Abstract
Amino acids related to neurotransmitters and the GABAergic/glutamatergic system were measured using a 3 T-MRI instrument in 12 patients with autism and 10 normal controls. All measurements were performed in the frontal lobe (FL) and lenticular nuclei (LN) using a conventional sequence for n-acetyl aspartate (NAA) and glutamate (Glu), and the MEGA-editing method for GABA. The GABA level and [GABA]/[NAA] ratio were significantly lower (p < 0.01) in the FL, but not the LN, in patients with autism compared to normal controls. The [GABA]/[Glu] ratio in the FL was also significantly lower (p < 0.05) in the patients than in the normal controls, thus suggesting a possible abnormality in the regulation between GABA and Glu.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism is a pervasive developmental disorder characterized by impaired social interaction, deficits in verbal and nonverbal communication, and stereotyped interests and behaviors. It is now widely accepted that autism is a neurobiological disorder, although specific biological markers have not been discovered as yet. Neuroanatomically, autistic individuals demonstrate a loss of pyramidal neurons in the frontal cortex, limbic system abnormalities and a significant loss of Purkinje cells in the cerebellum (Bauman and Kemper 1985; Lotspeich and Ciaranello 1993). These brain areas have specialized responses to the principal excitatory transmitter substance glutamate (Glu) as well as to gamma-aminobutyric acid (GABA), which is responsible for most of the inhibitory communication in the brain. GABA is converted from glutamate by the rate-limiting enzyme glutamic acid decarboxylase (GAD). Fatemi et al. (2002) showed a reduction of the GAD 65- and 67-kDa proteins in autistic parietal and cerebellar cortices, and Aldred et al. (2003) and Shinohe et al. (2006) demonstrated the presence of a high level of glutamic acid in patients’ serum. Furthermore, Cook et al. (1998) detected an abnormality in the GABA receptor subunit gene on chromosome 15q11–q13 in autistic disorder. These findings lead us to hypothesize that the excessive glutamatergic activity relative to the inhibitory control by the GABAergic system causes deterioration in the quality of sensory information, as related to autism-spectrum neurodevelopmental disorder.
Proton MR spectroscopy (MRS) allows for the non-invasive evaluation of various chemical metabolites, and the signals from amino acid neurotransmitters such as Glu and GABA are included in the proton MR spectrum, although they are overlapped by the high peaks derived from other major metabolites. Using a clinical MRI instrument at 1.5 T, it is not easy to differentiate the signals from amino acid neurotransmitters from those of the other major metabolites. In particular, the concentration of GABA is much lower than that of Glu, and the GABA peaks are hidden by large signals from the major metabolites. Because an increase in field strength causes not only elevation of the signal-to-noise ratio but also an increase in the frequency resolution of a spectrum, a 3 T instrument is expected to improve differentiation of the complex peaks and quality of the data for the signal editing technique. To increase the reliability of the signals from GABA, the MEGA-editing J difference technique was applied to a 3 T instrument (Meshcer et al. 1998) and the obtained spectra were analyzed by the LCModel software package (Provencher 1993) using our original basis set for the MEGA-editing method. An examination of the test–retest reliability to measure the GABA level in the brain was conducted ahead of this clinical study and the reproducibility was considered to be acceptable as a clinical investigation, showing an intraclass correlation coefficient of 0.72 (Harada et al. in press).
Because GABA concentration has been demonstrated to show a large difference between gray and white matter (Petroff et al. 1988), we conducted segmentation of the cerebrospinal fluid and gray and white matter regions, and also calculated the volume ratio of each segment in a measurement location.
The aims of this study were to measure the amino acids associated with neurotransmitters in patients with autistic spectrum disorder using a clinical 3 T instrument and to examine the possibility that GABAergic/glutamatergic system regulation might afford a method for evaluating autistic patients in the future.
