbs TT, Blatt GJ. Decreased GABA(B) receptors in the cingulate cortex and fusiform gyrus in autism. J Neurochem. 2010;114:1414–23.
Blatt GJ, Fatemi SH. Alterations in GABAergic biomarkers in the autism brain: research findings and clinical implications. Anat Rec (Hoboken). 2011;294:1646–52.
Sears SM, Hewett SJ. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp Biol Med (Maywood). 2021;246:1069–83.
Heinze K, Barron HC, Howes EK, Ramaswami M, Broome MR. Impaired inhibitory processing: a new therapeutic target for autism and psychosis? Br J Psychiatry. 2020;218:1–4.
Uzunova G, Hollander E, Shepherd J. The role of ionotropic glutamate receptors in childhood neurodevelopmental disorders: autism spectrum disorders and fragile x syndrome. Curr Neuropharmacol. 2014;12:71–98.
Zheng Z, Zhu T, Qu Y, Mu D. Blood glutamate levels in autism spectrum disorder: A systematic review and meta-analysis. PLoS One. 2016;11:e0158688.
Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD65 mRNA levels in select subpopulations of neurons in the cerebellar dentate nuclei in autism: an in situ hybridization study. Autism Res. 2009;2:50–59.
Fatemi SH. The hyperglutamatergic hypothesis of autism. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:912–3.
Myers SJ, Yuan H, Kang JQ, Tan FCK, Traynelis SF, Low CM. Distinct roles of GRIN2A and GRIN2B variants in neurological conditions. F1000 Res. 2019;8:F1000.
Tang W, Liu D, Traynelis SF, Yuan H. Positive allosteric modulators that target NMDA receptors rectify loss-of-function GRIN variants associated with neurological and neuropsychiatric disorders. Neuropharmacology. 2020;177:108247.
Aoki Y, Kasai K, Yamasue H. Age-related change in brain metabolite abnormalities in autism: a meta-analysis of proton magnetic resonance spectroscopy studies. Transl Psychiatry. 2012;2:e69.
Snyder J, Wilman A. Field strength dependence of PRESS timings for simultaneous detection of glutamate and glutamine from 1.5 to 7T. J Magn Reson. 2010;203:66–72.
Edden RA, Pomper MG, Barker PB. In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla. Magn Reson Med. 2007;57:977–82.
Ding XQ, Maudsley AA, Schweiger U, Schmitz B, Lichtinghagen R, Bleich S, et al. Effects of a 72 h fasting on brain metabolism in healthy women studied in vivo with magnetic resonance spectroscopic imaging. J Cereb Blood Flow Metab. 2018;38:469–78.
Mennecke A, Gossler A, Hammen T, Dorfler A, Stadlbauer A, Rosch J, et al. Physiological effects of cigarette smoking in the limbic system revealed by 3 tesla magnetic resonance spectroscopy. J Neural Transm (Vienna). 2014;121:1211–9.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82071676) and the High-Level Hospital Development Program for Foshan “Climbing” Project.
Author information
Authors and Affiliations
Contributions
YC conceived and designed this study; YD and MCY extracted the data; all authors analyzed and interpreted the data; and YD drafted the manuscript with critical revisions from YC, LC, and MCY.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Du, Y., Chen, L., Yan, MC. et al. Neurometabolite levels in the brains of patients with autism spectrum disorders: A meta-analysis of proton magnetic resonance spectroscopy studies (N = 1501). Mol Psychiatry 28, 3092–3103 (2023). https://doi.org/10.1038/s41380-023-02079-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02079-y
- Springer Nature Limited
This article is cited by
-
The diagnosis of ASD with MRI: a systematic review and meta-analysis
Translational Psychiatry (2024)
-
Identification of Immune Infiltration and Iron Metabolism–Related Subgroups in Autism Spectrum Disorder
Journal of Molecular Neuroscience (2024)
-
Transcriptome Analysis and Epigenetics Regulation in the Hippocampus and the Prefrontal Cortex of VPA-Induced Rat Model
Molecular Neurobiology (2024)
-
Facial emotion recognition function and white matter microstructural alterations in drug-naive, comorbidity-free autism
European Archives of Psychiatry and Clinical Neuroscience (2024)