Methods
Subjects
Subjects were 22 children, 12 of whom were diagnosed with autistic spectrum disorder (2–11 years of age; mean ± SD = 5.2 ± 3.0 years) and 10 who were recruited as normal control subjects (3–12 years of age; mean ± SD = 5.9 ± 3.2 years). All of the patients were determined by pediatricians with extensive clinical experience in autism to meet the DSM-IV criteria for a pervasive developmental disorder that showed six or more items from the categories of (a) qualitative impairment in social interaction, (b) qualitative impairments in communication and (c) restricted repetitive and stereotyped patterns of behavior, interests and activities. The normal controls were prospectively nominated subjects who complained of a non-specific temporal symptom such as headache or vertigo, but showed no neurological findings and were confirmed by experienced pediatricians to have no autistic traits. A routine MRI examination was conducted for all subjects to confirm the absence of organic disease, and the MRS measurement was conducted immediately afterwards. None of the nominated control subjects showed any abnormalities or symptoms during observation for more than one month and thus data from all control subjects were used as normal control data. Most of the subjects under 8 years of age (10 autistic patients, 9 normal controls) were sedated with triclofos sodium (Tricloryl, 0.2 mL/kg body weight) 20 min before the MR measurement, following the guidelines for monitoring and management of pediatric patients during and after sedation published by the American Academy of Pediatrics (Cote and Wilson 2006).
This study was approved by the Institutional Review Board (No. 207) and informed consent for additional MRS measurements and data analysis were obtained from all of the subjects and their parents, following the approved procedures.
MRS and MRI Measurements
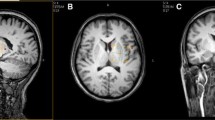
All measurements were performed using a clinical 3 T MRI device (Sigma 3 T HD, GE, Milwaukee, USA) and a standard volume birdcage head coil for the RF transmission and receiver. MEGA was incorporated into a spin echo sequence (PRESS) as described in a previous report by Meshcer et al. (1998). Briefly, two frequency selective pulses (180°) with 1.9 ppm frequency centered at the solvent resonance, which surround the broadband 180° pulse, and asymmetrically placed gradients were used to diphase spins within the bandwidth of the selective pulses. During the odd-numbered acquisitions, the GABA resonance at 1.9 ppm was flipped 180° by the double-banded pulses to refocus the J evolution at 3.02 ppm. During the even-numbered acquisitions, the J evolution of GABA resonance at 3.02 ppm was not refocused. Phase correction was conducted for each spectrum. Therefore, the difference of the acquired spectra provided an edited spectrum of GABA (3.02 ppm). The MEGA-PRESS editing sequences with repetition time (TR) = 2.5 s, echo time (TE) = 68 ms and sum of signals = 256 were acquired from a voxel (3 × 3 × 3 cm3) located in the lenticular nuclei (LN) and left frontal lobe (FL) as shown in Fig. 1. An axial image showing the genu of the corpus callosum and the anterior horn and atrium of the lateral ventricles was chosen as a reference slice for the LN, and the voxel was placed between the left Sylvian fissure and the lateral ventricles to encompass the left lenticular nuclei. For the reference slice of the voxel in the FL, an axial image approximately 1 cm above the upper end of the body of the lateral ventricles was selected, and the center of a voxel was positioned in the left centrum semiovale between the cingulate gyrus and middle or inferior frontal gyrus, and the posterior margin of a voxel was placed ahead of the central sulcus. Gradient map shimming was conducted in the measurement location by the high-order shim program, and the suppression of water was achieved at a level of <1% of the unsuppressed water signal.
Conventional proton MR spectra were obtained in the same measurement locations using the STEAM (Stimulated Echo Acquisition Mode) sequence with parameters of TR = 5 s, TE = 15 ms, sum of signals = 48 and a voxel size = 3 × 3 × 3 cm3 to diminish the influence of the longitudinal and transverse relaxation (Harada et al. 1999).
The segmentation of gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) within the voxel was carried out on 3D-SPGR images with TR = 10 ms, TE = 4.2 ms, slice thickness = 0.8 mm, matrix = 256 × 256, field of view = 24 × 24 cm and flip angle = 15° based on the intensity of each voxel, using imaging analysis software (Medx Ver. 3.4.2, Sensor Systems, USA).
Post-processing of MRS Data
For a single MEGA-PRESS measurement of each cerebral location, three spectra were obtained: a subtracted spectrum (Fig. 2a), a J evolution refocused spectrum (Fig. 2b) and a non-refocused conventional spectrum (Fig. 2c).
The post-processing of each spectrum was conducted using LCModel (Ver. 6.2). As preparation for a basis set for the LCModel, the in vitro spectrum of the following chemicals were obtained under the same conditions as for those in the human study: GABA, glutamine (Gln), glutamate (Glu), n-acetyl aspartate (NAA), n-acetylaspartylglutamate (NAAG), creatine (Cr), phosphocreatine (PCr), myo-inositol (mIns), glycerophophocholine (GPC), phosphorylcholine (PCh), glycine (Gly), taurine (Tau), glucose (Glc) and lactate (Lac). The in vitro spectra of GABA, Gln, Glu and NAA and the default data of MM around 0.9, 1.2, 1.4, 1.7 and 2.0 ppm in the LCModel package were incorporated into a basis set for J-difference editing spectra measured by MEGA-PRESS. Quantitation of the in vivo metabolite signals was based on the unsuppressed water signal obtained from the same voxel assuming a water content of 82% (Harada et al. 1999). Based on the findings of previous studies (Terpstra et al. 2003; Oz et al. 2006), the T2 relaxation effects were assumed to have a negligible effect on quantitation. The criteria for maintaining reliable metabolite concentrations were based on the %SD of the fit for each metabolite reflecting the Cramer-Rao lower bounds (CRLB). All of the GABA spectra showed %SD < 20% and were included in the analysis, and the curve fitting of the individual contribution of GABA was checked by visual estimation whether it was a suitable shape for the peak of GABA at 3.02 ppm as shown in Fig. 3.
For post-processing of the conventional STEAM spectrum with long TR and short TE, our original basis set was made using the in vitro spectra of all of the above metabolites measured using the same measurement parameter as for the STEAM sequence. The quantitation of each metabolite of NAA, Cr, Cho, mIns and Glu separated from Gln was conducted by the LCModel based on the unsuppressed water signal in the same manner as for MEGA-PRESS. The %SD of Glu was <15% for all of the obtained spectra and the curve fitting of the individual contribution of Glu was confirmed as shown in Fig. 4.
It is known that the quantified values of NAA from conventional proton MRS reflect the neuronal density (Baslow 2003). Using these values as a standard for normalization, the GABAergic or glutamatergic index per neuronal density ([GABA]/[NAA], [Glu]/[NAA]) = [quantitation value of GABA by MEGA-PRESS or Glu by STEAM]/[quantitation value of NAA by STEAM]. Furthermore, the index of the regulation between GABA and Glu was calculated as follows: the inhibitory control index ([GABA]/[Glu]) = [quantitation value of GABA by MEGA-PRESS]/[quantitation value of Glu by STEAM].
Statistical differences between the autistic patients and normal controls were evaluated by both an analysis of variance (ANOVA) and the Wilcoxon signed-rank test. The SPSS software package (Ver. 12) was used for all analyses. A power analysis was conducted to examine the lack of effect using GraphPad StatMate (Ver. 2.00).
Results
The result of the segmented volume ratio in measurement voxels is shown in Table 1. In both the FL and the LN, the volume ratio did not differ between normal volunteers and autistic patients.
The quantified values of NAA, Cr, Cho, mIns and Glu measured by the conventional STEAM sequence and of GABA measured by MEGA-PRESS are given in Table 2.
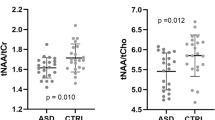
No statistically significant difference was found for [NAA], [Cr], [Cho], [mIns] or [Glu] in either the FL or LN between the autistic patients and the normal controls. The statistical power of these comparisons was calculated with a significance level of 0.05 (two-tailed), and powers <20% to detect a difference were found. The quantified value of GABA in the FL showed a statistical difference (p < 0.01) between the patients and the normal controls, but there was no remarkable difference in the value in the LN (p = 0.86). Figure 5 shows the calculated [Glu]/[NAA] index at the FL and LN for the patients and normal controls, and due to the small difference and large variation, no statistically significant difference was found for either the FL or LN.
The two GABAergic indices, the [GABA]/[NAA] and [GABA]/[Glu], in the FL and in the LN are shown in Figs. 6 and 7, respectively. The GABA level and [GABA]/[NAA] ratio were significantly lower (p < 0.01) in the FL, but not the LN, in patients with autism compared to normal controls.
Discussion
After Cook et al. (1998) found an abnormality in the GABA receptor subunit gene in autistic disorder, an epigenetic overlap and a gene–gene interaction of the GABA receptor subunit genes in autism were reported by Samaco et al. (2004) and Ma et al. (2005). Recently, the downregulation or alteration of both the GABAA and GABAB receptors in subjects with autism was reported (Fatemi et al. 2008, 2009), and an autoradiographic study using the [3H]flunitrazepam-labeled benzodiazepine site also suggested there is an alteration in the modulation of the GABAA receptors in the autistic brain (Guptill et al. 2007). This accumulating evidence of GABA receptor abnormality indicates that the GABAergic system in the autistic brain is affected. However, research on GABA concentrations in the brain in autism seems to be limited, although there are several reports that glutamic acid decarboylase (Fatemi et al. 2002) is reduced in the brain cortex in this disease. Our study demonstrated a low level of GABA in the FL in patients with autism, which seems to be consistent with the results of the study on the reduction of GABAA and GABAB receptors by Fatemi et al. (2008, 2009). Our results, which showed a decrease in the [GABA]/[Glu] ratio, are also highly supportive of the hypothesis that the GABAergic system is suppressed, resulting in excessive stimulation of the glutamate system (Lam et al. 2006). It is therefore speculated that the dysregulation of the GABAergic/glutamatergic system causes excessive glutamatergic hyperfunction which influences neuronal growth and connectivity during critical periods of development.
The observation of metabolites in patients with autism using proton MRS has thus far been studied by our group (Otsuka et al. 1999; Hisaoka et al. 2001) and by Levitt et al. (2003) and Friedman et al. (2003). The main findings of these studies were a reduction of NAA in the temporal gray matter, cingulated gyrus, frontal and parietal lobe, hippocampus-amydala complex and cerebellum. A recent study using spectroscopic imaging by Devito et al. (2007) that showed lower levels of NAA in widespread gray matter regions also supports the general results of previous MRS studies. However, Murphy et al. (2002) found an elevation in the level of prefrontal NAA in patients with Asperger syndrome. This contradictory result may reflect the heterogeneity of autistic-spectrum disorder. In our study, the NAA level in the FL and LN did not differ between the patients and normal controls. This may result from the large measurement location in our study including a large volume of white matter, making any such difference in the cerebral cortex obscure.
Two studies of proton MRS concerned with Glu+Gln (Glx) have been reported by Page et al. (2006) and Devito et al. (2007), but their results were inconsistent. The former group showed a higher concentration of Glx in the amygdala-hippocampal region but not in the parietal region, whereas the latter group demonstrated a lower level of Glx in patients’ gray matter than that in control subjects. The NAA level observed by Devito et al. (2007) was significantly decreased in the cerebral cortex, but Page et al. (2006) found no difference in the NAA level across brain regions. The different extent of the neuronal disorder may be the reason for the contradictory results obtained. Our results concerning Glu were very similar to those described by Page et al. (2006) and no large difference in Glu was observed in the autistic patients. These results suggest that the difference in Glu level relative to the extent of neuronal loss was not significant, and does not therefore play a major role in the neurodevelopmental disorder seen with autism.
The GABA measurements obtained in this study are consistent with those obtained in previous neurochemical studies; however, we recognize several limitations concerning the methodology of our study. The effect of the use of triclofos sodium for sedation on the metabolism of GABA and Glu was unclear. There are several reports that vigabatrin increases the brain GABA level by inhibiting GABA transaminase (Petroff et al. 1995, 1996; Weber et al. 1999; Novotny et al. 1999) although triclofos sodium does not change the activity of this enzyme. Aasly et al. (1999) reported that the GABA content did not appear to be correlated with antiepileptic therapy. In our study, the issue of sedation to neurochemicals detected by proton MRS remains an issue for future investigation.
Furthermore, the age range of the subjects in our study was large, from 2 to 12 years of age. Besides the significant changes in metabolites seen during the first year of life, age-dependent changes in NAA during infancy and childhood were observed in gray matter, the cerebellum and thalamus (Pouwels et al. 1999). However, it was reported that none of the investigated brain regions showed developmental changes in relation to Glu, and the concentration of GABA was highest in the basal ganglia, cerebellum and gray matter where its level remains constant during development (Pouwels et al. 1999). Pouwels et al. 1999 also found that no developmental changes occur for Cr after 1 year of age. Because their study was conducted using a 2.0 T instrument and the sequence was different from that used in our study, further study is needed to determine whether or not the Glu and GABA levels in children alter during development.
The T2 relaxation effects were assumed to have a negligible influence on quantitation in this study, but there remains the possibility of a difference in T2 relaxation between individuals with autism and normal controls. However, although Friedman et al. (2006) reported that T2 relaxation of Cho was prolonged in the gray matter of autistic patients compared to normal controls, they did not show significant a difference in NAA and Cr between the two groups. The difference in the relaxation effects for GABA and Glu between the patients and normal subjects was not evaluated in our study, and thus whether the relaxation effects are changed in autistic patients remains an issue for future study.
Another problem concerns the editing technique, specifically the coediting of the MM coherences and its contamination at the 3.0 ppm peak of GABA. Terpstra et al. (2002, 2003) showed the proportion of MM contamination to the total edited signal for GABA to only be 15% and that the post-processing using LCModel could diminish the MM contamination. However, the extent of MM contamination was not clear in our study. Finally, the difficulty in GABA measurement in relation to tissue separation (gray and white matter) due to the large measurement voxel should be noted. Although there have been several MRS studies of GABA measurement which involved tissue separation (Petroff et al. 1988; Jensen et al. 2005; Choi et al. 2006, 2007), the ratio between the gray and white matter showed a large variation, from 1.8 to 8.1. Because this variation may depend on the method used or the threshold for tissue segmentation, the reliability of the tissue separation in GABA measurement remains to be clarified.
In summary, the main result of this study was the decreased GABA level in the FL of autistic patients, which supports the findings of an abnormality in GABAergic/glutamatergic regulation as reported in neurochemical studies. Thus, it is suggested that MEGA-PRESS might be a useful method for evaluating the neurodevelopment disorders associated with autistic-spectrum disorder.
References
Aasly, J., Silfvenius, H., Aas, T. C., Sonnewald, U., Olivecrona, M., Juul, R., et al. (1999). Proton magnetic resonance spectroscopy of brain biopsies from patients with intractable epilepsy. Epilepsy Research, 35, 211–217.
Aldred, S., Moore, K. M., Fitzgerald, M., & Waring, R. H. (2003). Plasma amino acid levels in children with autism and their families. Journal of Autism and Developmental Disorders, 33, 449–454.
Baslow, M. H. (2003). N-acetylaspartate in the vertebrate brain: Metabolism and function. Neurochemical Research, 28, 941–953.
Bauman, M., & Kemper, T. L. (1985). Histoanatomic observations of the brain in early infantile autism. Neurology, 35, 866–874.
Choi, C., Bhardwaj, P. P., Kalra, S., Casault, C. A., Yasmin, U. S., Allen, P. S., et al. (2007). Measurement of GABA and contaminants in gray and white matter in human brain in vivo. Magnetic Resonance in Medicine, 58, 27–33.
Choi, I.-Y., Lee, S.-P., Merkle, H., & Shen, J. (2006). In vivo detection of gray and white matter differences in GABA concentration in the human brain. NeuroImage, 33, 85–93.
Cook, E. H., Courchesne, R. Y., Cox, N. J., Lord, C., Gonen, D., Guter, S. J., et al. (1998). Linkage-disequilibrium mapping of autistic disorder, with 15q11–13 Markers. American Journal of Human Genetics, 62, 1077–1083.
Cote, C. J., & Wilson, S. (2006). Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: An update. Pediatrics, 118, 2587–2602.
Devito, T. J., Drost, D. J., Neufeld, R. W., Rajakumar, N., Pavlosky, W., Williamson, P., et al. (2007). Evidence for cortical dysfunction in autism: A proton magnetic resonance spectroscopic imaging study. Biological Psychiatry, 61, 465–473.
Fatemi, S. H., Folsom, T. D., Reutiman, T. J., Thuras, P. D. (2008). Expression of GABAB receptors is altered in brains of subjects with autism. Cerebellum Nov 12 (Epub ahead of print).
Fatemi, S. H., Halt, A. R., Stary, J. M., Kanodia, R., Schulz, S. C., & Realmuto, G. R. (2002). Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biological Psychiatry, 52, 805–810.
Fatemi, S. H., Reutiman, T. J., Folsom, T. D., & Thuras, P. D. (2009). GABAA receptor downregulation in brains of subjects with autism. Journal of Autism and Developmental Disorders, 39, 223–230.
Friedman, S. D., Shaw, D. W. W., Artru, A. A., Dawson, G., Petropoulos, H., & Dager, S. R. (2006). Gray and white matter brain chemistry in young children with autism. Archives of General Psychiatry, 63, 786–794.
Friedman, S. D., Shaw, D. W., Artru, A. A., Richards, T. L., Gardner, J., Dawson, G., et al. (2003). Regional brain chemical alterations in young children with autism spectrum disorder. Neurology, 60, 100–107.
Guptill, J. T., Booker, A. B., Gibbs, T. T., Kemper, T. L., Bauman, M. L., & Blatt, G. J. (2007). [3H]-FLunitrazepam-labeled benzodiazepine binding sites in the hippocampal formation in autism: A multiple concentration autoradiographic study. Journal of Autism and Developmental Disorders, 37, 911–920.
Harada, M., Kubo, H., Nose, A., Nishitani, H., Matsuda, T. (in press) Measurement of variation in the human cerebral GABA level by in vivo MEGA-editing proton MR spectroscopy using a clinical 3 Tesla instrument and its dependence on brain region and the female menstrual cycle. Hum Brain Mapp.
Harada, M., Miyoshi, H., Uno, M., Okada, T., Hisaoka, S., Hori, A., et al. (1999). Neuronal impairment of adult moyamoya disease detected by quantified proton MRS and comparison with cerebral perfusion by SPECT with Tc-99m HM-PAO: A trial of clinical quantification of metabolites. Journal of Magnetic Resonance Imaging, 10, 124–129.
Hisaoka, S., Harada, M., Nishitani, H., & Mori, K. (2001). Regional magnetic resonance spectroscopy of the brain in autistic individuals. Neuroradiology, 43, 496–498.
Jensen, J. E., de Frederick, B., & Renshaw, P. F. (2005). Gray and white matter GABA level difference in the human brain using two-dimensional J-resolved spectroscopic imaging. NMR Biomed, 18, 570–576.
Lam, K. S. L., Aman, M. G., & Arnold, L. E. (2006). Neurochemical correlates of autistic disorder: A review of the literature. Research in Developmental Disabilities, 27, 254–289.
Levitt, J. G., O’Neill, J., McCracken, J. T., Guthrie, D., Toga, A. W., & Alger, J. R. (2003). Proton magnetic resonance spectroscopic imaging in childhood autism. Biological Psychiatry, 54, 1355–1366.
Lotspeich, L. J., & Ciaranello, R. D. (1993). The neurobiology and genetics of infantile autism. International Review of Neurobiology, 35, 87–129.
Ma, D. Q., Whitehead, P. L., Menold, M. M., etMartin, E. R., Ashley-Koch, A. E., Mei, H., et al. (2005). Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet, 77, 377–388.
Meshcer, M., Merkle, H., Kirsch, J., Garwood, M., & Gruetter, R. (1998). Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine, 11, 266–272.
Murphy, D. G., Critchley, H. D., Schmitz, N., MacAlonan, G., van Amelsvoort, T., Robertson, D., et al. (2002). Asperger syndrome: A proton magnetic resonance study of brain. Archives of General Psychiatry, 59, 885–891.
Novotny, E. J., Jr., Hyder, F., Shevell, M., & Rothman, D. L. (1999). GABA changes with vigabatrin in the developing human brain. Epilepsia., 40, 462–466.
Otsuka, H., Harada, M., Mori, K., Hisaoka, S., & Nishitani, H. (1999). Brain metabolites in the hippocampus-amygdala region and cerebellum in autism: An 1H-MR spectroscopy study. Neuroradiology, 41, 517–519.
Oz, G., Terpstra, M., Tkac, I., Aia, P., Lowary, J., Tuite, P. J., et al. (2006). Proton MRS of the unilateral substantia nigra in the human brain at 4 Tesla: Detection of high GABA concentrations. Magnetic Resonance in Medicine, 55, 296–301.
Page, L. A., Daly, E., Schmitz, N., Simmons, A., Toal, F., Deeley, Q., et al. (2006). In vivo 1H-magnetic resonance spectroscopy study of amygdale-hippocampal and parietal regions in autism. American Journal of Psychiatry, 163, 2189–2192.
Petroff, O. A. C., Behar, K. L., Mattson, R. H., & Rothman, D. L. (1996). Human brain γ-aminobutyric acid levels and seizure control following initiation of vigabatrin therapy. Journal of Neurochemistry, 67, 2399–2404.
Petroff, O. A. C., Ogino, T., & Alger, J. R. (1988). High-resolution proton magnetic resonance spectroscopy of rabbit brain: Regional metabolite levels and postmortem changes. Journal of Neurochemistry, 51, 163–171.
Petroff, O. A. C., Rothman, D. L., Behar, K. L., & Mattson, R. H. (1995). Initial observations on effect of vigabatrin on in vivo 1H spectroscopic measurements of γ-aminobutyric acid, glutamate, and glutamine in human brain. Epilepsia, 36, 457–464.
Pouwels, P. J. W., Brockmann, K., Kruse, B., Wilken, B., Wick, M., Hanefeld, F., et al. (1999). Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatric Research, 46, 474–485.
Provencher, S. E. (1993). Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine, 30, 672–679.
Samaco, R. C., Hogart, A., & Lasalle, J. M. (2004). Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Human Molecular Genetics, 14, 483–492.
Shinohe, A., Hashimoto, K., Nakamura Tsujii, M., Iwata, Y., Tsuchiya, K. J., Sekine, Y., et al. (2006). Increase serum levels of glutamate in adult patients with autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 30, 1472–1477.
Terpstra, M., Henry, P.-G., & Gruetter, R. (2003). Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magnetic Resonance in Medicine, 50, 19–23.
Terpstra, M., Ugurbil, K., & Gruetter, R. (2002). Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 Tesla. Magnetic Resonance in Medicine, 47, 1009–1012.
Weber, O. M., Verhagen, A., Duc, C. O., Meier, D., Leenders, K. L., & Boesiger, P. (1999). Effects of vigabatrin intake on brain GABA activity as monitored by spectrally edited magnetic resonance spectroscopy and positron emission tomography. Magnetic Resonance Imaging, 17, 417–425.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (B) No. 19390321 form the Japan Society for the Promotion of Science (JSPS) and a Grant from the Japanese Society of Neuroradiology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harada, M., Taki, M.M., Nose, A. et al. Non-Invasive Evaluation of the GABAergic/Glutamatergic System in Autistic Patients Observed by MEGA-Editing Proton MR Spectroscopy Using a Clinical 3 Tesla Instrument. J Autism Dev Disord 41, 447–454 (2011). https://doi.org/10.1007/s10803-010-1065-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-010-1065-0